1. Introduction
In the era of climate change, pearl millet (
Pennisetum glaucum L. R. Br) could be one of the most climate-resilient crops due to its better adaptability to marginal environments and high nutritional values [
1,
2,
3,
4,
5,
6]. Pearl millet is a subtropical grain and forage crop. As forage, it is grown in several sub-tropical countries as well as in the United States of America. Pearl millet is also advantageous as a dual-purpose crop because it is an excellent livestock feed, both as grain and fodder [
7]. Pearl millet outperforms all other cereals such as wheat, maize, rice, sorghum, and barley due to its high photosynthetic efficiency, higher dry matter production capacity, and ability to survive under adverse agro-climatic conditions with lower inputs and higher economic returns [
1,
2,
8,
9]. Due to its heat stress tolerance and water use efficiency, pearl millet is well adapted to harsh climates where other crops fail to produce economic yields [
10,
11].
Compared to maize, Sudan grass and sorghum, pearl millet is privileged with several desirable forage attributes, e.g. it is known to be more tolerant to abiotic stresses, such as drought, salinity, high temperature and soil nutrient deficiency, compared to other cereal crops such as sorghum, wheat, maize and rice [
12,
13], high leafiness and tillering capacity, high percentage of crude protein, and high forage yield [
14,
15], in addition to excellent regenerative ability, which permits multi-cut forage production and grazing [
16]. Moreover, it is free from prussic acid at all growth stages [
14].
The year 2023 has been declared by the United Nations General Assembly as the International Year of Millets [
17] because of their suitability for cultivation under adverse and changing climatic conditions. Millets in general, can grow on marginal areas such as arid lands with minimal inputs and are resilient to changes in climate. As such, they are an ideal solution for countries, especially in the arid areas, to increase self-sufficiency and reduce dependance on imported cereal grains [
17].
Crop yield is a function of genotype, environment and their interaction. Evaluation of genotype × environment interaction (GEI) is necessary when different genotypes respond differently to different environments [
18]. A significant GEI can seriously compromise efforts to select superior genotypes for crop adaptation and variety development programs [
18,
19,
20]
For successful variety selection, it is necessary to study the nature of association among different traits of interest. Therefore, several methods have been identified and used to evaluate the GEI in different crops including, pearl millet breeding [
21]. In this study, besides the additive main effects and multiplicative interaction (AMMI) analysis to identify superior and well adapted genotypes, graphical discrimination of the genotypes and environments using the GGE biplot analysis was performed [
18,
19,
21].
Pearl millet was probably domesticated 5000 years ago in Africa in the savannah south of the Sahara and west of the Nile [
9,
22]. In Sudan, it is well adapted to almost all over the country. Ironically however, it had never been considered as a forage crop to any degree. Instead, it is grown mainly for grain production in the warm and drier regions of western Sudan (Darfur and Kordofan), with little in the central clay plains, e.g. Gezira, Gadarif, Sennar and Blue Nile [
23]. It has desirable forage attributes as mentioned above [
16]; nevertheless, it has not been grown for forage production in Sudan.
Exception to few introduced grain varieties, research on pearl millet is limited in Sudan. In fact, more than 3200 accessions were collected from different parts of Sudan and available in the Agricultural Plant Genetic Resources for Research and Conservation Centre (APGRC) of the Agricultural Research Corporation in Wad Medani, Sudan (
https://www.genesys-pgr.org/a/overview/v2GajzMOG5e, accessed in November 6, 2023). Such a huge number of accessions could be utilized to improve the crop for both grain and forage production.
Differential fresh matter yield [
24], forage quality [
25] and genetic diversity [
26,
27] among the pearl millet genotypes were evident. However, not even a single forage pearl millet variety has been developed in Sudan. Therefore, the objectives of this study were to evaluate the field performance, stability and the GEI for forage yield and other related traits of selected pearl millet (
Pennisetum glaucum (L) R. Br) genotypes under different environments.
2. Materials and Methods
2.1. Experimental Sites and Plant Materials
The experiment was conducted for three consecutive seasons at Rahad site (2016/2017, R1; 2017/2018, R2 and 2018/2019, R3) and two seasons at Gezira and Sennar sites (2016/2017, G1, S1 and 2018/2019, G2, S2, respectively). The Gezira Research Station Farm (GRSF) is located in Wad Medani, Gezira State (latitude 14o 24' N, longitude 33o 29' E and altitude 406.9 m above sea level (asl)). The soil of the GRSF is heavy cracking clay and alkaline (clay 58%, pH 8.3, organic matter 0.02%, nitrogen 0.25%, phosphorus 0.06% and potash 3.0%). The second site was located in Rahad Research Station Farm (RRSF), Elgadarif state (latitude 13o 31' to 14 o 25' N, longitude 33o 31' to 34o 32' E). The soil of the RRSF is heavy, cracking clay (clay 67%, pH 7.8, organic carbon 0.74%, nitrogen 0.04% and phosphorus 1.5-2.2 ppm), while the third site was located in Sennar Research Station Farm (SRSF), Sennar state (latitude 13o 33' N, longitude 33o 34' E and Altitude 421 m asl). The soil of the SRSF is vertisol with 60% clay content, 7.8-8.5 pH, about 0.4-0.5% organic carbon and 0.05% total nitrogen.
The pearl millet genotypes used in this study were provided by Agricultural Plant Genetic Resources for Research and Conservation Centre (APGRC) of the Agricultural Research Corporation (ARC), Sudan. Two hundred pearl millet accessions, mostly collected from the Darfur and Kordofan regions of Sudan, were evaluated and one hundred of them were selected for having glabrous (non-hairy) leaves. Twenty-five genotypes were identified as top ranking for their yield. The pearl millet accessions were purified by pure line selection, so, the numerical suffix -1 was added to the original accession number to distinguish it from the original accession (
Table S1).
2.2. Experimentation and Data Collection
The selected
twenty-five genotypes were arranged in Alpha lattice design with three replications. In each season, the land was disc ploughed and harrowed, leveled and ridged to 80 cm. The plot size was 4 ridges of 5.25-meter length each with a spacing of 0.8 m between ridges. Planting rate was 5 seeds/hole and then thinned later to three plants/hole. The sowing in each season and location was done in the first week of July. Irrigation was done every 12-14 days according to weather conditions. Fertilizer was applied at the second irrigation (187 kg urea/ha). The experiments were kept free from weeds by hand weeding and the ridges were kept intact by earthing up after every weeding. Days to 50% flowering (DF) was recorded as the number of days from sowing to a stage when 50% of plants in the plot reached flowering. When the genotype reached 85% flowering stage, data were collected on plant height (cm), number of culms m
-2, leaf to stem ratio, stem girth (cm) and fresh forage yield (t/ha) as previously described [
24]. Briefly, number of culms m
-2 (CLM) was recorded as the average number of culms of three counts per square meter in each plot. Plant height (PH) was the average of five measurements of the main shoot, and leaf to stem ratio (LSR) was measured as the average of three plants on a dry matter basis. The accessions were harvested manually from the ground level in an area of 3.2 m
-2 and the fresh matter yield (FY) was weighed immediately in the field.
