1. Introduction
The correction of refractive errors (myopia, astigmatism, hyperopia) based upon reshaping of the corneal profile can be currently achieved by various techniques; the most used is laser in situ keratomileusis (LASIK), in which a superficial hinged corneal flap is created by a femtosecond laser, the flap is folded, and the underlying stromal bed is photoablated by excimer laser to change its curvature, thus modifying its refractive power. The flap is then replaced and let adhere by oncotic pressure [
1].
The edges of the corneal flap can be tapered or angled, the latter shape having several advantages: ease of reposition during surgery, resistance to tangential forces due to eyelid movement (hence resistance to early dislocation) [
2], stronger adhesion by scarring, barrier effect against the infiltration of epithelial cells in the interface (epithelial ingrowth) [
3].
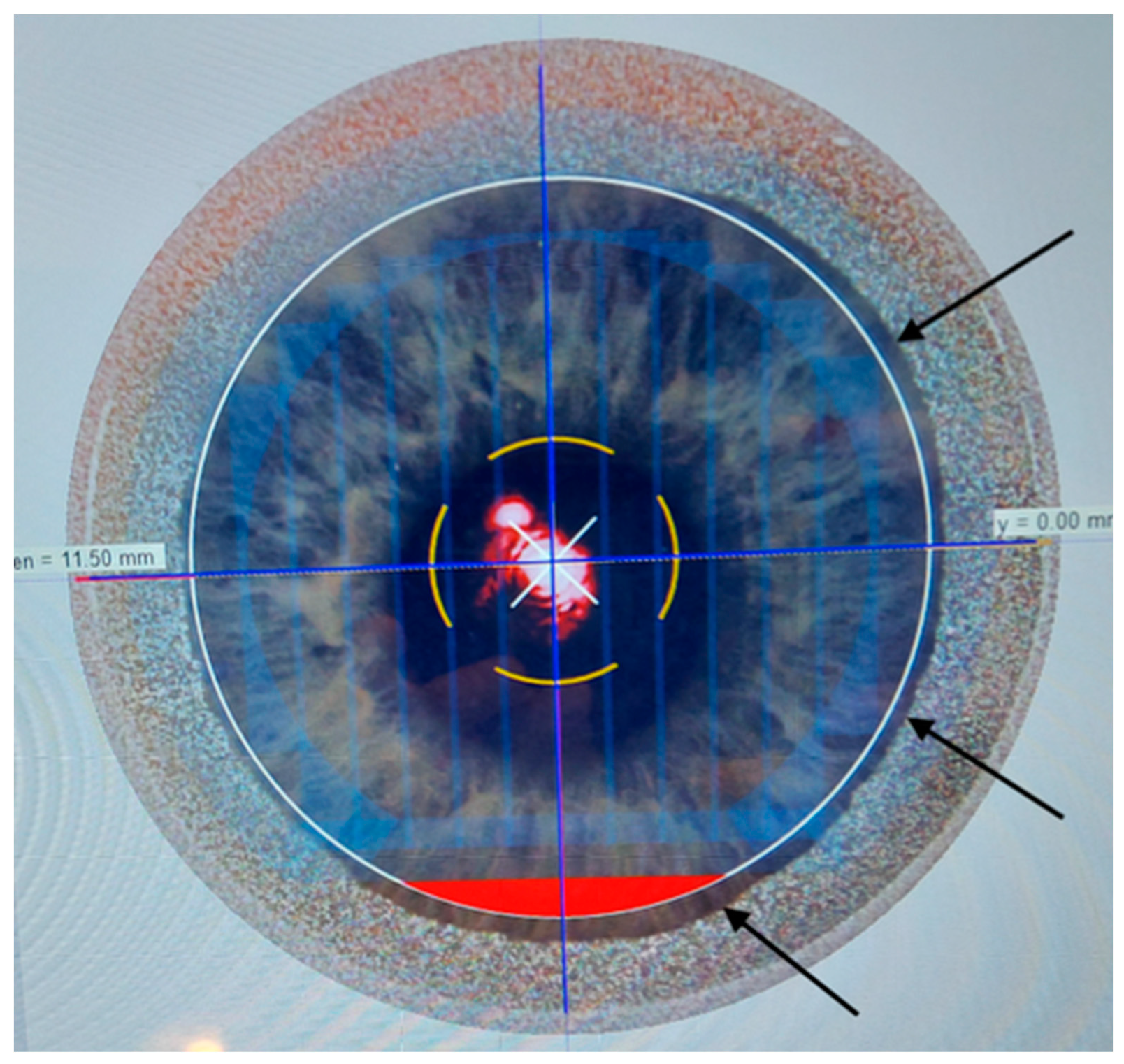
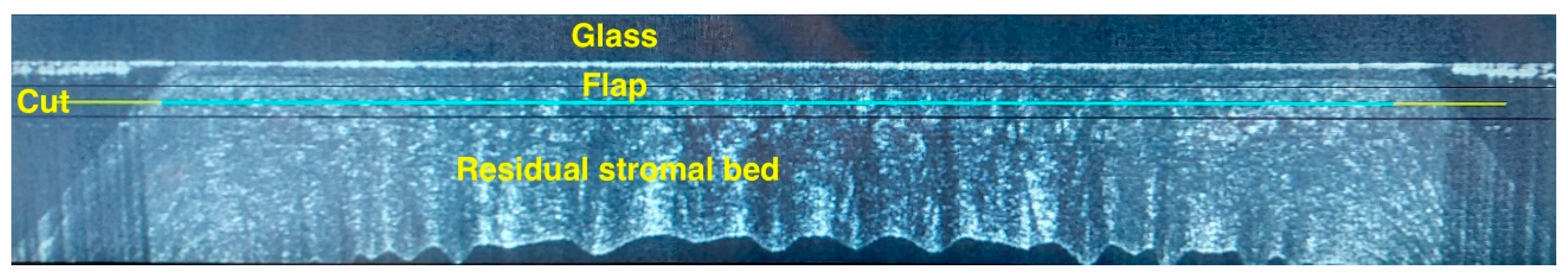
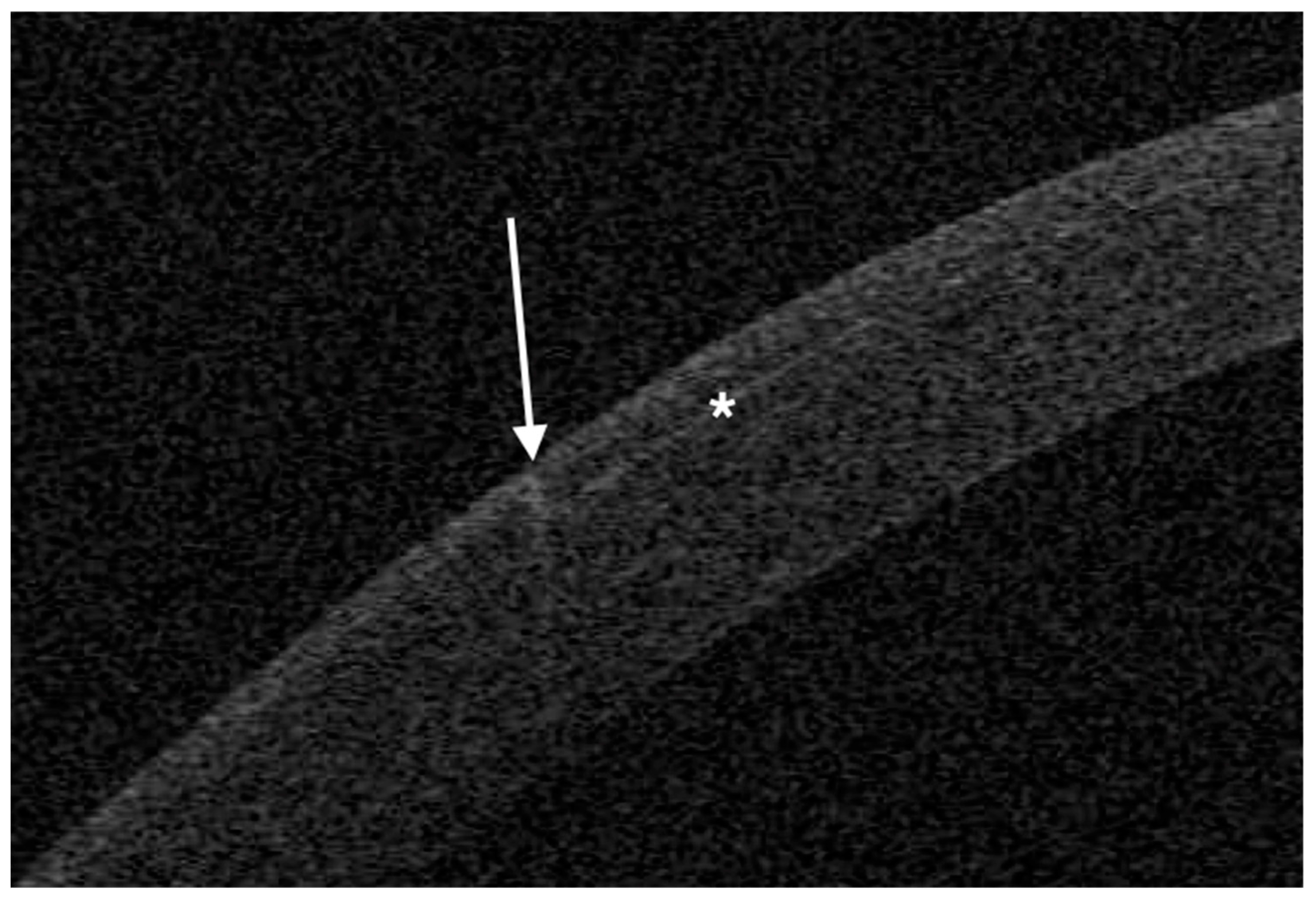
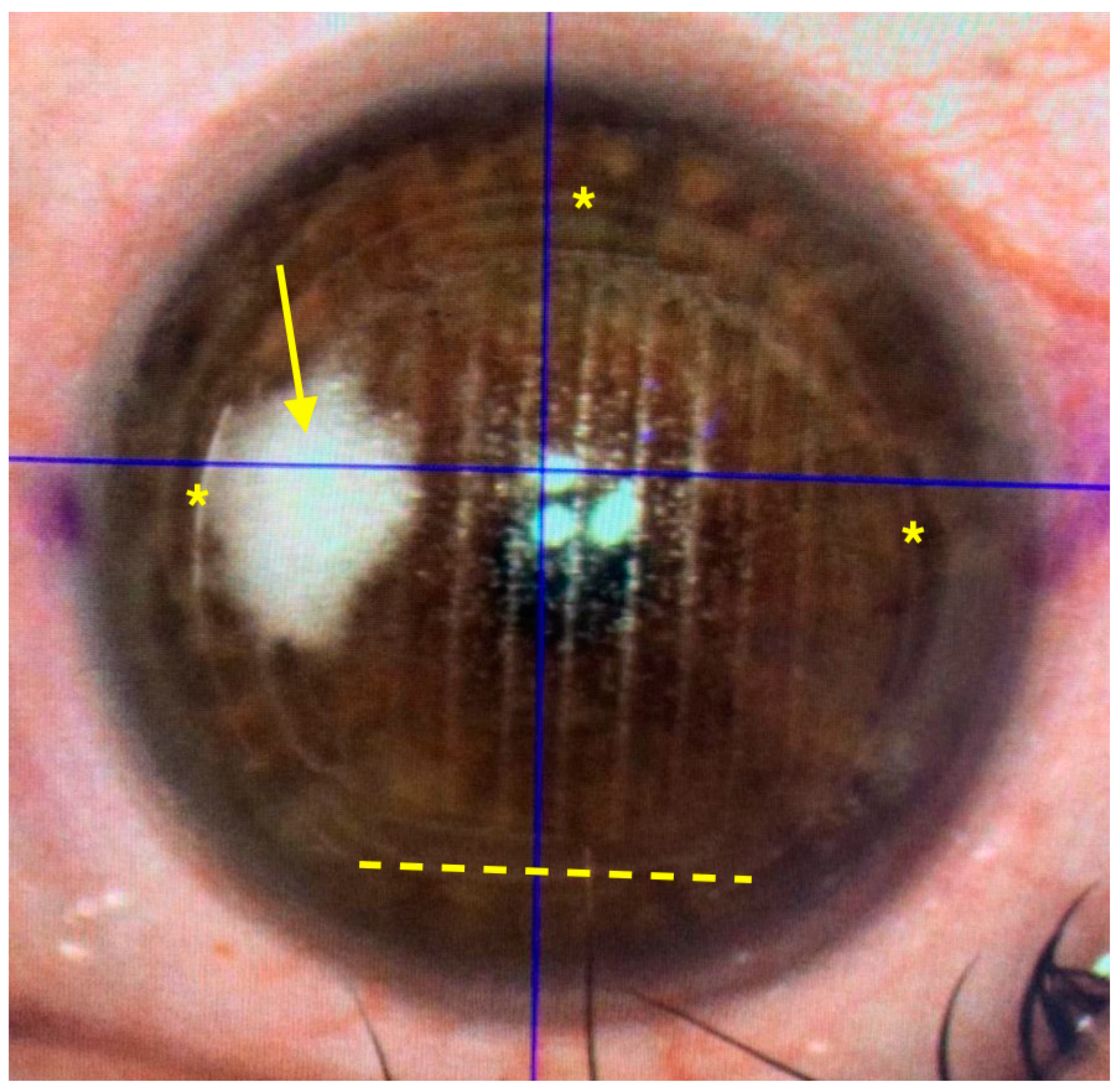
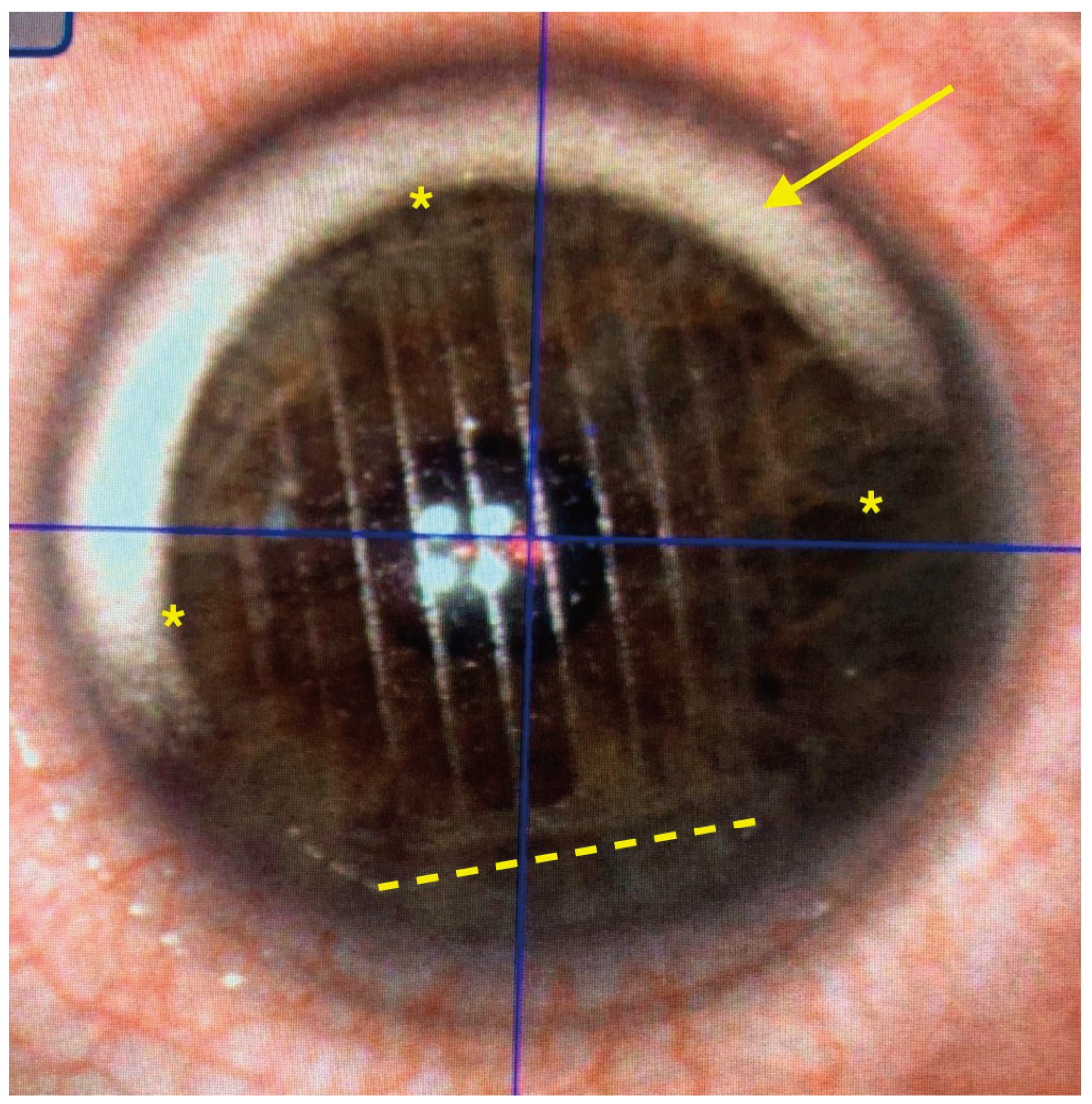
