1. Introduction
1.1. Biorefinery Concept
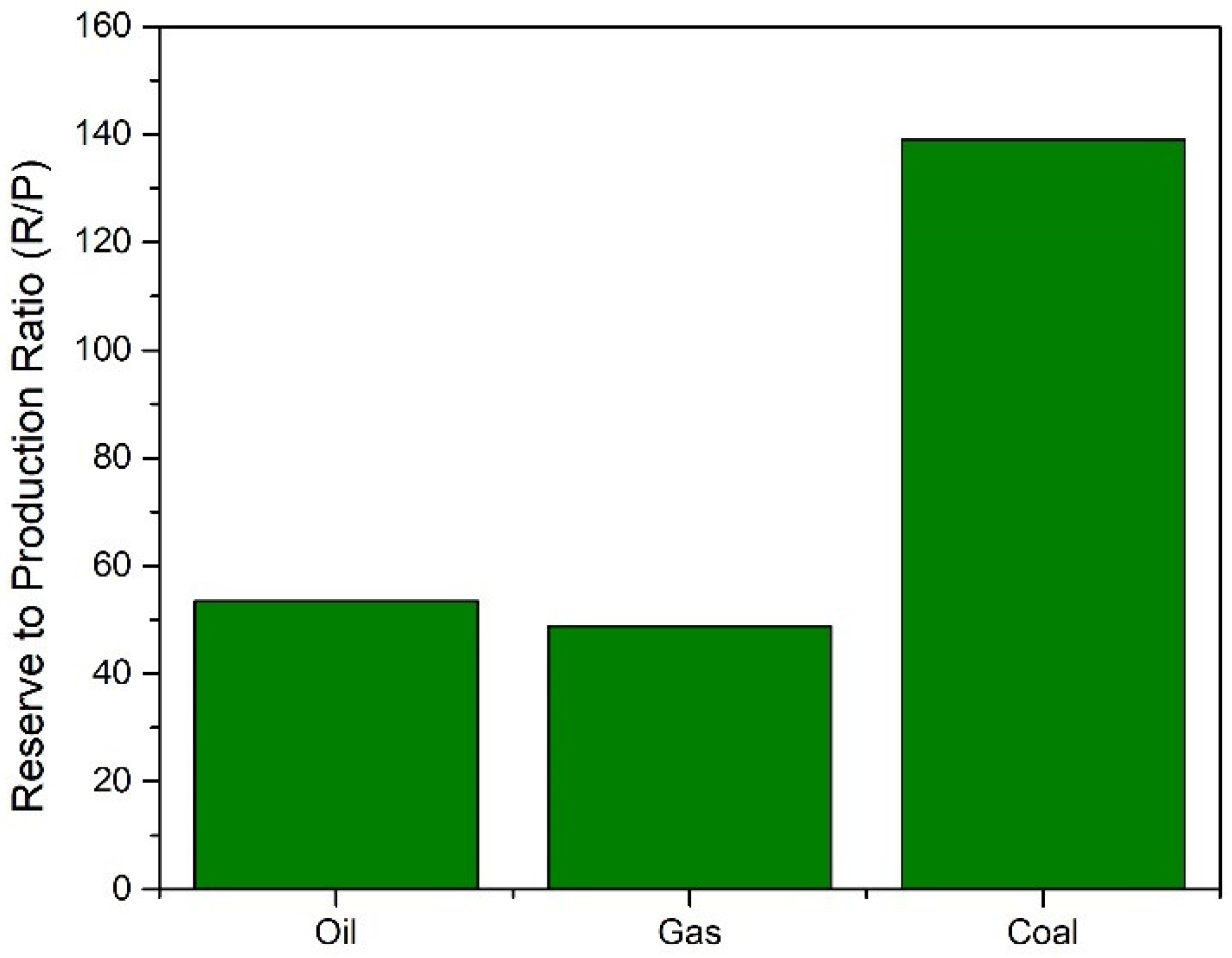
Historically the world has been reliant on conventionally used fossil fuels (oil, coal, and gas) for its supply of fuel, energy, and chemicals. This reliance however is not sustainable for several reasons. Firstly, fossil fuels are a finite resource and therefore will eventually run out. The reserve to production ratio of these fuels is shown in
Figure 1. These values show how long reserves would last if consumption continued at the same rate. Global reserves of crude oil were estimated to be at 1732.4 billion barrels, meaning it would take 53.5 years till exhaustion of supplies assuming current consumption rates [
1]. Whilst new reserves of oil are continuing to be explored, the reliance on fossil fuels must be limited due to its adverse effects on the environment. Emissions of greenhouse gases (GHG) such as carbon dioxide (CO
2), methane (CH
4) and nitrous oxide (N
2O) have been linked with negatively altering the earth’s climate. It has been well documented that the use of fossil fuels has resulted in global warming, with average surface temperatures reaching 1 °C above pre-industrial levels in 2017. This rise in global temperature can be correlated with the increased dependence on fossil fuels post industrial revolution. From 1750 to 2011 cumulative anthropogenic CO
2 emissions totalled 2040 ± 310 GtCO
2. Of the total increase in the greenhouse gas emissions from the combustion of fossil fuels and industrial processes, 78 % was directly from CO
2 emissions. In 2018, carbon emissions grew by 2 %, the highest rate for 7 years with natural gas fueling energy growth [
2]. With reserves of conventionally used fossil fuels depleting, increasing energy demand and improved awareness toward global warming and climate change, significant importance has been placed on finding sustainable, environmentally friendly, and economically viable alternative sources of fuels and chemicals.
In recent years, wind and solar have emerged as viable sources of electrical energy. In 2018 power generation by renewable energy increased by 16%, with wind contributing 142 TWh and solar 131 TWh [
2]. Overall wind accounts for 50 % of renewable generation, in comparison with the 24% provided by solar. Whilst wind and solar can address the need for electrical energy demand, alternative renewable sources are required for transportation and heating fuels, and sources of chemicals. Currently over half of a barrel of crude oil is refined into transportation fuels. One barrel gives 10.04 gallons of diesel (22 %) and 19.36 gallons of petrol (43 %). In terms of chemical production 4 % of oil produced worldwide is used for chemical and plastic production [
3]. Currently, the transport sector relies on petroleum, accounting for 96% of the transport energy. The use of biofuels could allow a reduction of annual GHG emissions by ~52 million metric tons (MT) by 2030 (19% reduction), and by ~194 million MT by 2050 (47% reduction). The EU directive stipulated that, by 2020, 10% transport energy must be derived from biofuels, however, according to the department of transport statistics, by 2023, the UK met only 3% of the renewable biofuels target.
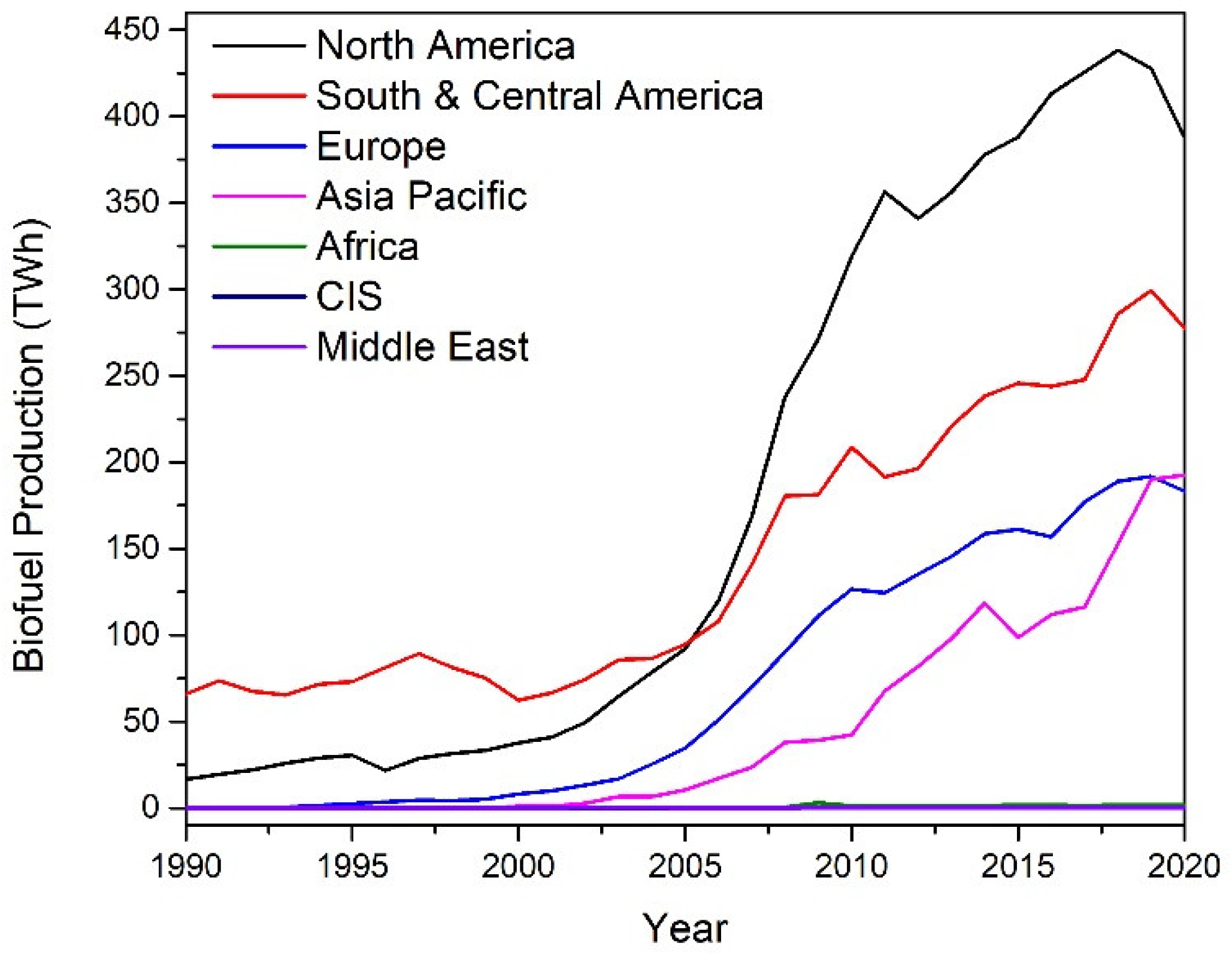
Biomass has emerged in recent years as a potential feedstock for the sustainable production of renewable fuels and chemicals. The biorefinery concept has come to the fore as a possible solution to this issue. The International Energy Association Bioenergy Task 42 has defined a biorefinery as “the sustainable processing of biomass into a spectrum of marketable products and energy”. The spectrum of marketable products and energy consist of intermediates and final products, and include food, feed, materials, chemicals, and energy (fuels, power and/or heat). Typically, biorefineries can produce a form of biofuel product such as bioethanol or biodiesel. The growth in biofuel production since 1990 is shown in
Figure 2. Biofuel production growth was above the 10-year average in 2018, with a 9.7 % increase in production [
2]. Bioethanol production totalled 60.4 mtoe (million tonnes oil equivalent), with North America the largest producer at 56 %. Biodiesel production totalled 34.9 mtoe in 2018, with Europe the largest contributor at 37 %. Combined bioethanol and biodiesel production are shown below in
Figure 2.
As a fuel source biodiesel has several advantages as it is renewable, non-toxic, and biodegradable. Upon combustion biodiesel produces no sulphur, no net CO
2, less carbon monoxide, zero particulate matters, no smoke, and no hydrocarbons [
4]. Biodiesel is attractive as it can be used in diesel engines with little to no modifications or performance decline. Within the European Union targets set by the Renewable Energy Directive (RED II) have increased the mandates of renewable transport fuels from 10 % in 2020 to 14 % in 2030. Similarly, in the United Kingdom the Renewable Transport Fuel Obligation (RTFO), has set a target of a 12.4 % biofuel blend by 2032. Hence it is evident that biodiesel production will continue to increase in the next decade, to meet these targets as countries push towards net zero CO
2.
