1. Introduction
Milk contains important nutrients and satisfies people’s nutritional needs. However, milk is an ideal medium for the growth and multiplication of diverse microorganisms, resulting in its early deterioration (Sarkar, 2015). Contaminating bacteria may multiply rapidly and render it unsuitable for processing and/or unfit for human consumption. The most commonly used preservation technology to stop or retard the deterioration of milk and reduce milk postharvest loss from producers to collection centers is cooling facilities. However, lack of available capital, lack of electricity, less developed road systems, high operational costs, frequent equipment breakdowns, a lack of spare parts, and difficulties in equipment repair in rural areas are major challenges to milk collection centers (Seifu et al., 2005). There is also the informal addition of formalin to raw milk for preservation by the producers to increase shelf life for long-distance transportation to reach milk collection centers in Ethiopia (Zebib et al., 2023). Formalin is highly toxic, causes liver and kidney damage, and is considered carcinogenic. To tackle this problem, the use of the LPS in areas where there is currently no adequate infrastructure for the collection of raw milk has been developed and applied (Codex Alimentarius, 1991; FAO, 1999; IDF, 1988).
Lactoperoxidase (LPO) is an enzyme naturally found in milk. One of its unique biological functions is an antibacterial effect in the presence of hydrogen peroxide (H2O2) and thiocyanate. Both of these substances are naturally present in milk in varying concentrations. The natural bacteriostatic effect of LPS lasts at least one hour after milking (Chamberlain 1993). For continued effect, the system has to be activated. This activation can be achieved by adding about 10 parts per million (ppm) (5 ppm is naturally present) of thiocyanate (preferably in powder form) to the raw milk to increase the overall level to 15 ppm (Bennet 2000).
LPS is effective against most microorganisms with the activation of thiocyanate and hydrogen peroxide to oxidize and produce the antimicrobial agent hypothiocyanite (OSCN), which exerts its action by oxidizing the sulphydryl groups of proteins to disulphides. Thus, LPS enhances the shelf life of raw milk from 7 to 26 hours at different storage temperatures (30 to 15 °C) (Codex Alimentarius, 1991). LPS also has a bactericidal effect on most milk-borne pathogenic bacteria (Escherichia coli, Salmonella spp., Campylobacter spp., Staphylococcus aureus, Listeria monocytogenes, Yersinia enterocolitica, and Brucella melitensis) (Moffat et al., 2016). However, the effectiveness of LPS depends on the raw milk's initial microbial load on fluid milk's quality and shelf life (Barrett et al., 1999). The spoilage microflora in pasteurized milk is completely different from that found in raw milk, which consists mainly of post-pasteurization contaminants (Marks et al., 2001). There is also an incidence of pathogens in pasteurized milk, and foodborne outbreaks due to inadequate pasteurization or post-pasteurization contamination have been reported in Ethiopia. Residual lacto-peroxidase activity plays a role in the quality of pasteurized milk and dairy products generally (Moffat et al., 2016).
Ideally, milk should be cooled to < 4 °C immediately after milking (2–3 hrs) and transported to dairy plants as soon as possible under the cold chain. However, in some countries, including Ethiopia, the establishment of cooling units is impractical because of a lack of capital, a lack of electricity, insufficient transportation systems, and high operational costs. Insufficient cold storage systems eventually lead to excessive multiplication of bacteria and increase the acidity of raw milk far beyond the level acceptable for processing (Zer, 2014). It is therefore important to look for alternative methods for retarding bacterial growth in raw milk during collection and transportation to the dairy processing plant.
The production of high-quality milk should be a priority for good-quality end products with a long shelf life and for marketing value-added products (Gemechu, 2016). But it is difficult to achieve due to a lack of cooling facilities, high ambient temperatures, and insufficient infrastructure for milk transportation to the market (Bille et al., 2010). The fact that the few dairy enterprises currently operating in and around Addis Abeba are operating below capacity due to a lack of milk supply does not imply a gap between milk production and demand, as only 5% of milk produced in rural areas is marketed (Getachew 2003).
Much work has been conducted on both the LPS in raw cow milk and the activation of the system prior to heat treatment in different countries under laboratory conditions (FAO, 2005: 2016). So far, in Ethiopia, few papers have been published on the evaluation of LPS during raw milk storage periods at different temperatures under laboratory conditions at a controlled temperature (Kassa et al., 2013; Fanta et al., 2019). These studies did not address the quality and shelf life of milk during transportation to processing plants under real-world conditions and did not evaluate important liquid milk quality parameters (methylene blue reduction test, thiocyanate concentration, resazurin, and titratable acidity). In addition to this limitation, there is no information on the effect of LPS on pasteurization efficiency or residual LPS on the keeping of pasteurized milk in the context of poor handling, unhygienic practices, or where there is a high microbial load in raw milk. Therefore, the objectives of this research were (i) to evaluate the effect of LPS on the quality of raw milk during storage and transportation at the farm under real conditions; (ii) to evaluate the effect of LPS during storage and/or transportation along collection centers under real conditions prior to processing; (iii) to evaluate the effect of LPS on the quality and shelf life of pasteurized milk during post-pasteurization storage; and (iv) to compare LPS-activated milk quality with Ethiopian standards.
2. Materials and Methods
2.1. Study Area
The study districts for sample collection were selected according to the Central Statistical Agency based on milk production potential. The study districts include urban and peri-urban areas of the Oromia region (Selale, Holeta, Bishoftu/Debrezeyit, and Asella) (CSA, 2019). In each study district, smallholder dairy farmers, collectors, and milk factories were included.
2.2. Study Design, Sample Size and Sampling Technique
A cross-sectional study was used to collect primary samples. Dairy farmers were selected using a simple random technique, whereas collectors and factories were selected purposefully. In this study, dairy farmers (n = 40), collectors (n = 4), and processors (n = 4) participated.
Table 1 shows the sample size for treatments along the value chain at each study site.
Sampling of raw milk from farmers, collectors, and factories was conducted according to ES ISO, 2012. Milk in the farmer's buckets (small vessels) was thoroughly mixed using a suitable dipper. Then about 500 ml of sample was taken from each farmer’s bucket. From this, about 250 ml of milk samples were used for LPS activation and control. At the collection centers, five different aluminum or plastic cans were randomly selected from the collected milk. Then each container was thoroughly mixed using suitable dippers. From this, about 250 ml of milk samples were used for LPS treatment and control. After milk was received by milk factories, composite samples (3 L) were sampled from different milk tankers for each treatment analysis and pasteurized milk shelf-life evaluation.
2.3. Collection of Laboratory Sample, Transportation and Storage
All samples were collected from January 2022 to October 2022. Raw milk samples were collected from farmers, collectors, and milk factories (prior to pasteurization). Laboratory samples for microbiological and physico-chemical examinations were taken separately using sterilized equipment, sampling apparatus, and containers in accordance with ES ISO, 2012. Those for microbiological analysis were taken first. Raw milk samples were collected in sterile, screw-capped, clean plastic bottles (250 ml capacity). Proper labeling was done for each sample. Morning samples were transported under real conditions, whereas overnight samples were transported in an ice box at 2–8 °C to the Addis Ababa University Center for Food Science and Nutrition laboratory. Samples were analyzed immediately for microbiological determination. Those samples for chemical quality were stored at 2–8 °C.
2.4. Activation of LPS in Raw Milk
LPS activation in raw milk was done according to Codex Alimentarius (1991). The LPS was activated by addition of 14 ml of freshly made sodium thiocyanate 1 mg/ml solution (General Chemical Division, New York, USA) per liter of milk in order to provide a source of the thiocyanate (SCN-) ion. 10 ml of freshly made, 1 mg/ml hydrogen peroxide solution (BDH Chemicals Ltd., Poole, England) was added to the milk after the mixture had been completely stirred for 1 minute.
2.5. Experimental Trial and Procedure
The raw milk experimental samples from farmers, collectors, and milk factories (prior to pasteurization) were grouped into two treatments: LPS-activated morning milk and LPS-activated overnight milk at farmers, collectors, and milk factories. Control consisted of LPS untreated morning and overnight milk samples in each value chain. Overnight milk samples from each value chain were activated with LPS and kept overnight (12 hours) at farmers, collectors, and milk factories under real conditions, and then laboratory samples were transported using a portable refrigerator (Dometic, CFX-50 W, China) to the laboratory, whereas morning milk samples were activated with LPS and then directly transported under real conditions to the laboratory. For pasteurization purposes, the LPS was activated overnight, and morning milk collected from milk factories was pasteurized and evaluated for shelf life. All analysis was run in duplicate.
2.6. Validation of Pasteurization Method
Pasteurization method validation can be accomplished in two ways: first, by validating the process equipment temperature and holding time, and second, by validating the product by pathogen reduction and growth. Pasteurization systems are designed to provide a 5 log reduction of the microbial load. With pasteurization, not only are pathogenic microorganisms killed but also a wide range of spoilage organisms are destroyed (Zer, 2014). Based on the above criteria, both process equipment and product validation were performed prior to the study by measuring the temperature with an electronic thermometer to determine whether the water bath display reading was correct or not, and product validation was also performed by detecting the TBC and TCC of samples before and after pasteurization to determine the effectiveness of the pasteurization.
2.7. Pasteurization and Shelf-Life Evaluation of Pasteurized Milk
Pasteurization of samples was done at laboratory scale using a water bath as described by Tetrapack (1995). All plastic containers containing samples (LPS-activated and control morning and overnight milk) were immersed in a water bath (Biobase, WB-82, China) and pasteurized at 65 °C for about 30 minutes. Then the samples were cooled in running water and kept in the refrigerator at 2–8 °C for 10 days. Afterwards, the samples were drawn at 0, 2, 4, 6, 8, and 10 days of storage to evaluate keeping quality and shelf life.
2.8. Assessment of Microbiological Quality of Milk
2.8.1. Total Bacterial Count (TBC)
The presence of mesophilic bacteria in mL of diluted milk samples was tested according to the FDA (2000) using the pour plate method with plate count agar. Milk samples were homogenized and serially diluted by adding 1 mL of the sample into 9 mL of 0.1% peptone water for initial dilution and by transferring 1 mL of the previous dilution into 9 mL of peptone water. Appropriate dilutions were placed on petri dishes and pour-plated with 10 to 15 mL of molten plate count agar for TPC. The sample and agar were gently mixed and permitted to harden on the bench for roughly 30 minutes using alternate clockwise and anti-clockwise rotations. The plates were inverted and incubated at 32 +/- °C for 48 hrs. Plates inoculated with a sample dilution yielding between 25 and 250 colonies were counted after incubation. The number of bacteria in a millilitre of milk was determined using the FDA (2000) method after colony counts were performed with a colony counter.
