1. Introduction
As life expectancy continues to rise, the prevalence of brain aging and cognitive-related diseases is increasing[
1,
2,
3]. Aging often accompanies changes in brain structure markers, which may contribute to the development of cognitive-related diseases[
4]. Existing research has indicated longitudinal changes in brain structural markers can to some extent reflect alterations in the progression of neurodegenerative diseases[
5,
6,
7,
8]. It is crucial to identify modifiable risk factors to slow down the changes in neurodegeneration-related brain structure markers.
Many studies have suggested that dietary factors, as a modifiable component of lifestyle, play a vital role in preserving brain health[
9,
10,
11]. Protein, as a primary component of the diet, has garnered widespread attention in this context. They constitute fundamental components of neurotransmitters and neurons, contributing to the maintenance of brain structure and function [
12]. Research suggested that moderate dietary protein intake may reduce the brain amyloid-beta (Aβ) plaques burden in older adults to delay the onset of Alzheimer's disease[
13,
14]. Therefore, we speculate that alterations in dietary protein intake may contribute to changes in brain structure markers.
However, few studies have examined the dietary protein intake and sources in combination with the longitudinal changes in brain structure markers. Moreover, in previous studies on brain structural markers, most were cross-sectional and used a single wave of brain image data.
Therefore, we employed a cohort study conducted by the UK Biobank (UKB), utilizing two waves of brain imaging data, to examine the association between dietary protein intake and different sources of dietary protein with the longitudinal rate of change in brain structural markers.
2. Materials and Methods
2.1. Study Population
The data used in this study were obtained from the UK Biobank (UKB), comprising participants aged 40–69 years at the time of recruitment in 2006–2010[
15]. It recruited over 500,000 UK residents through 22 assessment centers[
16]. Following the dietary assessments, the UKB initiated a multi-modal imaging sub-study[
17], with nearly 50,000 participants assessed by the end of 2019. From 2018 to 2022, participants from the initial assessment were invited to undergo repeat imaging assessments. All UKB participants provided written informed consent and ethical approval for this research was obtained from the North West-Haydock Research Ethics Committee, with reference number 16/NW/0274. The current study was conducted under application number 95715, utilizing the resources provided by the UKB. Details can be found on the UKB website (
http://www.ukbiobank.ac.uk/resources/).
2.2. Assessment of Dietary Protein Intake and Sources
Dietary information was obtained through a 24-hour dietary recall questionnaire. Participants in the UK Biobank (UKB) took part in 5 waves of online surveys during this period (2009-2012). Using the acquired dietary data in the UKB, the daily protein intake (g/d) for each participant was calculated, including both plant and animal protein. [
18,
19]. In addition, we calculated the ratio of animal protein to vegetable protein in relation to total protein intake. We also compared the consumption of animal protein to vegetable protein intake. These calculations were made to provide insights into the participants' dietary protein levels.
In addition, based on previous studies on protein sources in European diets[
20,
21], we adjusted the classification of dietary protein sources. We categorized into 8 types of animal protein sources and 3 types of plant protein sources. Detailed information and codes regarding food protein sources can be found in the Supplementary Table1.
2.3. The Rate of Change in Brain Structural Markers
MRI data were acquired during the third (2014+) and fourth assessment (2019+) visits at three imaging centers equipped with identical scanners (Siemens Skyra 3T running VD13A SP4 with a Siemens 32-channel RF receive head coil, Munich, Germany). The average interval between assessments is 3 years. Structural magnetic resonance imaging (MR) imaging was utilized to estimate total brain volume (TBV), gray matter volume (GMV), white matter volume (WMV), hippocampus volume (HV), and white matter high-intensity volume (WMHV). The MR imaging protocols have been detailed elsewhere[
17]. Full information on structural image segmentation and data normalization is available elsewhere[
22]. Publicly available image processing tools, primarily from the FMRIB Software Library, were employed for data processing, utilizing the output of the standard Biobank processing pipeline. All data were normalized for head size. Additionally, a new variable, representing the rate of change in brain structure markers, was calculated to depict the longitudinal changes in the brain structure of the participants.
The rate of longitudinal change in brain structural markers= [brain image data (2014+)- brain image data (2019+)]/brain image data (2014+)
2.4. Covariates
In our study, certain confounding factors were adjusted for[
23,
24]. Demographic characteristics were collected at recruitment. They included age, sex, energy, ethnicity, education and Townsend Deprivation Index (TDI). TDI represented social deprivation status and was categorized as low, medium, or high deprivation. Participants' education was categorized as college, above or below. Physical activity, smoking, alcohol consumption, and body mass index (BMI) were adjusted for as lifestyle factors. Participants' BMI was categorized as underweight (BMI < 18.5), normal weight (BMI ≥ 18.5 but < 25.0), and overweight/obese (BMI ≥ 25.0). Participants were grouped into low, moderate, and high activity levels based on metabolic equivalent minutes per week. The baseline disease status, encompassing cancer, cardiovascular diseases (CVDs), hypertension, and diabetes, was determined using electronic records. Additionally, we accounted for the Polygenic Risk Score for Alzheimer’s disease (AD-PRS) to control for genetic factors.
2.5. Statistical Analyses
The baseline characteristics of participants were described separately by gender. Continuous variables were presented as mean ± standard deviation. Categorical variables were expressed as percentages. We used a multiple linear regression model to investigate the relationship between dietary protein intake (animal protein, plant protein, and total protein), the ratio of animal protein to plant protein in total protein intake, and the relative comparison of animal protein to plant protein, with changes in brain structure. Additionally, data were transformed based on the distribution type of variables to approximate a normal or symmetrical distribution. To control for the influence of confounding factors, we established three models for adjustment. The β was adjusted for age and sex in model 1. Model 2 was additionally adjusted for TDI, education, energy, physical activity, smoking (ever smoked or not), alcohol intake (ever drunk or not), body weight status. Model 3 was additionally adjusted for baseline cancer, CVDs, hypertension, and diabetes. To address the issue of multicollinearity among the independent variables, we calculated variance inflation factors (VIF) and tolerances for collinearity diagnosis. In addition, to examine the presence of nonlinear relationships, we also introduced restricted cubic spline analysis to explore dose-response relationships in our analysis.
