2. Materials and Methods
2.1. Chickens
Rhode Island Red (RIR) chicks were obtained from an unvaccinated flock maintained in isolation accommodation at the Institute for Animal Health, Compton, UK. The experiments met with local ethical guidelines as well as those of the UK Home Office.
2.2. Viruses
Fowlpox virus FP9 derivative fpIBD1 [
9], expressing most of the IBDV F52/70 VP2 protein as a β-galactosidase fusion protein under the control of the Vaccinia virus p7.5 early/late promoter, from the BglII insertion site in ORF FPV002, was from laboratory stocks. fpIBD1 was grown on chicken embryo fibroblast (CEF) cells in the presence of 1X 199 medium (Sigma).
2.3. Experimental Design
Forty two chicks at one week of age were divided into three groups, 14 birds in each group as follows:
Group 1 were inoculated with 50 μl PBSa per bird (negative control).
Group 2 were inoculated with 250 μg/ml PHA per bird (positive control).
Group 3 were inoculated with fpIBD1.
The vaccination dose of fpIBD1 at one week of age was 107 pfu in a 50 μl volume. The inoculum was placed on both wing-webs of each bird and the skin punctured 30 times over an area of 2 mm2 with a 21-gauge hypodermic needle. Two birds from each group were killed each time point by cervical dislocation and skin samples from the site of inoculation were taken. The first sample was taken 2 days post-inoculation and sampling was then carried out every 2 days till 14 days post-inoculation. Two skin samples from each bird (both wing-webs) were taken, one of the wing-webs was taken for real-time quantitative PCR to measure viral load, and the other for immunohistochemical staining (for FPV, macrophages, CD4+ T cells, CD8+ T cells and B cells), to estimate the relative number of positively staining cells at the site of inoculation using Image-Pro® Plus soft-ware version 4.0. Two skin sections (from two birds) and three fields of view per skin section were used at each time-point for image analysis. This is a semi-quantity measurement, as it gives a percentage of the whole microscopic field (per area) that stained positive.
2.4. DNA Extraction
Up to 25 mg skin were cut into small pieces, placed in a 1.5 ml micro-centrifuge tube. DNeasy Tissue Kits from QIAGEN was used for rapid isolation of total DNA following the manufacturer’s instructions.
2.5. Frozen Sections for Immunohistochemical Staining
Each skin sample was put on a 2.5 cm2 cork tile and covered with Tissue-Tek® O.C.TTM Compound. The samples were then snap-frozen in a dry-ice/iso-pentane bath and transferred to liquid nitrogen. Frozen blocks were then removed from the liquid nitrogen, wrapped in aluminium foil and stored at -70oC. Sections (6-8 μm) were then cut from these blocks for immunohistochemistry staining using a cryostat, picked up onto glass slides, then fixed in acetone for 10 min and air-dried. Staining was then carried out using a Vectastain® ABC αmouse IgG HPR staining kit (Vector Laboratories, Burlingame, CA, USA), following the manufacturer’s instructions. The monoclonal antibodies used were DF6 [
10] for FPV, KUL01 [
11] for macrophages, AV14 [
12] for CD8, AV29 [Fred Davison, IAH] for CD4 and AV20 [
13] for B cells.
2.6. Real-Time Quantitative PCR
The fluorescently labelled probes were labelled with the reporter dye 5-carboxyfluoroscein (FAM) at the 5′ end and the quencher N,N,N’,N’–tetramethyl-6-carboxyrhodamine (TAMRA) at the 3′ end. Specific primers were designed to closely flank the probe. Primers and probes sequences are given in
Table 1.
Amplification and detection of specific products were undertaken using the ABI PRISM™ 7700 Sequence Detection System. Quantification was based on the increased fluorescence detected by the ABI PRISM™ 7700 Sequence Detection System (PE Applied Biosystems) due to hydrolysis of the target-specific probes by the 5′ nuclease activity of the rTth DNA polymerase during PCR amplification. Results are expressed in terms of the threshold cycle value (Ct), the cycle at which the change in the reporter dye (ΔRn) passes a significance threshold.
2.7. Construction of Standard Curves for Quantitative PCR Assays
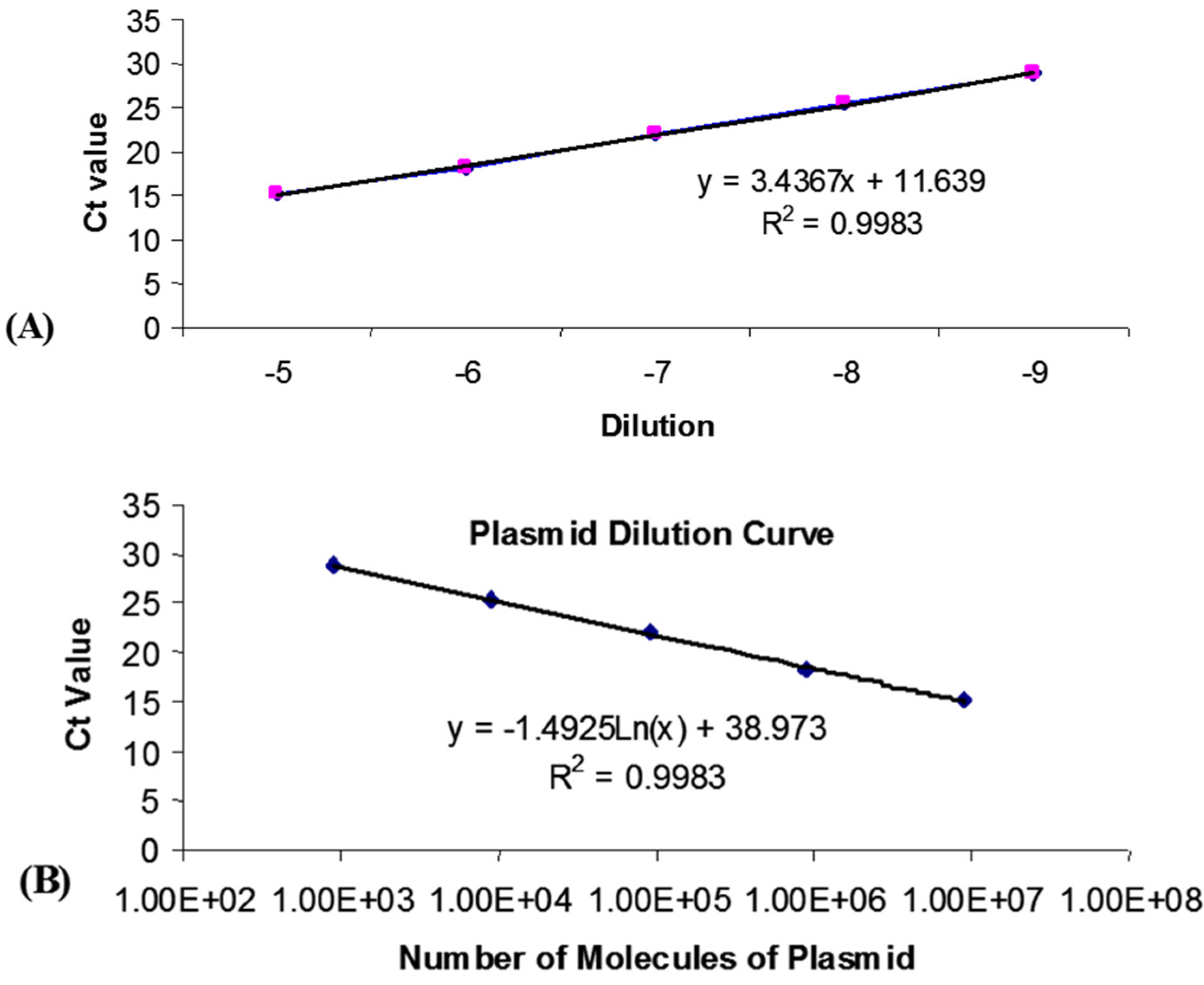
Standard curves for the DNA-specific reactions were generated from real-time PCR reactions on serial log10 dilutions of DNA standards (usually plasmid DNA containing same DNA template), and were included in every experiment. Dilutions of 10-1 to 10-9 were made in DEPC-H2O. Regression analysis of the mean values for the log10 diluted DNA plasmid was used to generate a standard curve (
