1. Introduction
AF is a common arrhythmia, with an estimated prevalence of 3% in adults [
1], and is associated with an elevated risk of stroke, heart failure, and premature death [
2]. However, early detection of AF, particularly paroxysmal AF, is very challenging due to its asymptomatic or infrequent nature. Even when patients present with symptoms such as palpitations or chest discomfort, standard ECG examinations often show SR. Some studies suggest that the progression of AF can induce electrical and structural changes, manifesting as subtle patterns on normal SR ECGs [
3]. However, currently it remains difficult for cardiologists to manually distinguish AF on ECGs with normal SR.
With the rapid progress and breakthrough brought from the artificial Intelligence (AI) technology, serval studies demonstrated that some subtle signals caused from clinically important phenomena can be detected with AI in ECG data that are imperceptible to the human eye [
4]. Some studies have reported promising results from well-trained AI models in extracting relevant features from subtle pattern changes in 12-lead ECGs [
3,
5,
6]. However, these studies often encountered imbalanced datasets between the positive and the negative classes. Furthermore, each patient had unequal numbers of ECG records included in training, validation and testing datasets, which could potentially mislead the prediction accuracy and the estimated area under the receiver operating characteristic (ROC) curve (AUC) [
7,
8]. Variations exist among various studies, particularly in four key aspects: dataset composition and pre-processing, types of model input, deep learning model architectures, and classification approaches [
9]. Despite these advancements, much of the existing literature remains confined to academic research and lacks exploration into the feasibility and efficiency of methods for deploying edge AI.
In this study, we proposed a novel approach to convert standard 10-second, 12-lead ECGs into binary images for model input and design a lightweight CNN model to enable real-time AF-risk detection on edge ECG devices. The dataset was well-balanced between the AF and non-AF groups, with each patient contributing an equal number of ECG data, specifically one ECG data per patient for testing. Performance evaluation and statistical analysis were conducted using internal and external testing datasets collected from diverse clinical facilities in Japan.
2. Methods
2.1. Ethics and Data Collection
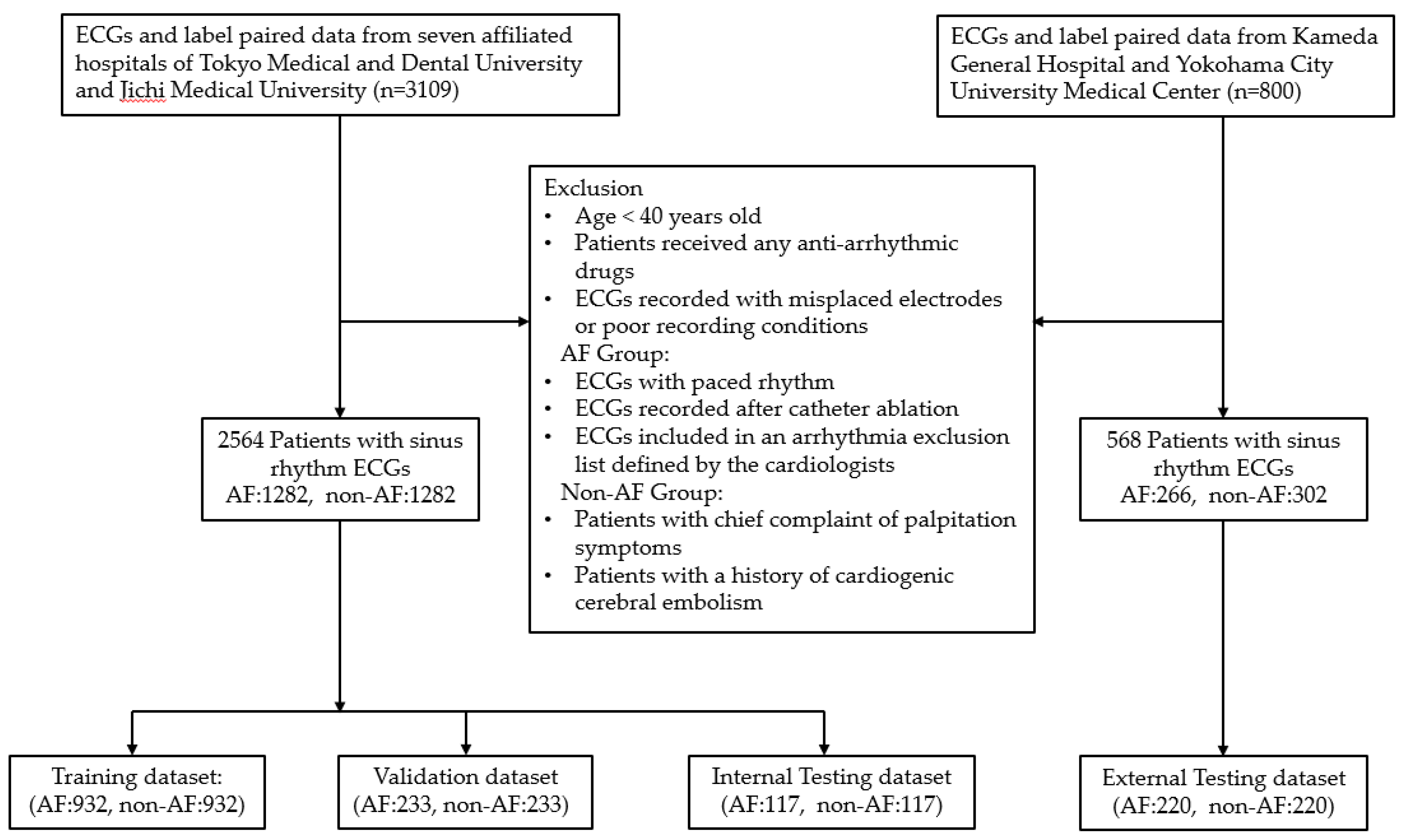
Approval for data collection was obtained from the Ethics Committees of Tokyo Medical and Dental University. A total of 3109 ECGs of 2930 patients aged over 40 years were retrospectively collected from seven affiliated hospitals between September 2019 and March 2022. The study adhered to the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Education of Japan in 2015. Only data from individuals who provided consent were used, and all records were anonymized. All ECGs were recorded at a sampling rate of 500Hz with 10-second length using FCP-8800 ECG machines manufactured by Fukuda Denshi, Tokyo, Japan. Diagnostic labels were assigned by trained physicians under the supervision of cardiologists.