We further analyzed dietary protein sources and examined the associations between various dietary protein sources and changes in brain structural biomarkers using multiple linear regression, with multiple adjustments made. Participants were categorized based on the source of dietary protein intake. Those with a dietary protein intake of 0 were classified into the none-intake group, while non-zero intake levels were divided into lower and higher intake groups based on median of intake.
In sensitivity analyses, we performed subgroup analyses based on gender to explore the impact of different genders on the results. Furthermore, to control for the influence of genetic factors, we additionally adjusted for AD-PRS. Additionally, we restricted the analysis to participants who had completed at least two dietary recalls.
Statistical analyses were performed using R 4.2.3, and 2-sided P values < 0.05 were considered statistically significant.
3. Results
3.1. Participant Characteristics
Among the 210,948 UKB participants who had at least one dietary assessment, we excluded participants who were missing data from two waves of imaging, as well as those with incomplete dietary data (total energy =0 MJ or ≥20 MJ[
25,
26]) and missing covariates. This resulted in a final analysis dataset comprising 2,723 and 2,679 participants, as dtailed in the flowchart (
Figure 1). The average follow-up time is 8 years.
Among the 2,723 participants in the study on TBV, WMV, GMV, and HV, the average (SD)age was 52.66(7.42), and 51.7% were female (
Table 1). In the additional group of 2,679 participants that included WMHV, the average (SD) age was 52.7 (7.41), with 51.7% being female (
Supplemental Table 2). Men are more likely than women to have higher intake of protein, animal protein, plant protein, and energy. They are also more likely to suffer from cardiovascular diseases, diabetes, and hypertension.
3.2. Dietary Protein and Brain Structure
Significant association was not observed between dietary protein intake (including animal and plant protein) and longitudinal changes in TBV, WMV, WMHV, and GMV. However, significant association was found in terms of HV (
Table 2,
Figure 2,
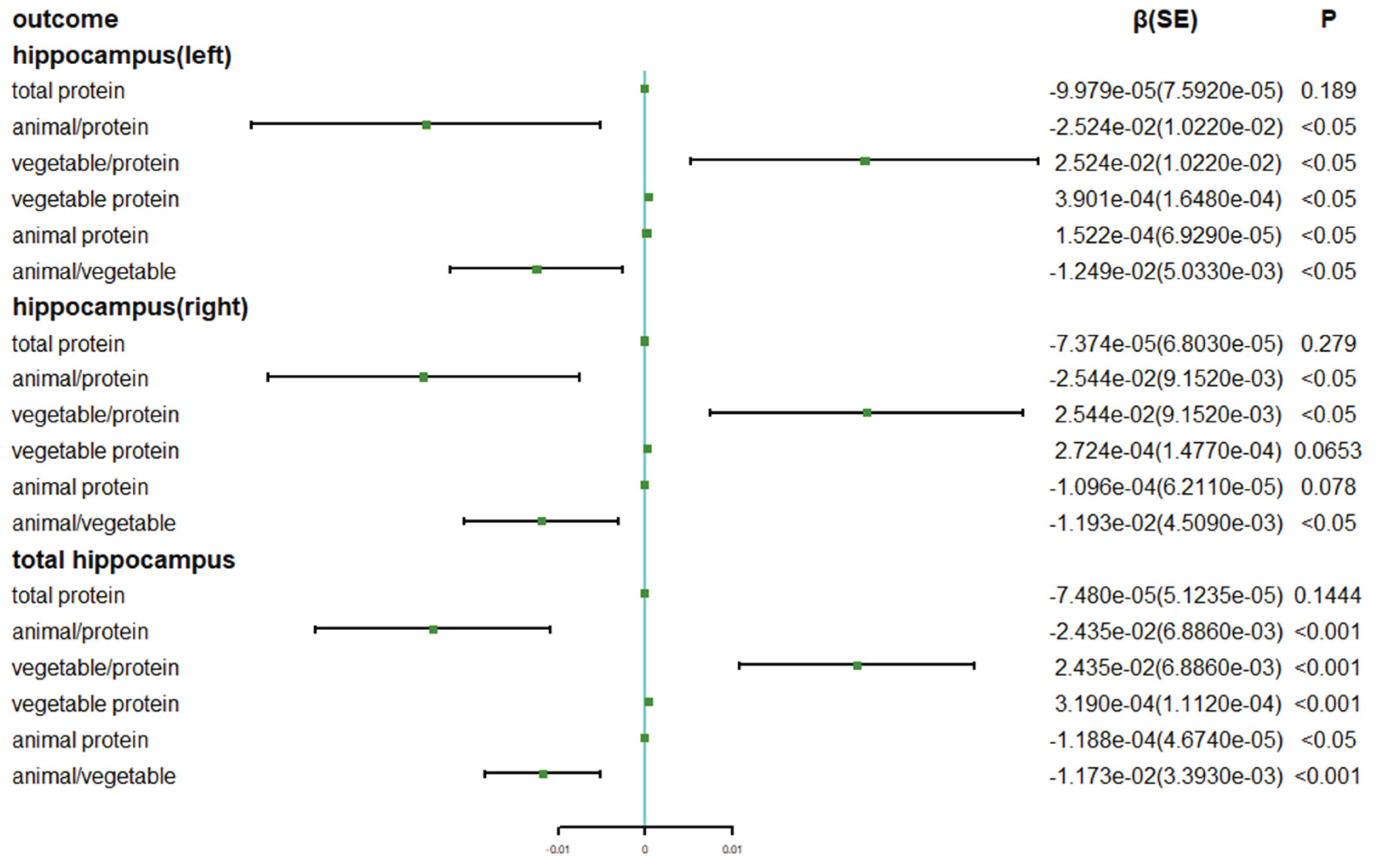
Supplemental Tables 3 and 4). In the overall adjustment model, increasing the proportion of animal protein in dietary protein intake or decreasing plant protein is associated with a slower reduction in the total hippocampus volume (THV, β: -0.02524, P<0.05), left hippocampus volume (LHV, β: -0.02435, P<0.01) and right hippocampus volume (RHV, β: -0.02544, P<0.05). Higher intake of animal protein relative to plant protein is linked to a lower atrophy rate in THV (β: -0.01249, P<0.05) and LHV (β: -0.01173, P<0.05) and RHV (β: -0.01193, P<0.05). However, there was no significant association observed between the total dietary protein intake and the longitudinal rate of change in hippocampus volume.