Figure 1A). Briefly, by knowing the plasmid size, and concentration, the number of plasmid copies can be easily quantified. The number of sample DNA copies could then be calculated from the sample Ct values. For example:
Plasmid pCI-neo::VP2 was used as a standard. The plasmid is 7105 bp in size (5474 bp pCI-neo + 1631 bp VP2), and the concentration of the DNA was 1400 μg/ml.
The average molecular weight of DNA is 660. Therefore, 7105 X 660 = 4689300 g which is the number of grams in one mole of plasmid.
So, number of grams in 1 ml / number of grams in 1 mole = number of moles of plasmid per ml of DNA. (1400 X 10-6)/4689300 = 2.985 X 10-10 moles/ml.
Avogadro’s number is the number of molecules in 1 mole = 6.022 X 1023. Therefore we have (2.985 X 10-10) X (6.022 X 1023) molecules per ml DNA = 1.798 X 1014.
5 μl DNA was used in each Taqman reaction, therefore [(1.798 X 1014) / (1000)] X 5 = 8.99 X 1011 molecules in 5 μl of undiluted DNA.
(8.99 X 1011) X dilution factor gives number of molecules in the reaction. This is graphed against Ct values for dilution (
Figure 1B).
The equation of the plasmid dilution curve regression line [y = -1.4925Ln(x) + 38.973] was used to estimate the number of viral copies per sample:
x = exp((y-38.973)/-1.4925), where x is VP2.
As there were two copies of VP2 inserted into fpIBD1 in the ITRs [
9], the resulting values were divided by two.
4. Discussion
It was showed that an intradermal inoculation of FPV (both challenge and vaccine strains) into the comb of birds resulted in primary lesions which were restricted to the site of inoculation [
14]. Local multiplication resulted in the development of lesions at the site of virus entry. Typically, primary lesions appeared by the fourth day and disappeared by the third week. No secondary lesions developed in any of the birds. No virus was detected in the blood, and the internal organs were free of virus during the infection [
14]. The challenge FPV was detected on the second day from the lesion samples collected from the comb (site of inoculation). Thereafter, the virus titre increased rapidly to a maximum on the ninth day, and then fell from the twelfth day onwards. No virus was detected on 27 dpi. Virus persistence was shorter and virus concentration was lower with the vaccine strain than the challenge strain [
14]. Another study [
15] showed that in chickens infected with fowlpox intradermally, the virus was detected in the lungs 4 dpi which was followed by viremia 5 dpi.
FPV strain FP9 was used as the recombinant vaccine in this study. Its genome has been completely sequenced [
16], allowing identification of all of the differences (including deletions totalling 22 kb) between it and the USDA standard challenge virus, which was described in the original sequence publication as “pathogenic” [
17]. FP9 (FPV plaque 9) was derived by plaque purification of virus that had been passaged some 438 times in CEF culture, the source isolate being HP-1 Munich [
18]. Any residual virulence (even for day-old chicks) had been lost by passage 350 [
19]. FP9 is highly attenuated and possibly has introduced genetic deletions/modifications that enhance the immune response elicited by this vector. Comparison of the FP9 genome sequence with the published sequence of a pathogenic FPV reference strain [
17] reveals several inserted as well as deleted sequences [
16]. Such deletions/insertions may account for the enhanced capacity of FP9 to elicit T cell responses. This may explain the short persistence of the recombinant FPV used in this study compared to the long persistence for the challenge FPV used by Minbay and Kreier [
14].
In terms of histopathology, hyperplasia of the epithelium, enlargement of cells and the presence of eosinophilic cytoplasmic inclusion bodies (Bollinger bodies) are common for FPV infection [
20]. It was interesting to determine which cell types were involved at the site of FP9 inoculation.
By virtue of their location at various sites in the body, macrophages are one of the first cells to encounter viruses [
21]. The immunological function of the epidermis is principally linked to the presence in this tissue of a distinct subpopulation of Langerhans cells (LC) or macrophages [
22]. In man, LC constitute 2-4% of the epidermal cell population and within the epidermis they are the only cells which express MHC class II antigens constitutively [
23]. LC play a key role in the initiation of T cell responses to cutaneous antigens by picking up antigen and migrating to the draining lymph node (in mammals), where they trigger specific T cell activation [
22].
