Submitted:
24 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
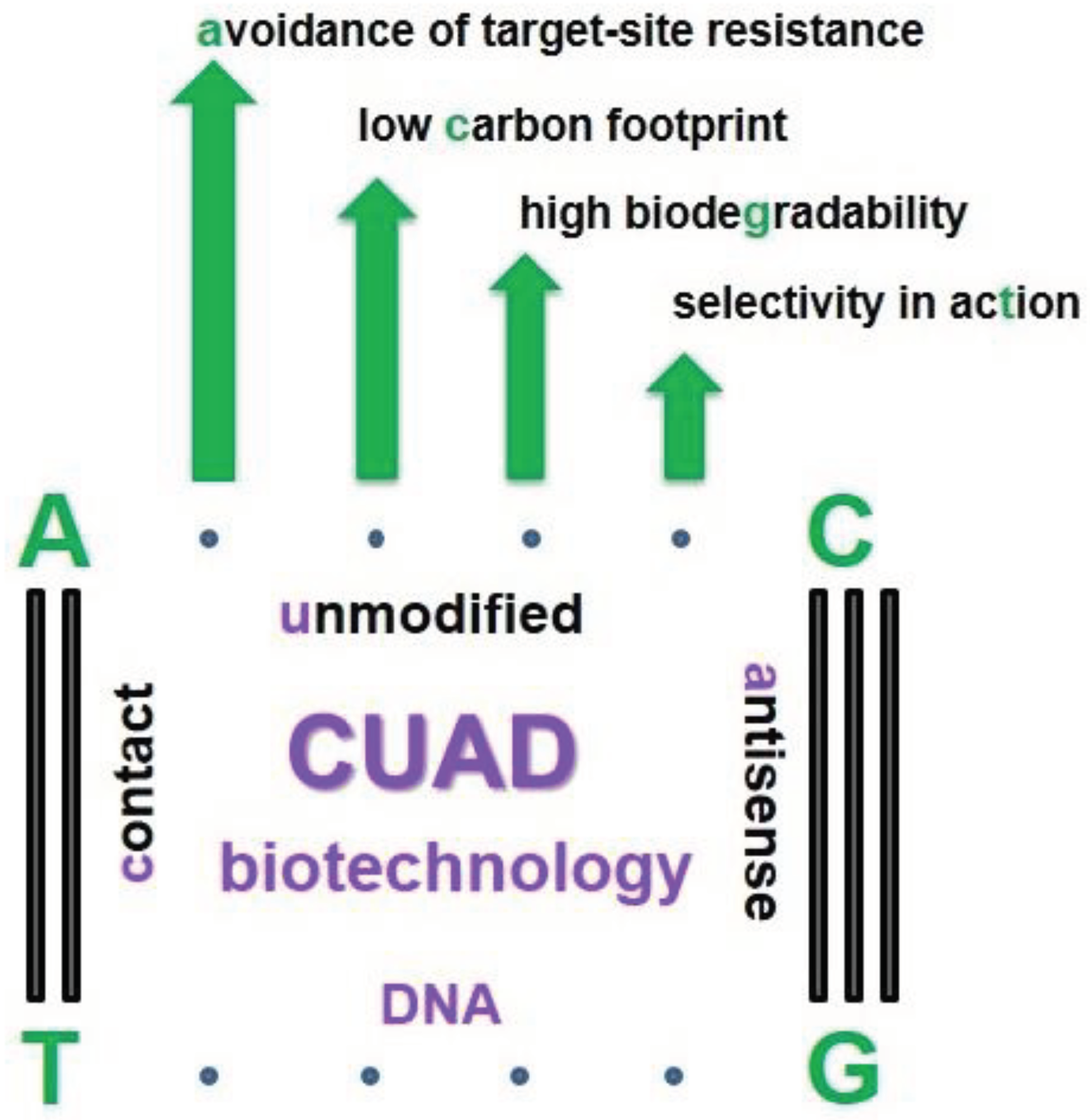
Introduction
Conclusion
Funding
Conflicts of Interest
References
- Aalaoui EI, Sbaghi M. 2023. Potential of parasitoids to control Diaspis echinocacti (Bouché) (Hemiptera: Diaspididae) on Opuntia spp. cactus pear. Egypt J. Biol. Pest. Control. 33:57. [CrossRef]
- Boukouvala MC, Kavallieratos NG, Skourti A et al. 2022. Lymantria dispar (L.) (Lepidoptera: Erebidae): Current Status of Biology, Ecology, and Management in Europe with Notes from North America. Insects. 13:854. [CrossRef]
- Ciampolini M, Lupi D, Süss L. 2002. Pseudococcus viburni (Signoret) (Hemiptera: Coccoidea) nocivo in frutticoltura nell’Italia centrale Boll. Zool. Agrar. Bachic. 34:97-108.
