Submitted:
26 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
- To summarize what is known about the role of premature cellular/neuronal senescence in the pathogenesis of SCZ and SLDs.
- To discuss potential strategies for improving sustained recovery in SCZ and SLDs via natural senotherapeutics, microbial phenazines, aryl hydrocarbon receptor (AhR) antagonists, membrane lipid replacement (MLR), and mitochondrial transplantation.
Premature Cellular Senescence in Schizophrenia
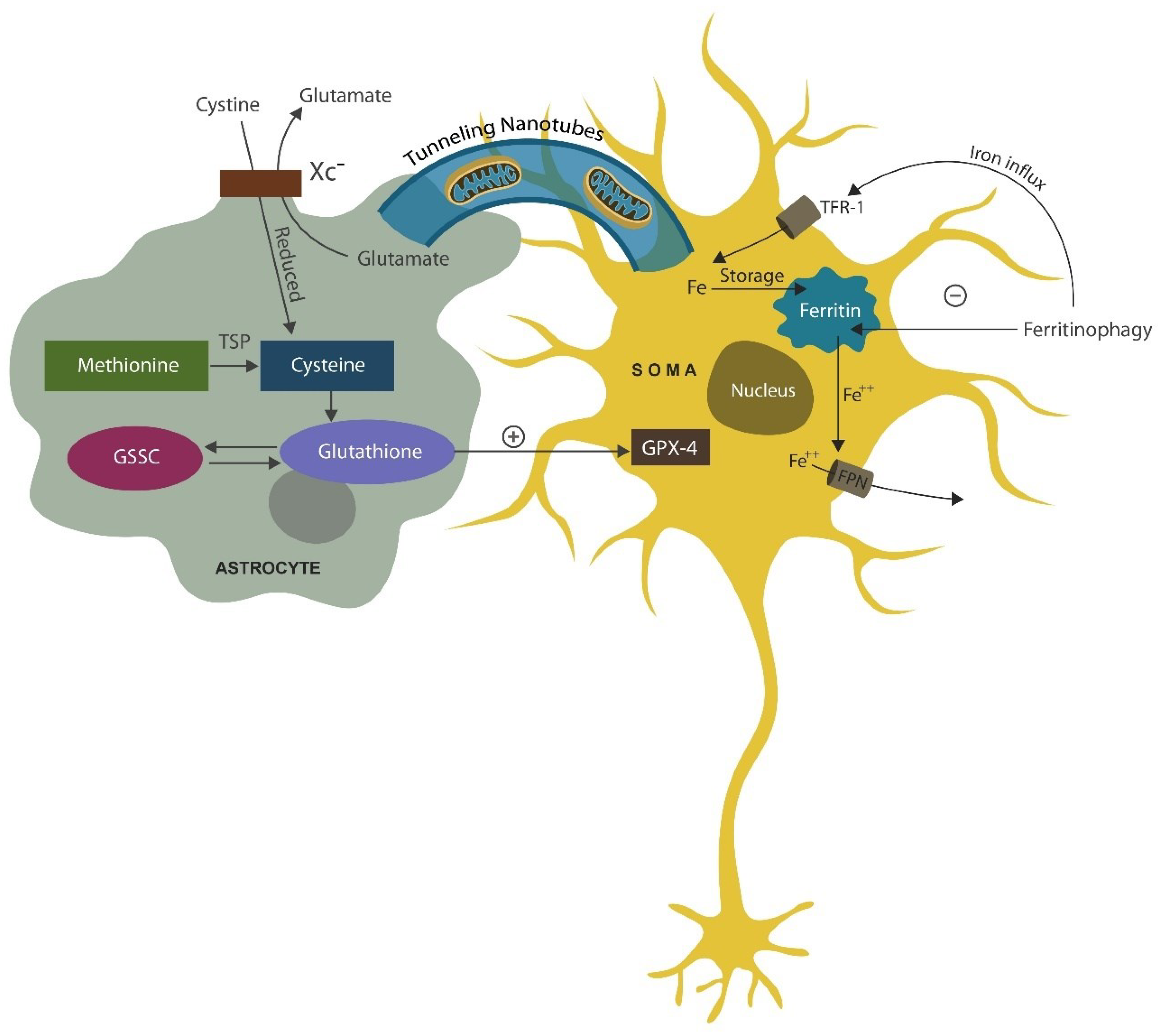
Ferrosenescence vs. Ferroptosis
Senescent Gut Barrier
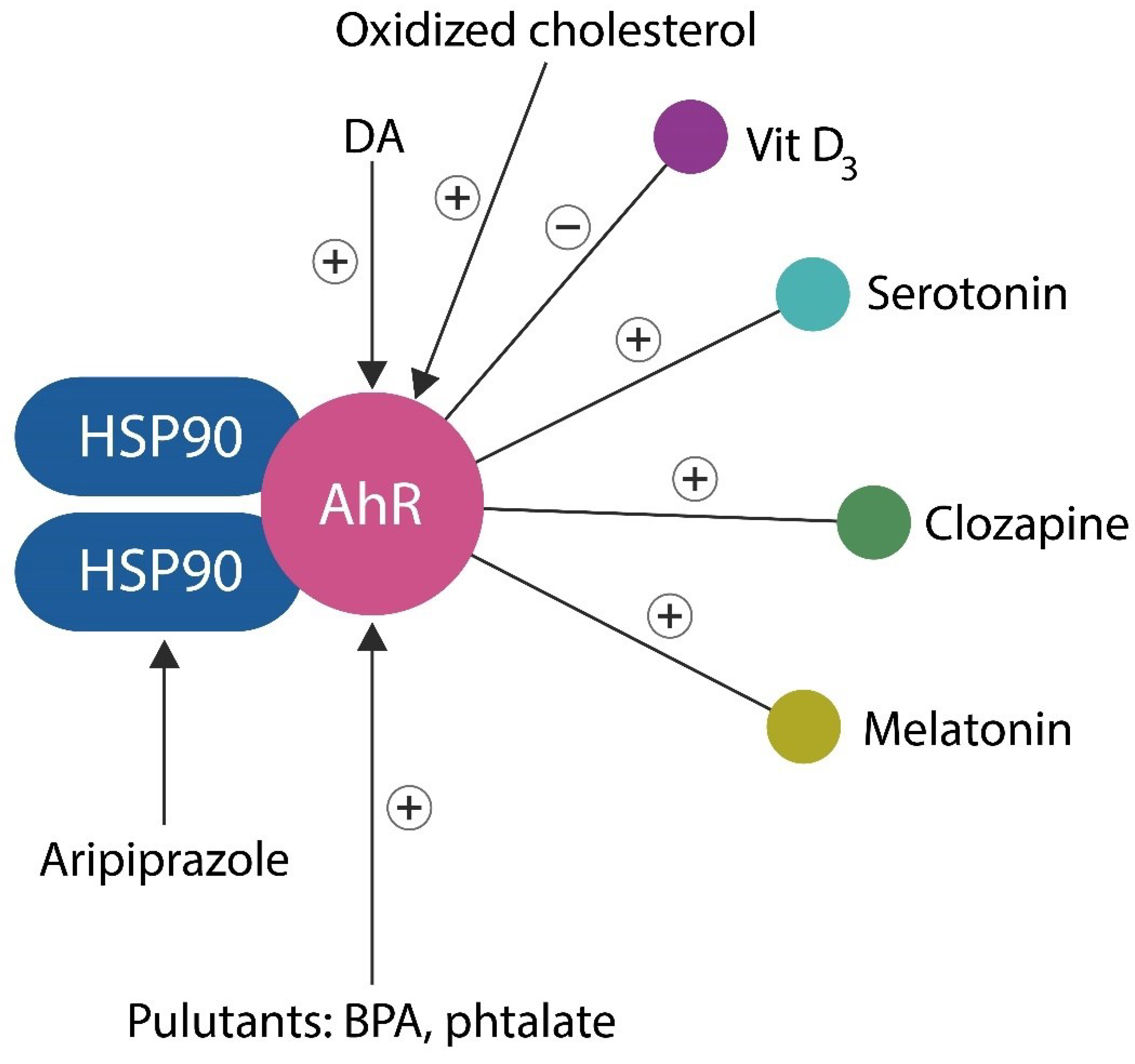
Aryl Hydrocarbon, the Master Regulator of Cellular Senescence
Gray and White Matter Loss
Dopamine-Sparing Antipsychotics
Mitochondrial Dysfunction and Loss of Gamma Band
Entrainment of Gamma Band Oscillations in Schizophrenia
Senotherapeutics
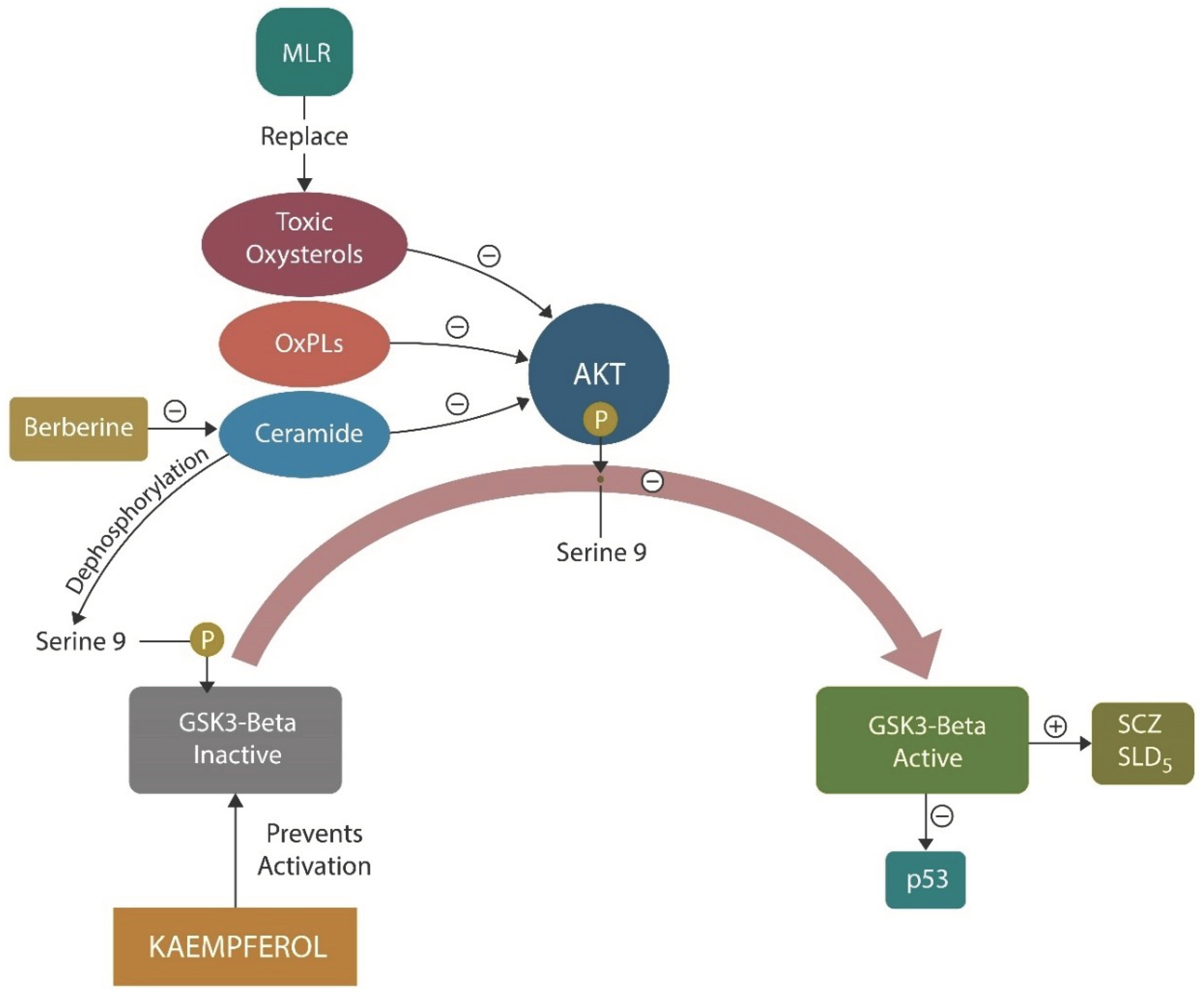
Membrane Lipid Replacement (MLR)
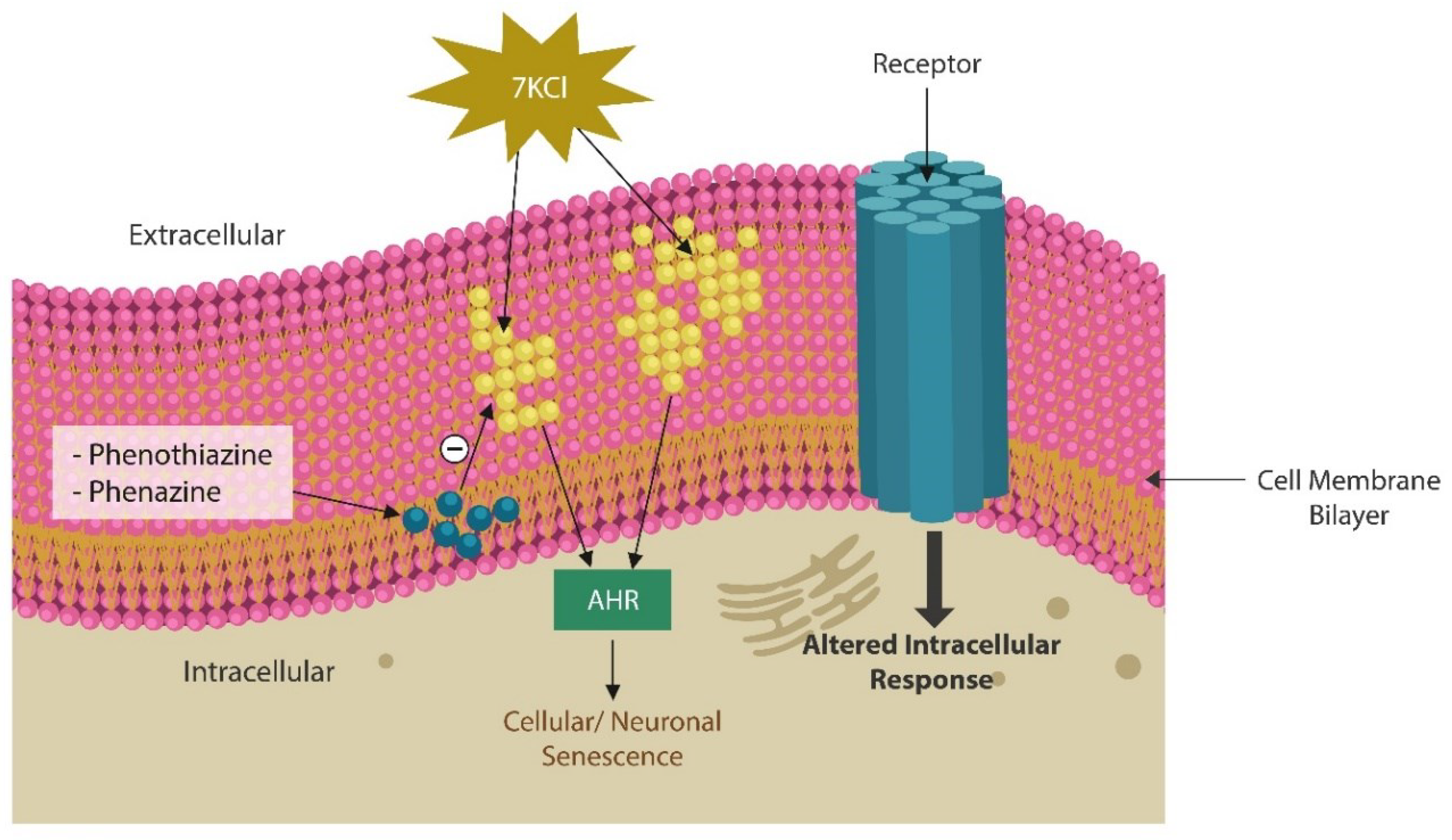
Phenazines and Antioxidant Phenothiazines
Mitochondrial Transfer and Transplantation
AhR Antagonists as Antipsychotics
- Quercetin is a natural flavonoid and plant pigment which exerts antioxidant and anticancer properties. In the CNS, quercetin is a negative allosteric modulator of GABARs as well as an enhancer of glutamatergic neurotransmission, a signaling pathway deficient in SCZ [210]. In addition, quercetin inhibits apoptosis of cortical neurons, likely preventing gray matter loss.
- Apigenin is a plant-based remedy extract from Elsholtzia rugulosa used by traditional practitioners from Africa for treating mental illness. Aside from antagonizing AhR, apigenin exhibits vasorelaxant, antioxidant, and antipsychotic properties [210].
- Alstonine is an indole alkaloid with antipsychotic effects which increases serotonergic, but not dopaminergic, signaling, possibly facilitating mitochondrial transfer [210].