In mammals, when an antigen has passed the epithelial barrier of the skin or mucosal surfaces it has to be processed and presented by accessory cells to lymphocytes. These reactions take place in lymphoid organs, such as the regional lymph nodes, Peyer’s patches and tonsils, but also in the spleen if the antigen entered the blood directly. The respective lymphocyte clone expands by proliferating, and primed lymphocytes of the B and T cell series emigrate from the lymphoid organs [
24].
As there are no lymph nodes in chickens, antigen presentation to lymphocytes may occur within the skin tissue [
25]. As shown in
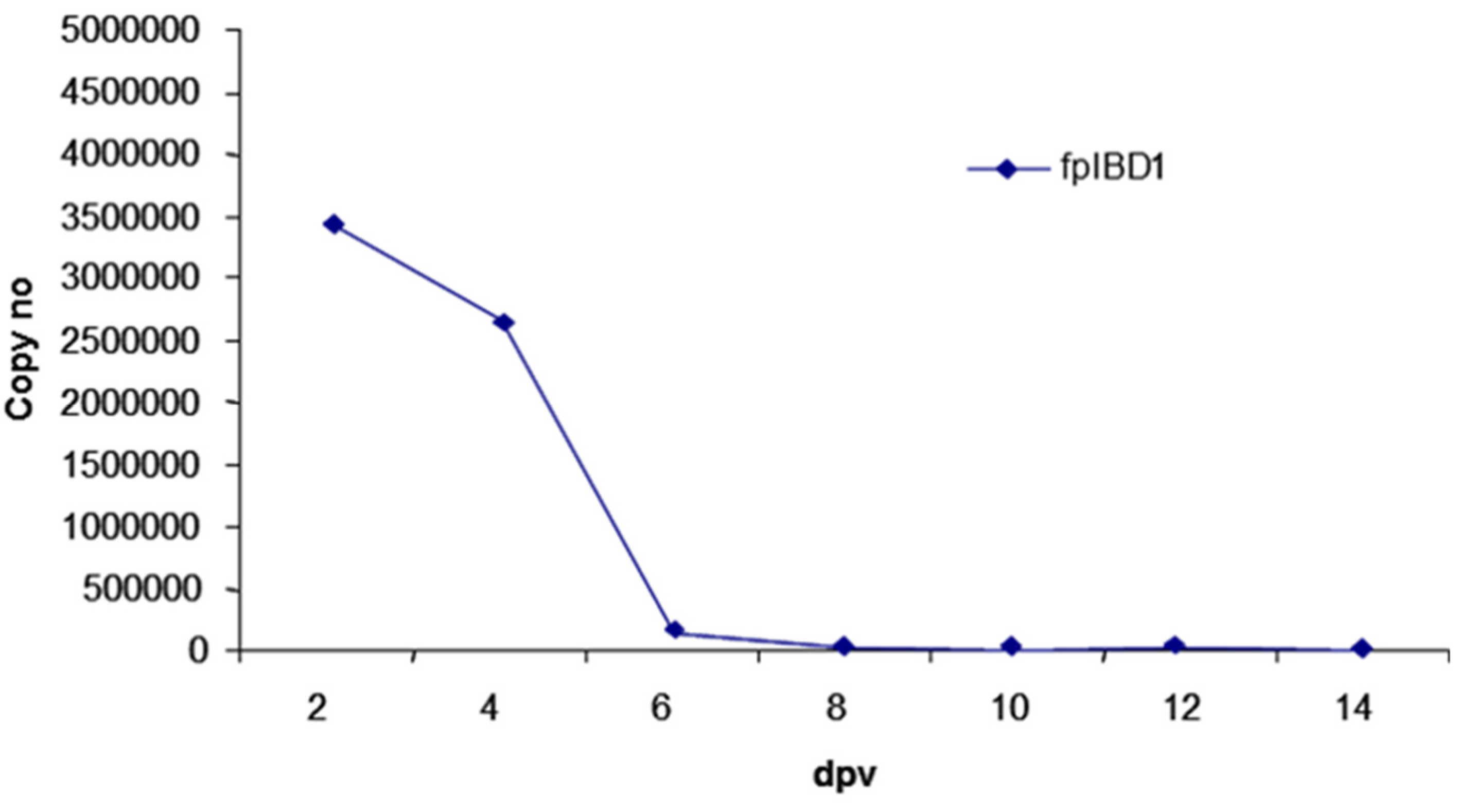
Figure 2 and
Figure 3, the virus was detected in skin tissue after vaccination at very high concentration at 2 dpv, to a lower degree at 4 dpv and was almost cleared from 6 dpv. The pattern of macrophage infiltration at the site of inoculation in the skin tissue was similar to that of the virus in that high numbers were seen at 2 dpv and 4 dpv, and these dropped from 6 dpv onwards (
Figure 4 and 5). The concentration of macrophages in the skin at the site of inoculation is higher than that for the birds which were inoculated with PBSa (negative control) and PHA (inflammatory agent), suggesting that the virus might be taken up by macrophages. In vitro, PHA stimulates T cell proliferation and differentiation, while in vivo the PHA-skin response is an inflammatory reaction, concerning complex interactions of cells - not only lymphocytes but also macrophages and basophils [
26].
