1. Introduction
The first attempts of posterior lumbar interbody fusion can be traced back to the 1940s, where their performance was marked by animosity due to high rates of failure. Initially, in the early 1940s, Ralph Cloward, during an open discectomy, observed a residual large void in the disc space after excision, and it occurred to him that this void should be filled with bone. However, he abandoned that idea since the patient died postoperatively due to pulmonary embolism [
1]. A large group of successful spine surgeons, a couple of years later, made new attempts at posterior interbody fusion due to lasting postoperative low back pain. Nevertheless, despite clinical success, the outcomes were still poor, as this method did not offer higher fusion rates compared to other available methods, while holding a higher risk of neurological impairment and blood loss [
2]. This concept persisted until the mid-1980s when Cloward reported fusion rates of over 92% for the same procedure [
3]. Nowadays, the use of interbody fusion involves a much more complicated consideration of factors such as global alignment, disc height, fusion rates, and operative risk, all combined at once [
4]. Despite the limitations of the posterior approach, expandable cages can be inserted through a minimal anatomical corridor while offering a significant amount of lordosis [
5]. Their biomechanical profile consists of expansion in a single plane to lengthen the anterior column, increase the foraminal space, and decrease the risk of endplate breach [
6]. Our study aims to examine all these effects using Flarehawk 9 as a means of interbody fusion.
2. Materials and Methods
2.1. Patients
A total of 58 patients underwent open posterior lumbar surgery using Flarehawk 9 between September 2021 and February 2023 at KAT General Hospital of Athens. All the patients were operated by the same surgical team. The mean age was 59.8 years old ( range between 33 and 79 years old), 36 of them are males while 22 of them are females. The diagnostic groups included spinal canal stenosis (n=40), failed back surgery syndrome-revision surgery (n=5),spondylolisthesis with slip percentage 25% (n=4),recurrent herniated disc (n=7) and adjacent segment disease (n=2). The fusion levels varied depending on the extent of the pathology but it strictly involved the lumbar spine. Sixteen patients had a history of previous spine surgery. Patients with a history of previous spine infection were excluded from this study.Ethical approval was obtained from both the scientific committee and the spinal surgery unit at the hospital where the study took place. Furthermore, a consent form was signed by all the participants in the study.
2.2. Imagistic Assessment
The imagistic assessment included postoperative standing x-rays and CT of the lumbar spine to evaluate the bone fusion at one year of follow-up. Simple standing x-rays were used to measure and compare the preoperative with the postoperative amount of lordosis added. The terms complete union, delayed union or absence of union were used to determine the quality of bone fusion. In our study we considered delayed union in any asymptomatic or symptomatic patient who did not present 360o fusion at 10 month follow-up. The follow up included 96.5% as 2 patients due to health-related problems could not undergo CT scan.
2.3. Clinical Outcome
The clinical outcome was assessed by means of Oswestry Disability Index (ODI). This is a patient-completed questionnaire regarding low back pain which provides a subjective percentage score indicating the level of function or disability in 10 daily routine activities (pain intensity, lifting, sitting, walking, standing, sleeping, personal care, social, sex if applicable and travelling). Each item comprises six statements, scored on a scale from 0 to 5, where 0 represents minimal disability and 5 represents severe disability. The total score is then calculated as a percentage, ranging from 0% denoting no disability to 100% indicating the utmost level of disability.
2.4. Statistical Analysis
All patients included in the study completed the SRS outcome survey, either during their latest follow-up appointment or remotely. Analysis of the survey results was conducted using IBM SPSS Statistics v12.0.1 (IBM Corp., Armonk, NY). This analysis included descriptive statistics, such as frequencies for categorical and ordinal variables, and measures like means, percentages and ranges for continuous variables calculated for each group separately and not totally. Additionally, independent t-tests were utilised for univariate analyses and determined that the sample was adequate for this study, with statistical significance set at a p-value ≤0.05. This study has several limitations such as a bigger sample size would be more reliable especially in certain diagnostic group categories as the percentages are calculated separately for each group (n) and the fact that the ODI results are self-reported.