- Luteolin is a natural antipsychotic that exerts its beneficial actions by reducing microglial inflammation [203]. Luteolin is currently in clinical trials for SCZ (NCT05204407)
Synthetic AhR Antagonists
Interleukin-22
- SCZ is often comorbid with IBD, conditions associated with increased gut barrier permeability and microbial translocation from the GI tract into host tissues, including the brain.
- Translocation markers, including soluble CD14 (sCD14) and lipopolysaccharide binding protein (LBP), are elevated in SCZ, suggesting bacterial translocation.
- Increased BBB permeability in SCZ enables translocated gut microbes to reach the brain.
- The Escherichia coli (E. coli) outbrake in 2011 in Germany has been associated with cases of new onset psychosis.
- Exacerbation or new onset psychosis in E. coli-associated UTIs.
Vehicles: Lipid Nanoparticles
Conclusions
References
- Lamb HR. Deinstitutionalization and the homeless mentally ill. Hosp Community Psychiatry. 1984 Sep;35(9):899-907. [CrossRef]
- Scott J. Homelessness and mental illness. Br J Psychiatry. 1993 Mar;162:314-24. PMID: 8453425. [CrossRef]
- Insel, T. Rethinking schizophrenia. Nature 468, 187–193 (2010). [CrossRef]
- Delport A, Harvey BH, Petzer A, Petzer JP. The monoamine oxidase inhibition properties of selected structural analogues of methylene blue. Toxicol Appl Pharmacol. 2017 Jun 15;325:1-8. [CrossRef]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A Systematic Review and Meta-Analysis of Recovery in Schizophrenia. Schizophr. Bull. 2012, 39, 1296–1306.
- Warner, R. Recovery from Schizophrenia Psychiatry and Political Economy, 3rd ed.; Brunner-Routledge: Hove, UK; New York, NY, USA, 1997; p. 74.
- Üçok, A.; Polat, A.; Çakır, S.; Genç, A. One year outcome in first episode schizophrenia: Predictors of relapse. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 256, 37–43.
