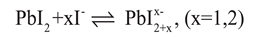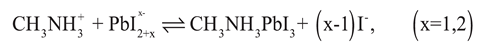1. Background
Photovoltaic devices based on hybrid perovskites, specifically (CH
3NH
3)PbI
3, are the subject of current studies because of their higher efficiencies and simplicity of production. [
1,
2] From the time when their pioneering from 2009 [
3], CH
3NH
3PbI
3 perovskite materials have fascinated substantial concentration owing to their potential applications in economical power conversion photovoltaics. Currently, solar cell device based on the hybrid organic-inorganic halide perovskites have achieved power conversion efficiency of 29.1% (
www.nrel.gov), [
4] the quickest-growing photovoltaic field up to now. [
5] This is recorded as nine-fold step-up in cell efficiency within seven years [
6] and the device has theoretical maximum beyond the 30% efficiency record. [
7] Its hypothetical limit is superior than 30% and this could hammer the competence of silicon, the hypothetical limit of which is bounded to 27%, and reasonably and nearly bounded to ~25%. [
8] Regardless of this promising efficiency, there are two key challenges for real applications of these types of solar cells: the long-term stability and toxicity during large scale production. Different research groups have been investigating to disclose the reasons for stability challenges in halide perovskites such as degradation of the organic part i.e sensitivity of methylamine group and/or its derivative to the ambient environment. [
9] In addition to this, it is important to study if any contributions to high power conversion efficiency and to many applications from the bonding and coordination chemistry of the constituent atoms as well as coordination engineering of the structure. It is highly essential to know whether the electrostatic energy is essentially liable for the stability of halide perovskites or the electron-electron interaction contributing to the stability and more to the electrical properties of such compounds.
The great halide perovskite material with suitable electronic and optical properties for various applications of photovoltaic and beyond photovoltaic is CH3NH3PbI3 perovskite. But, the toxicity of lead atom and its environmental impact during mass production of halide perovskites containing lead metal and waste disposals become challenging. The difficulty for removal of the toxic lead atom is due to the unsuccessful replacement of the toxic lead metal atom using other environmentally friendly metal atoms. Furthermore, why lead atom cannot be replaced and why the produced lead free perovskite materials could not be achieved the required properties and the performance like the lead halide perovskite materials become an ambiguous question. Is there any special behavior of this lead metal atom over the other post transition metal atoms such as Sn, In, Ge, Sb Bi and others? On the other way, does lead atom have special character of coordination engineering in forming the structure of the CH3NH3PbX3 so that lead halide perovskites achieve suitable electronic and optical properties in order to realize their current efficiency? How are the special bonding characters of the halide perovskite complex holding the structure, electronegativity properties of the constituent atoms and electronic interactions of the constituent atoms as well as their contribution expressed?
These halide perovskite materials have the generic ABX3 formula with atristotype (cubic) or hettotype (approximate cubic structures or posidocubic structures): tetragonal and orthorhombic. This sole blend is important for different applications. However, these halide perovskites especially the hybrid organic-inorganic halide perovskites such as CH
3NH
3PbX
3 are quite unstable and easy to degrade. [
9,
10,
11,
12,
13] Achieving high efficiency is not enough by itself unless the stability issue is overcomed. Moreover, how to make them stable becomes a great issue this time and is debatable to solve it even in the future. Although some reviews have been reported, [
14,
15,
16,
17,
18] nowadays, the coordination chemistry and coordination engineering of halide perovskites (both the hybrid organic-inorganic halide perovskites and fully inorganic halide perovskites) become more essential to understand. Similarly, understanding the electronic interactions forming the structure of halide perovskites should get more focus. Unless all these worries get solutions, there will no guarantee to realize the currently confirmed and promising application as well as the future of these halide perovskites materials. For this reason, the aspire of this article is to broadly organize the recent information about the families of halide perovskites; the coordination engineering; coordination chemistry and electronic interactions forming the structure. Subsequently, energy applications of halide perovskite beyond photovoltaic such as laser, light emitting devices, photodetectors and efficient nonlinear emission sources, CO
2 reduction and photocatalysis processes such as solar water splitting and HX splitting are reviewed thoroughly. Also, the purpose of this review is to share current status focusing on
what will be the future of halide perovskite materials with wide range applications. Furthermore, the its scope is to seriously review the current issues on the coordination engineering and coordination chemistry of halide perovskites; electronic interactions that help form halide perovskites structure followed by their energy applications beyond photovoltaic in order to assist the industries and scientific community. Finally, the
future direction of halide perovskite materials and a concluding remark are developed to address the major issues into the scientific community and to help readers improve their understanding on this perovskite field.
The development of this appraisal initiates from its background pursued by the outline in sequence of the families of halide perovskites materials and further refreshed by many of the subfamilies of the two broad families: lead free and lead halide halide perovskites. Furthermore, coordination engineering of the halide perovskite frameworks with an eye towards overcoming stability and toxicity challenges, as well as coordination chemistry and followed by the electronic interactions forming the structure of halide perovskite materials are comprehensively reviewed. Moreover, due to their remarkable optoelectronic properties, halide perovskite materials become revolutionizing the area of photovoltaic and many other wide range applications. Hence, interesting and appreciated energy applications of these halide perovskites beyond photovoltaic are discussed in depth. Finally, the organization of this review is completed with an open question to the scientific community and commercial enterprises: what will be the future of these materials? Their instability and toxicity create strong doubt that leads to the open question: will their commercialization and mass production successful in the future with the expected performance or not? Based on this essential information, a critical conclusion is drawn.
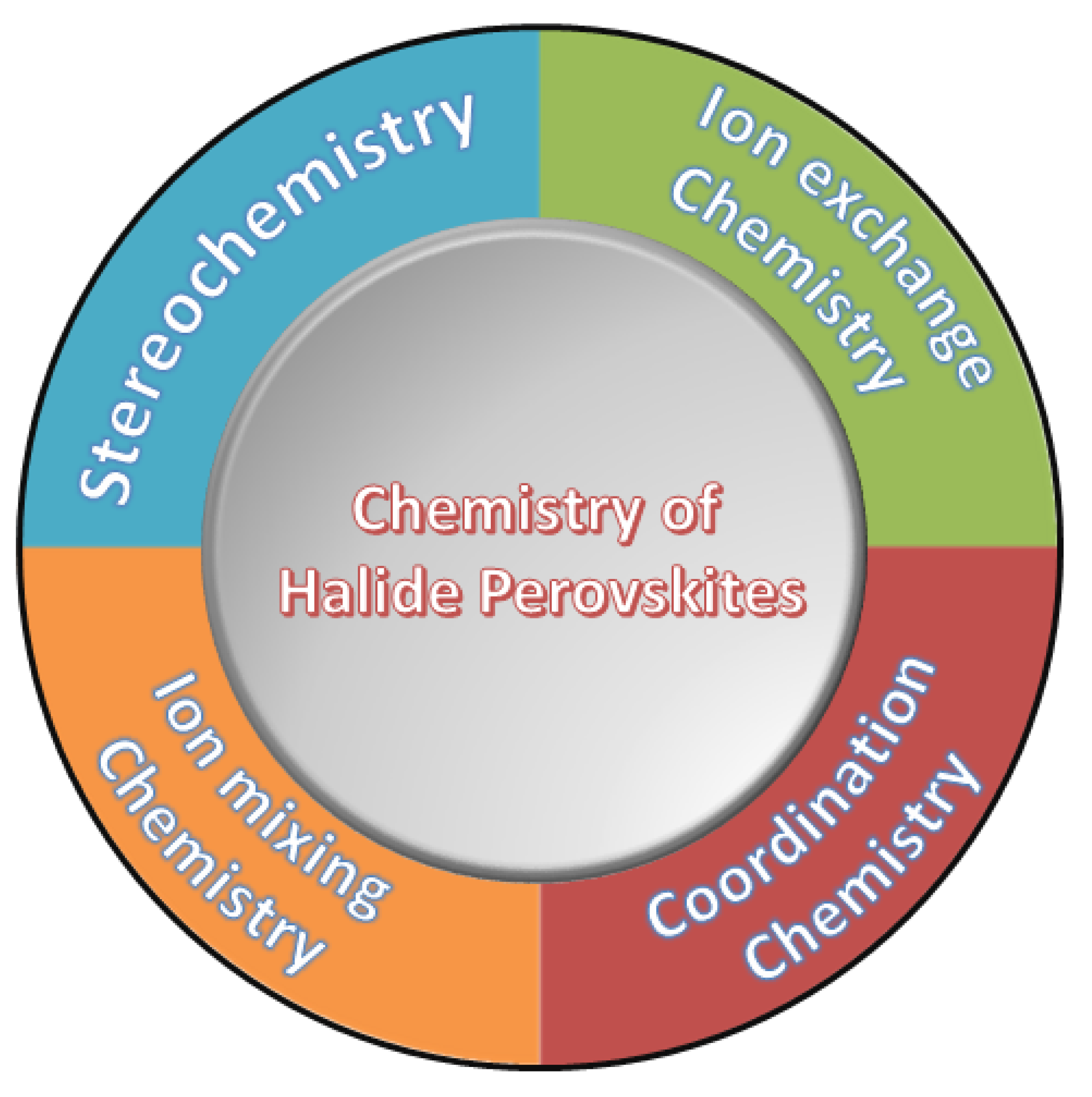
2. Halide Perovskites
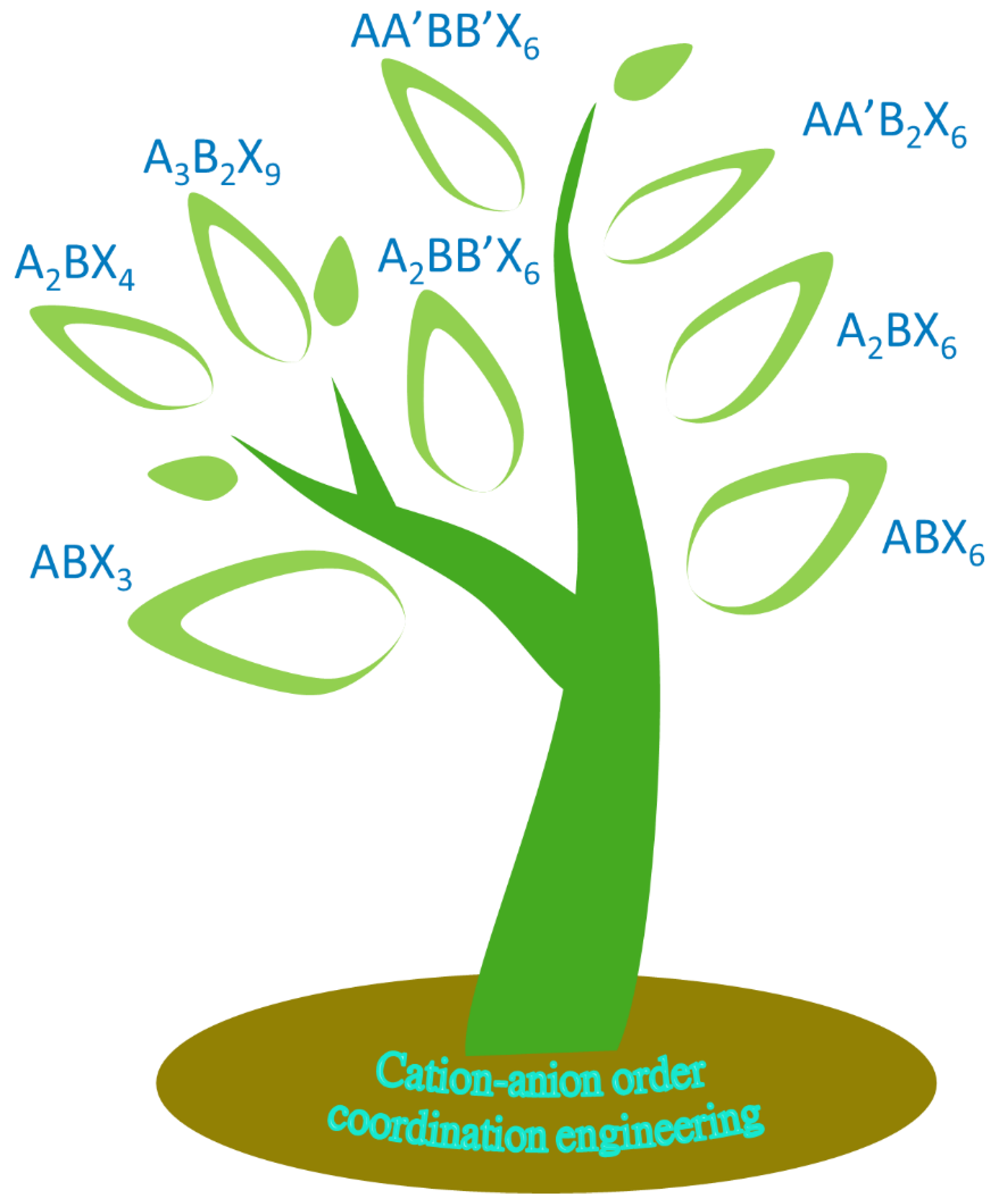
halide perovsite materials have been revolutionizing the area of photovoltaic area with remarable efficiency and this time its diversity with many perovsite derivative species become very common for researchers. In brief, those currently reported families and list of halide perovskite derivatives as well as the research development and expansion are summarized in
Scheme 1 and
Table 1.
Ordinarily, halide perovskite sensitizers depend on the 3D structure with universal recipe AMX
3, where X = Cl−, Br−, I−; A is CH
3NH
3+ (MA) or HC(NH
2)
2+ (FA); and M is Sn
2+ or Pb
2+. The 3D organization is a progression of corner-sharing MX
6 octahedra involving the cubohoctahedral cavities, keeping up electroneutrality of the framework. Along these lines, because of their fantastic tunability properties and probability of different substitutions, different sorts of halide perovskites have been building, which is the focal point of the accompanying subsections. Following the primary announced performance of 3.8% (2009), perovskite solar cells have risen to achieve performance beyond 22.1% in 2016. [
19] With such witnessed performance accomplished by straightforward construction forms, these devices are likewise extremely encouraging for supplementing silicon solar cells in a couple arrangement.
2.1. HC(NH2)2PbI3 and Its Derivatives
Among halide perovskite, CH
3NH
3PbI
3 and HC(NH
2)
2PbI
3 are right now the hero hybrid organic-inorganic halide perovskite materials with ~22.1% record efficiencies. The larger size of HC(NH
2)
2+ results in smaller bandgap compared to the smaller size of CH
3NH
3+, the reason is not known. Normally, when the R-group (carbon chain) increases, the bandgap becomes increased and this leads to discourage achieving higher efficiency but encourages achieving better stability. It is pondering that the utilization of HC(NH
2)
2+ expands proficiency and predominant photostability [
20,
21,
22,
23,
24,
25,
26] however bring down dampness stability [
27] of halide perovskites contrasted with CH
3NH
3+. Flimsiness of dark perovskite HC(NH
2)
2PbI
3 is expected to either precariousness of the polar formamidinium itself in nearness of water. Notwithstanding its dampness insecurity, HC(NH
2)
2PbI
3 displays sudden concealment of photovoltaic impact as the framework experiences cubic-to-hexagonal progress, after cooling.
2.2. (CH3NH3)x(HC(NH2)2)1-xPbI3 Perovskite
The paired cation perovskite of (CH
3NH
3)x(HC(NH
2)
2)
1-xPbI
3 was primary accounted for by Gr¨atzel. [
28] To acquire a high caliber and smooth halide perovskite film, this blended perovskite (CH
3NH
3)x(HC(NH
2)
2)
1-xPbI
3 was readied by means of a successive affidavit strategy by plunging PbI
2 in CH
3NH
3I + HC(NH
2)
2I blended arrangement.
2.3. (HC(NH2)2)1−xCsxPbI3 Perovskites
In order to improve photo and dampness dependability, (HC(NH
2)
2)
0.9Cs
0.1PbI
3 has been accounted for as an elective light safeguard to CH
3NH
3PbI
3 and HC(NH
2)
2PbI
3. [
29] (HC(NH
2)
2)
0.9Cs
0.1PbI
3 perovskite was shaped by means of Lewis base adduct of PbI
2. [
30] Optoelectronic properties and photovoltaic execution of Cs-joined HC(NH
2)
2PbI
3 were contrasted and those of flawless HC(NH
2)
2PbI
3. An efficiency of 19.0% estimated by invert sweep and normal efficiency of 16.5% as of forward output exhibited from (HC(NH
2)
2)
0.9Cs
0.1PbI
3 film in planar structure. [
29] More critically, it has been discovered that steadiness of (HC(NH
2)
2)
0.9Cs
0.1PbI
3 film against the light and mugginess was enhanced contrasted with HC(NH
2)
2PbI
3 film. Improved photo and dampness security of hybridized Cs with HC(NH
2)
2PbI
3 is begun from contracting of the cubo-octahedral volume and increment in the concoction association among HC(NH
2)
2+ and I. [
31] Li et al. detailed that blending Cs with HC(NH
2)
2+ generously brought down the stage change temperature from 165 °C to room temperature. By alloying the CsPbI
3, the resistance factor of the perovskite is tuned and the diminishing in stage progress temperature results from the adjustment of the perovskite structure. [
32]
2.4. The Difficulty of Replacing Lead Atom by Other Metals
The essential inquiry with respect to lead free materials is the reason lead is so difficult to supplant, to which there is no simple answer. Fundamentally the properties for a B-Site particle are:
- a)
Ionic radius: best outlined by the tolerance factor: Replacing the toxic Pb in perovskite crystals needs an atom with similar size. The excellent performance of Pb-based perovskites is mainly due to high structural symmetry and strong antibonding coupling between Pb and I.