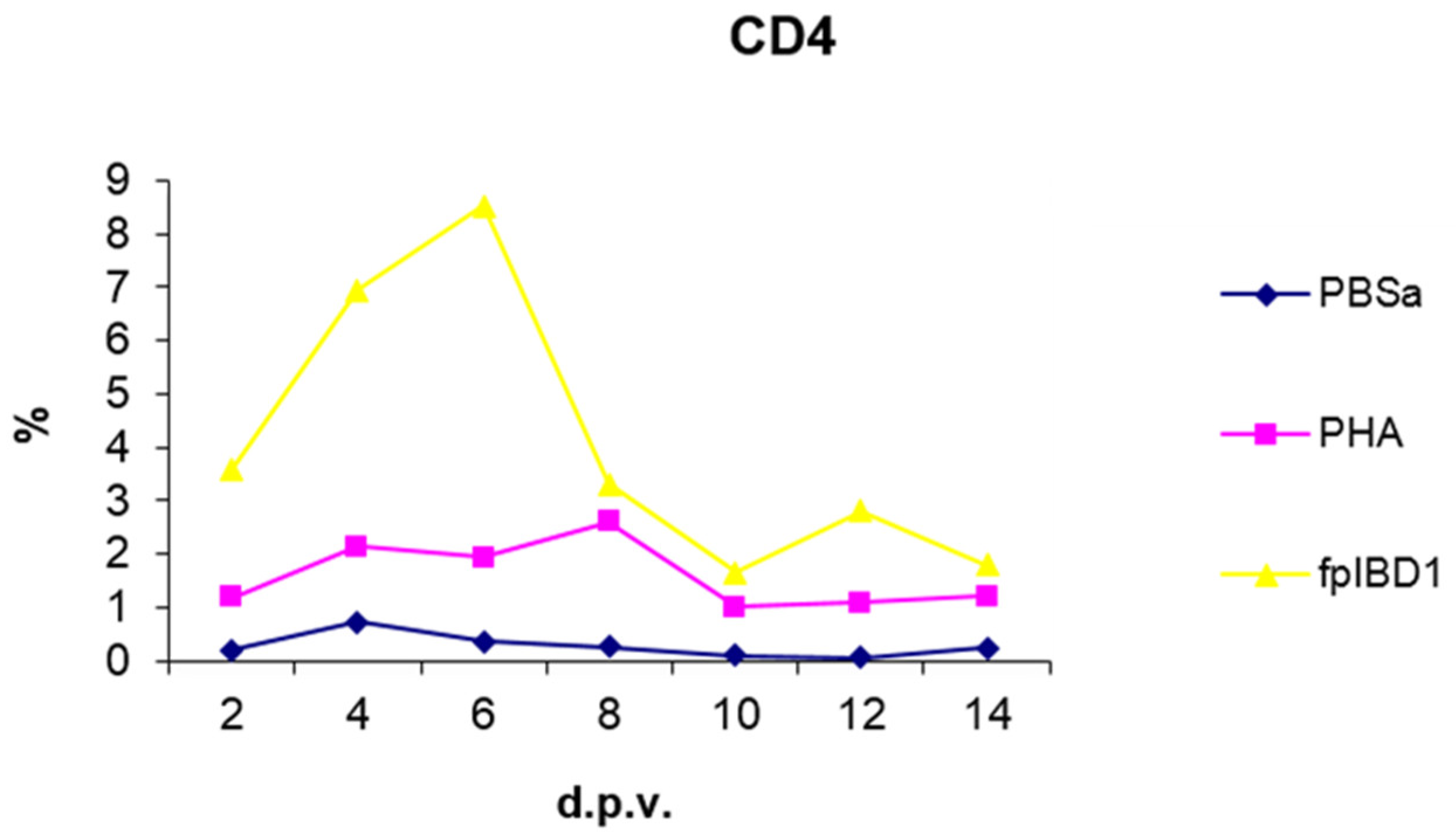
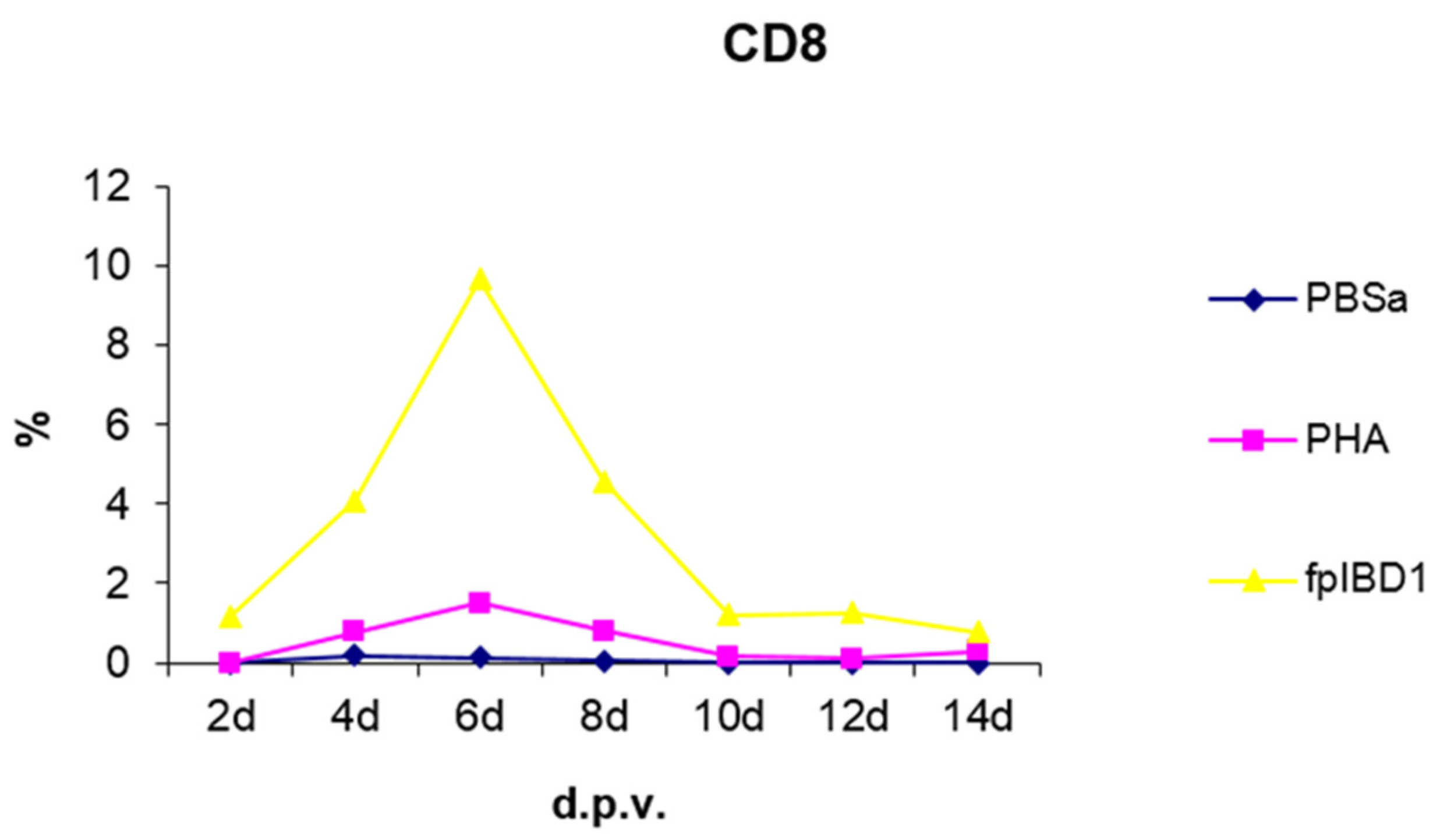
The use of specific monoclonal antibodies, coupled with image analysis (Image pro-plus software), made it easy to assess changes in the quantity of target cells at the site of vaccination. The results of the experiments in this paper suggested that both CD4+ and CD8+ T cells participated in the response.
