You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
Numerical Modeling of the Venous Outflow from the Cranial Cavity in the Supine Body Position
Altmetrics
Downloads
84
Views
22
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
Hemodynamic relevance of differently located stenoses of the internal jugular veins remains undetermined. It particularly concerns nozzle-like strictures in the upper parts of these veins and stenotic jugular valves located at the end of this vein. This study was aimed at understanding flow disturbances caused by such stenoses. Computational fluid dynamics software, Flowsquare+, was used. We constructed 3-dimensional models of venous outflow, comprising two alternative routes: tube representing the internal jugular vein and irregular network representing the vertebral veins. At the beginning of the tube representing the internal jugular vein, differently-shaped and sized short strictures, representing nozzle-like strictures were built in. At the end of this tube, differently-shaped membranes, representing the jugular valve, were built in. With the use of computational fluid dynamics modeling, we studied how these two obstacles influenced the outflow. We found that the most relevant outflow disturbances were evoked by nozzle-like strictures in the upper part of the internal jugular vein that were either small, long or asymmetrically positioned. Very tight stenotic valves and septum-like malformed valve were equally hemodynamically relevant. These findings suggest that both upper and lower strictures of the internal jugular vein can be of clinical significance.
Keywords:
Subject: Biology and Life Sciences - Biophysics
1. Introduction
The internal jugular veins (IJVs), the paired veins located in the neck, constitute the primary blood outflow route from the brain. Still, actually outflow routes from the head, particularly from the cranial cavity, depend on body posture. While in the horizontal (supine, prone and lateral decubitus) body positions at least one of the IJV is open, enabling an unrestricted venous outflow, in the upright (sitting and standing) body positions the IJVs collapse due to the gravitational effects (negative transmural pressure). Consequently, a substantial part of the outflow is shifted towards alternative routes, primarily to the vertebral venous plexus and vertebral veins, which are situated alongside the cervical part of the vertebral column [1,2,3,4,5,6,7]. This outflow pathway is composed of the vertebral veins, the epidural venous plexus and other adjacent tiny veins. Since the veins in the vertebral outflow route are of small dimeter, even if their total cross-sectional area is comparable to that of the IJVs, flow resistance in this pathway is much lower in comparison with the non-collapsed IJVs. Therefore, a majority of cerebral venous outflow utilizes the jugular route. This situation changes when the head is elevated (in the standing or sitting individual). In this body position about 80-90 % of blood flows out through the vertebral veins and adjacent venous plexuses, contrasting with approximately 30% or even less in the horizontal position. Of note, venous outflow in the upright body position is facilitated by the gravity. Therefore, a high flow resistance in the vertebral route does not substantially affect the cerebral venous outflow. On the other hand, at least theoretically, pathological strictures in the IJVs should compromise cerebral venous outflow in the horizontal body positions, even if the vertebral outflow route is not narrowed.
Currently, it remains controversial if such a compromised cerebral venous outflow is of any clinical relevance, considering the abundance of collateral venous channels in the neck. Still, several studies claimed for impaired venous outflow as a cause of neurodegenerative and neuroinflammatory diseases [8,9,10,11,12,13,14,15]. Therefore, understanding the physical background of the compromised venous outflow from the brain is important. A majority of the strictures in the IJV are either found in the upper part of this vein, at the level of the jugular foramen, or in the lower part, where stenosis is usually caused by abnormal morphology of the jugular valve (the only valve of this vein, located just above junction of the IJV with the brachiocephalic vein) [16,17,18]. There is an ongoing debate which of these strictures are more relevant. Since the investigations on blood vessel flow in living subjects are difficult to perform, are invasive and often not possible to conduct due to bioethical aspects, the computational flow modeling (CFM) can provide a surrogate insight into the biomechanics of blood flow. Our previous research, utilizing computational flow simulations, suggested that strictures located at the level of the jugular foramen are probably more hemodynamically relevant [19]. It is in line with the results of clinical studies that evaluated flow and morphology of the IJVs with the use of magnetic resonance imaging [20,21,22]. Yet, in this in silico study we used 3D models of the IJVs, still without alternative outflow pathways. In the current paper, we used more reliable models comprising both jugular and vertebral outflow pathways. Models comprising both jugular and vertebral outflow pathways have already been validated and described in another paper published by our team [23]. Here, we present the results of next step of this research, which evaluated flow disturbances caused by differently shaped, positioned and sized strictures in the upper and lower parts of the IJV.
2. Materials and Methods
For the purpose of this study, CFM software, the Flowsquare+ (Nora Scientific, Japan), was used. All the computations were executed in the Intel BOXNUC8i7BEH2 mini PC (Intel, Santa Clara, CF, USA) equipped with the Intel® CoreTM i7 processor and the Intel® Iris® Plus Graphics 655 graphic card.
The 3-dimensional models were 180 mm long along the axis of flow, and 60 mm and 30 mm along other axes. The mesh size of the models in any direction was 0.25 mm and contained about 7 million of active cells. It comprised the initial inflow field representing the cerebral circulation, which branched into two alternative outflow routes: the tube representing the internal jugular vein and the irregular network representing the vertebral veins and the epidural venous plexus that in humans form the vertebral outflow route. Then these two alternative pathways again joined together to form the outflow field. To facilitate the simulations, we modelled only one side of the cerebral venous outflow: one internal jugular vein and one side of the vertebral venous pathway. The tubular-shaped model of the internal jugular vein was 125 mm long and at the outflow had the cross-sectional diameters of 10 mm and 12 mm (cross-sectional area of 0.94 cm2). Model of the vertebral venous pathway had a similar length as the model of the internal jugular vein, and had at the outflow cross-sectional area of about 0.75 cm2, still main part of the vertebral pathway was divided into many thin irregular parallel channels. During simulations ten probes measuring flow parameters were positioned inside the models: two probes in the inflow field, two probes in the middle part of the IJV, three probes in the terminal part of the IJV, just above the jugular valve, and three probes in the terminal part of the vertebral pathway (Figure 1). Details of validation of this model were presented in our previous paper [23].
The flow was simulated during the 2500 consecutive steps, each of them lasting 0.28 ms, which in total was equivalent to 0.7 s of the real-time flow. Using our computer set, it took around 24 hours of computational time for each case. Parameters of the fluid were set up to be similar to those observed during blood flow in the IJV. Velocity component along the long axis of the model for the initial field, which area was 2.08 cm2, was set up at 8 cm/s, which enabled the inflow of fluid into the model without strictures in the jugular route at the level of 400 ml/min, which was equal to aproximatelly 50% of the physiological cerebral blood flow [23]. Initial pressure was 750 Pa (equivalent to 7.65 cm H2O; a physiological pressure in the IJV in the supine body position). Dynamic viscosity of the fluid: 2.78 x 10-3 kg/m/s (which is the dynamic viscosity of whole blood at 37o C). Density of the modelled fluid was constant, it was 1055 kg/m3, which is the density of blood at room temperature [24,25].
We augmented the model of cerebral venous outflow in the supine body position, which has been validated in our previous study [23], with two strictures (Figure 2). The first one was positioned at the beginning of the IJV, mimicking the nozzle-like stenosis evoked by a narrow jugular foramen, an enlarged transverse process of the atlas, or an elongated styloid process of the temporal bone. Such pathological strictures can be seen in some patients, particularly those ptresenting with neurological symptoms [12]. The second strictured was positioned at the end of the IJV, mimicking the stenotic jugular valve. Such aberrant jugular valves are also found in some patients [14].
We constructed models with following types of upper IJV strictures:
- no stenosis in the upper part of the IJV (cross-sectional area 0.94 cm2);
- minor elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.42 cm2);
- minor irregularly-shaped stenosis in the upper part of the IJV (cross-sectional area 0.60 cm2);
- major elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.24 cm2);
- major elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.24 cm2) with opening positioned eccentrically;
- major elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.24 cm2) with a longer narrowed segment (12 mm instead of 2,5 mm);
- very narrow elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.07 cm2);
- very narrow elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.07 cm2) with opening positioned eccentrically;
- major stenosis in the upper part of the IJV comprising 2 eccentrically positioned openings (cross-sectional area 0.48 cm2);
- major stenosis in the upper part of the IJV comprising eccentrically positioned short flattening (cross-sectional area 0.48 cm2);
We also constructed different types of valves in the lower part of the IJV:
- no valve;
- bicuspid valve, distance between leaflets: 4.3 mm (opening located centrally, 4.3 mm x 8..5 mm);
- bicuspid valve, distance between leaflets: 1.5 mm (opening located centrally, 1.5 mm x 8..5 mm);
- septum instead of a valve, almost completely obstructing the IJV (opening located next to the wall, 1.5 mm x 8.5 mm).
