Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Results
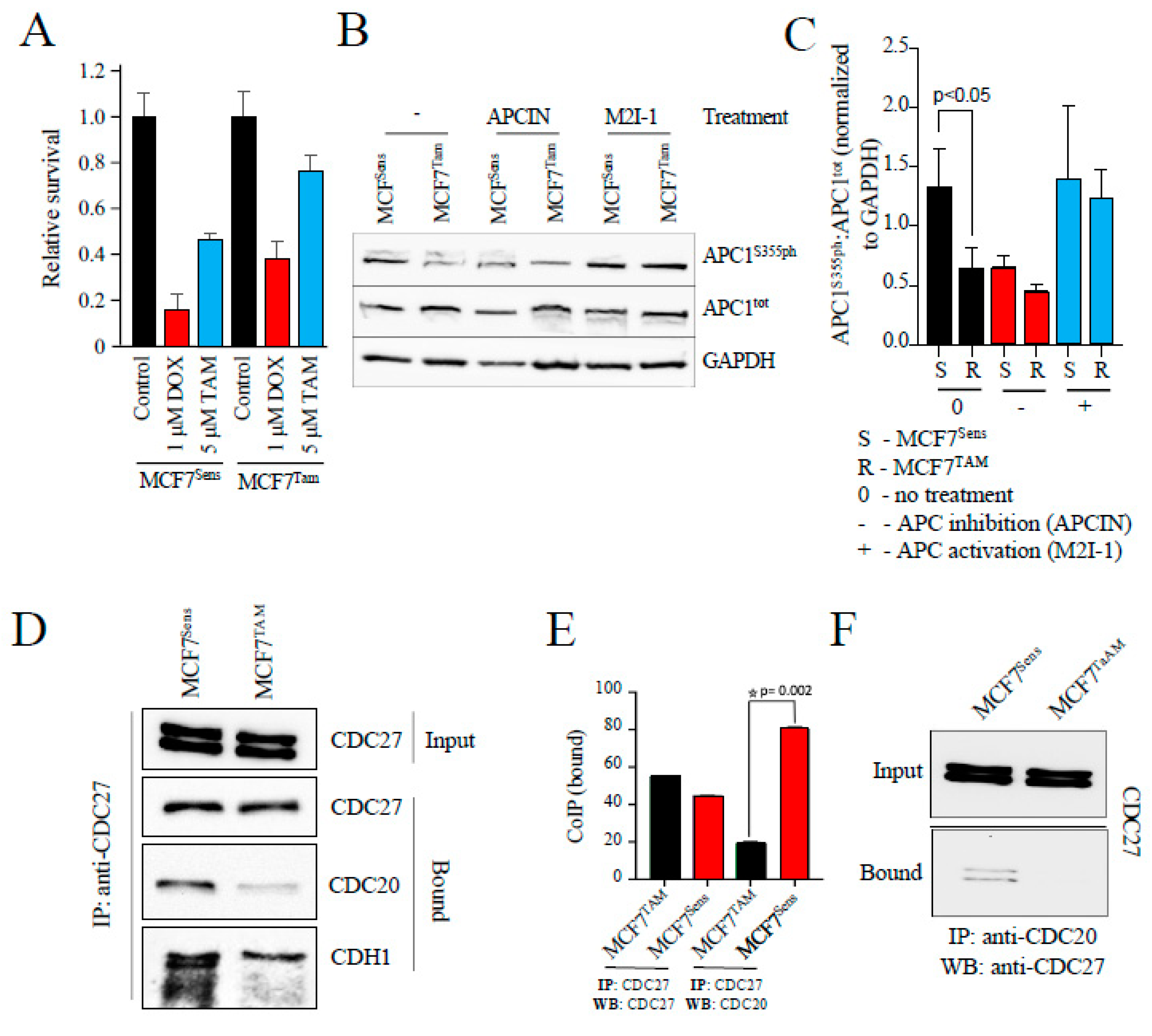
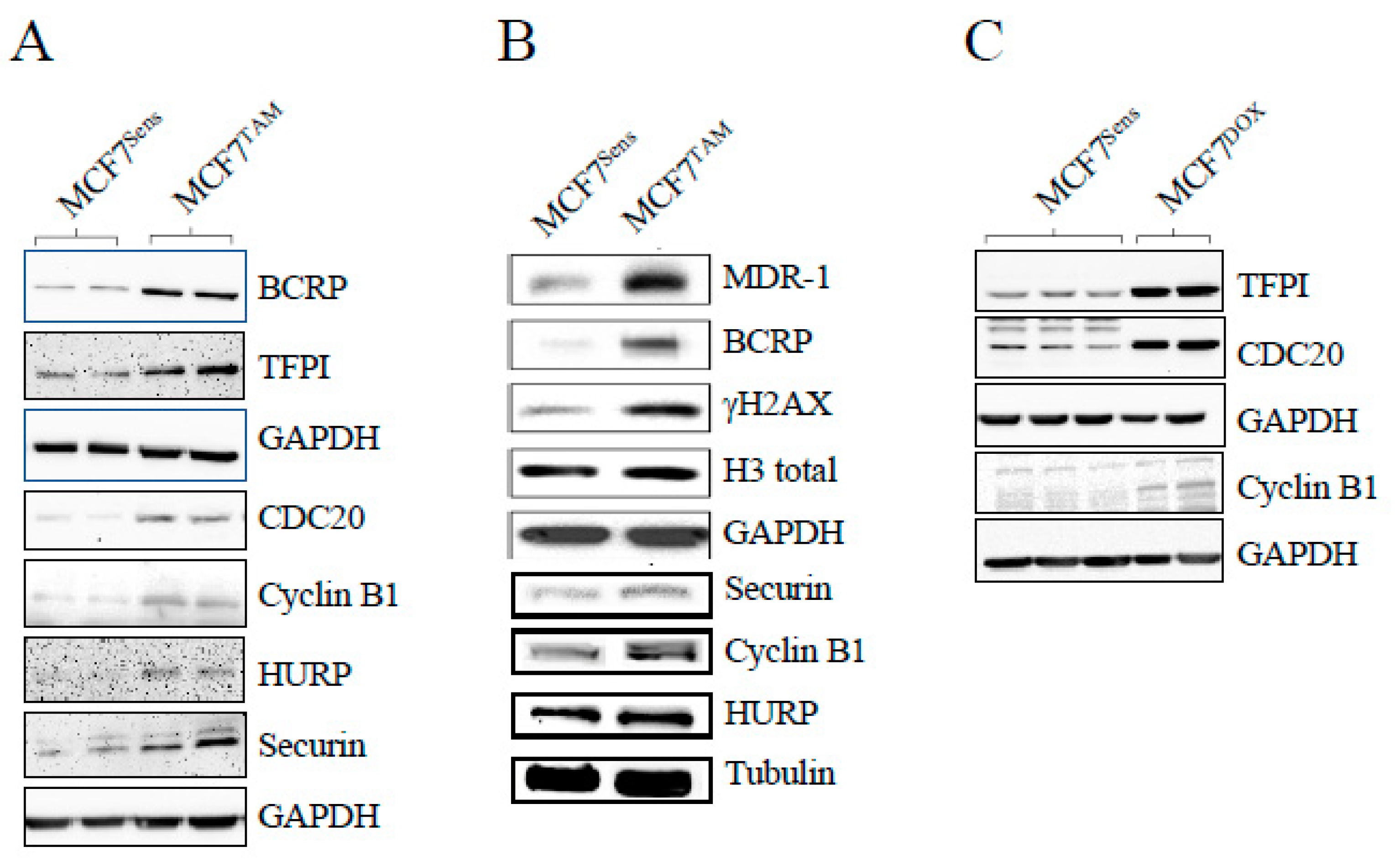
APC Activity Is Impaired in Drug Resistant MCF7 Human Breast Cancer Cells
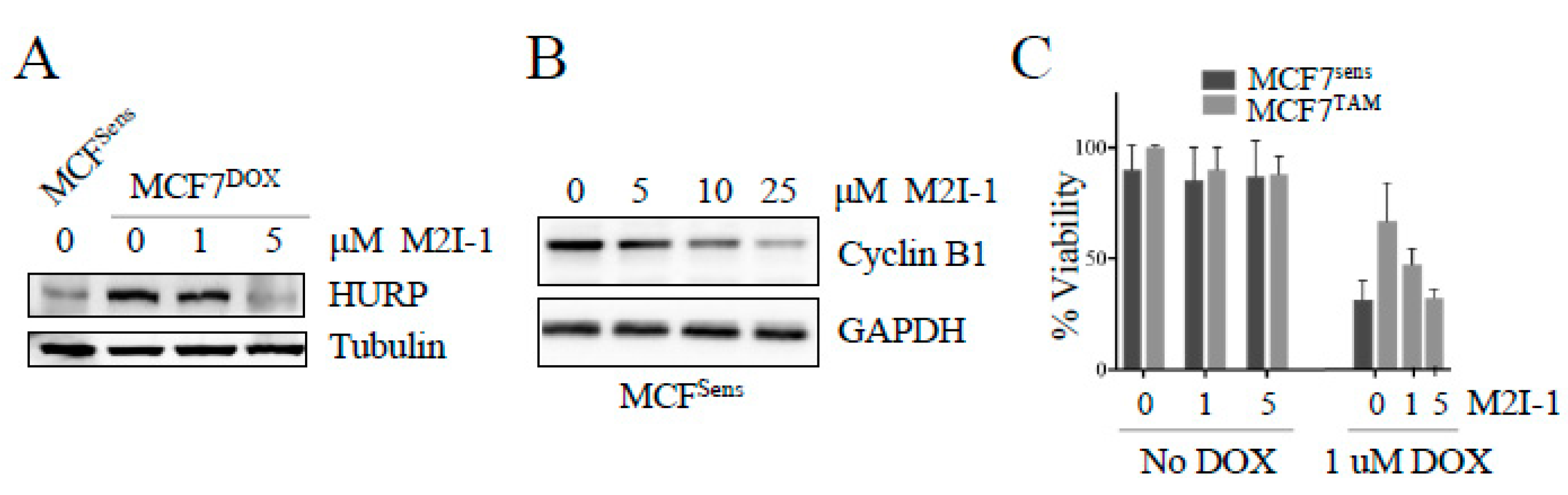
