1. Introduction
Lichen amyloidosis (LA) is the most common form of Primary Localized Cutaneous Amyloidosis (PLCA) [
1]. The latter is characterized by the extracellular amyloid deposition exclusively in the skin [
2,
3,
4,
5]. Primary localized cutaneous amyloidosis is divided into four subtypes, namely: lichen amyloidosis, macular amyloidosis (MA), biphasic amyloidosis (BA), and nodular amyloidosis (NA).
Clinically, LA presents with clusters of multiple brownish hyperkeratotic papules coalescing in plaques. They may be localized or generalized and are typically distributed on the lower leg, back, forearm or thigh [
2,
3,
4,
5]. At the onset, lesions are usually unilateral, but a bilateral symmetric distribution pattern can develop over time. Lesions are often associated with itching but may also be asymptomatic [
6].
LA is more common in women and among people from Asia and South America than in Europe or North America [
1,
2].
It can occur as an isolated finding or in association with other diseases, including atopic dermatitis (AD), mycosis fungoides, lichen planus (LP), HIV, multiple endocrine neoplasia type 2 (MEN2A), angiolymphoid hyperplasia with eosinophilia, ankylosing spondylitis, autoimmune thyroiditis, hyperthyroidism and connective tissue disorders [
7].
In light of the clinical presentation and the numerous pathologies with which it can be associated, the diagnosis of LA is not always straightforward.
It is in differential diagnosis with: keratosis pilare (KP), prurigo nodularis (PN) [
8], xanthomas, perforating dermatoses (collagenoses), lichen planus, mycosis fungoides [
2], papular mucinosis, myxedema, stasis dermatitis [
9], lichen simplex chronicus (LSC) and pretibial pruritic papular dermatitis [
6].
KP presents as multiple small grey-white folliculocentric keratotic papules measuring 1 mm, usually located on the extensor surfaces of the proximal extremities. The lesions are associated with less intense itching and, in fewer cases, than LA.
PN is characterized by numerous symmetrically distributed hyperkeratotic nodules of variable size (from several millimetres up to 2 cm). The diagnosis of PN is mostly clinical, and smaller hyperkeratotic nodules in PN may be misdiagnosed as LA. In such a case, the pigmentation and size of the papules, which in PN are associated with true nodules, must be considered to guide the diagnostic suspicion [
8].
In LSC, papules aggregate into irregular, scaly plaques with unclear borders with lichenification and peripheral hyperpigmentation, whereas in LA, the papules represent the dominant element.
LP presents as violaceous, pruritic papules and plaques with shiny surfaces with Wickham striae. Unlike LA, it also involves mucous membranes and nails. It enters into differential diagnosis with LA, especially in the lichen verrucous variant, which presents with brownish pigmentation and a verrucous appearance that is more similar to LA [
9].
A skin biopsy of the affected areas must be performed to obtain a definitive diagnosis, in addition to the analysis of the clinical aspects just mentioned. Where the case requires, obtaining a sampling of the rectal mucosa or periumbilical fat is recommended to exclude systemic involvement [
1].
Different types of PLCA are frequently recognized by common histological features such as PAS positive, affinity for thioflavin T and Congo Red, and metachromasia following staining with crystal violet or methyl violet. When examined under polarized light, amyloid exhibits distinctive apple green birefringence, also known as dichroism, due to the use of Congo Red. Frequently amyloid is located in lichenoid (papular) and macular patterns in the papillary dermis, beneath dermo-epidermal junction; acanthosis and hyperkeratosis of the underlying epidermis are frequently observed and both macular and lichenoid amyloidoses do not involve blood vessels [
10,
11]. A loose network of unbranched filaments with a diameter of 7.5–10 nm is visible under electron microscopy. The fibrillary anti-parallel beta-sheet structure of the various amyloid precipitates is what unites them ultrastructurally. Protofilaments make up the filaments, and filaments group together to form fibrils. Fibrils are present in the extracellular space, fibroblasts phagocytose minute amounts of them, and a degeneration of keratinocytes known as filamentous and pyknotic degeneration has been documented in the literature. It is possible that fibroblasts play a direct role in the production of amyloid, however particular studies on this matter are currently lacking. Unlike the corresponding amyloid protein, the amyloid P component exhibits a pentagonal ultrastructure and is non-fibrillary [
12].
Abdominal fat pad fine-needle aspiration biopsy has emerged as the gold standard for diagnosing systemic amyloidosis in recent years, owing in part to its excellent sensitivity and simplicity in smear preparation.
LA is a relatively rare condition, which is the reason why the therapeutic strategies reported in the literature are the result of minor studies and not major clinical trials. It significantly impacts patients' lives because of the blemish it generates and, when symptomatic, because of all the itch-related problems [
3].
The current therapeutic approach involves topical therapies in the first line aimed at extinguishing the probable underlying inflammatory process and itching where present. The topical drugs recommended to date are essentially topical corticosteroids or topical tacrolimus [
3]; Dimethyl sulfoxide (DMSO) has been used for several patients but presents some non-negligible side effects (contact urticaria, desquamation of the skin, and a burning sensation), while topical calcipotriol has not returned better effects than corticosteroids [
1,
3].
