Submitted:
27 March 2024
Posted:
27 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
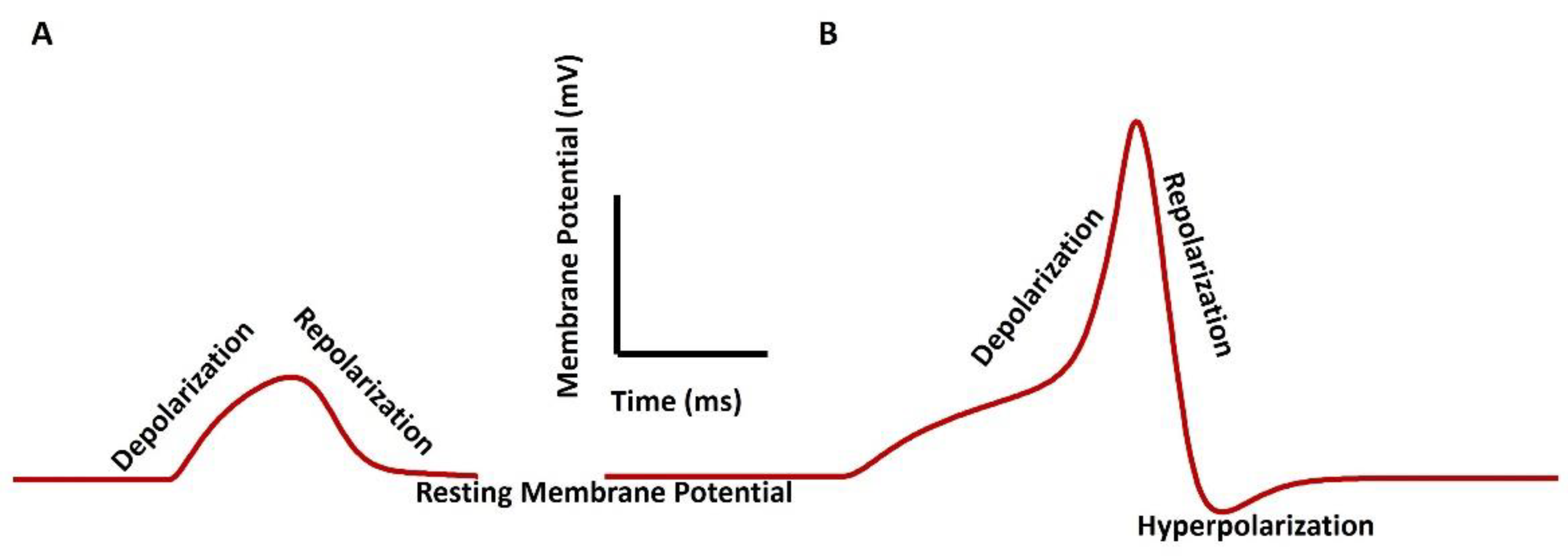
3. Membrane Potential in Smooth Muscle Contraction
4. Ion Channel Biophysics in Smooth Muscle
5. Calcium Dynamics in VSM Contraction
6. The Model of Generation of Tension Generation in VSM Cell
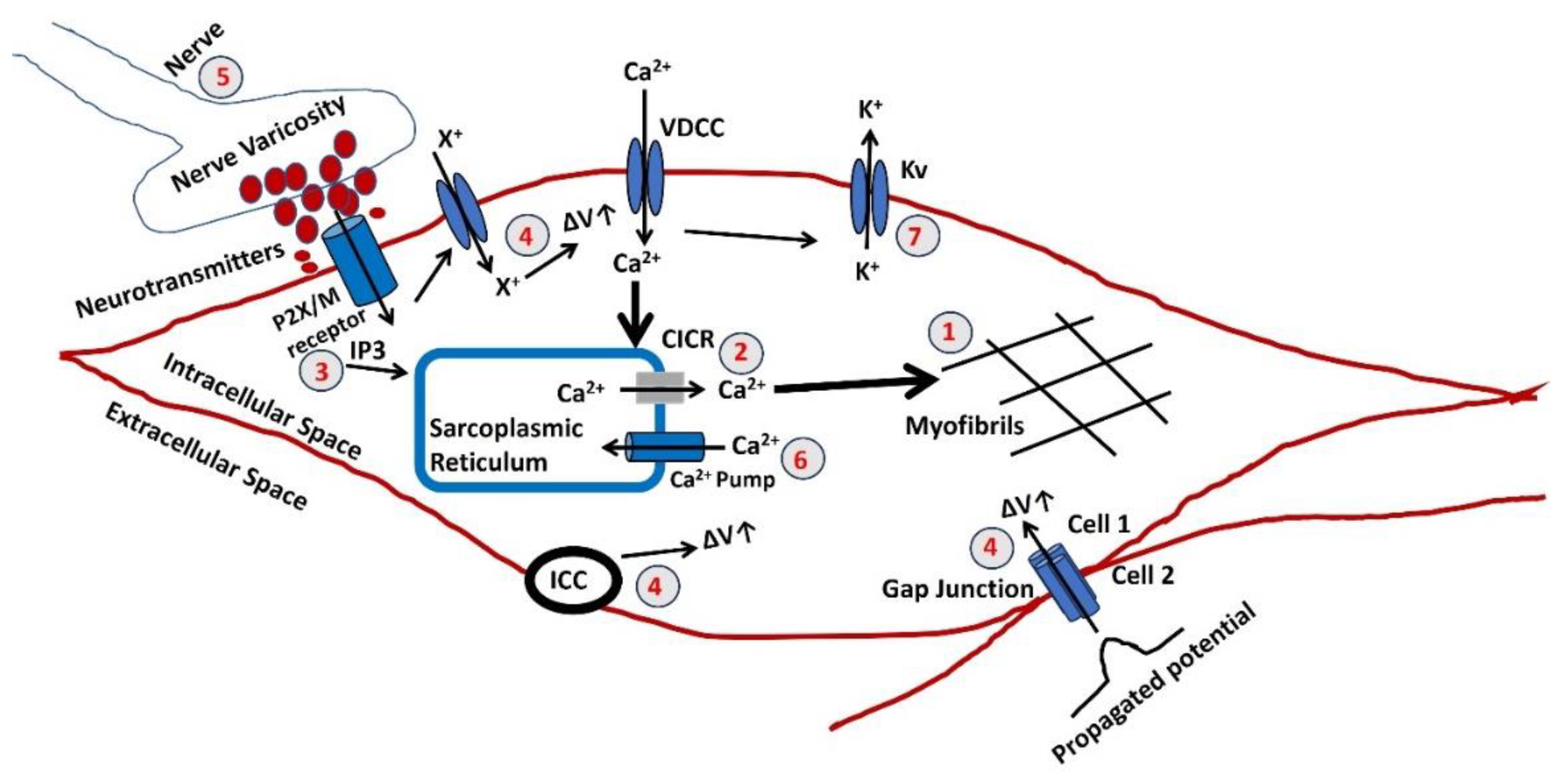
- The final stage in generating tension involves an increase in the sarcoplasmic concentration of Ca2+. The myofibrils exhibit a comparable sensitivity to Ca2+, as shown in other muscles, necessitating a Ca2+ concentration of around one mmol/L for half-maximal activation. Calcium ions Ca2+ form complexes with a soluble protein called calmodulin. This complex triggers a series of processes that activate a part of the myosin molecule by phosphorylation. As a result, actin and myosin can interact, requiring ATP.
- Sarcoplasmic Ca2+ is derived from the SR, an intracellular reservoir. Ca2+ ions are transported from the storage site to the sarcoplasm by Ca2+ channels, which intracellular agents’ control. The formation of tension is influenced by various factors that affect the buildup or release of calcium in the SR. Any disruption to the cellular metabolic mechanisms that produce ATP would undermine their effectiveness. The release of Ca2+ from the SR can often be accomplished through one of two methods. An increase in the Ca2+ concentration near the SR triggers further release of Ca2+. The CICR mechanism is usually initiated by a Ca2+ flux across the surface membrane, although this is not always true.
- There is a possibility of an elevation in the concentration of a diffusible second messenger, which connects the surface membrane with the release of intracellular Ca2+. The primary mechanism in typical human vagina smooth muscle involves the binding of purinergic or acetylcholine (ACh) to the P2X or M3 muscarinic receptor, which triggers a series of membrane-bound processes resulting in the synthesis of inositol trisphosphate (IP3). Alterations can significantly influence the release of intracellular Ca2+ in the sensitivity or gain of this mechanism.
