2. Materials and Methods
2.1. Generation of Dairy Based Fermentates
Skim Milk Powder (SMP) was used as a substrate for the generation of the fermentates used in this study. SMP was reconstituted at 10% w/v in distilled water to generate Reconstituted Skim Milk (RSM), autoclaved, cooled and stored at 4°C for a maximum of 7 days. An inoculum of frozen mother culture stocks of the individual strains Lb. helveticus SC232 and Lb. helveticus Lafti L10 SC234 (which were previously prepared in 10% w/v RSM) was added to 10% RSM and incubated for 24hrs at 37°C under aerobic conditions without agitation, to generate these individual fermentates derived from the above-mentioned individual strains. From these cultures, a further inoculum was added to 10% w/v RSM and incubated for 24hrs at 37°C under aerobic conditions again without agitation. These fermentates were subjected to a heat treatment step to generate the fermentates which contained one of the heat-killed LAB strains mentioned above. After cooling to room temperature, the pH of the fermentates was neutralized. These fermentates were aliquoted and immediately frozen at -80°C until further analysis. Non-fermented RSM samples subjected to the same heat-treatment mentioned above were used as negative controls for all experiments described herein.
2.2. Cell Culture
J774.A.1 Murine Macrophage, purchased from European Collection of Animal Cell Cultures (Salisbury, UK), were maintained in Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum (FBS), and 1% Penicillin-Streptomycin Antibiotic obtained from Bioscienes (Dublin, Ireland), and incubated at 37oC, with 5% CO2 and 95% humidified air. Cells were passaged every three to four days at a confluence of 80-90%. Cells were sub-cultured at a ratio 1:10. Bone Marrow Derived Macrophage cells (BMDMs), harvested from the bone marrow of a female BALB/c mouse of 6-8 weeks old obtained from Charles River (Margate, UK), were cultured in complete Roswell Park Memorial Institute (RPMI) 1640 medium, containing 25ng/ml rM-CSF (Merck, Haverhill, UK).
2.3. Cell Viability
Cell viability was determined using the CellTiter 96® AQueous One Solution Cell Proliferation Assay and conducted as per manufacturer’s instructions (MyBio, Kilkenny, Ireland). Macrophage were seeded at a concentration of 1 x 106 cells/mL in a flat bottom 96-well plate, incubated for 24 hours at 37°C in a 95% humidified air, and 5% CO2 atmosphere. Cells were treated with 25mg/mL of the fermentate sample for 1 hour and incubated under the same conditions, before stimulation with LOX 0.5mM and, LPS 100ng/mL for 24 hours. DMSO was included as a positive control to induce cell death. After 24 hours, 20μl of the thawed CellTiter96® Aqueous One Solution was added to each well of the 96-well plate, incubated at 37°C for 3 hours in a humidified, 5% CO2 atmosphere. Absorbance was read at 490 nm using VersamaxTM 96-well plate reader. Cell viability was expressed as the percentage viability of the treatment group relative to the control group.
2.4. Enzyme Linked ImmunoSorbent Assay (ELISA)
Determination of the effects of the fermentate samples, on cytokine and chemokine production in activated macrophage, required harvesting of the cell supernatants, and subsequent analysis using commercial DuoSet ELISA kits (R&D Systems Europe, Abdingdon, Oxon, UK), according to the manufacturer’s instructions. This allowed for the quantification of the cytokines, IL-1β, IL-6, IL-10, TNF-α, IL-12p40, and IL-27, as well as chemokines MCP, MIP-1, and MIP-2.
2.5. Nitric Oxide (NO) and Arginase Assay
NO production was determined by measuring the NO2− in the cell supernantants of the cultured macrophage via a Griess assay, carried out as per manufacturer’s instructions (MyBio, Kilkenny, Ireland). Cell lysates were prepared and analysed for arginase activity, via the proportional detection of urea, a direct result of arginase catalysing the conversion of arginine to urea and ornithine, using commercial kit and followed as per manufacteres instructions (Merck, Haverhill, UK). BMDM cells were seeded at a concentration of 5 × 105 cells per well in 24-well plates and incubated for 30 minutes at 37°C. BMDMs were stimulated with 25mg/mL fermentates for 3 hours and incubated under the same conditions. BMDM cells were polarised towards M1 macrophages by adding LPS (100 ng/mL) in the presence of 20 ng/ml rIFN-γ or towards M2 cells by adding 20 ng/ml rIL-4, 20 ng/mL IL-13, and 20 ng/ml rTGF-B and incubated for 24 hours at 37°C. After 24 hours, supernantant was harvested and Griess assay was carried out to quantify the NO2− present, while an Arginase assay was carried out to determine the arginase activity within the cell.
2.6. Cell Surface Marker Expression Analysis
The determination of cell surface markers present on J774.A.1 macrophage, was carried out via cell surface marker staining using fluorescently labelled antibodies FITC, APC, and PE. J774.A.1 macrophage were seeded at a concentration of 1 x 106 cells/mL in a 6 well plate, stimulated with appropriate treatments and incubated at 37oC, with 5% CO2 and 95% humidified air. Cells were blocked with FBS for 15 minutes, before being harvested via centrifugation at 2,000rpm for 5 minutes at 4oC. Cells were resuspended in FACS buffer. Cell suspension was plated in a 96 well round bottom plate and centrifuged. Supernatant was aspirated, and cells resuspended in 1:1000 dilutions of antibodies (FITC, APC, PE) and incubated for 30 minutes at 4oC. Cells were centrifuged and washed 3 times in FACS buffer. Cells were resuspended in FACS buffer and transferred to FACS loading tubes. Cells were analysed using BD FACSAria 1 system flow cytometer. Raw FCS files were analysed, and data was graphed using V10.0 FlowJo software. Cell surface marker expression was determined for cell surface markers MHCI, MHCII, TLR2, TLR4, CD40, CD14, CD80, and CD86.
2.7. Phagocytosis Assay
J774A.1 macrophages were seeded at 1x106 cells/mL in 6 well plates and incubated overnight at 37°C in a humidified, 5% CO2 atmosphere. Cells were stimulated with sample for 1 hour, incubated under the same conditions. Subsequently cells were stimulated with 100ng/mL LPS and LOX for 4 hours. Cells were incubated with 1µm fluorescent latex beads (Merck, Haverhill, UK) at a concentration of 20 beads per cell, for 1 hour at 37°C in a humidified, 5% CO2 atmosphere. Cells were scraped from the cell culture plate and pelleted via centrifugation at 4oC at 2,000rpm for 5mins. Cells were resuspended and washed twice in 1mL FACs buffer via centrifugation. Cells were resuspended in FACs buffer and added to FACs tubes. The uptake of beads was measured by flow cytometry on a FACSAria™ flow cytometer. Data was analysed using FlowJo software (Treestar). MFI and percentage phagocytosing cells were the two outputs measured.
Data was represented as the MPI. This is a representation to incorporate the MFI from the phagocytosed beads as well as the percentage of phagocytosing cells in the viable population of cells and comparing to the control, which represents baseline phagocytosis. The MPI is calculated as follows:
2.8. Statistical Analysis
Statistical analysis was carried out using a one-way ANOVA to compare variance among the means of different sample groups. A Newman-Keuls post-test was used to determine significance among the samples. The level of statistical significance was indicated by * (p<0.05), ** (p<0.01), and *** (p<0.001).
2.9. Ethical Statement
The care, treatment, and experiments involved in this study were approved by the Research Ethics Committee (REC), of Dublin City University (Approval ID: DCUREC/2011/008).
3. Results
3.1. Immune Boosting Effects of Fermentates on Cytokine Secretion
An MTS assay confirmed that the fermentates SC232 and SC234 in the presence/absence of LOX or LPS had no effect on the viability of either J774.A.1 cells and BMDMs (data not shown).
Initially, an ELISA was performed on the cell line J774.A.1 macrophage to assess bioactivity of fermentates SC232 and SC234 in the presence/absence of LOX or LPS. The novel fermentates altered the secretion of cytokines in response to LOX and LPS when compared to the respective controls in J774.A.1s (data not shown).