If the lesions persist, whether it is a localized LA, it is possible to substitute or add topical drugs to surgical removal (electrodesiccation, a special technique called 'scraping with the scalpel', and dermabrasion) or laser removal [
13,
14,
15]. If it is diffuse LA, on the other hand, phototherapy (narrow band UVB and photochemotherapy with PUVA), and/or systemic drug treatment (oral antihistamines, DMSO, acitretin or other retinoids, cyclophosphamide, cyclosporine) can be used [
3,
16,
17,
18]. In addition, satisfactory results have also been reported using amitriptyline and transcutaneous nerve stimulation [
3]. Finally, there are a small number of case reports in the literature in which biological drugs such as Upadacitinib [
19], Baricitinib [
20] and Dupilumab [
7,
21,
22,
23] have been used.
We report the case of a patient with LA associated with AD resistant to conventional therapies who responded to treatment with Dupilumab.
2. Case Presentation
A 52-year-old female patient came to our attention on 14th October 2021 with severe itchy symptoms associated with eczematous and lichenified lesions of the back and upper limbs, patches with brownish, itchy papules as in LA on the lower limbs and abdomen, and numerous scratching lesions (
Figure 1). The patient reports suffering from AD and LA for about 27 years. She reported a negative family history of both conditions. The first lesions of LA appeared in 1994 as brownish, itchy papules coalescing in patches on the lower limbs. In the following years, the patient went to various centres where the hypothesis of systemic amyloidosis was rejected, and the diagnosis of localized LA was made. The patient had been started on cycles of cyclosporine treatment for 4 years previously with little benefit.
The general evaluation showed: Eczema Area Severity Index (EASI) 22, pruritus Visual Analogue Scale (VAS) 10, Dermatology Life Quality Index (DLQI) 19.
A panel of analysis showed an increase in IgE and eosinophils. Prick tests revealed a sensitization to mites and cypress, while patch tests returned negative results, allowing the exclusion of contact dermatitis.
At our clinic for a diagnosis of certainty of LA, an incisional biopsy was performed at typical infiltrated plaques on the lower limbs. The histopathological investigations reported typical LA findings such as deposition of amyloid material at the papillary dermis, beneath to dermo-epidermal junction; there were also some areas with incontinence of melanic pigment and diffuse inflammatory infiltrate at the middle and superficial dermis. The epidermis was not interested by this infiltration and acts as an innocent bystander with acanthosis, hyperkeratosis and elongation of the epidermal ridges (
Figure 3 ,
Figure 4 and
Figure 5).
We confirmed the diagnosis of LA and AD. Therefore, it was necessary to find an alternative treatment to resolve the skin problem or at least the itching, given that cyclosporine had proved ineffective.
In light of the presence of AD and the significant itchy symptoms, it is decided to start the patient on Dupilumab treatment. Induction is therefore carried out with 2 fl of 300 mg and maintenance with 1 fl every other week starting on 4th March 2022.
The therapy proved to be effective, showing a marked improvement in the 3-month follow-up with a reduction in itching and the extension of the eczematous patches, as expected. In contrast, the lesions of LA of the legs persisted. The patient presented: EASI 15, VAS 0, DLQI 3.
One year after the beginning of the therapy, a total resolution of the pathology was found with the unexpected disappearance also of the amyloid papules and the achievement of EASI 0 and DLQI and VAS of 0 (
Figure 2).
To date, the patient's quality of life has exponentially improved thanks to the disappearance of itchy symptoms and skin blemishes related to AD but also, above all, to LA. At the last check-up, in fact, a total remission of the clinical presentation was appreciated, with the persistence of a slight residual skin xerosis and barely perceptible hyperpigmentation on the legs. The lower limbs, as well as the rest of the skin area, do not show any noticeable or scratching lesions, and the patient has not experienced any side effects, so she is still on Dupilumab 300 mg sc every other week.