- b)
High polarizability: lead(II) is considered a softer or borderline hard/soft cation, has plarizable outer electrons, large size, low electronegative and should interact most strongly'with donor types. Typically a soft cation will covalently bond with a soft donor atom which has low electronegativity, highly polarizable low-lying empty orbitals and is easily oxidized, and a hard cation will form an ionic bond with a donor atom which has high electronegativity, low polarizability, high energy empty orbitals and is hard to oxidize.
- c)
Valence: a B site atom has in a perfect world a 2+ valence, different configurations are conceivable, however they require remuneration to accomplish charge neutrality. Lead (II) has stable oxidation state of +2 with coordination number of 6. All six PbII-X bonds of the halogen ligands, the holodirected structures in which the ligand atoms are connected to each other are clearly ionic, but the ionic character of the bonds decreases as the atomic number of the halogen ligand increases and greater transfer of electron density from the ligands to the lead occurs as the electronegativity of the ligand decreases and the bond become covalent bond. If the arrangement is holodirected geometry, the PbII-ligand bonds are all similar.
- d)
Lone pairs: Ideally the B-site displays a contorted (non symmetric) 6s2 lone pair. When considering every one of these elements, of each of the 120+ elements just lead has this alluring mix of properties.
3. Coordination Engineering of Halide Perovskite Crystals
It is clear that energy materials having superior characterstics encompass the prospective to stimulate upcoming technological development. Hence, finding other new perovskites becomes important in order to overcome two vital challenges: stability and toxicity. This section provides an important concepts of coordination engineering framework for engineering new perovskite materials with a special aim to improve stability and avoiding toxicity as well as provide an insight for the future directions and new research horizons of this field.
3.1. Cation and Anion Order Engineering of New Halide Perovskite
Other than the previously mentioned structural flexibility, these halide perovskite materials have generous compositional flexibility, which is done by means of ion order engineering. Subsequently, from the viewpoint of particle arrange designing, concoction substitutions happens onto every one of the three locales of the aristotype structure. Following this, the anion site can house higher amount of vacancies and molecules exchange (e.g. halides) together while cation exchanges go up against either arbitrary or ordered orientations as appeared in
Scheme 2, which effectsly affect the attributes of the designed materials.
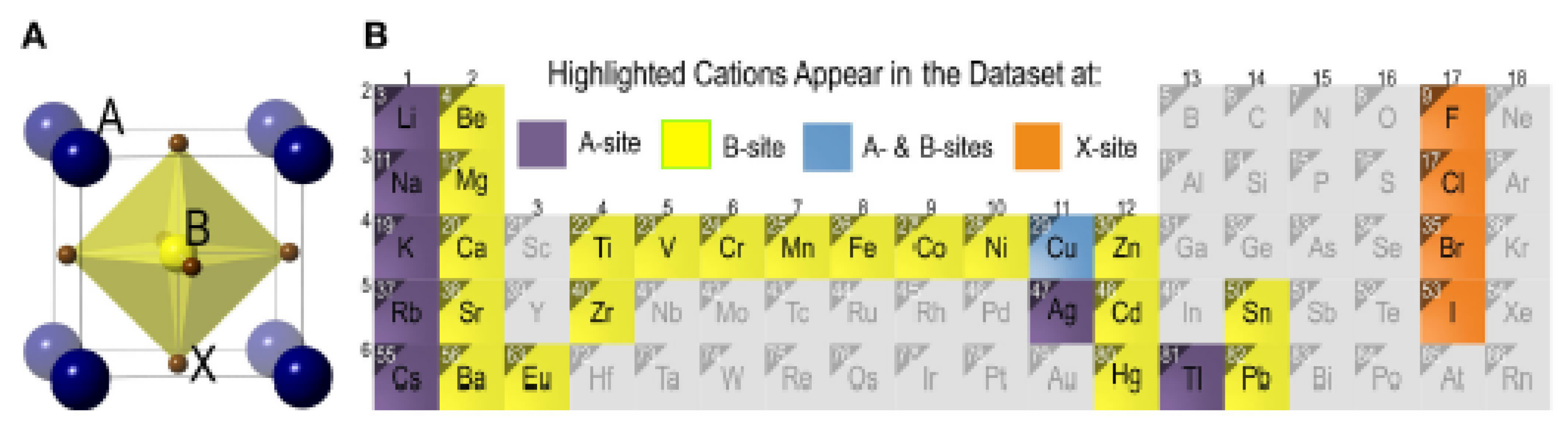
In the giant mass of A
2BB'X
6 perovskites the B and B' cations go up against an organized model that resembles cation and anion positions in the crystal salt structure (
Figure 1). In excess of 400 revealed points of reference of shake salt can be found in A
2BB'X
6 perovskites. [
33,
34] As a general rule when the oxidation states of B and B’ differ by less than two a disordered arrangement is observed (e.g. La
2CrFeO
6), while, a variation larger than two almost always constructs an ordered arrangement (e.g. Sr
2NiWO
6). When the variation in oxidation states is just two, disordered (e.g. Sr
2FeRuO
6), partially ordered (e.g. Sr
2AlTaO
6), or fully ordered (e.g. Sr
2YNbO
6) arrangements can consequence, depending on differences in size and/or bonding preference of the B and B’ cations. [
35,
36,
37] There have been various broad reports of B-site cation arrange in perovskites, and the forces that drive B-site cation ordering are normally understood and reported elsewhere [
35]
, [
38]
, [
34].
3.2. ABX3 Perovskite
The stability issues in halide perovskite materials become a default challenge for practicing. Thus, looking for other analogous materials with similar octahedral arrangement (i.e 3D arrangement of corner-sharing octahedral BX
6 units) or other materials that might fulfill the vision of perovskite community (i.e edge sharing octahedral arrangement) is a must. In this section, a representative cubic crystal structure was taken on by ABX
3 perovskite halides [
40] as shown in
Figure 2A, where A and B aretwelve- and six-fold organized, and have plus one, plus two supposed charge states, correspondingly, whereas X [
41]
I is a halide. On the other hand, edge sharing non-perovskite structures with a real so universal in arrangements with ABX
3 stoichiometry (for example CsNiF
3 and CsCoCl
3 crystal structures) [
42]. Furthermore, from the obtainable data on formability of ABX
3 structures, the possibility to create a model and forecast by means of an acceptable correctness whether a suggested structure by means of known option of cations with +1, +2 and halide with -1 charge ought to be halide perovskite or a non-perovskite as shown in
Figure 2B.
3.3. ABX6 Perovskite
The breakthrough with a novel ABX
6 crystal showing superior performance leads to a broad occasion for lucid representation for sophisticated optoelectronic and solar cell function. [
44] Whereas the photovoltaic characteristics of Sb(III) iodides well explored previously, halide complexes of Sb(V) continue uncultivated. Furthermore, these halide complexes of Sb(V) materials characterize seriously hued substances [
45,
46] and, subsequently, can be viewed as conceivably accommodating materials for photovoltaic cells. Substance arrangement and precious crystal structure of the items emphatically rely upon the idea of organic cations A, while no unmistakable relationships empowering objective material plan have been built up so far. [
47,
48,
49]
3.4. A2BX4 Perovskite
A
2BX
4 halide perovskites are two dimentional materials with BX
6 octahedra, [
50] prompting adaptable mechanical properties and valuable light emission. [
51,
52] Furthermore, these halide perovskites can be differed by consolidating either divalent metal or an extended organic cation chain suggestive of numerous other imaginative bearings that could strengthening enhance the usefulness of these materials.
3.5. A2BX6 Perovskite
A
2BX
6 framework is another material that is expected to play its contribution. For instance, in recent times Cs
2SnI
6 is introduced; its exceptional electronic and optical properties create it a capable applicant with novel efficiency. [
53] Furthermore, this outline technique has likewise propelled the comprehension of the basic security for the perovskite sunlight based device, since two dimentional [PEA]
2 [MA]
2Pb
3I
10 has demonstrated superior protection from moisture [
54] conceivably because of the hydrophobicity of the benzene ring.
3.6. A3B2X9 Perovskite-like 3D Framework
The majority of the new attempts have been spotlighted on the examination of halide complexes of the post transition group 15 elements for example Bi and Sb. Alongside the spearheading writes about BiI [
55] and A
3Bi
2I
9 (A = MA or Cs), [
56,
57,
58,
59] The scope of antimony (III) halides explored in photovoltaic cells is constrained to A
3Sb
2I
9. [
60,
61,
62] Furthermore, both Cs
3Bi
2I
9 and MA
3Bi
2I
9 are showing a different advantage over Pb-, or Sn-based perovskites. [
63,
64] This is on the grounds that the upsides of non-danger, encompassing stability, and low-temperature arrangement processability, which gives a promising answer for location the poisonous quality and stability issues.
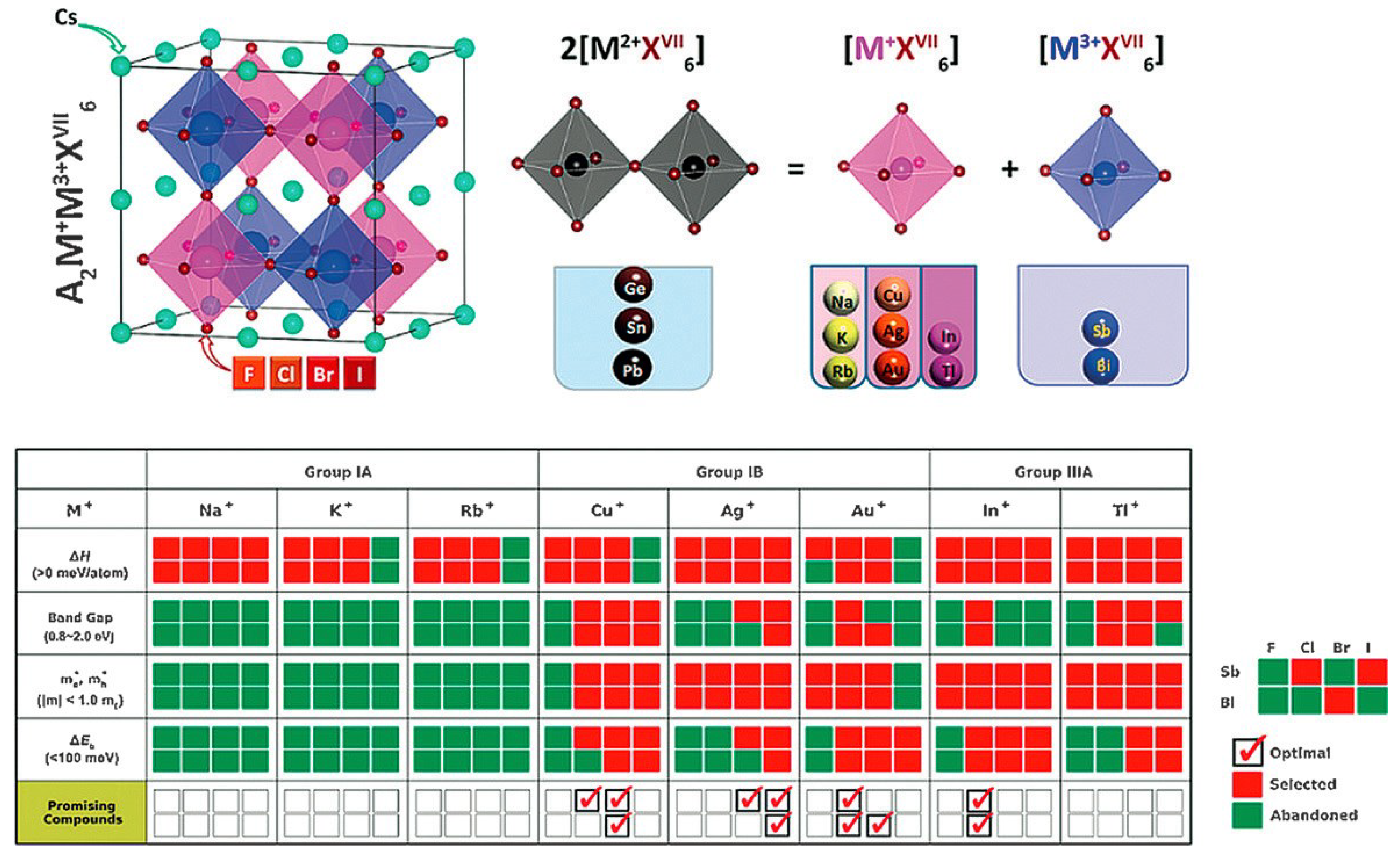
3.7. A2BB’X6 Double Perovskites
The journey for a totally without Pb yet continues a noteworthy objective in the halide perovskite photovoltaics. So as to understand the 3D perovskite design this has shown favorable circumstances for high efficiency, twofold perovskite with 3D structure gets incredible consideration this time. As of late, Zhao et al. [
65] found, through first-standards counts, a prosperous set of quaternary halide perovskites through A
2B
+B
3+X
6 in the course of the transformation of Pb
2+ particles into one monovalent particle (B
+) and one trivalent particle (B
3+), as appeared in
Figure 3. The new perovskite viably kept away from harmful Pb
2+ cations. Additionally, all these have inborn thermodynamic solidness, appropriate band holes, little bearer compelling masses, and low excitation restricting energies. This suggests us show a potential strategy for taking out harmful Pb in PSCs. It might likewise be important to dope different molecules into the CH
3NH
3PbI
3 cross section, or to change to different materials, for example, Cs
2InSbCl
6, Cs
2InBiCl
6, Cs
2BiAgCl
6, and Cs
2AgBiBr
6, to enhance the conventional CH
3NH
3PbI
3. [
65,
66,
67,
68,
69] However, it stays testing to lead point by point examinations on the systems of perovskite light assimilation through first principals computations.
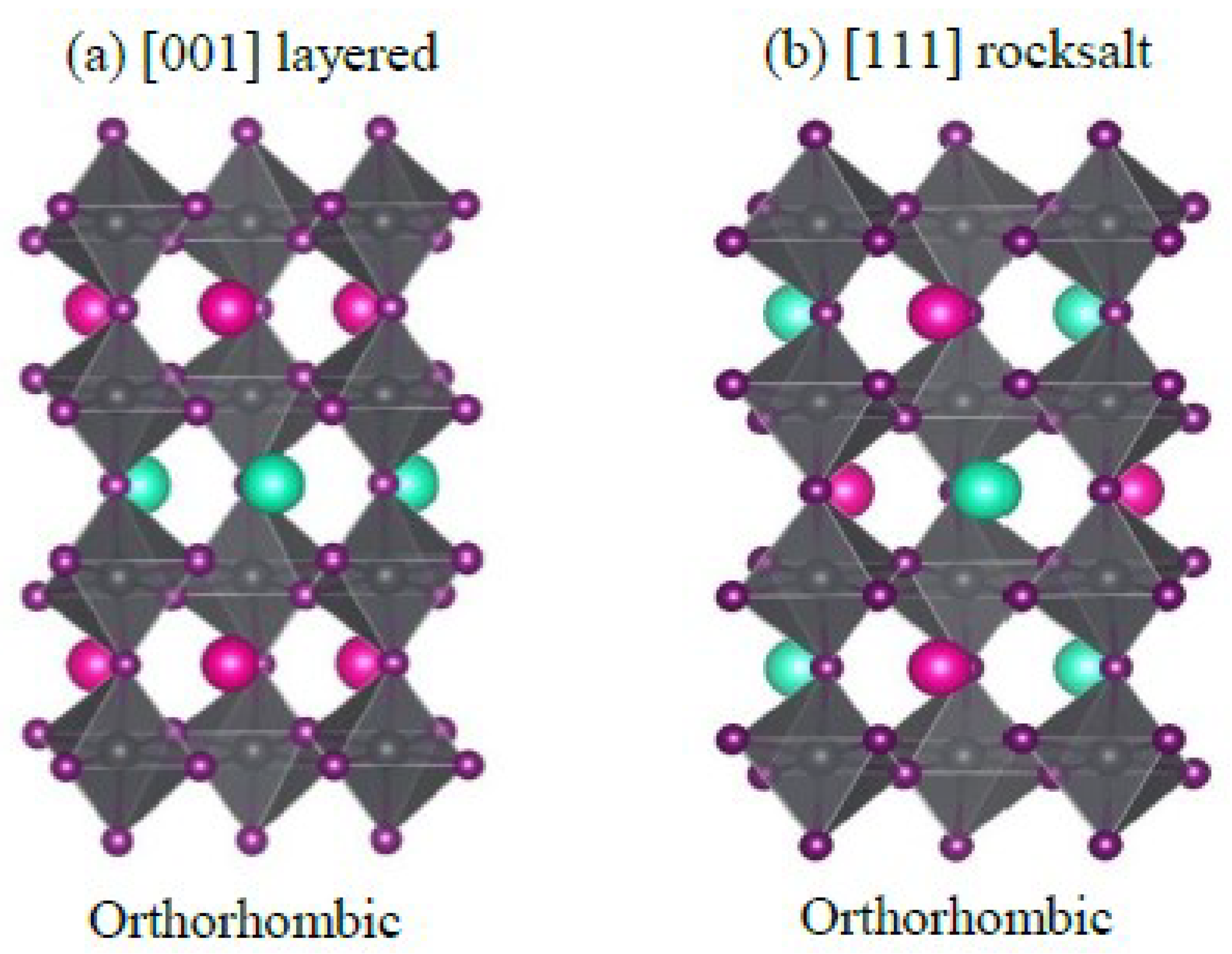
3.8. AA’B2X6 Double Perovskite
Gulzhanat
et.al. performed ab-initio calculations of CsRbPb
2I
6 halide perovskites with ferroelectric AA’B
2X
6 perovskite framework. [
70] The watched unconstrained polarization of AA’B
2X
6 perovskite materials is relied upon to be one of the critical properties which decide efficiency of perovskite based sunlight based cells. The unit cell of perovskite contains 20 atoms. Space group of [001] layered supercell is Pmc21, [111] rocksalt supercells space assemble is Pna21, this is really a polar space bunches appeared in
Figure 4.