In order to confirm the effects of our fermentates in macrophages, we assessed their effects in primary cells. SC234 and SC232 significantly affected the secretion of cytokines in response to LOX and LPS when compared to the respective controls in BMDMs (
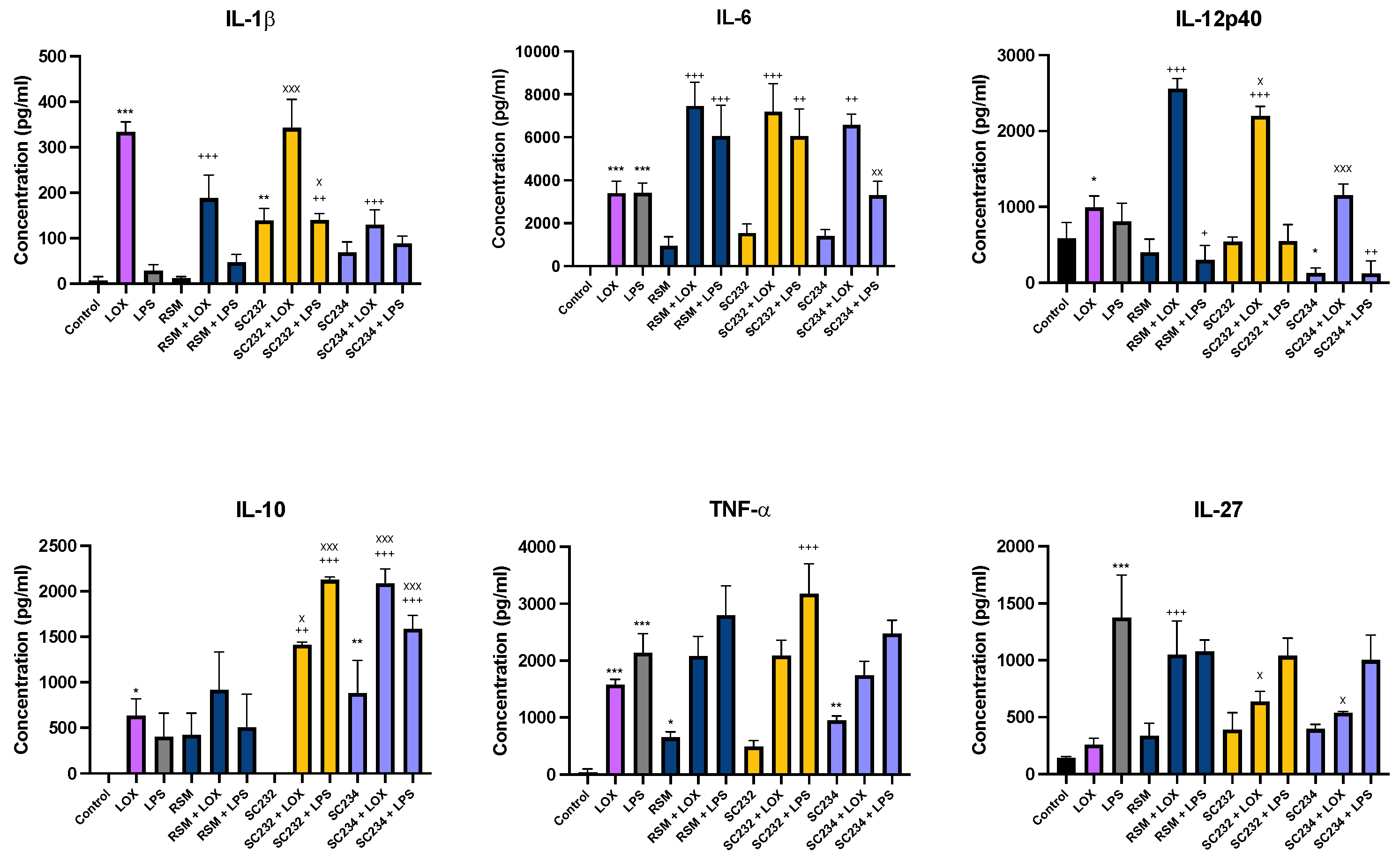
Figure 1). IL-1β (p<0.001), IL-6 (p<0.001), IL-12p40 (p<0.033), IL-10 (p<0.033), and TNF-α (p<0.001) were increased in the presence of LOX with only low levels of IL-27 detected. IL-6 (p<0.001), TNF-α (p<0.001), and IL-27 (p<0.001) were increased in the presence of LPS with only low levels of IL-1β, IL-10, IL-12p40.
In the presence of LOX, SC234 significantly enhanced IL-6, and IL-10 secretion (p<0.002; p<0.001), but decreased IL-1β secretion (p<0.001), with no significant effects on the other cytokines measured when compared to control cells. In contrast SC232 significantly increased IL-6 (p<0.001), IL-12p40 (p<0.001), and IL-10 (p<0.001), with no significant effect on the other cytokines. Exposure of cells to SC234 in the presence of LPS resulted in an increase in IL-10 (p<0.001), but a decrease in IL-12p40 (p<0.002), with no significant effect on the other cytokines. SC232 in the presence of LPS resulted in an increase in IL-1β (p<0.002), IL-6 (p<0.002), IL-10 (p<0.001), and TNF-α (p<0.001) but no change in IL-12p40 or IL-27. Interestingly, exposure of cells to SC234 alone, in the absence of either LOX or LPS stimulation, resulted in enhanced secretion of IL-10 (p<0.001), but decreased IL-12p40 (p<0.033), and TNF-α secretion (p<0.002) and exposure of cells to SC232 alone, resulted in decreased secretion of IL-1β (p<0.002).
The non-fermented RSM, used as a negative control, did affect cytokine secretion in the presence of LOX with an increase in IL-6 (p<0.001), IL-12p40 (p<0.001), and IL-27 (p<0.001), but a decrease in IL-1β (p<0.001). Furthermore, RSM enhanced IL-6 (p<0.001), but decreased IL-12p40 (p<0.033) in the presence of LPS. RSM in the absence of TLR stimulation, increased TNF-α (p<0.033).
Given that RSM itself had some effects, we also compared the fermentates to the RSM. In the absence of TLR stimulation SC232 increases IL-10 (p<0.002). In the presence of LOX SC234 increased IL-10 (p<0.001), but decreased IL-12p40 (p<0.001), and IL-27 (p<0.001), relative to the RSM. In the presence of LOX SC232 increased IL-1β (p<0.001), IL-10 (p<0.033), but decreased IL-12p40 (p<0.033), and IL-27 (p<0.001). In the presence of LPS, SC234 increased IL-10 (p<0.001), but decreased IL-6 (p<0.002), and SC232 increased IL-1β (p<0.033) and IL-10 (p<0.001), relative to the RSM.
Given that the fermentates had clear effects on macrophage, we sought to determine if they could exert specific effects on M1 and M2 polarised macrophage.
Figure 2A shows the cytokine secretion profile of M0, M1, and M2 polarised macrophage. M1 macrophage secrete high levels of IL-6 and TNF-α (p<0.001; p<0.001), while M2 macrophage secrete only small concentrations of IL-6 and TNF-α but secrete higher concentrations of IL-10 compared with the M0 unpolarised macrophage and the M1 polarised macrophage which secrete undetectable levels. The M0 macrophage secrete undetectable levels of IL-6, TNF-α, or IL-10.
SC234 increased IL-6 (p<0.001), IL-10 (p<0.001), and TNF-α (p<0.001), in M0 BMDMs (
Figure 2A-D). M0 BMDMs in the presence of SC232 had increased IL-6 (p<0.002), and IL-10 (p<0.001), relative to the M0 control. In M1 polarised BMDMs, SC234 increased IL-6 (p<0.001) and IL-10 (p<0.001), and TNF-α (p<0.001), and SC232 increased IL-6 (p<0.001), IL-10 (p<0.001), and TNF-α (p<0.002), relative to the M1 control. In M2 polarised BMDMs, SC234 increased IL-10 (p<0.001), and TNF-α (p<0.002) and SC232 increased IL-10 (p<0.001), relative to the M2 control.
3.2. Immune Boosting Effects of Fermentates on Nitric Oxide production and Arginase Activity
Nitric oxide production and arginase activity are classical markers of M1 and M2 macrophage.
Figure 3A exhibits that M1 macrophage secrete high levels of NO (p<0.001), while M0 and M2 macrophage secrete only small concentrations of NO.
Figure 3B shows that M2 macrophage have high levels of arginase activity (p<0.033), while M0 and M1 macrophage have much lower levels of arginase activity.
Figure 3C demonstrates that SC234 and SC232 significantly increased NO production in M0 (p<0.001; p<0.001), M1 (p<0.001; p<0.001), and M2 macrophages (p<0.001; p<0.001). However, it is the M1 BMDMs in the presence of SC234 and SC232 that produce the highest concentration of NO.
Figure 3D exhibits that SC234 and SC232 increased arginase activity in M0 (p<0.002; p<0.033). In M1 BMDMs, only SC234 increases arginase activity (p<0.033), and in M2 BMDMs there is no significant effect.
3.3. Immune Boosting Effects of Fermentates on Chemokine Secretion
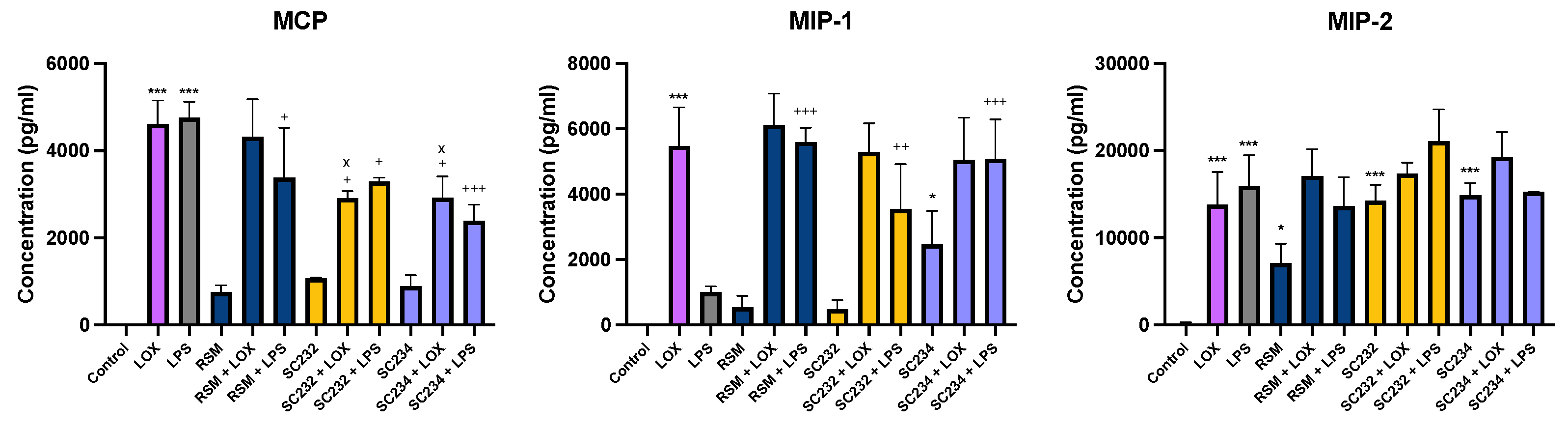
Figure 4 exhibits that our novel fermentates significantly affected the secretion of chemokines in response to LOX and LPS in BMDMs. MCP (p<0.001), MIP-1 (p<0.001) and MIP-2 (p<0.001) were increased in the presence of LOX, relative to LOX control.. MCP (p<0.001), and MIP-2 (p<0.001) were increased in the presence of LPS, with only a small increase seen in MIP-1, relative to LPS control.