2.3. Statistical Analysis
The statistical analysis was done using Genstat Twenty-second Edition (VSN International Ltd.) [
28]. The AMMI Stability Value (ASV) was used for the stability analysis of genotypes, in addition to the non-parametric index, genotype selection index (GSI), calculated by the following formula [
29]:
GSI = RASV + RY
Where RASV is the rank of ASV, and RY is the rank of mean fresh matter yield of genotypes across environments.
The violin plots were performed using GraphPad Prism version 10.1.2 for Windows, GraphPad Software, Boston, Massachusetts USA,
www.graphpad.com. The heatmap plots were created using the Pheatmap package in R software [
30].
3. Results
3.1. Genotypic and Environmental Effects
Table 1 summarizes the minimum, maximum and mean values of fresh matter yield (t/ha), days to 50% flowering, plant height (cm), leaf to stem ratio, number of culms m
-2 and stem gith (cm) of the 25 pearl millet genotypes grown across different environments (combination of location and season). The combined analysis revealed that environments and genotypes had highly significant effects on all traits studied except the genotypic effect on stem girth (
Table 1). The G × E interaction effects were also highly significant for all traits studied except for stem gith which was significant at P = 0.025.
3.2. Fresh Matter Yield (FY)
The FY of the twenty-five genotypes in the seven environments is shown in
Table 2. Across all genotypes, the mean FY was highest at Rahad 2018/19 (R3), followed by Gezira 2018/19 (G2), whereas the lowest FY was recorded at Rahad 2016/17 (R1). Across all environments, Genotypes G14, G01, G12 and G22 outyielded the check (G25) by 20.7, 16.5, 11 and 9.8%, respectively. Compared to the check, the FYs of genotype G14 were higher in the seven environments, whereas that of G01, G12 and G22 were higher in 5, 4 and 3 environments, respectively (
Table 2).
3.3. Growth Attributes
3.3.1. Days to Flowering
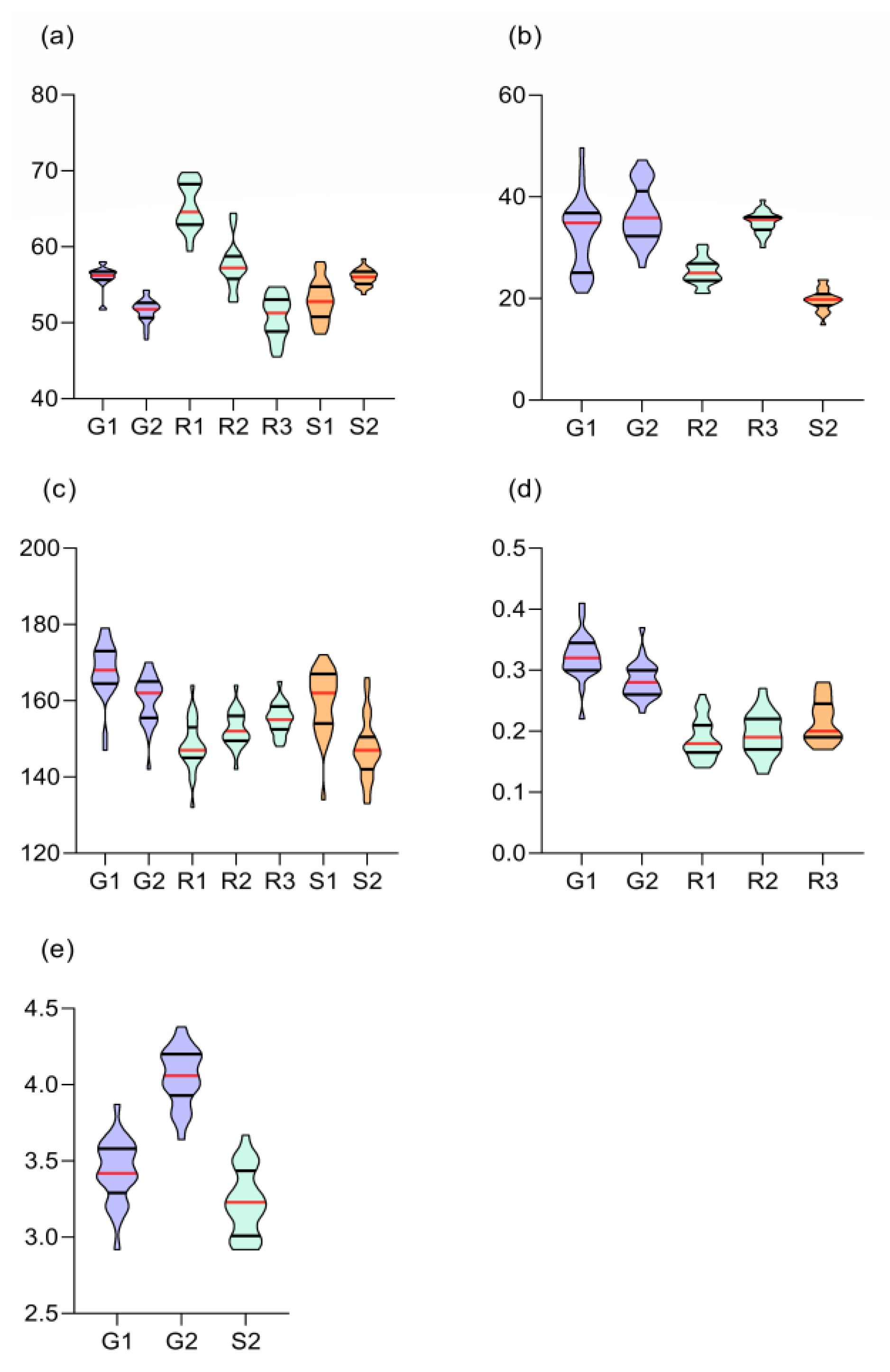
Across genotypes, the mean days to flowering was latest at R1, which showed the widest range of flowering together with R2. Narrow flowering ranges were observed at S2, G1 and G2 (
Figure 1a). In R1 and R2, the G12 (70 and 65 days, respectively) and G22 (69 and 57 days, respectively) were significantly late in attaining flowering compared to the check (60 and 53 days, respectively) (
Table S1). Genotypes G01 and G14 were comparable to the check in R1, but were significantly earlier (49 and 48 days, respectively) than the check (54 days) in R2. At Sinna, G01, G12, G14 and G22 were significantly different from the check in S1 but not in S2 (
Table S1).
3.3.2. Number of Culms
The highest numbers of culms m
-2 were recorded at G1 and G2, whereas the lowest number was recorded at S2. Genotypes showed wide ranges at both Gezira sites. In contrary, the S2 showed a narrow range among the genotypes in the number of culms m
-2 (
Figure 1b). At G1 and G2, genotypes G24, G05 and G12 showed consistently higher number of culms than the check (G25), whereas at R2, G18, G02 and G19 were the top-ranking genotypes. At R3, G17, G09 and G16 ranked top, whereas at S2, G13, G08 and G20 were the top-ranking genotypes. Across all environments, G24 and G09 showed 16.2 and 11.4% higher number of culms over the check, respectively (
Table S2).