- Three reasons can cause a rise in the membrane potential ΔV. The membrane potential can be propagated from cell 2 to cell one via the gap junction as the VSM behaves like a syncitium. Activating pacemaking cell ICC can also trigger a rise in membrane potential. The extracellular ATP might bind to the purinergic receptor (P2X) and open a non-specific cation channel to permit the influx of any positive ion (X+), which can cause a rise in membrane potential. The resultant depolarization can open L-type Ca2+ channels, initiate Ca2+ influx, and trigger AP.
- The parasympathetic nerves innervate the smooth muscle, and varicosites are the sites where the neurotransmitters are released. The number and distribution of excitatory nerves or the quantity of transmitter released modulate the membrane potential of the VSM. The neurotransmitted might be purinergic or cholinergic cotransmitters.
- The Ca2+ is filled in the SR lumen through a highly efficient ATP-dependent calcium pump, which transports calcium against a concentration gradient.
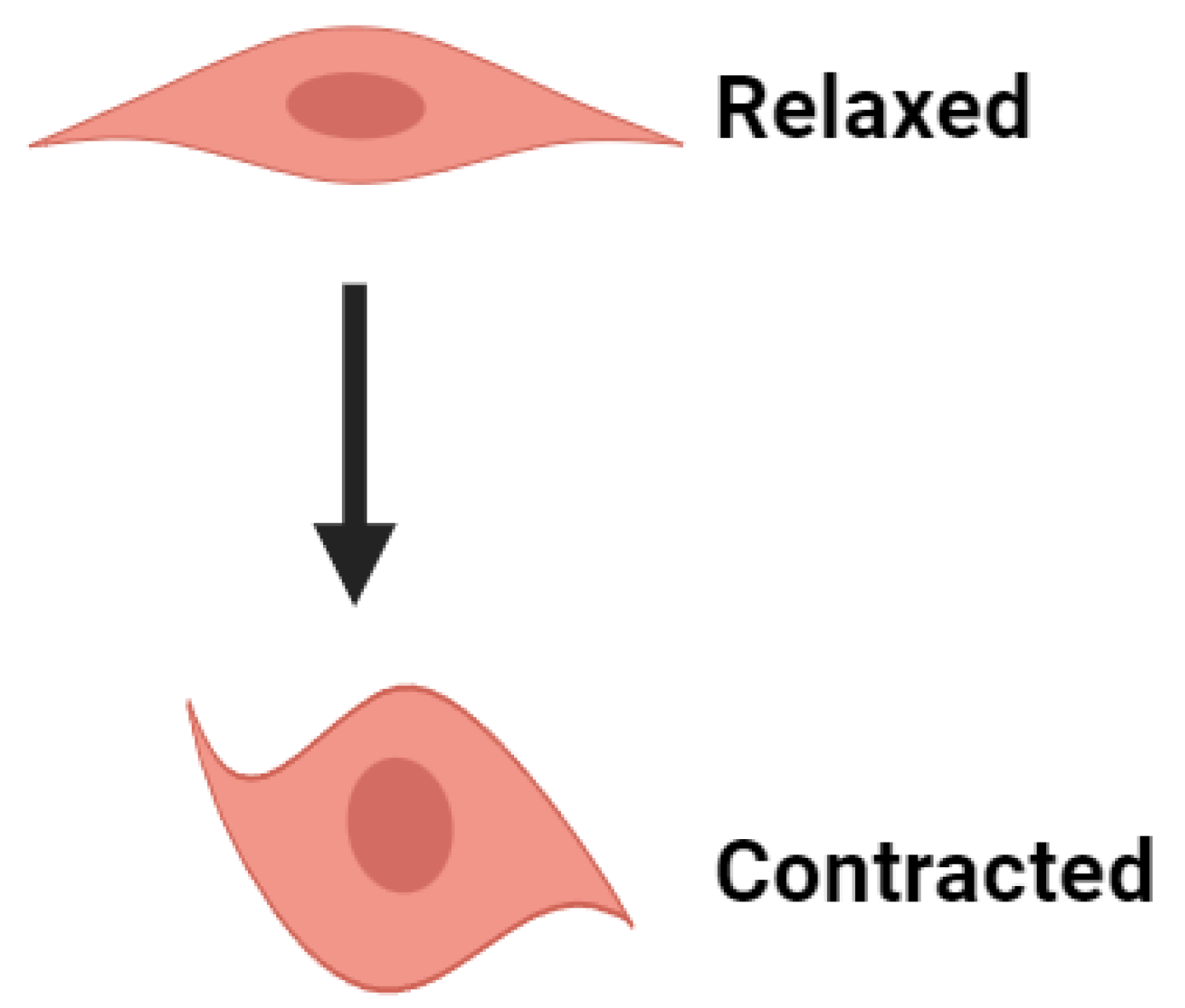
- The decaying of the Ca2+ transient, which occurs after the generation of AP or SW, ceases the VSM contraction. The activation of the Ca2+ channel and generation of AP/SW open the various K+ channels to repolarize the membrane, bringing the membrane potential to the RMP. After completing the contraction, the VSM cell returns to the relaxed state.