After adjusting for all factors, the longitudinal rate of change in THV and LHV shows a significant negative correlation with the absolute intake of dietary animal protein (LHV, β: -1.522e-04, P<0.05; THV: β: -1.188e-04, P<0.05), while plant protein exhibits a positive correlation (LHV: β: 0.0003901, P<0.05; THV: β: 0.000319, P<0.01. However, such an association was not observed in the analysis of the right hippocampus (
Table 2,
Figure 2).
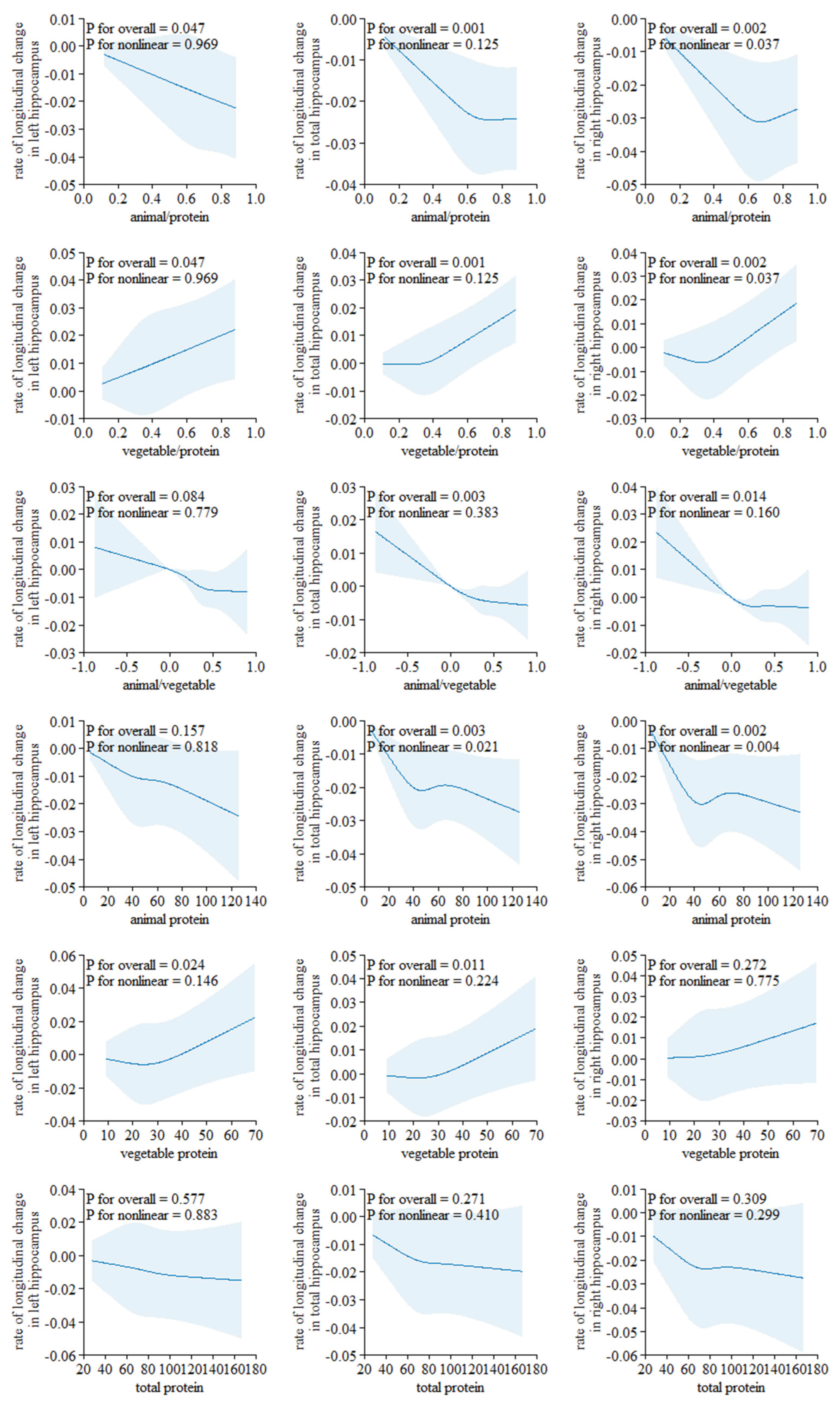
The results from the restricted cubic spline analysis indicate a clear non-linear association in right hippocampus (
Figure 3). Both the proportion of animal protein and plant protein to total protein, and the intake of animal protein show evident non-linear associations with the longitudinal rate of change in the volume of the right hippocampus (P for overall <0.05 and all P for nonlinearity <0.05). The proportion of plant protein to total protein shows an approximately "J"-shaped association with the rate of change in hippocampal volume. However, no non-linear associations were found between the longitudinal rate of change in other brain structure biomarkers and protein intake (P for overall >0.05 and all P for nonlinearity >0.05,
Supplemental Figures 1 and 2).
