1. Introduction
Vaccines are considered one of the most effective life-saving medical interventions in medical history [
1], playing a crucial role in preventing infections and diseases. However, the variability in vaccine efficacy among individuals underscores the importance of understanding more deeply the mechanisms that regulate immune responses to vaccination. Predicting who will respond optimally to a given vaccine is a critical aspect of vaccine development. The potential interaction between vaccines and infectious agents has lately attracted a lot of attention. A growing number of studies indicate that some vaccines possess significant non-specific protective effects, offering broader immunity beyond their primary targets. Vaccines such as Bacillus Calmette-Guérin (BCG) [
2], measles vaccines and the oral polio vaccine have demonstrated to have protective effects towards unrelated infectious diseases [
3,
4], highlighting the potential of using existing vaccines to provide protection against a range of pathogens.
In line with this concept, recent studies suggested a potential link between influenza vaccination and decreased COVID-19 incidence and severity [
5,
6,
7]. This suggests that influenza vaccination may potentially convey partial protection against COVID-19. A systematic review and meta-analysis of observational studies reveals an inverse association between influenza vaccination and COVID-19 risk and analyses the association with clinical outcomes such as mortality, hospitalization, and admissions in intensive care of SARS-CoV-2 infected subjects. However, more evidence-based studies are needed to investigate the underlying mechanisms that could explain this association [
8].
Despite being the most effective measure for preventing influenza and its complications, influenza vaccination coverage remains relatively low, particularly among fragile individuals [
9]. The response to influenza virus vaccination exhibits significant variability within populations, leaving certain individuals inadequately protected even when the seasonal vaccine aligns with the prevalent influenza viral strains [
10].
The increase of antibody titers against influenza antigens after the vaccination and the achievement of an antibody titer of 40 is generally regarded as a protective threshold level, beyond which there is a 50% or greater reduction in the possibility of contracting influenza infection. This is related to intrinsic factors such as age [
11,
12], gender [
13], pre-existing antibody titers [
11,
12] and vaccination history [
14,
15]. However, most of the variability in antibody responses to influenza virus vaccination remains unexplained [
12]. Identifying individuals with high or low responsiveness to immunization, and understanding the extent of this responsiveness, holds potential for optimizing existing vaccination strategies.
In this study, we explored the association between influenza vaccination and the immune responses to the booster dose of the anti-SARS-CoV-2 vaccine in a cohort of healthy individuals, exploring the hypothesis that the ability to respond to influenza vaccination has an impact on the capacity to respond to the anti-SARS-CoV-2 vaccination.
2. Materials and Methods
2.1. Cohort Description and Sampling
A cohort study of healthy individuals was recruited (N=113) between October 2021 and January 2022, by their general practitioners (GPs) located in the area of Rome municipality, Lazio region, Italy. Study participants received the booster dose of the SARS-CoV-2 mRNA vaccine and 54 of them were also vaccinated with the quadrivalent inactivated influenza vaccine (QIV) Flucelvax (Seqirus), which contained neuraminidase and hemagglutinin from the following viral strains: A/Wisconsin/588/2019, A/Cambodia/e0826360/2020, B/Phuket/3073/2013 and B/Washington/02/2019 [
16]. Study participants diagnosed with cancer, immunological disorders, receiving immunosuppressive therapy, subjected to transplant or to vaccinations in the last 6 months, suffering from chronic disease or viral or bacterial infections, and pregnant women were excluded from the study. Influenza vaccine was injected before the booster dose of the SARS-CoV-2 vaccine and the participants were bled before and after 28 days after receiving the anti-flu vaccination. Blood samples were collected in all participants before (T0), one (T1), and three (T3) months after receiving the booster dose of SARS-CoV-2 vaccination. After each vaccination, we tracked their humoral immune responses against the influenza antigens included in the vaccine and against SARS-CoV-2 virus.
2.2. Ethics Statement
The study was approved by the ethics committee of the Istituto Superiore di Sanità (A00ISS-15/03/2021-0010084). This study was conducted in accordance with applicable laws and regulations, including, without limitation, the International Conference on Harmonisation (ICH) Guideline for Good Clinical Practice (GCP) and the ethical principles derived from the Declaration of Helsinki. At the time of enrolment, patients were required to sign an informed consent that included the acceptance of sampling procedures, and of the collection and management of data for epidemiologic and scientific purposes. Patient data was anonymized.
2.3. Serum Samples
Human serum samples (n = 113) were collected anonymously and stored in compliance with Italian ethics law. Influenza sheep hyperimmune serum samples were provided by NIBSC and were used as positive controls: A/Wisconsin/588/2019, (H1N1, 09/152) (A/Wis); A/Cambodia/e0826360/2020 (H3N2, 13/110) (A/Cam), B/Phuket/3073/2013 (B/Yamagata lineage, 11/136) (B/Phu), and B/Washington/02/2019 (B/Victoria lineage, 19/218). Human serum without IgA, IgM, and IgG was used as negative controls (Sigma-Aldrich, S5393).
2.4. Hemagglutination Inhibition Assay
All serum samples, including the sheep hyperimmune serum samples and negative control, were pre-treated with receptor-destroying enzyme (RDE) (ratio 1:5) from Vibrio cholerae (C8772, Sigma-Aldrich, Milan, Italy) for 18 hours at 37°C in a water bath and then heat-inactivated for 1 hour at 56°C in a water bath with 8% sodium citrate (ratio 1:4). Fresh turkey red blood cells were centrifuged twice, washed with a saline solution (0.9%), and adjusted to a final dilution of 0.35%. From an initial dilution of 1:10, serum samples were 2-fold diluted in duplicate with saline solution (0.9%) in a 96-well plate; 25 μL of standardized viral antigen (4 HA units/25 μL) was added to each well, and the mixture was incubated at room temperature for 1 hour. Red blood cells were added and, after 1-hour incubation at room temperature, the plates were evaluated for the presence of agglutination inhibition. The antibody titer was expressed as the reciprocal highest serum dilution that showed complete inhibition of agglutination. As the starting dilution was 1:10, the lower limit of the detectable antibody titer was 10. When the titer was below the detectable threshold, the results were conventionally expressed as 5 for calculation purposes, half the lowest detection threshold.
2.5. Influenza virus Micro-Neutralization Assay
Madin Darby Canine Kidney (MDCK) cells were maintained for a maximum of 30 passages in EME medium containing 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, 1% non-essential amino acid solution, and 100 U/mL penicillin-streptomycin. The MDCK cell cultures were grown at 37°C in 5% CO2. Serum samples, previously heat-inactivated at 56°C for 30 minutes, were diluted 2-fold with EMEM culture medium supplemented with 0.5% FBS in a 96-well plate, mixed with an equal volume of virus (100 TCID50/well), and incubated for 1 hour at 37°C in 5% CO2. At the end of incubation, the MDCK cell suspension (1.5 × 105 cells/mL) was added to the plates, which were then stored in an incubator (37°C, 5% CO2) for 5 days. After incubation, the plates were observed by optical microscopy and evaluated for cytopathic effect (CPE). A CPE higher than 50% indicates infection. The titer was expressed as the inverse of the last dilution that showed inhibition of cytopathic effect.
2.6. SARS-CoV-2 IgG Immunoassay Testing
Serum samples were tested for the presence of SARS-CoV-2 IgG antibodies against the receptor binding domain (RBD) of the spike (S) protein and the nucleocapsid (NP) protein on the Abbott Architect i2000SR automated analyzer using the Abbott SARS-CoV-2 IgG assay (Abbott Park, USA) according to the manufacturer's instructions. Assay results higher than or equal to the cut-off index value of 50 AU for the anti-S and 1.4 AU for the anti-NP IgG antibody titer were interpreted as positive.
SARS-CoV-2 virus neutralization assay
The virus neutralization (VN) assay has been performed as previously reported [
17]. Briefly, serum samples were heat-inactivated for 30 minutes at 56°C and, starting from 1:10 dilution, were mixed with an equal volume of SARS-CoV-2 (2019-nCov/Italy-INMI1 strain) viral solution containing 100 Tissue Culture Infective Dose 50% (TCID50). After 1 hour of incubation at room temperature, 100μL of virus-serum mixture were added to a 96-well plate containing VERO E6 cells with 80% confluency. Plates were incubated for 3 days at 37°C, 5% CO2 in humidified atmosphere, then inspected for presence/absence of CPE by means of an inverted optical microscope. A CPE higher than 50% indicated infection. The VN titer was expressed as the reciprocal of the highest serum dilution showing protection from viral infection and CPE.
