1. Introduction
Efforts into the conservation of giraffes, formerly ‘
Giraffa camelopardalis’, have led to the potential restructuring of previously documented giraffe lineages. Upon initial classification, it was accepted that one giraffe species existed, divided into nine sub-species comprised of non-interbreeding populations [
1]. However, recent research suggests that it is more appropriate to consider four genetically distinct major species of giraffes, with five potential sub-species in discrete locations across Africa [
2,
3]. This restructuring of the giraffe genus has led to some consternation regarding current giraffe vulnerability assessments. Recent reports on the IUCN red list [
4] suggest that giraffes as a species should be classified as vulnerable due to extensive population decay, whereas Deacon and Tutchings [
5] argue that populations of the major South African sub-species ‘
Giraffa camelopardalis giraffa’ are stable and may be increasing.
Within the Mogalakwena Research Centre (MRC), the consensus is that research on giraffes should focus on ‘
Giraffa giraffa giraffa’; a sub-species from one of four recently proposed major species. This sub-species is referred to as the South African giraffe, or Cape giraffe. The South African giraffe has a range covering: south-eastern Angola, northern Botswana, southern Mozambique, northern South Africa, south-western Zambia, and areas in Zimbabwe [
6].
Thermoregulation, which comprises the body of this work, is defined generally as “the maintenance of a relatively constant core body temperature” [
7]. Several different mechanisms of thermoregulation have been previously identified. The use of different sets of mechanisms is used to divide organisms into ‘ectotherms’ and ‘endotherms’ [
8]. Ectotherms are defined as organisms that rely entirely on the external environment to maintain a stable internal temperature [
9,
10]. Endotherms, or warm-blooded animals, on the other hand, are defined as organisms that employ primarily metabolic heat production to maintain a stable internal temperature. Endothermy is a key characteristic of birds [
11] and mammals [
12], such as the giraffe.
According to Tan and Knight [
13], studies on thermoregulation often split the body into two key components: the external surface, which fluctuates with temperature, and the core, which maintains a stable temperature. The internal core is the target of the thermoregulatory response system (Hensel 1973, cited in Tan and Knight [
13], which employs the key components in two major ‘responses’, with a convergent goal of maintaining a set temperature.
Feedback responses are full-body responses which are enacted when core temperature undergoes a major fluctuation; These fluctuations are detected by thermoreceptors throughout the core of the body [
14]. Feed-forward responses, on the other hand, are pre-emptive actions, undertaken when a perceived thermal challenge that does not affect the core arises; for example, cutaneous sensation of temperature changes may lead to a thermoregulatory reflex, minimising the effect of ambient environmental temperature [
15].
In mammals, feedback and feed-forward responses can be separated into physiological and behavioural reflexes. Physiological reflexes consist of involuntary, primarily autonomic actions, which aim to generate or dissipate heat, opposing the external temperature [
13]; Two examples of this are cutaneous vasodilation and reduced thermogenesis [
16]. Behavioural reflexes, on the other hand, are goal-oriented actions, and are reinforced through attainment of rewards, such as relief from negative sensations attributed to hypo- or hyperthermia. The two most prominent behavioural reflexes are warm- and cold-seeking [
17,
18].
The giraffe, a large endothermic mammal, has been previously noted to have specialised body structures to aid physiological thermoregulation. These include a thin, long neck and legs, which aid in evaporative and convective heat loss, whilst maintaining a surface area to volume ratio that is characteristic of many mammals. In prior research [
19], it was also ascertained that giraffes employ behavioural reflexes to combat extreme weathers observed across Africa. Two major behaviours aiming to reduce heat absorption have been observed: facing the sun during direct exposure, and shade-seeking. Facing the sun is hypothesised to be a method of employing the neck of the giraffe, casting shade over the main bulk of the body to reduce net absorption of solar radiation.
This study aimed to examine the observation that giraffes use their neck to cast shade over the body as a behavioural thermoregulatory response, considering the effects of sun exposure and temperature on a local population of giraffes.
Project aims will be broken down into individual hypotheses, which will seek to determine how behaviours and different identities are affected by temperature and exposure. The hypotheses tested are as follows:
Giraffes spend more time facing the sun as temperature increases.
Adult giraffes spend more time facing the sun as thermoregulatory behaviours may be learned.
Male giraffes are predicted to have darker coat colours, so will spend more time facing the sun.
Individuals with darker coats, of any sex, are expected to spend more time facing the sun, due to increased heat absorption.
Giraffes will use more shade as temperature increases, to combat increased rates of heat absorption.
Giraffes will spend more time facing the sun during wet season, compared to dry season, due to increased temperatures.
4. Discussion
The results from the study on behavioural thermoregulation in giraffes demonstrated the main hypothesis that as temperature increases, time spent facing longitudinally (anterior or posterior [
19,
28]) to the sun increases. This was displayed by the significant increase in time spent facing toward or away from the sun, and the significant decrease in time sent facing partially away from the sun (
Figure 2).
During the study of Kuntsch and Nel [
28], the majority of observations were recorded at summer highs of 35°C, and winter highs of 16°C. Conversely, the summer period comprising the main dataset of this study displayed an average temperature of approximately 30°C (
Figure 1). This meant that less data was available for higher temperatures, especially those closer to the giraffe internal body temperature of 38.5°C [
29]. This may have weakened any observed trends regarding time spent facing the sun as temperature increases, however, the same trends were observed, showing that the change in behaviour occurs over a smaller temperature range than expected.
