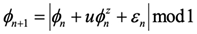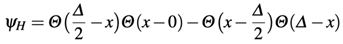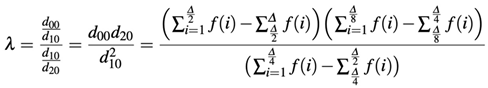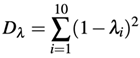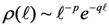1. Introduction
Cognitive decline is a central phenotypic manifestation of dementia. Alzheimer’s disease (AD) remains one of the most common neurodegenerative diseases and a leading cause of dementia worldwide [
1]. Because of its commonly slow progression, and since the diagnosis is mostly clinical, the time between symptom onset and diagnosis often remains long [
2]. The impact on the affected individuals and their families, as well as the socioeconomic burden of the disease demand for earlier identification of the disease, i.e., during the stage of Mild Cognitive Impairment (MCI) [
3], when cognitive deficits might still be subtle and thus missed by the clinician, if not carefully examined and regularly reassessed. The analysis of the cerebrospinal fluid (CSF) and other imaging modalities like FDG-PET have been studied for early detection of the disease [
1]. As the quest for viable disease-modifying therapies (DMTs), i.e., monoclonal antibodies, has gained momentum, there arises a need for reliable, highly reproducible, cheap, fast, easy-to-perform and less invasive methods that could contribute to early diagnosis or even provide additional measures that would facilitate the selection and screening of subjects undergoing cognitive training or cognitive rehabilitation [
4].
Among possible diagnostic modalities and biomarkers, electroencephalography (EEG), a simple, low-cost and non-invasive technique, has already provided robust results. The processing of resting-state EEG signals with various novel methodologies has shown potential in AD diagnosis and assessment of progression [
5]. Regarding spectral markers, increased delta and theta activity with a simultaneous decrease in alpha and beta as well as "slowing" and a "disconnection syndrome" have been described [
6]. Linear methods have demonstrated dysfunction of cortical connectivity in AD [
7], [
8].
Adding to previously methodologies, the theory of brain criticality has been examined in AD subjects in the recent years. Criticality is a dynamical state that occurs near the point of dynamic instability where entropy is decreased and scale-free oscillations in various spatiotemporal patterns are observed [
9]. Near the critical point, at a transition phase, criticality is demonstrated by high-amplitude fluctuations that slowly decrease along the characteristic in a logarithmic scale “slowing down” [
10], leading to long-range temporal correlations. In every scale-free network a power-law distribution is observed.
According to the theory of self-organized criticality (SOC) "neuronal avalanches" are created during a phase transition in the brain, and there is a great communication of neurons through the largest possible number of synapses [
11], i.e., connection of brain activity at various levels of organization and an increase in overall neuronal complexity [
12]. This is in line with computation models at the “edge of chaos” [
13]. Studying functional connectivity and fluctuations of synchronization levels, Stam et al. documented significant reduction of alpha and beta waves in AD patients, despite the maintenance of SOC [
14]. Based on previous data supporting dysfunction of neuronal integrity and connectivity, Vysata et al. compared the power-law exponents in patients with moderate to severe disease, noting a reduction of the system for SOqC and highlighting the need for further validation in patients with milder stages of the disease, like MCI [
15]. More recent research by Tait et al. and Kulkarni et al. has shown reduction of EEG complexity during the progression of dementia, providing measures of diagnostic accuracy for the detection of AD [
2].
After the formulation of the theory of self-organized quasi-criticality (SOqC) [
16], evidence of organization of brain dynamics in a quasi-critical manner has been presented, with exponents maintaining a scaling relation indicative of the proximity to criticality, even when external inputs push these networks away from a critical point [
17]. Furthermore, since the role of non-linear dynamics in the approach of human cognition studies has been highlighted [
18], research suggests that individuals with strong cognition present neural dynamics that are more closely associated with criticality, in comparison to subjects with weaker cognitive abilities [
19].
The purpose of this study is to explore the relation between criticality and homeostatic brain plasticity by investigating brain criticality changes in patients with MCI before and after memory training in association with the documented improvement in neuropsychological evaluations.
2. Materials and Methods
2.1. Study Design
EEG recordings of seventeen right-handed subjects (11 females, 6 males; chi square goodness of fit = 1.47, df = 1,
p = .225) with clinically diagnosed amnesic Mild Cognitive Impairment (aMCI) according to Petersen criteria [
3]were analyzed in our study. Based on evidence of pronounced prospective memory deficits in patients with AD neuropathology even at the stage of MCI, the patients had previously participated in a 6-month single-blind randomized clinical trial (RCT) of Prospective Memory training [
20]. Details on inclusion and exclusion criteria are mentioned in the paper by Agogiatou et al. [
21]. The RCT had a naturalistic design for the performance of daily and routine tasks based on visual stimuli. The 6-month training consisted of 48 sessions (1 hour per session, 2 sessions per week). After the completion of the intervention patients exhibited significant improvement in neuropsychological assessments of working memory, verbal memory, verbal fluency, and overall executive functions, as shown by the improvement of activities of daily life (ADL), compared to an age-, gender-, and education-matched control group. We used “before” and “after” EEGs to measure and compare changes in criticality indices. The RCT was approved by the Scientific and Ethics Committee of “Alzheimer Hellas”.