Where, C = the sum of colonies on all plates counted
V =the volume applied to each plate
n= the number of plates counted at first dilution.
n2= the number of plates counted at second dilution,
d = dilution from which first count was obtained.
N= the average plate count
2.8.2. Enumeration of Total Coliform Count (TCC) and E. coil
The protocols for counting total coliforms and E. coli counts in milk samples were followed according to 3M Food Safety, 2017. One millilitre of raw and pasteurized milk was serially diluted in nine milliliters of 0.1% peptone, up to five and three dilutions, respectively. Prior to applying the 1 ml diluent to the E. coli/coliform count plate Petri film for quantification of coliforms and E. coli, the diluent was vortexed to homogenize the serial dilution. One mL of diluent was taken from the supernatant for serial dilution. Then, the top film was lifted, and 1 mL of sample suspension was dispensed onto the inoculation area. The Petri film plates were incubated in a horizontal position at 35 °C ± 2°C for 24 hrs ± 2 hrs. The plates were incubated for an additional 24 hrs ± 2 hrs (48 hrs ± 4 hrs total) until colonies of sufficient size to count were observed. The total coliform count consisted of both red and blue colonies associated with gas, and blue colonies with a gas bubble were counted as E. coli (3M Food Safety, 2017).
Where, ∑C = s the sum of the colonies counted on the two petrifilms
V = s the volume of culture Plates
d = s the dilution corresponding to the first dilution retained [d = when the undiluted liquid product (test sample) retained.
2.9. Determination of Milk Quality Indicators
2.9.1. Determination of pH
The pH of samples was measured using a digital pH meter (HFCC, PHS-3DW, China) according to the manufacturer's manual. Before analyzing the sample, the pH meter was calibrated using 4.0 and 7.0 buffer solutions.
2.9.2. Determination of Titratable Acidity (TTA)
The TTA of milk samples was determined according to AOAC (2016) by measuring 10 ml and adding water (40 °C) to a volume of 105 ml. After vigorous shaking, the mixture was filtered, and an aliquot of the filtrate (25 ml) was titrated with 0.1 N sodium hydroxide solution using phenolphthalein as an indicator. The TTA was expressed as % lactic acid and calculated using the formula below.
2.9.3. Clot on Boiling (COB) Test
A 10-ml milk sample was boiled in a test tube over a spark lamp and allowed to cool for a few minutes. If there was clotting, coagulation, or precipitation, the milk sample failed the test due to its higher acidity (Arefin et al., 2017).
2.9.4. Determination of Alcohol Test
This was done according to Gutema (2019). For detection, 75% ethanol (2 ml) was mixed with an equal volume of milk (2 ml). The samples were mixed thoroughly and incubated at room temperature for a few minutes. No coagulation, clotting, or precipitation indicates good-quality milk.
2.9.5. Determination of Thiocyanate Concentration
This was measured using trichloroacetic acid (TCA), as the ferric complex, at 460 nm absorbance (Codex Alimentarius, 1991). Milk samples (4.0 ml) were mixed with 2.0 ml of a 20% TCA solution. The mixture was blended well and then allowed to stand for at least 30 minutes. It was thereafter filtered through a suitable filter paper (Whatman No. 40). The clear filtrate of 1.5 ml was then mixed with 1.5 ml of the ferric nitrate reagent (16.0 g Fe (NO3)). 3.9H2O was dissolved in 50 ml of 2M HNO3 and then diluted with distilled water to 100 ml, and the absorbance was measured at 460 nm using a UV spectrophotometer. As a blank, a mixture of 1.5 ml of ferric nitrate solution and 1.5 ml of water was used. The measurement was carried out within 10 minutes of the addition of the ferric nitrate solution, as the colored complex is not stable for any length of time. The concentration of thiocyanate was then determined by comparison with standard solutions of known thiocyanate concentrations (10, 15, 20, and 30 g/ml of thiocyanate).
2.9.6. Methylene Blue Reduction Test (MBRT)
MBRT were analyzed according to Thornton & Hastings (1930). 1 ml of the methylene blue thiocyanate solution (by dissolving one methylene blue tablet in 200 ml of distilled hot water) were added into a test tube. Then 10 ml of thoroughly mixed milk sample were added and tightened the stopper. Tubes were placed in the water bath immediately. When temperature reached 36 °C, tubes were slowly inverted a few times to assure uniform creaming and covered to keep out light. This time was recorded as the beginning of the incubation period. Samples were checked for decolorization after 30 minutes of incubation. The classification of sample color and quality of milk is listed below.
Class 1. Excellent, not decolorized in 8 hours.
Class 2. Good, decolorized in less than 8 hours but not less than 6 hours.
Class 3. Fair, decolorized in less than 6 hours but not less than 2 hours.
Class 4. Poor, decolorized in less than 2 hours.
2.9.7. Resazurin Reduction Test (RRT)
RRT was done according to Atherton and Newlander (1977). 1 ml of resazurin dye solution (by dissolving one resazurin tablet in 200 ml of hot distilled water) was placed in a sterile test tube. Then 10 ml of milk samples were added, tightened with a stopper, and placed in the water bath. When the temperature reaches 36 °C, the test tube is inverted to mix the milk and dye. Then it was incubated at 36 °C. Tubes were examined and classified at the end of an hour in the "one-hour test." The following relationships between color and quality were generally accepted:
1. Blue (no color change): Excellent
2. Blue to deep mauve: Good
3. Deep mauve to deep pink: Fair
4. Deep pink to whitish pink: Poor
5. White: Bad
2.10. Statistical Analysis
The obtained data were performed using STATA Version 20 and XLSTAT for Microsoft Excel Version 2015. 2.01. Independent t-tests were used to compare the quality of LPS-activated liquid milk and the control. One-way ANOVA and descriptive statistics were used to compare the mean results. DMRT was used to test between mean pairs and accepted at a probability of 0.05.
3. Results
3.1. Effect of LPS on Microbiological Quality of Raw Milk along Dairy Value Chain
3.1.1. Effect of LPS on Microbiological Quality of Producer’s Milk
The effects of LPS on the microbiological quality of raw milk collected from dairy producers are presented in Table 2. Results indicate that there is a significant difference (p<0.05) in the TBC between the different treatments in both the morning and overnight milk samples collected from farmers. In the morning milk samples, the mean TBC of the LPS-activated samples was 5.79 log cfu/ml, which was significantly lower than the control samples, which had a mean TBC of 6.73 log cfu/ml. This indicates a decrease in TBC of 0.94 log cfu/ml in the LPS-activated samples as compared to the control samples. Similarly, in the overnight milk samples, the mean TBC of the LPS-activated samples was 6.55 log cfu/ml, which was significantly lower than the TBC of the control-treated samples, which had a mean TBC of 7.31 log cfu/ml. This indicates a decrease in TBC of 0.76 log cfu/ml in the LPS activated samples as compared to the control samples.
In the present work, LPS indicates a significant reduction in the TCC of producer milk. The mean TCC values of morning and overnight milk with LPS activator were 3.77 log cfu/ml and 4.19 log cfu/ml, respectively. In comparison, the mean TCC values of the control samples were 4.7 log cfu/ml for morning milk and 5.04 log cfu/ml for overnight milk. This result suggests a reduction in TCC of 0.9 log units in morning milk and 0.86 log units in overnight milk when LPS activator was used.
Activation of LPS on morning and overnight milk significantly (P<0.05) reduced the growth of E. coli as compared to that of control activated morning and overnight milk samples produced, stored, and transported under real conditions. The mean E. coli count in both control morning and overnight milk samples was 0.84 and 0.52 log cfu/ml, respectively (
Table 2). The mean E-coli count in LPS-activated milk was lowered by 0.66 and 0.52 log cfu/ml as compared to control morning and overnight milk samples, respectively. This implies that LPS had a significant effect on reducing the E. coli count.
3.1.2. Effect of LPS on Microbiological Quality of Collector’s Milk
Table 2 summarizes the microbiological quality of collectors' milk samples with LPS activation and control samples. The presence of TBC was significantly (p<0.05) higher in the control samples compared to the LPS activated samples. The mean TBC of morning milk with LPS activation was 6.15 log cfu/ml, while the mean TBC of control morning milk was 6.99 log cfu/ml. Similarly, the mean TBC of overnight milk with LPS activation was 6.96 log cfu/ml, whereas the mean TBC of control overnight milk was 7.78 log cfu/ml.
There was a significant (p<0.05) difference in the TCC between the activated samples and control samples under the same conditions. In the study, a reduction of 1 log unit in TCC was observed in the LPS-activated morning milk samples compared to the control. Similarly, a reduction of 1.1 log units in TCC was observed in the LPS-activated overnight milk samples compared to the control samples. The mean TCC values reported in the study were 4.63 log cfu/ml for the activated morning milk sample, 5.27 log cfu/ml for the activated overnight milk sample, 5.63 log cfu/ml for the control morning milk sample, and 6.41 log cfu/ml for the control overnight milk sample.
Both morning and overnight milk samples with LPS activators had significantly (p< 0.05) lower E. coli counts than control samples. The mean E. coli count of morning milk with LPS activators was 0.2 log cfu/ml, and E. coli was not detected in activated overnight milk. E. coli was found in the control morning and overnight milk samples with concentrations of 0.93 log cfu/ml and 0.63 log cfu/ml, respectively.
3.2. Effect of LPS on Microbiological Shelf Life of Pasteurized Milk
3.2.1. Effect of LPS on TBC and TCC of Pasteurized Milk
LPS activated and control milk collected during overnight storage had no significant difference (p > 0.05) in TBC until the second day of storage; however, on the fourth day of storage, control overnight milk showed a significant increase in TBC as compared to LPS activated, and at the 6th day of storage, the control sample failed to meet EAS (2006), while activated samples were acceptable until the 10th day of storage. Similarly, the TBC of control morning pasteurized milk failed to meet EAS (not exceeding 30,000 log cfu/ml) (EAS, 2006) on the eighth day of storage, while LPS-activated morning pasteurized milk had 3.9 log cfu/ml on the tenth day of storage, which was below African standards (2006).