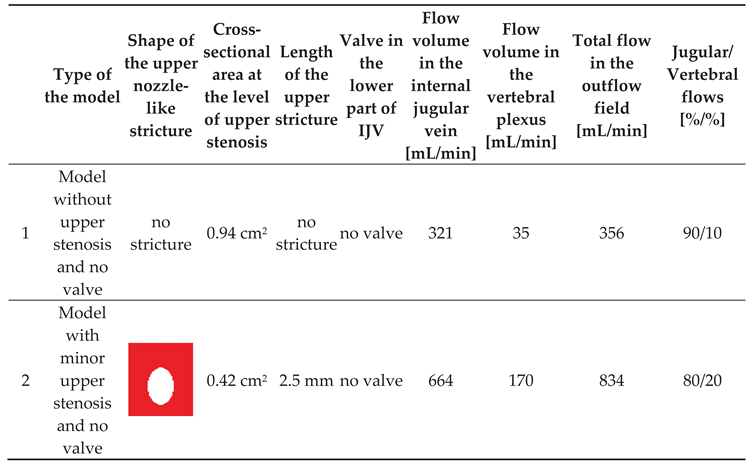
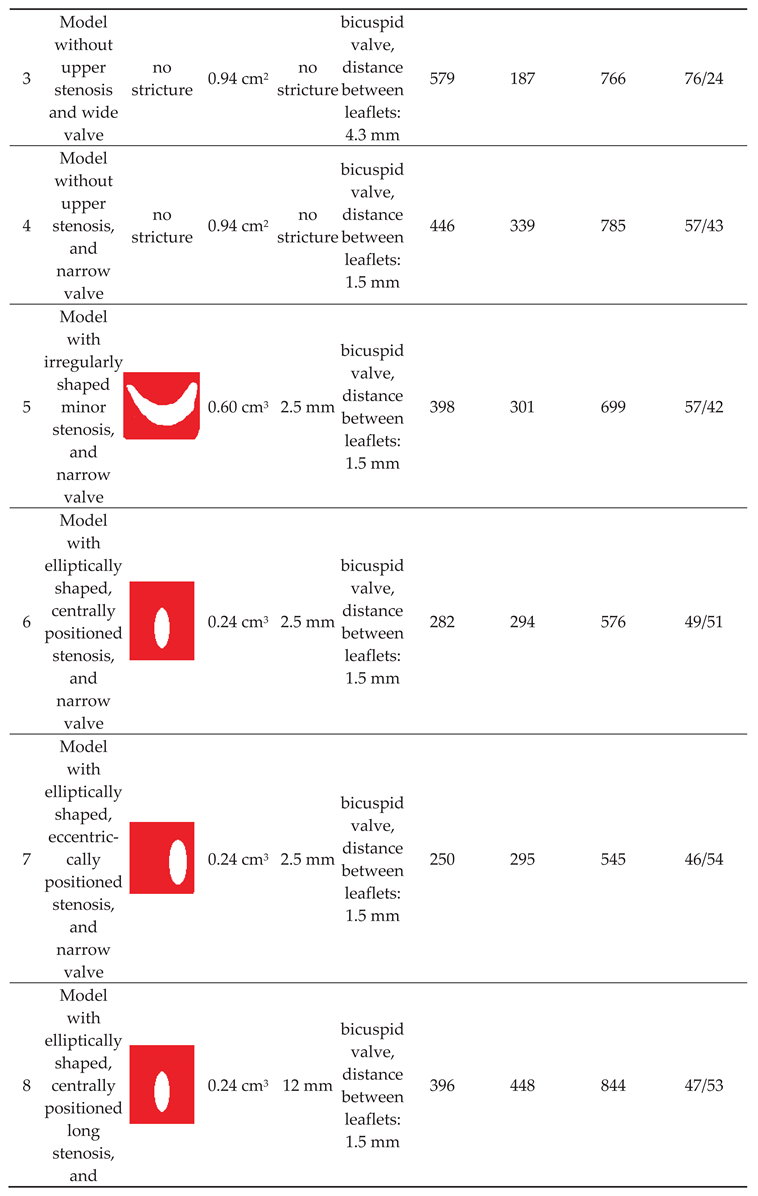
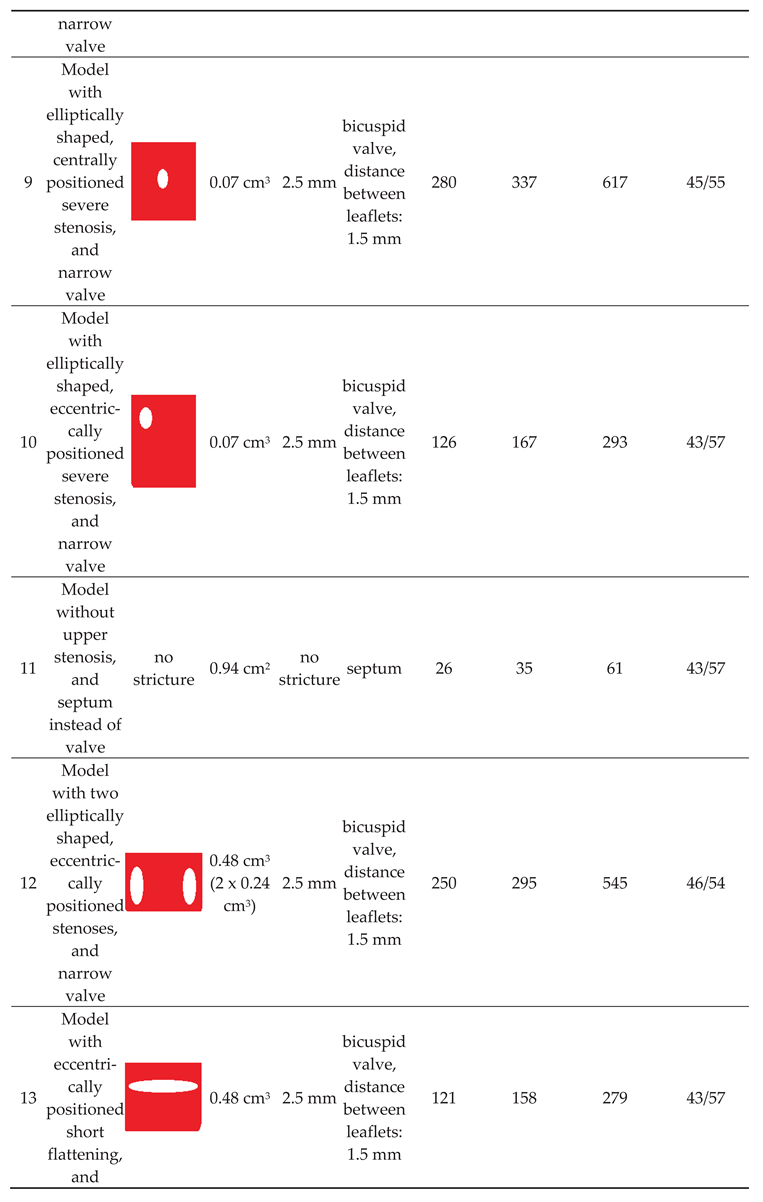
Of note, valve leaflets and the septum, unlike actual intraluminal structures located in the IJVs, in our study were immobile, since the CFD software used did not allow for the building a mobile and elastic material. In total, we examined 10 models exhibiting different types and shapes of the stricures; morphologies of these strictures are presented in Table 1. The total flow in the jugular and vertebral channels were measurted with the above-described probes. The model with collapsed IJV, which has been validated in our previous paper, was used as the reference (Model 11 in Table 1).
3. Results
Our previous flow simulations in the model, in which the IJV did no exhibit any strictures, revealed that 84% of the fluid flew through the IJV. In the current study, with a longer time of simulated flow, we got a similar result (90%). In our 3-D models with two alternative outflow pathways, a shift from the jugular to vertebral pathway was interpreted as the sign of hemodynamically relevant flow resistance evoked by stenotic lesions in the IJV (nozzle-like upper stricture or stenotic abnormal valve located downstream). We also interpreted the degree of such a shift as the level of hemodynamic relevance of the stenosis. Quantitative results of the simulations are presented in Table 1.
We found that a significant shift from the jugular to the vertebral pathway can be evoked by both upper and lower lesions (Table 1). Regarding the nozzle-like strictures located upstream, flow resistance resulting in shifting the flow towards the vertebral outflow route seemed to be more significant if such a stenosis was:
- narrower (models 6 and 9);
- longer (model 8);
- eccentrically positioned (models 7, 10 and 13);
- irregularly shaped (models 5 and 12).
Regarding stenoses of the jugular valve located downstream, the shift was more pronounced if the distance between stiff valve leaftets was smaller (model 3 vs. model 4). Abnormally shaped valve forming a septum (model 11) resulted in the most signficant flow disturbances.
4. Discussion
Usually, albeit the IJVs exhibit slightly irregular shape and there is asymmetry regarding their size (typically the right IJV is wider than the left one), there are no strictures in these veins that could significantly alter the cerebral venous outflow. Of note, the IJVs represent major outflow route from the brain in the horizontal body position, also during the sleep. Recently, is has been hypothesized that an impaired cerebral venous outflow can affect function of the glymphatic system, which is responsible for cleansing the brain from noxious metabolites, including pathological proteins [26,27]. Importantly, glymphatic system is mainly active during the sleep, therefore an unrestricted venous outflow from the brain during the sleep is potentially indispensable. Although, for the time being, direct observations proving that there is indeed a causative association between venous abnormalities and neurological pathologies are lacking, it is known that malformed IJVs are more often seen in neurological patients in comparison with healthy controls [13,15].