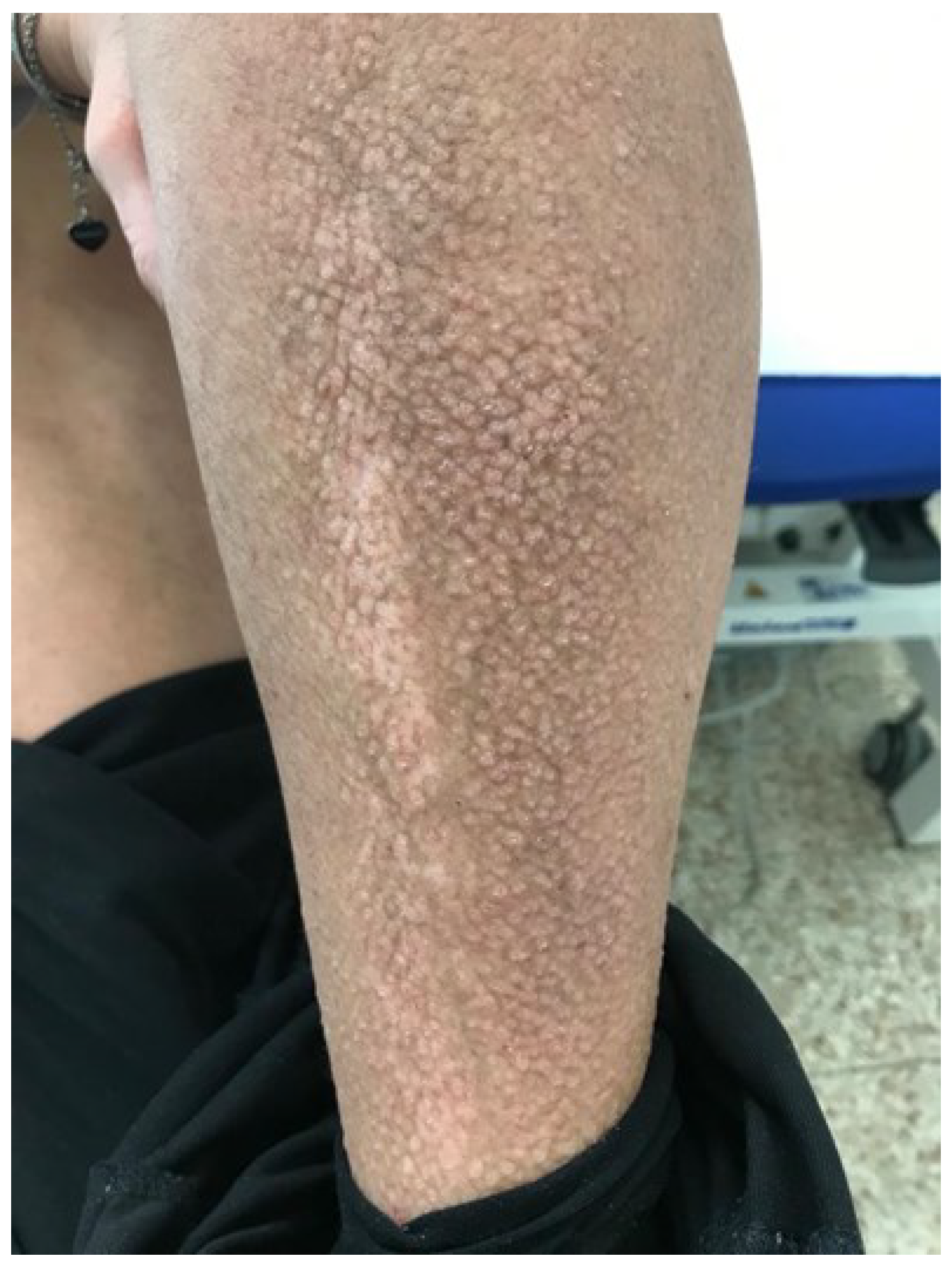
Figure 1.
Clinical photograph of lichen amyloidosis before the treatment with Dupilumab. Leg of the patient revealing generalized rippled, dyschromic brown and thin plaques.
Figure 1.
Clinical photograph of lichen amyloidosis before the treatment with Dupilumab. Leg of the patient revealing generalized rippled, dyschromic brown and thin plaques.
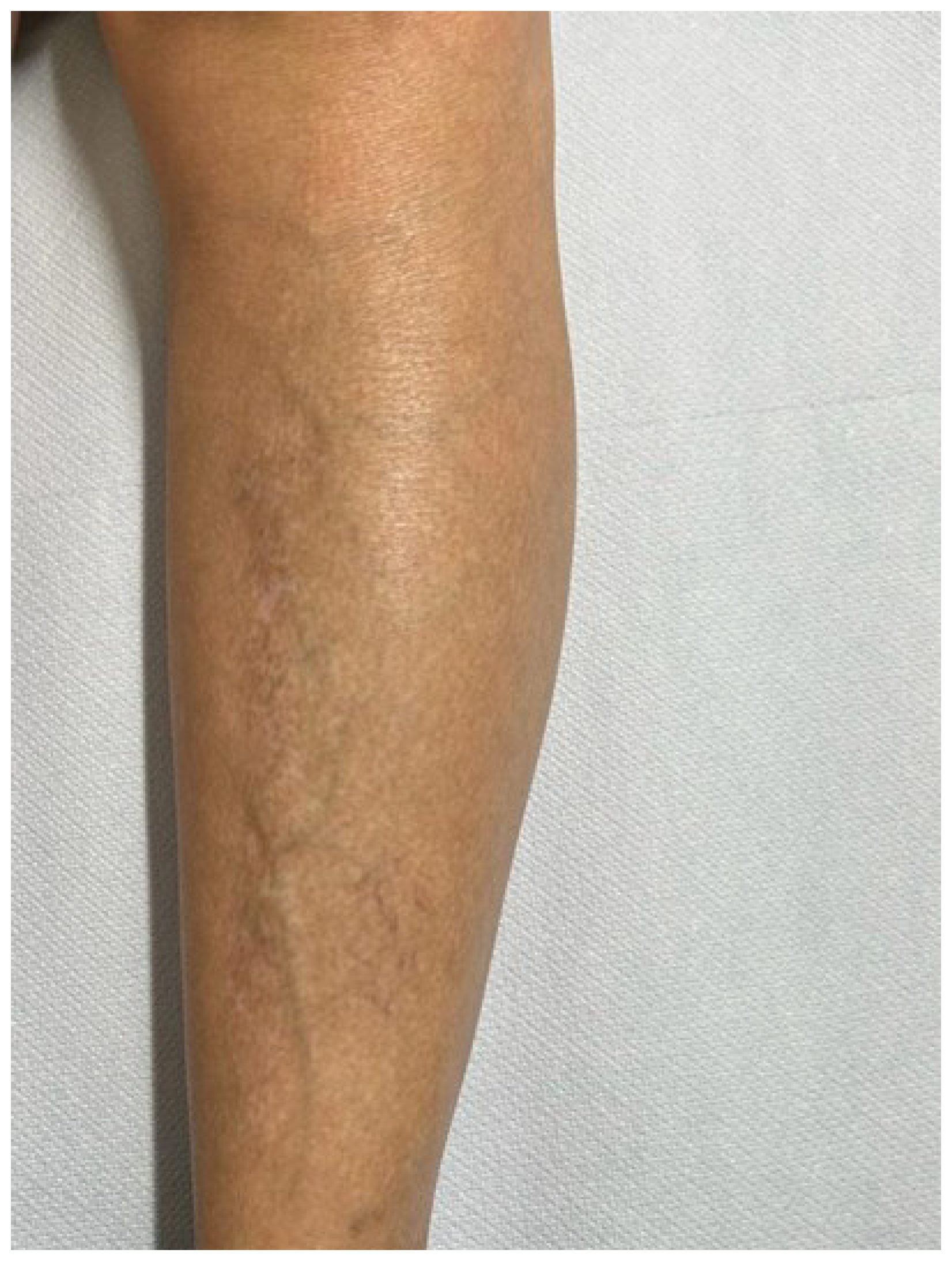
Figure 2.
Condition of the patient after one year of Dupilumab therapy showing skin smooth and free of lesions with mild dyscromic aftermaths.Figure 