3. Results
3.1. Imagistic Outcomes
The imagistic outcomes are demonstrated in
Table 1. The table categorises patients into different diagnostic groups, including spinal canal stenosis, failed back surgery syndrome (FBSS), recurrent herniated disc, spondylolisthesis, and adjacent segment disease where 5.2% of total cases presented with delayed union while only 1.7% presented with absence of union at the moment of follow-up.Patients are categorised based on whether they had previous surgery or not, and the percentages indicates that 12.5% of patients with a history of spine surgery presented with delayed union while a 6.25% with absence of union. Only 2.3% in the group without previous surgery presented with delayed union while at the same time the percentage of absence of union was 0%. Finally increased age and the number of levels fused seem to have an impact on the time of complete union. The rate and amount of cage subsidence or migration did not affect the bone fusion in our study group.
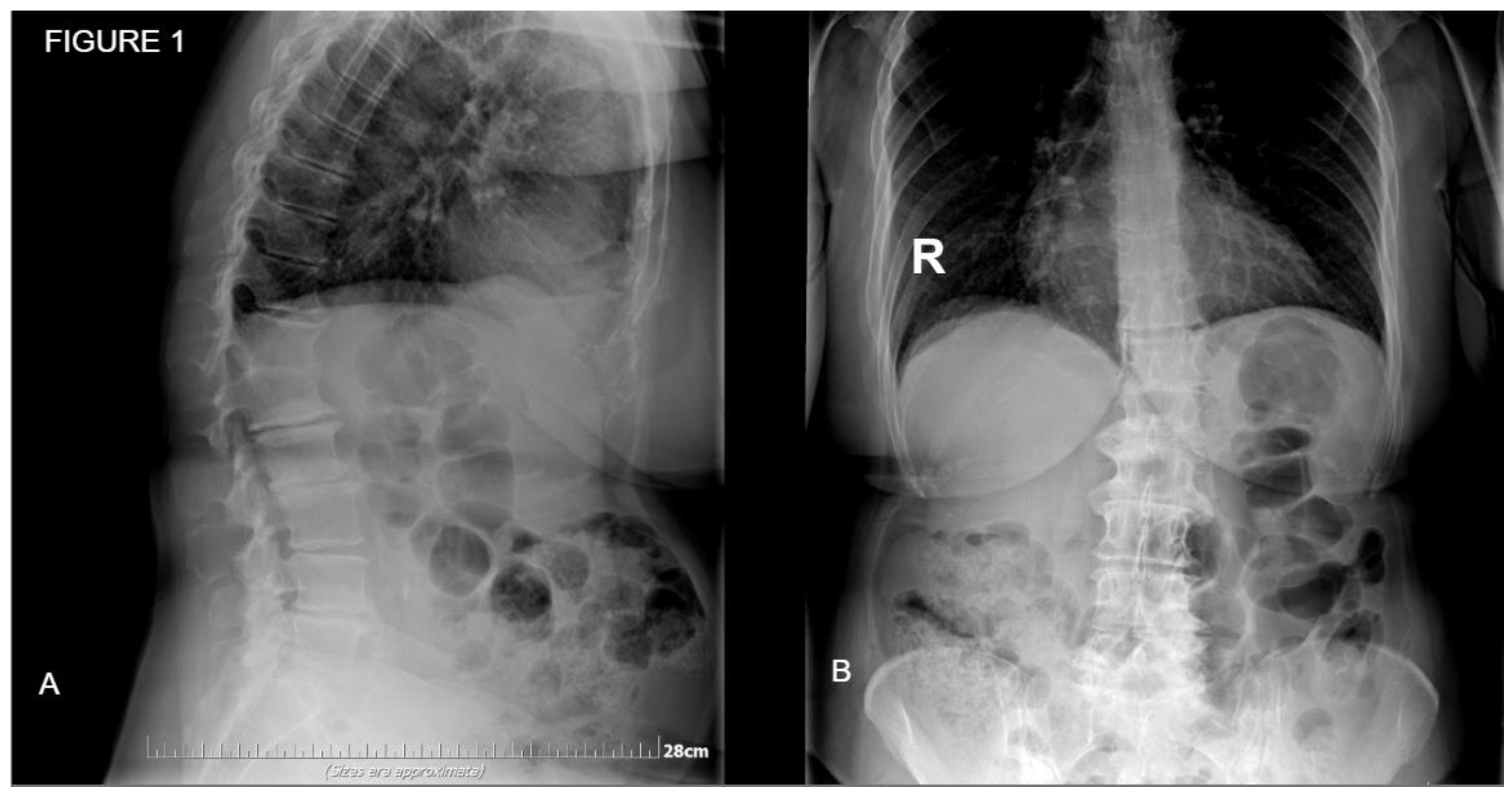
Figure 1.
