Submitted:
25 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Immune System in Pregnancy; A Focus on Regulatory T Cells (Tregs)
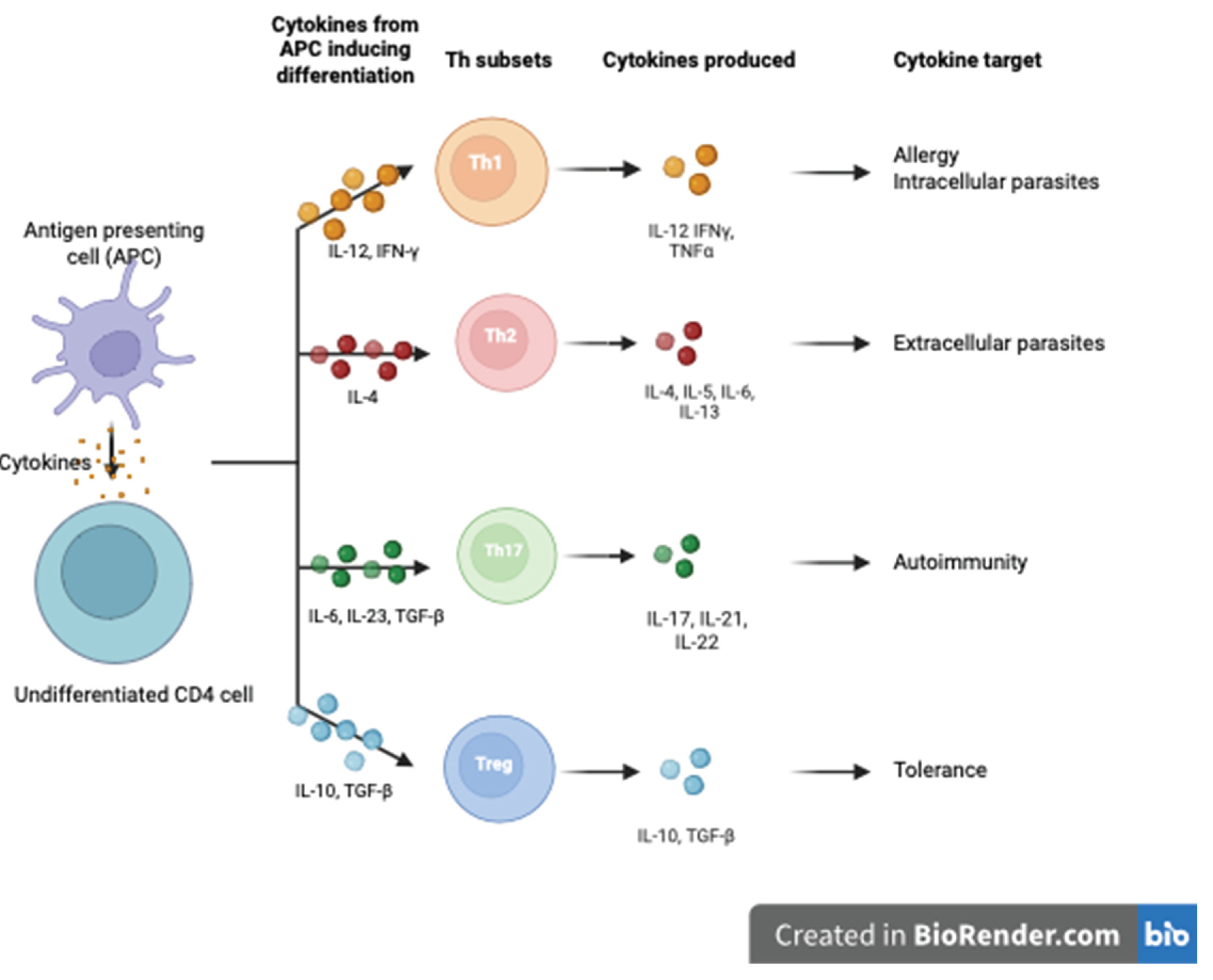
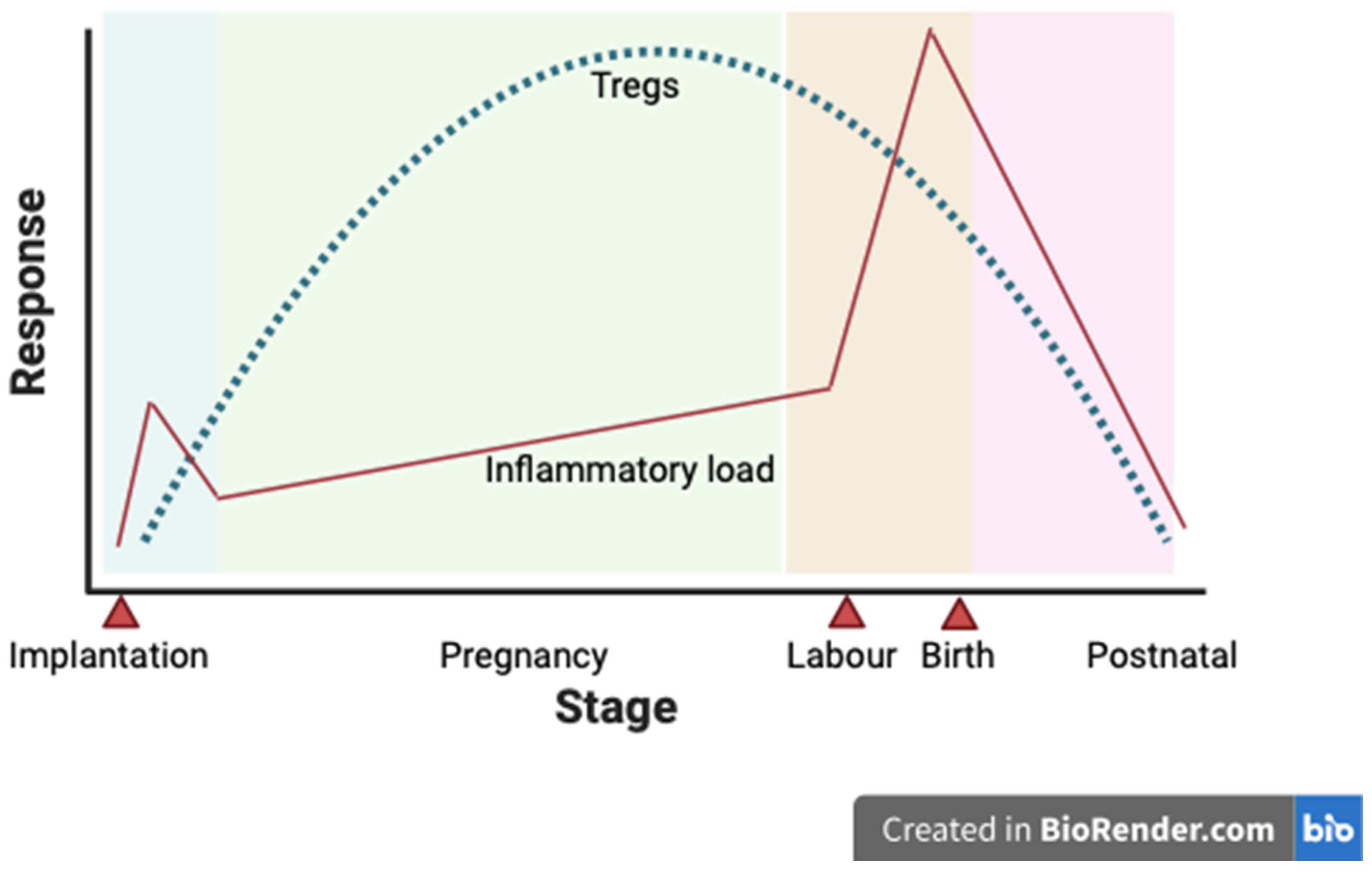