APC Activation In Vitro Slows MDR Cancer Cell Growth and Restores APC Substrate Protein Levels to Normal
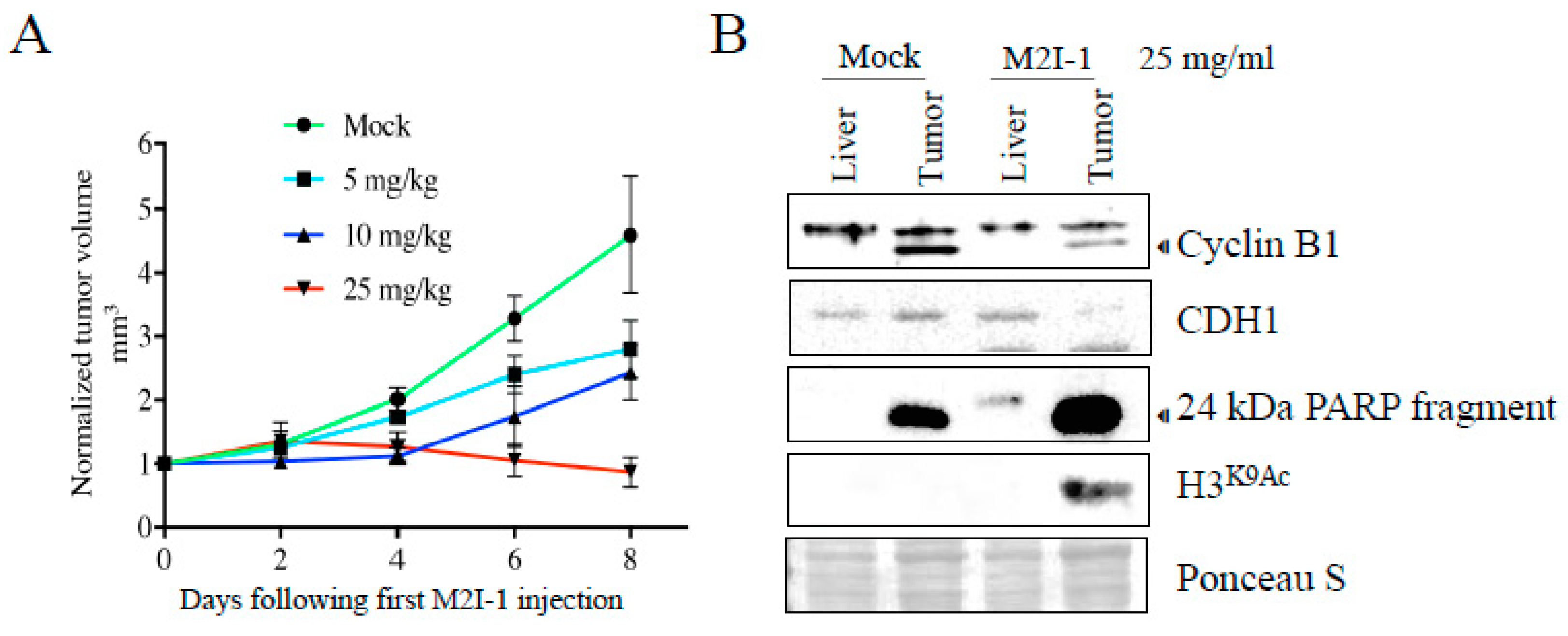
In Vivo APC Activation in Tumor-Bearing Mice Stalls Tumor Growth
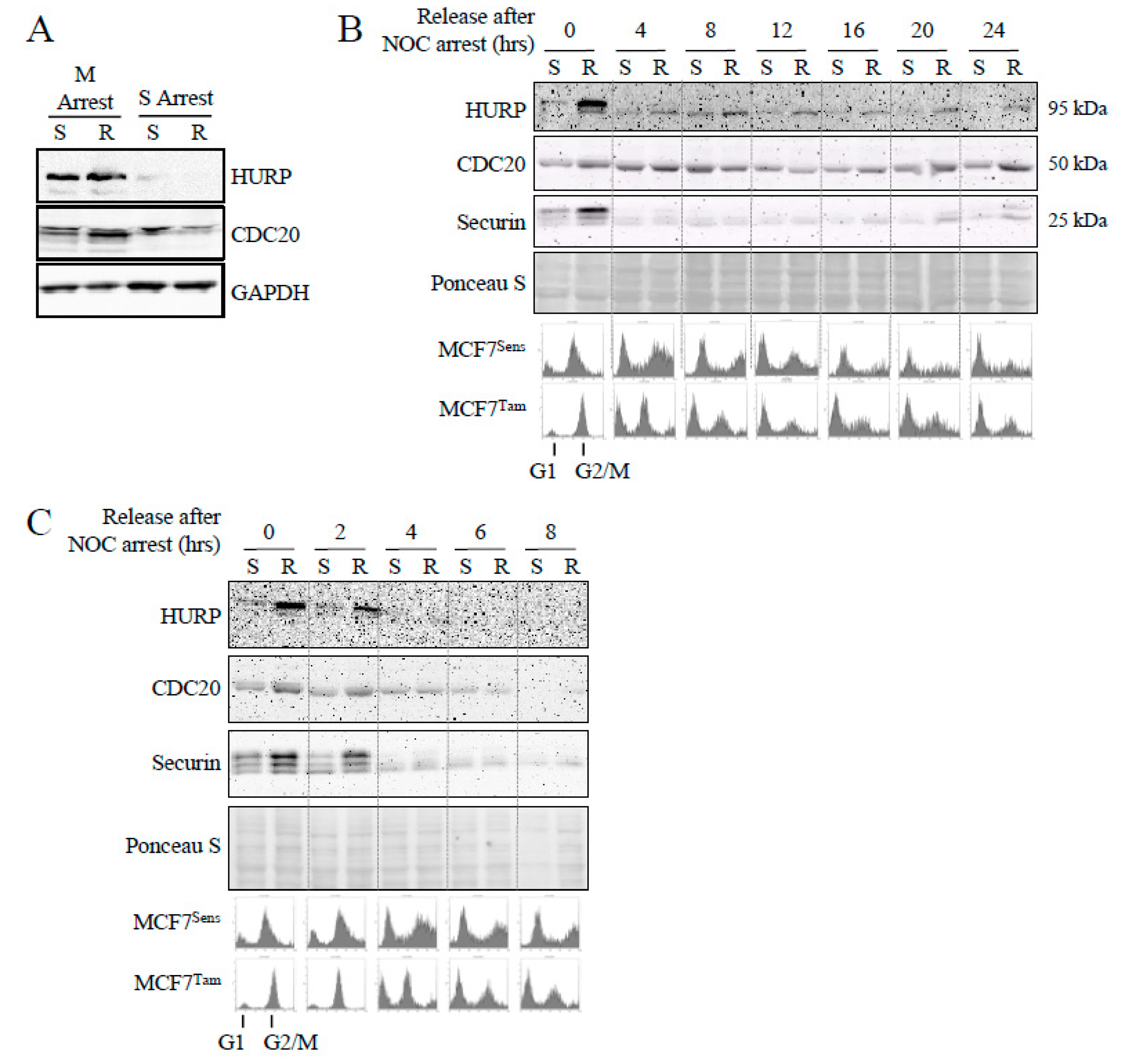
APC Substrate Degradation Is Delayed during Mitosis in Drug Resistant Cells