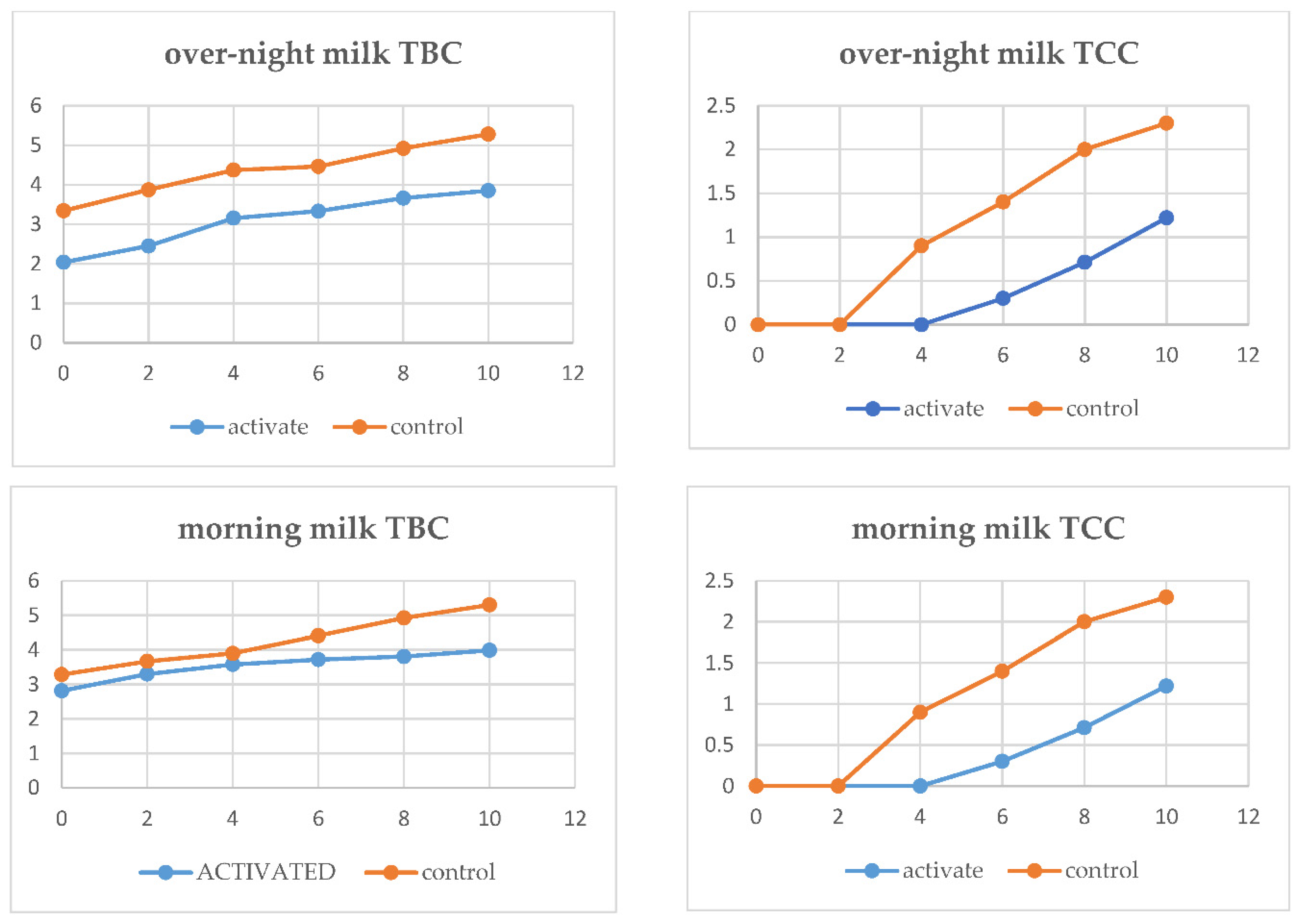
The effect of LPS on the TCC of overnight and morning milk is shown in
Figure 1. LPS activation prior to processing overnight milk during storage inhibits TCC growth significantly as compared to the control sample during the 10th day of storage. Overnight milk LPS activated had 0.7 log unit TCC, while that of control had 2.5 log CFU/ml at the 8th day of storage. The LPS-activated morning milk indicated only 0.5 log unit growth in TCC from 0 to the 8th day of storage, while 1.9 log unit growth was observed in the control activated sample.
According to ESA (2009) (no more than 10 cfu/ml) recommendations for pasteurized milk to contain TCC, the TCC of LPS-activated morning milk met the standard up to the 10th day of storage, while that of control-activated morning milk failed to meet the standard at the 6th day of storage. In the case of overnight milk, the control activated and stored over night for 12 hours under real conditions before processing did not meet ESA's 2009 recommendations at the 4th day of storage, while the LPS activated met the standard until the 10th day of storage. This indicates that LPS activation before processing can improve the quality of pasteurized milk until the 10th.
3.3. Comparison of Microbiological Quality with Ethiopian Standards
Comparison of microbiological quality of LPS activated and control samples with Ethiopian Standard Agency (ESA) is presented in
Table 3. The results show that 51% of LPS activated morning samples and 40% LPS overnight samples collected from producers met ESA for TBC, respectively. Both activated samples had higher percentage of passes than their control samples. Samples from collectors, only 45% of morning and 15% of overnight samples met the minimum requirement set by ESA (2009) limit of no more than 50,000 cfu/ml.
Of samples analysed from producers, TCC for LPS activated morning samples (82%), and control samples (51%) were found to fulfil the minimum requirements of ESA while for LPS activated overnight samples (60.55%) had passed the minimum requirements. The results also show that with the use of LPS activator in collectors’ milk, 70% of the morning milk samples and 30% of the overnight milk samples passed the ESA (2009) limit of no more than 50,000 cfu/ml.
According to the study's E. coli count from producers’ milk, 23.6% of morning control samples, 25.6% of overnight control samples, as well as 10.3% of LPS activated morning milk, did not meet the ESA safety requirement to be nil in marketable dairy products. From collectors’ milk, 90% morning activated morning, 65% morning control and 75% overnight control passed the ESA minimum requirement. Results also indicate that E. coli was not detected in overnight milk activated with the LPS from producers and collector’s milk.
3.4. Effect of LPS on Quality Test of Raw Milk along Dairy Value Chain
3.4.1. pH, TTA and Thiocyanate Concentration
The effect of the LPS on pH, TTA, and thiocyanate concentrations in milk samples is presented in Table 4. There were significant (P < 0.05) differences in pH values between LPS-activated and control samples collected from producers. The pH of the control morning 6.33 and overnight sample 6.04 was much lower than that of the LPS-activated morning milk 6.51 and overnight sample 6.31, though the pH values of all samples were lower than the normal milk pH value (6.6–6.7).
We also found a significant (P < 0.05) difference in TTA values between control and activated morning samples. However, statistical analysis did not show a significant (p > 0.05) difference between activated and control morning samples.
In this work, there was no significant difference (P < 0.05) between the average thiocyanate content of morning and overnight milk with LPS activator compared to control. The highest thiocyanate concentration recorded was 18.3 ppm, and the lowest was 8.92 ppm. The mean thiocyanate content of morning and overnight milk with LPS activator observed in this study was 13.64 ppm and 15.14 ppm, respectively.
Effect of LPS on pH, TTA and thiocyanate concentration of collector’s milk are show in
Table 4. In this work, the mean TTA of LPS-activated morning and overnight milk samples was 0.23 and 0.27, and the mean TTA of control morning and overnight milk samples was 0.26 and 0.35, respectively, which were higher values than the ESA (0.1 to 0.17%). The mean pH values of LPS-activated morning (6.4) and overnight collectors’ milk (6.3) were significantly higher than those of control morning (6.22) and overnight (6.06).
In the present work, the thiocyanate ion (SCN-) content of raw milk collected from collectors was lower in control samples than in LPS-activated samples. There was no statistically significant difference (p> 0.05) in all study samples. The thiocyanate level in LPS-activated samples increased by 0.08 ppm in the morning and 1.03 ppm in the overnight milk compared to control samples.
3.4.2. Alcohol and COB test
The alcohol test is based on the fact that milk with increased acidity (sourness) flocculates when mixed with an equal or double volume of alcohol (FAO 1999).
Table 5 presents the effect of LPS on producer’s milk quality based on alcohol and COB tests. We found no significant (p > 0.05) differences between LPS-activated and control samples collected from producers and collectors.
3.4.3. RRT
The data provided in
Table 6 shows the quality of LPS-activated and control samples based on RRT. It was observed that 20% of the LPS activated morning milk from producers had bad quality, where as 9% had excellent quality. On the other hand, only 3% of the control samples had excellent quality, 20% good quality, 52% poor quality, and 36% bad quality.
According to the resazurin test for collectors’ milk, 87% of control samples had bad or poor quality, while only 26% of activated samples under the same condition had poor or bad quality. Moreover, 30% and 24% of activated samples were categorized as having good or excellent quality, while only 4% and 1% of control-treated samples were categorized as having good or excellent quality, respectively.
3.4.4. MBRT
The methyl blue milk quality grading data for both LPS-activated and control samples collected along value chain is shown in
Table 7. The MBRT is also a common method used to assess the quality of milk. Results show that a high percentage (44%) of LPS-activated producer’s samples had passed the quality test (good or excellent quality), while only 21% of control samples had passes the test. There is a significant (p < 0.05) difference in the quality of milk between the two treatments of collector’s samples. The data indicates that 87% of control samples and 26% of activated samples had poor quality.
