Submitted:
22 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
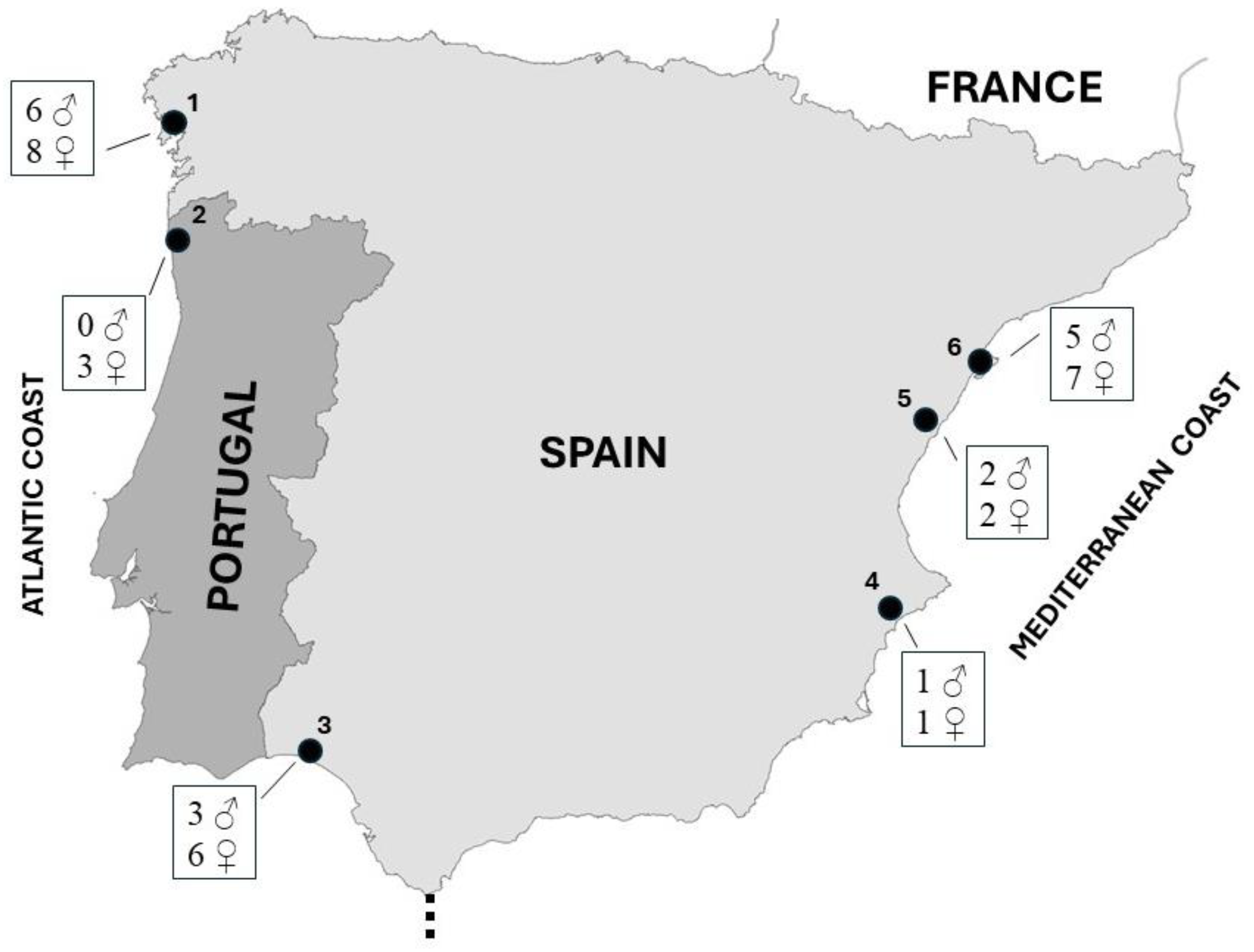
2.1. Study Area
2.2. Methods
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Delany, S.; Scott, D.A.; Dodman, T.; Stroud, D.A. An Atlas of Wader Populations in Africa and Western Eurasia.; Wageningen., 2009.
