Submitted:
22 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Fishery Production and Management
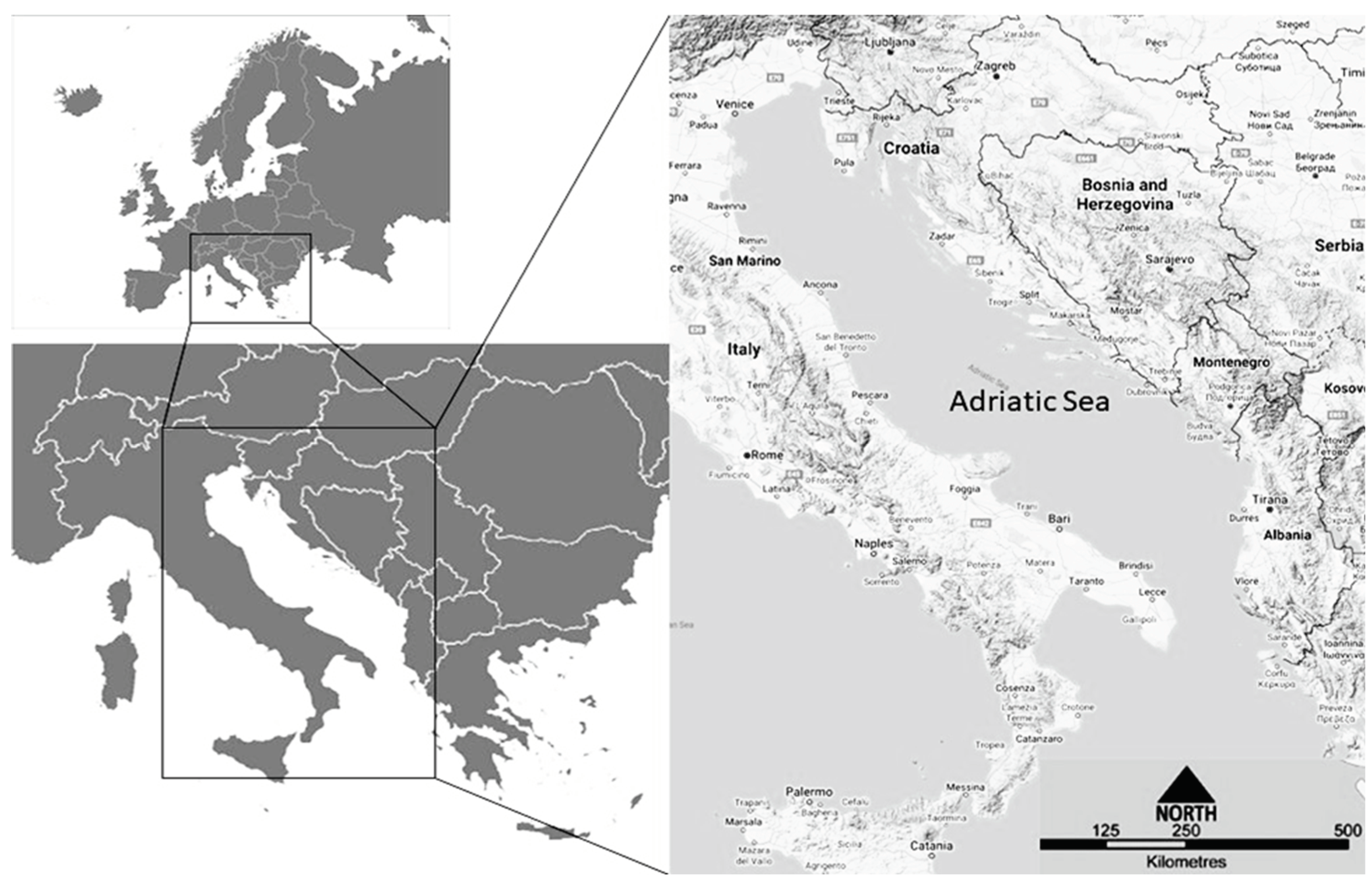
1.1. Adriatic Sea Specificities
1.2. Fishery Production
1.3. Fishery Management
1.4. Historical, Social and Political Context
2. Fishery Product Consumption and Potential Hazards
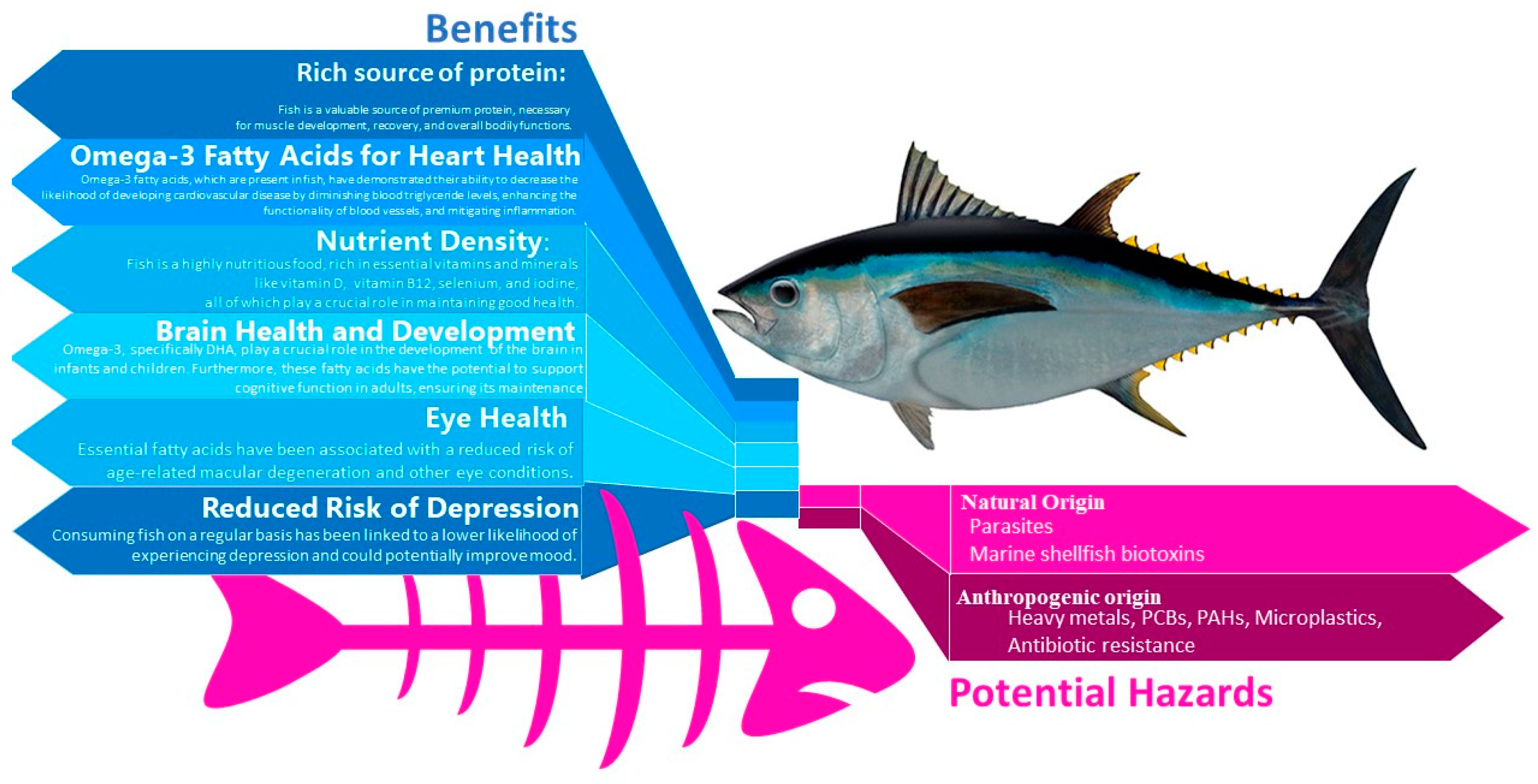
2.1. General Considerations
2.2. Heavy Metal Pollution in the Adriatic Sea
2.3. Contamination of Biota with POPs, OCPs PCBs
2.4. Contamination of Biota with PAHs
2.5. Antibiotic Resistance in Fish Farming
3. Effects of Climate Change on Fishery Products
4. Future Prospective and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dragičević, B.; Matić-Skoko, S.; Dulčić, J. Fish and Fisheries of The Eastern Adriatic Sea in The Light of Climate Change. Trends in Fisheries and Aquatic Animal Health, 2017,1-22.
- UNEP-MAP-RAC/SPA. Status and Conservation of Fisheries in the Adriatic Sea. 2014.
- Zore-Armanda, M.; Grbec, B.; Morović, M. Oceanographic properties of the Adriatic Sea-A point of view. Acta Adriat 1999; 40 (Suppl.): 39-54.
- Marini, M.; Campanelli, A. , Sanxhaku, M.; Kljajić, Z.; Grilli, F. Late spring characterization of different coastal areas of the Adriatic Sea. Acta Adriat 2015; 56(1): 27-46.
- Orlić, M.; Gačić, M.; La Violette, PE. The currents and circulation of the Adriatic Sea. Oceanol Acta 1992; 15(2): 109-24.