The flowchart of data collection and data composition is presented in
Figure 1. A total of 1668 ECGs from 1489 patients with AF records and 1441 ECGs from 1441 non-AF patients were initially collected. After applying exclusion criteria and selecting one 12-lead SR ECG data per patient, from both groups, three datasets were prepared: a training and validation dataset with a ratio of 8:2, comprising 2330 ECGs (AF:1165, non-AF:1165). The remaining 234 ECGs (AF:117, non-AF:117) were used as the internal testing dataset.
Additionally, to assess the generalization ability and external performance validation of AF-risk detection, 800 (AF:400, non-AF:400) more ECGs with paired label data were retrospectively collected from Kameda General Hospital and Yokohama City University Medical Center from April 2023 to July 2023. Approval for data collection was obtained from the Ethics Committees of these two facilities. All ECG records were anonymized and an opt-out form on a website was used as an acceptable method to obtain consent from the patients. These two distinct facilities did not contribute any data to the model training. The same SR ECGs inclusion and exclusion criteria described above were applied for data selection. According to the determined sample size for performance validation, a total of 220 ECGs from 220 patients in the AF group were randomly selected, and an equal number of SR-ECGs with similar patient characteristics were matched from the non-AF group, resulting in an external testing dataset comprising 440 ECGs (AF:220, non-AF:220).
2.2. Identifying Study Groups and Selecting SR ECGs
Both the digital SR ECGs and the extracted labels of the included patients were collected. The dataset was divided into two groups: one group labeled as AF, consisting of patients with at least one documented AF episode within the past 2 years before the collected SR ECGs, and the other labeled as non-AF, consisting of patients without any chief complaint of palpitation symptoms and without an AF diagnostic code in their electronic medical records. Patients with an AF diagnostic code but no corresponding ECG documentation of AF were excluded from the performance analysis to mitigate ambiguity.
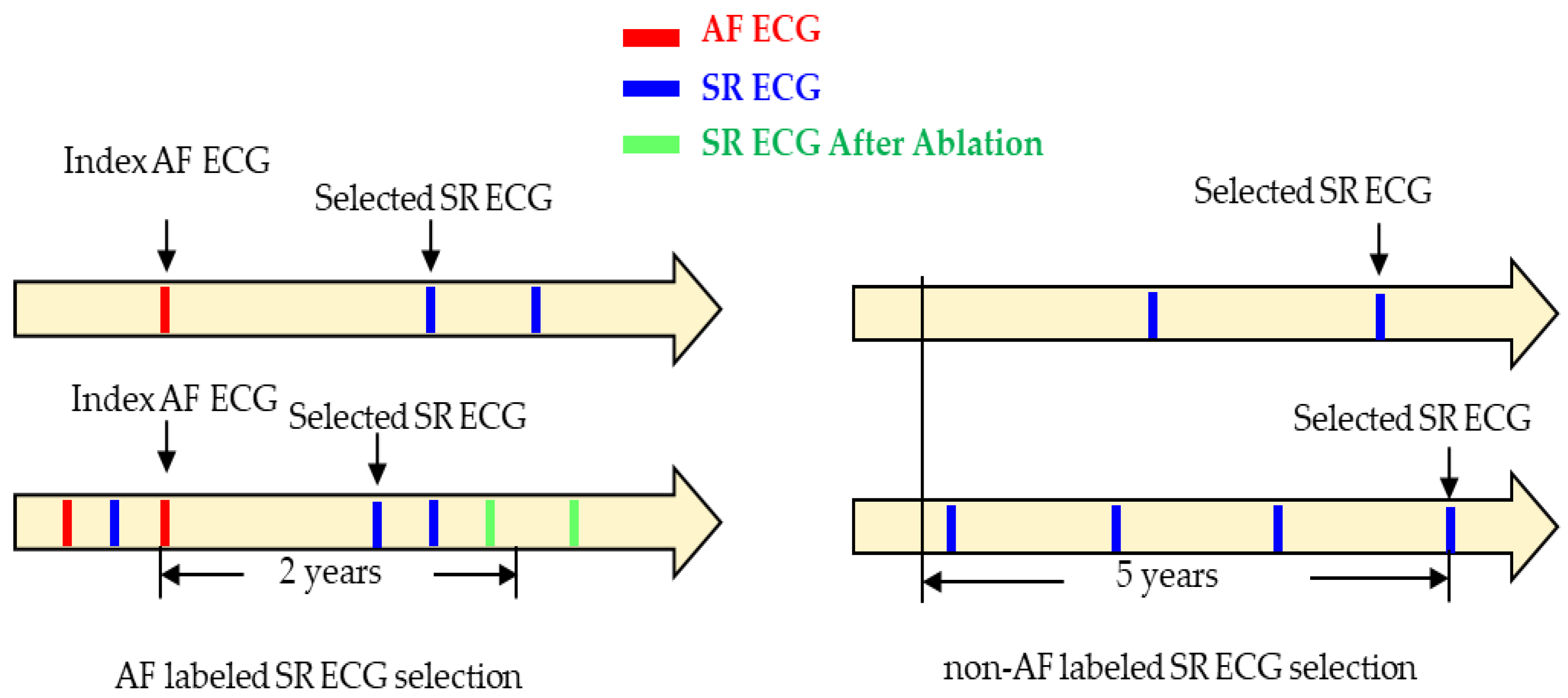
The inclusion criteria for selecting SR ECGs in both groups are illustrated in
Figure 2. For the AF group, the last event of AF ECG was served as an index, and SR ECGs within 2 years following this index were considered for selection. If multiple SR ECGs were available, the one closest to the index within the 2-year window was selected. SR ECGs recorded before the index or after catheter ablation were excluded. The figure on the right side illustrates examples of SR ECGs selection in the non-AF group. The latest SR ECG was served as an index. The window of interest was defined as a timeframe of 5 years before the index SR ECG. If the presence of at least one more SR ECG before it within the 5-year period, the SR ECG was selected. Otherwise, it was discarded.
