1. Introduction
Uveal melanoma (UM) is the most common primary intraocular cancer in adults characterized by high mortality observed in Poland [
1]. Our study on the incidence and survival of ocular melanoma in National Cancer Registry of Poland during 2010–2017 showed that in our country 8.39% patients diagnosed with UM died within one year, and 39.24% died within five years from the initial diagnosis, which gives the one-year and five-year mortality rates of 8.39% and 39.24%, respectively [
1]. The one-year overall survival (OS) was 91.61%, and the five-year OS was 60.76%. However, our mortality rate was higher than that found in the Isarael, Singapore, Sweden, Denmark and United Kingdom, it was comparable with data from an epidemiological study of uveal melanoma from US Surveillance, Epidemiology, and End Results Program for 2010–2015, where the five-year OS was 61.8% [
2,
3,
4,
5,
6,
7]. Previously published studies have shown that more than 50% of patients develop metastases within 15 years of initial diagnosis and found that older age at diagnosis, severe tumor stage, distant metastasis, and lack of radiation therapy were associated with a higher risk of cancer death [
4,
6,
7,
8,
9,
10,
11]. Given the poor prognosis of patients with uveal melanoma, early detection and treatment initiation are crucial for overall survival.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been reported in China since December 2019 and the global outbreak began in early 2020, with the first lockdowns in Europe implemented in February 2020. [
12] The ocular manifestations of SARS-CoV-2 (COVID-19) have been reported in anterior and posterior segments of the eye, including (but not limited to) blepharitis, eyelid dermatitis, conjunctivitis, keratitis, episcleritis, retinitis, optic neuritis, papillophlebitis, panuveitis, central retinal artery/vein occlusion and Purtchner-like retinopathy [
13,
14,
15,
16] But more importantly, restrictions put in place to prevent the spread of the virus have impacted the diagnosis and treatment of chronic eye diseases and uveal melanoma is no exemption. Despite the fact that COVID-19 pandemic has had an unprecedented impact on health care systems around the world, the number of publications on the impact of COVID-19 pandemic on characteristic and treatment of uveal melanoma is limited [
17,
18,
19,
20] and most of them included small patient samples.
The present study aimed to analyze the impact of SARS-CoV-2 (COVID-19) pandemic on characteristics and management of uveal melanoma (UM) in a large group of patients in National Referral Center in Poland.
2. Materials and Methods
2.1. Data sources, Patients and Definitions
The recruitment methods for this study have been described in details in our previous paper [
21]. In brief, the study design was a retrospective case series. Department of Ophthalmology and Ophthalmic Oncology, Jagiellonian University Collegium Medicum, Krakow, Poland is a national referral center for adult patients with eye cancers in Poland. The hospital database complies medical data including the diagnoses coded according to the International Classification of Diseases, 10th Revision (ICD-10) and the 3rd edition of the International Classification of Diseases for Oncology (ICD-O-3) codes, and all procedures performed coded using the International Classification of Diseases, 9th Revision (ICD-9) procedure codes and unique National Health Fund (NHF) codes corresponding to certain hospital procedures, as well as demographic features like personal identification number (PESEL), date of birth, sex of patients and place of residence.
All patients who were newly diagnosed with uveal melanoma and treated between January 1, 2018 and December 31, 2021 were extracted from the hospital database and included into this study. The demographical and clinical data were analyzed including: sex of patients, age at the time of diagnosis, year of diagnosis, laterality of tumor (right or left eye), intra-ocular localization and cancer stage according to TNM classification of malignant tumors (both at the time of diagnosis), as well as applied treatments including plaque radiotherapy (brachytherapy with iodine-125 or rhutenium-106), proton beam irradiation (PBI), local surgery and/or enucleation of the eye globe.
2.2. Statistical Analyses
The statistical analyses included the standard annual incidence analysis of uveal melanoma cases and analysis of fixed-base dynamics indicators for the incidence of uveal melanoma (base year 2018) in the national referral center in Poland during 2018-2021. It also included analyses of clinical features i.e., laterality of tumor, localizations of tumors, cancer stages, and treatment methods. The differences between time periods before and during SARS-CoV-2 (COVID-19) pandemic were explored by the Chi squared (χ2) test). The commercially available software STATISTICA v. 13.0 PL (StatSoft Polska, Krakow, Poland) was used to perform all statistical analyses. P values < 0.05 were considered statistically significant. The study adhered to the tenets of the Declaration of Helsinki for research involving human subjects and was approved by the Institutional Review Board of the Jagiellonian University Collegium Medicum (the informed consent was waived).