Preoperative x-ray of a 58 y.o. patient. A: Sagittal plane showing multilevel degenerative disc disease B: Coronal plane showing slight lateral bending.
Figure 1.
Preoperative x-ray of a 58 y.o. patient. A: Sagittal plane showing multilevel degenerative disc disease B: Coronal plane showing slight lateral bending.
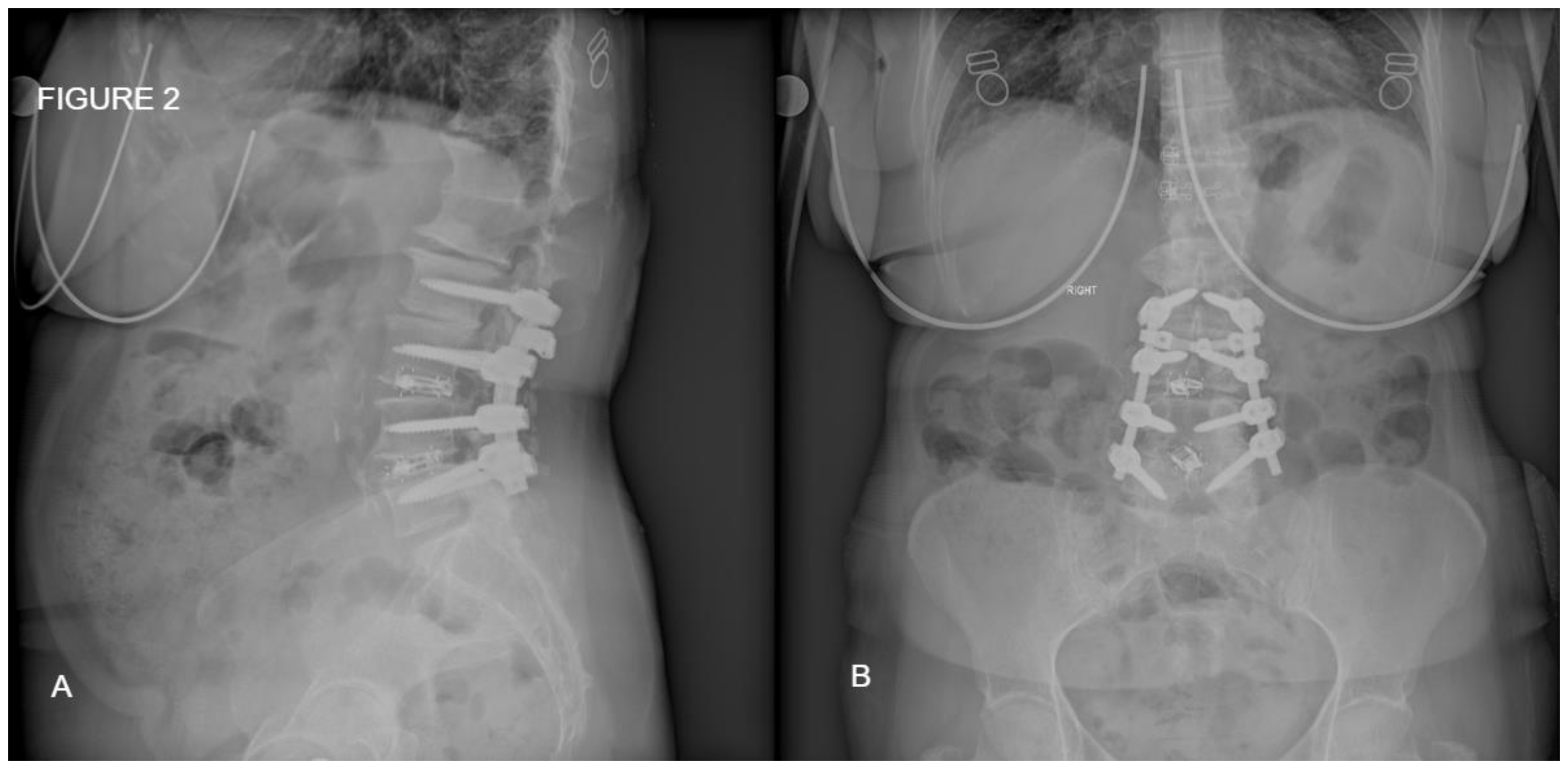
Figure 2.