3.3.3. Plant Height
The mean plant height at G1 and G2 was 168 and 160 cm, respectively. Plants were shorter at the Rahad sites, with the mean plant heights of 148, 152 and 155 cm, at R1, R2 and R3, respectively. Narrow ranges in plant height among the genotypes were observed at R2 and R3, whereas the widest range was observed at S1, followed by S1 and G1 (
Figure 1c). Plant height at S1 was taller (160 cm) than that at S2 (147 cm). Except for R3, the 5 tallest genotypes were taller than the check variety in all environments (
Table S3).
3.3.4. Leaf to Stem Ratio
The Gezira environments showed higher leaf to stem ratio (LSR) values compared to the Rahad environments (
Figure 1d). Within the same location at Gezira, the LSR values ranged from 0.283 to 0.323, whereas at Rahad the range was from 0.189 to 0.217. Across all environments, G16 showed the highest LSR values, whereas G01 showed the lowest value. At all environments, the five top ranking genotypes had higher LSR than the check genotype. Across the five environments, G16, G22, G21 and G24 were the top-ranking genotypes (
Table S4).
3.3.5. Stem Girth
The stem girth was measured in only three environments (G1, G2 and S2). As shown earlier, genotypes had no effect on stem girth; however, the effect of the environment was highly significant. The stem girth at G2 was significantly higher than that at both G1 and S2 (
Figure 1e). The mean stem girth at G2 was 4.05 cm compared to 3.43 and 3.23 cm at G1 and S2, respectively (
Table S5).
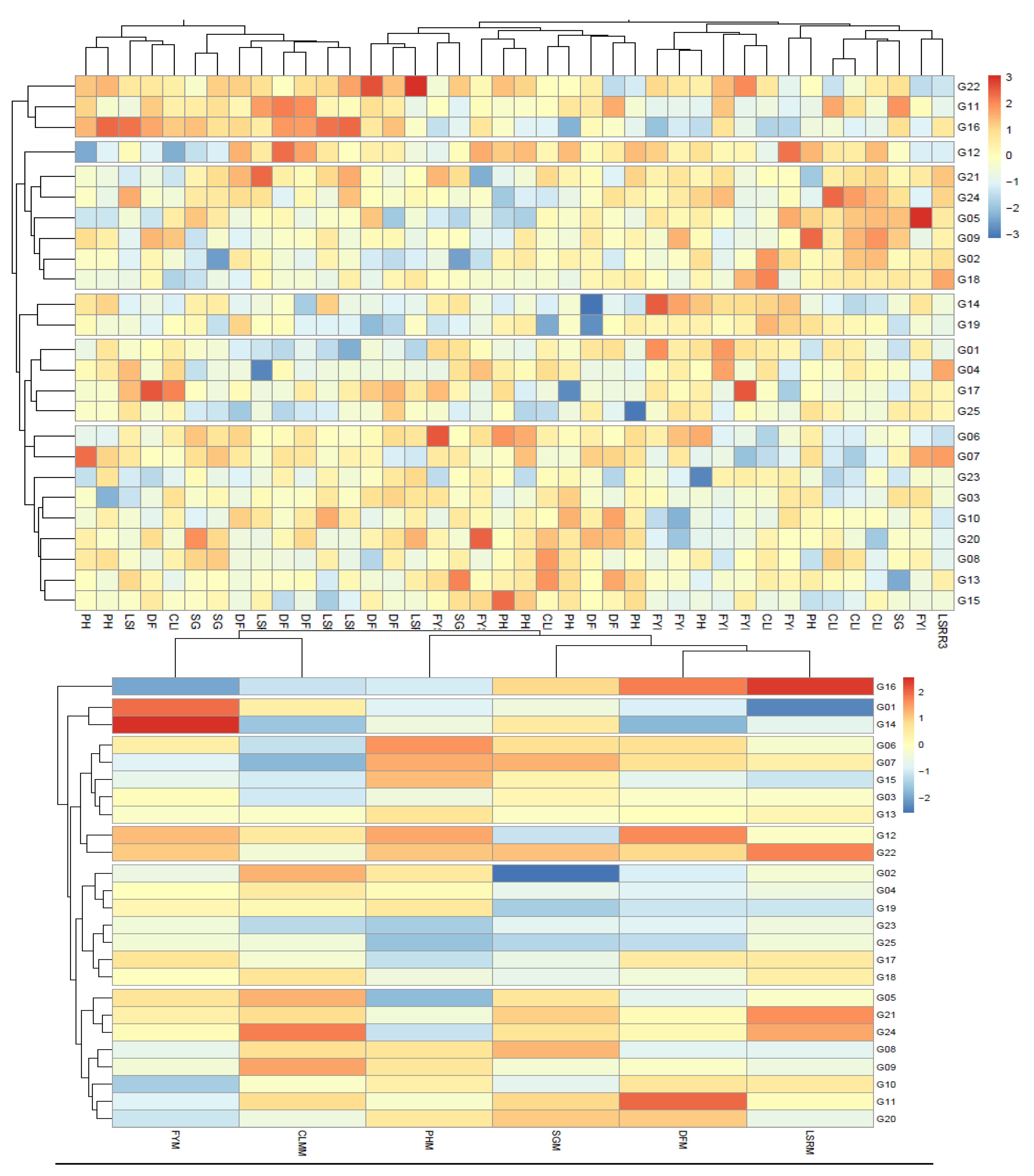
3.4. Heatmap Clustering Analysis
The cluster heatmap analysis illustrated the responses of different forage-related traits of the 25 pearl millet genotypes grown in seven environments. The 40 trait combinations clustered the 25 pearl millet genotypes into six groups (
Figure 2a). The largest group consisted of nine genotypes, followed by a group consisted of six genotypes. The check variety was grouped with G01, G04 and G17. Genotypes G14 and G19 were placed in a separate group. Genotypes G11, G19 and G22 were grouped together, while G12 was clustered alone (
Figure 2a).
When the means of the six traits were used, the 25 pearl millet genotypes were also clustered into six groups (
Figure 2b). However, there were differences in the distribution of the genotypes among these clusters. The largest group consisted of eight genotypes, including genotypes with forage yield (FY) around or below the mean, followed by a group consisted of seven genotypes, including the check variety, which had FY around the mean and with low SG values. The third group consisted of five genotypes, most of which had below average CLM. Genotypes G14 and G01 were placed in a separate group as they were the top fresh matter yielders. Similarly, genotypes G12 and G22 were grouped together because of their high FY and tall height. The genotype with the lowest FY and highest LSR (G16) was clustered alone (
Figure 2b).
3.5. Genotype x Environment Interaction and Stability of Fresh Matter Yield
The analysis of variance of the additive Main effects and Multiplicative Interaction (AMMI) showed that, genotypes (G), environment (E) and their interaction (GEI) significantly (P≤0.001) affected the fresh matter yield of the pearl millet genotypes (
Table 3). Based on the total treatment sum squares, E, G, and GEI accounted for 70.4%, 4.3%, and 25.5% of the variance, respectively. The GEI was partitioned into first, second, and third interaction principal component axes (IPCA1, IPCA2, and IPCA3). The IPCA1, IPCA2, and IPCA3 were highly significant and contributed 31.8, 29.2, and 13.4%, respectively, to the total GEI sum of squares (
Table 3).