3. Results
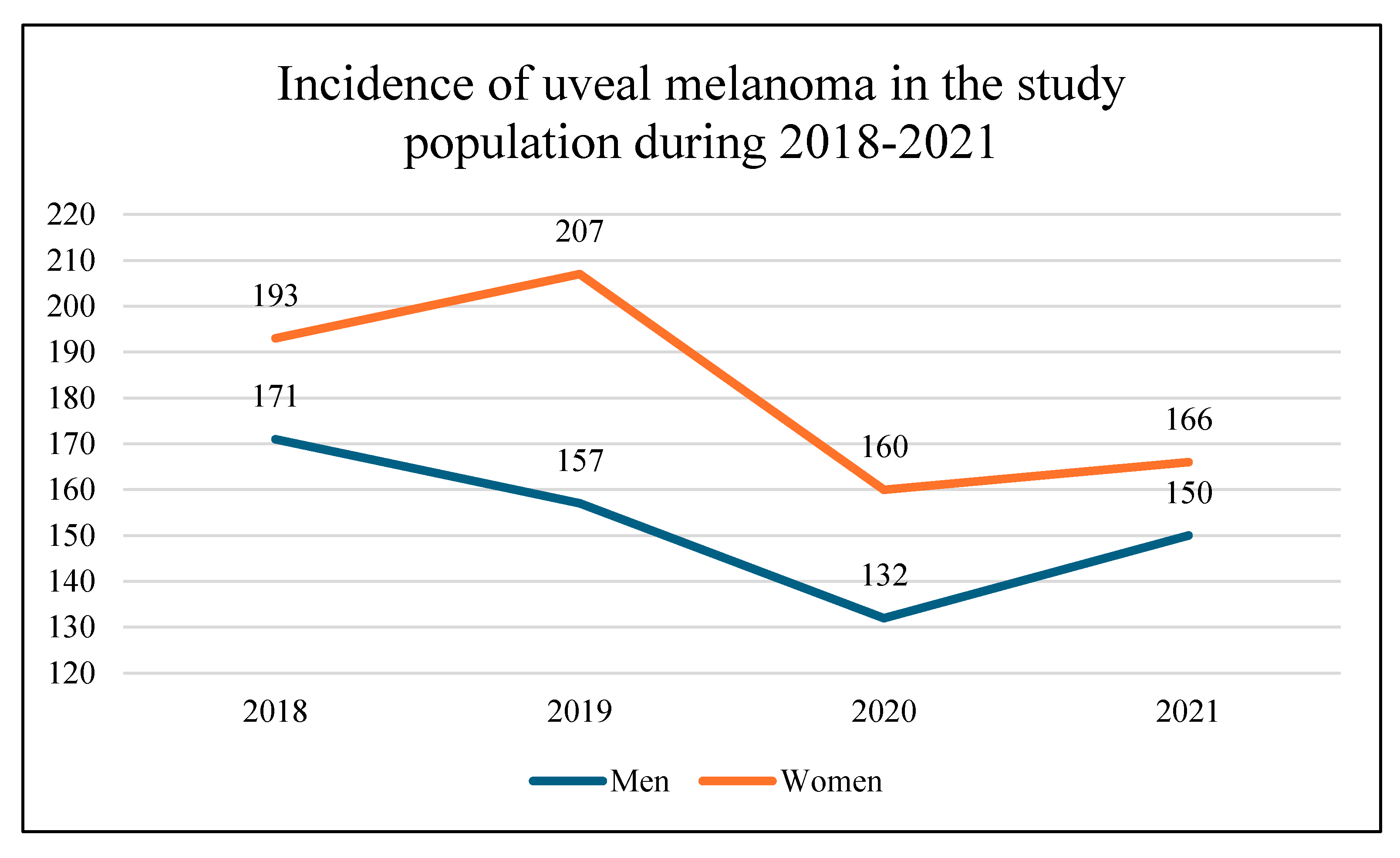
In total, 1,336 patients with uveal melanoma (UM) were identified and included into this study in national referral center in Poland between January 1, 2018 and December 31, 2021 (
Table 1,
Figure 1). Of them, 728 were included before SARS-CoV-2 (COVID-19) pandemic in years 2018-2019 and 608 were included during COVID-19 pandemic in years 2020-2021. There were 726 women (54.34%) and 610 men (45.66%) in the study population. The sex distribution was similar to that found among patients with uveal melanoma in the National Cancer Registry of Poland (statistical analysis- chi square test:
χ2 = 0.20, p= 0.6570) [
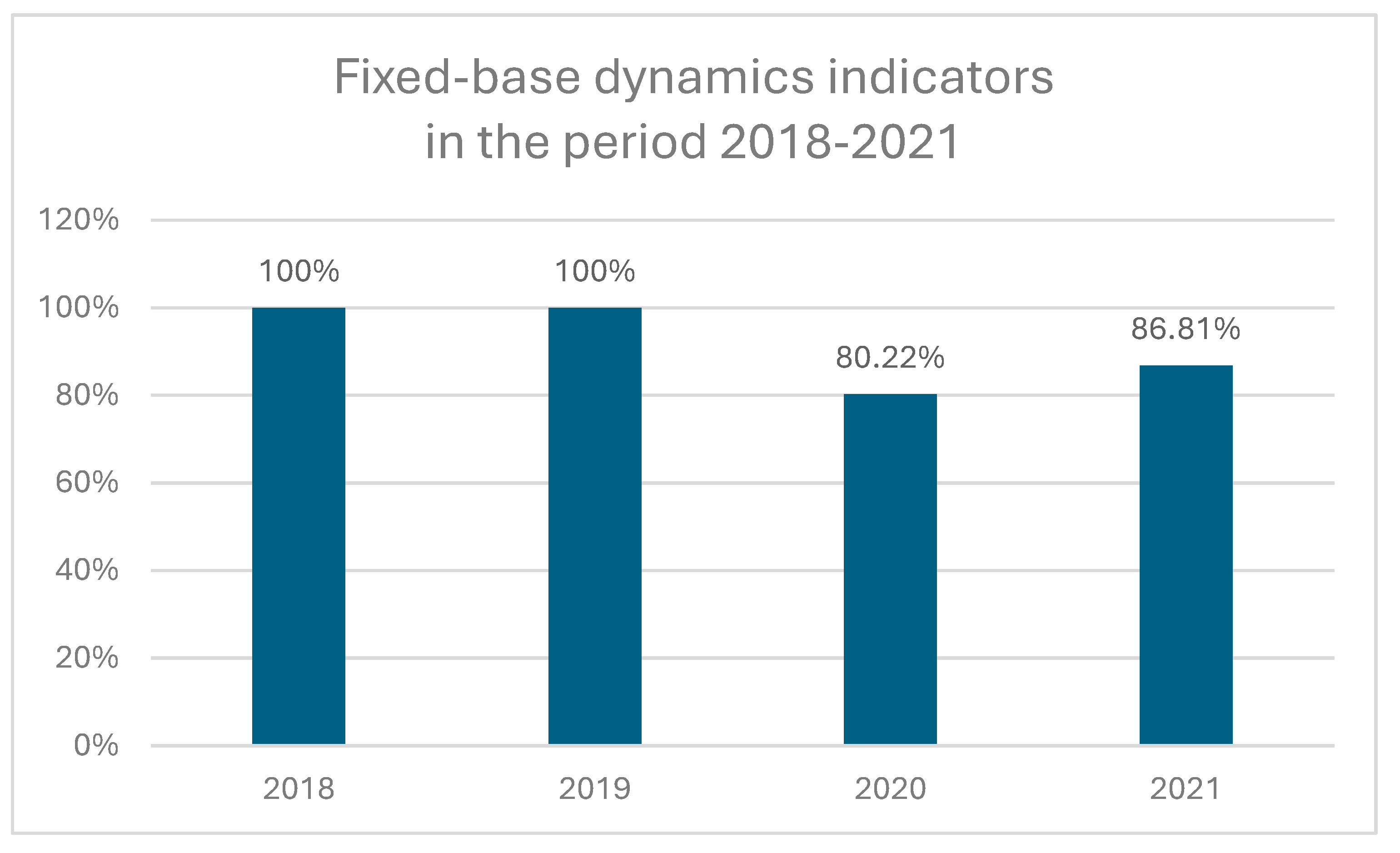
1]. The mean age of patient was 63.84 ± 13.86 years, at the time of diagnosis. Fixed-base dynamics indicators for the incidence of uveal melanoma (base year 2018) in the national referral center in Poland during 2018-2021 are presented in
Figure 2. Comparing to year 2018, the number of patients with diagnosis of uveal melanoma decreased during COVID-19 pandemic to 80.22% and 86.81% in year 2020 and 2021, respectively. However, we did not find statistically significant differences in sex distribution and age of patients before and during COVID-19 pandemic (
Table 1).