3. Role of T Regs in Healthy Pregnancy
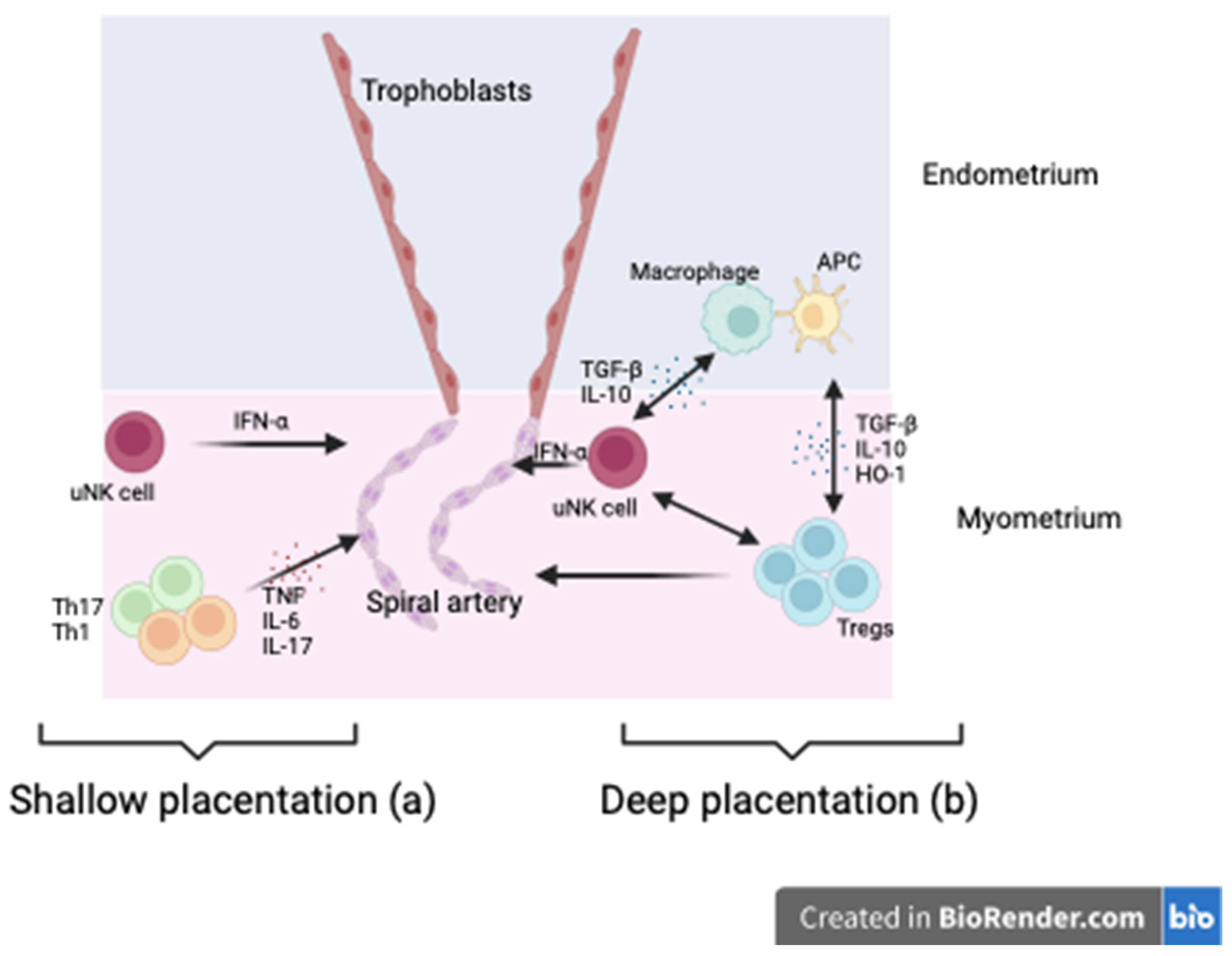
4. Role of T Regs in GH/PE
- (a)
- Tregs modulate uterine natural killer cells (uNK) and macrophages while suppressing inflammation via TGF-βIL-10 and HO-1 release. uNK cells promote vascular remodelling via IFN-γ essential to trophoblast invasion through the decidual later of the endometrium into the myometrium. This improved blood flow to the placenta.