In the presence of LOX, SC234 and SC232 significantly decreased MCP (p<0.033), Exposure of cells to SC234 in the presence of LPS resulted in an increase in MIP-1 (p<0.001), but a decrease in MCP (p<0.001), relative to LOX control. Similarly, SC232 in the presence of LPS resulted in an increase in MIP-1 (p<0.002), but a decrease in MCP (p<0.033), relative to LPS control. Interestingly, exposure of cells to SC234 alone, in the absence of either LOX or LPS stimulation, resulted in enhanced secretion of MIP-1 (p<0.033) and MIP-2 (p<0.001), relative to control cells. Exposure of cells to SC232 alone, in the absence of either LOX or LPS stimulation, resulted in increased secretion of MIP-2 (p<0.001), relative to control cells.
The RSM control itself did affect chemokine secretion in the presence of LPS with an increase in MIP-1 (p<0.001), but decreased MCP (p<0.033), relative to LPS control. Furthermore, RSM in the absence of TLR stimulation enhanced MIP-2 (p<0.033), relative to the control cells.
Given that RSM itself had some effects, we also compared the fermentates to RSM. In the presence of LOX, SC234 and SC232 decreased MCP (p<0.033).
3.4. Immune Boosting Effects of Fermentates on Cell Surface Marker Expression
Figure 5 exhibits that fermentates significantly affected the expression of cell surface markers in response to LOX and LPS. LOX significantly increased the expression of CD86, CD14, CD40, TLR4 and CD80 (p<0.001), and LPS significantly increased the expression of CD86, CD14, CD40, TLR4, CD80 and MHCI (p<0.001).
In the presence of LOX, SC234 further enhanced the expression of MHCII (p<0.033), CD86 (p<0.001), TLR2 (p<0.001), MHCI (p<0.001), CD14 (p<0.001), CD40 (p<0.001), and CD80 (p<0.001). In the presence of LPS, SC234 further enhanced the expression of MHCII (p<0.033), TLR4 (p<0.033), CD86 (p<0.001), TLR2 (p<0.001), CD14 (p<0.001), CD40 (p<0.001), and CD80 (p<0.001). In the absence of TLR SC234 increased MHCII (p<0.002), CD86 (p<0.001), CD14 (p<0.001), CD40 (p<0.001), CD80 (p<0.001), TLR2 (p<0.001), and MHCI (p<0.001).
In the presence of LOX, SC232 increased MHCII (p<0.033) and TLR4 (p<0.033) expression, but decreased CD14 (p<0.002), CD40 (p<0.033), and MHCI (p<0.002) expression.
In the presence of LPS, R00352further enhanced the expression of MHCII (p<0.001), TLR4 (p<0.001), CD14 (p<0.001), CD86 (p<0.001), CD80 (p<0.001), TLR2 (p<0.001), CD40 (p<0.001), and MHCI (p<0.033). In the absence of TLR, SC232 increased expression of MHCII, CD14, CD80, TLR2, and MHCI (p<0.001), and further enhanced TLR4, CD40, CD86 (p<0.001).
In the presence of LOX, RSM further enhanced the expression of MHCII (p<0.001), TLR4 (p<0.001), CD14 (p<0.001), CD40 (p<0.001), CD86 (p<0.001), CD80 (p<0.001), TLR2 (p<0.001), and MHCI (p<0.001). In the presence of LPS, RSM further enhanced the expression of MHCII (p<0.001), TLR4 (p<0.001), CD14 (p<0.001), CD40 (p<0.002), CD86 (p<0.001), CD80 (p<0.001), and TLR2 (p<0.001). In the absence of TLR RSM increased expression of MHCII, TLR4, CD14, CD40, CD86, CD80, TLR2, and MHCI (p<0.001).
Given that RSM itself had some effects, we also compared the fermentates to the RSM control. In the absence of TLR, SC234 decreased TLR4, and MHCI (p<0.001; p<0.002), but further increased CD14 (p<0.001), CD40 (p<0.001), CD80 (p<0.001), and TLR2 (p<0.033). In the absence of TLR, SC232 decreased TLR4 (p<0.033), and CD86 (p<0.033), but further increased CD40 (p<0.033). In the presence of LOX, SC234 decreased TLR4, CD86, and TLR2 (p<0.001). In the presence of LOX, SC232 decreased TLR4, CD14, CD40, CD86, CD80, TLR2, and MHCI (p<0.001). In the presence of LPS, SC234 decreased TLR4 (p<0.002), and CD86 (p<0.001), but further increased CD40 (p<0.001), CD80 (p<0.002), CD14 (p<0.033). In the presence of LPS, SC232 decreased CD86 (p<0.001), CD40 (p<0.033), and MHCI (p<0.033).
3.5. Stimulation of LOX and LPS Activated J774 with 25mg/ml Fermentates Affect Phagocytosis
The procedure for phenotypic analysis of cell phagocytosis when stimulated with TLR ligands was carried out as previously described using 1µm fluorescent FITC latex beads. MFI, percentage phagocytosing cells and MPI were measured.
MFI:
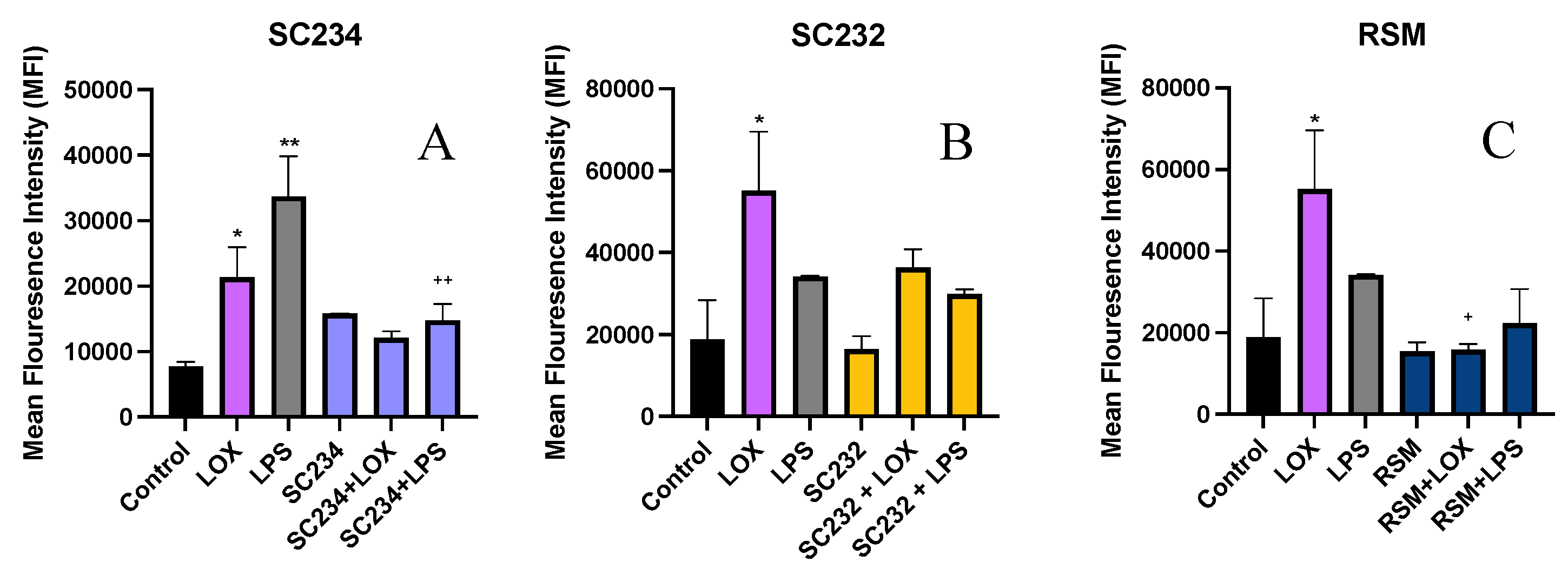
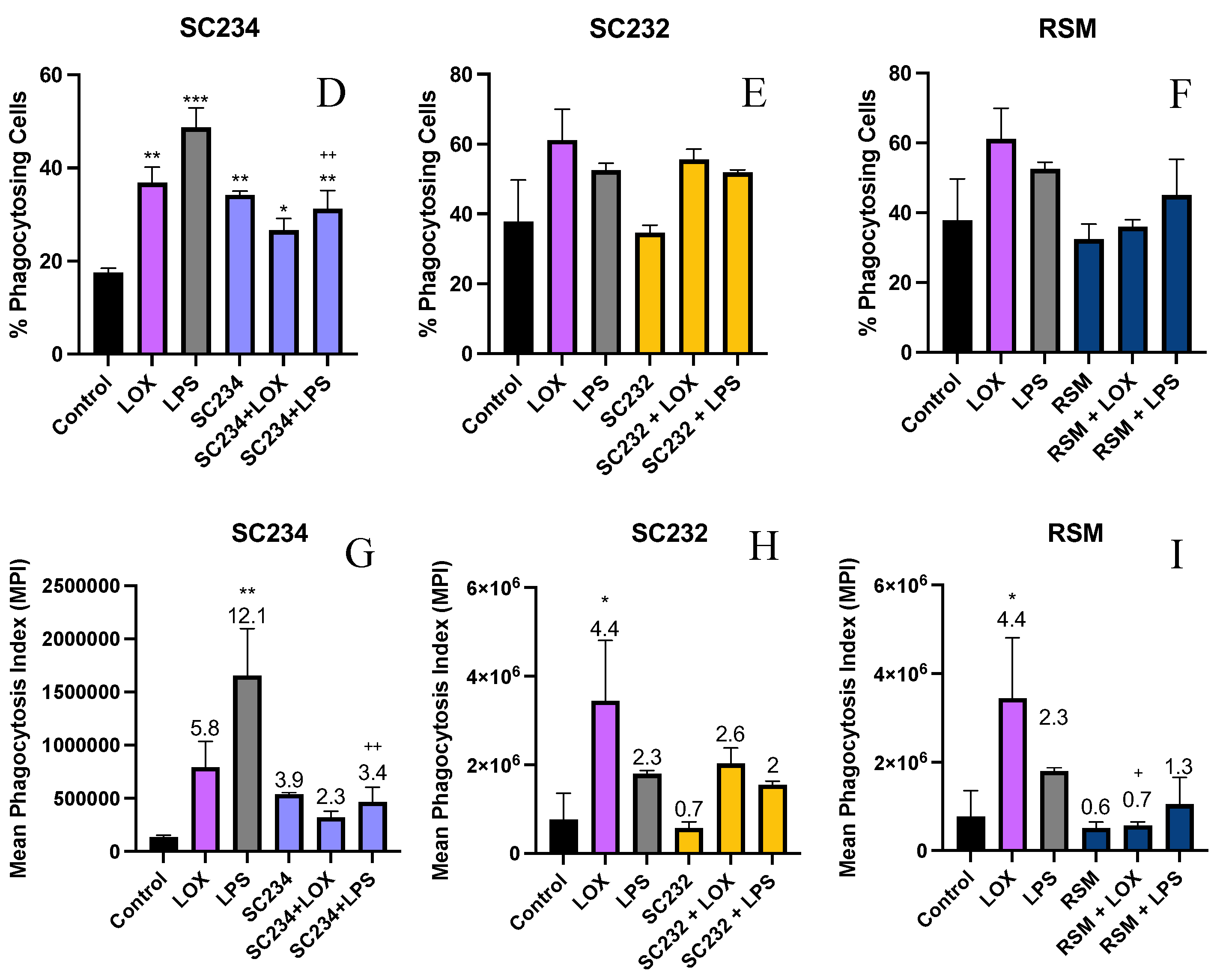
Figure 6A-C demonstrates that stimulation of J774 cells with LOX and LPS significantly increased the MFI (p<0.033; p<0.002) and that the addition of SC234 or SC232 to LOX and LPS stimulated cells suppressed this increased MFI. SC234 and SC232 alone had no effect.
Figure 6C demonstrates that the presence of RSM had a similar effect on LOX and LPS stimulated cells to SC234 and SC232 and RSM alone had no effect.
Percentage phagocytes:
Figure 6D-F demonstrates that LOX and LPS increased the percentage of phagocytosing cells (p<0.002; p<0.001) which was suppressed by the presence of SC234 but not SC232. Interestingly, SC234 alone also increased the percentage of phagocytosing cells and RSM alone or in the presence of LOX or LPS had no significant effect.
MPI:
Figure 6G-I demonstrates that stimulation of J774 cells with LOX and LPS significantly increased the MPI (p<0.033; p<0.001) which was suppressed by the addition of SC234, however SC234 alone enhanced the MPI. The addition of SC232 to LOX stimulated cells also suppressed the increased MPI, in contrast to the maintained MPI in LPS activated cells. SC232 alone had no effect and RSM had a similar effect on LOX and LPS stimulated cells to SC234.
4. Discussion