3.5.1. AMMI Stability Value and Genotype Selection Index
Based on the ASV of fresh forage yield, genotypes G11, G13, G19, G25, and G14 showed the lowest ASVs of 0.129, 0.184, 0.198, 0.397, and 0.450, respectively (
Table 4). Genotypes G01 and G12, with their high grain yield, showed moderate ASVs, whereas G22 was one of the most unstable genotypes according to ASV. However, based on GSI, which combines the rank of ASV with the rank of the genotypes according to their fresh yield, G14, G19, G12, G06, and G01 showed low values and ranked 1st to 5th, respectively. Genotypes G11, G13, and G25, which had low ASVs, were ranked 7th, 6th, and 12th, respectively, by their GSI values (
Table 4).
3.5.2. AMMI Estimate
The best four pearl millet genotypes were arranged based on AMMI estimate in the seven environments. The genotype G14 was among the best four genotypes in four environments, whereas G03, G17 and G22 were among the top four genotypes in three environments. Each of G01, G04, G05, G06 and G18 appeared in two environments among the best four genotypes. The G25 (check) was not among the best four ranking genotypes in any environment (
Table 5).
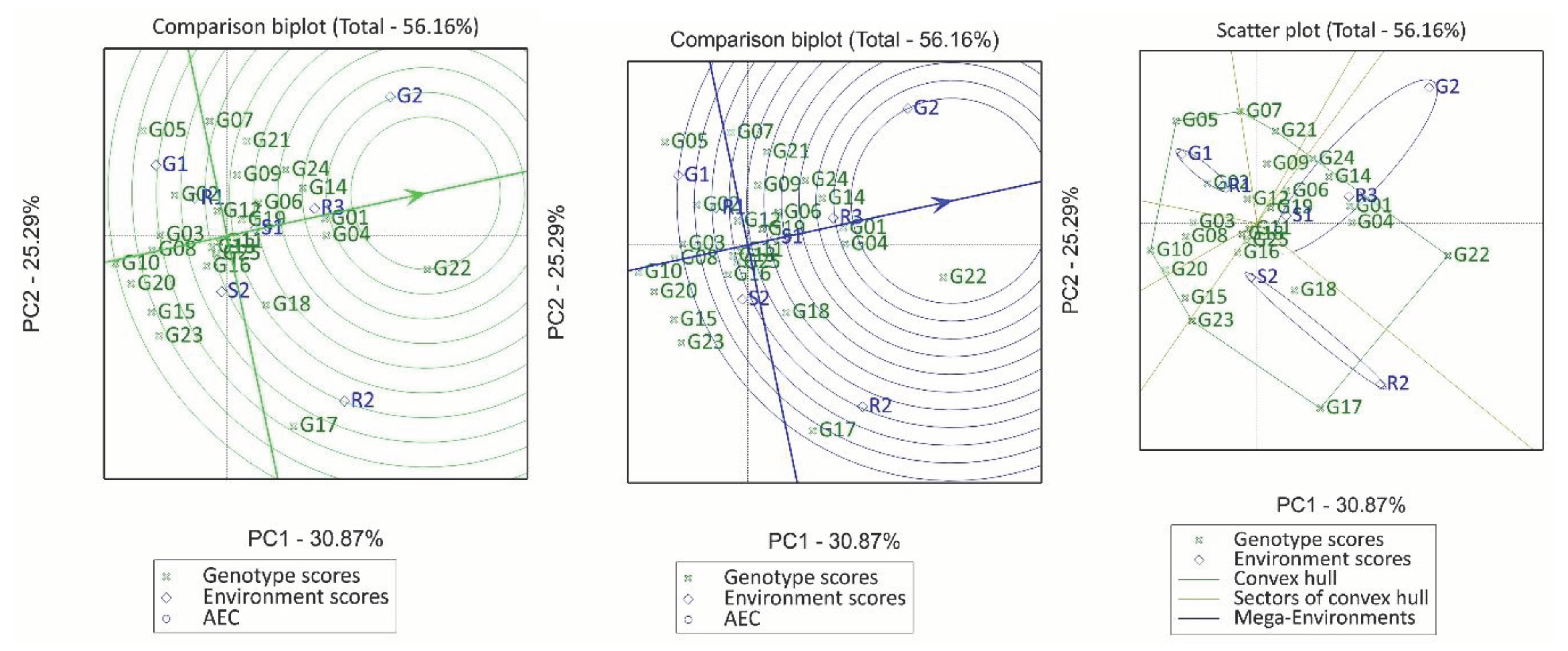
3.5.3. GGE Scattered Biplot
The scattered biplot of the GGE analysis showed that 56.16% of the variance was due to PC1 (30.87%) and PC2 (25.29%) (
Figure 3a). The seven environments were grouped into three mega-environments. The first mega-environment comprised S1 (Sennar 2016/17), G2 (Gezira 2016/17), R3 (Rahad 2018/19), with G22 being the winning genotype. The second mega environment included G1 (Gezira 2016/17) and R1 (Rahad 2016/17) where G05 performed well, whereas the third mega-environment included R2 (Rahad 2017/18) and S2 (Sennar 2018/19) with G17 being the winning genotype. Environments G2, R2 and R3 were the most discriminating environments, while S1, R1 and S2 were the least discriminating. Both S1 and R3 were more representative, but R3 was a representative and discriminating environment (
Figure 3b). On the other hand, R2 and G2 were less representative but highly discriminative and thus are good for identifying specific adapted genotypes. Relative to the ideal genotype (with high mean yield across all environments and high stability), G22, G01, G04, and G14 were the most desirable genotypes (
Figure 3c). On the other hand, G12, G20, G15 and G23 were the least desirable genotypes due to their poor performance across all environments. Although G25 (the check) was more stable, its fresh matter yield was just similar to the grand mean of the fresh matter yield.
4. Discussion
The importance of the current study stems from the fact that it is the first multi-location evaluation of some high-ranking forage-based selected pearl millet genotypes from the first exploratory study for forage related variations among some accessions of Sudan collection of pearl millet [
24,
31]. In this study, it was evident that some pearl millet genotypes produced fresh matter yield significantly higher than the check at three locations. This, in turn, encourages further exploration of forage suited accessions among Sudan pearl millet collections.
Sudan is endowed with a huge livestock number as well as very vast range lands. Paradoxically, however, the livestock products are neither cheap in the local markets nor contribute as much as their potential to exports. This paradox could largely be attributed to the fact that the animal production system is more extensive rather than intensive. Hence, livestock are mainly reared on natural rangelands, crop residues, and little cultivated forage crops. A practical approach to improve the situation of the animal production is through establishing intensive production farms for both dairy and meat production. This is highly plausible due to the increasing adoption of modern irrigation systems, such as central pivot irrigation, by several farmers. Currently, the most popular forages under these systems are long-standing perennial crops, mainly, Rhodes grass, and alfalfa. Adopting a two-course legume/grass rotation by introducing crops with short life cycle could be a more feasible and productive system, and improve forage yield and nutritive value [
15,
32]. The current study to explore the potential of pearl millet as an irrigated forage crop could provide a short duration of multiple cut forage grass. As an endogenous crop in Sudan, pearl millet showed a huge number of accessions with wide variations.