Interestingly, a decade ago, many multiple sclerosis patients were managed using endovascular angioplasty of their narrowed IJVs. Unfortunately, although early results of such a treatment were encouraging, positive outcomes in a long run were seen only in some subgroups of these patients [28,29,30]. Therefore, this treatment modality is no longer a standard one. However, majority of aforementioned endovascular procedures comprised balloon angioplasty of pathological jugular valves. External compressions at the level of the jugular foramen were not addressed, while it is known that such strictures are quite common in these patients [13,20,21]. This may account for unfavorable results of clinical trials in multiple sclerosis patients. In addition, stenotic lesions of the IJVs are found in other neurological pathologies, including Alzheimer’s and Parkinson’s diseases, Ménière disease and lateral amyotrophic sclerosis. Thus, addressing these venous abnormalities potentially may have a prophylactic or therapeutic potential.
Yet, hemodynamic relevance of different types of strictures in the IJV are difficult to assess in living subject. Firstly, all investigations aimed at such an evaluation are more or less invasive and not free from severe side effects. Therefore, clinical investigations are very difficult to conduct, considering bioethical issues. Secondly, proper interpretation of these investigations (like catheter venography, Doppler ultrasonography or magnetic resonance imaging) is not obvious [31]. Therefore, in silico flow modeling can provide a surrogate evaluation of hemodynamic significance of particular types of these strictures.
In our previous paper, based on the results of computational flow simulations using the same CFD package, we suggested that strictures located at the level of the jugular foramen are probably more clinically relevant than pathological jugular valves [19]. Yet, in this study we evaluated the flow only in models of the IJV, without alternative outflow through the vertebral pathway. Also, flow disturbances evoked by stenoses were evaluated qualitatively, by an assessment of flow characteristic. In the current study, we evaluated the flow quantitatively and with the alternative outflow route, thus more similarly to real patients. In contrast to the former study, in the present one we found that both types of strictures, located upstream and downstream, can significantly compromise the outflow. Importantly, since severe flow impairments were caused by short nozzle-like stenoses, especially those located eccentrically, probably flow separation and flow reversal were the main source of an increased flow resistance and the shift of outflow towards the vertebral pathway (Figure 3 and Figure 4). But stenotic jugular valves, especially membrane-shaped (Figure 5 and Figure 6), can equally evoke significant flow impairments.
Currently, the significance of irregular flows in blood vessels in medical literature is not adequately understood. Doctors acknowledge that in most of blood vessels energy losses resulting in drop of pressure come from friction. But in some blood vessels frictional energy losses are accompanied by other types of losses, in physics commonly referred to as minor losses [32,33]. These energy losses primarily result from flow separation that typically develops when a blood vessel bends or suddenly expands. Except for aneurysms (pathologically enlarged arteries), the significance of flow separation and associated minor losses has been largely ignored by medical research. But flow abnormalities resulting in flow separation can severely impair blood flow, and IJVs are one of such blood vessels where these phenomena could have clinical consequences [34].
We acknowledge that there are limitations to our study. Firstly, to facilitate simulations, we built models comprising only one side of the cerebral outflow. Models with two IJVs could provide further insight into this problem, especially if only one IJV is affected. Secondly, valve leaflets in our models were stiff and immobile. Actually, they exhibit different levels of elasticity and movability. In our another in silico study, we demonstrated that there is a dynamic interplay between stenosis located upstream and the valve located downstream, and that a relevant stenosis changes the valve geometry to the unfavorable one [35]. Thirdly, flow volume measurements using the “probes” were not very precise. Yet, even such an approximate assessment could provide a useful insight into the problem studied. Besides, flow modeling using our CFD package was probably prone to significant errors developing when the flow resistance in the fields studied were high; thus we got very different total flow volumes in particular models (see: Table 1).
Other limitations of this study are associated with the use of simplified models instead of real morphology-based ones. Besides, an interplay between the veins and surrounding tissues should also be modeled. It should also be mentioned that in this study the fluid was considered Newtonian. Actually, blood is a non-Newtonian fluid. Since blood is shear-thinning fluid, slowing down its flow is associated with a higher flow resistance than that of a Newtonian fluid, which probably resulted in non-adequate flow modeling. However, the level of errors associated with non-Newtonian characteristics of blood is difficult to estimate. Probably, at least regarding the problem studied in this paper, it is not much relevant.
5. Conclusions
Numerical flow simulations of the flow in the IJVs suggest that both upper and lower strictures of these veins can be of clinical significance. Therefore, clinical studies on these veins, especially in the context of neurological pathologies, should comprise morphological and functional assessment of the entire course of the IJVs, from their origin in the cranial cavity to their junction with the brachiocephalic veins in the neck. It particularly regards possible stenosis at the level of the jugular foramen, as well as the jugular valve and other intraluminal structures located in the lower part of the IJV.
Author Contributions
Conceptualization, M.S; methodology, M.S. and J.C..; software, M.S.; validation, M.S., and P.L.; formal analysis, M.S. and A.K.; investigation, J.C., U.K.; resources, M.S.; data curation, M.S., A.K., P.L; writing—original draft preparation, M.S.; writing—review and editing, P.L.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by research grant of the University of Opole.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cirovic, S.; Walsh, C.; Fraser, W.D.; Gulino, A. The effect of posture and positive pressure breathing on the hemodynamics of the internal jugular vein. Aviat Space Environ Med 2003, 74, 125–131. [Google Scholar]
- Ciuti, G.; Righi, D.; Forzoni, L.; Fabbri, A.; Pignone, A.M. differences between internal jugular vein and vertebral vein flow examined in real time with the use of multigate ultrasound color doppler. Am J Neuroradiol 2013, 34, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Doepp, F.; Schreiber, S.J.; von Műnster, T.; et al. How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns. Neuroradiol 2004, 46, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B. Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain Res Rev 2004, 46, 243–260. [Google Scholar] [CrossRef]
- Schreiber, S.J.; Lürtzing, F.; Götze, R.; Doepp, F.; Klingbiel, R.; Valdueza, J.M. Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound. J Appl Physiol 2003, 94, 1802–1805. [Google Scholar] [CrossRef]
- Simka, M.; Czaja, J.; Kowalczyk, D. Collapsibility of the internal jugular veins in the lateral decubitus body position: a potential role of the cerebral venous outflow against neurodegeneration. Med Hypothes 2019, 133, 109397. [Google Scholar] [CrossRef]