- Civitarese, G.; Gačić, M.; Lipizer, M.; Borzelli, G.L.E. On the impact of the Bimodal Oscillating System (BiOS) on the biogeochemistry and biology of the Adriatic and Ionian Seas (Eastern Mediterranean). Biogeosciences 2010, 7, 3987–3997. [Google Scholar] [CrossRef]
- Batistić, M.; Garić, R.; Molinero, J. Interannual variations in Adriatic Sea zooplankton mirror shifts in circulation regimes in the Ionian Sea. Clim. Res. 2014, 61, 231–240. [Google Scholar] [CrossRef]
- Buljan, M. Fluctuations of salinity in the Adriatic, Institut za Oceanografiju i Ribarstvo—Split (Croatia),Reports.1953; 2(2): 64.
- Pallaoro, A. On the possibilites of the occurrence of certain fish species in the Middle Adriatic connected with the Adriatic ingressions in years 1986/1987. Morsko Ribarstvo 1988; 3: 82-7. [In Croatian].
- The State of Mediterranean and Black Sea Fisheries 2023; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023.
- Carpi, P.; Scarcella, G.; Cardinale, M. The Saga of the Management of Fisheries in the Adriatic Sea: History, Flaws, Difficulties, and Successes toward the Application of the Common Fisheries Policy in the Mediterranean. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Mackelworth, P.; Holcer, D.; Jovanović, J.; Fortuna, C. Marine Conservation and Accession: The Future for the Croatian Adriatic. Environ. Manag. 2010, 47, 644–655. [Google Scholar] [CrossRef] [PubMed]
- AdriaMed. General Outline of Marine Capture Fisheries Legislation and Regulations in the Adriatic Sea Countries. FAO-MiPAF Scientific Cooperation to Support Responsible Fisheries in the Adriatic Sea. GCP/RER/010/ITA/TD14 (rev. 2). AdriaMed Technical Documents 2007, 14, 70.
- Colloca, F.; Garofalo, G.; Bitetto, I.; Facchini, M.T.; Grati, F.; Martiradonna, A.; Mastrantonio, G.; Nikolioudakis, N.; Ordinas, F.; Scarcella, G.; et al. The Seascape of Demersal Fish Nursery Areas in the North Mediterranean Sea, a First Step Towards the Implementation of Spatial Planning for Trawl Fisheries. PLOS ONE 2015, 10, e0119590. [Google Scholar] [CrossRef] [PubMed]
- EU. Facts and Figures on the Common Fisheries Policy. Publications Office of the European Union, 2016.
- Grbec, B.; Dulcic, J.; Morovic, M. Long-term changes in landings of small pelagic fish in the eastern Adriatic-possible influence of climate oscillations over the Northern Hemisphere. Clim. Res. 2002, 20, 241–252. [Google Scholar] [CrossRef]
- Allan, G. “Fish for feed vs fish for food,” in Fish, Aquaculture and Food Security: Sustaining Fish as Food Supply Conference. 2004, (Canberra, ACT).
- Muñoz-Benavent, P.; Andreu-García, G.; Martínez-Peiró, J.; Puig-Pons, V.; Morillo-Faro, A.; Ordóñez-Cebrián, P.; Atienza-Vanacloig, V.; Pérez-Arjona, I.; Espinosa, V.; Alemany, F. Automated Monitoring of Bluefin Tuna Growth in Cages Using a Cohort-Based Approach. Fishes 2024, 9, 46. [Google Scholar] [CrossRef]
- Mozaffarian, D. and Rimm, E.B.,. Fish intake, contaminants, and human health: evaluating the risks and the benefits. Jama, 2006, 296(15), 1885-1899.
- Innis, S.M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991, 30, 39–103. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Lees, M.J.; Carson, B.P. The Potential Role of Fish-Derived Protein Hydrolysates on Metabolic Health, Skeletal Muscle Mass and Function in Ageing. Nutrients 2020, 12, 2434. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nashimoto, M.; Okuda, Y.; Ota, T.; Yamamoto, M. Fish as a major source of vitamin D in the Japanese diet. Nutrition 2002, 18, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F. Vitamin B12 sources and bioavailability. Experimental biology and medicine, 2007, 232(10), pp.1266-1274.