2.7. Statistical Analysis
We summarized categorical variables by frequency and percent, while continuous variables by median and interquartile ranges (IQR). We performed the description of the study population by main study groups, i.e. individuals who received both vaccines (flu-vaccine and SARS-CoV-2 vaccine) vs those who received only SARS-CoV-2 vaccine. Double vaccinated individuals were stratified by considering as a reference the median of HI titer of each specific antigen 28 days post vaccination (D28). The study population was stratified in five groups: individuals with no specific HI titer above the specific median at D28 (0) and individuals with respectively, one (1), two (2), three (3) and four (4) specific HI titer above the specific median. Participants belonging to the group 0 or 1 were classified as low responders (LR) and participants belonging to the groups 2, 3, or 4 were classified as high responders (HR). We assessed differences between two groups by non-parametric Mann-Whitney U test. We applied Kruskal-Wallis test when the number of the groups was higher than two; we applied also Bonferroni adjustment when appropriate. In order to investigate the relationship between the response to the S protein and to the four flu-antigens, we applied a multiple regression model after one (T1) and three (T3) months post SARS-CoV-2 vaccination. In details, we considered the natural logarithmic Ln of anti-S titer as the response-variable, whilst as the covariates the Ln of anti-flu for all four antigens, as well as its interaction with each antigen. Further, other interactions were considered in the model when resulting statistically significant, with one of the possible covariates: anti-spike at baseline (≤its median vs > its median), age (≤60 vs >60) and sex (females vs males). We used SAS/STAT version 9.4 (SAS Institute, Cary, NC) for the statistical analyses.
3. Results
3.1. Characteristics of the Study Population
To evaluate the effects of the 2021/2022 seasonal influenza vaccine on immune response to SARS-CoV-2 vaccination, we recruited participants from a cohort study of healthy individuals (N=113) who received or not QIV in autumn 2021 and the booster dose of SARS-CoV-2 vaccine. Healthy participants without previous COVID-19 diagnosis and without significant co-morbidities were considered for the analysis (N=74) (
Table 1). In particular, study participants diagnosed with cancer, immunological disorders, receiving immunosuppressive therapy, subjected to transplant or to vaccinations in the last 6 months, suffering from chronic disease or viral or bacterial infections, and pregnant women were excluded from the study. Among the 74 participants, 50 (68%) were females and 24 (32%) males, with similar proportion between the two sexes in participants who received two vaccines (67%
vs 33%) and the ones who received one vaccine (70%
vs 30%) (
Table 1); overall, median age was 54 years (range: 31-72 years; interquartile range: 49-60 years) with similar distribution among individuals who received two (median: 54, range: 49-60; interquartile 35-70) or one (median: 51, range: 49-56; interquartile 31-72) vaccine. Most participants have higher (72%) or middle/lower (28%) education, with higher proportion of higher education among participants who received two vaccines as compared with the ones who received only one vaccine (76% vs 60%). Regarding frailty, the majority (69%) of study participants declared to be fit at the time of enrolment, part of them declared to be very fit (20%) and a minority (11%) declared to manage well with no substantial differences between participants who received two vaccines or one vaccine (
Table 1). Regarding BMI 57% had a normal weigh, 35% were overweight and 7% were obese. However, no significant differences in the proportion of overweight/obese participants were observed between participants who received two vaccines or one vaccine (
Table 1). Smoking at the time of vaccination was declared by 16% participants with higher number of smokers among participants who received only one vaccine (25% vs 13%). The majority of study participants (62.3%) did not have respiratory symptoms during the current season. The most frequent diseases found in the study population are chronic cardiac diseases (28%), asthma (21%), thyroiditis (15%), hemoglobinopathy (9%), essential thrombocythemia, diabetis or anemia (6%), renal diseases or coagulopathy (3%). Part of the participants enrolled in the study received the seasonal influenza vaccine before the booster dose of the SARS-CoV-2 vaccine and they were bled before and after 28 days after receiving the flu vaccination. Blood samples were collected in all participants before (T0), one (T1), and three (T3) months after receiving the boosting dose of SARS-CoV-2 vaccination. After each vaccination, we tracked their humoral immune responses against the influenza antigens included in the vaccine and against SARS-CoV-2 virus.
3.2. Subjects Who Received Influenza and COVID-19 Vaccinations Are More Responsive to the SARS-CoV-2 Vaccine
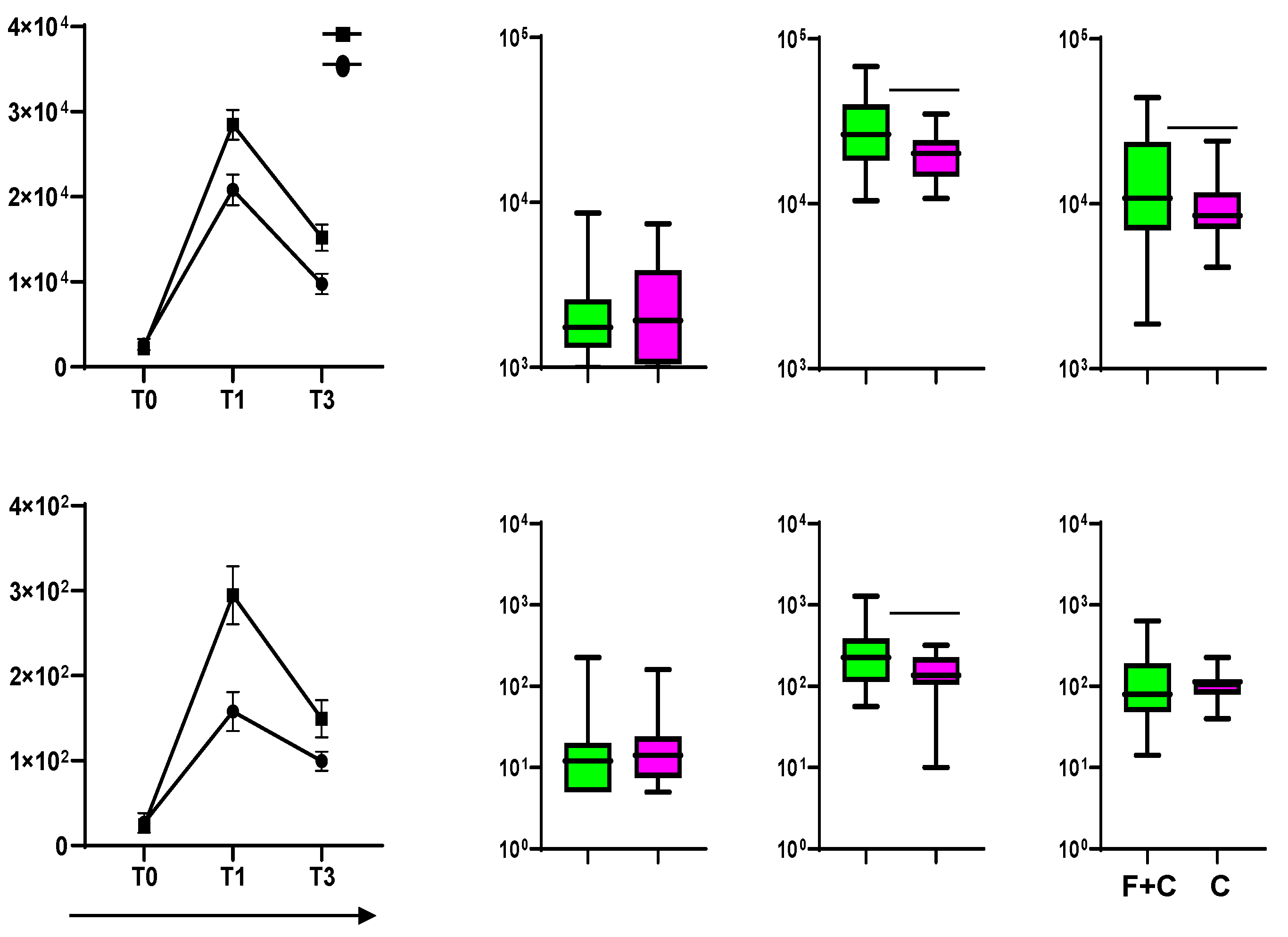
All participants who received two vaccines received the 2021/2022 seasonal anti-influenza tetravalent vaccine before the mRNA-based anti-SARS-CoV-2 vaccine. Time between the two vaccinations ranged between 1 and 70 days. Blood was collected by venous puncture before and 4 weeks after each vaccination and 12 weeks after SARS-CoV-2 vaccination. Subjects who received both influenza and COVID-19 vaccinations were more responsive to the SARS-CoV-2 vaccine after 4 weeks from the injection of the SARS-CoV-2 vaccine (T1) compared with individuals who received only the SARS-CoV-2 vaccine (
Figure 1). The responses were evaluated in terms of the anti-S-specific antibody titers by an immunometric assay (
Figure 1A). Furthermore, an in vitro neutralization assay using authentic SARS-CoV-2 virus, revealed higher titers of functional antibodies towards SARS-CoV-2 infection in individuals who received two vaccines (
Figure 1B). To assess whether an asymptomatic COVID-19 infection occurred before or during the trial, the anti- NP antibody titers were evaluated at each time point considered. Participants infected with SARS-CoV-2, showing an anti-NP antibody titer higher than 1.4 according to the assay used were excluded from the analysis (23% of the enrolled participants). The anti-S antibody titers decreased after the SARS-CoV-2 vaccine injection in all participants in accordance with the waning of the antibody titers observed after vaccination [
18]. However, after three months (T3) from the injection the antibody titer was higher in the individuals who received both influenza and COVID-19 vaccines, suggesting that influenza vaccination also affects the durability of the immune response to the SARS-CoV-2 vaccine.