This study demonstrates the potential of the novel fermentates, SC232 and SC234, in modulating key macrophage functions, which are central to protection and clearance of viral infections. Macrophages act as scavengers, enabled by the presence of pattern recognition receptors, to alert the immune system through chemokine and cytokine secretion and antigen presentation, and to engulf and destroy invading pathogens via phagocytosis [
5]. Macrophage play a critical role in viral immunity and so are an important cell to target to enhance their capabilities.
Following confirmation that the fermentates and the starting substrate, RSM, did not affect cell viability, we demonstrated that cytokine secretion in J774.A.1 and BMDM macrophage are positively affected by the presence of SC232 and SC234, when compared to the effects of the RSM. Furthermore, these effects differ depending on the mode of activation of the cell. J774.A.1 and BMDM cells when activated with LOX and LPS, in the presence of SC232 and SC234 show enhanced levels of secretion of IL-1β, IL-6, IL-27, and IL-10. BMDM cells when activated with LOX and LPS, in the presence of SC232 and SC234 show enhanced levels of secretion of IL-12p40, and IL-27 following LOX exposure, but decreased IL-12p40, and IL-27 secretion following LPS exposure. This suggests that they may have specific effects on the immune system in the presence of a viral ligand.
In polarised BMDMs SC232 and SC234 show a similar profile of activity. M0 and M1 polarised BMDMs in the presence of SC232 and SC234 secrete high levels of IL-6, and TNF-α and M0, M1, and M2 polarised BMDMs in the presence of SC232 and SC234 secrete high levels of IL-10.
Given the importance of IL-6, TNF-α, IL-12p40 and IL-27 in aiding the immune system during viral infections such as influenza, vaccinia virus, HIV and herpes simplex [
6,
7,
8,
9,
10,
11,
12] and supporting viral immunity, a fermentate that can enhance these cytokines could be beneficial. In response to a viral activation, the novel fermentates SC234 and SC232 can enhance not only IL-6, IL-12p40, and IL-27 in BMDMs, but also support IL-6 and TNF-α secretion in polarised BMDMs, thus they have potential to support viral immunity. These effects are not the same in the presence of LPS with decreased IL-12p40 and IL-27 and so the unique bioactivity we see in the fermentates ability to enhance cytokine response to viral ligands further supports their possible specificity in enhancing viral immunity.
Similarly, this anti-viral profile is seen in other classic markers of M1 and M2 macrophage, NO production and arginase activity. M0, M1, and M2 BMDMs in the presence of SC232 and SC234 produce high levels of NO. M0, and M1 BMDMs in the presence of SC232 and SC234 promoted low levels of arginase activity. This emphasises the largely proinflammatory M1 profile of SC232 and SC234. The effect on NO production is of particular interest given that NO production is necessary for viral clearance, via inducible NO synthase (iNOS), and depending on the virus can have direct antiviral properties, limiting severity of virus induced disease [
13,
14]. NO production is linked to the M1 phenotype, killing/fighting, whereby arginine is metabolised via iNOS, to NO and citrulline, to aid M1 macrophage in the production of Th1 responses for fighting infection, and aiding in the drive and recruitment of pro-inflammatory cytokines useful for the defence of the immune system against viruses [
15,
16].
It has previously been demonstrated that an isolated acidic polysaccharide from the fungus
Cordyceps militaris enhanced mRNA expression of IL-1β, IL-6, IL-10, and TNF-α, increased NO production and induced iNOS mRNA and protein expression in RAW 264.7 macrophage cells, as well as increasing TNF-α and IFN-γ in mice, to decrease virus titres in the bronchoalveolar lavage fluid and the lung of mice infected with influenza A virus to increase survival rate [
17]. Similarly a studying involving
Lactobacillus helveticus has been found to show a trending decrease in influenza like illness in an elderly population, warranting further investigation in at risk populations, this suggesting a similar role for both SC232, and SC234 [
18]. Another study showed that germinated
Rhynchosia nulubilis fermented with
Pediococcus pentosaceus SC11, isolated from a salted small octopus, has immune-enhancing and anti-viral effects, inhibiting 3CL protease activity in SARS-CoV in immunocompromised mice, increased T lymphocyte production and spleenocyte proliferation, increased phagocytic activity, NO production via induction of iNOS, mRNA expression of IFN-γ, IFN-α and ISG15 in RAW 264.7 macrophage, and subsequent increase in expression of TNF-α [
19], suggesting the role of GRC-SC11 in immunosuppressed patients for the support against SARS-CoV. Similarly,
Grifola frondosa extract can induce the expression of TNF-α mRNA in Madin-Darby canine kidney cells leading to production of TNF-α, with subsequent inhibition of viral growth of influenza A/Aichi/2/68 virus [
20]. TNF-α possesses antiviral activity through its synergy with IFNs to induce resistance to DNA and RNA of viruses in diverse cell types, selectively killing the virus, necessary for initiation and continuation of inflammation and immunity, adhesion molecule expression and recruitment of leukocytes [
21,
22]. Other studies by Takeda et al. showed that LAB, in particular strain
Lactiplantibacillus plantarum 06CC2, from Cow cheese, increased the production of IL-12, and IL-12p40 in vitro and in vivo [
23].