7. Experimental and Computational Techniques for Studying VSM Contraction
8. Clinical Implications and Future Directions
9. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gold, Joann M., and Isha Shrimanker. "Physiology, vaginal." (2019).
- Hafen, Brant B., Micah Shook, and Bracken Burns. "Anatomy, Smooth Muscle." (2018).
- Banerjee D, Das PK. Check for updates Muscular System Dipak Banerjee, Pradip Kumar Das, and Joydip Mukherjee. Textbook of Veterinary Physiology. 2023 Aug 31:235.
- Clark-Patterson GL, Buchanan LM, Ogola BO, Florian-Rodriguez M, Lindsey SH, De Vita R, Miller KS. Smooth muscle contribution to vaginal viscoelastic response. Journal of the mechanical behavior of biomedical materials. 2023 Apr 1;140:105702. [CrossRef]
- Wong PY, Fong Z, Hollywood MA, Thornbury KD, Sergeant GP. Regulation of nerve-evoked contractions of the murine vas deferens. Purinergic Signalling. 2024 Feb 20:1-1. [CrossRef]
- Mahapatra, Chitaranjan, and Inna Samuilik. "A Mathematical Model of Stochastic Synaptic Noise Dynamics Based Spontaneous Action Potential in Non-neural Cell." (2024).
- Clark GL, Pokutta-Paskaleva AP, Lawrence DJ, Lindsey SH, Desrosiers L, Knoepp LR, Bayer CL, Gleason Jr RL, Miller KS. Smooth muscle regional contribution to vaginal wall function. Interface Focus. 2019 Aug 6;9(4):20190025.
- Webb RC. Smooth muscle contraction and relaxation. Advances in physiology education. 2003 Dec;27(4):201-6.
- Huntington A, Abramowitch SD, Moalli PA, De Vita R. Strains induced in the vagina by smooth muscle contractions. Acta Biomaterialia. 2021 Jul 15;129:178-87. [CrossRef]
- Mahapatra C, Brain KL, Manchanda R. A biophysically constrained computational model of the action potential of mouse urinary bladder smooth muscle. PloS one. 2018 Jul 26;13(7):e0200712. [CrossRef]
- Maleiner B, Spadiut O, Fuchs C. The importance of biophysical and biochemical stimuli in dynamic skeletal muscle models. Frontiers in Physiology. 2018 Aug 22;9:342859. [CrossRef]
- Jorge S, Chang S, Barzilai JJ, Leppert P, Segars JH. Mechanical signaling in reproductive tissues: mechanisms and importance. Reproductive Sciences. 2014 Sep;21(9):1093-107. [CrossRef]
- Mahapatra C, Dave V, Manchanda R. A Mathematical Modeling of Voltage gated Calcium ion channel based Calcium Transient Response in UrinaryBladder Smooth Muscle Cell. International Journal of Pure and Applied Mathematics. 2017;117(9):71-5.
- Berridge MJ. Smooth muscle cell calcium activation mechanisms. The Journal of physiology. 2008 Nov 1;586(21):5047-61. [CrossRef]
- Dave V, Mahapatra C, Manchanda R. A mathematical model of the calcium transient in urinary bladder smooth muscle cells. In2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2015 Aug 25 (pp. 5359-5362). IEEE.
- KS T. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:215-42.
- Pereira da Silva EA, Martín-Aragón Baudel M, Navedo MF, Nieves-Cintrón M. Ion channel molecular complexes in vascular smooth muscle. Frontiers in Physiology. 2022 Aug 26;13:999369.
- Mahapatra C, Brain K, Manchanda R. Biophysically Realistic Modles of Detrusor Ion Channels: role in shaping spike and excitavility. InUrinary Bladder Physiology: Computational Insights 2024 Jan 1. Publ Narosa Publishing House.