In the study period, 49.70% of tumors were localized in right eye and 50.30% in left eye. Detailed localizations of uveal melanoma in the national referral center in Poland during 2018-2021 are presented in
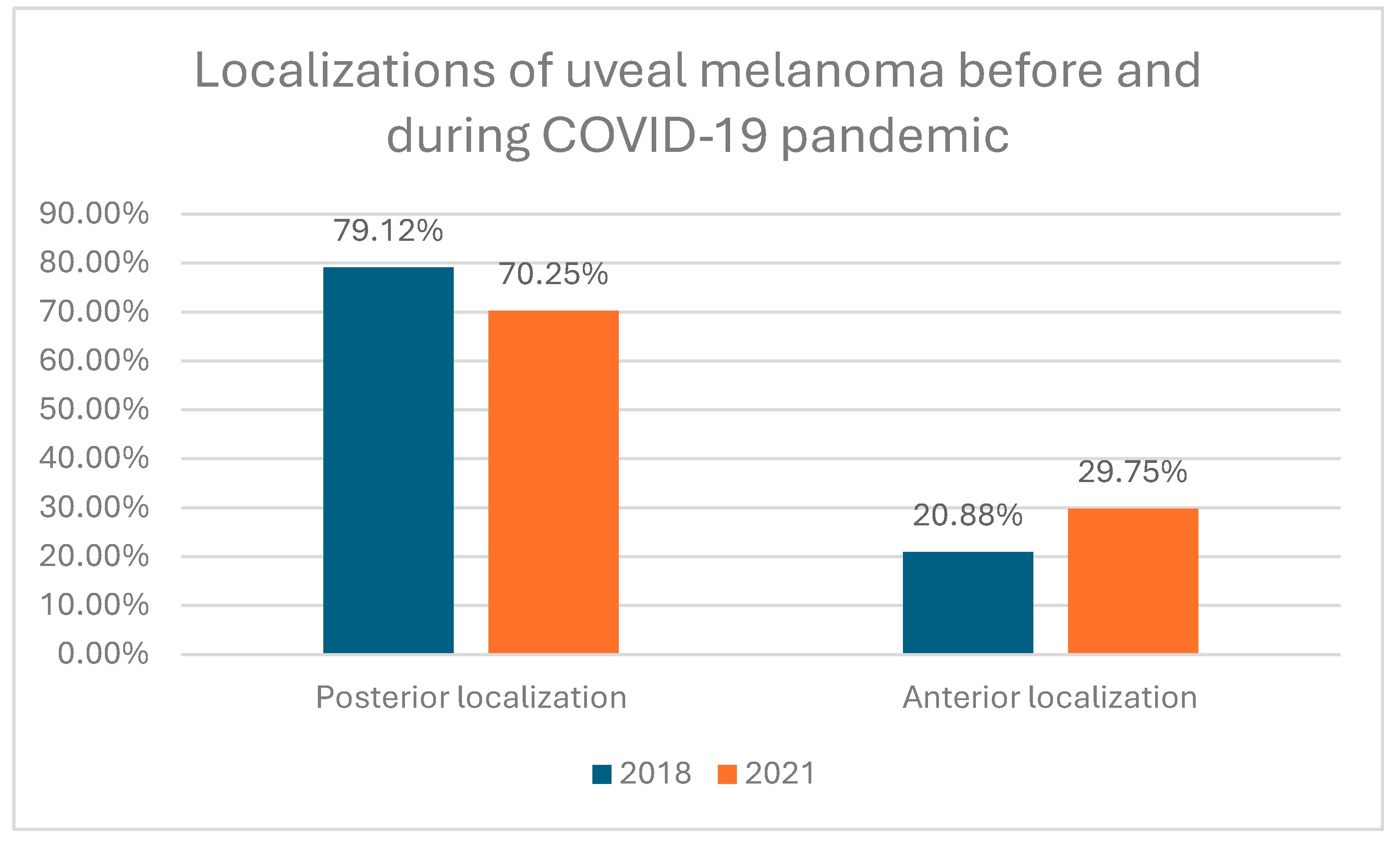
Table 2. At the time of diagnosis, 1024 (76.65%) of all UMs were localized in choroid, 151 (11.30%) in choroid and ciliary body, 49 (3.67%) in iris, 71 (5.31%) in iris and ciliary body, 29 (2.17%) in ciliary body and 12 (0.90%) in iris, ciliary body and choroid. Statistical analysis revealed that during COVID-19 pandemic UMs were statistically significantly more frequently localized anterior to the equator of the eye globe than before COVID-19 pandemic: 29.75% in year 2021 vs 20.88% in year 2018 (Chi^2 Pearson test p= 0.0077)(
Figure 3).