1.2. Glycerol: A Liability from Biodiesel Industry
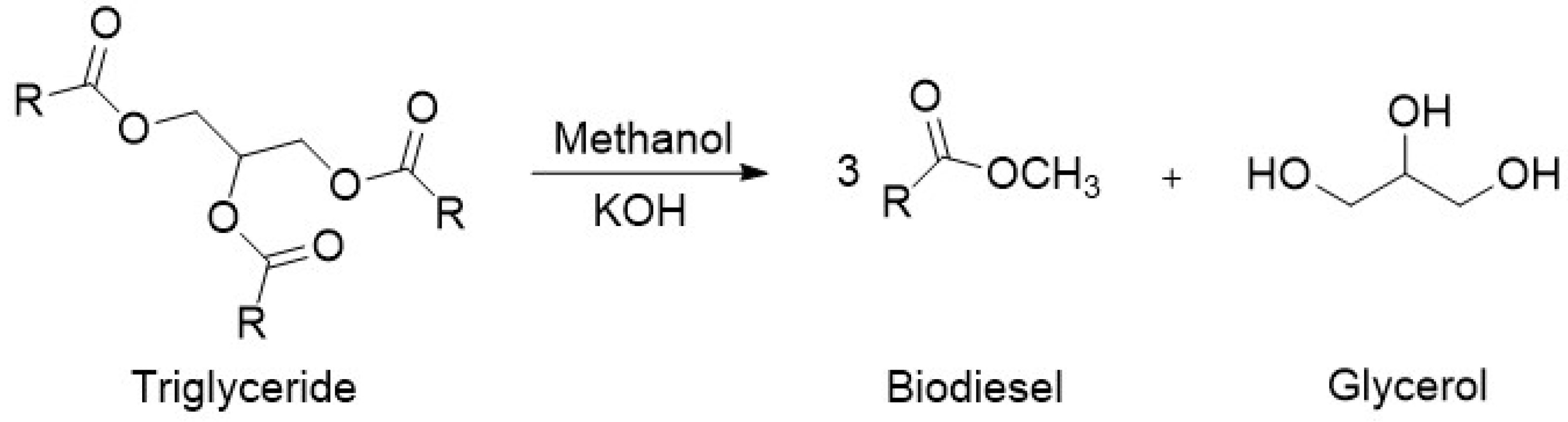
Typically, biodiesel is produced through the transesterification of triglycerides, contained in vegetable oils, with methanol to produce fatty acid methyl esters (FAME), as shown below in
Figure 3 [
5]. The reaction is catalyzed by alkalis such as sodium or potassium hydroxide. Due to its reversible nature, the reaction is normally performed with an excess of alcohol to ensure complete conversion of the vegetable oil.
A major problem associated with the production of biodiesel is the formation of a by-product glycerol, which accounts for 10 wt% of all biodiesel production. Glycerol or glycerine (IUPAC propane-1,2,3-triol, CAS:56-81-5) is a simple polyol with a molecular formula of C
3H
8O
3. It consists of a propane molecule substituted with 3 hydroxyl groups at positions 1, 2 and 3. The structure of glycerol is shown in
Figure 3. In its pure form glycerol is colourless, non-toxic, odourless, and viscous. Properties of glycerol are shown in
Table 1.
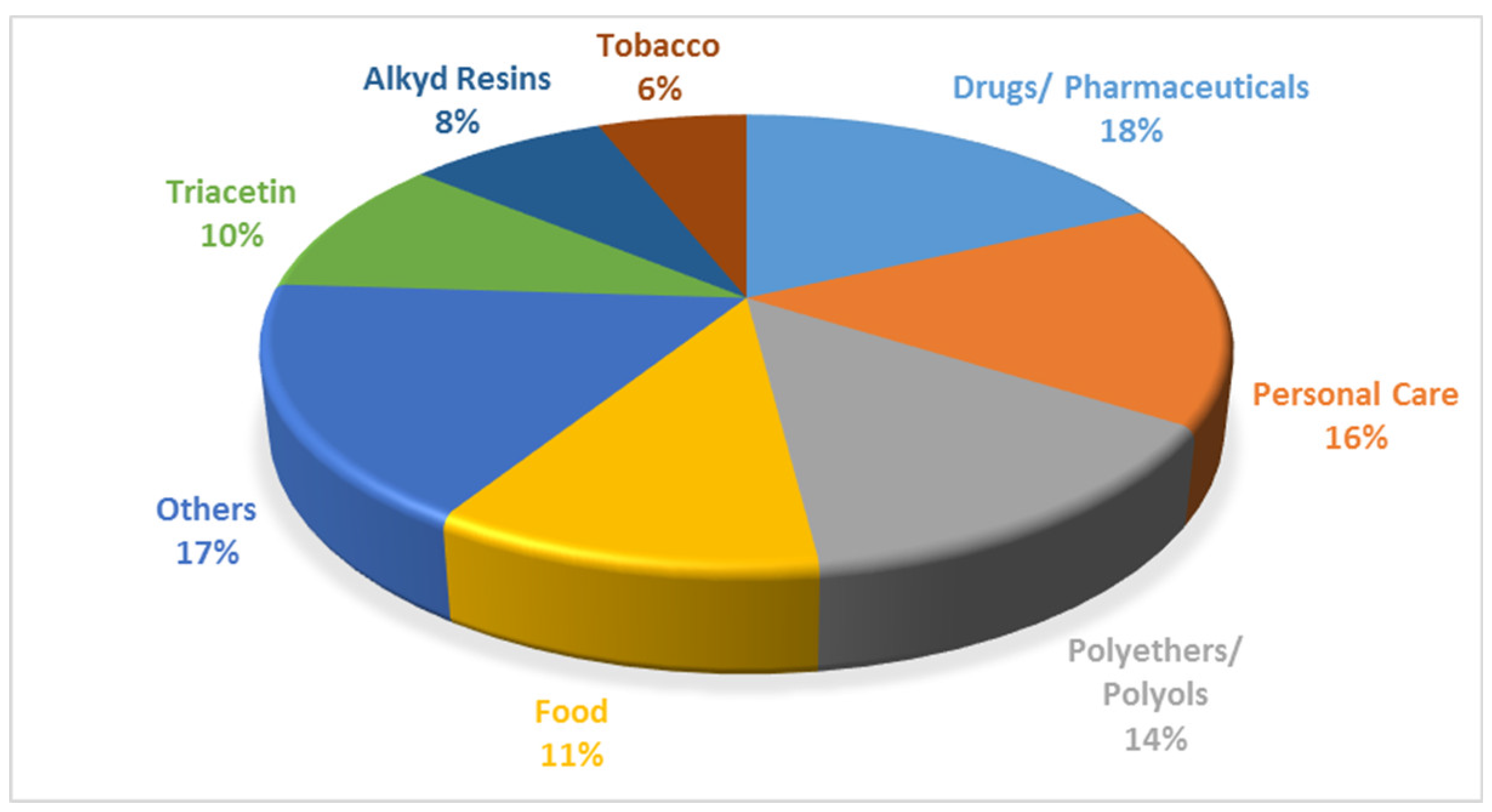
Pure glycerol has a wide range of uses including the manufacture of drugs, cosmetics, toothpastes, urethane foam, synthetic resins, and ester gums [
8]. It is also used as a miscellaneous or general-purpose food additive due to its non-toxic nature. The various applications of glycerol are shown in
Figure 4.
The problem associated with the increase in biodiesel production is two-fold. Firstly, the increase in biodiesel production over the last 20 years has naturally led to an increase in the amount of by-product formation. The surplus of glycerol resulting from this increase has led to a market where the supply of glycerol is independent of the demand, resulting in a marked decrease in the price of glycerol [
10]. In 1999, the oleochemical industry supplied 47% of the world’s glycerol, changing dramatically from 2009, where 64% of glycerol was supplied from the biodiesel industry. In 2014, the price of 80% crude glycerol was
$0.24 kg
-1 and United State Pharmacopeia grade was
$0.9 kg
-1 [
11]. One positive aspect of the decrease in glycerol price is that it makes it an attractive feedstock to create value added products, for instance some of the traditional applications are as shown in
Figure 4.
1.3. Upgrading Glycerol to Value Added Glycerol Esters: The Key to Convert Liability into Assets
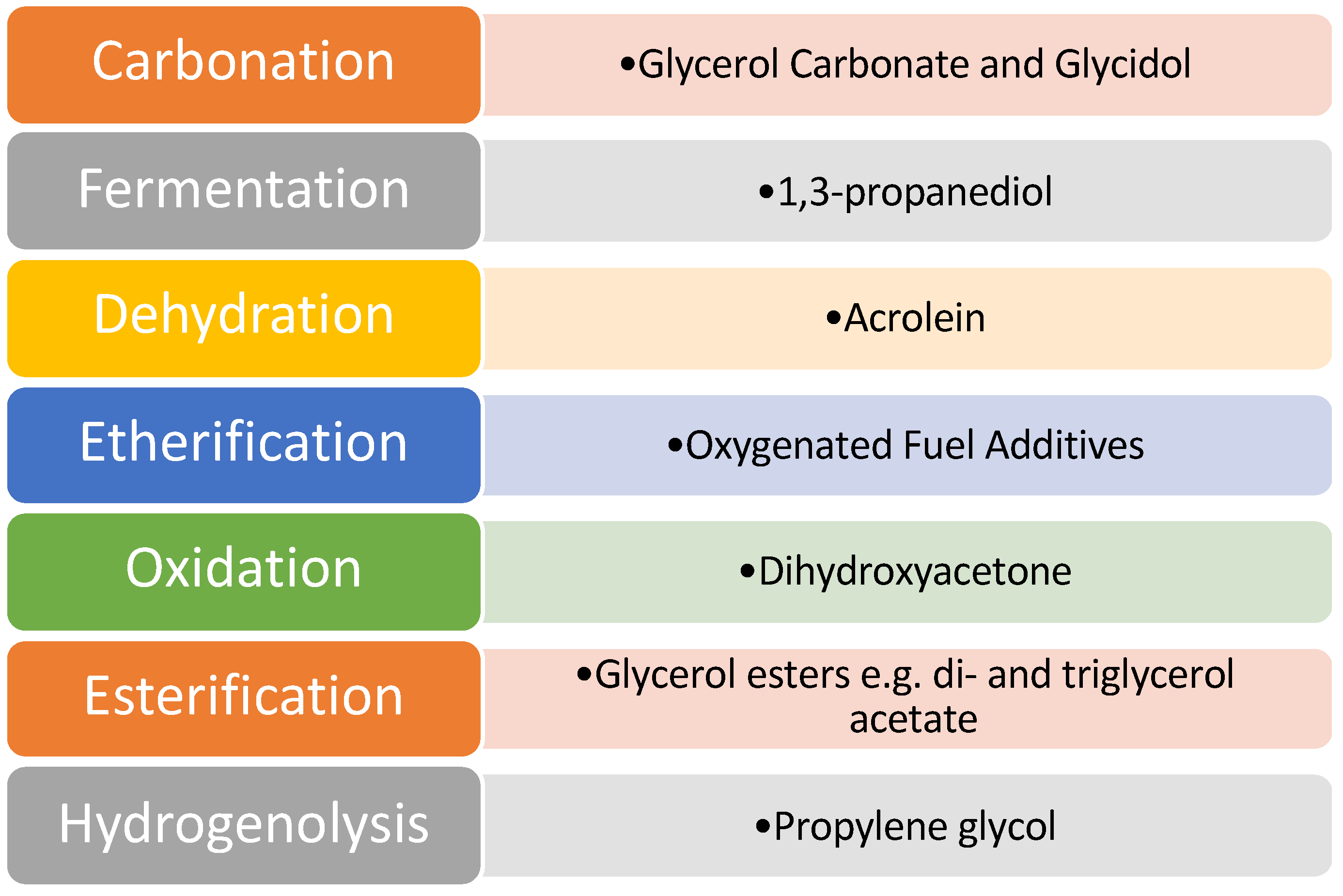
Despite the issues associated with glycerol, it was identified as one of the top twelve platform chemicals by the United States Department of Energy. As a result of this, a great deal of scientific research has been directed towards this area to develop effective catalysts and efficient pathways of value addition. Various pathways that have been explored for glycerol valorisation are shown in
Figure 5.
The purity of glycerol produced from biodiesel industry as a by-product, is quite low and therefore unsuitable for most traditional applications. Whilst the composition of crude glycerol varies from producer to producer, most of the crude glycerol includes impurities such as methanol, water, soap, and FAME [
12]. Crude glycerol will also contain smaller amounts of glycerides, unreacted free fatty acids, and ash. Also depending on the efficacy of post-treatment in the plants residual alkali, such as NaOH or KOH, can remain in the crude glycerol resulting in a high pH level. Characterization of crude glycerol is important as it can affect which applications it is appropriate for. Often the large cost associated with refining crude glycerol can only be afforded by large scale manufacturers, and not an economically viable option for small or medium scale manufacturers. It is therefore important to find ways of adding value to this waste product, not only to promote a circular economy and improve sustainability, but also to improve the economic viability of the biorefinery industry [
13]. In this context, esterification of glycerol is amongst most employed organic transformations for upgrading glycerol to glycerol esters. The key advantage here being the application of glycerol esters as high energy density “drop-in” fuel additive, which can be blended back into the biodiesel pool. Thus, converting the liability from biodiesel industry into the asset, adhering to the principles of circular economic approach, while at the same time improving the process economics and profitability of the overall biorefinery concept. In continuation of our group’s interest in biomass derived drop in fuels [14–23], and environmental catalysis [24–36], herein we have critically reviewed the upgrading glycerol to glycerol esters as the products.