- Riemer RK, Heymann MA. Regulation of uterine smooth muscle function during gestation. Pediatric research. 1998 Nov;44(5):615-27. [CrossRef]
- Brading A. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. The Journal of physiology. 2006 Jan;570(1):13-22. [CrossRef]
- Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. American journal of physiology-cell physiology. 2003 Mar 1;284(3):C583-95.
- Jackson WF. Potassium channels in regulation of vascular smooth muscle contraction and growth. Advances in Pharmacology. 2017 Jan 1;78:89-144.
- Jallah Z, Liang R, Feola A, Barone W, Palcsey S, Abramowitch SD, Yoshimura N, Moalli P. The impact of prolapse mesh on vaginal smooth muscle structure and function. BJOG: An International Journal of Obstetrics & Gynaecology. 2016 Jun;123(7):1076-85. [CrossRef]
- Hille B. A Life of Biophysics. Annual Review of Biophysics. 2022 May 9;51:1-7.
- Motschall E, Falck-Ytter Y. Searching the MEDLINE literature database through PubMed: a short guide. Oncology Research and Treatment. 2005 Aug 19;28(10):517-22. [CrossRef]
- Armstrong CM, Hille B. Voltage-gated ion channels and electrical excitability. Neuron. 1998 Mar 1;20(3):371-80. [CrossRef]
- Seidl AH. Regulation of conduction time along axons. Neuroscience. 2014 Sep 12;276:126-34. [CrossRef]
- Hille B. Evolutionary origin of electrical excitability. InCellular Mechanisms of Conditioning and Behavioral Plasticity 1988 (pp. 511-518). Boston, MA: Springer US.
- Abdul Kadir L, Stacey M, Barrett-Jolley R. Emerging roles of the membrane potential: action beyond the action potential. Frontiers in physiology. 2018 Nov 21;9:419544. [CrossRef]
- Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem cell reviews and reports. 2009 Sep;5:231-46. [CrossRef]
- Endresen LP, Hall K, Høye JS, Myrheim J. A theory for the membrane potential of living cells. European Biophysics Journal. 2000 May;29:90-103. [CrossRef]
- Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. British journal of pharmacology. 2003 Sep;140(1):146-58. [CrossRef]
- Mahapatra C, Manchanda R. Modeling Vas Deferens Smooth Muscle Electrophysiology: Role of Ion Channels in Generating Electrical Activity. InSoft Computing for Problem Solving: SocProS 2017, Volume 2 2019 (pp. 655-663). Springer Singapore.
- Holman ME, Tonta MA, Parkington HC, Coleman HA. Tetrodotoxin-sensitive action potentials in smooth muscle of mouse vas deferens. Journal of the autonomic nervous system. 1995 Apr 8;52(2-3):237-40. [CrossRef]
- Mahapatra C. Computational Study of Action Potential Generation in Urethral Smooth Muscle Cell. InComputational Advances in Bio and Medical Sciences: 10th International Conference, ICCABS 2020, Virtual Event, December 10-12, 2020, Revised Selected Papers 10 2021 (pp. 26-32). Springer International Publishing.
- Brading A. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. The Journal of physiology. 2006 Jan;570(1):13-22. [CrossRef]
- Mahapatra C, Manchanda R. Computational studies on ureter smooth muscle: modeling ion channels and their role in generating electrical activity. InProceedings of the 2019 Summer Simulation Conference 2019 Jul 22 (pp. 1-6).
- Burdyga T, Wray S. Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels. Nature. 2005 Jul 28;436(7050):559-62. [CrossRef]
- Sanders KM. Spontaneous electrical activity and rhythmicity in gastrointestinal smooth muscles. Smooth muscle spontaneous activity: Physiological and pathological modulation. 2019:3-46.
- Van Helden DF, Laver DR, Holdsworth J, Imtiaz MS. Generation and propagation of gastric slow waves. Clinical and Experimental Pharmacology and Physiology. 2010 Apr;37(4):516-24.
- WAKUI M, FUKUSHI Y. Evidence for suppression of potassium conductance by noradrenaline in smooth muscle of guinea-pig vas deferens. The Tohoku Journal of Experimental Medicine. 1986;150(4):365-71. [CrossRef]
- Kobayashi M, Irisawa H. Effect of sodium deficiency of the action potential of the smooth muscle of ureter. American Journal of Physiology-Legacy Content. 1964 Jan 1;206(1):205-10. [CrossRef]
- Shmigol AV, Eisner DA, Wray S. Properties of voltage-activated [Ca2+] i transients in single smooth muscle cells isolated from pregnant rat uterus. The Journal of physiology. 1998 Sep;511(3):803-11.