- Zaniewski, M.; Simka, M. Biophysics of venous return from the brain from the perspective of the pathophysiology of chronic cerebrospinal venous insufficiency. Rev Recent Clin Trials 2012, 7, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Alpini, D.C.; Bavera, P.M.; Hahn, A.; et al. Chronic cerebrospinal venous insufficiency (CCSVI) in Meniere disease: case or cause? ScienceMED 2013, 4, 9–15. [Google Scholar]
- Beggs, C.; Chung, C.P.; Bergsland, N.; et al. Jugular venous reflux and brain parenchyma volumes in elderly patients with mild cognitive impairment and Alzheimer's disease. BMC Neurol 2013, 13, 157. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chang, F.C.; Chao, A.C.; et al. Internal jugular venous abnormalities in transient monocular blindness. BMC Neurol 2013, 13, 94. [Google Scholar] [CrossRef]
- Chung, C.P.; Beggs, C.; Wang, P.N.; et al. Jugular venous reflux and white matter abnormalities in Alzheimer's disease: a pilot study. J Alzheimer Dis 2014, 39, 601–609. [Google Scholar] [CrossRef]
- Ding, J.Y.; Zhou, D.; Pan, L.Q.; et al. Cervical spondylotic internal jugular venous compression syndrome. CNS Neurosci Ther 2020, 26, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.K.; Utriainen, D.T.; Daugherty, A.M.; et al. Jugular venous flow abnormalities in multiple sclerosis patients compared to normal controls. J Neuroimaging 2015, 25, 600–607. [Google Scholar] [CrossRef]
- Simka, M.; Latacz, P.; Ludyga, T.; Kazibudzki, M.; Świerad, M.; Janas, P.; Piegza, J. Prevalence of extracranial venous abnormalities: results from a sample of 586 multiple sclerosis patients. Funct Neurol 2011, 26, 197–203. [Google Scholar] [PubMed]
- Zivadinov, R.; Marr, K.; Cutter, G.; Ramanathan, M.; Benedict, R.H.; Kennedy, C.; Elfadil, M.; Yeh, A.E.; Reuther, J.; Brooks, C.; Hunt, K.; Andrews. M.; Carl, E.; Dwyer, M.G.; Hojnacki, D.; Weinstock-Guttman, B. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011, 77, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Hubbard, D.; Siddiqui, A.H.; Dake, M.D.; Sclafani, S.J.; Al-Omari, M.; Eisele, C.G.; Haskal, Z.J.; Ludyga, T.; Miloševič, Z.V.; Sievert, H,; Stehling, M. K;, Zapf, S.; Zorc, M. Catheter venography for the assessment of internal jugular veins and azygous vein: position statement by expert panel of the International Society for Neurovascular Disease. Vasa 2013, 42, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Morovic, P.; Menegatti, E.; et al. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound - recommendations for a protocol. Int Angiol 2011, 30, 571–597. [Google Scholar]
- Zivadinov, R.; Bastianello, S.; Dake, M.D.; et al. Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. J Vasc Interv Radiol 2014, 25, 1785–94. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Latacz, P. Numerical modeling of blood flow in the internal jugular vein with the use of computational fluid mechanics software. Phlebology 2021, 36, 541–548. [Google Scholar] [CrossRef]
- Feng, W.; Utriainen, D.; Trifan. G.; et al. Characteristics of flow through the internal jugular veins at cervical C2/C3 and C5/C6 levels for multiple sclerosis patients using MR phase contrast imaging. Neurol Res 2012, 34, 802–809. [Google Scholar] [CrossRef]
- Feng, W.; Utriainen, D.; Trifan, G.; Elias, S.; Sethi, S.; Hewett, J.; Haacke, E.M. Characteristics of flow through the internal jugular veins at cervical C2/C3 and C5/c6 levels for multiple sclerosis patients using MR phase contrast imaging. Neurol Res 2012, 34, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Feng, W.; Utriainen, D.; Trifan, G.; Wu, Z.; Latif, Z.; Katkuri, Y.; Hewett, J.; Hubbard, D. Patients with multiple sclerosis with structural venous abnormalities on MR Imaging exhibit an abnormal flow distribution of the internal jugular veins. J Vas Inter Radiol 2012, 23, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Skuła, M.; Bielaczyc, G. Validation of models of venous outflow from the cranial cavity in the supine and upright body positions. Phlebol Rev 2022, 30, 8–12. [Google Scholar] [CrossRef]
- Phillips, R.A.; Van Slyke, D.D.; Hamilton, P.B.; et al. Measurement of specific gravities of whole blood and plasma by standard copper sulfate solutions. J Biol Chem 1950, 183, 305–330. [Google Scholar] [CrossRef]
- Trudnowski, R.J.; Rico, R.C. Specific gravity of blood and plasma at 4 and 37 °C. Clin Chem 1974, 20, 20–615. [Google Scholar] [CrossRef]
- Zivadinov, R.; Chung, C.P. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med 2013, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Simka, M. Activation of the glymphatic system during sleep – is the cerebral venous outflow a missing piece of the puzzle? Phlebol Rev 2019, 7, 1–2. [Google Scholar] [CrossRef]
- Zamboni, P.; Tesio, L.; Galimberti. S.; et al. Efficacy and safety of extracranial vein angioplasty in multiple sclerosis. JAMA Neurol 2018, 75, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Galeotti, R.; Salvi, F.; Giaquinta, A.; Setacci, C.; Alborino, S.; Guzzardi, G.; Sclafani, S.J.; Maietti, E.; Veroux, P. Brave Dreams Research Group. Effects of Venous Angioplasty on Cerebral Lesions in Multiple Sclerosis: Expanded Analysis of the Brave Dreams Double-Blind, Sham-Controlled Randomized Trial. J Endovasc Ther 2020, 27, 1526602819890110. [Google Scholar] [CrossRef]
- Simka, M. An overview of randomized controlled trials on endovascular treatment for chronic cerebrospinal venous insufficiency in multiple sclerosis patients. Phlebologie 2021, 50, 76–80. [Google Scholar] [CrossRef]
- Simka, M. Chronic cerebrospinal venous insufficiency: current perspectives. J Vasc Diagn 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Rend, R.R.; Sparrow, E.M.; Bettenhausen, D.W.; et al. Parasitic pressure losses in diffusers and in their downstream piping systems for fluid flow and heat transfer. Int J Heat Mass Transfer 2013, 61, 56–61. [Google Scholar] [CrossRef]
- Li, L.; Walker, A.M.; Rival, D.E. The characterization of a non-Newtonian blood analog in natural- and shear-layer-induced transitional flow. Biorheology 2014, 51, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Latacz, P.; Redelbach, W. Blood flow in the internal jugular veins during the spaceflight – Is it actually bidirectional? Life Sci Space Res 2020, 25, 103–106. [Google Scholar] [CrossRef]
- Rashid, A.; Iqrar, S.A.; Rashid, A.; Simka, M. Results of numerical modeling of blood flow in the internal jugular vein exhibiting different types of strictures. Diagnostics 2022, 12. [Google Scholar] [CrossRef]
Figure 1.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes – in these areas flow characteristics was measured. Flow is from the left to the right. In this model there are no stenoses in the internal jugular vein, consequently most of the outflow utilizes the jugular pathway.
Figure 1.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes – in these areas flow characteristics was measured. Flow is from the left to the right. In this model there are no stenoses in the internal jugular vein, consequently most of the outflow utilizes the jugular pathway.
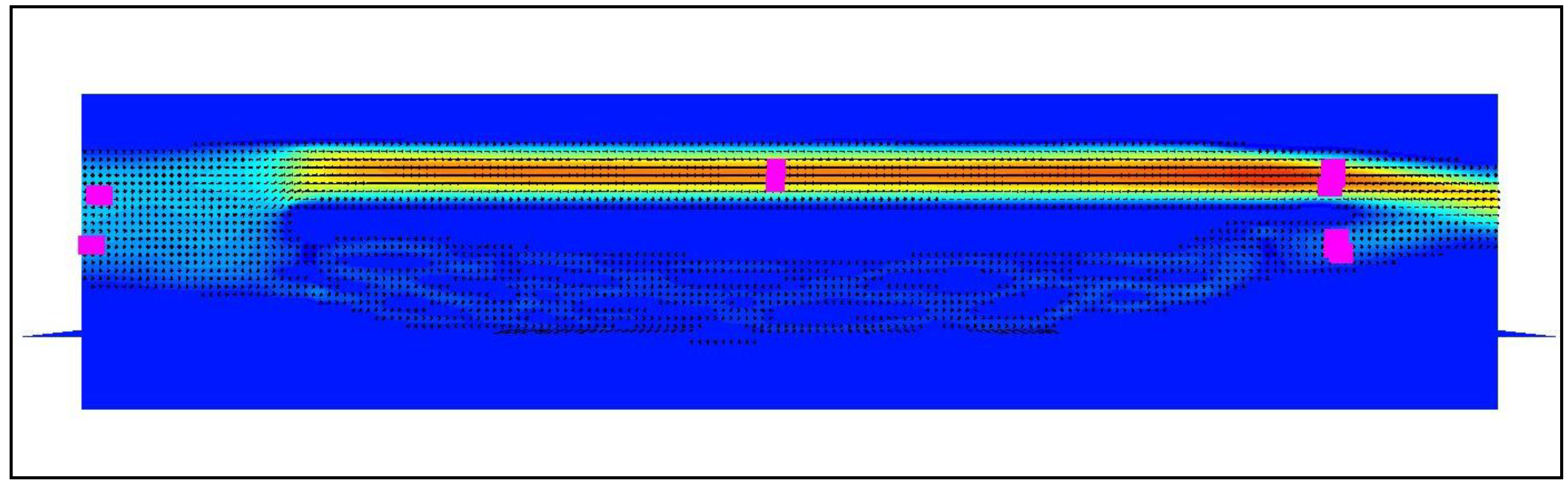
Figure 2.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes. Flow is from the left to the right. There are 2 strictures in the internal jugular vein: in the beginning (white arrow) and at its end (black arrow). In the model with stenoses part of the flow is shifted towards the vertebral pathway.
Figure 2.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes. Flow is from the left to the right. There are 2 strictures in the internal jugular vein: in the beginning (white arrow) and at its end (black arrow). In the model with stenoses part of the flow is shifted towards the vertebral pathway.

Figure 3.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and stenotic valve downstream.
Figure 3.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and stenotic valve downstream.