Kuntsch and Nel [
28] also found that there were significant differences between the sex or age of a giraffe, and the time spent facing the sun longitudinally. Juveniles were found to spend significantly less time facing the sun compared to adult individuals. This finding acts in agreement with the current study, which found that adults and sub-adults spend significantly more time facing the sun than juveniles (
Table 3). There was some overlap of the boxplot results, and noticeable standard deviations (
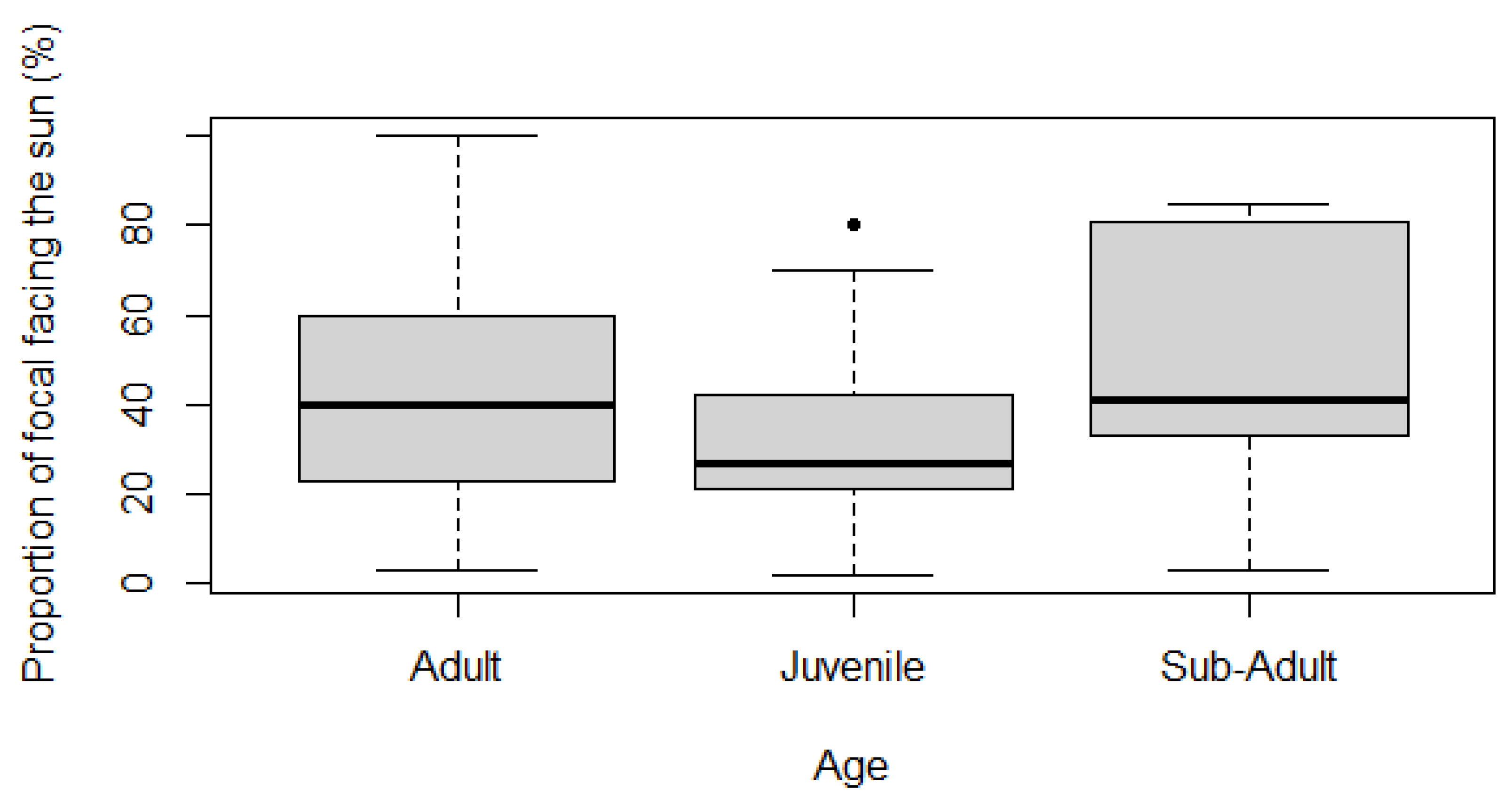
Figure 3). The overlap of results suggests that differences between the groups may only be small, and the large standard deviation was expected, due to the collection of data across a large temperature range; providing a proportion of time facing the sun that is not clustered around a mean value.
Despite concurrence, the conclusion reached in the current study may be subject to scrutiny, due to sample sizes. Adult individuals made up 376 of the total observations, whereas Juveniles made up 74, and Sub-Adults only 37. Without secondary literature, the unequal and small number of samples may have led to inaccurate trends, most notably within the sub-adult category. This issue arose due to the small number of sub-adult giraffes on the reserve; however, this could be solved by studying a community with more sub-adults or correcting for the smallest sample number. Similarly, multiple individuals within the MRR had approximated ages, with greater accuracy for juveniles born during the time of the study. This approximation may have led to the incorrect age categorisation, allowing for reduced sample sizes of smaller age groups, such as sub-adults. In future works focusing on the age of individuals, precise ages may be advisable to maintain accuracy in conclusions.
Regarding the sex of giraffes, Kuntsch and Nel [
28] ventured that males spend significantly more time facing the sun. The current results also found that males spent significantly more time facing the sun (
Table 4;
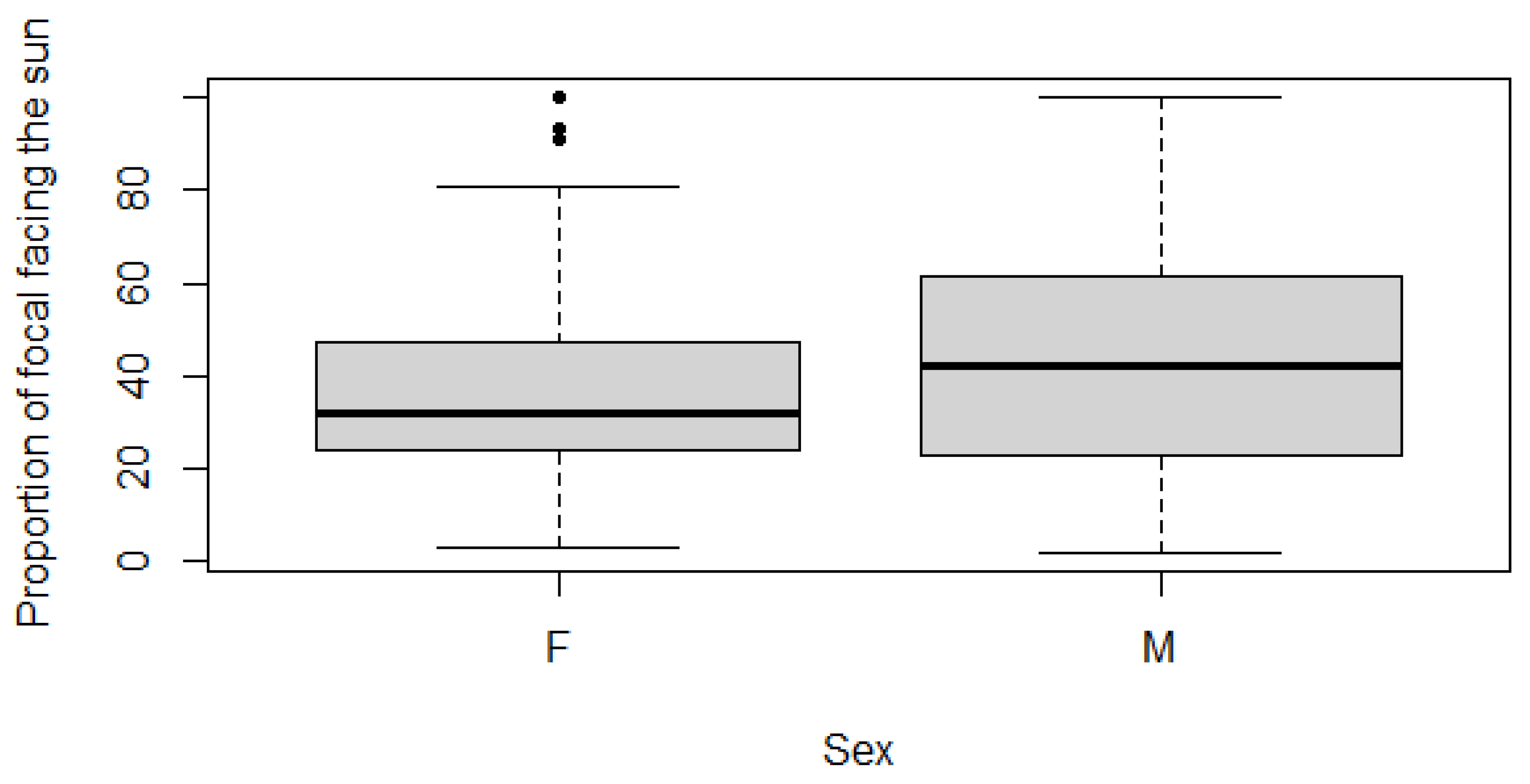
Figure 4). There is a large overlap within the boxplots which suggests that the effect is minimal.
The work of Kuntsch and Nel [
28] excluded non-adult individuals from sex-based conclusions, whereas the current work included every age category, even assigning sexes to juveniles that could not be sexed reliably. This was done by assessing the proportion of males and females born to each family tree, and image analysis of individuals. This method may have led to false attributions of sex, which in turn gave the weak significant difference observed. To combat any errors this may cause in future works, the use of only known-sex individuals may be advisable.
