Introduction
Plants and their essential oils are often used as medicine to cure or prevent diseases. Medicinal plants are preferred as medication due to their low risk of causing genetic mutagenicity, carcinogenicity, or teratogenicity [
1]. These plants are a significant source of therapeutic drug molecules as they contain secondary metabolites that can serve as potential drugs. Aromatic plants are commonly used in traditional medicine [
2]. In several countries worldwide, especially in rural areas, aromatic plants are used in primary healthcare [
3].
Eucalyptus is a type of flowering tree belonging to the Myrtaceae family, with over 900 species and subspecies [
3]. These trees are known for their fast growth and ability to tolerate harsh environments such as wildfires, droughts, and acidic soils. The wood industry uses various species of
Eucalyptus to produce pulp, timber, and paper. Additionally, the barks, leaves, and branches of these trees are utilized as by-products to generate energy [
4].
There are several species of Eucalyptus, such as E. camaldulensis, E. saligna, E. citriodera, Eucalyptus globule, Eucalyptus maculate, E. staigeriana, E. radiate, E. laxophleba, E. cinerea, E. tereticornis, and E. leocoxylon [
5,
6]. One of the most popular species is Eucalyptus globulus Labill or blue gum tree, which is an evergreen tree widely grown in Australia, South Africa, India, Southern Europe, and Ethiopia [
7,
8,
9]. E. globulus is known for its many medical benefits, such as treating wounds, tuberculosis (TB), sore throats, arthritis, asthma, boils, colds, coughs, diabetes, diarrhea, dyspepsia, and bronchitis [
10]. These plants produce secondary metabolites such as terpenoids, alkaloids, flavonoids, phenols, and saponins as a defense mechanism [
2].
Eucalyptus trees are extensively researched due to their essential oil, which has several health benefits, including anti-inflammatory, antimalarial, antibacterial, analgesic, anti-diabetic, antiviral, anti-cancer, antiseptic, antioxidant, and antifungal properties [
6,
11].
Eucalyptus leaves are a great source of antioxidants, particularly flavonoids, which offer protection against oxidative stress and free radical damage [
4].
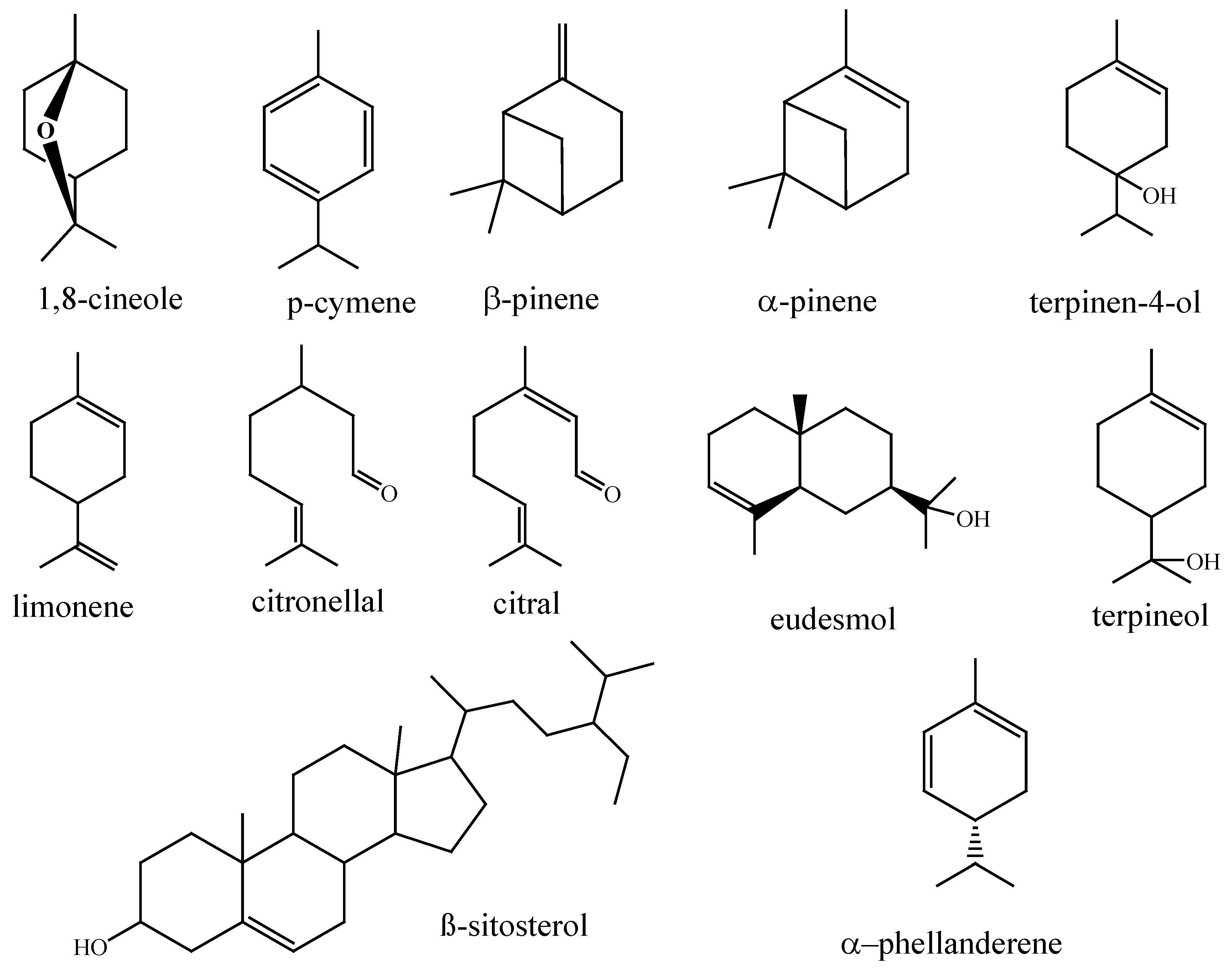
Figure 1.
Chemical structures of the main components of Eucalyptus fruits, branch tips, and leaves essential oils.
Figure 1.
Chemical structures of the main components of Eucalyptus fruits, branch tips, and leaves essential oils.
Eucalyptus trees are a rich source of essential oils that can be extracted from their fruits, branch tips, and leaves. These oils contain a variety of beneficial phytochemicals such as 1,8-cineole, p-cymene, α and β-pinene, limonene, citronellal, citral, eudesmol, terpinen-4-ol, terpineol, α–phellanderene, and 9β-sitosterol. Different methods, like hydro-distillation, supercritical fluid extraction, and solvent extraction, can be used to extract these chemicals [
6]. Among these compounds, 1,8-cineole is the most abundant in all
Eucalyptus species [
12]. To treat illnesses,
Eucalyptus extracts are used both alone and in conjunction with certain other plants.
Eucalyptus essential oils are safe and non-toxic. They are used as preservatives, flavoring agents, and in a wide variety of consumer goods [
1]. Researchers have recently shown increased interest in essential oils due to their therapeutic properties, which make them useful for preservation and drug formulations. They’re also used as flavoring substances in the food, nutraceutical, and pharmaceutical industries.
Eucalyptus essential oils have been used in medicine to treat a wide range of human and animal ailments. They contain phenolic and flavonoid compounds, which have antioxidant and antibacterial properties [
13]. This review aims to provide scientific information on the phenolic and flavonoid contents, antioxidant, and antibacterial activity of selected
Eucalyptus species.
Eucalyptus Species
Eucalyptus is a non-native and most popular tree species classified in the Myrtaceae family. It is a genus of over 900 species [
3] and has become the most planted genus of tree species in the world [
14].
