1. Introduction
3D printing as part of additive manufacturing has had huge impacts on different parts of technological advancements. With the beginning of research on the use of 3D printing in medical and pharmaceutical disciplines, the hopes for using the benefits of these techniques have risen. With Spritam
® and the used ZipDose
® technology (Aprecia Pharmaceuticals, LLC, USA) the first 3D printed tablet using a combination of powder layering and inkjet technology was approved by the FDA in 2015 [
1]. Different extrusion-based technologies such as semi-solid extrusion (SSE) [
2,
3] and fused deposition modeling (FDM) were considered in the research of medical devices and pharmaceutical products. So much, that recently Triastek (Triastek, Inc, Nanjing, Jiangsu, China) has received IND (Investigational New Drug) clearance for their second 3D printed product T20 using melt extrusion deposition (MED
TM) technology [
4]. With the rise of extrusion-ready pharmaceutical grade excipients tablets with different release patterns were produced using FDM-based 3D printing technique. In terms of orally administered dosage forms immediate release [
5], modified release [
6,
7], bilayer [
8], and even intra-gastric floating tablets [
9,
10] have been developed. The main advantage in 3D printed medicines is seen in the ability to produce customizable and patient-oriented geometries and strengths of tablets as well as in rapid prototyping [
11]. While the general acceptability of 3D printing has been shown, concerns were found regarding different shapes. Already established shapes such as capsule or disc-shaped printlets are regarded as acceptable for swallowing, more intricate shapes such as tilted diamonds were harder to “sell” to the testing group [
7]. First approaches of 3D printing used commercially available 3D printers and filaments. The filament was loaded with the API (active pharmaceutical ingredient) using different techniques such as loading by soaking [
12]. Disadvantages found in the control of the loading and general concerns about solvent usage over time rendered this method obsolete. The next step was to extrude the filament needed directly from raw materials and to include the API in the desired concentration [
13]. The production of filament is challenging since an additional heating step is needed, which can show a negative impact on thermo-degradable drugs and polymers. Also, production must be strictly controlled to result in a sufficiently uniform filament. To avoid these problems the extrusion and printing must take place in a single step. The company FabRx (FabRx Ltd., UK) developed a 3D printer especially for the use in development of pharmaceutical products. The filament printhead is exchanged for a printhead consisting of a small-scale single-screw extruder with a detachable nozzle which enables so-called direct powder extrusion (DPE) [
14]. This opened the door to the printing of many of the excipients already developed for hot melt extrusion (HME). While printing with filaments requires extensive knowledge of excipients and devices to produce filaments with relevant properties such as tensile strength, these parameters are not relevant when printing directly from the powder. Thus, printing temperature can be reduced for the use of thermo-degradable drugs and polymers by incorporating plasticizers.
The aim of our research was to show that biopharmaceutics classification system (BCS) class II [
15] drugs can be directly printed from powdered excipients into tablets with different strengths while forming amorphous solid dispersions (ASD). BCS class II compounds show low (water-) solubility and high permeability, so improvement of the solubility is often searched for. Polyvinylalcohol (PVA) is a well-researched pharmaceutical excipient that shows appropriate thermal behavior (no thermal degradation up to at least 230°C, appropriate melt viscosity) as well as water solubility [
16]. The synthetic opioid agonist loperamide (hydrochloride) [
17] was used as a thermally stable BCS class II compound. One of the main challenges was to be able to form ASDs while performing the printing at temperatures well below the melting point of loperamide (227°C) since thermal degradation of PVA starts below the melting temperature of loperamide. Information about requirements of excipients for extrusion/3D printing using direct powder extrusion is scarce. Thus, a rheological model for PVA showing minimum melt viscosity needed as well as temperature and shear rate dependency was investigated. Regarding the release behavior, the aim was set to be equivalent to typical formulations with immediate to sustained release.
2. Materials and Methods
2.1. Materials
PVA (Parteck® MXP (Polyvinylalcohol), Merck KGaA, Germany) was used as a water-soluble pharma-grade excipient available for (HME). Fumed silica (Aerosil® R 972 Pharma, Evonik, Germany) was added to improve flowability of the powder mixtures. As model API loperamide hydrochloride (Merck KGaA, Germany) was selected. As a commonly used plasticizer for PVA sorbitol (Parteck SI 400, Merck KGaA, Germany) was used. All solvents used (Methanol, Acetonitrile, ammonium acetate) were HPLC-grade.
2.2. Methods
2.2.1. Thermal Analysis
Differential Scanning Calorimetry (DSC)
Pure substances were analyzed via DSC (Mettler DSC 820, Mettler-Toledo GmbH, Germany) regarding their melting point, glass transition temperature, and possible recrystallization. Printed tablets were measured after 2-4 weeks of storage. Approximately 10-15 mg samples were accurately weighed into sealed aluminum pans with punctured lids. Measurements used a heat-cool-heat cycle to determine the melting point in the first heating and glass transition temperature during the second heating. Information about the miscibility of loperamide and PVA was expected to be found during DSC trials. One of the approaches for estimation of glass transition temperature is described by the Gordon-Taylor equation, which can be applied to miscible blends.
with T(g,mix) and T(g,i) representing the glass transition temperature of the mixture and the components, ωi is the mass fraction component I and K is an adjustable fitting parameter.
It is expected for two miscible substances to show one glass transition temperature instead of two individual glass transition temperatures. The detected temperature should be partially composed of the individual glass transition temperatures [
18].
Simultaneous Thermal Analyzer (STA)
Thermal degradation as well as loss of water taken up from air humidity during printing and storage was tested using a Netzsch STA 409 PG/1/G Luxx (Erich NETZSCH GmbH & Co. Holding KG, Germany). All samples were treated the same way, measuring the mass of a sample using Al2O3 as a reference during heating of the samples up to 250°C. Pure substances and printed tablets were measured at a mass of approximately 22 mg.
2.2.2. XRD-Measurements
Wide angle X-ray (powder) diffraction patterns were obtained in an angular range of 10-50° 2θ with a stepwise size of 0.02° on a Bruker D8 Advance diffractometer (Bruker Corp., USA) using monochromatic CuKα radiation (λ = 0.15406 nm). Pure substances were measured in powdered form, while printed tablets were measured intact.
2.2.3. Meltrheology
To measure the viscosity of the molten mixtures and thus link the rheological properties to the printing process, the shear rate occurring during printing was determined. Two different methods related to different extrusion processes were compared.
The first method is usually used in FDM 3D printers that run on filament. Here, only the shear rate in the nozzle is considered [
19].
Volume flow (Q) was determined empirically by accurately weighing printed tablets with 100% infill and measuring their volume (V) using a gas displacement pycnometer (Accupyc 1330, Micromeritics Instrument Corporation, USA). Temperature was set to 25°C while the remaining volume was flushed with Helium. During printing, the time (t) for each individual tablet was recorded. The following equation was employed afterwards:
The apparent shear rate at the nozzle wall (
) can be calculated as:
with a radius (r) of the equipped nozzle of 0.04 cm.
The second method is applied to extrusion processes and used to calculate shear rates inside single-screw extruders. Approximation of the shear rate γ ̇ in the screw channel can be achieved from the Couette shear rate:
with v (velocity), H (channel depth = 0.55 mm), D (diameter = 8.0 mm) and N (screw speed) [
20].
Melt rheology was conducted using compact rheometer (Anton Paar Physica MCR 501, Anton Paar GmbH, Germany) equipped with single-use stainless steel plates with a radius of 10 mm in a plate-plate configuration. Sample specimens used for the melt rheology were produced using the MeltPrep device (MeltPrep GmbH, Austria) with a 20 mm disc geometry at temperatures 10°C above printing temperature yielding ASDs with consistent geometric shape [
21]. Since the viscosity of non-newtonian fluids is a product of temperature and shear rate [
22], samples were measured at a range of temperatures after determination of the linear viscoelastic region (LVR). Determination of LVR was conducted for every measured temperature individually by performing amplitude sweeps from 0.01% to 100% deformation (0.0103 to 103 mrad) at 10 rad/s. Frequency sweeps from 100 Hz to 0.1 Hz were performed afterwards at 1% amplitude.
2.2.4. HME of PVA/Sorbitol Mixtures
Apart from PVA/loperamide mixtures, also plasticized mixtures were used. Sorbitol was used as the plasticizer. Direct powder extrusion of PVA/sorbitol mixtures was not possible due to the different melting points. To be able to still print this mixture a single screw extruder (Noztek Pro, Noztek, England) was used to extrude these two excipients before adding the API and printing the mixture. Afterward, it was milled using an ethanol–cooled mill and sieved while the fraction < 0.400 mm was used in further printing steps.
2.2.5. Preparation of Powder Mixtures
All powder mixtures for printing were produced by sieving (0.400 mm mesh size) and accurately weighing the compounds needed and subsequent mixing in a tumbler mixer (Turbula Type T2C, Willy A. Bachofen AG, Switzerland) for 20 min.
Table 1.
Physical mixtures for 3D printing.
Table 1.
Physical mixtures for 3D printing.
| Batch |
Parteck MXP |
Parteck MXP/Sorbitol 15% Extrudate |
Loperamide |
Aerosil |
| PAR-LOP5%-AER1% |
94.0% |
|
5.0% |
1.0% |
| PAR_LOP10%_AER0.5% |
89.0% |
|
10.0% |
1.0% |
| PAR-SOR15%E_LOP5%_AER1.5% |
|
93.5% |
5.0% |
1.5% |
2.2.6. 3D Printing Using Direct Powder Extrusion Tool of M3dimaker
A computer-aided design (CAD) tablet-shaped geometry was created using Autodesk® Fusion 360 (Autodesk GmbH, München). The .stl file was sliced by Repetier-Host (Hot-World GmbH & Co. KG, Germany). Objects were printed with 2 outer perimeters, 3 bottom and top layers using varying infills to change the dosage of printed tablets on the M3dimaker 3D printer (FabRx Ltd., London) equipped with the direct powder extrusion (DPE) tool. Print speed and temperature were adjusted according to the properties of the mixtures in molten state.
Figure 1.
stl file of tablet shaped geometry (d = 5 mm; h = 5mm).
Figure 1.
stl file of tablet shaped geometry (d = 5 mm; h = 5mm).
Figure 2.
stl file of improved geometry (d = 5 mm; h = 5mm; edges rounded with r = 2mm).
Figure 2.
stl file of improved geometry (d = 5 mm; h = 5mm; edges rounded with r = 2mm).
2.2.7. Confocal Raman Microspectroscopy
Witec’s alpha 300R system (WITec GmbH, Ulm, Germany) was employed for the measurements. Pure substances were measured with 30 mW power of the laser (λ = 532 nm) and the evaluated spectra were used for the true component analysis to show the distribution of loperamide in the tablet.
Printed tablets were measured on the outer surface to find information on potential demixing processes, varying concentrations, and recrystallization on the outer surface.
