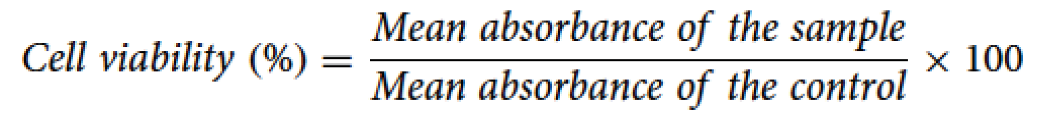1. Introduction
Cancer is one of the most challenging health problems of XXI century. Worldwide, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred in 2020 [
1]. The same study showed that lung cancer remained the leading cause of cancer death (18%), followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%) cancers.
New innovative solutions are being developed to address cancer disease. In addition to strategies designed by researchers, often a highly effective solution for solving health problems is to find the model where nature itself has already evolved such a solution. For the case of cancer, nature seems to have evolved anticancer mechanism in the growing deer antler. Antlers are bony cranial appendages that are cast each year and grow (in red deer) in about 4 months [
2,
3]. They constitute the only case of full regeneration in mammals, reaching weights of more than 13 kg and 116 cm in length [
4,
5]. This represents an astonishing growth rate in the tips (where they grow) of up to 2.75 cm/d in length [
6]. Such fast growth led to unique characteristics with potential medical applications [
7] being the anticancer mechanisms one of them. Wang et al. (2019) showed that antlers evolved a speed of growth faster than cancer based on high expression of proto-oncogenes [
8]. Indeed, the study found that gene expression profiles between antlers and that of osteosarcoma are more correlated (r = 0.67 to 0.78) than between antlers and normal growth in bone tissues (r = 0.33 to 0.47). As a result, the study postulated that deer evolved several tumour suppression genes and mechanisms to reduce the high risk of developing cancer.
This is most likely the reason why the extract of the growing antler (called deer velvet antler, henceforth indicated as DVA) shows anticancer properties both in an array of human and mouse tumours. Fan et al. (1998) published the first evidence of the anti-tumour activity of DVA in mice inoculated with sarcoma 180 cells [
9]. They observed that DVA treated mice intraperitoneally significantly prolonged the life of the cancer-bearing mice from 15 to 20 days. These results were confirmed later in the same animal model with DVA extracted from the tip (henceforth DVA-TIP) [
10]. Furthermore, DVA showed proliferation reductions in cell cultures of human prostate cancer similar to those of chemotherapy [
11] whereas in xenograft mice, DVA achieved a 65% tumour volume reduction, again similar to that of cisplatin chemotherapy [
12]. In malignant glioblastoma (GBM), the most prevalent and aggressive malignant brain tumour, DVA showed a reduced proliferation (37.5%) and colony-formation capacity (84%), inhibited migration (39%), induced changes in cell cycle progression and promoted apoptosis, whereas it did not affect non-cancerous human (NCH) cell lines [
13]. Other studies have shown that DVA or its proteins exert anticancer effects in human breast cancer (BRCA) [
14,
15] and mouse tumours such as colorectal cancer (CRC) [
16] or sarcoma [
17]. Since the growing antlers have a variety of tissues (among others, skin, cartilage, bone, blood vessels, and nerves), Landete-Castillejos et al. (2022) suggested that deer antlers may show a general anticancer activity, particularly in the tips, the growing section [
18]. Furthermore, because these mechanisms are acting in a live mammal, this explains why it has no negative secondary effects in other tissues and cells reproducing according to healthy physiological processes, and no such effect is expected in future studies. This makes a very promising line of research.
How could such anticancer effects be achieved? The limited research so far shows direct effects on tumour cells, and others derived from DVA potentiating the immune system, as shown in the paper by Cao et al. [
17]. In this study DVA was given as preventive anticancer treatment to mice for 15 days before injecting murine sarcoma 180 (S180) cells. The weight of the sarcoma was smaller the higher the velvet antler protein (VA-pro) dose (one of the components of DVA): ranging from 25% to 55% tumour weight reduction from lowest to highest dose of VA-pro. The reduction in tumour weight seems to be explained, among other mechanisms, by the results in the study showing that tumour cell proliferation was arrested in phase S. The apoptosis test (Annexin V-FITC/PI) showed that tumour apoptotic cells increased from 6% (control) to 76% (highest DVA dose), whereas normal sarcoma cells went down from 91% (control) to 19%. All this would explain the direct anticancer effects of DVA shown in vitro in other studies (although in vivo, apoptosis can be induced by cytotoxic T cells, see below).
However, this study and others showed also immunomodulatory properties [
19] and indirect anticancer effects mediated by the mouse immune system [
20]. To understand the role of immune system in cancer, it is important to discern between effects aiming at killing the tumour cells, and the opposite effects induced by the tumour to create a immunosuppressive microenvironment in the tumour to avoid precisely immune attacks on cancer cells [
21,
22]. The tumour is infiltrated by cytotoxic CD8+ T cells to kill tumour cells, a response that is often mediated by CD4+ T cells, which are trained for the immune response in the thymus [
23,
24]. Both Li et al. [
15] and Cao et al. [
17] showed that DVA could increase in mice the number of CD4+ and CD8+ T lymphocytes, in addition to IL-2 and other interleukins involved in the immune response against cancer. Equally, the size of thymus increased with increasing dose of DVA, likely paralleling the rise in lymphocytes involved in anticancer response [
17]. To successfully develop, tumours must escape immune surveillance by developing an immunosuppressive microenvironment that induces immune tolerance [
20]. This tumour microenvironment contains various immunosuppressive cells, including tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and tumour-associated neutrophils (TANs), which contribute to immune tolerance and tumour progression [
24,
25,
26].
From these, TAMs account for most of the proportion, particularly M2 type which is dominant in most solid tumours [
27,
28]. In fact, M2 protects the tumour cells from chemotherapy reducing apoptosis, whereas another one, M1, is pro-inflammatory and cytotoxic, increasing the apoptosis normally produced by chemotherapy alone [
29]. Surprisingly, these types can be polarized from one type to another [
22,
28], so that one way of fighting cancer is to re-polarize M2 into M1 [
30].
Another immune organ involved in fighting cancer, the spleen, was also influenced in mice with tumours treated with DVA [
17]. Previous studies have demonstrated that the spleen is an important site of extramedullary haematopoiesis and, in tumour-bearing mice, the spleen generates immunosuppressive myeloid cells [
31,
32]. These cells also induce the tumour immune tolerance mentioned above [
21,
33]. These myeloid cells promote tumour progression by regulating the anti-tumour immune activity of T lymphocytes, natural killer T (NKT) cells, natural killer (NK) cells, dendritic cells (DCs) and various other cell types [
34]. The study by Cao et al. [
17] showed that the greater the dose of DVA, the smaller the spleen and the closer its weight to that of the healthy mice (whereas the greatest spleen was that of control mice with tumour). Thus, spleen size may be related to the production of the immunosuppressive myeloid cells, thus promoting tumour growth, but also monocytes that infiltrate the tumour, differentiate into macrophages (called M0), and then polarized into M2 or M1 [
27].
Thus, the aims of this study were to assess direct anticancer effects of DVA in several tumour cell lines as compared with healthy cells and examine in detail the modulation of these effects plus its potential mechanisms in the immune system in GBM xenograft mice. In addition, we aimed to assess whether DVA can modulate the M1/M2 effect on GBM (as an example for other cancer cells). Thus the specific aims were: 1) to assess the anticancer effects of DVA in vitro in cell cultures of GBM (cell lines U87 and U251), CRC (lines DLD1, HT29, SW480, and SW620) and BRCA (lines PA00, SKBR3 and MCF7) and, additionally, if these effects are also exerted in tumours such as THP-1 leukaemia, which affect the immune cells; 2) to assess the validity of these direct effects in vivo as assessed in the weight of the human GBM xenograft tumour in mice and its histopathology; 3) to assess the potential mechanisms of these effects modulated by the immune system via assessment of the weight of the spleen, cytokines, and, in particular, 4) whether DVA can affect the immune microenvironment of the tumour by assessing the viability of GBM in vitro with supernatant of M1 and M2 with or without DVA.