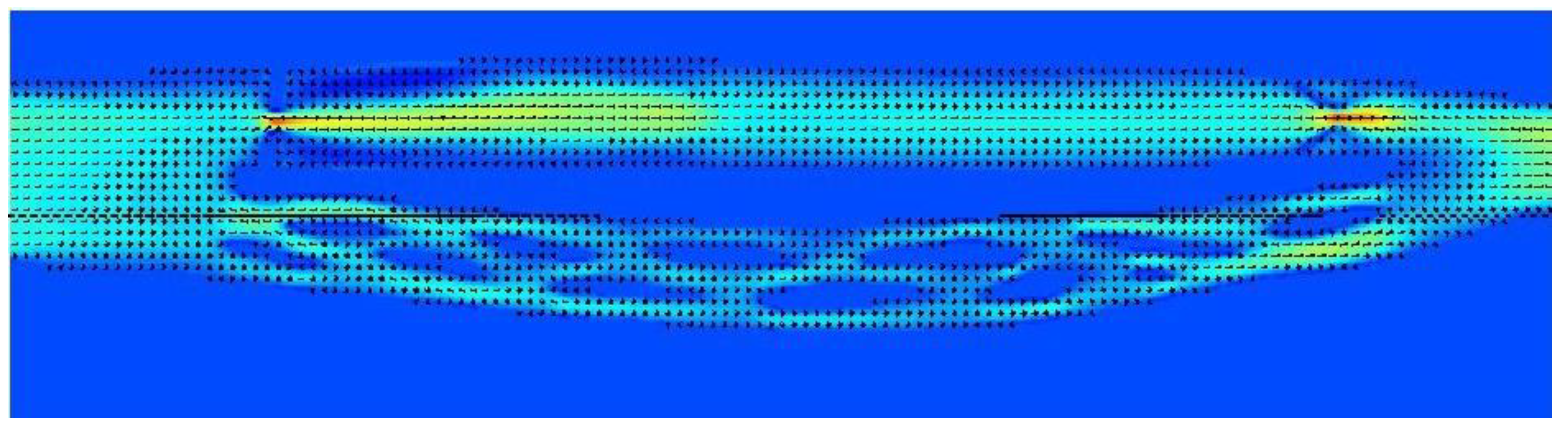
Figure 4.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and no valve downstream.
Figure 4.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and no valve downstream.
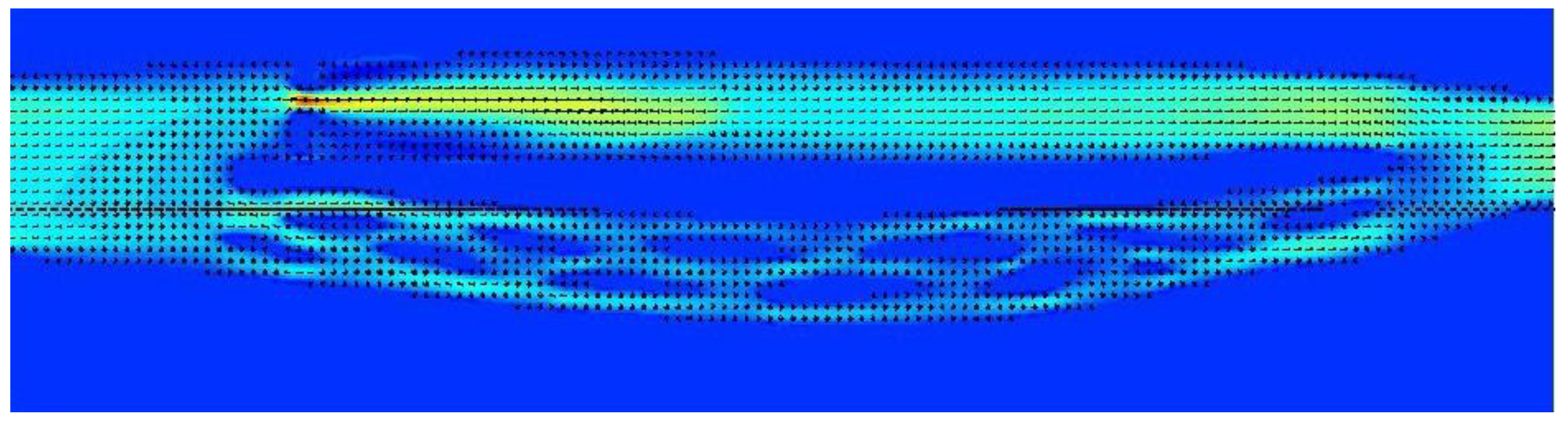
Figure 5.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted and there is a stenotic valve downstream.
Figure 5.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted and there is a stenotic valve downstream.
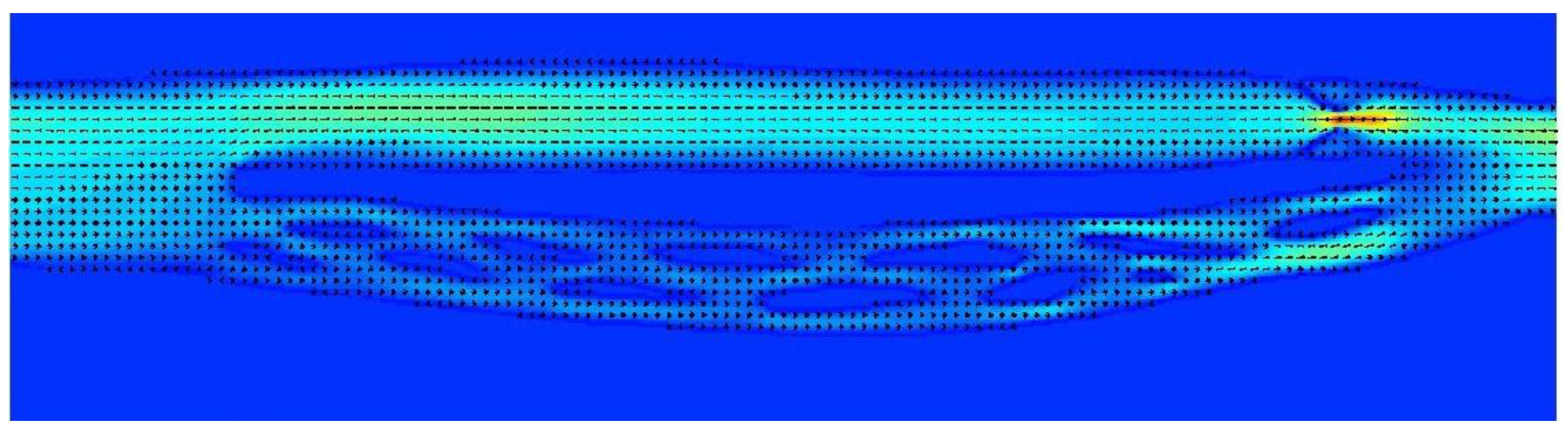
Figure 6.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted, while downstream there is a septum nearly completely obstructing the outflow.
Figure 6.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted, while downstream there is a septum nearly completely obstructing the outflow.
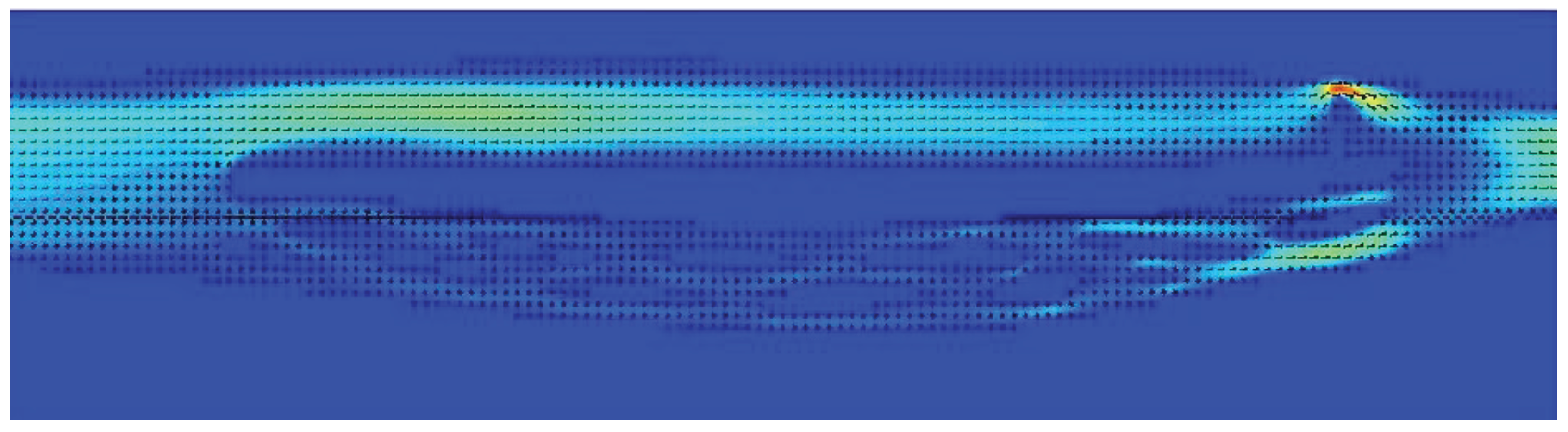
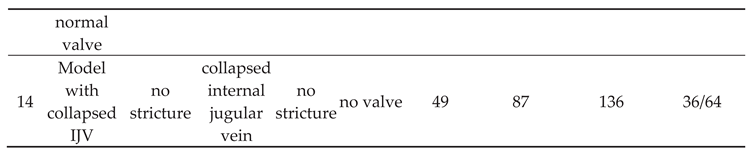
Table 1.
Characteristic of the strictures in the modelled internal jugular vein, flow volumes in the jugular and vertebral pathways.
Table 1.
Characteristic of the strictures in the modelled internal jugular vein, flow volumes in the jugular and vertebral pathways.