One printed tablet of each mixture was measured using 30 mW laser (λ = 532 nm). The surface of the measurement area was evaluated and corrected by the TrueSurface Mk II module. An area of 20 x 2000 µm with 4 x 400 pixels was measured using the autofocus function.
For the evaluation of drug content, the true component analysis was employed using the previously obtained spectra of individual substances. The software calculates based on individual spectra the amount of drug found in each pixel measured and reports a colored picture of the distribution and intensity of the signal found.
2.2.8. Drug Content Analysis by HPLC
Evaluation of the drug content was performed on the powdered mixtures as well as on the printed tablets. From powdered samples, an equivalent of 5 mg loperamide was accurately weighed and dissolved in 100 ml of the mobile phase used for the subsequent HPLC analysis (purified water/acetonitrile/0.5% ammonium acetate solution 29/36/35 (v/v)). Tablets were also dissolved in the mobile phase using stirring and an ultrasonic bath. Drug contents were determined by HPLC-UV/VIS (System 20A, Shimadzu Ltd., Japan) at a wavelength of 254 nm. The method showed a linearity of R2=0.99680 between 0.0023 mg/ml and 0.07 mg/ml with a tested LOQ of 0.00166 mg/ml and an estimated LOD of 0.0005488 mg/ml calculated according to ICH guidelines [
23]. All experiments were performed in triplicate.
2.2.9. In Vitro Dissolution
Dissolution testing was adapted from standard procedures of the FDA [
24] used for loperamide capsules using a Pharma Test PT-DT7 (Pharma Test Apparatebau AG, Germany) manual dissolution tester. 0.1M HCl was used as dissolution medium (500 or 900 ml, depending on the tablet strength) at 37°C with 100 RPM paddle speed. Sampling of 1 ml took place every 30 min for 5.5 hours and a last sample was drawn after 24 hours. Samples were measured using UV-HPLC as described in 2.2.8. For each formulation and infill setting printed, 4 tablets were measured and the mean value including the standard deviation is shown. For each formulation, a linear regression of the correlation between mass and infill is calculated and the best-fit values are given. Slopes are compared using two-tailed testing with the null hypothesis that the slopes are identical.
3. Results and Discussion
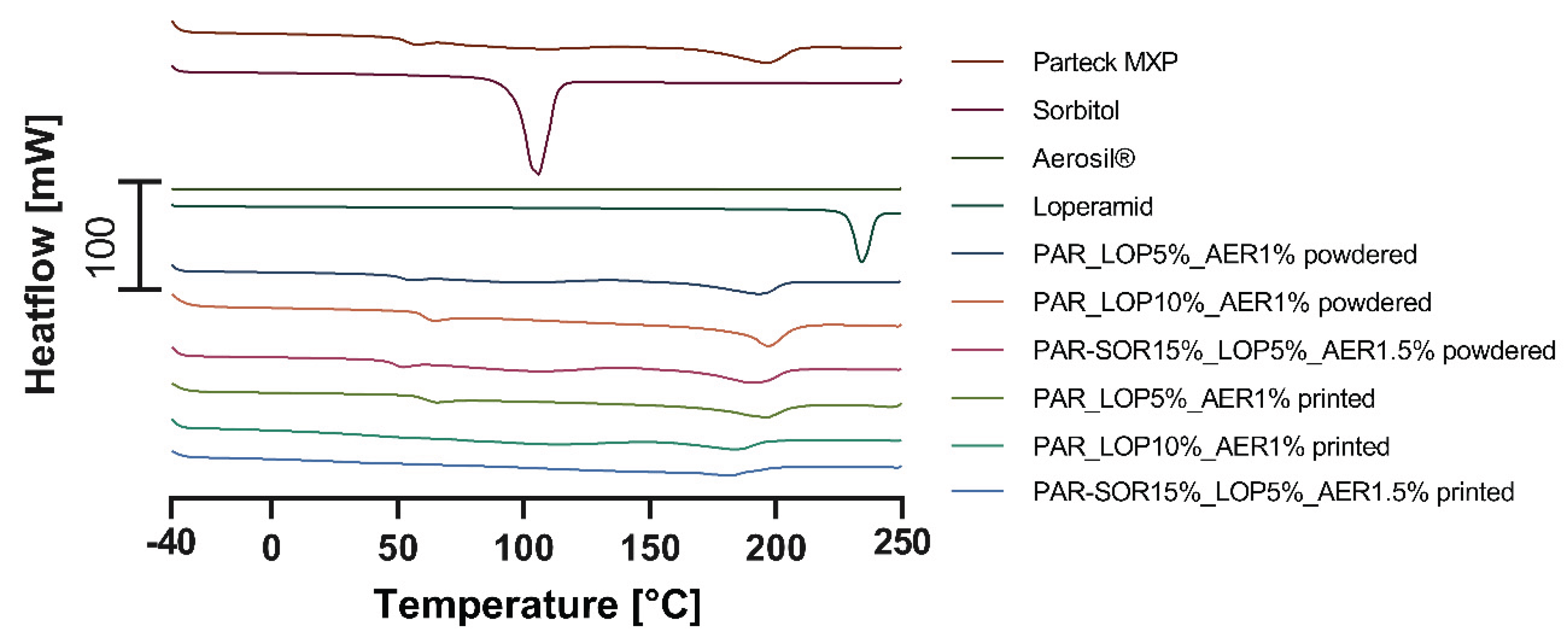
3.1. Results of Thermal Analysis
Thermal properties of the excipients, mainly the melting point and the glass transition temperature (DSC) and degradation (STA), were measured. From powdered samples information about the solubility was expected, while printed tablets were controlled for signs of crystallization of the API.
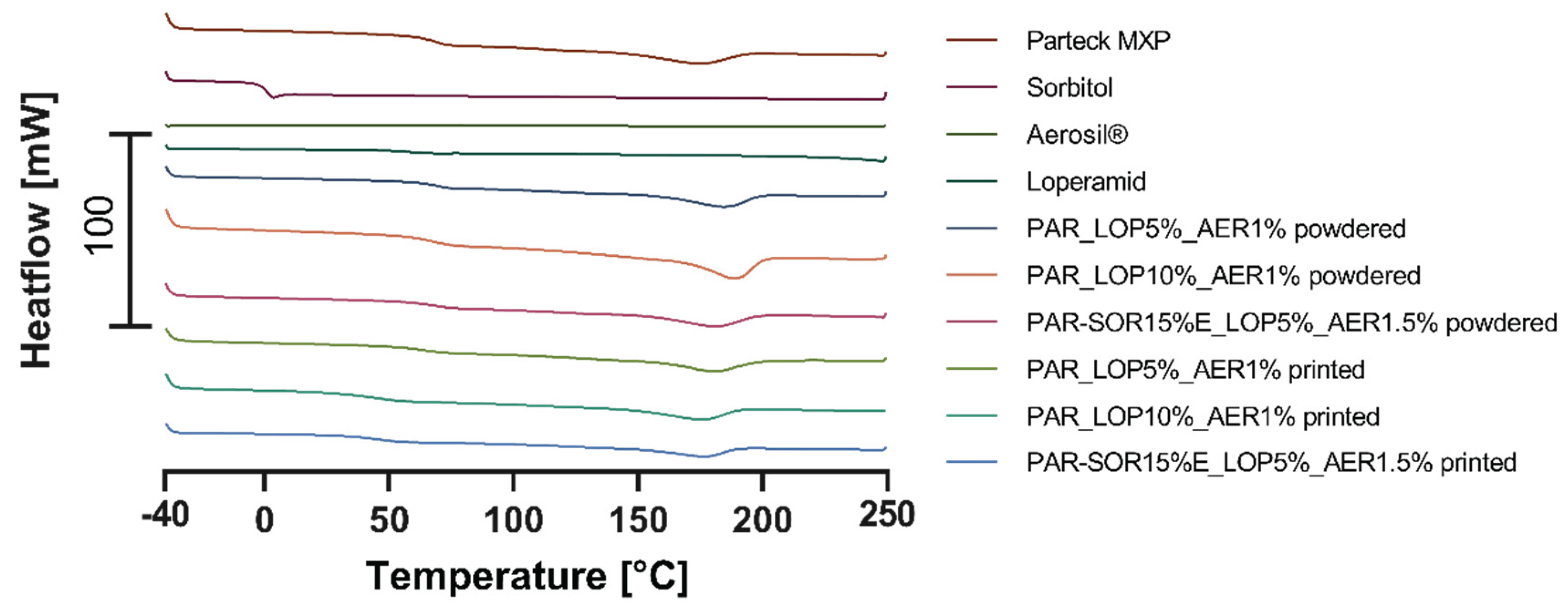
In the following diagrams, the thermograms of the measured samples (pure substances, powder mixtures, and printed tablets) are shown.
3.1.1. Physical Characterization Using DSC
Figure 3 contains the curves of the first heating run, where crystalline substances showed their melting point, while amorphous substances showed their glass transition temperature. Glass transition temperature of printed tablets was used as an indicator for the formation of ASDs.
Figure 4 contains the thermograms of the second heating run.
The second heating run is used to determine the glass transition temperature of previously crystalline substances and information about the solubility of the API and polymer for powdered mixtures.
Table 2 gives an overview of the measured melting and glass transition temperatures.
During the first heating, the melting point of sorbitol and loperamide was detected as well as the semicrystalline nature of PVA indicated by a glass transition at 48-55°C and a melting point of ∼180°C. During the second heating run the glass transition temperatures of sorbitol (-2.48°C – 2.50°C) and loperamide (50.92°C – 64.51°C) were found.
In the powdered samples, there were no signs of crystalline loperamide, or sorbitol found even during the first heating where crystalline loperamide was contained. This indicates a good solubility of loperamide in PVA at least at elevated temperatures. The missing individual glass transition of loperamide and sorbitol in the second heating indicates a miscible system formed from the API and the polymer and sorbitol, respectively.
Mixtures containing 15% sorbitol showed decreased glass transition temperature (31.95°C – 53.47°C for the powder and 34.42°C – 53.99°C for the printlet) indicating that miscible systems were formed from PVA and sorbitol. In these thermograms, only one glass transition temperature was found. This temperature is composed of the two individual temperatures one would find for non-miscible systems.
Concluding the DSC experiments, sorbitol showed to be a good choice for the use as a plasticizer and loperamide showed miscibility with the polymer in the measured concentrations.