4. Discussion
Our results show that the extract of DVA has a direct anticancer effect that is general in all cell lines tested: two GBM, four CRC and three BRCA, as well as the leukaemia ones, showing in all of them a remarkable reduction in viability and a reduction in mobility in the subsample of lines tested (GBM, CRC and BRCA are shown on
Figure 2). The most impacting results (i.e., the most important for future steps towards applicability), is that both the modulation of the direct anticancer effects in live mice models of GBM, as well as indirect effects potentially exerted via immune system, showed a two-thirds reduction of the weight of the tumour, as well as the histological evidence of damage in the remaining part. Furthermore, the gene expression showed that this effect was achieved by DVA modulating the expression profile affecting genes mainly involved in cytokine activity and inflammatory/immune response. Thus, our results, together with the few published evidence both in cell cultures and in vivo models of other types of tumours, confirms the hypothesis of Landete-Castillejos et al. (2022) and Wang and Landete-Castillejos (2023) who have suggested that DVA may have a general anticancer effect, whereas evidence found through different assays in the experiment in mice shows how such effect is achieved [
7,
18]. We will discuss direct effects assessed in vitro and the experiment on glioblastoma in vivo separately.
One of the strongest pieces of evidence supporting a general anticancer effect of DVA is the direct effects shown on reduction of viability and mobility in cell cultures of several types of tumours. Obviously, the evidence is widest here because to assess direct effects on cell cultures is easier than assessing direct and indirect effects on the most complex animal models (which, on the other hand, are closer to a medical application). Our study showed further evidence on the reduction on viability of glioblastoma cancer cells on both lines (high proliferative U87, and slower one U251) in a dose-dependent way. This is similar to results by Chonco et al. [
13] in GBM in which DVA produced a reduction of 37.5% in viability at the highest dose, whereas it did not produce such effect in non-tumour cells (HaCaT), in contrast to the damage produced by chemotherapy (temozolomide) [
13]. Our results show a 36.6% (U87) and 31% (U251) reduction in viability precisely at the same dose of 1 mg/mL tested in Chonco et al.’s [
13] paper. Our results show a similar reduction in viability at this dose when the DVA extract came from the middle sections of the antler (35.6% and 37.8%, respectively for U87 and U251). We observed previously a similar effect in A172 cell line, a human GBM cell line sensitive to TMZ that expresses low levels of MGMT and MPG enzymes (data not shown in Chonco et al.). Due to the close proximity of the TIP-MID sections (2.5 cm), the anti-tumour activity in another TMZ-sensitive GBM cell lines such as U87 and U251 is an added value to our findings.
Our results provide the widest evidence published so far in a single article for the hypothesis that DVA has a general anticancer activity. DVA reduced the viability of tumour lines by 21% at 0.5 mg/mL, and 25-29% at 1 mg/mL (reaching up to 49% in some cases) tested in colorectal and breast cancer lines DLD1, SW620, HT29, SW480, MCF7, SKBR3 and PA00. The DLD-1 cell line is particularly susceptible to the effect of the DVA compound, and this sensitivity is attributed to various molecular characteristics that influence its cellular behaviour. Among these characteristics, microsatellite instability stands out, a condition that compromises the efficiency of DNA repair. This vulnerability to the accumulation of genetic damage may enhance the response of DLD-1 to DVA treatment, as cells become less able to correct lesions in their genetic material. Furthermore, the mutation in the APC gene present also plays a crucial role in its response to DVA. The mutation in APC results in decreased regulation of key processes, such as cell division and migration. This lack of control may make DLD-1 cells more prone to DVA action, as normal cellular regulatory mechanisms are compromised. It is important to note that the presence of KRAS wild type may also contribute to its susceptibility to DVA. While activating mutations in KRAS often confer resistance to certain treatments, the wild-type version of this gene could be associated with increased receptivity to DVA [
37].
The SW480 cell line shares molecular similarities with the DLD-1 cell line, including the presence of a mutation in the APC gene, microsatellite instability, and KRAS wild-type status. These characteristics are fundamental in the context of colorectal cancer, influencing the regulation of cell proliferation and genomic stability. Unlike the DLD-1 cell line, both SW480 and its metastatic derivative, SW620, exhibit notable disparities in the expression of the CD26 enzyme. This enzyme, with a significantly reduced presence in SW480 and SW620 cells, plays a crucial role in modulating substrates relevant to DVA treatment. The decrease in CD26 could influence the bioavailability of effects with anticancer action, highlighting the complexity of cellular responses to specific treatments [
38].
The decreased sensitivity observed in the HT-29 cell line could be attributed in part to the presence of a mutation in the p53 gene [
39]. This mutation plays a crucial role in modulating the apoptotic response to DNA damage, which could confer resistance to the induction of apoptosis and limit the efficacy of treatments that depend on this pathway. Despite sharing the feature of microsatellite instability with DLD-1 and other colorectal cell lines, instability in HT-29 is not manifested as pronounced. This variation in the degree of microsatellite instability between cell lines suggests that, although they share similarities in DNA repair capacity, the magnitude of this phenomenon may be a determining factor in the response to treatment.
In the context of breast cancer tumour lines, it is crucial to consider the distinctive molecular characteristics of MCF7, PA00, and SKBR3. MCF7, classified as luminal A, stands out for its expression of hormone receptors, which makes it especially receptive to treatments that take advantage of these pathways, such as DVA, which benefits from its complex with growth hormones [
40]. On the other hand, the PA00 line, classified as luminal B, presents an intriguing paradox. Despite the presence of hormone receptors, it exhibits greater proliferation and aggressiveness compared to MCF7. This phenomenon suggests a complexity in the regulation of intracellular signalling pathways, influencing their response to therapies such as DVA [
41]. On the other hand, SKBR3, is characterized by the overexpression of HER2. Its absence of hormone receptors may confer resistance to DVA compared to luminal lines. However, overexpression of HER2 gives it an exceptional ability to invade tissues [
42]. More interestingly, it was effective in both primary tumours and secondary ones, in those sensitive to chemotherapy and those multi-resistant to it. In contrast, and in line with evidence of our previous study in GBM [
13], DVA had no significant general reduction in viability in non-cancerous lines 293T and HaCaT, except in one case for each line, only from one of the two sections at the highest dose. Considering this has not been found either in non-tumour cells in the study of Chonco et al. [
13] or Yang et al. [
11] it is reasonable to conclude that DVA does not reduce viability in healthy cells. In addition to the evidence previously published in GBM lines T98 and A172, the study by Yang et al. [
11] showed that DVA (here tested as sika deer growing antler protein) is as effective as chemotherapy in cell cultures of prostate cancer. This is very interesting, as the growing antler has tissues such as skin, cartilage, bone, blood vessels and nerves growing, but not like those in a prostate.
DVA also reduced the mobility of both U87 and U251 GBM cell lines. This effect is cumulative (i.e., stronger at 48 than at 24 h) so that cells did not compensate after an initial slow down (it does not disappear on the second day tested). Not only that, the effect was greater at 48 h (greater difference with control) for DVA-TIP. In the study of Chonco et al. [
13] who also examined DVA-TIP at 1 mg/mL in a scratch test, T98 GBM lines reduced their mobility by 39% at 6 h, whereas it had no effect on healthy cells (HaCaT). As with viability, the results found here in other types of tumours (colorectal and breast cancer lines), DVA also showed a reduced mobility that was clearer in some lines over others, but the most general effect was the tip reduction in mobility at 72 h.