Postoperative standing x-ray of the same patient from
Figure 1. A: Sagittal place demonstrating satisfactory amount of lordosis and disc height. B: Coronal plane of the posterior fixation and interbody fusion.
Figure 2.
Postoperative standing x-ray of the same patient from
Figure 1. A: Sagittal place demonstrating satisfactory amount of lordosis and disc height. B: Coronal plane of the posterior fixation and interbody fusion.
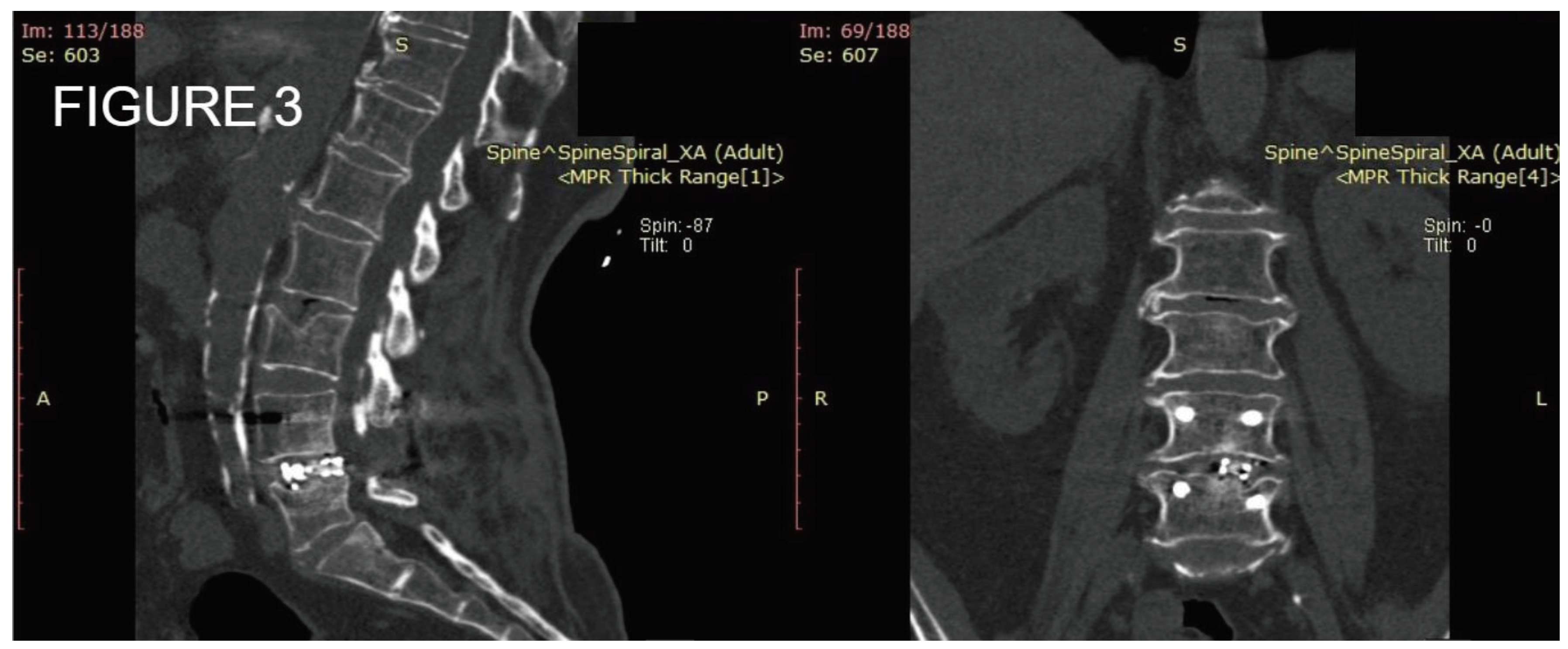
Figure 3.