- Holm, M.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Mitterdorfer-Rutz, E. Employment among people with schizophrenia or bipolar disorder: A population-based study using nationwide registers. Acta Psychiatr. Scand. 2020, 143, 61–71.
- Lévesque, I.S.; Abdel-Baki, A. Homeless youth with first-episode psychosis: A 2-year outcome study. Schizophr. Res. 2019, 216, 460–469.
- Lai CH, Wu YT, Chen CY, Hou YC. Gray matter increases in fronto-parietal regions of depression patients with aripiprazole monotherapy: An exploratory study. Medicine (Baltimore). 2016 Aug;95(34):e4654. [CrossRef]
- Rollema H, Lu Y, Schmidt AW, Zorn SH. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur J Pharmacol. 1997 Nov 5;338(2):R3-5. [CrossRef]
- Papanastasiou E, Gaughran F, Smith S. Schizophrenia as segmental progeria. J R Soc Med. 2011 Nov;104(11):475-84. [CrossRef]
- Russo P, Prinzi G, Proietti S, Lamonaca P, Frustaci A, Boccia S, et al. Shorter telomere length in schizophrenia: evidence from a real-world population and meta-analysis of Most recent literature. Schizophr Res. (2018) 202:37–45. Epub 2018/07/14, PMID. [CrossRef]
- Dada O, Adanty C, Dai N, Jeremian R, Alli S, Gerretsen P, Graff A, Strauss J, De Luca V. Biological aging in schizophrenia and psychosis severity: DNA methylation analysis. Psychiatry Res. 2021 Feb;296:113646. [CrossRef]
- Schnack HG, van Haren NE, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am J Psychiatry. 2016 Jun 1;173(6):607-16. [CrossRef]
- Langhi Prata LGP, Tchkonia T, Kirkland JL. Cell senescence, the senescence-associated secretory phenotype, and cancers. PLoS Biol. 2023 Sep 21;21(9):e3002326. [CrossRef]
- Huang, W., Hickson, L.J., Eirin, A. et al. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol 18, 611–627 (2022). [CrossRef]
- Stec A, Maciejewska M, Zaremba M, Paralusz-Stec K, Michalska M, Rudnicka L, Sikora M. The Clinical Significance of Serum Biomarkers of the Intestinal Barrier in Systemic Sclerosis: A Cross-Sectional Study. J Pers Med. 2023 Apr 18;13(4):678. [CrossRef]
- Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021 Mar 26;371(6536):eabc4552. [CrossRef]
- White MG, Wargo JA. Gut Microbes' Impact on Oncogenic Drivers: Location Matters. Mol Cell. 2020 Sep 17;79(6):878-880. PMID: 32946762; PMCID: PMC7717102. [CrossRef]
- Ağagündüz D, Cocozza E, Cemali Ö, Bayazıt AD, Nanì MF, Cerqua I, Morgillo F, Saygılı SK, Berni Canani R, Amero P, Capasso R. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front Pharmacol. 2023 Jan 24;14:1130562. [CrossRef]
- Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules. 2020 Mar 8;10(3):420. PMID: 32182711; PMCID: PMC7175209. [CrossRef]
- Ni X, Trakalo J, Valente J, Azevedo MH, Pato MT, Pato CN, Kennedy JL. Human p53 tumor suppressor gene (TP53) and schizophrenia: case-control and family studies. Neurosci Lett. 2005 Nov 18;388(3):173-8. [CrossRef]
- Dono A, Nickles J, Rodriguez-Armendariz AG, McFarland BC, Ajami NJ, Ballester LY, Wargo JA, Esquenazi Y. Glioma and the gut-brain axis: opportunities and future perspectives. Neurooncol Adv. 2022 Apr 14;4(1):vdac054. [CrossRef]
- Palacios E, Lobos-González L, Guerrero S, Kogan MJ, Shao B, Heinecke JW, Quest AFG, Leyton L, Valenzuela-Valderrama M. Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa B activation and cause neuronal damage in vivo in a murine model. J Neuroinflammation. 2023 Mar 9;20(1):66. [CrossRef]
- Zhuo, C., Tian, H., Song, X. et al. Microglia and cognitive impairment in schizophrenia: translating scientific progress into novel therapeutic interventions. Schizophr 9, 42 (2023). [CrossRef]
- Niraula A., Sheridan J.F., Godbout J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacol. Rev. 2017;42:318–333. [CrossRef]
- Wiwanitkit V. Hemolysis in E. coli O104:H4 Infection. Indian J Hematol Blood Transfus. 2012 Jun;28(2):127. Epub 2011 Sep 4. PMID: 23730024; PMCID: PMC3332272. [CrossRef]
- Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 2017 Sep;57:36-46. [CrossRef]
- Yang L, Kim TW, Han Y, Nair MS, Harschnitz O, Zhu J, Wang P, Koo SY, Lacko LA, Chandar V, et al. SARS-CoV-2 infection causes dopaminergic neuron senescence. Cell Stem Cell. 2024 Feb 1;31(2):196-211.e6. [CrossRef]
- Fettucciari K, Fruganti A, Stracci F, Spaterna A, Marconi P, Bassotti G. Clostridioides difficile Toxin B Induced Senescence: A New Pathologic Player for Colorectal Cancer? Int J Mol Sci. 2023 May 2;24(9):8155. [CrossRef]
- Vinithakumari AA, Padhi P, Hernandez B, Lin SJ, Dunkerson-Kurzhumov A, Showman L, Breitzman M, Stokes C, Sulaiman Y, Tangudu C, et al. Clostridioides difficile Infection Dysregulates Brain Dopamine Metabolism. Microbiol Spectr. 2022 Apr 27;10(2):e0007322. [CrossRef]
- Feng Y, Shen J, He J, Lu M. Schizophrenia and cell senescence candidate genes screening, machine learning, diagnostic models, and drug prediction. Front Psychiatry. 2023 Apr 11;14:1105987. [CrossRef]
- Ling, E., Nemesh, J., Goldman, M. et al. A concerted neuron–astrocyte program declines in ageing and schizophrenia. Nature (2024). [CrossRef]
- Ishida I, Ogura J, Aizawa E, Ota M, Hidese S, Yomogida Y, Matsuo J, Yoshida S, Kunugi H. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol Rep. 2022 Mar;42(1):70-76. [CrossRef]
- Scheurink TAW, Borkent J, Gangadin SS, El Aidy S, Mandl R, Sommer IEC. Association between gut permeability, brain volume, and cognition in healthy participants and patients with schizophrenia spectrum disorder. Brain Behav. 2023 Jun;13(6):e3011. [CrossRef]
- Wasiak J, Gawlik-Kotelnicka O. Intestinal permeability and its significance in psychiatric disorders - A narrative review and future perspectives. Behav Brain Res. 2023 Jun 25;448:114459. [CrossRef]
- Lotan, A., Luza, S., Opazo, C.M. et al. Perturbed iron biology in the prefrontal cortex of people with schizophrenia. Mol Psychiatry 28, 2058–2070 (2023). [CrossRef]
- Dichtl S, Demetz E, Haschka D, Tymoszuk P, Petzer V, Nairz M, Seifert M, Hoffmann A, Brigo N, Würzner R, Theurl I, Karlinsey JE, Fang FC, Weiss G. Dopamine Is a Siderophore-Like Iron Chelator That Promotes Salmonella enterica Serovar Typhimurium Virulence in Mice. mBio. 2019 Feb 5;10(1):e02624-18. [CrossRef]
- Gregory J Anderson, David M Frazer, Lactate as a regulator of iron homeostasis, Life Metabolism, Volume 2, Issue 5, October 2023, load033. [CrossRef]
- Cai, Z.; Deng, L.; Fan, Y.; Ren, Y.; Ling, Y.; Tu, J.; Cai, Y.; Xu, X.; Chen, M. Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in the Aorta of Atherosclerotic Miniature Pigs. Antioxidants 2024, 13, 4. [CrossRef]
- de la Monte SM. Triangulated mal-signaling in Alzheimer's disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis. 2012;30 Suppl 2(0 2):S231-49. [CrossRef]
- Ravanfar P, Syeda WT, Jayaram M, Rushmore RJ, Moffat B, Lin AP, Lyall AE, Merritt AH, Yaghmaie N, Laskaris L, Luza S, Opazo CM, Liberg B, Chakravarty MM, Devenyi GA, Desmond P, Cropley VL, Makris N, Shenton ME, Bush AI, Velakoulis D, Pantelis C. In Vivo 7-Tesla MRI Investigation of Brain Iron and Its Metabolic Correlates in Chronic Schizophrenia. Schizophrenia (Heidelb). 2022 Oct 26;8(1):86. [CrossRef]
- Feng S, Chen J, Qu C, Yang L, Wu X, Wang S, Yang T, Liu H, Fang Y, Sun P. Identification of Ferroptosis-Related Genes in Schizophrenia Based on Bioinformatic Analysis. Genes (Basel). 2022 Nov 20;13(11):2168. [CrossRef]
- Lian K, Li Y, Yang W, Ye J, Liu H, Wang T, Yang G, Cheng Y, Xu X. Hub genes, a diagnostic model, and immune infiltration based on ferroptosis-linked genes in schizophrenia. IBRO Neurosci Rep. 2024 Feb 3;16:317-328. [CrossRef]
- Go S, Kang M, Kwon SP, Jung M, Jeon OH, Kim BS. The Senolytic Drug JQ1 Removes Senescent Cells via Ferroptosis. Tissue Eng Regen Med. 2021 Oct;18(5):841-850. [CrossRef]
- Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos AT, Haupt S, Haupt Y, Denoyer D, Adlard PA, Bush AI, Cater MA. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018 Apr;14:100-115. [CrossRef]
- Sfera A, Osorio C, Maguire G, Rahman L, Afzaal J, Cummings M, Maldonado JC. COVID-19, ferrosenescence and neurodegeneration, a mini-review. Prog Neuropsychopharmacol Biol Psychiatry. 2021 Jul 13;109:110230. [CrossRef]
- Sfera A, Bullock K, Price A, Inderias L, Osorio C. Ferrosenescence: The iron age of neurodegeneration? Mech Ageing Dev. 2018 Sep;174:63-75. [CrossRef]
- Dean DC 3rd, Sojkova J, Hurley S, Kecskemeti S, Okonkwo O, Bendlin BB, Theisen F, Johnson SC, Alexander AL, Gallagher CL. Alterations of Myelin Content in Parkinson's Disease: A Cross-Sectional Neuroimaging Study. PLoS One. 2016 Oct 5;11(10):e0163774. [CrossRef]
- Belaidi, A.A., Masaldan, S., Southon, A. et al. Apolipoprotein E potently inhibits ferroptosis by blocking ferritinophagy. Mol Psychiatry (2022). [CrossRef]
- Arnold SE, Joo E, Martinoli MG, et al. Apolipoprotein E genotype in schizophrenia: frequency, age of onset, and neuropathologic features. Neuroreport. 1997;8:1523–6.