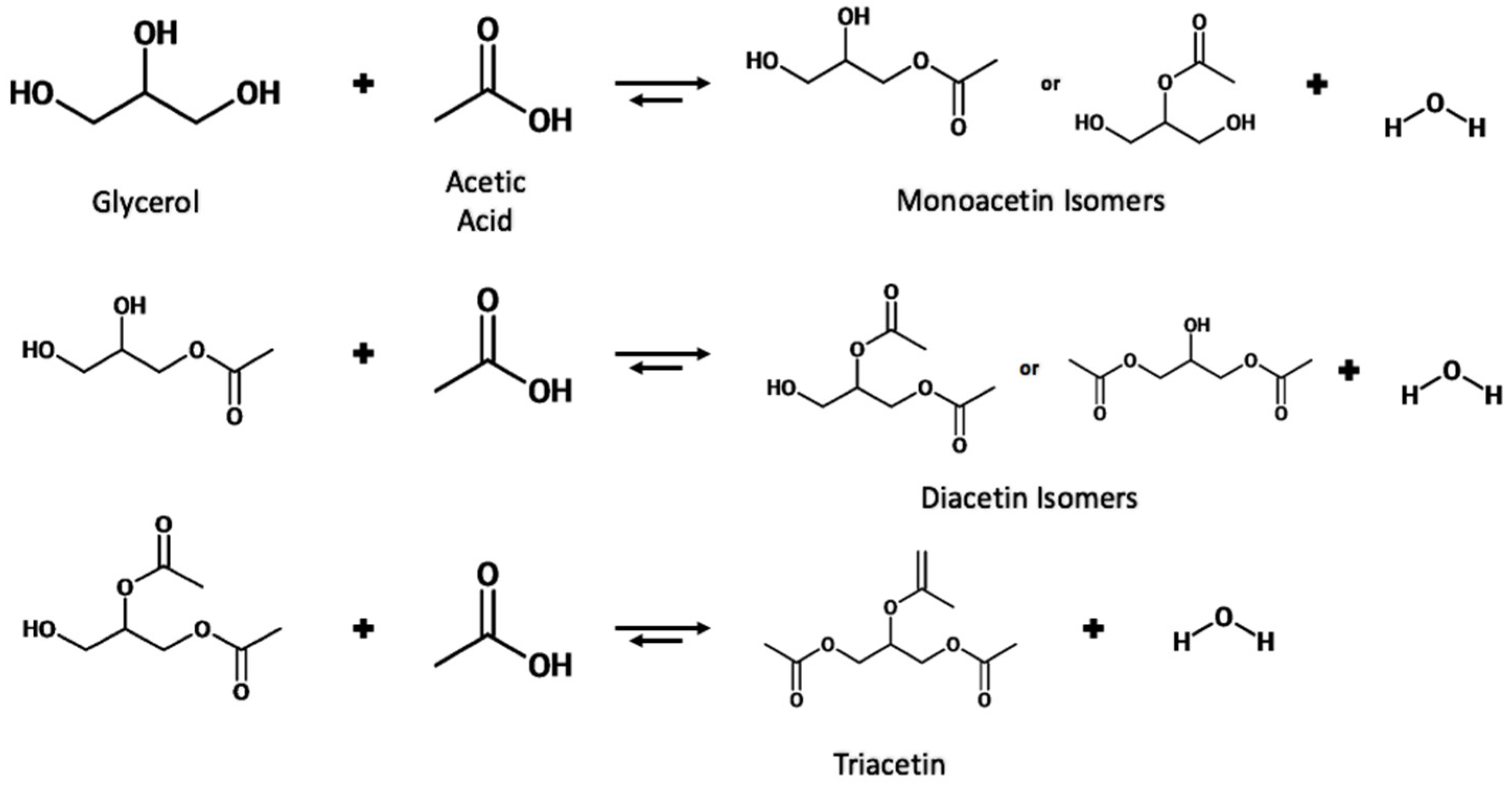
The esterification of glycerol with acetic acid produces monoacetin (MA), diacetin (DA) and triacetin (TA) acetyl esters, which have added economic value compared to the crude glycerol waste from biorefinery. The various uses of these esters are summarized in
Table 2. The reaction can also be performed using acetic anhydride as an acetylating agent; however, safety issues can arise due to the formation of explosive vapor/air mixtures [
11]. Acetic acid is also cheaper when compared to acetic anhydride, at 0.5 US
$ kg
-1 and 0.98 US
$ kg
-1 respectively [
41].
The reaction proceeds stepwise with the substitution an acetyl group with the hydrogen of a hydroxyl group to form the ester and water. Due to the three hydroxyl groups present in glycerol, the substitution can occur for each group producing a water molecule each time. The reaction scheme is shown in
Figure 6.
Due to the reversible nature of the reaction various techniques have been utilized to shift the equilibrium towards the right-hand side. These techniques include increasing the temperature of the reaction, removal of in-situ water and increasing the molar ratio of glycerol to acetic acid.
1.4. Reaction Mechanism for Esterification of Glycerol
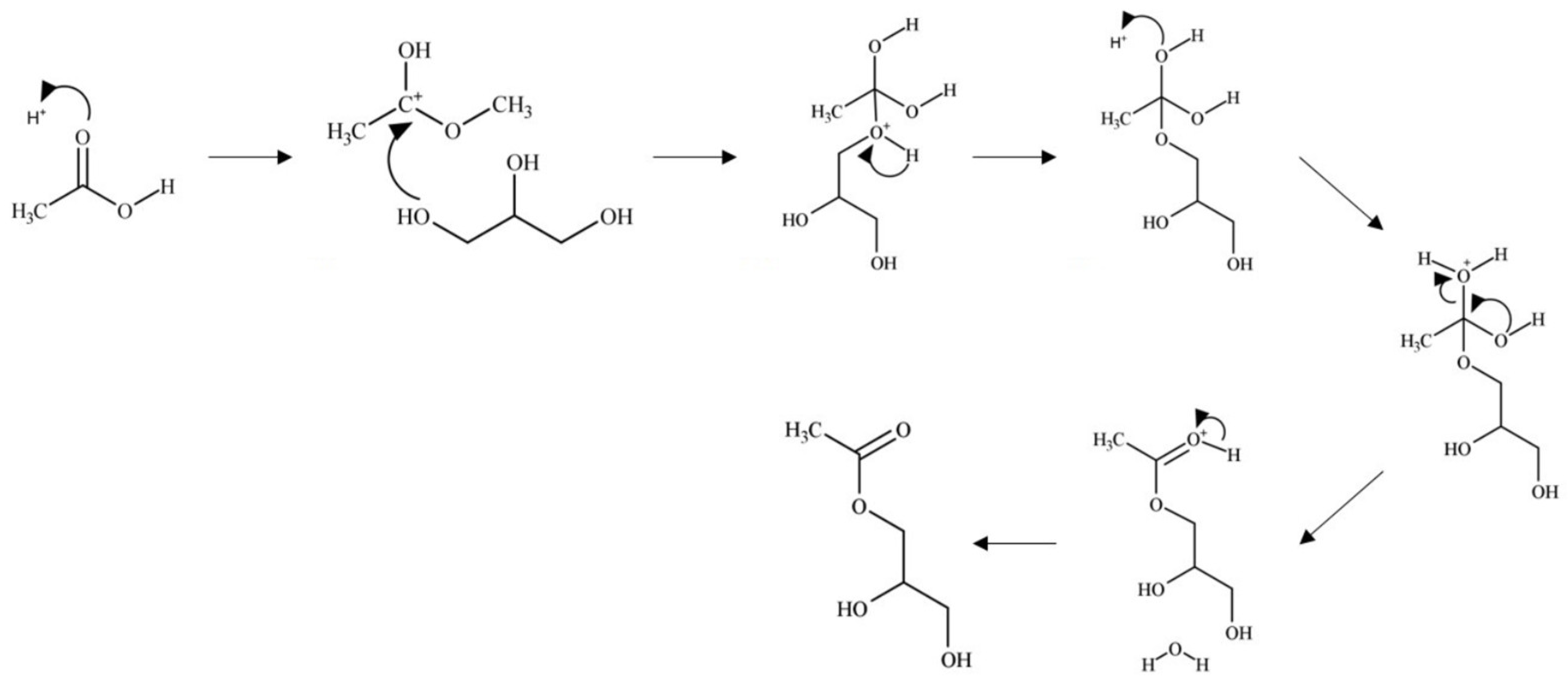
The reaction can occur through a Brønsted-acid or Lewis-acid catalyzed mechanism, although both mechanisms are similar in nature.
In the Brønsted-acid mechanism, as shown in
Figure 7 protonation of the acetic acid carbonyl occurs via a proton from the catalyst. The resulting carbocation formed undergoes nucleophilic attack from an oxygen of a glycerol hydroxyl leading to loss of a proton. An ester bond is formed through the hydroxyl groups of acetic acid which undergo fast equilibrium proton exchanges resulting in the elimination of water. The catalyst is regenerate through elimination of the excess proton. This mechanism occurs similarly with the remaining hydroxyl groups of glycerol.
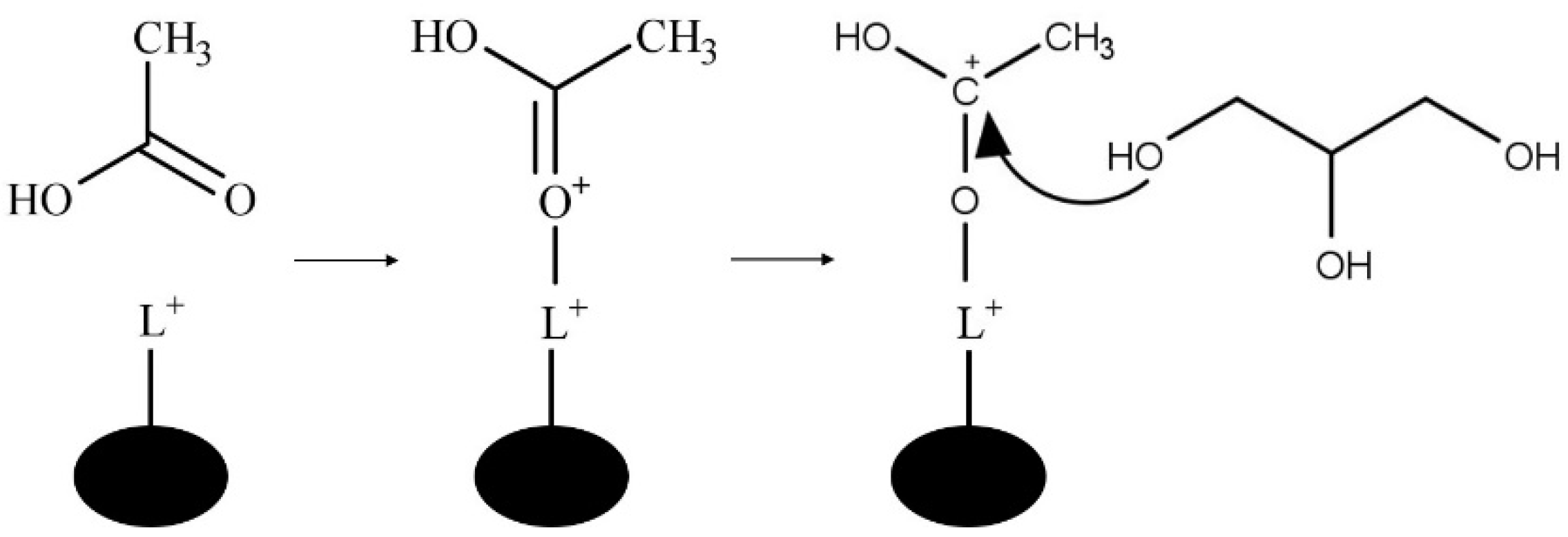
The Lewis-Acid catalyzed mechanism is shown in
Figure 8. In the Lewis-acid catalyzed mechanism a metal cation acts as an electrophile to form the carbocation via acetic acid carbonyl oxygen and Lewis-acid site of the catalyst [
11].
2. Nanostructured Solid/Liquid Acid Catalysts for Glycerol Esterification
2.1. Homogeneous Catalysts Used in the Esterification of Glycerol
Mineral acid catalysts, such as H2SO4 and HCl, have typically been used in esterification reactions. These catalysts are associated with several drawbacks. These are hazardous to handle, corrosive, and lead to large volumes of process waste due to the need for quenching and separation of acids, and the acids are normally destroyed in quenching and neutralisation so are non-reusable. With increased importance placed upon green chemistry practices, the need to develop catalysts, which can overcome these drawbacks is high.