Intracellular organisms (usually viruses) will synthesize proteins inside the infected cells, and these antigens therefore gain ready access to the MHC class I pathway; as a result, they often induce strong MHC class I restricted CD8+ T cell responses (these cells are usually cytotoxic, although they also secrete a variety of cytokines in response to antigen) [
27].
As shown in
Figure 9, there was a rapid increase of CD8+ T cells in skin sections from 4 dpv, which reached a peak at 6 dpv, at which the virus was almost cleared. Furthermore, the antigens synthesized during intracellular infections usually will be released from the infected cells into the extracellular matrix, and these soluble materials are taken up by specialized antigen presenting cells (APC). Inside these APC, the antigens enter the MHC class II pathway, permitting them to induce MHC class II restricted CD4+ T cells, which usually provide “help” to B cells. The soluble antigens will also encounter B lymphocytes, and therefore should (in concert with CD4+ T cell “help”) induce antibody responses. Therefore, we would expect most intracellular organisms to induce strong CD4+ and CD8+ cell responses, and B cell responses (Figs. 7, 9 and 11 respectively).
Recombinant FPV expressing tumour [
28,
29,
30] and HIV antigens [
31,
33] elicit CD8+ T cell responses in rodents. In addition, studies with nonhuman primates have shown that recombinant FPV encoding HIV antigens can boost the immune response primed by a DNA vaccine, leading to enhanced cytotoxic T cell responses and protection against viral challenge [
33]. Recombinant FPV vectors can be used to elicit CD8+ T cell responses against Plasmodium berghei Malaria by prime boost immunization regimens using a novel attenuated FPV [
34]. Interestingly, FP9 was more effective in eliciting a response against the Plasmodium berghei circumsporozoite protein than the commercially available FPV vaccine strain.
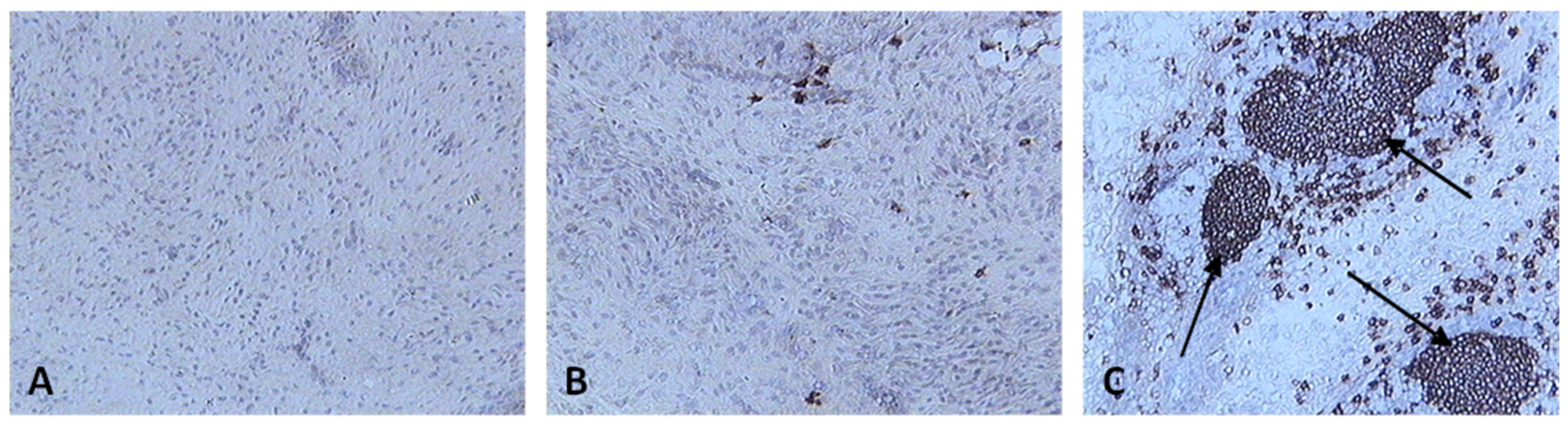
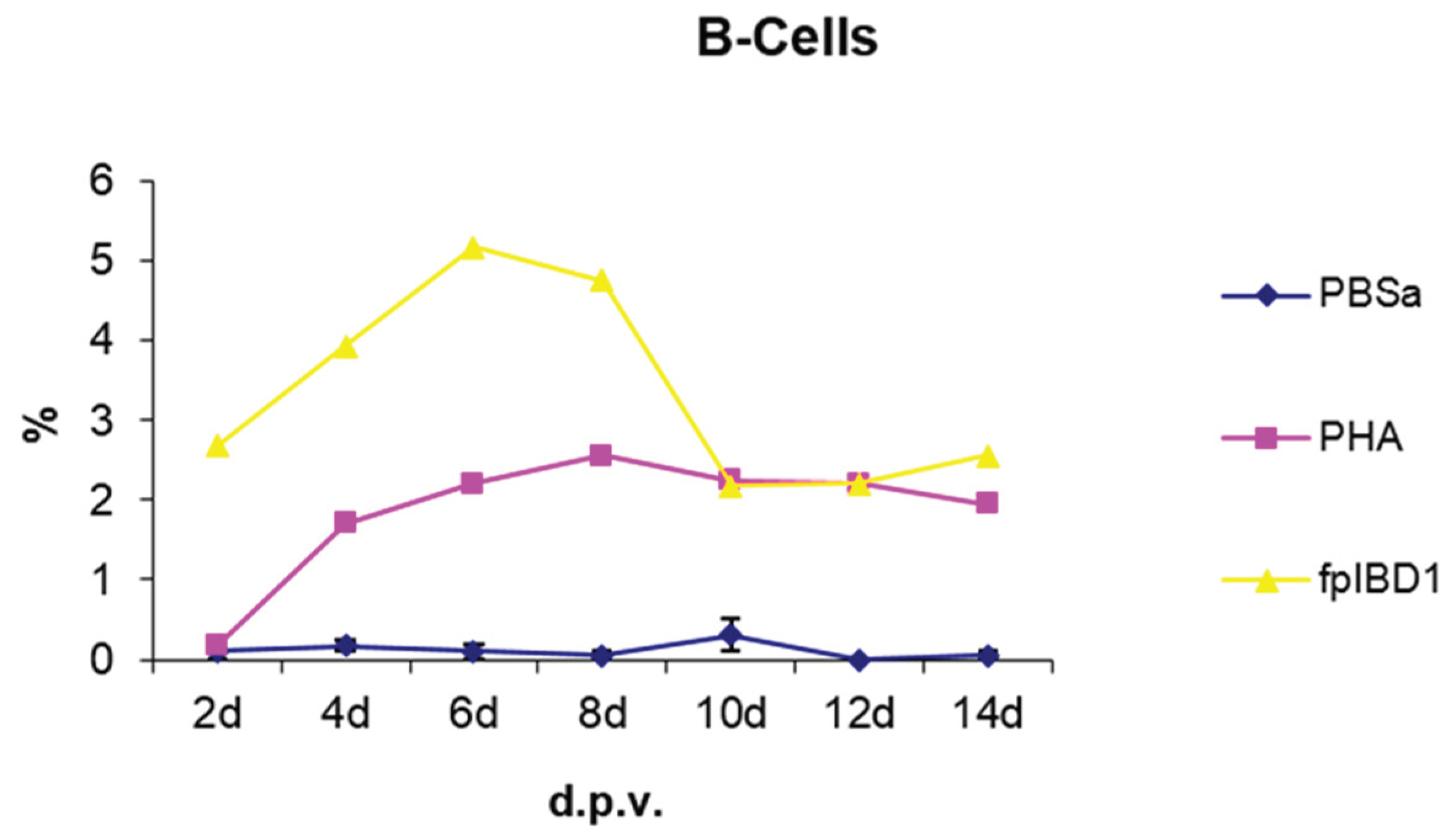
B cells were not present in normal human skin [