3.3. Quantitative and Qualitative Analysis of the Immune Responses to Seasonal 2021/2022 Influenza Vaccine
Healthy participants received the 2021/2022 seasonal quadrivalent influenza vaccine following the Italian public health authority’s recommendation [
16,
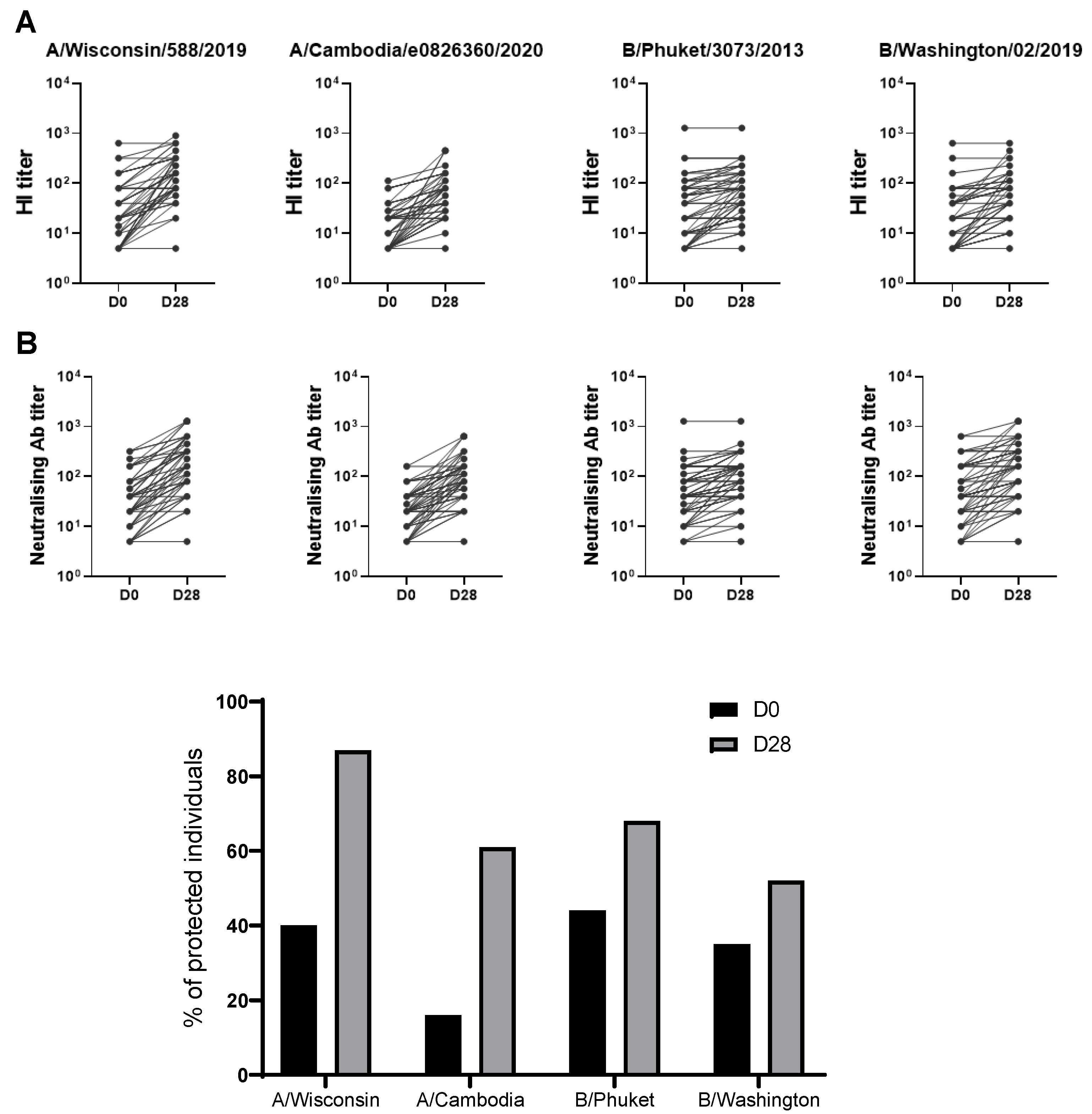
19]. To evaluate the response to the vaccine, blood was collected at baseline before receiving the injection (D0) and 28 days after (D28). The hemagglutination-inhibition (HI) antibody titer at baseline (D0) and at D28 post-vaccination was evaluated (
Figure 2A). The participants responded to vaccination with a significant increase in HI titers against all four antigens included in the vaccines tested at day 28 (
Figure 2A). Following the current international guidelines for seroconversion (>4-fold increase in HI titers over baseline) and seroprotection (HI titers ≥40) [
19,
20], a high number of participants were already seroprotected at the baseline against the four antigens included in the vaccine. Differences between the baseline percentage of protection were observed for the different viral strains and less percentage of protection was found for the A/Cambodia/H3N2 strain (20%), overall the percentage of protection increases for all strains included in the vaccine even if at different extension after 28 days post vaccination (
Figure 2C). The high baseline titers can result from previous exposure to the influenza virus or to earlier vaccinations. Furthermore, the HI results were supported by the microneutralization assays (
Figure 2B) showing that the influenza-specific antibodies produced following vaccination are functional and have virus neutralization capacity.