- Correa MCG, Germain J-F, Malausa T et al. 2012. Molecular and morphological characterization of mealybugs (Hemiptera: Pseudococcidae) from Chilean vineyards Bull. Entomol. Res. 102:524-530.
- Danzig EM, Pellizzari G. 1998. Diaspididae. In: Kozár, F. (Eds.), Catalogue of Palaearctic Coccoidea. Budapest, Hungary: Hungarian Academy of Sciences, 172-370 p.
- Dapoto GL, Olave A, Bondoni M et al. 2011. Obscure mealybug (Pseudococcus viburni) in pear trees in the Alto Valle of Rio Negro and Neuquen, Argentina Acta Hortic. 909:497-504.
- Donis-Keller, H. 1979. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 7(1):179-192. 10.1093/nar/7.1.179.
- El-Sayed SM, Ahmed N, Selim S et al. 2022. Acaricidal and antioxidant activities of anise oil (Pimpinella anisum) and the oil’s effect on protease and acetylcholinesterase in the two-spotted spider mite (Tetranychus urticae Koch). Agriculture. 12:224.
- Gal'chinsky N, Useinov R, Yatskova E et al. 2020. A breakthrough in the efficiency of contact DNA insecticides: rapid high mortality rates in the sap-sucking insects Dynaspidiotus britannicus Comstock and Unaspis euonymi Newstead. Journal of Plant Protection Research. 60(2):220-223. [CrossRef]
- Gal'chinsky NV, Yatskova EV, Novikov IA et al. 2023. Icerya purchasi Maskell (Hemiptera: Monophlebidae) Control Using Low Carbon Footprint Oligonucleotide Insecticides. Int J Mol Sci. 24(14):11650. [CrossRef]
- Gal'chinsky NV, Yatskova EV, Novikov IA et al. 2024. Mixed insect pest populations of Diaspididae species under control of oligonucleotide insecticides: 3′-end nucleotide matters. Pesticide Biochemistry and Physiology. 105838. [CrossRef]
- García Morales M, Denno B, Miller DR et al. 2017. ScaleNet: a literature-based model of scale insect biology and systematics. www.scalenet.info (accessed 19 Jul 2017).
- García Morales M, Denno BD, Miller DR et al. 2016. ScaleNet: A literature-based model of scale insect biology and systematics. Database J. Biol. Database Curation. bav118. [CrossRef]
- Hoose A, Vellacott R, Storch M et al. 2023. DNA synthesis technologies to close the gene writing gap. Nat Rev Chem. 7:144–161. [CrossRef]
- Kapranas A, Morse JG, Pacheco P et al. 2007. Survey of brown soft scale Coccus hesperidum L. parasitoids in southern California citrus. Biol. Control. 42:288–299.
- Kaydan M, Ülgentürk S, Özdemir I et al. 2014. Coccoidea (Hemiptera) species in Bartın and Kastamonu Provinces. Bulletin of Plant Protection. 54(1):11–44.