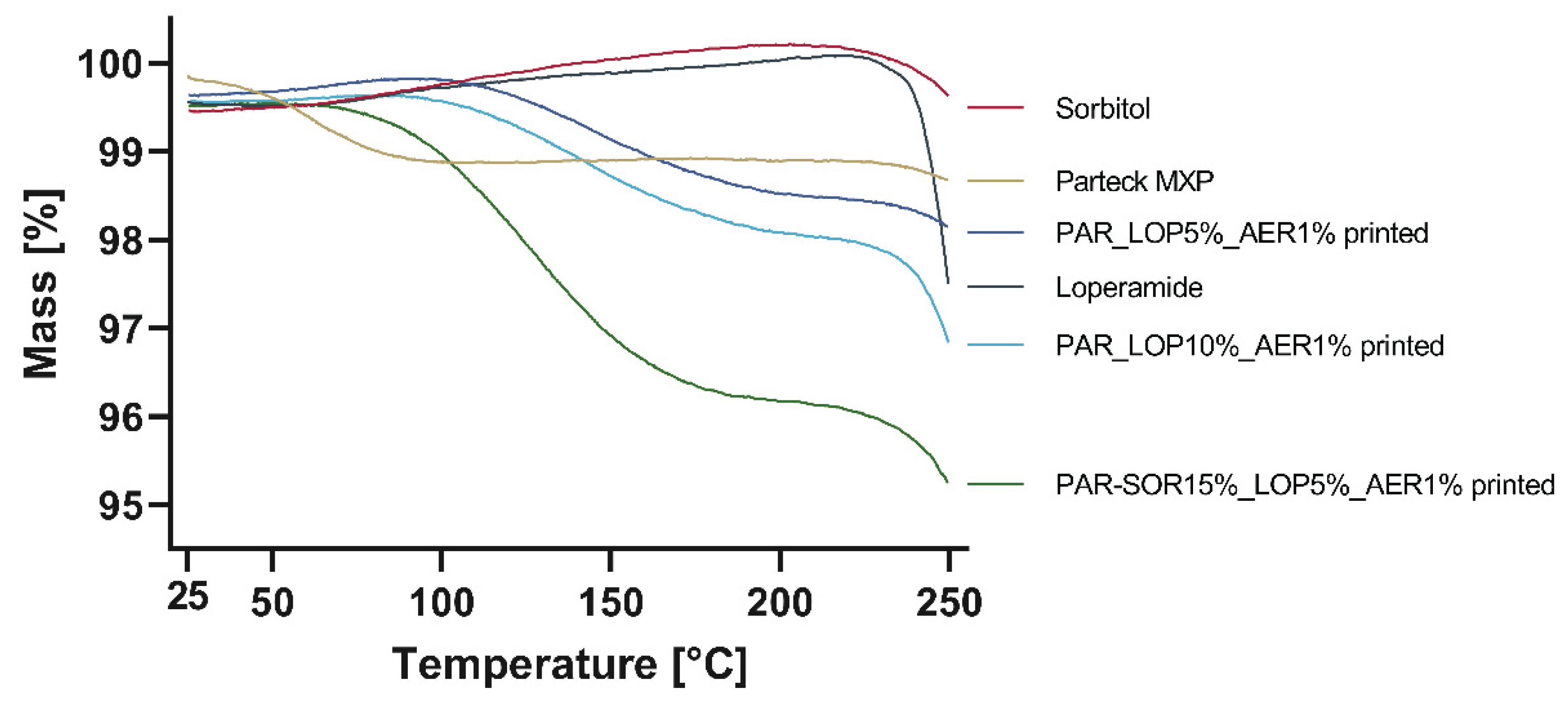
3.1.2. Results of Thermal Stability Using STA
Powdered samples were accurately weighed, while printed tablets were broken down and then weighed into the crucibles for analysis of thermal stability and potential water loss. Results are shown in
Figure 5.
Significant mass loss was found for Parteck MXP (1%), as well as for the printed tablets. An increased mass loss was found for the sample containing 15% sorbitol, which might be a result of water uptake from humidity during printing or storage. Loperamide and sorbitol were thermally stable up to at least 230°C as no degradation could be found.
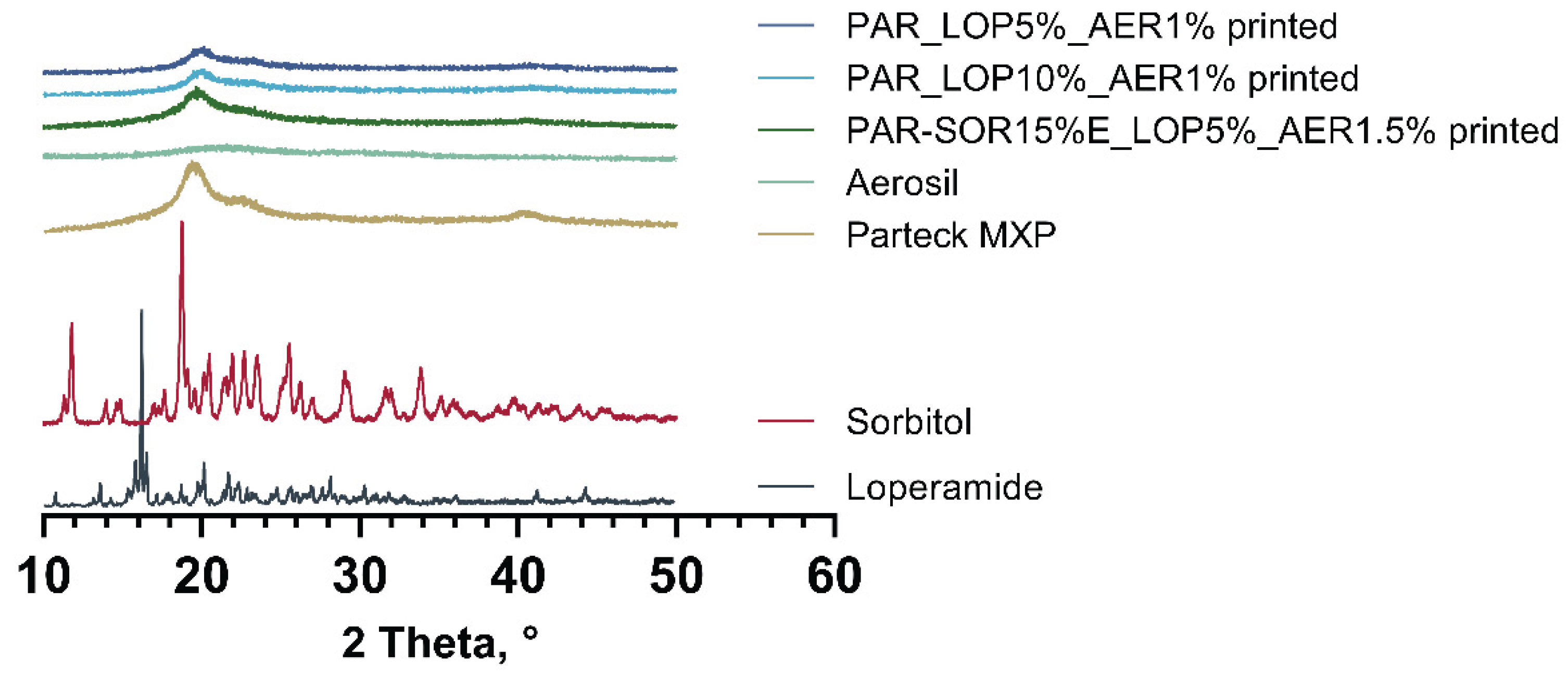
3.2. Physical State Examination - XRD
DSC measurements indicated the amorphous state of loperamide in PVA. To further harden this assumption, a second method to gain information about the physical state of the API at room temperature was implemented.
Using XRD whole printed tablets were measured, and the obtained spectra were compared to the spectra of pure substances. Results are given in
Figure 6.
Spectra obtained from loperamide, and sorbitol showed distinct peaks at a variety of angles which was expected from crystalline substances. In the printed tablets no signs of crystalline loperamide or sorbitol were found. The semicrystalline nature of PVA was found in the pure PVA as well as the printed tablets. This complies with results from thermal analysis, underpinning the amorphous nature of loperamide in the printed tablets.
3.3. 3D Printing
3.3.1. PVA without Plasticizer
Apart from the suitable temperature regarding melt viscosity, there are further parameters of interest for extrusion using a small-scale single-screw extruder. Powder flowability is a key characteristic since the powder must flow into the barrel before it can be melted and pushed through. As the mixtures showed insufficient flowability, adjustments were made by adding 1% of Aerosil® to ensure sufficient flow behavior.
Direct printing of Batch 1 (
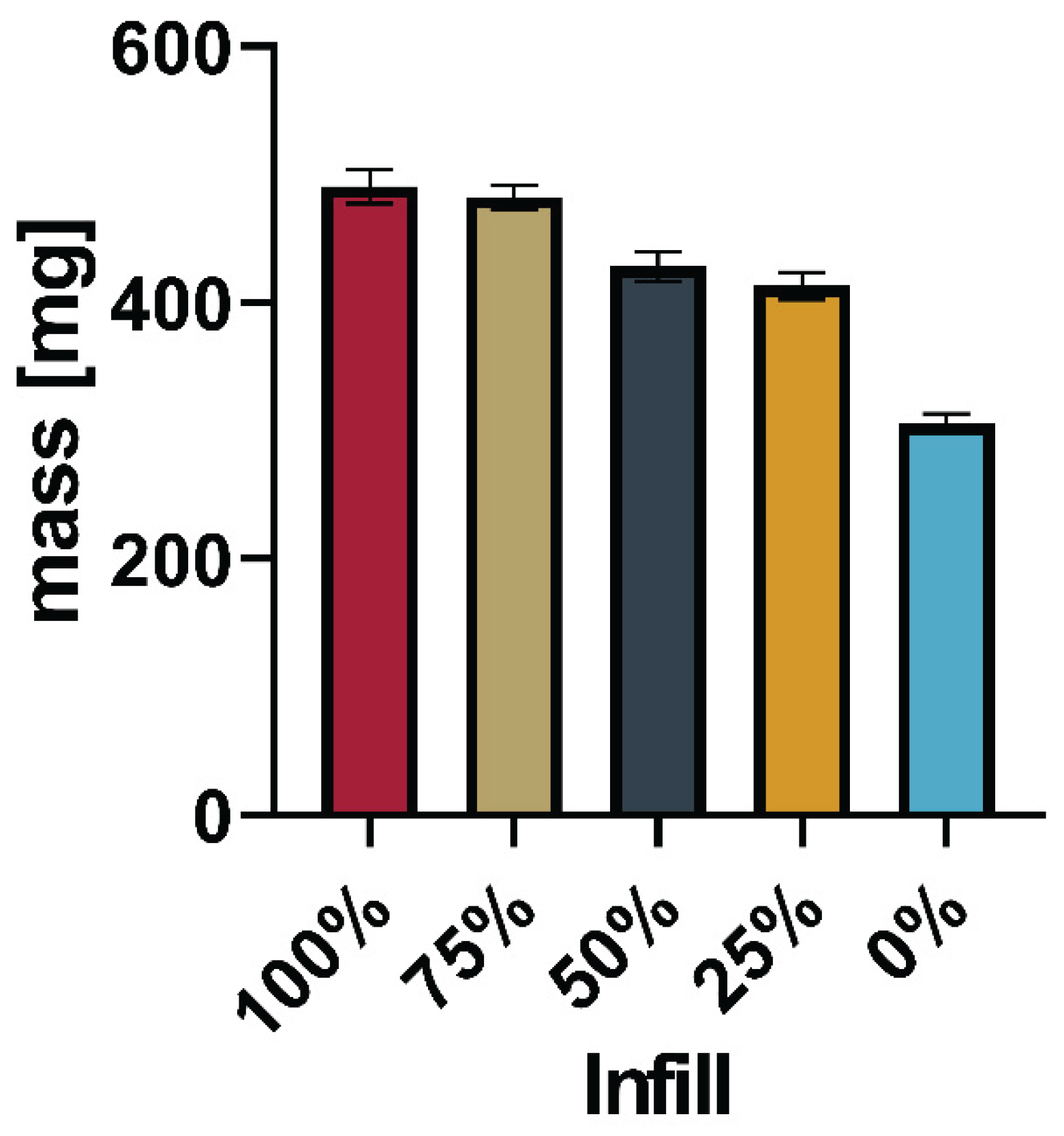
Table 1) was successful using 200°C nozzle temperature and 40°C print bed temperature. Tablets of different strengths were produced by varying the infill in 25% increments (100%, 75%, 50%, 25%, 0%). In the next iteration, the drug load was increased by doubling the API concentration in the powder mixture (Batch 2,
Table 1). Printing was successful at 205°C/40°C with tablets printed from 100% - 0% infill in 25% increments.