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 Fatty Acids and Depression: Scientific Evidence and Biological Mechanisms. Oxidative Med. Cell. Longev. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Telli-Karakoç, F.; Tolun, L.; Henkelmann, B.; Klimm, C.; Okay, O.; Schramm, K.-W. Polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) distributions in the Bay of Marmara sea: İzmit Bay. Environ. Pollut. 2002, 119, 383–397. [Google Scholar] [CrossRef]
- Capodiferro, M.; Marco, E.; Grimalt, J.O. Wild fish and seafood species in the western Mediterranean Sea with low safe mercury concentrations. Environ. Pollut. 2022, 314, 120274. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Conti, G.O.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals in fish from the Mediterranean Sea: potential impact on diet. In The Mediterranean Diet. Academic Press. 2015, 547–562. [Google Scholar]
- Traven, L.; Furlan, N.; Cenov, A. Historical trends (1998–2012) of nickel (Ni), copper (Cu) and chromium (Cr) concentrations in marine sediments at four locations in the Northern Adriatic Sea. Mar. Pollut. Bull. 2015, 98, 289–294. [Google Scholar] [CrossRef]
- DI Leo, A.; Annicchiarico, C.; Cardellicchio, N.; Spada, L.; Giandomenico, S. Trace metal distributions in Posidonia oceanica and sediments from Taranto Gulf (Ionian Sea, Southern Italy). Mediterr. Mar. Sci. 2013, 14, 204. [Google Scholar] [CrossRef]
- Gallmetzer, I.; Haselmair, A.; Tomašových, A.; Stachowitsch, M.; Zuschin, M. Responses of molluscan communities to centuries of human impact in the northern Adriatic Sea. PLOS ONE 2017, 12, e0180820. [Google Scholar] [CrossRef] [PubMed]
- Joksimović, D.; Perošević, A.; Castelli, A.; Pestorić, B.; Šuković, D.; Đurović, D. Assessment of heavy metal pollution in surface sediments of the Montenegrin coast: a 10-year review. J. Soils Sediments 2019, 20, 2598–2607. [Google Scholar] [CrossRef]
- Igwe, C.O.; Saadi, A.A.; Ngene, S.E. Optimal options for treatment of produced water in offshore petroleum platforms. J Pollut Eff Cont, 2013, 1(2), pp.1-5.
- Spada, L.; Annicchiarico, C.; Cardellicchio, N.; Giandomenico, S.; DI Leo, A. Heavy metals monitoring in mussels Mytilus galloprovincialis from the Apulian coasts (Southern Italy). Mediterr. Mar. Sci. 2013, 14. [Google Scholar] [CrossRef]
- Bille, L.; Binato, G.; Cappa, V.; Toson, M.; Pozza, M.D.; Arcangeli, G.; Ricci, A.; Angeletti, R.; Piro, R. Lead, mercury and cadmium levels in edible marine molluscs and echinoderms from the Veneto Region (north-western Adriatic Sea—Italy). Food Control. 2015, 50, 362–370. [Google Scholar] [CrossRef]
- Bogdanović, T.; Ujević, I.; Sedak, M.; Listeš, E.; Šimat, V.; Petričević, S.; Poljak, V. As, Cd, Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem. 2014, 146, 197–203. [Google Scholar] [CrossRef]
- Tanaskovski, B.; Jović, M.; Mandić, M.; Pezo, L.; Degetto, S.; Stanković, S. Elemental analysis of mussels and possible health risks arising from their consumption as a food: The case of Boka Kotorska Bay, Adriatic Sea. Ecotoxicol. Environ. Saf. 2016, 130, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Varrà, M.O.; Husáková, L.; Patočka, J.; Ianieri, A.; Ghidini, S.; Zanardi, E. Cadmium, lead, and mercury in two commercial squid species from the north Adriatic Sea (central Mediterranean): contamination levels and health risk assessment. Ital. J. Food Saf. 2023, 12, 11037. [Google Scholar] [CrossRef] [PubMed]
- Rožič, P. .; Dolenec, T.; Baždarić, B.; Karamarko, V.; Kniewald, G.; Dolenec, M. Element levels in cultured and wild sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) from the Adriatic Sea and potential risk assessment. Environ. Geochem. Heal. 2013, 36, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Kucaj, E.; Abazi, U.; Abazi, E.Z. Bioaccumulation of heavy metals in Bass fish (Morone Saxatilis) at Rodoni Cape, in Adriatik sea, Albania. IOSR Journal of Engineering (IOSRJEN). 2015, 5(8), 28–31. [Google Scholar]
- Di Lena, G.; Casini, I.; Caproni, R.; Orban, E. Total mercury levels in crustacean species from Italian fishery. Food Addit. Contam. Part B 2018, 11, 175–182. [Google Scholar] [CrossRef]
- Antović, I.; Šuković, D.; Andjelić, S.; Svrkota, N. HEAVY METALS AND RADIONUCLIDES IN MUSCLES OF FISH SPECIES IN THE SOUTH ADRIATIC—MONTENEGRO. RAP Conference. LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE;
- Brkić, D.; Bošnir, J.; Bošković, A.G.; Miloš, S.; Šabarić, J.; Lasić, D.; Jurak, G.; Cvetković, B.; Racz, A.; Ćuić, A.M. Determination of heavy metals in different fish species sampled from markets in Croatia and possible health effects. Med. Jadertina, 2017, 47, 89–105. [Google Scholar]
- Grgec, A.S.; Kljaković-Gašpić, Z.; Orct, T.; Tičina, V.; Sekovanić, A.; Jurasović, J.; Piasek, M. Mercury and selenium in fish from the eastern part of the Adriatic Sea: A risk-benefit assessment in vulnerable population groups. Chemosphere 2020, 261, 127742. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Storelli, A.; Garofalo, R.; Mallamaci, R.; Storelli, M.M. Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health. Toxics 2022, 10, 223. [Google Scholar] [CrossRef]
- Bettoso, N.; Pittaluga, F.; Predonzani, S.; Zanello, A.; Acquavita, A. Mercury Levels in Sediment, Water and Selected Organisms Collected in a Coastal Contaminated Environment: The Marano and Grado Lagoon (Northern Adriatic Sea, Italy). Appl. Sci. 2023, 13, 3064. [Google Scholar] [CrossRef]
- Walker, C.H.; Hopkin, S.P.; Sibly, R.M.; Peakall, D.B. Principles of Ecotoxicology. Taylor & Francis, London, UK, 1996.