3.5. Effect of LPS on Keeping Quality of Pasteurized Milk
3.5.1. Effect of LPS on pH and TTA of Pasteurized Milk
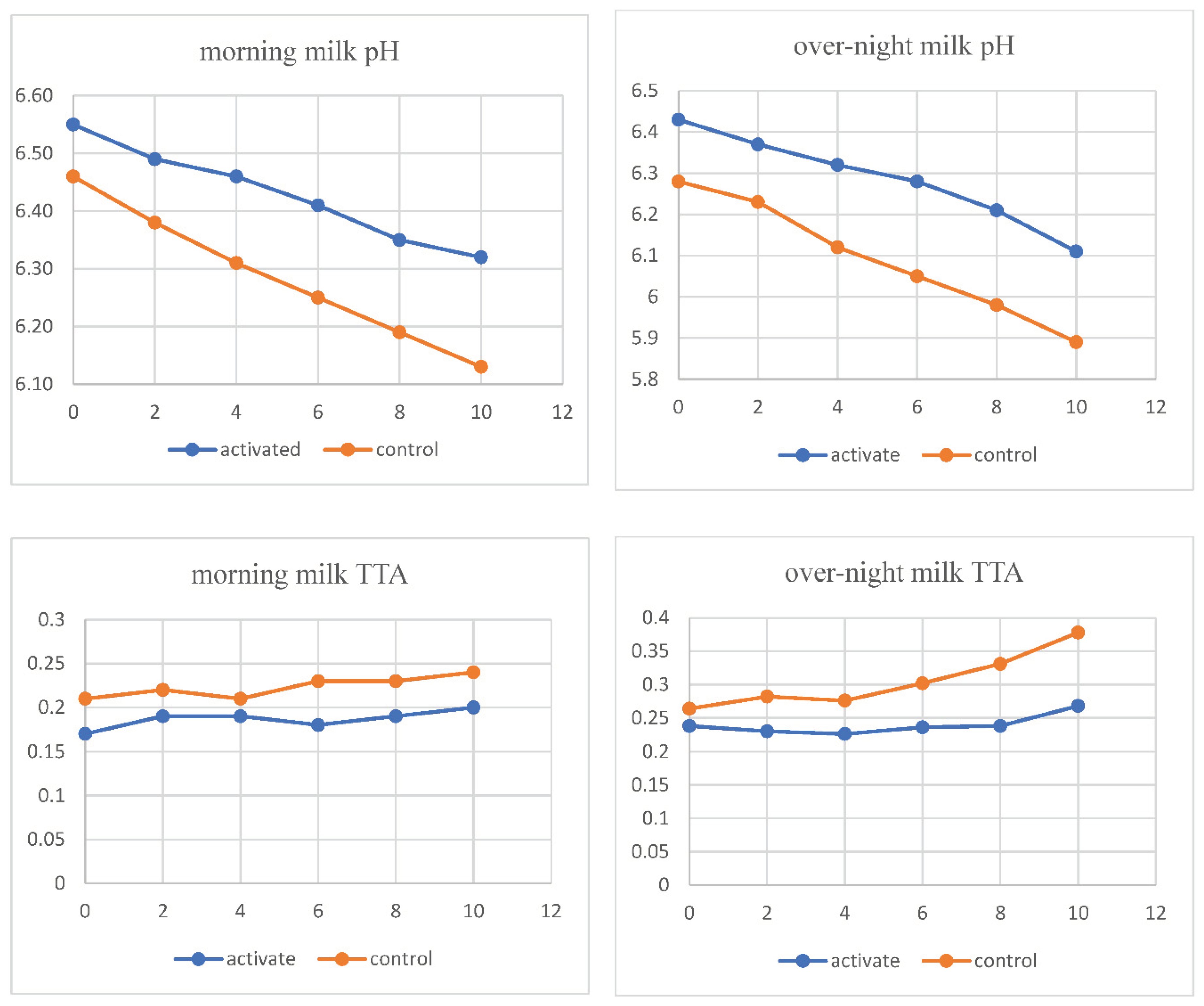
The effect of LPS activation on the pH and TTA of pasteurized morning and overnight milk during storage is depicted in
Figure 2. The pH of both types of morning milk samples did not differ significantly (p<0.05) until the 4th day of storage, after which control milk showed a significant drop in pH compared to LPS-activated milk (Figure 3). On the other hand, the TTA of both types of morning milk samples differed significantly (p<0.05) starting from the 0th day of storage until the 10th day. The TTA of pasteurized morning milk control samples was significantly (p 0.05) higher than that of LPS-activated milk.
4. Discussion
4.1. Effect of LPS on Microbiological Quality of Producer’s Milk
In the present work, the microbiological quality of raw milk samples varied when treated with LPS. TBC of raw milk from farmers between LPS-activated morning milk and activated overnight milk when compared to control. These reductions suggest that the use of the LPS had a positive effect on retarding and multiplying bacterial loads. The present results were consistent with the findings of Sepulveda and Munoz (2003), who discovered significant differences in the bacterial count between samples of milk with and without the LPS activator after 12 hours of storage at ambient temperature. The study by Ponce (2007) also demonstrated that activation of the LPS had an effect on viable mesophiles due to the bacteriostatic effect with a light reduction of bacterial load at first and increasing its bactericidal potential after 4 hrs of activator inoculation, reaching its maximum expression at 9 hrs and later decreasing upon reaching 12 hrs. The study by Amenu et al. (2017) found a 1.07 log cycle TBC reduction after 6 hours of storage at 30°C in raw milk. Similarly, Nigussie and Seifu (2007) reported 8.57 log cfu/ml TBC for samples without LPS activator after 7 hours of storage at 22–24 ℃ storage temperature, whereas samples with LPS activator in the same condition had 7.5 log cfu/ml TBC.
In present research, the TCC in morning LPS activated milk was lower than control. This indicates that the LPS exhibited a bacteriostatic effect against TCC in milk under real milk production, storage, and transportation. The present result is in agreement with Amenu et al. (2017), who reported 1.28 log unit TCC reduction at 7 hr of storage at 30 ℃ and 1.73 log cfu/ml reduction by Nigussie and Seifu (2007) at 7 hrs of storage at 22-23 ℃. The effectiveness of the LPS for raw milk preservation on TCC can be influenced by initial milk quality and the circumstances of the experiment (Reiter 1985; Zapico et al., 1993; FAO 1999).
Activation of LPS in morning and overnight milk reduced the growth of E. coli as compared to that of the control. This implies that LPS had a significant effect on reducing the E. coli count. Thomas and Aune (1978) stated that LPS inhibited succinate-dependent respiration in E. coli which correlated with the loss of bacterial viability. The study in Ethiopia by Amenu et al. (2017) significantly reduced the growth of E. coli as compared to control samples at 6 hours of storage. E. coli have the high metabolic activity, and thus the oxidation product of the LPS may not have been able to counteract E. coli multiplication at ambient temperature (Seifu et al., 2003) which may have been the cause of LPS failure to have bactericidal effects in morning milk. The research done by Pruitt and Njage (1991) also reported that the variability of the bactericidal properties of milk can be caused by variations of the quantities of peroxidases contained in different milk samples.
4.2. Effect of LPS on Microbiological Quality of Collector’s Milk
In this study, the presence of TBC was higher in control samples than in LPS-activated samples (
Table 2). This implies that LPS activation had a positive effect on the retardation of bacterial multiplication as compared to the control groups. However, a relatively higher initial bacterial load, which was beyond the recommended standard, was observed in both LPS-activated and control samples. This could be due to large-volume mixing of different initial densities of bacteria in raw milk from different farmers, through formal and informal means, at the milk collectors, thereby elevating the load of bacteria in the mixed raw milk.
The higher TBC could be influenced by a lack of knowledge about clean milk production, milk contamination from the hands of handlers, the use of plastic containers for collecting and keeping milk, further contamination of the milk during transportation, and the absence of cooling systems at milk selling points. Poor quality can be attributed to the milkmen's lack of cleanliness, the insecurity of the water used for cleaning purposes, the udder of the cow, the milking environment, and the milking equipment, which could be the primary sources of the initial milk contamination (Welearegay et al., 2012).
In present investigation, reduction of TCC of activated samples were observed as compared to its control samples under the same real condition. This reduction suggests that activation of the LPS can maintain the initial quality of raw milk by inhibiting the growth and multiplication of TCC. The present finding values were harmonious with the results obtained by Amenu et al. (2017), who found differences with respect to the coliform count between samples of milk with and without the activator of the LPS at 7 and 12 hours of storage, since said samples with the activator of the LPS showed 1.23 and 1 log unite coliform count reduction at 6 and 12 hours of storage, respectively.
The current finding was also in agreement with the finding of Nigusse and Seifu (2007), who reported a decrease in TCC in cow milk after 7 hours of activation of LPS as compared to the control treatment at ambient storage temperatures between 22 and 23 °C. The study by Campos-Vallejo et al. (2017) also reported 0.1, 0.56, 1.67, 2.66, and 1.8 log units of total coliform reduction at 0, 3, 6, 9, and 12 hours of storage at ambient temperature, respectively. LPS activation affects the TCC of cow milk, confirming the ability to preserve milk quality and the combination of bactericidal and bacteriostatic effects on mesophilic bacteria, including coliforms (Schlorke et al., 2016). According to FAO/WHO (2005), the hygiene of milk plays an important role in extending the shelf life of LPS (Pokhrel & Das, 2014). A high microbial load in normal milk showed the contamination of milk. Likewise, a high load of coliform indicates contamination from manure or soil. Other possible causes of contamination might be the hands and arms of the milking person, the water, and the milking environment (Frazier and Westhoff, 1995).
In the present work, activated milk samples collected from collectors had a lower E. coli count than samples without the LPS activator. This could be due to the bactericidal effect of LPS in overnight milk samples and the inhibitory effect of LPS in morning milk samples. The mean E-coli counts of morning and overnight milk samples without LPS activator were 0.93 log cfu/ml and 0.63 log cfu/ml, respectively, whereas morning milk samples with LPS activator had 0.2 log cfu/ml. However, E. coli was not detected in overnight milk with LPS activator.
Compared to the morning sample without LPS activator, the E. coli count was 0.73 log lower with LPS activated. zer (2014) stated that both gram-positive and gram-negative bacteria can be affected by the LP system reversibly or irreversibly. The capacity of cells to recover from inhibition depends mainly on environmental conditions (temperature and pH) and on the particular strain. In a study conducted at Haramaya University, Amenu et al. (2017) reported that after 6 hours of storage at 30 °C, LPS in a camel milk sample with E. coli significantly inhibited E. coli growth. Since E. coli is a mesophilic bacteria that thrives in temperatures between 21 and 49 °C, it is possible that the ambient temperature and contamination during transportation under real conditions can explain why the bactericidal effect of LPS against E. coli was not noticed in morning milk in the current study.