The femtosecond laser Ziemer Z8 (Ziemer Group, Port, Switzerland) can create a LASIK flap on the applanated cornea with a contact flat glass fixated by scleral suction, through which the laser is delivered at a defined depth. In the classical 2-dimension (2D) method, the flap size is determined by the applanated area, and its edges are defined by the border of the applanated cornea, resulting in a tapered shape (
Figure 1,
Figure 2 and
Figure 3). In the new 3-dimension (3D) method, a sidecut is actively created at the desired angle (
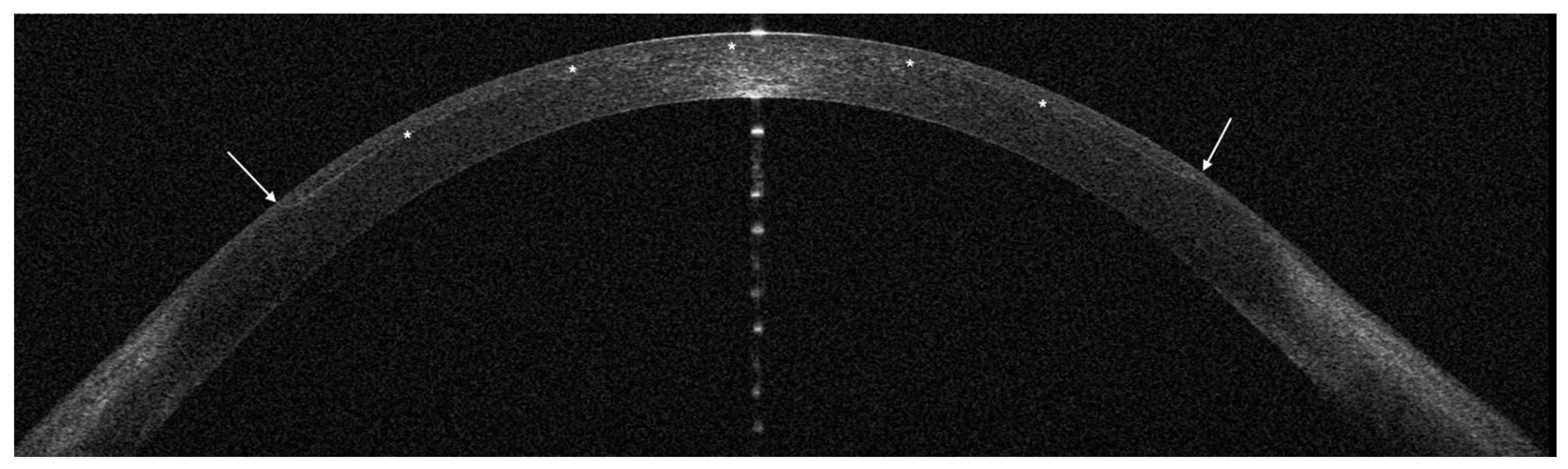
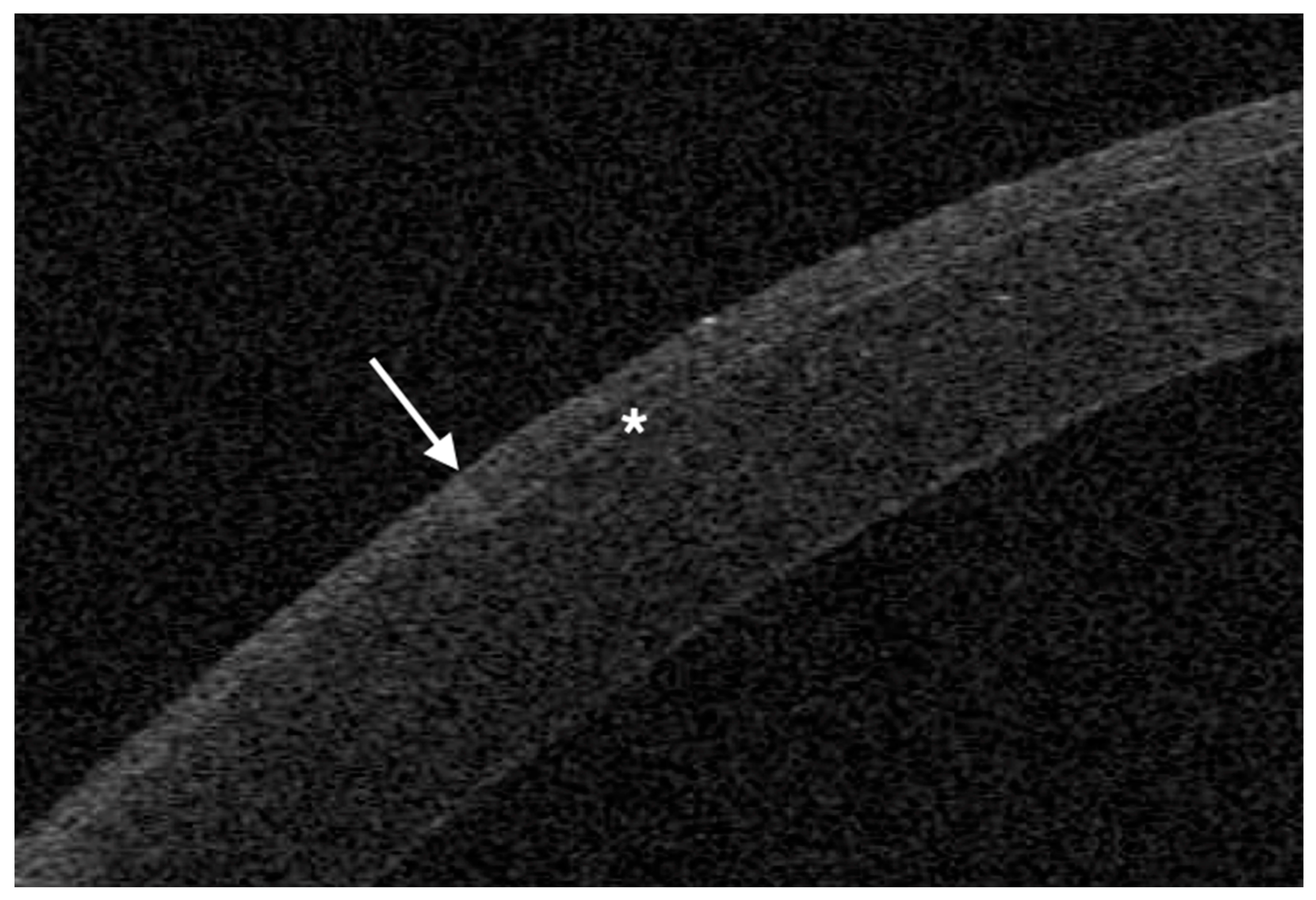
Figure 4 and
Figure 5), so that the flap size and edges are planned on a dedicated software, requiring however additional surgical time.