According to the research conducted by Alemu M. M in 2016 [
15], it has been determined that Brazil possesses the most extensive expanse of
Eucalyptus plantations globally, with India and China following suit in terms of coverage. In Africa, South Africa proudly claims the initial position in establishing such plantations, securing a notable fifth place in the worldwide ranking. Ethiopia, on the other hand, stands adjacent to South Africa and holds the seventh position on a global scale.
Eucalyptus covers 90% of the total planted forest area in the central highlands of Ethiopia. These species of trees are the first non-native tree species formally introduced to Ethiopia by Emperor Minilik II from Australia in the 1890s [
16].
Around 70 species of Eucalyptus are accessible in Ethiopia, most of which are broadly spread in numerous locales of the nation, mostly within the central highlands, where population density is higher. The most common and widespread
Eucalyptus species include
E. globulus, E.camaldulensis, E. saligna, E. grandis, E. citriodora, E. regnans, and
E. tereticornis [
15,
17].
Numerous products and services, including fuel wood, charcoal, building materials, home and agricultural tools, pulp,
Eucalyptus oil, lumber, and poles, are known to be provided by
Eucalyptus trees. The tree proved to be highly helpful for the production of paper because of its rapid growth tendency. Additionally, the tree has traditional and modern medical uses. For example, in Ethiopia, the steam from water-boiled
E. globulus tree leaves is known to cure the flu. Australia, Brazil, Chile, Portugal, South Africa, Spain, and Swaziland are among the nations recognized for their production of
Eucalyptus oil [
15].
Benefits of Eucalyptus Species for Health
The medicinal properties of
Eucalyptus species have been reported in various studies. Olawore NO and Ololade ZS [
1] studied the therapeutic properties of the essential oils derived from the seeds of
E. camaldulensis var. nancy and
E. camaldulensis var. petford, including antioxidant, anti-inflammatory, antinociceptive, and antimicrobial activities.
E. Citriodora fruit essential oil has natural antioxidant and antimicrobial activity [
13].
The
E. globulus Labill species is one of the most well-known. Its leaves have been used as traditional medicine to cure a variety of illnesses, including diabetes, influenza, fungus infections, and pulmonary tuberculosis. The leaf extracts of
E. globulus have been also used as food additives due to their antioxidant activity [
7].
The
Eucalyptus plant was traditionally used as an antiseptic and antibacterial effect for treating respiratory tract infections such as colds, flu, sore throats, and chest infections including bronchitis and pneumonia. This effect on bacteria may be attributed to the dominant presence of eucalyptol (1,8-cineole), which has demonstrated strong antimicrobial activity against many pathogens [
3].
Eucalyptus contains phenolic compounds that possess anti-inflammatory, antimicrobial, and antioxidant properties. Moreover, it has neuroprotective effects and could be effective in preventing or delaying the onset of Alzheimer’s disease. Due to their diverse bioactivities,
Eucalyptus phenolic compounds have potential uses in various industries, such as food, cosmetics, and pharmaceuticals [
18].
Traditional Use of Eucalyptus in Ethiopia
Ethiopia has a wide range of aromatic and medicinal plant species from which essential oil can be produced for a variety of uses. The most prevalent of these is the
Eucalyptus plant [
19]. Reforestation of
Eucalypt is a popular practice in many countries, particularly in developing countries like Ethiopia. Ethiopian farmers cultivate a lot of
Eucalyptus trees on tiny plots of land, and they maintain them well to provide a range of goods, such as small branches and leaves for fuel wood, as well as poles and posts for building houses and other agricultural purposes.
Eucalyptus trees provide fuel and building materials for a large number of people in Ethiopia [
20].
Eucalyptus extracts have been used traditionally to cure a wide range of ailments in various nations, including Ethiopia. For example, hot water extracts of both fresh and dried
E. globulus leaves are used as analgesic, anti-inflammatory, and antipyretic treatments for sinus infections, the common cold, and the flu. This is because
E. globulus is a unique natural product with antiseptic properties that also help to clear the bronchial tubes and nasal passages, which makes breathing easier. Additionally, they inhale the vapor released when boiling
Eucalyptus leaves in water to relieve colds, particularly common ones. It has significant biological activities of the essential oils of
Eucalyptus species, including their diaphoretic, disinfecting, antimalarial, antiseptic, analgesic, anti-inflammatory, antibacterial, and antioxidant qualities.
Eucalyptus oil is one of the ingredients to make laundry detergents and toiletries with good deodorizing and antiseptic properties [
19].
E. globulus is used to treat respiratory tract infections; any illness in the respiratory tract that may affect the lungs, bronchi, or nasal passages is referred to as a respiratory tract infection. Because of antimicrobial resistance is developing quickly and reducing the effectiveness of currently available drugs, these illnesses are becoming major sources of death and morbidity [
21].
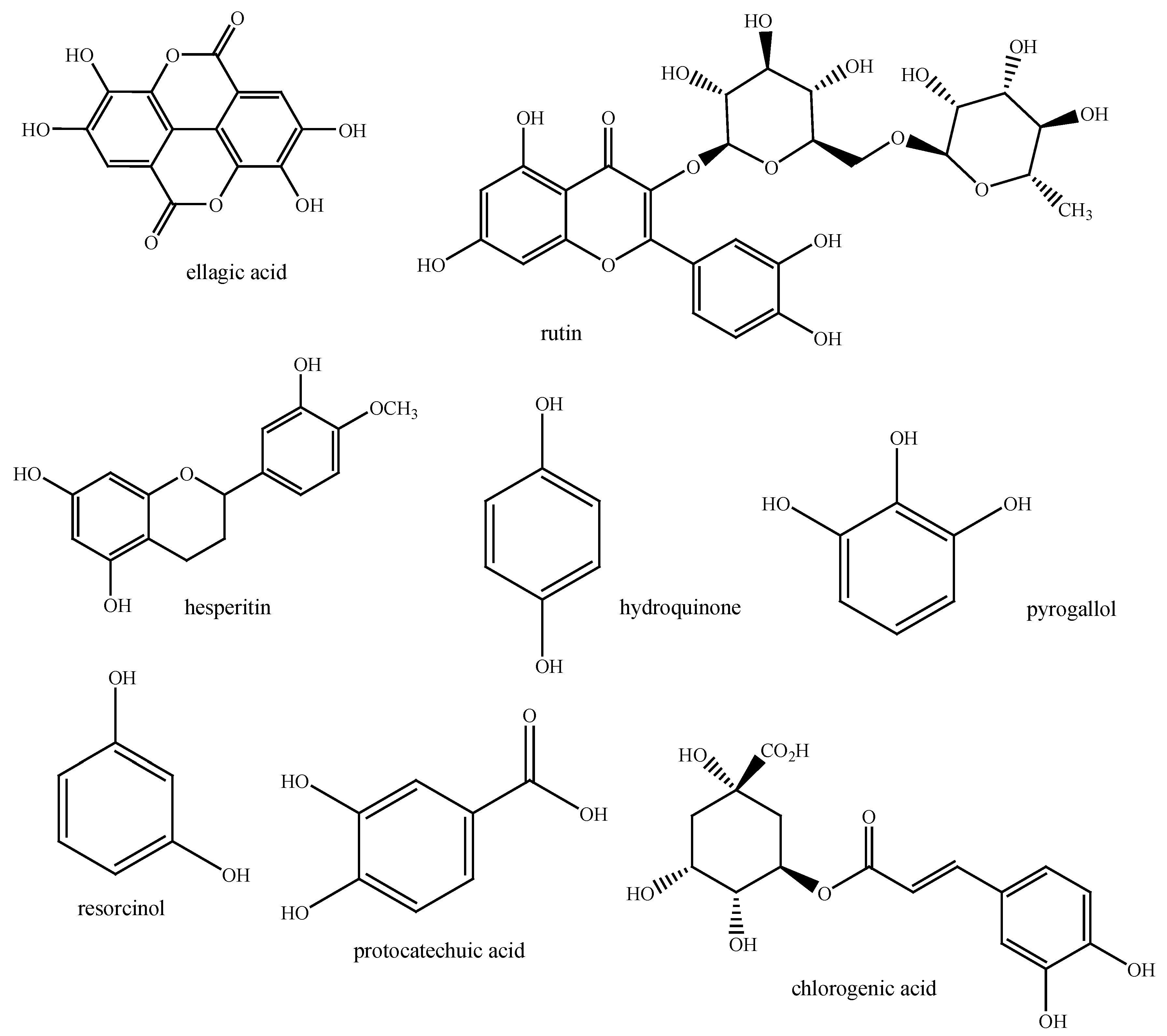
Phenolic Contents
A common structural feature of phenolic compounds is an aromatic ring with one or more hydroxyl substituents. These compounds can be classified into many classes, with flavonoids, phenolic acids, tannins, stilbenes, and lignans being the main groups [
4,
22]. Various phenolic chemicals (
Figure 2) have been extracted from different
Eucalyptus extracts, including quercetin, rutin, ellagic acid, hydroquinone, protocatechuic acid, naringenin, chlorogenic acid, hesperitin, pyrogallol, resorcinol, and catechin [
23].