- Klinčić, D.; Romanić, S.H.; Katalinić, M.; Zandona, A.; Čadež, T.; Sarić, M.M.; Šarić, T.; Aćimov, D. Persistent organic pollutants in tissues of farmed tuna from the Adriatic Sea. Mar. Pollut. Bull. 2020, 158, 111413. [Google Scholar] [CrossRef] [PubMed]
- Krivokapić, M. Assessment of PCBs and OCPs in anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) from theAdriatic Sea, Bay of Herceg Novi (alongside Kumbor Marine Channel). Acta Adriat. 2020, 61, 27–38. [Google Scholar] [CrossRef]
- Nuro, A.; Murtaj, B. , October. Levels of Some Priority Substances on Adriatic Sea, Albania. In Fourth International Scientific Conference on Recent Advances in Information Technology, Tourism, Economics, Management and Agriculture 2020, 277.
- Capriotti, M.; Cocci, P.; Bracchetti, L.; Cottone, E.; Scandiffio, R.; Caprioli, G.; Sagratini, G.; Mosconi, G.; Bovolin, P.; Palermo, F.A. Microplastics and their associated organic pollutants from the coastal waters of the central Adriatic Sea (Italy): Investigation of adipogenic effects in vitro. Chemosphere 2020, 263, 128090. [Google Scholar] [CrossRef]
- Spognardi, S.; Bravo, I.; Rea, R.; Cappelli, L.; Papetti, P. A perspective on the potential health risks from PCBs and heavy metals contamination of M. merluccius from Mediterranean Sea. International Journal of Food Safety, Nutrition and Public Health, 2021,6(2), pp.85-103.
- Milićević, T.; Romanić, S.H.; Popović, A.; Mustać, B.; Đinović-Stojanović, J.; Jovanović, G.; Relić, D. Human health risks and benefits assessment based on OCPs, PCBs, toxic elements and fatty acids in the pelagic fish species from the Adriatic Sea. Chemosphere 2021, 287, 132068. [Google Scholar] [CrossRef]
- Campanelli, A.; Grilli, F.; Paschini, E.; Marini, M. The influence of an exceptional Po River flood on the physical and chemical oceanographic properties of the Adriatic Sea. Dyn. Atmos. Oceans 2011, 52, 284–297. [Google Scholar] [CrossRef]
- Zhang YanYan, Z.Y.; Dong SiJun, D.S.; Wang HongOu, W.H.; Tao Shu, T.S.; Kiyama Ryoiti, K.R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors, 2016.
- Frapiccini, E.; Cocci, P.; Annibaldi, A.; Panfili, M.; Santojanni, A.; Grilli, F.; Marini, M.; Palermo, F.A. Assessment of seasonal relationship between polycyclic aromatic hydrocarbon accumulation and expression patterns of oxidative stress-related genes in muscle tissues of red mullet (M. barbatus) from the Northern Adriatic Sea. Environ. Toxicol. Pharmacol. 2021, 88, 103752. [Google Scholar] [CrossRef]
- Frapiccini, E. Polycyclic Aromatic Hydrocarbon (PAH) pollution in wild Adriatic fish-from the main determining factors of PAH accumulation to some biological responses of fish, 2021.
- Frapiccini, E.; Panfili, M.; Guicciardi, S.; Santojanni, A.; Marini, M.; Truzzi, C.; Annibaldi, A. Effects of biological factors and seasonality on the level of polycyclic aromatic hydrocarbons in red mullet (Mullus barbatus). Environ. Pollut. 2019, 258, 113742. [Google Scholar] [CrossRef] [PubMed]
- Frapiccini, E.; Pellini, G.; Gomiero, A.; Scarcella, G.; Guicciardi, S. , Annibaldi, A. ; Betti, M.; Marini, 2020, 226-233., M.. Microplastics and Polycyclic Aromatic Hydrocarbons Occurrence in a Demersal Fish (Solea solea) in the Adriatic Sea. In Proceedings of the 2nd International Conference on Microplastic Pollution in the Mediterranean Sea. Springer International Publishing.