Postoperative CT scan of one level posterior fixation with interbody fusion after one year of follow up demonstrating satisfactory bone formation with bone union.
Figure 3.
Postoperative CT scan of one level posterior fixation with interbody fusion after one year of follow up demonstrating satisfactory bone formation with bone union.
3.2. Clinical Outcome
The clinical outcomes are demonstrated based on ODI in
Table 2. Significant improvement after spinal surgery can be observed in all ranges of the ODI index. Upon comparing all the percentages, the highest rate of least improvement can be seen within the range of 51-60%, where the number of patients is larger. In this group, we had 2 patients with surgical site infection who underwent successful irrigation and debridement followed by intravenous and oral antibiotics. Additionally, in the range of 61-70%, we had one patient with FBSS, in whom at the one-year follow-up with CT scan, union was incomplete, but the patient was asymptomatic.
4. Discussion
The alignment of the spinopelvic region following lumbar fusion significantly affects the long-term outcomes. In recent years,there has been great focus on achieving optimal fusion angles, even in single-segment fusions, as a crucial aspect of surgical planning to correct or maintain an optimal sagittal and coronal plane [
7].The reasons for realignment following lumbar fusion surgery can be broadly divided into the impacts of decompression and the correction of segmental alignment at the fused segment [
8]. Certain studies have recorded reactive lumbar and overall sagittal improvement following decompression without fusion when discussing decompression effects.[
9]. Particularly in individuals with degenerative lumbar stenosis, protective mechanisms such as anterior displacement of the C-7 plumb line and loss of lordosis occur to mitigate neurological symptoms such as spinal canal stenosis where the forward body bending can increase the canal space and ameliorate the symptoms [
10].However correction with fusion alone most of the times can fail or prove challenging, Gödde et al. in a retrospective radiographic evaluation of 42 patients who underwent short-segment fusion reported that the impact of posterior lumbar interbody fusion in the restoration of spinal alignment was positive [
11].Additionally Hong et al in a retrospective study of 67 patients reported that more lordotic cages are more likely to maintained the amount of correction given intraoperatively without subsidence [
12].
Lumbar interbody fusion (LIF) typically requires bone grafting to stimulate bony fusion and the insertion of a lordotic cage to maintain the disc height and increase the foraminal space[
13]. The attainment of a solid bony fusion within the disc space hinges on successful osteogenesis in the empty disc space[
14]. An early sufficient volume of osteogenesis can stabilise the lumbar segment and prevent a potential cage migration and endplate breach[
15]. However, some patients may lack the required biologic mechanisms due to comorbidities to create bone tissue to bridge the disc space, thereby increasing the risk of nonunion and implant failure[
16]. Various approaches have been utilised for LIF, including anterior (A), oblique (O), lateral (X), transforaminal (T), and posterior (P) approaches.