2.2. EEG Recordings – Data Acquisition
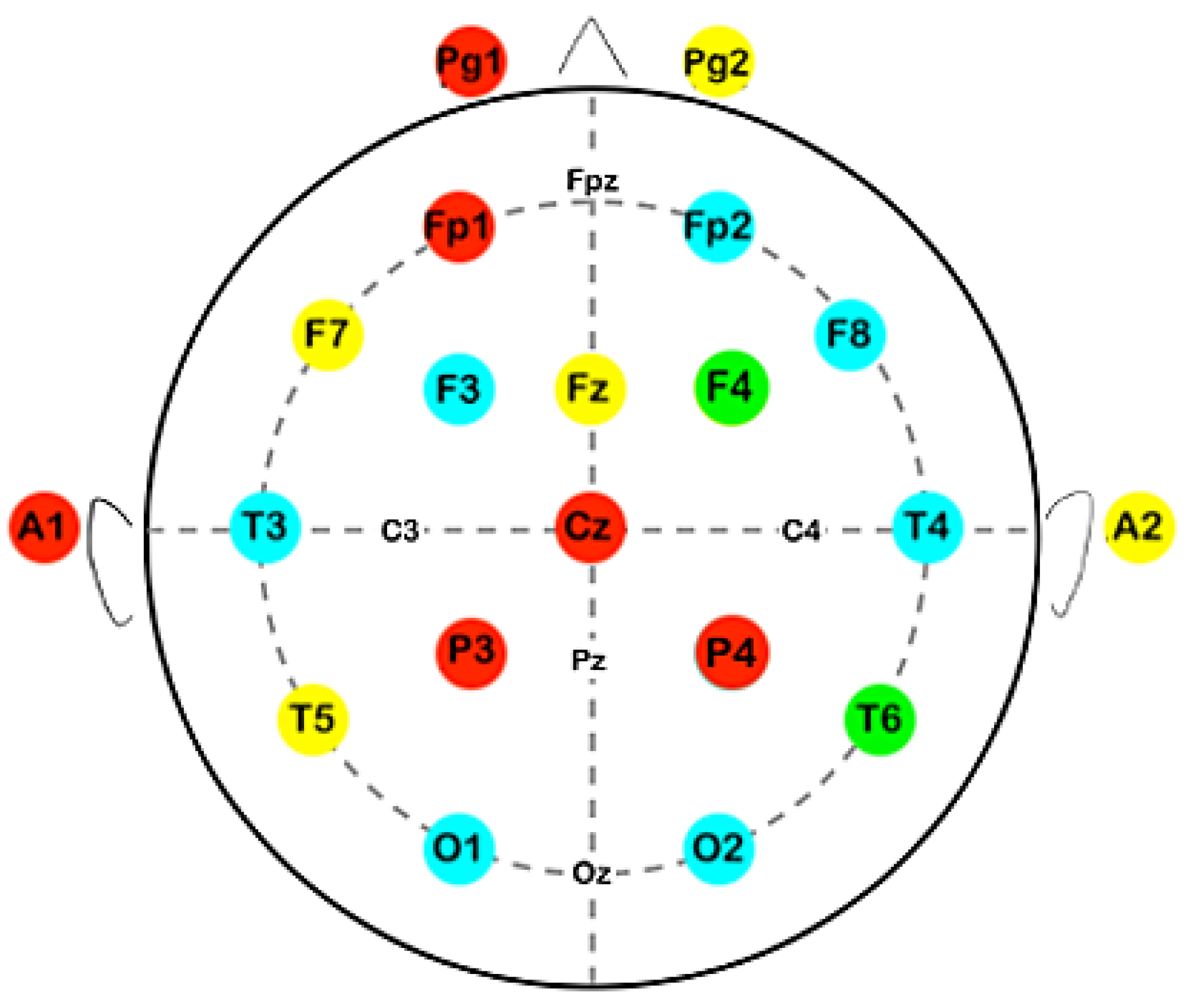
We examined resting-state EEG recordings collected via a 21-electrode Nihon Kohden Neurofax J 921A, using the international 10-20 system (i.e., Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2). Where possible the number of electrodes was extended, beyond the basic arrangement of the 10-20 system. The signals were digitized with the Neurofax EEG-12200 Ver. 01-93.
The frequency was set as follows: fs = 500 Hz and the EEG electrode resistance was less than 5 KOhm. Recordings were taken without the use of filters. The protocol for EEG data acquisition included a 10-minute resting state (5 minutes with eyes open and 5 minutes with eyes closed, while seated upright and with no voluntary movements).
All subjects included in the present study had formally agreed to the conduction of an EEG study both at baseline and after the completion of the RCT. It is noted that all EEGs had been examined by two neurologists and all diagrams were normal.
2.3. Data Analysis
2.3.1. Method of Critical Fluctuations
The method of critical fluctuations (MCF), originally described by Contoyiannis et al., was employed for the analysis of the EEG time-series [
22], [
23]. MCF is thought to reveal the critical state, as well as to detect a system’s distance to criticality [
22], [
24]. The main points of the analysis are herein presented.
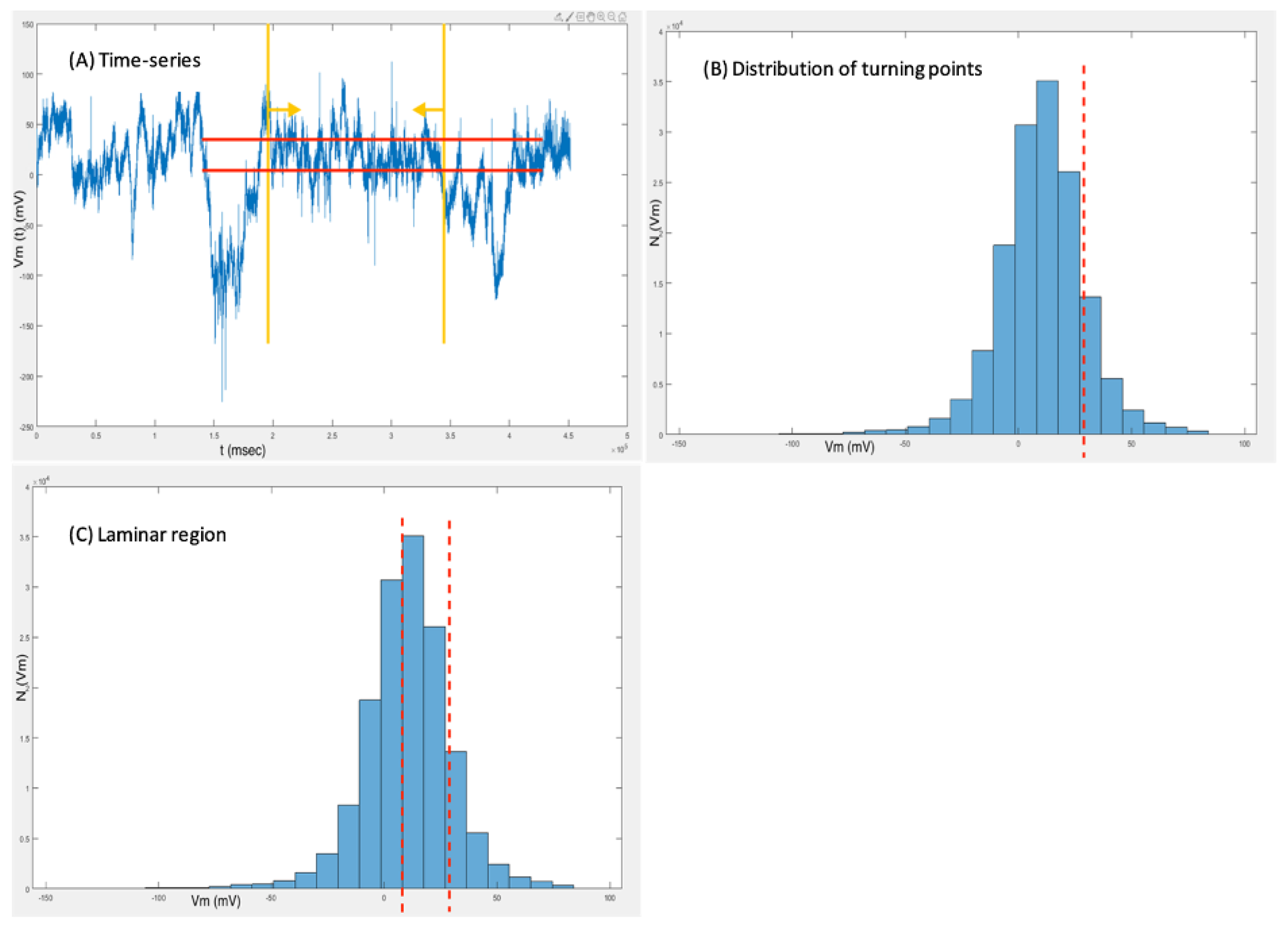
As seen in
Figure 1A, only time-series with stationary sections with at least 1*105 points before and after the intervention were considered for analysis. The histogram of
Figure 1B depicts the distribution of turning points, marking the area of turning point discontinuation as a possible fixed point (red dashed line). Fixed points are related to neurons’ resting states and are thus investigated for a possible operation at or near a critical state. Consequently, the fixed point’s laminar region can then be used for fluctuation analysis (
Figure 1C). It is noted, that laminar behavior will be detected, if present, without strict determination of the laminar region.