35]. After vaccination, there was an aggregation of B cells at the site of vaccination. After antigen challenge, GC are formed within the secondary lymphoid organs (lymph nodes, Peyer’s patches, spleen or tonsils) and in this experiment GC-like structures were formed in the skin tissue too (
Figure 10). At 2 and 4 dpv, B cells in skin sections were diffuse. However, the formation of GC-like structures started at 6 dpv (
Figure 10). At 8 dpv onwards, almost all B cells were aggregated in these GC-like structures.
GC are the site where B cells grow and differentiate to immunoglobulin-producing plasma cells, generate high affinity antigen-specific B-cell receptors by affinity maturation and differentiate into memory cells. However, after fpIBD1 vaccination, detectible antibodies were produced against FPV but not against IBDV [
9,
36]. One possibility is that fpIBD1 may not express correctly-folded VP2, due to the absence of VP4, or express it at very low levels so as not to stimulate an antibody response. The other possibility is that VP2 does stimulate cell-mediated immune responses which is crucial for protection against IBDV infection following vaccination with fpIBD1.
For successful vaccination, “pock” lesions should appear at the site of inoculation within the first week after vaccination. Immunity will normally develop at 10-14 dpv.
It was suggested that CMI plays a more important role in the recovery from FPV infection than humoral immunity [
37]. On the other hand, humoral immunity was not excluded since mortality was significantly higher in bursectomised-thymectomised chickens than in thymectomised chickens following FPV infection [
37].
The regulation of the entry of activated T and B lymphocytes into skin tissue is not well understood. There is also evidence that keratinocytes participate in immune responses in the skin since these cells produce a wide variety of cytokines that can modulate T cell responses [
38].
Figure 1.
Real-time quantitative PCR. (A): Standard curve for pCI-neo::VP2. (B): Number of molecules of plasmid per Ct value.
Figure 1.
Real-time quantitative PCR. (A): Standard curve for pCI-neo::VP2. (B): Number of molecules of plasmid per Ct value.
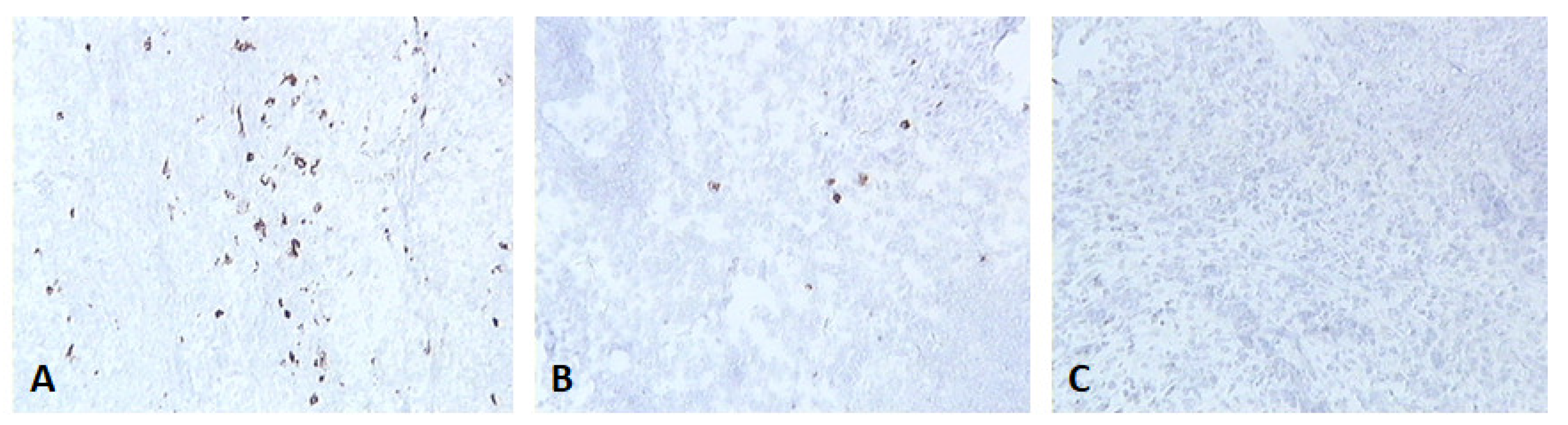
Figure 2.