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
26 March 2024
Posted:
27 March 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
26 March 2024
Posted:
27 March 2024
You are already at the latest version
Alerts
Abstract
Hemodynamic relevance of differently located stenoses of the internal jugular veins remains undetermined. It particularly concerns nozzle-like strictures in the upper parts of these veins and stenotic jugular valves located at the end of this vein. This study was aimed at understanding flow disturbances caused by such stenoses. Computational fluid dynamics software, Flowsquare+, was used. We constructed 3-dimensional models of venous outflow, comprising two alternative routes: tube representing the internal jugular vein and irregular network representing the vertebral veins. At the beginning of the tube representing the internal jugular vein, differently-shaped and sized short strictures, representing nozzle-like strictures were built in. At the end of this tube, differently-shaped membranes, representing the jugular valve, were built in. With the use of computational fluid dynamics modeling, we studied how these two obstacles influenced the outflow. We found that the most relevant outflow disturbances were evoked by nozzle-like strictures in the upper part of the internal jugular vein that were either small, long or asymmetrically positioned. Very tight stenotic valves and septum-like malformed valve were equally hemodynamically relevant. These findings suggest that both upper and lower strictures of the internal jugular vein can be of clinical significance.
Keywords:
Subject: Biology and Life Sciences - Biophysics
1. Introduction
The internal jugular veins (IJVs), the paired veins located in the neck, constitute the primary blood outflow route from the brain. Still, actually outflow routes from the head, particularly from the cranial cavity, depend on body posture. While in the horizontal (supine, prone and lateral decubitus) body positions at least one of the IJV is open, enabling an unrestricted venous outflow, in the upright (sitting and standing) body positions the IJVs collapse due to the gravitational effects (negative transmural pressure). Consequently, a substantial part of the outflow is shifted towards alternative routes, primarily to the vertebral venous plexus and vertebral veins, which are situated alongside the cervical part of the vertebral column [1,2,3,4,5,6,7]. This outflow pathway is composed of the vertebral veins, the epidural venous plexus and other adjacent tiny veins. Since the veins in the vertebral outflow route are of small dimeter, even if their total cross-sectional area is comparable to that of the IJVs, flow resistance in this pathway is much lower in comparison with the non-collapsed IJVs. Therefore, a majority of cerebral venous outflow utilizes the jugular route. This situation changes when the head is elevated (in the standing or sitting individual). In this body position about 80-90 % of blood flows out through the vertebral veins and adjacent venous plexuses, contrasting with approximately 30% or even less in the horizontal position. Of note, venous outflow in the upright body position is facilitated by the gravity. Therefore, a high flow resistance in the vertebral route does not substantially affect the cerebral venous outflow. On the other hand, at least theoretically, pathological strictures in the IJVs should compromise cerebral venous outflow in the horizontal body positions, even if the vertebral outflow route is not narrowed.
Currently, it remains controversial if such a compromised cerebral venous outflow is of any clinical relevance, considering the abundance of collateral venous channels in the neck. Still, several studies claimed for impaired venous outflow as a cause of neurodegenerative and neuroinflammatory diseases [8,9,10,11,12,13,14,15]. Therefore, understanding the physical background of the compromised venous outflow from the brain is important. A majority of the strictures in the IJV are either found in the upper part of this vein, at the level of the jugular foramen, or in the lower part, where stenosis is usually caused by abnormal morphology of the jugular valve (the only valve of this vein, located just above junction of the IJV with the brachiocephalic vein) [16,17,18]. There is an ongoing debate which of these strictures are more relevant. Since the investigations on blood vessel flow in living subjects are difficult to perform, are invasive and often not possible to conduct due to bioethical aspects, the computational flow modeling (CFM) can provide a surrogate insight into the biomechanics of blood flow. Our previous research, utilizing computational flow simulations, suggested that strictures located at the level of the jugular foramen are probably more hemodynamically relevant [19]. It is in line with the results of clinical studies that evaluated flow and morphology of the IJVs with the use of magnetic resonance imaging [20,21,22]. Yet, in this in silico study we used 3D models of the IJVs, still without alternative outflow pathways. In the current paper, we used more reliable models comprising both jugular and vertebral outflow pathways. Models comprising both jugular and vertebral outflow pathways have already been validated and described in another paper published by our team [23]. Here, we present the results of next step of this research, which evaluated flow disturbances caused by differently shaped, positioned and sized strictures in the upper and lower parts of the IJV.
2. Materials and Methods
For the purpose of this study, CFM software, the Flowsquare+ (Nora Scientific, Japan), was used. All the computations were executed in the Intel BOXNUC8i7BEH2 mini PC (Intel, Santa Clara, CF, USA) equipped with the Intel® CoreTM i7 processor and the Intel® Iris® Plus Graphics 655 graphic card.
The 3-dimensional models were 180 mm long along the axis of flow, and 60 mm and 30 mm along other axes. The mesh size of the models in any direction was 0.25 mm and contained about 7 million of active cells. It comprised the initial inflow field representing the cerebral circulation, which branched into two alternative outflow routes: the tube representing the internal jugular vein and the irregular network representing the vertebral veins and the epidural venous plexus that in humans form the vertebral outflow route. Then these two alternative pathways again joined together to form the outflow field. To facilitate the simulations, we modelled only one side of the cerebral venous outflow: one internal jugular vein and one side of the vertebral venous pathway. The tubular-shaped model of the internal jugular vein was 125 mm long and at the outflow had the cross-sectional diameters of 10 mm and 12 mm (cross-sectional area of 0.94 cm2). Model of the vertebral venous pathway had a similar length as the model of the internal jugular vein, and had at the outflow cross-sectional area of about 0.75 cm2, still main part of the vertebral pathway was divided into many thin irregular parallel channels. During simulations ten probes measuring flow parameters were positioned inside the models: two probes in the inflow field, two probes in the middle part of the IJV, three probes in the terminal part of the IJV, just above the jugular valve, and three probes in the terminal part of the vertebral pathway (Figure 1). Details of validation of this model were presented in our previous paper [23].
The flow was simulated during the 2500 consecutive steps, each of them lasting 0.28 ms, which in total was equivalent to 0.7 s of the real-time flow. Using our computer set, it took around 24 hours of computational time for each case. Parameters of the fluid were set up to be similar to those observed during blood flow in the IJV. Velocity component along the long axis of the model for the initial field, which area was 2.08 cm2, was set up at 8 cm/s, which enabled the inflow of fluid into the model without strictures in the jugular route at the level of 400 ml/min, which was equal to aproximatelly 50% of the physiological cerebral blood flow [23]. Initial pressure was 750 Pa (equivalent to 7.65 cm H2O; a physiological pressure in the IJV in the supine body position). Dynamic viscosity of the fluid: 2.78 x 10-3 kg/m/s (which is the dynamic viscosity of whole blood at 37o C). Density of the modelled fluid was constant, it was 1055 kg/m3, which is the density of blood at room temperature [24,25].
We augmented the model of cerebral venous outflow in the supine body position, which has been validated in our previous study [23], with two strictures (Figure 2). The first one was positioned at the beginning of the IJV, mimicking the nozzle-like stenosis evoked by a narrow jugular foramen, an enlarged transverse process of the atlas, or an elongated styloid process of the temporal bone. Such pathological strictures can be seen in some patients, particularly those ptresenting with neurological symptoms [12]. The second strictured was positioned at the end of the IJV, mimicking the stenotic jugular valve. Such aberrant jugular valves are also found in some patients [14].
We constructed models with following types of upper IJV strictures:
- no stenosis in the upper part of the IJV (cross-sectional area 0.94 cm2);
- minor elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.42 cm2);
- minor irregularly-shaped stenosis in the upper part of the IJV (cross-sectional area 0.60 cm2);
- major elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.24 cm2);
- major elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.24 cm2) with opening positioned eccentrically;
- major elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.24 cm2) with a longer narrowed segment (12 mm instead of 2,5 mm);
- very narrow elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.07 cm2);
- very narrow elliptically-shaped stenosis in the upper part of the IJV (cross-sectional area 0.07 cm2) with opening positioned eccentrically;
- major stenosis in the upper part of the IJV comprising 2 eccentrically positioned openings (cross-sectional area 0.48 cm2);
- major stenosis in the upper part of the IJV comprising eccentrically positioned short flattening (cross-sectional area 0.48 cm2);
We also constructed different types of valves in the lower part of the IJV:
- no valve;
- bicuspid valve, distance between leaflets: 4.3 mm (opening located centrally, 4.3 mm x 8..5 mm);
- bicuspid valve, distance between leaflets: 1.5 mm (opening located centrally, 1.5 mm x 8..5 mm);
- septum instead of a valve, almost completely obstructing the IJV (opening located next to the wall, 1.5 mm x 8.5 mm).
Of note, valve leaflets and the septum, unlike actual intraluminal structures located in the IJVs, in our study were immobile, since the CFD software used did not allow for the building a mobile and elastic material. In total, we examined 10 models exhibiting different types and shapes of the stricures; morphologies of these strictures are presented in Table 1. The total flow in the jugular and vertebral channels were measurted with the above-described probes. The model with collapsed IJV, which has been validated in our previous paper, was used as the reference (Model 11 in Table 1).