- Rocha, A.D.; Fonseca, D.; Masero, J.A.; Ramos, J.A. Coastal saltpans are a good alternative breeding habitat for Kentish plover Charadrius alexandrinus when umbrella species are present. J. Avian Biol. 2016, 47, 824–833, . [CrossRef]
- Robin, F.; Delaporte, P.; Rousseau, P.; Meunier, F.; Bocher, P. Tracing changes in the diet and habitat use of black-tailed godwits in Western France, using a stable isotope approach. Isot. Environ. Heal. Stud. 2018, 54, 288–303, . [CrossRef]
- Brown, A.C.; McLachlan, A. The ecology of sandy shores; Elsevier: Amsterdam, 2006.
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuarine, Coast. Shelf Sci. 2009, 81, 1–12, . [CrossRef]
- Vousdoukas, M.I.; Ranasinghe, R.; Mentaschi, L.; Plomaritis, T.A.; Athanasiou, P.; Luijendijk, A.; Feyen, L. Sandy coastlines under threat of erosion. Nat. Clim. Chang. 2020, 10, 260–263, . [CrossRef]
- Dangendorf, S.; Hay, C.; Calafat, F.M.; Marcos, M.; Piecuch, C.G.; Berk, K.; Jensen, J. Persistent acceleration in global sea-level rise since the 1960s. Nat. Clim. Chang. 2019, 9, 705–710, . [CrossRef]
- Galbraith, H.; Jones, R.; Park, R.; Clough, J.; Herrod-Julius, S.; Harrington, B.; Page, G. Global Climate Change and Sea Level Rise: Potential Losses of Intertidal Habitat for Shorebirds. Waterbirds 2002, 25, . [CrossRef]
- Ferreira, .; Kupfer, S.; Costas, S. Implications of sea-level rise for overwash enhancement at South Portugal. Nat. Hazards 2021, 109, 2221–2239, . [CrossRef]
- Smart, J.; Gill, J.A. Non-intertidal habitat use by shorebirds: a reflection of inadequate intertidal resources? Biol. Conserv. 2003, 111, 359-369. [CrossRef]
- Yasué, M.; Patterson, A.; Dearden, P. Are saltflats suitable supplementary nesting habitats for Malaysian Plovers Charadrius peronii threatened by beach habitat loss in Thailand? Bird Conserv. Int. 2007, 17, 211-223. [CrossRef]
- Jackson, M.V.; Choi, C.-Y.; Amano, T.; Estrella, S.M.; Lei, W.; Moores, N.; Mundkur, T.; Rogers, D.I.; Fuller, R.A. Navigating coasts of concrete: Pervasive use of artificial habitats by shorebirds in the Asia-Pacific. Biol. Conserv. 2020, 247, . [CrossRef]
- Lei, W.; Masero, J.A.; Dingle, C.; Liu, Y.; Chai, Z.; Zhu, B.; Peng, H.; Zhang, Z.; Piersma, T. The value of coastal saltpans for migratory shorebirds: conservation insights from a stable isotope approach based on feeding guild and body size. Anim. Conserv. 2021, 24, 1071–1083, . [CrossRef]
- Jourdan, C.; Fort, J.; Robin, F.; Pinaud, D.; Delaporte, P.; Desmots, D.; Gentric, A.; Lagrange, P.; Gernigon, J.; Jomat, L.; et al. Combination of marine and artificial freshwater habitats provides wintering Black-tailed Godwits with landscape supplementation. Wader Study 2022, 129, . [CrossRef]
- Iwamura, T.; Possingham, H.P.; Chadès, I.; Minton, C.; Murray, N.J.; Rogers, D.I.; Treml, E.A.; Fuller, R.A. Migratory connectivity magnifies the consequences of habitat loss from sea-level rise for shorebird populations. Proc. R. Soc. B: Biol. Sci. 2013, 280, 20130325, . [CrossRef]