- Kidd NAC. 1988. The Large Pine Aphid on Scots Pine in Britain. In: Berryman, A.A. (Eds), Dynamics of Forest Insect Populations. Population Ecology. Springer, Boston, MA. [CrossRef]
- Kollar J, Bakay L, Pastor M. 2016. First record of cottony cushion scale Icerya purchasi (Hemiptera, Monophlebidae) in Slovakia. Plant. Prot. Sci. 52:217–219.
- Kumar H, Sharma M, Chandel A. 2022. DNA Insecticides: Future of Crop Protection. ICAR- Indian Agricultural Research Institute, New Delhi-110012. Article ID: 37444. www.agrifoodmagazine.co.in (accessed 28 Feb 2024).
- Kuzio J, Pearson MN, Harwoo SH et al. 1999. Sequence and analysis of the genome of a baculovirus pathogenic for Lymantria Dispar. Virology. 253:17–34.
- Liu Y, Shi J. 2020. Predicting the Potential Global Geographical Distribution of Two Icerya Species under Climate Change. Forests. 11:684.
- Manju M, Nirosha V, Tullika T et al. 2022. DNA insecticides: an emerging tool in pest management. 4(9). https://agriallis.com/issue/volume-4-issue-9-september-2022/ (accessed 28 Feb 2024).
- Martemyanov V, Bykov R, Demenkova M et al. 2019. Genetic evidence of broad spreading of Lymantria dispar in the West Siberian Plain. PLOS ONE. 14(8):e0220954. [CrossRef]
- Migeon A, Dorkeld F. 2022. Spider Mites Web: a comprehensive database for the Tetranychidae. Available from https://www1.montpellier.inrae.fr/CBGP/spmweb/index.php (accessed 5 Dec 2022).
- Miller D, Davidson J. 1990. Armored scale insects as pests. In: Rosen, D. (Eds.), Armored scale insects, their biology, natural enemies and control. In World Crop Pests, 299-306 p.
- Morales MG, Denno BD, Miller DR et al. 2016. ScaleNet: A literature-based model of scale insect biology and systematics. Database, 2016, article ID bav118. [CrossRef]
- Mrabti I, Haddad N, Moghaddam M et al. 2022. Current status of the cactus scale Diaspis echinocacti Bouché (Hemiptera: Diaspididae) on Opuntia ficus-indica and its first morphological and microscopic description in Morocco. EPPO Bulletin. 52:718-724. 10.1111/epp.12902.
- Novikov A, Yatskova E, Bilyk A et al. 2023. Efficient Control of the Obscure Mealybug Pseudococcus viburni with DNA Insecticides. In Vitro Cellular & Developmental Biology-Animal; Springer: New York, NY, USA. 59:92–108. [CrossRef]
- Novikov IA, Yatskova EV, Useinov RZ et al. 2022. Efficient Bay Sucker (Trioza Alacris) Control with DNA Insecticides. In Vitro Cellular & Developmental Biology-Animal; Springer: New York, NY, USA. 58:43. [CrossRef]
- Oberemok VV, Gal’chinsky NV, Useinov RZ et al. 2023. Four Most Pathogenic Superfamilies of Insect Pests of Suborder Sternorrhyncha: Invisible Superplunderers of Plant Vitality. Insects. 14:462. [CrossRef]
- Oberemok VV, Gal’chinsky NV. 2024. Oligonucleotide insecticides (contact unmodified antisense DNA biotechnology) and RNA biocontrols (double-stranded RNA technology): newly born fraternal twins in plant protection. bioRxiv 584797. [CrossRef]
- Oberemok VV, Laikova KV, Gal'chinsky NV et al. 2019a. DNA insecticide developed from the Lymantria dispar 5.8S ribosomal RNA gene provides a novel biotechnology for plant protection. Sci Rep. 9(1):6197. [CrossRef]
- Oberemok VV, Laikova KV, Useinov RZ et al. 2019b. Insecticidal activity of three 10–12 nucleotides long antisense sequences from 5.8S ribosomal RNA gene of gypsy moth Lymantria dispar L. against its larvae. Journal of Plant Protection Research. 59(4):561-564. [CrossRef]
- Oberemok VV, Laikova KV, Zaitsev A.S et al. 2017. Molecular Alliance of Lymantria dispar Multiple Nucleopolyhedrovirus and a Short Unmodified Antisense Oligonucleotide of Its Anti-Apoptotic IAP-3 Gene: A Novel Approach for Gypsy Moth Control. Int. J. Mol. Sci. 18:2446. [CrossRef]
- Oberemok VV, Laikova KV, Zaitsev AS et al. 2016. The RING for gypsy moth control: Topical application of fragment of its nuclear polyhedrosis virus anti-apoptosis gene as insecticide. Pest. Biochem. Physiol. 131:32–39.