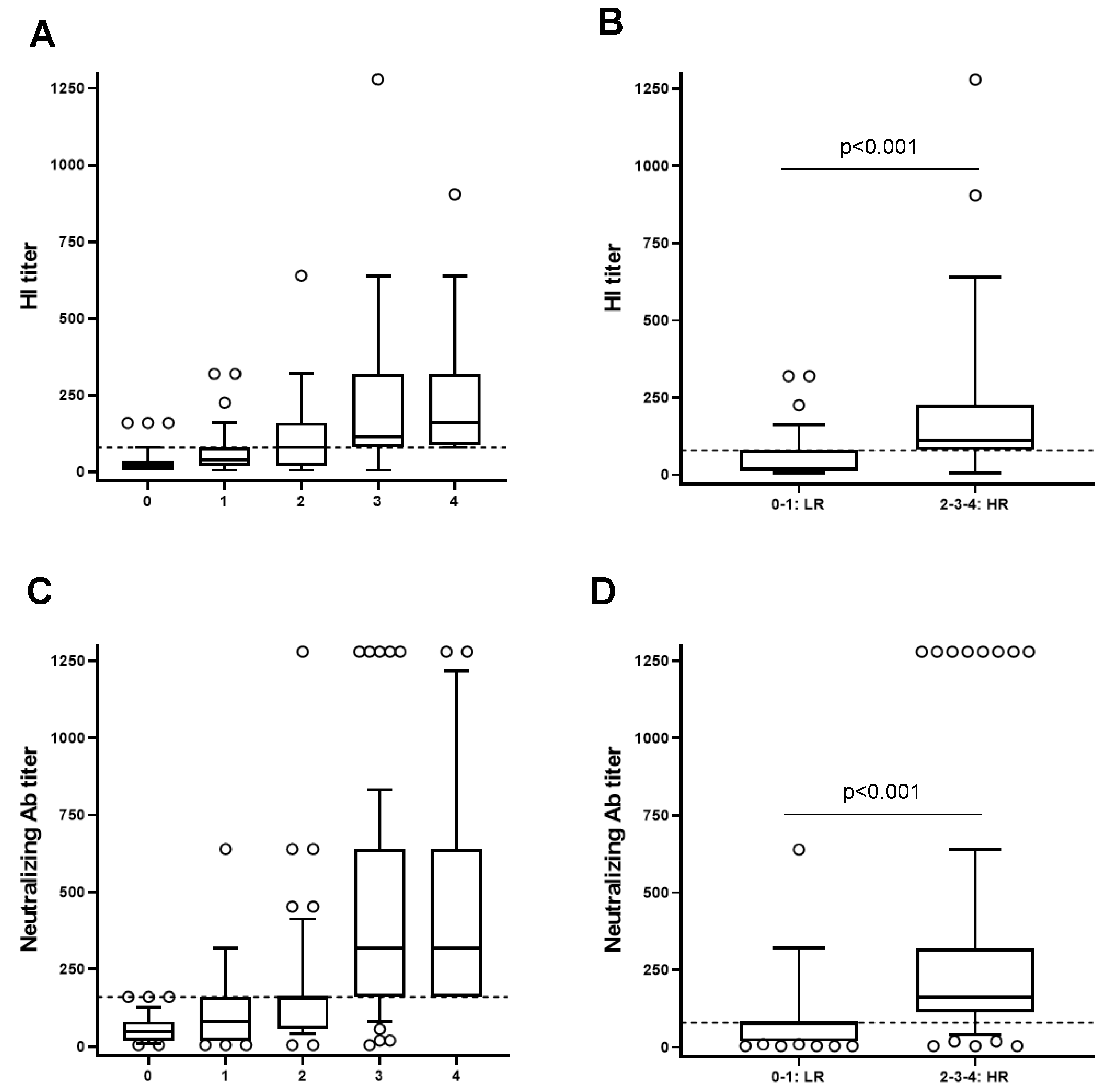
Due to the elevated variability of the response to the influenza vaccine among individuals, double vaccinated individuals were stratified according to their capacity to respond to the flu-vaccination by considering as a reference the median of HI titer of each post vaccination specific antigen. The study population was stratified in five groups: individuals with no specific HI titer above the specific median post vaccination (D28) (0) and individuals with respectively, one (1), two (2), three (3) and four (4) specific HI titer above the specific median at D28 post vaccination (
Figure 3A). Participants belonging to the group 0 or 1 were classified as low responders (LR) and participants belonging to the groups 2, 3, or 4 were classified as high responders (HR) (
Figure 3B). The differences between these groups were statistically significant (p<0.001).
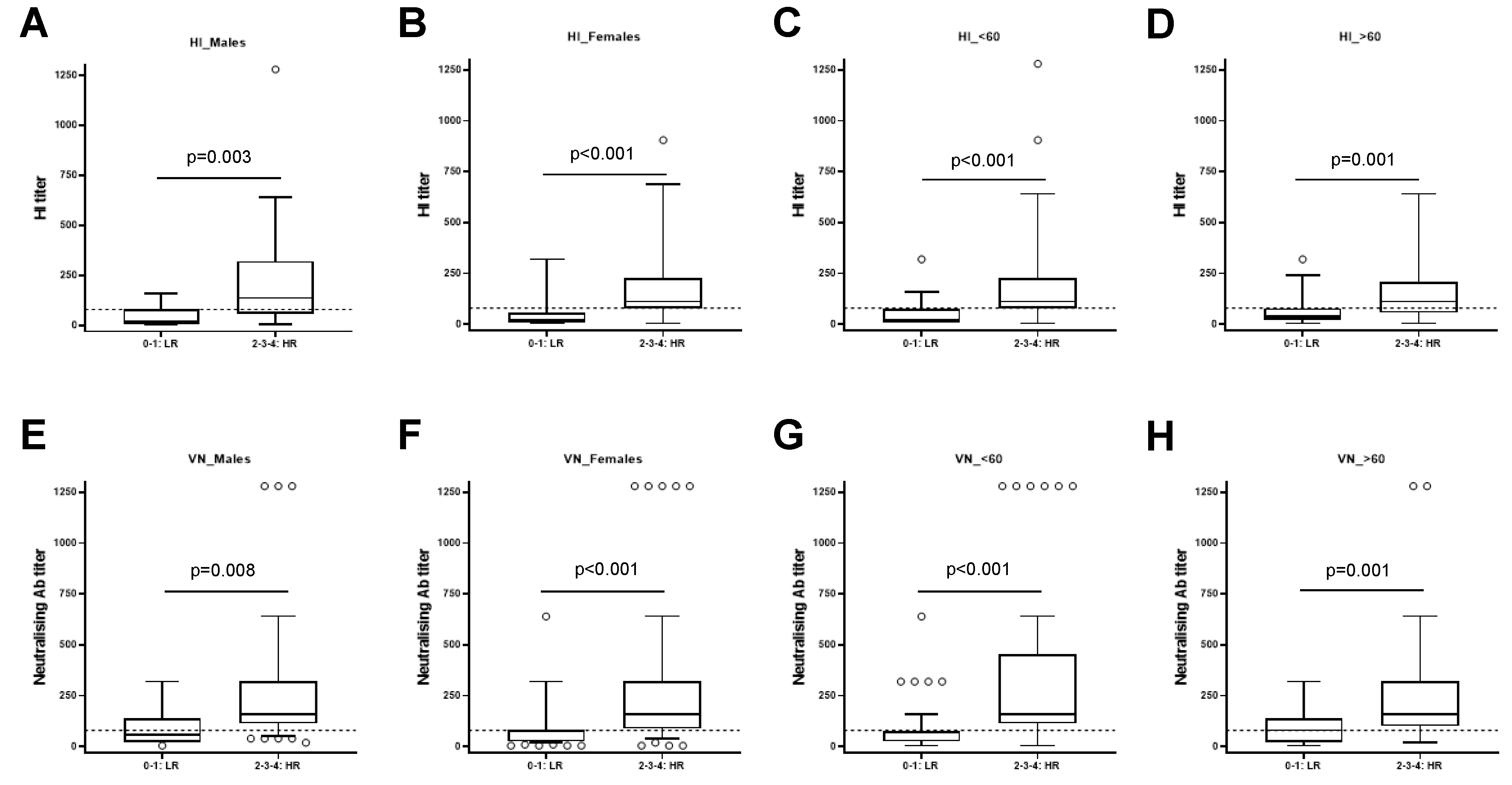
The same analysis was applied to the study population stratified by sex or age. We found that the differences between the HI titer of HR and LR were significant in males (p=0.003) (
Figure 4A) and females (p<0.001) (
Figure 4B). Same results were observed stratifying the population in individual with age below 60 (p<0.001) (
Figure 4C) and individuals with more than 60 years (p<0.001,
Figure 4D). These data suggest that the results were similar independently of sex and age.