- Moon, Y.M.; Kim, K.; Ki, J.; Kim, H.; Yoo, J.C. Use of stable isotopes (δ2H, δ13C and δ15N) to infer the migratory connectivity of Terek Sandpipers (Xenus cinereus) at stopover sites in the East Asian-Australasian Flyway. Avian Biol. Res. 2020, 13, 10-17. [CrossRef]
- Catry, T.; Lourenço, P.M.; Lopes, R.J.; Bocher, P.; Carneiro, C.; Alves, J.A.; Delaporte, P.; Bearhop, S.; Piersma, T.; Granadeiro, J.P. Use of stable isotope fingerprints to assign wintering origin and trace shorebird movements along the East Atlantic Flyway. Basic Appl. Ecol. 2016, 17, 177–187, . [CrossRef]
- Ulman, S.E.; Van Wilgenburg, S.L.; Morton, J.M.; Williams, C.K. Geographic Origins of Shorebirds Using an Alaskan Estuary during Migration. Waterbirds 2023, 46, 47–56, . [CrossRef]
- Hobson, K.A. Isotopic ornithology: a perspective. J. Ornithol. 2011, 152, 49–66, . [CrossRef]
- Bronskov, O.; Keller, V. Charadrius alexandrinus, Kentish Plover. In European Breeding Bird Atlas 2: Distribution, Abundance and Change, Keller, V., Herrando, S., Voříšek, P., Franch, M., Kipson, M., Milanesi, P., Martí, D., Anton, M., Klvaňová, A., Kalyakin, M.V., et al., Eds.; European Bird Census Council & Lynx Edicions: Barcelona, 2020; pp. 300-301.
- Meininger, P.; Székely, T.; Scott, D.A. Kentish Plover (Charadrius alexandrinus). In An Atlas of Wader Populations in Africa and Western Eurasia, Delany, S., Scott, D., Dodman, T., Stroud, S., Eds.; Wetlands International: Wageninger, 2009; pp. 230-235.
- De Juana, E.; García, E. The Birds of the Iberian Peninsula.; Christopher Helm: London, 2015.
- Gómez-Serrano, M.A.; Hortas, F. Chorlitejo patinegro Charadrius alexandrinus. In III Atlas de las aves en época de reproducción en España, Molina, B., Nebreda, A., Muñoz, R.A., Seoane, J., Real, R., Bustamante, J., Del Moral, J.C., Eds.; SEO/BirdLife: Madrid, 2022.
- Rocha, A. Censo nacional de Borrelho-de-coleira-interrompida. In O estado das aves em Portugal, 2022, Alonso, H., Andrade, J., Teodósio, J., Lopes, A., Eds.; Sociedade Portuguesa para o Estudo das Aves: Lisboa, 2022; pp. 70-75.
- Catry, P.; Costa, H.; Elias, G.; Matías, R. Aves de Portugal. Ornitología do território continental; Assirio & Alvim.: Lisboa., 2010.
- Toral, G.M.; Figuerola, J. Nest success of Black-winged Stilt Himantopus himantopus and Kentish Plover Charadrius alexandrinus in rice fields, southwest Spain. Ardea 2012, 100, 29-36. [CrossRef]
- Amat, J.A. Chorlitejo patinegro. In Enciclopedia Virtual de los Vertebrados Españoles, Salvador, A., Morales, M.B., Eds.; Museo Nacional de Ciencias Naturales: Madrid, 2016.
- Gómez-Serrano, M.Á.; Castro, E.M.; Domínguez, J.; Pérez-Hurtado, A.; Tejera, G.; Vidal, M. Chorlitejo patinegro Charadrius alexandrinus. In Libro Rojo de las Aves de España, López-Jiménez, A.N., Ed.; SEO/Birdlife: Madrid, 2021; pp. 375-385.