4.3. Effect of LPS on Microbiological Shelf Life of Pasteurized Milk
In this study, LPS activated samples had a higher shelf life of pasteurized milk. The TBC and TCC of LPS activated samples were lower. This can be explained by the inhibition effect of the combination of LPS and low refrigeration temperature. The prolonged shelf life of LPS activated pasteurized milk is also explained by the fact that certain bacteria become weakened by the effect of LP treatment, making them more susceptible to heat treatment (Mwaikambo et al., 2003). LPS has the greatest impact on pschrotrophic bacteria and certain heat resistant spore-forming bacteria, which normally are the cause of spoilage of pasteurized milk under refrigeration storage.
The residual LPS in pasteurized milk in standard pasteurization was sufficient to catalyze the reactions between thiocyanate and hydrogen peroxide in pasteurized milk (FAO/WHO 2005; Seifu et al. 2005; Trujillo et al. 2007). However, time taken for storage, transportation, and improper field-level practices contributed to the high bacterial density of raw milk, which can impair the microbial quality of pasteurized milk and also the effectiveness of LPS in pasteurized milk. The LPS has been shown to improve the shelf life of bovine milk when activation is done prior to pasteurization (Kamau et al., 1991).
The LPS can continue to function under normal pasteurization temperature and time (Barrett et al., 1999). This is because lactoperoxidase is the most heat-stable enzyme in milk and is only inactivated above 78°C for 15 seconds. There was sufficient residual enzyme activity for the LPS to be operational and exert an effect on the keeping quality of the milk pasteurized in standard pasteurization temperatures. Moreover, LPS-activated milk produces pasteurized milk of better bacteriological and storage quality due to enhanced thermal destruction of milk spoilage bacteria (Kamau et al., 1990; 1991).
4.4. Effect of LPS on Quality of Producer’s Milk
In the present work, the pH of control samples was much lower than that of LPS-activated samples. This can be explained by the higher pH-maintaining capability of LPS compared to milk without the activator. The study by Fonteh et al. (2005) reported the shelf life of LPS-activated milk kept fresh without a considerable drop in pH, while the LPS-control milk stored at room temperature (21–23 °C) got spoiled only after an additional 3 hours of storage.
TTA results indicate that the speed of acid production was lower in activated overnight milk than in control overnight milk. The decreased acid production rate might be due to inhibitory compounds such as hypo-thiocyanate formation during oxidation of thiocyanate and hydrogen peroxide during activation of LPS in milk (Njage and Wangoh, 2008; Seifu, 2005). The acidity difference observed between LPS-activated and control samples in the current study was much lower than the study done by Asaah (2007). He found 29% lower lactic acid content in activated milk than that of control milk after 16 hours of activation at ambient temperature and 15% lower in a water bath (20 °C).
The thiocyanate content of the present result is similar to the finding of Fonthe (2006), who reported an average value of 13.60 ppm for cow milk in Cameroon. The thiocyanate concentration obtained in this study was higher than that of Nigussie and Seifu's (2007) findings, who found an average value of 7.38 ppm in Kombolcha, Eastern Ethiopia. The concentration of thiocyanate in milk can be influenced by the animal's age, health, species, breed, lactation stage, and feed type (Korhonen, 1980; FAO, 2005). The range of thiocyanate ion concentrations in milk from individual cows was 0.05–0.62 mmol/L, and the range of concentrations of thiocyanate in bulk milk was 0.1–1.18 mmol/L, with an average of 0.14 mmol/L (Ponce, 2010).
The LPS activation of milk has a significant impact on its quality. Evaluation of non-activated milk samples with RRT and MBRT had a higher percentage of poor and bad quality which indicate that non-activated milk may contain higher levels of bacteria, which can degrade its quality. Activated milk samples had a higher percentage of good and excellent quality, indicating that the activation process improves the quality of milk. On the other hand,
4.5. Effect of LPS on Quality of Collector’s Milk
The mean pH values of LPS-activated morning and overnight collectors’ milk were significantly higher than those of control samples. These values imply that the use of the activator of the LPS maintained a stable pH in morning milk. In general, the mean pH of both the LPS-activated and control values obtained from the current study was below the recommended range for fresh cows’ milk (6.6–6.7). This might be due to the 12-hour storage time between milking and analysis under real conditions and the further exposure of milk to high contamination during transportation under real conditions. The pH values of the LPS-activated milk samples dropped slowly, while the control milk showed a faster drop in pH (Kassa et al., 2013).
In this study we observed a significant increase in acid production in control morning milk samples during real transportation as compared to the LPS-activated milk acidity, which indicates the positive effect of LPS on retarding the growth of lactic acid bacteria during storage under real conditions. Kumar and Mathur (1989) reported an increase in the TTA of milk stored in similar conditions of elevated temperatures.
In this work, the thiocyanate level in both LPS-activated samples was higher than in control samples. This result is similar to the finding of Fonthe (2006), who reported an average value of 13.60 ppm for cow milk in Cameroon. The thiocyanate concentration obtained in this study was higher than that of Nigusse and Seifu's (2007) findings, who found an average value of 7.38 ppm in Kombolcha, Eastern Ethiopia. The concentration of thiocyanate in milk can be influenced by the animal's age, health, species, breed, lactation stage, and feed type (Korhonen, 1980; FAO, 2005).
In the current study, activation of LPS retained the freshness of raw milk based on alcohol and COB tests and overnight, compared to the control groups. This result showed that application of the LPS can extend the keeping quality of milk, and this agreed with other studies (Barrett et al., 1999; Marks et al., 2001. FAO (1999) states that fresh milk can be diluted with alcohol without flocculation, but if it flocculates when mixed with an equal amount of alcohol, there is an increase in acidity. This milk may not clot on boiling, but it has to be heat treated as soon as possible.
4.6. Effect of LPS on Keeping Quality of Pasteurized Milk
In this study, control samples showed a significant drop in pH values compared to LPS-activated milk. This suggests that LPS activation could help maintain the pH of pasteurized milk during storage by retarding the multiplication and growth of bacteria. The TTA of pasteurized morning milk control samples was significantly higher than that of LPS-activated milk. This indicates that LPS activation retards acid production in pasteurized milk and hence could be useful in extending the shelf life of pasteurized milk.
The LPS activation had no significant effect on the pH or TTA of overnight milk. Therefore, the study suggests that LPS-activated milk samples should be processed before storage for 12 hours or more. This is because storage time could significantly decrease the effectiveness of LPS in retarding acid production in pasteurized milk. The results suggest that LPS activation can be useful in extending the shelf life of pasteurized morning milk by maintaining the pH and retarding acid production. However, the effectiveness of LPS could vary with the type of milk and storage time.
In Ethiopia, pasteurized milk is predicted to have a shelf life of 5 days when kept in a refrigerator and a pH of 6.6 when freshly processed. According to Ethiopian customers, pasteurized milk should pass the sweet test and be re-heatable, which means that the milk's pH should be above 6.4 and its lactic acid content should be below 0.22%. Based on this criterion, the results suggest that LPS activation enhanced the shelf life of morning pasteurized milk under refrigerator storage.
In this study, the LPS-activated morning milk remained acceptable until the 10th day of storage, while the control sample failed on the 6th day in TTA and the activated sample failed on the 8th day in pH, with the control sample failing on the 4th day of storage. However, in the case of overnight milk, the LPS-activated sample failed on the second day of storage, whereas the control sample failed on the first day of processing. Both LPS-activated and control samples failed on the first day of processing in TTA. The increase in shelf life by LPS is less evident in overnight pasteurized milk, where the slight increase in the shelf life of the milk is not commercially significant
5. Conclusions
In this work, activation of LPS can significantly reduce the growth of TBC, TCC, and E. coli in raw milk. Moreover, the study shows that the activation of the system has a notable impact on the quality of milk, as assessed by methyl blue and resazurin tests. In the present research, treatment of LPS raw milk at processors (prior to pasteurization) enhanced the shelf life of morning pasteurized milk by up to 10 days. The overall finding of the current study indicates that LPS was successful in reducing bacterial growth in both raw and pasteurized milk under real-world conditions (dairy environment, milk production, handling, and transportation practices). However, LPS application alone is inadequate for enhancing the quality and accessibility of fresh milk from the dairy industry. Instead, a combination of hygienic practices and LPS application should be implemented at the farmer and collector levels to have a significant impact. Further studies should be conducted to evaluate the cost and benefit of implementing the LPS at the level of farmers, collectors, and processors.
Author Contributions
T.A.: conceptualization; investigation; formal analysis; data collection; data analysis; writing—original draft, writing—review and editing; H.Z.: conceptualization, methodology, supervision; writing—review and editing; A.Z.W.: conceptualization; methodology; data curation; fund acquisition; supervision; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Addis Ababa University-Center for Food Science and Nutrition (Grant Number INV-008459).
Data Availability Statement
All raw data used in the analyses presented here are available in the Supplementary Material.
Acknowledgments
The project is supported by the Bill and Melinda Gates Foundation and the Foreign, Common-wealth & Development Office of the UK through the ENSURE project, grant number INV-008459, awarded to Addis Ababa University. The authors greatly thank Addis Ababa University-Center for food Science and Nutrition for providing facilities for sample collection and analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 3M Food Safety. 2017. PetrifilmE. coli / Coliform Count Plate Interpretation Guide. 3M Food Safety. United States.
- Abera, Y., &Angaw, M. (2015). Handling practice and microbial quality of raw cow’s milk produced and marketed in Adigrat Town, North Eastern Tigray. Journal of Biology, Agriculture and Healthcare, 5, 15.AOAC. 2016. Official Methods of Analysis, 20th Ed: Association Of Official Analytical Chemists International.
- Adane, B. T., Getu, A. A., Akililu, Z., &Feleke, F. B. (2020). Value chain analysis of smallholder dairy production in debark district, Ethiopia.
- Alimentarius, C. (1991). Guidelines for the preservation of raw milk by use of the lactoperoxidase system. CAC/GL, 13-1991.
- Amenu, B., Eshetu, M., Hailu, Y., & Hansen, E. B. (2017). Activation Of Lactoperoxidase System: Evaluation Of The Acidification Rate, Microbial Quality, And Shelf Life Of Camel And Cow Milk. East African Journal of Sciences, 11(2), 107-116.
- AOAC. 2016. Official Methods of Analysis, 20th ed: Association of Official Analytical Chemists International.