3. Results
Our previous flow simulations in the model, in which the IJV did no exhibit any strictures, revealed that 84% of the fluid flew through the IJV. In the current study, with a longer time of simulated flow, we got a similar result (90%). In our 3-D models with two alternative outflow pathways, a shift from the jugular to vertebral pathway was interpreted as the sign of hemodynamically relevant flow resistance evoked by stenotic lesions in the IJV (nozzle-like upper stricture or stenotic abnormal valve located downstream). We also interpreted the degree of such a shift as the level of hemodynamic relevance of the stenosis. Quantitative results of the simulations are presented in Table 1.
We found that a significant shift from the jugular to the vertebral pathway can be evoked by both upper and lower lesions (Table 1). Regarding the nozzle-like strictures located upstream, flow resistance resulting in shifting the flow towards the vertebral outflow route seemed to be more significant if such a stenosis was:
- narrower (models 6 and 9);
- longer (model 8);
- eccentrically positioned (models 7, 10 and 13);
- irregularly shaped (models 5 and 12).
Regarding stenoses of the jugular valve located downstream, the shift was more pronounced if the distance between stiff valve leaftets was smaller (model 3 vs. model 4). Abnormally shaped valve forming a septum (model 11) resulted in the most signficant flow disturbances.
4. Discussion
Usually, albeit the IJVs exhibit slightly irregular shape and there is asymmetry regarding their size (typically the right IJV is wider than the left one), there are no strictures in these veins that could significantly alter the cerebral venous outflow. Of note, the IJVs represent major outflow route from the brain in the horizontal body position, also during the sleep. Recently, is has been hypothesized that an impaired cerebral venous outflow can affect function of the glymphatic system, which is responsible for cleansing the brain from noxious metabolites, including pathological proteins [26,27]. Importantly, glymphatic system is mainly active during the sleep, therefore an unrestricted venous outflow from the brain during the sleep is potentially indispensable. Although, for the time being, direct observations proving that there is indeed a causative association between venous abnormalities and neurological pathologies are lacking, it is known that malformed IJVs are more often seen in neurological patients in comparison with healthy controls [13,15].
Interestingly, a decade ago, many multiple sclerosis patients were managed using endovascular angioplasty of their narrowed IJVs. Unfortunately, although early results of such a treatment were encouraging, positive outcomes in a long run were seen only in some subgroups of these patients [28,29,30]. Therefore, this treatment modality is no longer a standard one. However, majority of aforementioned endovascular procedures comprised balloon angioplasty of pathological jugular valves. External compressions at the level of the jugular foramen were not addressed, while it is known that such strictures are quite common in these patients [13,20,21]. This may account for unfavorable results of clinical trials in multiple sclerosis patients. In addition, stenotic lesions of the IJVs are found in other neurological pathologies, including Alzheimer’s and Parkinson’s diseases, Ménière disease and lateral amyotrophic sclerosis. Thus, addressing these venous abnormalities potentially may have a prophylactic or therapeutic potential.
Yet, hemodynamic relevance of different types of strictures in the IJV are difficult to assess in living subject. Firstly, all investigations aimed at such an evaluation are more or less invasive and not free from severe side effects. Therefore, clinical investigations are very difficult to conduct, considering bioethical issues. Secondly, proper interpretation of these investigations (like catheter venography, Doppler ultrasonography or magnetic resonance imaging) is not obvious [31]. Therefore, in silico flow modeling can provide a surrogate evaluation of hemodynamic significance of particular types of these strictures.
In our previous paper, based on the results of computational flow simulations using the same CFD package, we suggested that strictures located at the level of the jugular foramen are probably more clinically relevant than pathological jugular valves [19]. Yet, in this study we evaluated the flow only in models of the IJV, without alternative outflow through the vertebral pathway. Also, flow disturbances evoked by stenoses were evaluated qualitatively, by an assessment of flow characteristic. In the current study, we evaluated the flow quantitatively and with the alternative outflow route, thus more similarly to real patients. In contrast to the former study, in the present one we found that both types of strictures, located upstream and downstream, can significantly compromise the outflow. Importantly, since severe flow impairments were caused by short nozzle-like stenoses, especially those located eccentrically, probably flow separation and flow reversal were the main source of an increased flow resistance and the shift of outflow towards the vertebral pathway (Figure 3 and Figure 4). But stenotic jugular valves, especially membrane-shaped (Figure 5 and Figure 6), can equally evoke significant flow impairments.
Currently, the significance of irregular flows in blood vessels in medical literature is not adequately understood. Doctors acknowledge that in most of blood vessels energy losses resulting in drop of pressure come from friction. But in some blood vessels frictional energy losses are accompanied by other types of losses, in physics commonly referred to as minor losses [32,33]. These energy losses primarily result from flow separation that typically develops when a blood vessel bends or suddenly expands. Except for aneurysms (pathologically enlarged arteries), the significance of flow separation and associated minor losses has been largely ignored by medical research. But flow abnormalities resulting in flow separation can severely impair blood flow, and IJVs are one of such blood vessels where these phenomena could have clinical consequences [34].
We acknowledge that there are limitations to our study. Firstly, to facilitate simulations, we built models comprising only one side of the cerebral outflow. Models with two IJVs could provide further insight into this problem, especially if only one IJV is affected. Secondly, valve leaflets in our models were stiff and immobile. Actually, they exhibit different levels of elasticity and movability. In our another in silico study, we demonstrated that there is a dynamic interplay between stenosis located upstream and the valve located downstream, and that a relevant stenosis changes the valve geometry to the unfavorable one [35]. Thirdly, flow volume measurements using the “probes” were not very precise. Yet, even such an approximate assessment could provide a useful insight into the problem studied. Besides, flow modeling using our CFD package was probably prone to significant errors developing when the flow resistance in the fields studied were high; thus we got very different total flow volumes in particular models (see: Table 1).
Other limitations of this study are associated with the use of simplified models instead of real morphology-based ones. Besides, an interplay between the veins and surrounding tissues should also be modeled. It should also be mentioned that in this study the fluid was considered Newtonian. Actually, blood is a non-Newtonian fluid. Since blood is shear-thinning fluid, slowing down its flow is associated with a higher flow resistance than that of a Newtonian fluid, which probably resulted in non-adequate flow modeling. However, the level of errors associated with non-Newtonian characteristics of blood is difficult to estimate. Probably, at least regarding the problem studied in this paper, it is not much relevant.
5. Conclusions
Numerical flow simulations of the flow in the IJVs suggest that both upper and lower strictures of these veins can be of clinical significance. Therefore, clinical studies on these veins, especially in the context of neurological pathologies, should comprise morphological and functional assessment of the entire course of the IJVs, from their origin in the cranial cavity to their junction with the brachiocephalic veins in the neck. It particularly regards possible stenosis at the level of the jugular foramen, as well as the jugular valve and other intraluminal structures located in the lower part of the IJV.