In national referral center in Poland, during the study period 2018-2021, 347 (25.97%) of all UMs were classified as T1, 392 (29.35%) as T2, 382 (28.59%) as T3 and 215 (16.09%) as T4, at the time of diagnosis. During COVID-19 pandemic the number of T4 tumors significantly increased to 26.26% in year 2021 (from 8.78% in year 2018) and simultaneously the number of T1 tumors significantly decreased to 30.38% in year 2021 (from 34.07% in year 2018)( Chi^2 Pearson test p= 0.0001).
The analysis of medical management of UMs in our patients is presented in
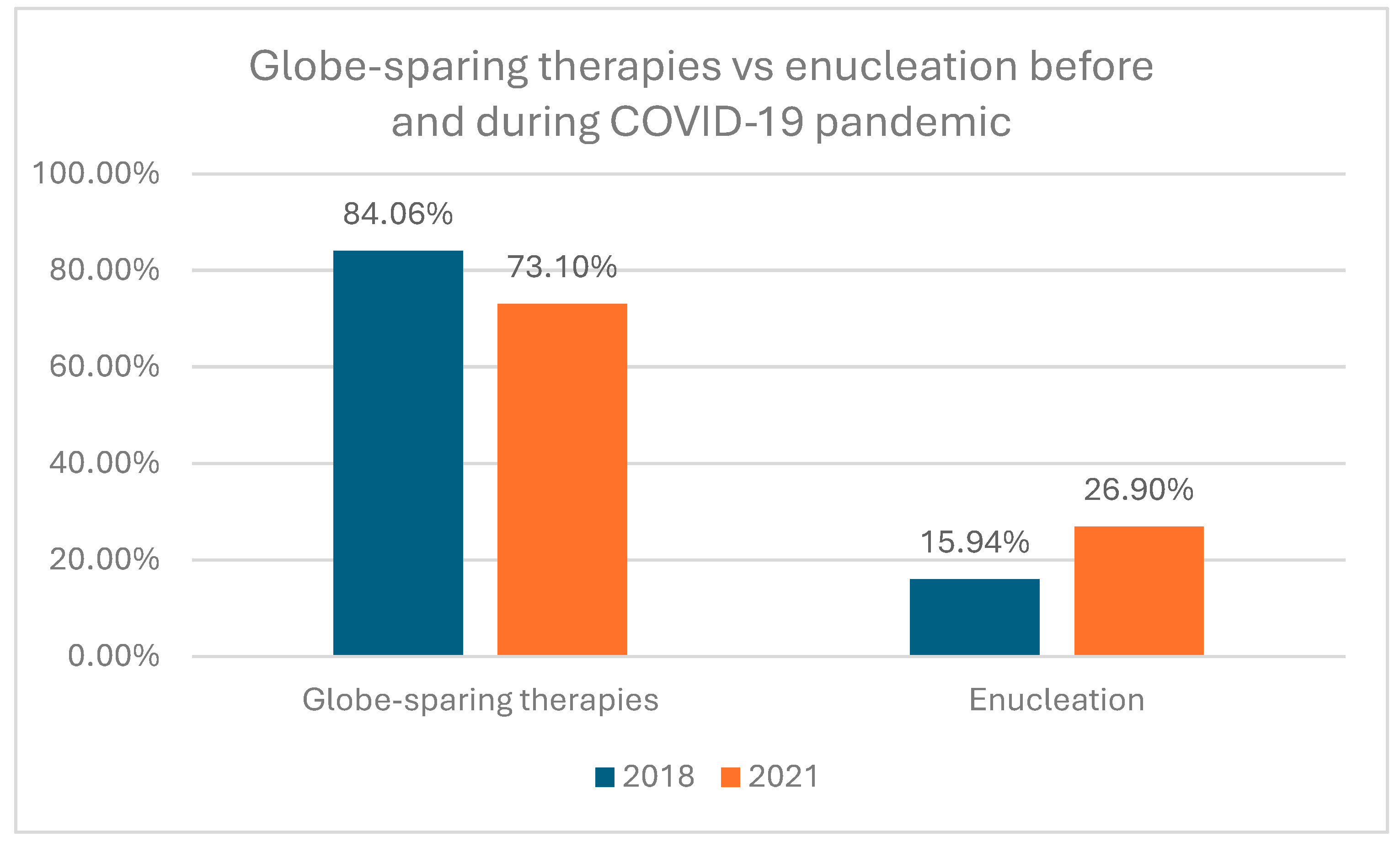
Table 3. In the study group, 909 (68.03%) of all tumors were treated with plaque brachytherapy including 405 (30.31%) with iodine-125 and 504 (37.72%) with rhutenium-106, 36 (2.70%) of tumors were treated with local surgery combined with plaque brachytherapy (either with iodine-125 or rhutenium-106) and 117 (8.76%) were treated with proton beam irradiation (PBI). Enucleation as primary treatment was provided in 274 (20.51%) of tumors. Statistical analysis revealed that during COVID-19 pandemic UMs were statistically significantly more frequently enucleated than before COVID-19 pandemic: 26.90% in year 2021 vs 15.94% in year 2018 (Chi^2 Pearson test p= 0.0005)(
Figure 4).