3.3. Dietary Protein Sources and Brain Structure
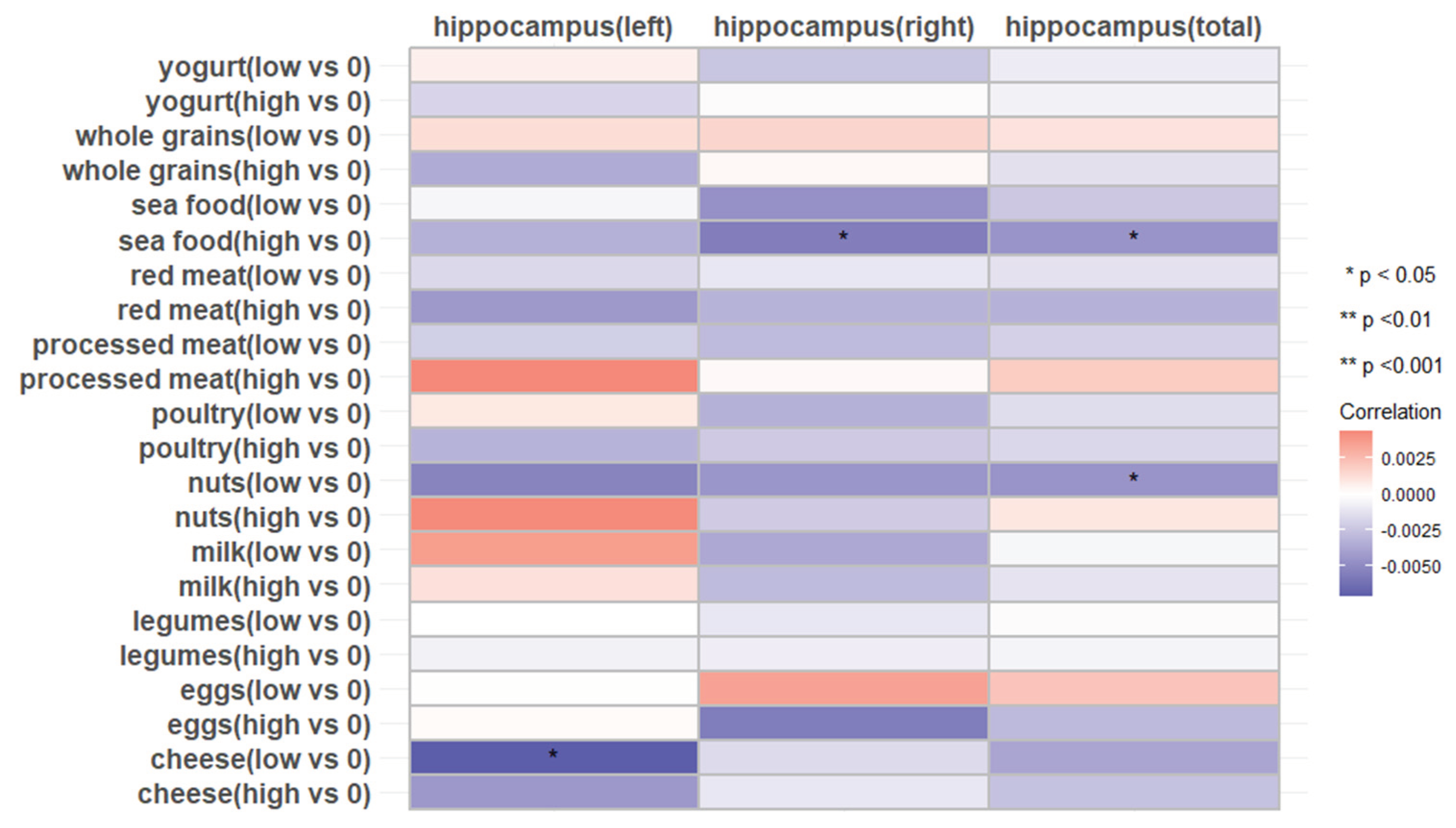
In the total hippocampus and the right hippocampus, individuals with higher intake of seafood exhibit lower longitudinal rates of change compared to those with no consume (THV, β: -0.004514; P<0.05; RHV, β: -0.005527, P<0.05), but this was not observed in individuals with lower seafood intake(P>0.05). Similarly, Individuals with relatively lower nuts intake exhibit a lower rate of atrophy in the total hippocampus compared to those who didn’t consume nuts, while no significant association was observed at higher intake level. Lower cheese intake is associated with a lower longitudinal rate of change in the left hippocampus (
Figure 4 and
Supplemental Table 5).
3.4. Subgroup and Sensitivity Analyses
In males, the reduction in plant protein intake and proportion was associated with a lower rate of volume atrophy in the total hippocampus and the left hippocampus (P<0.05,
Supplemental Table 6). In females, after adjusting for covariates, there was a significant negative correlation between the proportion of animal protein to total protein and the rate of volume change in the hippocampus (including total, left, and right, P<0.05). However, a significant negative association with animal protein intake was only found in the total volume of the hippocampus (
Supplemental Table 7). We further adjusted for AD-PRS. The association between dietary protein intake and the longitudinal change rate of the hippocampus did not show significant changes (
Supplemental Table 8). Additionally, we repeated the analysis by including participants with at least two waves of dietary data. We found that the results did not significantly alter (
Supplemental Table 9).
4. Discussion
In this prospective study conducted in the UKB, we observed that the increase in animal protein intake and proportion (or a decrease in plant protein intake and proportion) were associated with a lower longitudinal rate of atrophy in hippocampus volume. In gender subgroups, these associations were similar, and there was not a significantly alteration by the genetic risk of dementia. Additionally, higher intake of seafood and cheeses were associated with a lower longitudinal rate of change in hippocampus volume.
Our study did not find any association between total protein intake and the longitudinal change rate of brain structural markers. Conversely, higher intake of animal protein and the proportion of animal protein to total protein intake showed a positive association with the longitudinal change rate of hippocampus volume. Findings from previous studies suggested that Participants with a higher proportion of protein in their diet had a lower risk of dementia [
27,
28,
29]. However, in another study, it was found that patients with neurodegenerative diseases had similar protein intake compared to the control group. Seafood is a good source of dietary protein. We hypothesize that, concerning the longitudinal change rate of hippocampus volume, the impact on brain health may not be solely related to the absolute dietary intake of protein but is more likely influenced by altering the proportion of animal protein in the diet. The association may also be explained through dietary protein sources such as seafood. Additionally, a cross-sectional study showed a significant association between seafood consumption (at least one meal per week) and reduced pathological changes in the brain[
30]. Further in-depth research and exploration are warranted.