According to the prevalence analysis of atrial fibrillation in the general population of Japan [
10,
11], all patients included in both study groups were required to be over 40 years old at the date the selected SR ECG was recorded. Additionally, none of the patients in either group received any anti-arrhythmic drugs. The following six criteria were applied for data exclusion: 1) ECGs with paced rhythms. 2) ECGs recorded after catheter ablation or heart surgery. 3) Patients with mitral stenosis or artificial valve replacement. 4) Patients with a history of cardiogenic cerebral embolism in the control group. 5) ECGs included in an arrhythmia exclusion list defined by the cardiologists (
Supplementary material online, Table S1). 6) ECGs recorded with misplaced electrodes or poor recording conditions.
2.3. Data Pre-processing and Model Input Type
ECG signals often contain various types of noises and artifacts, such as power line interference, myoelectric noise, base-line drift, and high frequency noise components that arise from the device or environment. The corresponding digital filters are provided on the ECG device. Clinicians may apply different filters during ECG recording to remove noise, and information about the applied filters is recorded in the saved ECG data. To standardize the conditions of all collected ECGs, the unused filters among the provided four filters were applied to the ECG signals for uniform noise removal of all ECGs.
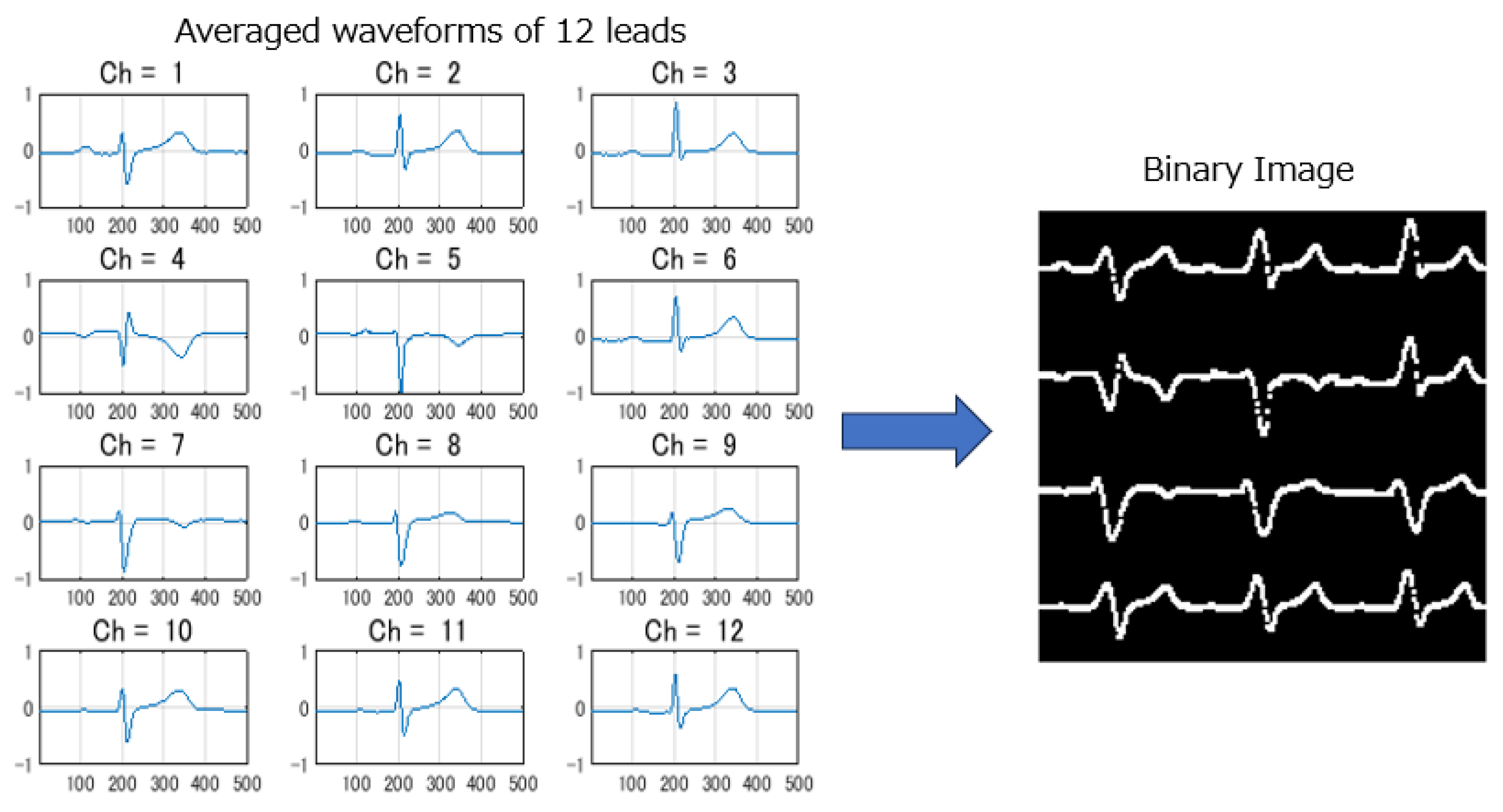
Since all ECGs were collected during SR with 10-second long and 12 leads, the data dimensionality was high for 12-lead ECG signals. Some researchers used only a subset of 12-leads or part of signal segments to reduce the computation cost, but still a quite deep AI model needs to be used for a good performance. This increases the difficulty of a high memory usage for edge AI deployment on resource-constrained