3.4. The High Responders to Influenza Vaccine as Compared to the Low Responders Show a Strong Capacity to Respond to the SARS-CoV-2 Vaccine
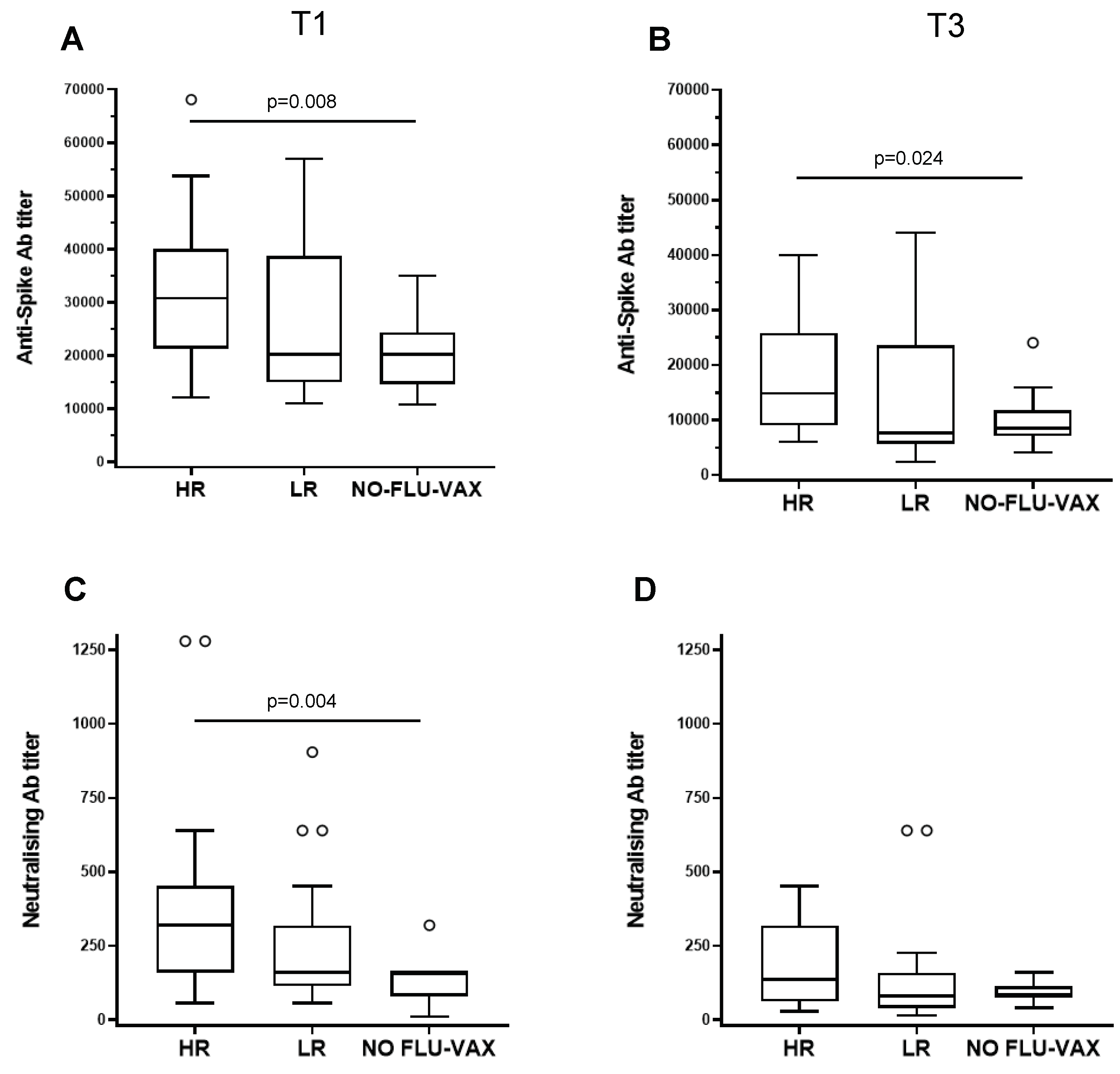
To evaluate if the capacity to respond to the flu vaccine had any effect on the response to the booster dose of the SARS-CoV-2 vaccine, we compared the responses to the S protein of the HR or LR to the influenza vaccine and the ones who received only the SARS-CoV-2 vaccine either after one and three months post vaccine injection. We found that the HR have a strong capacity to respond to the SARS-CoV-2 vaccine after one month, whereas the LR do not show any significant differences compared to the individuals who received only one vaccine (
Figure 5A). Furthermore, the HR to the influenza vaccine showed a significant higher response also after three months from the vaccine injection (
Figure 5B), suggesting that the duration of the immune responses to the SARS-CoV-2 vaccine was longer when people received the influenza vaccine.
3.5. Analysis of the Effects of the Flu Specific Antibody Response on Anti-Spike Antibody Levels in Double-Vaccinated Individuals
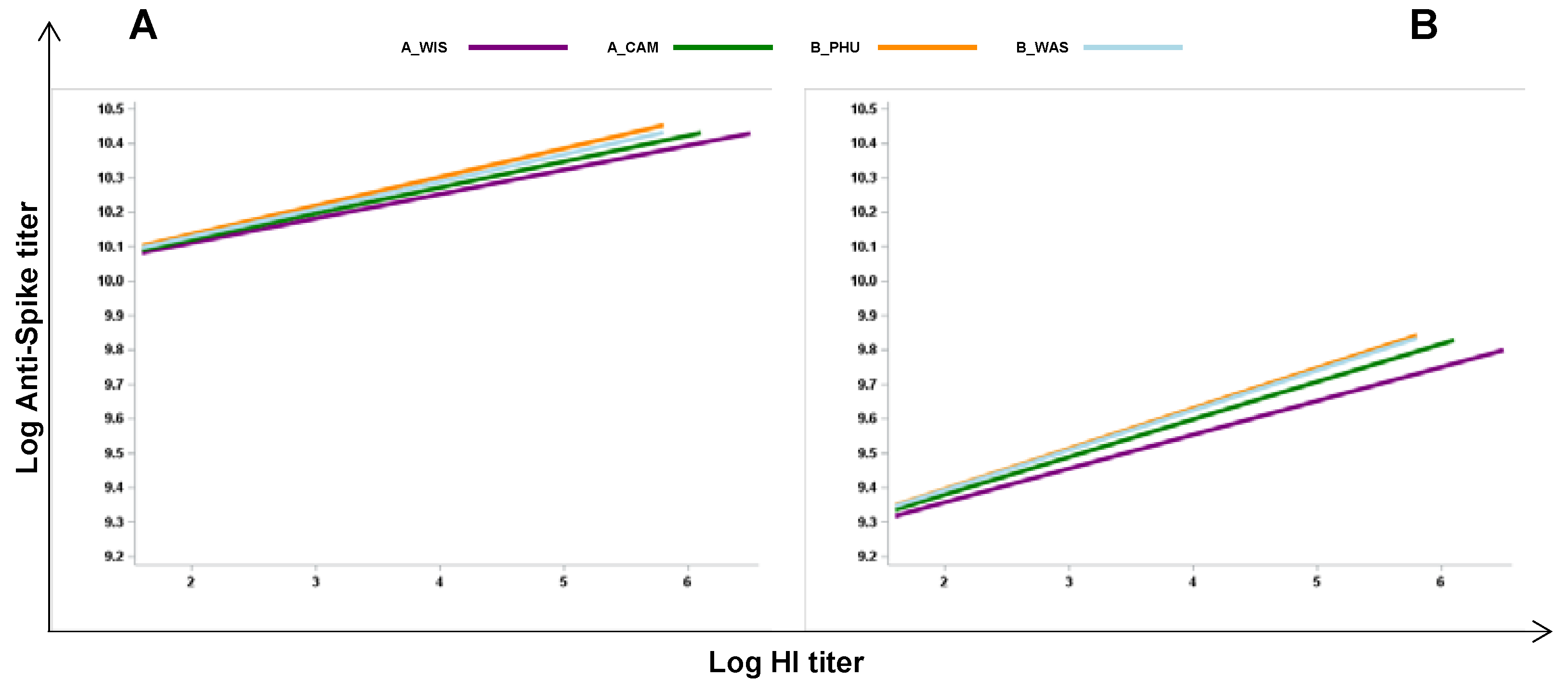
Next, to evaluate whether the extension of the response to the influenza vaccination could affect the capacity to respond to the SARS-CoV-2 vaccine by comparing the vaccines responses in the same subjects, we analyzed the associations of the responses in individuals who received two vaccines. A linear regression model was applied considering values relative to samples collected after one (
Figure 6A) and three months (
Figure 6B) post SARS-CoV-2 vaccination. We found that there was a positive association between anti-S antibody titers and each specific flu antigens, with A/Wis: β = 0.071, p=0.004; A/Cam: β = 0.075, p= 0.010; B/Phu: β = 0.083 p = 0.003; B/Was: β = 0.080 p=0.007, one month after vaccination. Furthermore, there was a positive association between anti-S and each flu specific antigens with A/Wis: β = 0.098, p=0.023; A/Cam: β =0.109, p= 0.035; B/Phu: β =0.11, p =0.003; B/Was: β = 0.116 p=0.023 also after three months following SARS-CoV-2 vaccination. These findings further suggest that influenza vaccination has a positive effect on the capacity to mount a good immune response to an unrelated vaccine.