Animal protein and plant protein exhibit significant differences in their amino acid composition[
31,
32,
33,
34]. Animal protein especially from sea food typically contains all essential amino acids (EAA). These amino acids are essential for the human body, and the composition and proportion of amino acids in animal protein are similar to those in the human body. In contrast, plant protein may sometimes lack or have lower levels of certain EAA such as branched-chain amino acids (BCAA, especially leucine), tryptophan, and lysine[
35,
36,
37]., making it an incomplete protein. On the other hand, plant proteins often come with some antinutrients, such as lectins, phytic acid, and saponins. Furthermore, animal protein is generally more easily absorbed and utilized by the human body, with a higher biological availability. In addition, low BCAA levels may impair brain structure and function[
38,
39]. Current research suggested that BCAAs, particularly leucine, may serve as crucial donors of nitrogen in the brain, participating in the cycle of glutamate and glutamine[
40]. Glutamate, a major excitatory neurotransmitter in the brain, regulates various functions within the brain[
41,
42]. Secondly, the cycle between glutamate and glutamine in the brain may be restricted due to low brain BCAA levels. This could lead to the accumulation of glutamate and ammonia in the brain, resulting in neurotoxicity and neurodegenerative changes that impair brain health [
38,
42]. Thirdly, reduced levels of brain BCAAs may diminish protein synthesis, impacting the repair of brain tissue, synaptic growth, and remodeling [
38,
43]. Fourth, supplementation of branched-chain amino acids may potentially reduce the levels of neuroinflammation in the brain to protect its structure[
44]. These may explain our results between animal protein intake and the rate of brain atrophy.
To the best of our knowledge, there has been limited exploration into the longitudinal relationship between dietary factors, and markers of brain structure. We adjusted for multiple covariates to control for the influence of confounding factors and stratified the analysis by gender to examine differences between different gender groups. Furthermore, we utilized both absolute and relative indicators to evaluate the extent of dietary protein intake. Previous studies investigating the association of lifestyle or dietary factors with brain structural markers have predominantly relied on single-wave brain imaging data as outcome measures [
9,
11,
26], introducing variability due to individual differences and the influence of different imaging centers. In contrast, our study employed two waves of brain imaging data to calculate longitudinal change rates. This approach aimed to comprehensively understand the influence of dietary factors on brain structure while mitigating potential confounding factors.
However, there are still some limitations. Firstly, a 24-hour dietary recall may not accurately represent long-term dietary habits, and the data, being self-reported, may introduce recall bias. Although we averaged multiple waves of dietary data to reduce random errors and mitigate the impact of individual variations. Secondly, due to potential residual confounding and reverse causation, our study results may not necessarily reflect a causal relationship, even though the imaging assessment was conducted long after the dietary assessment. Thirdly, imaging examinations may have the potential to exert health-promoting effects on participants. Last, in the current study, the majority of the study population consists of individuals of white. Prudence is advised when extrapolating the results to different populations.
5. Conclusions
In conclusion, current research findings indicate that a moderate increase in the intake and proportion of animal protein in the diet, especially from seafood, is associated with a lower longitudinal change rate in hippocampus volume. These associations are consistent across subgroups and are not altered by genetic susceptibility to dementia. Additional extensive longitudinal studies involving diverse populations are required to validate research findings and inform public health initiatives focused on enhancing brain health.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplemental Table 1: Dietary Protein Sources Classification and Codes; Supplemental Table 2: Baseline characteristics of study participants on white matter hyperintensities(N=2679); Supplemental Table 3: Association between dietary protein intake and longitudinal change rate of total brain, white matter, grey matter volume; Supplemental Table 24: Association between dietary protein intake and longitudinal change rate of white matter hyperintensities(N=2679); Supplemental Figure 1: Non-linear associations of dietary protein with the longitudinal change rate of brain structure markers Using a Restricted Cubic Spline Regression Model (N= 2723); Supplemental Figure 2:Non-linear associations of dietary protein with the longitudinal change rate of white matter hyperintensities Using a Restricted Cubic Spline Regression Model (N= 2679); Supplemental Table 5: Association between dietary protein sources with the longitudinal change rate of hippocampus volume (N=2723); Supplemental Table 6: Association between dietary protein intake with the longitudinal change rate of hippocampus volume in male(N=1337); Supplemental Table 7: Association between dietary protein intake with the longitudinal change rate of hippocampus volume in female(N=1386); Supplemental Table 8: Association between dietary protein intake with the longitudinal change rate of hippocampus volume adjusted for AD-PRS(N=1386); Supplemental Table 9: Association between dietary protein intake with the longitudinal change rate of hippocampus volume among participants with at least two waves of dietary data (N=2009)
Author Contributions
Conceptualization, F.C. and D.Z.; methodology, F.C.; software, F.C.; validation, W.W., F.C., H.L., Y.C. and D.Z.; data curation, F.C.; writing—original draft preparation, F.C..; writing—review and editing, F.C. and D.Z.; visualization, F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82073641)
Institutional Review Board Statement
This study was covered by the generic ethical approval for UK Biobank studies from the National Research Ethics Service Committee North West–Haydock (Ref 16/NW/0274)
Informed Consent Statement
All participants gave written informed consent.
Data Availability Statement
The data that support the findings of this study are available from UK Biobank (
http://www.ukbiobank.ac.uk/about-biobank-uk/ accessed on Feb 2023). Restrictions apply to the availability of these data, which were used under license for the current study (Project ID: 95715). Data are available for bona fide researchers upon application to the UK Biobank.