- (b)
5. Tregs as a Therapeutic Tool in Adverse Pregnancy Outcomes
6. Therapeutic Potential of Tregs in Hypertensive Disorders of Pregnancy
7. Limitations of This Review Article
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.; Tang, C.; Gong, Y.; Liu, Y.; Rao, J.; Chen, S.; et al. PD-1/PD-L1 regulates Treg differentiation in pregnancy-induced hypertension. Brazilian Journal of Medical and Biological Research. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013, 209, 544–e1. [Google Scholar] [CrossRef] [PubMed]
- Vatish, M.; Powys, V.R.; Cerdeira, A.S. Novel therapeutic and diagnostic approaches for preeclampsia. Curr Opin Nephrol Hypertens. 2023, 32, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P. Preeclampsia. N Engl J Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Wright, A.; Nicolaides, K.H. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol. 2020, 223, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Vol. 72, Hypertension. Lippincott Williams and Wilkins; 2018. p. 24–43.
- Lambert, G.; Brichant, J.F.; Hartstein, G.; Bonhomme, V.; Dewandre, P.Y. Preeclampsia: an update. Acta Anaesthesiol Belg. 2014, 65, 137–149. [Google Scholar] [PubMed]
- Gathiram, P.; Moodley, J. Preeclampsia: Its pathogenesis and pathophysiolgy. Vol. 27, Cardiovascular Journal of Africa. Clinics Cardive Publishing (PTY)Ltd; 2016. p. 71–8.
- Huang, C.; Wei, K.; Lee, P.M.Y.; Qin, G.; Yu, Y.; Li, J. Maternal hypertensive disorder of pregnancy and mortality in offspring from birth to young adulthood: national population based cohort study. BMJ. 2022, 379, e072157. [Google Scholar] [CrossRef]
- Zakiyah, N.; Tuytten, R.; Baker, P.N.; Kenny, L.C.; Postma, M.J.; van Asselt, A.D.I. Early cost-effectiveness analysis of screening for preeclampsia in nulliparous women: A modelling approach in European high-income settings. PLoS One. 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- NICE Hypertension in pregnancy: diagnosis and management. NICE Guideline 133. 2019.
- Green, S.; Politis, M.; Rallis, K.S.; Saenz de Villaverde Cortabarria, A.; Efthymiou, A.; Mureanu, N.; et al. Regulatory T Cells in Pregnancy Adverse Outcomes: A Systematic Review and Meta-Analysis. Vol. 12, Frontiers in Immunology. Frontiers Media S.A.; 2021.
- McElwain, C.; McCarthy, F.; McCarthy, C. Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far. Int J Mol Sci. 2021, 22, 4261. [Google Scholar] [CrossRef]
- Pellerin, L.; Jenks, J.A.; Bégin, P.; Bacchetta, R.; Nadeau, K.C. Regulatory T cells and their roles in immune dysregulation and allergy. Vol. 58, Immunologic Research. Humana Press Inc.; 2014. p. 358–68.
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061, J Immunol. 2017 Feb 1;198(3):981–5. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. Reconsideration of the Role of Regulatory T Cells During Pregnancy: Differential Characteristics of Regulatory T Cells Between the Maternal-Fetal Interface And Peripheral Sites and Between Early and Late Pregnancy. Medical Principles and Practice. 2022 Oct 4.
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4 + T Cells: Differentiation and Functions. Clin Dev Immunol. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, M.M.; Stern, L.J. Class II MHC antigen processing in immune tolerance and inflammation. Immunogenetics. 2019, 71, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Giganti, G.; Atif, M.; Mohseni, Y.; Mastronicola, D.; Grageda, N.; Povoleri, G.A.; et al. Treg cell therapy: How cell heterogeneity can make the difference. Eur J Immunol. 2021, 51, 39–55. [Google Scholar] [CrossRef]
- Kanhere, A.; Hertweck, A.; Bhatia, U.; Gökmen, M.R.; Perucha, E.; Jackson, I.; et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012, 3, 1268. [Google Scholar] [CrossRef] [PubMed]
- Dhamne, C.; Chung, Y.; Alousi, A.M.; Cooper, L.J.N.; Tran, D.Q. Peripheral and thymic foxp3(+) regulatory T cells in search of origin, distinction, and function. Front Immunol. 2013, 4, 253. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.S.; Schumacher, A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 2016, 148, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M.; Thornton, A.M. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef]