- Kampman O, Anttila S, Illi A, et al. Apolipoprotein E polymorphism is associated with age of onset in schizophrenia. J Hum Genet. 2004;49:355–9.
- Xu M, Guo Y, Cheng J, Xue K, Yang M, Song X, Feng Y, Cheng J. Brain iron assessment in patients with First-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736. [CrossRef]
- Sabbatinelli J, Prattichizzo F, Olivieri F, Procopio AD, Rippo MR, Giuliani A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front Physiol. 2019 Dec 18;10:1523. [CrossRef]
- Pruett B.S., Meador-Woodruff J.H. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: A focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr. Res. 2020;223:29–42. [CrossRef]
- Chou SM, Yen YH, Yuan F, Zhang SC, Chong CM. Neuronal Senescence in the Aged Brain. Aging Dis. 2023 Oct 1;14(5):1618-1632. PMID: 37196117; PMCID: PMC10529744 . [CrossRef]
- Murty DVPS, Manikandan K, Kumar WS, Ramesh RG, Purokayastha S, Javali M, Rao NP, Ray S. Gamma oscillations weaken with age in healthy elderly in human EEG. Neuroimage. 2020 Jul 15;215:116826. [CrossRef]
- Sharma R. Emerging Interrelationship Between the Gut Microbiome and Cellular Senescence in the Context of Aging and Disease: Perspectives and Therapeutic Opportunities. Probiotics Antimicrob Proteins. 2022 Aug;14(4):648-663. Epub 2022 Jan 5. PMID: 34985682. [CrossRef]
- Frey, N., Venturelli, S., Zender, L. et al. Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics. Nat Rev Gastroenterol Hepatol 15, 81–95 (2018). [CrossRef]
- Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF. In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotox Res. 2019 Apr;35(3):684-698. [CrossRef]
- Hennigar SR, McClung JP. Nutritional Immunity: Starving Pathogens of Trace Minerals. Am J Lifestyle Med. 2016 Feb 4;10(3):170-173. [CrossRef]
- Pretorius L, Kell DB, Pretorius E. Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer's Type Dementia. Front Neurosci. 2018 Nov 16;12:851. [CrossRef]
- Link CD. Is There a Brain Microbiome? Neurosci Insights. 2021 May 27;16:26331055211018709. PMID: 34104888; PMCID: PMC8165828. [CrossRef]
- Dworkin J, Shah IM. Exit from dormancy in microbial organisms. Nat Rev Microbiol. 2010 Dec;8(12):890-6. [CrossRef]
- Peyrusson F, Nguyen TK, Najdovski T, Van Bambeke F. Host Cell Oxidative Stress Induces Dormant Staphylococcus aureus Persisters. Microbiol Spectr. 2022 Feb 23;10(1):e0231321. [CrossRef]
- Berthelot JM, de la Cochetière MF, Potel G, Le Goff B, Maugars Y. Evidence supporting a role for dormant bacteria in the pathogenesis of spondylarthritis. Joint Bone Spine. 2013 Mar;80(2):135-40. [CrossRef]
- Sienkiewicz M, Sroka K, Binienda A, Jurk D, Fichna J. A new face of old cells: An overview about the role of senescence and telomeres in inflammatory bowel diseases. Ageing Res Rev. 2023 Nov;91:102083. [CrossRef]
- Qian, L., He, X., Gao, F. et al. Estimation of the bidirectional relationship between schizophrenia and inflammatory bowel disease using the mendelian randomization approach. Schizophr 8, 31 (2022). [CrossRef]
- Bartocci, B.; Dal Buono, A.; Gabbiadini, R.; Busacca, A.; Quadarella, A.; Repici, A.; Mencaglia, E.; Gasparini, L.; Armuzzi, A. Mental Illnesses in Inflammatory Bowel Diseases: mens sana in corpore sano. Medicina 2023, 59, 682. [CrossRef]
- Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016 Mar;11(2):182-90. [CrossRef]
- Adonis Sfera, Nyla Jafri and Leah Rahman. F-652 (Recombinant Human Interleukin-22) For Schizophrenia. Arch Phar &Pharmacol Res. 3(3): 2023. APPR.MS.ID.000564. [CrossRef]
- Schubert KO, Föcking M, Cotter DR. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: potential roles in GABAergic interneuron pathology. Schizophr Res. 2015 Sep;167(1-3):64-72. [CrossRef]
- Ludmila Juricek, Xavier Coumoul. The Aryl Hydrocarbon Receptor and the Nervous System. International Journal of Molecular Sciences, 2018, 19 (9), pp.2504. ff10.3390/ijms19092504ff. ffhal-01955.
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. [CrossRef]
- Murray, I., Nichols, R., Zhang, L. et al. Expression of the aryl hydrocarbon receptor contributes to the establishment of intestinal microbial community structure in mice. Sci Rep 6, 33969 (2016). [CrossRef]
- Park H, Jin UH, Karki K, Jayaraman A, Allred C, Michelhaugh SK, Mittal S, Chapkin RS, Safe S. Dopamine is an aryl hydrocarbon receptor agonist. Biochem J. 2020 Oct 16;477(19):3899-3910. [CrossRef]
- Fehsel K, Schwanke K, Kappel BA, Fahimi E, Meisenzahl-Lechner E, Esser C, Hemmrich K, Haarmann-Stemmann T, Kojda G, Lange-Asschenfeldt C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J Psychopharmacol. 2022 Feb;36(2):191-201. [CrossRef]
- Moura-Alves P, Faé K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, Skrahina T, Guhlich-Bornhof U, Klemm M, Koehler AB, Bandermann S, Goosmann C, Mollenkopf HJ, Hurwitz R, Brinkmann V, Fillatreau S, Daffe M, Tümmler B, Kolbe M, Oschkinat H, Krause G, Kaufmann SH. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014 Aug 28;512(7515):387-92. [CrossRef]
- Bai, Z., Yang, P., Yu, F. et al. Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis 13, 305 (2022). [CrossRef]
- Yurchenko OP, Grigoriev NG, Turpaev TM, Konjević D, Rakić L. Intracellular injection of dopamine enhances acetylcholine responses of neuron R2 in the Aplysia abdominal ganglion. Comparative Biochemistry and physiology. C, Comparative Pharmacology and Toxicology. 1987 ;87(2):389-391. PMID: 2888583. [CrossRef]
- Nakao M, Tanaka H, Koga T. Cellular Senescence Variation by Metabolic and Epigenomic Remodeling. Trends Cell Biol. 2020 Dec;30(12):919-922. [CrossRef]
- Xuebin Zhang, Fanju Meng, Wencong Lyu, Jianuo He, Ran Wei, Zhehao Du, Chao Zhang. Histone lactylation antagonizes senescence and skeletal muscle aging via facilitating gene expression reprogramming. bioRxiv preprint; this version posted May 26, 2023. [CrossRef]
- Xie Y, Hu H, Liu M, Zhou T, Cheng X, Huang W, Cao L. The role and mechanism of histone lactylation in health and diseases. Front Genet. 2022 Aug 23;13:949252. [CrossRef]
- Wei L, Yang X, Wang J, Wang Z, Wang Q, Ding Y, Yu A. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer's disease through the NFκB signaling pathway. J Neuroinflammation. 2023 Sep 11;20(1):208. [CrossRef]
- Ng PY, McNeely TL, Baker DJ. Untangling senescent and damage-associated microglia in the aging and diseased brain. FEBS J. 2023 Mar;290(5):1326-1339. [CrossRef]
- Tay TL, Béchade C, D'Andrea I, St-Pierre MK, Henry MS, Roumier A, Tremblay ME. Microglia Gone Rogue: Impacts on Psychiatric Disorders across the Lifespan. Front Mol Neurosci. 2018 Jan 4;10:421. [CrossRef]
- Zhu, H., Guan, A., Liu, J. et al. Noteworthy perspectives on microglia in neuropsychiatric disorders. J Neuroinflammation 20, 223 (2023). [CrossRef]
- Galle E, Wong CW, Ghosh A, Desgeorges T, Melrose K, Hinte LC, Castellano-Castillo D, Engl M, de Sousa JA, Ruiz-Ojeda FJ, De Bock K, Ruiz JR, von Meyenn F. H3K18 lactylation marks tissue-specific active enhancers. Genome Biol. 2022 Oct 3;23(1):207. [CrossRef]
- Hagihara H, Shoji H, Otabi H, Toyoda A, Katoh K, Namihira M, Miyakawa T. Protein lactylation induced by neural excitation. Cell Rep. 2021 Oct 12;37(2):109820. [CrossRef]
- Melanie Föcking, Benjamin Doyle, Nayla Munawar, Eugene T. Dillon, David Cotter, Gerard Cagney; Epigenetic Factors in Schizophrenia: Mechanisms and Experimental Approaches. Molecular Neuropsychiatry 15 March 2019; 5 (1): 6–12. [CrossRef]
- Miwa S, Kashyap S, Chini E, von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. 2022 Jul 1;132(13):e158447. [CrossRef]
- Wiley CD, Campisi J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. 2016 Jun 14;23(6):1013-1021. [CrossRef]
- Chen AN, Luo Y, Yang YH, Fu JT, Geng XM, Shi JP, Yang J. Lactylation, a Novel Metabolic Reprogramming Code: Current Status and Prospects. Front Immunol. 2021 Jun 10;12:688910. [CrossRef]
- Howes, O.D.; Cummings, C.; Chapman, G.E.; Shatalina, E. Neuroimaging in schizophrenia: An overview of findings and their implications for synaptic changes. Neuropsychopharmacology 2022, 48, 151–167.