- Thorneloe KS, Nelson MT. Properties of a tonically active, sodium-permeable current in mouse urinary bladder smooth muscle. American Journal of Physiology-Cell Physiology. 2004 Jun;286(6):C1246-57. [CrossRef]
- Kuriyama H, Ohshima K, Sakamoto Y. The membrane properties of the smooth muscle of the guinea-pig portal vein in isotonic and hypertonic solutions. The Journal of Physiology. 1971 Aug;217(1):179.
- Clapp LH, Gurney AM. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Experimental Physiology: Translation and Integration. 1991 Sep 1;76(5):677-93. [CrossRef]
- Cauvin C, Lukeman S, Cameron J, Hwang O, Meisheri K, Yamamoto H, Van Breemen C. Theoretical bases for vascular selectivity of Ca2+ antagonists. Journal of Cardiovascular Pharmacology. 1984;6: S630-8. [CrossRef]
- Furness JB. An electrophysiological study of the innervation of the smooth muscle of the colon. The Journal of Physiology. 1969; 205:549-62.
- Kajimoto N, Kirpekar SM, Wakade AR. An investigation of spontaneous potentials recorded from the smooth-muscle cells of the guinea-pig seminal vesicle. The Journal of Physiology. 1972 Jul 1;224(1):105-19. [CrossRef]
- van Helden DF, Kamiya A, Kelsey S, Laver DR, Jobling P, Mitsui R, Hashitani H. Nerve-induced responses of mouse vaginal smooth muscle. Pflügers Archiv-European Journal of Physiology. 2017; 469:1373-85. [CrossRef]
- Parkington HC, Tonta MA, Davies NK, Brennecke SP, Coleman HA. Hyperpolarization and slowing of the rate of contraction in human uterus in pregnancy by prostaglandins E2 and F2α: involvement of the Na+ pump. The Journal of physiology. 1999; 514:229-43.
- Shafik A, El Sibai O, Shafik AA, Ahmed I, Mostafa RM. The electrovaginogram: study of the vaginal electric activity and its role in the sexual act and disorders. Archives of Gynecology and Obstetrics. 2004; 269:282-6. [CrossRef]
- Shafik A, Shafik IA, El Sibai O, Shafik AA. An electrophysiologic study of female ejaculation. Journal of sex & marital therapy. 2009; 35:337-46. [CrossRef]
- Sarmento AL, Sá BS, Vasconcelos AG, Arcanjo DD, Durazzo A, Lucarini M, Leite JR, Sousa HA, Kückelhaus SA. Perspectives on the Therapeutic Effects of Pelvic Floor Electrical Stimulation: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19:14035. [CrossRef]
- Blanks AM, Eswaran H. Measurement of uterine electrophysiological activity. Current Opinion in Physiology. 2020; 13:38-42. [CrossRef]
- Kuo IY, Ehrlich BE. Signaling in muscle contraction. Cold Spring Harbor perspectives in biology. 2015;7(2): a006023.
- Camerino DC, Tricarico D, Desaphy JF. Ion channel pharmacology. Neurotherapeutics. 2007; 4:184-98. [CrossRef]
- KS T. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005; 83:215-42.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Ion channels and the electrical properties of membranes. InMolecular Biology of the Cell. 4th edition 2002. Garland Science.
- LEWIS DL, LECHLEITER JD, KIM D, NANAVATI C, CLAPHAM DE. Intracellular regulation of ion channels in cell membranes. InMayo Clinic Proceedings 1990, 65, 1127-1143. [CrossRef]
- Balse E, Steele DF, Abriel H, Coulombe A, Fedida D, Hatem SN. Dynamic of ion channel expression at the plasma membrane of cardiomyocytes. Physiological reviews. 2012; 92:1317-58. [CrossRef]
- Daghbouche-Rubio N, Pérez-García MT, Cidad P. Vascular smooth muscle ion channels in essential hypertension. Frontiers in physiology. 2022; 13:1016175. [CrossRef]
- Firth AL, Remillard CV, Platoshyn O, Fantozzi I, Ko EA, Yuan JX. Functional ion channels in human pulmonary artery smooth muscle cells: Voltage-dependent cation channels. Pulmonary circulation. 2011; 1:48-71. [CrossRef]
- Syed AU, Le T, Navedo MF, Nieves-Cintrón M. Ion channels and their regulation in vascular smooth muscle. InBasic and Clinical Understanding of Microcirculation 2019. IntechOpen.