4. Discussion
The COVID-19 pandemic resulted in unprecedented disruption to healthcare and restrictions taken to limit the exposure of patients to virus contagion had notable impact for non-COVID-19 pathologies including eye cancers [
17,
18,
19,
20]. The incidence of uveal melanoma varies among ethnic groups and regions around the world and has remained stable over recent decades. [
1,
8,
22]. Our previously published study revealed that the total incidence of uveal melanoma in the overall population of Poland during 2010-2017 was 6.67/1,000,000 person-years and the mean age at the time of diagnosis was 62.73 ± 14.43 years [
1]. The present study showed, that the number of patients (in national referral center in Poland) with diagnosis of uveal melanoma decreased significantly during COVID-19 pandemic (to 80.22% and 86.81% in year 2020 and 2021, respectively), compared to years 2018-2019. Our results were similar to data from the United Kingdom (UK), which saw a 43.2% reduction in uveal melanoma diagnoses during the first four months of the COVID-19 pandemic (March-June 2020). [
18]. However, other studies did not confirmed this finding but those studies comprised of low number of patients [
17,
19,
20].
Our study also showed that during COVID-19 pandemic uveal melanomas were statistically significantly larger and more frequently localized anterior to the equator of the eye globe. The number of T4 tumors increased from 8.78% in year 2018 to 26.26% in year 2021 and number of uveal melanomas localized anterior to the equator of the eye globe increased from 20.88% in year 2018 to 29.75% in year 2021, respectively. Our results were consistent with those from studies conducted in Spain and Ireland, where increased tumor size at diagnosis was found during the COVID-19 pandemic [
17,
19]. A study in the UK also found that more patients presented with more advanced cancers post lockdown [
18]. In contrast to these results, a study conducted in Texas (United States of America) showed that the Covid-19 pandemic had no impact on the presentation of patients with uveal melanoma in terms of all tumor characteristics, including size, stage and gene expression data [
20]. The difference in results may be attributed to the regional context of the studies, with Texas having a different approach to the Covid-19 pandemic: the Texas government has never issued an isolation order [
20]. Although these studies did not analyze the specific localization of uveal melanomas, researchers from Spain and Ireland confirmed a greater number of patients diagnosed with extraocular extension of tumors compared to the pre-pandemic era [
17,
19].
Treatment of uveal melanoma depends on the tumor localization, size, local extension, visual acuity and systemic status. Most patients are currently treated with globe sparing therapies including plaque brachytherapy, laser photocoagulation, transpupillary thermotherapy, particle beam radiotherapy, gamma knife radiosurgery and local surgical resection [
1,
8,
9,
10,
11,
23] . Contrary to this trend, our study showed that during COVID-19 pandemic the number of enucleations increased significantly from 15.94% in year 2018 to 26.90% in year 2021. Our results were consistent with studies conducted in Spain and Ireland, where the number of enucleations increased significantly, from 11.9% and 9.3%, respectively, before the Covid-19 pandemic to 47.5% and 21.6% during the Covid-19 pandemic [
17,
19]. A study conducted in Spain also showed that patients diagnosed during the pandemic had a statistically significantly increased risk of treatment with the enucleation method. A study in the UK also found increased number of enucleations among patients diagnosed during first lockdown (March-June 2020). However, they believe that there was no conscious intention to favor enucleation over globe sparing therapies in the UK or elsewhere and this increase was due to a trend towards reducing the risk of transmitting the virus during isolation [
20,
24,
25]. The largest decline in the number of globe sparing therapies, in national referral center in Poland was observed in the first year of COVID-19 pandemic in proton beam radiotherapy, which is consistent with the studies from United Kingdom and Ireland [
18,
19]. Proton beam radiotherapy requires multiple hospitalizations which was difficult during strict lockdowns in the first year of pandemic and could also increase the risk of contracting the virus.