Figure 7,
Figure 8,
Figure 9 and
Figure 10 show the average mass including the standard deviation of the printed tablets as well as pictures of one tablet from each infill (FLTR: 100 – 0% Infill).
3.3.2. PVA with 15% Sorbitol
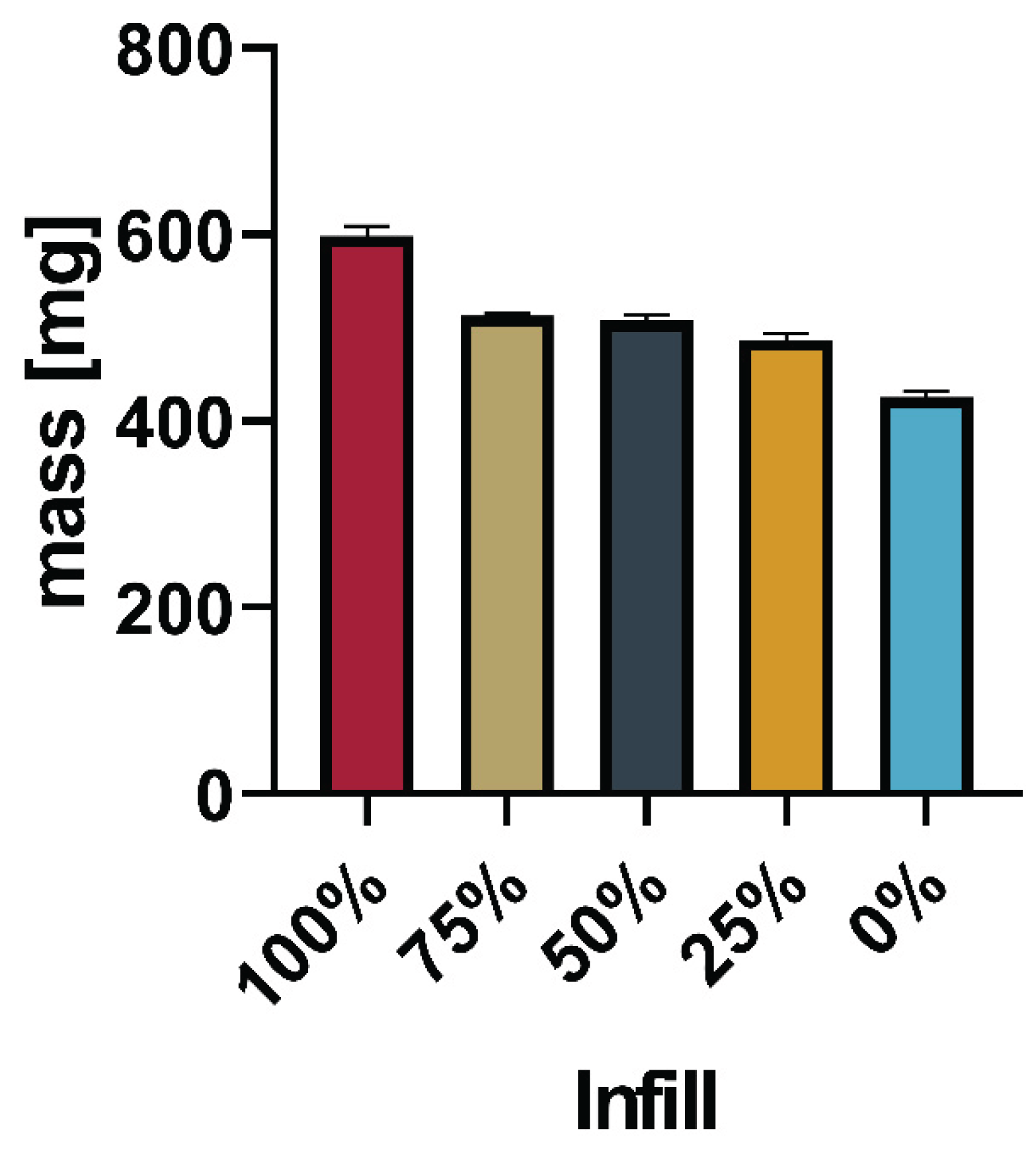
To decrease the dissolution times and reduce the printing temperature 15% sorbitol was added to the PVA. Batch 3 (
Table 1) was successfully printed using 185°C/40°C to print tablets varying in infill as seen in 3.4.1.
Figure 11 and
Figure 12 show the mass and standard deviation of the printed tablets and exemplary pictures of tablets from each infill percentage using the updated shape.
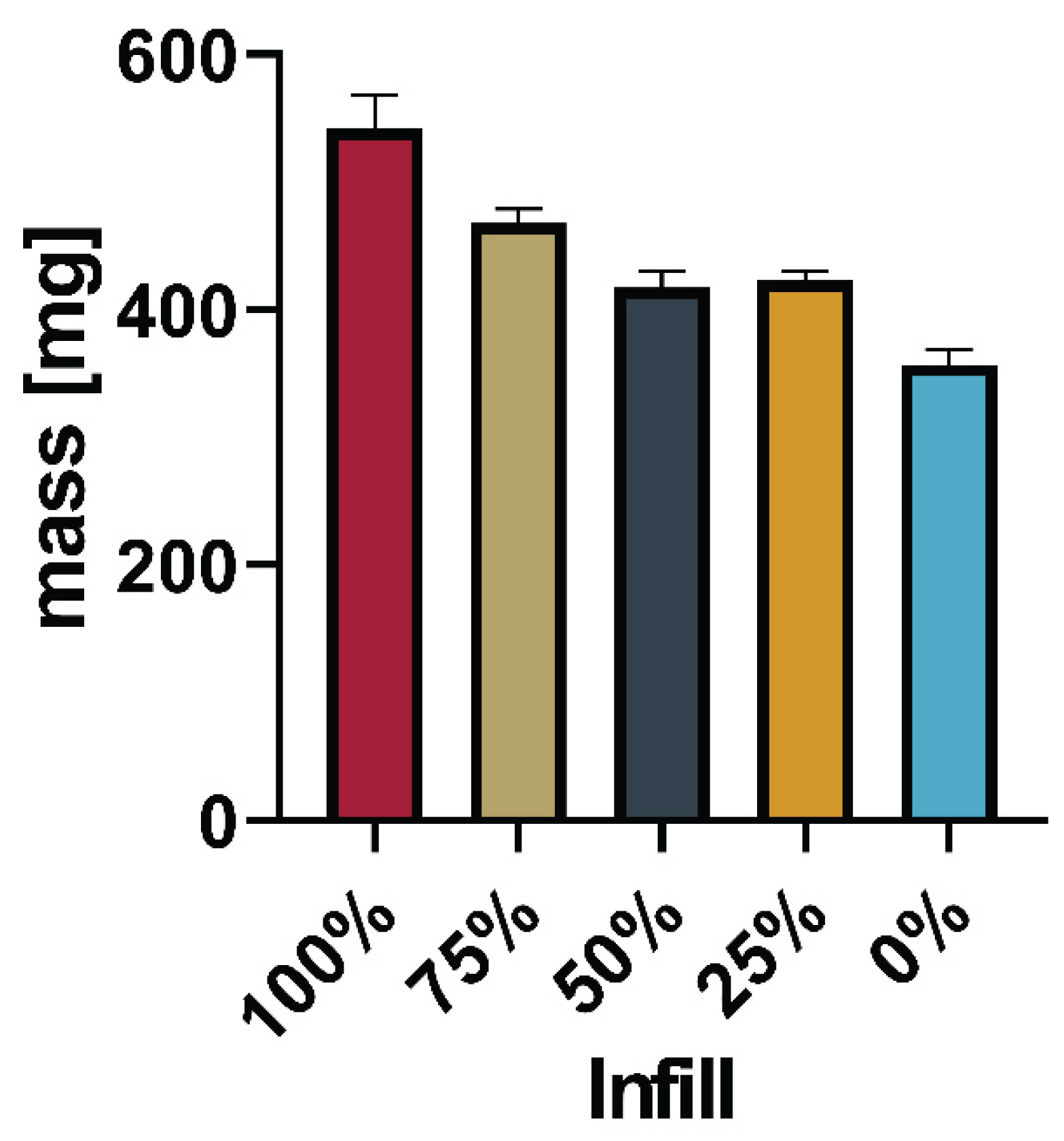
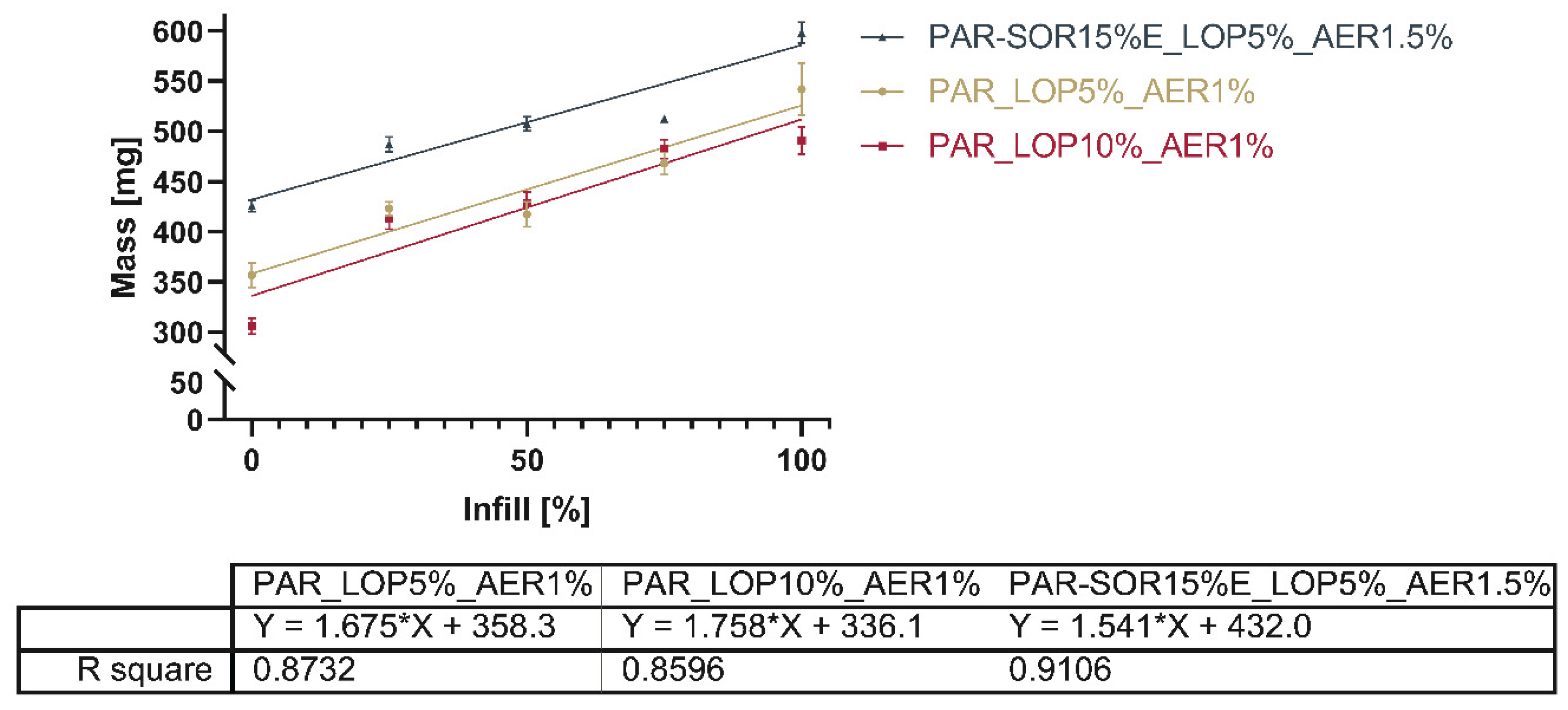
Figure 13 gives a comprehensive overview of the mass of all printed tablets.