Phenolic compounds are a large class of plant secondary metabolites, existing in various higher plant organs such as vegetables, fruits, spices, grains, legumes, and nuts, playing important roles in diverse physiological processes such as plant quality, coloring, flavor, and stress resistance [
22]. Because of their beneficial effects on human health, polyphenols are very valuable phytochemicals in the food and pharmaceutical industries [
13].
Plants with high phenolic content are highly nutritious and beneficial to health. In addition, they display defense mechanisms against reactive oxygen species (ROS). Natural phenolic compounds play a critical role in both illness prevention and treatment. Phenolic compounds have chemopreventive effects because of a range of therapeutic activities that also regulate carcinogen metabolism and ontogenesis expression, differentiation, inhibiting DNA binding and cell adhesion, migration, proliferation, and blocking signaling pathways [
1].
Phenolic compounds are frequently linked to a number of advantageous health outcomes, including as anti-inflammatory, antioxidant effects, anti-cancer, and decreases in the risk of cardiovascular diseases [
7]. Phenolic chemicals exert protective effects against oxidative stress and inflammation caused by airborne particulate matter and also play a significant role in protecting plants from Ultraviolet radiation and disease attacks [
24].
Olawore NO and Ololade ZS [
1] reported that the total phenolic contents (TPC) value of
E. camaldulensis var. nancy and
E. camaldulensis var. petford essential oils was 156.25 ± 0.00 and 167.93 ± 0.00 µg GAE/mg respectively, which showed that relatively the same phenolic contents as compared with
E. citriodera essential oil extracts (175.84±0.00 µg GAE/mg) reported by Ololade et al. (2021).
As shown
Table 1, the total phenolic content of
E. saligna leaves extract with pressurized 95% ethanol is higher than the other
Eucalyptus species which is 618.57 mg GAE/g plant material. Fischer et al. [
25] reported that the total phenolic content of 80% methanol extract of
E. globulus is lower than the other
Eucalyptus species (50.00 mg GAE/g plant material) in (
Table 1) showed.
Park et al. [
18] reported that in
Table 2, the highest TPC values were reported in the different concentrations of ethanol
E. globulus leaf extracts with 422.0, 492.7, 497.7, and 448.5 mg GAE/g extract in 10, 30, 50, and 70% ethanol extracts, respectively. The next highest TPC was 384.5 mg GAE/g extract in the 90% ethanol extract. On the other hand, the TPC of 100% ethanol extract was 273.2 mg GAE/g extract. The lowest TPC value was estimated in the 0% ethanol extract, 126.7 ± 8.5 mg GAE/g extract. From the extraction condition,
E. globulus leaves 50% ethanol extract has the highest TPC relative to the other extracts, as shown in
Table 2.
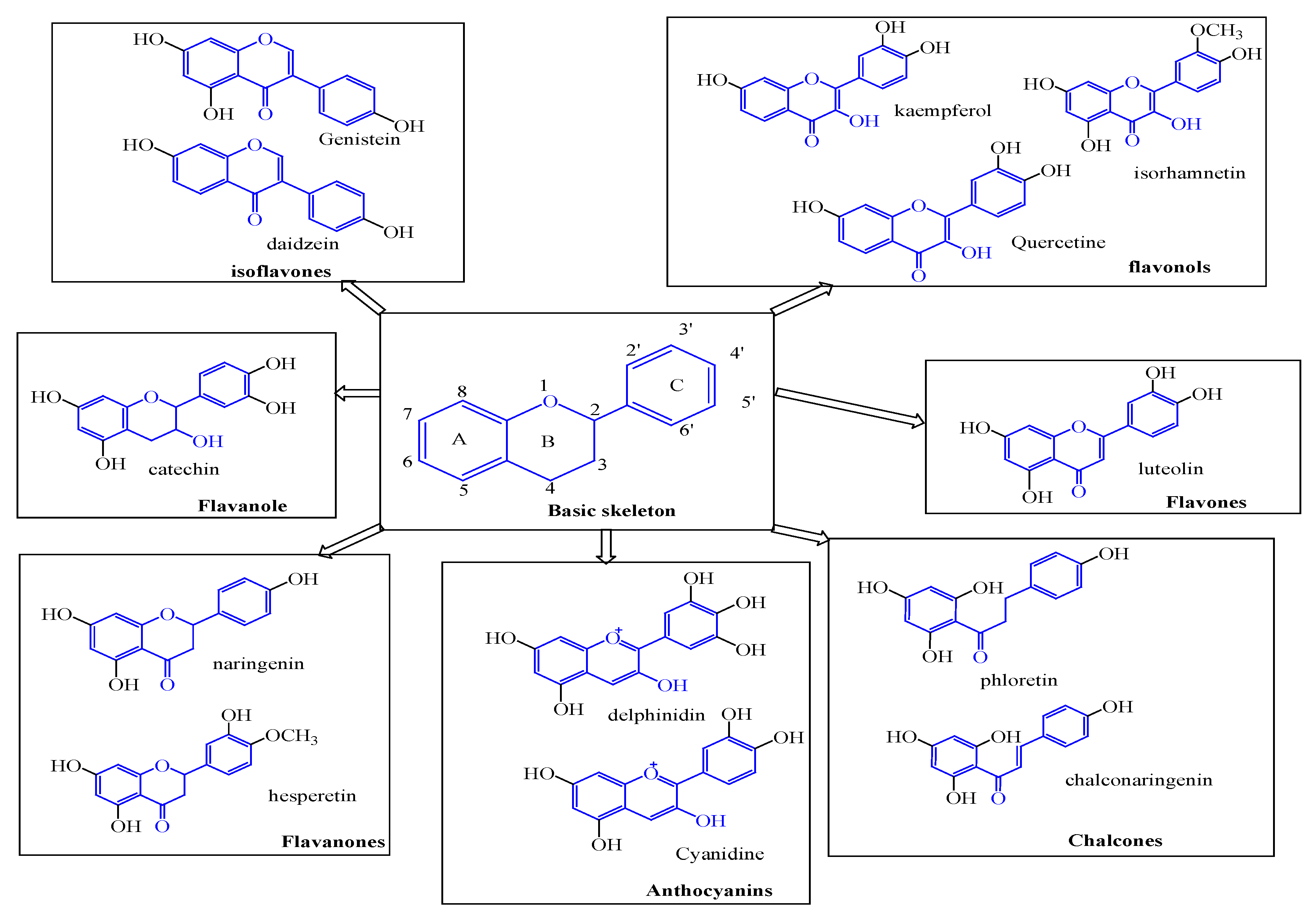
Flavonoid Contents
Flavonoids are classes of secondary metabolites. Their main structural element is a benzopyrone ring that has variously positioned phenolic or polyphenolic groups. Based on the degree of unsaturation and oxidation of the C ring as well as the carbon of the C ring to which the B ring is attached, flavonoids are divided into subgroups, as shown in
Figure 3. Flavonoids in which the B ring is linked to position 3 of the C ring are called isoflavones. Those in which the B ring is linked in position 4 are called neoflavonoids, whereas those in which the B ring is linked in position 2 can be further separated into multiple subgroups according to the structural characteristics of the C ring. These subgroups include anthocyanins, chalcones, flavones, flavonols, flavanones, and flavanonols, as well as flavanols or catechins [
4,
27]. Quercetin, kaempferol, isorhamnetin, luteolin, phloretin, and catechins are the primary flavonoids found in
Eucalyptus [
4].