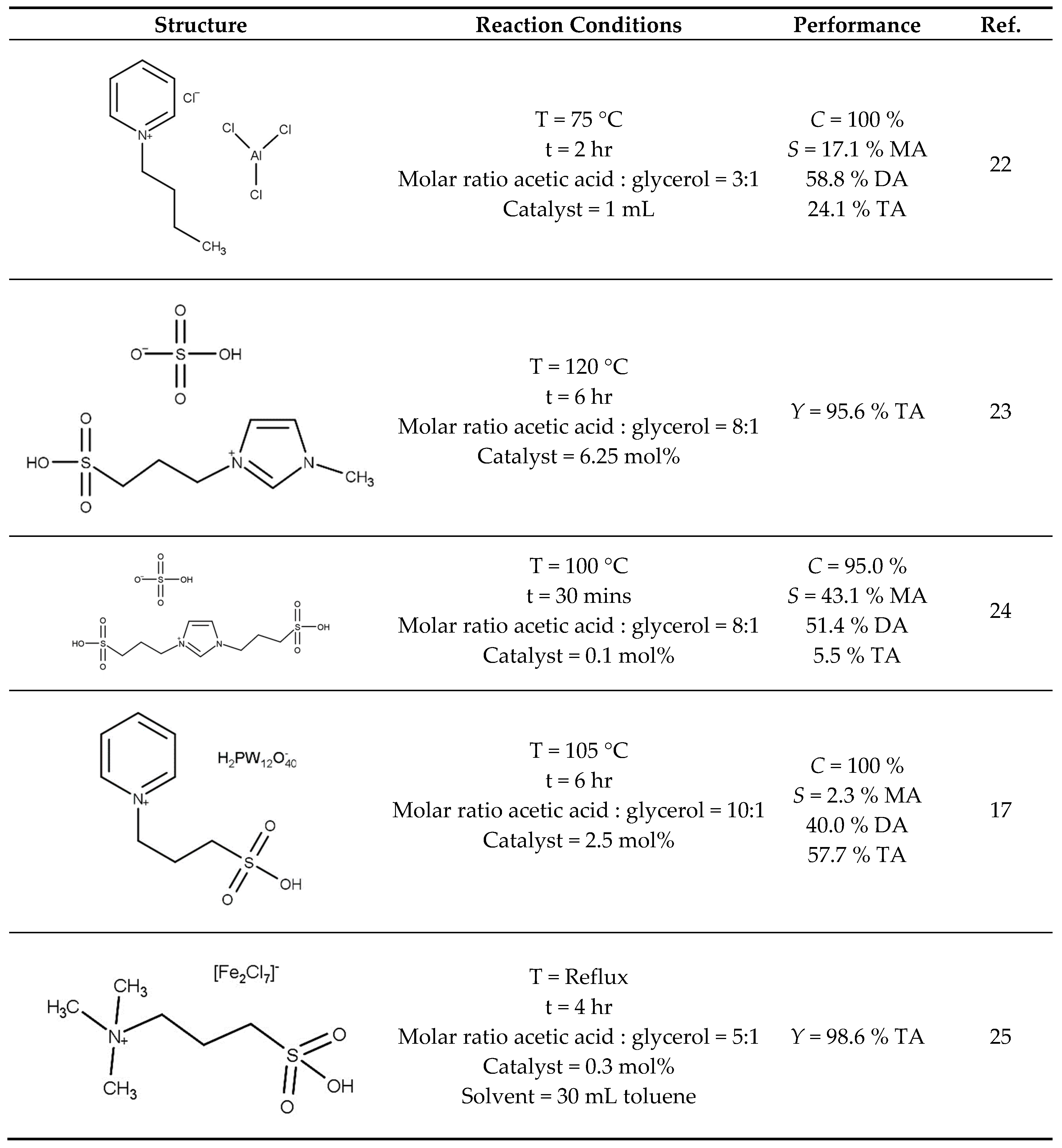
Ionic liquids (ILs) have emerged as potential replacements of mineral acid catalysts as they have several benefits such as good thermal stability, ease of handling and importantly good recyclability. The structure and performance of various ILs are reported in
Table 3. As ILs are composed of a cation and anion, the chemical and physical properties of the ILs can be changed by adjusting the composition of the ions to produce functionalized ionic liquids. One such form of functionalized ionic liquids is the Brønsted acidic ionic liquids (BAILs), which gain functionality through covalently bonded sulfonic acid species (-SO
3H) or Brønsted acidic counter anions (HSO
4-, H
2PO
4-) [
48].
The use of ionic liquids for the esterification of glycerol with acetic acid was first reported by Deng et al. [
49]. Using a combination of aluminum (III) chloride and 1-butylpyridinium chloride, conversion of glycerol and selectivity of the products was found to be comparable to sulphuric acid.
Li et al. first reported the use of SO
3H-functionalized ionic liquids, which were composed of [HSO
3-pmim] as cation, and a range of counter anions such as [HSO
4]
-, [PTSA]
-, [H
2PO
4]
-, [BF
4]
- and Cl
- [
55]. The use of [PTSA] provided the most active catalyst, [HSO
4] also provided excellent activity whilst remaining active upon recycling. The effect of double SO
3H-functionalized ionic liquids was investigated by Liu et al. [
56]. The double SO
3H-functionalised ionic liquids outperformed those with only one SO
3H-group, due to a higher level of Brønsted acidity. Similarly, [HSO
4]
- was found to be the most active counter anion against [NTf
2]
- and [tos]
-. Huang et al. reported the use of heteropolyacid-based ionic liquids consisting of pyridinium propyl sulfonate, tungstophosphoric acid, and acetic acid achieving 85.9 % selectivity to triacetin after 4 hr at 105 °C with continuous water removal [
45].
Keogh et al. reported the use of a range of nitrogen based Brønsted-acidic ionic liquids based on alkyl-pyrrolidone and alkyl-amine cations [
52]. Amongst all ionic liquids studied, N-methyl-2-pyrrolidinium hydrogen sulfate [H-NMP][HSO
4] was found to be the most active catalyst. The effect of significant reaction parameters on selectivity to the tri-substituted product, triacetin, was modelled using a Design of Experiment (DoE) approach with a response surface methodology involving a central composite design. Amongst the reaction parameters evaluated, temperature had the highest influence on product selectivity, followed by the glycerol to acetic acid molar ratio, and the model also showed dependence on the synergistic interaction between temperature and mole ratio.
In a separate study, Liu et al. showed the synergistic effect of both Brønsted and Lewis sites where a Brønsted-Lewis acidic ionic liquid outperformed solely Brønsted acidic and solely Lewis acidic ionic liquids [
53]. Sun et al. prepared rod-like carbon-based ionic liquids which were functionalized with sulfonic acid [
43]. The prepared ionic liquids were evaluated in their ability to produce triacetin from glycerol. The [PrSO
3HN][SO
3CF
3]/C-2 ionic liquid was the most active, giving a high yield of triacetin of 74.8 % after 8 hr.
Heteropolyacids are a class of strong Brønsted acids consisting of (i) metal e.g., tungsten, molybdenum or vanadium, (ii) oxygen, (iii) a p-block element e.g., silicon, phosphorus or arsenic, and (iv) acidic hydrogen atoms [
47]. Typically, tungstophosphoric acid (TPA), silicotungstic acid (STA) and phosphomolybdic acid (PMA) are used. Whilst heteropolyacids tend to be highly soluble in the reaction mixture, they can be reused in a series of recycling steps. Gonçalves et al. reported the use of TPA which offered comparable activity to that of sulfuric acid and p-toluenesulfonic acid [
54]. The protons of heteropolyacids can be exchanged with metal ions to improve the activity, thermal stability and also tune the solubility of the heteropolyacid in the reaction media. Although exchange with metal ions can often lead to insoluble heteropolyacid salts, it can also have limited effect on the solubility. Da Silva et al. investigated the effect of exchanging the protons of TPA, PMA and STA with Lewis-acidic metals such as Cu, Co, Mn and Fe [
55]. STA was found to be the most active heteropolyacid, followed by TPA and finally PMA. Iron was found to be the most active metal regardless of the heteropolyacid. Complete exchange of the protons of STA with iron, resulted in a partial soluble catalyst with increased activity. When exchanging with Sn, Chaves et al. found TPA to be the more active than STA and PMA [
56].
Soluble tin (II) chloride has also been reported for the reaction [
57]. The less corrosive Lewis-acid was found to have comparable activity to sulphuric acid and produced less reaction by-products.
2.2. Heterogeneous Catalysts Used in the Esterification of Glycerol
Solid acid catalysts can offer easier separation when compared to reusable homogeneous catalysts. Often a simple filtration can separate the catalyst from the reaction mixture. Solid acids also give generally clean and selective reactions with high purity products [
47]. The characteristics of solid acid catalysts such as the acidity, catalyst texture and surface morphology, can be tuned to offer high conversion of glycerol and high selectivity of DA and TA products [
11].
2.2.1. Metal Oxide Catalysts
Hu et al. investigated the abilities of a wide range of metal oxides to catalyst the esterification on glycerol with acetic acid, with high selectivity to diacetin [
58]. When compared to a blank experiment which gave 45.2 % conversion of glycerol and selectivity to diacetin of 12.6 %, only marginal improvements were observed with Sb
2O
3, Bi
2O
3, SnO
2, TiO
2 and Sb
2O
5. Higher glycerol conversion of 94.5 % and selectivity to diacetin of 46.8 % was observed using antimony pentoxide (Sb
2O
5). Good selectivity to diacetin is often hard to achieve, as low activity catalysts will mainly show high selectivity to monoacetin, with high activity catalysts showing high selectivity to triacetin. The Sb
2O
5 catalyst also showed good reusability with no change in conversion or selectivity after 6 runs.
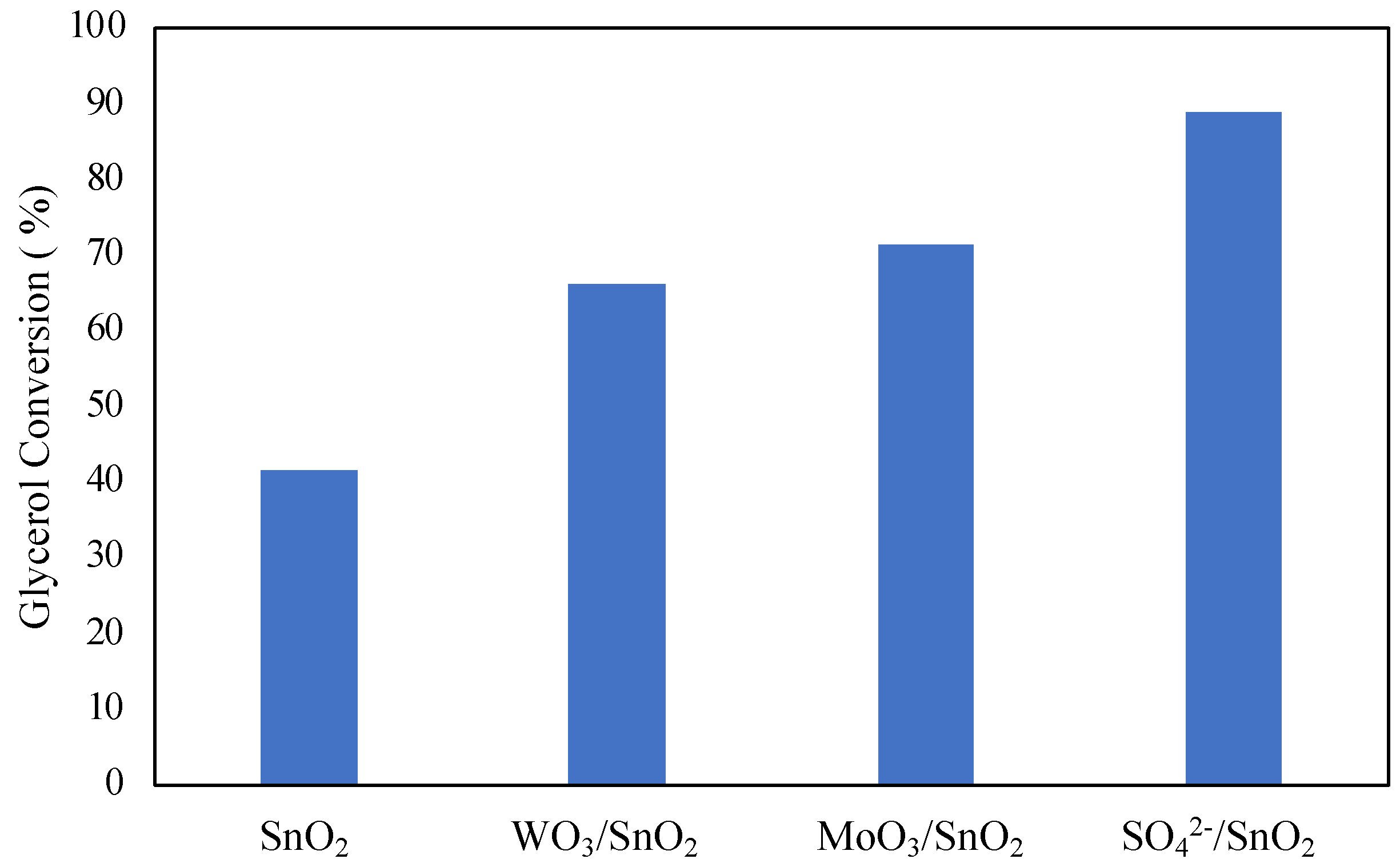
The effect of adding promoting species to metal oxides was investigated by Mallesham et al. [
59]. Promoting species SO
42-, MoO
3 and WO
3 were added to tin oxide using a wet-impregnation method. The addition of promoting species was found to improve glycerol conversion, as shown in
Figure 9. The highest glycerol conversion was observed with the SO
42- promoted tin chloride at 89 %. The enhanced performance of the catalyst can be explained by the high number of acidic sites at 186.98 μmol/g with an abundance of superacidic sites. Reusability of the catalyst was found to be quite low, with glycerol conversion decreasing to 51 % on the 4th cycle, also leading to decreased selectivity to diacetin and triacetin with each cycle. The quick decline in catalytic activity was attributed to decreased acidic sites and BET surface area of the catalyst after each cycle. It can then be noted from the results of Mallesham et al. that a high number of acidic sites on the catalyst is imperative to high conversion of glycerol in the reaction.
Reaction conditions: 70 °C, 120 min, molar ratio of acetic acid to glycerol of 1:1 and 5 wt% catalyst.
Sulfate ions (SO
42-) have also been used by Reddy et al. to improve the activity of a ceria and zirconia mixed oxide species [
60]. The sulfonated mixed oxide showed an increased surface area (49 to 92 m
2/g) and increased strength and number of acid sites from the pristine mixed oxide. Sulfonated ceria-zirconia has also been reported by Kulkarni et al. [
61]. Sulfate ions have been shown to improve the activity of titania and silica mixed oxides [
62]. The mixture of these two oxides, consisting of 13.8 wt% TiO
2, generated a higher number and strength of acid sites.