devices. In this study, we proposed a novel approach to transform standard 10-second, 12-lead ECGs into binary images for model input and to design a lightweight CNN model for real-time AF-risk detection on edge ECG devices. Five steps of signal pre-processing were conducted: 1) R wave-triggered signal averaging method was used to generate averaged ECGs with a length of 1 second for each lead. 2) The averaged waveforms were compressed along the time and amplitude axes to an appropriate size suitable for deployment. 3) The compressed averaged waveform was converted into a binary image using brightness processing. 4) Binary images from 12 leads were arranged into a composite image with a layout of 4 rows and 3 columns. 5) The total image resolution was adjusted to align with the depth of the CNN model and suitable for deployment.
Figure 3 illustrates an example of the 12 generated average waveforms being converted into a composite binary image.
2.4. Model Architecture and Deployment
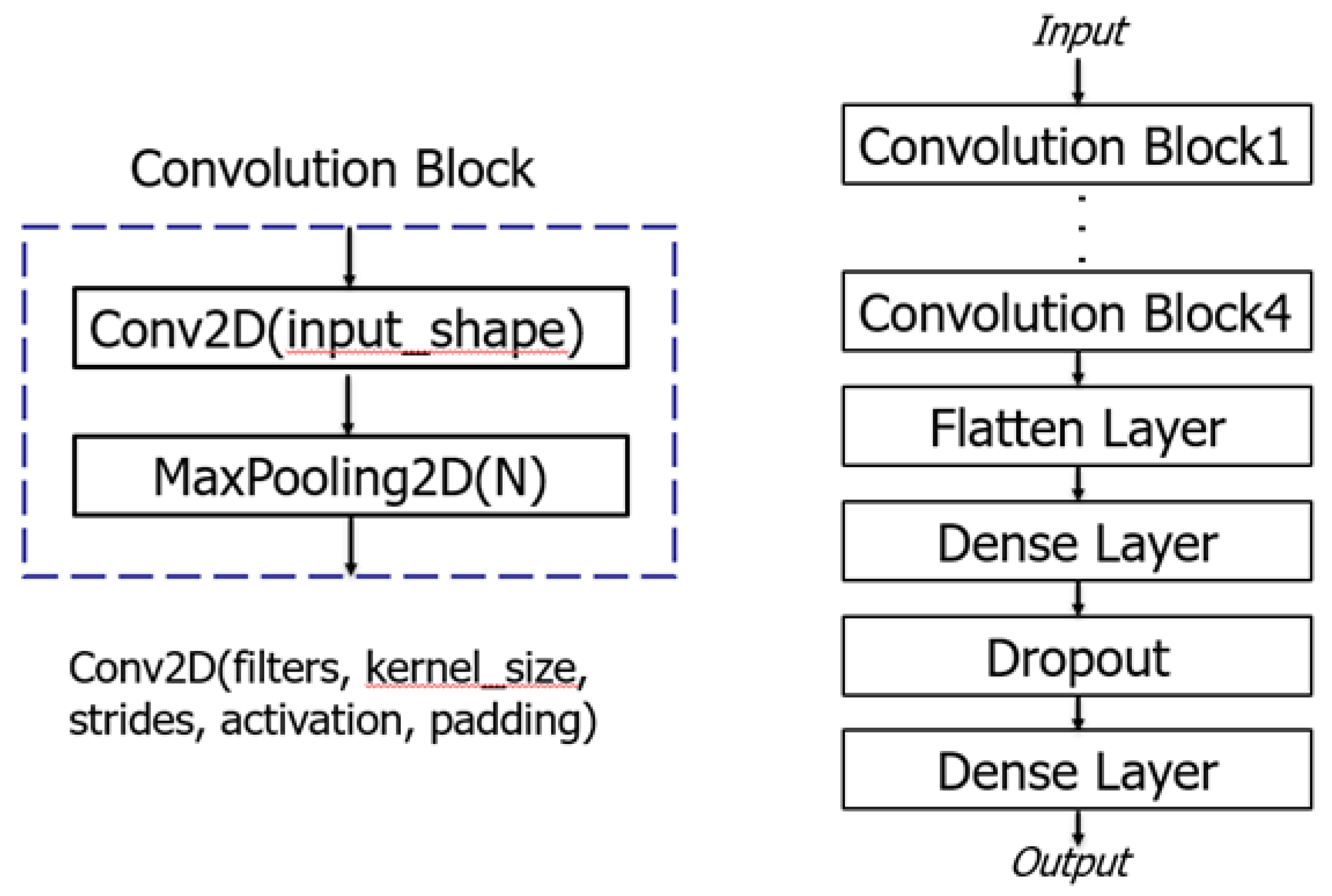
To ensure that the AI model remains compact, efficient and accurate, a convolutional neural network with a small number of layers was implemented using the Keras package with a TensorFlow backend in Python. The architecture of the model consisted of four convolution blocks, each comprising a two-dimensional convolution layer with kernel size of 3×3, ReLU activation function, different filters, and a max-pooling layer as illustrated in
Figure 4. After the final max-pooling layer, the extracted ECG features were input to a fully connected layer (Flatten layer), two dense layers and a dropout layer, before being fed into an output layer activated with softmax function for AF classification. The batch size was set to 64, and the Adam optimizer was employed to iteratively update network weights trained on a computer equipped with an NVIDIA GeForce GTX1080 GPU(8GB).