- Oberemok VV, Useinov RZ, Skorokhod OA 2022. Oligonucleotide Insecticides for Green Agriculture: Regulatory Role of Contact DNA in Plant–Insect Interactions. Int. J. Mol. Sci. 23:15681. [CrossRef]
- Oberemok, VV. 2008. Method of Elimination of Phyllophagous Insects from Order Lepidoptera. Ukraine Patent UA # 36, 445.
- Pellizzari G, Germain J. 2010. Scales (Hemiptera, Superfamily Coccoidea). Chapter 9.3. BioRisk 4:475-510. [CrossRef]
- Plugatar YuV, Chichkanova ES, Yatskova EV et al. 2021. An innovative method of Diaspis echinocacti Bouche control using DNA insecticide on Opuntia ficus-indica (L.) Mill. in the Nikitsky Botanical Garden, Crimea. South of Russia: ecology, development. 16(2):119–128. [CrossRef]
- Puzanova YV, Novikov IA, Bilyk AI et al. 2023. Perfect Complementarity Mechanism for Aphid Control: Oligonucleotide Insecticide Macsan-11 Selectively Causes High Mortality Rate for Macrosiphoniella sanborni Gillette. Int. J. Mol. Sci. 24:11690. [CrossRef]
- Salisbury A, Malumphy C, Halstead AJ. 2013. Euonymus scale Unaspis euonymi (Hemiptera:Diaspididae); an introduced pest of spindle (Euonymus)in Britain. Brit. J. Entomol. Nat. Hist. 26:211‒218.
- TriLink BioTechnologies, 2023. Feasibility of Antisense Oligonucleotides as DNAInsecticides. https://www.trilinkbiotech.com/blog/feasibility-of-antisense-oligonucleotides-as-dna-insecticides/ (accessed 28 Feb 2024).
- Ülgentürk S, Şahin Ö, Ayhan B et al. 2012. Scale insects species of Taurus cedar in Turkey. Turkish Journal of Entomology. 36:113–121.
- Useinov RZ, Gal'chinsky N, Yatskova E et al. 2020. To bee or not to bee: creating DNA insecticides to replace non-selective organophosphate insecticides for use against the soft scale insect Ceroplastes japonicus Green. Journal of Plant Protection Research. 60(4):406-409. [CrossRef]
- Villanueva RT, Gauthier N, Ahmed ZM. 2021. First Record of Coccus hesperidum L. (Hemiptera: Coccidae) in Industrial Hemp in Kentucky. Fla. Entomol. 103:514–515.
- Zamecnik PC, Stephenson ML. 1978. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl Acad. Sci. 75:280–284.
- Zeity, M. 2018. First record of the bay sucker Trioza alacris Flor (Triozidae: Hemiptera) in Syria. EPPO Bulletin. 48(3):586–588. [CrossRef]
- Zhong J, Wang Y, Lu Y et al. 2022. Identification and Expression Analysis of Chemosensory Genes in the Antennal Transcriptome of Chrysanthemum Aphid Macrosiphoniella sanborni. Insects. 13:597. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