Similarly, Reddy et al. investigated the effect of promoting species (TiO
2, WO
x and MoO
x) on zirconium oxide [
63]. Of the catalysts prepared nearly 100 % conversion after 3 hr at 120 °C was observed with WO
x/TiO
2-ZrO
2 and MoO
x/TiO
2-ZrO
2. High selectivity towards diacetin was also observed with 40.01 % and 40.45 % respectively.
2.2.2. Ion-Exchange Resins
One class of solid acid catalysts are ion-exchange resins, which exchange ions between itself and the reaction media [
47]. The resins are usually copolymers of divinylbenzene or styrene and ion-exchanging functional groups. Dosuna-Rodriguez et al. evaluated the ability of several ion exchange resins to catalyse the reaction [
64]. Different to other literature based on this topic, reactions were carried out with an excess of glycerol. As glycerol is the low value molecule this keeps costs of reactions low, but also results in the desired shift of equilibrium to the right to promote formation of the products. The results of the various ion-exchange resins are shown in
Table 4. Amberlyst-36 was tested to determine the reusability of the catalyst, no significant change in activity was observed after 4 cycles.
Zhou et al. showed that Amberlyst-15 could obtain high conversions and selectivity to DA and TA when combined with an excess of acetic acid [
65]. Amberlyst-15 and 70 were investigated by Kale et al. with the use of toluene as an entrainer aiming for high selectivity to triacetin [
66]. The use of toluene as an entrainer was found to be key for conversion and product selectivity. Without toluene, A-70 gave only 9.3 % selectivity to TAG, increasing markedly to 45.8 % with the use of toluene. A maximum TAG selectivity of 95.3 % was observed after 24 hr using A-70.
A polysulfone catalyst which was 2 times as acidic as Amberlyst-15 was developed by Wang et al. [
67]. The catalyst was more active due to its increased acidity and swelling properties. Moreover, the catalyst showed good stability upon reuse, whilst Amberlyst-15 underwent deactivation upon reuse. This was attributed to the unstable bonding of acid groups via the post-sulfonation method.
Other commercial ion-exchange resins such as Dowex Monosphere 650C and Purolite CT-275 have also been reported [
68,
69,
70].
2.2.3. Zeolite-Based Solid Acids
Zeolite-based solid acids are aluminium silicates which form a regular crystal lattice, catalysing reactions in their internal cavities [
47]. Gonçalves et al. compared the zeolites HUSY and HZSM-5 against the acidic catalysts of amberlyst-15, K-10 montmorillonite and Niobic acid 45. The results showed poor activity of the zeolite catalysts with HZSM-5 and HUSY giving 30 % and 14 % glycerol conversion respectively after 30 minutes. For both, monoacetin was the major component with small amounts of diacetin, however no triacetin was detected for either catalyst. The low conversion was attributed to diffusion problems of the esters within the catalyst pores, and deactivation of acid sites on the catalyst.
The effects of Zr-modification on mordenite (M) and hierarchical mordenite (M1) for catalysing the reaction was investigated by Popova et al. [
73]. H-mordenite was prepared with acidic treatment of the parent mordenite, with Zr added by incipient wetness impregnation. The highest conversion of glycerol observed after 3 hr at 100 °C was with Zr/M1 of 93.5 %, with an impressive yield of triacetin of 69.2 %. Under the same conditions it was observed that acidic treatment of mordenite gave higher activity than the parent mordenite, which can be attributed to the increase in pore size from 0.9 nm to 1.6 nm. Higher conversions of glycerol to valuable triacetin were also observed for the M1 catalysts. The Zr-modified catalysts exhibited increases in glycerol conversion from their parent catalysts, which can be attributed to the increased number of Brønsted and Lewis-acid sites. Results from this experiment are summarised in
Table 5.
Gao et al. compared the activity of a graphene oxide catalyst against zeolites ZSM-48, ZSM-5 and H-mordenite [
74]. After 1 hr at 120 °C with a glycerol to acetic acid ratio of 1:10 the catalyst produced average conversion of glycerol, with the activity following the trend ZSM-5 > H-mordenite > ZSM-48. Despite average conversion of glycerol, these catalysts produced high yields of diacetin, with ZSM-5 giving a diacetin yield of 62.2 %. The activity of ZSM-5 could be improved through the incorporation of 5 wt% cerium in the structure, with glycerol conversion increasing from 76.43 to 98.32 % under the same conditions. However, only monoacetin and diacetin were reported.
2.2.4. Silica-Based Solid Acids
Silica-based solid acids are also widely investigated as a support in catalysis as it is easily available and inexpensive [
47]. The addition of sulfonic groups to mesoporous materials, commonly MCM-41, HMS and SBA-15, produce solid acid catalysts. The acid catalysts have properties of high surface area (≥1000 m
2/g), large pore sizes (2 nm – 20 nm) and relatively high acid strengths [
75,
76]. Melero et al. investigated acidic mesoporous silica for the acetylation of glycerol [
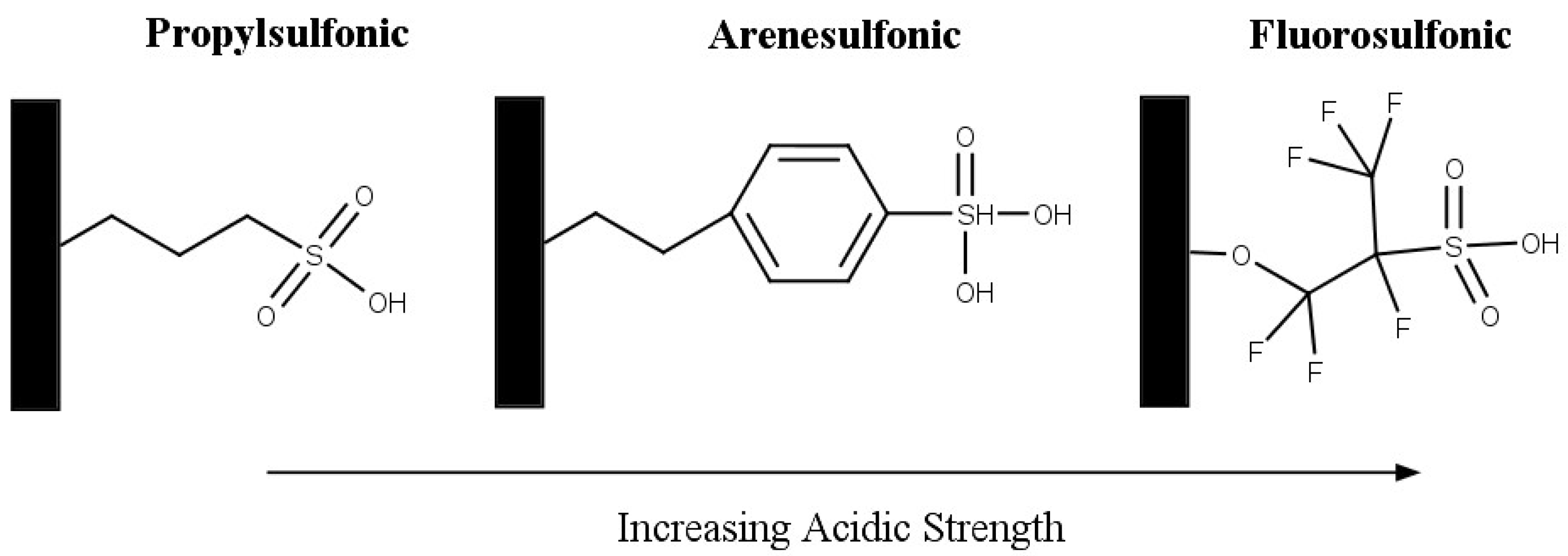
75]. Three materials were prepared by incorporating phenyl, propyl and fluorosulfonic groups with SBA-15 material. The structure of the sulfonic acid groups is shown in
Figure 10. The incorporation of more electron withdrawing groups, such as phenyl and fluoro, results in an increased acid strength. The activity that was observed followed the same trend of sulfonic group acid strength: fluorosulfonic > arenesulfonic > propylsulfonic. After 2 hr the highest selectivity to di and triacetin was shown by Ar-SBA-15, however it was noted that after 4 hr most of the materials achieved around 80% selectivity to di and triacetin.
The effect of niobium on the formation and stability of sulphonic species in these materials was examined by Trejda et al. who prepared silicate and niobiosilicate SBA-15 type catalysts modified with MPTMS (3-mercaptropropyl)trimethoxysilane [
77]. It was found that incorporation of niobium into SBA-15 improves the oxidation of –SH by hydrogen peroxide to sulphonic species, however it did not increase the stability of the species. A maximum conversion of 94 % was found with MP-Nb-SBA-15-32 after 4 hr, with selectivity to di and triacetin of 52 % and 37 % respectively.
Khayoon et al. investigated the promotional effect of yttrium on the activity of SBA-3 [
78]. Incorporation of yttrium was found to increase the surface area of SBA-3 from 1462 m
2/g to 1568 m
2/g. The increased activity of the 3 wt% Y/SBA-3 catalyst was attributed to the combination of a higher surface area and increased stability of the crystalline SBA-4 material after yttrium grafting.
Other silica based materials include mesostructured cellular foams (MCF) which have walls formed from silica [
79]. MCFs are uniform spherical cells with large surface areas up to ca 900 m
2g
-1 interconnected by uniform windows (7-20 nm) forming a continuous porous system. Stawicka et al. synthesised niobium and tantalum containing MCFs modified with MPTMS. Highest conversion of glycerol was achieved with MP-TaMCF, but MP-NbMCF gave the highest yield of triacetin (38 % after 4 hr at 398 K). The choice of metal was found to not affect the amount of MPTMS anchored, instead affecting the number of Brønsted-acid sites. Whilst MP-TaMCF had the highest number of Brønsted-acid sites, MP-NbMCF was found to have the strongest Brønsted-acid sites. Stawicka and co-workers found that the strength of Brønsted-acid sites was the most important factor in determining the yield of the valuable product triacetin.
2.2.5. Heteropolyacids (HPAs)
To combat the disadvantages associated with the solubility of heteropolyacids in the reaction mass, heteropolyacids can be supported on an appropriate carrier. Similarly exchanging the protons of the heteropolyacids with a metal ion can result in a heteropolyacid salt which is insoluble in the reaction mass.
Metal oxides have been used as supports for heteropolyacids. Zhu et al. investigated glycerol esterification using three zirconia supported HPAs; TPA, STA and PMA [
80]. Previously Zhu et al. reported that ZrO
2 supported STA was the most active and had the highest stability when compared to supports such as y-Al
2O
3, activated carbon, TiO
2 and SiO
2 [
81]. From results it was shown that the acid strength of the HPAs followed the trend of TPA > STA > TMA. STA had the highest Brønsted acidity at 92.2 𝜇mol/gcat with the results of the glycerol esterification reflecting this. With STA/ZrO
2 conversion of glycerol reached 96.4 % after only 1 hr at 120 °C, with selectivity of 60.5 % and 11.2 % to di and triacetin respectively. Conversion increased to 100 % after 4 hr with selectivity increasing to 61.3 % and 32.3 % respectively. The catalyst also exhibited good reusability after 4 runs with negligible change in conversion, whereas TPA and PMA exhibited decreased conversion.
Jagadeeswaraiah et al. doped zirconia with cesium, and used it as a support for TPA [
82]. The loading of TPA onto the cesium doped zirconia, resulted in partial exchange of the TPA protons with cesium ions. The presence of cesium was found to increase the activity of the catalyst as a result of the increase in strength and number of acid sites. Full exchange (TPA/Cs
3-ZrO
2) was the least active exchanged catalyst, due to the absence of residual protons. The optimal catalyst was found to be TPA/Cs
2-ZrO
2 which has two protons exchanged with two cesium ions. TPA has also been supported on niobium pentoxide [
83].
Silica-based materials have been widely used as supports for the incorporation of HPAs. Patel et al. have also investigated HPAs anchored onto supports with 12-tungstophosphoric acid on MCM-41 and zirconia [
45]. TPA
3/MCM-41 gave the highest yield of 87 % after 6 hr at 100 °C, with selectivity to di- and triacetin of 60 % and 15 % respectively. Ferreira et al. prepared TPA on a silica matrix prepared by sol-gel and wet impregnation methods [
84]. Catalysts prepared by sol-gel were more active than those prepared by wet-impregnation. Loading of TPA by sol-gel method resulted in an increase in the SA from 223 to 254 m
2/g, with the presence of very strong acid sites. SBA-15 was found to be an effective support for PMA [
85]. A 15 wt% loading of PMA/SBA-15 gave complete glycerol conversion after 1 hour and a combined DA and TA selectivity of 86 % after 3 hours.
Magar et al. investigated the activity of different HPAs using polyvinylpyrrolidone as a support [
86]. The activity of the HPAs was found to be TPA > STA > PMA, with the activity corresponding well with the acidic strength of the catalysts. Zeolites such as USY and activated carbon have also been used as supports [
87,
88].
Zhu et al. synthesis Ag exchanged TPA (or HPW) catalysts using an ion-exchange method [
89]. The trend for the catalyst activity from highest to lowest was Ag
1PW > Ag
2PW > Ag
3PW. Glycerol conversion with Ag
1PW reached 100 % within only 45 minutes. The conversions after 15 min at 120 °C with 1 wt% catalyst and a glycerol to acetic acid mole ratio of 1:10 are shown in
Table 6. The Ag1PW showed similar activity after 5 cycles, exhibiting good reusability.