4. Discussion
In this study, we found that the vaccination with the QIV improves the immune responses to the booster dose of the anti-SARS CoV-2 vaccine in a cohort of healthy individuals. Furthermore, we observed that the intrinsic ability to respond to influenza vaccination correlates to the capacity to mount a strong antibody response to the anti SARS-CoV-2 vaccination. In addition, the virus neutralization assay with an authentic SARS-CoV-2 virus showed that the anti-S antibodies are functional and confer protection against infection. These data are in accordance with several recent observational studies, which suggest a link between influenza vaccination and decreased COVID-19 incidence and severity [
6,
7,
8]. The possibility of bias in epidemiological observations should be taken into account due to the inherent possibility of interfering factors that can cause over- or underestimation of outcomes. However, as a support of epidemiological observations, immunological evidence showed that the quadrivalent inactivated influenza vaccination is able to induce trained immunity by inducing a transcriptional and functional reprogramming of innate immune cells [
20]. Indeed, it has been suggested that trained immunity might be an important mechanism underlying protective heterologous effects of vaccines [
21]. BCG is the most studied vaccine that induces trained immunity, and its putative protective effects against COVID-19 severity was studied in several clinical trials since before the vaccines against SARS-CoV-2 were released [
22], [
23]. Here, we found that influenza vaccination is able to potentiate SARS-CoV-2 vaccine efficacy, which has an impact on the protection from the disease in accordance with the epidemiological and immunological studies [
24,
25,
26]. On the other hand, whether influenza vaccination induces trained immunity in our cohort is currently under investigation.
The responses to vaccinations are highly variable in the population, with same individuals who respond promptly and at high levels of antibodies and others who don’t develop a protective responses and this is a common feature of different vaccines [
27,
28,
29,
30,
31]. According to this, the participants included in the study responded with a significant increase in HI titers against all four antigens included in the vaccines, with high variability among individuals, as expected [
10,
32]. A high number of participants were already seroprotected at the baseline against the four antigens included in the vaccine. Differences between percentage of protection at baseline were observed for the different viral strains and less percentage of baseline protection was found for the A/Cambodia/H3N2 strain (20%). The high baseline titers are the results of previous exposures to the influenza virus or to earlier vaccinations. It has been widely reported that the baseline titers have an effect on the responses to the flu vaccine, with controversial results [
14,
33,
34,
35,
36,
37,
38]. Here, we found that the baseline titer correlates with the response (data not shown). Due to the different baseline titers among individuals, the identification of responders and non-responders following influenza vaccination is complex in both young and elderly population [
39,
40]. Therefore, here the population was stratified in five groups according to the capacity of the individuals to respond to the vaccination with an HI titer below or above the median of the HI of each viral antigen. Participants were classified as LR and HR. High responders to flu vaccine have a stronger capacity to respond to the SARS-CoV-2 vaccine after one and three months after vaccination, as compared to LR, which do not show any significant differences if compared to the individuals who received only one vaccine. In addition, a positive association between influenza specific antibody response and anti-S antibody levels was found in double-vaccinated individuals, further suggesting that influenza vaccination has a positive effect on the response to unrelated antigens. On the other hand, these findings are in accordance with the important role played by the intrinsic factors of the host, in the responses to vaccinations and highlight the importance to analyze the immune signatures measured prior to vaccination in order to identify potential biomarkers able to predict the vaccine immune responses [
35]. Identifying the immune signature of individuals with high or low responsiveness to immunization holds potential for optimizing existing vaccination strategies.
Author Contributions
Conceptualization, S.V., C.V. and P.P.; methodology, A.R., N.S., F.F., R.G., U.V., C.M. and S.M.; visualization, M.D., F.F. and A.C.; funding acquisition, S.V., P.P., E.M. and A.R.C.; project administration S.V. and P.P; supervision, S.V.; writing—original draft preparation, S.V. and D.D.P.; writing—review and editing, S.V., C.M.T., M.D., A.C. and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
The VITAL project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No. 806776. The study was partly supported by project n.7S06 from the Ministry of Health and by institutional funds of Istituto Superiore di Sanità".
Institutional Review Board Statement
The study was approved by the ethics committee of the Istituto Superiore di Sanità (A00ISS-15/03/2021-0010084). This study was conducted in accordance with applicable laws and regulations, including, without limitation, the International Conference on Harmonisation (ICH) Guideline for Good Clinical Practice (GCP) and the ethical principles derived from the Declaration of Helsinki.
Informed Consent Statement
At the time of enrolment, patients were required to sign an informed consent that included the acceptance of sampling procedures, and of the collection and management of data for epidemiologic and scientific purposes. Patient data was anonymized. Written informed consent has been obtained from the patients for publication on scientific journals.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Due to the ongoing nature of the study, the data are not publicly available at this time.
Acknowledgments
the authors thank the FIMMG Group participants for their contribution.
Conflicts of Interest
C.M.T. is an external consultant of VisMederi Research srl. E.M. is founder and Chief Scientific Officer of VisMederi srl and VisMederi Research srl.
References
- Plotkin, S. History of Vaccination. Proceedings of the National Academy of Sciences 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Lee, A.; Floyd, K.; Wu, S.; Fang, Z.; Tan, T.K.; Froggatt, H.M.; Powers, J.M.; Leist, S.R.; Gully, K.L.; Hubbard, M.L.; et al. BCG Vaccination Stimulates Integrated Organ Immunity by Feedback of the Adaptive Immune Response to Imprint Prolonged Innate Antiviral Resistance. Nat Immunol 2023. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Ahmed, S.S.; Curtis, N.; Kollmann, T.R.; Levy, O.; Netea, M.G.; Pollard, A.J.; Van Crevel, R.; Wilson, C.B. Harnessing the Beneficial Heterologous Effects of Vaccination. Nat Rev Immunol 2016, 16, 392–400. [Google Scholar] [CrossRef]
- Nielsen, S.; Sujan, H.M.; Benn, C.S.; Aaby, P.; Hanifi, S.M.A. Oral Polio Vaccine Campaigns May Reduce the Risk of Death from Respiratory Infections. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef]
- Paolo Matteo, A.; Serena, M.; Claudia Maria, T.; Emma, A. Flu Vaccine Administration in the Period before SARS-CoV-2 Infection and Its Outcomes: An Umbrella Review. Prev Med Rep 2023, 102575. [Google Scholar] [CrossRef]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated Trivalent Influenza Vaccination Is Associated with Lower Mortality among Patients with COVID-19 in Brazil. BMJ Evid Based Med 2021, 26, 192–193. [Google Scholar] [CrossRef]
- Candelli, M.; Pignataro, G.; Torelli, E.; Gullì, A.; Nista, E.C.; Petrucci, M.; Saviano, A.; Marchesini, D.; Covino, M.; Ojetti, V.; et al. Effect of Influenza Vaccine on COVID-19 Mortality: A Retrospective Study. Intern Emerg Med 2021, 16, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, M.; Liu, J. The Association between Influenza Vaccination and Covid-19 and Its Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huedo, M.A.; Lopez-De-Andrés, A.; Mora-Zamorano, E.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; Zamorano-Leon, J.J.; Jiménez-García, R. Decreasing Influenza Vaccine Coverage among Adults with High-Risk Chronic Diseases in Spain from 2014 to 2017. Hum Vaccin Immunother 2020, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Cobey, S. Immune History and Influenza Vaccine Effectiveness. Vaccines (Basel) 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Avey, S.; Cheung, F.; Fermin, D.; Frelinger, J.; Gaujoux, R.; Gottardo, R.; Khatri, P.; Kleinstein, S.H.; Kotliarov, Y.; Meng, H.; et al. Multicohort Analysis Reveals Baseline Transcriptional Predictors of Influenza Vaccination Responses. Sci Immunol 2017, 2. [Google Scholar] [CrossRef]
- Tsang, J.S.; Schwartzberg, P.L.; Kotliarov, Y.; Biancotto, A.; Xie, Z.; Germain, R.N.; Wang, E.; Olnes, M.J.; Narayanan, M.; Golding, H.; et al. Global Analyses of Human Immune Variation Reveal Baseline Predictors of Postvaccination Responses. Cell 2014, 157, 499–513. [Google Scholar] [CrossRef]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems Analysis of Sex Differences Reveals an Immunosuppressive Role for Testosterone in the Response to Influenza Vaccination. Proceedings of the National Academy of Sciences 2014, 111, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Skowronski, D.M.; McLean, H.Q.; Chambers, C.; Sundaram, M.E.; De Serres, G. Repeated Annual Influenza Vaccination and Vaccine Effectiveness: Review of Evidence. Expert Rev Vaccines 2017, 16, 723–736. [Google Scholar] [CrossRef]
- Nienen, M.; Stervbo, U.; Mölder, F.; Kaliszczyk, S.; Kuchenbecker, L.; Gayova, L.; Schweiger, B.; Jürchott, K.; Hecht, J.; Neumann, A.U.; et al. The Role of Pre-Existing Cross-Reactive Central Memory CD4 T-Cells in Vaccination with Previously Unseen Influenza Strains. Front Immunol 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) Recommended Composition of Influenza Virus Vaccines for Use in the 2021-2022 Northern Hemisphere Influenza Season Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in- the-2021-2022-northern-hemisphere-influenza-season (accessed on 26 January 2024).
