Submitted:
22 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Gut Microbiota in Health and Disease
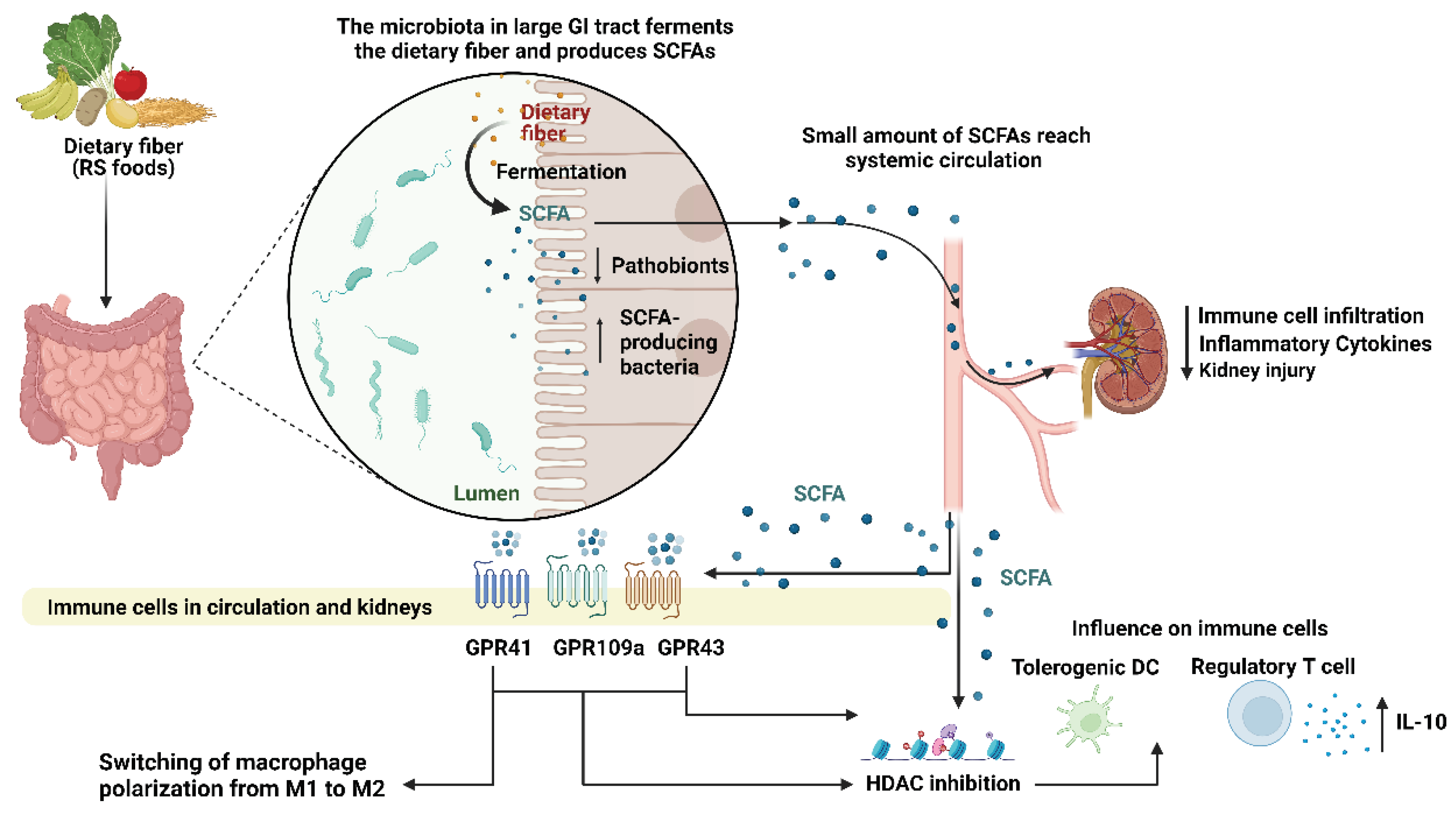
3. Role of SCFAs in Modulating the Immune Response
4. Gut-Kidney Axis
5. Role of Microbiota in Specific Inflammatory Kidney Diseases
5.1. ANCA-Associated Vasculitis
5.2. Goodpasture’s Syndrome
5.3. IgA Vasculitis
5.4. IgA Nephropathy
5.5. Lupus Nephritis
5.6. Acute Kidney Injury
5.7. Diabetic Nephropathy
6. Gut Microbiota and Interactions with Standard of Care Immunosuppressants
7. The Role of SCFAs and Innate Cells
8. SCFAs Modulate T Regulatory Cells
9. Use of Animal Models in Microbiome Studies - Considerations for Translation into Human Studies
9.1. Differences between Human and Mouse Gut Anatomy as Gut Microbiota Diversity
9.2. Use of Germ Free/Gnotobiotic Animal Models
9.3. Housing Consideration When Using Mouse Models for Microbiota Studies
9.4. The Requirement for Guidelines for Studying the Microbiome
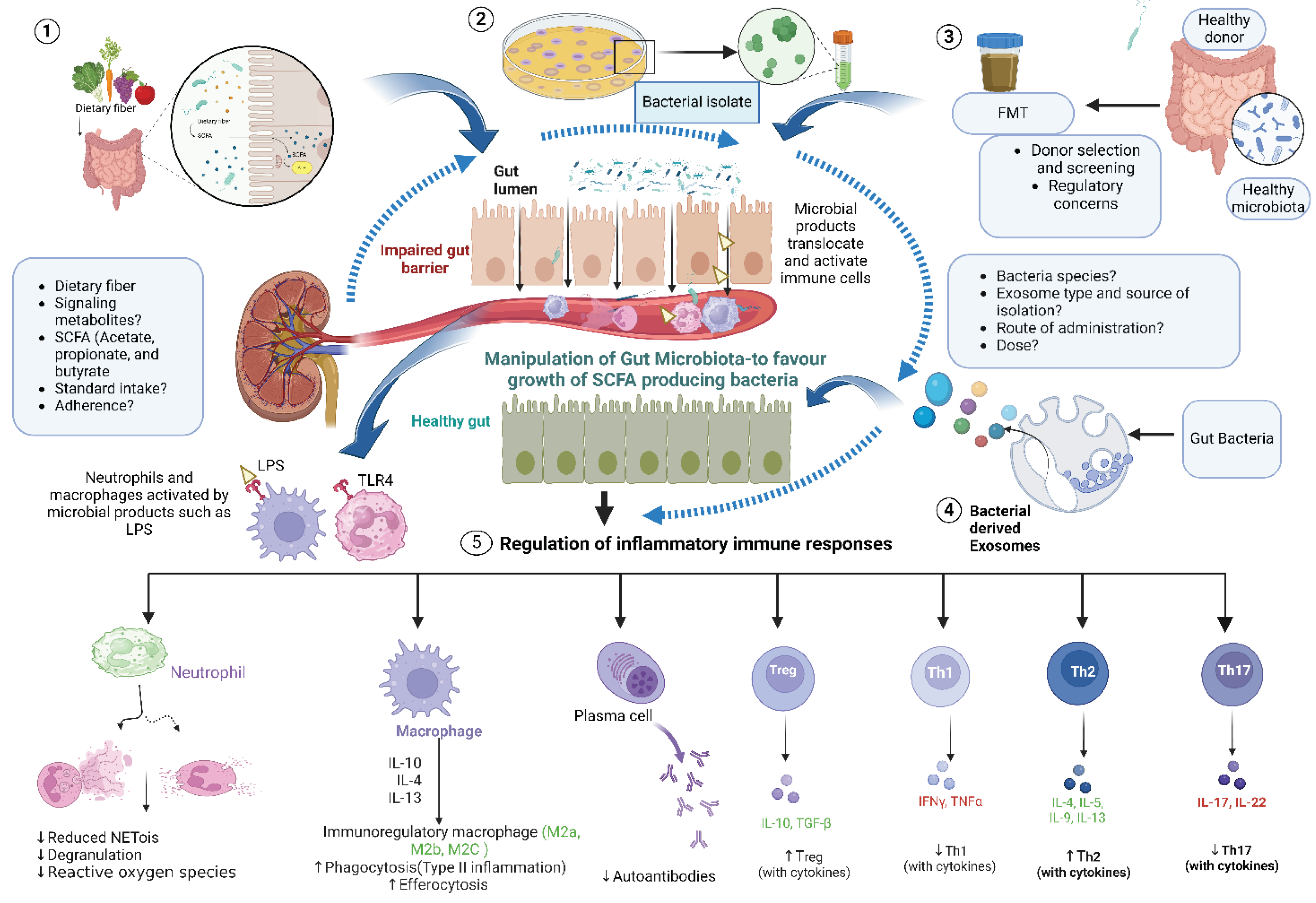
10. Modulation of Gut Microbiota as Potential Therapy
10.1. Dietary Intervention
10.2. SCFAs as Potential Therapeutic Agents
10.3. Fecal Microbiota Transplants as Therapy
10.4. Transfer of Immunomodulatory Microbial Isolates as Therapy
10.5. Bacterial Exosomes
11. Conclusion
References
- Marino, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 2017, 18, 552–562. [Google Scholar] [CrossRef]
- Valiente, G.R.; Munir, A.; Hart, M.L.; Blough, P.; Wada, T.T.; Dalan, E.E.; Willis, W.L.; Wu, L.C.; Freud, A.G.; Jarjour, W.N. Gut dysbiosis is associated with acceleration of lupus nephritis. Sci Rep 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.D.; Fernandes, G.R.; Calderaro, D.C.; Mendonca, S.M.S.; Silva, J.M.; Albiero, M.L.; Cunha, F.Q.; Xiao, E.; Ferreira, G.A.; Teixeira, A.L.; et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep 2019, 9, 8379. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol 2018, 15, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Kellow, N.J.; Coughlan, M.T. Modulation of the Gut Microbiota by Resistant Starch as a Treatment of Chronic Kidney Diseases: Evidence of Efficacy and Mechanistic Insights. Adv Nutr 2019, 10, 303–320. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation 2020, 141, 1393–1403. [Google Scholar] [CrossRef]
- Tanti, J.F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne) 2012, 3, 181. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr Rev 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J Am Soc Nephrol 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Chen, J.; Yue, Y.; Wang, L.; Deng, Z.; Yuan, Y.; Zhao, M.; Yuan, Z.; Tan, C.; Cao, Y. Altered gut microbiota correlated with systemic inflammation in children with Kawasaki disease. Sci Rep 2020, 10, 14525. [Google Scholar] [CrossRef] [PubMed]
- R, R.M.; Marques, F.Z. Diet-related gut microbial metabolites and sensing in hypertension. J Hum Hypertens 2021, 35, 162–169. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Im, S.H. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med 2020, 52, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Tan, S.; Clarke, R.; de Pasquale, C.; Thallas-Bonke, V.; Nguyen, T.; Penfold, S.; Harcourt, B.; Sourris, K.; Lindblom, R.; et al. Processed Foods drive Intestinal Barrier permeability and Microvascular Diseases. Science Advances 2021, 7. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Kusnadi, Y.; Saleh, M.I.; Ali, Z.; Hermansyah, H.; Murti, K.; Hafy, Z.; Yuristo, N.S.E. Firmicutes/Bacteroidetes Ratio of Gut Microbiota and Its Relationships with Clinical Parameters of Type 2 Diabetes Mellitus: A Systematic Review. Open Access Macedonian Journal of Medical Sciences 2023, 11, 67–72. [Google Scholar] [CrossRef]
- Polidori, I.; Marullo, L.; Ialongo, C.; Tomassetti, F.; Colombo, R.; di Gaudio, F.; Calugi, G.; Marrone, G.; Noce, A.; Bernardini, S.; et al. Characterization of Gut Microbiota Composition in Type 2 Diabetes Patients: A Population-Based Study. International Journal of Environmental Research and Public Health 2022, 19, 15913. [Google Scholar] [CrossRef]
- Chow, J.; Tang, H.; Mazmanian, S.K. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol 2011, 23, 473–480. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, L.; Ren, Q.; Feng, H.; Tao, S.; Cheng, L.; Ma, L.; Gou, S.J.; Fu, P. Understanding the Gut-Kidney Axis in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: An Analysis of Gut Microbiota Composition. Front Pharmacol 2022, 13, 783679. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.L.; Barnich, N.; Bringer, M.A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, T.; Nakashima, Y.; Murata, K.; Kanno, S.; Nishio, H.; Saito, M.; Tanaka, T.; Yamamura, K.; Sakai, Y.; Takada, H.; et al. Kawasaki disease-specific molecules in the sera are linked to microbe-associated molecular patterns in the biofilms. PLoS One 2014, 9, e113054. [Google Scholar] [CrossRef]