M.K. Manzur et al. performed a systematic review to evaluate the rate of fusion for stand alone ALIF and they reported that anterior approach offers high rates of fusion but still there are cases of pseudarthrosis especially in the smoking population [
17]. Tanaka et al in a retrospective cohort study of 54 patients where he compared L5-S1 OLIF vs L5-S1 TLIF for adult spinal deformity, reported that the clinical outcomes were similar but OLIF created more lordosis [
18]. Additionally Aono et al in a retrospective study of 48 patients reported that the fusion rates of a two-level PLIF was 85% while all incidents of delayed union or non-union was at the caudal level [
19].
Various studies have analysed and compared the preoperative and postoperative functional status of the patients who underwent lumbar interbody fusion [
20]. Marques et al in a recent retrospective study of 33 patients who underwent posterior lumbar interbody fusion reported that patients who have a poorer score based on ODI scale preoperatively the more they are likely to benefit from a surgical operation [
21]. On the contrary,
Abduljabbar FH et al reported that there is no correlation between ODI and the preoperative status [
22]
. Since revision surgeries are often demanding, in the hands of inexperienced spine surgeons can sometimes yield poor outcomes. Montenegro TS et al studied the clinical outcomes in revision lumbar surgery [
23]
.The findings from this study on a prospective quality demonstrate that primary lumbar fusions yield superior outcomes compared to revision surgeries. Nevertheless, revisions performed in accordance with evidence-based medicine (EBM) guidelines showed greater changes in ODI scores, suggesting that adhering to specific EBM criteria for reoperations can reinforce the clinical outcomes of revision lumbar fusions.In our study indeed statistically the rates of delayed union or non-union were higher in the revision surgery group. Moreover, 100% of the patients who did not present with bone union in the CT scan were above 60 years old.Finally our study is also in accord with the findings of Aono et al since 75% of the patients with delayed or no-union had more than 2 levels fused.
5. Conclusions
Even though anterior approaches become more and more popular and advanced nowadays, posterior fusion with interbody cage still remains a safe and effective method to treat various spine surgical pathologies with optimal results. Posterior lumbar interbody fusion may be inferior comparing to anterior techniques in deformity cases due to the decreased amount of lordosis it offers but it still remains a golden tool for degeneratives cases.
Author Contributions
Conceptualization, Κ.A. and K.Z.; methodology, K.D.; software, P.I.; validation, C.I, and K.A.; formal analysis, K.Z..; investigation, M.S.; resources, P.I.; data curation, C.I..; writing—original draft preparation, K.Z.; writing—review and editing, K.Z.; visualization, M.S.; supervision, K.D.; project administration, K.A.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of KAT General Hospital of Athens.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All data are available upon reasonable request.
Acknowledgments
This study was not supported by any group or any means.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cloward, RB. History of PLIF: Forty years of personal experience. In: Lin PM, editor. Posterior lumbar interbody fusion. Springfield: Charles C Thomas; 1982. p. 58–70.
- Lin PM, editor. Posterior lumbar interbody fusion. Springfield: Charles C Thomas; 1982.
- Cloward, R.B. Posterior lumbar interbody fusion updated. Clin Orthop Relat Res 1985, 16–9. [Google Scholar] [CrossRef]
- Hey, H.W.; Hee, H.T. Lumbar degenerative spinal deformity: Surgical options of PLIF, TLIF and MI-TLIF. Indian J Orthop. 2010, 44, 159–62. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U.; Ferrara, L.; Cheng, B. Expandable Interbody Fusion Cages: An Editorial on the Surgeon's Perspective on Recent Technological Advances and Their Biomechanical Implications. Int J Spine Surg. 2020, 14, S56–S62. [Google Scholar] [CrossRef] [PubMed]