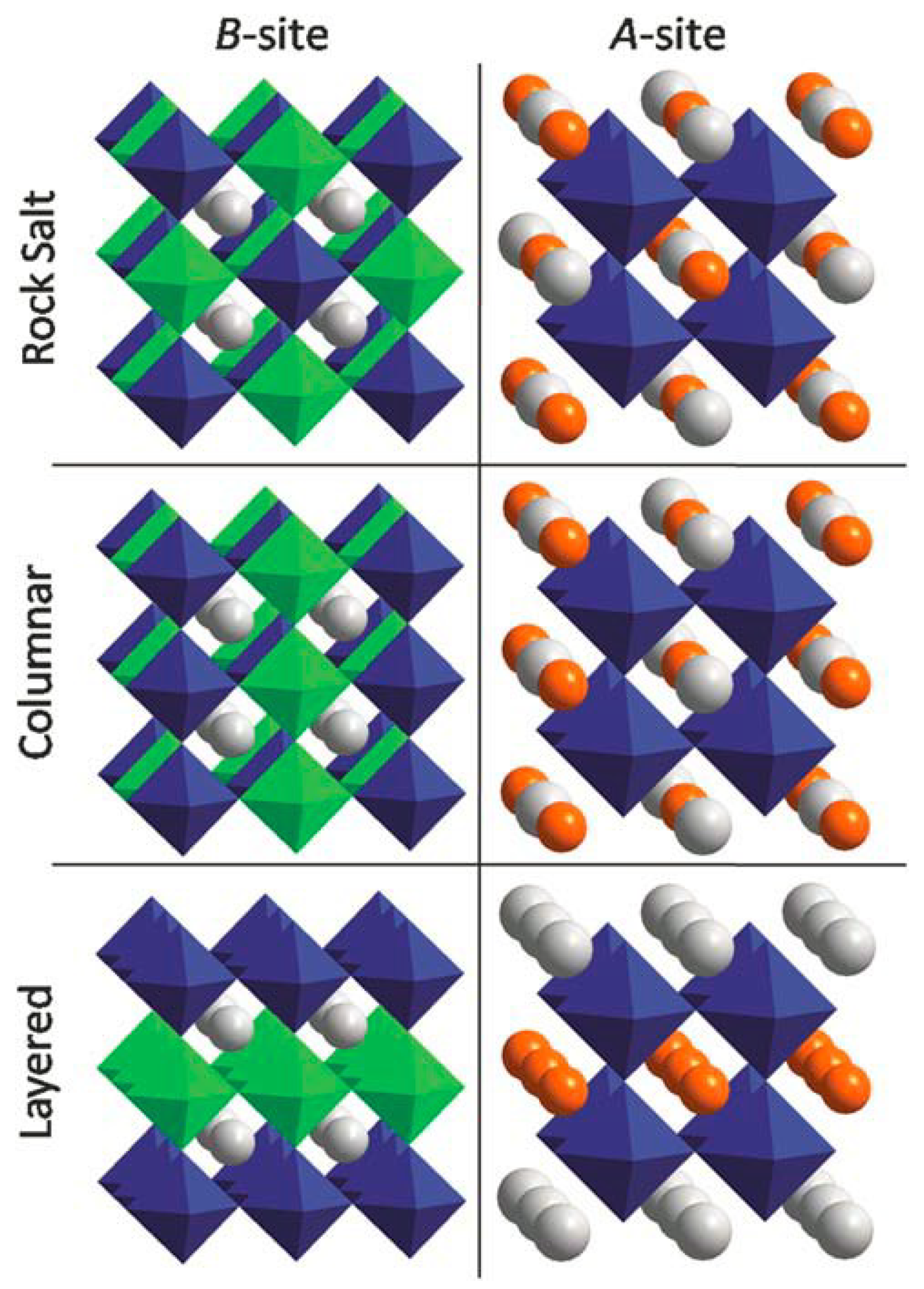
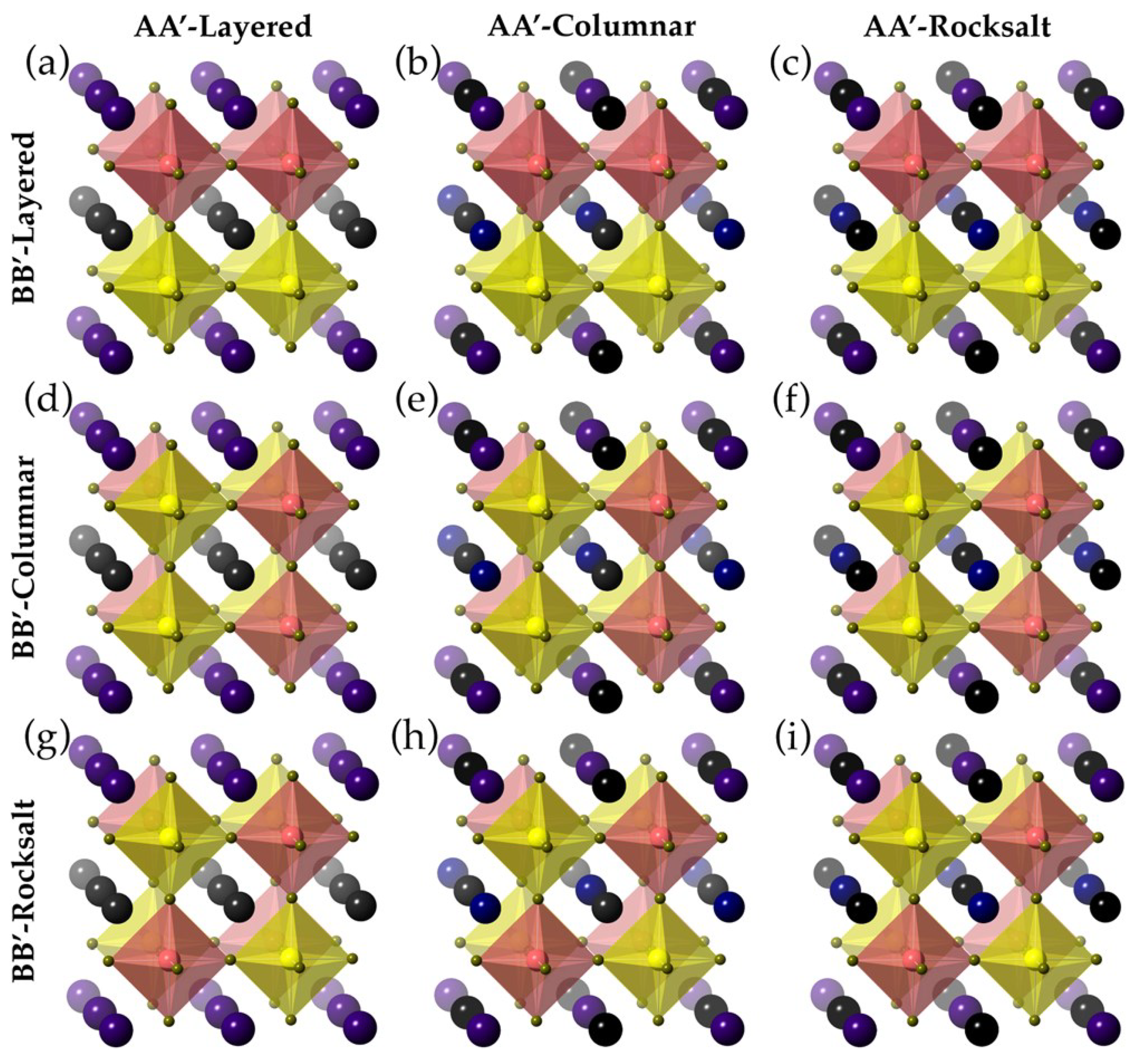
3.9. AA’BB’X6 Double Perovskite
Within twofold AA’BB’X
6 crystal structures through one:one proportion of its components, layered, columnar, and rocksalt become unique potential outcomes for requesting on every one of the A- and the B-site cation sub-cross sections. On account of columnar requesting cations of a similar kind are ceaseless just along one measurement and shape sections of interfacing Cl octahedra, while for the instance of layered requesting cations of every sort frame on the other hand stacked 2D planes. At last, the rocksalt requesting, which speaks to the the majority of symmetric one out of the three conceivable outcomes, known as in light of the fact that the example of A-site or B-site is comparable to the anion and cation arrangement observed from rocksalt structures. For this situation, cations substitute in every one of the three symmetrical headings. The concurrent requesting of the halfway replaced cations at A- and B-destinations consequences an aggregate of 9 unique potential outcomes for CsRbCaZnCl
6 (
Scheme 3).
4. Coordination Chemistry of Halide Perovskite Structures
The investigation of lead halide structures (
Scheme 4) is well established. [
73] Furthermore, change metals with a s
2 electron setup (e.g., Ti
4+, Sn
2+, Pb
2+, Sb
3+) speedily encounter complexation with halide particles. Lead buildings are typically implied as "plumbates" (e.g., triiodoplumbate (PbI
3−)) and fill in as forerunners in arrangement. Right when separated in DMSO, PbI
2 is dreary yet winds up darker unending supply of plenitude iodide particles. As planning dissolvable ligands are superseded by I
− course of action of PbI
3− and PbI
42− buildings, cutting down imperativeness charge-trade retention groups is observed. [
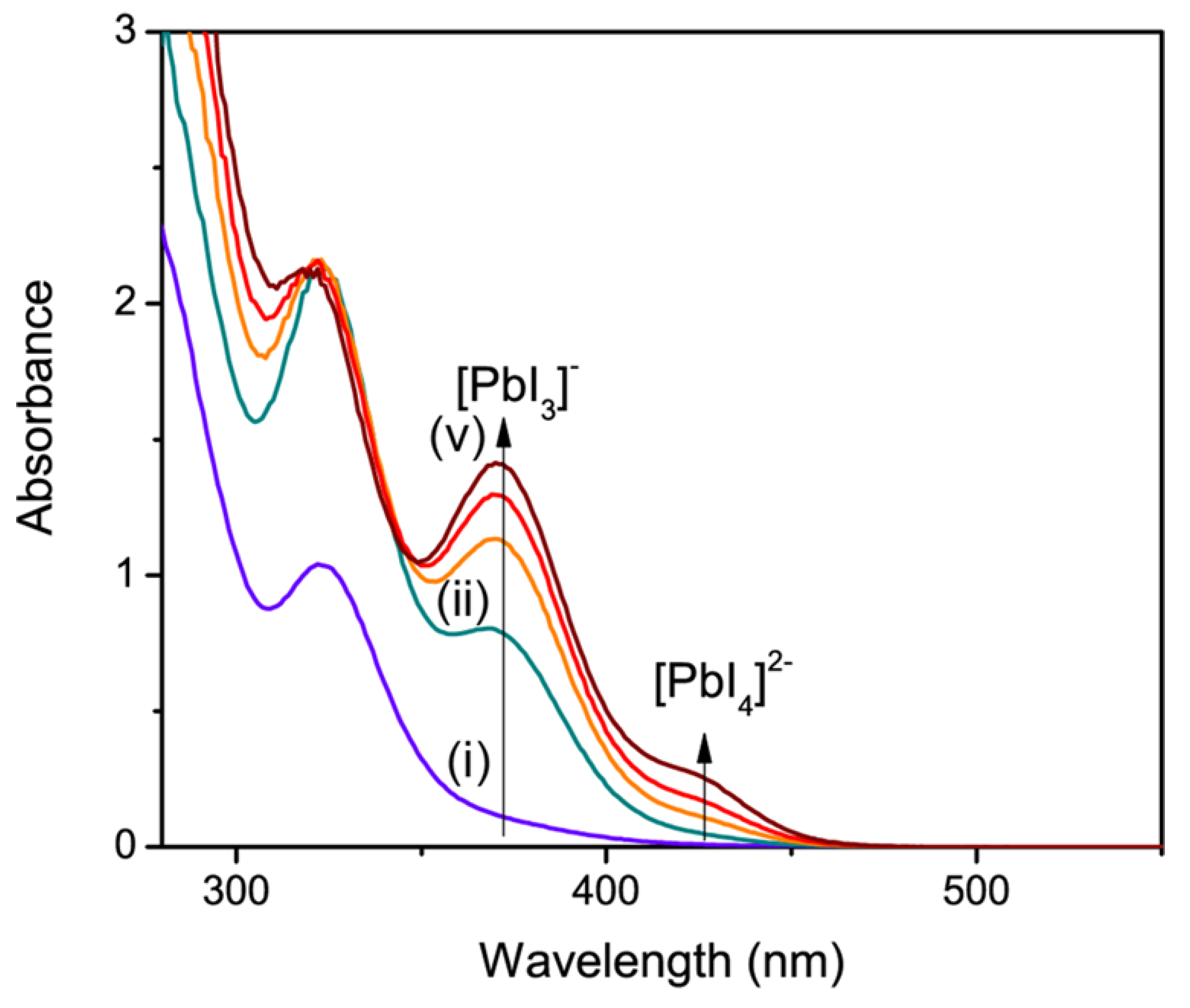
73] These new advances can be quickly pursued and give understanding into complexation events in the forerunner arrangement.