One reason hypothesised as to why males face the sun more often was that of coat colour. It was assumed that on average, male giraffes would have darker coats than females. This would increase the time spent facing the sun to combat the increased absorption of solar radiation associated with darker colours [
30]. This conclusion considered secondary literature, however these commonly focused exclusively on the male giraffe. Similarly, disagreements on how coat colour changes with time, primarily in males, occurred frequently [
31,
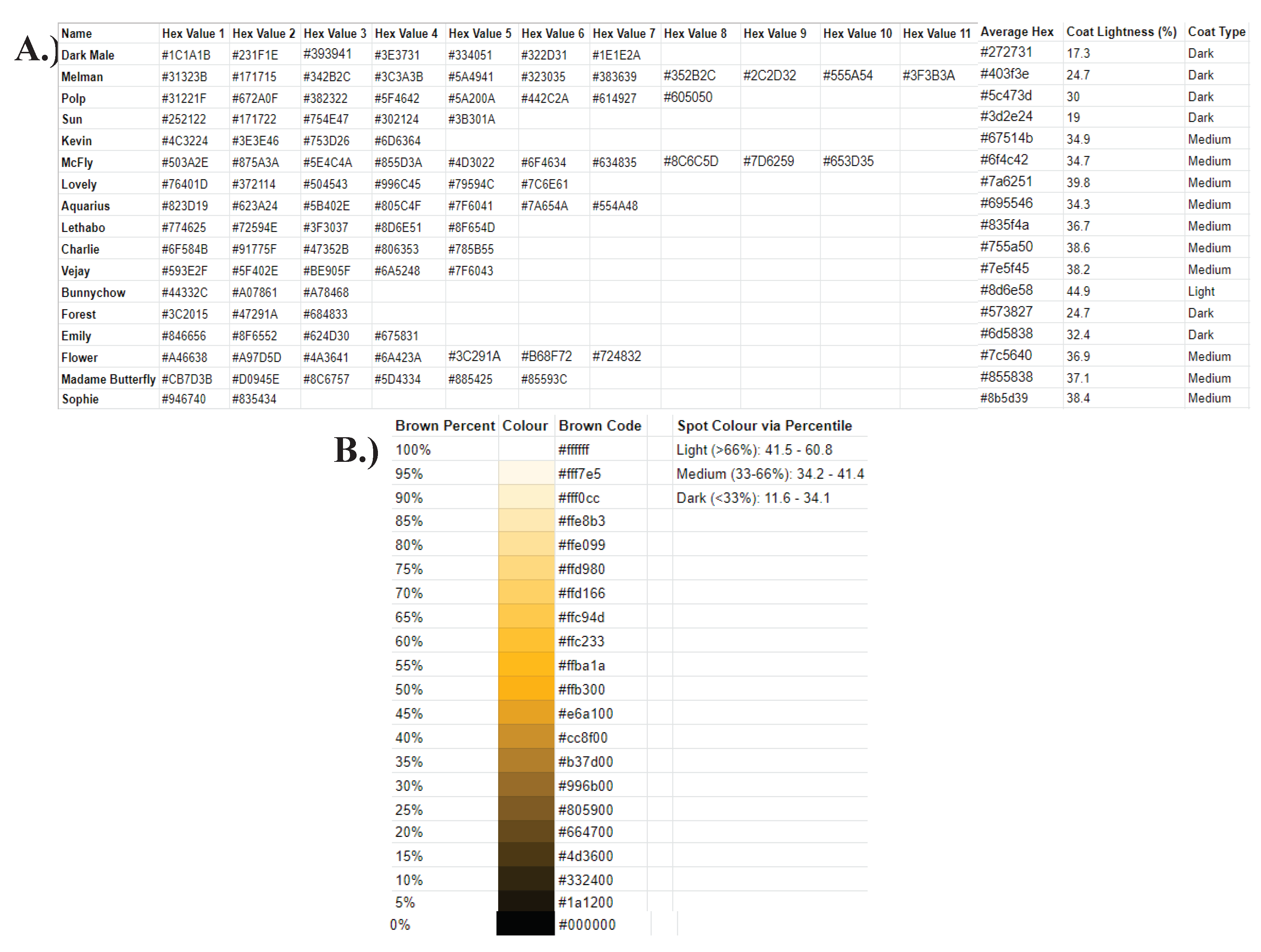
32]. To assess how the coat colour differs between males and females on the MRR, the average coat colour of each sex group was determined. It was found that the average coat colour, represented by Percentage Coat Lightness, was similar between sexes (Males = 37.7%, Females = 38.8%). The lack of difference between the average coat colours may suggest that coat colour has a negligible effect on the time spent facing the sun regarding the sex of an individual.
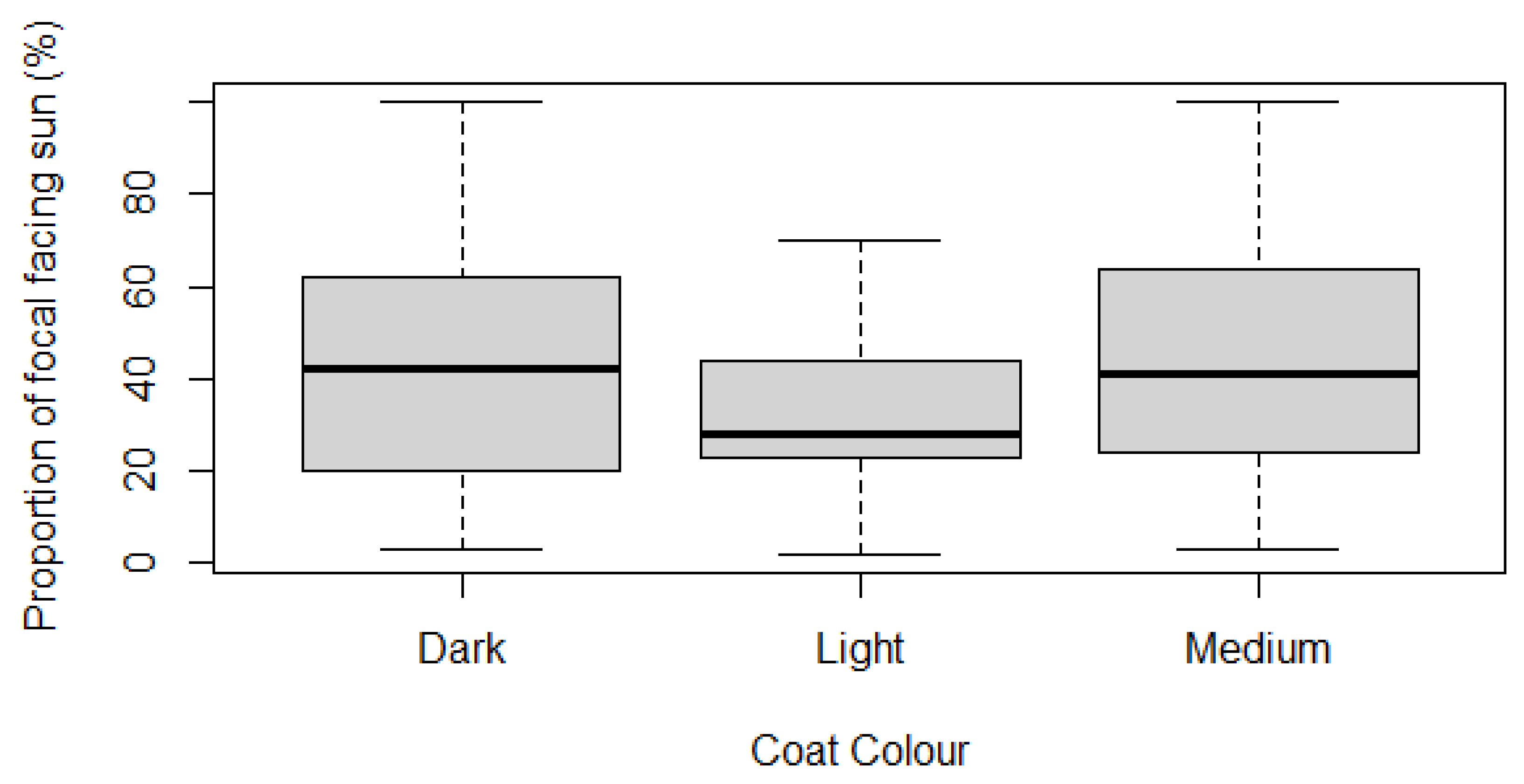
Independently of sex, coat colour was found to have a significant effect on time spent facing the sun, with the data proving that darker individuals spend more time facing the sun (
Table 6;
Figure 5). The boxplots show partial overlap, which may be due to the inclusion of individuals with coat colours that lie on the boundary between two coat colour groups. The large standard deviations observed in the medium and dark colour groups may be due to the larger number of individuals classified in these groups, providing a more random spread of data compared to the smaller light category.