- Leung, M.; Cheung, C.; Yu, K.; Yip, B.; Sham, P.; Li, Q.; Chua, S.; McAlonan, G. Gray Matter in First-Episode Schizophrenia Before and After Antipsychotic Drug Treatment. Anatomical Likelihood Estimation Meta-analyses With Sample Size Weighting. Schizophr. Bull. 2009, 37, 199–211.
- Fusar-Poli, P.; Smieskova, R.; Kempton, M.; Ho, B.; Andreasen, N.; Borgwardt, S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013, 37, 1680–1691. [Google Scholar] [CrossRef] [Green Version].
- Ho, B.C.; Andreasen, N.C.; Ziebell, S.; Pierson, R.; Magnotta, V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 2011, 68, 128–137.
- Cahn, W.; Pol HE, H.; Lems, E.B.; van Haren, N.E.; Schnack, H.G.; van der Linden, J.A.; Schothorst, P.F.; van Engeland, H.; Kahn, R.S. Brain volume changes in first-episode schizophrenia: A 1-year follow-up study. Arch. Gen. Psychiatry 2002, 59, 1002–1010.
- Nishijo M., Tawara K., Nakagawa H., Honda R., Kido T., Nishijo H., Saito S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin in maternal breast milk and newborn head circumference. J. Expo. Sci. Environ. Epidemiol. 2008;18:246–251. [CrossRef]
- Keo A, Dzyubachyk O, van der Grond J, Hafkemeijer A, van de Berg WDJ, van Hilten JJ, Reinders MJT, Mahfouz A. Cingulate networks associated with gray matter loss in Parkinson's disease show high expression of cholinergic genes in the healthy brain. Eur J Neurosci. 2021 Jun;53(11):3727-3739. Epub 2021 May 4. PMID: 33792979. [CrossRef]
- Chung SJ, Kim YJ, Kim YJ, Lee HS, Jeong SH, Hong JM, Sohn YH, Yun M, Jeong Y, Lee PH. Association Between White Matter Networks and the Pattern of Striatal Dopamine Depletion in Patients With Parkinson Disease. Neurology. 2022 Dec 12;99(24):e2672-e2682. [CrossRef]
- Dean DC 3rd, Sojkova J, Hurley S, Kecskemeti S, Okonkwo O, Bendlin BB, Theisen F, Johnson SC, Alexander AL, Gallagher CL. Alterations of Myelin Content in Parkinson's Disease: A Cross-Sectional Neuroimaging Study. PLoS One. 2016 Oct 5;11(10):e0163774. [CrossRef]
- Brown JS Jr. Effects of bisphenol-A and other endocrine disruptors compared with abnormalities of schizophrenia: an endocrine-disruption theory of schizophrenia. Schizophr Bull. 2009 Jan;35(1):256-78. [CrossRef]
- Newbury JB, Stewart R, Fisher HL, Beevers S, Dajnak D, Broadbent M, Pritchard M, Shiode N, Heslin M, Hammoud R, Hotopf M, Hatch SL, Mudway IS, Bakolis I. Association between air pollution exposure and mental health service use among individuals with first presentations of psychotic and mood disorders: retrospective cohort study. Br J Psychiatry. 2021 Dec;219(6):678-685. [CrossRef]
- Domínguez-Acosta O, Vega L, Estrada-Muñiz E, Rodríguez MS, Gonzalez FJ, Elizondo G. Activation of aryl hydrocarbon receptor regulates the LPS/IFNγ-induced inflammatory response by inducing ubiquitin-proteosomal and lysosomal degradation of RelA/p65. Biochem Pharmacol. 2018 Sep;155:141-149. [CrossRef]
- Kinney DK, Teixeira P, Hsu D, Napoleon SC, Crowley DJ, Miller A, Hyman W, Huang E. Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: a role for prenatal vitamin d deficiency and infections? Schizophr Bull. 2009 May;35(3):582-95. [CrossRef]
- Youngren, K., Inglis, F., Pivirotto, P. et al. Clozapine Preferentially Increases Dopamine Release in the Rhesus Monkey Prefrontal Cortex Compared with the Caudate Nucleus. Neuropsychopharmacol 20, 403–412 (1999). [CrossRef]
- Tronchin G, Akudjedu TN, Ahmed M, Holleran L, Hallahan B, Cannon DM, McDonald C. Progressive subcortical volume loss in treatment-resistant schizophrenia patients after commencing clozapine treatment. Neuropsychopharmacology. 2020 Jul;45(8):1353-1361. Epub 2020 Apr 8. Erratum in: Neuropsychopharmacology. 2021 Sep;46(10):1857-1858 . [CrossRef]
- van Erp TGM, Walton E, Hibar DP, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry 2018; 84: 644–654.
- Zhang X, Zhang Y, Liao J, Jiang S, Yan J, Yue W, Zhang D, Yan H. Progressive Grey Matter Volume Changes in Patients with Schizophrenia over 6 Weeks of Antipsychotic Treatment and Their Relationship to Clinical Improvement. Neurosci Bull. 2018 Oct;34(5):816-826. [CrossRef]
- Liu, N., Xiao, Y., Zhang, W. et al. Characteristics of gray matter alterations in never-treated and treated chronic schizophrenia patients. Transl Psychiatry 10, 136 (2020). [CrossRef]
- Voineskos AN, Mulsant BH, Dickie EW, Neufeld NH, Rothschild AJ, Whyte EM, Meyers BS, Alexopoulos GS, Hoptman MJ, Lerch JP, Flint AJ. Effects of Antipsychotic Medication on Brain Structure in Patients With Major Depressive Disorder and Psychotic Features: Neuroimaging Findings in the Context of a Randomized Placebo-Controlled Clinical Trial. JAMA Psychiatry. 2020 Jul 1;77(7):674-683. [CrossRef]
- Vita A, De Peri L, Deste G, et al. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry 2015; 78: 403–412.
- Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001 Dec;58(12):1118-25. [CrossRef]
- Winkler TE, Lederer SL, Kim E, Ben-Yoav H, Kelly DL, Payne GF, Ghodssi R. Molecular processes in an electrochemical clozapine sensor. Biointerphases. 2017 May 1;12(2):02B401. PMID: 28460529. [CrossRef]
- Abdel Majid A. Adam, Hosam A. Saad, Moamen S. Refat, Mohamed S. Hegab .Charge-transfer complexes of antipsychotic drug sulpiride with inorganic and organic acceptors generated through two different approaches: Spectral characterization jJournal: Journal of Molecular Liquids, 2022, p. 118819. [CrossRef]
- Chartoumpekis DV, Zaravinos A, Apidianakis Y, Lagoumintzis G. Editorial: Microbiota and mitochondria: Impact on cell signaling, physiology, and disease. Front Microbiol. 2022 Oct 18;13:1056499. [CrossRef]
- Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The Similarities between Human Mitochondria and Bacteria in the Context of Structure, Genome, and Base Excision Repair System. Molecules 2020, 25, 2857. [CrossRef]
- Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013 Apr 2;17(4):491-506. [CrossRef]
- Johnson, E.L., Heaver, S.L., Waters, J.L. et al. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat Commun 11, 2471 (2020). [CrossRef]
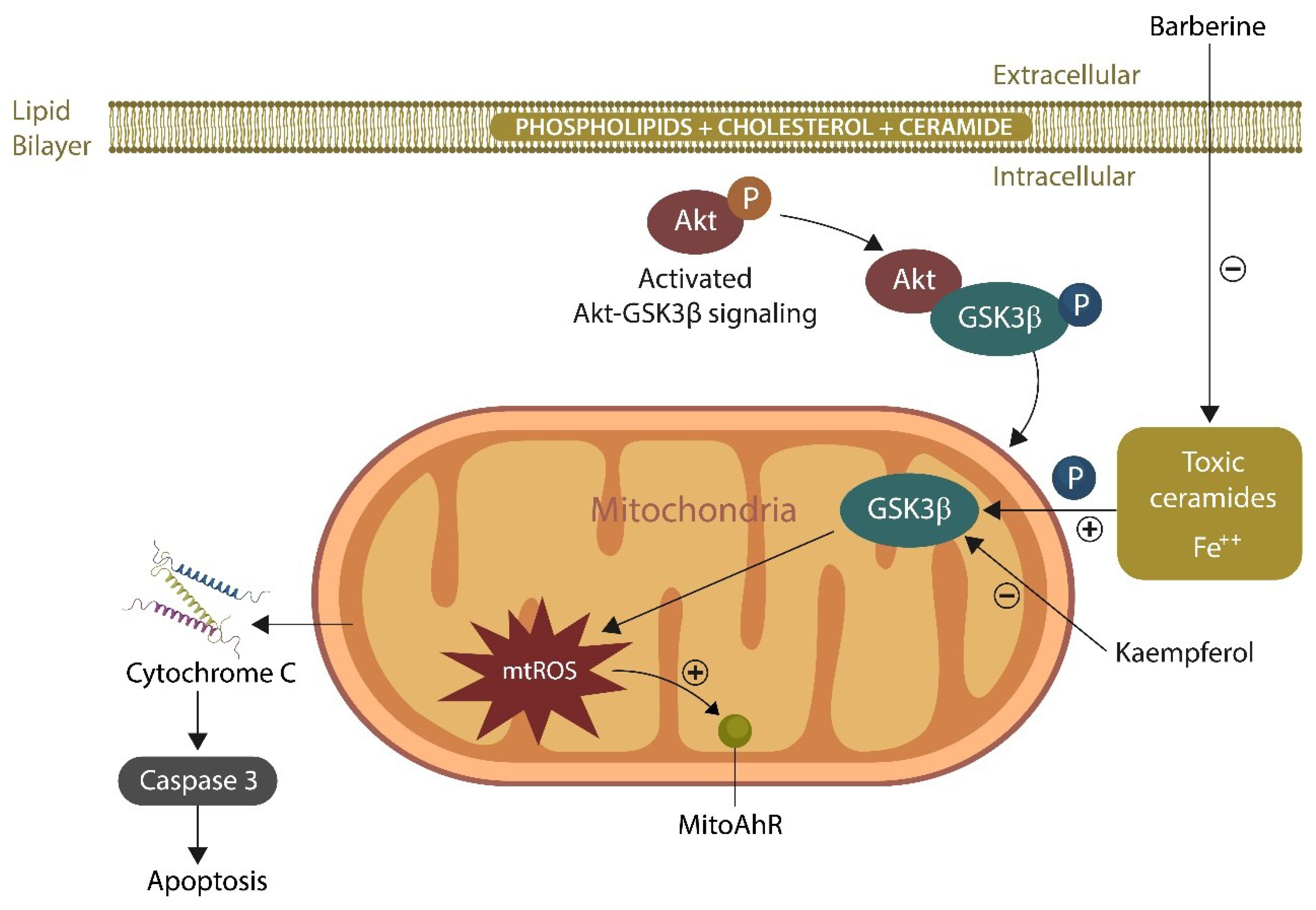
- Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995 Dec 22;270(51):30701-8. [CrossRef]
- Dadsena, S., Bockelmann, S., Mina, J.G.M. et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun 10, 1832 (2019). [CrossRef]
- Colombini M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1239-44. [CrossRef]
- Debdeep Dutta et al, A defect in mitochondrial fatty acid synthesis impairs iron metabolism and causes elevated ceramide levels, Nature Metabolism (2023). [CrossRef]
- Zietzer A, Düsing P, Reese L, Nickenig G, Jansen F. Ceramide Metabolism in Cardiovascular Disease: A Network With High Therapeutic Potential. Arterioscler Thromb Vasc Biol. 2022 Oct;42(10):1220-1228. [CrossRef]
- Smesny S, Schmelzer CE, Hinder A, Köhler A, Schneider C, Rudzok M, Schmidt U, Milleit B, Milleit C, Nenadic I, Sauer H, Neubert RH, Fluhr JW. Skin ceramide alterations in first-episode schizophrenia indicate abnormal sphingolipid metabolism. Schizophr Bull. 2013 Jul;39(4):933-41. [CrossRef]
- Zhuo C, Zhao F, Tian H, Chen J, Li Q, Yang L, Ping J, Li R, Wang L, Xu Y, Cai Z, Song X. Acid sphingomyelinase/ceramide system in schizophrenia: implications for therapeutic intervention as a potential novel target. Transl Psychiatry. 2022 Jun 23;12(1):260. [CrossRef]
- Yuan X, Bhat OM, Zou Y, Li X, Zhang Y, Li PL. Endothelial Acid Sphingomyelinase Promotes NLRP3 Inflammasome and Neointima Formation During Hypercholesterolemia. J Lipid Res. 2022 Dec;63(12):100298. [CrossRef]
- Xia QS, Wu F, Wu WB, Dong H, Huang ZY, Xu L, Lu FE, Gong J. Berberine reduces hepatic ceramide levels to improve insulin resistance in HFD-fed mice by inhibiting HIF-2α. Biomed Pharmacother. 2022 Jun;150:112955. [CrossRef]
- D. Torralba, F. Baixauli, F. Sanchez-Madrid. Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front. Cell. Dev. Biol., 4 (107 (2016), 10.3389/fcell.2016.00107.
- L.H. Fairley, A. Grimm, A. Eckert. Mitochondria transfer in brain injury and disease.Cells., 11 (22) (2022), 10.3390/cells11223603.
- Jackson JG, Robinson MB. Regulation of mitochondrial dynamics in astrocytes: Mechanisms, consequences, and unknowns. Glia. 2018 Jun;66(6):1213-1234. [CrossRef]
- Hogan DB, Jetté N, Fiest KM, Roberts JI, Pearson D, Smith EE, Roach P, Kirk A, Pringsheim T, Maxwell CJ. The Prevalence and Incidence of Frontotemporal Dementia: a Systematic Review. Can J Neurol Sci. 2016 Apr;43 Suppl 1:S96-S109. [CrossRef]
- Course MM, Wang X. Transporting mitochondria in neurons. F1000Res. 2016 Jul 18;5:F1000 Faculty Rev-1735. PMID: 27508065; PMCID: PMC4955021. [CrossRef]
- Nuñez MT, Chana-Cuevas P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals (Basel). 2018 Oct 19;11(4):109. [CrossRef]
- Thayyullathil, F., Cheratta, A.R., Alakkal, A. et al. Acid sphingomyelinase-dependent autophagic degradation of GPX4 is critical for the execution of ferroptosis. Cell Death Dis 12, 26 (2021). [CrossRef]
- Abdel-Salam OME, Morsy SMY, Sleem AA. The effect of different antidepressant drugs on oxidative stress after lipopolysaccharide administration in mice. EXCLI J. 2011 Dec 8;10:290-302.
- Kann O. The energy demand of fast neuronal network oscillations: insights from brain slice preparations. Front Pharmacol. 2012 Jan 10;2:90. [CrossRef]
- Nakao, K., Singh, M., Sapkota, K. et al. GSK3β inhibition restores cortical gamma oscillation and cognitive behavior in a mouse model of NMDA receptor hypofunction relevant to schizophrenia. Neuropsychopharmacol. 45, 2207–2218 (2020). [CrossRef]
- Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004 Nov 3;24(44):9993-10002. [CrossRef]
- McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry. 2016 May;29(3):202-10. [CrossRef]
- Tada, M., Kirihara, K., Koshiyama, D. et al. Alterations of auditory-evoked gamma oscillations are more pronounced than alterations of spontaneous power of gamma oscillation in early stages of schizophrenia. Transl Psychiatry 13, 218 (2023). [CrossRef]
- Williams S, Boksa P. Gamma oscillations and schizophrenia. J Psychiatry Neurosci. 2010 Mar;35(2):75-7. [CrossRef]
- Veit J, Handy G, Mossing DP, Doiron B, Adesnik H. Cortical VIP neurons locally control the gain but globally control the coherence of gamma band rhythms. Neuron. 2023 Feb 1;111(3):405-417.e5. [CrossRef]
- Antonoudiou P, Tan YL, Kontou G, Upton AL, Mann EO. Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations. J Neurosci. 2020 Sep 30;40(40):7668-7687. [CrossRef]
- Betterton, R., Mellor, J. & Tsaneva-Atanasova, K. Modulation of hippocampal gamma oscillations by acetylcholine: insights from mathematical and in vitro optogenetic models. BMC Neurosci 16 (Suppl 1), P267 (2015). [CrossRef]
- Çalışkan G, French T, Enrile Lacalle S, Del Angel M, Steffen J, Heimesaat MM, Rita Dunay I, Stork O. Antibiotic-induced gut dysbiosis leads to activation of microglia and impairment of cholinergic gamma oscillations in the hippocampus. Brain Behav Immun. 2022 Jan;99:203-217. [CrossRef]
- Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021 Jun 19;13(6):2099. [CrossRef]
- Klaver R, De Vries HE, Schenk GJ, Geurts JJ. Grey matter damage in multiple sclerosis: a pathology perspective. Prion. 2013 Jan-Feb;7(1):66-75. [CrossRef]
- Keo A, Dzyubachyk O, van der Grond J, Hafkemeijer A, van de Berg WDJ, van Hilten JJ, Reinders MJT, Mahfouz A. Cingulate networks associated with gray matter loss in Parkinson's disease show high expression of cholinergic genes in the healthy brain. Eur J Neurosci. 2021 Jun;53(11):3727-3739. [CrossRef]
- Yohn SE, Weiden PJ, Felder CC, Stahl SM. Muscarinic acetylcholine receptors for psychotic disorders: bench-side to clinic. Trends Pharmacol Sci. 2022 Dec;43(12):1098-1112. [CrossRef]
- Sahu PP, Tseng P. Gamma sensory entrainment for cognitive improvement in neurodegenerative diseases: opportunities and challenges ahead. Front Integr Neurosci. 2023 Apr 17;17:1146687. [CrossRef]
- Yan L, Li H, Qian Y, Zhang J, Cong S, Zhang X, Wu L, Wang Y, Wang M, Yu T. Transcutaneous vagus nerve stimulation: a new strategy for Alzheimer's disease intervention through the brain-gut-microbiota axis? Front Aging Neurosci. 2024 Feb 27;16:1334887. [CrossRef]
- Karpiński P, Żebrowska-Różańska P, Kujawa D, Łaczmański Ł, Samochowiec J, Jabłoński M, Plichta P, Piotrowski P, Bielawski T, Misiak B. Gut microbiota alterations in schizophrenia might be related to stress exposure: Findings from the machine learning analysis. Psychoneuroendocrinology. 2023 Sep;155:106335. [CrossRef]
- Attademo L, Bernardini F, Garinella R, Compton MT. Environmental pollution and risk of psychotic disorders: A review of the science to date. Schizophr Res. 2017 Mar;181:55-59. [CrossRef]
- Breno S. Diniz BS, Seitz-Holland J, Sehgal R, Kasamoto J, Higgins-Chen AT, Lenze E. Geroscience-Centric Perspective for Geriatric Psychiatry: Integrating Aging Biology With Geriatric Mental Health Research. Geriatric Psychiatry (2024). VOLUME 32, ISSUE 1, P1-16, JANUARY 2024.