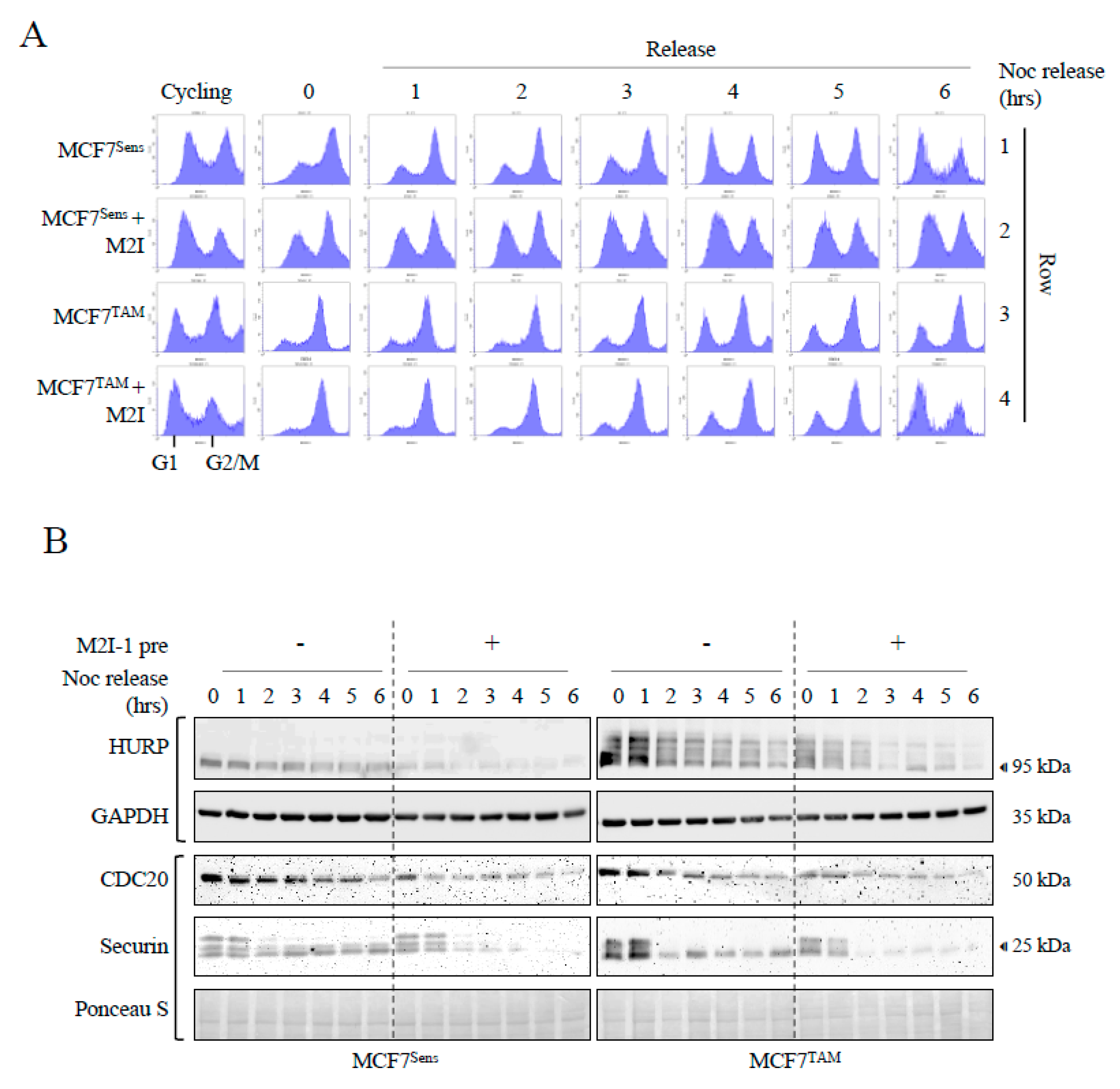
APC Activation Increases Cell Cycle Progression through Mitosis in MCFTAM Cells in Coordination with Enhanced APC Substrate Degradation
Discussion
Materials And Methods
Cell Lines and Materials:
DOX-Selection of MDR Cell Lines
Western Blot Analysis
Coimmunoprecipitation
Animals
Murine Xenograft Experiments
Cell Cycle Arrest
Flow Cytometry
Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Cancer statistics at a glance. Canadian cancer Society; 2023. Available: https://cancer.ca/en/research/cancer-statistics/cancer-statistics-at-a-glance (accessed March 6, 2024).