The trained AI model, developed in the Python environment, was saved in JSON format and uploaded, along with the necessary header library, into the app package written in C++ for on-board AF-risk detection. The model was optimized to ensure low memory usage, making it suitable for deployment on resource-constrained ECG devices.
2.5. Outcome Assessments
Performance metrics refer to mathematical formulas that are used for assessing how well an AI model predicts clinical or other health outcomes from the data. In binary classification tasks, where outcomes are classified into two categories, several metrics such as accuracy, sensitivity, specificity, precision and AUC are commonly used. While accuracy and AUC are suitable for well-balanced datasets, they may not be appropriate for datasets with class imbalances. To address potential bias and ensure robust evaluation, all datasets in this study, including those used for training, validation, internal testing, and external testing, were well-balanced between AF and non-AF groups. This allows for the comprehensive assessment of model performance using all the metrics mentioned above.
2.6. Statistical Analysis
Statistical analysis involves collecting and analyzing large volumes of data to identify trends and develop insights. Once the final fitted model was obtained, a statistical analysis plan was designed and specified in advance for external testing. The plan included the following steps: 1) Descriptive and Inferential analysis of the clinical characteristics of patients included in AF and non-AF groups. Mean, standard deviation and independent t-tests for continuous variables, and percentages and Fisher exact tests for categorical variables were calculated and performed to verify if there were statistically significant differences (p < 0.05) in clinical variables between the patients in the two groups. 2) Measurement of outcomes and estimation of their 95% confidence intervals. 3) Special data testing for non-AF identification. All the statistical analyses were performed using EZR version 1.55 and R version 4.3.1 software.
3. Results
3.1. Internal Testing
The model was input with the binary ECG images and trained using the dataset (n=2330, AF: 1165, non-AF:1165) as described in
Section 2.3. To enrich the training dataset, a representative waveform, termed the dominant waveform, was extracted from each lead of the recorded 10-second, 12-lead ECGs. These dominant waveforms exhibited less noise and matched the 1-second length of the averaged waveforms used for training. Following the signal pre-processing described in
Section 2.4, two binary ECG images were generated per patient, effectively doubling the dataset size to n=4660 (AF: 2330, non-AF: 2330) for model training.
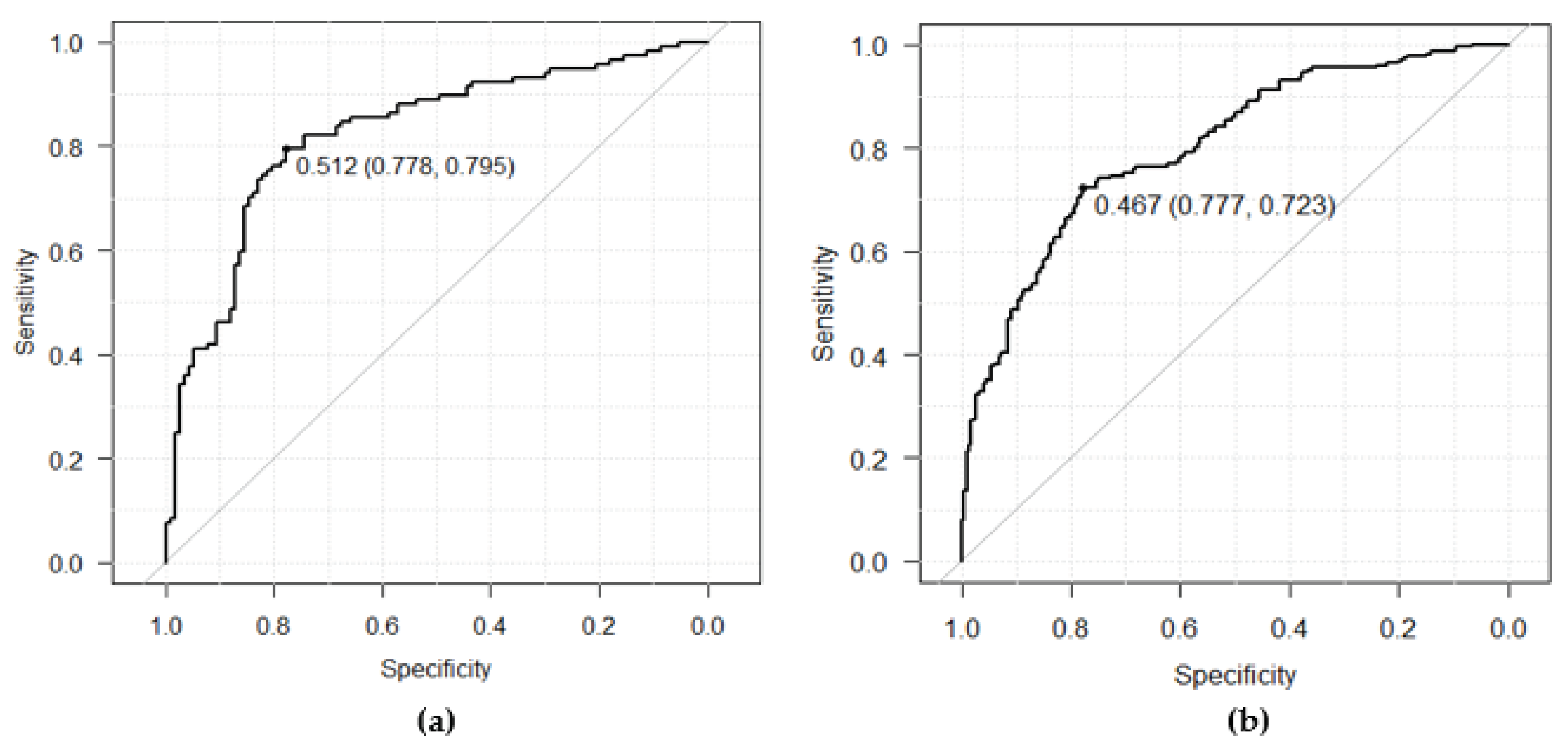
For internal testing, a dataset comprising 234 ECGs (AF:117, non-AF:117) was utilized. Each patient contributed one binary image using the averaged waveforms, and no dominant ECG waveforms were used for testing. The outcome metrics of the internal testing were measured as follows: AUC, 0.82 (95% CI 0.77-0.88); sensitivity, 79.5% (95% CI 71.0-86.4); specificity, 77.8% (95% CI 69.2-84.9); precision, 78.2% (95% CI 69.6-85.2), and accuracy, 78.6% (95% CI 72.8-83.7). The ROC curve was depicted on the left side in
Figure 5 (
a), shown in
Section 3.2.4 to compare with the ROC curve obtained from the following external testing.
3.2. External Testing
3.2.1. External Dataset Analysis
The external testing and statistical analysis were further conducted using the external dataset (n=440, AF:220, non-AF:220) as described in
Section 2.3. Each patient contributed one binary image using the averaged waveforms, and no dominant waveforms were used as the same for internal testing. The age distribution of patients was analyzed and compared with that of the training + validation datasets, as shown in