Figure 5 indicates retention spectra of Pb
I2 recorded at various I
− focuses in DMF. The reliance of ingestion on the I
− fixation empowers estimation of complexation constants, as appeared in Equation 1 and 2, respectively. [
74]
It is spellbinding to see that the complexation of Pb(II) with iodide particles is free of the likelihood of the iodide counter molecule. Close spooky highlights relating to lead halide structures were in like way observed when CH
3NH
3I was supplanted with KI. An issue by and large examined in considering the coordination and stereochemistry of liberal metals is that of the 'stereochemical movement' of valence shell lone electron sets. [
75,
76,
77,
78,
79]
4.1. Coordination Chemistry of Post Transition Metal Atoms
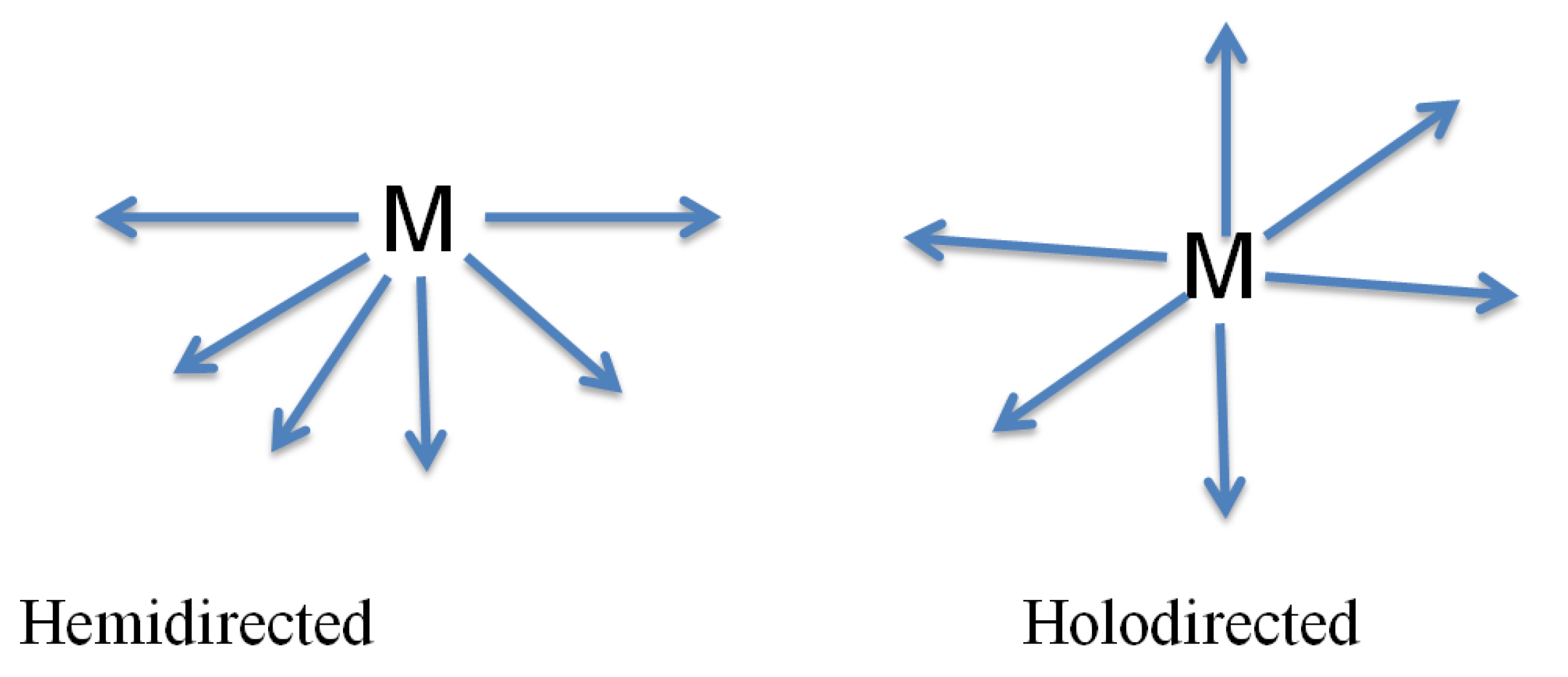
Of all p-block elements, lead(II) has a specific interest for coordination chemists, [
80] as it can receive a wide range of geometries in its complexes, permitting a degree of tolerance for ligand arrangements which isn't seen in, for instance, d-block components. The capacity to tie well to both hard and delicate benefactor iotas make lead(II) an intriguing metal to think about. Lead(II) structures have also pulled in incredible intrigue in light of lead's huge particle span, changeable coordination number, and the conceivable event of a stereochemically dynamic solitary combine of 6s
2 external electrons and in addition novel system topologies. [
81] Consistent with the hard-delicate corrosive base hypothesis, the moderate coordination capacity of Pb(II) implies that it can adaptably facilitate little nitrogen or oxygen iotas and additionally huge sulfur atoms. [
82] The examination of "stereo-synthetic action" of valence shell electron solitary matches in polymeric and supramolecular mixes might be additionally intriguing. [
83] Lead(II) has an electronic structure [Xe]4f
145d
106s
2. Because of relativistic impacts the 6s orbital is contracted and settled. This settled 6s sets lessens its cooperation in the science of the component (turning into an “inert-pair”) and this clarifies why inorganic Pb frames mixes in a lower oxidation state (less by two) than would be normal from its gathering number. [
84] The evident hesitance of the 6s electrons to assume a role in the science of the component may likewise influence the stereochemistry of Pb(II) edifices. This impact can be comprehended as far as basic hybridization or valence shell electron-match shock arguments. [
85] It appears that the 6s orbital, despite its adjustment, can hybridize with the 6p orbitals to give a "stereochemically dynamic" 6s electron combine possessing one position in the coordination circle of the metal. Since the match is not specifically discernible, its quality is typically recognized by a void in the conveyance of the coordination bonds (asymmetrical coordination (hemidirected), as see in
Scheme 5). On the off chance that hybridization does not happen and the combine has just s character, at that point it is "stereochemically latent" and the complex does not demonstrate a hole or void in the security appropriation (symmetrical coordination (holodirected) in
Scheme 5). [
86]
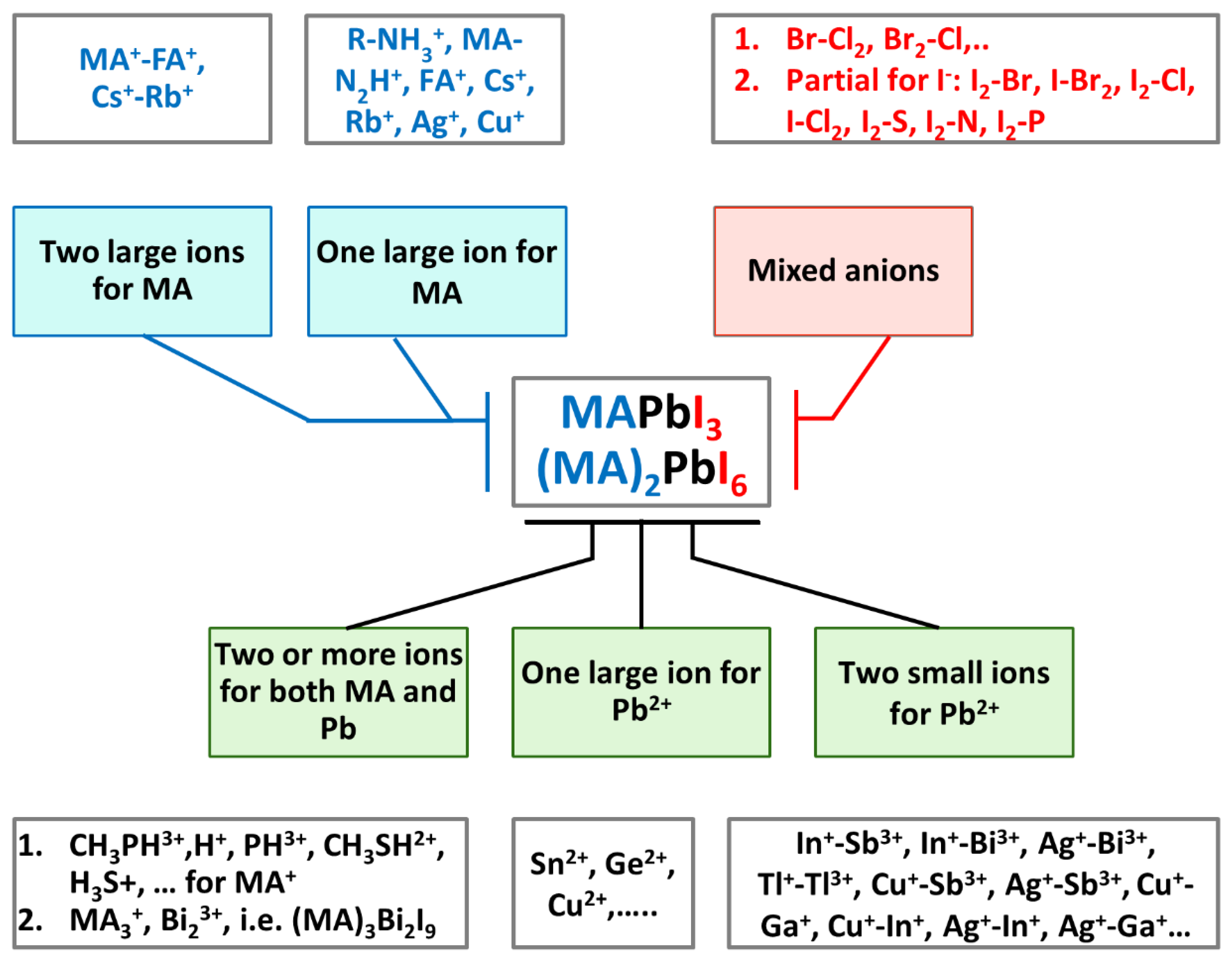
4.2. Proposed Ion Exchange and Ion Mixing Chemistry in Perovskites
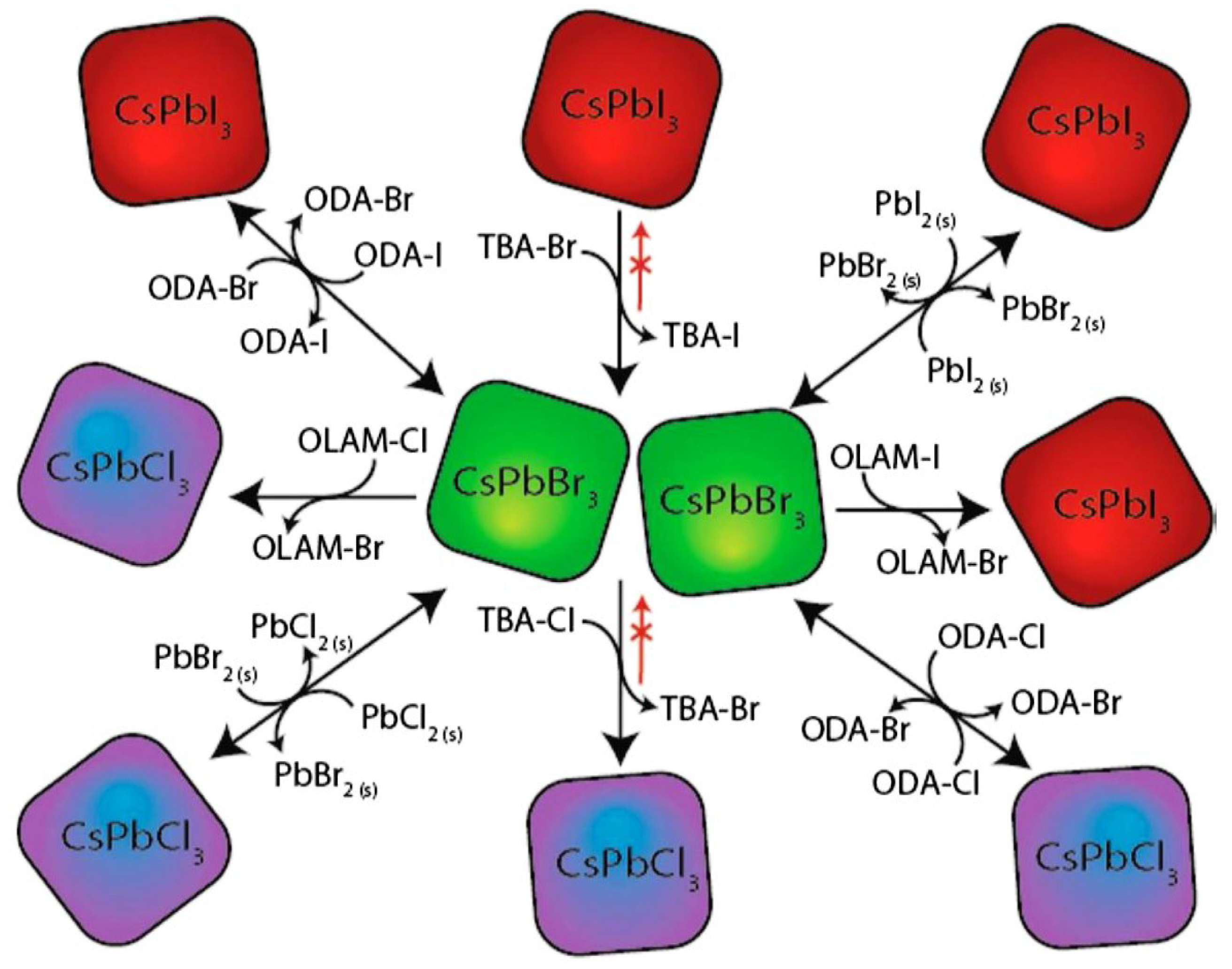
Figure 6 shows the various types of ion exchange, substitutions and ion mixing attempted for engineering new perovskite families. The figure provides a simple basis for a discussion and is an indicative of many perovskite structures for many optoelectronic applications. If the proposed cation and anion exchange and substitution become successful, there will be a chance of improving the stability and overcoming the toxicity: the two most challenging issues for future commercialization of halide perovskites. These exchanges and substitutions and mixing of ions take place at the A site, B site and X site and the ion mixing can take place in all sites.
Figure 7 shows the total viewpoint of the strategy used for a halide substitute method of cesium lead halide quantum specks. Halide substitute is useful for getting the cubic CsPbI
3 which was normally gained at higher temperatures. Hoffman et al. [
87] used a CsPbBr
3 quantum dots film and transformed it to a cubic CsPbI
3 arrange by diving the CsPbBr
3 quantum dots layer (~75-nm thickness) into the iodide forerunner. In this way, the technique for halide trade process in cesium lead halide quantum specks is greatly direct and this strategy can swear off using the surface functionalization. [
88]
4.3. Coordination Chemistry of Single Crystal Complexes Formation
Grasping crystal development system is extraordinarily significance for headway and manufactured stratagies for additional functions. Typically, valuable precious crystal development in solution can be segregated via three central forms: in situ change and dissolution−crystallization. [
90] Furthermore, It has been established that the vitality of such valuable precious processes of crystal advancement decidedly relied upon the CH
3NH
3I concentration. [
91] Then, lead iodide completely organized with iodine particles in order to frame in an iodine prosperous condition ( Equation 3): [
92]
At that point lead complex additionally went about as building units to recrystallize into a thermodynamically supported morphology within the sight of ammonium cations (Equation 4).
4.4. Coordination Chemistry Limits Crystallization of Halide Perovskites
Photovoltaic perovskites field is slowly shifting from highly disordered [
93] perovskite solar cells towards generation of single crystal [
94] devices which can ultimately provide lower energy losses. Competition between iodides with solvent molecules to coordinate lead atoms will determine the species present during perovskite nucleation and growth. Low complexity DFT calculations help to understand solvent coordination ability and can be used as a tool to screen suitable solvents. Highly coordinating solvents such as DMSO will form partially covalent bonds with lead retarding crystallization kinetics and its subsequent removal may be difficult. Poorly coordinating solvents like GBL will not be able to stabilize PbI
3- moieties enabling a fast reaction with the methyl ammonium cation. Importantly, water present in environmental humidity can be regarded as an additive which retards perovskite crystallization.
5. Electronic Interaction during Coordination Chemistry
In this section we have discussed about bonding and complex bonding ideas, hydrogen bonding, electronegativity, electron localization, cation-anion orbital interaction and spin orbit coupling on the electronic structure as shown in
Scheme 6. All these essential concepts are the results of electronic interactions and provide important electronic characterstics for many electronic functionalities as discussed in next section 6.
5.1. Bonding Idea in Lead Halide Perovskites
The lead halide perovskites are direct bandgap materials. Despite the fact that this direct bandgap nature is a substantial speculation, there are ongoing computations recommending special cases that happen in noncentrosymmetric halide perovskite on account of splitting. [
95,
96,
97,
98,
99,
100] Furthermore, electronic structure close to the band edge is primarily expressed as the fundamental BX
6 units. [
101,
102,
103,
104,
105,
106] Consequently, orbital outlines of segregated [BX
6]
4− bunches, similar to those in the zero dimension (CH
3NH
3)
4 [PbI
6]•2H
2O [
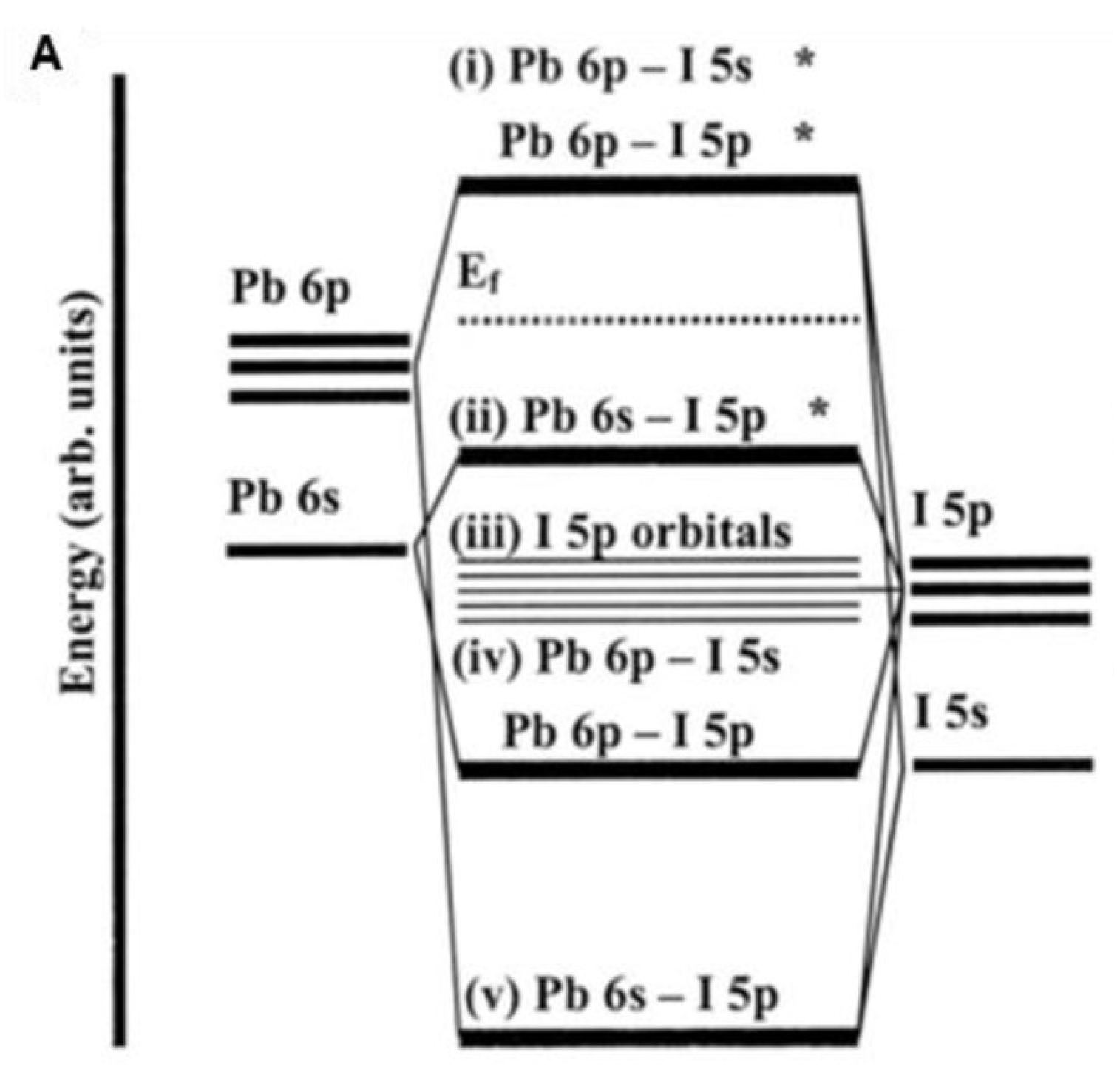
107] give a base for thoughtful more multifaceted band structures. For [PbI
6]
4− units specifically, a Pb 6s−I 5p σ-antibonding orbital contains the most noteworthy involved sub-atomic orbital (HOMO), while Pb 6p−I 5p π-antibonding and Pb 6p−I 5s σ-antibonding orbitals include the least abandoned sub-atomic orbital (LUMO) (
Figure 8A). [
103]
The chemical bonds of the ions directly impact the perovskite structure; however, it can be challenging to directly link experimental results to theoretical calculations. Consequently, Brown [
110] developed
bond valence theory as a way of linking empirical bond lengths to theoretical bond valences. Bond valence theory also apparently predicts both bond length and stability (Equation 5). The number resulting from Equation 4 is the total bond valence sum of that ion:
where
BV is the valence of the A-X or B-X bond,
R0 and
b are empirically determined parameters, and
R(A-X or B-X) is the experimentally determined A-X or B-X bond length.