Skin sections of birds vaccinated with fpIBD1 and stained with monoclonal antibody DF6 against FPV. (A) 2 dpv, (B) 4 dpv and (C) 6 dpv. The positive stained cells (brown) are the cells infected with the FPV (100x magnification).
Figure 2.
Skin sections of birds vaccinated with fpIBD1 and stained with monoclonal antibody DF6 against FPV. (A) 2 dpv, (B) 4 dpv and (C) 6 dpv. The positive stained cells (brown) are the cells infected with the FPV (100x magnification).
Figure 3.
Copy number of the viral vaccine (fpIBD1) in the skin following vaccination, using real-time quantitative PCR.
Figure 3.
Copy number of the viral vaccine (fpIBD1) in the skin following vaccination, using real-time quantitative PCR.
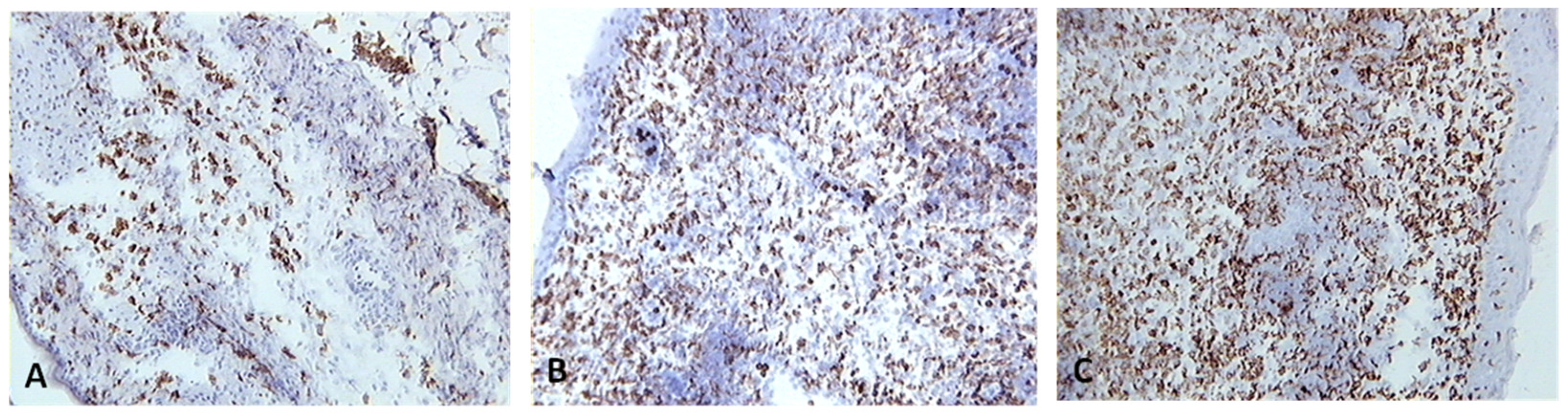
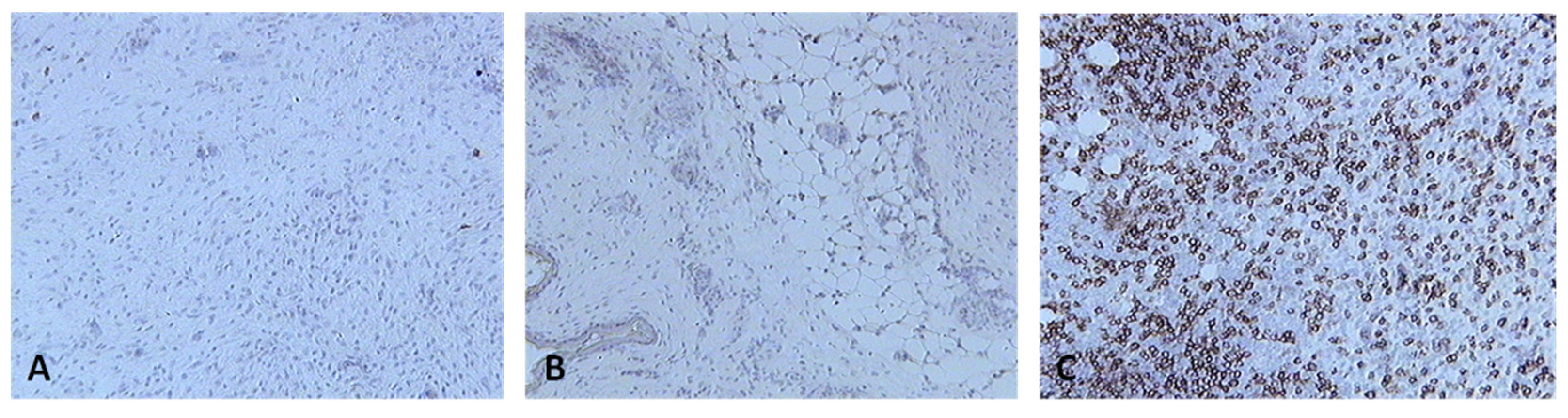
Figure 4.
Skin sections of birds stained with monoclonal antibody KUL01 against macrophages. 2 days post inoculation with (A) PBSa, (B) PHA and (C) fpIBD1. Positive staining for macrophages is seen as brown (100x magnification).
Figure 4.
Skin sections of birds stained with monoclonal antibody KUL01 against macrophages. 2 days post inoculation with (A) PBSa, (B) PHA and (C) fpIBD1. Positive staining for macrophages is seen as brown (100x magnification).