Flavonoids are found in many plants, fruits, vegetables, and leaves. These compounds have potential biological and medicinal uses. It offers several health benefits, including the potential to prevent age-related neurodegenerative diseases Preventing Alzheimer’s disease. They also possess antioxidant, antiviral, and anticancer properties [
26,
28].
Flavonoids play many important role in plants including protect against reduction, fertility and reproduction, protect against infection, and rhizosphere. Flavonoids function as signaling molecules, detoxifying agents, phytoalexins and promote seed germination, temperature acclimatization, and drought resistance. Flavonoids are also used to reduce reactive oxygen species in plant tissue, another role they play in the fragrance, color, and taste of fruits, flowers, or seeds. This fragrance and color attract pollinators that help pollination and dispersal of seeds [
29]. The relative importance of flavonoids in humans and plants as signaling molecules and reducing agents [
30]. Flavonoids facilitate colonization of tomato roots and the germination of Rhizophagus irregularis spores [
31].
Flavonoids have shown their ability to control or prevent inflammation. Due to their anti-inflammatory, anti-oxidative, and immune-modulatory qualities, flavonoids are essential for pharmacological, medical, and nutraceutical applications [
32]. The number and position of free-OH groups on the flavonoid skeleton appear to determine the antioxidant potential of naturally occurring polyphenolic compounds. The B-ring substitution pattern plays a key role in flavonols capacity to scavenge free radicals. Flavonoids with multiple hydroxyl groups are more potent antioxidants than those with only one [
33].
As shown in
Table 3, the flavonoid content of
E. saligna leaves extracted by pressurized 95% ethanol which is 160.27 mg QE/g of extract. Sharma, Chahal and Kaur [
2] reported that the flavonoid content of 80% methanol extract of
E. globulus leaf is lower, which is 23 mg RE/g plant material.
Park et al. [
18] reported that
Table 4, the total flavonoid content (TFC) values of
E. globulus leaf extract was increased as the concentration of ethanol increases from 0% to 100% ethanol. In the range of 0 and 50% ethanol extraction conditions, TFC values ranged from 32.5 to 41.2 mg QE/g extract, with similar values. The 70% ethanol extract showed a value of 66.5 mg QE/g extract, which is about 1.5-fold larger than the 0, 10, 30, and 50% ethanol conditions. The highest TFC values is 156.5 and 169.3 mg QE/g extract in 90% and 100% ethanol conditions, respectively. The best extract conditions for TFC is 100% ethanol conditions.
Antioxidant Activities
Antioxidants are molecules able to scavenge ROS or free radicals, protecting cells from damage and death. In biological systems, free radicals are crucial for the synthesis of some biomolecules, energy production, phagocytosis, and cell development. An imbalance between free radical generation and unfavorable antioxidant defenses leads to oxidative stress, resulting in DNA or tissue damage [
3].
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH), Ferric reducing antioxidant power (FRAP) and 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays are commonly used to measure antioxidant capacity. The main differences between are their reaction mechanisms and type of radicals [
18].
Olawore NO and Ololade ZS [
1] reported that
Table 5, the two seed essential oils of
E. camaldulensis vars. nancy and
petford had Half maximal inhibitory concentration (IC
50) values of 3.50 μg/ml, indicating more efficacy as antioxidants and free radical scavengers than ascorbic acid, which had an IC
50 value of 9.00 μg/ml. The essential oil of the seed of two
Eucalyptuses under investigation shows two times higher reduction antioxidant activity (5.0 μg/ml) than ascorbic acid (11.0 μg/ml). This is because, unlike ascorbic acid, which only contained one molecule, the seed essential oils include several significant terpinoids in addition to phenolic chemicals. According to Ololade et al. [
13] the antioxidant IC
50 value of the fruit essential oil
E.citriodera is 2.00 μg/ml and ascorbic acid gave an IC
50 value of 11.00 μg/ml. This shows that the antioxidant activity of the essential oil is more potent than ascorbic acid.
The lower the IC
50 value, the higher the antioxidant potential [
13]
. The presence of terpenoids in the essential oil of
Eucalyptus species played an active role in the antioxidant potential and its higher reduction antioxidant effect [
1,
13]. The presence of terpenoids in the oil contributed to its higher reduction antioxidant effect since these compounds are known to form chelate metal ions [
13].
Park et al. [
18]reported that the antioxidant ability of
E. globulus leaves using DPPH and ABTS radicals. As shown in
Table 6, the highest radical scavenging activity against DPPH radicals was 30% ethanol with 188.2 µg/ml of scavenging capacity (SC
50) value, followed by 10%, 50%, and 70% ethanol conditions having SC
50 values of 357.9, 505.3, and 509.3 µg/ml, respectively. Among the extraction conditions, 0% ethanol, 90% ethanol, 100% ethanol the SC
50 value is 5841.7, 1008.4, and 1304.7 µg/ml, respectively, which exhibits little antioxidant ability. However, compared to the DPPH assay, the ABTS radical scavenging activity assay often produced better findings. The results indicated that the
E. globulus leaf extracts with 30% and 50% ethanol extract had the highest antioxidant effectiveness, with SC
50 values of 14.2 and 18.0 µg/ml, respectively. The results showed that the ethanol extracts at 100% and 0% had the lowest antioxidant effectiveness, with corresponding SC
50 values of 34.9 and 171.3 µg/ml. These findings verified that extracting
E. globulus leaves with the right ratio of water to ethanol produced a more potent antioxidant effect than extracting the leaves with either water or ethanol alone. The findings showed that, in order to optimize the antioxidant potential, a mixture of extract solvents was necessary.
Antioxidant Mode and Mechanism of Action
The term “oxidative stress” describes the imbalance between antioxidants and oxidants in the body as a result of either an excess of reactive sulfur species (RSS), reactive nitrogen species (RNA), or ROS that have the ability to damage cells [
34]. Under various pathophysiological circumstances, oxidative stress is caused by ROS and RNS. Oxidative stress circumstances modify the cellular components of our body, leading to different disease states. Antioxidants are a useful tool for strengthening cellular defenses against oxidative stress. Certain compounds act as in vivo antioxidants by raising the levels of endogenous antioxidant defenses. Expression of genes encoding the enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSHPx) increases the level of endogenous antioxidants [
35].
All highly reactive forms of oxygen, including free radicals, are collectively referred to as ROS. ROS categories include hypochlorous acid (HOCl), hypochlorite radical (OCl
•), peroxynitrite (ONOO), hydrogen peroxide (H
2O
2), per hydroxyl radical (HO
2•), hydroxyl radical (OH
•), superoxide anion radical (O
2•¯), singlet oxygen (
1O
2), nitric oxide radical (NO
•), and different lipid peroxides. RSS are easily produced from thiols through a reaction with ROS, while RNS are derived from nitric oxide by the reaction with O
2•¯ to form ONOO [
34].