After estimating the laminar region, it is necessary to estimate the distribution of laminar lengths, i.e., the number of consecutive points in the time series that are within the selected laminar region and consist of intervals with a length from 1 to several tens or hundreds. According to previous studies, laminar lengths follow a power-law distribution when there are intermittent dynamical components in the system [
22], [
23], a property that can also be quantified at the critical point by creating a criticality map [
25], where the potentials at the neighboring points of each fixed point are described by the following function:
However, these potentials are also reflected in the distribution of laminar lengths given by the following function:
where parameters p and q describe the long and short range temporal correlations respectively, and are expected to have values p=[
1,
2] and q≈0 in a critical system [
22].
2.3.2. Haar Wavelet Analysis
The most common causes of noise in the EEG are eye movement artifacts, often leading to wrong conclusions [
26]. Noise can affect the discrimination of the power law as well as the point and range of the distribution in which it is identified, leading to miscalculation of the exponent p, and there is no commonly accepted tool that reliably identifies it over a range of scales [
27].
The particularity of this work is the analysis based on the Haar wavelet method, which ignores the noise and reveals the power-law distribution and the critical state, if present. Wavelets have been used in this way in the past both to calculate patterns that lead to a power law and to reduce noise [
28]. We followed the process introduced by Contoyiannis et al., starting the wavelet processing at a later stage, when the target distribution has already been identified, demonstrating that the information about the existence of the power law lies in the low-scale coefficients that are not affected by noise [
27]. Therefore, calculations can be done smoothly even in the presence of noise, bypassing the fitting process. An in-depth review of the methodology is beyond the scope of this study, as it is thoroughly described in the respective paper mentioned above. Therefore, we shall only briefly report the main steps and functions of Haar wavelet analysis.
First, a slightly different approach to the afore-mentioned MCF is introduced. For the extraction of laminar lengths histograms are no longer needed.
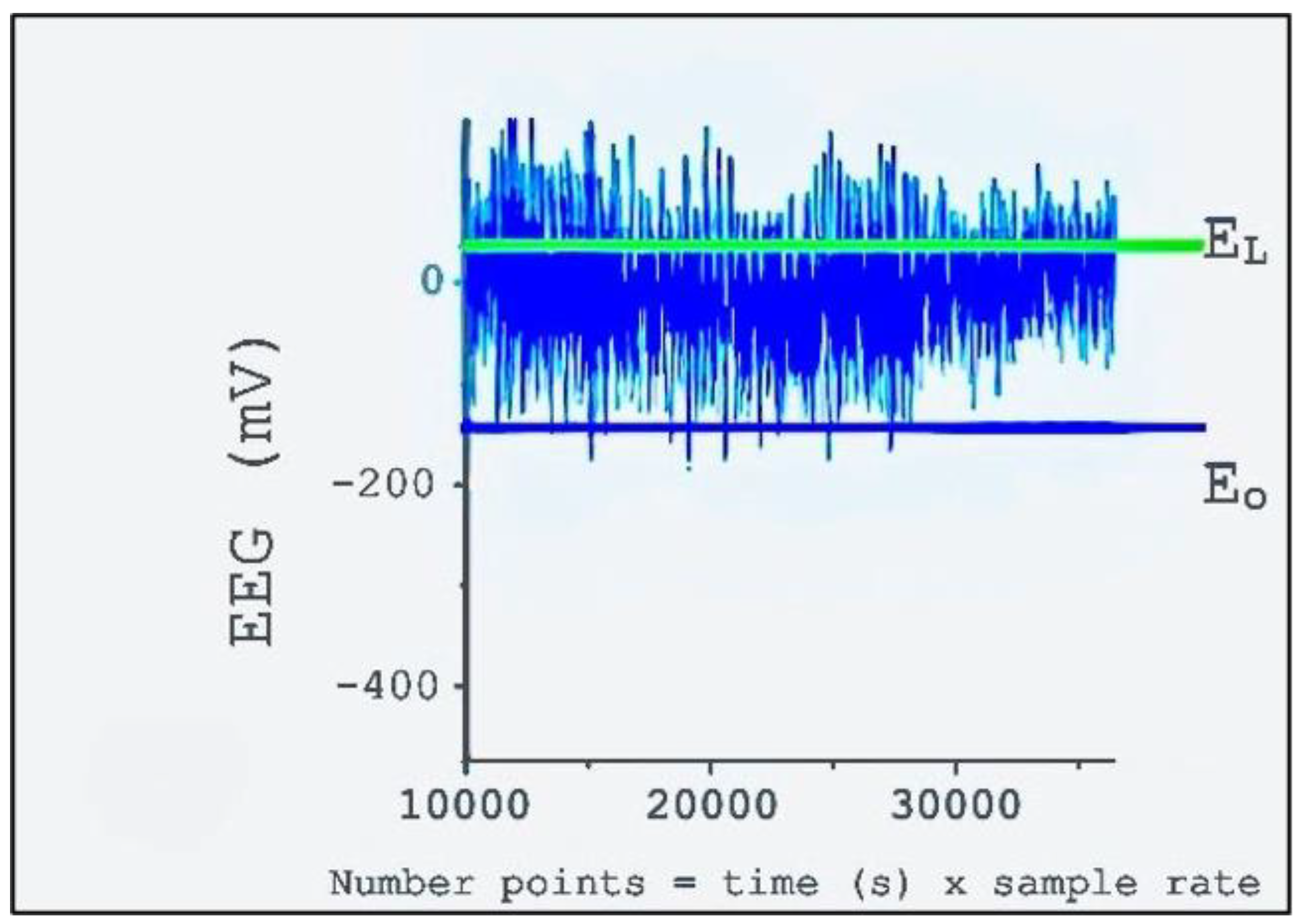
Figure 2 presents the selection of two points in order to determine the laminar region: Eo (blue line) is a fixed point representing the lower values; EL is a free parameter that signals the end of the laminar region, around the symmetric distribution of EEG values (green line).