- Weiss, J.M.; Bilate, A.M.; Gobert, M.; Ding, Y.; Curotto de Lafaille, M.A.; Parkhurst, C.N.; et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012, 209, 1723–1742. [Google Scholar] [CrossRef]
- Karim, M.; Feng, G.; Wood, K.J.; Bushell, A.R. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood 2005, 105, 4871–4877. [Google Scholar]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Oderup, C.; Cederbom, L.; Makowska, A.; Cilio, C.M.; Ivars, F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006, 118, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. Foxp3 + natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proceedings of the National Academy of Sciences. 2008, 105, 10113–10118. [Google Scholar] [CrossRef]
- Kennedy, A.; Waters, E.; Rowshanravan, B.; Hinze, C.; Williams, C.; Janman, D.; et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat Immunol. 2022, 23, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Workman, C.; Lee, J.; Chew, C.; Dale, B.M.; Colonna, L.; et al. Regulatory TCells Inhibit Dendritic Cells by Lymphocyte Activation Gene-3 Engagement of MHCClass, I. I. The Journal of Immunology. 2008, 180, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; et al. Role of LAG-3 in Regulatory T Cells. Immunity. 2004, 21, 503–513. [Google Scholar] [CrossRef]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; et al. CTLA-4 Control over Foxp3 + Regulatory T Cell Function. Science (1979). 2008, 322, 271–275. [Google Scholar]
- Sojka, D.K.; Huang, Y.H.; Fowell, D.J. Mechanisms of regulatory T-cell suppression – a diverse arsenal for a moving target. Immunology. 2008, 124, 13–22. [Google Scholar] [CrossRef]
- Kamanaka, M.; Huber, S.; Zenewicz, L.A.; Gagliani, N.; Rathinam, C.; O’Connor, W.; et al. Memory/effector (CD45RBlo) CD4 T cells are controlled directly by IL-10 and cause IL-22–dependent intestinal pathology. Journal of Experimental Medicine. 2011, 208, 1027–1040. [Google Scholar] [CrossRef]
- Sojka, D.K.; Fowell, D.J. Regulatory T cells inhibit acute IFN-γ synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalised requirement for IL-10. Proceedings of the National Academy of Sciences. 2011, 108, 18336–18341. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007, 450, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Melzer, F.L.; Würzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Cho, I.; Ali, N. Tissue regulatory T cells. Immunology. 2020, 161, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell. 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, J.Y.; Hur, S.E.; Kim, C.J.; Na, B.J.; Lee, M.; et al. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Krop, J.; Heidt, S.; Claas, F.H.J.; Eikmans, M. Regulatory T Cells in Pregnancy: It Is Not All About FoxP3. Vol. 11, Frontiers in Immunology. Frontiers Media S.A.; 2020.
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, M.L.; Sollwedel, A.; Bertoja, A.Z.; Ritter, T.; et al. Abnormal T-Cell Reactivity against Paternal Antigens in Spontaneous Abortion. Am J Pathol. 2005, 166, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Salvany-Celades, M.; van der Zwan, A.; Benner, M.; Setrajcic-Dragos, V.; Bougleux Gomes, H.A.; Iyer, V.; et al. Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell Rep. 2019, 27, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Sasaki, Y.; Itoh, M.; Nakashima, A.; Ishii, N.; Sugamura, K.; et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010, 85, 121–129. [Google Scholar] [CrossRef]
- Nevers, T.; Kalkunte, S.; Sharma, S. Uterine Regulatory T cells, IL-10 and Hypertension. American Journal of Reproductive Immunology 2011, 66, 88–92. [Google Scholar] [CrossRef]
- Rowe, J.H.; Ertelt, J.M.; Aguilera, M.N.; Farrar, M.A.; Way, S.S. Foxp3+ Regulatory T Cell Expansion Required for Sustaining Pregnancy Compromises Host Defense against Prenatal Bacterial Pathogens. Cell Host Microbe. 2011, 10, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Green, E.S.; Care, A.S.; Moldenhauer, L.M.; Prins, J.R.; Hull, M.L.; et al. Therapeutic Potential of Regulatory T Cells in Preeclampsia-Opportunities and Challenges. Front Immunol. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Maganto-García, E.; Bu, D.X.; Tarrio, M.L.; Alcaide, P.; Newton, G.; Griffin, G.K.; et al. Foxp3+-Inducible Regulatory T Cells Suppress Endothelial Activation and Leukocyte Recruitment. The Journal of Immunology. 2011, 187, 3521–3529. [Google Scholar]