Lactiplantibacillus plantarum 06CC2 has been associated with the enhancement of the Th1 response, and resulted in the alleviation of influenza virus infection in mice [
24]. Melatonin exhibits anti influenza H1N1 properties via the induction of IL-10 by melatonin caused by the upregulation of IL-27 in DCs [
25].
Rapanea melanophloeos has been shown to increase IL-27 production ultimately increasing IL-10 production, to decrease the viral titre of influenza A virus in MDCK cells, suggesting its role as an anti-influenza treatment [
26]. IL-27 activate and promote production of IFNs which are associated with various antiviral activities, support plasmacytoid DCs to sense viral DNA and RNA, promote macrophage differentiation and polarisation, increases TLR expression, and promote IL-10 cytokine production [
6]. Therefore not only is IL-27 important in viral immunity but IL-27 leads to the subsequent enhanced IL-10 production for viral clearance, and thus where IL-27 is increased, IL-10 will often reflect this. IL-10 is a CD4 produced Th2 cytokine with the ability to indirectly suppress Th1 responses, downregulates antigen presenting capacities of APC, inhibits activation and effector function of T cells, monocytes, and macrophage, therefore limiting host immune response to invading pathogens, ultimately preventing damage to the host from over activation of the pro-inflammatory molecules, and provides a supportive role in effective virus clearance [
27,
28,
29,
30].
Having established SC234 and SC232 as potential anti-viral fermentates that enhance viral immunity through their increased secretion of cytokines important for viral immune responses, we then extended our analysis to chemokines which are critical in supporting the immune system in response to viral infection and host protection. We demonstrate that chemokine secretion in BMDM macrophage are positively affected by the presence of SC232 and SC234, when compared to the activity of the RSM. Chemokines are critical in order to mediate macrophage chemotaxis, cell trafficking, and in the regulation of M1 and M2, and regulate differentiation of monocytes into dendritic cells (DCs), to attract macrophage towards the site of injury or infection [
31,
32,
33]. MCP-1 (CCL2) is a key chemokine for the regulation of migration and infiltration of monocytes, playing a critical role in inflammation [
32,
34]. MIP-1 α (CCL3) on the other hand plays a key role in viral immunity, being a chemotactic chemokine secreted by macrophage to aid in the recruitment of cells, wound healing, inhibition of stem cells, maintaining effector immune responses, and is a key mediator of virus induced inflammation [
35,
36]. Similarly, MIP-2 (CXCL2) plays a role in viral immunity, aiding in neutrophil recruitment and activation, and is a potent chemoattractant secreted by macrophage and epithelial cells, that plays a critical role in LPS induced inflammation, as well as aiding in suppressing of viral replication [
37,
38,
39]. Therefore, while MCP is important for inflammation, it is MIP-1 and MIP-2 that play critical roles in the context of viral infection, with their roles in virus induced inflammation and suppressing viral replication [
32,
35,
36,
37,
38,
39]. It is important, however, that these chemokines are not over expressed as this leads to pathogenesis of many inflammatory diseases including cancers, rheumatoid arthritis, as well as viruses such as coronavirus [
33,
35].
BMDMs, when activated with LOX in the presence of SC232 and SC234, decrease MCP. LOX activated BMDMs in the presence of RSM, SC232, and SC234 maintain MIP-1, and MIP-2 concentrations. MCP, MIP-1, and MIP-2 are all enhanced in the presence of the fermentates alone. This highlights again the potential for SC232 and SC234 to be anti-viral. as they maintain concentrations of viral associated chemokines MIP-1, and MIP-2, and decrease MCP which is often associated with pathogenesis of viral infections [
33,
35].
It is well established that decreases in MCP can be linked with the ability to inhibit viruses such as HIV [
40]. The use of the Chinese herbal medicine Shikonin, from the dried root of
Lithospermum erythrorhizon, has been linked to the ability to inhibit HIV-1 through its interactions inhibiting MCP and MIP-1 [
40]. Extract of Indigo Naturalis from the leaves of
Baphicacanthus cusia (Nees)
Bremek. (Fam.
Acanthaceae),
Polygonum tinctorium Ait. (Fam.
Polygonaceae) or
Isatis indigotica Fort. (Fam
Cruciferae), has been shown to be effective in the treatment of acute lung injury induced by influenza A virus, via the reduction of high levels of MCP-1, as well as regulation of other pro-inflammatory markers [
41]. Indeed, Indigo Naturalis inhibited viral adhesion to cells
in vitro, [
41]. Lianhuaqingwen Capsule from traditional Chinese medicine prescription, Maxing Shigan Tang and Yinqiao San, decreased the expression of MCP-1, resulting in antiviral activity for the treatment of influenza viral infection [
42]. The similar activities of SC234 and SC232 on chemokines may highlight their anti-viral potential.
Next we went on to assess the effect of fermentates SC234 and SC232 on cell surface marker expression in J774.A.1. These cell surface proteins play a critical role in host immunity by enabling the cell to respond and interact with the environment around them, thus playing a critical role in intracellular signalling [
43]. Therefore enhancing any of these cells surface markers would suggest further anti-viral activity for SC234 and SC232.
J774.A.1 cells when activated with LOX or LPS, in the presence of SC234 and SC232 positively impact cell surface marker expression. LOX and LPS activated J774.A.1 in the presence of SC234 showed increases in CD80, CD86, CD40, MHCII, TLR2, and CD14. LOX activated J774.A.1 in the presence of SC234 also had increased MHCI, highlighting the specificity in bioactivity whereby SC234 has unique specificity in increasing viral associated cell surface marker MHCI, not seen in LPS activated cells. LOX and LPS activated J774.A.1 in the presence of SC232 increase MHCII, and TLR4. LOX activated J774.A.1 cells in the presence of SC232 decrease CD14, CD40, and MHCI. Our results clearly demonstrate different effects on cells depending on either Lox or LPS activation.
MHC class molecules function to bind peptide fragments from pathogen and display them on cell surface for recognition by T cells and thus integral to the signalling of the adaptive immune system [
44]. MHCI plays a particularly important role in viral immunity, for detection of virally infected cytotoxic T lymphocytes [
45]. CD80 and CD86 interact on APC and CD28 on T cells as costimulatory signals for the activation of T cells, and are key players in antiviral humoral and cellular immune responses, and play a critical role in the control of chronic and latent infections [
46]. CD40 is a costimulatory molecule expressed by T cells, B cells, professional APCs, as well as non-immune cells and tumours, that plays pivotal roles in cellular immune processes of DCs, B cells, and endothelial cells, and many other cells including hematopoietic and non-hematopoietic, in both humoral and cellular immune responses [
47]. CD40 in particular is important for the restriction of infection of a broad range of RNA viruses and is critical for the control of RNA viruses over the first 24 hours of infection [
48].
Additional cell surface markers MHCII, CD14, TLR2, and TLR4 are included for their overall effect on general immunity. MHCII major role is in general immunity to support and initiate antigen presentation, for antigen specific immune responses [
49]. CD14 is a monocyte differentiation antigen expressed on the surface of myeloid lineages, that plays a critical role in immune recognition via its interactions with TLR4 [
50]. TLR2 and TLR4 have the ability to identify unique molecular patterns from invading pathogens via PRRs, acting as innate sensors against invading pathogens, while also bridging the innate and adaptive immune responses, and regulating the balance between Th1 and Th2 responses essential for host survival [
51]. Of note, however, TLR2 has been identified to play a role in viral immunity with protective roles against viruses such as varicella zoster virus, hepatitis C virus, vaccinia virus, cytomegalovirus and respiratory syncytial virus [
52]. Our data demonstrates clear positive effects on these markers and therefore suggests that these fermentates may possess anti-viral properties.
Ginseng berry extract has been shown to have antiviral, anti-tumour and immune activating effects, including the upregulation of costimulatory molecules CD86, MHCI and MHCII
in vivo, promotion of IL-6, IL-12 and TNF-α, and promotion of DCs and T cell activation even in tumour bearing mice, resulting in inhibition of tumour cell growth [
53]. M1 and M4 metabolites of ginsenosides of root Panax ginseng C.A. Meyer have also been shown to increase cell surface expression of CD80, CD83, CD86 in human monocyte derived DCs, making DCs the potential targets for the immunomodulatory capacity of the ginseng components via the enhancement of differentiation and proliferation of lymphocytes [
54,
55]. Exopolysaccharide (EPS) from Cordyceps sinensis induce expression of MHCII, CD40, CD80, and CD86 in DC sarcoma cells, enhance their ability of antigen uptake, as well as increase secretion of IL-12 and TNF-α, thus suggesting that EPS have a critical in initiating antitumor immunity and pro-inflammatory immune modulation [
56]. Carrot pomace has been found to increase the expression of co-stimulatory molecules CD40 and CD80, and the fraction of cells CD11c+MHCII+ cells in BMDCs, increase proinflammatory cytokine production, and in cyclophosphamide immunosuppressed mice administered with influenza vaccine challenge, significantly enhanced the efficacy of the influenza vaccine [
57]. Resveratrol has been shown to enhance antigen presentation of peritoneal macrophage via the upregulation of CD86, MHCII and TLR4 levels, suggesting its role as pseudorabies virus vaccine adjuvant therapy, aiding in the host protection against viral infection [
58]. The similar effects of our fermentates on key cell surface markers involved in viral immunity further support their potential as anti-viral ingredients.