Similarly, TPA can be exchanged with be exchanged with cesium to produce an insoluble cesium phosphotungstate salt [
90]. The CsTPA catalyst outperformed H-beta, K-10 and sulphated zirconia due to a high number of acid sites (1.87 mmol/g). The catalyst also out exhibited higher selectivity to triacetin than Amberlyst-15. Sun et al. reported the use of an indium exchanged TPA catalyst [
91]. The catalyst was found to exist in a nanotube like structure, which combined with the presence of Lewis and Brønsted-acid sites lead to selectivity formation of MA.
Keogh et al. investigated the kinetics of esterification of glycerol with acetic acid using partial tin exchanged TPA supported on montmorillonite K-10 as catalysts [
17]. Partially, exchanging the H
+ ion of TPA with Sn (x = 1) increased the acidity of the catalyst and showed an increase in the catalytic activity as compared to supported TPA/K-10 catalyst. Among various catalysts, Sn
1-TPA/K-10 proved to be the most active catalyst for glycerol esterification. The Langmuir-Hinshelwood (L-H) dual-site model was able to describe the experimental data with high agreement between the experimental and calculated results. The tin exchanged TPA supported on montmorillonite K-10 catalysts were found to be robust and shown to recycle four times without loss of activity.
2.2.6. Carbon-Based
In 2015 Gao et al. reported the esterification of glycerol and acetic acid using a graphene oxide catalyst [
73]. Under the reaction conditions of 120 °C, 1:10 molar ratio of glycerol to acetic acid and 0.1 g catalyst, glycerol conversion reached 98.5 % after 1 hr, with selectivity to di and triacetin of 60 % and 24.5 % respectively. The high catalytic activity of graphene oxide for this reaction can be directly attributed to the high number of –SO
3H groups on the catalyst surface, which was measured to be 0.378 mmol/g. The catalyst also showed good reusability with no decline in conversion or variation in the distribution of products.
Sanchez et al. prepared porous carbon-based catalysts by sulfonation of carbonised sucrose [
93]. Direct synthesis carbonisation (DC) and template assisted carbonisation (TAC) were used followed by functionalisation of the carbon with –SO
3H groups. TAC-673 was observed to have the highest density of sulfonate groups at 1.35 mmol/g. In the esterification of glycerol with acetic acid (1:9), in the reaction temperature range of 378 to 473 K, all reactions using the DC and TAC gave conversions of higher than 99.6 %, with a significant increase in the selectivity to triacetin from 17% (at 378 K) to 50% (at 473 K). Willow catkins, a low-cost biomass, has also undergone carbonisation to produce a catalyst [
94]. Sulfonation of activated carbon was also reported by Khayoon et al. [
95].
Okoye et al. had a novel solution for the excess of crude glycerol, using it in the synthesis of an acid catalyst involving sulfonation and carbonisation which could then catalyse the acetylation of glycerol [
96]. The carbon catalyst is irregularly shaped with few pores, and it contains both Brønsted-acidic sulfonate groups and Lewis-acidic carboxylic groups. After seven recycles the catalyst showed constant acid density, indicating good reusability of the catalyst.
Carbon spheres and xerogels can be modified with sulfonic acid groups to produce active acidic catalysts [
97]. Both decreased in surface area upon sulfonation but it was most dramatic with carbon spheres, decreasing from 371 to 11 m
2/g. Sulfonated xerogel had an acidity of 1.19 mmol/g, and carbon spheres 2.77 mmol/g. As a result sulfonated carbon spheres were more active, providing a similar level of activity to Amberlyst-15.
2.2.7. Others
Troncea et al. reported the use of hydroxylated magnesium fluoride catalysts [
46]. The mesoporous catalyst contained a mixture of Lewis and Brønsted-acid sites. A higher Lewis to Brønsted-acid site ratio was found to favour the formation of DA and TA due to the two-fold effect of Lewis-acid sites – acting as a catalyst and dehydrating site. Tangestanifard et al. investigated the use of bentonite which was functionalised with arenesulfonic acid [
97]. The modified clay exhibited a marked decrease in the SA and pore volume but an increase in number of acidic sites (1.7 mmol/g). As such an increase in conversion was found when compared to H-bentonite from 67 % conversion to 100 %. Utilising toluene as an entrainer complete conversion could be achieved, with selectivity of 26 % DA and 74 % TA. The functionalisation of phenolic resins and polyphenylene sulfide fabrics with SO
3H groups have been reported [98,99].
2.2.8. Comparison of Homogeneous and Heterogeneous Catalysts
The performance and reaction conditions of various homogeneous and heterogeneous acid catalysts in the esterification of glycerol with acetic acid is shown below in
Table 7. It can be noted that homogeneous catalysts tend to outperform heterogeneous catalysts in this reaction at relatively lower catalytic loading. Ionic liquid catalysts can provide the benefits of homogeneous catalysis whilst also being reusable and recyclable. The ionic liquid [HSO
3-pmim][HSO
4] was among the most active yielding 95.6 % TA (Reaction conditions: 120 °C, 8:1 acetic acid to glycerol mole ratio, 6.25 mol% catalyst loading, 360 mins). This has shown that ionic liquids can be highly efficient catalysts for this reaction. However there are disadvantages to ionic liquids such as [HSO
3-pmim][HSO
4]. The use of expensive components and multistep synthesis methods limit the industrial use of these catalysts. To overcome these disadvantages the development of more cost-effective acidic ionic liquid catalysts for the reaction should be pursued further. Cost-effective and easily synthesised ionic liquids such as those based upon alkyl pyrrolidone and alkylamine cations with a hydrogen sulphate anion have not yet been explored in the research.
Similarly, heteropolyacids have been shown to be effective catalysts for the reaction. Tin exchanged tungstophosphoric acid (Sn
1.5PW
12O
40) gave 96 % conversion and 40 % selectivity to TA after 180 minutes (Reaction conditions: 70 °C, 12:1 acetic acid to glycerol mole ratio, 0.78 mol% catalyst loading) [
17]. Disadvantages of this catalytic system occur from difficulties in recycling of the catalyst after the reaction. The use of a support has shown to be effective in catalyst heterogenization. Further investigation should focus on the use of more acidic catalyst supports such as K-10 montmorillonite clay. The effect of cost-effective metal ion substitution such as tin, should also be considered to tailor the strength of acid site.
2.2.9. Techno-Economic Assessment and Sensitivity Analysis of Glycerol Esterification
Recently from our group, Keogh et al. investigated the economic feasibility of production of DA and TA via a two-stage process using Aspen Plus
® [
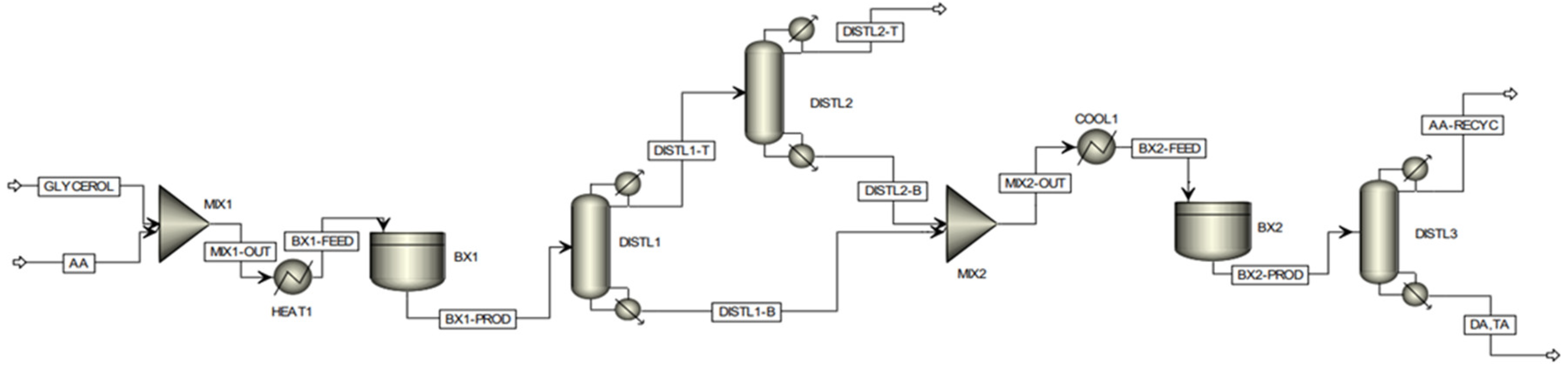
23]. The assessment of the commercial viability of the partial tin exchanged TPA supported on montmorillonite K-10 catalyst at scale was conducted by detailed techno-economic analysis, considering a plant with a fixed annual capacity for processing 100,000 tonnes of crude glycerol. The proposed batch modelling flowsheet of the process is shown in the
Figure 11. Based on the experimental data it was not feasible to achieve complete selectivity to di- and triacetin by using a single batch reactor stage, hence a two-stage reaction process was considered. Following the first batch reactor, the product enters a distillation column, defined as ‘DISTL1′. The purpose of this preliminary column is to remove all water co-generated by the esterification reactions and thus remove the inhibiting presence of water from the reaction medium, which restricts the position of equilibrium. Due to the proximity in boiling points of acetic acid and water, a secondary column, ‘DISTL2′, is required to effectively recover the acetic acid lost in the distillate of the primary column; such acetic acid is recovered efficiently in this column, leaving with high purity within the bottoms stream where it is subsequently utilised in the second stage reaction. The distillate of the secondary distillation column consists of an essentially pure water stream, with only trace quantities of acetic acid, which can subsequently be disposed of safely, posing no threat to the environment. Due to the high acetic acid demand required to assist in driving the position of equilibrium toward the formation of the desired higher esters, an effective acetic acid recovery system is imperative from a sustainability and economic viability perspective. The distillation sequence proposed above was developed considering distillation heuristics for favourable separations and economic operations. Within the second stage batch reaction, occurring within ‘BX2′, the bottoms stream from the primary distillation column, consisting of a mixture of acetin species only, is fed with the recovered acetic acid. Following this second phase reaction, complete selectivity to the desired higher esters (diacetin and triacetin) could be attained, with all glycerol and monoacetin effectively converted. The product stream leaving the secondary batch reactor is fed into a final distillation column, whereby the desired product could be effectively isolated within the bottoms stream with high purity, with the excess acetic acid recovered within the distillate stream, which can be recycled and reused in subsequent batches.
The capital costs were estimated from the Aspen Process Economic Analyzer® software. The analysis indicated the capital costs were 71 M$ while the operating costs were 303 M$/year. The gross profit was 60.5 M$/year and the net present value (NPV) of the project was 235 M$ with a payback period of 1.7 years. Sensitivity analysis indicated that the product price has the most impact on the NPV.
The economic analysis performed by Keogh et al. revealed the process to be highly profitable and thus definitively confirmed the commercial viability of the novel catalyst at an industrial manufacturing scale. The economic analysis has shown that the project could be highly profitable with a NPV of 235M$ for a project lifetime of 20 years. As shown by the sensitivity analysis the project is stable as there are no major price changes predicted in the near future.
3. Conclusions
A variety of acid-catalysts have been shown to facilitate the production of glycerol esters through the esterification of glycerol with acetic acid. The use of acetic acid compared with acetic anhydride offers a more cost-effective and safer reaction pathway. To produce higher selectivity towards di- and triacetin a number of factors need to be considered. Higher selectivity can be facilitated through the use of a higher acetic acid to glycerol mole ratio. From a catalyst design perspective, higher overall catalyst acidity results in better glycerol conversion and higher selectivity. Specifically, for solid catalysts, larger pore sizes facilitate the movement of the bulkier di- and tri- substituted products to and away from the catalyst active sites. From scale up and commercialization perspective, easy availability of catalysts at large scale, high stability, facile recovery, good recyclability, and low cost are key criteria. Based on these criteria, both SO3H-functionalized ionic liquids, for example [H-NMP][HSO4] and supported heteropoly acids, for example tin exchanged TPA supported on montmorillonite K-10 catalysts are potential catalysts, with excellent fit to above catalyst design criteria.
Ionic liquids are exciting homogeneous catalysts, with potential for customisation to tailor the strength of acid sites, and good reusability. The ionic liquids reported for the esterification of glycerol with acetic acid have shown good activity and selectivity. However, the use of expensive components and multistep synthesis methods limit the industrial use of these catalysts. The development of more cost-effective acidic ionic liquid catalysts for the reaction should be pursued further.
Heteropolyacids have shown to be capable catalysts for the reaction. The use of support has been shown to be effective in catalyst heterogenization. Further work in this area should focus on the use of more acidic catalyst supports to increase the overall catalyst acidity. The effect of cost effectivity metal ion substitution to tailor the strength of acid site should also be considered.
The detailed techno-economic assessment and sensitivity analysis have shown the process to be highly profitable and thus assertively confirmed the economic viability of the glycerol esterification process at an industrial manufacturing scale.