Considering that, as shown by Cao et al. [
17] and Li et al. [
15] in other types of tumours, the effects of DVA or its derived proteins exert part of their effects via immune system cells and organs (such as spleen and thymus) [
15,
17], we tested whether DVA could reduce the viability of tumour cells precisely in those originated from immune cells: THP-1 monocytes and differentiated macrophages (M0). Our prediction was confirmed by results with similar 40% reduction in viability in both cases (a figure very similar to that of Chonco et al., [
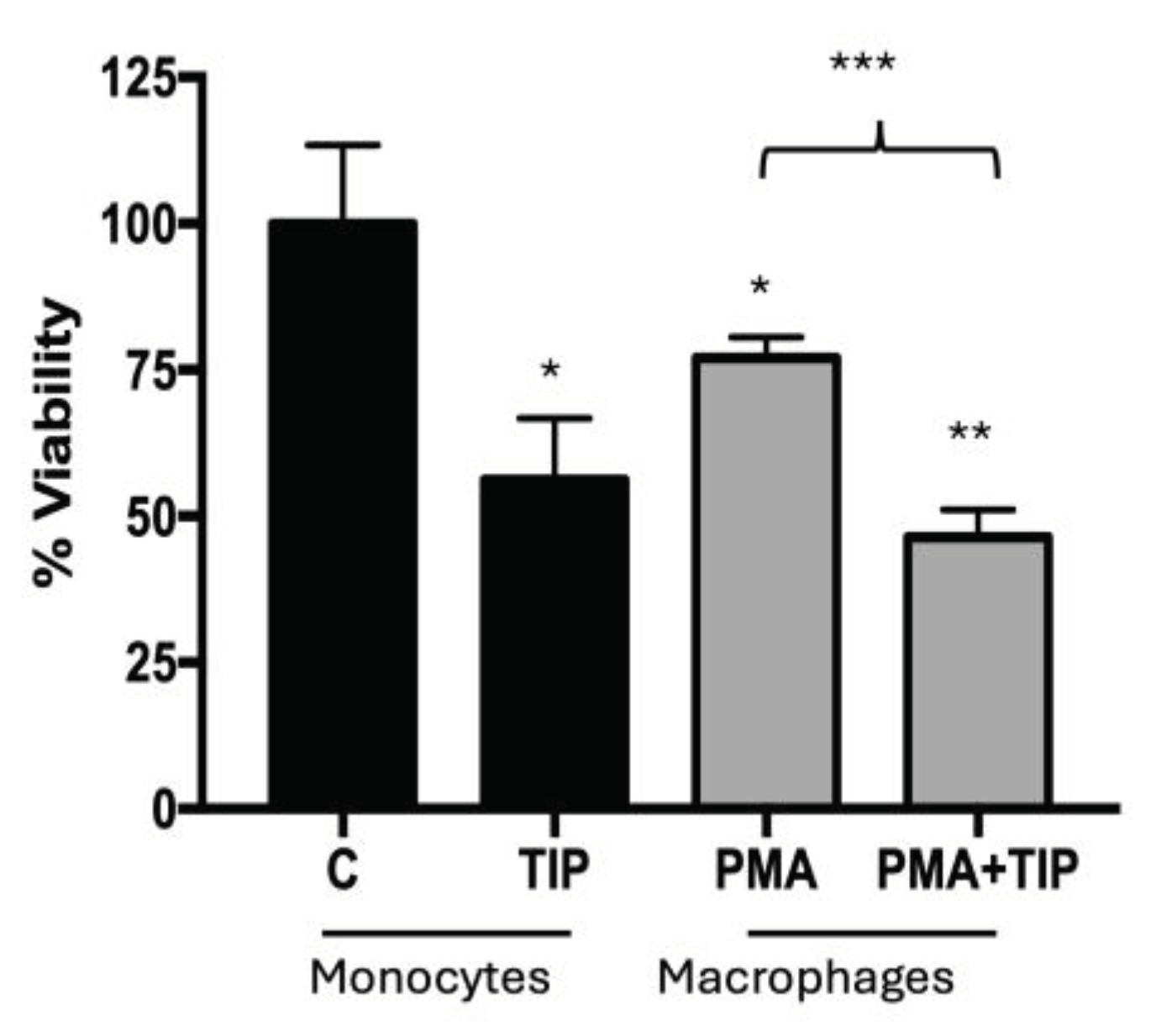
13], in GBM). This result has also potential implications to explain the effect found in tumours in mice: as indicated in the introduction, M0 can be polarized into TAMs, of which M2 is responsible for a great part of the immune system tolerance to the tumour. DVA may reduce the M0 that have infiltrated into the tumour, killing a proportion of them, and potentially reducing the M2 available to protect the tumour from M1 and cytotoxic T cells.
The assays on the M1/M2 environment showed a reduction in GBM viability with both types of macrophages. Actually, the reduction in viability was greater than if DVA was added, although DVA also had, as in previous tests, an effect. The surprising fact is that M2 induces a tolerant effect towards the tumour. Genin et al. (2015) found that co-cultures of THP-1-M2 macrophages and HepG2 or A549 cancer cells, M2 reduced the level of chemotherapy induced apoptosis in these cancer cell lines, whereas THP-1-M1 macrophages increased the cell death above the chemotherapy alone [
29]. In our case, DVA showed an unexpected protective effect in co-cultures with M2 with respect to the effect of M2 alone (despite the fact that DVA alone kills tumour cells), whereas for M1 there is an apparent synergistic effect of DVA and M1 to reduce further the viability, but at the small sample size used this was not significant. However, in the complex environment of the tumour, with cytotoxic T cells infiltrating the tumour to kill it and a whole array of cytokines and immune cells interacting, the size of the effect and synergies of DVA may be different (as the results in vivo further below suggest).
All the above-mentioned evidence on GBM cell lines adds to published studies on other direct effects produced by DVA: thus, Chonco et al. [
13] found an 84% reduction in colony formation capacity, much greater than the 40% achieved with temozolomide at the lower dose 20 μg/mL and somewhat similar to the 95% achieved at the high dose of 200 μg/mL. This paper suggests that DVA could promote apoptosis in GBM, as they found a non-significant trend of 3-fold increase in the number of apoptotic cells. The effect was confirmed in other types of tumours as Cao et al. [
17] found, in mice with sarcoma, that apoptotic tumour cells increased from 6% (untreated mice with sarcoma) to 76% (at the highest DVA dose), whereas the number of normal sarcoma cells decreased from 91% (untreated) to 19% (highest DVA dose) [
17]. Obviously, in a living mouse the apoptosis may be produced by several mechanisms (including the apoptosis induced by cytotoxic T cells), whereas in a cell plate the DVA effect can only be direct but results in both studies suggest that DVA induce apoptosis in the tumours tested. Why would DVA induce high levels of apoptosis? In a very recent paper, Li et al. [
15] proposed that the possible strategy to prevent tumour growth in antlers is the highly efficient cell apoptosis mechanism, and that this would be the reason why inner (IR) layer of the reserve mesenchyme in the antler tip (the layer proliferating fastest in the antler) shows a 64% level of apoptosis which is the highest in the antler and higher than in any other adult tissue [
20]. Thus, our hypothesis here is that at least one of the direct anticancer effects of DVA on tumour cells is the use of the highly effective signalling of apoptosis (evolved for the development of the antler to induce apoptosis specifically in the tumour cells). This points to a very interesting line of research to fight cancer, as the induced cell death is specifically aimed at cancer cells and not healthy proliferating cells.
One of the most interesting results were those produced in the experiment with the xenograft model. Previous research had shown similar effects of a dose of 200 and 400 mg/kg live mice, inhibiting around 65% the growth in weight or volume of the human prostate cancer xenograft [
12]. That is why we selected this dose in our experiment. We tried two administrations expecting that the oral one would show either very reduced or no effects of DVA on GBM tumour. The results show a reduction in tumour weight surprisingly similar to that in the study of Tang et al. [
12]: 66% reduction, despite using another cervid species (sika deer,
Cervus nippon vs.
Cervus elaphus here). To ensure comparability, we prepared the samples freeze-dried and milled in a similar way to Tang et al. [
12]. Contrary to our expectations, the oral administration produced an effect rather similar to the intraperitoneal one (only a slight reduction of 4.9 percent units less than intraperitoneal administration), despite DVA was digested in the former. Interestingly, the study by Cao et al. [
17], which used only one protein from the remains of producing a velvet antler alcoholic drink (called antler wine in China), the VA-pro (acronym for velvet antler protein) showed a reduction of mice sarcoma not very far from the data shown above: 55% reduction at the 100 mg/kg dose, and this despite the treatment was given for a shorter time (16 vs. 28 days in our study), and more importantly, as a preventive treatment before the sarcoma was introduced [
17]. A similar 50% reduction was produced by DVA in Li et al. [
15] in mice growing another type of human tumour: triple-negative breast cancer. This occurred despite using a commercial powder of DVA, which likely included the whole antler (as top sections reach a much higher price in the market [
43]), therefore it should be less effective. Thus, DVA seems to have a wide and strong anticancer effect in live mice too, whether they have their full immune system in work (as in Cao et al. [
17]) or, as in our case, Tang et al. [
12] and Li et al. [
15], partly compromised in order to grow a human tumour.