- Park, J.; Goergen, C.J.; HogenEsch, H.; Kim, C.H. Chronically Elevated Levels of Short-Chain Fatty Acids Induce T Cell-Mediated Ureteritis and Hydronephrosis. J Immunol 2016, 196, 2388–2400. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol 2018, 831, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Coady, M.J.; Chang, M.H.; Charron, F.M.; Plata, C.; Wallendorff, B.; Sah, J.F.; Markowitz, S.D.; Romero, M.F.; Lapointe, J.Y. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol 2004, 557, 719–731. [Google Scholar] [CrossRef]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun 2019, 10, 760. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.A.; Andrusaite, A.; Andersen, P.; Lawson, M.; Alcon-Giner, C.; Leclaire, C.; Caim, S.; Le Gall, G.; Shaw, T.; Connolly, J.P.R.; et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci Transl Med 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the Gut-Kidney Axis in Health and Disease. Front Med (Lausanne) 2020, 7, 620102. [Google Scholar] [CrossRef]
- Cummings, J.H.; Hill, M.J.; Bone, E.S.; Branch, W.J.; Jenkins, D.J. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 1979, 32, 2094–2101. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 2014, 10, 463–473. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J. Small-vessel vasculitis. N Engl J Med 1997, 337, 1512–1523. [Google Scholar] [CrossRef]
- Jennette, J.C.; Hoidal, J.R.; Falk, R.J. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood 1990, 75, 2263–2264. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G. Glomerulonephritis. Lancet 1999, 353, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Pearce, F.A.; Craven, A.; Merkel, P.A.; Luqmani, R.A.; Watts, R.A. Global ethnic and geographic differences in the clinical presentations of anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford) 2017, 56, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat Rev Dis Primers 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Ball, G.V. The history of ANCA-associated vasculitis. Rheum Dis Clin North Am 2010, 36, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D. Review article: Progress of treatment in ANCA-associated vasculitis. Nephrology (Carlton) 2009, 14, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hamour, S.; Salama, A.D.; Pusey, C.D. Management of ANCA-associated vasculitis: Current trends and future prospects. Ther Clin Risk Manag 2010, 6, 253–264. [Google Scholar] [CrossRef]
- Salama, A.D. Pathogenesis and treatment of ANCA-associated systemic vasculitis. J R Soc Med 1999, 92, 456–461. [Google Scholar] [CrossRef]
- Little, M.A.; Smyth, C.L.; Yadav, R.; Ambrose, L.; Cook, H.T.; Nourshargh, S.; Pusey, C.D. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood 2005, 106, 2050–2058. [Google Scholar] [CrossRef]
- Nachman, P.H.; Hogan, S.L.; Jennette, J.C.; Falk, R.J. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996, 7, 33–39. [Google Scholar] [CrossRef]
- Menahem, S.; Hiremagalur, B.; Mudge, D.; Toussaint, N.; Walters, G.; Caring for Australians with Renal, I. The CARI guidelines. Induction and maintenance therapy in ANCA-associated systemic vasculitis. Nephrology (Carlton) 2008, 13 Suppl 2, S24–S36. [Google Scholar] [CrossRef]
- Keogh, K.A.; Wylam, M.E.; Stone, J.H.; Specks, U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005, 52, 262–268. [Google Scholar] [CrossRef]
- de Lind van Wijngaarden, R.A.; Hauer, H.A.; Wolterbeek, R.; Jayne, D.R.; Gaskin, G.; Rasmussen, N.; Noel, L.H.; Ferrario, F.; Waldherr, R.; Hagen, E.C.; et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 2006, 17, 2264–2274. [Google Scholar] [CrossRef]
- Jayne, D.R.; Gaskin, G.; Rasmussen, N.; Abramowicz, D.; Ferrario, F.; Guillevin, L.; Mirapeix, E.; Savage, C.O.; Sinico, R.A.; Stegeman, C.A.; et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007, 18, 2180–2188. [Google Scholar] [CrossRef]
- O’Sullivan, K.M.; Snelson, M.; Nguyen, J.; Le, A.C.; Coughlan, M. Resistant starch supplementation alters the gut microbial consortium and attenuates kidney inflammation in an experimental model of autoimmune vasculitis. The Journal of Immunology 2023, 210, 165.08. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis Model Mech 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Linnenbrink, M.; Wang, J.; Hardouin, E.A.; Kunzel, S.; Metzler, D.; Baines, J.F. The role of biogeography in shaping diversity of the intestinal microbiota in house mice. Mol Ecol 2013, 22, 1904–1916. [Google Scholar] [CrossRef]