- Brenner D.R., Poirier A., Woods R.R., Ellison L.F, Billette J.M., Demers A.A., Zhang S.X., Yao C., Finley C., Fitzgerald N., et al. Canadian Cancer Statistics Advisory Committee. Projected estimates of cancer in Canada in 2022. CMAJ. 2022;194:E601-E607. [CrossRef] [PubMed] [PubMed Central]
- https://www.cancertherapyadvisor.com/home/tools/fact-sheets/cancer-recurrence-statistics/divide (ER/PR positive, HER2 negative ER/PR positive, HER2 negative negative) subtypes.
- Voduc K.D., Cheang M.C., Tyldesley S., Gelmon K., Nielsen T.O., Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684-91. [CrossRef] [PubMed]
- Sleightholm R., Neilsen B.K., Elkhatib S., Flores L., Dukkipati S., Zhao R., Choudhury S., Gardner B., Carmichael J., Smith L., et al. Percentage of Hormone Receptor Positivity in Breast Cancer Provides Prognostic Value: A Single-Institute Study. J Clin Med Res. 2021;13:9-19. [CrossRef] [PubMed] [PubMed Central]
- Yi M., Huo L., Koenig K.B., Mittendorf E.A., Meric-Bernstam F., Kuerer H.M., Bedrosian I., Buzdar A.U., Symmans W.F., Crow J.R., et al. Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann Oncol. 2014;25:1004-11. [CrossRef] [PubMed] [PubMed Central]
- Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P., Peto R., Pritchard K.I., Bergh J., Dowsett M., et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med. 2017;377:1836-1846. [CrossRef] [PubMed] [PubMed Central]
- Cree, I.A., Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer. 2017;17:10. [CrossRef] [PubMed] [PubMed Central]
- Arnason T.G., MacDonald-Dickinson V., Gaunt M.C., Davies G.F., Lobanova L., Trost B., Gillespie Z.E., Waldner M., Baldwin P., Borrowman D., et al. Activation of the Anaphase Promoting Complex Reverses Multiple Drug Resistant Cancer in a Canine Model of Multiple Drug Resistant Lymphoma. Cancers. 2022;14:4215. [CrossRef] [PubMed] [PubMed Central]
- Sinha D., Duijf P.H.G., Khanna K.K. Mitotic slippage: an old tale with a new twist. Cell Cycle. 2019;18:7-15. [CrossRef] [PubMed] [PubMed Central]
- Thu K.L., Silvester J., Elliott M.J., Ba-Alawi W., Duncan M.H., Elia A.C., Mer A.S., Smirnov P., Safikhani Z., Haibe-Kains B., et al. Disruption of the anaphase-promoting complex confers resistance to TTK inhibitors in triple-negative breast cancer. Proc Natl Acad Sci U S A. 2018;115:E1570-E1577. [CrossRef] [PubMed] [PubMed Central]
- De K., Grubb T.M., Zalenski A.A., Pfaff K.E., Pal D., Majumder S., Summers M.K., Venere M. Hyperphosphorylation of CDH1 in Glioblastoma Cancer Stem Cells Attenuates APC/CCDH1 Activity and Pharmacologic Inhibition of APC/CCDH1/CDC20 Compromises Viability. Mol Cancer Res. 2019;17:1519-1530. [CrossRef] [PubMed] [PubMed Central]
- Zhang Y., Li J., Yi K., Feng J., Cong Z., Wang Z., Wei Y., Wu F., Cheng W., Samo A.A., et al. Elevated signature of a gene module coexpressed with CDC20 marks genomic instability in glioma. Proc Natl Acad Sci U S A. 2019;116:6975-6984. Epub 2019 Mar 15. Erratum in: Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):1234. [CrossRef] [PubMed] [PubMed Central]
- Murphy J.M., Jeong K., Ahn E.E., Lim S.S. Nuclear focal adhesion kinase induces APC/C activator protein CDH1-mediated cyclin-dependent kinase 4/6 degradation and inhibits melanoma proliferation. J Biol Chem. 2022;298:102013. [CrossRef] [PubMed] [PubMed Central]
- Gong R.H., Chen M., Huang C., Wong H.L.X., Kwan H.Y., Bian Z. Combination of artesunate and WNT974 induces KRAS protein degradation by upregulating E3 ligase ANACP2 and β-TrCP in the ubiquitin-proteasome pathway. Cell Commun Signal. 2022;20:34. [CrossRef] [PubMed] [PubMed Central]
- Russell P., Hennessy B.T., Li J., Carey M.S., Bast R.C, Venkitaraman A.R. Cyclin G1 regulates the outcome of taxane-induced mitotic checkpoint arrest. Oncogene. 2012;31:2450-60. [CrossRef] [PubMed] [PubMed Central]
- Simonetti G., Bruno S., Padella A., Tenti E., Martinelli G. Aneuploidy: Cancer strength or vulnerability? Int J Cancer. 2019;144:8-25. [CrossRef] [PubMed] [PubMed Central]
- Pernicone N., Peretz L., Grinshpon S., Listovsky T. MDA-MB-157 Cell Line Presents High Levels of MAD2L2 and Dysregulated Mitosis. Anticancer Res. 2020;40:5471-5480. [CrossRef] [PubMed]
- Sansregret L., Patterson J.O., Dewhurst S., López-García C., Koch A., McGranahan N., Chao W.C.H., Barry D.J., Rowan A., Instrell R., et al. APC/C Dysfunction Limits Excessive Cancer Chromosomal Instability. Cancer Discov. 2017;7:218-233. [CrossRef] [PubMed] [PubMed Central]
- VanGenderen C., Harkness T.A.A., Arnason T.G. The role of Anaphase Promoting Complex activation, inhibition and substrates in cancer development and progression. Aging. 2020;12:15818-15855. [CrossRef] [PubMed] [PubMed Central]