Despite using the nude mice to be able to grow a human tumour, the results show evidence of DVA effects in the immune system to the partial extent that using this strain of mice can show. Nude mice do not have thymus and therefore, our experiment cannot show the effects of DVA on this organ as it showed in the study by Cao et al. [
17]. However, they do have a spleen, and the results showed a 44% and 30% reduction in spleen size when DVA was administered IP or orally, respectively. As indicated in the introduction, spleen weight varies with tumour progression and may be a predictor of tumour recurrence. In tumour-bearing mice (which is our case), the spleen generates immuno-suppressive myeloid cells [
31,
32] that are also involved in inducing immune tolerance towards the tumour [
21,
33]. Spleen is also involved in the proliferation of monocytes that infiltrate the tumour [
27], and then differentiate into macrophages (called M0), that may polarize into M1 or M2. Either if spleen grows to produce more cells that attack the tumour, or it is induced by the tumour to produce more cells to protect it, the result is that, as we found in our study, the greatest variability explained by spleen size is tumour size. Thus, the reduction in tumour produced by DVA results in smaller spleen size. Cao et al. [
17] also showed that the greater the dose of DVA, the smaller the spleen and the closer its weight to that of the healthy mice (whereas the greatest spleen was that of control mice with tumour).
The effect of DVA on the tumour was further clarified by the histochemistry and the assays on differential gene expression profiles. The microscopy exam showed evidence of direct effects of DVA inducing necrosis directly on tumour cells as well as in the supportive tissue such as in blood vessels. Part of such direct effect may be achieved by the activity of immune cells. Although, due to the lack of a thymus, nude mice cannot generate mature T lymphocytes and therefore are unable to carry out many types of adaptive immune responses, it must be considered that most of the nude mouse strains used are slightly immunosuppressed and have some T cells, especially as they age. The histopathological results of the study by Li et al. [
15] examining the effect of DVA on human triple negative breast cancer treated with chemotherapy (neoadjuvant or NAC) also showed, as in our study, signs of cell death at tissue level [
15]. This DVA effect appears to have been achieved by promoting the immune system because CD4
+ and CD8
+ T cells when DVA was added to NAC treatment in the study by Li et al. were higher than numbers in mice with tumours or those treated with NAC without DVA. Unfortunately, we did not measure numbers of CD4
+ or CD8
+ T cells in our study to confirm an increase in numbers when treated with DVA.
In any case, we believe that part of the anti-tumour effect of DVA could have been exerted through changes in the tissue microenvironment in which immune cells can localize. We know that increasing numbers of tumour-infiltrating TAMs is correlated to poor survival among recurrent GBM patients [
44]. Thus, DVA would reduce the tumour as a mixture of different actions: killing the cancer cells, reducing the tumour-associated macrophages M2 (or also repolarizing them into M1 killers), reducing myeloid-derived suppressor cells (MDSCs) and tumour-associated neutrophils (TANs) that grow to create this permissive immune environment for the tumour [
24,
25,
26], and increasing the numbers of T killer cells found by Cao et al. [
17] and Li et al. [
15]. Certainly, the reduction of viability of both monocytes and related macrophages from tumour cell lines produced by DVA in this study may explain the effects on the GBM xenograft mouse model found.
Also, we analysed the concentration of cytokines from the serum of the mice to understand how DVA may influence the inflammatory state of the tumour. The results showed a down-regulation of some factors after the treatment with DVA extract, like FasL, that is associated with the increase of tumour progression [
45]; id est, DVA produces the opposite effect). Other chemokine found down-regulated, and which, therefore, DVA reduces their functions are: CX3CL1 (Fractalkine), which with its axis stimulates cancer cell migration [
46]; GM-CSF, that when it is very high it can exhaust immune cells and promote cancer growth [
47]; some ILs, such as IL1-b, which is able to induce angiogenesis and cancer cell proliferation [
48]. We also found that DVA produced upregulation of some chemokines which constitute promising targets for cancer immunotherapy. One of them, for example, was CD30L, that is a molecule which regulated the glioma microenvironment so that when it is deficient (i.e., the opposite effect produced by DVA), leads to a pro-tumorigenic phenotype [
49]. Another interesting result is that DVA enhanced the expression of CD40, which seems to produce anti-tumour effects in several tumour models [
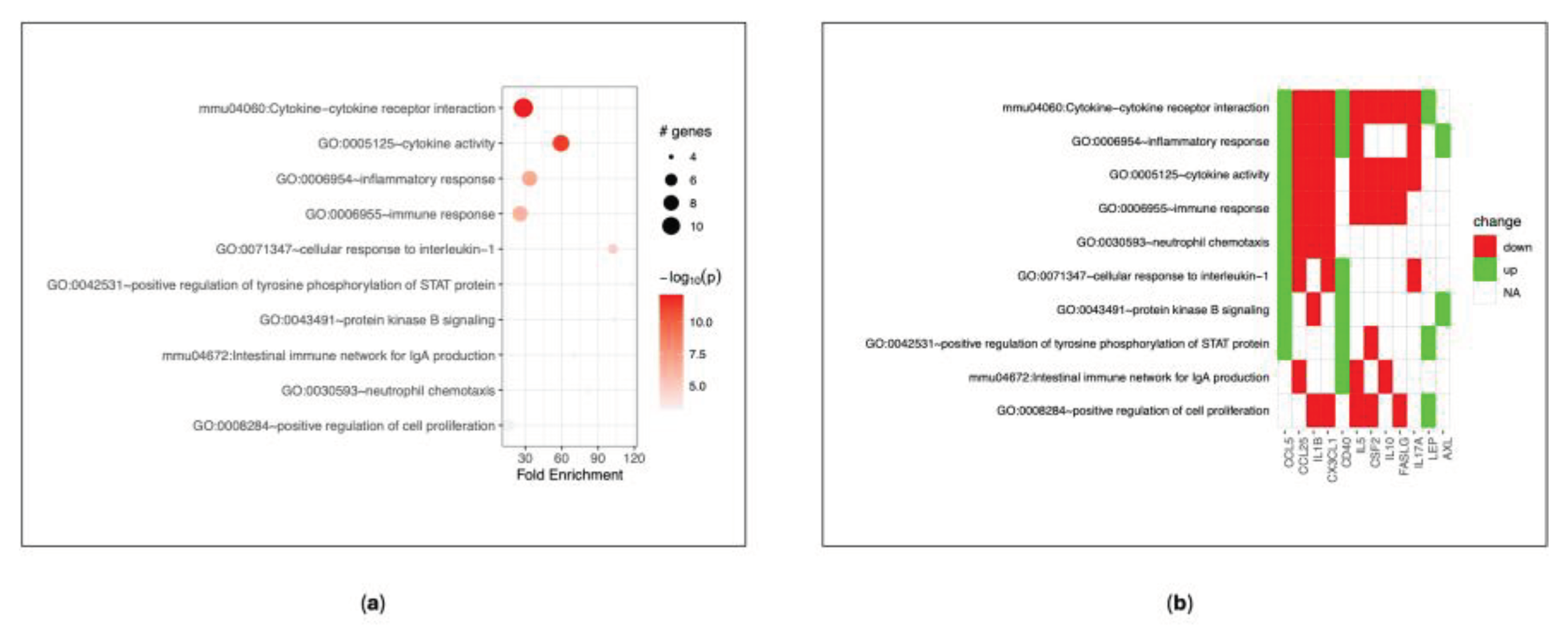
50]. Altogether, these findings show that DVA induce a different expression profile in GBM mouse models producing an interference with cytokine-cytokine receptor interaction and activity of what would be the normal progression of GBM tumour had the mice not been treated with DVA. As a consequence, this causes a different immune response in treated mice that modulates the inflammatory response regulating the tumoral micro-environment in a way that leads to anti-proliferative tumour conditions.
Figure 1.
Viability assay for glioblastoma (GBM), colorectal cancer (CRC), breast cancer (BRCA) and non-cancerous human (NCH) cell lines. (a) U87 (GBM) cell line has a neuronal-like phenotype with a high proliferative capacity, while (b) U251 (GBM) cell line has a mesenchymal-like phenotype with a lower proliferative activity. From left to right DVA doses after 72 h increase from 0.1, 0.5 to 1 mg/mL, whereas in the rest it shows only 0.5 mg/mL and 1 mg/mL (to strengthen validity of results in CRC lines, whilst saving DVA extract, tests at 0.5 mg/mL were repeated in 3 experiments). (c) SW480 (CRC) cell line is sensitive to chemotherapy and derive from primary tumour. Cell lines (d) SW620 (CRC derived from metastasis), (e) DLD1 (CRC) and (f) HT29 (CRC) are lines of fast-growing and relapsing tumours, in addition to being multi-resistant to chemotherapy. In contrast, (g) SKBR3 (BRCA) and (i) PA00 (BRCA) are a fast growing, chemotherapy sensitive lines, whereas (h) MCF7 (BRCA) grows more slowly and is chemotherapy resistant. NCH cell lines (j) 293T and (k) HaCaT are, respectively, human embryonic kidney and human keratinocyte cells. Bars show DVA cytotoxicity on cancer or control cell lines whereas asterisks show significant differences with the control (black). Error lines show SD. The probability indicated by *, **, and *** corresponds to a t-test at levels p < 0.05 , p < 0.01 and p < 0.001 against control (black).