The total mass of the printed tablets showed a reduction with the reduced infill, as expected.
Figure 13 shows the relation between the infill set in the slicing software and the actual mass of the printed tablets.
Linearity of the correlation between infill and total mass (as shown in
Figure 11) is found to be between 0.8596 and 0.9106. Comparison of the slopes showed no significant difference between the slopes (P=0.5053). This indicates that it is possible to dictate the drug load from the infill independent of the powder mixture used. The slicing software determined based on the shape of the object and nozzle size at which position additional material would be deposited. Differences between 25%, 50%, and 75% were not as predictable as the software suggested e.g., the difference in mass between the tablets with 25% and 50% infill was way smaller than the correlation seemed to predict.
3.4. Measurement of rheological properties of the mixtures in molten state
Determination of shear rate was conducted using tablets consisting of PAR-SOR15%E/LOP5%/AER1% printed at 185°C and 6 mm/s print speed. The print speed represents a consideration between decreasing the time needed to print a tablet and the torque capacity of the motor as well as finding a temperature where material could be printed without oozing from the nozzle.
Individual printing times of 558 ± 2.45 s were measured for a batch of five consecutive printed tablets. During this time 15.8 screw turns were recorded and an actual volume of the printlet of 0.461 ± 0.005 cm3 was measured, compared to the theoretical volume of 0.4936 cm3.
This results in a volume flow Q of 8.26*10-4 ± 7.80*10-6 cm3/s, which gives a shear rate in the nozzle of 16.43 ± 0.155 1/s.
For the same tablets couette shear rate was calculated using the aforementioned dimensions of the extruder screw used. With N (screw rotational speed) of 2.83*10*-2 ± 1.24 *10-4 1/s a Couette shear rate of 1.294 ± 5.68 *10-3 1/s was calculated. The calculated shear rates were intended to be used in combination with the rheological data to make a claim about the viscosity of a polymer melt suitable for printing.
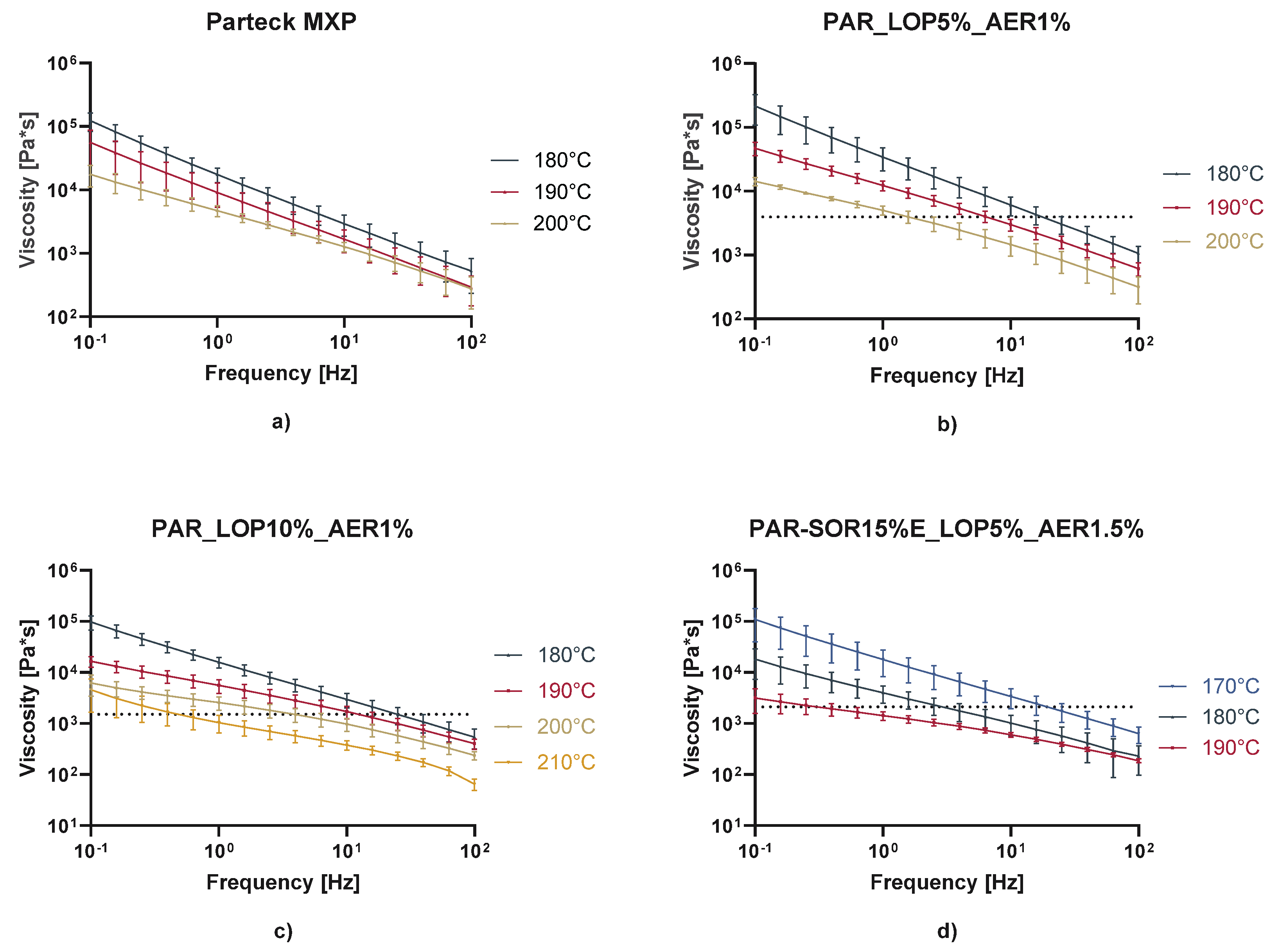
In rheologic measurements, the linear viscoelastic range (LVR) was determined via amplitude sweeps. A deformation of 1% was found to be in the LVR for all the samples at the measured temperatures. Afterwards frequency sweeps from 100 to 0.1 Hz were performed at temperature around the printing temperature (
Figure 14). As expected, viscosity decreased with increasing temperature, increasing frequency, and with the addition of the plasticizer sorbitol. Prior research indicates that usually viscosities from 100,000 to 10,000 Pa*s are found during HME processes [
25]. Maximum viscosities as seen in
Figure 14 needed at the lower calculated shear rate (Couette: 1.294 1/s) were found at ranges from 1500 – 4000 Pa*s, which indicates that a rather low melt viscosity is needed to print successfully with the M3dimaker. The dashed lines represent the viscosity of the mixture at the calculated shear rate and temperature used to print.
This correlates well with the fact, that compared to lab-scale extruders this printer comprises a very small screw and a motor of lower torque to keep the weight down and increase the precision of the printing. We propose that the selection of polymer and its expected printing temperature should include frequency sweeps with increasing temperature until a viscosity of a maximum of 5000 Pa*s is measured.