Anterior segment optical coherence tomography (OCT) is the ideal imaging technique to depict the corneal details after laser surgery [
4]. We therefore reviewed 2D- and 3D-generated LASIK flaps in a large series, to compare their OCT features, surgical time, intra- and early post-operative complications.
2. Materials and Methods
A retrospective, comparative case series study was designed, including consecutive myopic patients undergone femtosecond LASIK in our institute between February and March 2024. In patients receiving bilateral treatment, a single eye was randomized to be included. The Institutional Review Board provided approval on March 1, 2024. The research followed the tenets of the Declaration of Helsinki; patients provided a signed informed consent.
Inclusion criteria for surgery were:
a) myopia or compound myopic astigmatism with spherical equivalent (SE) -1 to -12 diopters (D), with at least 18 months of refractive stability, and a refractive astigmatism ≤3 D.
b) age: between 25 and 50 years;
c) general health status: absence of collagen vascular disease, no pregnancy;
d) ocular disease: no previous surgery; absence of scars or epithelial irregularities; absence of macular or lens abnormality; no topical treatment for ocular hypertension; absence of dry eye symptoms, non-invasive tear film break-up time ≥10 seconds (MS-39, Costruzione Strumenti Oftalmici), lacrimal fluid osmolarity ≤300 mOsm/l (I-PEN, Imedpharma);
e) corneal features on OCT and Placido topography (MS-39): central pachymetry ≥480 µm; regular posterior elevation, anterior and posterior tangential topography; no signs of ectasia;
f) corrected distance visual acuity (CDVA) ≥ 20/40 Snellen;
h) minimum follow-up: 1 month from treatment.
Pre-operative assessment consisted of UDVA, CDVA, manifest and cycloplegic refraction (by tropicamide eye drops), undilated and dilated slit-lamp evaluation, Placido corneal topography, OCT tomography with epithelial and stromal thickness evaluation, computer-assisted scotopic pupillography, tonometry, tear function evaluation.
Soft contact lens use was interrupted 1 month before examination and surgery; rigid contact lens use was interrupted 3 months before examination and surgery. All patients were informed about the surgical procedure and provided written consent.
Treatment allocation was determined by the exclusive use of 3D software since February 8, 2024: therefore 200 eyes of the last 200 consecutive patients treated until February 7, 2024 formed the 2D group. The 3D group was then formed by 200 eyes of the first 200 consecutive patients treated since February 8, 2024.
Our technique for femtosecond LASIK has been described in detail [
5]. Briefly, full manifest spherical and cylindrical corrections were set. The planned thickness of the flap was 100 µm in both groups. In all cases, it was programmed to leave a stromal bed thick ≥ 300 µm. The suction ring diameter was chosen according to horizontal corneal diameter measured by OCT. Femtosecond laser power and velocity were adjusted to obtain a uniform pattern of tiny, non-confluent plasma bubbles. After topical anesthesia with oxybuprocaine, a drop of unpreserved 0.2% sodium hyaluronate was dripped on the cornea, and the applanation glass applied on the cornea; centration was initiated as soon as scleral suction was activated.
Flap centration on the cornea can be achieved by addressing patient voluntary eye movements towards a reference aiming light, then adjusting the centration with arrows on the laser touchscreen. The new 3D software includes a self-centration option, where the pupil is automatically recognized and the flap centered on it. In all 3D eyes, the self-centration option was used. After centration, the femtosecond treatment was initiated and the total time under activated suction measured.