- Matrougui, K.; Zakaria, A.E.; Kassan, M.; Choi, S.; Nair, D.; Gonzalez-Villalobos, R.A.; et al. Natural Regulatory T Cells Control Coronary Arteriolar Endothelial Dysfunction in Hypertensive Mice. Am J Pathol. 2011, 178, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Liu , B.; Li, Q.; Wang, Z.; Fan, S.; et al. Functional Defects of Regulatory T Cell Through Interleukin 10 Mediated Mechanism in the Induction of Gestational Diabetes Mellitus. DNA Cell Biol. 2018, 37, 278–285. [Google Scholar]
- Bennett, W.A.; Lagoo-Deenadayalan, S.; Whitworth, N.S.; Brackin, M.N.; Hale, E.; Cowan, B.D. Expression and production of interleukin-10 by human trophoblast: relationship to pregnancy immunotolerance. Early Pregnancy. 1997, 3, 190–198. [Google Scholar] [PubMed]
- Kwak-Kim, J.; Bao, S.; Lee, S.K.; Kim, J.W.; Gilman-Sachs, A. Immunological Modes of Pregnancy Loss: Inflammation, Immune Effectors, and Stress. American Journal of Reproductive Immunology. 2014, 72, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Kusanovic, J.P.; Chaiworapongsa, T.; Hassan, S.S. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011, 25, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Tinsley, J.H.; Chiasson, V.L.; Mahajan, A.; Young, K.J.; Mitchell, B.M. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens. 2009, 22, 1314–1319. [Google Scholar] [CrossRef]
- Thaxton, J.E.; Romero, R.; Sharma, S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009, 183, 1144–1154. [Google Scholar]
- Lai, Z.; Kalkunte, S.; Sharma, S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011, 57, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Mutter, W.P.; Wolf, M.; Levine, R.J.; Taylor, R.N.; Sukhatme, V.P.; et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004, 89, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai , J.i.; Mammoto, T.; Kim, Y.M.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006, 12, 642–649. [Google Scholar]
- Barhoumi, T.; Kasal, D.A.; Li, M.W.; Shbat, L.; Laurant, P.; Neves, M.F.; et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011, 57, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, A.; Trevelin, S.C.; Mongue-Din, H.; Becker, P.D.; Ortiz, C.; Smyth, L.A.; et al. Nox2 in regulatory T cells promotes angiotensin II–induced cardiovascular remodeling. Journal of Clinical Investigation. 2018, 128, 3088–3101. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.; Norouzian, M.; Arabpour, F.; Naderi, N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol. 2016, 12, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Darmochwal-Kolarz, D.; Suzuki, D.; Sakai, M.; Ito, M.; Shima, T.; et al. Proportion of peripheral blood and decidual CD4+ CD25bright regulatory T cells in preeclampsia. Clin Exp Immunol. 2007, 149, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Fazekas de St Groth, B.; et al. Systemic Increase in the Ratio between Foxp3+ and IL-17-Producing CD4+ T Cells in Healthy Pregnancy but Not in Preeclampsia. The Journal of Immunology. 2009, 183, 7023–7030. [Google Scholar] [CrossRef]
- Quinn, K.H.; Lacoursiere, D.Y.; Cui, L.; Bui, J.; Parast, M.M. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. 2011, 91, 76–82. [Google Scholar] [CrossRef]
- Hsu, P.; Santner-Nanan, B.; Dahlstrom, J.E.; Fadia, M.; Chandra, A.; Peek, M.; et al. Altered Decidual DC-SIGN+ Antigen-Presenting Cells and Impaired Regulatory T-Cell Induction in Preeclampsia. Am J Pathol. 2012, 181, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, A.; Haensch, G.M.; Mahnke, K.; Schmitt, E.; Toermer, A.; Meuer, S.; et al. Distinct subsets of regulatory T cells during pregnancy: Is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clinical Immunology. 2008, 129, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.H.; Lacoursiere, D.Y.; Cui, L.; Bui, J.; Parast, M.M. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. 