3. Histological photomicrograph showing an area with hyperkeratosis, some degree of acantosis and amyloid deposition in the papillary dermis (Hematoxylin-Eosin, Original Magnification 4x).
Figure 2.
Condition of the patient after one year of Dupilumab therapy showing skin smooth and free of lesions with mild dyscromic aftermaths.Figure 3. Histological photomicrograph showing an area with hyperkeratosis, some degree of acantosis and amyloid deposition in the papillary dermis (Hematoxylin-Eosin, Original Magnification 4x).
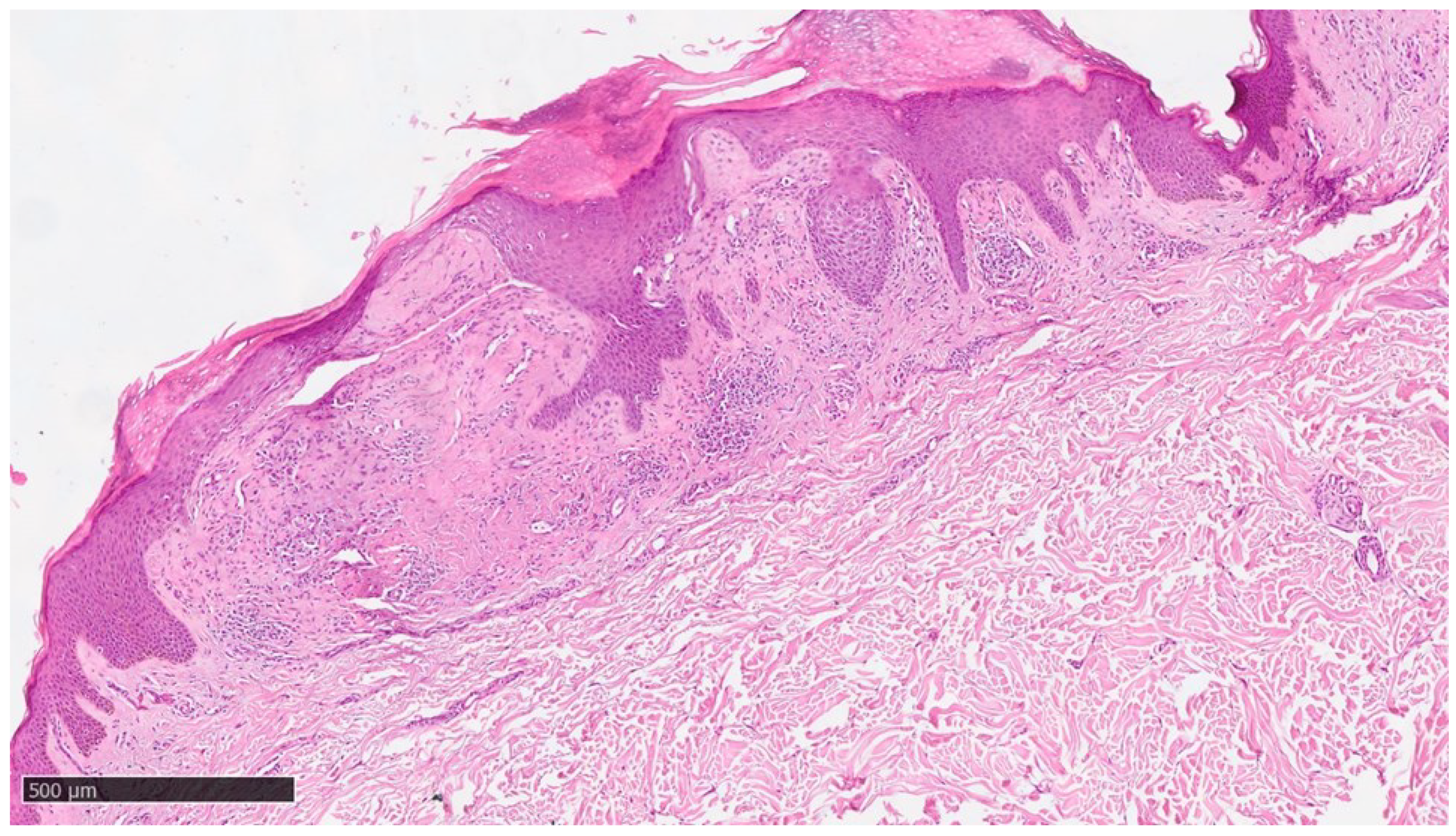
Figure 3.