Antioxidants are substances or systems that slow down the autoxidation by preventing the production of free radicals or by stopping the spread of free radicals through one or more of a number of different mechanisms [
33]. Antioxidant chemicals have multiple chemical modes of action, including single electron transfer, hydrogen atom transfer, and transition metal chelation [
36].
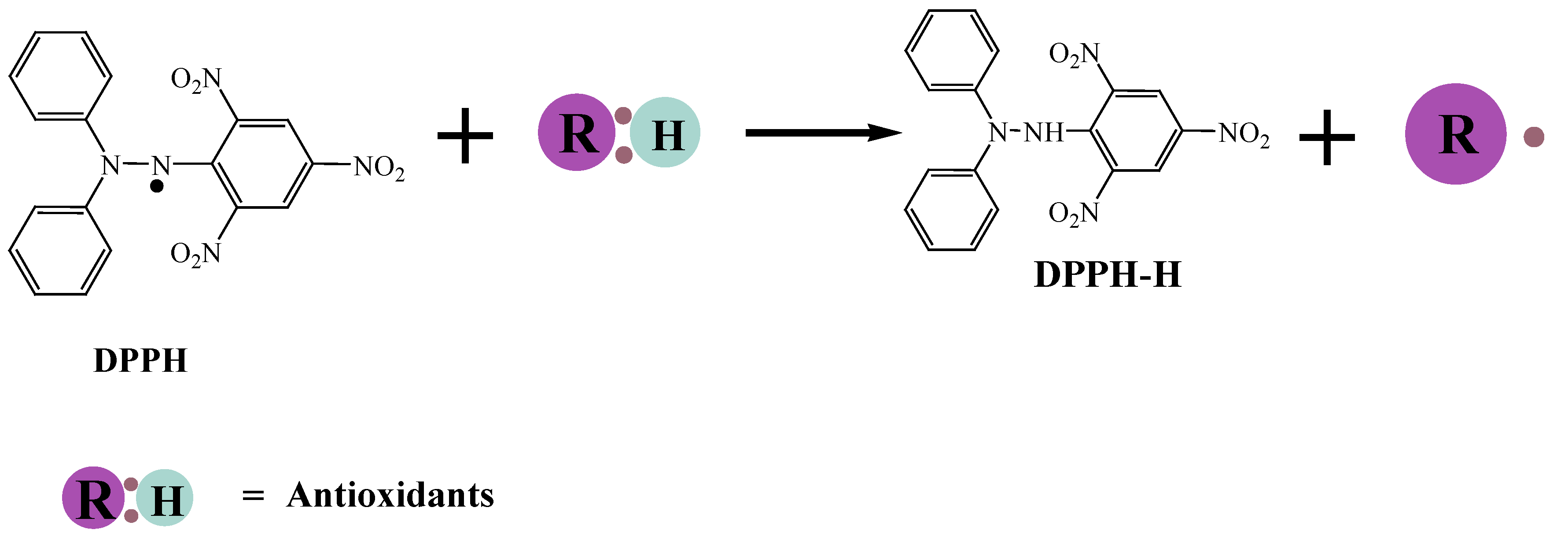
Figure 4.
Reaction mechanism of DPPH with an antioxidant.
Figure 4.
Reaction mechanism of DPPH with an antioxidant.
DPPH is a stable radical by nature compared with the ABTS
•+ radical, it is a radical that should be generated by chemical reactions (Equation 1). Generally, the improved method generates the ABTS
•+ radical in only one reaction by reacting ABTS with ammonium persulfate ((NH
4)
2S
2O
3) or potassium persulfate (K
2S
2O
3) prior to the addition of antioxidants (
Figure 5) [
37].
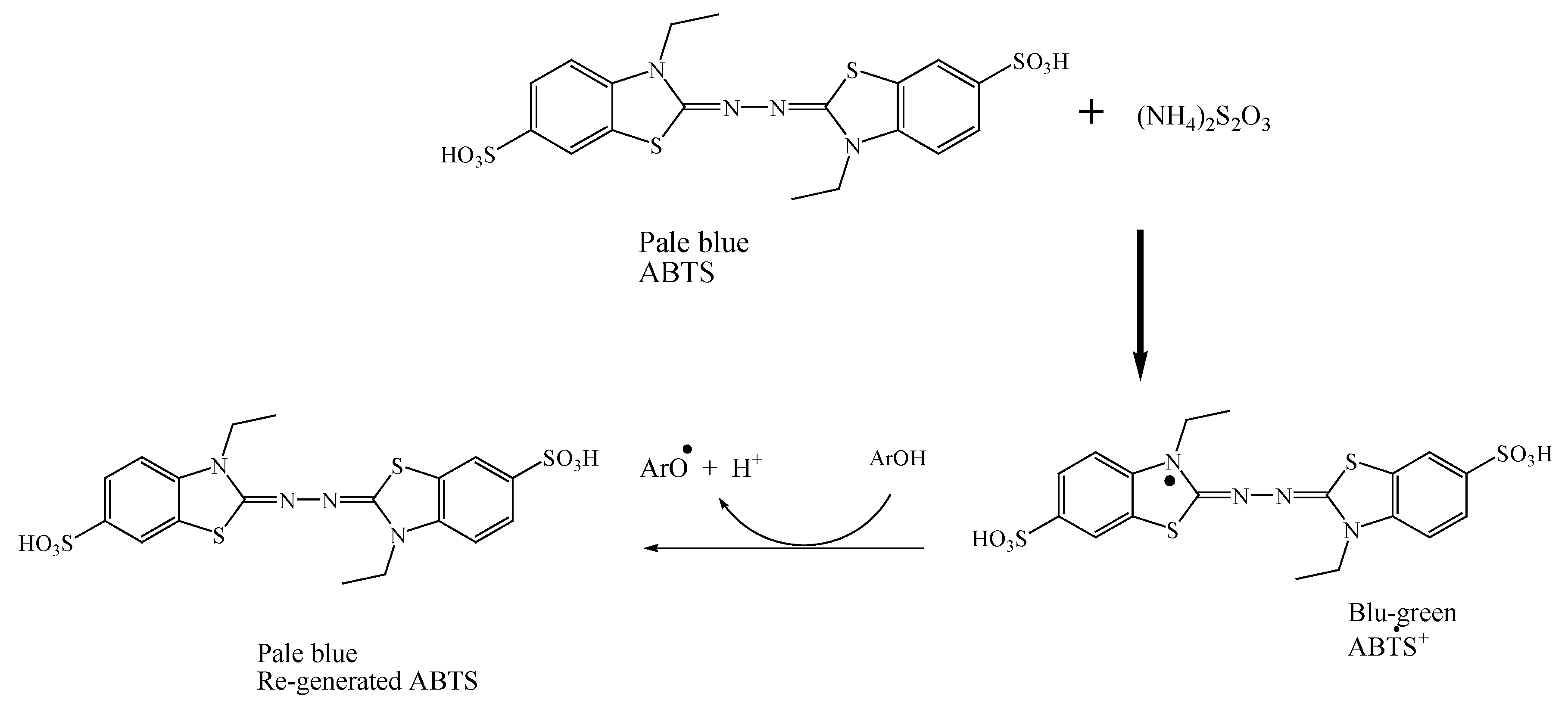
Chemical reaction of generating ABTS•+ radical
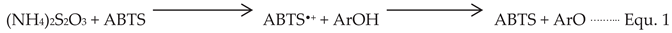
ArOH = represent phenolic antioxidant
Figure 5.