Figure 1.
Viability assay for glioblastoma (GBM), colorectal cancer (CRC), breast cancer (BRCA) and non-cancerous human (NCH) cell lines. (a) U87 (GBM) cell line has a neuronal-like phenotype with a high proliferative capacity, while (b) U251 (GBM) cell line has a mesenchymal-like phenotype with a lower proliferative activity. From left to right DVA doses after 72 h increase from 0.1, 0.5 to 1 mg/mL, whereas in the rest it shows only 0.5 mg/mL and 1 mg/mL (to strengthen validity of results in CRC lines, whilst saving DVA extract, tests at 0.5 mg/mL were repeated in 3 experiments). (c) SW480 (CRC) cell line is sensitive to chemotherapy and derive from primary tumour. Cell lines (d) SW620 (CRC derived from metastasis), (e) DLD1 (CRC) and (f) HT29 (CRC) are lines of fast-growing and relapsing tumours, in addition to being multi-resistant to chemotherapy. In contrast, (g) SKBR3 (BRCA) and (i) PA00 (BRCA) are a fast growing, chemotherapy sensitive lines, whereas (h) MCF7 (BRCA) grows more slowly and is chemotherapy resistant. NCH cell lines (j) 293T and (k) HaCaT are, respectively, human embryonic kidney and human keratinocyte cells. Bars show DVA cytotoxicity on cancer or control cell lines whereas asterisks show significant differences with the control (black). Error lines show SD. The probability indicated by *, **, and *** corresponds to a t-test at levels p < 0.05 , p < 0.01 and p < 0.001 against control (black).
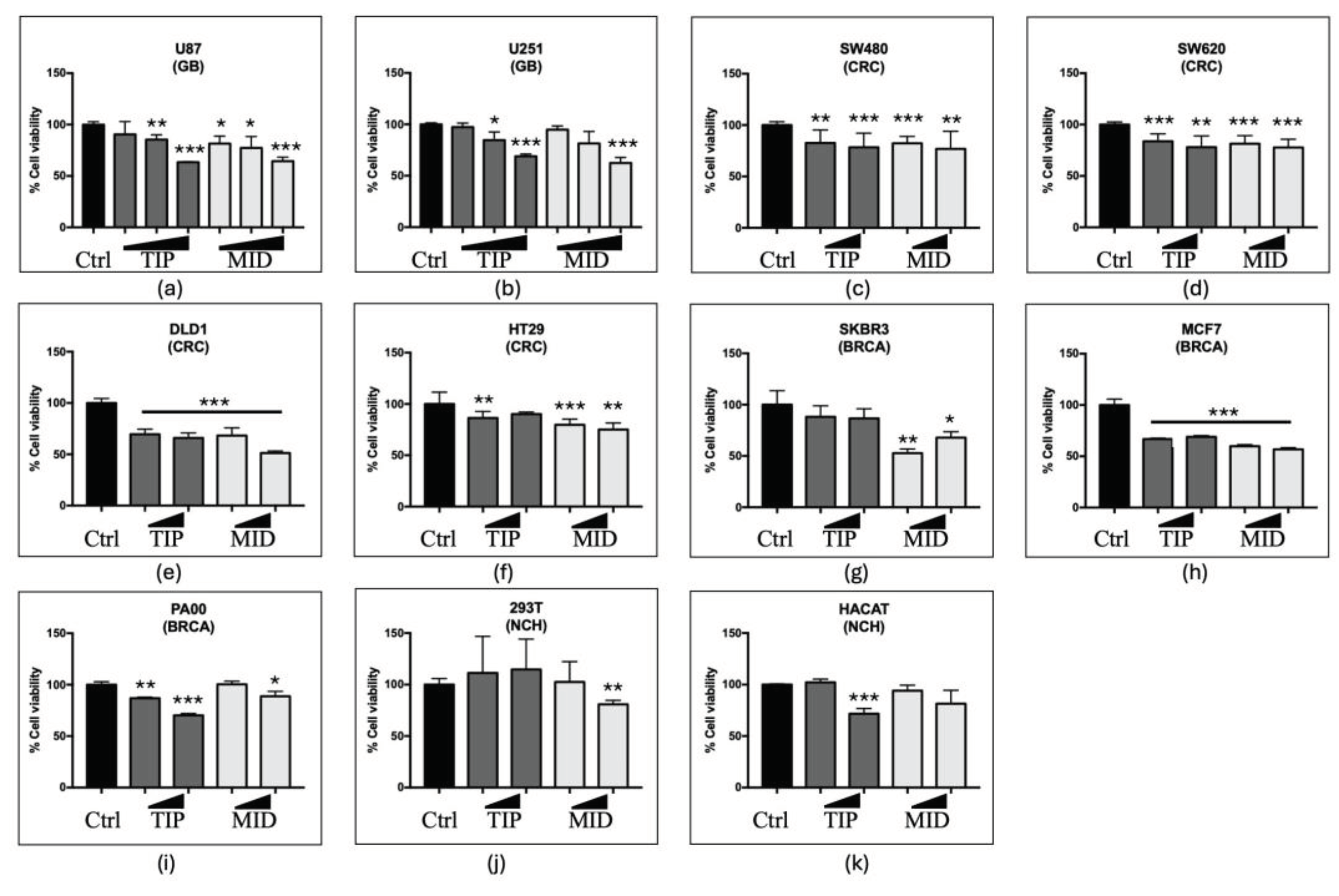
Figure 2.
Scratch test showing how DVA affects cell mobility. Bars show how the percentage of surface void of GBM cell lines (a) U87 and (b) U251 changes from 100% (void) to 0% (fully colonized) after a central line of a plate is scratched to remove all the cells and treated with DVA at 1 mg/mL. The U87 cell line has a neuronal-like phenotype with a high proliferative capacity, while the U251 cell line has a mesenchymal-like phenotype with a lower proliferative activity. Lower graphs show the same test at 1 mg/mL in the colorectal lines tested in the previous assay: lines (f) SW620 (derived from metastasis), (c) DLD1 and (d) HT29 are lines of fast growth and relapsing tumours, in addition to being multi-resistant to chemotherapy. For breast cancer, the bottom line shows the results for line (h) PA00 and (g) SKBR3, both fast growing, chemotherapy sensitive lines. Time 0h shows the 100% surface removed from cells. The rest of the bars shows how the void space is reduced by colonization of after subjected them either to no treatment (c) or DVA-TIP or DVA-MID one at 24, 48 or 72 h. The advance of cell proliferation was measured on the images using NIH ImageJ software. Higher bars at any given time show slower mobility from unscratched areas (estimate of lower metastasis ability). In general, DVA-TIP reduced the mobility of tumour cells as compared to control, although in lines (e) SW480 and SW620 the effect is only found at 72 h.
Figure 2.
Scratch test showing how DVA affects cell mobility. Bars show how the percentage of surface void of GBM cell lines (a) U87 and (b) U251 changes from 100% (void) to 0% (fully colonized) after a central line of a plate is scratched to remove all the cells and treated with DVA at 1 mg/mL. The U87 cell line has a neuronal-like phenotype with a high proliferative capacity, while the U251 cell line has a mesenchymal-like phenotype with a lower proliferative activity. Lower graphs show the same test at 1 mg/mL in the colorectal lines tested in the previous assay: lines (f) SW620 (derived from metastasis), (c) DLD1 and (d) HT29 are lines of fast growth and relapsing tumours, in addition to being multi-resistant to chemotherapy. For breast cancer, the bottom line shows the results for line (h) PA00 and (g) SKBR3, both fast growing, chemotherapy sensitive lines. Time 0h shows the 100% surface removed from cells. The rest of the bars shows how the void space is reduced by colonization of after subjected them either to no treatment (c) or DVA-TIP or DVA-MID one at 24, 48 or 72 h. The advance of cell proliferation was measured on the images using NIH ImageJ software. Higher bars at any given time show slower mobility from unscratched areas (estimate of lower metastasis ability). In general, DVA-TIP reduced the mobility of tumour cells as compared to control, although in lines (e) SW480 and SW620 the effect is only found at 72 h.