After the completion of the femtosecond phase, the flap was separated and folded in a “taco” fashion with a flap spatula (MMSU1171, Malosa Surgical). After the refractive treatment with a Teneo 317 excimer laser (Bausch+Lomb) in Planoscan mode, the flap was repositioned, interface washed with balanced salt solution for 2 seconds through a single use 25-G cannula, and the flap finally smoothed down with a wet microsponge. A drop of unpreserved netilmicin 0.3% + dexamethasone 0.1% was dripped on the cornea. Netilmicin and dexamethasone were continued 4 times daily for a week.
Only eyes where a 9 mm flap was planned (by the suction ring diameter with 2D; with laser setting on 3D) were included. In the 3D group, a 90° angle was chosen for the sidecut.
OCT was performed the day after surgery with a horizontal high-resolution section; the sidecut angle was measured on the tangent line of the corneal curvature at the temporal aspect of the cornea with the MS-39 software tool.
Statistical analysis was performed with the SPS software, available online at
www.statisticsfordataanalysis.com (accessed March 7, 2024). The mean ± standard deviation was used to describe quantitative variables, and a
p value less than 0.05 considered statistically significant.
3. Results
A total of 400 eyes were finally included, 200 for each group.
Table 1 reports the preoperative features of the 2 groups and the outcome measures. Mean age, male/female ratio, right/left eye ratio did not differ significantly between groups.
Mean suction time was 31.1 seconds (standard deviation, SD 3.3) in the 2D group and 36.3 seconds (SD 4.2) in the 3D group; the difference between the means (5.2 seconds) is statistically significant (p=0).
Irregular edges or difficult dissection were not encountered in either group.
An opaque bubble layer (OBL, temporary stromal collection of plasma) was observed in 2 cases (2 peripheral, 0 central) after 2D treatment and in 9 cases (6 peripheral, 3 in the flap area) after 3D treatment; the difference is statistically significant (p=0.03 at Chi
2 test of independence; Chi
2 value 4.58). In no cases OBL interfered with the completion of the treatment, being the 3 in the flap area not central (
Figure 6 and
Figure 7).
The mean angle of the temporal edge of the flap was 21° (SD 5°) in the 2D group and 92° (SD 3°) in the 3D group.
Visually non-significant flap folds at day 1 were found in 13 eyes post-2D treatment and in 7 eyes post-3D treatment (p=0.25 at Chi2 test of independence; Chi2 value 1.89).
Interface inflammation (diffuse lamellar keratitis, DLK) was encountered only in 1 eye in the 2D group, at grade 1 (peripheral), resolving with topical steroids in 2 weeks without complications.
No flap dislocations occurred in either group.
4. Discussion
In our series, the use of the new 3D software allowed precise flap cut in femtosecond LASIK, with no complications. The only significant differences with the 2D program were a slightly increased occurrence of OBL and an increased suction time (5.2 seconds longer). The flap edge was, as programmed, angled at 90°. A non-significant tendency to reduce flap folds was also observed in the 3D group.
The importance of suction duration is determined by the raise of intraocular pressure (IOP), which may damage the eye. During suction with the Ziemer laser, the IOP reached 184 mmHg in an experimental model [
6]; acute IOP increase can induce optic neuropathy and retinal detachment [
7,
8,
9]. Is unknown whether a 5-second difference poses a further risk of complication.
OBL is induced by the photodisruption caused by the femtosecond laser in the cornea, generating plasma, composed by water vapor and carbon dioxide [
10]. Dense OBL may interfere with the subsequent excimer laser phase [
11]. Different patterns of treatment during femtosecond flap creation may influence the formation of OBL, as the plasma may escape earlier and laterally from the applanated area, thus avoiding accumulation [
12]. The occurrence of central OBL covering the pupil may affect the centration tracking systems and therefore require to delay the treatment until the gas is reabsorbed. In the 2D program, the peripheral part is treated first, thus creating an escape route for the plasma during the whole procedure. In the 3D program, 2 vents are created near the hinge at the beginning of the treatment, but the flap edge is only completed at the end of the procedure, so that plasma may still unduly accumulate. The cases of OBL that we observed were not central, not posing any challenge to the completion of the LASIK procedure.
Flap folds (striae) are a common complication of LASIK, that is some cases may become visually significant; flap malposition at surgery is one of the causes [
13]. The cases in our series were mild and did not require treatment; although non statistically significant, a slight reduction of their incidence was seen in the 3D group. A sharp flap edge may contribute to better intrasurgical alignment.