Darker individuals likely face the sun more as compensation for the increased rate of heat absorption that comes alongside the benefits of a darker coat, such as the perceived dominance of males. Further work is necessary to understand the mechanisms of coat colour in giraffes, independently and regarding the sex of an individual. This work is especially important in females, which are often excluded from research on coat colour.
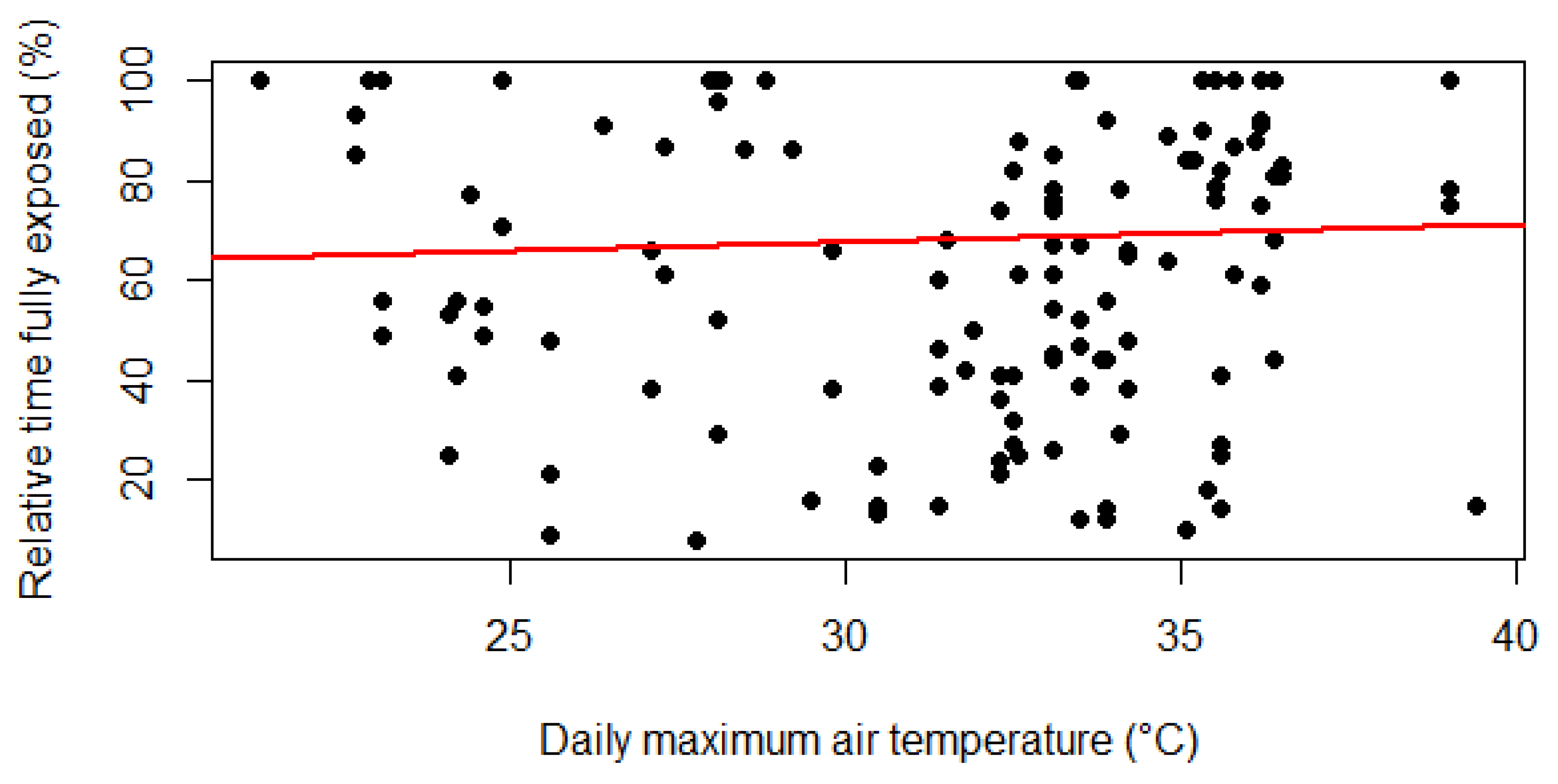
When observing the shade usage of giraffes, it was found that individuals spend significantly more time in full sunlight as temperature increases (
Table 7;
Figure 6). This may suggest that thermoregulatory behaviours such as facing the sun are sufficient to maintain a stable internal temperature as external temperatures increase. This data opposes previous works by Brand [
27] and Kuntsch and Nel [
28] which suggested that as temperature increases, time spent facing the sun and time spent seeking shade increases. This may also have links presence of cloud cover in the 2022 study.
Kuntsch and Nel [
28] observed that females and juvenile individuals spent more time seeking shade, whereas the current study looked at shade usage in giraffes as a whole and did not split the data into sex or age with regard to shade-seeking. Kuntsch and Nel [
28] also suggested that as the availability of direct water and water from food sources increases, certain behaviours such as facing the sun and seeking shade would decrease, as non-behavioural methods to mediate temperature have been introduced. This may have led to the differences in results, as the reserve provides a constant source of water in the form of man-made waterholes, and rainfall has been spread over a longer period of the year, into the dry season. This increased availability of water may have drawn giraffes in the study group to spend extended time without seeking shade, especially in open areas, where shade was not present. In these areas water was obtained directly and indirectly, thus the necessity for shade usage was decreased, opposing the increased requirement for facing toward or away from the sun.
The difference between shade-seeking and identity is also noted by Brand [
27]. Pale males were found to have sought more shade than dark males and females during hot summer months, and dark males and females were found to seek more shade in colder winter months. However, Brand [
27] also identified that giraffes as a whole sought more shade during cloudy days, “possibly because of the reduced directionality of incident sunlight”. This restricted the analysis to days where the cloud cover was a quarter or less. In the current study, analysis of shade usage completely excluded cloudy days, as an attempt to keep consistent with analysis of position and time spent facing the sun, which also included only sunny days. The differences between the division of giraffe identity and inclusion of cloud cover in separate works may have caused the differences observed between results. These differences may be resolved by dividing the current data regarding shade-seeking collected on the reserve into age, sex, and coat colour. Further research with a greater focus on weather conditions such as cloud-cover may also be undertaken.