Histological photomicrograph showing an area with hyperkeratosis, some degree of acantosis and amyloid deposition in the papillary dermis (Hematoxylin-Eosin, Original Magnification 4x).
Figure 4.
Scanning magnification of the previous picture showing the amyloid deposition beneath the dermo-epidermal junction with the typical cleft (white spaces) and moderate lymphocytic infiltration in the middle dermis (Hematoxylin-Eosin, Original Magnification 10x).
Figure 4.
Scanning magnification of the previous picture showing the amyloid deposition beneath the dermo-epidermal junction with the typical cleft (white spaces) and moderate lymphocytic infiltration in the middle dermis (Hematoxylin-Eosin, Original Magnification 10x).
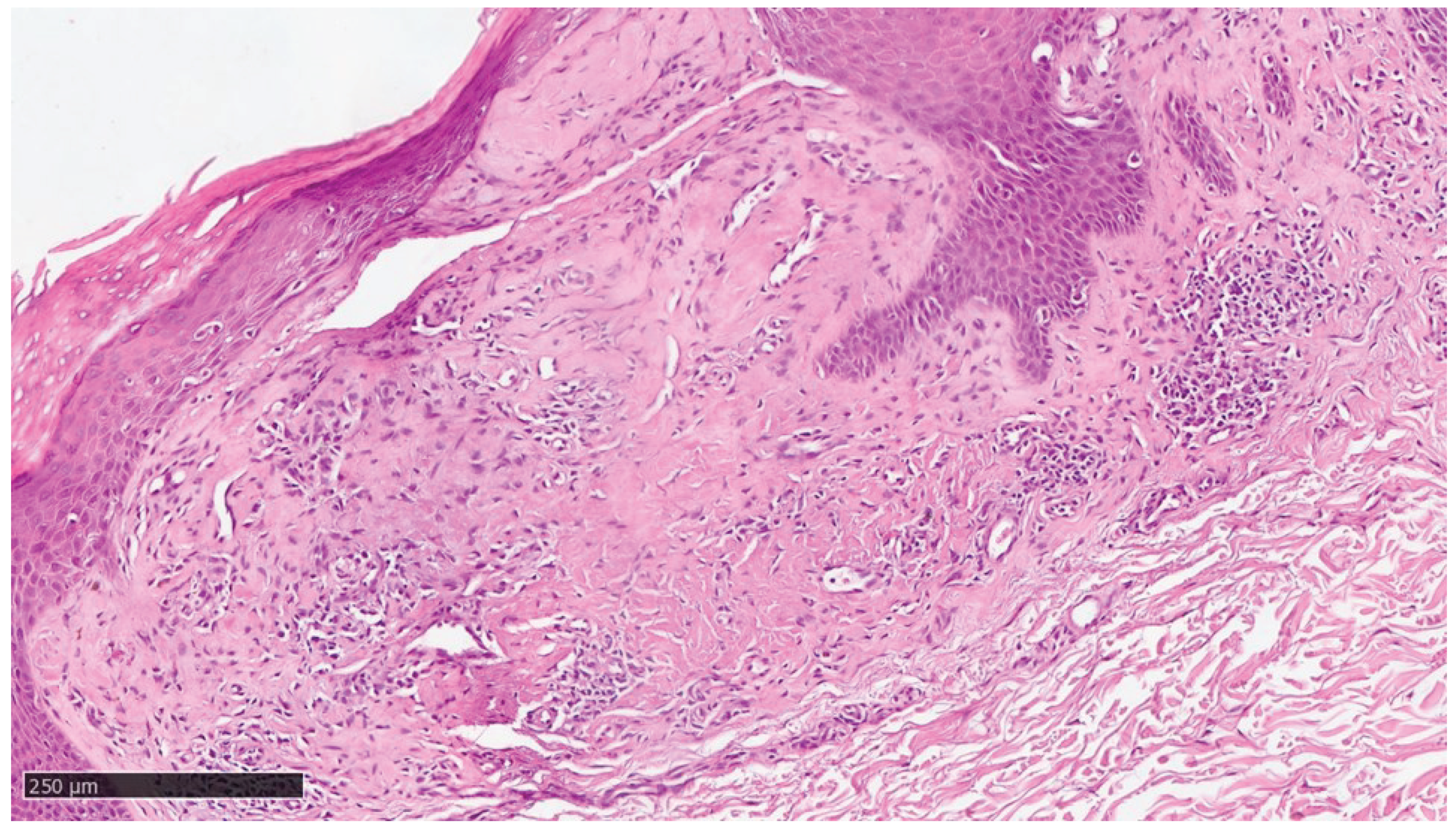
Figure 5.