- Molina, B. Chorlitejo Patinegro. In Aves acuáticas reproductoras. Población en 2007 y método de censo, Palomino, D., Molina, B., Eds.; SEO/BirdLife: Madrid, 2009; pp. 115-125.
- Cramp, S.; Simmons, K.E.L. The birds of the Western Palearctic, vol. III.; Oxford University Press: Oxford, 1982.
- Pienkowski, M.W.; Knight, P.J.; Stanyard, D.J.; Argyle, F.B. THE PRIMARY MOULT OF WADERS ON THE ATLANTIC COAST OF MOROCCO. Ibis 1976, 118, 347–365, . [CrossRef]
- Blasco-Zumeta, J.; Heinze, G.M. Chorlitejo patinegro. Sociedad de Ciencias Aranzadi. 2023.
- Pinheiro, J.C.; Bates, D.M. Linear mixed-effects models: basic concepts and examples. In Mixed-effects models in S and S-Plus; Springer: New York, 2000; pp. 3-56.
- Sabat, P.; Martínez del Rio, C. Inter-and intraspecific variation in the use of marine food resources by three Cinclodes (Furnariidae, Aves) species: carbon isotopes and osmoregulatory physiology. Zoology 2002, 105, 247-256. [CrossRef]
- del Rio, C.M.; Sabat, P.; Anderson-Sprecher, R.; Gonzalez, S.P. Dietary and isotopic specialization: the isotopic niche of three Cinclodes ovenbirds. Oecologia 2009, 161, 149–159, . [CrossRef]
- Schoeninger, M.J.; DeNiro, M.J.; Tauber, H. Stable Nitrogen Isotope Ratios of Bone Collagen Reflect Marine and Terrestrial Components of Prehistoric Human Diet. Science 1983, 220, 1381–1383, . [CrossRef]
- Chmura, G.; Aharon, P. Stable carbon isotope signatures of sedimentary carbon in coastal wetlands as indicators of salinity regime. J. Coast. Res. 1995, 124-135.
- Jiménez-Morillo, N.T.; Moreno, J.; Moreno, F.; Fatela, F.; Leorri, E.; De la Rosa, J.M. Composition and sources of sediment organic matter in a western Iberian salt marsh: Developing a novel prediction model of the bromine sedimentary pool. Sci. Total. Environ. 2024, 907, 167931, . [CrossRef]
- Bouillon, S.; Dehairs, F.; Velimirov, B.; Abril, G.; Borges, A.V. Dynamics of organic and inorganic carbon across contiguous mangrove and seagrass systems (Gazi Bay, Kenya). 2007, 112, . [CrossRef]
- Gonçalves, M.S.S.; Gil-Delgado, J.A.; Gosálvez, R.U.; López-Iborra, G.M.; Ponz, A.; Velasco, A. Spatial synchrony of wader populations in inland lakes of the Iberian Peninsula. Ecol. Res. 2016, 31, 947–956, . [CrossRef]
- De Souza, J.A.; Caeiro, M.L.; Rosende, F.; Monteagudo, A.; Fafián, J.M. Estacionamientos, estructura y patrones de residencia de la población invernante del Chorlitejo patinegro (Charadrius alexandrinus) en Galicia: un análisis preliminar. Chioglossa 1999, 1, 23-45.
- Sardá, R.; Ariza, E.; Jimenez, J.A. Buscando el uso sostenible de las playas. In La gestión integrada de playas y dunas: experiencias en Latinoamérica, Norte de Africa y Europa, Rodríguez-Perea, A., Pons, G.X., Roig-Munar, F.X., Martín-Prieto, J.A., Mir-Gual, M., Cabrera, J.A., Eds.; Palma de Mallorca: Mon. Sociedad Historia Natural Balears, Spain, 2012; Volume 19, pp. 33-44.