Having assessed and confirmed the immuno-supportive roles of SC234 and SC232 as immune boosting compounds for the defence against viral infection, we also assessed the effect of these novel fermentates on phagocytosis. Phagocytosis is another critical function of the macrophage in the defence against viral infection. Hindering the cells abilities to carry out this function could impact on host immune function. The mechanisms by which viral and bacterial phagocytosis occur are however different. Viral phagocytosis occurs through apoptosis-dependent phagocytosis [
59]. This means that the host cell infected with the virus is killed via apoptosis and subsequently engulfed by the phagocyte for virus removal, antigen presentation, inflammation resolution, and trained immunity [
59]. Bacterial phagocytosis on the other hand simply involves the engulfing of the invading pathogen by the phagocyte, for pathogen internalisation, destruction, and subsequent antigen presentation and immune signalling [
60]. In setting up the model for phagocytosis, the MFI of the latex beads, and the percentage of phagocytosing cells within the population were measured. These two parameters were then combined in order to form the overall mean MPI, the combined effect of the MFI and percentage of phagocytosing cells, for a collective outlook of sample effect on phagocytosis.
In contrast to the viral immune supportive roles identified for SC234 and SC232 so far, these fermentates negatively impact on the MPI. There is a small decrease in the MPI, meaning that the macrophages ability to phagocytose is negatively impacted in the presence of SC234 and SC232. This negative impact on phagocytosis is something to consider if these novel fermentates are to be considered for commercial development. However, it must be noted that the MPI for SC234 and R003, is enhanced in comparison to the RSM. This means that in comparison to the fermentate starting substrate, these novel fermentates in fact increase the ability of the macrophage to phagocytose. Phagocytosis is traditionally known for its role in bacterial and fungal clearance [
61]. This is supported by the literature whereby patients with defects in phagocytic function are found to be more susceptible to common bacterial and fungal infections [
61,
62]. This critical role of phagocytosis in bacterial and fungal clearance highlights the importance of phagocytosis in the context of bacterial and fungal infections, opposed to viral infections, thus suggesting that in the context of viral immunity the role of phagocytosis may not be deemed as important as in the context of bacterial or fungal infection. Therefore the decrease seen in the MPI as a result of the presence of fermentates SC234 and SC232, may still support the role of such novel fermentates in the context of viral immunity, as they provide an increase in MPI above the RSM non-fermented control.
It is clear from the literature that increases in phagocytotic activity through the use of functional foods can be linked with enhanced immunity. When RAW 264.7 murine macrophage are treated with wild simulated ginseng, increased phagocytotic activity is observed, along with increased production of immunostimulators such as NO, iNOS, COX-2, IL-1β, IL-6 and TNF-α, via LR2/4-dependent MAPK, NF-κB and PI3K/AKT signalling pathways [
63]. Panax ginseng Meyer when administered to BALB/c mice enhanced innate and adaptive immunity via the improved cell-mediated and humoral immunity, macrophage phagocytosis capacity and NK cell activity [
64]. In that study He et al. hypothesise that the increased immunomodulating activity is due to the increased macrophage phagocytosis capacity, along with increased NK cell activity, enhancement of T and Th cells, as well as IL-2, IL-6 and IL-12 secretion and IgA, IgG1 and IgG2b production [
64]. A study in RAW 264.7 macrophage established that a water extract from Lycium chinense Miller root enhanced the innate immune system through increased induction of pro-inflammatory cytokines TNF-α and IL-6, chemokines RANTES and MIP-1α, NO, iNOS expression, and activated the Akt, NF-κB, and MAPKs ERK and p38 signalling proteins [
65]. Lycium chinense Miller root was able to enhance phagocytic activity not only in RAW 264.7 cells but also in BMDMs [
65]. Fermenting
C. militaris with
Pediococcus pentosaceus ON89A (GRC-ON89A) can enhance phagocytosis in RAW 264.7 cells and primary peritoneal macrophage from normal mice and cyclophosphamide immunosuppressed mice, via the activation of MAPK and Lyn pathways [
66]. It is suggested that GRC-ON89A has the potential to act as an immunostimulant for the use as an immune boosting therapy in immunosuppressed patients [
66].
Figure 1.
Exposure of LOX and LPS activated BMDM to 25mg/ml fermentates results in the secretion of cytokines. BMDM cells were seeded at 1x106 cells/ml and incubated overnight at 37°C in 5% CO2. The following day cells were stimulated with 25mg/ml fermentate, incubated for 1 hour at 37°C in 5% CO2 2. and subsequently exposed to LOX 0.5mM; LPS 100ng/ml before incubating overnight under the same conditions. Non-fermented RSM is the fermentate control. Supernatants were removed after 24 hours and ELISA was performed for cytokines IL-1β, IL-6, IL-10, TNF-α, IL-12p40, and IL-27. Data is presented as mean ± SEM of three replicates. Significance determined using one- way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing control cells, to LOX and LPS, and unstimulated samples”*”, 2) comparing TLR, to sample + TLR “+” and 3) comparing RSM +/- TLR, to sample +/-TLR “x”.
Figure 1.
Exposure of LOX and LPS activated BMDM to 25mg/ml fermentates results in the secretion of cytokines. BMDM cells were seeded at 1x106 cells/ml and incubated overnight at 37°C in 5% CO2. The following day cells were stimulated with 25mg/ml fermentate, incubated for 1 hour at 37°C in 5% CO2 2. and subsequently exposed to LOX 0.5mM; LPS 100ng/ml before incubating overnight under the same conditions. Non-fermented RSM is the fermentate control. Supernatants were removed after 24 hours and ELISA was performed for cytokines IL-1β, IL-6, IL-10, TNF-α, IL-12p40, and IL-27. Data is presented as mean ± SEM of three replicates. Significance determined using one- way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing control cells, to LOX and LPS, and unstimulated samples”*”, 2) comparing TLR, to sample + TLR “+” and 3) comparing RSM +/- TLR, to sample +/-TLR “x”.
Figure 2.
Exposure of M1/M2 polarised BMDMs to 25mg/ml fermentates results in the secretion of cytokines IL-6, TNF-α, and IL-10. BMDM cells were seeded at 5x105 cells/mL and incubated for 1 hour at 37°C in 5% CO2. Cells were stimulated with 25mg/ml fermentates, incubated for 3 hours at 37°C in 5% CO2 The cells were either polarised to M1 phenotype by stimulating with LPS (100 ng/ml) in the presence of 20 ng/ml rIFN-γ or towards M2 cells by adding 20 ng/ml rIL-4, 20 ng/mL IL-13, and 20 ng/ml rTGF-B and incubate for 24 h at 37°C. Supernatants were removed after 24 hours and ELISA was performed for IL-6, IL-10, and TNF-α. Data is presented as mean ± SEM of three replicates. Figure A represent the M0, M1, and M2 profiles for each cytokine. Figure B-D represents cytokine output in response to sample presence. Significance determined using one way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002 (**), and <.001 (***),; 1comparing fermentates, to polarised control cells ‘*”, and 2) comparing M0, to M1, and M2 controls “x”.
Figure 2.
Exposure of M1/M2 polarised BMDMs to 25mg/ml fermentates results in the secretion of cytokines IL-6, TNF-α, and IL-10. BMDM cells were seeded at 5x105 cells/mL and incubated for 1 hour at 37°C in 5% CO2. Cells were stimulated with 25mg/ml fermentates, incubated for 3 hours at 37°C in 5% CO2 The cells were either polarised to M1 phenotype by stimulating with LPS (100 ng/ml) in the presence of 20 ng/ml rIFN-γ or towards M2 cells by adding 20 ng/ml rIL-4, 20 ng/mL IL-13, and 20 ng/ml rTGF-B and incubate for 24 h at 37°C. Supernatants were removed after 24 hours and ELISA was performed for IL-6, IL-10, and TNF-α. Data is presented as mean ± SEM of three replicates. Figure A represent the M0, M1, and M2 profiles for each cytokine. Figure B-D represents cytokine output in response to sample presence. Significance determined using one way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002 (**), and <.001 (***),; 1comparing fermentates, to polarised control cells ‘*”, and 2) comparing M0, to M1, and M2 controls “x”.