Figure 5.
Kinetics of macrophage numbers in skin sections of birds vaccinated with fpIBD1. % represents the percentage of field of view staining positive with an anti-macrophage monoclonal antibody (KUL01). PBSa - negative control. PHA - positive control.
Figure 5.
Kinetics of macrophage numbers in skin sections of birds vaccinated with fpIBD1. % represents the percentage of field of view staining positive with an anti-macrophage monoclonal antibody (KUL01). PBSa - negative control. PHA - positive control.
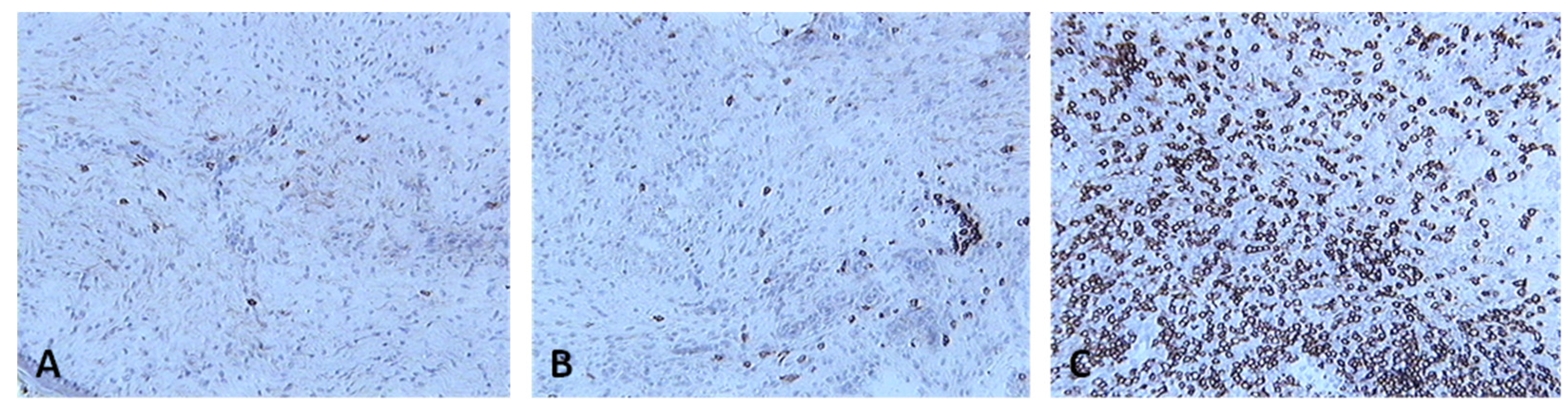
Figure 6.
Skin sections of birds stained with the monoclonal antibody AV29, which recognizes CD4+ cells, 6 days post inoculation with (A) PBSa, (B) PHA, (C) fpIBD1. Positive staining for CD4+ cells is seen as brown (100x magnification).
Figure 6.
Skin sections of birds stained with the monoclonal antibody AV29, which recognizes CD4+ cells, 6 days post inoculation with (A) PBSa, (B) PHA, (C) fpIBD1. Positive staining for CD4+ cells is seen as brown (100x magnification).
Figure 7.
Kinetics of CD4+ T cell numbers in skin sections of birds vaccinated with fpIBD1. % represents the percentage of field of view staining positive with an anti-CD4+ T cell monoclonal antibody (AV29). PBSa - negative control. PHA - positive control.
Figure 7.
Kinetics of CD4+ T cell numbers in skin sections of birds vaccinated with fpIBD1. % represents the percentage of field of view staining positive with an anti-CD4+ T cell monoclonal antibody (AV29). PBSa - negative control. PHA - positive control.
Figure 8.
Skin sections of birds stained with the monoclonal antibody AV14, which recognizes CD8+ cells, 6 days post inoculation with (A) PBSa, (B) PHA, (C) fpIBD1. Positive staining for CD8+ cells is seen as brown (100x magnification).
Figure 8.
Skin sections of birds stained with the monoclonal antibody AV14, which recognizes CD8+ cells, 6 days post inoculation with (A) PBSa, (B) PHA, (C) fpIBD1. Positive staining for CD8+ cells is seen as brown (100x magnification).
Figure 9.
Kinetics of CD8+ T cell numbers in skin sections of birds vaccinated with fpIBD1. % represents the percentage of field of view staining positive with an anti-CD8+ T cell monoclonal antibody (AV14). PBSa - negative control. PHA - positive control.
Figure 9.
Kinetics of CD8+ T cell numbers in skin sections of birds vaccinated with fpIBD1. % represents the percentage of field of view staining positive with an anti-CD8+ T cell monoclonal antibody (AV14). PBSa - negative control. PHA - positive control.
Figure 10.
Skin sections of birds stained with the monoclonal antibody AV20, which recognizes Bu-1 on B cells, 6 days post inoculation with (A) PBSa, (B) PHA, (C) fpIBD1. Positive staining for B cells is seen as brown. Arrows indicate formation of GC-like structures (100x magnification).
Figure 10.
Skin sections of birds stained with the monoclonal antibody AV20, which recognizes Bu-1 on B cells, 6 days post inoculation with (A) PBSa, (B) PHA, (C) fpIBD1. Positive staining for B cells is seen as brown. Arrows indicate formation of GC-like structures (100x magnification).
Figure 11.
Kinetics of B cell numbers in skin sections of birds vaccinated fpIBD1. % represents the percentage of field of view staining positive with an anti-B cell monoclonal antibody (AV20). PBSa - negative control. PHA - positive control.
Figure 11.
Kinetics of B cell numbers in skin sections of birds vaccinated fpIBD1. % represents the percentage of field of view staining positive with an anti-B cell monoclonal antibody (AV20). PBSa - negative control. PHA - positive control.
Table 1.
Primers and probes for real-time quantitative PCR.
Table 1.
Primers and probes for real-time quantitative PCR.
| RNA target |
Primer/Probe* |
Sequence (5′-3′) |
| IBDV (VP2) |
F |
GAG GTG GCC GAC CTC AAC T |
| R |
AGC CCG GAT TAT GTC TTT GAA G |
| Probe |
TCC CCT GAA GAT TGC AGG AGC ATT TG |