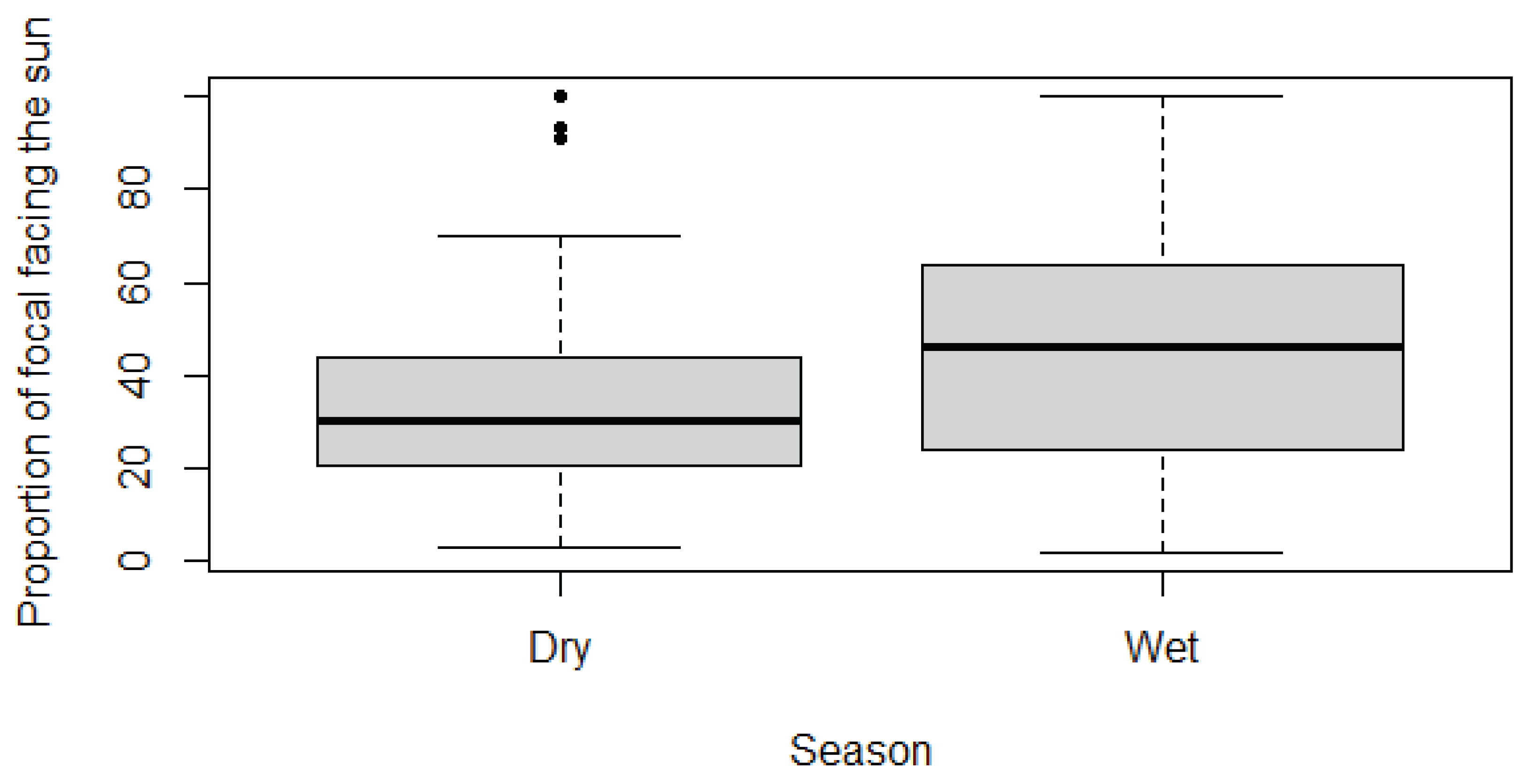
Seasonal differences were observed to assess any annual trends in giraffe thermoregulatory behaviours. The results found that giraffes spent a significantly higher proportion of time facing the sun in summer months, compared to winter months (
Table 8;
Figure 7). This follows the established hypotheses and can be explained by the increased temperatures observed during the summer season (
Figure 1); This links to the first hypothesis, that giraffes face the sun more as temperature increases.
The observed seasonal trend may be useful in predicting how the proportion of time spent facing the sun will change in future, when temperatures have been altered via the action of global warming. Similarly, this trend may allow for effective comparisons between previous works, and future studies.
As the current study was carried out over 8 months, the time available for data collection during winter months was reduced. This increased difference in sample numbers between summer and winter may reduce confidence in seasonal trends. This calls for further research to cover the entire course of a year, or more, to obtain equal samples across full seasons.
This winter data was also affected by the increased cloud cover during the winter months of 2022. Many days during winter were recorded as ‘cloudy days’, where determining the position of an individual relative to the sun was difficult, if not impossible. The increased cloud cover may also make the comparison to alternative research such as that of Brand [
27], who factored in cloud cover to their work, less reliable.
One issue that affected data collection was the use of AppSheet. This application allowed data to be automatically uploaded to a spreadsheet, making collection more efficient and providing parity to methods employed in previous works on the MRR. However, due to bugs, ongoing maintenance of the data collection application was required, and individual behaviours often took time to record, making short-lived behaviours difficult to collect. This may have affected results by decreasing the number of observed behaviours and increasing the length of others. This may be solved by using field-notes or identifying better software for data collection.
Another complication was the subjectivity of variables. Steps such as mimicking the direction of the giraffe to accurately identify the position relative to the sun were taken, however, the study observed several variables differently to previous research undertaken on the reserve [
33,
34,
35], making comparison between works difficult. Similarly, the time taken to determine the position and weather made the collection of short-lived behaviours challenging. This led to the decision to remove the wind category, which was assumed to have little effect on thermoregulatory behaviour, due to the lack of wind observed across the study period. These problems may be minimised by considering the opinions of multiple field researchers when recording subjective data categories.
The number of individuals on the reserve was also thought to have had an effect on behaviour. The carrying capacity of giraffes on the reserve, based on size consideration, was found to be 6 individuals [
36]. Throughout the study, the number of giraffes on the reserve increased from 50 to 57 individuals, 55 of which were studied. This may have had an effect on the behaviour of the study group, as giraffes were often found in highly social groups of up to 15 individuals. These large groups could have had an impact on the types of behaviours observed. Prioritisation of social behaviours, for example necking and mating, and a reduction in vigilance behaviours in these large groups [
37] may have been encouraged by the close proximity of so many dominant individuals.
The time spent facing the sun may have been affected by the vigilance behaviour of giraffes. A habituation session was put in place to reduce the overall vigilance of giraffes to the observer, and a reasonable distance was maintained from individuals during focal sessions, however, there was no feasible means to completely prevent wariness towards the researcher. The initiation of vigilance behaviour to the researchers often meant that other thermoregulatory behaviours were cut short, and during vigilant periods, the individual often ‘froze’ in place or fled, leading to significant proportions of a focal session spent in a position that would otherwise not have occurred, or cutting the focal session short.
5. Conclusions
The field collection and data analysis on behavioural thermoregulation in giraffes displayed ample concordance with previous research, with a handful of differences that may be explained by the context of the research, such as recent weather patterns and the location of the work.