- Martinac B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2014; 1838:682-91. [CrossRef]
- Bulley S, Jaggar JH. Cl− channels in smooth muscle cells. Pflügers Archiv-European Journal of Physiology. 2014; 466:861-72.
- Reinl EL, Cabeza R, Gregory IA, Cahill AG, England SK. Sodium leak channel, non-selective contributes to the leak current in human myometrial smooth muscle cells from pregnant women. MHR: Basic science of reproductive medicine. 2015 Oct 1;21(10):816-24. [CrossRef]
- Brading AF, Brain KL. Ion channel modulators and urinary tract function. Urinary Tract. 2011:375-93.
- Mahapatra C, Manchanda R. Modulating Properties of Hyperpolarization-Activated Cation Current in Urinary Bladder Smooth Muscle Excitability: A Simulation Study. InRecent Findings in Intelligent Computing Techniques: Proceedings of the 5th ICACNI 2017, Volume 1 2019 (pp. 261-266). Springer Singapore.
- Mahapatra C, Brain KL, Manchanda R. Computational study of Hodgkin-Huxley type calcium-dependent potassium current in urinary bladder over activity. In2018 IEEE 8th international conference on computational advances in bio and medical sciences (ICCABS) 2018 Oct 18 (pp. 1-4). IEEE.
- Mahapatra C, Brain KL, Manchanda R. Computational study of ATP gated Potassium ion channel in urinary bladder over activity. In2016 International Conference on Inventive Computation Technologies (ICICT) 2016,2, 1-4.
- Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007; 132:1852-65. [CrossRef]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F. Calcium signalling—an overview. InSeminars in cell & developmental biology 2001,12, 3-10. Academic Press.
- Bootman MD, Bultynck G. Fundamentals of cellular calcium signaling: a primer. Cold Spring Harbor perspectives in biology. 2020;12: a038802. [CrossRef]
- Ureshino RP, Erustes AG, Bassani TB, Wachilewski P, Guarache GC, Nascimento AC, Costa AJ, Smaili SS, da Silva Pereira GJ. The interplay between Ca2+ signaling pathways and neurodegeneration. International journal of molecular sciences. 2019; 20:6004. [CrossRef]
- Matthew A, Shmygol A, Wray S. Ca2+ entry, efflux and release in smooth muscle. Biological research. 2004;37(4):617-24. [CrossRef]
- Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiological reviews. 2010; 90:113-78. [CrossRef]
- Collier ML, Ji G, Wang YX, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. The Journal of general physiology. 2000; 115:653-62.
- Park YJ, Yoo SA, Kim M, Kim WU. The role of calcium–calcineurin–NFAT signaling pathway in health and autoimmune diseases. Frontiers in immunology. 2020; 11:455685.
- Lin W, Wang Y, Chen Y, Wang Q, Gu Z, Zhu Y. Role of calcium signaling pathway-related gene regulatory networks in ischemic stroke based on multiple WGCNA and single-cell analysis. Oxidative medicine and cellular longevity. 2021;2021:1-35. [CrossRef]
- Cellai I, Filippi S, Comeglio P, Cipriani S, Maseroli E, Di Stasi V, Todisco T, Marchiani S, Tamburrino L, Villanelli F, Vezzani S. Testosterone positively regulates vagina NO-induced relaxation: an experimental study in rats. Journal of Endocrinological Investigation. 2022; 45:1161-72. [CrossRef]
- Mahapatra C, Manchanda R. Simulation of In Vitro-Like Electrical Activities in Urinary Bladder Smooth Muscle Cells. Journal of Biomimetics, Biomaterials and Biomedical Engineering. 2017; 33:45-51. [CrossRef]
- Mahapatra C, Brain KL, Manchanda R. Computational studies on urinary bladder smooth muscle: Modeling ion channels and their role in generating electrical activity. In2015 7th International IEEE/EMBS Conference on Neural Engineering (NER) 2015 Apr 22 (pp. 832-835). IEEE.