- Seeman, M.V. Subjective Overview of Accelerated Aging in Schizophrenia. Int. J. Environ. Res. Public Health 2023, 20, 737. [CrossRef]
- Marin, I., Serrano, M. & Pietrocola, F. Recent insights into the crosstalk between senescent cells and CD8 T lymphocytes. npj Aging 9, 8 (2023). [CrossRef]
- Harris MJ, Jeste DV, Gleghorn A, Sewell DD. New-onset psychosis in HIV-infected patients. J Clin Psychiatry. 1991 Sep;52(9):369-76.
- Kozato N, Mishra M, Firdosi M. New-onset psychosis due to COVID-19. BMJ Case Rep. 2021 Apr 26;14(4):e242538. [CrossRef]
- Luís C, Maduro AT, Pereira P, Mendes JJ, Soares R, Ramalho R. Nutritional senolytics and senomorphics: Implications to immune cells metabolism and aging - from theory to practice. Front Nutr. 2022 Sep 8;9:958563. [CrossRef]
- An S, Cho SY, Kang J, Lee S, Kim HS, Min DJ, Son E, Cho KH. Inhibition of 3-phosphoinositide-dependent protein kinase 1 (PDK1) can revert cellular senescence in human dermal fibroblasts. Proc Natl Acad Sci U S A. 2020 Dec 8;117(49):31535-31546. [CrossRef]
- Gregory M SolisRozina KardakarisElizabeth R ValentineLiron Bar-PeledAlice L ChenMegan M BlewettMark A McCormickJames R WilliamsonBrian KennedyBenjamin F CravattMichael Petrascheck (2018) Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms eLife 7:e40314. [CrossRef]
- Deakin B, Suckling J, Dazzan P, Joyce E, Lawrie SM, Upthegrove R, Husain N, Chaudhry IB, Dunn G, Jones PB, Lisiecka-Ford D, Lewis S, Barnes TRE, Williams SCR, Pariante CM, Knox E, Drake RJ, Smallman R, Barnes NM. Minocycline for negative symptoms of schizophrenia and possible mechanistic actions: the BeneMin RCT. Southampton (UK): NIHR Journals Library;
- Abir MH, Mahamud AGMSU, Tonny SH, Anu MS, Hossain KHS, Protic IA, Khan MSU, Baroi A, Moni A, Uddin MJ. Pharmacological potentials of lycopene against aging and aging-related disorders: A review. Food Sci Nutr. 2023 Jun 27;11(10):5701-5735. [CrossRef]
- Perrott KM, Wiley CD, Desprez PY, Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience. 2017 Apr;39(2):161-173. [CrossRef]
- Elsallabi O, Patruno A, Pesce M, Cataldi A, Carradori S, Gallorini M. Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection. Molecules. 2022 Jan 23;27(3):738. [CrossRef]
- Kumar R, Sharma A, Kumari A, Gulati A, Padwad Y, Sharma R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology. 2019 Apr;20(2):171-189. [CrossRef]
- Li W, He Y, Zhang R, Zheng G, Zhou D. The curcumin analog EF24 is a novel senolytic agent. Aging (Albany NY). 2019 Jan 28;11(2):771-782. [CrossRef]
- Dang Y, An Y, He J, Huang B, Zhu J, Gao M, Zhang S, Wang X, Yang B, Xie Z. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression. Aging Cell. 2020 Jan;19(1):e13060. [CrossRef]
- Islam MT, Tuday E, Allen S, Kim J, Trott DW, Holland WL, Donato AJ, Lesniewski LA. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell. 2023 Feb;22(2):e13767. [CrossRef]
- von Kobbe C. Targeting senescent cells: approaches, opportunities, challenges. Aging (Albany NY). 2019 Nov 30;11(24):12844-12861. [CrossRef]
- Suda M, Shimizu I, Katsuumi G, Yoshida Y, Hayashi Y, Ikegami R, Matsumoto N, Yoshida Y, Mikawa R, Katayama A, Wada J, Seki M, Suzuki Y, Iwama A, Nakagami H, Nagasawa A, Morishita R, Sugimoto M, Okuda S, Tsuchida M, Ozaki K, Nakanishi-Matsui M, Minamino T. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat Aging. 2021 Dec;1(12):1117-1126. [CrossRef]
- Poblocka, M., Bassey, A.L., Smith, V.M. et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci Rep 11, 20358 (2021). [CrossRef]
- Nicolson GL, Ferreira de Mattos G, Ash M, Settineri R, Escribá PV. Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging. Membranes (Basel). 2021 Nov 29;11(12):944. [CrossRef]
- Zhou M, Ren H, Han J, Wang W, Zheng Q, Wang D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3β. Oxid Med Cell Longev. 2015;2015:481405. [CrossRef]
- Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006 Nov;7(11):1421-34. [CrossRef]
- Jin S, Zhang L, Wang L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. Biomed Pharmacother. 2023 Sep;165:115215. [CrossRef]
- Nicolson GL, Ash ME. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim Biophys Acta Biomembr. 2017 Sep;1859(9 Pt B):1704-1724. [CrossRef]
- Schmidt NW; Mishra A; Lai GH; Davis M; Sanders LK ; Dat T; Garcia A; Tai KP; McCray J; Paul B; Ouellette AJ; Selsted ME; Wong GCL Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J. Am. Chem. Soc 2011, 133, 6720–6727.
- Blankenfeldt W, Parsons JF. The structural biology of phenazine biosynthesis. Curr Opin Struct Biol. 2014 Dec;29:26-33. Epub 2014 Sep 15. PMID: 25215885; PMCID: PMC4268259. [CrossRef]
- Pierson LS 3rd, Pierson EA. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol. 2010 May;86(6):1659-70. [CrossRef]
- Abdelaziz, A.A., Kamer, A.M.A., Al-Monofy, K.B. et al. Pseudomonas aeruginosa’s greenish-blue pigment pyocyanin: its production and biological activities. Microb Cell Fact 22, 110 (2023). [CrossRef]
- Ohlendorf B, Schulz D, Erhard A, Nagel K, Imhoff JF. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J Nat Prod. 2012 Jul 27;75(7):1400-4. [CrossRef]
- Paul SM, Yohn SE, Popiolek M, Miller AC, Felder CC. Muscarinic Acetylcholine Receptor Agonists as Novel Treatments for Schizophrenia. Am J Psychiatry. 2022 Sep;179(9):611-627. [CrossRef]
- Wang X, Abbas M, Zhang Y, Elshahawi SI, Ponomareva LV, Cui Z, Van Lanen SG, Sajid I, Voss SR, Shaaban KA, Thorson JS. Baraphenazines A-G, Divergent Fused Phenazine-Based Metabolites from a Himalayan Streptomyces. J Nat Prod. 2019 Jun 28;82(6):1686-1693. [CrossRef]
- Cha JW, Lee S, Kim MC, Thida M, Lee JW, Park JS, et al. Pontemazines a and B, phenazine derivatives containing a methylamine linkage from Streptomyces sp. UT1123 and their protective effect to HT-22 neuronal cells. Bioorganic & Medicinal Chemistry Letters. 2015;25:5083-5086. [CrossRef]
- Kim WG, Ryoo IJ, Yun BS, Shin-ya K, Seto H, Yoo ID. Phenazostatin C, a new diphenazine with neuronal cell protecting activity from Streptomyces sp. J Antibiot (Tokyo). 1999 Aug;52(8):758-61. [CrossRef]
- Kato S., Shindo K., Yamagishi Y., Matsuoka M., Kawai H., Mochizuki J. Phenazoviridin, a novel free radical scavenger from Streptomyces sp. taxonomy, fermentation, isolation, structure elucidation and biological properties. J. Antibiot. 1993;46:1485–1493. doi:.
- Nadeem A., Meijler MM. Unraveling the Antibacterial and Iron Chelating Activity of N-Oxide Hydroxy-Phenazine natural Products and Synthetic Analogs against Staphylococcus aureus. Israel Journal of Chemistry (2023) Volume63, Issue 5-6. [CrossRef]
- Heitmann ASB, Zanjani AAH, Klenow MB, Mularski A, Sønder SL, Lund FW, Boye TL, Dias C, Bendix PM, Simonsen AC, Khandelia H, Nylandsted J. Phenothiazines alter plasma membrane properties and sensitize cancer cells to injury by inhibiting annexin-mediated repair. J Biol Chem. 2021 Aug;297(2):101012. [CrossRef]
- Boonnoy, P., Jarerattanachat, V., Karttunen, M. & Wong-ekkabut, J. Bilayer Deformation, Pores, and Micellation Induced by Oxidized Lipids. J. Phys. Chem. Lett. 6, 4884–4888 (2015).