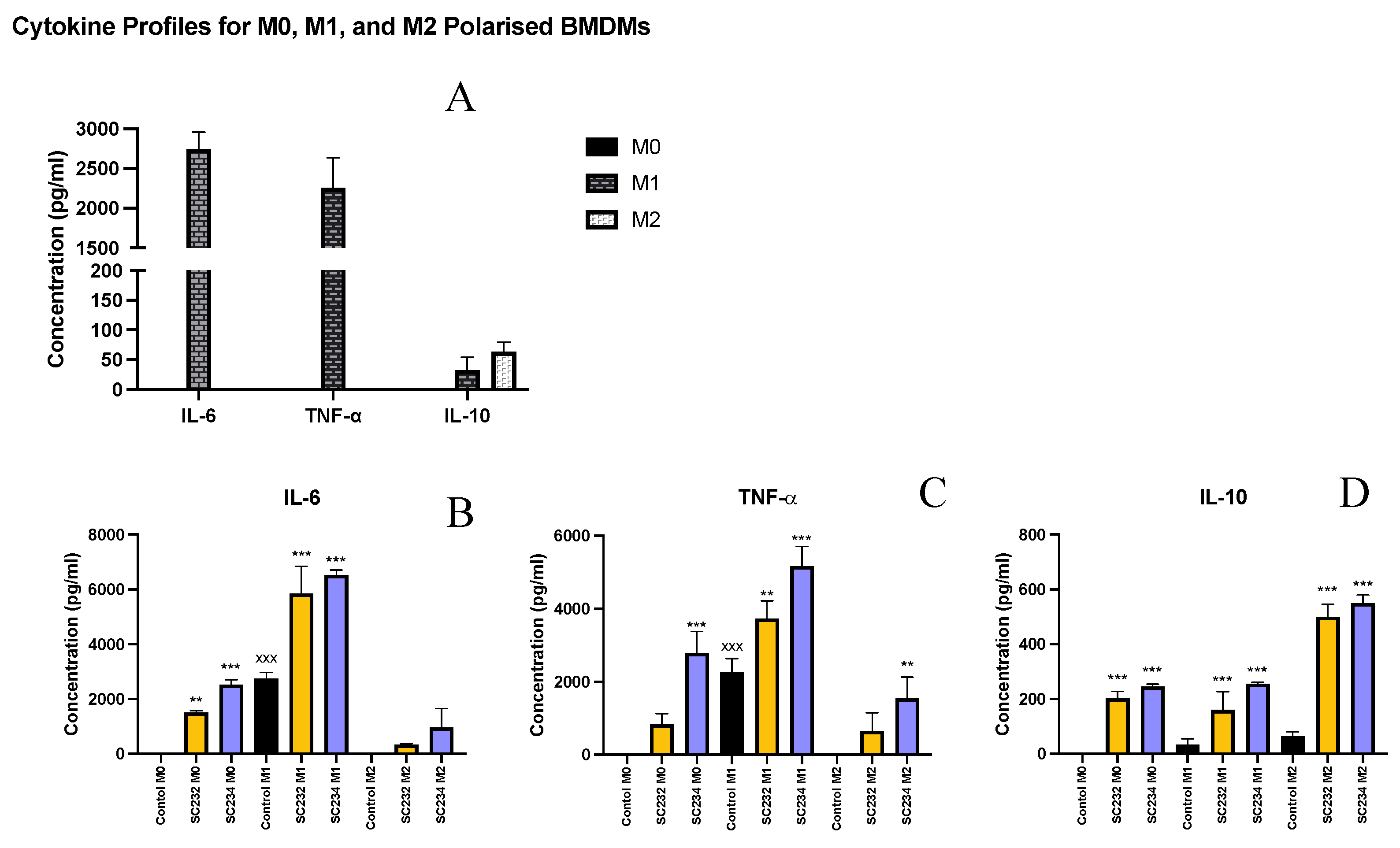
Figure 3.
Exposure of M0, M1, and M2 BMDM to 25mg/ml fermentates affects production of NO production and arginase activity. BMDM cells were seeded at 5x105 cells/ml and incubated for1 hour at 37°C in 5% CO2. Cells were stimulated with 25mg/ml fermentates, incubated for 3 hour at 37°C in 5% CO2 The cells were either polarised to M1 phenotype by stimulating with LPS (100 ng/ml) in the presence of 20 ng/ml rIFN-γ or towards M2 cells by adding 20 ng/ml rIL-4, 20 ng/ml IL-13, and 20 ng/ml rTGF-B and incubate for 24 h at 37 °C. Supernatants were removed after 24 hours and Griess assay was performed as per manufacturer’s instructions for determination of NO production (A,C). Cell lysates were prepared, and arginase assay carried out to determine arginase activity (B, D). Data is presented as mean ± SEM of three replicates. Figure A and B represent the NO and arginase activity profiles for M0, M1, and M2 polarised cells respectively. Significance determined using one way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing fermentates, to polarised control cells ”*” and, 2) comparing M0, to M1 and M2 controls “x”.
Figure 3.
Exposure of M0, M1, and M2 BMDM to 25mg/ml fermentates affects production of NO production and arginase activity. BMDM cells were seeded at 5x105 cells/ml and incubated for1 hour at 37°C in 5% CO2. Cells were stimulated with 25mg/ml fermentates, incubated for 3 hour at 37°C in 5% CO2 The cells were either polarised to M1 phenotype by stimulating with LPS (100 ng/ml) in the presence of 20 ng/ml rIFN-γ or towards M2 cells by adding 20 ng/ml rIL-4, 20 ng/ml IL-13, and 20 ng/ml rTGF-B and incubate for 24 h at 37 °C. Supernatants were removed after 24 hours and Griess assay was performed as per manufacturer’s instructions for determination of NO production (A,C). Cell lysates were prepared, and arginase assay carried out to determine arginase activity (B, D). Data is presented as mean ± SEM of three replicates. Figure A and B represent the NO and arginase activity profiles for M0, M1, and M2 polarised cells respectively. Significance determined using one way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing fermentates, to polarised control cells ”*” and, 2) comparing M0, to M1 and M2 controls “x”.
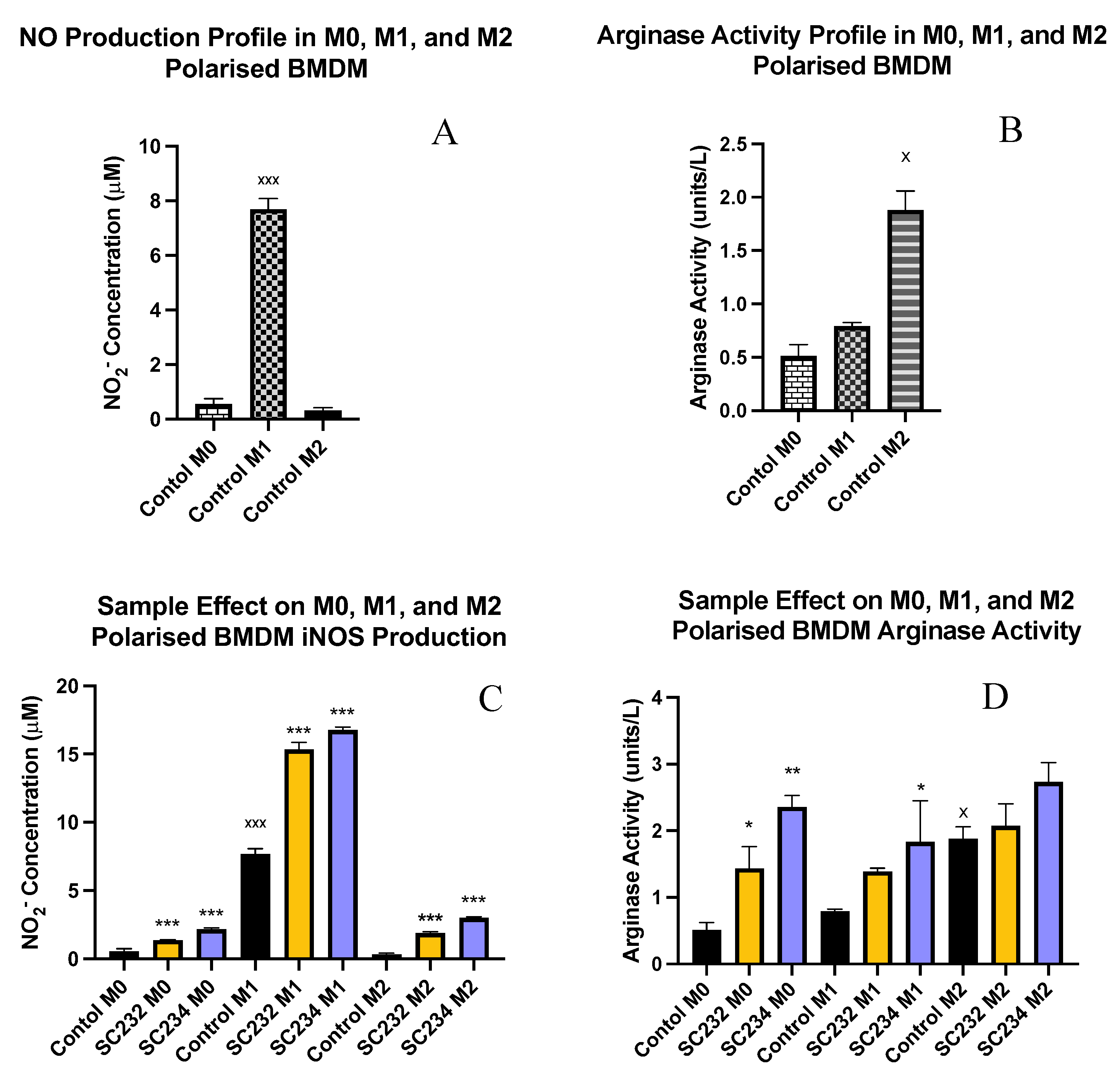
Figure 4.