- Aliev RR, Richards W, Wikswo JP. A simple nonlinear model of electrical activity in the intestine. Journal of theoretical biology. 2000; 204:21-8. [CrossRef]
- Bursztyn L, Eytan O, Jaffa AJ, Elad D. Mathematical model of excitation-contraction in a uterine smooth muscle cell. American Journal of Physiology-Cell Physiology. 2007;292:C1816-29. [CrossRef]
- Rihana S, Terrien J, Germain G, Marque C. Mathematical modeling of electrical activity of uterine muscle cells. Medical & biological engineering & computing. 2009; 47:665-75. [CrossRef]
- Tong WC, Choi CY, Karche S, Holden AV, Zhang H, Taggart MJ. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PloS one. 2011;6:e18685. [CrossRef]
- Poh, Yong Cheng, Alberto Corrias, Nicholas Cheng, and Martin Lindsay Buist. "A quantitative model of human jejunal smooth muscle cell electrophysiology." (2012): e42385. [CrossRef]
- Corrias A, Buist ML. A quantitative model of gastric smooth muscle cellular activation. Annals of biomedical engineering. 2007; 35:1595-607. [CrossRef]
- Corrias A, Buist ML. Quantitative cellular description of gastric slow wave activity. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;294: G989-95.
- Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: agonist and NO stimulation. Journal of theoretical biology. 2008; 253:238-60. [CrossRef]
- Miftakhov RN, Abdusheva GR, Wingate DL. Model predictions of myoelectrical activity of the small bowel. Biological cybernetics. 1996; 74:167-79.
- Cha CY, Earm KH, Youm JB, Baek EB, Kim SJ, Earm YE. Electrophysiological modelling of pulmonary artery smooth muscle cells in the rabbits—special consideration to the generation of hypoxic pulmonary vasoconstriction. Progress in biophysics and molecular biology. 2008; 96:399-420. [CrossRef]
- Jacobsen JC, Aalkjær C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH. A model of smooth muscle cell synchronization in the arterial wall. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H229-37. [CrossRef]
- Yang M, Chen C, Wang Z, Long J, Huang R, Qi W, Shi R. Finite element analysis of female pelvic organ prolapse mechanism: current landscape and future opportunities. Frontiers in Medicine. 2024;11. [CrossRef]
- Hutchings CJ, Colussi P, Clark TG. Ion channels as therapeutic antibody targets. InMAbs 2019, 11, 265-296. Taylor & Francis. [CrossRef]
- Lang F, Stournaras C. Ion channels in cancer: future perspectives and clinical potential. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014 Mar 19;369(1638):20130108.
- Szabo, I., Zoratti, M. and Biasutto, L., Targeting mitochondrial ion channels for cancer therapy. Redox Biology, 2021 42, p.101846. [CrossRef]
- Haustrate A, Hantute-Ghesquier A, Prevarskaya N. Monoclonal antibodies targeting ion channels and their therapeutic potential. Frontiers in pharmacology. 2019; 10:435761. [CrossRef]
- Mei S, Ye M, Gil L, Zhang J, Zhang Y, Candiotti K, Takacs P. The role of smooth muscle cells in the pathophysiology of pelvic organ prolapse. Urogynecology. 2013 Sep 1;19(5):254-9. [CrossRef]
- Huntington A, Abramowitch SD, Moalli PA, De Vita R. Strains induced in the vagina by smooth muscle contractions. Acta Biomaterialia. 2021; 129:178-87. [CrossRef]
| Smooth muscle type | RMP (mV) | AP/SW | Reference | |
|---|---|---|---|---|
| Urinary bladder | -45 to -55 | AP | [10] | |
| Vas deferens | -60 | AP | [41] | |
| Ureter | -45 | AP | [42] | |
| Uterine | -50 | AP | [43] | |
| Urethra | -40 | AP | [44] | |
| Portal vein | -50 | SW | [45] | |
| Pulmonary artery | -55 | SW | [46] | |
| Aorta | -50 | SW | [47] | |
| Colon (GI tract) | -60 | SW | [48] | |
| Seminal vesicles | -50 | SW | [49] |
| Ion channel type | Role in AP/SW | |
|---|---|---|
| Ca2+ channels | Depolarization, RMP, AP firing | |
| Na+ channels | Depolarization, AP firing | |
| K+ channels | Repolarization, Hyperpolarization, RMP | |
| Cl- channels | Repolarization, RMP | |
| TRP channels | Depolarization, RMP, AP firing | |
| Leak channels | RMP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