Giraffes displayed interest in positioning themselves laterally to the sun on higher temperature days, with a predicted preference for facing anteriorly to the sun, casting shade over the main bulk of the body during full exposure. This behaviour was divided according to hypotheses regarding the identity of giraffes, with males and darker individuals facing the sun for longer periods than females and lighter individuals. The only identity that showed unexpected results was sub-adults, which were found to spend more time facing the sun, which were explained by the sample number of the study. The thermoregulatory behaviours studied were also found to follow a seasonal course, with more time spent facing the sun during the winter, where temperatures were lower.
Finally, the shade usage of giraffes was found to be negligible, with a slight preference for full exposure on hot days. This suggested that the behaviours studied were crucial in maintaining a stable internal body temperature, above other options.
These results may be instrumental in the commencement of further works on giraffes as a species, determining further taxonomic differences between individuals that may aid in settling the question on the existing number of species and sub-species, and the action through which these sub-species are formed. Similarly, future works may find it important to place greater focus on identities less represented in general research, such as females and sub-adults.
This understanding may also help in the conservation of groups of giraffes that are vulnerable or endangered. The creation of environments where the need for thermoregulatory behaviours is reduced may alleviate the stress on individuals, leading to more efficient breeding programmes, and reduced mortality rates. This may provide a necessary boost to local population numbers, which in turn could allow for transfer of individuals into separate locations, where genetic disparity could be bolstered. This would lend further resilience to struggling populations, where alteration of the environment is not possible, or would damage the biodiversity organisms other than the giraffe.
Acknowledgments
To begin my acknowledgements, I would first like to thank my supervisor Grietjie Stander for her unquestioning assistance in my research and the creation of the materials that will be used in my academic assessment. Throughout my extended time at the Mogalakwena Research Centre, there were times where my work was attempted as independently as possible, however, in so many cases where any questions formed, or the focus and motivation within my research became uncertain, Grietjie provided all the help that could possibly be asked for, and even gave advice intuitively when it became outwardly apparent that I had an issue that I thought I should solve; seeing as it was of my own creation. Similarly, I would also like to thank other supervisors, Dr Tanja van de Ven, and Berenger Laurent, for providing further insights into the work I have carried out, making me feel like a true scientific peer, over a temporary intern. Secondly, I’d like to thank the Coetsee family for creating the River Reserve, and other protected areas that aim to contribute to the ongoing fight for conservation. These areas have been transformed into amazing environments for the study of wild animals, which very few get the chance to visit and complete research on. Each member of the family has been very caring to me and other students on the reserve, offering ample insights into the various different roles it takes to maintain a reserve in the harsh South African bush. On top of this, many personal anecdotes have been provided by each individual within the family, defining them not only as the creators and caretakers of the reserve, but passionate people with captivating stories and ideals inside and outside of their lives’ work. Next, I’d like to thank the students who surrounded me on a day-to-day basis, supporting me in both social and professional contexts. My special thanks go to Camille Chalas, for providing quality images of some of the new juveniles born within my time on the reserve, helping me to edit the Mogalakwena giraffe ID kit for future students. Similarly, I thank Bastien Macé, Elisa Vassas, Elodie Courtel, and Elsa Marçon, for all of the help they provided at the beginning of my work with giraffes, it would have been impossible without them. Special thanks are also in order for every other individual I met during my time within the Mogalakwena research centre, however, due to the length of my placement, I have met so many amazing people, with the most immense variation of journeys and destinations; so, I take this time to thank you all individually for providing something different to my life, shaping it in the most important ways. Additionally, I would like to express my gratitude to the University of Cardiff and my personal tutor, Isa-Rita Russo. Isa-Rita provided a great deal of help in finding a placement, suggesting the Mogalakwena Research Centre amongst other locations. I’d like to believe that she is the main reason I ended up at the research centre, where I have had the most amazing experience. My university also provided a lot of help with the financial and planning aspects of my placement year, helping to lessen any anxieties I had with the extensive preparations for a placement relatively far from home. Finally, I would like to thank my family and friends in England. I have had constant support from a multitude of people throughout my life, pushing me to carry on and fight to get to the point where I was able to undertake such an immense experience. The support has continued, even from so far away and I cannot stress the strength that I have drawn from all of these individuals who have made my regular life worth living.
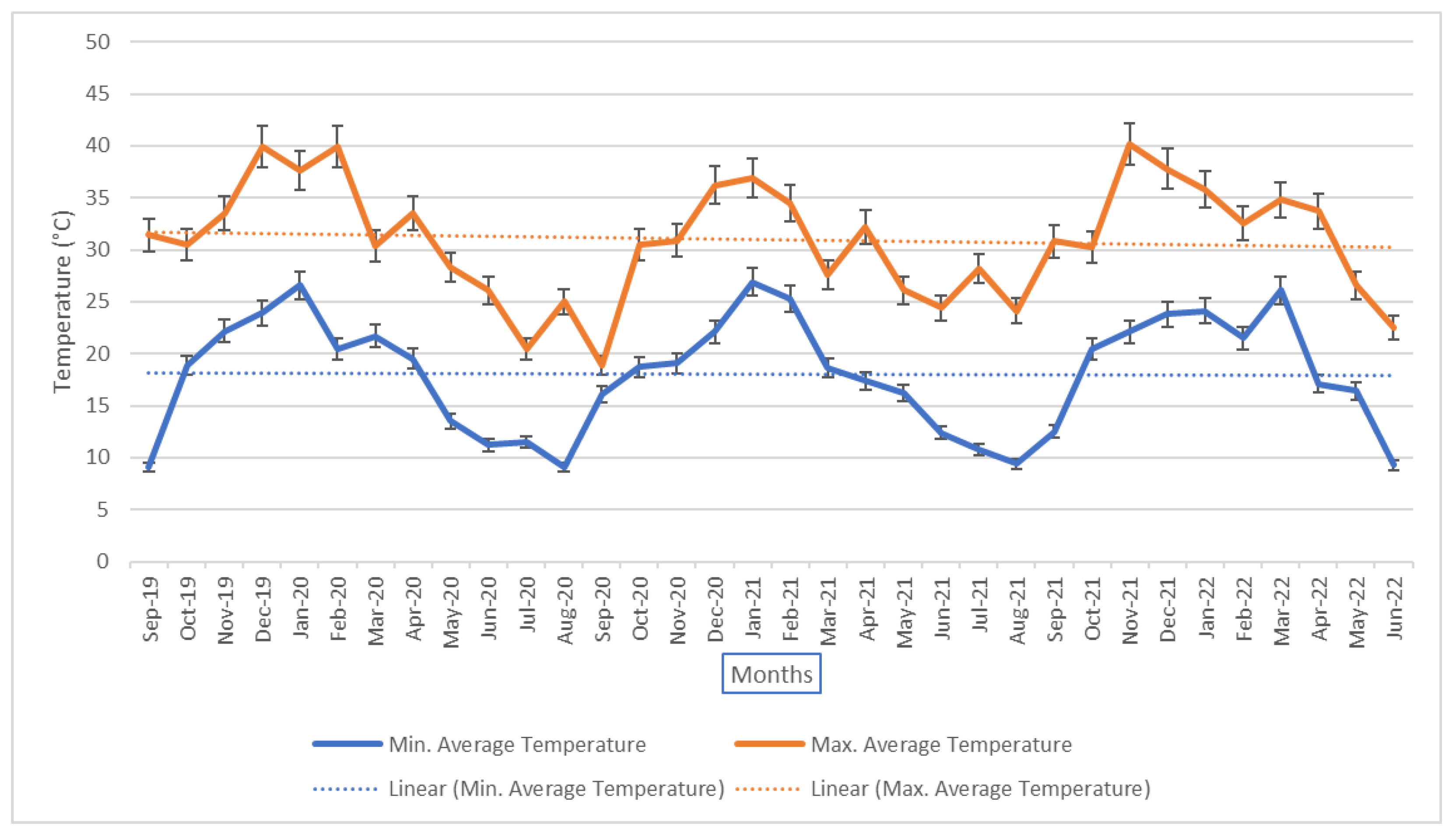
Figure 1.