- Moroni, G.; Ponticelli, C. Rapidly progressive crescentic glomerulonephritis: Early treatment is a must. Autoimmun Rev 2014, 13, 723–729. [Google Scholar] [CrossRef]
- Reynolds, J.; Preston, G.A.; Pressler, B.M.; Hewins, P.; Brown, M.; Roth, A.; Alderman, E.; Bunch, D.; Jennette, J.C.; Cook, H.T.; et al. Autoimmunity to the alpha 3 chain of type IV collagen in glomerulonephritis is triggered by ‘autoantigen complementarity’. J Autoimmun 2015, 59, 8–18. [Google Scholar] [CrossRef]
- Marques, C.; Plaisier, E.; Cacoub, P.; Cadranel, J.; Saadoun, D. [Review on anti-glomerular basement membrane disease or Goodpasture’s syndrome]. Rev Med Interne 2020, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, Q.-h.; Cui, Z.; Zhao, M.-h.; Jia, X.-y. Short-chain fatty acids ameliorate experimental anti-glomerular basement membrane disease. Clinical Immunology 2024, 259, 109903. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, X.; Yu, G.; Qiao, J.; Cheng, J.; Wu, J.; Chen, J. Pathogenesis of IgA Vasculitis: An Up-To-Date Review. Front Immunol 2021, 12, 771619. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Tu, L.; Zou, J.S.; Zhu, S.Q.; Zhao, Y.J.; Qin, Y.H. The Involvement of Neutrophil Extracellular Traps in Disease Activity Associated With IgA Vasculitis. Front Immunol 2021, 12, 668974. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.S.; Zandbergen, M.; Wolterbeek, R.; Baelde, H.J.; van Es, L.A.; de Fijter, J.W.; Bruijn, J.A.; Bajema, I.M. Complement-mediated microangiopathy in IgA nephropathy and IgA vasculitis with nephritis. Mod Pathol 2019, 32, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Tsai, I.J.; Chang, C.J.; Chuang, Y.H.; Hsu, H.Y.; Chiang, B.L. The interaction between circulating complement proteins and cutaneous microvascular endothelial cells in the development of childhood Henoch-Schönlein Purpura. PLoS One 2015, 10, e0120411. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, R.; Khan, M.A.; Echaide, M.; Pérez-Gil, J.; Palaniyar, N. SP-D attenuates LPS-induced formation of human neutrophil extracellular traps (NETs), protecting pulmonary surfactant inactivation by NETs. Communications Biology 2019, 2, 470. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, X.; Pan, P.; Zhang, L.; Hu, Y.; Tan, H.; Wu, D.; Liu, B.; Li, H.; Li, H.; et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Scientific Reports 2016, 6, 37252. [Google Scholar] [CrossRef] [PubMed]
- Aubé, F.A.; Bidias, A.; Pépin, G. Who and how, DNA sensors in NETs-driven inflammation. Front Immunol 2023, 14, 1190177. [Google Scholar] [CrossRef]
- Mallavia, B.; Liu, F.; Lefrancais, E.; Cleary, S.J.; Kwaan, N.; Tian, J.J.; Magnen, M.; Sayah, D.M.; Soong, A.; Chen, J.; et al. Mitochondrial DNA Stimulates TLR9-Dependent Neutrophil Extracellular Trap Formation in Primary Graft Dysfunction. Am J Respir Cell Mol Biol 2020, 62, 364–372. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Lin, X.; Bian, X.; Jing, R.; Frelinger, A.; Zhang, A. Comparison and Analysis of Gut Microbiota in Children With IgA Vasculitis With Different Clinical Symptoms. Front Pediatr 2021, 9, 800677. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, C.; Zhao, L.; Sheng, D.; Chen, B.; Zhao, G.; Wang, Q.; Zhang, L. Taxonomic and functional shifts of gut microbiome in immunoglobulin A vasculitis children and their mothers. Front Pediatr 2024, 12, 1356529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, X.; Lai, P.; Wang, K.; Bao, Y.; Li, X. Relevance of Intestinal Microbiota in Immunoglobulin A Vasculitis With Abdominal Involvement. Front Pediatr 2022, 10, 943267. [Google Scholar] [CrossRef] [PubMed]
- Rizk, D.V.; Saha, M.K.; Hall, S.; Novak, L.; Brown, R.; Huang, Z.Q.; Fatima, H.; Julian, B.A.; Novak, J. Glomerular Immunodeposits of Patients with IgA Nephropathy Are Enriched for IgG Autoantibodies Specific for Galactose-Deficient IgA1. J Am Soc Nephrol 2019, 30, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, Z.; Suzuki, H.; Reily, C.; Satake, K.; Novak, L.; Xu, N.; Huang, Z.Q.; Knoppova, B.; Khan, A.; Hall, S.; et al. Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J Autoimmun 2021, 118, 102593. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Montemurno, E.; Piccolo, M.; Vannini, L.; Lauriero, G.; Maranzano, V.; Gozzi, G.; Serrazanetti, D.; Dalfino, G.; Gobbetti, M.; et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One 2014, 9, e99006. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Tan, J.; Tan, L.; Tang, Y.; Qiu, Z.; Pei, G.; Qin, W. Modifications of gut microbiota are associated with the severity of IgA nephropathy in the Chinese population. Int Immunopharmacol 2020, 89, 107085. [Google Scholar] [CrossRef]