The algorithm on which Haar wavelet analysis is based on the function:
The calculation is then done with the following functions that determine the following parameters (presented in detail by Contoyiannis et al. [
27]
Finally, the results are quantified. Considering the value λ = 1 the perfect power law, the distance of λ is calculated using the function:
Every step of MCF/Haar wavelet analysis was performed twice for the sake of verification. The analyst remained blinded during analysis, with no evidence of the group (before or after) from which each electrode originated.
MATLAB (RRID:SCR_001622) R2019b Update 7 (9.7.0.1471314) was used for the analysis. The codes that we used are given as supplementary material.
2.4. Statistical Analysis
Electrode pairs that presented stationarity both before and after the training were included in the statistical analysis. Descriptive statistics, the Shapiro-Wilk normality test and plot observation were used for data exploration. Means of the λ values before and after prospective memory training were compared for each separate electrode. Mean before and after values of the sum of all electrodes were compared, as well. Additionally, frontotemporal electrodes were isolated and their before and after values were compared. A paired t-test or a Wilcoxon signed-rank test was used, depending on the normality of the data. The level of statistical significance was set to 5%. R version 4.1.2 (
http://www.R-project.org) was used for the analysis.
3. Results
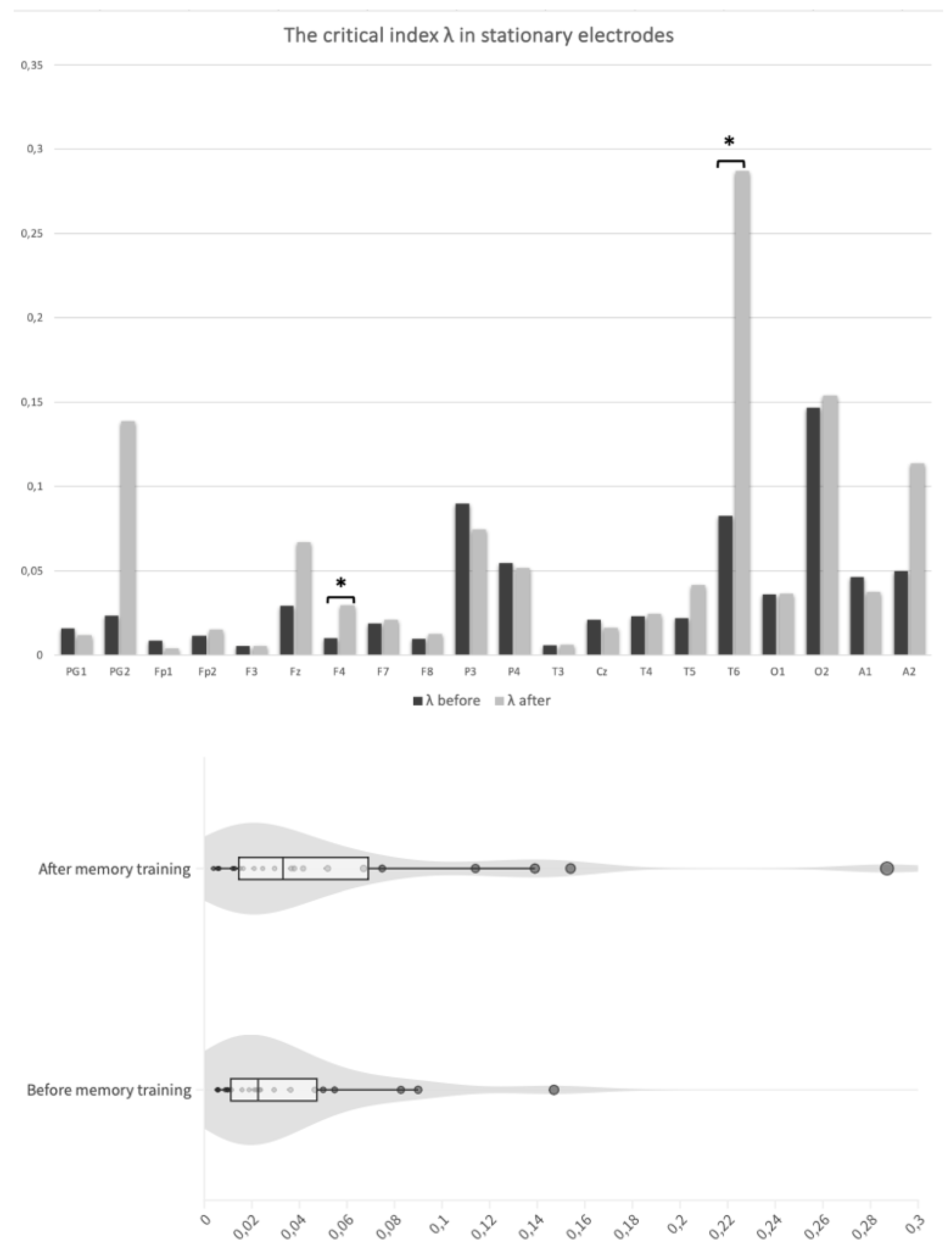
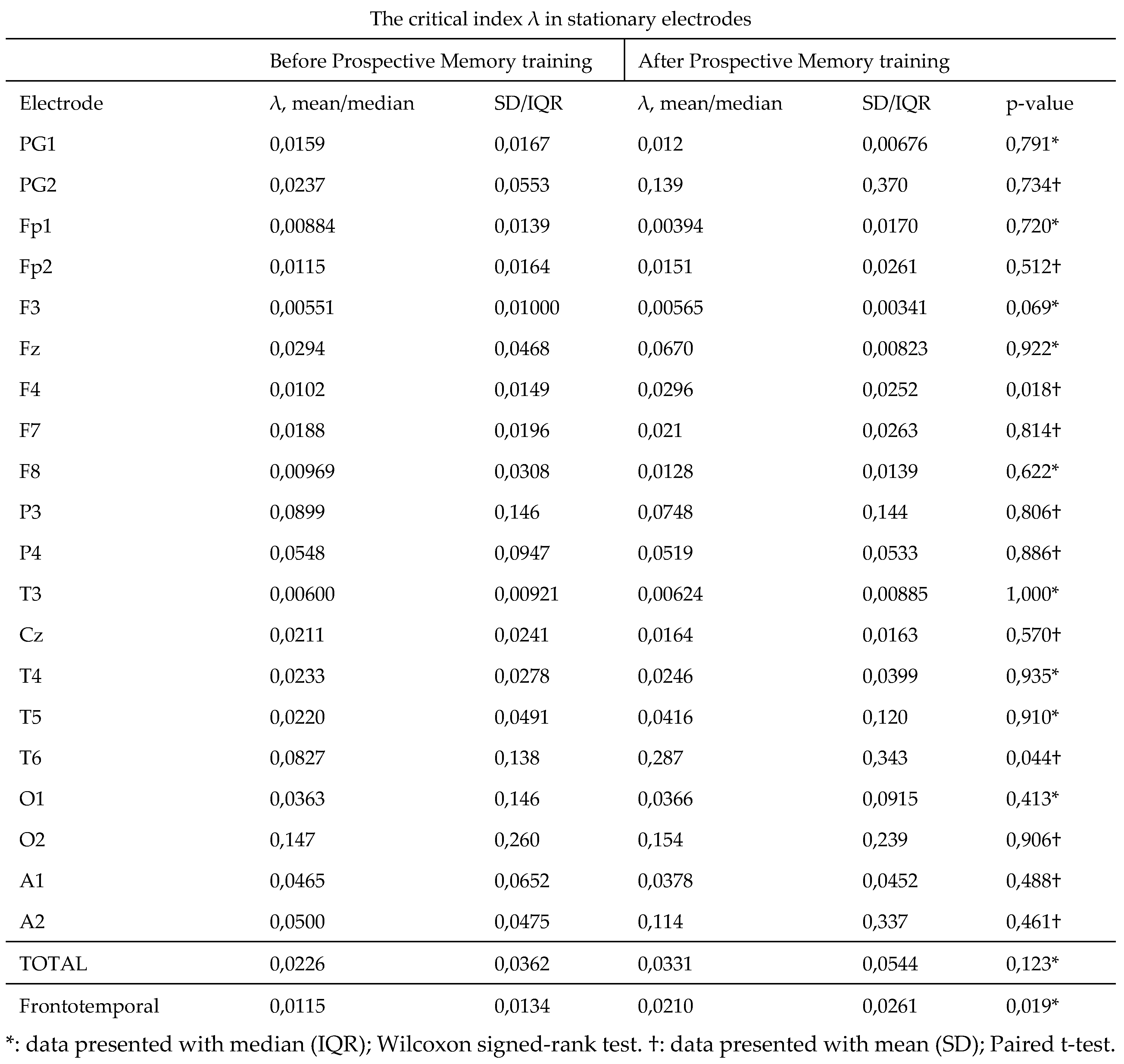
Our results concerning critical index λ in each separate electrode are presented
Table 1. In most electrodes an improvement of mean values after the intervention was noted, although not always statistically significant. In electrode T6, that is placed over the temporo-parieto-occipital (TPO) junction, a statistically significant difference was observed between pre- (M = 0.0827; SD = 0. 138) and post-intervention recordings (M = 0.287; SD = 0.343); t(10) = -2.3,
p value = .044. A statistically significant improvement was also presented in the electrode F4, that covers an area of the right frontal lobe, (mdn = 0.0102; IQR = 0.0149 in recordings taken before and mdn = 0.0296; IQR = 0.0252 after the intervention); t(10) = -2.82,
p value = .018 (
Figure 3A). Values in electrodes PG1, Fp1, P3, P4, Cz and A1, although not statistically significant, showed a lower mean in the λ index after memory training, compared to the initial values (
Figure 4).