Table 1. Notably, in the external testing dataset presented on the right side of
Table 1, there was a 17% decrease in patients aged 40 to 59, and a 14% increase in patients aged 70 to 89, compared with the training and validation dataset. This distribution trend more closely resembled the prevalence proportion observed in the age group of the AF population, and the proportion of female patients was observed 5% increase in the external testing dataset as well. Additionally, in addition to collecting ECGs from patients visiting the Department of Cardiovascular Medicine for training, validation, and internal testing datasets, we also included ECGs from patients transported by emergency or other departments in the external testing dataset. This broader sample allows for a more comprehensive validation of the generalization ability of the developed AI model.
3.2.2. Additional Measures of Bias Minimization
To minimize the influence of bias from patient characteristics between AF and non-AF groups on performance evaluation, additional steps were taken. After selecting the necessary ECGs of AF patients randomly from the available data, an equal number of non-AF ECGs were selected. These selections were not only matched with the clinical characteristics but also the age distribution of the patients in the AF group. The results, as presented in
Table 2, indicate that apart from a higher number of Diabetes patients in the non-AF group compared to the AF group, other patient characteristic items were quite similar between the two groups. Furthermore, patients with both normal (borderline normal included) and abnormal (borderline abnormal included) ECGs during ECG automatic interpretation, were well-balanced as well. Therefore, no bias effect existed during external performance evaluation. The disparity in the occurrence of Diabetes, similar to that observed in the training dataset, may be attributed to patients without a history of AF predominantly visiting the hospital for periodic inspections. This approach helps ensure the robustness and reliability of the model's performance evaluation process by mitigating potential biases.
3.2.3. Statistical Analysis of Patients Characteristics
According to the first step of the statistical analysis plan outlined in Section 2.7, patient characteristics were statistically analyzed. The results are summarized in
Table 3. It was observed that several data points were missing in the Smoking and Hypertension items. The mean values of age, height, and weight for patients in both groups were approximately 70 years, 160cm, and 61kg, respectively. Additionally, approximately 61% of patients were male.
P-values for continuous variables and categorical variables were obtained from F-test, student t-tests and Fisher exact tests, respectively. With the exception of the Diabetes item, all p-values were greater than the 0.05 significance level. This indicates that there were no statistically significant differences in clinical characteristics between patients in the AF and the non-AF groups.
3.2.4. Performance Validation
In the second step of the statistical analysis plan, the performance of the fitted model used for external validation was assessed. The following performance metrics were evaluated: AUC, 0.80 (95% CI 0.76-0.84); sensitivity, 72.3% (95% CI 65.9-78.1); specificity, 77.7% (95% CI 71.6-83.0); precision, 76.4% (95% CI 70.1-82.0), and accuracy, 75.0% (95% CI 70.7-79.0), respectively. Two-sided 95% confidence intervals for the measured metrics were estimated with the Delong method for AUC and the Clopper-Pearson method for the other metrics. The ROC curve obtained from the external testing is depicted on the right side in
Figure 5b. The mere 2% difference compared to the AUC from the internal testing suggests a strong generalizability of the fitted model.