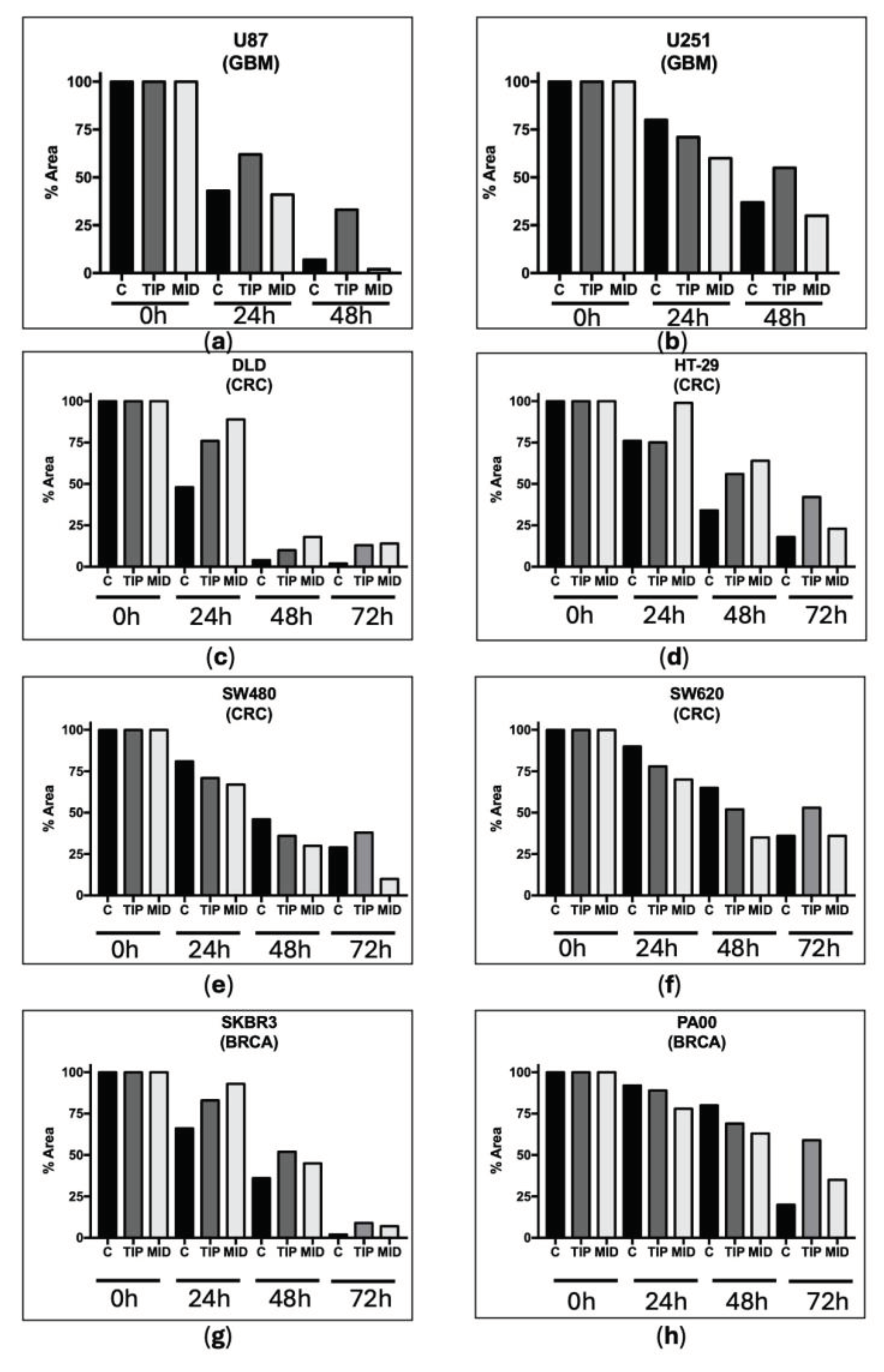
Figure 3.
Monocyte/Macrophage viability assay. Viability reduction of leukemia cell cultures (THP-1) after treatment with DVA-TIP at 24 h. The two bars at the left show the control (C) viability of monocytes (label at the base), and the percentage of viability reduced by applying DVA-TIP at a concentration of 1 mg/mL. The two bars at the right show, respectively, the percentage of leukemia monocytes differentiated and activated into macrophages after administration of PMA (M0, differentiated-THP-1 macrophages), and the percentage of the latter surviving after the treatment with DVA-TIP. Error lines show SD. The probability indicated by *, **, and *** corresponds to a t-test at levels p < 0.05 , p < 0.01 and p < 0.001. Asterisks on top of bars show differences with control monocytes (C), whereas those above brackets indicate the significance of differences between the bars at both ends of the bracket.
Figure 3.
Monocyte/Macrophage viability assay. Viability reduction of leukemia cell cultures (THP-1) after treatment with DVA-TIP at 24 h. The two bars at the left show the control (C) viability of monocytes (label at the base), and the percentage of viability reduced by applying DVA-TIP at a concentration of 1 mg/mL. The two bars at the right show, respectively, the percentage of leukemia monocytes differentiated and activated into macrophages after administration of PMA (M0, differentiated-THP-1 macrophages), and the percentage of the latter surviving after the treatment with DVA-TIP. Error lines show SD. The probability indicated by *, **, and *** corresponds to a t-test at levels p < 0.05 , p < 0.01 and p < 0.001. Asterisks on top of bars show differences with control monocytes (C), whereas those above brackets indicate the significance of differences between the bars at both ends of the bracket.
Figure 4.
Modulation of macrophages M1 (pro-inflammatory, anticancer, top) and M2 (tolerant and protective of tumour, bottom) effect by DVA in assays with glioblastoma. The bars show the viability of GBM line U87 alone (control), treated with DVA-TIP (1 mg/mL), treated with supernatant of macrophage M1 or M2, and the macrophage supernatant with DVA. (a, b) M1 and (c, d) M2 are differentiated from THP-1 after treatment with PMA towards M0, then polarized towards M1 (LPS) or M2 (IL4 and IL13). The graphic on the left shows the effects at 24 h, whereas the one on the right is at 48 h. Data are shown as mean value ± SD. The brackets show the probability indicated of a t-test comparing U87 (left end of bracket) with treatment at the other end of the bracket (p < 0.001 in all cases). Despite the further reduction in viability of adding DVA-TIP to M1 supernatant at 48 h (compared to M1 supernatant alone: bar next to the left), the test between them did not achieve significance. Error lines show SD. The probability indicated by *, **, and *** corresponds to a t-test at levels p < 0.05 , p < 0.01 and p < 0.001. Asterisks on top of bars show differences with control (C), whereas those above brackets indicate the significance of differences between the bars at both ends of the bracket.
Figure 4.
Modulation of macrophages M1 (pro-inflammatory, anticancer, top) and M2 (tolerant and protective of tumour, bottom) effect by DVA in assays with glioblastoma. The bars show the viability of GBM line U87 alone (control), treated with DVA-TIP (1 mg/mL), treated with supernatant of macrophage M1 or M2, and the macrophage supernatant with DVA. (a, b) M1 and (c, d) M2 are differentiated from THP-1 after treatment with PMA towards M0, then polarized towards M1 (LPS) or M2 (IL4 and IL13). The graphic on the left shows the effects at 24 h, whereas the one on the right is at 48 h. Data are shown as mean value ± SD. The brackets show the probability indicated of a t-test comparing U87 (left end of bracket) with treatment at the other end of the bracket (p < 0.001 in all cases). Despite the further reduction in viability of adding DVA-TIP to M1 supernatant at 48 h (compared to M1 supernatant alone: bar next to the left), the test between them did not achieve significance. Error lines show SD. The probability indicated by *, **, and *** corresponds to a t-test at levels p < 0.05 , p < 0.01 and p < 0.001. Asterisks on top of bars show differences with control (C), whereas those above brackets indicate the significance of differences between the bars at both ends of the bracket.