Major limitation of our study is the lack of survival analysis of patients with uveal melanoma. However, we believe that the follow-up period was influenced by the Covid-19 pandemic, which may have influenced the cause of death in some patients; the large population size is the major strength of the present study.
In summary, to the best of our knowledge, this is the largest study on the impact of the COVID-19 pandemic on the characteristics and treatment of uveal melanoma. Advice on staying at home, while reducing the risk of contracting the virus, resulted in the increase in tumor size seen in uveal melanoma patients during the Covid-19 pandemic. It could also cause delays in cancer diagnosis and treatment, which could negatively impact patient survival. There is a potential risk that large number of patients with uveal melanoma remained undiagnosed in the community and it is likely that there would be a surge of UMs cases after COVID-19 pandemic is finished [
17,
18,
19,
20].
5. Conclusions
Statistically significant differences were found in the characteristics and management of uveal melanoma in National Referral Center in Poland during COVID-19 pandemic with tumors being larger, more frequently localized anterior to the equator of the eye globe and more often enucleated. The present study also showed, the number of patients with diagnosis of uveal melanoma decreased significantly during COVID-19 pandemic, when compared to the pre-pandemic era.
Author Contributions
Bozena Romanowska-Dixon and Michal S. Nowak conceived and designed the experiments. Data was colected by Magdalena Debicka-Kumela. The results were analyzed by Bozena Romanowska-Dixon, Michal S. Nowak and Janusz Smigielski. The first and final drafts were written by Michal S. Nowak and Bozena Romanowska-Dixon. The defects of the draft were reviewed by Bozena Romanowska-Dixon. All authors agreed on the final draft of this study.
Institutional Review Board Statement
The study adhered to the tenets of the Declaration of Helsinki for research involving human subjects and the study protocol was approved by the Institutional Review Board of the Jagiellonian University Collegium Medicum (the informed consent was waived).
Data Availability Statement
not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nowak, M.S.; Romanowska-Dixon, B.; Grabska-Liberek, I.; Żurek, M. Incidence and survival of ocular melanoma in National Cancer Registry of Poland in 2010-2017. Adv Clin Exp Med. 2022, 31, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.Y.; Hong, J.; Goh, W.L.; Chang, E.W.Y.; Yang, V.S.; Poon, E.; Somasundaram, N.; Farid, M.; Chan, A.S.Y.; Chan, J.Y. Clinical features and survival outcomes of ocular melanoma in a multi-ethnic Asian cohort. Sci Rep. 2020, 10, 16367. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Hendler, K.; Peer, J. Uveal melanoma in Israel in the last two decades: Characterization, treatment and prognosis. Isr Med Assoc J. 2009, 11, 280–285. [Google Scholar] [PubMed]
- Xu, Y.; Lou, L.; Wang, Y.; Miao, Q.; Jin, K.; Chen, M.; Ye, J. Epidemiological Study of Uveal Melanoma from US Surveillance, Epidemiology, and End Results Program (2010-2015). J Ophthalmol. 2020, 2020, 3614039. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E.; EUROCARE Working Group. Survival in patients with uveal melanoma in Europe. Arch Ophthalmol. 2008, 126, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Rajeshuni, N.; Zubair, T.; Ludwig, C.A.; Moshfeghi, D.M.; Mruthyunjaya, P. Evaluation of racial, ethnic, and socioeconomic associations with treatment and survival in uveal melanoma, 2004–2014. JAMA Ophthalmol. 2020, 138, 876–884. [Google Scholar] [CrossRef]
- Radivoyevitch, T.; Zabor, E.C.; Singh, A.D. Uveal melanoma: Long-term survival. PLoS One. 2021, 16, e0250939. [Google Scholar] [CrossRef]