- Aprodu, I., Stǎnciuc, N., Dumitraşcu, L., Râpeanu, G., & Stanciu, S. (2014). Investigations Towards Understanding The Thermal Denaturation Of Lactoperoxidase. International Dairy Journal, 38(1), 47–54. [CrossRef]
- Arefin, S., Sarker, M. A. H., Islam, M. A., Harun-ur-Rashid, M., & Islam, M. N. (2017). Use of Hydrogen Peroxide (H2O2) in raw cow’s milk preservation. Journal of Advanced Veterinary and Animal Research, 4(4), 371-377.
- Asaah, N. O., Fonteh, F., Kamga, P., Mendi, S., &Imele, H. (2007). Activation of the lactoperoxidase system as a method of preserving raw milk in areas without cooling facilities. African Journal of Food, Agriculture, Nutrition and Development, 7(2), 1-15.
- Atherton, H. V. and J. A. Newlander. 1977. Chemistry and testing of dairy products. 4th Edn. AVI, Westport, CT.
- Ay M., and K..Bostan. 2017: Effects of activatedlactoperoxidase system on microbiological quality of raw milk. Kafkas Univ Vet Fak Derg, 23, 131-136. [CrossRef]
- Ay, M., &Bostan, K. (2017). Effects of activatedlactoperoxidase system on microbiological quality of raw milk. KafkasUniv Vet Fak Derg, 23, 131-136.
- Aydin, A., Sudagidan, M., &Muratoglu, K. (2011). Prevalence of staphylococcal enterotoxins, toxin genes and genetic-relatedness of foodborne Staphylococcus aureus strains isolated in the Marmara Region of Turkey. International journal of food microbiology, 148(2), 99-106.
- Azeze, T., & Haji, B. (2016). Assessment of post-harvest loss of milk and milk products and traditional mitigation systems in Southern Ethiopia. Assessment, 48.
- Bafort, F., Parisi, O., Perraudin, J. P., & Jijakli, M. H. (2014). Mode of action of lactoperoxidase as related to its antimicrobial activity: A review. Enzyme Research, 2014. [CrossRef]
- Barrett N. E., A. S. Grandison and M. J. Lewis. 1999. Contribution of the lactoperoxidase system to the keeping quality of pasteurized milk. J. Dairy Res. 66: 73-80. [CrossRef]
- Barrett, N. E., Grandison, A. S., & Lewis, M. J. (1999). Contribution of the lactoperoxidase system to the keeping quality of pasteurized milk. Journal of dairy research, 66(1file:///c:/users/pc/desktop/new/newww/litratures/corrected/fao and idf/fao 1999 good.pdf), 73–80. [CrossRef]
- Belitz, H. D., Grosch, W., & Schieberle, P. (2009). Food chemistry. food chemistry, may, 1–1070. [CrossRef]
- Bennet A 2000. The Lactoperoxidase System (LPs) of milk preservation. FAO e-mail conference on “Small-scale milk collection and processing in developing countries”. June 6 – August 3, 2000.
- Bereda, A., Nurfeta, A., & Yilma, Z. (2012). Hygienic and microbial quality of raw whole cow’s milk produced in ezha district of the gurage zone, southern ethiopia. Article In Journal Of Agricultural Research, 1(11), 459–465.Http://Www.Wudpeckerresearchjournals.Org.
- Beyene, B. (2015). Review on value chain analysis of dairy products in Ethiopia college of agriculture and veterinary medicine. J. Econ. Sustain, 26-37.
- Bille, P., Haradoeb, B., & Shigwedha, N. (2010). Evaluation of chemical and bacteriological quality of raw milk from neudamm dairy farm in namibia. African Journal Of Food, Agriculture, Nutrition And Development, 9(7), 1511–1523. [CrossRef]
- Bonfoh, B., Wasem, A., Traoré, A. N., Fané, A., Spillmann, H., Simbé, C. F., Alfaroukh, I. O., Nicolet, J., Farah, Z., & Zinsstag, J. (2003). Microbiological quality of cows’ milk taken at different intervals from the udder to the selling point in bamako (mali). food control, 14(7), 495–500. [CrossRef]
- Bosch, E. H., Van Doorne, H., & De Vries, S. (2000). The lactoperoxidase system: the influence of iodide and the chemical and antimicrobial stability over the period of about 18 months.Journal Of Applied Microbiology, 89(2), 215–224. [CrossRef]
- Boulares, M., Mankai, M., & Hassouna, M. (2011). Effect of activating lactoperoxidase system in cheese milk on the quality of saint-paulin cheese.International Journal Of Dairy Technology, 64(1), 75–83. [CrossRef]
- Campos-Vallejo, M., Puga-Torres, B., Núñez-Naranjo, L., Torre-Duque, D. D. La, Morales-Arciniega, S., & Vayas, E. (2017). Evaluation of the use of sodium thiocyanate and sodium percarbonate in the activation of the lactoperoxidase system in the conservation of raw milk without refrigeration in the ecuadorian tropics.Food And Nutrition Sciences, 08(05), 526–534. [CrossRef]
- Chamberlain A. 1993. Milk production in the tropics. Intermediate Tropical Agriculture Series. Longman scientific and technical. UK, Malaysia. pp. 178.
- Chin, V. T. S. (2017). Microbiological quality of street food and knowledge and practice of street food handlers in kualalumpur (Doctoral dissertation, International Medical University).
- Cifelli, C. J., Maples, I. S., & Miller, G. D. (2010). Pasteurization: implications for food safety and nutrition. Nutrition Today, 45(5), 207-213.
- Codex Alimentarius. 1991. Guidelines for the preservation of raw milk by use of the Lactoperoxidase system. CAC/GL 13-1991. 5 pp.
- CSA (2019). Livestock Sample Survey (Agsslv 2010-2011). Central Statistical Agency Of Ethiopia (CSA).
- CSA, (2010). Agricultural sample survey. Report on crop and livestock product utilization. The Federal Democratic republic of Ethiopia, Central Statistical Agency (CSA). Private Peasant Holdings. Statistical Bulletin 468, Addis Ababa, Ethiopia, 2010.
- Davidson, P. M., &Zivanovic, S. (2003). The use of natural antimicrobials. In Food preservation techniques (pp. 5-30). Woodhead Publishing.
- De Spiegeleer, P., Vanoirbeek, K., Lietaert, A., Sermon, J., Aertsen, A., & Michiels, C. W. (2005). Investigation into the resistance of lactoperoxidase tolerant Escherichia coli mutants to different forms of oxidative stress. FEMS microbiology letters, 252(2), 315-319.
- De Wit, J. N., & VAN HOOYDONK, A. M. (1996). Structure, functions and applications of lactoperoxidase in natural antimicrobial systems. NederlandsmelkenZuiveltijdschrift, 50(2), 227-244.
- Deneke, T. T., Bekele, A., Moore, H. L., Mamo, T., Almaw, G., Mekonnen, G. A.,... & Berg, S. (2022). Milk and meat consumption patterns and the potential risk of zoonotic disease transmission among urban and peri-urban dairy farmers in Ethiopia. BMC public health, 22(1), 222.
- Didanna, H. (N.D.). Livestock in food prod and nut production and nutrition in Ethiopia.
- Dolango, A., Woreda, K. S., Argaw, S., Tolosa, T., &Deresa, B. (2021). Quality of Assessment Raw Cows’ Milk at Different Sampling Points Using Bacteriological Parameters and Other Techniques in Jimma Town, South Western Ethiopia. safety, 104.
- Draaiyer, J., Dugdill, B., Bennett, A., &Mounsey, J. (2009). Milk testing and payment systems. Resource book: a practical guide to assist milk producer groups. Milk testing and payment systems. Resource book: a practical guide to assist milk producer groups.
- Dugdill B. (1999). low cost milk packaging-pasteurising-chilling systems. animal production services-Dairy Page. FAO. Pp 1-2.
- El Zubeir, I. E., & Ahmed, M. I. (2007). The hygienic quality of raw milk produced by some dairy farms in Khartoum State, Sudan. Research Journal of Microbiology, 2(12), 988-991.
- El-ahwal, R. E., El-kher, A. B. O., Sala, E., &Hattem, H. E. (2018). Activation of lactoperoxidase (lp) system in milk and its effect on the quality of kareish cheese. Egyptian Journal of Agricultural Research, 96(3), 1127-1138.
- Eshetu, M., Seyoum, M., & Mummed, Y. Y. (2019). Milk production, marketing practices and qualities along milk supply chains of Haramaya District, Ethiopia. African Journal of Agricultural Research, 14(35), 1990-2005.FAO. (2005). Developing Countries And The Global Dairy Sector Part I Global Overview, Pro-Poor Livestock Policy, PPLPI Working Paper No.30. 30.
- Ethiopian Standard, (2009). Unprocessed Whole/Raw Cow Milk Specification. ES, 3460, 2009.
- Ethiopian Standard. 2009. Pasteurized liquid milk-Specification. ES 3462. 2nd ed. 1-5.
- ES ISO 707; Milk and Milk Products-Guidance on Milk Sampling. 1st ed. Ethiopian Standards Agency: Addis Ababa, Ethiopia, 2012; pp. 1–45.
- F.A.O. (1957). Report On The Meeting Of Experts On The Use Of Hydrogen Peroxide And Other Preservatives In Milk. FAO/57/11/8655. Interlaken, September.Google Scholar.
- Fanta D. G., S. Hani, D. Fufa, B. T. Takele and A. Dinka. 2019. Assessment of the Effect of ActivatedLactoperoxidase System on keeping quality of raw cow milk under different climatic zones of Ethiopia. Dairy and Vet Sci J. 10: 555796. [CrossRef]
- FAO 1999 Manual on the use of the lp-system in milk handling and preservation. rome: Food And Agriculture Organization Of The United Nations (FAO) Http://Www.Fao.Org/Ag/Againfo/Subjects/Documents/LPM/LPMCOVER.Htm.
- FAO. 1999. Manual on the use of the LP-system in milk handling and preservation. Rome: Food and Agriculture Organization of the United Nations.
- FAO. 2005. Benefits and iv Potential Risks of the Lactoperoxidase System of Raw Milk Preservation. Report of FAO/ technical meeting FAO/WHO headquarters, room Italy. Pp:73.