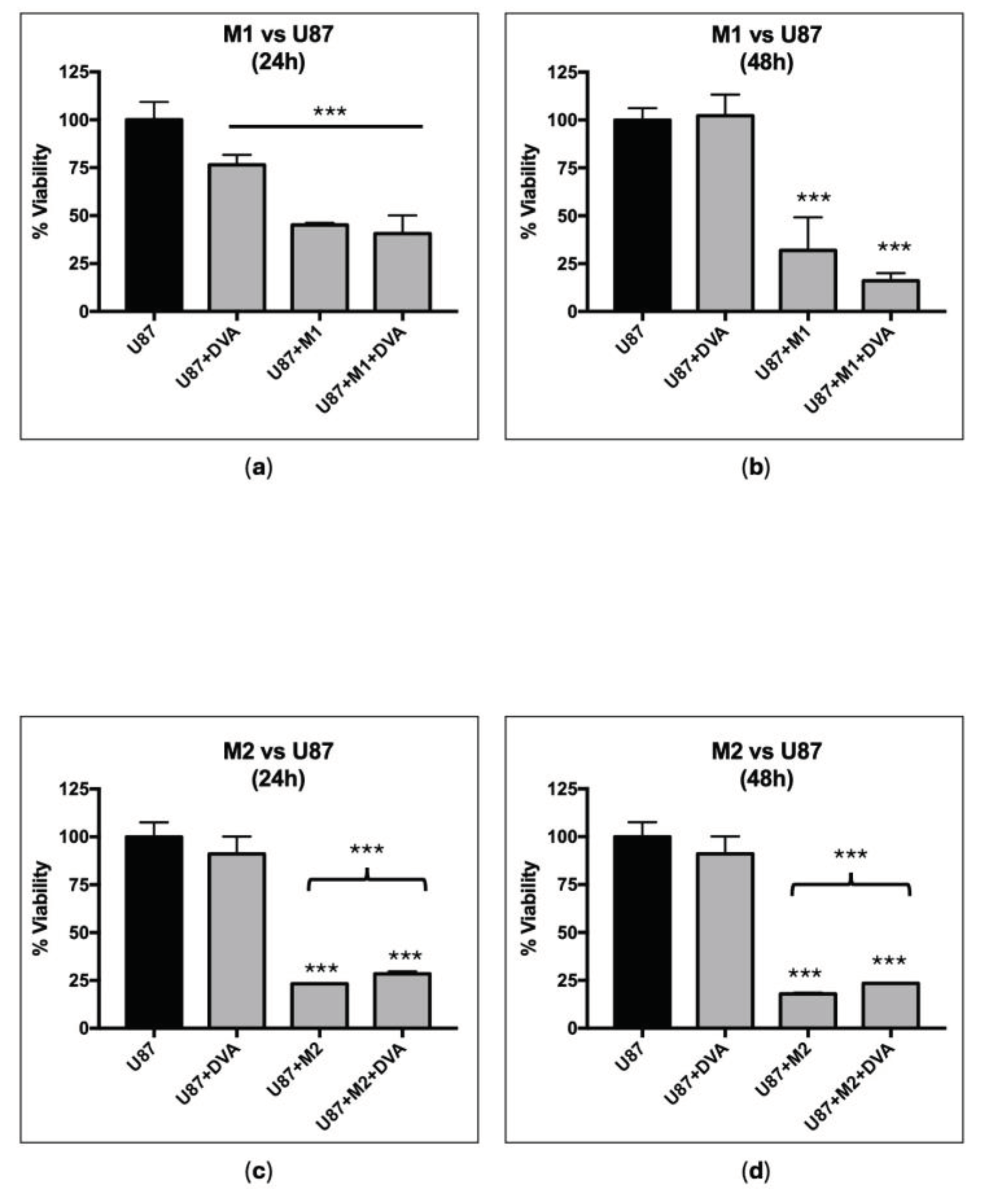
Figure 5.
Xenograft human glioblastoma growing in CD1-nu/nu mice treated with DVA-TIP. When the U87MG tumour reached 80 mm3 in volume, mice were randomly assigned 3 treatments: Control as non-treated mice (C); Oral administration of 200 mg DVA/kg live weight and d-1 for 28 consecutive days (Oral); intraperitoneal injections of the same amount for the same period (IP). Vertical axis shows weight in grams. GBM (black colour) is the tumour weight for each treatment (control, oral or IP), whereas Spleen is the weight of this organ for each treatment. A GLM for tumour weight was: GBM tumour weight = 0.706 ± 0.069*** -0.430 ± 0.098*** (Oral) -0.464 ± 0.098*** (IP); R2 = 60.6%. Intercept corresponds to the weight of control tumours, and each treatment reduced it as shown by the coefficients oral or IP (asterisks show the significance level of the coefficient). For Spleen weight, the GLM obtained was: Spleen weight = 0.251 ± 0.024*** -0.076 ± 0.024* (Oral) -0.110 ± 0.024** (IP); R2 = 38.3%. Intercept corresponds to the weight of spleen in control mice, and coefficients are interpreted as for tumour weight.
Figure 5.
Xenograft human glioblastoma growing in CD1-nu/nu mice treated with DVA-TIP. When the U87MG tumour reached 80 mm3 in volume, mice were randomly assigned 3 treatments: Control as non-treated mice (C); Oral administration of 200 mg DVA/kg live weight and d-1 for 28 consecutive days (Oral); intraperitoneal injections of the same amount for the same period (IP). Vertical axis shows weight in grams. GBM (black colour) is the tumour weight for each treatment (control, oral or IP), whereas Spleen is the weight of this organ for each treatment. A GLM for tumour weight was: GBM tumour weight = 0.706 ± 0.069*** -0.430 ± 0.098*** (Oral) -0.464 ± 0.098*** (IP); R2 = 60.6%. Intercept corresponds to the weight of control tumours, and each treatment reduced it as shown by the coefficients oral or IP (asterisks show the significance level of the coefficient). For Spleen weight, the GLM obtained was: Spleen weight = 0.251 ± 0.024*** -0.076 ± 0.024* (Oral) -0.110 ± 0.024** (IP); R2 = 38.3%. Intercept corresponds to the weight of spleen in control mice, and coefficients are interpreted as for tumour weight.
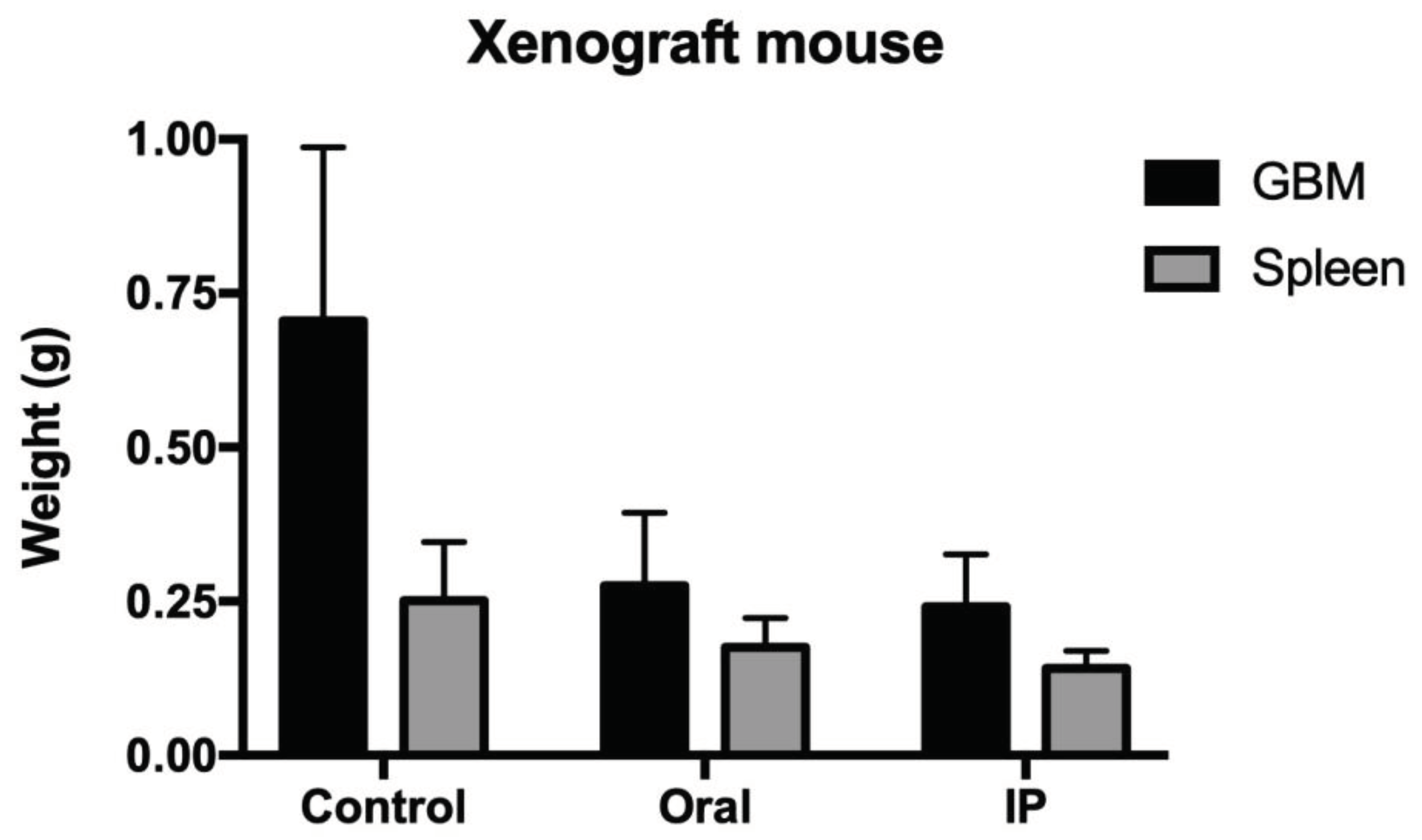
Figure 6.