- Losada, I.J.; Méndez, F.J.; Olabarrieta, M.; Liste, M.; Menéndez, M.; Tomás, A.; Abascal, A.J.; Agudelo, P.; Guanche, R. Fase II: Evaluación de efectos en la costa española. In Impactos en la costa española por efecto del cambio climático, Ministerio de Medio Ambiente, Ed.; Madrid, 2004.
- Caetano, E.; Innocentini, V.; Magaña, V.; Martins, S.; Méndez, B. Cambio climático y el aumento del nivel del mar. In Vulnerabilidad de las zonas costeras mexicanas ante el cambio climático Botello, A.V., Villanueva-Fragoso, S., Gutiérrez, J., Rojas-Galaviz, J.L., Eds.; Semarnat-INE, UNAM-ICMYL, Universidad Autónoma de Campeche.: México, 2011; pp. 283-304.
- Cooley, S.; Schoeman, D.; Bopp, L.; Boyd, P.; Donner, S.; Ito, S.-I.; Kiessling, W.; Martinetto, P.; Ojea, E.; Racault, M.-F.; et al. Oceans and coastal ecosystems and their services. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2022.
- Melet, A.; Van de Wal, R.; Amores, A.; Arns, A.; Chaigneau, A.A.; Dinu, I.; Haigh, I.D.; Hermans, T.H.; Lionello, P.; Marcos, M. Sea level rise in Europe: observations and projections. State of the Planet Discussions 2023, 1-106, in review. [CrossRef]
- Vousdoukas, M.I.; Mentaschi, L.; Voukouvalas, E.; Verlaan, M.; Feyen, L. Extreme sea levels on the rise along Europe's coasts. Earth's Future 2017, 5, 304-323. [CrossRef]
- Phillips, R.A.; Bearhop, S.; Mcgill, R.A.R.; Dawson, D.A. Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 2009, 160, 795–806, . [CrossRef]
- Dias, M.P.; Granadeiro, J.P.; Phillips, R.A.; Alonso, H.; Catry, P. Breaking the routine: individual Cory's shearwaters shift winter destinations between hemispheres and across ocean basins. Proc. R. Soc. B: Biol. Sci. 2011, 278, 1786-1793. [CrossRef]
- Navarro, A.B.; Magioli, M.; Bogoni, J.A.; Moreira, M.Z.; Silveira, L.F.; Alexandrino, E.R.; Apolinario da Luz, D.T.; Pizo, M.A.; Silva, W.R.; de Oliveira, V.C.; et al. Human-modified landscapes narrow the isotopic niche of neotropical birds. Oecologia 2021, 196, 171–184, doi:10.1007/s00442-021-04908-9.
- Domínguez, J.; Vidal, M. Plan de Conservación del Chorlitejo patinegro (Charadrius alexandrinus) en Galicia; Consellería de Medio Ambiente e Desenvolvemento Sostible: Santiago de Compostela, 2008.