Acknowledgments
This research has been conducted using the UK Biobank Resource under Application Number ‘95715’.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murray, C.J.L.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, H.; Wardlaw, J.M.; Wang, Y. A new dawn of preventing dementia by preventing cerebrovascular diseases. BMJ 2020, 371. [Google Scholar] [CrossRef] [PubMed]
- Gauthier Sea. World Alzheimer Report 2022: Life After Diagnosis: Navigating Treatment, Care And Support. Alzheimer’s Disease International.
- Fjell, A.M.; Walhovd, K.B. Structural Brain Changes in Aging: Courses, Causes and Cognitive Consequences. Prog. Neurobiol. 2010, 21, 187–222. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Bakkour, A.; Salat, D.H.; Feczko, E.; Pacheco, J.; Greve, D.N.; et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex (New York, NY : 1991) 2009, 19, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; et al. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimer's & dementia: the journal of the Alzheimer's Association 2016, 12, 292–323. [Google Scholar]
- Elias, M.F.; Beiser, A.; Wolf, P.A.; Au, R.; White, R.F.; D'Agostino, R.B. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Archives of Neurology 2000, 57, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shen, J.; Cai, X.; Chen, H.; Zong, G.; Zhu, W.; Jing, J.; Liu, T.; Jin, A.; Wang, Y.; et al. Adherence to a healthy lifestyle and brain structural imaging markers. Eur. J. Epidemiology 2023, 38, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.E.; Leoni, V.; Klein-Flügge, M.C.; Ebmeier, K.P.; Suri, S. Associations of dietary markers with brain volume and connectivity: A systematic review of MRI studies. Ageing Res. Rev. 2021, 70, 101360. [Google Scholar] [CrossRef] [PubMed]
- Croll, P.H.; Voortman, T.; Ikram, M.A.; Franco, O.H.; Schoufour, J.D.; Bos, D.; et al. Better diet quality relates to larger brain tissue volumes: The Rotterdam Study. Neurology 2018, 90, e2166–e2173. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Tyrovolas, S.; Panagiotakos, D.B. Red meat consumption and healthy ageing: A review. Maturitas 2015, 84, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Hill, E.; Zhu, Z.; Liu, J.; Ge, Z.; Wang, W.; He, M. Macronutrient Intake and Risk of Dementia in Community-Dwelling Older Adults: A Nine-Year Follow-Up Cohort Study. J. Alzheimer's Dis. 2022, 85, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.; Rainey-Smith, S.R.; Gardener, S.L.; Villemagne, V.L.; Burnham, S.C.; Macaulay, S.L.; et al. Associations of Dietary Protein and Fiber Intake with Brain and Blood Amyloid-β. Journal of Alzheimer's disease : JAD 2018, 61, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Lin L, Xiong M, Wu S. [A review on the application of UK Biobank in neuroimaging]. Sheng wu yi xue gong cheng xue za zhi = Journal of biomedical engineering = Shengwu yixue gongchengxue zazhi 2021, 38, 594–601. [Google Scholar]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Alfaro-Almagro, F.; Bangerter, N.K.; Thomas, D.L.; Yacoub, E.; Xu, J.; Bartsch, A.J.; Jbabdi, S.; Sotiropoulos, S.N.; Andersson, J.L.R.; et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 2016, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; A Spencer, E.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. PhotoniX 2011, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Pollard, Z.; Young, H.; van Uden, M.; Andrews, C.; Piernas, C.; Key, T.J.; Mulligan, A.; Lentjes, M. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur. J. Nutr. 2021, 60, 4019–4030. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Glenn, A.J.; Yang, Q.; Ding, D.; Zheng, L.; Bao, W.; et al. Dietary Protein Sources, Mediating Biomarkers, and Incidence of Type 2 Diabetes: Findings From the Women's Health Initiative and the UK Biobank. Diabetes Care 2022, 45, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, J.; Olsen, A.; Bjerregaard, L.J.; Deharveng, G.; Tjønneland, A.; A Welch, A.; Crowe, F.L.; Wirfält, E.; Hellstrom, V.; Niravong, M.; et al. Intake of total, animal and plant proteins, and their food sources in 10 countries in the European Prospective Investigation into Cancer and Nutrition. Eur. J. Clin. Nutr. 2009, 63, S16–S36. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Almagro, F.; Jenkinson, M.; Bangerter, N.K.; Andersson, J.L.; Griffanti, L.; Douaud, G.; Sotiropoulos, S.N.; Jbabdi, S.; Hernandez-Fernandez, M.; Vallee, E.; et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 2017, 166, 400–424. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Himali, J.J.; Beiser, A.S.; Aparicio, H.J.; Satizabal, C.L.; Vasan, R.S.; et al. Sugar- and Artificially Sweetened Beverages and the Risks of Incident Stroke and Dementia: A Prospective Cohort Study. Stroke 2017, 48, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hypponen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J.; Gray, S.R.; Welsh, P.; Mackay, D.F.; Celis-Morales, C.A.; Lyall, D.M.; et al. The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: a prospective cohort study. BMC Medicine 2020, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, J.; Cao, Y.; Sun, Y.; Huang, L.; Ji, J.S.; Voortman, T.; Vernooij, M.W.; Shen, J.; Zheng, Y.; et al. Sugary beverages and genetic risk in relation to brain structure and incident dementia: a prospective cohort study. Am. J. Clin. Nutr. 2023, 117, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Roberts, L.A.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; O'Connor, H.M.; Knopman, D.S.; Petersen, R.C. Relative Intake of Macronutrients Impacts Risk of Mild Cognitive Impairment or Dementia. J. Alzheimer's Dis. 2012, 32, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kawashima, R. Nutrients and Dementia: Prospective Study. Nutrients 2023, 15, 842. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, H.; Pan, S.; Bai, X.; Zhu, Z.; Zhang, R.; Li, C.; Chen, Y.; Bao, M.; Zhang, K.; et al. Causal Relationships Between Relative Intake from the Macronutrients and Alzheimer’s Disease: A Two-Sample Mendelian Randomization Study. J. Alzheimer's Dis. 2022, 87, 665–673. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of Fish and n-3 Fatty Acids and Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Marinangeli, C.P.F.; House, J.D. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr. Rev. 2017, 75, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Rehm, C.D.; Rogers, G. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality among US Adults, 1999–2016. JAMA: J. Am. Med Assoc. 2019, 322, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Dietary protein quality evaluation in human nutrition. Report of an FAQ Expert Consultation. FAO food and nutrition paper 2013, 92, 1–66.