Higher magnification showing some incontinence of melanin within amyloid deposition (Hematoxylin-Eosin, Original Magnification 20x).
Figure 5.
Higher magnification showing some incontinence of melanin within amyloid deposition (Hematoxylin-Eosin, Original Magnification 20x).
4. Discussion
As mentioned in the introduction, LA is an underrepresented condition in general, but especially within the Caucasian population. For this reason, no particularly effective treatment procedure has been devised to date [
3]. Often, in fact, good clinical control is not achieved, or the itching goes away without the lesions regressing [
7]. In this case, Dupilumab led to a complete resolution of both AD and LA by achieving not only the disappearance of itching but also of the typical lesions. It is also interesting to note that ours is the first case to report follow-up data one year after the start of dupilumab therapy. This last finding reflects not only the efficacy of Dupilumab, but also the absence of relapse obtained with continued therapy. Therefore, based on the reported results, this drug could be introduced in the treatment of LA.
Our case fits into the context of a small number of minor studies and case reports, which, in the absence of publications with a larger number of patients, are extremely valuable and useful for further pathology investigation. While the etiopathogenetic mechanism AD has been thoroughly investigated, at least in its main features, that of LA has not yet been clarified. To understand the efficacy of Dupilumab, the pathophysiological association between the two diseases must first be investigated.
It is well known that IL-4, IL-13 and IL-31 in AD patients are associated with sensory nerve sensitisation, itching and the perpetuation of chronic type II inflammation. In particular, in AD there is an alteration of the normal physiology of the skin barrier, which leads to an increase in transepidermal water loss (TEWL) and the triggering of Th2-mediated cutaneous inflammatory phenomena. As part of this inflammation, dysregulation of various cytokine patterns occurs, including that of IL-4, IL-13 and IL-31, which interests us. On the one hand, IL-4 and 13 exacerbate changes in the skin barrier, and on the other hand, they determine the itchy stimulus either directly or by stimulating IL-31 production. Increased expression of this cytokine is, therefore, one of the main mediators of itching in AD [
24]. The aetiopathogenetic mechanism of LA has not yet been fully elucidated.
According to the most widely accepted hypothesis, the first pathogenic moment is apoptosis of basal keratinocytes. During the cellular end programme, they release cytokeratins, which are then coated by autoantibodies, phagocytosed by macrophages and enzymatically degraded into amyloid K, which triggers an itchy stimulus. Amyloid K is derived in most cases from cytokeratin 5, but cytokeratin 1, 10, and 14 [
25] have also been found, and in one study also deposits of ApoE [
1]. The primum movens of keratinocyte apoptosis has not yet been identified. Still, some possible triggers, such as itching and dysregulation of cytokine signalling (particularly of IL-13 and 31 and certain elements regulating their expression), have been proposed [
2,
26,
27,
28,
29,
30,
31]. Therefore, the cause of this apoptosis would be found in the first instance in mechanical trauma from scratching, which accounts for the strong association with AD and the initiation of a scratch-itch-scratch circle [
1,
2,
32]. Chronic inflammation (not necessarily related to itching) could play a role in the genesis of LA as well as in that of AD. Indeed, both IL-13 and 31 have been found in lesions of LA [
30] and, furthermore, in cases of familial cutaneous amyloidosis, mutations have been identified in the gene encoding two subunits (IL31RA and OSMR β ) of the IL 31 receptor [
28,
29,
31]. Given this, the abnormality of the IL-31 signalling pathway may constitute the molecular substrate of both AD and LA. In this sense, these pathologies could represent two aspects, sometimes isolated, sometimes coexisting, of the phenotypic expression of the aforementioned alteration [
33]. Comparing the current management of the two diseases, corticosteroids, calcineurin inhibitors, phototherapy and immunosuppressors are used in both, including cyclosporine; it should be emphasised that the treatment of patients with LA and AD is even more complex, as acitretin and other systemic retinoids cannot be used as they would lead to a worsening of AD [
33]. Dupilumab fits perfectly into this context because it acts by antagonising several elements of the signalling just described. It is an all-human monoclonal IgG4 antibody that binds to the shared alpha subunit of both type I (IL-4Rα/γc) and type II (IL-4Rα/IL-13Rα) IL-4 receptor, inhibiting IL-4 and 13 signal transduction and with them other elements, such as IL-31, that are responsible for the dysregulation of the type II inflammatory reaction [
33]. It is currently used for treating AD, and its efficacy and good safety profile have been amply demonstrated. It causes an early reduction in itching and AD lesions by acting on several levels 30. Firstly, it directly blocks the action of IL-4 and IL-13 on sensory neurons. In addition, IL-4 stimulates Th2-cells to produce IL-31, so Dupilumab, by antagonising the action of IL-4, indirectly reduces the action of IL-31. Dupilumab has also received Food and Drug Administration (FDA) approval for use in PN, and there are several off-label studies concerning its application in additional dermatological disorders [
33]. Concerning LA, there are only 7 other cases of LA and AD effectively treated with Dupilumab in the literature [
21,
22,
23,
24] and one case of isolated generalized LA [
7].