- Manenti, A.; Maggetti, M.; Casa, E.; Martinuzzi, D.; Torelli, A.; Trombetta, C.M.; Marchi, S.; Montomoli, E. Evaluation of SARS-CoV-2 Neutralizing Antibodies Using a CPE-Based Colorimetric Live Virus Micro-Neutralization Assay in Human Serum Samples. J Med Virol 2020, 92, 2096–2104. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of MRNA BNT162b2 COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. The Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Seasonal Influenza - Annual Epidemiological Report for 2021−2022 Available online: https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-annual-epidemiological-report-2021-2022 (accessed on 21 February 2023).
- Wimmers, F.; Donato, M.; Kuo, A.; Ashuach, T.; Gupta, S.; Li, C.; Dvorak, M.; Foecke, M.H.; Chang, S.E.; Hagan, T.; et al. The Single-Cell Epigenomic and Transcriptional Landscape of Immunity to Influenza Vaccination. Cell 2021, 184, 3915–3935.e21. [Google Scholar] [CrossRef]
- Geckin, B.; Konstantin Föhse, F.; Domínguez-Andrés, J.; Netea, M.G. Trained Immunity: Implications for Vaccination. Curr Opin Immunol 2022, 77, 102190. [Google Scholar] [CrossRef]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; van Eyk, J.E.; Cheng, S.; Arditi, M. BCG Vaccination History Associates with Decreased SARS-CoV-2 Seroprevalence across a Diverse Cohort of Health Care Workers. Journal of Clinical Investigation 2021, 131. [Google Scholar] [CrossRef]
- Ten Doesschate, T.; Moorlag, S.J.C.F.M.; Van Der Vaart, T.W.; Taks, E.; Debisarun, P.; Ten Oever, J.; Bleeker-Rovers, C.P.; Verhagen, P.B.; Lalmohamed, A.; Ter Heine, R.; et al. Two Randomized Controlled Trials of Bacillus Calmette-Guérin Vaccination to Reduce Absenteeism among Health Care Workers and Hospital Admission by Elderly Persons during the COVID-19 Pandemic: A Structured Summary of the Study Protocols for Two Randomised Controlled Trials. Trials 2020, 21. [Google Scholar] [CrossRef]
- Ragni, P.; Marino, M.; Formisano, D.; Bisaccia, E.; Scaltriti, S.; Bedeschi, E.; Grilli, R. Association between Exposure to Influenza Vaccination and COVID-19 Diagnosis and Outcomes. Vaccines (Basel) 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Yang, M.J.; Rooks, B.J.; Le, T.T.T.; Santiago, I.O.; Diamond, J.; Dorsey, N.L.; Mainous, A.G. Influenza Vaccination and Hospitalizations among COVID-19 Infected Adults. Journal of the American Board of Family Medicine 2021, 34, S179–S182. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between Influenza Vaccination and Hospitalisation or All-Cause Mortality in People with COVID-19: A Retrospective Cohort Study. BMJ Open Respir Res 2021, 8. [Google Scholar] [CrossRef]
- Visalli, G.; Laganà, A.; Lo Giudice, D.; Calimeri, S.; Caccamo, D.; Trainito, A.; Di Pietro, A.; Facciolà, A. Towards a Future of Personalized Vaccinology: Study on Individual Variables Influencing the Antibody Response to the COVID-19 Vaccine. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev 2019, 32. [Google Scholar] [CrossRef]
- Meireles, L.C.; Marinho, R.T.; Van Damme, P. Three Decades of Hepatitis B Control with Vaccination. World J Hepatol 2015, 7, 2127–2132. [Google Scholar] [CrossRef]
- Camous, X.; Visan, L.; Ying, C.T.T.; Abel, B.; Nyunt, M.S.Z.; Narang, V.; Poidinger, M.; Carre, C.; Sesay, S.; Bosco, N.; et al. Healthy Elderly Singaporeans Show No Age-Related Humoral Hyporesponsiveness nor Diminished Plasmablast Generation in Response to Influenza Vaccine. Immunity and Ageing 2018, 15. [Google Scholar] [CrossRef]
- Carre, C.; Wong, G.; Narang, V.; Tan, C.; Chong, J.; Chin, H.X.; Xu, W.; Lu, Y.; Chua, M.; Poidinger, M.; et al. Endoplasmic Reticulum Stress Response and Bile Acid Signatures Associate with Multi-Strain Seroresponsiveness during Elderly Influenza Vaccination. iScience 2021, 24. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Ng, T.; Flores-Malavet, V.; Mansoor, M.A.M.; Arvelo, A.C.; Dhume, K.; Prokop, E.; McKinstry, K.K.; Strutt, T.M. Intermediate Levels of Pre-Existing Protective Antibody Allow Priming of Protective T Cell Immunity against Influenza. The Journal of Immunology 2023, 210, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Hachim, A.; Cowling, B.J.; Valkenburg, S.A. Repeated Influenza Vaccination Provides Cumulative Protection from Distinct H3N2 Viruses. Clin Transl Immunology 2021, 10. [Google Scholar] [CrossRef]
- Cevirgel, A.; Shetty, S.A.; Vos, M.; Nanlohy, N.M.; Beckers, L.; Bijvank, E.; Rots, N.; van Beek, J.; Buisman, A.; van Baarle, D. Pre-vaccination Immunotypes Reveal Weak and Robust Antibody Responders to Influenza Vaccination. Aging Cell 2023. [Google Scholar] [CrossRef]
- Künzel, W.; Glathe, H.; Engelmann, H.; Van Hoecke, C. Elsevier Kinetics of to Trivalent Humoral Antibody Response Inactivated Split Influenza Vaccine in Subjects Previously Vaccinated or Vaccinated for the First Time; 1996; Vol. 14;
- Kim, S.S.; Flannery, B.; Foppa, I.M.; Chung, J.R.; Nowalk, M.P.; Zimmerman, R.K.; Gaglani, M.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Effects of Prior Season Vaccination on Current Season Vaccine Effectiveness in the United States Flu Vaccine Effectiveness Network, 2012-2013 Through 2017-2018. Clinical Infectious Diseases 2021, 73, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, Y.; Nakayama, T.; Sugawara, T.; Ohkusa, Y. Negative Effect on Immune Response of Repeated Influenza Vaccination and Waning Effectiveness in Interseason for Elderly People. Vaccine 2020, 38, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Fukushima, W.; Morikawa, S.; Fujioka, M.; Matsushita, T.; Kubota, M.; Yagi, Y.; Takasaki, Y.; Shindo, S.; Yamashita, Y.; et al. Influence of Prior Influenza Vaccination on Current Influenza Vaccine Effectiveness in Children Aged 1 to 5 Years. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef]
- Kitamura, S.; Matsushita, M.; Komatsu, N.; Yagi, Y.; Takeuchi, S.; Seo, H. Impact of Repeated Yearly Vaccination on Immune Responses to Influenza Vaccine in an Elderly Population. Am J Infect Control 2020, 48, 1422–1425. [Google Scholar] [CrossRef]
Figure 1.