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S. High Protein Foods: A Comparison of Animal Origin. In Plant Protein Foods; Manickavasagan, A., Lim, L.-T., Ali, A., Eds.; Springer International Publishing: Cham, 2022; pp. 1–25. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones' Factors. Critical Reviews in Food Science and Nutrition 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- García-Espinosa, M.A.; Wallin, R.; Hutson, S.M.; Sweatt, A.J. Widespread neuronal expression of branched-chain aminotransferase in the CNS: implications for leucine/glutamate metabolism and for signaling by amino acids. J. Neurochem. 2006, 100, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Costa, A.; Maestri, R.; Cotta Ramusino, M.; Perini, G.; Boselli, M.; et al. Is the Brain Undernourished in Alzheimer's Disease? Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Platell, C.; Kong, S.E.; McCauley, R.; Hall, J.C. Branched-chain amino acids. J Gastroenterol Hepatol 2000, 15, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Hutson, S.M.; Islam, M.M.; Zaganas, I. Interaction between glutamate dehydrogenase (GDH) and L-leucine catabolic enzymes: intersecting metabolic pathways. (1872-9754 (Electronic)).
- Conway, M. Hutson SJTGG-GCAANH. BCAA metabolism and NH 3 homeostasis. 2016, 99-132.
- Cole, J.T.; Mitala, C.M.; Kundu, S.; Verma, A.; Elkind, J.A.; Nissim, I.; Cohen, A.S. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc. Natl. Acad. Sci. 2009, 107, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, F.; Sun, L.; Yu, J.; Sun, L.; Si, Y.; Yao, L. Role of gut microbiota-derived branched-chain amino acids in the pathogenesis of Parkinson’s disease: An animal study. Brain, Behav. Immun. 2022, 106, 307–321. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Participant inclusion.
Figure 1.
Participant inclusion.
Figure 2.
Associations of dietary protein with the longitudinal change rate of hippocampus volume (N= 2723). Beta coefficients and SE were calculated using general linear models adjusted for age, sex, ethnicity, energy, Townsend deprivation index, education level, physical activity, smoking, body weight status, total energy intake, baseline cancer, CVDs, hypertension, and diabetes.
Figure 2.
Associations of dietary protein with the longitudinal change rate of hippocampus volume (N= 2723). Beta coefficients and SE were calculated using general linear models adjusted for age, sex, ethnicity, energy, Townsend deprivation index, education level, physical activity, smoking, body weight status, total energy intake, baseline cancer, CVDs, hypertension, and diabetes.
Figure 3.
Non-linear associations of dietary protein with the longitudinal change rate of hippocampus volume Using a Restricted Cubic Spline Regression Model (N= 2723). The model was adjusted for age, sex, ethnicity, Townsend deprivation index, education level, physical activity, smoking, body weight status, total energy intake, baseline cancer, CVDs, hypertension, and diabetes.
Figure 3.
Non-linear associations of dietary protein with the longitudinal change rate of hippocampus volume Using a Restricted Cubic Spline Regression Model (N= 2723). The model was adjusted for age, sex, ethnicity, Townsend deprivation index, education level, physical activity, smoking, body weight status, total energy intake, baseline cancer, CVDs, hypertension, and diabetes.
Figure 4.
Associations of 11 dietary protein sources with the longitudinal change rate of hippocampus volume (N= 2723). Beta coefficients and SE were calculated using general linear models adjusted for age, sex, ethnicity, Townsend deprivation index, education level, physical activity, smoking, body weight status, total energy intake, baseline cancer, CVDs, hypertension, and diabetes.
Figure 4.
Associations of 11 dietary protein sources with the longitudinal change rate of hippocampus volume (N= 2723). Beta coefficients and SE were calculated using general linear models adjusted for age, sex, ethnicity, Townsend deprivation index, education level, physical activity, smoking, body weight status, total energy intake, baseline cancer, CVDs, hypertension, and diabetes.
Table 1.
Baseline characteristics of study participants(N=2723).
Table 1.
Baseline characteristics of study participants(N=2723).