In the meantime, the mechanism by which Dupilumab leads to an improvement in LA remains unclear. In light of the above, it seems that interrupting the vicious circle of itchy scratching, but also its direct and indirect effects on the cytokines involved in the Th2 inflammatory response and consequently in the possible pathophysiology of this disorder, are primarily involved.
5. Conclusions
LA is a condition that significantly impacts the quality of life of affected patients. It is a relatively uncommon disorder. Hence, several fundamental aspects of this pathology, such as its pathophysiology, diagnostic investigation and, consequently, therapeutic approach, still need to be fully acknowledged.
Our case report is interesting because it offers insights into aspects of LA that have not yet been fully investigated.
Chronic scratching (whether or not related to AD) could be confirmed as one of the main pathogenetic factors of LA. Patients with abnormalities in IL-31,13 and 4 signalling might present with coexisting LA and AD phenotypes.
Dupilumab in dermatology already has an indication for AD and PN and has been used off-label for numerous other clinical conditions. In light of its effect on several inflammatory cytokines and its usefulness against pruritus, it could be an effective new therapy in patients with DA-related LA and probably in patients with isolated LA.
To confirm this, it proved to be a drug with an excellent safety profile and a good clinical response in our case as well.
The application of Dupilumab in patients with LA is certainly a scientifically relevant topic. Further studies should be done focusing on the changes in skin lesions after discontinuing the drug and the efficacy and safety of long-term application.
Author Contributions
Conceptualization, B.T. and F.A.; methodology, G.C.; software, F.A.; validation, G.C., F.A. and C.F.; formal analysis, G.C.; investigation, B.T.; resources, F.A.; data curation, B.T.; writing—original draft preparation, B.T., F.A. and G.C.; writing—review and editing, B.T., G.C., F.A., C.F. and M.B.; visualization, M.B.; supervision, C.F. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schreml, S.; Szeimies, R.M.; Vogt, T.; Landthaler, M.; Schroeder, J.; Babilas, P. Cutaneous amyloidoses and systemic amyloidoses with cutaneous involvement. Eur J Dermatology. 2010, 20, 152–160. [Google Scholar] [CrossRef]
- Rook’s Textbook of Dermatology.; 2016.
- Weidner, T.; Illing, T.; Elsner, P. Primary Localized Cutaneous Amyloidosis: A Systematic Treatment Review. Am J Clin Dermatol. 2017, 18, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Steinmann, S.; Maul, J.T.; Kolm, I. Primary Localized Cutaneous Amyloidosis: A Retrospective Study of an Uncommon Skin Disease in the Largest Tertiary Care Center in Switzerland. Dermatology. 2022, 238, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Pálla, S.; Kuroli, E.; Tóth, E.A.; Hidvégi, B.; Holló, P.; Medvecz, M. Primary Localized Cutaneous Amyloidosis in Central Europe: A Retrospective Monocentric Study on Epidemiology and Therapy. J Clin Med. 2023, 12. [Google Scholar] [CrossRef]
- Rousseau, M.A.; Valek, S.A.; Rashid, R.M. A Case Report of Generalised Non-pruritic Lichen Amyloidosis. Cureus. 2023, 15, 6–8. [Google Scholar] [CrossRef]
- Humeda, Y.; Beasley, J.; Calder, K. Clinical resolution of generalised lichen amyloidosis with dupilumab: A new alternative therapy. Dermatol Online J. 2020, 26, 0–4. [Google Scholar] [CrossRef]
- Oh, S.M.; Ahn, H.J.; Shin, M.K. Clinical Characteristics of Lichen Amyloidosis Associated with Atopic Dermatitis: A Single Center, Retrospective Study. Ann Dermatol. 2023, 35, 432–438. [Google Scholar] [CrossRef]
- Kecelj, B.; Kecelj Leskovec, N.; Žgavec, B. A case report and differential diagnosis of pruritic pretibial skin lesions. Acta dermatovenerologica Alpina, Pannonica, Adriat. 2020, 29, 157–159. [Google Scholar] [CrossRef]
- Frew, J.W.; Fallah, H. Lichen amyloidosis involving the scalp. Australas J Dermatol. 2017, 58, e260–e261. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Romero, P.L.; Ballestin-Carcavilla, C.; Lopez-Estebaranz, J.L.; Iglesias-Diez, L. Clinicopathologic and immunohistochemical studies on lichen amyloidosis and macular amyloidosis. Arch Dermatol. 1994, 130, 1559–1560. [Google Scholar] [CrossRef] [PubMed]