Histochemistry micrographs of glioblastoma treated with DVA intraperitoneally (DVA (IP)) or untreated (CTRL). (a) Shows a haematoxylin and eosin stain as a basic stain, whereas (b) shows a trichrome stain to see the reaction of the tumour parenchyma (cancer cells) and the stroma (supportive tissue of the tumour) to the treatment. (a) Shows that tumours collected from untreated control mice consist of compact cell clusters with little fibrous stroma, and the presence of inflammatory cells being mainly monocytes and neutrophilic granulocytes. In contrast, the tumours collected from mice treated with DVA (c), which are much smaller in size, have a different consistency with harder fibrotic portions that are coloured blue/light blue with trichrome staining and hyaline portions that are very soft to the touch with evidence of necrosis, especially of the colliquative/liquefactive necrosis type. This is likely evidence of the direct effects of DVA producing cell death on GBM tumour. (d) In treated tumours, necrosis is also abundant with red blood cells due to a weak perivascular matrix, fragile vessels, and frequent ruptures indicating an additional effect of DVA on tumour angiogenesis. As the tumour reduction was similar, tumours of oral treatments were not examined.
Figure 6.
Histochemistry micrographs of glioblastoma treated with DVA intraperitoneally (DVA (IP)) or untreated (CTRL). (a) Shows a haematoxylin and eosin stain as a basic stain, whereas (b) shows a trichrome stain to see the reaction of the tumour parenchyma (cancer cells) and the stroma (supportive tissue of the tumour) to the treatment. (a) Shows that tumours collected from untreated control mice consist of compact cell clusters with little fibrous stroma, and the presence of inflammatory cells being mainly monocytes and neutrophilic granulocytes. In contrast, the tumours collected from mice treated with DVA (c), which are much smaller in size, have a different consistency with harder fibrotic portions that are coloured blue/light blue with trichrome staining and hyaline portions that are very soft to the touch with evidence of necrosis, especially of the colliquative/liquefactive necrosis type. This is likely evidence of the direct effects of DVA producing cell death on GBM tumour. (d) In treated tumours, necrosis is also abundant with red blood cells due to a weak perivascular matrix, fragile vessels, and frequent ruptures indicating an additional effect of DVA on tumour angiogenesis. As the tumour reduction was similar, tumours of oral treatments were not examined.
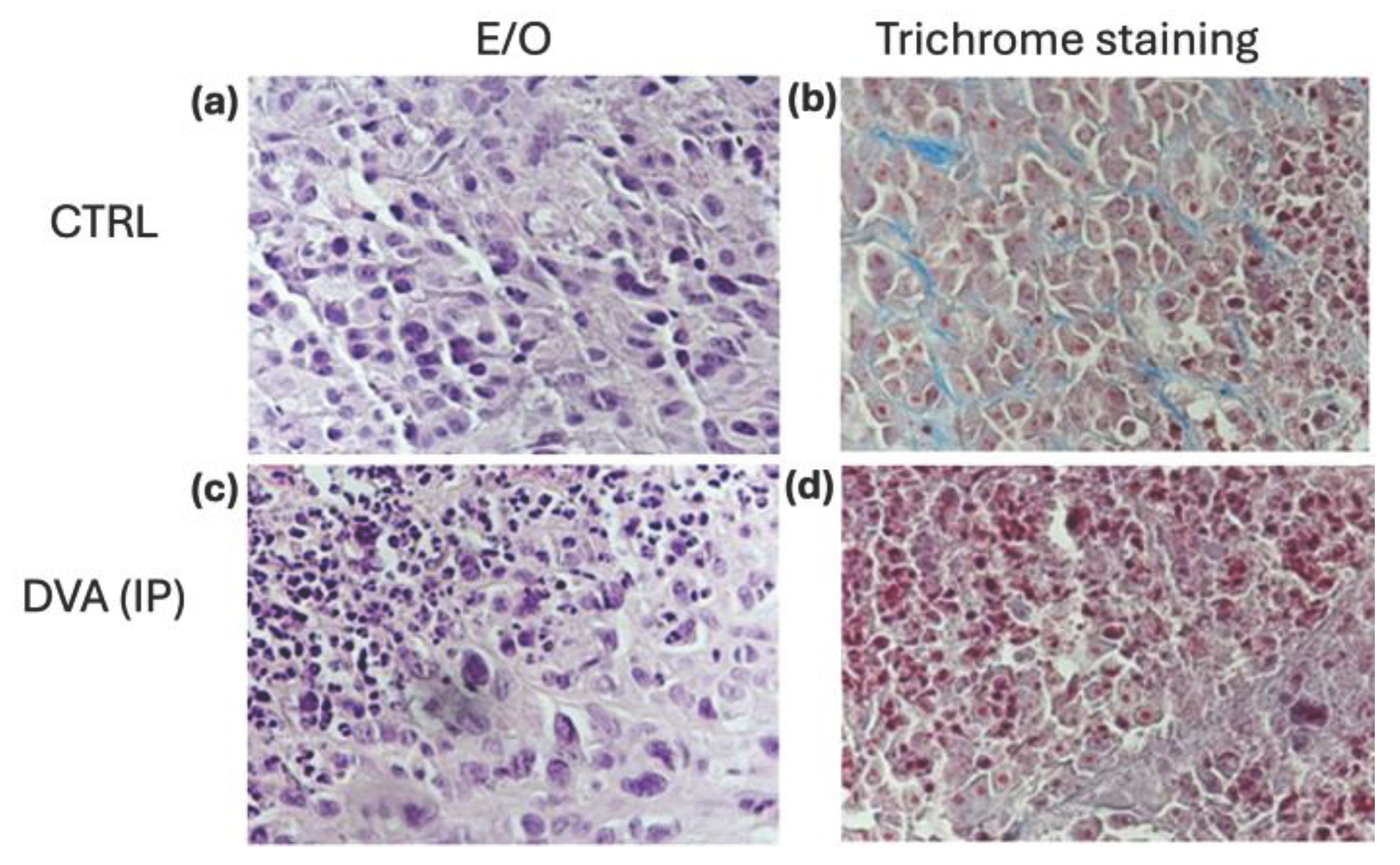
Figure 7.
Functional enrichment analysis. Bubble chart (a) and heatmap (b) showing the top ten significant Gene Ontology terms/Pathways derived from the analysis of the down-regulated (N=9) and up-regulated (N=5) genes in the serum samples of the two GBM groups of DVA treated mice (oral and intraperitoneal) as compared to that of untreated controls.
Figure 7.
Functional enrichment analysis. Bubble chart (a) and heatmap (b) showing the top ten significant Gene Ontology terms/Pathways derived from the analysis of the down-regulated (N=9) and up-regulated (N=5) genes in the serum samples of the two GBM groups of DVA treated mice (oral and intraperitoneal) as compared to that of untreated controls.