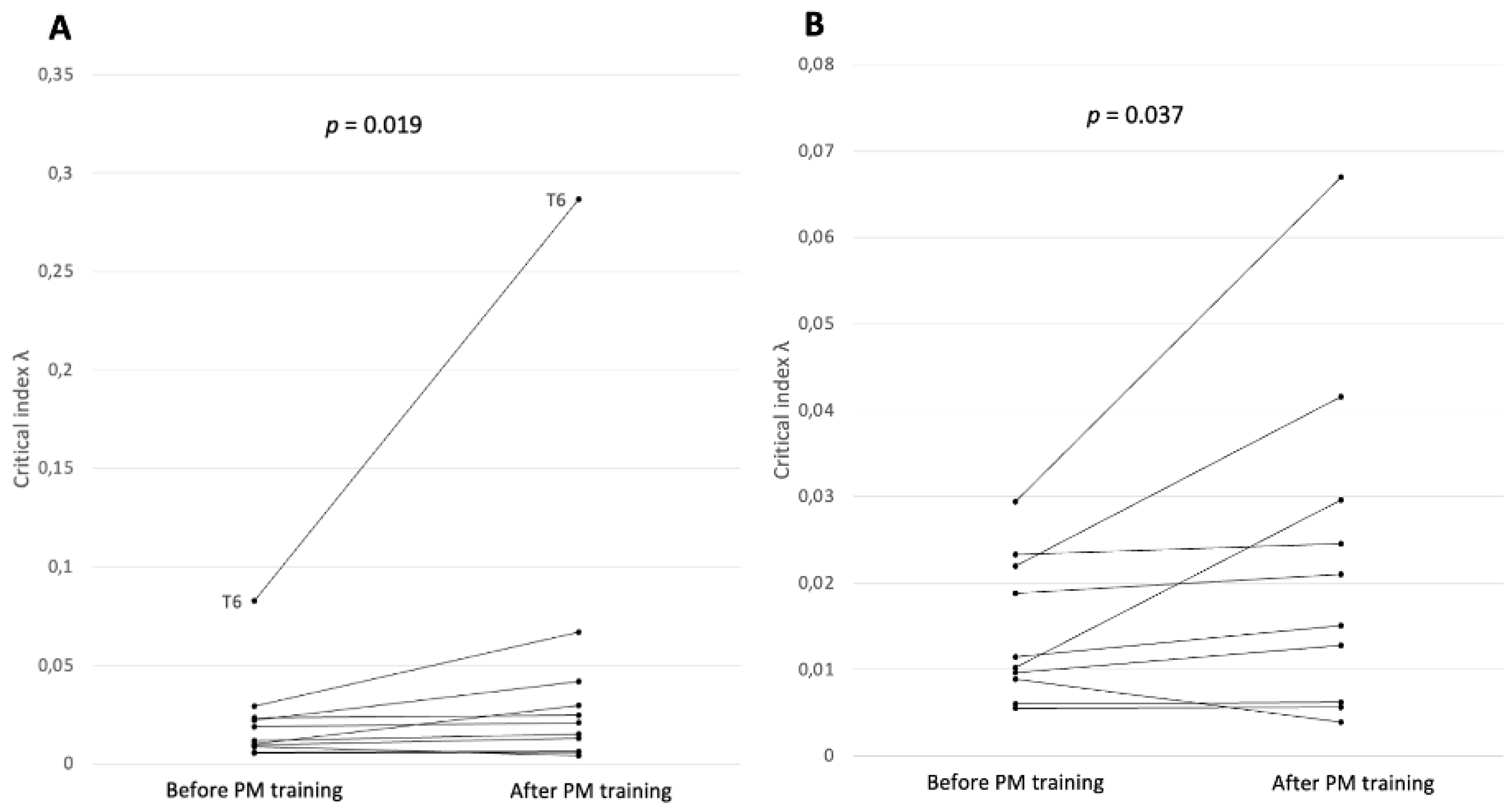
Pooled λ for all electrodes after prospective memory training was higher (mdn = 0.0331; IQR = 0.0544) than the respective value for electrodes before the intervention (mdn = 0.0226; IQR = 0.0362), but the difference was not significant; Z = 79,
p value = .123 (
Figure 3B). However, a significant increase was found when only focusing on the comparison of critical index λ from frontotemporal stationary electrodes (before training: mdn = 0.0115; IQR = 0.0134 and after training: mdn = 0.0210; IQR = 0.0261; Z = 7,
p value = .019) (
Figure 5). The significant increase persisted even when the electrode with the highest increase, T6, was considered a possible outlier and was omitted in a sensitivity analysis (Z = 7,
p value = .037).
*: data presented with median (IQR); Wilcoxon signed-rank test. †: data presented with mean (SD); Paired t-test.
4. Discussion
Our findings support significant enhancement of criticality in frontotemporal electrodes in MCI patients that underwent prospective memory training. Isolated electrode analysis indicated improvement in T6 and F4. It is emphasized that for all patients the traditional EEG examination by two neurologists had shown normal diagrams, both before and after the intervention. Our EEG time-series analysis combined the principles of MCF with Haar wavelet analysis. The limitation of noise was overcome, enabling a scale-free approach in the analysis of laminar lengths, since there is no more a need to restrict to small scales that are theoretically invulnerable to noise. We analyzed electrodes with stationary recordings or stationary sections of at least 1*105 points. Contoyiannis et al. examined the methodology in healthy and epileptic subjects, demonstrating absence of a power law distribution in the latter [
27]. To our knowledge, this specific methodology was applied for the first time in data deriving from subjects with cognitive decline. Moreover, this is the first study to encompass an EEG criticality analysis in the setting of cognitive training/rehabilitation.
MCI patients that received prospective memory training demonstrated a favorable clinical outcome in working memory, verbal memory, verbal fluency and activities of daily living [
21]. These skills have a special neuroanatomic correlation with the topography of the electrodes, in which with criticality enhancement was documented. More specifically, the areas of the brain related to prospective memory, the ability to carry out an action in the future, belong to the frontal, parietal and temporal lobes. The frontal lobe, and specifically the rostral prefrontal cortex and Brodmann area 10, has been associated with event-based prospective memory [
29], the temporal lobe has been linked to recognition of stimuli and cues related to the future task [
30]. Last, the parietal lobe, and particularly the temporo-parieto-occipital junction (TPO), possibly plays a role in decision making related to time-based prospective memory, by processing and monitoring stimuli related to the performance of a task in the future [
31]. Moreover, T6 reflects the neuronal activity of the right temporo-parieto-occipital junction (TPO) of the association cortex (Brodmann areas 37, 39 and 19), a brain region that assembles somatosensory, visual and auditory information and is involved in language, working memory [
32], self-processing and recognition of visuo-spatial patterns [
33]. F4 reflects the activity of the right premotor cortex and dorsolateral prefrontal cortex (Brodmann areas 9, 8 and 6), that are associated with motor planning working memory [
34], speech and verbal description [
35]. Therefore, our findings are in accordance with the patients’ clinical improvement, expressed through established neuropsychological tests, adding to the internal validity of the methodology.
Interestingly, apart the overall criticality enhancement in frontotemporal regions, significant results from the isolated electrode analysis came from the non-dominant hemispheres of our subjects, since all of them are right-handed. This observation is in line with previously reported findings of rightward dominance and loss of left-hemisphere laterality in right-handed MCI and AD patients, that may suggest activation of right-hemisphere neural resources as a compensatory mechanism [
36]. Regarding the rest of the recordings, an improvement was detected in most of the electrodes after the intervention, although not statistically significant. On the contrary, notably but still not significantly lower values were observed in electrodes PG1, Fp1, P3, P4, Cz and A1, which could also be partly explained by compensatory mechanisms and increased recruitment in prefrontal and parietal regions at the initial stages of AD neuropathology, that would justify higher values prior to the intervention [
37], [
38], [
39].