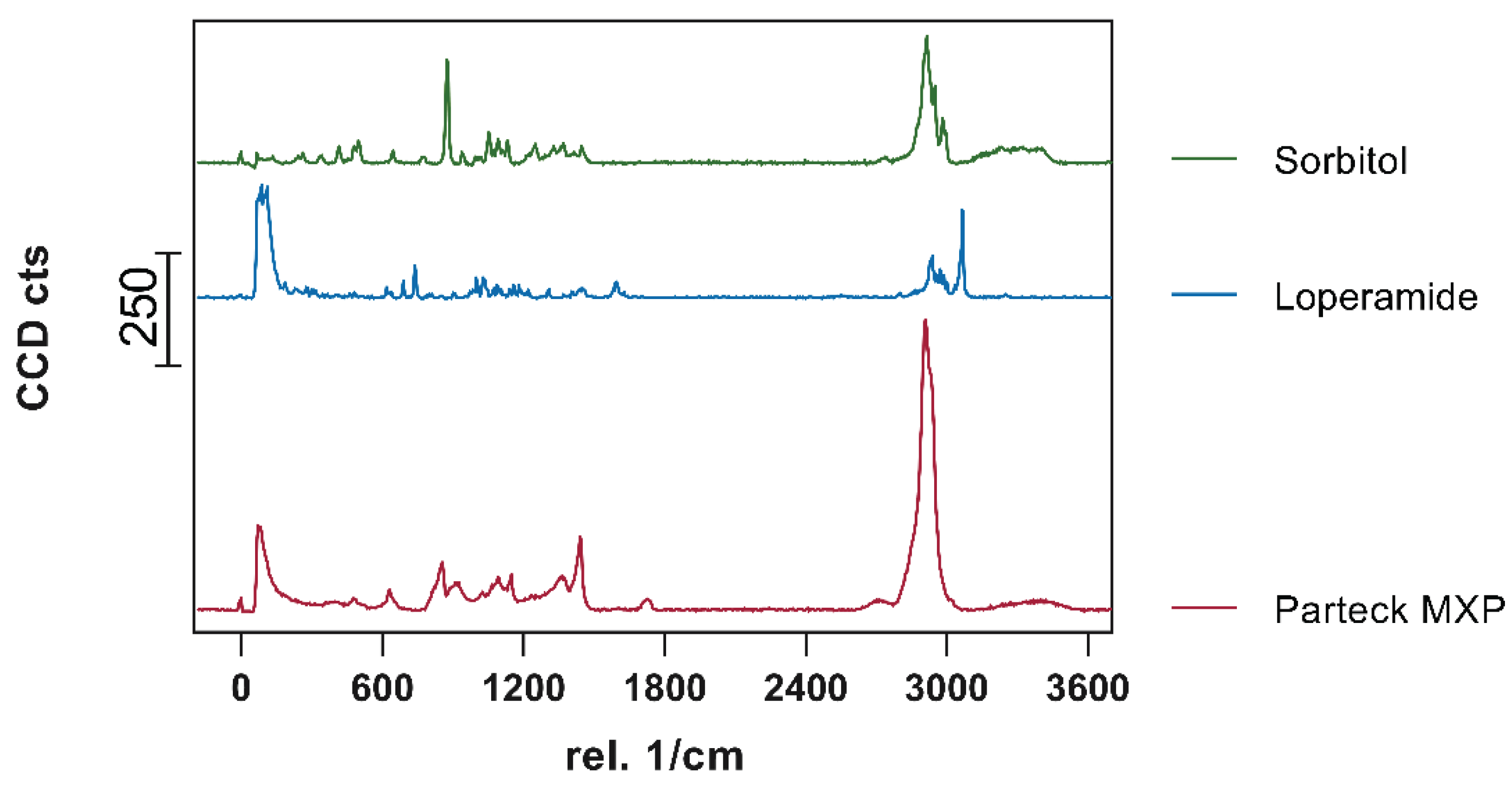
3.5. Confocal Raman Microspectroscopy
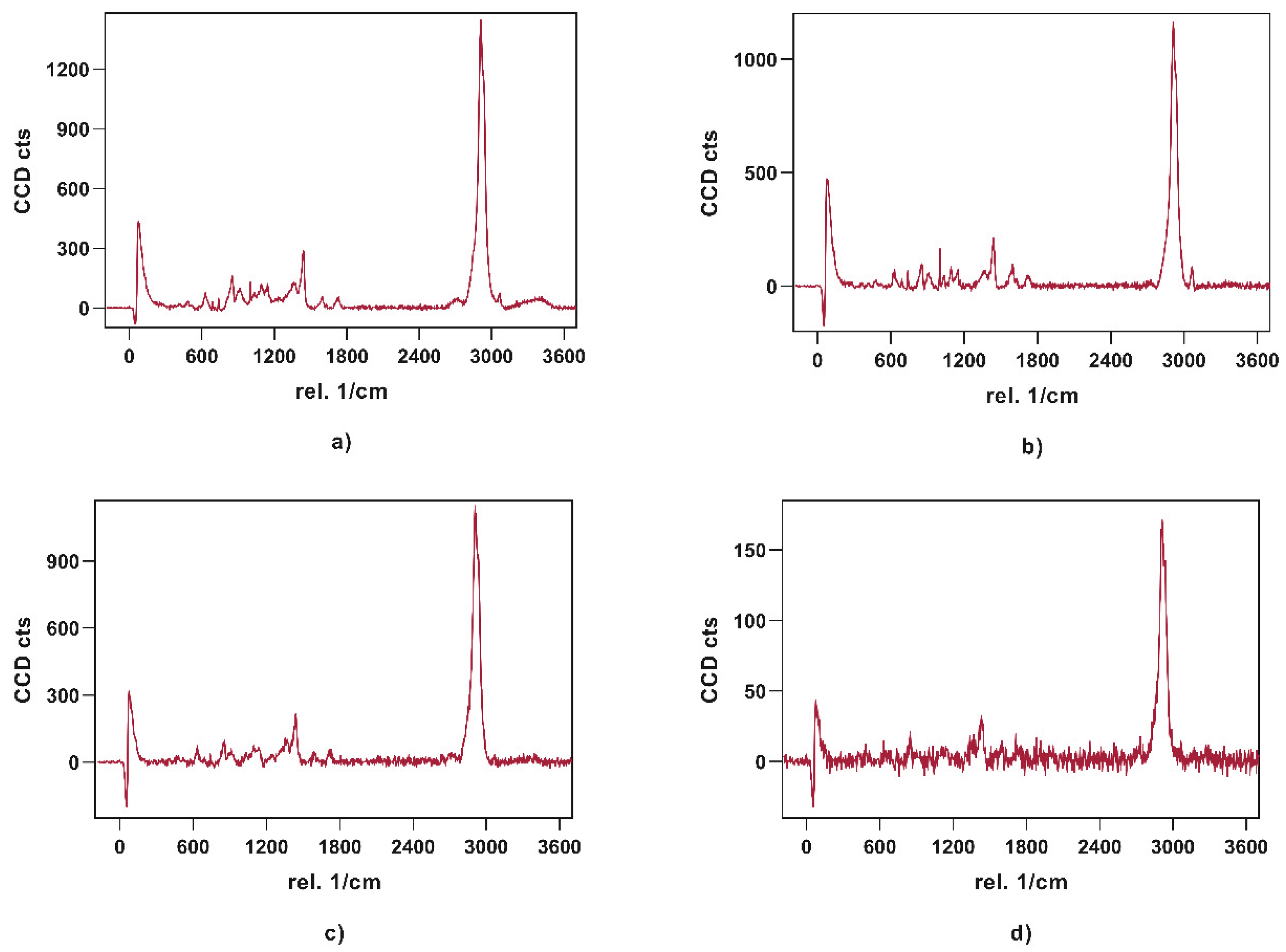
Visualization of API content, distribution, and potential recrystallization can be achieved via confocal Raman microspectroscopy (CRM). The overlay of the single spectra of loperamide, sorbitol, and PVA showed peaks that can be used for the evaluation of contained API in the printed tablets. The peak in the high wavenumber range (3040 – 3090 1/cm) as well as the peak in the fingerprint region (1575 – 1615 1/cm) have no significant overlapping with peaks from other substances and can be used to identify loperamide. The low drug load of 5 and 10%, respectively lead to a decreased signal intensity of these peaks in the resulting spectra of printed tablets relative to the single spectra obtained from pure substances.
Figure 15.
Obtained single spectra (0.5 s; 10 Acc.) red = Parteck MXP, blue = loperamide, green = sorbitol.
Figure 15.
Obtained single spectra (0.5 s; 10 Acc.) red = Parteck MXP, blue = loperamide, green = sorbitol.
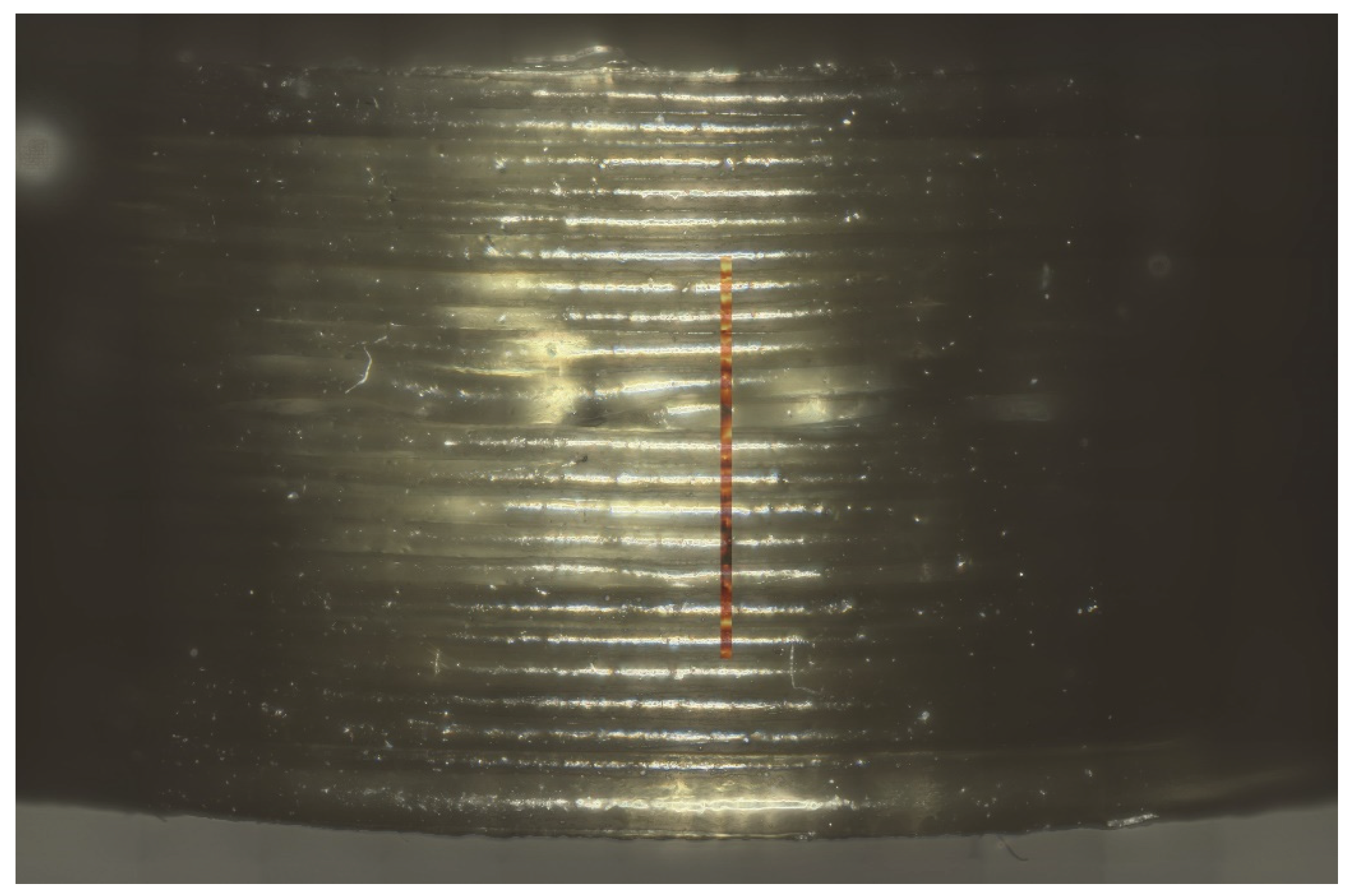
Figure 16 shows an example of the measured area on the side of the printed tablet. The true surface module was able to cover most of the differences in the height of the surface area. Fine-tuning to achieve good spectra was done via auto-focus.