- Derman, P.B.; Yusufbekov, R.; Braaksma, B. Device profile of the FlareHawk interbody fusion system, an endplate-conforming multi-planar expandable lumbar interbody fusion cage. Expert Review of Medical Devices 2023, 20, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okuda, S.; Maeno, T.; et al. Spinopelvic sagittal imbalance as a risk factor for adjacent-segment disease after single-segment posterior lumbar interbody fusion. J Neurosurg Spine 2017, 26, 435–40. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okuda, S.; Nagamoto, Y.; Takahashi, Y.; Furuya, M.; Iwasaki, M. Spinopelvic sagittal realignment and incidence of adjacent segment disease after single-segment posterior lumbar inter-body fusion using 12° lordotic cages-a 2-year prospective cohort study. J Spine Surg. 2023, 9, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Kawamura, N.; Ikegami, M.; et al. Radiological improvements in global sagittal alignment after lumbar decompression without fusion. Spine (Phila Pa 1976) 2015, 40, 703–9. [Google Scholar] [CrossRef] [PubMed]
- Barrey, C.; Jund, J.; Noseda, O. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J 2007, 16, 1459–67. [Google Scholar] [CrossRef]
- Gödde, S.; Fritsch, E.; Dienst, M.; et al. Influence of cage geometry on sagittal alignment in instrumented posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2003, 28, 1693–9. [Google Scholar] [CrossRef]
- Hong, T.H.; Cho, K.J.; Kim, Y.T.; et al. Does Lordotic Angle of Cage Determine Lumbar Lordosis in Lumbar Interbody Fusion? Spine (Phila Pa 1976) 2017, 42, E775–80. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, B.; Chen, F.; Dai, Z.; Kang, Y. Effect of Trabecular Microstructure of Spinous Process on Spinal Fusion and Clinical Outcomes After Posterior Lumbar Interbody Fusion: Bone Surface/Total Volume as Independent Favorable Indicator for Fusion Success. World Neurosurg. 2020, 136, e204–e213. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Lee, S.H.; Lee, Y.H.; Bae, S.C.; Suk, K.S. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine (Phila Pa 1976) discussion 1358. 2006, 31, 1351–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.H. Factors associated with intervertebral cage subsidence in posterior lumbar fusion. J Orthop Surg Res. 2024, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Menger, M.M.; Ehnert, S.; Nüssler, A.K.; Histing, T.; Laschke, M.W. Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions-Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors. Bioengineering (Basel) 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Manzur, M.; Virk, S.S.; Jivanelli, B.; Vaishnav, A.S.; McAnany, S.J.; Albert, T.J.; Iyer, S.; Gang, C.H.; Qureshi, S. The rate of fusion for stand-alone anterior lumbar interbody fusion: a systematic review. Spine J. 2019, 19, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sonawane, S.; Meena, U.; Lu, Z.; Fujiwara, Y.; Taoka, T.; Uotani, K.; Oda, Y.; Sakaguchi, T.; Arataki, S. Comparison of C-Arm-Free Oblique Lumbar Interbody Fusion L5-S1 (OLIF51) with Transforaminal Lumbar Interbody Fusion L5-S1 (TLIF51) for Adult Spinal Deformity. Medicina (Kaunas) 2023, 59, 838. [Google Scholar] [CrossRef]
- Aono, H.; Takenaka, S.; Nagamoto, Y.; Tobimatsu, H.; Yamashita, T.; Furuya, M.; Iwasaki, M. Fusion Rate and Clinical Outcomes in Two-Level Posterior Lumbar Interbody Fusion. World Neurosurg. 2018, 112, e473–e478. [Google Scholar] [CrossRef]
- Arnold, P.M.; Robbins, S.; Paullus, W.; Faust, S.; Holt, R.; McGuire, R. Clinical outcomes of lumbar degenerative disc disease treated with posterior lumbar interbody fusion allograft spacer: a prospective, multicenter trial with 2-year follow-up. Am J Orthop (Belle Mead NJ) 2009, 38, E115–22. [Google Scholar] [PubMed]
- Marques, R.; Gomes, S.; Nogueira, J.; Afonso, M.; Duarte, N. Assessment of Functional Outcome Predictors in Patients Undergoing Lumbar Interbody Fusion Surgery: A Single-Centre Analysis. Cureus. 2022, 14, e23529. [Google Scholar] [CrossRef]
- Abduljabbar, F.H.; Makhdom, A.M.; Rajeh, M.; Tales, A.R.; Mathew, J.; Ouellet, J.; Weber, M.; Jarzem, P. Factors Associated With Clinical Outcomes After Lumbar Interbody Fusion With a Porous Nitinol Implant. Global Spine J. 2017, 7, 780–786. [Google Scholar] [CrossRef]
- Montenegro, T.S.; Gonzalez, G.A.; Saiegh, F.A.; Philipp, L.; Hines, K.; Hattar, E.; Franco, D.; Mahtabfar, A.; Keppetipola, K.M.; Leibold, A.; Atallah, E.; Fatema, U.; Thalheimer, S.; Wu, C.; Prasad, S.K.; Jallo, J.; Heller, J.; Sharan, A.; Harrop, J. Clinical outcomes in revision lumbar spine fusions: an observational cohort study. J Neurosurg Spine. 2021, 35, 437–445. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Imagistic and demographic data using Flarehawk 9 as an interbody cage.