In 2001, Lufaso and Woodward [
111]used the bond-valence method to back-calculate ionic radii to calculate a so-called bond-valence tolerance factor. The bond valence tolerance factor (t
BV) may be a powerful method for predicting perovskite stability, but foreknowledge of the bond valence parameters is needed. It also fails to account for stoichiometric structural vacancies. In 2009, Ubic [
112] derived a tolerance factor model as a function of the cubic/pseudocubic lattice constant,
rB and
rX (Equation 8), which accounts for A-site point defects.
where
R0(A-X) and
R0(B-X) are the unit valence bond lengths of the A-X and B-X bonds,
VA and
VB are the ideal valence states of the A and B cations,
NA and
NB are their coordinations, and B = 0.37. The ratios V
A/N
A and V
B/N
B are bond strengths for A-X and B-X, respectively. [
113]
Where, and is cubic lattice constant
The
apc (average relative error = 0.60%) is given by equation 8:
Where,
effective ionic radii of A, B nd are X ions in six fold coordination. For the pseudocubic lattice, t
1 can be calculated as in equation 9
Therefore, these bonding parameters are helpful to understand how the bonding interaction, electronic interaction and coordination chemistry takes place which gives information on the distortion and tolerance factor of the structure formed. It also provides information on what bonding parameters are important during electronic interactions. It is primarily important in validating chemical structures.
5.2. Complex Bonding Idea in Lead Halide Perovskites
The leading complex bonding between the A site cation and BX
3- complex anion is electrostatic. Furthermore, there is a strong electrostatic potential (~8 V) holding the cation at its lattice site. For instance, the positively charged ion, CH
3NH
3+, is within a negatively charged cage, PbI
3-. Moreover, further electrostatic role to the chemical bonding between the molecular dipole and the PbI
6 octahedra is the charge−dipole interaction, which depends on the dipole orientation. Similarly, there is also the consequence of prime polarization. An induced dipole interaction is expected owing to the substantial polarizability of the iodide ions (ca. 7 × 10
−24 cm
3). Because of these interactions, a molecular orientation correlation with octahedral deformation in molecular dynamic simulations is strongly expected [
114] and more comprehensive investigations are continuing. Additionally, the van der Waals interactive forces together explains the intermolecular and Debye force interactions.
5.3. Electronegativity and Electronic Bandgap Tuning
From the perspective of band structure, bandgap tuning and structure factor, it is highly recommended to consider all electronic interactions such as electronegativity of each atoms making the semiconductor materials. For instance, the band gap for CH
3NH
3SnI
3 ranges from 1.2−1.4 eV, while the 1.5−1.6 eV band gap values for CH
3NH
3PbI
3. [
115,
116] Thus, Pb (1.87) has a smaller Pauling electronegativity contrasted to Sn (1.96), [
116] indicating the band structure of Pb states have to be higher and thus larger bandgap values. Hence, Sn is less metallic in contrast to Pb and therefore, Sn−I interactions have to be less ionic in contrast to Pb−I interactions, suggesting smaller band gap belongs to CH
3NH
3SnI
3. As confirmed from the conduction band edges of CH
3NH
3SnI
3 and CH
3NH
3PbI
3 at −4.17 eV and −3.90eV, correspondingly, i.e. Pb states are positioned higher in the band structure of CH
3NH
3PbI
3. [
117] Hence, considering the influence of electronegativity on the band structure is vital.
5.4. Cation-Anion Orbital Interaction
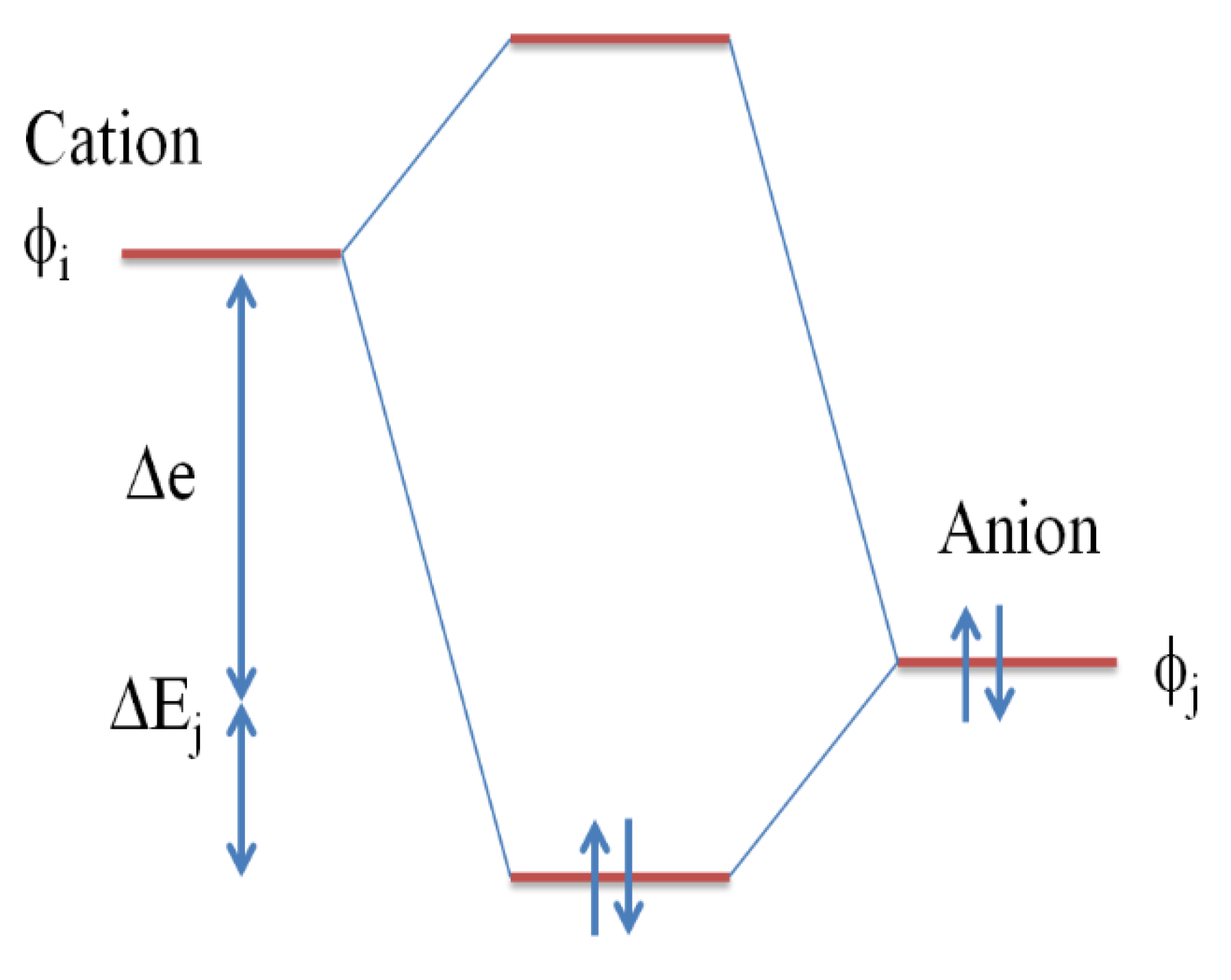
A cooperation between any two orbitals φ
i and φ
j, be it degenerate or nondegenerate, prompts two new vitality levels that look as though the collaboration repulses the associating levels from one another. As needs be, the orbital connection of an anion with a cation is naturally balancing out, on the grounds that it includes the cooperation of an unfilled level φ
i of a cation with a filled level φ
j (often, a solitary combine orbital) of an anion (
Figure 9).
This vitality adjustment is corresponding to the square of the cover indispensable S
ij = <φ
i|φ
j> and is contrarily relative to the vitality distinction Δe
ij = |e
i – e
j| between the two orbitals, S
ij2/Δ
eij. [
118] When a cation is encased in an enclosure of anions (e.g., the A cation of ABO
3 in a B
8 block and proportionately in a confine of 12O
2– anions), the aggregate adjustment vitality ΔEtot related with the cation-anion cooperations is gotten by summing up every individual commitment as in Equation 10,
The extent of the cover vital Sij diminishes exponentially as the interatomic remove rij increments. This prompts some stretched and contracted cation-anion bonds inside the enclosure.
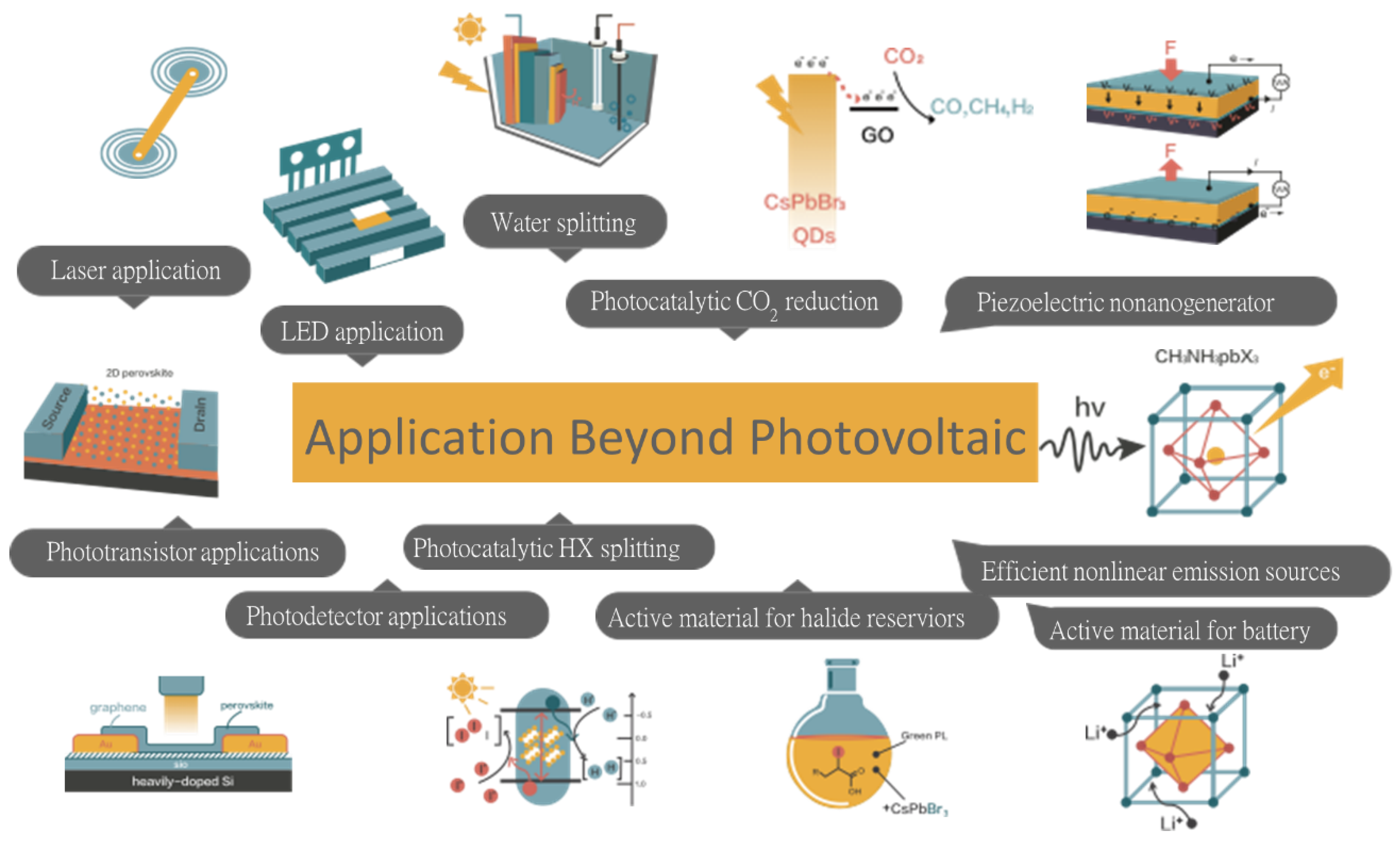
6. Energy Applications of Halide Perovskites Beyond Photovoltaic
In recent times, the energy function of halide perovskite beyond photovoltaic has been lengthened with excellent results to light-emitting devices, [
119] opportunity for innovative and cutting edges for perovskite based lasers, [
120] light-emitting diodes (LEDs), [
121] and field-effect light-emitting transistors (FETs), [
122] photodetectors, nonlinear emission sources, efficient water, CO
2 and HX splitting, photocatalytic activities, active material in lithium and sodium ion batteries, halide reservoir in catalysis system and piezoelectric generators (
Scheme 7), which are the main focus of this section.