The estimated annual forage gap in Sudan is more than 28 million tons [
25]. However, about 23 million tons (82%) is in the form of production rations (i.e., concentrates). Expansion of concentrate production in Sudan is highly unlikely under the current crop production system. The situation is further exacerbated by the growing trend to export alfalfa hay. An alternative way to minimize the concentrate gap in Sudan should be based on the horizontal and vertical expansion in the production of high-quality forages. From this point of view, the importance of pearl millet as a forage crop to play such a role could be appreciated.
Pearl millet is privileged over the common cereal forages in Sudan, such as sorghum, barley, and maize, for its high yields and quality, in addition to its high regenerative ability, suitability to more than one season and its freedom of prussic acid [
14,
16]. Compared to forage sorghum in Sudan, the fresh forage yield of the higher yielding pearl millet genotypes of this study, are either similar or slightly lower [
33,
34].
The analysis of variance of growth attributes revealed highly significant differences among the pearl millet genotypes. This indicated that the genotypes used in this study, although screened and selected in previous studies [
24,
25], had sufficient variability. The results also showed that growth attributes (Days to 50 % flowering, plant height, number of culms m
-2, leaf/stem ratio) were significantly affected by genotype, environment, and their interaction. The significant difference among the tested pearl millet genotypes in the growth attributes may reflect their differential responses to environment. The existence of genotype x environment interaction is a challenging for crop improvement and could hamper genetic gain, especially under stress conditions such as those usually face pearl millet production [
10,
11,
19,
35]. Understanding the environmental causes of the GEI could facilitate the identification of adaptive plant traits, and may lead to a more rational choice of test environments for the crop breeding programs [
19,
20,
35].
The cluster heatmap analysis conducted on 25 pearl millet genotypes grown in seven environments using various forage-related traits illustrated the grouping of genotypes based on similarities or dissimilarities in these forage-related traits. The 25 pearl millet genotypes were grouped into six distinct clusters based on these trait combinations. Despite the fact that the pearl millet genotypes used in this study were selected from a larger population on the basis of forage-attributed traits [
24,
25], the cluster analysis provided insights into the diversity of trait responses among the 25 pearl millet genotypes. A better understanding of the pattern of the trait variation could provide useful information to the forage breeding programs aiming at developing cultivars with desired forage-attributed traits under different environmental conditions.
Information on GEI is important for plant breeders to develop improved, stable and better adapted varieties. In this study, the statistical analysis showed that the genotype and environment main effects and the genotype by environment interaction were very highly significant (P≤0.001) for forage yield and all other traits except stem girth, indicating differential response of genotypes a cross testing environments [
19].
The AMMI analysis also revealed significant effects of genotype, environment, and their interaction (GEI) on fresh matter yield. The substantial proportion of the variance attributed to the environmental effect indicated the great impact of the environment on the fresh matter yield of pearl millet. The interaction between the environment and the genotype was also an important source of variation, which was further partitioned into three IPCAs, all of which were found to be significant.
The combined use of the AMMI Stability Value (ASV) and the Genotype Selection Index (GSI) to evaluate the genotype stability and adaptability across the environments provided better insights into genotype performance. Genotype G14, with lower values of both ASV and GSI, displayed desirable stability and performance across environments compared to genotypes, demonstrating a consistent high yield across different conditions. Other genotypes, such as G12, G01, also showed varying levels of stability and performance.
The ranking of the genotypes according to the AMMI estimate, based on their performance across different environments, reinforced the superiority of G14, which was among the best four genotypes in four environments. Genotypes G03, G17 and G22 were among the top four genotypes in three environments, whereas G01, G04, G05, G06 and G18 were among the top four ranking genotypes in two environments. On the other hand, the check genotype, G25, was not among the top four ranking genotypes in any of the seven environments.
5. Conclusions
The wide range of performance observed among the tested pearl millet genotypes tested in this study indicated the possibility of developing pearl millet varieties for forage production directly through evaluation of the wealth available collections or indirectly through a crop breeding program. However, the existence of highly significant genotype by environment interaction needs to be carefully addressed. Across all environments, genotypes G14, G01, G12 and G22 out-yielded the check by 20.7, 16.5, 11.0 and 9.8%, respectively. The results of ASV and the GSI combined with the AMMI estimate-based selection showed that genotypes G14, G22 and G01 were the most stable and adapted genotypes, demonstrating their superiority over the check genotype, which exhibited average values for the above attributes.
Currently, most of the crop breeding programs in Sudan depend mainly on introducing genotypes from abroad. Henceforth, we believe that the significant variations found in this study could greatly impact the future breeding efforts. This is particularly true for indigenous crops such as pearl millet and sorghum, due to the large number of accessions available in the Sudan National Genebank.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1. Days to 50% flowering of 25 pearl millet genotypes grown in seven environments at Gezira (G1 and G2), Rahad (R1, R2 and R3) and Sennar (S1 and S2) in Sudan; Table S2. Number of culms m-2 of 25 pearl millet genotypes grown at Gezira (G1 and G2) Rahad (R2 and R3) and Sennar (S2); Table S3. Plant height (cm) of 25 pearl millet genotypes grown in seven environments at Gezira (G1 and G2), Rahad (R1, R2 and R3) and Sennar (S1 and S2); Table S4. Leaf to stem ratio of 25 pearl millet genotypes grown during 2016/2017 and 2018/2019 at Gezira (G1 and G2) and during 2016/17-2018/2019 at Rahad; Table S5. Stem girth (cm) of 25 pearl millet genotypes grown during the seasons of 2016/2017 and 2018/2019 at Gezira site and 2018/2019 at Sennar site.
Author Contributions
“Conceptualization, S.A.E.B., M.A.M.K., and I.S.A.T.; methodology, S.A.E.B,.; software, I.S.A.T., N.M.K; validation, S.A.E.B, and I.S.A.T.; formal analysis, S.A.E.B, and I.S.A.T.; investigation and data curation, S.A.E.B, A.A.A., M.A.M., A.M.E.H.; resources, M.A.M.K., E.I.M., and S.A.E.B.; writing—original draft preparation, S.A.E.B, and I.S.A.T..; writing—review and editing, S.A.E.B, M.A.M.K., A.A.A., M.A.M., A.M.E.H., E.I.M., N.M.K, H.T., and I.S.A.T.; supervision, M.A.M.K. All authors have read and agreed to the published version of the manuscript.”
Data Availability Statement
All data used in this study are provided in tables, figures, and supplementary materials.
Acknowledgments
The original pearl millet accessions were kindly provided by Agricultural Plant Genetic Resources for Research and Conservation Centre (APGRC), Agricultural Research Corporation (ARC), Sudan. The authors are grateful to the all technical and supporting staff at different research stations who assisted in conducting the experiments and collecting the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jukanti, A.K. ..; Gowda, C.L.L.; Rai, K.N.; Manga, V.K.; Bhatt, R.K. Crops that feed the world 11. Pearl Millet (Pennisetum glaucum L.): an important source of food security, nutrition and health in the arid and semi-arid tropics. Food Secur. 2016, 8, 307–329. [Google Scholar] [CrossRef]
- Satyavathi, C.T.; Ambawat, S.; Khandelwal, V.; Srivastava, R.K. Pearl Millet: A Climate-Resilient Nutricereal for Mitigating Hidden Hunger and Provide Nutritional Security. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Lauriault, L.M.; Darapuneni, M.K.; Martinez, G.K. Pearl Millet-Cowpea Forage Mixture Planting Arrangement Influences Mixture Yield and Nutritive Value in Semiarid Regions. Crops 2023, 3, 266–275. [Google Scholar] [CrossRef]
- Andrews DJ, Kumar KA. Pearl millet for food, feed, and forage. Advances in Agronomy 1992, 48, 89–139.