In the context of novel biomarker research in the field of impaired cognition, our results provide evidence for the augmentation of the diagnostic and screening value of the common and easy-to-perform EEG, through the application of brain criticality analysis. Previous work on criticality and the use of EEG has already exhibited promising results. Vysata et al. described a loss of functional connectivity in AD patients, by examining differences from healthy controls regarding power-law exponents on EEG spectrogram, highlighting however the need for future study of this parameter in patients with MCI [
15]]. Tait et al. and Kulkarni at al. have shown reduction of EEG complexity during dementia progression [
2]. Flores-Sandoval et al. demonstrated a lower EEG spectral power ratio in patients with amyloid-positive amnestic mild cognitive impairment compared with cognitively normal subjects [
40]. Trihn et al. evaluated task-induced intra-subject spectral power variability of resting-state EEGs and suggested its use in the early detection of MCI [
41]. Our findings are in line with previously conducted research and indicate the possible use of EEG criticality as a screening tool in patients undergoing cognitive training or rehabilitation. We underscore the need for experimental implementation of this parameter in future clinical studies of cognitive training, so that more could be explored regarding critical states and the patients’ performance in various tests. Combined with the analysis fluid or neuroimaging biomarkers, criticality could shed light on the mechanisms of synaptic brain plasticity or even provide insights about AD theragnostics in patients who will undergo disease-modifying treatments. Furthermore, standardization of the methodology in a wide range of individuals, both healthy and with cognitive impairment, could provide an easy-to-obtain, quantifiable biomarker, that could promote early disease identification and early shaping of treatment strategies in selected patients.
Our study had an explanatory pilot character and its design implies the existence of limitations. Regarding limitations in the analysis, the need for stationarity should be noted, since the methodology could only be performed in electrodes with stationary recordings. Methodologic limitations also include the discrepancy of our results from the theoretically expected values in previous reports of the methodology, highlighting the need for future research in order to standardize expected values. The small number of patients also restrict the external validity of our findings. Moreover, no EEG data were available for MCI patients who abstained from the training, serving as a control group in the RCT. Plus, no comparison was made between MCI patients and healthy individuals.
5. Conclusions
EEG criticality is a promising monitoring biomarker of Alzheimer disease and may have a role in cognitive rehabilitation/training, providing insights of brain plasticity. Furthermore, criticality might be of importance in early disease identification. Future research on large patient cohorts at different disease stages is imperative, with direct comparisons to healthy controls or individuals with other cortical dementias, using standardized methods. Of special interest would be the study of criticality alongside established blood/CSF or neuroimaging biomarkers and in conjunction with amyloid β burden in patients under disease-modifying therapies, that could unlock new paths in Alzheimer disease theragnostics.
Supplementary Materials
MATLAB codes.
Author Contributions
VST; concept framing, data collection, study design, data manipulation/analysis, manuscript drafting/revising. MT; idea conception, concept framing, data collection, study design and manuscript revising. GV; data analysis, manuscript drafting/revising. MS; concept framing, data collection, study design, manuscript drafting. EK; supervision, data manipulation/analysis, manuscript drafting/revising.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The MATLAB codes used for the analysis are publicly available, as a supplementary material.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Y. A. R. Mahaman et al., “Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention,” Ageing Res Rev, vol. 74, Feb. 2022. [CrossRef]
- L. Tait et al., “EEG microstate complexity for aiding early diagnosis of Alzheimer’s disease,” Sci Rep, vol. 10, no. 1, Dec. 2020. [CrossRef]
- R. C. Petersen and J. C. Morris, “Mild cognitive impairment as a clinical entity and treatment target,” Arch Neurol, vol. 62, no. 7, pp. 1160–1163, Jul. 2005. [CrossRef]
- B. M. Hampstead, C. B. Mosti, and T. Swirsky-Sacchetti, “Cognitively-based methods of enhancing and maintaining functioning in those at risk of Alzheimer’s disease,” J Alzheimers Dis, vol. 42 Suppl 4, pp. S483–S493, 2014. [CrossRef]
- R. Cassani, M. Estarellas, R. San-Martin, F. J. Fraga, and T. H. Falk, “Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment,” Dis Markers, vol. 2018, 2018. [CrossRef]
- L. C. Fonseca, G. M. A. S. Tedrus, L. R. Prandi, and A. C. A. de Andrade, “Quantitative electroencephalography power and coherence measurements in the diagnosis of mild and moderate Alzheimer’s disease,” Arq Neuropsiquiatr, vol. 69, no. 2B, pp. 297–303, 2011. [CrossRef]
- C. Babiloni et al., “Functional cortical source connectivity of resting state electroencephalographic alpha rhythms shows similar abnormalities in patients with mild cognitive impairment due to Alzheimer’s and Parkinson’s diseases,” Clin Neurophysiol, vol. 129, no. 4, pp. 766–782, Apr. 2018. [CrossRef]
- U. Smailovic et al., “Regional Disconnection in Alzheimer Dementia and Amyloid-Positive Mild Cognitive Impairment: Association Between EEG Functional Connectivity and Brain Glucose Metabolism,” Brain Connect, vol. 10, no. 10, p. 555, Dec. 2020. [CrossRef]
- L. Cocchi, L. L. Gollo, A. Zalesky, and M. Breakspear, “Criticality in the brain: A synthesis of neurobiology, models and cognition,” Prog Neurobiol, vol. 158, pp. 132–152, Nov. 2017. [CrossRef]
- M. Scheffer et al., “Early-warning signals for critical transitions,” Nature, vol. 461, no. 7260, pp. 53–59, Sep. 2009. [CrossRef]
- J. M. Beggs and N. Timme, “Being critical of criticality in the brain,” Front Physiol, vol. 3 JUN, 2012. [CrossRef]