- FAO. 2016. Technical and investment guidelines for milk cooling centers, by Moffat, F., S. Khanal, A. Bennett, T.B. Thapa and S. Malakaran George. Rome, Italy.
- FAO/WHO (Food And Agriculture Organization Of The United Nations, And World Health Organization). 2005. benefits and potential risks of the lactoperoxidase system of raw milk preservation: Report of an FAO/WHO Technical Meeting, FAO, Rome, Italy, 28th Nove.
- FAO/WHO Expert Committee On Food Additives. Meeting. (1992). Compendium Of Food Additive Specifications: Addendum 1 (No. 52). Food & Agriculture Org.
- Fathi, S. S., Mohamed, A. S., & El-Sayed, M. S. (2019). Coliforms Contamination in Raw Milk and Some Dairy Products with Special Reference to Comparative Identification of Enterobacter spp. Zagazig Veterinary Journal, 47(4), 388-397.
- Fonteh, F. A., Grandison, A. S., Lewis, M. J., &Niba, A. T. (2005). The keeping quality of LPS-activated milk in the western highlands of Cameroon. Livestock Research Rural Development, 17, 114.
- Ford, J. E. (1960). A microbiological method for assessing the nutritional value of proteins. British Journal of Nutrition, 14(4), 485-497.
- Gaya, P., Medina, M., &Nuñez, M. (1991). Effect of the lactoperoxidase system on Listeria monocytogenes behavior in raw milk at refrigeration temperatures. Applied and Environmental Microbiology, 57(11), 3355-3360.
- Gemechu, A. T. (2016). Assessment of safety and quality of raw whole cow milk produced and marketed by smallholders in central highlands of Ethiopia. Food Sci Qual Manag, 49.
- Gemechu, T., Beyene, F., & Eshetu, M. (2014). Handling practices and microbial quality of Raw Cow’s milk produced and marketed in Shashemene Town, Southern Ethiopia. Int J Agric Soil Sci, 2, 153-62.
- Guetouache, M., Guessas, B., &Medjekal, S. (2014). Composition and nutritional value of raw milk. J Issues Biol Sci Pharm Res, 2350, 1588.
- Gutema, F. D., Solomon, H., Dawo, F., Tufa, T. B., & Ayana, D. (2019). Assessment of the effect of activatedlactoperoxidase system on keeping quality of raw cow milk under different climatic zones of Ethiopia. Dairy and Veterinary Science of Journal, 10(5).
- Guya, M. E., Adugna, M. M., &Mumed, Y. Y. (2019). Milk production, marketing and quality in meta district of eastern Hararghe Zone, Ethiopia. Journal of Agricultural Science, 11(5), 535.
- Haddadin, M. S., Ibrahim, S. A., & Robinson, R. K. (1996). Preservation of raw milk by activation of the natural lactoperoxidase systems. Food Control, 7(3), 149-152.
- Holsinger, V. H., Rajkowski, K. T., &Stabel, J. R. (1997). Milk pasteurisation and safety: a brief history and update. Revue scientifique et technique-Office international des epizooties, 16(2), 441-466.
- IDF 1988 Code Of Practices For Preservation Of Raw Milk By The Lactoperoxidase System. Bulletin Of The International Dairy Federation 234: 1-15.
- IDF. 1988. Code of practices for the preservation of raw milk by the lactoperoxidase system. Bulletin of the International Dairy Federation, 234: 1–15.
- ISO and IDF 2008. Milk and milk products sampling- guidance on sampling. 3rd ed.
- Isobe, N., Kubota, H., Yamasaki, A., & Yoshimura, Y. (2011). Lactoperoxidase activity in milk is correlated with somatic cell count in dairy cows. Journal of Dairy Science, 94(8), 3868-3874.
- Jayarao, B. M., Pillai, S. R., Sawant, A. A., Wolfgang, D. R., & Hegde, N. V. (2004). Guidelines for monitoring bulk tank milk somatic cell and bacterial counts. Journal of Dairy Science, 87(10), 3561-3573.
- Kamau, D. N., Doores, S., & Pruitt, K. M. (1990). Antibacterial activity of the lactoperoxidase system against Listeria monocytogenes and Staphylococcus aureus in milk. Journal of Food Protection, 53(12), 1010-1014.
- Kamau, D. N., Doores, S., & Pruitt, K. M. (1991). Activation of the lactoperoxidase system prior to pasteurization for shelf-life extension of milk. Milchwissenschaft (Germany, FR).
- Kameni, A., Imele, H., &Mbanya, N. J. (2002). An alternative heat treatment for milk pasteurization in Cameroon. International Journal of Dairy Technology, 55(1), 40-43.
- Kassa F, Z.Yilma, G. Assefa, T. Bekele, Y. Gojam R. Nebiyu and B Kassa 2013. Evaluation of Lactoperoxidase system as raw milk preservative at different storage temperature conditions in the central highlands of Ethiopia. Livestock Research Rural Development 25: 4.
- Kassa, F., Yilma, Z., Assefa, G., Bekele, T., Gojam, Y., Nebiyu, R., &Kassa, B. (2013). Evaluation of Lactoperoxidase system as raw milk preservative at different storage temperature conditions in the central highlands of Ethiopia. Development, 25(4).
- Keba, A., Rolon, M. L., Tamene, A., Dessie, K., Vipham, J., Kovac, J., &Zewdu, A. (2020). Review of the prevalence of foodborne pathogens in milk and dairy products in Ethiopia. International dairy journal, 109, 104762.
- Kelly, P., Woonton, B. W., & Smithers, G. W. (2009). Improving the sensory quality, shelf-life and functionality of milk. In Functional and speciality beverage technology (pp. 170-231). Woodhead Publishing.
- Kelly, P., Woonton, B. W., & Smithers, G. W. (2009). Improving the sensory quality, shelf-life and functionality of milk. In Functional and speciality beverage technology (pp. 170-231). Woodhead Publishing.
- Korhonen H Rintamaki O Antila M Tuori M and Poutiainen E 1977. A polyo mixture of molasses treatment beet pulpin the silage based diet of dairy cows. II the effect on lactoperoxidase and thiocyanate content of milk and the udder health. J. Sci. Agri. Society Finland. 49: 330.
- Korhonen, H. (1980). A new method for preserving raw milk: The lactoperoxidase antibacterial system. Revue Mondiale de Zootechnie (FAO)-Revista Mundial de Zootecnia (FAO).
- Korhonen, H. (1980). A new method for preserving raw milk: The lactoperoxidase antibacterial system. Revue Mondiale de Zootechnie (FAO)-Revista Mundial de Zootecnia (FAO).
- Kuma, A., Tolossa, D., &Abdisa, M. (2015). Assessment of raw milk microbial quality at different critical points of oromia to milk retail centers in Addis Ababa. Food Science and Quality Management, 38.
- Kumssa, G. (2018). Effect of milking procedure and handling on its quality. Journal of Dairy & Veterinary Sciences, 7(5), 1-6.
- Kurwijila, L. R. (2006). Hygienic milk handling, processing and marketing: reference guide for training and certification of small-scale milk traders in Eastern Africa.
- Kussendrager, K. D., & Van Hooijdonk, A. C. M. (2000). Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and applications. British Journal of Nutrition, 84(SUPPL. 1). [CrossRef]
- Lara-Aguilar, S., &Alcaine, S. D. (2019). Lactose oxidase: A novel activator of the lactoperoxidase system in milk for improved shelf life. Journal of Dairy Science, 102(3), 1933-1942.
- Lijalem, T., &Zereu, G. (2015). Hygienic milk handling and processing at farmer level in Wolaita Zone, Southern Ethiopia. Food Science and Quality Management www. iiste. org, 41.
- Lore, T. A., Omore, A. O., &Staal, S. J. (2005). Types, levels and causes of post-harvest milk and dairy losses in sub-Saharan Africa and the Near East: Phase two synthesis report.
- Lues, J. F., Rasephei, M. R., Venter, P., & Theron, M. M. (2006). Assessing food safety and associated food handling practices in street food vending. International journal of environmental health research, 16(5), 319-328.
- Mahari, A. T., &Yemane, H. (2016). Cow Milk Handling Practices and Factors Contributing to Quality Deterioration in Ethiopia. Food Sci. Qual. Man, 48, 14-17.
- Marks N. E., A. S. Grandison and M. J. Lewis. 2001. Challenge testing of the lactoperoxidase system in pasteurized milk. J. Appl. Microbio., 91:735-741. [CrossRef]
- Marks, N. E., Grandison, A. S., & Lewis, M. J. (2001). Challenge testing of the lactoperoxidase system in pasteurized milk. Journal of Applied Microbiology, 91(4), 735-741.
- Marks, N. E., Grandison, A. S., & Lewis, M. J. (2001). Challenge testing of the lactoperoxidase system in pasteurized milk. Journal of Applied Microbiology, 91(4), 735-741.
- Mpatswenumugabo, J. P. M., Bebora, L. C., Gitao, G. C., Mobegi, V. A., Iraguha, B., &Shumbusho, B. (2019). assessment of bacterial contamination and milk handling practices along the raw milk market chain in the north-western region of rwanda. African Journal of Microbiology Research, 13(29), 640-648.
- Muleta, T. (2016). Research article the microbiology of ethiopian milk and milk products : review*.
- Mwaikambo, J. J., Kurwijila, R. L., &Ryoba, R. Z. (2003). The effect of activation of lactoperoxide system (LPS) on the quality and shelf life of in-pouch pasteurised milk. Tanzania Journal of Agricultural Sciences, 6(1).
- Narkowicz, S., Jaszczak, E., Polkowska, Ż., Kiełbratowska, B., Kotłowska, A., &Namieśnik, J. (2018). Determination of thiocyanate as a biomarker of tobacco smoke constituents in selected biological materials of human origin. Biomedical Chromatography, 32(3), e4111.
- Ndambi, O. A., Kamga, P. B., Imele, H., Mendi, S. D., &Fonteh, F. A. (2008). Effects of milk preservation using the lactoperoxidase system on processed yoghurt and cheese quality. African Journal of Food, Agriculture, Nutrition and Development, 8(3), 358-374.
- Negash, F., Tadesse, E., &Woldu, T. (2012). Microbial quality and chemical composition of raw milk in the mid-rift Valley of Ethiopia. African Journal of Agricultural Research, 7(29), 4167-4170.Gaya, P.,.