- Shah, N.B.; Nigwekar, S.U.; Kalim, S.; Lelouvier, B.; Servant, F.; Dalal, M.; Krinsky, S.; Fasano, A.; Tolkoff-Rubin, N.; Allegretti, A.S. The Gut and Blood Microbiome in IgA Nephropathy and Healthy Controls. Kidney360 2021, 2, 1261–1274. [Google Scholar] [CrossRef]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat Rev Dis Primers 2020, 6, 7. [Google Scholar] [CrossRef]
- Zhang, L.; Qing, P.; Yang, H.; Wu, Y.; Liu, Y.; Luo, Y. Gut Microbiome and Metabolites in Systemic Lupus Erythematosus: Link, Mechanisms and Intervention. Front Immunol 2021, 12, 686501. [Google Scholar] [CrossRef]
- Zhang, S.X.; Wang, J.; Chen, J.W.; Zhang, M.X.; Zhang, Y.F.; Hu, F.Y.; Lv, Z.Q.; Gao, C.; Li, Y.F.; Li, X.F. The level of peripheral regulatory T cells is linked to changes in gut commensal microflora in patients with systemic lupus erythematosus. Ann Rheum Dis 2021, 80, e177. [Google Scholar] [CrossRef]
- Azzouz, D.; Omarbekova, A.; Heguy, A.; Schwudke, D.; Gisch, N.; Rovin, B.H.; Caricchio, R.; Buyon, J.P.; Alekseyenko, A.V.; Silverman, G.J. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis 2019, 78, 947–956. [Google Scholar] [CrossRef]
- Vahidi, Z.; Samadi, M.; Mahmoudi, M.; RezaieYazdi, Z.; Sahebari, M.; Tabasi, N.; Esmaeili, S.-A.; Sahebkar, A.; Rastin, M. Lactobacillus rhamnosus and Lactobacillus delbrueckii ameliorate the expression of miR-155 and miR-181a in SLE patients. Journal of Functional Foods 2018, 48, 228–233. [Google Scholar] [CrossRef]
- Jang, H.R.; Gandolfo, M.T.; Ko, G.J.; Satpute, S.; Racusen, L.; Rabb, H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol 2009, 297, F1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol 2017, 28, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Cho, W.Y.; Chung, S.M.; Choi, Y.E.; Fang, Y.; Park, M.S.; Park, S.J.; Ko, Y.S.; Lee, H.Y.; Yang, J.; et al. Altered gut microbiome plays an important role in AKI to CKD transition in aged mice. Front Med (Lausanne) 2023, 10, 1238960. [Google Scholar] [CrossRef] [PubMed]
- Gharaie, S.; Lee, K.; Newman-Rivera, A.M.; Xu, J.; Patel, S.K.; Gooya, M.; Arend, L.J.; Raj, D.S.; Pluznick, J.; Parikh, C.; et al. Microbiome modulation after severe acute kidney injury accelerates functional recovery and decreases kidney fibrosis. Kidney International 2023, 104, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; de Pasquale, C.; Ekinci, E.I.; Coughlan, M.T. Gut microbiome, prebiotics, intestinal permeability and diabetes complications. Best Practice & Research Clinical Endocrinology & Metabolism 2021, 35, 101507. [Google Scholar] [CrossRef]
- Tao, S.; Li, L.; Li, L.; Liu, Y.; Ren, Q.; Shi, M.; Liu, J.; Jiang, J.; Ma, H.; Huang, Z.; et al. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol 2019, 56, 581–592. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Liu, C.; Yu, X.; Li, H.; Zhang, W.; Li, Y.; Geng, Y.; Wang, Z. Compositional Alterations of Gut Microbiota in Patients with Diabetic Kidney Disease and Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes 2022, 15, 755–765. [Google Scholar] [CrossRef]
- Du, X.; Liu, J.; Xue, Y.; Kong, X.; Lv, C.; Li, Z.; Huang, Y.; Wang, B. Alteration of gut microbial profile in patients with diabetic nephropathy. Endocrine 2021, 73, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Xu, K.-Y.; Xia, G.-H.; Lu, J.-Q.; Chen, M.-X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.-W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Scientific Reports 2017, 7, 1445. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney International 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. American Journal of Nephrology 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.B.; Allegretti, A.S.; Nigwekar, S.U.; Kalim, S.; Zhao, S.; Lelouvier, B.; Servant, F.; Serena, G.; Thadhani, R.I.; Raj, D.S.; et al. Blood Microbiome Profile in CKD : A Pilot Study. Clin J Am Soc Nephrol 2019, 14, 692–701. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunology 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Ibrahim, R.; Lambert, K. The gut microbiota profile of adults with kidney disease and kidney stones: a systematic review of the literature. BMC Nephrology 2020, 21, 215. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernandez-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Salosensaari, A.; Laitinen, V.; Havulinna, A.S.; Meric, G.; Cheng, S.; Perola, M.; Valsta, L.; Alfthan, G.; Inouye, M.; Watrous, J.D.; et al. Taxonomic signatures of cause-specific mortality risk in human gut microbiome. Nature Communications 2021, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J Clin Med 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, Y.; Tamura, H.; Furuie, K.; Kuraoka, S.; Nagata, H.; Nakazato, H. Cyclosporine therapy could be considered for membranoproliferative glomerulonephritis with immunoglobulin A deposits: a case report. BMC Nephrology 2022, 23, 358. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, D.; Law, H.K.-W.; Wu, Y.; Zhu, G.-h.; Huang, W.-y.; Kang, Y. Integrative Analysis of Gut Microbiota and Fecal Metabolites in Rats after Prednisone Treatment. Microbiology Spectrum 2021, 9, e00650–00621. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol 2018, 16, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol Biochem 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wu, S.; Yan, C.; Zhao, C.; Jin, H.; Yan, N.; Xu, J.; Wu, Y.; Li, C.; Shao, Q.; et al. Butyrate upregulates the TLR4 expression and the phosphorylation of MAPKs and NK-kappaB in colon cancer cell in vitro. Oncol Lett 2018, 16, 4439–4447. [Google Scholar] [CrossRef]
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res 2008, 28, 321–328. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-kappaB pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef]
- Lepenies, J.; Eardley, K.S.; Kienitz, T.; Hewison, M.; Ihl, T.; Stewart, P.M.; Cockwell, P.; Quinkler, M. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-beta(1) in patients with chronic kidney disease. Nephron Clin Pract 2011, 119, c97–c104. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Lawson, B.R. Toll-like receptors and kidney diseases. Inflamm Allergy Drug Targets 2009, 8, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; Steinmetz, O.M.; Ooi, J.D.; Gan, P.Y.; O’Sullivan, K.M.; Visvanathan, K.; Akira, S.; Kitching, A.R.; Holdsworth, S.R. Toll-like receptor 9 enhances nephritogenic immunity and glomerular leukocyte recruitment, exacerbating experimental crescentic glomerulonephritis. Am J Pathol 2010, 177, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; van der Veen, B.S.; O’Sullivan, K.M.; Gan, P.Y.; Ooi, J.D.; Heeringa, P.; Satchell, S.C.; Mathieson, P.W.; Saleem, M.A.; Visvanathan, K.; et al. Intrinsic renal cell and leukocyte-derived TLR4 aggravate experimental anti-MPO glomerulonephritis. Kidney international 2010, 78, 1263–1274. [Google Scholar] [CrossRef]
- O’Sullivan, K.M.; Ford, S.L.; Longano, A.; Kitching, A.R.; Holdsworth, S.R. Intrarenal Toll-like receptor 4 and Toll-like receptor 2 expression correlates with injury in antineutrophil cytoplasmic antibody-associated vasculitis. Am J Physiol Renal Physiol 2018, 315, F1283–F1294. [Google Scholar] [CrossRef]
- Rutting, S.; Xenaki, D.; Malouf, M.; Horvat, J.C.; Wood, L.G.; Hansbro, P.M.; Oliver, B.G. Short-chain fatty acids increase TNFalpha-induced inflammation in primary human lung mesenchymal cells through the activation of p38 MAPK. Am J Physiol Lung Cell Mol Physiol 2019, 316, L157–L174. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Zhang, D.; Frenette, P.S. Cross talk between neutrophils and the microbiota. Blood 2019, 133, 2168–2177. [Google Scholar] [CrossRef]
- Ruth, A.J.; Kitching, A.R.; Kwan, R.Y.; Odobasic, D.; Ooi, J.D.; Timoshanko, J.R.; Hickey, M.J.; Holdsworth, S.R. Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 2006, 17, 1940–1949. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Hamam, H.J.; Khan, M.A.; Palaniyar, N. Histone Acetylation Promotes Neutrophil Extracellular Trap Formation. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- O’Sullivan, K.M.; Holdsworth, S.R. Neutrophil Extracellular Traps: A Potential Therapeutic Target in MPO-ANCA Associated Vasculitis? Front Immunol 2021, 12, 635188. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Ferguson, G.J.; Kulkarni, S.; Damoulakis, G.; Anderson, K.; Bohlooly, Y.M.; Stephens, L.; Hawkins, P.T.; Curi, R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One 2011, 6, e21205. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.E.; Shim, R.; Nicholls, A.J.; Oliveira, A.C.; Mason, L.J.; Binge, L.; Mackay, C.R.; Wong, C.H. G Protein-Coupled Receptor 43 Modulates Neutrophil Recruitment during Acute Inflammation. PLoS One 2016, 11, e0163750. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-alpha-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun 2017, 486, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Liu, J.; Gu, Q.H.; Cui, Z.; Zhao, M.H.; Jia, X.Y. Short-chain fatty acids ameliorate experimental anti-glomerular basement membrane disease. Clin Immunol 2024, 259, 109903. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal immunology 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol Invest 2016, 45, 205–222. [Google Scholar] [CrossRef]