Effect of influenza vaccination on the response to the booster dose of anti-SARS-CoV-2 vaccine. Anti-spike antibody titers (A) and neutralizing antibody titers (B) in individuals who received both anti-flu and anti-SARS-CoV-2 vaccines (F+C) and those who only received the anti-SARS-CoV-2 vaccine (C) before receiving the mRNA-based SARS-CoV-2 vaccine (T0), after one month (T1) and after 3 months post vaccination. Statistically significant differences between groups are shown with their relative p values.
Figure 1.
Effect of influenza vaccination on the response to the booster dose of anti-SARS-CoV-2 vaccine. Anti-spike antibody titers (A) and neutralizing antibody titers (B) in individuals who received both anti-flu and anti-SARS-CoV-2 vaccines (F+C) and those who only received the anti-SARS-CoV-2 vaccine (C) before receiving the mRNA-based SARS-CoV-2 vaccine (T0), after one month (T1) and after 3 months post vaccination. Statistically significant differences between groups are shown with their relative p values.
Figure 2.
Humoral immunity to influenza vaccination. (A) Graph showing the basal (D0) HI titers and the response at day 28 (D28) for each one of the four influenza virus strains after vaccination. (B) Results from the microneutralization assays performed on the same individuals shown in A. The neutralization capacity is expressed as the reciprocal of the highest dilution of the donor’s serum at which virus infection is blocked. (C) Percentages of seroprotection in vaccinees at baseline (D0) and day 28 (D28) for each one of the four influenza virus strains.
Figure 2.
Humoral immunity to influenza vaccination. (A) Graph showing the basal (D0) HI titers and the response at day 28 (D28) for each one of the four influenza virus strains after vaccination. (B) Results from the microneutralization assays performed on the same individuals shown in A. The neutralization capacity is expressed as the reciprocal of the highest dilution of the donor’s serum at which virus infection is blocked. (C) Percentages of seroprotection in vaccinees at baseline (D0) and day 28 (D28) for each one of the four influenza virus strains.
Figure 3.
Stratification of the subjects based on the response to the four influenza virus strains. The study population was stratified in five groups considering as a reference the median of HI titer (A, B) and the microneutralization titer (MN) (C-D) of each post-vaccination specific viral antigen: individuals with no specific HI or MN titer above the specific median post vaccination (D28) (0) and individuals with respectively, one (1), two (2), three (3) and four (4) specific HI or MN titers above the specific median at D28 post vaccination. Participants belonging to the group 0 or 1 were classified as low responders (LR) and participants belonging to the groups 2, 3, or 4, were classified as high responders (HR). The differences between LR and HR are statistically significant (p<0.001).
Figure 3.
Stratification of the subjects based on the response to the four influenza virus strains. The study population was stratified in five groups considering as a reference the median of HI titer (A, B) and the microneutralization titer (MN) (C-D) of each post-vaccination specific viral antigen: individuals with no specific HI or MN titer above the specific median post vaccination (D28) (0) and individuals with respectively, one (1), two (2), three (3) and four (4) specific HI or MN titers above the specific median at D28 post vaccination. Participants belonging to the group 0 or 1 were classified as low responders (LR) and participants belonging to the groups 2, 3, or 4, were classified as high responders (HR). The differences between LR and HR are statistically significant (p<0.001).
Figure 4.
Stratification of subjects as low responders (LR) and high responders (HR) based on sex and age. The study population was stratified according to sex, male (A, HI and E, MN) and female (B, HI and F, MN) and to age, < 60 (C, HI and G, MN) and ≥ 60 (D, HI and H, MN) years old. The differences between LR and HR are significant in all analyzed subgroups (p<0.01).
Figure 4.
Stratification of subjects as low responders (LR) and high responders (HR) based on sex and age. The study population was stratified according to sex, male (A, HI and E, MN) and female (B, HI and F, MN) and to age, < 60 (C, HI and G, MN) and ≥ 60 (D, HI and H, MN) years old. The differences between LR and HR are significant in all analyzed subgroups (p<0.01).
Figure 5.
Effect of influenza vaccination on the response to the booster dose of SARS-CoV-2 vaccine in high (HR) and low (LR) responders. Anti-spike antibody titers (A, B) and neutralizing antibody titers (C, D) in HR, LR, and individuals who received only the anti-SARS-CoV-2 vaccine (NO-FLU-VAX) are shown at one month (T1) and 3 months post SARS-CoV-2 vaccination. Statistically significant differences between groups are shown with their relative p values.
Figure 5.
Effect of influenza vaccination on the response to the booster dose of SARS-CoV-2 vaccine in high (HR) and low (LR) responders. Anti-spike antibody titers (A, B) and neutralizing antibody titers (C, D) in HR, LR, and individuals who received only the anti-SARS-CoV-2 vaccine (NO-FLU-VAX) are shown at one month (T1) and 3 months post SARS-CoV-2 vaccination. Statistically significant differences between groups are shown with their relative p values.
Figure 6.
Positive association between the influenza-specific antibody response and anti-spike antibody levels in double vaccinated participants. A linear regression model was applied considering values relative to samples collected after one (A) and three months (B) post SARS-CoV-2 vaccination in participants who received both the quadrivalent influenza vaccine and the anti-SARS-CoV-2 vaccine. Each line represents the levels of anti-spike antibodies for the four different flu-antigens. Statistical analysis reveals significant effects of flu-antigen on anti-spike antibodies at both time points T1 and T3 (1- and 3-months post-vaccination, respectively).
Figure 6.
Positive association between the influenza-specific antibody response and anti-spike antibody levels in double vaccinated participants. A linear regression model was applied considering values relative to samples collected after one (A) and three months (B) post SARS-CoV-2 vaccination in participants who received both the quadrivalent influenza vaccine and the anti-SARS-CoV-2 vaccine. Each line represents the levels of anti-spike antibodies for the four different flu-antigens. Statistical analysis reveals significant effects of flu-antigen on anti-spike antibodies at both time points T1 and T3 (1- and 3-months post-vaccination, respectively).
Table 1.
Sociodemographic characteristics of the study population.
Table 1.
Sociodemographic characteristics of the study population.
| |
two vaccines (n=54, 73%) |
one vaccine (n=20, 27%) |
total (n=74) |
| Gender |
n. |
% |
n. |
% |
n. |
% |
| male |
18 |
33 |
6 |
30 |
24 |
32 |
| female |
36 |
67 |
14 |
70 |
50 |
68 |
| Age |
|
|
|
|
|
|
| median |
57 |
51 |
54 |
| IQR; range |
(49-60) ; (35-70) |
(49-56) ; (31-72) |
(49-60) ; (31-72) |
| Education |
|
|
|
|
|
|
| Higher |
41 |
76 |
12 |
60 |
53 |
72 |
| Middle or lower |
13 |
24 |
8 |
40 |
21 |
28 |
| Fraility |
|
|
|
|
|
|
| Very fit |
8 |
15 |
7 |
35 |
15 |
20 |
| Fit |
39 |
72 |
12 |
60 |
51 |
69 |
| Managing well |
7 |
13 |
1 |
5 |
8 |
11 |
| Body mass index |
|
|
|
|
|
|
| Underweight |
1 |
2 |
0 |
0 |
1 |
1 |
| Healthy Weight |
30 |
55 |
12 |
60 |
42 |
57 |
| Overweight |
20 |
37 |
6 |
30 |
26 |
35 |
| Obesity |
3 |
6 |
2 |
10 |
5 |
7 |
| Smoking |
|
|
|
|
|
|
| yes |
7 |
13 |
5 |
25 |
12 |
16 |
| no |
47 |
87 |
15 |
75 |
62 |
84 |
| Medical conditions |
|
|
|
|
|
|
| yes |
23 |
43 |
11 |
55 |
34 |
46 |
| no |
31 |
57 |
9 |
45 |
40 |
54 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).