- Melloy P.G. The anaphase-promoting complex: A key mitotic regulator associated with somatic mutations occurring in cancer. Genes Chromosomes Cancer. 2020;59:189-202. [CrossRef] [PubMed]
- Kotani S., Tugendreich S., Fujii M., Jorgensen P.M., Watanabe N., Hoog C., Hieter P., Todokoro K. PKA and MPF activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371-80. [CrossRef] [PubMed]
- Zhang S., Chang L., Alfieri C., Zhang Z., Yang J., Maslen S., Skehel M., Barford D. Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature. 2016;533:260-264. [CrossRef] [PubMed] [PubMed Central]
- Gao D., Inuzuka H., Tseng A., Chin R.Y., Toker A., Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397-408. [CrossRef] [PubMed] [PubMed Central]
- Song M.S., Carracedo A., Salmena L., Song S.J., Egia A., Malumbres M., Pandolfi P.P. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187-99. [CrossRef] [PubMed] [PubMed Central]
- Choppara S., Malonia S.K., Sankaran G., Green M.R., Santra M.K. Degradation of FBXO31 by APC/C is regulated by AKT- and ATM-mediated phosphorylation. Proc Natl Acad Sci USA. 2018115:998-1003. [CrossRef] [PubMed] [PubMed Central]
- Davies G., Lobanova L., Dawicki W., Groot G., Gordon J.R., Bowen M., Harkness T., Arnason T. Metformin inhibits the development, and promotes the resensitization, of treatment-resistant breast cancer. PLoS One. 2017;12(12):e0187191. [CrossRef] [PubMed] [PubMed Central]
- Loddo M., Kingsbury S.R., Rashid M., Proctor I., Holt C., Young J., El-Sheikh S., Falzon M., Eward K.L., Prevost T., et al. Cell-cycle-phase progression analysis identifies unique phenotypes of major prognostic and predictive significance in breast cancer. Br. J. Cancer. 2009;100(6):959-70. [CrossRef] [PubMed] [PubMed Central]
- Bakhoum S.F., Kabeche L., Compton D.A., Powell S.N., Bastians H. Mitotic DNA Damage Response: At the Crossroads of Structural and Numerical Cancer Chromosome Instabilities. Trends Cancer. 2017;3:225-234. [CrossRef] [PubMed] [PubMed Central]
- Levine M.S., Holland A.J. The impact of mitotic errors on cell proliferation and tumorigenesis. Genes Dev. 2018;32:620-638. [CrossRef] [PubMed] [PubMed Central]
- Sarwar S., Morozov V.M., Purayil H., Daaka Y., Ishov A.M. Inhibition of Mps1 kinase enhances taxanes efficacy in castration resistant prostate cancer. Cell Death Dis. 2022;13:868. [CrossRef] [PubMed] [PubMed Central]
- Davies G.F., Berg A., Postnikoff S.D., Wilson H.L., Arnason T.G., Kusalik A., Harkness T.A. TFPI1 mediates resistance to doxorubicin in breast cancer cells by inducing a hypoxic-like response. PLoS One. 2014;9:e84611. [CrossRef] [PubMed] [PubMed Central]
- Kraft C., Herzog F., Gieffers C., Mechtler K., Hagting A., Pines J., Peters J.M. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22:6598-609. [CrossRef] [PubMed] [PubMed Central]
- Qiao R., Weissmann F., Yamaguchi M., Brown N.G., VanderLinden R., Imre R., Jarvis M.A., Brunner M.R., Davidson I.F., Litos G., et al. Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc Natl Acad Sci U S A. 2016;113:E2570-8. [CrossRef] [PubMed] [PubMed Central]
- Kastl J., Braun J., Prestel A., Möller H.M., Huhn T., Mayer T.U. Mad2 Inhibitor-1 (M2I-1): A Small Molecule Protein-Protein Interaction Inhibitor Targeting the Mitotic Spindle Assembly Checkpoint. ACS Chem Biol. 2015;10:1661-6. [CrossRef] [PubMed]
- Sackton K.L., Dimova N., Zeng X., Tian W., Zhang M., Sackton T.B., Meaders J., Pfaff K.L., Sigoillot F., Yu H., et al. Synergistic blockade of mitotic exit by two chemical inhibitors of the APC/C. Nature. 2014;514:646–649. [CrossRef] [PubMed] [PubMed Central]
- Garrido D., Bourouh M., Bonneil É., Thibault P., Swan A., Archambault V. Cyclin B3 activates the Anaphase-Promoting Complex/Cyclosome in meiosis and mitosis. PLoS Genet. 2020;16:e1009184. [CrossRef] [PubMed] [PubMed Central]
- Davies G.F., Juurlink B.H., Harkness T.A. Troglitazone reverses the multiple drug resistance phenotype in cancer cells. Drug Des Devel Ther. 2009;3:79-88. [CrossRef] [PubMed] [PubMed Central]
- Georgoulis A., Vorgias C.E., Chrousos G.P., Rogakou E.P. Genome Instability and γH2AX. Int J Mol Sci. 2017;18:1979. [CrossRef] [PubMed] [PubMed Central]
- Tokumoto T., Yamashita M., Tokumoto M., Katsu Y., Horiguchi R., Kajiura H., Nagahama Y. Initiation of cyclin B degradation by the 26S proteasome upon egg activation. J Cell Biol. 1997;138:1313-22. [CrossRef] [PubMed] [PubMed Central]
- Soldani C., Scovassi A.I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321-8. [CrossRef] [PubMed]