- Schiweck, C.; Edwin Thanarajah, S.; Aichholzer, M.; Matura, S.; Reif, A.; Vrieze, E.; Weigert, A.; Visekruna, A. Regulation of CD4(+) and CD8(+) T Cell Biology by Short-Chain Fatty Acids and Its Relevance for Autoimmune Pathology. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: from bench to bedside. Int Immunol 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, C.T.; Seibert, C.; Peterson, F.C.; Sakmar, T.P.; Volkman, B.F. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12). J Mol Biol 2006, 359, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Strazza, M.; Azoulay-Alfaguter, I.; Silverman, G.J.; Mor, A. T cell chemokine receptor patterns as pathogenic signatures in autoimmunity. Discovery medicine 2015, 19 103, 117–125. [Google Scholar]
- García-Cuesta, E.M.; Santiago, C.A.; Vallejo-Díaz, J.; Juarranz, Y.; Rodríguez-Frade, J.M.; Mellado, M. The Role of the CXCL12/CXCR4/ACKR3 Axis in Autoimmune Diseases. Frontiers in Endocrinology 2019, 10. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Ragusa, F.; Ruffilli, I.; Elia, G.; Paparo, S.R.; Antonelli, A. Th1 chemokines in autoimmune endocrine disorders. The Journal of clinical endocrinology and metabolism 2019. [Google Scholar] [CrossRef] [PubMed]
- Sato, W. Chemokine receptors on T cells in multiple sclerosis. Clinical and Experimental Neuroimmunology 2014, 5. [Google Scholar] [CrossRef]
- Tan, D.S.; Gan, P.Y.; O’Sullivan, K.M.; Hammett, M.V.; Summers, S.A.; Ooi, J.D.; Lundgren, B.A.; Boyd, R.L.; Scott, H.S.; Kitching, A.R.; et al. Thymic deletion and regulatory T cells prevent antimyeloperoxidase GN. J Am Soc Nephrol 2013, 24, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Mendez-Garcia, C.; Rojo, D.; Barbas, C.; Moya, A. Antibiotic use and microbiome function. Biochem Pharmacol 2017, 134, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Aden, L.A.; Barbaro, N.R.; Van Beusecum, J.P.; Xiao, L.; Simmons, A.J.; Warden, C.; Pasic, L.; Himmel, L.E.; Washington, M.K.; et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight 2019, 5. [Google Scholar] [CrossRef]
- Treuting, P.M.; Dintzis, S.; Montine, K.S. Comparative anatomy and histology: a mouse, rat, and human atlas; Academic Press, 2017. [Google Scholar]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Krych, L.; Hansen, C.H.; Hansen, A.K.; van den Berg, F.W.; Nielsen, D.S. Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS One 2013, 8, e62578. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-human Primate, and Human Feces. Front Microbiol 2018, 9, 2897. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, S.; Kim, B.; Holzapfel, W.H.; Hyun, C.K. Disturbance of lipid metabolism in germ-free mice transplanted with gut microbiota of DSS-induced colitis mice. PLoS One 2023, 18, e0280850. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int J Neuropsychopharmacol 2016, 19. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.E.; Ritz, N.L.; Martin, J.A.; Wardill, H.R.; Cryan, J.F.; Clarke, G. Investigating causality with fecal microbiota transplantation in rodents: applications, recommendations and pitfalls. Gut Microbes 2021, 13, 1941711. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Kaiser, T.; Beura, L.K.; Hamilton, M.J.; Weingarden, A.R.; Bobr, A.; Kang, J.; Masopust, D.; Sadowsky, M.J.; Khoruts, A. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome 2017, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Nicolson, S.W.; Jackson, S. The effect of different oral antibiotics on the gastrointestinal microflora of a wild rodent (Aethomys namaquensis). Comp Biochem Physiol A Mol Integr Physiol 2004, 138, 475–483. [Google Scholar] [CrossRef]
- Shekhar, S.; Petersen, F.C. The Dark Side of Antibiotics: Adverse Effects on the Infant Immune Defense Against Infection. Front Pediatr 2020, 8, 544460. [Google Scholar] [CrossRef]
- Muralitharan, R.R.; Snelson, M.; Meric, G.; Coughlan, M.T.; Marques, F.Z. Guidelines for microbiome studies in renal physiology. Am J Physiol Renal Physiol 2023, 325, F345–F362. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Copio, J.N.; Shi, Y.; Kang, S.; Franklin, C.L.; Ericsson, A.C. Reduced housing density improves statistical power of murine gut microbiota studies. Cell Rep 2022, 39, 110783. [Google Scholar] [CrossRef] [PubMed]
- Hylander, B.L.; Qiao, G.; Cortes Gomez, E.; Singh, P.; Repasky, E.A. Housing temperature plays a critical role in determining gut microbiome composition in research mice: Implications for experimental reproducibility. Biochimie 2023, 210, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Shen, T.D.; Chen, E.Z.; Bittinger, K.; Bailey, A.; Roggiani, M.; Sirota-Madi, A.; Friedman, E.S.; Chau, L.; Lin, A.; et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Secombe, K.R.; Al-Qadami, G.H.; Subramaniam, C.B.; Bowen, J.M.; Scott, J.; Van Sebille, Y.Z.A.; Snelson, M.; Cowan, C.; Clarke, G.; Gheorghe, C.E.; et al. Guidelines for reporting on animal fecal transplantation (GRAFT) studies: recommendations from a systematic review of murine transplantation protocols. Gut Microbes 2021, 13, 1979878. [Google Scholar] [CrossRef] [PubMed]