| |
Total |
Female |
Male |
P-value |
| n |
2723 |
1407 |
1316 |
|
| age, mean (SD) |
52.66 (7.42) |
51.65 (7.16) |
53.74 (7.54) |
<0.001 |
| sex (%) |
|
|
|
|
| female |
1407 (51.7) |
1407 (100.0) |
|
|
| male |
1316 (48.3) |
|
1316 (100.0) |
|
| MET (%) |
|
|
|
0.376 |
| low |
474 (17.4) |
233 (16.6) |
241 (18.3) |
|
| medium |
1118 (41.1) |
592 (42.1) |
526 (40.0) |
|
| high |
1131 (41.5) |
582 (41.4) |
549 (41.7) |
|
| TDI, mean (SD) |
-1.99 (2.64) |
-1.91 (2.68) |
-2.07 (2.59) |
0.110 |
| smoke (%) |
|
|
|
0.234 |
| never |
1742 (64.0) |
915 (65.0) |
827 (62.8) |
|
| ever smoked |
981 (36.0) |
492 (35.0) |
489 (37.2) |
|
| race (%) |
|
|
|
0.706 |
| others |
80 (2.9) |
43 (3.1) |
37 (2.8) |
|
| white |
2643 (97.1) |
1364 (96.9) |
1279 (97.2) |
|
| drink (%) |
|
|
|
0.101 |
| never |
56 (2.1) |
35 (2.5) |
21 (1.6) |
|
| ever drunk |
2667 (97.9) |
1372 (97.5) |
1295 (98.4) |
|
| education (%) |
|
|
|
0.133 |
| below |
1226 (45.0) |
614 (43.6) |
612 (46.5) |
|
| college or above |
1497 (55.0) |
793 (56.4) |
704 (53.5) |
|
| BMI (%) |
|
|
|
<0.001 |
| Underweight |
16 (0.6) |
13 (0.9) |
3 (0.2) |
|
| Normal weight |
1140 (41.9) |
728 (51.7) |
412 (31.3) |
|
| Overweight and obesity |
1567 (57.5) |
666 (47.3) |
901 (68.5) |
|
| cancer (%) |
226 (8.3) |
139 (9.9) |
87 (6.6) |
0.003 |
| CVDs (%) |
78 (2.9) |
8 (0.6) |
70 (5.3) |
<0.001 |
| hypertension (%) |
529 (19.4) |
163 (11.6) |
366 (27.8) |
<0.001 |
| DM (%) |
80 (2.9) |
25 (1.8) |
55 (4.2) |
<0.001 |
| animal protein, mean (SD) |
53.03 (20.18) |
50.71 (18.59) |
55.50 (21.47) |
<0.001 |
| vegetable protein, mean (SD) |
28.67 (9.65) |
27.30 (9.11) |
30.14 (10.00) |
<0.001 |
| proportion of animal protein, mean (SD) |
0.64 (0.12) |
0.64 (0.12) |
0.64 (0.11) |
0.844 |
| Proportion of vegetable protein, mean (SD) |
0.36 (0.12) |
0.36 (0.12) |
0.36 (0.11) |
0.844 |
| animal/vegetable, mean (SD) |
0.26 (0.24) |
0.26 (0.25) |
0.25 (0.23) |
0.822 |
| total protein, mean (SD) |
81.70 (22.81) |
78.02 (20.23) |
85.64 (24.70) |
<0.001 |
Table 2.
the association between dietary protein intake and longitudinal change rate of hippocampus volume.
Table 2.
the association between dietary protein intake and longitudinal change rate of hippocampus volume.
| |
hippocampus(left) |
hippocampus(right) |
hippocampus(total) |
| |
β(SE) |
P |
β(SE) |
P |
β(SE) |
P |
| total protein |
|
|
|
|
|
|
| model1 |
-8.278e-06 (5.342e-05) |
0.877 |
-2.899e-05 (4.785e-05) |
0.545 |
-8.653e-06(3.606e-05) |
0.81 |
| model2 |
-9.956e-05 (7.586e-05) |
0.19 |
-7.375e-05 (6.8e-05) |
0.268 |
-7.547e-05(5.118e-05) |
0.1405 |
| model3 |
-9.979e-05 (7.592e-05) |
0.189 |
-7.374e-05 (6.803e-05) |
0.279 |
-7.48e-05(5.1235e-05) |
0.1444 |
| animal/protein |
|
|
|
|
|
|
| model1 |
-2.581e-02(1009e-02) |
0.0106 |
-2.403e-02(9.034e-03) |
0.008 |
-2.399e-02(6.7998e-03) |
0.00425 |
| model2 |
-2.528e-02(1.021e-02) |
0.0133 |
-2.558e-02(9.148e-03) |
0.005 |
-2.443e-02(6.881e-03) |
0.000392 |
| model3 |
-2.524e-02(1.022e-02) |
0.0135 |
-2.544e-02(9.152e-03) |
0.005 |
-2.435e-02(6.886e-03) |
0.000412 |
| vegetable/protein |
|
|
|
|
|
|
| model1 |
2.581e-02 (1.009e-02) |
0.0106 |
2.403e-02(9.034e-03) |
0.008 |
2.399e-02(6.71e-03) |
0.00425 |
| model2 |
2.528e-02 (1.021e-02) |
0.0133 |
2.558e-02(9.148e-03) |
0.005 |
2.443e-02(6.881e-03) |
0.000392 |
| model3 |
2.524e-02 (1.022e-02) |
0.0135 |
2.544e-02(9.152e-03) |
0.005 |
2.435e-02(6.886e-03) |
0.000412 |
| vegetable protein |
|
|
|
|
|
| model1 |
3.243e-04(1.257e-04) |
0.01 |
1.644e-04(1.127e-04) |
0.145 |
2.457e-04(8.479e-05) |
0.00378 |
| model2 |
3.909e-04(1.646e-04) |
0.018 |
2.731e-04(1.476e-04) |
0.0645 |
3.194e-04(1.111e-04) |
0.0041 |
| model3 |
3.901e-04(1.648e-04) |
0.018 |
2.724e-04(1477e-04) |
0.0653 |
3.19e-04(1.112) |
0.00414 |
| animal protein |
|
|
|
|
|
|
| model1 |
-8.415e-05(5.996e-05) |
0.161 |
1.644e-05 (1.127e-04) |
0.169 |
-6.676e-05(4.046e-05) |
0.0991 |
| model2 |
-1.522e-04(6.924e-05) |
0.0281 |
-1.111e-04 (6.208e-05) |
0.074 |
-1.194e-04(4.671e-05) |
0.0106 |
| model3 |
-1.522e-04(6.929e-05) |
0.0282 |
-1.096e-04(6.211e-05) |
0.078 |
-1.188e-04(4.674e-05) |
0.011 |
| animal/vegetable |
|
|
|
|
|
|
| model1 |
-1.281e-02(5e-03) |
0.01 |
-1.127e-02(4.451e-03) |
0.011 |
-1.16e-02 (3.3e-03) |
0.001 |
| model2 |
-1.251e-02(5.03e-03) |
0.013 |
-1.199e-02(4.508e-03) |
0.008 |
-1.176e-02(3.391e-03) |
0.001 |
| model3 |
-1.249e-02(5.033e-03) |
0.013 |
-1.193e-02(4.509e-03) |
0.008 |
-1.173e-02(3.393e-03) |
0.001 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).