Obtained signals were color-coded (
Figure 18) and exemplary spectra were given for the individual colors used (
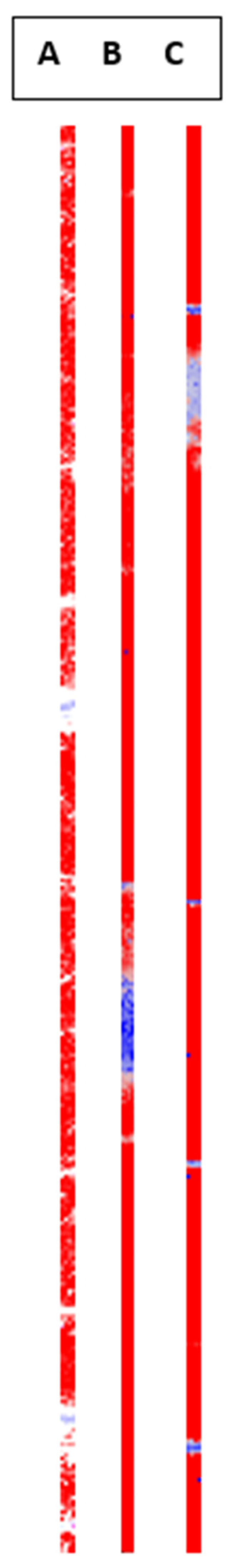
Figure 17). Red shows signal where loperamide was found, and spectrum intensity was sufficient. With the loss in signal intensity (as seen in the lighter and blue-colored regions) the signal-to-noise ratio of the loperamide peaks gets worse, although loperamide concentration remains constant.
Raman imaging was able to show consistent spectra containing polymer and API over a range of points distributed over roughly 40% of the printing time. No “hotspots” or crystalline API were found during these measurements, which supports findings from the XRPD measurements of a uniform ASD.
3.6. Drug Content
Since the powder mixtures contained 5% (m/m) and 10% of API respectively, the expected drug content was calculated as 5% and 10% respectively, of the total mass of the printed tablets The actual drug content was assessed to make sure the correct amount was referred to as 100% release during in vitro-measurements. By dissolving the whole tablet in a suitable medium the actual drug content was determined. The results of this experiment are given in
Table 3.
The drug content shown in
Table 3 deviates from the expected amount. Deviations in content from the expected amount in the powdered samples can be attributed to adhesion to mixing vessels and spatulas used. The reduction in the measured content of the printed tablets can partially be explained by the water uptake, which increases the determined total mass of the tablet without adding further API. Also, adhesion to the screw in the extruder is a possible explanation.
3.7. In-Vitro Dissolution Testing of Printed Tablets
While four tablets were tested at once to show the average ± SD. Total drug content was calculated from the mass of the printed tablet as well as the measured content shown in
Table 3. The cumulative released drug was calculated and put into relation to the measured content to give the percentage released.
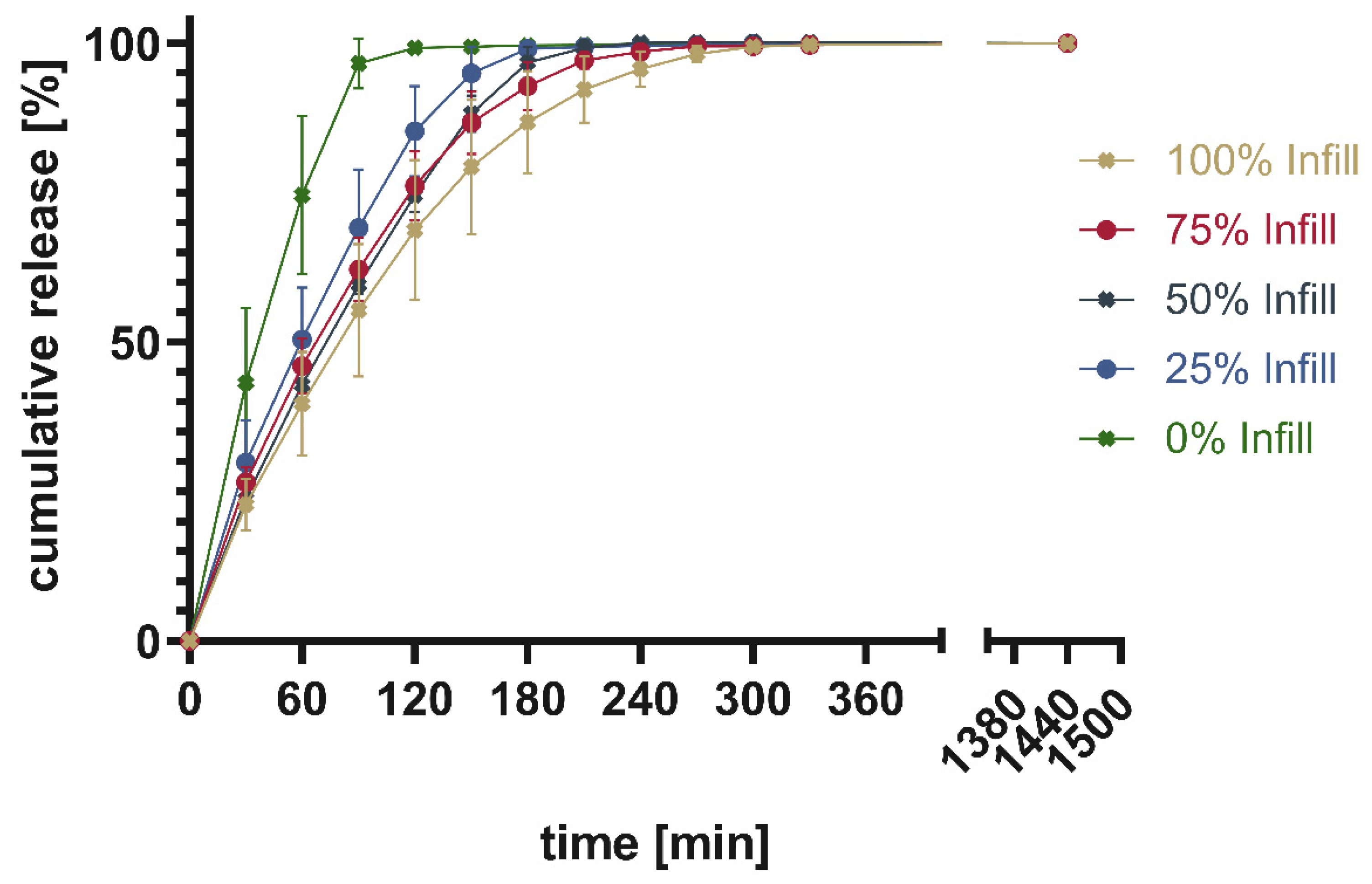
Figure 19 shows the percentage of cumulative drug release from PAR_LOP5%_AER1%, while
Figure 20 and
Figure 21 show the release of PAR_LOP10%_AER1% and PAR-SOR15%E_LOP5%_AER1.5%, respectively.
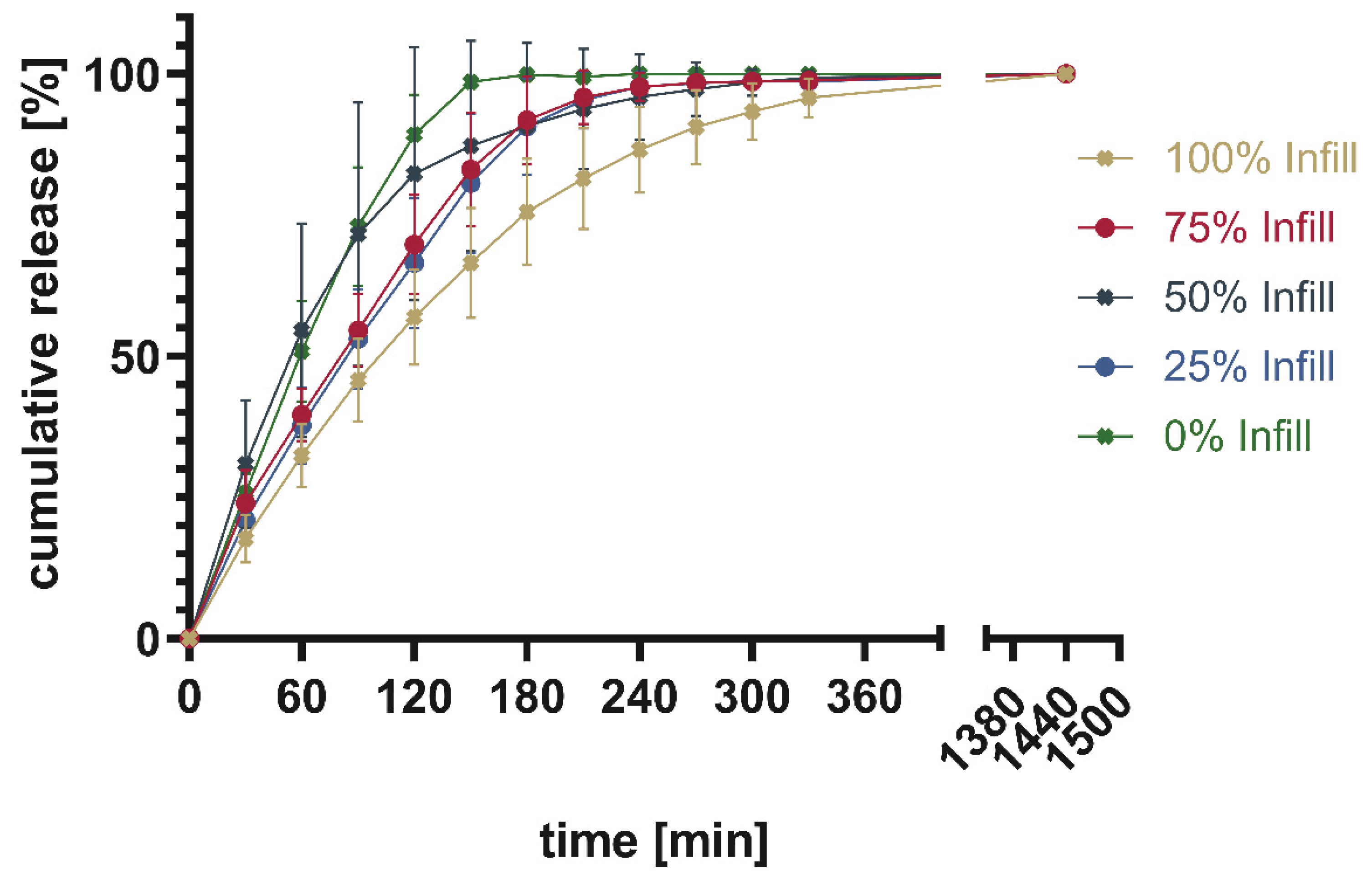
The release patterns of loperamide from printed PVA or PVA/sorbitol mixtures show the higher the percentage infill of the tablets the slower the release. This matches the expectations based on existing research [
26]. Unexpectedly, overall, a very slow release was found for PVA without a plasticizer. This indicates that the incorporation of a poorly water-soluble drug into PVA resulted in slower release. The addition of 15% sorbitol improved the dissolution time of the printed tablets. Still, the amount of infill showed a larger impact on the dissolution time, than the addition of the highly water-soluble plasticizer. This can be explained by the different infill resulting in changes in the area-to-volume ratio. As soon, as the outer layers of the tablet are penetrated by media, tablets with lower infill offer a larger area, and thus, the dissolution rate increases.