Author Contributions
John Keogh: Methodology, formal analysis, data curation, writing—original draft preparation, Nancy Artioli: Methodology, validation, writing—review and editing, Haresh Manyar: Conceptualisation, Methodology, validation, resources, data curation, writing—review and editing, funding acquisition, project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Funding and Acknowledgments
The authors gratefully acknowledge the financial support from “The Bryden centre for advanced marine and bio-energy research” funded through the INTERREG VA Programme for PhD studentship to JK. HM thankfully acknowledges the funding and support provided by the Leverhulme Trust research grant RPG-2020-301, as well as the UK Catalysis hub via our membership of the UK Catalysis Hub Consortium funded by EPSRC grant: EP/R026645/1.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- BP sustainability report 2020. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/sustainability/group-reports/bp-sustainability-report-2020.pdf.
- BP statistical Review of World Energy, 2019. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf.
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Conversion and Management, 2010, 51, 1412-1421. [CrossRef]
- Mishra, V. K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels, 2018, 9, 273-289. [CrossRef]
- Meher, L. C.; Vidya Sagar, D.; Naik, S.N.; Technical aspects of biodiesel production by transesterification—a review. Renewable and Sustainable Energy Reviews, 2006, 10, 248-268. [CrossRef]
- Chemical Book - Glycerol. https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5339206.htm.
- PubChem – Glycerol.
- Morrison, L.R.; Kirk-Othmer Encyclopedia of Chemical Technology, 2000.
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From Glycerol to Value-Added Products. Angewandte Chemie International Edition, 2007, 46, 4434-4440. [CrossRef]
- Ciriminna, R.; Pina, C. D.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. European Journal of Lipid Science and Technology, 2014, 116, 1432-1439. [CrossRef]
- Kong, P. S.; Aroua, M. K.; Daud, W. M. A. W.; Lee, H. V.; Cognet, P.; Pérès, Y. Catalytic role of solid acid catalysts in glycerol acetylation for the production of bio-additives: a review. RSC Advances, 2016, 6, 68885-68905. [CrossRef]
- Chozhavendhan, S.; Praveen Kumar, R.; Elavazhagan, S.; Barathiraja, B.; Jayakumar, M.; Varjani, S. J. in Waste to Wealth, eds. Singhania, R. R.; Agarwal, R. A.; Kumar, R. P.; Sukumaran, R. K. Springer Singapore, 2018, pp. 65-82.
- Hu, S.; Luo, X.; Wan, C.; Li, Y.; Characterization of Crude Glycerol from Biodiesel Plants. Journal of Agricultural and Food Chemistry, 2012, 60, 5915-5921. [CrossRef]
- Inrirai, P.; Keogh, J.; Centeno-Pedrazo , A.; Artioli, N.; Manyar, H. Recent advances in processes and catalysts for glycerol carbonate production via direct and indirect use of CO2, J. of CO2 Utilization 2024, 80, 102693, doi.org/10.1016/j.jcou.2024.102693.
- Keogh, J.; Deshmukh, G.; Manyar, H. Green Synthesis of Glycerol Carbonate via Transesterification of Glycerol Using Mechanochemically Prepared Sodium Aluminate Catalysts. Fuel 2022, 310, 122484. [CrossRef]
- Ralphs, K.; Collins, G.; Manyar, H.; James, S.L.; Hardacre, C. Selective Hydrogenation of Stearic Acid Using Mechanochemically Prepared Titania-Supported Pt and Pt–Re Bimetallic Catalysts. ACS Sustainable Chem. Eng. 2022, 10, 6934–6941. [CrossRef]
- Keogh, J.; Jeffrey, C.; Tiwari, M.S.; Manyar, H. Kinetic Analysis of Glycerol Esterification Using Tin Exchanged Tungstophosphoric Acid on K-10. Ind. Eng. Chem. Res. 2023, 62, 45, 19095–19103. [CrossRef]
- Tiwari, M.S.; Wagh, D.; Dicks, J.S.; Keogh, J.; Ansaldi, M.; Ranade, V.V.; Manyar, H.G. Solvent Free Upgrading of 5-Hydroxymethylfurfural (HMF) with Levulinic Acid to HMF Levulinate Using Tin Exchanged Tungstophosphoric Acid Supported on K-10 Catalyst. ACS Org. Inorg. Au 2023, 3, 27–34. [CrossRef]
- Mazumdar, N.J.; Kumar, P.; Arredondo-Arechavala, M.; Artioli, N.; Manyar, H. Structure sensitivity of Cu supported on manganese oxide catalysts in levulinic acid hydrogenation. Catal. Sci. and Tech., 2023, 14, 4, 840–849. [CrossRef]
- Mazumdar, N.J.; Kumar, P.; Arredondo-Arechavala, M.; Artioli, N.; Manyar, H. Intensifying levulinic acid hydrogenation using mechanochemically prepared copper on manganese oxide catalysts. Chem. Eng. J., 2023, 478, 147479. [CrossRef]
- Manyar, H; Yadav, G.D.; Synthesis of a Novel Redox Material UDCaT-3: An Efficient and Versatile Catalyst for Selective Oxidation, Hydroxylation and Hydrogenation Reactions. Advanced Synthesis and Catalysis 2008, 350, 2286. [CrossRef]
- Skillen, N.; Ralphs, K.; Craig, D.; McCalmont, S.; Muzio, A.F.V.; O’Rourke, C.; Manyar, H.; Robertson, P. Photocatalytic Reforming of Glycerol to H2 in a Thin Film Pt-TiO2 Recirculating Photo Reactor. Journal of Chemical Technology and Biotechnology 2020, 95, 2619–26272620. [CrossRef]
- Pandit, K.; Jeffrey, C.; Keogh, J.; Tiwari, M.S.; Artioli, N.; Manyar, H. Techno-Economic Assessment and Sensitivity Analysis of Glycerol Valorization to Biofuel Additives via Esterification, Ind. Eng. Chem. Res., 2023, 62, 23, 9201–9210. [CrossRef]
- Jakubek, T.; Ralphs, K.; Kotarba, A.; Manyar, H. Nanostructured Potassium-Manganese Oxides Decorated with Pd Nanoparticles as Efficient Catalysts for Low-Temperature Soot Oxidation. Catal Lett 2019, 149, 100–106. [CrossRef]
- Salisu, J.; Gao, N.; Quan, C.; Yanik, J.; Artioli, N. Co-Gasification of Rice Husk and Plastic in the Presence of CaO Using a Novel ANN Model-Incorporated Aspen plus Simulation. Journal of the Energy Institute 2023, 108, 101239. [CrossRef]
- Quan, C.; Zhang, G.; Gao, N.; Su, S.; Artioli, N.; Feng, D. Behavior Study of Migration and Transformation of Heavy Metals during Oily Sludge Pyrolysis. Energy Fuels 2022, 36, 8311–8322. [CrossRef]
- Byrne, E.L.; O’Donnell, R.; Gilmore, M.; Artioli, N.; Holbrey, J.D.; Swadźba-Kwaśny, M. Hydrophobic Functional Liquids Based on Trioctylphosphine Oxide (TOPO) and Carboxylic Acids. Phys. Chem. Chem. Phys. 2020, 22, 24744–24763. [CrossRef]
- Castoldi, L.; Matarrese, R.; Kubiak, L.; Daturi, M.; Artioli, N.; Pompa, S.; Lietti, L. In-Depth Insights into N2O Formation over Rh- and Pt-Based LNT Catalysts. Catalysis Today 2019, 320, 141–151. [CrossRef]
- Yilleng, M.T.; Gimba, E.C.; Ndukwe, G.I.; Bugaje, I.M.; Rooney, D.W.; Manyar, H.G. Batch to Continuous Photocatalytic Degradation of Phenol Using TiO2 and Au-Pd Nanoparticles Supported on TiO2. Journal of Environmental Chemical Engineering 2018, 6, 6382–6389. [CrossRef]
- O’Donnell, R.; Ralphs, K.; Grolleau, M.; Manyar, H.; Artioli, N. Doping Manganese Oxides with Ceria and Ceria Zirconia Using a One-Pot Sol–Gel Method for Low Temperature Diesel Oxidation Catalysts. Top Catal 2020, 63, 351–362. [CrossRef]
- Coney, C.; Hardacre, C.; Morgan, K.; Artioli, N.; York, A.P.E.; Millington, P.; Kolpin, A.; Goguet, A. Investigation of the oxygen storage capacity behaviour of three way catalysts using spatio-temporal analysis. Appl. Catal. B: Environ. 2019, 258, 117918. [CrossRef]
- Lietti, L.; Righini, L.; Castoldi, L.; Artioli, N.; Forzatti, P. Labeled 15NO study on N2 and N2O formation over Pt-Ba/Al2O3 NSR catalysts. Topics in Catalysis 2013, 56, 7-13, doi.org/10.1007/s11244-013-9920-9.
- Shah, S.R.; Mazumdar, N.J.; Centeno-Pedrazo, A.; Artioli, N.; Manyar, H. Recent Advances in Catalyst Design for Carboxylation Using CO2 as the C1 Feedstock. Catalysts, 2023, 13, 12, 1489. [CrossRef]
- Maddaloni, M.; Centeno-Pedrazo, A.; Avanzi, S.; Mazumdar, N.J.; Manyar, H.; Artioli , N.; Novel Ionic Liquid synthesis of highly selective catalysts for the direct hydrogenation of CO2 to short chain hydrocarbons, Catalysts 2023, 13, 12, 1499;. [CrossRef]
- Yilleng, M.T.; Artioli, N.; Rooney, D.; Manyar, H.; Continuous Flow Photocatalytic Degradation of Phenol Using Palladium@Mesoporous TiO2 Core@Shell Nanoparticles, Water, 2023, 15, 16, 2975. [CrossRef]
- Maddaloni, M.; Marchionni, M.; Abbá, A.; Mascia, M.; Tola, V.; Carpanese, M.P.; Bertanza, G.; Artioli, N. Exploring the Viability of Utilizing Treated Wastewater as a Sustainable Water Resource for Green Hydrogen Generation Using Solid Oxide Electrolysis Cells (SOECs), Water, 2023, 15, 14, 2569. [CrossRef]
- Johnson, D. T.; Taconi, K. A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environmental Progress, 2007, 26, 338-348. [CrossRef]
- Casas, A.; Ruiz, J. R.; Ramos, M. J.; Pérez, Á. Effects of Triacetin on Biodiesel Quality Energy & Fuels, 2010, 24, 4481-4489. [CrossRef]
- Triacetin/Glyceral Triacetate Market To Reach USD 255.6 Million By 2026 | Reports And Data. https://www.globenewswire.com/news-release/2019/07/15/1882588/0/en/Triacetin-Glyceral-Triacetate-Market-To-Reach-USD-255-6-Million-By-2026-Reports-And-Data.html#:~:text=According%20to%20the%20current%20analysis,of%20glycerol%20and%20acetic%20acid., (accessed 07th March 2024).