- Voronova O, Zhuravkov S, Korotkova E, Artamonov A, Plotnikov E. Antioxidant Properties of New Phenothiazine Derivatives. Antioxidants (Basel). 2022 Jul 14;11(7):1371. [CrossRef]
- Keynes RG, Karchevskaya A, Riddall D, Griffiths CH, Bellamy TC, Chan AWE, Selwood DL, Garthwaite J. N10 -carbonyl-substituted phenothiazines inhibiting lipid peroxidation and associated nitric oxide consumption powerfully protect brain tissue against oxidative stress. Chem Biol Drug Des. 2019 Sep;94(3):1680-1693. [CrossRef]
- Philot EA, da Mata Lopes D, de Souza AT, Braz AS, Nantes IL, Rodrigues T, Perahia D, Miteva MA, Scott LP. Binding of phenothiazines into allosteric hydrophobic pocket of human thioredoxin 1. Eur Biophys J. 2016 Apr;45(3):279-86. [CrossRef]
- Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68: 128–137.
- Veijola J, Guo JY, Moilanen JS, et al. Longitudinal changes in total brain volume in schizophrenia: relation to symptom severity, cognition antipsychotic medication. PLoS One 2014; 9: 101689.
- Engwa G.A., Ayuk E.L., Igbojekwe B.U., Unaegbu M. Potential Antioxidant Activity of New Tetracyclic and Pentacyclic Nonlinear Phenothiazine Derivatives. Biochem. Res. Int. 2016;2016:9896575. [CrossRef]
- Clark MA, Shay JW. Mitochondrial transformation of mammalian cells. Nature. (1982) 295:605–7. [CrossRef]
- Katrangi E, D’Souza G, Boddapati SV, Kulawiec M, Singh KK, Bigger B, et al. Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function. Rejuvenation Res. (2007) 10:561–70. [CrossRef]
- Pacak AP, Preble JM, Kondo H, Seibel P, Levitsky S, del Nido PJ, et al. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open. (2015) 4:622–6. [CrossRef]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. (2016) 535:551–5. [CrossRef]
- Ali Pour P, Hosseinian S, Kheradvar A. Mitochondrial transplantation in cardiomyocytes: foundation, methods, and outcomes. Am J Physiol Cell Physiol. (2021) 321:C489–503. [CrossRef]
- Sasaki, D., Abe, J., Takeda, A. et al. Transplantation of MITO cells, mitochondria activated cardiac progenitor cells, to the ischemic myocardium of mouse enhances the therapeutic effect. Sci Rep 12, 4344 (2022). [CrossRef]
- Chen T, Majerníková N, Marmolejo-Garza A, Trombetta-Lima M, Sabogal-Guáqueta AM, Zhang Y, Ten Kate R, Zuidema M, Mulder PPMFA, den Dunnen W, Gosens R, Verpoorte E, Culmsee C, Eisel ULM, Dolga AM. Mitochondrial transplantation rescues neuronal cells from ferroptosis. Free Radic Biol Med. 2023 Nov 1;208:62-72. [CrossRef]
- Um, J.-H.; Lee, K.-M.; Kim, Y.-Y.; Lee, D.-Y.; Kim, E.; Kim, D.-H.; Yun, J. Berberine Induces Mitophagy through Adenosine Monophosphate-Activated Protein Kinase and Ameliorates Mitochondrial Dysfunction in PINK1 Knockout Mouse Embryonic Fibroblasts. Int. J. Mol. Sci. 2024, 25, 219. [CrossRef]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016 Jul 28;535(7613):551-5. Erratum in: Nature. 2016 Sep 14;539(7627):123. [CrossRef]
- Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003 Dec;111(16):1877-82. PMID: 14644660; PMCID: PMC1241760. [CrossRef]
- Linck VM, Ganzella M, Herrmann AP, Okunji CO, Souza DO, Antonelli MC, Elisabetsky E. Original mechanisms of antipsychotic action by the indole alkaloid alstonine (Picralima nitida). Phytomedicine. 2015 Jan 15;22(1):52-5. [CrossRef]
- Cordaro, M.; Cuzzocrea, S.; Crupi, R. An Update of Palmitoylethanolamide and Luteolin Effects in Preclinical and Clinical Studies of Neuroinflammatory Events. Antioxidants 2020, 9, 216. [CrossRef]
- McGovern K, Castro AC, Cavanaugh J, Coma S, Walsh M, Tchaicha J, Syed S, Natarajan P, Manfredi M, Zhang XM, Ecsedy J. Discovery and Characterization of a Novel Aryl Hydrocarbon Receptor Inhibitor, IK-175, and Its Inhibitory Activity on Tumor Immune Suppression. Mol Cancer Ther. 2022 Aug 2;21(8):1261-1272. [CrossRef]
- Kang S, Lee AG, Im S, Oh SJ, Yoon HJ, Park JH, Pak YK. A Novel Aryl Hydrocarbon Receptor Antagonist HBU651 Ameliorates Peripheral and Hypothalamic Inflammation in High-Fat Diet-Induced Obese Mice. Int J Mol Sci. 2022 Nov 28;23(23):14871. [CrossRef]
- Zai W, Chen W, Liu H, Ju D. Therapeutic Opportunities of IL-22 in Non-Alcoholic Fatty Liver Disease: From Molecular Mechanisms to Clinical Applications. Biomedicines. 2021 Dec 14;9(12):1912. [CrossRef]
- Sfera, A. Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2? Reports 2023, 6, 36. [CrossRef]
- Shi C, Su C, Cen L, Han L, Tang J, Wang Z, Shi X, Ju D, Cao Y, Zhu H. Vunakizumab-IL22, a Novel Fusion Protein, Promotes Intestinal Epithelial Repair and Protects against Gut Injury Induced by the Influenza Virus. Biomedicines. 2023 Apr 12;11(4):1160. [CrossRef]
- Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY, Grin A, Kandel G, Loutfy M, Ostrowski M, Gommerman JL, Kaushic C, Kaul R. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012 Nov;5(6):670-80. [CrossRef]
- Nsairat H, Khater D, Odeh F, Al-Adaileh F, Al-Taher S, Jaber AM, Alshaer W, Al Bawab A, Mubarak MS. Lipid nanostructures for targeting brain cancer. Heliyon. 2021 Sep 16;7(9):e07994. [CrossRef]
- Aldosari BN, Alfagih IM, Almurshedi AS. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics. 2021 Feb 2;13(2):206. [CrossRef]
- Sun D, Lu ZR. Structure and Function of Cationic and Ionizable Lipids for Nucleic Acid Delivery. Pharm Res. 2023 Jan;40(1):27-46. [CrossRef]
- Brannagan TH 3rd, Berk JL, Gillmore JD, Maurer MS, Waddington-Cruz M, Fontana M, Masri A, Obici L, Brambatti M, Baker BF, Hannan LA, Buchele G, Viney NJ, Coelho T, Nativi-Nicolau J. Liver-directed drugs for transthyretin-mediated amyloidosis. J Peripher Nerv Syst. 2022 Dec;27(4):228-237. [CrossRef]
| Senolytics | Source | References |
| Lycopene | Grape skin, guava, grapefruit, blueberries, tomatoes | [166] |
| Apigenin | Cabbage, blueberries, acai berries | [167] |
| Fisetin | Strawberries, onions, apples, mangoes, persimmons, kiwis. | [168] |
| Curcumin and analog EF24 | chicken, beef, tofu, vegetables | [169] |
| Epigallocatechin gallate | Apples, blackberries, broad beans, cherries, black grapes, pears, raspberries, and chocolate | [170] |
| Berberine | Oregon grape, phellodendron, and tree turmeric. | [171] |
| Quercetin | Fruits, apples, onions, parsley, sage, tea, and red wine | [172] |
| Kaempferol | Fruits and vegetables. | [173] |
| Compound | Naturally occurring | Synthetic |
|---|---|---|
| Phenazines | geranyl-phenazine, bara-phenazines A–G | Pontemazines A and B; Halogenated phenazines |
| Phenothiazines | Propenyl-phenothiazine; N10-carbonyl-substituted phenothiazines |
|
| GSK-3β inhibitors | Kaempferol; Curcumin | Lithium Valproic acid Clozapine; Olanzapine |
| AhR inhibitors | quercetin, apigenin, alstonine, luteolin | IK-175; HBU651 |
| Acid sphingomyelinase (ASM) inhibitors | fluvoxamine rosuvastatin tricyclic antidepressants |
|
| Dopamine D1R agonists | A68930; A77636; Dihydrexidine |
|
| Mitochondrial export | SSRIs | |
| ACh agonists |
Catharanthus roseus; Salvia spp. (Lamiaceae) |
Cholinesterase inhibitors: donepezil, galantamine, rivastigmine |
| Senotherapeutics | Please see Table 1 | Senotherapeutic antibiotics |
| Ferroptosis inhibitors | Natural flavonoids; Berberine |
Fluvoxamine; SSRI; N acetyl cysteine (NAC) |
| Recombinant IL-22 | ||
| Membrane lipid replacement | ||
| Mitochondrial transplantation | ||
| 40 HZ entrainment with sensory stimuli | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