Mechanism of 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) reaction.
Figure 5.
Mechanism of 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) reaction.
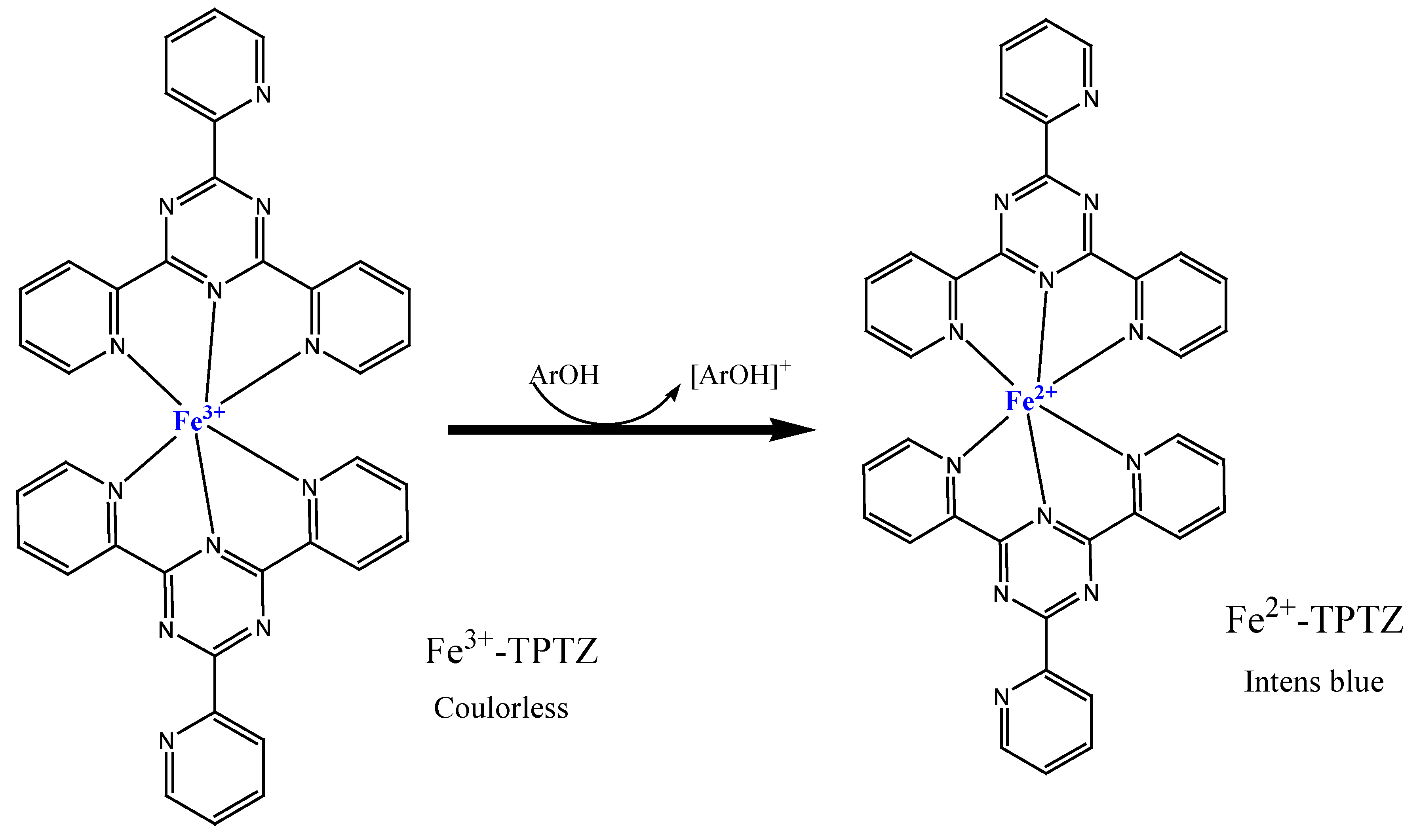
The FRAP test is a typical SET-based method measuring the reduction of the complex of ferric ions (Fe
3+)-ligand to the intensely blue ferrous complex (Fe
2+) by means of antioxidants in acid environments (
Figure 6). The original FRAP test employed tripyridyltriazine (TPTZ) as the connecting ligand to the iron ion. Alternative ligands, such as ferrozine, were also employed to bind the iron ion in order to evaluate the reducing power of ascorbic acid. Potassium ferricyanide has emerged as the most often utilized ferric reagent in FRAP experiments in recent times [
38].
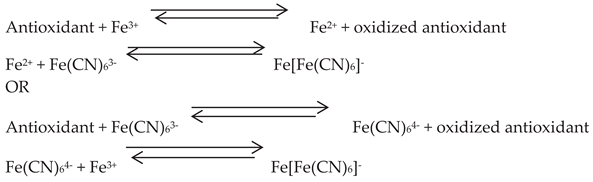
Figure 6.
The mechanism of ferric reducing antioxidant power (FRAP) reaction.
Figure 6.
The mechanism of ferric reducing antioxidant power (FRAP) reaction.
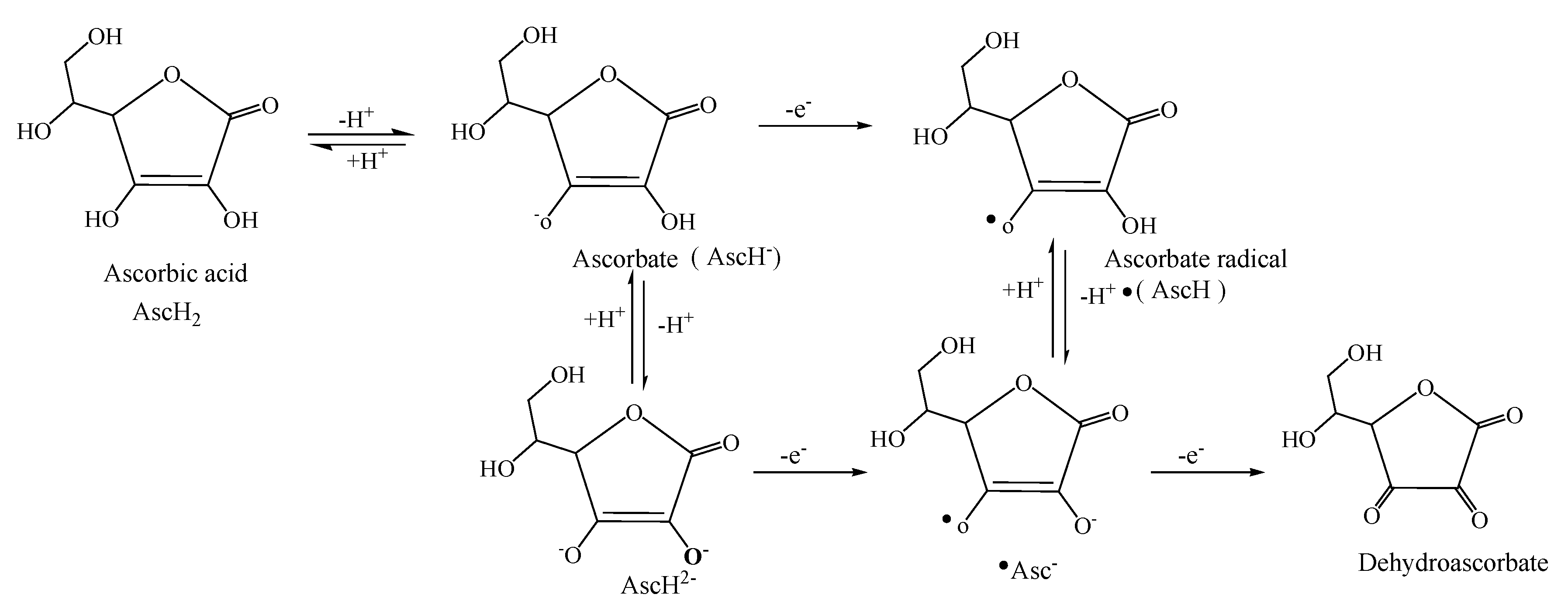
Vitamin C (ascorbic acid) is a water-soluble free radical scavenger. Vitamin C, changes to the ascorbate radical (
Figure 7) by donating an electron to the lipid radical in order to terminate the lipid peroxidation chain reaction. One ascorbate and one dehydroascorbate molecule are produced by the quick reaction of the pairs of ascorbate radicals. There is no antioxidant capacity in dehydroascorbate. Thus, the addition of two electrons transforms dehydroascorbate back into ascorbate. It has been suggested that oxidoreductase completes the final step of adding two electrons to the dehydroascorbate [
35].