- Ethiraj, J.; Wagh, D.; Manyar, H. Advances in Upgrading Biomass to Biofuels and Oxygenated Fuel Additives Using Metal Oxide Catalysts, Energy & Fuels, 2022, 36, 1189-1204. [CrossRef]
- Konwar, L. J.; Mäki-Arvela, P.; Begum, P.; Kumar, N.; Thakur, A. J.; Mikkola, J.-P.; Deka, R. C.; Deka, D. Shape selectivity and acidity effects in glycerol acetylation with acetic anhydride: Selective synthesis of triacetin over Y-zeolite and sulfonated mesoporous carbons, Journal of Catalysis, 2015, 329, 237-247. [CrossRef]
- Nebel, B.; Mittelbach, M.; Uray, G. Determination of the Composition of Acetylglycerol Mixtures by 1H NMR Followed by GC Investigation. Analytical Chemistry, 2008, 80, 8712-8716. [CrossRef]
- Sun, Y.; Hu, J.; An, S.; Zhang, Q.; Guo, Y.; Song, D.; Shang, Q. Selective esterification of glycerol with acetic acid or lauric acid over rod-like carbon-based sulfonic acid functionalized ionic liquids. Fuel, 2017, 207, 136-145. [CrossRef]
- Huang, M.-Y.; Han, X.-X.; Hung, C.-T.; Lin, J.-C.; Wu, P.-H.; Wu, J.-C.; Liu, S.-B. Heteropolyacid-based ionic liquids as efficient homogeneous catalysts for acetylation of glycerol, Journal of Catalysis, 2014, 320, 42-51. [CrossRef]
- Sun, Z.; Duan, X.; Tao, M.; Wang, X.; Zhou, D. Design of a Highly Efficient Indium-Exchanged Heteropolytungstic Acid for Glycerol Esterification with Acetic Acid. Catalysis Surveys from Asia, 2014, 118, 358-364. [CrossRef]
- Troncea, S. B.; Wuttke, S.; Kemnitz, E.; Coman, S. M.; Parvulescu, V. I. Hydroxylated magnesium fluorides as environmentally friendly catalysts for glycerol acetylation. Applied Catalysis B: Environmental, 2011, 107, 260-267. [CrossRef]
- Gupta, P.; Paul, S. Solid acids: Green alternatives for acid catalysis. Catalysis Today, 2014, 236, 153-170. [CrossRef]
- Deng, Y.; Shi, F.; Beng, J.; Qiao, K. Ionic liquid as a green catalytic reaction medium for esterifications J. of Molecular Catalysis A: Chemical, 2001, 165, 33–36. [CrossRef]
- Da Silva, M. J.; Liberto, N. A.; De Andrade Leles, L. C.; Pereira, U. A. Fe4(SiW12O40)3-catalyzed glycerol acetylation: Synthesis of bioadditives by using highly active Lewis acid catalyst Journal of Molecular Catalysis A: Chemical, 2016, 422, 69-83. [CrossRef]
- Chaves, D. M.; Ferreira, S. O.; Chagas da Silva, R.; Natalino, R.; Da Silva, M. J. Glycerol Esterification over Sn(II)-Exchanged Keggin Heteropoly Salt Catalysts: Effect of Thermal Treatment Temperature. Energy & Fuels, 2019, 33, 7705-7716. [CrossRef]
- Keogh, J.; Tiwari, M. S.; Manyar, H. Esterification of Glycerol with Acetic Acid Using Nitrogen-Based Brønsted-Acidic Ionic Liquids. Ind. Eng. Chem. Res. 2019, 58, 37, 17235–17243. [CrossRef]
- Vafaeezadeh, M.; Alinezhad, H. Brønsted acidic ionic liquids: Green catalysts for essential organic reactions, Journal of Molecular Liquids, 2016, 218, 95-105. [CrossRef]
- Deng, Y.; Shi, F.; Beng, J.; Qiao, K. Ionic liquid as a green catalytic reaction medium for esterifications. Journal of Molecular Catalysis A: Chemical, 2001, 165, 33-36. [CrossRef]
- Li, L.; Yu, S.-T.; Xie, C.-X.; Liu, F.-S.; Li, H.-J. Synthesis of glycerol triacetate using functionalized ionic liquid as catalyst. Journal of Chemical Technology & Biotechnology, 2009, 84, 1649-1652. [CrossRef]
- Liu, X.; Ma, H.; Wu, Y.; Wang, C.; Yang, M.; Yan, P.; Welz-Biermann, U. Esterification of glycerol with acetic acid using double SO3H-functionalized ionic liquids as recoverable catalysts. Green Chemistry, 2011, 13, 697-701. [CrossRef]
- Liu, S.; Wang, A.; Liu, Z.; Li, L.; Yu, S.; Xie, C.; Liu, F. Synthesis of Glycerol Triacetate Using a Brønsted–Lewis Acidic Ionic Liquid as the Catalyst. Journal of the American Oil Chemists’ Society, 2015, 92, 1253-1258. [CrossRef]
- Gonçalves, C. E.; Laier, L. O.; Silva, M. Novel Esterification of Glycerol Catalysed by Tin Chloride (II): A Recyclable and Less Corrosive Process for Production of Bio-Additives. Catalysis Letters, 2011, 141, 1111-1117. [CrossRef]
- Hu, W.; Zhang, Y.; Huang, Y.; Wang, J.; Gao, J.; Xu, J. Selective esterification of glycerol with acetic acid to diacetin using antimony pentoxide as reusable catalyst. Journal of Energy Chemistry, 2015, 24, 632-636. [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy B. M., Production of Biofuel Additives from Esterification and Acetalization of Bioglycerol over SnO2-Based Solid Acids Industrial and Engineering Chemistry Research, 2014, 53, 18775-18785. [CrossRef]
- Reddy, P. S.; Sudarsanam, P.; Raju, G.; Reddy, B. M., Selective acetylation of glycerol over CeO2–M and SO42−/CeO2–M (M = ZrO2 and Al2O3) catalysts for synthesis of bioadditives Journal of Industrial and Engineering Chemistry, 2012, 18, 648-654. [CrossRef]
- Kulkarni, R. M.; Britto, P. J.; Narula, A.; Saqline, S.; Anand, D.; Bhagyalakshmi, C.; Herle, R. N., Kinetic studies on the synthesis of fuel additives from glycerol using CeO2–ZrO2 metal oxide catalyst Biofuel Research Journal, 2020, 7, 1100-1108. [CrossRef]
- Yang, H.; Lu, R.; Zhao, J.; Yang, X.; Shen, L.; Wang, Z., Sulfated binary oxide solid superacids Materials Chemistry and Physics, 2003, 80, 68-72. [CrossRef]
- Reddy, P. S.; Sudarsanam, P.; Raju, G.; Reddy, B. M., Synthesis of bio-additives: Acetylation of glycerol over zirconia-based solid acid catalysts Catalysis Communications, 2010, 11, 1224-1228. [CrossRef]
- Dosuna-Rodríguez, I.; Gaigneaux, E. M., Glycerol acetylation catalysed by ion exchange resins Catalysis Today, 2012, 195, 14-21. [CrossRef]
- Zhou, L.; Al-Zaini, E.; Adesina, A. A., Catalytic characteristics and parameters optimization of the glycerol acetylation over solid acid catalysts Fuel, 2013, 103, 617-625. [CrossRef]
- Kale, S.; Umbarkar, S. B.; Dongare, M. K.; Eckelt, R.; Armbruster, U.; Martin, A., Selective formation of triacetin by glycerol acetylation using acidic ion-exchange resins as catalyst and toluene as an entrainer Applied Catalysis A: General, 2015, 490, 10-16. [CrossRef]
- Wang, Z.-Q.; Zhang, Z.; Yu, W.-J.; Li, L.-D.; Zhang, M.-H.; Zhang, Z.-B., A swelling-changeful catalyst for glycerol acetylation with controlled acid concentration Fuel Processing Technology, 2016, 142, 228-234. [CrossRef]
- Reinoso, D. M.; Tonetto, G. M., Bioadditives synthesis from selective glycerol esterification over acidic ion exchange resin as catalyst Journal of Environmental Chemical Engineering, 2018, 6, 3399-3407. [CrossRef]
- Reinoso, D. M.; Boldrini, D. E., Kinetic study of fuel bio-additive synthesis from glycerol esterification with acetic acid over acid polymeric resin as catalyst Fuel, 2020, 264, 116879. [CrossRef]
- Banu, I.; Bumbac, G.; Bombos, D.; Velea, S.; Gălan, A.-M.; Bozga, G., Glycerol acetylation with acetic acid over Purolite CT-275. Product yields and process kinetics Renewable Energy, 2020, 148, 548-557. [CrossRef]
- Gonçalves, V. L. C.; Pinto, B. P.; Silva, J. C.; Mota, C. J. A., Acetylation of glycerol catalyzed by different solid acids Catalysis Today, 2008, 133-135, 673-677. [CrossRef]
- Popova, M.; Lazarova, H.; Kalvachev, Y.; Todorova, T.; Szegedi, Á.; Shestakova, P.; Mali, G.; Dasireddy, V. D. B. C.; Likozar, B., Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification Catalysis Communications, 2017, 100, 10-14. [CrossRef]
- Gao, X.; Zhu, S.; Li, Y., Graphene oxide as a facile solid acid catalyst for the production of bioadditives from glycerol esterification Catalysis Communications, 2015, 62, 48-51. [CrossRef]
- Gautam, P.; Barman, S.; Ali, A., Catalytic performance of cerium-modified ZSM-5 zeolite as a catalyst for the esterification of glycerol with acetic acid International Journal of Chemical Reactor Engineering, 2020, 18, 9, 20200081. [CrossRef]
- Melero, J. A.; van Grieken, R.; Morales, G.; Paniagua, M., Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol Fuel Energy Fuels, 2007, 21, 1782-1791. [CrossRef]
- Hasan, Z.; Yoon, J. W.; Jhung, S. H., Esterification and acetylation reactions over in situ synthesized mesoporous sulfonated silica Chemical Engineering Journal, 2015, 278, 105-112. [CrossRef]
- Trejda, M.; Stawicka, K.; Dubinska, A.; Ziolek, M., Development of niobium containing acidic catalysts for glycerol esterification Catalysis Today, 2012, 187, 129-134. [CrossRef]
- Khayoon, M. S.; Triwahyono, S.; Hameed, B. H.; Jalil, A. A., Improved production of fuel oxygenates via glycerol acetylation with acetic acid Chemical Engineering Journal, 2014, 243, 473-484. [CrossRef]
- Stawicka, K.; Trejda, M.; Ziolek, M., The production of biofuels additives on sulphonated MCF materials modified with Nb and Ta—Towards efficient solid catalysts of esterification Applied Catalysis A: General, 2013, 467, 325-334. [CrossRef]
- Zhu, S.; Zhu, Y.; Gao, X.; Mo, T.; Zhu, Y.; Li, Y., Production of bioadditives from glycerol esterification over zirconia supported heteropolyacids Bioresource Technology, 2013, 130, 45-51. [CrossRef]
- Zhu, S.; Zhu, Y.; Hao, S.; Chen, L.; Zhang, B.; Li, Y., Aqueous-Phase Hydrogenolysis of Glycerol to 1,3-propanediol Over Pt-H4SiW12O40/SiO2 Catalysis Letters, 2012, 142, 267-274. [CrossRef]
- Jagadeeswaraiah, K.; Balaraju, M.; Prasad, P. S. S.; Lingaiah, N., Selective esterification of glycerol to bioadditives over heteropoly tungstate supported on Cs-containing zirconia catalysts Applied Catalysis A: General, 2010, 386, 166-170. [CrossRef]
- Balaraju, M.; Nikhitha, P.; Jagadeeswaraiah, K.; Srilatha, K.; Sai Prasad, P. S.; Lingaiah, N., Acetylation of glycerol to synthesize bioadditives over niobic acid supported tungstophosphoric acid catalysts Fuel Processing Technology, 2010, 91, 249-253. [CrossRef]
- Ferreira, P.; Fonseca, I. M.; Ramos, A. M.; Vital, J.; Castanheiro, J. E., Glycerol acetylation over dodecatungstophosphoric acid immobilized into a silica matrix as catalyst Applied Catalysis B: Environmental, 2009, 91, 416-422. [CrossRef]
- Khayoon, M. S.; Hameed, B. H., Synthesis of hybrid SBA-15 functionalized with molybdophosphoric acid as efficient catalyst for glycerol esterification to fuel additives Applied Catalysis A: General, 2012, 433-434, 152-161. [CrossRef]
- Magar, S.; Mohanraj, G. T.; Jana, S. K.; Rode, C. V., Synthesis and characterization of supported heteropoly acid: Efficient solid acid catalyst for glycerol esterification to produce biofuel additives Inorganic and Nano-Metal Chemistry, 2020, 50, 1157-1165. [CrossRef]
- Ferreira, P.; Fonseca, I. M.; Ramos, A. M.; Vital, J.; Castanheiro, J. E., Esterification of glycerol with acetic acid over dodecamolybdophosphoric acid encaged in USY zeolite Catalysis Communications, 2009, 10, 481-484. [CrossRef]
- Ferreira, P.; Fonseca, I. M.; Ramos, A. M.; Vital, J.; Castanheiro, J. E., Acetylation of glycerol over heteropolyacids supported on activated carbon Catalysis Communications, 2011, 12, 573-576. [CrossRef]
- Zhu, S.; Gao, X.; Dong, F.; Zhu, Y.; Zheng, H.; Li, Y., Design of a highly active silver-exchanged phosphotungstic acid catalyst for glycerol esterification with acetic acid Journal of Catalysis, 2013, 306, 155-163. [CrossRef]
- Sandesh, S.; Manjunathan, P.; Halgeri, A. B.; Shanbhag, G. V., Glycerol acetins: fuel additive synthesis by acetylation and esterification of glycerol using cesium phosphotungstate catalyst RSC Advances, 2015, 5, 104354-104362. [CrossRef]
- Sun, Z.; Duan, X.; Tao, M.; Wang, X.; Zhou, D., Design of a Highly Efficient Indium-Exchanged Heteropolytungstic Acid for Glycerol Esterification with Acetic Acid Catalysis Surveys from Asia, 2016, 20, 82-90. [CrossRef]
- Sánchez, J. A.; Hernández, D. L.; Moreno, J. A.; Mondragón, F.; Fernández, J. J., Alternative carbon based acid catalyst for selective esterification of glycerol to acetylglycerols Applied Catalysis A: General, 2011, 405, 55-60. [CrossRef]
- Tao, M.-L.; Guan, H.-Y.; Wang, X.-H.; Liu, Y.-C.; Louh, R.-F., Fabrication of sulfonated carbon catalyst from biomass waste and its use for glycerol esterification Fuel Processing Technology, 2015, 138, 355-360. [CrossRef]
- Khayoon, M. S.; Hameed, B. H., Acetylation of glycerol to biofuel additives over sulfated activated carbon catalyst Bioresource Technology, 2011, 102, 9229-9235. [CrossRef]
- Okoye, P. U.; Abdullah, A. Z.; Hameed, B. H., Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst Fuel, 2017, 209, 538-544. [CrossRef]
- Malaika, A.; Kozłowski, M., Glycerol conversion towards valuable fuel blending compounds with the assistance of SO3H-functionalized carbon xerogels and spheres Fuel Processing Technology, 2019, 184, 19-26. [CrossRef]
- Tangestanifard, M.; Ghaziaskar, H. S., Arenesulfonic Acid-Functionalized Bentonite as Catalyst in Glycerol Esterification with Acetic Acid Catalysts 2017, 7, 7, 211; [CrossRef]
- Jiang, Y.; Li, X.; Zhao, H.; Hou, Z., Esterification of glycerol with acetic acid over SO3H-functionalized phenolic resin Fuel, 2019, 255, 115842. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).