- Jambrosic, J.; From, L.; Hanna, W. Lichen amyloidosus. Ultrastructure and pathogenesis. Am J Dermatopathol. 1984, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Korbi, M.; Akkari, H.; Soua, Y.; et al. Lichen amyloidosis successfully treated with fractional ablative laser CO 2 : A new alternative therapeutic. J Cosmet Laser Ther. 2019, 21, 1–3. [Google Scholar] [CrossRef]
- Ahramiyanpour, N.; Akbari, Z.; Sarasyabi, M.S.; Aflatoonian, M.; Saki, N.; Shafie'ei, M. The therapeutic role of lasers in primary localised cutaneous amyloidosis: a systematic review. Lasers Med Sci. 2022, 37, 799–813. [Google Scholar] [CrossRef]
- Panchaprateep, R.; Tusgate, S.; Munavalli, G.S.; Noppakun, N. Fractional 1,550nm Ytterbium/Erbium fiber laser in the treatment of lichen amyloidosis: Clinical and histological study. Lasers Surg Med. 2015, 47, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Atacan, D.; Ergin, C.; Çelik, G.; Gönül, M.; Adabağ, A. Oral isotretinoin: A new treatment alternative for generalised lichen amyloidosis. Australas J Dermatol. 2016, 57, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.S.; Oh, E.H.; Kim, J.E.; Ro, Y.S. Alitretinoin treatment of lichen amyloidosis. Dermatol Ther. 2017, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vasani, R. Response to oral acitretin in lichen amyloidosis. Indian Dermatol Online J. 2014, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Dilling, A.; Ghoreschi, F.C.; Nast, A.; Ghoreschi, K.; Meier, K. Upadacitinib for treatment-resistant Lichen amyloidosis. J Eur Acad Dermatology Venereol. 2023, 37, e633–e635. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Xiao, Y.; Li, M.; Li, W. Refractory cutaneous lichen amyloidosis coexisting with atopic dermatitis responds to the Janus Kinase inhibitor baricitinib. Dermatol Ther. 2022, 35, 2–3. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Zhu, W.-J.; Mou, Y.; Xu, M.; Xia, J.-X. Dupilumab for treatment of severe atopic dermatitis accompanied by lichenoid amyloidosis in adults: Two case reports. World J Clin Cases. 2023, 11, 2301–2307. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, B.-Q.; Zhang, J.-F.; Shi, L.-P.; Zhang, G.-Q. Successful treatment of lichen amyloidosis coexisting with atopic dermatitis by dupilumab: Four case reports. World J Clin Cases. 2023, 11, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Saussine, A.; Calugareanu, A.; et al. Dramatic response to dupilumab in papular amyloidosis. J Eur Acad Dermatology Venereol. 2022, 36, e1071–e1072. [Google Scholar] [CrossRef] [PubMed]
- Seegräber M, Srour J, Walter A, Knop M, Wollenberg A. Dupilumab for treatment of atopic dermatitis. Expert Rev Clin Pharmacol. 2018, 11, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Cai D, Li Y, Zhou C, Jiang Y, Jiao J, Wu L. Comparative proteomics analysis of primary cutaneous amyloidosis. Exp Ther Med. 2017, 14, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Dousset L, Seneschal J, Boniface K, et al. A th2 cytokine interleukin-31 signature in a case of sporadic lichen amyloidosis. Acta Derm Venereol. 2015, 95, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Tey HL, Cao T, Nattkemper LA, Tan VWD, Pramono ZAD, Yosipovitch G. Pathophysiology of pruritus in primary localised cutaneous amyloidosis. Br J Dermatol. 2016, 174, 1345–1350. [Google Scholar] [CrossRef]
- Arita K, South AP, Hans-Filho G, et al. Oncostatin M Receptor-β Mutations Underlie Familial Primary Localized Cutaneous Amyloidosis. Am J Hum Genet. 2008, 82, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu H, Qiu B, Yang H, et al. AHNAK, regulated by the OSM/OSMR signaling, involved in the development of primary localised cutaneous amyloidosis. J Dermatol Sci. 2023, 110, 53–60. [Google Scholar] [CrossRef] [PubMed]
- He A, Zampella JG, Kwatra SG. Interleukin-31 receptor and pruritus associated with primary localised cutaneous amyloidosis. Br J Dermatol. 2016, 175, 433. [Google Scholar] [CrossRef]
- Tanaka A, Arita K, Lai-Cheong JE, Palisson F, Hide M, McGrath JA. New insight into mechanisms of pruritus from molecular studies on familial primary localised cutaneous amyloidosis. Br J Dermatol. 2009, 161, 1217–1224. [Google Scholar] [CrossRef]
- Behr FD, Levine N, Bangert J. Lichen amyloidosis associated with atopic dermatitis: Clinical resolution with cyclosporine. Arch Dermatol. 2001, 137, 553–555. [Google Scholar]
- Lee DD, Huang CK, Ko PC, Chang YT, Sun WZ, Oyang YJ. Association of primary cutaneous amyloidosis with atopic dermatitis: A nationwide population-based study in Taiwan. Br J Dermatol. 2011, 164, 148–153. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).