- Vidal, M.; Domínguez, J. Long-term population trends of breeding Kentish Plovers (Charadrius alexandrinus) in Northwestern Spain under the effects of a major oil spill. Bird Conserv. Int. 2013, 23, 386-397. [CrossRef]
- AlRashidi, M.; Shobrak, M.; Al-Eissa, M.S.; Székely, T. Integrating spatial data and shorebird nesting locations to predict the potential future impact of global warming on coastal habitats: A case study on Farasan Islands, Saudi Arabia. Saudi J. Biol. Sci. 2012, 19, 311–315, . [CrossRef]
- Aiello-Lammens, M.E.; Chu-Agor, M.L.; Convertino, M.; Fischer, R.A.; Linkov, I.; Akcakaya, H.R. The impact of sea-level rise on Snowy Plovers in Florida: integrating geomorphological, habitat, and metapopulation models. Glob. Change Biol. 2011, 17, 3644-3654. [CrossRef]
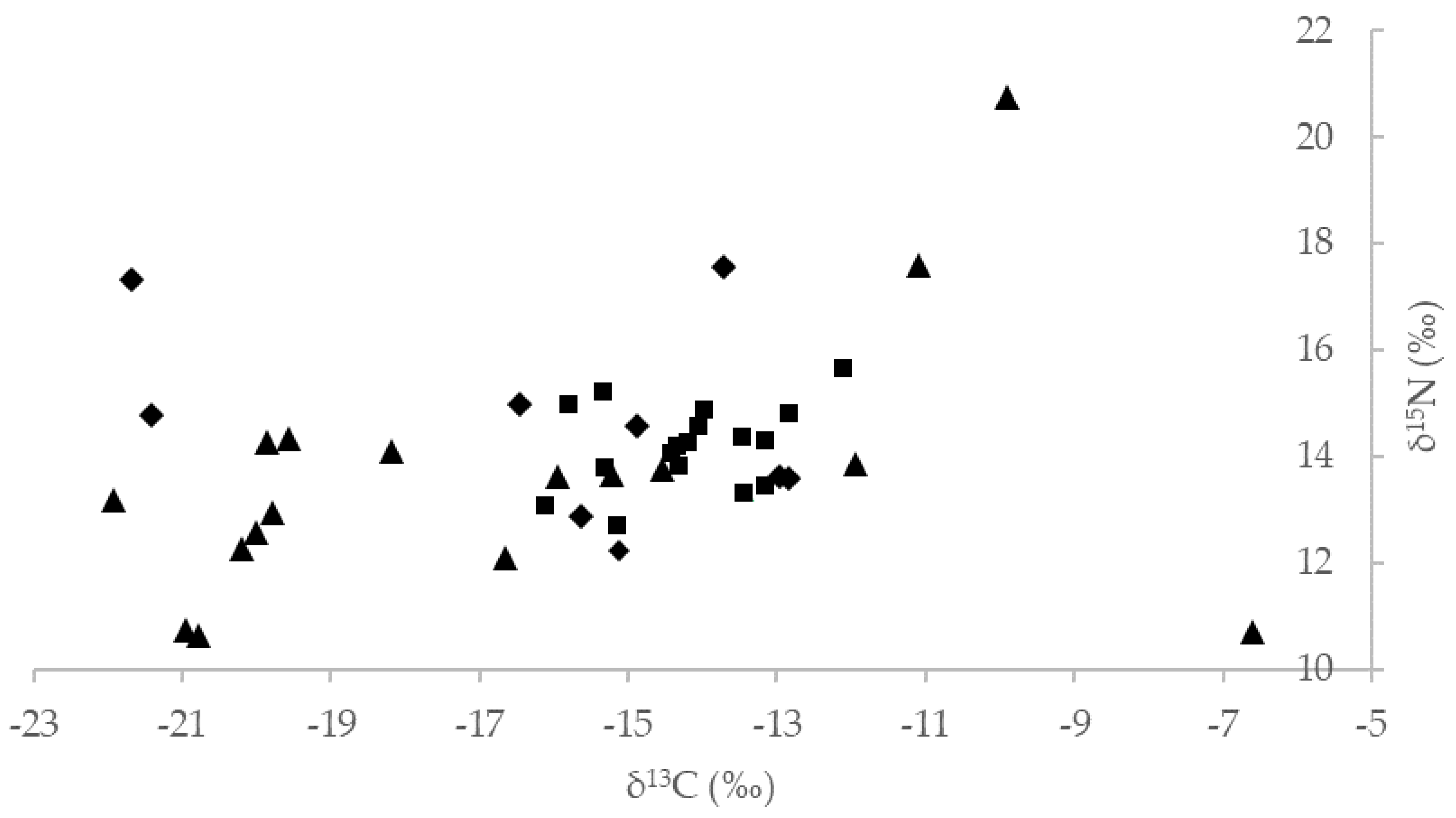
| Predictors | δ13C | δ15N |
|---|---|---|
| Coastal section | 0.325 | 0.013 |
| Sex | 0.612 | 2.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