3.2.5. Special Data Testing
To minimize the risk of mislabeling patients in the non-AF group who may have undetected AF, several measures were implemented. Patients presenting with a chief complaint of palpitations or subjective symptoms were excluded from the non-AF data collection. Moreover, at least two SR ECGs recorded in the past five years based on the latest selected SR ECG were required for inclusion. In the third step of the statistical analysis plan, patients with palpitations but diagnosed with inappropriate sinus tachycardia (IST), or atrioventricular nodal reentrant tachycardia (AVNRT) after catheter ablation, had their SR ECGs collected before the catheter ablation and utilized as special data for non-AF identification.
A total of 29 patients were collected, comprising 12 males and 17 females, with ages ranging from 40s to 80s. The detection rate of non-AF in this subset was 75.9% (95% CI 56.5-89.7), which was slightly lower (1.8%) than the specificity of 77.7% measured from the external testing data (n=440). This outcome further indicates a successful detection rate on the special data with similar accuracy for non-AF identification.
Each patient contributed one binary image using the averaged waveforms, ensuring no data duplication occurred in the special data testing. Furthermore, we utilized the arrhythmia exclusion list described in
Section 2.2 to exclude patients with heart diseases unrelated to AF.
3.3. Successful Deployment
The app package with the edge AI model was successfully deployed on an edge ECG device, where the time for detecting AF-risk on board was measured to be approximately 2 seconds, nearly in real-time following an automated diagnosis of routine standard 10-second, 12-lead ECGs. Additionally, the prediction results obtained on the edge device after deployment were confirmed completely the same compared with the results predicted on a PC in a Python environment. These comparative results validated the low-cost and successful deployment of the method.
4. Discussion
4.1. Major Findings and Key Outcomes
Existing screening methods for AF often miss cases due to the condition's paroxysmal and asymptomatic nature. This under-detection can lead to serious consequences such as stroke and premature death. The findings of this study highlight the potential of deep learning-based edge AI models in the early detection of AF during normal SR using standard 10-second 12-lead ECGs.
The inclusion and exclusion criteria for data collection, such as age over 40 years old, the presence of normal SR, and exclusion of the ECGs included in a defined arrhythmia exclusion list, aimed to capture a representative sample of patients who may have undetected AF in the past 2 years but are not currently experiencing symptomatic episodes.
The well-balanced data collection, with each patient contributing an equal number of ECG data, specifically one ECG data per patient for testing, additional measures of bias minimization in the two groups, and the rigorous labeling process conducted by trained physicians under cardiologist supervision, ensured the reliability of the datasets for model training, testing, and accurate performance evaluation.
The proposed method involved converting the averaged waveform from each lead of a standard 10-second, 12-lead ECGs into a binary image and then composing them. This approach facilitated the training of a lightweight CNN model for AF-risk detection during SR.
The performance metrics of the deployed model, including sensitivity, specificity, precision, overall accuracy, and AUC, demonstrate its effectiveness and generalization capability in detecting AF-risk during SR in both internal and external testing datasets.
The model maintains comparable performance and improves its suitability for deployment on resource-constrained devices, thereby expanding its potential impact to a wide range of healthcare settings. Its successful deployment enables real-time AF-risk detection during SR in clinical settings where immediate intervention is crucial.
4.2. AUC and Methods Comparison
The estimated AUC for the fitted model in internal and external testing was 0.82 (95% CI: 0.77-0.88) and 0.80 (95% CI: 0.76-0.84), respectively. These values outperform those of other medical screening tests, such as B-type natriuretic peptide (BNP) for heart failure and cardiovascular disease diagnosis (AUC: 0.60-0.70) [
12], Papanicolaou smear for cervical cancer screening (AUC: 0.70) [
13], and the CHA2DS2-VASc score for stroke risk assessment (AUC: 0.57-0.72) [
14].
Several studies have been reported for AF-risk detection during SR ECGs in the recent 5 years. The difference between this study and others are summarized in
Table 4.
The achieved sensitivity and specificity of the external testing were balanced at the optimized cutoff of 0.467. This threshold can be adjusted depending on clinical needs. A low cutoff with high sensitivity may be useful in excluding healthy individuals who do not require further inspection, while a high cutoff with high specificity may be beneficial for identifying patients with a high pretest probability for intensive monitoring.
By employing the R wave-triggered signal averaging method to generate averaged waveforms from SR ECGs and then converting them into binary images, a lightweight CNN model was trained. This approach proved to be efficient and feasible for AF-risk detection on resource-constrained ECG devices, with an approximate time of 2 seconds after automatic 12-lead ECGs interpretation.
4.3. Limitations and Future Directions
Several limitations were identified in this study. First, all ECG data was retrospectively collected from general or university affiliated hospitals, necessitating further evaluation in a broader, ostensibly healthy population. Second, although a total of 1502 AF-labeled ECGs, with one data per patient, were collected, which is more than some other studies, the relatively moderate scale of the ECG dataset for AI model training may limit model performance and robustness. This warrants further analysis with additional data. Finally, being a multi-center retrospective study, prospective, large-scale studies are required to validate the model’s performance in the future.
5. Conclusions
The proposed method, which involved extracting averaged waveforms from standard 10-second, 12-lead SR ECGs and converting them into binary images, facilitated the training of a lightweight CNN model for AF-risk detection during SR. The achieved performance, as evaluated from internal and external datasets, demonstrated the effectiveness and generalization capability of the trained model in detecting undiagnosed AF.
Moreover, the successful deployment of the app package on edge ECG devices enables the practical application of undiagnosed AF detection in real-time during SR. This development marks a significant contribution to the advancement of AI in healthcare and holds important implications for early AF screening and the management of patients with unexplained stroke.
Moving forward, further improvements can be explored through the utilization of large-scale data. Continual refinement and validation of the model's performance will be essential for its continued effectiveness and reliability in clinical practice.