- Picard, K.; Barreto Silva, M.I.; Mager, D.; Richard, C. Dietary Potassium Intake and Risk of Chronic Kidney Disease Progression in Predialysis Patients with Chronic Kidney Disease: A Systematic Review. Adv Nutr 2020, 11, 1002–1015. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.M.; Pagovich, O.E.; Kriegel, M.A. Diet, microbiota and autoimmune diseases. Lupus 2014, 23, 518–526. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacological research 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Cigarrán Guldris, S.; Latorre Catalá, J.A.; Sanjurjo Amado, A.; Menéndez Granados, N.; Piñeiro Varela, E. Fibre Intake in Chronic Kidney Disease: What Fibre Should We Recommend? Nutrients 2022, 14, 4419. [Google Scholar] [CrossRef]
- Bell, K.J.; Saad, S.; Tillett, B.J.; McGuire, H.M.; Bordbar, S.; Yap, Y.A.; Nguyen, L.T.; Wilkins, M.R.; Corley, S.; Brodie, S.; et al. Metabolite-based dietary supplementation in human type 1 diabetes is associated with microbiota and immune modulation. Microbiome 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Jama, H.A.; Rhys-Jones, D.; Nakai, M.; Yao, C.K.; Climie, R.E.; Sata, Y.; Anderson, D.; Creek, D.J.; Head, G.A.; Kaye, D.M.; et al. Prebiotic intervention with HAMSAB in untreated essential hypertensive patients assessed in a phase II randomized trial. Nature Cardiovascular Research 2023, 2, 35–43. [Google Scholar] [CrossRef]
- Su, G.; Qin, X.; Yang, C.; Sabatino, A.; Kelly, J.T.; Avesani, C.M.; Carrero, J.J. Fiber intake and health in people with chronic kidney disease. Clin Kidney J 2022, 15, 213–225. [Google Scholar] [CrossRef] [PubMed]
- El Amouri, A.; Snauwaert, E.; Foulon, A.; Vande Moortel, C.; Van Dyck, M.; Van Hoeck, K.; Godefroid, N.; Glorieux, G.; Van Biesen, W.; Vande Walle, J.; et al. Dietary Fibre Intake Is Associated with Serum Levels of Uraemic Toxins in Children with Chronic Kidney Disease. Toxins (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, X.; Riserus, U.; Krishnamurthy, V.M.; Cederholm, T.; Ärnlöv, J.; Lindholm, B.; Sjögren, P.; Carrero, J.J. Dietary fiber, kidney function, inflammation, and mortality risk. Clinical journal of the American Society of Nephrology: CJASN 2014, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, M.; Song, J.; Liu, S.; Wang, Y.; Su, X.; Wei, K.; Xu, Z.; Li, H.; Wang, Z. The prebiotic effects of soluble dietary fiber mixture on renal anemia and the gut microbiota in end-stage renal disease patients on maintenance hemodialysis: a prospective, randomized, placebo-controlled study. J Transl Med 2022, 20, 599. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, N.; Baid-Agrawal, S.; Grams, S.; Nadal, J.; Schmid, M.; Schneider, M.P.; Eckardt, K.U.; Floege, J.; Bergmann, M.M.; Schlieper, G.; et al. Low adherence to CKD-specific dietary recommendations associates with impaired kidney function, dyslipidemia, and inflammation. European journal of clinical nutrition 2021, 75, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Mun, K.H.; Yu, G.I.; Choi, B.Y.; Kim, M.K.; Shin, M.H.; Shin, D.H. Association of Dietary Potassium Intake with the Development of Chronic Kidney Disease and Renal Function in Patients with Mildly Decreased Kidney Function: The Korean Multi-Rural Communities Cohort Study. Med Sci Monit 2019, 25, 1061–1070. [Google Scholar] [CrossRef]
- Dobranowski, P.A.; Stintzi, A. Resistant starch, microbiome, and precision modulation. Gut microbes 2021, 13, 1926842. [Google Scholar] [CrossRef]
- Kalmokoff, M.; Zwicker, B.; O’Hara, M.; Matias, F.; Green, J.; Shastri, P.; Green-Johnson, J.; Brooks, S.P. Temporal change in the gut community of rats fed high amylose cornstarch is driven by endogenous urea rather than strictly on carbohydrate availability. J Appl Microbiol 2013, 114, 1516–1528. [Google Scholar] [CrossRef]
- Karaduta, O.; Glazko, G.; Dvanajscak, Z.; Arthur, J.; Mackintosh, S.; Orr, L.; Rahmatallah, Y.; Yeruva, L.; Tackett, A.; Zybailov, B. Resistant starch slows the progression of CKD in the 5/6 nephrectomy mouse model. Physiol Rep 2020, 8, e14610. [Google Scholar] [CrossRef]
- Meng, Y.; Bai, H.; Yu, Q.; Yan, J.; Zhao, L.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. High-Resistant Starch, Low-Protein Flour Intervention on Patients With Early Type 2 Diabetic Nephropathy: A Randomized Trial. J Ren Nutr 2019, 29, 386–393. [Google Scholar] [CrossRef]