Early flap dislocation after LASIK usually occur spontaneously, without traumatic events; their incidence is 0.012% and higher with meniscus shaped flaps [
2]. Being a rare event, our sample is too small to compare its occurrence in the 2 groups. Late flap dislocation is instead usually traumatic, and often caused by a tangential trauma [
14]; an angled flap edge may theoretically provide a more solid cicatricial adhesion and less susceptibility to tangential traumas.
Epithelial ingrowth in caused by corneal epithelial either introduced during surgery or infiltrating through the flap edge [
15]. In an experimental model, angled flap edges reduce its incidence by blocking epithelial cell migration [
3].
The limitations of the present study include the retrospective design and the relative rarity of possible complications. This study was however conceived to assess the safety of the surgical procedure and the effective shape of the flap with the new 3D software.
In conclusion, the creation of a LASIK flap by a 3D-femtosecond laser cut was safe and effective. Long term studies are required to evaluate the impact of an angled flap edge on epithelial ingrowth and spontaneous or traumatic flap dislocations.
Author Contributions
Conceptualization, AL, SF, GDB; Methodology, AL, SF; Software, CC; Validation, GDB, MP, CC; Formal Analysis, AL, MP; Investigation, AL; Resources, MP.; Data Curation, SF, CC; Writing – Original Draft Preparation, AL, MP; Writing – Review & Editing, AL, SF, GDB, CC, MP; Visualization, SF; Supervision, AL. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Institutional Review Board provided approval on March 1, 2024. The research followed the tenets of the Declaration of Helsinki; patients provided a signed informed consent.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kymionis, G.D.; Kankariya, V.P.; Plaka, A.D.; Reinstein, D.Z. Femtosecond laser technology in corneal refractive surgery: a review. J Refract Surg 2012, 28, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Clare, G.; Moore, T.C.B.; Grills, C.; et al. Early flap displacement following LASIK. Ophthalmology 2011, 118, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, V.; Chan, T.C.; Li, W.Y.; et al. Conventional versus inverted sidecut flaps for femtosecond laser-assisted LASIK: laboratory and clinical evaluation. J Refract Surg 2017, 33, 96–103. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.; Rhee, K.I. Flap thickness reproducibility in laser in situ keratomileusis with a femtosecond laser: optical coherence tomography measurement. J Cataract Refract Surg 2008, 34, 132–136. [Google Scholar] [CrossRef]
- Leccisotti, A.; Fields, S.V.; De Bartolo, G. Femtosecond-LASIK retreatments after primary myopic photorefractive keratectomy. Cornea 2023, 42, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J.M.; Holzer, M.P.; Teping, C.; et al. Intraocular pressure during corneal applanation: comparison among four femtosecond lasers in porcine eyes. J Refract Surg 2011, 27, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cameron, B.D.; Sara, N.A.; Strominger, M.B. Laser in situ keratomileusis-induced optic neuropathy. Ophthalmology 2001, 108, 660–665. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Azar-Arevalo, O. Retinal detachment in myopic eyes after laser in situ keratomileusis. Am J Ophthalmol 2000, 129, 825–826. [Google Scholar] [CrossRef]
- Mirshahi, A.; Kohnen, T. Effect of microkeratome suction during LASIK on ocular structures. Ophthalmology 2005, 112, 645–649
. [Google Scholar] [CrossRef]
- Farjo, A.A.; Sugar, A.; Schallhorn, S.C.; et al. Femtosecond lasers for LASIK flap creation: a report by the American Academy of Ophthalmology. Ophthalmology 2013, 120, e5–20. [Google Scholar] [CrossRef]
- Marino, G.K.; Santhiago, M.R.; Wilson, S.E. OCT study of the femtosecond laser opaque bubble layer. J Refract Surg 2017, 33, 18–22. [Google Scholar] [CrossRef]
- He, X.; Li, S.M.; Zhai, C.; et al. Flap-making patterns and corneal characteristics influence opaque bubble layer occurrence in femtosecond laser-assisted laser in situ keratomileusis. BMC Ophthalmol 2022, 22, 300. [Google Scholar] [CrossRef] [PubMed]
- Melki, S.A.; Azar, D.T. LASIK complications: etiology, management, and prevention. Surv Ophthalmol 2001, 46, 95–116. [Google Scholar] [CrossRef]
- Leccisotti, A.; Fields, S.V.; De Bartolo, G.; Malandrini, A. Traumatic flap complications after femtosecond LASIK. Cornea 2022, 41, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Asano-Kato, N.; Toda, I.; Hori-Komai, Y.; et al. Epithelial ingrowth after laser in situ keratomileusis: clinical features and possible mechanisms. Am J Ophthalmol 2002, 134, 801–807. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).