Average monthly maximum and minimum temperatures between September 2019 - June 2022. Temperature is indicated with percentage deviation, as represented by vertical bars. The highest average maximum temperatures are observed annually between December and February, with a maximum monthly average recorded in November 2021, at 40.2°C. The lowest minimum average temperatures are observed annually between June and August, with a minimum monthly average recorded in August 2020, at 9.1°C. A trend of decreasing annual temperatures has been observed across temperature collection, as represented by decreasing graduated trend lines.
Figure 1.
Average monthly maximum and minimum temperatures between September 2019 - June 2022. Temperature is indicated with percentage deviation, as represented by vertical bars. The highest average maximum temperatures are observed annually between December and February, with a maximum monthly average recorded in November 2021, at 40.2°C. The lowest minimum average temperatures are observed annually between June and August, with a minimum monthly average recorded in August 2020, at 9.1°C. A trend of decreasing annual temperatures has been observed across temperature collection, as represented by decreasing graduated trend lines.
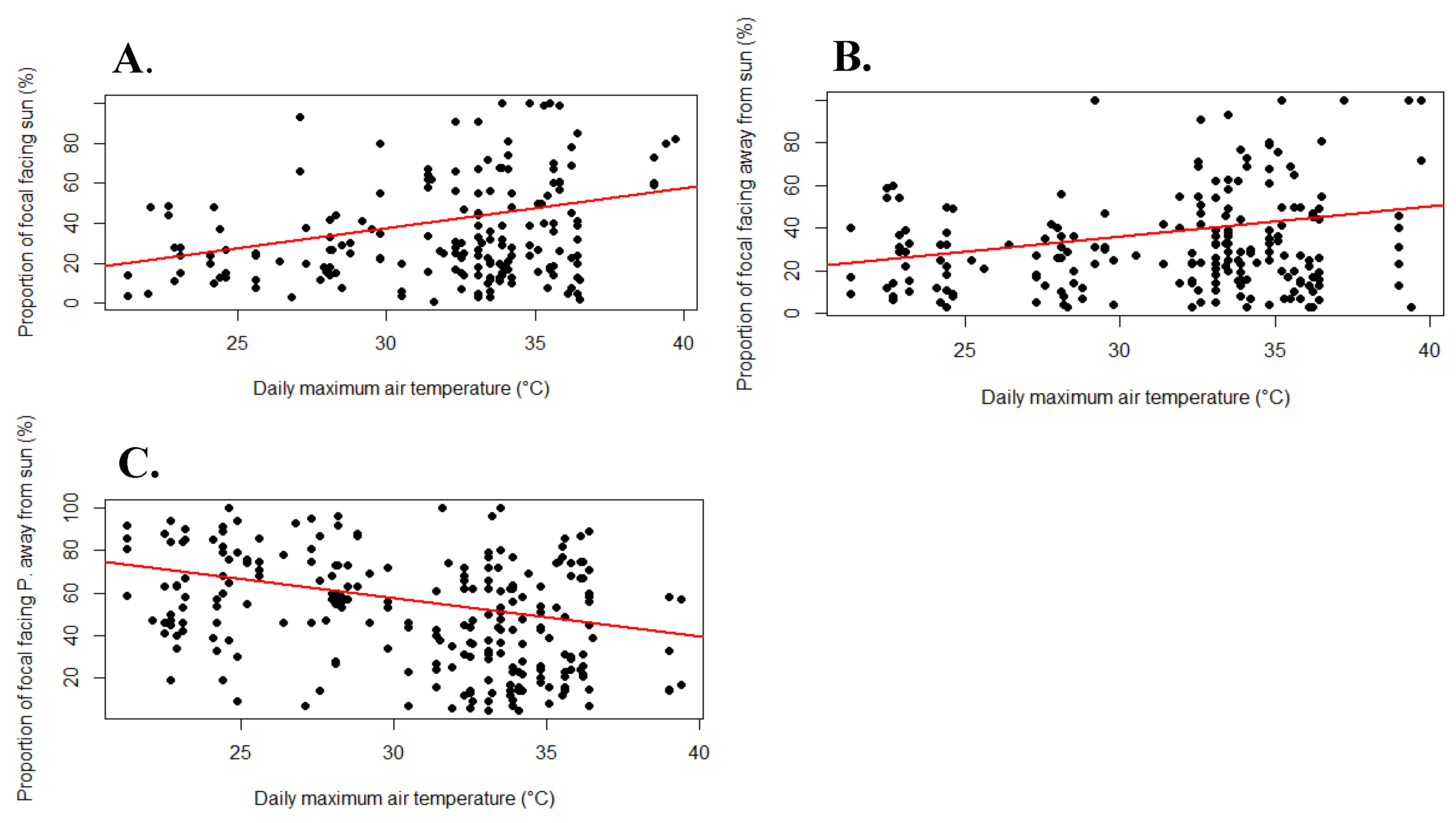
Figure 2.
Linear regression for significant positions as a function of maximum daily air temperature. Each plot displays the trend for a Relative Position Duration, found to be significant through a Spearman correlation against the maximum daily air temperature. A.) The relative time per focal facing the sun is plotted as a function of maximum temperature. B.) The relative time per focal facing directly away from the sun is plotted as a function of maximum temperature. C.) The relative time per focal facing partially away from the sun is plotted as a function of maximum temperature.
Figure 2.
Linear regression for significant positions as a function of maximum daily air temperature. Each plot displays the trend for a Relative Position Duration, found to be significant through a Spearman correlation against the maximum daily air temperature. A.) The relative time per focal facing the sun is plotted as a function of maximum temperature. B.) The relative time per focal facing directly away from the sun is plotted as a function of maximum temperature. C.) The relative time per focal facing partially away from the sun is plotted as a function of maximum temperature.
Figure 3.
Boxplots for relative time spent facing the sun among three age categories. The percentage proportions of each 15-minute focal spent facing the sun were divided into the Adult, Juvenile, and Sub-Adult age categories.
Figure 3.
Boxplots for relative time spent facing the sun among three age categories. The percentage proportions of each 15-minute focal spent facing the sun were divided into the Adult, Juvenile, and Sub-Adult age categories.
Figure 4.
Boxplot visualisation of Relative Time Facing the Sun against Sex.
Figure 4.
Boxplot visualisation of Relative Time Facing the Sun against Sex.
Figure 5.
Boxplot visualisation of Relative Time Facing the Sun against coat colour.
Figure 5.
Boxplot visualisation of Relative Time Facing the Sun against coat colour.
Figure 6.
Significant regression plot for the proportion of each focal fully exposed as a function of maximum temperature. The percentage proportion of each 15-minute focal session in any given exposure (Full, None, Partial) was plotted against the maximum daily air temperature, using a trendline to visualise correlation. Only significant plots were reported.
Figure 6.
Significant regression plot for the proportion of each focal fully exposed as a function of maximum temperature. The percentage proportion of each 15-minute focal session in any given exposure (Full, None, Partial) was plotted against the maximum daily air temperature, using a trendline to visualise correlation. Only significant plots were reported.
Figure 7.
Boxplot visualisation of Relative Time Facing the Sun against season.
Figure 7.
Boxplot visualisation of Relative Time Facing the Sun against season.