Overall, the deployment of the model on edge AI ECG devices represents a significant step towards enhancing healthcare outcomes and addressing the challenges associated with undiagnosed AF, ultimately improving patient care and management strategies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. The arrhythmia exclusion list.
Author Contributions
Conceptualization, H.W., Ta.S., Te.S., K.A.; Methodology, H.W. and T.Y.; Software, H.W.; Validation, H.W. ; Formal analysis, H.W.; Investigation, Te.S., K.A., H.W.; Data curation, T.G. and H.W.; Visualization, H.W.; Writing-original draft preparation, H.W.; Writing-review and editing, Ta.S., T.G., T.Y., Te.S. and K.A.; Supervision, Te.S.; Project administration, Ta.S.; Funding action, Ta.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the Japan Agency for Medical Research and Development (AMED) (Grant Number: JP21he2102002h0503).
Institutional Review Board Statement
The study adhered to the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Education of Japan in 2015. Approval for data collection was obtained from the Ethics Committees of Tokyo Medical and Dental University (02019-006, October 8, 2019), Kameda General Hospital (22-138, April 7,2023), and Yokohama City University Medical Center (F231100031(revised), November 20, 2023).
Informed Consent Statement
For model training, validation and internal testing, only data from individuals who provided consent were used. For external testing, an opt-out form on a website was used as an acceptable method to obtain consent from the patients. All ECG records were anonymized.
Data Availability Statement
The datasets used for training, validation, and internal testing were provided by AMED. Information regarding the data management guidelines and access platform can be found at the following link:
https://www.amed.go.jp/koubo/datamanagement.html. While the construction of the platform for data reuse is ongoing, researchers can visit the link to check its progress and submit requests for dataset access. External testing data were collected by Fukuda Denshi, Tokyo, Japan, with consent obtained through an opt-out form on a website. As participants were informed that the data would be used solely for this study, we regret that it cannot be shared publicly.
Acknowledgments
We express our gratitude to Akihiko Matsumura and Daisuke Ueshima from Kameda General Hospital and Kozo Okada from Yokohama City University Medical Center for supervising the data collection process. Special thanks to Tetsuya Ito, Kyohei Fukatani and Takashi Kuwayama for data collection and support in statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haim, M.; Hoshen, M.; Reges, O.; Rabi, Y.; Balicer, R.; Leibowitz, M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc. 2015, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.; Russak, A.J.; Richter, F.; Zhao, S.; Vaid, A.; Chaudhry, F.; De Freitas, J.K.; Naik, N.; Miotto, R.; Nadkarni, G.N.; et al. Deep learning and the electrocardiogram: Review of the current state-of-the-art. Europace 2021, 23, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Lee, S.; Choi, W.; Kim, D. A new deep learning algorithm of 12-lead electrocardiogram for identifying atrial fibrillation during sinus rhythm. Scientific Reports 2021, 11, 12818. [Google Scholar] [CrossRef] [PubMed]
- Melzi, P.; Tolosana, R.; Cecconi, A.; Sanz-Garcia, A.; Ortega, G.J.; Jimenez-Borreguero, L.J.; Vera-Rodriguez, R. Analyzing artificial intelligence systems for the prediction of atrial fibrillation from sinus-rhythm ECGs including demographics and feature visualization. Scientific Reports 2021, 11, 22786. [Google Scholar] [CrossRef] [PubMed]
- Jeni, L.A.; Cohn, J.F.; Torre, F.D.L. Facing Imbalanced Data Recommendations for the Use of Performance Metrics. Int Conf Affect Comput Intell Interact Workshops 2013, 245–251. [Google Scholar] [CrossRef]
- Jason Brownlee. Imbalanced Classification with Python, eBook 1.2 ed. Victoria, Australia, 2020, pp. 37-74.
- Murat, F.; Sadak, F.; Yildirim, O.; Talo, M.; Murat, E.; Karabatak, M.; Demir, Y.; Tan, R.; Acharya, R. Review of Deep Learning-Based Atrial Fibrillation Detection Studies. Int. J. Environ. Res. Public Health 2021, 18, 11302. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Fujiki, A.; Origasa, H.; Ogawa, S.; Okumura, K.; Kubota, I.; Aizawa, Y.; Yamashita, T.; Atarashi, H.; Horie, M.; et al. Prevalence of atrial fibrillation in the general population of Japan: An analysis based on periodic health examination. Int. J. Cardiol. 2009, 137, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Kimura, K.; Aoki, J.; Kobayashi, K.; Terasawa, Y.; Sakai, K.; Shibazaki, K. Prevalence of Atrial Fibrillation in Community-Dwelling Japanese Aged 40 Years or Older in Japan. Circ J, 2008, 72, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Isakson, S.; Bhalla, M.A.; Lin, J.P.; Clopton, P.; Gardetto, N.; Maisel, A.S. Diagnostic ability of B-type natriuretic peptide and impedance cardiography: testing to identify left ventricular dysfunction in hypertensive patients. Am J Hypertens 2005, 18, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, Z.; Xiao, Z.; Hu, M.; Jiang, C.; Lin, Y.; Chen, Y. PAX1 and SOX1 methylation as an initial screening method for cervical cancer: a meta-analysis of individual studies in Asians. Ann Transl Med 2016, 4, 365. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, S.; Chu, Y.; Long, D.; Dong, J.; Fan, X.; Yang, H.; Duan, H.; Yan, L.; Qian, P. CHADS2 and CHA2DS2-VASc scores predict the risk of ischemic stroke outcome in patients with interatrial block without atrial fibrillation. J Atheroscler Thromb 2017, 24, 176–84. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).