4. Conclusion
Direct powder extrusion and 3D printing of tablets was achieved with formulations containing PVA, sorbitol, and loperamide using the M3dimaker 3D printer. In printed tablets, loperamide appeared to form amorphous solid dispersions as characterized by DSC and XRPD. Confocal Raman microspectroscopy showed homogenous distribution of the API during the print. Printing temperature was sufficiently low to encounter no thermal degradation of API, polymer, or other excipients. By adding the API after extruding the other excipients, one heating step and therefore potential thermal degradation for the API could be eliminated. Control over the drug content was realized by changing the amount of infill. While this is a proven way to change drug content one must keep in mind, that changes in the area/volume ratio also can impact the release behavior. If the acceleration of dissolution time is aimed for, the addition of (super)disintegrants could be a promising approach.
From the characterization of the melt properties of the mixtures, a system for the selection of polymers is proposed. Extrudability as well as printability seem to work sufficiently with melt viscosity below 4000 Pa*s. This value could be used in future trials to reduce material use during preliminary tests. The range of usable polymers and even working temperatures for these polymers could be evaluated by conducting rheological tests.
Author Contributions
Conceptualization, J.L. and D.J.L; methodology, J.L.; investigation, J.L., and F.P.; data curation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, D.J.L.; supervision, D.J.L, and K.G.W.; project administration, D.J.L; All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support from the Open Access Publication Fund of the University of Tübingen.
Acknowledgments
Special thanks to Dr. Yucang Liang (Institute of Inorganic Chemistry, University of Tuebingen) for measuring XRD-Data. Also special thanks to Dominik Fauser (Institute of Applied Mechanics (civil engineering), University of Stuttgart) for support with rheological studies above 200°C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- West, T.G.; Bradbury, T.J. 3D Printing: A Case of ZipDose® Technology - World’s First 3D Printing Platform to Obtain FDA Approval for a Pharmaceutical Product. In 3D and 4D Printing in Biomedical Applications; Maniruzzaman, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 53–79. ISBN 9783527813704. [Google Scholar]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. International Journal of Pharmaceutics 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. International Journal of Pharmaceutics 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, F.; Wang, B.; Wu, Y.; Luo, Q.; Zuo, X.; Liu, X.; Cao, L.; Li, M.; Lu, H.; et al. Melt extrusion deposition (MED™) 3D printing technology - A paradigm shift in design and development of modified release drug products. International Journal of Pharmaceutics 2021, 602, 120639. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W. Entwicklung und Charakterisierung 3D-gedruckter, wirkstoffhaltiger Darreichungsformen am Beispiel von Tabletten und Implantaten; Universität Greifswald, 2018.
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. International Journal of Pharmaceutics 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. International Journal of Pharmaceutics 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Molecular pharmaceutics 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. International Journal of Pharmaceutics 2018, 549, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef] [PubMed]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: a review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. International Journal of Pharmaceutics 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. International Journal of Pharmaceutics 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. International Journal of Pharmaceutics 2019, 567, 118471. [Google Scholar] [CrossRef] [PubMed]
- Chavda, H.V.; Patel, C.N.; Anand, I.S. Biopharmaceutics classification system. Syst Rev Pharm 2010, 1, 62. [Google Scholar] [CrossRef]
- Wei, C.; Solanki, N.G.; Vasoya, J.M.; Shah, A.V.; Serajuddin, A.T.M. Development of 3D Printed Tablets by Fused Deposition Modeling Using Polyvinyl Alcohol as Polymeric Matrix for Rapid Drug Release. J. Pharm. Sci. 2020, 109, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M.; Artursson, P.; Bergström, C.A.S. A modified physiological BCS for prediction of intestinal absorption in drug discovery. Molecular pharmaceutics 2010, 7, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, E.S.; Neumann, D.; Gryczke, A.; Wagner, K.G. Micro-scale prediction method for API-solubility in polymeric matrices and process model for forming amorphous solid dispersion by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2016, 107, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Boetker, J.; Water, J.J.; Aho, J.; Arnfast, L.; Bohr, A.; Rantanen, J. Modifying release characteristics from 3D printed drug-eluting products. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences 2016, 90, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Rauwendaal, C. Polymer extrusion, 5th edition; Hanser Publications; Hanser Publication: Munich, Cincinnati, 2014; ISBN 9781569905166. [Google Scholar]
- Treffer, D.; Troiss, A.; Khinast, J. A novel tool to standardize rheology testing of molten polymers for pharmaceutical applications. International Journal of Pharmaceutics 2015, 495, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Jaluria, Y. Heat and Mass Transfer in the Extrusion of Non-Newtonian Materials. In Transport Phenomena in Materials Processing; Elsevier: Amsterdam, The Netherlands, 1996; pp. 145–230. ISBN 9780120200283. [Google Scholar]
- CHMP/ICH. Q 2 (R1) Validation of Analytical Procedures: Text and Methodology.
- FDA/CDER/”Yeaton, A. Guidance for Industry. In Managing the Documentation Maze; Gough, J., Nettleton, D., Eds.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2010; pp. 431–450. ISBN 9780470597507. [Google Scholar]
- LaFountaine, J.S.; McGinity, J.W.; Williams, R.O. Challenges and Strategies in Thermal Processing of Amorphous Solid Dispersions: A Review. AAPS PharmSciTech 2016, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
Figure 3.
Thermograms of the first heating.
Figure 3.
Thermograms of the first heating.
Figure 4.
Thermograms of the second heating.
Figure 4.
Thermograms of the second heating.
Figure 5.
Change of mass during heating up to 250°C.
Figure 5.
Change of mass during heating up to 250°C.
Figure 6.
XR(P)D-Spectra of pure substances and printed tablets.
Figure 6.
XR(P)D-Spectra of pure substances and printed tablets.
Figure 7.
Masses of printed tablets from PAR_LOP5%_AER1% (N=5 ± SD).
Figure 7.
Masses of printed tablets from PAR_LOP5%_AER1% (N=5 ± SD).
Figure 8.
Printed tablets from PAR_LOP5%_AER1% (fltr.: 100-0% infill).
Figure 8.
Printed tablets from PAR_LOP5%_AER1% (fltr.: 100-0% infill).
Figure 9.
Masses of printed tablets from PAR_LOP10%_AER1% (N=5 ± SD).
Figure 9.
Masses of printed tablets from PAR_LOP10%_AER1% (N=5 ± SD).
Figure 10.
Printed tablets from PAR_LOP10%_AER1% (fltr.: 100–0% infill).
Figure 10.
Printed tablets from PAR_LOP10%_AER1% (fltr.: 100–0% infill).
Figure 11.
Masses of printed tablets from PAR-SOR15%E_LOP5%_AER1.5% (N=5 ± SD).
Figure 11.
Masses of printed tablets from PAR-SOR15%E_LOP5%_AER1.5% (N=5 ± SD).
Figure 12.
Printed tablets from PAR-SOR15%E_LOP5%_AER1.5% (fltr.: 100-0% infill).
Figure 12.
Printed tablets from PAR-SOR15%E_LOP5%_AER1.5% (fltr.: 100-0% infill).
Figure 13.
Linearity of the relation between set infill and measured mass.
Figure 13.
Linearity of the relation between set infill and measured mass.
Figure 14.
Melt viscosity of neat Polymer and printed formulations.
Figure 14.
Melt viscosity of neat Polymer and printed formulations.
Figure 16.
Picture of tablet printed from PAR_LOP5%_AER1% with an overlay of the measured area by true surface model.
Figure 16.
Picture of tablet printed from PAR_LOP5%_AER1% with an overlay of the measured area by true surface model.
Figure 17.
Exemplary spectra obtained from the measured area; A: PAR_LOP5%_AER1%, B: PAR_LOP10%_AER1%, C: PAR-SOR15%E_LOP5%_AER1.5%, D: failed focus or bad spectrum found in the light and blue areas.
Figure 17.
Exemplary spectra obtained from the measured area; A: PAR_LOP5%_AER1%, B: PAR_LOP10%_AER1%, C: PAR-SOR15%E_LOP5%_AER1.5%, D: failed focus or bad spectrum found in the light and blue areas.
Figure 18.
Distribution of loperamide based on the true component analysis is shown for all three printed tablets.
Figure 18.
Distribution of loperamide based on the true component analysis is shown for all three printed tablets.
Figure 19.
Cumulative release given in percentage of calculated drug load for PAR_LOP5%_AER1%.
Figure 19.
Cumulative release given in percentage of calculated drug load for PAR_LOP5%_AER1%.
Figure 20.
Cumulative release given in percentage of calculated drug load for PAR_LOP10%_AER1%.
Figure 20.
Cumulative release given in percentage of calculated drug load for PAR_LOP10%_AER1%.
Figure 21.
Cumulative release given in percentage of calculated drug load for PAR-SOR15%E_LOP5%_AER1.5%.
Figure 21.
Cumulative release given in percentage of calculated drug load for PAR-SOR15%E_LOP5%_AER1.5%.
Table 2.
Comprehensive results of glass transition temperatures found during DSC experiments.
Table 2.
Comprehensive results of glass transition temperatures found during DSC experiments.
Glass transition
first Heating |
Onset [°C] |
Endset [°C] |
Glass transition
second Heating |
Onset [°C] |
Endset [°C] |
| PVA |
48.72 |
55.86 |
PVA |
61.11 |
72.52 |
| SOR |
crystalline, Mp: 89.76 °C |
SOR |
-2.48 |
2.5 |
| AER |
N/A |
AER |
N/A |
| LOP |
crystalline, Mp: 227.53 °C |
LOP |
50.92 |
64.51 |
| P1 powder |
46.92 |
54.66 |
P1 powder |
61.81 |
73.42 |
| P1 printed |
56.35 |
62.68 |
P1 printed |
59.04 |
74.22 |
| P2 powder |
44.6 |
50.75 |
P2 powder |
58.44 |
74.61 |
| P2 printed |
55.89 |
63.85 |
P2 printed |
54.81 |
72.74 |
| P3 powder |
N/A |
P3 powder |
40.62 |
62.51 |
| P3 printed |
N/A |
P3 printed |
33.97 |
52.53 |
Table 3.
Loperamide content in powdered and printed samples.
Table 3.
Loperamide content in powdered and printed samples.
| |
PAR_LOP5%_AER1% |
PAR_LOP10%_AER1% |
PAR-SOR15%E_LOP5%_AER1% |
| |
Powdered |
Printed |
Powdered |
Printed |
Powdered |
Printed |
| Mean API content |
92.63% |
95.13% |
88.49% |
82.99% |
95.85% |
85.40% |
| Standard deviation |
2.74% |
12.88% |
1.50% |
1.30% |
5.65% |
0.92% |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).