- Laffin, M.R.; Tayebi Khosroshahi, H.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodialysis International 2019, 23, 343–347. [Google Scholar] [CrossRef]
- Machado, M.G.; Sencio, V.; Trottein, F. Short-Chain Fatty Acids as a Potential Treatment for Infections: a Closer Look at the Lungs. Infection and immunity 2021, 89, e0018821. [Google Scholar] [CrossRef]
- Lin, X.B.; Farhangfar, A.; Valcheva, R.; Sawyer, M.B.; Dieleman, L.; Schieber, A.; Ganzle, M.G.; Baracos, V. The role of intestinal microbiota in development of irinotecan toxicity and in toxicity reduction through dietary fibres in rats. PLoS One 2014, 9, e83644. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? Journal of Atherosclerosis and Thrombosis 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol 2019, 10, 277. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv Nutr 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol Dial Transplant 2019, 34, 783–794. [Google Scholar] [CrossRef]
- Gill, P.A.; Muir, J.G.; Gibson, P.R.; van Zelm, M.C. A randomized dietary intervention to increase colonic and peripheral blood SCFAs modulates the blood B- and T-cell compartments in healthy humans. The American Journal of Clinical Nutrition 2022, 116, 1354–1367. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J Autoimmun 2023, 141, 103038. [Google Scholar] [CrossRef]
- Zhang, X.; Ishikawa, D.; Ohkusa, T.; Fukuda, S.; Nagahara, A. Hot topics on fecal microbiota transplantation for the treatment of inflammatory bowel disease. Frontiers in medicine 2022, 9, 1068567. [Google Scholar] [CrossRef]
- Chi, M.; Ma, K.; Wang, J.; Ding, Z.; Li, Y.; Zhu, S.; Liang, X.; Zhang, Q.; Song, L.; Liu, C. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. Journal of immunology research 2021, 2021, 5516035. [Google Scholar] [CrossRef]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nature reviews. Gastroenterology & hepatology 2016, 13, 508–516. [Google Scholar] [CrossRef]
- Lauriero, G.; Abbad, L.; Vacca, M.; Celano, G.; Chemouny, J.M.; Calasso, M.; Berthelot, L.; Gesualdo, L.; De Angelis, M.; Monteiro, R.C. Fecal Microbiota Transplantation Modulates Renal Phenotype in the Humanized Mouse Model of IgA Nephropathy. Front Immunol 2021, 12, 694787. [Google Scholar] [CrossRef]
- Zhong, H.J.; Xie, X.; Chen, W.J.; Zhuang, Y.P.; Hu, X.; Cai, Y.L.; Zeng, H.L.; Xiao, C.; Li, Y.; Ding, Y.; et al. Washed microbiota transplantation improves renal function in patients with renal dysfunction: a retrospective cohort study. J Transl Med 2023, 21, 740. [Google Scholar] [CrossRef]
- Benech, N.; Koppe, L. Is there a place for faecal microbiota transplantation in chronic kidney disease? Nephrol Dial Transplant 2022, 37, 2303–2306. [Google Scholar] [CrossRef]
- Bian, J.; Liebert, A.; Bicknell, B.; Chen, X.-M.; Huang, C.; Pollock, C.A. Faecal Microbiota Transplantation and Chronic Kidney Disease. Nutrients 2022, 14, 2528. [Google Scholar] [CrossRef]
- Gargari, G.; Taverniti, V.; Balzaretti, S.; Ferrario, C.; Gardana, C.; Simonetti, P.; Guglielmetti, S. Consumption of a Bifidobacterium bifidum Strain for 4 Weeks Modulates Dominant Intestinal Bacterial Taxa and Fecal Butyrate in Healthy Adults. Appl Environ Microbiol 2016, 82, 5850–5859. [Google Scholar] [CrossRef]
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8. [Google Scholar] [CrossRef]
- Zara, M.; Guidetti, G.F.; Camera, M.; Canobbio, I.; Amadio, P.; Torti, M.; Tremoli, E.; Barbieri, S.S. Biology and Role of Extracellular Vesicles (EVs) in the Pathogenesis of Thrombosis. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Ailuno, G.; Baldassari, S.; Lai, F.; Florio, T.; Caviglioli, G. Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells 2020, 9. [Google Scholar] [CrossRef]
- Peng, Y.; Yin, S.; Wang, M. Extracellular vesicles of bacteria as potential targets for immune interventions. Hum Vaccin Immunother 2021, 17, 897–903. [Google Scholar] [CrossRef]
- Thongboonkerd, V. Roles for Exosome in Various Kidney Diseases and Disorders. Frontiers in pharmacology 2019, 10, 1655. [Google Scholar] [CrossRef]
- Lv, L.L.; Feng, Y.; Tang, T.T.; Liu, B.C. New insight into the role of extracellular vesicles in kidney disease. J Cell Mol Med 2019, 23, 731–739. [Google Scholar] [CrossRef]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One 2013, 8, e76520. [Google Scholar] [CrossRef]
- Choi, J.H.; Moon, C.M.; Shin, T.S.; Kim, E.K.; McDowell, A.; Jo, M.K.; Joo, Y.H.; Kim, S.E.; Jung, H.K.; Shim, K.N.; et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med 2020, 52, 423–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