Antibacterial Activities
Medicinal Plants are important source of potentially useful structures for the development of novel chemotherapy drugs. Evaluating their antibacterial activity in vitro is the initial step towards achieving this goal [
7].
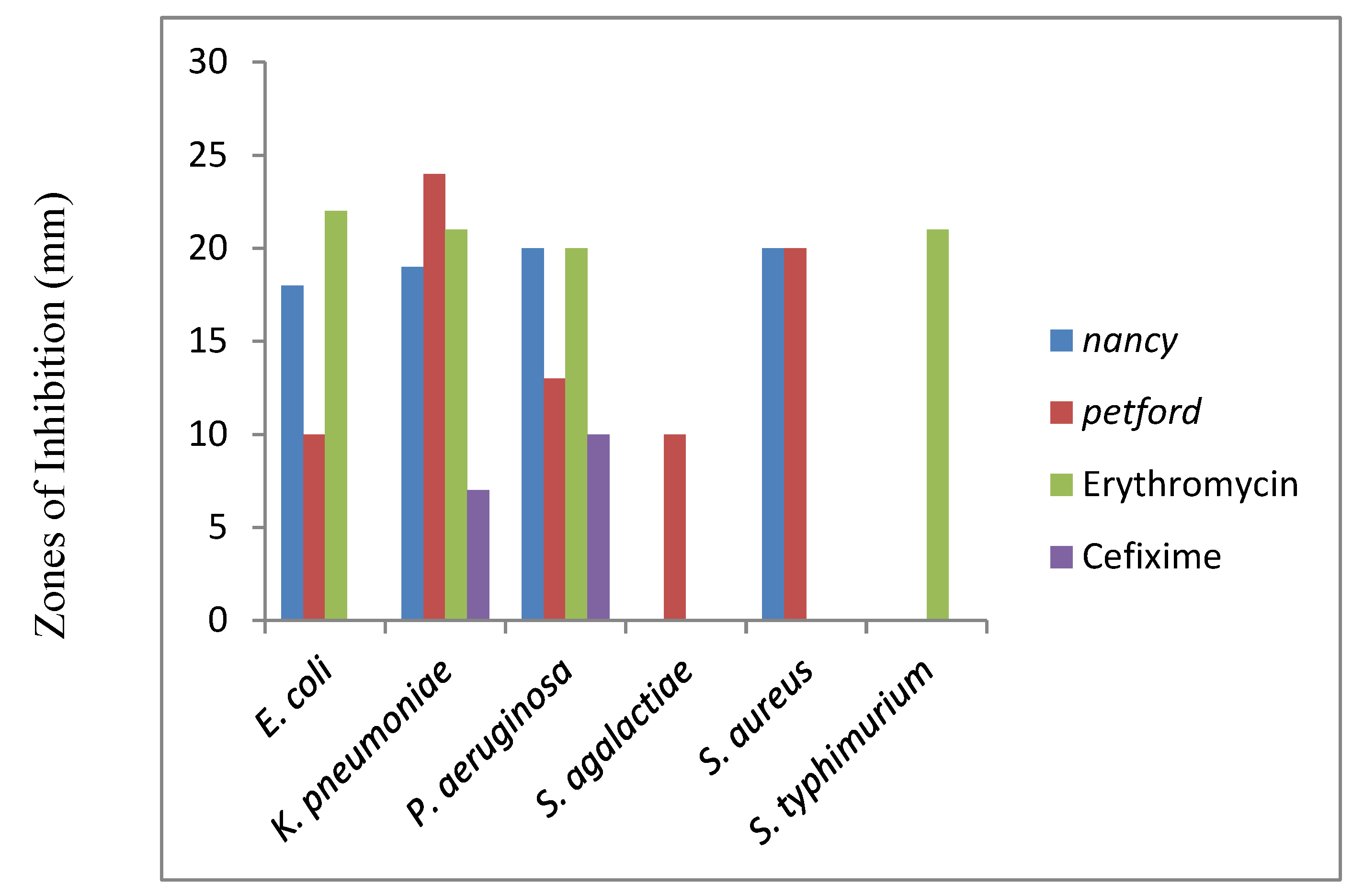
The antibacterial activities of the two seed essential oils of
E. camaldulensis varieties against tested bacteria were showed variable activities (
Figure 8). The highest inhibition zone of the seed essential oil of
E. camaldulensis var. nancy was showed against
P. aeruginosa (20 mm) and
S. aureus (20 mm),
K. pneumoniae (19 mm), and
E. coli (18 mm) but resistant to
S. agalactiae and S
. typhimurium while the inhibitory effects of
E. camaldulensis var. petford were showed against
K. pneumoniae (24 mm),
S. aureus (20 mm),
S. agalactiae (15 mm),
P. aeruginosa (13 mm) and
E. coli (10 mm) but resistant to S
. typhimurium. The tested bacteria and
S. agalactiae and
S. aureusthe were found to be resistant to Erythromycin and also
E. coli, S. agalactiae, S. aureus, and
S. typhimurium were found to be resistant to Cefixime antibiotics [
1].