6.1. MAPbI3 as a Photocatalytic Material for HI Splitting
The simultaneous oxidation response engaged with HX part delivers esteem included synthetic substances, for example, I
2/I
3-, Br
2/Br
3-, or Cl
2, which have an assortment of employments in the vitality and cleanliness industries. [
123,
124,
125,
126,
127,
128] Indeed, many spearheading works show fruitful HX splitting. [
126,
127,
128]
, [
129] By using a Nafion-isolated silicon microwire cathode, HI part has been accomplished with a 0.6% proficiency and unadulterated products. [
130] Furthermore, Park et.al. [
131] utilized MAPbI
3 as a photocatalytic material in unique balance with fluid HI arrangement. The vibrant harmony between the MAPbI
3 hastens and the soaked fluid arrangement is affirmed by means of replacement of I with Br. MAPbI
3 experiences a stage change to hydrated stages or PbI
2 at various particle exercises in the watery arrangement, and is steady just in particular fixation scopes of I
- and H
+. It has been discovered that the MAPbI
3 powder in the watery HI arrangement could adequately part HI into H
2 and I
3- under obvious light illumination, the proficiency of which could be expanded by means of warm toughening in a polar dissolvable environment and by utilizing a Pt cocatalyst. [
131]
6.2. Perovskite QD-GO Nanocomposite for Photocatalytic Reduction of CO2
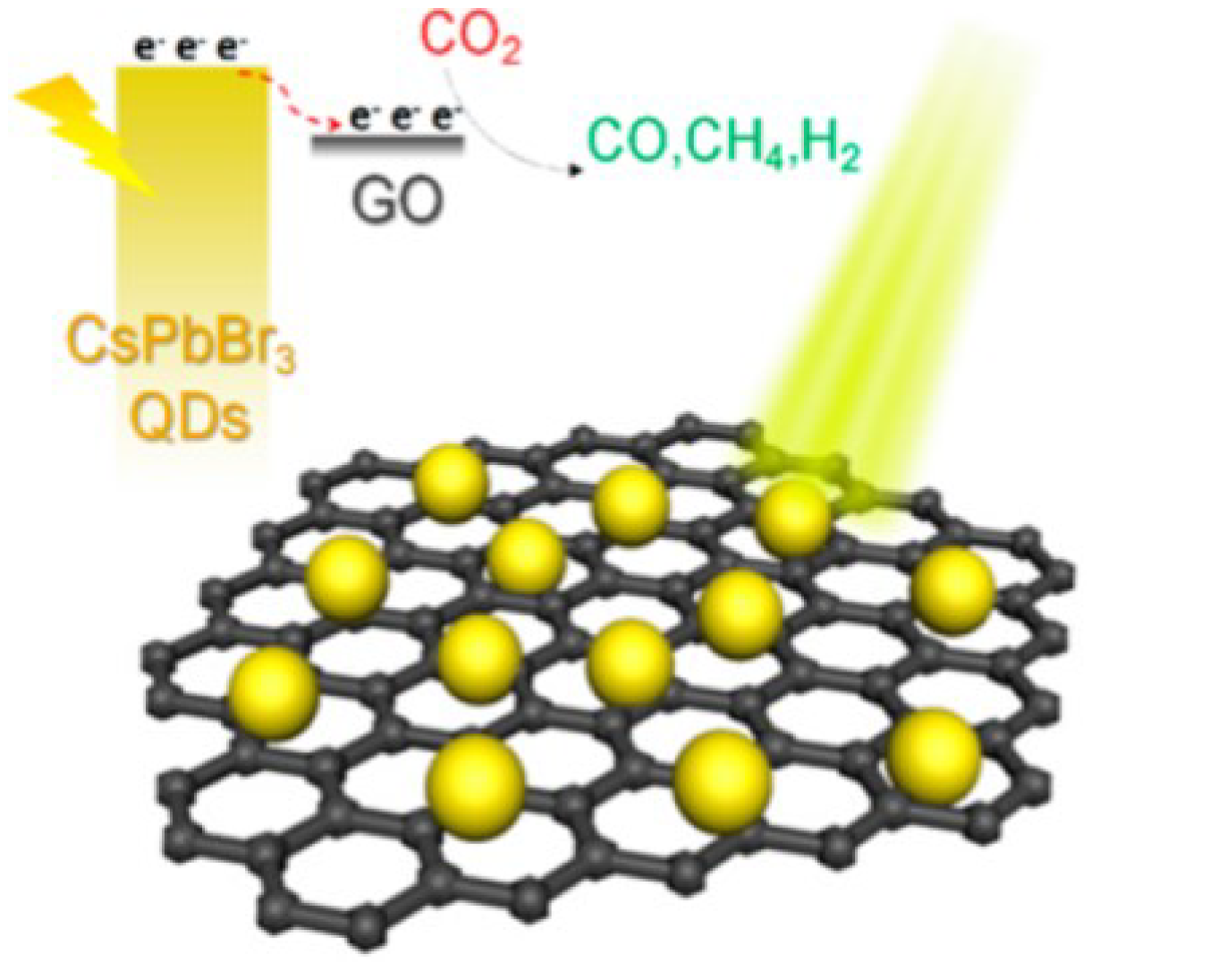
Reduction of CO
2 can be achieved either electrochemical or photocatalytic reduction process. The look for a superior contender has not yet rested. Current and fast growths in halide perovskite materials have activated huge attention amongst investigators for optoelectronic functions, particularly, solar cells. [
132,
133,
134] Furthermore, encouraged from the accomplishments of photovoltaics, these semiconductors are main contenders for performing proficient photosynthesis if the tremendous feature instability concerns of halide perovskites can be decided primarly. [
135,
136] Because of its improved stability, a CsPbBr
3 Quantum Dot/Graphene Oxide Composite was drawing for the photocatalytic reduction of CO
2 to ethyl acetate (
Figure 10). [
137]
6.3. Halide Perovskite as Active Material for Battery
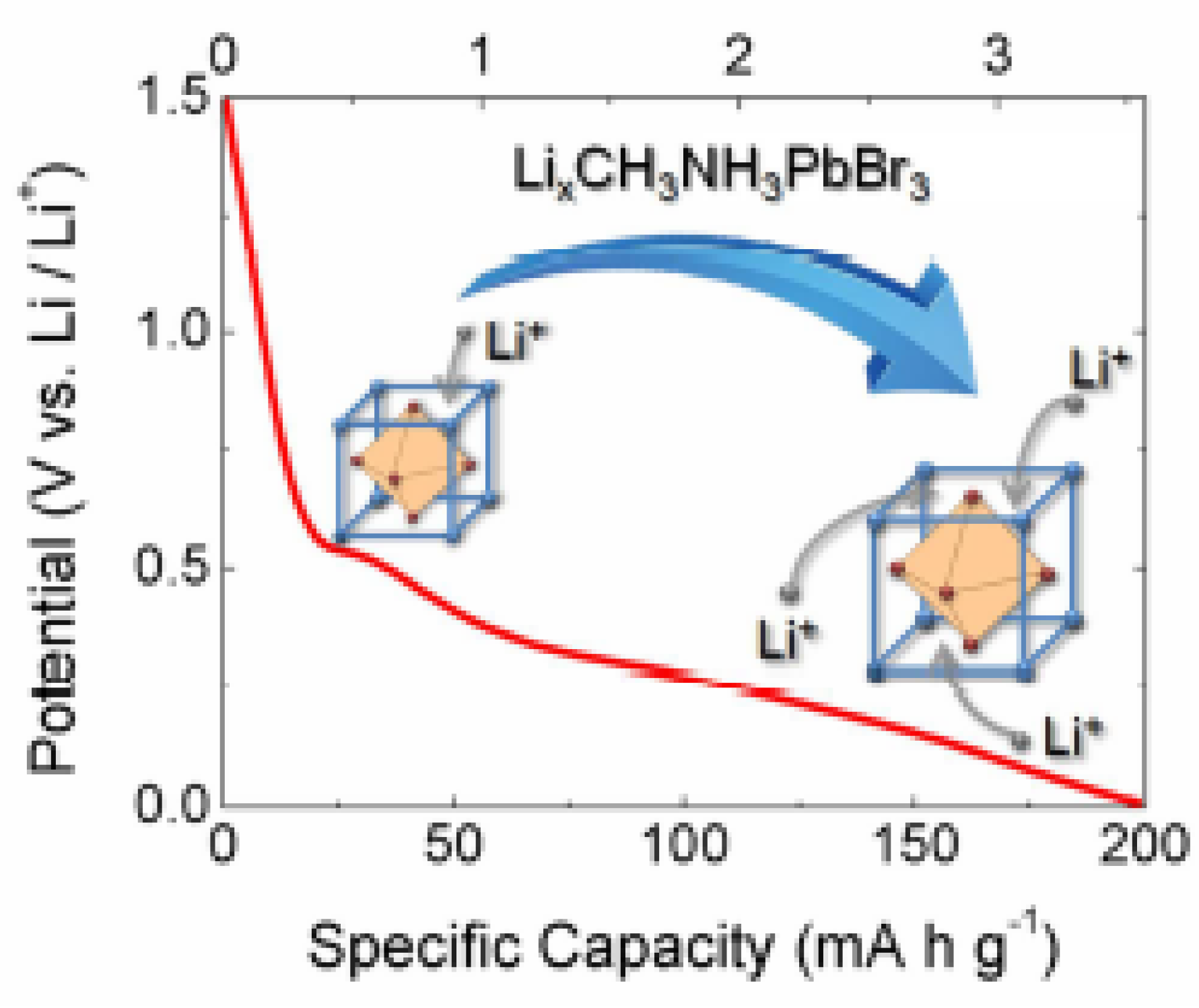
In parallel to photovoltaic uses, halide perovskites have been proposed as their use as active material for lithium-ion battery (LIB) anodes. Hybrid organic-inorganic halide perovskites such as methylammonium lead bromide (MAPbBr
3) exhibits reliable values of ≈200 mA h g
-1 with an outstanding rate potential as shown in
Figure 11. [
138] These preliminary results are comparable to current commercial anodes capacities. Furthermore, Xu
et.al. was also demonstrated the utilization of perovskite powered charging batteries of lithium amassed with a LiFePO
4 cathode and a Li
4Ti
5O
12 anode. [
139] This gadget demonstrated a high generally speaking photograph electric change and capacity effectiveness of 7.80% and superb cycling dependability, which outflanks other revealed lithium-particle batteries. The newly introduced self-chargeable power units based on integrated halide perovskite solar cells and lithium-ion batteries hold promise for various possible functions. Note that CH
3NH
3PbBr
3 accumulates then two principal preferences: (i) it considers high inclusion fixations with x>>1, and at the same time (ii) it displays little auxiliary mutilations. Critically, the rate ability does not show huge decrease for charging flows between 1
0C and 0.25
0C, demonstrating great probability for functionalites in the field of energy storage devices such as battery.
6.4. Halide Reservoir in Catalysis Applications
From the perspectives of catalysis, it is important to find a material that reserve halides. In supporting this idea, in recent times, perovskite nanoparticles (P-NPs) were prepared at the nanoscale through extraordinary size- and halide-tuned optical properties. [
140,
141] Of curiosity to the synthetic chemist is the apparently effortlessness where P-NPs experience composition alteration by swap using halides, as revealed at bulk [
142] and at nanointerfaces. [
143,
144,
145] Besides, Doane
et.al. at that point found the capability of the P-NPs to continue as halide repositories for Finkelstein operation responses in halide perovskite, which give an uncommon colorimetric report of response kinetics. [
146] Furthermore, it was hypothesized [
146] that P-NPs might have the capacity to (1) fill in as a wellspring of high centralizations of halides synergist reservoir, [
147] (2) screen free halide spotlight alteration of amid halide disposal reactions, [
148] and (3) fill in as a quick subjective/quantitative colorimetric examine of free particles in arrangement.
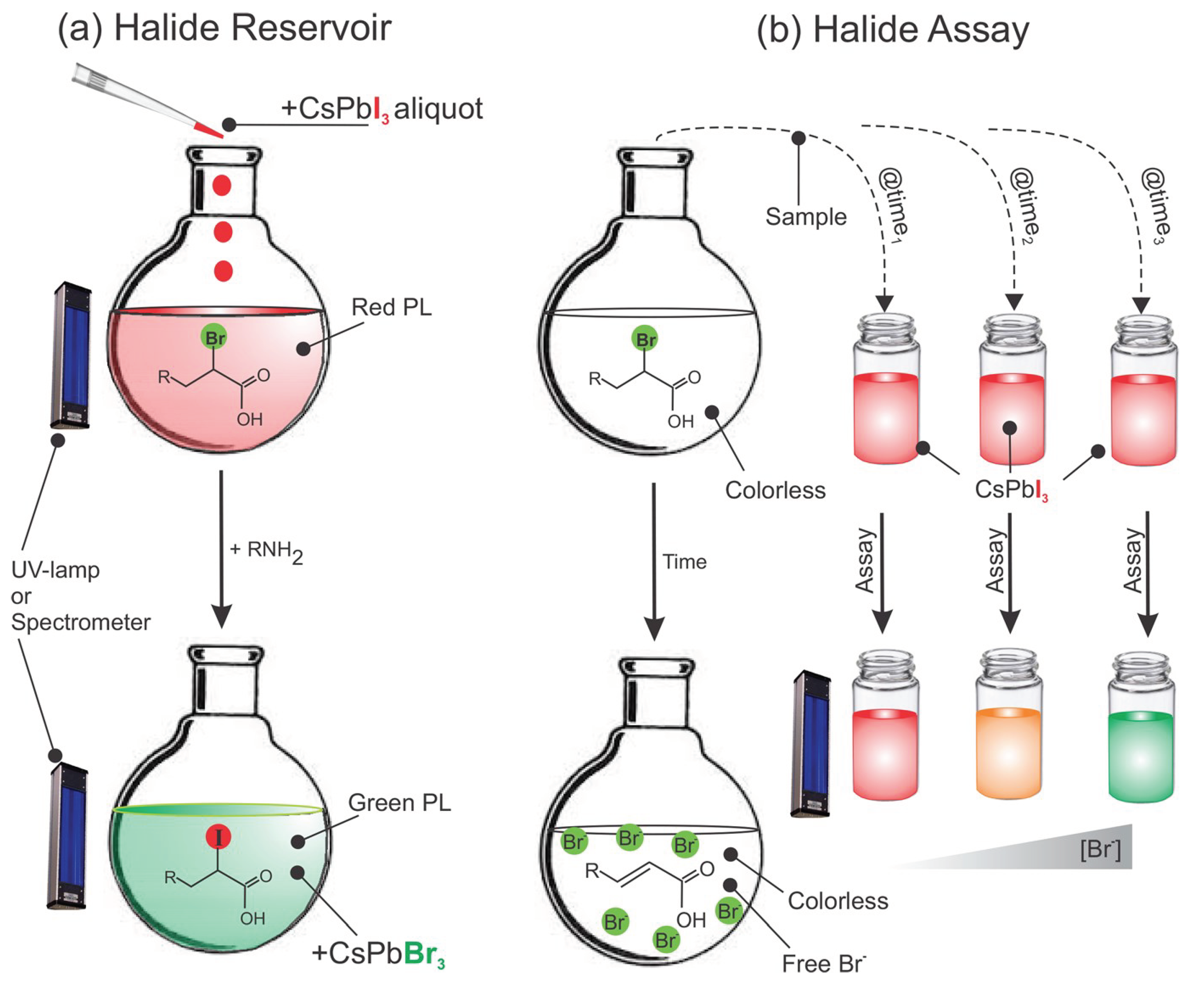
Figure 12 delineates these thoughts. As a halide supply (
Figure 12a), the P-NP and its dynamic halide−ligand complex [
149] supply halides in nonpolar environment that can respond and operated with halide perovskites, whereas in the meantime demonstrating colorimetric input. As a measure (
Figure 12b), tested responses or questions ions are acquainted by means of P-NPs aliquots of recognized fixation whose shading change can give quantitative colorimetric reaction and consolidating, path a and b can give an immediate methodology toward colorimetric observing of compound responses continuously.
6.5. Piezoelectric Generators
It is very intriguing that a half and half piezoelectric nanogenerator in view of combinations of piezoelectric HC(NH
2)
2PbBr
3 nanoparticles and polydimethylsiloxane polymer has been manufactured. [
150] Furthermore, the HC(NH
2)
2PbBr
3 nanoparticles contain all around created ferroelectric properties with high piezoelectric charge coefficient (d
33) of 25 pmV
−1. [
150] The adaptable gadget showed elite with a greatest recordable piezoelectric yield voltage of 8.5 V and current thickness of 3.8 μAcm
−2 under occasionally vertical pressure and discharge activities. The exchanging vitality produced from nanogenerators can be utilized to charge a capacitor and light up a red light-transmitting diode through an extension rectifier. This outcome inventively grows the attainability of halide perovskites for function in a broad assortment of superior vitality collecting gadgets.
6.6. What Could Happen in the Future of Halide Perovskites?
The future of halide perovskites should answer three challenging issues i.e 1) will perovskite solar cells achieve new breakthroughs beyond their current status of performance? 2) Will the future mass production and commercialization of these types of materials achieve the three ultimate goals of materials for optoelectronic applications: energy competent, low cost, and environmentally friendly. 3) Will the currently proposed new framework of single and double perovskite materials achieve enough efficiency like the efficiency of CH3NH3PbI3 perovskites with improved stability and toxicity free new research horizon? So that the commercialization and production processes become more economical, sustainable, and environmentally friendly and its application may be realizable too. In order to solve all these concerns, the coordination chemistry of the various organic and inorganic halid materials forming both single and double perovskites with their related electronic properties should be carefully studied. Moreover, the strategic frameworks stated in this review article (section 3) should get especial attention.
Another wondering thing in these halide perovskite materials is their wide range application! It is highly applicable in photovoltaic devices to meet the aim of energy demand, electronic devices such as lasers, photodetectors, phototransistors, LED and nonlinear emission sources to meet the goal of optoelectronic engineering, such as efficient water, CO2 and HX splitting to meet photocatalytic goals, energy storage devices such as battery, and in efficient catalysis in order to achieve the purpose of halide reservoirs.
After all, which field of study is not enjoying with the application of these highly essential materials? Since halide anions are excellent redox mediators, halide perovskites may also be important in fields of membrane and reaction engineering due to their ability as halide reservoir. Moreover, they can be useful to reduce global warming by reducing and splitting CO2. On the other hand, if we rise applications in biological system and life science in addition to the physical and chemical sciences: Growth of microorganism which need materials that can absorb light at infrared and near infrared regions, halide perovskites fulfill this criterion. But the lead atom is toxic and may affect the growth of microorganism. Other environmentally friendly metal atoms, which could be important to achieve this goal should replace this toxic metal atom. On another account, being toxic should also be important for some reasons. For instance, it would also be wondering that if halide perovskite materials are applicable for agricultural aspects such as pesticides for killing some insects and organisms since lead atom is toxic, indicating that halide perovskites are not only used as halide reservoirs but also toxic metal such as lead metal reservoirs.
7. Concluding Remarks
The halide perovskite field is fastly growing research area with improved device efficiency and the photophysics properties for wide range applications, but less stable. A consistently imperative research themes which have not been getting more attention are fundamental understanding of the coordination chemistry and coordination engineering as well as electronic interactions forming the halide perovskite structures in addition to their photophysics properties. Generally, grasping these fundamental concepts is quite relevant in five main concerns of this field: 1) stability improvements, 2) toxicity reduction, 3) discovery of new materials with multifunctionalities, 4) remarkable semiconducting properties and performance improvements and finally, 5) realizing the existing and new potential applications of halide perovskite materials. All these enhancements take place as a result of the modifications at either A, B or X sites and modifications at all A, B and X sites sites.