Author Contributions
Conceptualization, M.S; methodology, M.S. and J.C..; software, M.S.; validation, M.S., and P.L.; formal analysis, M.S. and A.K.; investigation, J.C., U.K.; resources, M.S.; data curation, M.S., A.K., P.L; writing—original draft preparation, M.S.; writing—review and editing, P.L.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by research grant of the University of Opole.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cirovic, S.; Walsh, C.; Fraser, W.D.; Gulino, A. The effect of posture and positive pressure breathing on the hemodynamics of the internal jugular vein. Aviat Space Environ Med 2003, 74, 125–131. [Google Scholar]
- Ciuti, G.; Righi, D.; Forzoni, L.; Fabbri, A.; Pignone, A.M. differences between internal jugular vein and vertebral vein flow examined in real time with the use of multigate ultrasound color doppler. Am J Neuroradiol 2013, 34, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Doepp, F.; Schreiber, S.J.; von Műnster, T.; et al. How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns. Neuroradiol 2004, 46, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B. Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain Res Rev 2004, 46, 243–260. [Google Scholar] [CrossRef]
- Schreiber, S.J.; Lürtzing, F.; Götze, R.; Doepp, F.; Klingbiel, R.; Valdueza, J.M. Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound. J Appl Physiol 2003, 94, 1802–1805. [Google Scholar] [CrossRef]
- Simka, M.; Czaja, J.; Kowalczyk, D. Collapsibility of the internal jugular veins in the lateral decubitus body position: a potential role of the cerebral venous outflow against neurodegeneration. Med Hypothes 2019, 133, 109397. [Google Scholar] [CrossRef]
- Zaniewski, M.; Simka, M. Biophysics of venous return from the brain from the perspective of the pathophysiology of chronic cerebrospinal venous insufficiency. Rev Recent Clin Trials 2012, 7, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Alpini, D.C.; Bavera, P.M.; Hahn, A.; et al. Chronic cerebrospinal venous insufficiency (CCSVI) in Meniere disease: case or cause? ScienceMED 2013, 4, 9–15. [Google Scholar]
- Beggs, C.; Chung, C.P.; Bergsland, N.; et al. Jugular venous reflux and brain parenchyma volumes in elderly patients with mild cognitive impairment and Alzheimer's disease. BMC Neurol 2013, 13, 157. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chang, F.C.; Chao, A.C.; et al. Internal jugular venous abnormalities in transient monocular blindness. BMC Neurol 2013, 13, 94. [Google Scholar] [CrossRef]
- Chung, C.P.; Beggs, C.; Wang, P.N.; et al. Jugular venous reflux and white matter abnormalities in Alzheimer's disease: a pilot study. J Alzheimer Dis 2014, 39, 601–609. [Google Scholar] [CrossRef]
- Ding, J.Y.; Zhou, D.; Pan, L.Q.; et al. Cervical spondylotic internal jugular venous compression syndrome. CNS Neurosci Ther 2020, 26, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.K.; Utriainen, D.T.; Daugherty, A.M.; et al. Jugular venous flow abnormalities in multiple sclerosis patients compared to normal controls. J Neuroimaging 2015, 25, 600–607. [Google Scholar] [CrossRef]
- Simka, M.; Latacz, P.; Ludyga, T.; Kazibudzki, M.; Świerad, M.; Janas, P.; Piegza, J. Prevalence of extracranial venous abnormalities: results from a sample of 586 multiple sclerosis patients. Funct Neurol 2011, 26, 197–203. [Google Scholar] [PubMed]
- Zivadinov, R.; Marr, K.; Cutter, G.; Ramanathan, M.; Benedict, R.H.; Kennedy, C.; Elfadil, M.; Yeh, A.E.; Reuther, J.; Brooks, C.; Hunt, K.; Andrews. M.; Carl, E.; Dwyer, M.G.; Hojnacki, D.; Weinstock-Guttman, B. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011, 77, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Hubbard, D.; Siddiqui, A.H.; Dake, M.D.; Sclafani, S.J.; Al-Omari, M.; Eisele, C.G.; Haskal, Z.J.; Ludyga, T.; Miloševič, Z.V.; Sievert, H,; Stehling, M. K;, Zapf, S.; Zorc, M. Catheter venography for the assessment of internal jugular veins and azygous vein: position statement by expert panel of the International Society for Neurovascular Disease. Vasa 2013, 42, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Morovic, P.; Menegatti, E.; et al. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound - recommendations for a protocol. Int Angiol 2011, 30, 571–597. [Google Scholar]
- Zivadinov, R.; Bastianello, S.; Dake, M.D.; et al. Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. J Vasc Interv Radiol 2014, 25, 1785–94. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Latacz, P. Numerical modeling of blood flow in the internal jugular vein with the use of computational fluid mechanics software. Phlebology 2021, 36, 541–548. [Google Scholar] [CrossRef]
- Feng, W.; Utriainen, D.; Trifan. G.; et al. Characteristics of flow through the internal jugular veins at cervical C2/C3 and C5/C6 levels for multiple sclerosis patients using MR phase contrast imaging. Neurol Res 2012, 34, 802–809. [Google Scholar] [CrossRef]
- Feng, W.; Utriainen, D.; Trifan, G.; Elias, S.; Sethi, S.; Hewett, J.; Haacke, E.M. Characteristics of flow through the internal jugular veins at cervical C2/C3 and C5/c6 levels for multiple sclerosis patients using MR phase contrast imaging. Neurol Res 2012, 34, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Feng, W.; Utriainen, D.; Trifan, G.; Wu, Z.; Latif, Z.; Katkuri, Y.; Hewett, J.; Hubbard, D. Patients with multiple sclerosis with structural venous abnormalities on MR Imaging exhibit an abnormal flow distribution of the internal jugular veins. J Vas Inter Radiol 2012, 23, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Skuła, M.; Bielaczyc, G. Validation of models of venous outflow from the cranial cavity in the supine and upright body positions. Phlebol Rev 2022, 30, 8–12. [Google Scholar] [CrossRef]
- Phillips, R.A.; Van Slyke, D.D.; Hamilton, P.B.; et al. Measurement of specific gravities of whole blood and plasma by standard copper sulfate solutions. J Biol Chem 1950, 183, 305–330. [Google Scholar] [CrossRef]
- Trudnowski, R.J.; Rico, R.C. Specific gravity of blood and plasma at 4 and 37 °C. Clin Chem 1974, 20, 20–615. [Google Scholar] [CrossRef]
- Zivadinov, R.; Chung, C.P. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med 2013, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Simka, M. Activation of the glymphatic system during sleep – is the cerebral venous outflow a missing piece of the puzzle? Phlebol Rev 2019, 7, 1–2. [Google Scholar] [CrossRef]
- Zamboni, P.; Tesio, L.; Galimberti. S.; et al. Efficacy and safety of extracranial vein angioplasty in multiple sclerosis. JAMA Neurol 2018, 75, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Galeotti, R.; Salvi, F.; Giaquinta, A.; Setacci, C.; Alborino, S.; Guzzardi, G.; Sclafani, S.J.; Maietti, E.; Veroux, P. Brave Dreams Research Group. Effects of Venous Angioplasty on Cerebral Lesions in Multiple Sclerosis: Expanded Analysis of the Brave Dreams Double-Blind, Sham-Controlled Randomized Trial. J Endovasc Ther 2020, 27, 1526602819890110. [Google Scholar] [CrossRef]
- Simka, M. An overview of randomized controlled trials on endovascular treatment for chronic cerebrospinal venous insufficiency in multiple sclerosis patients. Phlebologie 2021, 50, 76–80. [Google Scholar] [CrossRef]
- Simka, M. Chronic cerebrospinal venous insufficiency: current perspectives. J Vasc Diagn 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Rend, R.R.; Sparrow, E.M.; Bettenhausen, D.W.; et al. Parasitic pressure losses in diffusers and in their downstream piping systems for fluid flow and heat transfer. Int J Heat Mass Transfer 2013, 61, 56–61. [Google Scholar] [CrossRef]
- Li, L.; Walker, A.M.; Rival, D.E. The characterization of a non-Newtonian blood analog in natural- and shear-layer-induced transitional flow. Biorheology 2014, 51, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Latacz, P.; Redelbach, W. Blood flow in the internal jugular veins during the spaceflight – Is it actually bidirectional? Life Sci Space Res 2020, 25, 103–106. [Google Scholar] [CrossRef]
- Rashid, A.; Iqrar, S.A.; Rashid, A.; Simka, M. Results of numerical modeling of blood flow in the internal jugular vein exhibiting different types of strictures. Diagnostics 2022, 12. [Google Scholar] [CrossRef]
Figure 1.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes – in these areas flow characteristics was measured. Flow is from the left to the right. In this model there are no stenoses in the internal jugular vein, consequently most of the outflow utilizes the jugular pathway.
Figure 1.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes – in these areas flow characteristics was measured. Flow is from the left to the right. In this model there are no stenoses in the internal jugular vein, consequently most of the outflow utilizes the jugular pathway.

Figure 2.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes. Flow is from the left to the right. There are 2 strictures in the internal jugular vein: in the beginning (white arrow) and at its end (black arrow). In the model with stenoses part of the flow is shifted towards the vertebral pathway.
Figure 2.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); magenta squares represent the probes. Flow is from the left to the right. There are 2 strictures in the internal jugular vein: in the beginning (white arrow) and at its end (black arrow). In the model with stenoses part of the flow is shifted towards the vertebral pathway.

Figure 3.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and stenotic valve downstream.
Figure 3.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and stenotic valve downstream.

Figure 4.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and no valve downstream.
Figure 4.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. There is a significant stenosis at the beginning of the jugular pathway and no valve downstream.

Figure 5.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted and there is a stenotic valve downstream.
Figure 5.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted and there is a stenotic valve downstream.

Figure 6.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted, while downstream there is a septum nearly completely obstructing the outflow.
Figure 6.
Model of the cerebral venous outflow comprising the internal jugular vein (above) and the vertebral venous plexus (below); flow is from the left to the right. Upper part of the jugular pathway is unrestricted, while downstream there is a septum nearly completely obstructing the outflow.

Table 1.
Characteristic of the strictures in the modelled internal jugular vein, flow volumes in the jugular and vertebral pathways.
Table 1.
Characteristic of the strictures in the modelled internal jugular vein, flow volumes in the jugular and vertebral pathways.
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Results of Numerical Modeling of Blood Flow in the Internal Jugular Vein Exhibiting Different Types of Strictures
Anas Rashid
et al.
Diagnostics,
2022
Application of Recursive Theory of Slow Viscoelastic Flow to the Hydrodynamics of Second-Order Fluid Flowing through a Uniformly Porous Circular Tube
Kaleemullah Bhatti
et al.
Mathematics,
2020
Partial Obstruction of Ventricular Catheters Affects Performance in a New Catheter Obstruction Model of Hydrocephalus
Seunghyun Lee
et al.
Children,
2022
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