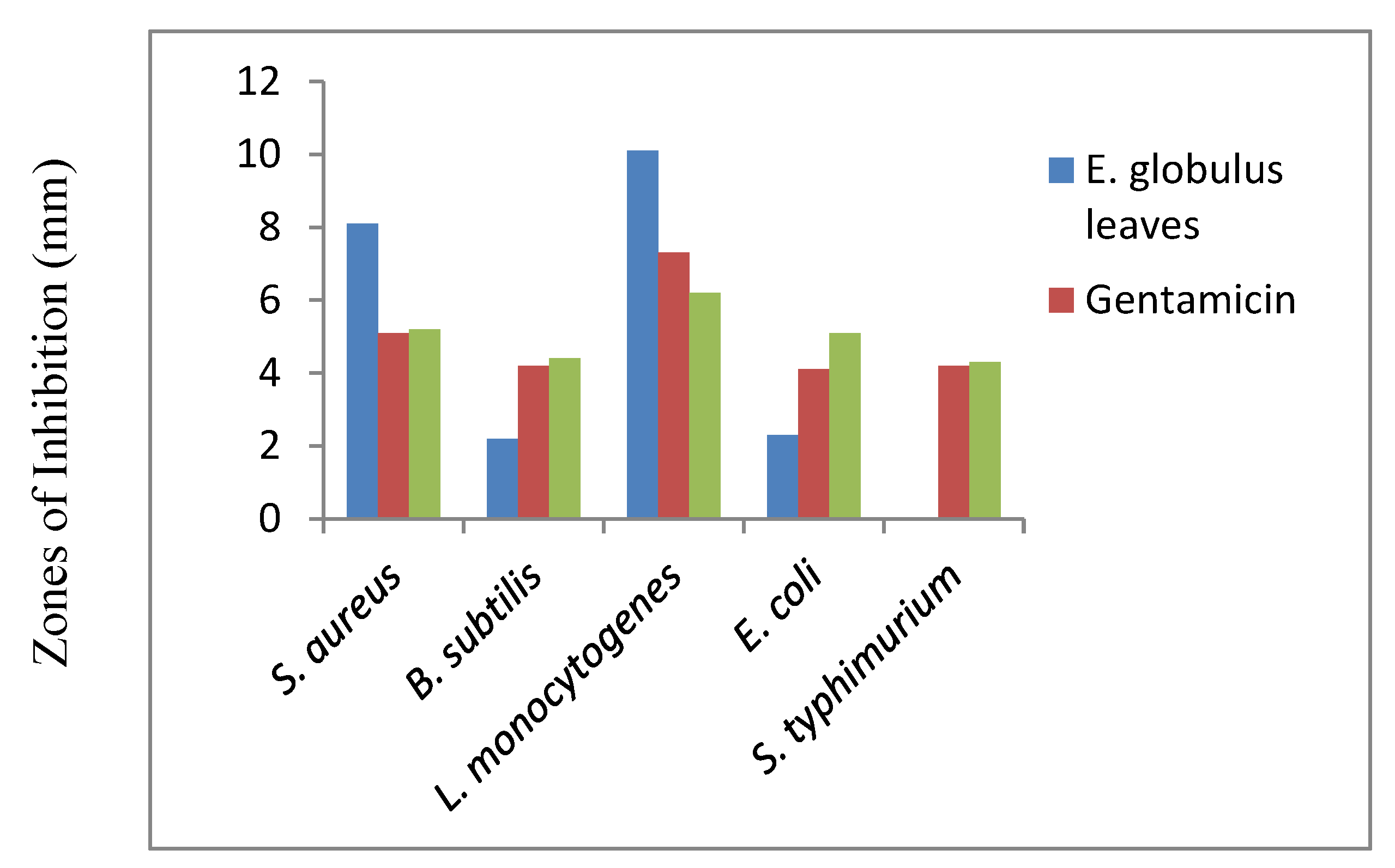
As shown in
Figure 9, the anti-bacterial activity of
E. globulus leaf extracts against
L. monocytogenes (10.1 ± 0.4 mm) and
S. aureus (8.1 ± 0.1mm) is higher than the standard antibiotics Gentamicin and Ciprofloxacin in the bacterial strains of
L. monocytogenes and
S. aureus [
7].
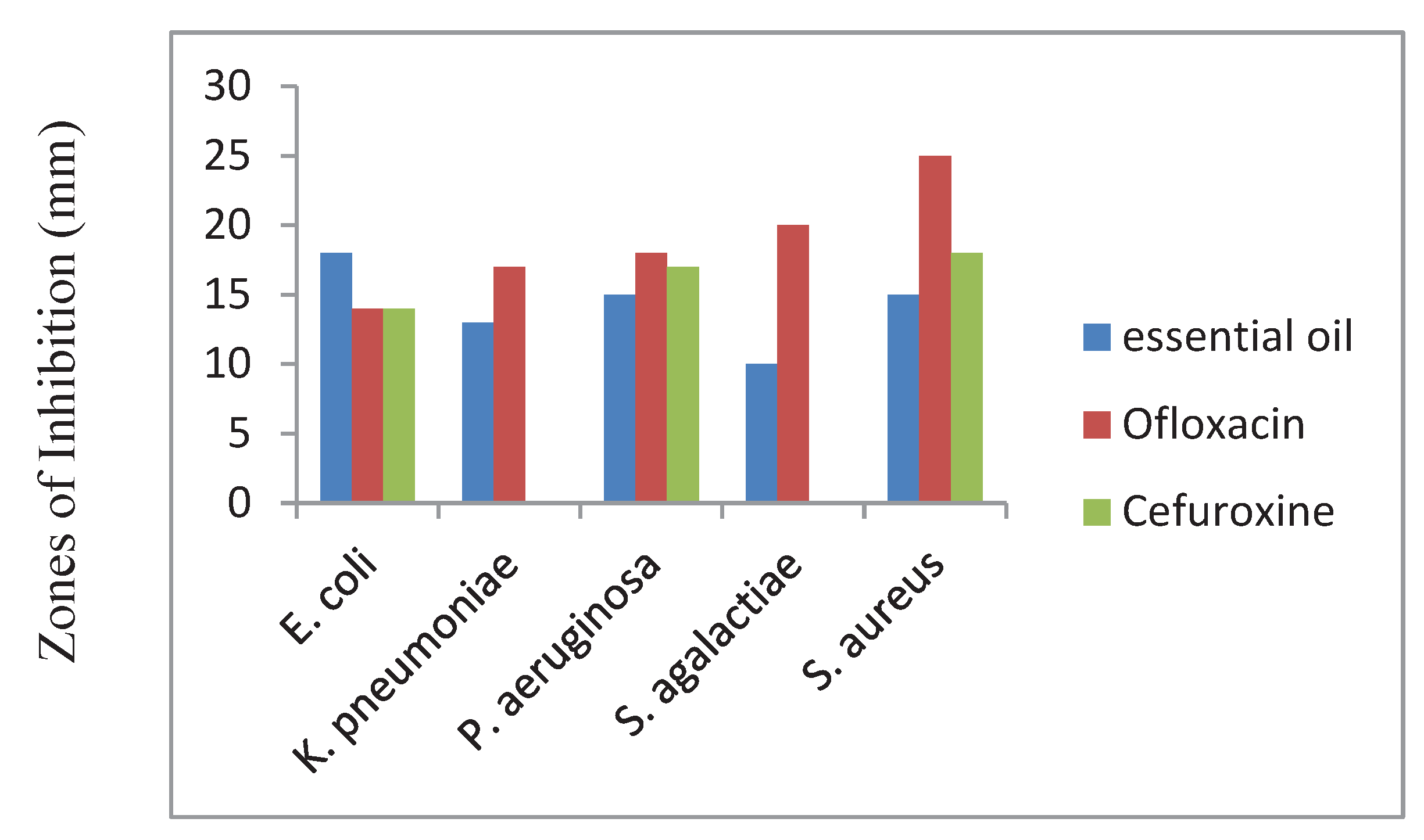
The antibacterial activities of the fruit essential oil of
E. Citriodora were determined against five bacteria
E. coli, K. pneumoniae, P. aeruginosa, S. agalactiae, and
S. aureus were found to be the highest inhibitory effect against
E. coli (18 mm),
P. aeruginosa (15.00 mm),
S. aureus (15.00 mm),
S. agalactiae (10.00 mm) and
K. pneumoniae (13.00 mm). The bacteria were found to be sensitive to Ofloxacin and some are resistant to Cefuroxine conventional antibiotics as shown in
Figure 10 [
13].
Conclusion
Medicinal plants are used to cure a variety of diseases and ailments in both humans and animals. These plants are an important source of therapeutic drug molecules they contain secondary metabolites, which are potential sources of drugs. The major bioactive components in medicinal plants are secondary metabolites such as alkaloids, flavonoids, phenols, and saponins, which have several broad biological activities. These include antibacterial, antiseptic, antioxidant, and antifungal properties. Eucalyptus plants have several medical benefits, such as antioxidant, anti-inflammatory, antinociceptive, and antimicrobial activities. They also have the potential to prevent age-related neurodegenerative and Alzheimer’s disease. Additionally, these plants possess antiviral and anticancer properties due to the presence of polyphenols and flavonoids. Research on aromatic and medicinal plants has a bright future thanks to the application of advanced technologies, interdisciplinary methods, and sustainable practices. This may result in the creation of novel and more effective plant-based medications, as well as new therapies.