- Pattanashetti SK, Upadhyaya HD, Dwivedi SL, Vetriventhan M, Reddy KN. Pearl millet. In: Genetic and Genomic Resources for Grain Cereals Improvement. Elsevier; 2016. p. 253–289.
- Tonapi VA, Thirunavukkarasu N, Gupta SK, Gangashetty PI, Yadav OP. Pearl Millet in the 21st Century. Singapore: Springer Nature Singapore; 2024.
- Yadav, O.P.; Gupta, S.K.; Govindaraj, M.; Sharma, R.; Varshney, R.K.; Srivastava, R.K.; Rathore, A.; Mahala, R.S. Genetic Gains in Pearl Millet in India: Insights Into Historic Breeding Strategies and Future Perspective. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Nambiar VS, Sareen N, Shahu T, Desai R, Dhaduk JJ, Nambiar S. Potential Functional Implications of Pearl millet (Pennisetum glaucum) in Health and Disease. J Appl Pharm Sci 2011, 01, 62–67.
- Andrews DJ, Kumar KA. Pearl millet for food, feed, and forage. Advances in Agronomy 1992, 48, 89–139.
- Garin, V.; Choudhary, S.; Murugesan, T.; Kaliamoorthy, S.; Diancumba, M.; Hajjarpoor, A.; Chellapilla, T.S.; Gupta, S.K.; Kholovà, J. Characterization of the Pearl Millet Cultivation Environments in India: Status and Perspectives Enabled by Expanded Data Analytics and Digital Tools. Agronomy 2023, 13, 1607. [Google Scholar] [CrossRef]
- Yadav, O.P.; Rai, K.N. Genetic Improvement of Pearl Millet in India. Agric. Res. 2013, 2, 275–292. [Google Scholar] [CrossRef]
- Vadez, V.; Hash, T.; Bidinger, F.R.; Kholova, J. II. 1.5 Phenotyping pearl millet for adaptation to drought. Front. Physiol. 2012, 3, 386. [Google Scholar] [CrossRef]
- Hoffman GT, Maas E V, Meyer JL, Prichard TL, Lancaster DR. Salt tolerance of corn in the Delta. 1979.
- Sedivec Kevin, K. , Schatz Blaine G. Pearl millet Forage production in North Dakota. 1991.
- Lauriault, L.M.; Darapuneni, M.K.; Martinez, G.K. Pearl Millet-Cowpea Forage Mixture Planting Arrangement Influences Mixture Yield and Nutritive Value in Semiarid Regions. Crops 2023, 3, 266–275. [Google Scholar] [CrossRef]
- Miller, DA. Forage crops. McGraw-Hill College; 1984.
- FAO. Millets recipe book - International Year of Millets 2023. Rome: FAO; 2023.
- Yan W, Kang MS, Ma B, Woods S, Cornelius PL. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci 2007, 47, 643–655.
- Lagat, N.; Kimurto, P.; Kiplagat, O.; Towett, B.K.; Jeptanui, L.; Gatongi, I.; Njogu, N.; Ojulong, H.; Manyasa, E. Siambi Evaluation of Genotype x Environment Interaction and Stability of Grain Yield and Related Yield Components in Pearl Millet (Pennisetum glaucum (L.) R.Br.). J. Exp. Agric. Int. 2018, 21, 1–18. [Google Scholar] [CrossRef]
- Gangashetty, P.I.; Yadav, C.B.; Riyazaddin, M.; Vermula, A.; Asungre, P.A.; Angarawai, I.; Mur, L.A.J.; Yadav, R.S. Genotype-by-environment interactions for starch, mineral, and agronomic traits in pearl millet hybrids evaluated across five locations in West Africa. Front. Plant Sci. 2023, 14, 1171773. [Google Scholar] [CrossRef] [PubMed]
- Crossa, J. Statistical Analyses of Multilocation Trials. Advances in Agronomy 1990, 44, 55–85. [Google Scholar]
- Brunken J, De JMJ, Harlan JR. The Morphology and Domestication of Pearl Millet. ECONOMIC BOTANY 163-174 April-June 1977 1977, 31, 163–174.
- FAO. Special Report – 2020 FAO Crop and Food Supply Assessment Mission (CFSAM) to the Republic of the Sudan. 2021,.
- Babiker, S.A.; Khair, M.A.M.; Tahir, I.S.A. Exploitation of forage attribute-based variations in Sudan pearl millet [Pennisetum glaucum (L.) R. Br.] collections. Plant Genet. Resour. Charact. Util. 2013, 12, 83–90. [Google Scholar] [CrossRef]
- Babiker, S.A.; Khair, M.A.M.; Tahir, I.S.A.; Elhag, F.M.A. Forage Quality Variations among Some Sudan Pearl Millet [Pennisetum glaucum (L.) R. Br] Collection. Annu. Res. Rev. Biol. 2015, 5, 293–298. [Google Scholar] [CrossRef]
- Bashir, E.M.A.; Ali, A.M.; Ali, A.M.; Mohamed, E.T.I.; Melchinger, A.E.; Parzies, H.K.; Haussmann, B.I.G. Genetic diversity of Sudanese pearl millet (Pennisetum glaucum (L.) R. Br.) landraces as revealed by SSR markers, and relationship between genetic and agro-morphological diversity. Genet. Resour. Crop. Evol. 2014, 62, 579–591. [Google Scholar] [CrossRef]
- Babiker, S. A., Khair, M. A. M., Tahir, I. S. A., Mohamed, E. I., Ali, A. M. Mustafa NS. Exploitation of Simple Sequence Repeat Markers to Assess the Genetic Diversity in Sudan Pearl Millet [Pennisetum Glaucum (L.) R. Br.] Collections. 2016, 4, 1071–1075.
- VSN International. Genstat for Windows 22nd Edition. VSN International. 2022.
- Farshadfar, E. Incorporation of AMMI Stability Value and Grain Yield in a Single Non-Parametric Index (GSI) in Bread Wheat. Pak. J. Biol. Sci. 2008, 11, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. pheatmap: Pretty Heatmaps. 2022,.
- Babiker Sara Abd Elraheem. Enhancement of forage suited accessions in Sudan pearl millet collection via exploitation of inter-and intra-seasonal, phenotypic, genotypic and quality variations. PhD Thesis. Sudan Academy of Sciences (SAS). 2012.