- 1)
Stability improvement as a way for flexible practical applications: currently, this is the first challenge that blocks practical applications of halide perovskites materials. This limitation is not only for device but also the material itself is easily prone to degrade. As a key parameter for any optoelectronic applications, materials environmental stability and durability determine the lifespan of the device. Hence, in depth sympathetic of the chemistry and engineering of halide perovskites is helpful to enhance stability in two ways: a) enhancing the hydrophobic character of halide perovskites to overcome the solubility and dissolution of these materials. This can be done by increasing the carbon chain in the organic tail to reduce its hydrophilic character or to increase inorganic character of the halide perovskites by completely replacing CH3NH3+ by water resistant in metal atoms such as Cs, Rb, etc. Using stoichiometric composition engineering of the organic tail with smaller amount of the organic tail could also enhance the hydrophobicity of these materials. b) coordination engineering framework of halide perovskite structure that can overcome the stability issues in these materials.
- 2)
Toxicity reduction for mass production of halide perovskites: Toxicity is the second most challenging issue that hinders the commercialization and mass production of halide perovskites. Understanding the chemistry and engineering of halide perovskites is highly to partially or completely avoid the toxicity in these materials. This can be done by a) complete removal of lead atom and replacing it with environmentally friendly metal atoms such as Ti, Sb, Bi, etc. b) completely replacing lead atom by at least lass toxic metal; atoms such as Sn and Ge, which could not affect the environment significantly. c) if both mechanism may not be successful, mixing metal ions can be the least alternative to optimize the degree of toxicity in lead based halide perovskites. d) if all these modifications may not be successful, engineering other perovskite materials with new framework and new stoichoimetric composition as well as structure could be the least alternative to avoid toxicity.
- 3)
Enhanced semiconducting properties such as optical and electrical properties as well as efficiency enhancement. This basic intention of coordination chemistry and coordination engineering of halide perovskite materials is to improve optical and electrical properties and to design new material with better semiconducting properties for better performance.
- 4)
Another exciting behavior of halide perovskites is their wide range potential applications resulted due to enhanced semiconducting properties that may be benefited from and require the fundamental concepts of chemistry and engineering in addition to their photophysics properties: It is wondering that halide perovskites are highly applicable beyond photovoltaic applications, for instance, a) many optoelectronic devices such as laser, LED, photodetectors, transistors and nonlinear emission sources, b) photocatalytic activities such as efficient water, CO2 and HX splitting, c) storage devices such as active materials for LIB and Na ion battery as well as halide reservoirs for catalysis purpose. Moreover, discovery of new perovskite materials with multifunctionalities and improved semiconducting properties: this point of view may be important to fabricate new device that fulfill the ‘Triple E’ rule: efficient, economical and environmental friendly.
Finally, in order to provide direction in to the future continuity of this field, we have drawn a great concern: ‘what will happen in the future of halide perovskites?’ Would the new approaches, coordination chemistry and coordination engineering of halide peroivskites, bring new research horizon for the future or not? This concern indicates the future fighting among the challenges and promising opportunities of halide perovskite materials i.e will this field stop or realized in the commercial enterprises and industries so that the future energy applications become powered by these materials. Moreover, this concern may also be important to draw new predictions on this field.
Acknowledgements
This work was financially supported by the Ministry of Science and Technology (MoST) (106-2923-E 011-005, 105-3113-E-011-001, 105-ET-E-011-004-ET, 104-2923-M-011-002-MY3, 104-2911-1-011-505-MY2, 103-2221-E-011-156-MY3), the Top University Projects (100H45140), the Global Networking Talent 3.0 Plan (NTUST 104DI005) from the Ministry of Education of Taiwan, Taiwan’s Deep Decarbonization Pathways toward a Sustainable Society Project (AS-KPQ-106- DDPP) from Academia Sinica as well as the facilities of support from National Taiwan University of Science and Technology (NTUST) and National Synchrotron Radiation Research Centre (NSRRC) are also acknowledged.
References
- Cahen, D.; D. G.; Kahn, A.; Berry, J.; Buonassisi, T.; Egger, D.; Hodes, G.; Kronik, L.; Loo, L.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; Mitzi, D.B.; Paz, Y.; Rappe, A.; Riess, I.; Rybtchinski, B.; Stafsudd, O.; Stevanovic, S.; Toney, M.; Zitoun, D. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar]
- Cai, M.; Y. W.; Chen, H.; Yang, X.; Qiang, Y.; Han, L. Adv. Sci. 2017, 4, 1600269. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. Journal of American Chemical Society 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J. A.; Morales Vilches, A. B.; Kasparavicius, E.; Smith, J. A.; Phung, N.; Menzel, D.; Grischek, M.; Kegelmann, L.; Skroblin, D.; Gollwitzer, C.; Malinauskas, T.; Jošt, M.; Matič, G.; Rech, B.; Schlatmann, R.; Topič, M.; Korte, L.; Abate, A.; Stannowski, B.; Neher, D.; Stolterfoht, M.; Unold, T.; Getautis, V.; Albrecht, S. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef]
- Collavini, S.; Völker, S. F.; Delgado, J. L. Understanding the Outstanding Power Conversion Efficiency of Perovskite-Based Solar Cells. Angewandte Chemie International Edition 2015, 54, 9757–9759. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-b.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Bailie, C. D.; Christoforo, M. G.; Mailoa, J. P.; Bowring, A. R.; Unger, E. L.; Nguyen, W. H.; Burschka, J.; Pellet, N.; Lee, J. Z.; Gratzel, M.; Noufi, R.; Buonassisi, T.; Salleo, A.; McGehee, M. D. Semi-transparent perovskite solar cells for tandems with silicon and CIGS. Energy & Environmental Science 2015, 8, 956–963. [Google Scholar]
- Kim, Y.-J.; T. -V. D.; Choi, H.-J.; Park, B.-J.; Eom, J.-H.; Song, H.-A.; Seol, D.; Kim, Y.; Shin, S.-H.; Nah, J.; Yoon, S.-G. J. Mater. Chem. A 2016, 4, 756. [Google Scholar] [CrossRef]
- Berhe, T. A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A. A.; Hwang, B.-J. Organometal halide perovskite solar cells: degradation and stability. Energy & Environmental Science 2016, 9, 323–356. [Google Scholar]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef]
- Ashurov, N.; Oksengendler, B. L.; Maksimov, S.; Rashiodva, S.; Ishteev, A. R.; Saranin, D. S.; Burmistrov, I. N.; Kuznetsov, D. V.; Zakhisov, A. A. Current state and perspectives for organo-halide perovskite solar cells. Part 1. Crystal structures and thin film formation, morphology, processing, degradation, stability improvement by carbon nanotubes. A review. Modern Electronic Materials 2017, 3, 1–25. [Google Scholar] [CrossRef]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; Chen, X. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Hee Joon Jung, D. K. , Sungkyu Kim, Joonsuk Park, Vinayak P. Dravid and Byungha Shin. Stability of Halide Perovskite Solar Cell Devices: In Situ Observation of Oxygen Diffusion under Biasing. Adv. Mater. 8027. [Google Scholar]
- Guo, X.; Burda, C. Coordination engineering toward high performance organic–inorganic hybrid perovskites. Coord. Chem. Rev.
- Gao, P.; Bin Mohd Yusoff, A. R.; Nazeeruddin, M. K. Dimensionality engineering of hybrid halide perovskite light absorbers. Nature Communications 2018, 9, 5028. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Kopyl, S.; Kholkin, A.; Rocha, J. Hybrid organic-inorganic perovskites: Polar properties and applications. Coord. Chem. Rev. 2019, 387, 398–414. [Google Scholar] [CrossRef]
- Mohd Yusoff, A. R. B.; Gao, P.; Nazeeruddin, M. K. Recent progress in organohalide lead perovskites for photovoltaic and optoelectronic applications. Coord. Chem. Rev. 2018, 373, 258–294. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Z.; Xu, J.; Cao, G. Synergistic combination of semiconductor quantum dots and organic-inorganic halide perovskites for hybrid solar cells. Coord. Chem. Rev. 2018, 374, 279–313. [Google Scholar] [CrossRef]
- Robert, F. Service, Perovskite solar cells gear up to go commercial. science 2016, 354. [Google Scholar]
- Koh, T.M.; K. F.; Fang, Y.; Chen, S.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G.; Boix, P.P.; Baikie, T. J. Phys. Chem. C 2014, 118, 16458. [Google Scholar] [CrossRef]
- Eperon, G.E.; S. D. S.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Energy Environ. Sci. 2014, 7, 982. [Google Scholar] [CrossRef]
- Pang, S.; H. H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. Chem. Mater. 2014, 26, 1485. [Google Scholar] [CrossRef]
- Pellet, N.; P. G.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Angew. Chem., Int. Ed. 2014, 53, 3151. [Google Scholar] [CrossRef]
- Lee, J.-W.; D. -J. S.; Cho, A.-N.; Park, N.-G. Adv. Mater. 2014, 26, 4991. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; L. L.; Mei, A.; Yang, Y.; Liu, T.; Han, H. J. Mater. Chem. A 2014, 2, 17115. [Google Scholar] [CrossRef]
- Binek, A.; F. C. H.; Docampo, P.; Bein, T. J. Phys. Chem. Lett. 2015, 6, 1249. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C. C.; Malliakas, C. D.; Kanatzidis, M. G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorganic chemistry 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Pellet, N.; P. G.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Gratzel, M. Angew. Chem., Int. Ed. Engl. 2014, 53, 3151–3157. [Google Scholar] [CrossRef]
- Jin-Wook Lee, D.-H. K. , Hui-Seon Kim, Seung-Woo Seo, Sung Min Cho, and Nam-Gyu Park *, Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar]
- Ahn, N.; D. -Y. S.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. J. Am. Chem. Soc. 2015, 137, 8696. [Google Scholar] [CrossRef]
- Lee, J.-W.; D. -H. K.; Kim, H.-S.; Seo, S.-W.; Cho, S.M.; Park, N.-G. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Li, Z.; M. Y.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Chem. Mater. 2016, 28, 284. [Google Scholar] [CrossRef]
- M. W. Lufaso, P. W. B. a. P. M. W., Acta Crystallogr., Sect. B: Struct. Sci. 2006, 62, 397–410.
- C. J. Howard, B. J. K. a. P. M. W., Acta Crystallogr., Sect. B: Struct. Sci. 2003, 59, 463–471.
- M. T. Anderson, K. B. G., G. A.. Taylor and K. R. Poeppelmeier. Prog. Solid State Chem. 1993, 22, 197–233. [Google Scholar]
- P. W. Barnes, M. W. L. a. P. M. W., Acta Crystallogr., Sect. B: Struct. Sci. 2006, 62, 384–396.
- P. Woodward, R. D. H. a. A. W. S. J. Mater. Res. 1994, 9, 2118–2127. [Google Scholar]
- P. K. Davies, H. W., A. Y. Borisevich, I. E. Molodetsky and L. Farber. Annu. Rev. Mater. Res., 2008, 38, 369–401. [Google Scholar]
- Woodward*, G. K. a. P. M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar]
- Muller, O. , andRoy,R., The Major Ternary Structural Families. NewYork: Springer.
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Barea, E. M.; Palomares, E. , A review of recent results on electrochemical determination of the density of electronic states of nanostructured metal-oxide semiconductors and organic hole conductors. Inorganica Chimica Acta 2008, 361, 684–698. [Google Scholar] [CrossRef]
- Muller, O.; Roy, R. , The Major Ternary Structural Families. NewYork: Springer.
- Ghanshyam Pilania1*, P. V. B. , Chiho Kim3 and Turab Lookman2, Finding New Perovskite Halides via Machine Learning. Frontiers in Materials 2016. [Google Scholar]
- Sergey, A. Adonin, L. A. F., Maxim N. Sokolov, Gennady V. Shilov, Denis V. Korchagin, Vladimir P. Fedin, Sergey M. Aldoshin, Keith J. Stevenson, and Pavel A. Troshin*, Antimony (V) Complex Halides: Lead-Free Perovskite-Like Materials for Hybrid Solar Cells Adv. Energy Mater. 2017, 1701140.
- S. L. Lawton, R. A. J. J. Am. Chem. Soc. 1966, 88, 616. [Google Scholar]
- S. L. Lawton, R. A. J. Inorg. Chem. 1971, 10, 709. [Google Scholar]
- S. L. Lawton, R. A. J. Inorg. Chem. 1968, 7, 2124. [Google Scholar]
- S. L. Lawton, R. A. J. Inorg. Chem. 1966, 5, 743. [Google Scholar]
- S. Lawton, R. J. Inorg. Chem. 1971, 10, 2813. [Google Scholar]
- Cheng, Z. L., J. , Layered Organic-Inorganic Hybrid Perovskites: Structure, Optical Properties, Film Preparation, Patterning and Templating Engineering. CrystEngComm. 2010, 12, 2646–2662. [Google Scholar] [CrossRef]
- Dohner, E. R. H., E. T.; Karunadasa, H. I., Self-Assembly of Broadband White-Light Emitters. J. Am. Chem. Soc. 2014, 136, 1718–1721. [Google Scholar] [CrossRef]
- Dohner, E. R. J., A.; Bradshaw, L. R.; Karunadasa, H. I. ,, Intrinsic White-Light Emission from Layered Hybrid Perovskites. J. Am. Chem. Soc. 2014, 136, 13154–13157. [Google Scholar] [CrossRef]
- Lee, B. S., C. C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.-Y.; Marks, T. J.; Kanatzidis, M. G.; Chang, R. P. H., Air-Stable Molecular Semiconducting Iodosalts for Solar Cell Applications: Cs2SnI6 as a Hole Conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Smith, I. C. H., E. T.; Solis-Ibarra, D.; McGehee, M. D.; Karunadasa, H. I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. 2014, 126, 11414–11417. [Google Scholar] [CrossRef]
- R. E. Brandt, R. C. K., R. L. Z. Hoye, J. R. Poindexter, M. W. B. Wilson, S. Sulekar, F. Lenahan, P. X. T. Yen, V. Stevanovic´, J. C. Nino, M. G. Bawendi, T. Bounassisi,, J. Phys. Chem. Lett. 2015, 6, 4297.
- N. T. Hahn, A. J. E. R., S. K. Beal, R. R. Fullon, C. B. Mullins,, J. Phys. Chem. C 2012, 116, 24878.
- S. Sfaelou, D. R., V. Dracopoulos, P. Lianos,, RSC Adv. 2015, 5, 95813.
- E. T. McClure, M. R. B., W. Windl, P. M. Woodward,, Chem. Mater. 2016, 28, 1348.
- Y. Kim, Z. Y., A. Jain, O. Voznyy, G.-H. Kim, M. Liu, L. N. Quan, F. P. G. de Arquer, R. Comin, J. Z. Fan, E. H. Sargent,, Angew. Chem., Int. Ed. 2016, 55, 9586.
- B. Saparov, F. H., J.-P. Sun, H.-S. Duan, W. Meng, S. Cameron, I. G. Hill, Y. Yan, D. B. Mitzi,, Chem. Mater. 2015, 27, 5622.
- P. C. Harikesh, H. K. M., B. Ghosh, T. W. Goh, Y. T. Teng, K. Thirumal, M. Lockrey, K. Weber, T. M. Koh, S. Li, S. Mhaisalkar, N. Mathews,, Chem. Mater. 2016, 28, 7496.
- C. Hebig, I. K., J. Flohre, T. Kirchartz,, ACS Energy Lett. 2016, 1, 309.
- Huang X, H. S. , Biswas P, et al., Band gap insensitivity to large chemical pressures in ternary bismuth iodides for photovoltaic applications. J Phys Chem C. 2016, 120, 28924–28932. [Google Scholar] [CrossRef]
- Lyu MQ, Y. J. , Cai ML, et al., Organic-inorganic bismuth (III)-based material: A lead-free, air-stable and solution-processable light-absorber beyond organolead perovskites. Nano Research. 2016, 9(3, 692–702. [Google Scholar]
- X. -G. Zhao, J.-H. Y., Y. Fu, D. Yang, Q. Xu, L. Yu, S.-H. Wei, L. Zhang,, J. Am. Chem. Soc. 2017, 139, 2630–2638.
- M. R. Filip, S. H., A. A. Haghighirad, H. J. Snaith, F. Giustino,, J. Phys. Chem. Lett. 2016, 7, 2579–2585.
- A. H. Slavney, T. H., A. M. Lindenberg, H. I. Karunadasa,, J. Am. Chem. Soc. 2016, 138, 2138–2141.
- Z. Xiao, K. Z. D., T. Hu, W. Meng, J. Wang, D. B. Mitzi, Y. Yan,, J. Am. Chem. Soc. 2017, 139, 6054–6057.
- Z. Xiao, W. M., J. Wang, D. B. Mitzi, Y. Yan,, Mater. Horiz. 2017, 4, 206–216.
- Amirbekova Gulzhanat1, A. B. a. T. N. Z. , Ferroelectricity of CsRbPb2I6 superlattice. Physics department, al-Farabi Kazakh National University, Kazakhstan.
- Abdykadyrov, B. , ANM abstracts 2015.