2011, 91, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; et al. The role of immune cells and mediators in preeclampsia. Nat Rev Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ghaemi, M.S.; Ando, K.; Peterson, L.S.; Ganio, E.A.; Tsai, A.S.; et al. Differential Dynamics of the Maternal Immune System in Healthy Pregnancy and Preeclampsia. Front Immunol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.; Cornelius, D.; Amaral, L.; Paige, A.; Herse, F.; Ibrahim, T.; et al. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy. 2015, 34, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Liu, F.J.; Xin-Liu Hao, C.F.; Bao, H.C.; Qu, Q.L.; et al. Adoptive transfer of pregnancy-induced CD4+CD25+ regulatory T cells reverses the increase in abortion rate caused by interleukin 17 in the CBA/J×BALB/c mouse model. Human Reproduction 2014, 29, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Idali, F.; Rezaii-nia, S.; Golshahi, H.; Fatemi, R.; Naderi, M.M.; Goli, L.B.; et al. Adoptive cell therapy with induced regulatory T cells normalises the abortion rate in abortion-prone mice. Reprod Fertil Dev. 2020. [CrossRef] [PubMed]
- Yin, Y.; Han, X.; Shi, Q.; Zhao, Y.; He, Y. Adoptive transfer of CD4+CD25+ regulatory T cells for prevention and treatment of spontaneous abortion. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2012, 161, 177–181. [Google Scholar]
- Woidacki, K.; Meyer, N.; Schumacher, A.; Goldschmidt, A.; Maurer, M.; Zenclussen, A.C. Transfer of regulatory T cells into abortion-prone mice promotes the expansion of uterine mast cells and normalises early pregnancy angiogenesis. Sci Rep. 2015, 5, 13938. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Preeclampsia. The Lancet. 2010, 376, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.E.; Broady, R.; Gregori, S.; Himmel, M.E.; Locke, N.; Roncarolo, M.G.; et al. CD4 + T-regulatory cells: toward therapy for human diseases. Immunol Rev. 2008, 223, 391–421. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.R.; Holvast, F.; van ’t Hooft, J.; Bos, A.F.; Ganzevoort, J.W.; Scherjon, S.A.; et al. Development of a core outcome set for immunomodulation in pregnancy (COSIMPREG): a protocol for a systematic review and Delphi study. BMJ Open. 2018, 8, e021619. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.G.; Ronchetti, S.; Ricci, E.; Alunno, A.; Gerli, R.; Nocentini, G.; et al. GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmun Rev. 2015, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Eastaff-Leung, N.; Bresatz-Atkins, S.; Warner, N.; Ruitenberg, J.; Krumbiegel, D.; et al. Inhibition of activation induced CD154 on CD4 + CD25 − cells: a valid surrogate for human Treg suppressor function. Immunol Cell Biol. 2012, 90, 812–821. [Google Scholar] [CrossRef]
- Saftlas, A.F.; Rubenstein, L.; Prater, K.; Harland, K.K.; Field, E.; Triche, E.W. Cumulative exposure to paternal seminal fluid prior to conception and subsequent risk of preeclampsia. J Reprod Immunol. 2014, 101–102, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Green, E.S.; Tan, T.C.Y.; Gonzalez, M.B.; Rumbold, A.R.; Hull, M.L.; et al. Periconception onset diabetes is associated with embryopathy and fetal growth retardation, reproductive tract hyperglycosylation and impaired immune adaptation to pregnancy. Sci Rep. 2018, 8, 2114. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ulrich, B.; Cho, J.; Park, J.; Kim, C.H. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011, 187, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lydon, J.P.; Kim, C.H. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012, 42, 2683–2696. [Google Scholar] [CrossRef]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. The Lancet. 2020, 395, 1627–1639. [Google Scholar] [CrossRef]
- Sánchez-Fueyo, A.; Whitehouse, G.; Grageda, N.; Cramp, M.E.; Lim, T.Y.; Romano, M.; et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. American Journal of Transplantation. 2020, 20, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.O.; Hester, J.; Petchey, W.; Rombach, I.; Dutton, S.; Bottomley, M.J.; et al. Transplantation Without Overimmunosuppression (TWO) study protocol: a phase 2b randomised controlled single-centre trial of regulatory T cell therapy to facilitate immunosuppression reduction in living donor kidney transplant recipients. BMJ Open. 2022, 12, e061864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