- Nigussie, H., &Seifu, E. (2007). Effect of the lactoperoxidase system and container smoking on the microbial quality of cows’ milk produced in Kombolcha woreda, eastern Ethiopia. Livestock Research for Rural Development, 19(10), 157.
- Njage, K. M. P., &Wangoh, J. (2008). Impact of the lactoperoxidase system on activity of selected lactic starter cultures in camel milk. Food, 14, 70-74.
- O’Connor, C. B. (1994). Rural diary technology ILCA training manual. Addis Ababa Ethiopia: International lifestock Research Institute, 133..
- O'Lakes, L. (2010). dairy development for ethiopia. dairy value chains, end markets and food security: Cooperative Agreement 663-A-00-05-0043-00. Addis Ababa, Ethiopia.
- Özer, B. (2014). Natural Anti-Microbial Systems Lactoperoxidase and Lactoferrin.
- Özer, B., Grandison, A., Robinson, R., &Atamer, M. (2003). Effects of lactoperoxidase and hydrogen peroxide on rheological properties of yoghurt. Journal of Dairy Research, 70(2), 227-232.
- Pandey, G. S., &Voskuil, G. C. S. (2011). Manual on milk safety, quality and hygiene. Golden Valley agricultural Research Trust, Zambia, 52.
- Parveen, R., Kausar, R., Sameen, A., Khan, M. I., & Sana, N. (2016). Effect of activating lacto peroxidase system (LPS) on quality and storage stability of soft cheese. Journal of Biotechnology Biomaterials, 6(2), 224.
- Pokhrel, P., & Das, S. K. L. (2012). Study on the extension of shelf-life by activation of inherent lactoperoxidase system in raw cow milk. Journal of Food Science and Technology Nepal, 7, 57-60.
- Ponce, P. (2010). Lactoperoxidase system under tropical conditions: use, advantages and limitations in conservation of raw milk and potential applications. Revista de Salud Animal, 32(3), 146-146.
- Pruitt, K. M. (1991). The lactoperoxidase systems of bovine and human milk. Oxid. Enzymes Foods, 138-174.
- Pruitt, K. M. And Njage, D. N. 1991. the lactoperoxidase system of bovine and human milk. in: Robinson, D.S. And Eskin, N.A.M. (Eds.), Oxidative Enzymes In Foods (Pp. 133–174). London: Elsevier Applied Science.
- Radu-Rusu, R. M., Usturoi, M. G., Vacaru-Opriş, I., Usturoi, A., Cojocariu, C. L., Aioanei, N., & Radu-Rusu, C. G. (2013). Study on certain freshness and hygiene parameters of edible milk marketed by small size farmers in Iasi City. LucrăriStiintifice, SeriaZootehnie, 60, 160-164.
- Reiter, B. (1985). The biological significance of the non-immunoglobulin protective proteins in milk: lysozyme, lactoferrin, lactoperoxidase. Developments in Dairy Chemistry—3: Lactose and Minor Constituents, 281-336.
- Reiter, B., &Härnulv, G. (1984). Lactoperoxidase antibacterial system: natural occurrence, biological functions and practical applications. Journal of Food Protection, 47(9), 724-732.
- Reiter, B., &Perraudin, J. P. (1991). Lactoperoxidase: biological functions. Peroxidases in chemistry and biology, 1, 143-180.
- Robinson, R. K. (1995). Microbiological and technological aspects of milks fermented by bifidobacteria. Journal Of Dairy Research, 62(1), 151–187. [CrossRef]
- Sarkar S. 2015. Microbiological considerations: pasteurized milk. I Int. J. Dairy. Sci. 10: 206-218. [CrossRef]
- Schlorke, D., Atosuo, J., Flemmig, J., Lilius, E. M., &Arnhold, J. (2016). Impact of cyanogen iodide in killing of Escherichia coli by the lactoperoxidase-hydrogen peroxide-(pseudo) halide system. Free radical research, 50(12), 1287-1295.
- Schlorke, D., Atosuo, J., Flemmig, J., Lilius, E. M., &Arnhold, J. (2016). Impact of cyanogen iodide in killing of Escherichia coli by the lactoperoxidase-hydrogen peroxide-(pseudo) halide system. Free radical research, 50(12), 1287-1295.
- Seifu E., E. M. Buys and E. F. Donkin. 2005. Significance of the lactoperoxidase system in the dairy industry and its potential applications: a review. Trends in Food. Sci. Technol. 16: 137–154. [CrossRef]
- Seifu, E., Buys, E. M., & Donkin, E. F. (2003). Effect of the lactoperoxidase system on the activity of mesophilic cheese starter cultures in goat milk. International Dairy Journal, 13(12), 953-959.
- Seifu, E., Buys, E. M., & Donkin, E. F. (2004). Quality aspects of Gouda cheese made from goat milk preserved by the lactoperoxidase system. International Dairy Journal, 14(7), 581-589.
- Seifu, E., Buys, E. M., & Donkin, E. F. (2005). Significance of the lactoperoxidase system in the dairy industry and its potential applications: a review. Trends in Food Science & Technology, 16(4), 137-154.
- Sepúlveda, N., Muñoz, A., &Jara, R. (2003). Evaluacion de un activador del Sistema Lactoperoxidasaen leche sin refrigerarrecolectada de pequenosproductores. RevistaCientífica de la Facultad de CienciasVeterinarias, 13(1), 12-18.
- Shin, K., Hayasawa, H., &Lönnerdal, B. (2001). Inhibition of Escherichia coli respiratory enzymes by the lactoperoxidase-hydrogen peroxide-thiocyanate antimicrobial system. Journal of applied Microbiology, 90(4), 489-493.
- Silva, E., Oliveira, J., Silva, Y., Urbano, S., Sales, D., Moraes, E.,... & Anaya, K. (2020). Lactoperoxidase system in the dairy industry: Challenges and opportunities. Czech Journal of Food Sciences, 38(6), 337-346.
- Sisay, T., Alemayehu, K., & Haile, M. (2018). Handling and marketing of dairy products in and around Bahir Dar Milkshed Areas, Ethiopia. International Journal of Tropical Drylands, 2(2), 48-58.
- Sitamahalakshmi S. and P.R. Rao. 2019. Microbial load, microflora and quality of pasteurized milk. Int. J. Innov. Sci. Res. Technol.. 4: 7.
- Tamime, A. Y., Marshall, V. M., & Robinson, R. K. (1995). Microbiological and technological aspects of milks fermented by bifidobacteria. Journal of Dairy Research, 62(1), 151-187.
- Tassew, A., &Seifu, E. (2011). Microbial quality of raw cow’s milk collected from farmers and dairy cooperatives in Bahir Dar Zuria and Mecha district, Ethiopia. Agriculture and Biology Journal of North America, 2(1), 29-33.
- Tegegne, A., Gebremedhin, B., & Hoekstra, D. (2010). Livestock input supply and service provision in Ethiopia: Challenges and opportunities for market-oriented development. IPMS Working Paper.
- Tekliye, M., &Gizaw, M. (2017). Handling practices, evaluation of adulteration and microbial quality of raw cow milk around Bahir Dar, Ethiopia. Food Science and Quality Management, 61, 1-9.
- Tetra Pack. 1995. Dairy Processing Handbook. Tetra Pak Processing Systems AB S-221 86 Lund, Sweden. Pp: 442.
- Thomas, E. L., & Aune, T. M. (1978). Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infection and immunity, 20(2), 456-463.
- Thomas, E. L., & Aune, T. M. (1978). Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infection and immunity, 20(2), 456-463.
- Thornton, H. R. and E.G. Hastings. 1930. Studies on oxidation-reduction in milk: The Methylene Blue Reduction Test. J. Dairy Sci. 13, 221-245. Doi: 10.3168/jds.S0022-0302 (30)93520-5.
- Trujillo, A. J., Pozo, P. I., &Guamis, B. (2007). Effect of heat treatment on lactoperoxidase activity in caprine milk. Small Ruminant Research, 67(2-3), 243-246.
- USAID Feed the Future, 2014. Scaling up the adoption and use of agricultural technologies – synthesis report Global Learning and Evidence Exchange (GLEE) – Ethiopia and Thailand. Pp 1-24.
- van der Valk, O. M. C., &Tessema, A. (2010). The formal dairy chain of Addis Ababa; An analysis of the integration of small-scale farmers. LEI Wageningen UR.
- Welearegay, H., Yilma, Z., &Tekle-Giorgis, Y. (2012). Hygienic practices and microbiological quality of raw milk produced under different farm size in Hawassa, southern Ethiopia. Agricultural Research and Review, 1(4), 1132-142.
- Wijkstrom-Frei, C., El-Chemaly, S., Ali-Rachedi, R., Gerson, C., Cobas, M. A., Forteza, R., & Conner, G. E. (2003). Lactoperoxidase and human airway host defense. American journal of respiratory cell and molecular biology, 29(2), 206-212.
- Wijkstrom-Frei, C., El-Chemaly, S., Ali-Rachedi, R., Gerson, C., Cobas, M. A., Forteza, R.,... & Conner, G. E. (2003). Lactoperoxidase and human airway host defense. American journal of respiratory cell and molecular biology, 29(2), 206-212.
- Wolfson, L. M., & Sumner, S. S. (1993). Antibacterial activity of the lactoperoxidase system: a review. Journal of Food Protection, 56(10), 887-892.
- World Health Organization. (2006). Benefits and potential risks of the lactoperoxidase system of raw milk preservation: report of an FAO. World Health Organization.
- Yilma, Z. (2010). Quality factors that affect ethiopian formal milk business; experiences from selected dairy potential areas. Addis Ababa, Ethiopia. Netherlands Development Organization (SNV).
- Yilma, Z. (2012). Microbial properties of Ethiopian marketed milk and milk products and associated critical points of contamination: An epidemiological perspective. Epidemiology insights, 15, 298-322.
- Zapico, P., Gaya, P., Nuñez, M., & Medina, M. (1993). Goats' milk lactoperoxidase system against Listeria monocytogenes. Journal of food protection, 56(11), 988-990.
- Zebib, H., Abate, D., &Woldegiorgis, A. Z. (2022). Nutritional quality and adulterants of raw milk, pasteurized milk and cottage cheese collected along value chain from three regions of Ethiopia. Heliyon, 9,15922. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).