- Switonski P.M., Delaney J.R., Bartelt L.C., Niu C., Ramos-Zapatero M., Spann N.J., Alaghatta A., Chen T., Griffin E.N., Bapat J., et al. Altered H3 histone acetylation impairs high-fidelity DNA repair to promote cerebellar degeneration in spinocerebellar ataxia type 7. Cell Rep. 2021;37:110062. [CrossRef] [PubMed] [PubMed Central]
- Bakhoum S.F., Kabeche L., Murnane J.P., Zaki B.I., Compton D.A. DNA-Damage Response during Mitosis Induces Whole-Chromosome Missegregation. Cancer Discovery. 2014;4:1281–1289. [CrossRef] [PubMed] [PubMed Central]
- Burrell R.A., McClelland S.E., Endesfelder D., Groth P., Weller M.C., Shaikh N., Domingo E., Kanu N., Dewhurst S.M., Gronroos E. et al. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494:492–496. [CrossRef]
- Bakhoum S.F., Kabeche L., Compton D.A., Powell S.N., Bastians H. Mitotic DNA Damage Response: At the Crossroads of Structural and Numerical Cancer Chromosome Instabilities. Trends Cancer. 2017;3:225-234. [CrossRef] [PubMed] [PubMed Central]
- Bansal S., Tiwari S. Mechanisms for the temporal regulation of substrate ubiquitination by the anaphase-promoting complex/cyclosome. Cell Div. 2019;14:14. [CrossRef] [PubMed] [PubMed Central]
- Pfleger C.M., Kirschner M.W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655-65. [PubMed] [PubMed Central]
- Robbins J.A., Cross F.R. Regulated degradation of the APC coactivator Cdc20. Cell Div. 2010;5:23. [CrossRef] [PubMed] [PubMed Central]
- Wu J.M., Chen C.T., Coumar M.S., Lin W.H., Chen Z.J., Hsu J.T., Peng Y.H., Shiao H.Y., Lin W.H., Chu C.Y., et al. Aurora kinase inhibitors reveal mechanisms of HURP in nucleation of centrosomal and kinetochore microtubules. Proc Natl Acad Sci U S A. 2013;110:E1779-87. [CrossRef] [PubMed] [PubMed Central]
- Song L., Rape M. Regulated degradation of spindle assembly factors by the anaphase-promoting complex. Mol Cell. 2010;38:369-82. [CrossRef] [PubMed] [PubMed Central]
- Hu X., Jin X., Cao X., Liu B. The Anaphase-Promoting Complex/Cyclosome Is a Cellular Ageing Regulator. Int J Mol Sci. 2022;23:15327. [CrossRef] [PubMed] [PubMed Central]
- Schrock M.S., Stromberg B.R., Scarberry L., Summers M.K. APC/C ubiquitin ligase: Functions and mechanisms in tumorigenesis. Semin Cancer Biol. 2020;67:80-91. [CrossRef] [PubMed] [PubMed Central]
- Li J., Dang N., Martinez-Lopez N., Jowsey P.A., Huang D., Lightowlers R.N., Gao F., Huang J.Y. M2I-1 disrupts the in vivo interaction between CDC20 and MAD2 and increases the sensitivities of cancer cell lines to anti-mitotic drugs via MCL-1s. Cell Div. 2019;14:5. [CrossRef] [PubMed] [PubMed Central]
- Jamal-Hanjani M., A’Hern R., Birkbak N.J., Gorman P., Grönroos E., Ngang S., Nicola P., Rahman L., Thanopoulou E., Kelly G., et al. Extreme chromosomal instability forecasts improved outcome in ER-negative breast cancer: a prospective validation cohort study from the TACT trial. Ann Oncol. 2015;26:1340-6. [CrossRef] [PubMed]
- Roylance R., Endesfelder D., Gorman P., Burrell R.A., Sander J., Tomlinson I., Hanby A.M., Speirs V., Richardson A.L., Birkbak N.J., et al. Relationship of extreme chromosomal instability with long-term survival in a retrospective analysis of primary breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2183-94. [CrossRef] [PubMed] [PubMed Central]
- Birkbak N.J., Eklund A.C., Li Q., McClelland S.E., Endesfelder D., Tan P., Tan I.B., Richardson A.L., Szallasi Z., Swanton C. Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res. 2011;71:3447-52. [CrossRef] [PubMed] [PubMed Central]
- Komlodi-Pasztor E., Sackett D.L., Fojo A.T. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18(1):51-63. [CrossRef] [PubMed]
- Du R., Huang C., Liu K., Li X., Dong Z. Targeting AURKA in Cancer: molecular mechanisms and opportunities for Cancer therapy. Mol Cancer. 2021;20(1):15. [CrossRef] [PubMed] [PubMed Central]
- Su S., Chhabra G., Singh C.K., Ndiaye M.A., Ahmad N. PLK1 inhibition-based combination therapies for cancer management. Transl Oncol. 2022;16:101332. [CrossRef] [PubMed] [PubMed Central]
- Zhang J., Zhang L., Wang J., Ouyang L., Wang Y. Polo-like Kinase 1 Inhibitors in Human Cancer Therapy: Development and Therapeutic Potential. J Med Chem. 2022;65:10133-10160. [CrossRef] [PubMed]
- Kidokoro T., Tanikawa C., Furukawa Y., Katagiri T., Nakamura Y., Matsuda K. CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene. 2008;27:1562–1571. [CrossRef] [PubMed]
- Jiang J., Jedinak A., Sliva D. Ganodermanontriol (GDNT) exerts its effect on growth and invasiveness of breast cancer cells through the down-regulation of CDC20 and uPA. Biochem Biophys Res Commun. 2011;415:325–329. [CrossRef] [PubMed]