- Hansen, P.K.; Lunestad, B.T.; Samuelsen, O.B. Effects of oxytetracycline, oxolinic acid, and flumequine on bacteria in an artificial marine fish farm sediment. Canadian Journal of Microbiology, 1992, 38(12),1307-1312.
- Alderman, D.J.; Hastings, T.S. Antibiotic use in aquaculture: development of antibiotic resistance—potential for consumer health risks*. Int. J. Food Sci. Technol. 1998, 33, 139–155. [Google Scholar] [CrossRef]
- DePaola, A.; Peeler, J.T.; E Rodrick, G. Effect of oxytetracycline-medicated feed on antibiotic resistance of gram-negative bacteria in catfish ponds. Appl. Environ. Microbiol. 1995, 61, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Mog, M.; Ngasotter, S.; Tesia, S.; Waikhom, D.; Panda, P.; Sharma, S.; Varshney, S. Problems of antibiotic resistance associated with oxytetracycline use in aquaculture: A review. J. Entomol. Zool. Stud, 2020, 8, 1075–1082. [Google Scholar]
- Kapetanović, D.; Vardić Smrzlić, I.; Gavrilović, A.; Jug-Dujaković, J.; Perić, L.; Kazazić, S.; Mišić Radić, T.; Kolda, A.; Čanković, M.; Žunić, J.; Listeš, E. Characterization of Vibrio Populations from Cultured European Seabass and the Surrounding Marine Environment with Emphasis on V. anguillarum. Microorganisms, 2022, 10(11), 2159.
- Kapetanović, D.; Smrzlić, I.V.; Kazazić, S.; Omanović, D.; Cukrov, N.; Cindrić, A.-M.; Rapljenović, A.; Perić, L.; Orlić, K.; Mijošek, T.; et al. A preliminary study of the cultivable microbiota on the plastic litter collected by commercial fishing trawlers in the south-eastern Adriatic Sea, with emphasis on Vibrio isolates and their antibiotic resistance. Mar. Pollut. Bull. 2023, 187, 114592. [Google Scholar] [CrossRef] [PubMed]
- Fonti, V.; Di Cesare, A.; Šangulin, J.; Del Negro, P.; Celussi, M. Antibiotic resistance genes and potentially pathogenic bacteria in the central Adriatic Sea: are they connected to urban wastewater inputs? Water, 2021, 13(23), p.3335.
- Zago, V.; Veschetti, L.; Patuzzo, C.; Malerba, G.; Lleo, M.M. Shewanella algae and Vibrio spp. strains isolated in Italian aquaculture farms are reservoirs of antibiotic resistant genes that might constitute a risk for human health. Mar. Pollut. Bull. 2020, 154, 111057. [Google Scholar] [CrossRef] [PubMed]
- Pavlinec, Ž.; Zupičić, I.G.; Oraić, D.; Lojkić, I.; Fouz, B.; Zrnčić, S. Biochemical and molecular characterization of three serologically different Vibrio harveyi strains isolated from farmed Dicentrarchus labrax from the Adriatic Sea. Scientific Reports, 2022, 12(1), 7309.
- Purgar, M.; Kapetanović, D.; Gavrilović, A.; Hackenberger, B.K.; Kurtović, B.; Haberle, I.; Ilić, J.P.; Geček, S.; Hackenberger, D.K.; Djerdj, T.; et al. Dataset AqADAPT: Physicochemical Parameters, Vibrio Abundance, and Species Determination in Water Columns of Two Adriatic Sea Aquaculture Sites. Data 2023, 8, 55. [Google Scholar] [CrossRef]
- Palladino, G.; Rampelli, S.; Scicchitano, D.; Nanetti, E.; Iuffrida, L.; Wathsala, R.H.G.R.; Interino, N.; Marini, M.; Porru, E.; Turroni, S.; et al. Seasonal dynamics of the microbiome-host response to pharmaceuticals and pesticides in Mytilus galloprovincialis farmed in the Northwestern Adriatic Sea. Sci. Total. Environ. 2023, 887, 163948. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.E.; Alessiani, A.; Donatiello, A.; Didonna, A.; D’attoli, L.; Faleo, S.; Occhiochiuso, G.; Carella, F.; Di Taranto, P.; Pace, L.; et al. Systematic Survey of Vibrio spp. and Salmonella spp. in Bivalve Shellfish in Apulia Region (Italy): Prevalence and Antimicrobial Resistance. Microorganisms 2023, 11, 450. [Google Scholar] [CrossRef]
- Ramljak, A.; Vardić Smrzlić, I.; Kapetanović, D.; Barac, F.; Kolda, A.; Perić, L.; Balenović, I.; Klanjšček, T.; Gavrilović, A. Skin Culturable Microbiota in Farmed European Seabass (Dicentrarchus labrax) in Two Aquacultures with and without Antibiotic Use. Journal of Marine Science and Engineering, 2022, 10(3),303.