- G. Tononi, O. Sporns, and G. M. Edelman, “A measure for brain complexity: relating functional segregation and integration in the nervous system.,” Proc Natl Acad Sci U S A, vol. 91, no. 11, p. 5033, May 1994. [CrossRef]
- R. Legenstein and W. Maass, “Edge of chaos and prediction of computational performance for neural circuit models,” Neural Netw, vol. 20, no. 3, pp. 323–334, Apr. 2007. [CrossRef]
- C. J. Stam et al., “Disturbed fluctuations of resting state EEG synchronization in Alzheimer’s disease,” Clin Neurophysiol, vol. 116, no. 3, pp. 708–715, 2005. [CrossRef]
- Vyšata et al., “Change in the characteristics of EEG color noise in Alzheimer’s disease,” Clin EEG Neurosci, vol. 45, no. 3, pp. 147–151, 2014. [CrossRef]
- J. A. Bonachela and M. A. Muñoz, “Self-organization without conservation: true or just apparent scale-invariance?,” Journal of Statistical Mechanics: Theory and Experiment, vol. 2009, no. 9, May 2009. [CrossRef]
- L. J. Fosque, R. V. Williams-García, J. M. Beggs, and G. Ortiz, “Evidence for Quasicritical Brain Dynamics,” Phys Rev Lett, vol. 126, no. 9, Mar. 2021. [CrossRef]
- N. J. Kopell, H. J. Gritton, M. A. Whittington, and M. A. Kramer, “Beyond the connectome: the dynome,” Neuron, vol. 83, no. 6, pp. 1319–1328, 2014. [CrossRef]
- T. Ezaki, E. Fonseca dos Reis, T. Watanabe, M. Sakaki, and N. Masuda, “Closer to critical resting-state neural dynamics in individuals with higher fluid intelligence,” Commun Biol, vol. 3, no. 1, Dec. 2020. [CrossRef]
- L. Spíndola and S. M. D. Brucki, “Prospective memory in Alzheimer’s disease and Mild Cognitive Impairment,” Dement Neuropsychol, vol. 5, no. 2, pp. 64–68, 2011. [CrossRef]
- C. Agogiatou, N. Markou, E. Poptsi, and M. Tsolaki, “Is it Possible the Training of Prospective Memory to Enhance Activities of Daily Living and Executive Function in People with Mild Cognitive Impairment? A Single-blind Randomized Controlled Trial,” Acta Scientific Medical Sciences, vol. 4, no. 10, pp. 102–113, Sep. 2020. [CrossRef]
- Y. F. Contoyiannis, F. K. Diakonos, and A. Malakis, “Intermittent dynamics of critical fluctuations,” Phys Rev Lett, vol. 89, no. 3, 2002. [CrossRef]
- Y. F. Contoyiannis, F. K. Diakonos, C. Papaefthimiou, and G. Theophilidis, “Criticality in the relaxation phase of a spontaneously contracting atria isolated from a frog’s heart,” Phys Rev Lett, vol. 93, no. 9, Aug. 2004. [CrossRef]
- E. K. Kosmidis, Y. F. Contoyiannis, C. Papatheodoropoulos, and F. K. Diakonos, “Traits of criticality in membrane potential fluctuations of pyramidal neurons in the CA1 region of rat hippocampus,” Eur J Neurosci, vol. 48, no. 6, pp. 2343–2353, Sep. 2018. [CrossRef]
- Y. F. Contoyiannis and F. K. Diakonos, “Criticality and intermittency in the order parameter space,” Phys Lett A, vol. 268, no. 4–6, pp. 286–292, Apr. 2000. [CrossRef]
- S. Romero, M. A. Mañanas, and M. J. Barbanoj, “A comparative study of automatic techniques for ocular artifact reduction in spontaneous EEG signals based on clinical target variables: a simulation case,” Comput Biol Med, vol. 38, no. 3, pp. 348–360, Mar. 2008. [CrossRef]
- Y. F. Contoyiannis, S. M. Potirakis, and F. K. Diakonos, “Wavelet-based detection of scaling behavior in noisy experimental data,” Phys Rev E, vol. 101, no. 5–1, May 2020. [CrossRef]
- D. L. Donoho and J. M. Johnstone, “Ideal spatial adaptation by wavelet shrinkage,” Biometrika, vol. 81, no. 3, pp. 425–455, Sep. 1994. [CrossRef]
- T. Martin et al., “Brain regions and their dynamics in prospective memory retrieval: A MEG study,” International Journal of Psychophysiology, vol. 64, no. 3, pp. 247–258, Jun. 2007. [CrossRef]
- D. L. Schacter and D. R. Addis, “The cognitive neuroscience of constructive memory: remembering the past and imagining the future,” Philos Trans R Soc Lond B Biol Sci, vol. 362, no. 1481, pp. 773–786, May 2007. [CrossRef]
- S. Shomstein, “Object-based attention: strategy versus automaticity,” Wiley Interdiscip Rev Cogn Sci, vol. 3, no. 2, pp. 163–169, Mar. 2012. [CrossRef]
- S. Deprez, M. Vandenbulcke, R. Peeters, L. Emsell, F. Amant, and S. Sunaert, “The functional neuroanatomy of multitasking: combining dual tasking with a short term memory task,” Neuropsychologia, vol. 51, no. 11, pp. 2251–2260, Sep. 2013. [CrossRef]
- M. P. Van Den Heuvel, R. C. W. Mandl, R. S. Kahn, and H. E. Hulshoff Pol, “Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain,” Hum Brain Mapp, vol. 30, no. 10, pp. 3127–3141, Oct. 2009. [CrossRef]
- B. van Vugt, T. van Kerkoerle, D. Vartak, and P. R. Roelfsema, “The Contribution of AMPA and NMDA Receptors to Persistent Firing in the Dorsolateral Prefrontal Cortex in Working Memory,” J Neurosci, vol. 40, no. 12, pp. 2458–2470, Mar. 2020. [CrossRef]
- H. Shibata and K. Ogawa, “Dorsal premotor cortex is related to recognition of verbal and visual descriptions of actions in the first-person perspective,” Neurosci Lett, vol. 687, pp. 71–76, Nov. 2018. [CrossRef]
- H. Liu et al., “Changes in Brain Lateralization in Patients with Mild Cognitive Impairment and Alzheimer’s Disease: A Resting-State Functional Magnetic Resonance Study from Alzheimer’s Disease Neuroimaging Initiative,” Front Neurol, vol. 9, no. FEB, Feb. 2018. [CrossRef]
- T. Montez et al., “Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease,” Proc Natl Acad Sci U S A, vol. 106, no. 5, pp. 1614–1619, Feb. 2009. [CrossRef]
- R. L. Buckner, “Memory and executive function in aging and ad: Multiple factors that cause decline and reserve factors that compensate,” Neuron, vol. 44, no. 1, pp. 195–208, Sep. 2004. [CrossRef]
- F. Clment and S. Belleville, “Compensation and disease severity on the memory-related activations in mild cognitive impairment,” Biol Psychiatry, vol. 68, no. 10, pp. 894–902, Nov. 2010. [CrossRef]
- Flores-Sandoval A. A. et al., “Spectral power ratio as a measure of EEG changes in mild cognitive impairment due to Alzheimer’s disease: a case-control study,” Neurobiol Aging, vol. 130, pp. 50–60, Oct. 2023. [CrossRef]
- T. T. Trinh, C. F. Tsai, Y. T. Hsiao, C. Y. Lee, C. Te Wu, and Y. H. Liu, “Identifying Individuals With Mild Cognitive Impairment Using Working Memory-Induced Intra-Subject Variability of Resting-State EEGs,” Front Comput Neurosci, vol. 15, Aug. 2021. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).