- Kato T., Daigo Y., Aragaki M., Ishikawa K., Sato M., Kaji M. Overexpression of CDC20 predicts poor prognosis in primary non-small cell lung cancer patients. J Surg Oncol. 2012;106:423–430. [CrossRef] [PubMed]
- Xian F., Zhao C., Huang C., Bie J., Xu G. The potential role of CDC20 in tumorigenesis, cancer progression and therapy: A narrative review. Medicine. 2023;102:e35038. [CrossRef] [PubMed] [PubMed Central]
- Zhao S.F., Leng J.F., Xie S.S., Zhu L.Q., Zhang M.Y., Kong L.Y., Yin Y. Design, synthesis and biological evaluation of CDC20 inhibitors for treatment of triple-negative breast cancer. Eur J Med Chem. 2024;268:116204. [CrossRef] [PubMed]
- Cheevapruk K., Ueno M., Sungwan P., Sittithumcharee G., Kariya R., Sampattavanich S., Okada S. Novel Midkine Inhibitor Induces Cell Cycle Arrest and Apoptosis in Multiple Myeloma. Anticancer Res. 2024;44:1023-1031. [CrossRef] [PubMed]
- Wan L., Tan M., Yang J., Inuzuka H., Dai X., Wu T., Liu J., Shaik S., Chen G., Deng J., et al. APC(Cdc20) suppresses apoptosis through targeting Bim for ubiquitination and destruction. Dev Cell. 2014;29:377-91. [CrossRef] [PubMed] [PubMed Central]
- Bhuniya R., Yuan X., Bai L., Howie K.L., Wang R., Li W., Park F., Yang C.Y. Design, Synthesis, and Biological Evaluation of Apcin-Based CDC20 Inhibitors. ACS Med Chem Lett. 2022;13:188-195. [CrossRef] [PubMed] [PubMed Central]
- Song C., Lowe V.J., Lee S. Inhibition of Cdc20 suppresses the metastasis in triple negative breast cancer (TNBC). Breast Cancer. 2021;28:1073-1086. [CrossRef] [PubMed]
- Gao Y., Guo C., Fu S., Cheng Y., Song C. Downregulation of CDC20 suppressed cell proliferation, induced apoptosis, triggered cell cycle arrest in osteosarcoma cells, and enhanced chemosensitivity to cisplatin. Neoplasma. 2021;68:382-390. [CrossRef] [PubMed]
- Maes A., Maes K., De Raeve H., De Smedt E., Vlummens P., Szablewski V., Devin J., Faict S., De Veirman K., Menu E., et al. The anaphase-promoting complex/cyclosome: a new promising target in diffuse large B-cell lymphoma and mantle cell lymphoma. Br J Cancer. 2019;120:1137-1146. [CrossRef] [PubMed] [PubMed Central]
- Hu Q., Liu Q., Zhao Y., Zhang L., Li L. SGOL2 is a novel prognostic marker and fosters disease progression via a MAD2-mediated pathway in hepatocellular carcinoma. Biomark Res. 2022;10:82. [CrossRef] [PubMed] [PubMed Central]
- Zhan S.J., Liu B., Linghu H. Identifying genes as potential prognostic indicators in patients with serous ovarian cancer resistant to carboplatin using integrated bioinformatics analysis. Oncol. Rep. 2018;39:2653–2663. [CrossRef] [PubMed] [PubMed Central]
- Vriend J., Thanasupawat T., Sinha N., Klonisch T. Ubiquitin Proteasome Gene Signatures in Ependymoma Molecular Subtypes. Int J Mol Sci. 2022;23:12330. [CrossRef] [PubMed] [PubMed Central]
- Weng Y., Liang W., Ji Y., Li Z., Jia R., Liang Y., Ning P., Xu Y. Key Genes and Prognostic Analysis in HER2+ Breast Cancer. Technol Cancer Res Treat. 2021;20:1533033820983298. [CrossRef] [PubMed] [PubMed Central]
- Zhang Q., Wang Y., Xue F. ASPM, CDC20, DLGAP5, BUB1B, CDCA8, and NCAPG May Serve as Diagnostic and Prognostic Biomarkers in Endometrial Carcinoma. Genet Res. 2022;2022:3217248. [CrossRef] [PubMed] [PubMed Central]
- Zheng W., Zhao Y., Wang T., Zhao X., Tan Z. Identification of hub genes associated with bladder cancer using bioinformatic analyses. Transl Cancer Res. 2022;11:1330-1343. [CrossRef] [PubMed] [PubMed Central]
- Hu Z., Chen H., Li H., Xu S., Mu Y., Pan Q., Tong J., Xu G. Lysosome-related genes: A new prognostic marker for lung adenocarcinoma. Medicine. 2023;102:e34844. [CrossRef] [PubMed] [PubMed Central]
- Li M., York J.P., and Zhang P. Loss of Cdc20 Causes a Securin-Dependent Metaphase Arrest in Two-Cell Mouse Embryos. Molecular and Cellular Biology. 2007;27:3481–3488. [CrossRef] [PubMed] [PubMed Central]
- Li M., Shin Y.H., Hou L., Huang X., Wei Z., Klann E., Zhang P. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol. 2008;10:1083-9. [CrossRef] [PubMed] [PubMed Central]
- García-Higuera I., Manchado E., Dubus P., Cañamero M., Méndez J., Moreno S., Malumbres M. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10:802-11. [CrossRef] [PubMed]
- Davies G.F., Roesler W.J., Juurlink B.H., Harkness T.A. Troglitazone overcomes doxorubicin-resistance in resistant K562 leukemia cells. Leuk Lymphoma. 2005;46:1199-206. [CrossRef] [PubMed]
- Chen G., Deng X. Cell Synchronization by Double Thymidine Block. Bio Protoc. 2018;8:e2994. [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