- Azzurro, E. The advance of thermophilic fishes in the Mediterranean Sea: overview and methodological questions. In: Briand F, Ed. Climate warming and related changes in Mediterranean marine biota. In: No:35. CIESM Workshop Monographs; 2008; 39-46.
- Dulčić, J.; Grbec, B. Climate change and Adriatic ichthyofauna. 1: Fish Oceanogr 2000, 9, 2000. [Google Scholar] [CrossRef]
- Stephens, JS.; Hose, JH.; Love, MS. Fish assemblages as indicators of environmental change in nearshore environments.Marine Organisms as Indicators. New York: Springer-Verlag 1988; 91-105. [CrossRef]
- Bakiu, R.; Santovito, G.; Hoda, A.; Shehu, J.; Durmishaj, S.; Irato, P.; Piccinni, E. Metallothionein (MT): a good biomarker in marine sentinel species like sea bream (Sparus aurata). Albanian Journal of Agricultural Sciences, 2013, 12, 247–253. [Google Scholar]
- Eissa, A.E.; Zaki, M.M. The impact of global climatic changes on the aquatic environment. Procedia Environ. Sci. 2011, 4, 251–259. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, S.; Cassardo, C.; Elmi, A.; et al. Air-sea interactions in the Adriatic basin: simulations of Bora and Sirocco wind events. Geofizika 2009; 26(2): 157-70.
- Shaltout, M.; Omstedt, A. Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia 2014, 56, 411–443. [Google Scholar] [CrossRef]
- Vilibić, I.; Šepić, J.; Proust, N. Weakening thermohaline circulation in the Adriatic Sea. Clim. Res. 2013, 55, 217–225. [Google Scholar] [CrossRef]
- Bakiu, R. . Innovation towards the sustainability of Mediterranean blue economy—New Technologies for Marine Aquaculture. European Week, Brussels, 2017.
- Hidalgo, M.; E El-Haweet, A.; Tsikliras, A.C.; Tirasin, E.M.; Fortibuoni, T.; Ronchi, F.; Lauria, V.; Ben Abdallah, O.; Arneri, E.; Ceriola, L.; et al. Risks and adaptation options for the Mediterranean fisheries in the face of multiple climate change drivers and impacts. ICES J. Mar. Sci. 2022, 79, 2473–2488. [Google Scholar] [CrossRef]
- Cavraro, F.; Monti, M.A.; Matić-Skoko, S.; Caccin, A.; Pranovi, F. Vulnerability of the Small-Scale Fishery to Climate Changes in the Northern-Central Adriatic Sea (Mediterranean Sea). Fishes 2022, 8, 9. [Google Scholar] [CrossRef]
- Lasram, F.B.R.; Guilhaumon, F.; Albouy, C.; Somot, S.; Thuiller, W.; Mouillot, D. The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob. Chang. Biol. 2010, 16, 3233–3245. [Google Scholar] [CrossRef]
- Bakiu, R.; Kamberi, E. ; Expected Climate Change Effects on Gilthead Seabream and European Seabass Abundance and Catch in Albanian Waters –Albanian j. agric. sci. 1: 2021;20 (2), 2021. [Google Scholar]
- Dulčić, J.; Grbec, B.; Lipej, L.; Beg Paklar, G.; Supić, N.; Smirčić, A. The effect of the hemispheric climatic oscillations on the Adriatic ichthyofauna. Fresenius Environ Bull, 2004; 13(3b): 293-8.
- Glamuzina, B.; Cukteras, M.; Dulcic, J. (2012, July). Present changes and predictions for fishery and mariculture in the eastern Adriatic (Croatia) in the light of climate change/cambiamenti attuali? Previsioni per la pesca? La maricoltura nell’Adriatico Orientale (Croazia) alla luce del cambiamento climatico. In Annales: Series Historia Naturalis Scientific and Research Center of the Republic of Slovenia, 2012, 22, 2, 105. [Google Scholar]
- Kamberi, E.; Beqiri, K.; Luli, K.; Bakiu, R. Tracking Changes in Fish Diversity in the South-Eastern Adriatic Sea (Albania) Based on Local Ecological Knowledge. Croat. J. Fish. 2022, 80, 17–25. [Google Scholar] [CrossRef]
- Crocetta, F.; Al Mabruk, S.A.; Azzurro, E.; Bakiu, R. , Bariche, M.; Batjakas, I.E.; Bejaoui, T.; Ben Souissi, S.J.; Cauchi, J.; Corsini-Foka, M.; Deidun, A. 2021. New alien Mediterranean biodiversity records, 2021.