- Jovanovic, P.; Mihajlovic, M.; Djordjevic-Jocic, J.; Vlajkovic, S.; Cekic, S.; Stefanovic, V. Ocular melanoma: An overview of the current status. Int J Clin Exp Pathol. 2013, 6, 1230–1244. [Google Scholar]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye (Lond). 2017, 31, 241–2. [Google Scholar] [CrossRef]
- Nichols, E.E.; Richmond, A.; Daniels, A.B. Tumor characteristics, genetics, management, and the risk of metastasis in uveal melanoma. Semin Ophthalmol. 2016, 31, 304–309. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer. 2016, 122, 2299–2312. [Google Scholar] [CrossRef]
- Kanclerz, P.; Lanca, C.; Radomski, S.A.; Nowak, M.S. The outdoor time in non-myopic children has decreased to that of myopic children during the SARS-CoV-2 pandemic. Rom J Ophthalmol. 2023, 67, 33–40. [Google Scholar]
- Nowak, M.; Nowak, W. Ocular manifestations in SARS-CoV-2 infection and pre-exposure prophylaxis of ophthalmic medical staff. Klinika Oczna / Acta Ophthalmologica Polonica. 2023, 125, 79–83. [Google Scholar] [CrossRef]
- Akbari, M.; Dourandeesh, M. Update on overview of ocular manifestations of COVID-19. Front Med (Lausanne). 2022, 9, 877023. [Google Scholar] [CrossRef] [PubMed]
- Dolar-Szczasny, J.; Toro, M.D.; Dworzanska, A.; Wojtowicz, T.; Korona-Glowniak, I.; Sawicki, R.; Boguszewska, A.; Polz-Dacewicz, M.; Tomasiewicz, K.; Zaluska, W.; et al. Ocular Involvement of SARS-CoV-2 in a Polish Cohort of COVID-19-Positive Patients. Int J Environ Res Public Health. 2021, 18, 2916. [Google Scholar] [CrossRef] [PubMed]
- Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578.
- Bermudez-Castellanos, I.; Saornil Álvarez, M.A.; Almaraz Gómez, A.; Villoria-Diaz, S.; García Álvarez, C. Impact of COVID-19 on a rare disease (uveal melanoma) in a national reference unit of intraocular tumors in Spain. Arch Soc Esp Oftalmol (Engl Ed). 2023, 98, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Elsheikh, M.; Gilmour, K.; Cohen, V.; Sagoo, M.S.; Damato, B.; Anguita, R.; Heimann, H.; Hussain, R.; Cauchi, P.; et al. Impact of COVID-19 pandemic on eye cancer care in United Kingdom. Br J Cancer. 2021, 124, 1357–1360. [Google Scholar] [CrossRef]
- Mc Glacken-Byrne, A.; Murtagh, P.; O’Neill, V.; Horgan, N. Ocular oncology service during the COVID-19 outbreak: uveal melanoma characteristics presenting in 2019 compared to 2020. Ir J Med Sci. 2023, 192, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Rusakevich, A.; Bernicker, E.; Teh, B.S.; Schefler, A. Comparison of Tumor Size and Gene Expression at Presentation in Uveal Melanoma Patients before and during the COVID-19 Pandemic. Ocul Oncol Pathol. 2022, 8, 156–160. [Google Scholar] [CrossRef]
- Romanowska-Dixon, B.; Dębicka-Kumela, M.; Śmigielski, J.; Nowak, M.S. Sex Differences in the Treatment of Uveal Melanoma in a Group of 1336 Patients. J Pers Med. 2023, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E.; EUROCARE Working Group. Incidence of uveal melanoma in Europe. Ophthalmology. 2007, 114, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.S.; Romanowska-Dixon, B.; Grabska-Liberek, I.; Żurek, M. Incidence and Characteristics of Retinoblastoma in Poland: The First Nationwide Study 2010-2017. Int J Environ Res Public Health. 2021, 18, 6539. [Google Scholar] [CrossRef] [PubMed]
- Skalet, A.H.; Allen, R.C.; Shields, C.L.; Wilson, M.W.; Mruthyunjaya, P.; Gombos, D.S. Considerations for the Management and Triage of Ocular Oncology Cases during the COVID-19 Pandemic. Ocul Oncol Pathol. 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Manjandavida, F.P.; Honavar, S.G.; Kim, U.; Singh, U.; Menon, V.; Das, S.; Kaliki, S.; Palanivelu, M.S.; Khetan, V.; Shah, P.K.; et al. Ocular oncology practice guidelines during COVID-19 pandemic-An expert consensus. Indian J Ophthalmol. 2020, 68, 1281–1291. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).