- Iqbal, M.A.; Hamid, A.; Hussain, I.; Siddiqui, M.H.; Ahmad, T.; Khaliq, A.; Ahmad, Z. Competitive Indices in Cereal and Legume Mixtures in a South Asian Environment. Agron. J. 2019, 111, 242–249. [Google Scholar] [CrossRef]
- Khair MAM, S. A. Salih SA, Elhag FMA, Eltayeb EI. Dry matter yield and quality of some winter sown forage crop in the Sudan. University of Khartoum J of Agric Sci 2007, 15, 204–219. [Google Scholar]
- Kambal, AE. Comparative performance of some varieties of sorghum, maize and pearl millet for forage production in different seasons. Sudan Agric Journal 1983, 10. [Google Scholar]
- van Oosterom, E.J.; Mahalakshmi, V.; Bidinger, F.R.; Rao, K.P. Effect of water availability and temperature on the genotype-by-environment interaction of pearl millet in semi-arid tropical environments. Euphytica 1996, 89, 175–183. [Google Scholar] [CrossRef]
Figure 1.
Growth attributes of 25 pearl millet genotypes grown in seven environments in Sudan. The traits were: days to 50% flowering (a), number of culms m-2 (b), plant height (c), leaf to stem ratio (d), and stem girth (e). Violin plots show the median (black line) and upper and lower quartiles (red lines), whereas the width of each violin is proportional of frequency.
Figure 1.
Growth attributes of 25 pearl millet genotypes grown in seven environments in Sudan. The traits were: days to 50% flowering (a), number of culms m-2 (b), plant height (c), leaf to stem ratio (d), and stem girth (e). Violin plots show the median (black line) and upper and lower quartiles (red lines), whereas the width of each violin is proportional of frequency.
Figure 2.
Heatmap clustering using phenotypic data of 25 pearl millet genotypes grown in multi-environments, using 40 trait combinations at different environment (a), using the mean of the six traits across the environments (b). Changes in the values of the traits are indicated by colour intensity and variation. The red colour indicates higher scores, while the blue colour indicates lower scores. FY, fresh yield; DF, days to 50% flowering; CLM, number of culms m-2; PH, plant height (cm); LSR, leaf to stem ratio and SG, stem girth (cm). A specific trait in a specific environment was denoted by the combination of the trait and environment abbreviation, while the mean of the trait across the environments was denoted by the letter "M" added to the trait abbreviation.
Figure 2.
Heatmap clustering using phenotypic data of 25 pearl millet genotypes grown in multi-environments, using 40 trait combinations at different environment (a), using the mean of the six traits across the environments (b). Changes in the values of the traits are indicated by colour intensity and variation. The red colour indicates higher scores, while the blue colour indicates lower scores. FY, fresh yield; DF, days to 50% flowering; CLM, number of culms m-2; PH, plant height (cm); LSR, leaf to stem ratio and SG, stem girth (cm). A specific trait in a specific environment was denoted by the combination of the trait and environment abbreviation, while the mean of the trait across the environments was denoted by the letter "M" added to the trait abbreviation.
Figure 3.
GGE biplots for the fresh matter yield of 25 pearl millet genotypes grown in seven environments in Sudan. Mega-environments scattered plot (a), comparison biplot of environments (b), and comparison biplot of genotypes (c).
Figure 3.
GGE biplots for the fresh matter yield of 25 pearl millet genotypes grown in seven environments in Sudan. Mega-environments scattered plot (a), comparison biplot of environments (b), and comparison biplot of genotypes (c).
Table 1.
Minimum, maximum and mean values of fresh matter yield (t/ha), days to 50% flowering, plant height (cm), leaf to stem ratio, number of culms m-2 and stem gith (cm) of the 25 pearl millet genotypes grown across different environments.
Table 1.
Minimum, maximum and mean values of fresh matter yield (t/ha), days to 50% flowering, plant height (cm), leaf to stem ratio, number of culms m-2 and stem gith (cm) of the 25 pearl millet genotypes grown across different environments.
| |
Fresh matter yield (t ha-1) |
Days to flowering |
Number of culms m-1
|
Plant height (cm) |
Leaf to stem ratio |
Stem girth (cm) |
| No. of environment |
7 |
7 |
5 |
7 |
5 |
3 |
| Min |
6.5 (R1) |
46 (R1) |
14.8 (S2) |
133 (S2) |
0.131 (R2) |
2.90 (G1) |
| Max |
26.5 (R3) |
70 (R1) |
49.6 (G1) |
179 (G1) |
0.411 (G1) |
4.40 (G2) |
| Mean |
15.6 |
56 |
31.7 |
156 |
0.241 |
3.57 |
| Chi probability |
|
|
|
|
|
|
| Environment (E) |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
| Genotype (G) |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
0.728 |
| G x E |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
0.082 |
| SE ± |
|
|
|
|
|
|
| E |
0.66 |
0.7 |
1.53 |
1.7 |
0.0094 |
0.065 |
| G |
0.62 |
0.6 |
1.05 |
1.4 |
0.0104 |
0.113 |
| G x E |
1.63 |
1.5 |
2.57 |
3.8 |
0.0255 |
0.196 |
Table 2.
Fresh matter yield (t/ha) of twenty-five genotypes of pearl millet (Pennisetum glacum L) grown across seven environments at Gezira, Rahad and Sennar sites, Sudan.
Table 2.
Fresh matter yield (t/ha) of twenty-five genotypes of pearl millet (Pennisetum glacum L) grown across seven environments at Gezira, Rahad and Sennar sites, Sudan.
| Genotype (G) |
Environment (E) |
Mean (G) |
Relative performance (%) |
| Gezira 2016/17 (G1) |
Gezira 2018/19 (G2) |
Rahad 2016/17 (R1) |
Rahad 2017/18 (R2) |
Rahad 2018/19 (R3) |
Sennar 2016/17 (S1) |
Sennar 2018/19 (S2) |
| G01 |
20.29 |
22.60 |
10.21 |
18.68 |
26.54 |
12.81 |
12.73 |
17.70 |
116.5 |
| G02 |
22.80 |
19.70 |
9.90 |
14.39 |
19.23 |
8.89 |
9.94 |
14.98 |
98.6 |
| G03 |
21.72 |
17.74 |
10.96 |
13.73 |
19.47 |
12.37 |
13.37 |
15.62 |
102.8 |
| G04 |
15.64 |
22.26 |
7.07 |
13.83 |
26.33 |
8.87 |
15.18 |
15.60 |
102.7 |
| G05 |
24.86 |
20.97 |
15.59 |
11.74 |
21.72 |
7.79 |
11.59 |
16.32 |
107.4 |
| G06 |
17.99 |
25.69 |
6.94 |
12.14 |
18.82 |
16.28 |
14.20 |
16.01 |
105.4 |
| G07 |
16.22 |
22.98 |
12.67 |
7.11 |
19.72 |
11.91 |
12.08 |
14.67 |
96.6 |
| G08 |
19.14 |
19.60 |
9.84 |
15.21 |
18.22 |
10.25 |
11.53 |
14.83 |
97.6 |
| G09 |
19.36 |
26.52 |
7.62 |
12.57 |
18.82 |
10.42 |
10.93 |
15.18 |
99.9 |
| G10 |
22.31 |
11.03 |
9.70 |
13.92 |
18.95 |
8.77 |
12.94 |
13.95 |
91.8 |
| G11 |
16.11 |
17.02 |
9.27 |
11.88 |
25.05 |
10.32 |
12.72 |
14.62 |
96.3 |
| G12 |
27.18 |
21.42 |
6.90 |
16.23 |
21.98 |
8.60 |
15.69 |
16.86 |
111.0 |
| G13 |
20.15 |
19.16 |
8.06 |
14.35 |
20.73 |
12.60 |
12.70 |
15.39 |
101.3 |
| G14 |
23.80 |
27.36 |
10.67 |
19.23 |
24.08 |
11.51 |
11.70 |
18.34 |
120.7 |
| G15 |
17.70 |
16.70 |
8.01 |
17.27 |
19.20 |
10.63 |
14.62 |
14.88 |
97.9 |
| G16 |
13.98 |
15.46 |
7.01 |
12.29 |
23.74 |
7.27 |
14.04 |
13.40 |
88.2 |
| G17 |
13.22 |
20.38 |
10.04 |
25.70 |
19.79 |
13.73 |
11.25 |
16.30 |
107.3 |
| G18 |
17.09 |
17.44 |
10.04 |
21.36 |
20.29 |
10.30 |
12.02 |
15.51 |
102.1 |
| G19 |
23.15 |
21.76 |
8.41 |
16.65 |
21.64 |
7.53 |
11.37 |
15.79 |
103.9 |
| G20 |
19.27 |
12.40 |
8.63 |
12.73 |
19.34 |
11.17 |
17.25 |
14.40 |
94.8 |
| G21 |
19.27 |
23.11 |
10.48 |
12.90 |
23.91 |
13.83 |
8.34 |
15.98 |
105.2 |
| G22 |
16.54 |
22.91 |
6.03 |
23.40 |
25.43 |
9.50 |
12.92 |
16.68 |
109.8 |
| G23 |
21.02 |
15.91 |
8.51 |
17.26 |
22.57 |
7.15 |
13.44 |
15.12 |
99.6 |
| G24 |
19.65 |
22.80 |
7.02 |
12.15 |
25.42 |
10.55 |
12.32 |
15.70 |
103.4 |
| G25 |
17.23 |
23.56 |
10.40 |
15.43 |
18.67 |
9.86 |
11.19 |
15.19 |
100.0 |
| Mean (E) |
19.43 |
20.26 |
9.20 |
15.29 |
21.59 |
10.52 |
12.64 |
15.56 |
|
| SE± |
for environment = 0.663, for genotype = 0.616, for G x E = 1.629 |
|
|
Table 3.