- Md. Nazmul Islam, Kusay Faisal Al-tabatabaie, Md. Ahasan Habib, Sheikh Sharif Iqbal, Khurram Karim Qureshi and Eid M. Al-Mutairi, Design of a Hollow-Core Photonic Crystal Fiber Based Edible Oil Sensor, Crystals 2022, 12, 1362. [CrossRef]
- Dulčić, J.; Tutman, P.; Ćaleta, M. Northernmost occurrence of the white grouper, Epinephelus aeneus (Perciformes: Serranidae), in the Mediterranean area. Acta Ichthyol. Et Piscat. 2006, 36, 73–75. [Google Scholar] [CrossRef]
- Bakiu, R. Climate Change Effects to Aquaculture Production Based on Social and Economic Indicators of the Albanian Marine Aquaculture. Albanian j. agric. sci. 2023, 21 (1):37-42.
- Agnetta, D.; Badalamenti, F.; Colloca, F.; Cossarini, G.; Fiorentino, F.; Garofalo, G.; Patti, B.; Pipitone, C.; Russo, T.; Solidoro, C.; et al. Interactive effects of fishing effort reduction and climate change in a central Mediterranean fishing area: Insights from bio-economic indices derived from a dynamic food-web model. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Gamito, R.; Costa, M.J.; Cabral, H.N. Fisheries in a warming ocean: trends in fish catches in the large marine ecosystems of the world. Reg. Environ. Chang. 2014, 15, 57–65. [Google Scholar] [CrossRef]
- Matić-Skoko, S.; Pavičić, M.; Šepić, J.; Janeković, I.; Vrdoljak, D.; Vilibić, I.; Stagličić, N.; Šegvić-Bubić, T.; Vujević, A. Impacts of Sea Bottom Temperature on CPUE of European Lobster Homarus gammarus (Linnaeus, 1758; Decapoda, Nephropidae) in the Eastern Adriatic Sea. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Hidalgo, M.; Mihneva, V.; Vasconcellos, M.; Bernal, M. Climate change impacts vulnerabilities adaptations: Mediterranean Sea the Black Sea marine fisheries. In Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options; Barange, M., Bahri, T., Beveridge, M.C., Cochrane, K.L., Funge-Smith, S., Poulain, F., Eds.; FAO: Kanagawa, Japan, 2018. [Google Scholar]
- Valencia-Gasti, J.A.; Baumgartner, T.; Durazo, R. Effects of ocean climate on life cycles and distribution of small pelagic fishes in the California Current System off Baja California. Cienc. Mar. 2015, 41, 315–348. [Google Scholar] [CrossRef]
- Valente, S.; Moro, S.; Di Lorenzo, M.; Milisenda, G.; Maiorano, L.; Colloca, F. Mediterranean fish communities are struggling to adapt to global warming. Evidence from the western coast of Italy. Mar. Environ. Res. 2023, 191, 106176. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.W.Y.; Cheung, W.W.L.; Reygondeau, G.; Sumaila, U.R. Projected change in global fisheries revenues under climate change. Sci. Rep. 2016, 6, 32607. [Google Scholar] [CrossRef] [PubMed]
- Bakiu, R. Albania: Climate change impacts become ever more obvious. Eurofish Magazine 5 2022.
- Kibria, G.; Nugegoda, D.; Rose, G.; Haroon, A.Y. Climate change impacts on pollutants mobilization and interactive effects of climate change and pollutants on toxicity and bioaccumulation of pollutants in estuarine and marine biota and linkage to seafood security. Mar. Pollut. Bull. 2021, 167, 112364. [Google Scholar] [CrossRef]
- Mol, S.; Coşansu, S. Seafood safety, potential hazards and future perspective. Turkish Journal of Fisheries and Aquatic Sciences, 2022, 22(6).
- Tirado, M.; Clarke, R.; Jaykus, L.; McQuatters-Gollop, A.; Frank, J. Climate change and food safety: A review. Food Res. Int. 2010, 43, 1745–1765. [Google Scholar] [CrossRef]
- FAO. Climate Change Adaptation Roadmap and Implementation Action Plan of the Agriculture Forestry and Other Land Use (AFOLU) sector of Albania, 2022, 1- 93.
- Coro, G.; Vilas, L.G.; Magliozzi, C.; Ellenbroek, A.; Scarponi, P.; Pagano, P. Forecasting the ongoing invasion of Lagocephalus sceleratus in the Mediterranean Sea. Ecol. Model. 2018, 371, 37–49. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Giorgi, F. Climate change hotspot. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