- Uberuaga, G. P. a. B. P. , Cation ordering and effect of biaxial strain in double perovskite CsRbCaZnCl6. JOURNAL OF APPLIED PHYSICS, 2015, 117, 114103. [Google Scholar]
- Stamplecoskie, K. G. M., J. S.; Kamat, P. V., Dual Nature of the Excited State in Organic−Inorganic Lead Halide Perovskites. Energy Environ. Sci. 2015, 8, 208–215. [Google Scholar]
- Joseph, S. Manser, ‡ Makhsud I. Saidaminov,∥ Jeffrey A. Christians,†,‡ Osman M. Bakr,∥ and Prashant V. Kamat*,†,‡,§, Making and Breaking of Lead Halide Perovskites. Acc. Chem. Res. 2016, 49, 330–338. [Google Scholar]
- R. D. Hancock, i. P. i. C. C. (Ed.) R. D. Hancock, i. P. i. C. C., ed. A. F. Williams, C. Floriani and A. E. Merbach,, VCHA:VCH, Basel, 1992, 129.
- R. D. Hancock, M. S. S., S. M. Dobson and J. C. A. Boeyens,, Inorg. Chim. Acta, 1988, 154, 229.
- P. Pyykkö, Chem. Rev. 1988, 88, 563.
- P. Schwerdtfeger, G. A. H., M. Dolg and M. A. Bennett,, J. Am. Chem. Soc. 1992, 114, 7518.
- A. Andrés, A. B., A. A. Andrés, A. B., A. Carachalios, A. Bianchi, P. Dapporto, E. Garcia-España, P. Paoletti and P. Paoli,, J. Chem. Soc., Dalton Trans., 1993, 3507.
- Parr, J. , Some recent coordination chemistry of lead(ll). Po(vhedron 1997, 16, 551–566. [Google Scholar]
- J. -H. Chung, B.-K. M., Y.K. Kim, K.-H. Kim and T.-Y. Kwon,, J Mol Struct 2014, 1076, 698–703.
- C. Platas-Iglesias, D. E.-G., T. Enriquez-Perez, F. Avecilla, A. deBlas and T. Rodriguez-Blas,, Inorg Chem 2005, 44, 2224–2233.
- J. W. Nugent, H. L., J.H. Reibenspies and R.D. Hancock,, 2015, 91, 120–127.
- L. Shimonni-Livny, J. P. G. a. C. W. B., Inorg Chem, 1998, 17, 1853.
- A. Walsh and G.W. Watson, J Solid State Chem 2005, 178, 1422.
- L. Shimoni-Livny, J. P. G. a. C. W. B., Inorg Chem, 1998, 37, 1853–1867.
- J. B. Hoffman, A. L. S., and P. V. Kamat,, “Transformation of sintered CsPbBr3 nanocrystals to cubic CsPbI3 and gradient CsPbBrxI3−x through halide exchange,” J. Am. Chem. Soc. 2016, 138, 8603–8611.
- G. Li et al., “Reversible anion exchange reaction in solid halide perovskites and its implication in photovoltaics,”. J. Phys. Chem. C 2015, 119, 26883–26888. [Google Scholar]
- Quinten, A. Akkerman, V. D. I., ‡ Sara Accornero,† Alice Scarpellini,† Annamaria Petrozza,‡ Mirko Prato,*,† and Liberato Manna*,†, Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar]
- I. V. Markov, C. G. f. B. I. V. Markov, C. G. f. B., World Scientifi c, Singapore 1995.
- S. Yang, Y. C. Z., Y. Hou, X. Chen, Y. Chen, Y. Wang, H. Zhao,H. G. Yang,, Chem. Mater. 2014, 26, 6705.
- O. Horváth, I. M., J. Photochem. Photobiol. A 1998 95, 114.
- al, G. e. , APL, 2014, 133902.
- al. , S. e., Nat. Commun. 2015, 6.
- Brivio, F. B., K. T.; Walsh, A.; van Schilfgaarde, M., Relativistic Quasiparticle Self-Consistent Electronic Structure of Hybrid Halide Perovskite Photovoltaic Absorbers. Phys. Rev. B: Condens. Matter Mater. Phys. 2014, 89, 155204. [Google Scholar] [CrossRef]
- Kepenekian, M. R., R.; Katan, C.; Sapori, D.; Pedesseau, L.; Even, J. , Rashba and Dresselhaus Effects in Hybrid Organic−Inorganic Perovskites: From Basics to Devices. ACS Nano 2015, 9, 11557–11567. [Google Scholar] [CrossRef] [PubMed]
- Kim, M. I., J.; Freeman, A. J.; Ihm, J.; Jin, H. , Switchable S = 1/2 and J = 1/2 Rashba Bands in Ferroelectric Halide Perovskites. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 6900–6904. [Google Scholar] [CrossRef] [PubMed]
- Even, J. P. , Guide to Symmetry Properties of the Reference Cubic Structure of 3D All-Inorganic and Hybrid Perovskites. J. Phys. Chem. Lett. 2015, 6, 2238–2242. [Google Scholar] [CrossRef] [PubMed]
- Amat, A. M., E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M. K.; Grätzel, M.; De Angelis, F. , Cation-Induced Band-Gap Tuning in Organohalide Perovskites: Interplay of Spin-Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Stroppa, A. D. S., D.; Barone, P.; Bokdam, M.; Kresse, G.; Franchini, C.; Whangbo, M.-H.; Picozzi, S. , Tunable Ferroelectric Polarization and Its Interplay with Spin−orbit Coupling in Tin Iodide Perovskites. Nat. Commun. 2014, 5, 5900. [Google Scholar] [CrossRef] [PubMed]
- Koutselas, I. B. D., L.; Papavassiliou, G. C. , Electronic Properties of Three- and Low-Dimensional Semiconducting Materials with Pb Halide and Sn Halide Units. J. Phys.: Condens. Matter. 1996, 8, 1217–1227. [Google Scholar]
- Chiarella, F. Z., A.; Licci, F.; Borriello, I.; Cantele, G.; Ninno, D.; Cassinese, A.; Vaglio, R. , Combined Experimental and Theoretical Investigation of Optical, Structural, and Electronic Properties of CH3NH3SnX3 Thin Films (X = Cl,Br). Phys. Rev. B: Condens. Matter Mater. Phys. 2008, 77, 045129. [Google Scholar] [CrossRef]
- Umebayashi, T. A., K.; Kondo, T.; Nakao, A. , Electronic Structures of Lead Iodide Based Low-Dimensional Crystals. Phys. Rev. B: Condens. Matter Mater. Phys. 2003, 67, 155405. [Google Scholar] [CrossRef]
- Brivio, F. W., A. B.; Walsh, A., Structural and Electronic Properties of Hybrid Perovskites for High-Efficiency Thin-Film Photovoltaics from First-Principles. APL Mater. 2013, 1, 042111. [Google Scholar] [CrossRef]
- Chang, Y. H. P., C. H.; Matsuishi, K., First-Principles Study of the Structural and the Electronic Properties of the Lead-Halide-Based Inorganic-Organic Perovskites (CH3NH3)PbX3 and CsPbX3 (X = Cl, Br, I). J. Korean Phys. Soc. 2004, 44, 889–893. [Google Scholar]
- Borriello, I. C., G.; Ninno, D. , Ab Initio Investigation of Hybrid Organic-Inorganic Perovskites Based on Tin Halides. Phys. Rev. B: Condens. Matter Mater. Phys. 2008, 77, 235214. [Google Scholar] [CrossRef]
- Vincent, B. R. R., K. N.; Cameron, T. S.; Knop, O., Alkylammonium Lead Halides. Part 1. Isolated PbI6 4− Ions in (CH3NH3)4PbI6•2H2O. Can. J. Chem. 1987, 65, 1042–1046. [Google Scholar] [CrossRef]
- Umebayashi, T. A., K.; Kondo, T.; Nakao, A. , Electronic Structures of Lead Iodide Based Low-Dimensional Crystals. Phys. Rev. B: Condens. Matter Mater. Phys. 2003, 67, 155405. [Google Scholar] [CrossRef]
- Brivio, F. B., K. T.; Walsh, A.; van Schilfgaarde, M., Relativistic Quasiparticle Self-Consistent Electronic Structure of Hybrid Halide Perovskite Photovoltaic Absorbers. Phys. Rev. B: Condens. Matter Mater. Phys. 2014, 89, 155204. [Google Scholar] [CrossRef]
- S. García-Martína, E. U.-G., M. S. García-Martína, E. U.-G., M.C. Knapp, G. King, P.M. Woodward,, Structural complexity in AA′MM′O6 Perovskites. A Transmission Electron Microscopy Study, Materials Research Society Symposium Proceedings. 2009, 1148.
- K. Kobayashi, T. K., H. 111. K. Kobayashi, T. K., H. Sawada, K. Terakura, Y. Tokura, , Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure, Nature.
- R. Ubic, G. S., M.T. Sebastian,, Effective size of vacancies in the Sr1-3x/2CexTiO3 superstructure, Ceramic Transactions 2009, 204, 177–185. [Google Scholar]
- Brown*, I. D. , Recent Developments in the Methods and Applications of the Bond Valence Model Chem. Rev. 2009, 6858–6919. [Google Scholar]
- Frost, J. M. B., K. T.; Brivio, F.; Hendon, C. H.; van Schilfgaarde, M.; Walsh, A., Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, T. A., C.; Prajongtat, P.; Rech, B.; Lux-Steiner, M. C. , Temperature Dependence of the Band Gap of CH3NH3PbI3 Stabilized with PMMA: A Modulated Surface Photovoltage Study. J. Phys. Chem. C 2015, 119, 23968–23972. [Google Scholar] [CrossRef]
- Pauling, L. , The Nature of the Chemical Bond. Cornell University Press: Ithaca, NY.
- Hao, F. S., C. C.; Chang, R. P. H.; Kanatzidis, M. G., Anomalous Band Gap Behavior in Mixed Sn and Pb Perovskites Enables Broadening of Absorption Spectrum in Solar Cells. J. Am. Chem. Soc., 2014, 136, 8094–8099. [Google Scholar] [CrossRef]
- T. A. Albright, J. K. B., M. 118. T. A. Albright, J. K. B., M.-H. Whangbo, , Orbital Interactions in Chemistry. 2nd ed., Wiley, New York,.
- Stranks, S. D. S., H. J. , Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Xing, G. M., N.; Lim, S.; Yantara, N.; Liu, X.; Sabba, D.; Graetzel, M.; Mhaisalkar, S.; Sum, T., Nat. , Nat. Mater. 2014, 13, 476–480. [Google Scholar]
- Tan, Z.-K. M., R. S. ; Lai, M. L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L. M.; Credgington, D.; Hanusch, F.; Bein, T.; Snaith, H. J.; Friend, R. H., Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar]
- Chin, X. Y. C., D.; Yin, J.; Bruno, A.; Soci, C., Nat. , Nat. Commun. 2015, 6, 7383. [Google Scholar]
- Huskinson, B. , Rugolo, J., Mondal, S. K. & Aziz, M. J., A high power density, high e ciency hydrogen chlorine regenerative fuel cell with a low precious metal content catalyst. Energy Environ. Sci. 2012, 5, 8690–8698. [Google Scholar]
- Taylor, G. R. B., M. , A comparison of the virucidal properties of chlorine, chlorine dioxide, bromine chloride and iodine. J. Hyg. 1982, 89, 321–328. [Google Scholar] [CrossRef]
- Yeo, R. S. C., D. -T., A hydrogen bromine cell for energy storage applications. J. Electrochem. Soc. 1980, 127, 549–555. [Google Scholar] [CrossRef]
- Baglio, J. A. e. a. , Characterization of n-type semiconducting tungsten disulfide photoanodes in aqueous and nonaqueous electrolyte solutions photo-oxidation of halides with high e ciency. J. Electrochem. Soc. 1982, 129, 1461–1472. [Google Scholar] [CrossRef]
- Singh, N. e. a. , Stable electrocatalysts for autonomous photoelectrolysis of hydrobromic acid using single-junction solar cells. Energy Environ. Sci. 2014, 7, 978–981. [Google Scholar] [CrossRef]
- Powers, D. C. , Hwang, S. J., Zheng, S.-L. & Nocera, D. G., Halide-bridged binuclear HX-splitting catalysts. Inorg. Chem. 2014, 53, 9122–9128. [Google Scholar]
- McKone, J. R. , Potash, R. A., DiSalvo, F. J. & Abruña, H. D., Unassisted HI photoelectrolysis using n-WSe2 solar absorbers. Phys. Chem. Chem. Phys. 2015, 17, 13984–13991. [Google Scholar]
- Ardo, S. , Park, S. H.,Warren, E. L. & Lewis, N. S., Unassisted solar-driven photoelectrosynthetic HI splitting using membrane-embedded Si microwire arrays. Energy Environ. Sci. 2015, 8, 1484–1492. [Google Scholar]
- Sunghak Park1†, W. J. C. , ChanWoo Lee1, Sangbaek Park1, Hyo-Yong Ahn1 and Ki Tae Nam1,2*, Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. NATURE ENERGY | www.nature.com/natureenergy 1©.
- Kazim, S. N., M. K. ; Grätzel, M.; Ahmad, S., Angew. Chem., Int. Ed. 2014, 53, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- He, M. Z., D.; Wang, M.; Lin, C.; Lin, Z., J. , J. Mater. Chem. A 2014, 2, 5994–6003. [Google Scholar] [CrossRef]
- He, M. P., X.; Liu, X.; Jiang, B.; He, Y.; Snaith, H.; Lin, Z. , Angew. Chem., Int. Ed. 2016, 55, 4280−4284. 55.
- Kim, Y. Y., E.; Voznyy, O.; Comin, R.; Walters, G.; Gong, X.; Kanjanaboos, P.; Nogueira, A. F.; Sargent, E. H. , ACS Appl. Mater. Interfaces 2015, 7, 25007–25013. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L. C. P. s., A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H. J.; Galian, R. E.; Pérez-Prieto, J., J. , J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Yang-Fan Xu, M.-Z. Y. , † Bai-Xue Chen, Xu-Dong Wang, Hong-Yan Chen, Dai-Bin Kuang,* and Cheng-Yong Su, A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 5660. [Google Scholar]
- Nuria Vicente, a. G. G.-B. , Methylammonium Lead Bromide Perovskite Battery Anodes Reversibly Host High Li-ion Concentrations. J. Phys. Chem. Lett. 2017. [Google Scholar] [CrossRef]
- Jiantie Xu1, Yonghua Chen1,* & Liming Dai1, Efficiently photo-charging lithium-ion battery by perovskite solar cell. nature comm. 2015, 6, 8103.
- Protesescu, L. Y., S.; Bodnarchuk, M. I.; Krieg, F.; Caputo, R.; Hendon, C. H.; Yang, R. X.; Walsh, A.; Kovalenko, M. V. , Nanocrystals of Cesium Lead Halide Perovskites (CsPbX 3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett.,, 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Wong, A. B. L., M.; Eaton, S. W.; Yu, Y.; Lin, E.; Dou, L.; Fu, A.; Yang, P. , Growth and Anion Exchange Conversion of CH3NH3PbX3 Nanorod Arrays for Light-Emitting Diodes. Nano Lett. 2015, 15, 5519–5524. [Google Scholar] [CrossRef] [PubMed]
- Pellet, N. T., J.; Maier, J.; Grätzel, M. , Transforming Hybrid Organic Inorganic Perovskites by Rapid Halide Exchange. Chem. Mater. 2015, 27, 2181–2188. [Google Scholar] [CrossRef]
- Jang, D. M. P., K.; Kim, D. H.; Park, J.; Shojaei, F.; Kang, H. S.; Ahn, J.-P.; Lee, J. W.; Song, J. K. , Reversible Halide Exchange Reaction of Organometal Trihalide Perovskite Colloidal Nanocrystals for Full-Range Band Gap Tuning. Nano Lett. 2015, 15, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, G. P., L.; Yakunin, S.; Bodnarchuk, M. I.; Grotevent, M. J.; Kovalenko, M. V. , Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q. A. D. I., V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. , Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- Tennyson, L. Doane, † Kayla L. Ryan,† Laxmikant Pathade,† Kevin J. Cruz,† Huidong Zang,‡ Mircea Cotlet,‡ and Mathew M. Maye*,†, Using Perovskite Nanoparticles as Halide Reservoirs in Catalysis and as Spectrochemical Probes of Ions in Solution. ACS Nano 2016, 10, 5864–5872. [Google Scholar]
- Starks, C. M. L., C. L., Phase Transfer Catalysis: Principles and Techniques; . Academic Press: New York, 1978. [Google Scholar]
- Gribble, G. W. , The Diversity of Naturally Occurring Organobromine Compounds. Chem. Soc. Rev. 1999, 28, 335–346. [Google Scholar] [CrossRef]
- De Roo, J. I. n. e., M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J. C.; Van Driessche, I.; Kovalenko, M. V.; Hens, Z. , Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef]
- Ran Ding, H. L. , Xiaoli Zhang,* Juanxiu Xiao, Rahul Kishor, Huaxi Sun, Bowen Zhu, Geng Chen, Fei Gao, Xiaohua Feng, Jingsheng Chen, Xiaodong Chen, Xiaowei Sun,* and Yuanjin Zheng*, Flexible Piezoelectric Nanocomposite Generators Based on Formamidinium Lead Halide Perovskite Nanoparticles. Adv. Funct. Mater. 2016. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).