Table 1.
Imagistic and demographic data using Flarehawk 9 as an interbody cage.
| Diagnostic Group |
Complete union
N(%) |
Delayed union
N(%) |
Absence of union
N(%) |
P-value |
| Spinal canal stenosis (n=40) |
37(92.5%) |
2(5%) |
1(2.5%) |
<0.001 |
| FBSS (n=5) |
4(80%) |
1(20%) |
0 |
N/S |
| Recurrent herniated disc (n=7) |
7(100%) |
0 |
0 |
N/S |
| Spondylolisthesis (n=4) |
4(100%) |
0 |
0 |
N/S |
| Adjacent segment disease (n=2) |
2(100%) |
0 |
0 |
N/S |
| Previous Surgery |
|
|
|
|
| Yes (n=16) |
13(81.2%) |
2(12.5%) |
1(6.3%) |
<0.001 |
| No (n=42) |
41 |
1 |
0 |
<0.001 |
| Fused levels |
|
|
|
|
| L5-S1 (n=8) |
8(100%) |
0 |
0 |
N/S |
| L4-L5 or L3-L4 (n=14) |
13(92.8%) |
1(7.2%) |
0 |
<0.001 |
| 2 or more fused levels (n=36) |
33(91.6%) |
2(5.5%) |
1(2.9%) |
<0.001 |
| Age |
|
|
|
|
| 33-50 years (n=17) |
17(100%) |
0 |
0 |
<0.001 |
| 51-79 years (n=41) |
37(90.2%) |
3(7.3%) |
1(2.5%) |
<0.001 |
| Sex |
|
|
|
|
| Male (n=36) |
35( 97.2%) |
1(2.8%) |
N/A |
<0.001 |
| Female (n=22) |
19(86.3%) |
2(9%) |
1(4.7%) |
<0.001 |
| Lordosis |
Degrees of lumbar lordosis |
|
| Preoperative |
23±8.6 degrees |
<0.001 |
| Postoperative |
25±9 degrees |
<0.001 |
Table 2.
Comparison of Oswestry disability index domains, before and after spinal surgery.
Table 2.
Comparison of Oswestry disability index domains, before and after spinal surgery.
| Range |
OSWESTRY DISABILITY INDEX |
| PRE-OP |
POST-OP 12 MONTHS |
| N% |
% Improved |
% Unchanged |
%
Worse |
| 0%-10% |
1 (1.7%) |
100% |
0% |
0% |
| 11%-20% |
3 (5.2%) |
100% |
0% |
0% |
| 21%-30% |
2 (3.4%) |
50% |
50% |
0% |
| 31%-40% |
8 (13.8%) |
75% |
12.5% |
12.5% |
| 41%-50% |
17 (29.3%) |
82.3% |
11.7% |
6% |
| 51%-60% |
19 (32.7%) |
78.9% |
10.5% |
10.5% |
| 61%-70% |
4 (6.8%) |
75% |
25% |
0% |
| 71%-80% |
4 (6.8%) |
50% |
25% |
25% |
| 81%-90% |
0 (0) |
N/A |
N/A |
N/A |
| 91%-100% |
0 (0) |
N/A |
N/A |
N/A |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).