Exposure of LOX and LPS activated BMDM to 25mg/ml fermentates results in the secretion of chemokines. BMDM cells were seeded at 1x106 cells/ml and left overnight at 37°C in 5% CO2. The following day cells were stimulated with 25mg/ml raw sample fermentate, incubated for 1 hour at 37°C in 5% CO2 and subsequently exposed to LOX 0.5mM; LPS 100ng/ml before incubating overnight under the same conditions. RSM is the fermentate control. Supernatants were removed after 24 hours and ELISA was performed MCP, MIP-1 and MIP-2. Data is presented as mean ± SEM of three replicates. Significance determined using one- way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing control cells, to LOX and LPS, and unstimulated samples”*”, 2) comparing TLR, to sample + TLR “+” and 3) comparing RSM +/- TLR, to sample +/-TLR “x”.
Figure 4.
Exposure of LOX and LPS activated BMDM to 25mg/ml fermentates results in the secretion of chemokines. BMDM cells were seeded at 1x106 cells/ml and left overnight at 37°C in 5% CO2. The following day cells were stimulated with 25mg/ml raw sample fermentate, incubated for 1 hour at 37°C in 5% CO2 and subsequently exposed to LOX 0.5mM; LPS 100ng/ml before incubating overnight under the same conditions. RSM is the fermentate control. Supernatants were removed after 24 hours and ELISA was performed MCP, MIP-1 and MIP-2. Data is presented as mean ± SEM of three replicates. Significance determined using one- way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing control cells, to LOX and LPS, and unstimulated samples”*”, 2) comparing TLR, to sample + TLR “+” and 3) comparing RSM +/- TLR, to sample +/-TLR “x”.
Figure 5.
Exposure of LOX and LPS activated J774.A.1 to 25mg/ml fermentates affect Cell Surface Marker Expression. J774.A.1 cells were seeded at 1x106 cells/ml, incubated overnight at 370C in 5% CO2. After 24hours cells were stimulated with 25mg/ml fermentates, incubated for 1 hour at 37°C in 5% CO2. before stimulating with LOX 0.5mM or LPS 100ng/ml. Cell suspensions were retained, and cell staining protocol was carried to assess the presence of cell surface markers MHCII, TLR4, CD86, CD80, CD14, CD40, TLR2 and MHCI in the presence of fermentate sample. Cells were analysed using BD FACSAria 1 system flow cytometer, raw FCS files analysed, data graphed using V10.0 FlowJo software. Data is presented as mean ± SEM of two replicates. Significance determined using one- way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***). 1) comparing control cells, to TLR controls, and unstimulated samples”*”, 2) comparing TLR controls, to sample + TLR “+” and 3) comparing RSM +/- TLR, to sample +/-TLR “x”.
Figure 5.
Exposure of LOX and LPS activated J774.A.1 to 25mg/ml fermentates affect Cell Surface Marker Expression. J774.A.1 cells were seeded at 1x106 cells/ml, incubated overnight at 370C in 5% CO2. After 24hours cells were stimulated with 25mg/ml fermentates, incubated for 1 hour at 37°C in 5% CO2. before stimulating with LOX 0.5mM or LPS 100ng/ml. Cell suspensions were retained, and cell staining protocol was carried to assess the presence of cell surface markers MHCII, TLR4, CD86, CD80, CD14, CD40, TLR2 and MHCI in the presence of fermentate sample. Cells were analysed using BD FACSAria 1 system flow cytometer, raw FCS files analysed, data graphed using V10.0 FlowJo software. Data is presented as mean ± SEM of two replicates. Significance determined using one- way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***). 1) comparing control cells, to TLR controls, and unstimulated samples”*”, 2) comparing TLR controls, to sample + TLR “+” and 3) comparing RSM +/- TLR, to sample +/-TLR “x”.
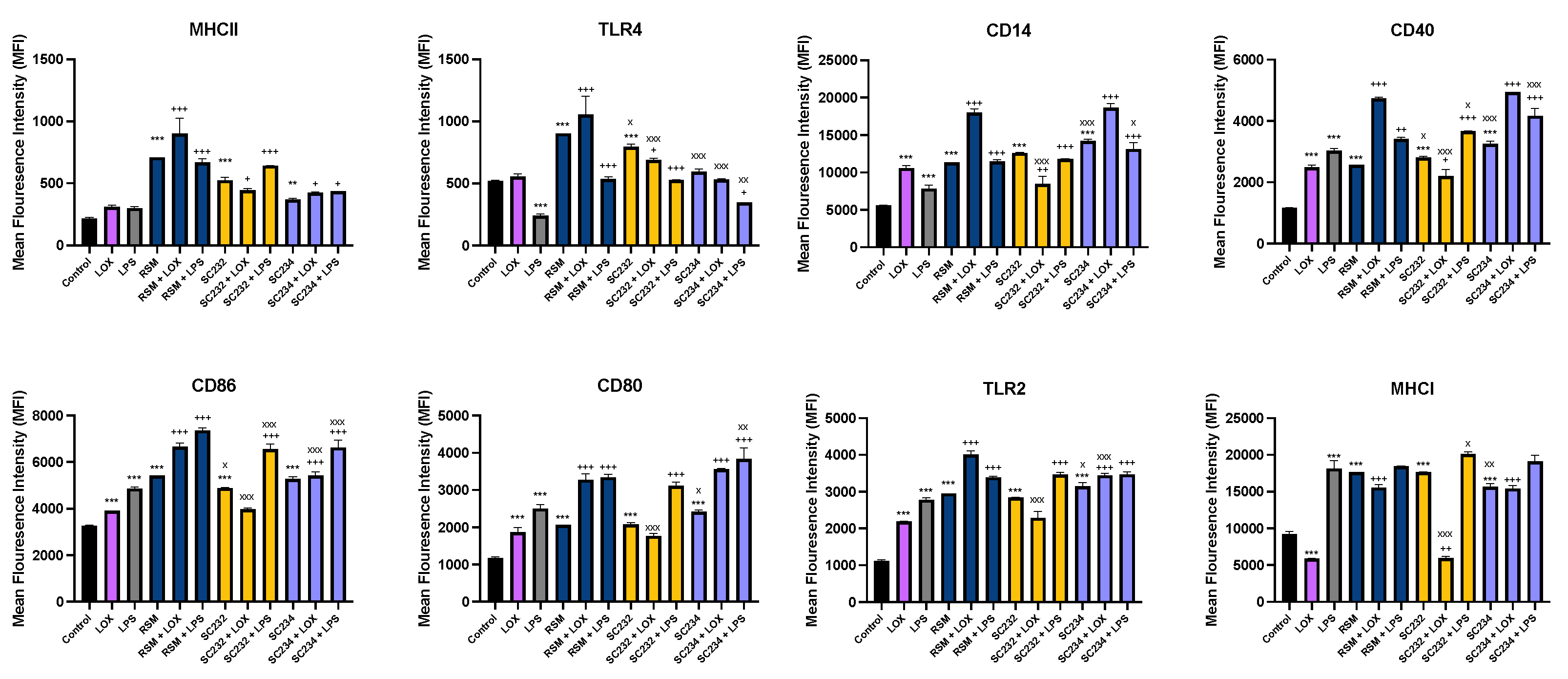
Figure 6.
Exposure of LOX and LPS activated J774.A.1 to 25mg/ml fermentates affects MFI, % phagocytosing cells, and MPI. J774.A.1. cells were seeded at 1x106 cells/ml and incubated overnight at 37°C in 5% CO2. The following day cells were stimulated with 25mg/ml fermentates for 1 hour before activating with LPS 100ng/ml, LOX 0.5mM, and incubated for 4 hours at 37°C in 5% CO2 . Cell suspensions were retained, and cells were stimulated with 1µm fluorescent latex beads at a concentration of 20 beads per cell, for 1 hour at 37°C in 5% CO2. Cells were analysed using BD FACSAria 1 system flow cytometer, raw FCS files analysed, data graphed using V10.0 FlowJo software. Data is presented as mean ± SEM of two replicates. Figure A-C represents MFI, D-F represents percentage phagocytes, and G-I represents MPI. Significance determined using one way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing control cells to each test cell ”*”, 2) comparing each corresponding sample+TLR to TLR alone “+”.MPI data analysed as a product of the percentage phagocytosing cells and MFI. Number indicated above bar is the MPI compared to the control cells represented as 1.
Figure 6.
Exposure of LOX and LPS activated J774.A.1 to 25mg/ml fermentates affects MFI, % phagocytosing cells, and MPI. J774.A.1. cells were seeded at 1x106 cells/ml and incubated overnight at 37°C in 5% CO2. The following day cells were stimulated with 25mg/ml fermentates for 1 hour before activating with LPS 100ng/ml, LOX 0.5mM, and incubated for 4 hours at 37°C in 5% CO2 . Cell suspensions were retained, and cells were stimulated with 1µm fluorescent latex beads at a concentration of 20 beads per cell, for 1 hour at 37°C in 5% CO2. Cells were analysed using BD FACSAria 1 system flow cytometer, raw FCS files analysed, data graphed using V10.0 FlowJo software. Data is presented as mean ± SEM of two replicates. Figure A-C represents MFI, D-F represents percentage phagocytes, and G-I represents MPI. Significance determined using one way ANOVA with a Newman-Keuls post-test. Output P value style APA: .12 (ns), .033 (*), .002(**) and <.001 (***); 1) comparing control cells to each test cell ”*”, 2) comparing each corresponding sample+TLR to TLR alone “+”.MPI data analysed as a product of the percentage phagocytosing cells and MFI. Number indicated above bar is the MPI compared to the control cells represented as 1.