Table 1.
Results of Spearman Correlation between maximum daily air temperature and Relative Position Duration. The Relative Position Duration (arb. unit) is divided into three positions: towards, away, and partially away from the sun. These are correlated against maximum daily air temperature. The values for S, ρ, and p are recorded in the table; significant p-values are marked with an ‘*’.
Table 1.
Results of Spearman Correlation between maximum daily air temperature and Relative Position Duration. The Relative Position Duration (arb. unit) is divided into three positions: towards, away, and partially away from the sun. These are correlated against maximum daily air temperature. The values for S, ρ, and p are recorded in the table; significant p-values are marked with an ‘*’.
| |
Maximum Temperature |
| |
|
S |
p |
p-value |
Relative
Position
Duration
|
Towards |
13231004 |
0.31 |
<0.01* |
| Away |
35391291 |
0.19 |
<0.01* |
| Partially away |
248012875 |
-0.36 |
<0.01* |
Table 2.
Reported values for the Shapiro-Wilk, Levene’s and Kruskal-Wallis tests on the Relative Time Facing the Sun against age. Values for normality, equality of variance, and independence of groups signify that a Kruskal-Wallis test is required to test for significance between Relative Time Facing the Sun and age. A significant difference between age and Relative Time Facing the Sun was detected (p-value ≤0.05).
Table 2.
Reported values for the Shapiro-Wilk, Levene’s and Kruskal-Wallis tests on the Relative Time Facing the Sun against age. Values for normality, equality of variance, and independence of groups signify that a Kruskal-Wallis test is required to test for significance between Relative Time Facing the Sun and age. A significant difference between age and Relative Time Facing the Sun was detected (p-value ≤0.05).
| |
Shapiro-Wilk p-value |
Levene’s Test
p-value |
Kruskal-Wallis Test |
| |
Juvenile Sub-Adult Adult |
|
χ2 Df p-value |
| Relative Time Facing Sun |
<0.01 <0.01 <0.01 |
0.02 |
13.35 2 <0.01* |
Table 3.
Reported Z and p-values for a Dunn test comparing three separate age categories. The Z-value result of the test displays the direction of the trend, with a positive value denoting a positive trend, and a negative value denoting a negative trend. Adjusted p-values show where significance occurs, between each combination of age groups, with significant results marked with ‘*’ (p-value ≤0.05).
Table 3.
Reported Z and p-values for a Dunn test comparing three separate age categories. The Z-value result of the test displays the direction of the trend, with a positive value denoting a positive trend, and a negative value denoting a negative trend. Adjusted p-values show where significance occurs, between each combination of age groups, with significant results marked with ‘*’ (p-value ≤0.05).
| Comparison |
Z |
Adjusted p-value |
Adult – Juvenile
Adult – Sub-Adult
Juvenile – Sub-Adult |
2.70
-2.11
-3.52 |
0.02*
0.10
<0.01* |
Table 4.
Reported values for the Shapiro-Wilk, Levene’s and Wilcoxon-Mann-Whitney tests for Relative Time Facing the Sun and sex. The normality of variables was determined by a Shapiro-Wilk test, with p≤0.05 representing non-normal data. The equality of variance was determined with a Levene’s test, with p≤0.05 displaying unequal variance among the data. The Significance was tested using a Wilcoxon-Mann-Whitney test, with significance being identified by p≤0.05 (‘*’).
Table 4.
Reported values for the Shapiro-Wilk, Levene’s and Wilcoxon-Mann-Whitney tests for Relative Time Facing the Sun and sex. The normality of variables was determined by a Shapiro-Wilk test, with p≤0.05 representing non-normal data. The equality of variance was determined with a Levene’s test, with p≤0.05 displaying unequal variance among the data. The Significance was tested using a Wilcoxon-Mann-Whitney test, with significance being identified by p≤0.05 (‘*’).
| |
Shapiro-Wilk p-value |
Levene’s Test
p-value |
Wilcoxon-Mann-Whitney p-value |
| |
Male Female |
|
|
| Relative Time Facing Sun |
<0.01 <0.01 |
<0.01 |
0.01* |
Table 5.
Reported values for the Shapiro-Wilk, Levene’s and Kruskal-Wallis tests on Relative Time Facing the Sun against coat colour. Values for normality, equality of variance, and independence of groups signify that a Kruskal-Wallis test is required to test for significance between Relative Time Facing the Sun and coat colour. A significant difference between coat colour and Relative Time Facing the Sun was detected (p-value ≤0.05).
Table 5.
Reported values for the Shapiro-Wilk, Levene’s and Kruskal-Wallis tests on Relative Time Facing the Sun against coat colour. Values for normality, equality of variance, and independence of groups signify that a Kruskal-Wallis test is required to test for significance between Relative Time Facing the Sun and coat colour. A significant difference between coat colour and Relative Time Facing the Sun was detected (p-value ≤0.05).
| |
Shapiro-Wilk p-value |
Levene’s Test
p-value |
Kruskal-Wallis Test |
| |
Dark Medium Light |
|
χ2 Df p-value |
| Relative Time Facing Sun |
<0.01 <0.01 <0.01 |
<0.01 |
261.17 72 <0.01* |
Table 6.
Reported values for a Dunn test between coat colour and Relative Time Facing the Sun.
Table 6.
Reported values for a Dunn test between coat colour and Relative Time Facing the Sun.
| Comparison |
Z |
Adjusted p-value |
Dark - Light
Dark - Medium
Light - Medium |
3.26
-0.96
-4.06 |
<0.01*
0.34
<0.01* |
Table 7.
Reported values for the Spearman correlation between Relative Exposures per Focal and the maximum temperature. The relative time spent in each exposure per 15-minute session was divided into Full, Partial, and No exposure. The S, ρ, and p values of the correlation are displayed in the table, with significant p-values denoted by ‘*’.
Table 7.
Reported values for the Spearman correlation between Relative Exposures per Focal and the maximum temperature. The relative time spent in each exposure per 15-minute session was divided into Full, Partial, and No exposure. The S, ρ, and p values of the correlation are displayed in the table, with significant p-values denoted by ‘*’.
| |
Maximum Temperature |
| |
Exposure |
S |
ϱ |
p-value |
Relative
Exposure per Focal
|
Full
None
Partial |
4029771
47888
969382 |
0.16
<0.01
<0.05 |
<0.01*
1.00
0.52 |
Table 8.
Reported values for the Shapiro-Wilk, Levene’s and Wilcoxon-Mann-Whitney tests for Relative Time Facing the Sun against season. The normality of variables was determined by a Shapiro-Wilk test, with p≤0.05 representing abnormally distributed data. The equality of variance was determined with a Levene’s test, with p≤0.05 displaying unequal variance amongst the data. The significance was tested using a Wilcoxon-Mann-Whitney test, with significance being identified by p≤0.05 (‘*’).
Table 8.
Reported values for the Shapiro-Wilk, Levene’s and Wilcoxon-Mann-Whitney tests for Relative Time Facing the Sun against season. The normality of variables was determined by a Shapiro-Wilk test, with p≤0.05 representing abnormally distributed data. The equality of variance was determined with a Levene’s test, with p≤0.05 displaying unequal variance amongst the data. The significance was tested using a Wilcoxon-Mann-Whitney test, with significance being identified by p≤0.05 (‘*’).
| |
Shapiro-Wilk p-value |
Levene’s Test
p-value |
Wilcoxon-Mann-Whitney p-value |
| |
Dry Wet |
|
|
| Relative Time Facing Sun |
<0.01 <0.01 |
<0.01 |
0.01* |