The ANOVA of AMMI for fresh matter yield of 25 pearl millet genotypes in seven environments.
Table 3.
The ANOVA of AMMI for fresh matter yield of 25 pearl millet genotypes in seven environments.
| Source |
DF |
SS |
MS |
VR |
F pr |
% explained |
| Total |
524 |
20054 |
38.3 |
|
|
|
| Treatments |
174 |
15786 |
90.7 |
8.01 |
<0.001 |
|
| Genotypes |
24 |
681 |
28.4 |
2.51 |
<0.001 |
4.3 |
| Environments |
6 |
11087 |
1847.8 |
56 |
<0.001 |
70.2 |
| Block |
14 |
462 |
33 |
2.91 |
<0.001 |
|
| Interactions |
144 |
4018 |
27.9 |
2.46 |
<0.001 |
25.5 |
| IPCA 1 |
29 |
1279 |
44.1 |
3.89 |
<0.001 |
31.8 |
| IPCA 2 |
27 |
1173 |
43.4 |
3.84 |
<0.001 |
29.2 |
| IPCA 3 |
25 |
539 |
21.6 |
1.9 |
0.0064 |
13.4 |
| Residuals |
63 |
1027 |
16.3 |
1.44 |
0.0231 |
|
| Error |
336 |
3805 |
11.3 |
|
|
|
Table 4.
AMMI estimates of fresh yield (t/ha), AMMI stability value (ASV), and genotype selection index (GSI) of 25 pearl millet genotypes grown in seven environments in Sudan.
Table 4.
AMMI estimates of fresh yield (t/ha), AMMI stability value (ASV), and genotype selection index (GSI) of 25 pearl millet genotypes grown in seven environments in Sudan.
| Genotype code |
Mean FY |
Rank (FY) |
Stability (ASV) |
Rank (ASV) |
GSI value |
Rank (GSI) |
| G01 |
17.21 |
3 |
1.079 |
13 |
16 |
5 |
| G02 |
15.06 |
16 |
0.971 |
11 |
27 |
11 |
| G03 |
16.07 |
8 |
1.237 |
15 |
23 |
9 |
| G04 |
15.67 |
11 |
1.793 |
22 |
33 |
19 |
| G05 |
15.83 |
10 |
2.058 |
23 |
33 |
18 |
| G06 |
16.29 |
6 |
0.487 |
6 |
12 |
4 |
| G07 |
14.91 |
18 |
1.675 |
21 |
39 |
24 |
| G08 |
14.25 |
23 |
0.885 |
9 |
32 |
16 |
| G09 |
14.67 |
19 |
0.957 |
10 |
29 |
13 |
| G10 |
14.5 |
20 |
1.629 |
19 |
39 |
25 |
| G11 |
15.27 |
15 |
0.129 |
1 |
16 |
7 |
| G12 |
16.72 |
4 |
0.757 |
8 |
12 |
3 |
| G13 |
15.32 |
14 |
0.184 |
2 |
16 |
6 |
| G14 |
18.39 |
1 |
0.45 |
5 |
6 |
1 |
| G15 |
14.33 |
22 |
1.278 |
16 |
38 |
23 |
| G16 |
13.49 |
25 |
0.55 |
7 |
32 |
17 |
| G17 |
15.94 |
9 |
2.782 |
25 |
34 |
20 |
| G18 |
16.35 |
5 |
1.049 |
12 |
17 |
8 |
| G19 |
16.17 |
7 |
0.198 |
3 |
10 |
2 |
| G20 |
14.49 |
21 |
1.157 |
14 |
35 |
21 |
| G21 |
15.63 |
12 |
1.352 |
18 |
30 |
14 |
| G22 |
17.75 |
2 |
2.506 |
24 |
26 |
10 |
| G23 |
15.03 |
17 |
1.665 |
20 |
37 |
22 |
| G24 |
15.47 |
13 |
1.347 |
17 |
30 |
15 |
| G25 |
14.18 |
24 |
0.397 |
4 |
28 |
12 |
Table 5.
The four best genotypes in each of the seven environments as per AMMI selections.
Table 5.
The four best genotypes in each of the seven environments as per AMMI selections.
| Environment |
Mean |
Score |
1st
|
2nd
|
3rd
|
4th
|
| Gezira 2016/17 (G1) |
19.43 |
3.144 |
G05 |
G12 |
G14 |
G10 |
| Gezira 2018/19 (G2) |
20.26 |
-1.662 |
G22 |
G24 |
G01 |
G04 |
| Rahad 2016/17 (R1) |
9.20 |
1.362 |
G03 |
G05 |
G14 |
G06 |
| Rahad 2o17/18 (R2) |
15.29 |
-2.228 |
G17 |
G22 |
G18 |
G23 |
| Rahad 2018/19 (R3) |
21.59 |
-1.031 |
G22 |
G14 |
G01 |
G04 |
| Sennar 2016/17 (S1) |
10.52 |
0.088 |
G06 |
G07 |
G17 |
G03 |
| Sennar 2018/19 (S2) |
12.64 |
0.327 |
G17 |
G03 |
G14 |
G18 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).