1. Introduction
Intradural extramedullary (IDEM) tumors account the 80% of all intraspinal tumors in adults. Schwannomas are the most common IDEM tumors (30% of cases), followed by meningiomas (25% of cases) [
1].
Schwannomas, which are considered benign tumors, are slow-growing lesions that commonly arise from the sensory dorsal roots of the cervical and lumbar spine with less frequent involvement of the thoracic region. They tend to develop an hourglass shape due to bony impression at the neural foramen during their growth and so they are called also dumb-bell tumors [2)] Meningiomas are generally well circumscribed and slow-growing lesions that occur most likely in the thoracic spine, less commonly in the cervical or lumbar spine, in a postero-lateral position [
2]. The first symptom of IDEM tumors in adults is often pain, especially in a recumbent position or at night. Neurological deficit develop over time once the spinal cord is compressed and depend on the location of the lesion. Due to the late onset and non-specific neurologic symptoms, the diagnosis of these tumors is often delayed [
3]. Fortunately, IDEM tumors are almost always benign lesions and gross total resection is therefore the treatment of choice with an excellent functional outcome and low recurrence rate [
4,
5,
6,
7].
The main goals of surgical excision of intraspinal tumors are to completely remove the lesion, to maintain spinal stability and to restore neurological functions. The traditional surgical approach includes total laminectomy extending to levels above and below the tumor through a midline skin incision and bilateral subperiosteal muscle dissection from the posterior spinal elements [
8]. This approach provides a wide working area, but it is associated with significant tissue trauma and it may results in greater postoperative pain, spinal instability and/or deformity with resulting persistent pain, especially after a multilevel laminectomy [
9,
10]. With the advent of concept of minimally invasive surgery, there has been an increasing interest in reducing the amount of bone and ligament removal during spinal surgery. These techniques have been applied successfully in the treatment of degenerative disease [
11,
12,
13,
14]. Recently, minimally invasive surgery with open unilateral hemilaminectomy has been used to resect spinal tumors. Unilateral hemilaminectomy approach for resection of spinal tumors results in reduced intraoperative blood loss and reduced postoperative pain, preservation of spinal stability with favourable clinical outcomes [
15,
16,
17,
18]. However, sufficient surgical indications have not yet been completed evaluated.
In this paper, we report the results of a surgical series of patients affected by spinal schwannomas and meningiomas who underwent surgical resection by laminectomy or hemilaminectomy comparing the clinical results between the two approaches.
2. Materials and Methods
We retrospectively reviewed our surgical series of 41 consecutive patients with IDEM tumors who underwent surgical resection via total laminectomy or via unilateral hemilaminectomy in our Institute between January 2013 and January 2023. In our study population we included patients with spinal schwannoma and meningioma for which surgery was indicated for the treatment of the symptoms (neurologic deficit, intractable pain and numbness). The diagnosis was obtained by contrast-enhanced magnetic resonance imaging (MR) and it was confirmed by histopathological examination.
Baseline medical data were collected. Clinical symptoms were reviewed using patients’ medical charts. Preoperative and postoperative neurological status were assessed by the American Spinal Injury Association (ASIA) Impairment Scale [
19], that is classified into grades A-E. Grades A to C are defined as a severe neurological disability; grade D or E is defined as a mild neurological disability. All patients underwent spinal MR preoperatively and during follow up.
Tumor size was measured on contrast-enhanced T1-weighted MR imaging using the widest diameter in three planes and tumor volume was calculated (ellipsoid methods volume= D1xD2xD3/2). Operative and perioperative details recorded included the extent of resection, operative time, amount of blood loss, need for transfusion, length of postoperative bed rest and hospitalization. These data were obtained from surgical and medical records. We also collected 1-month postoperative back pain through Numeric Pain Rating Scale (NRS score) [
20]. Additionally, postoperative complications were recorded.
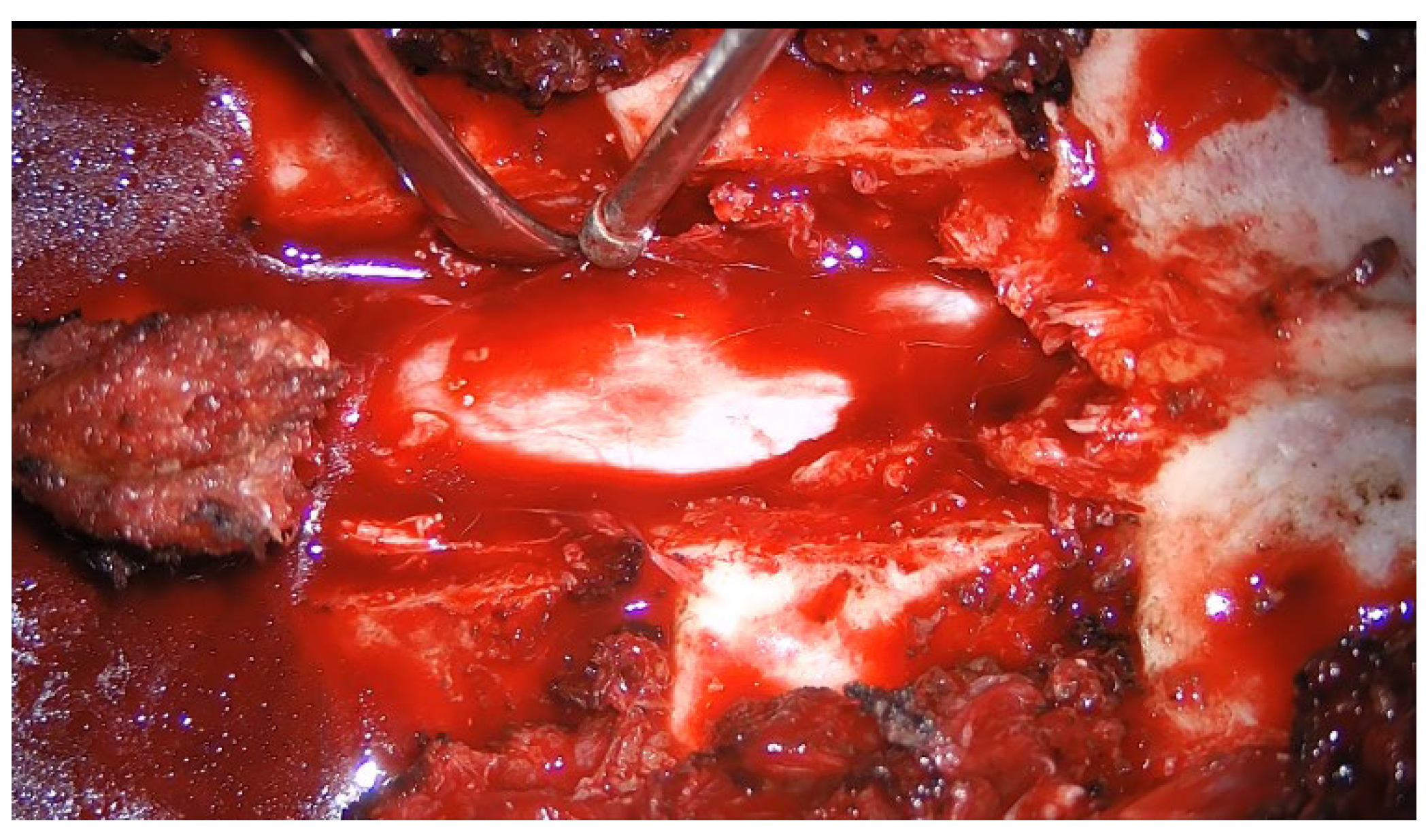
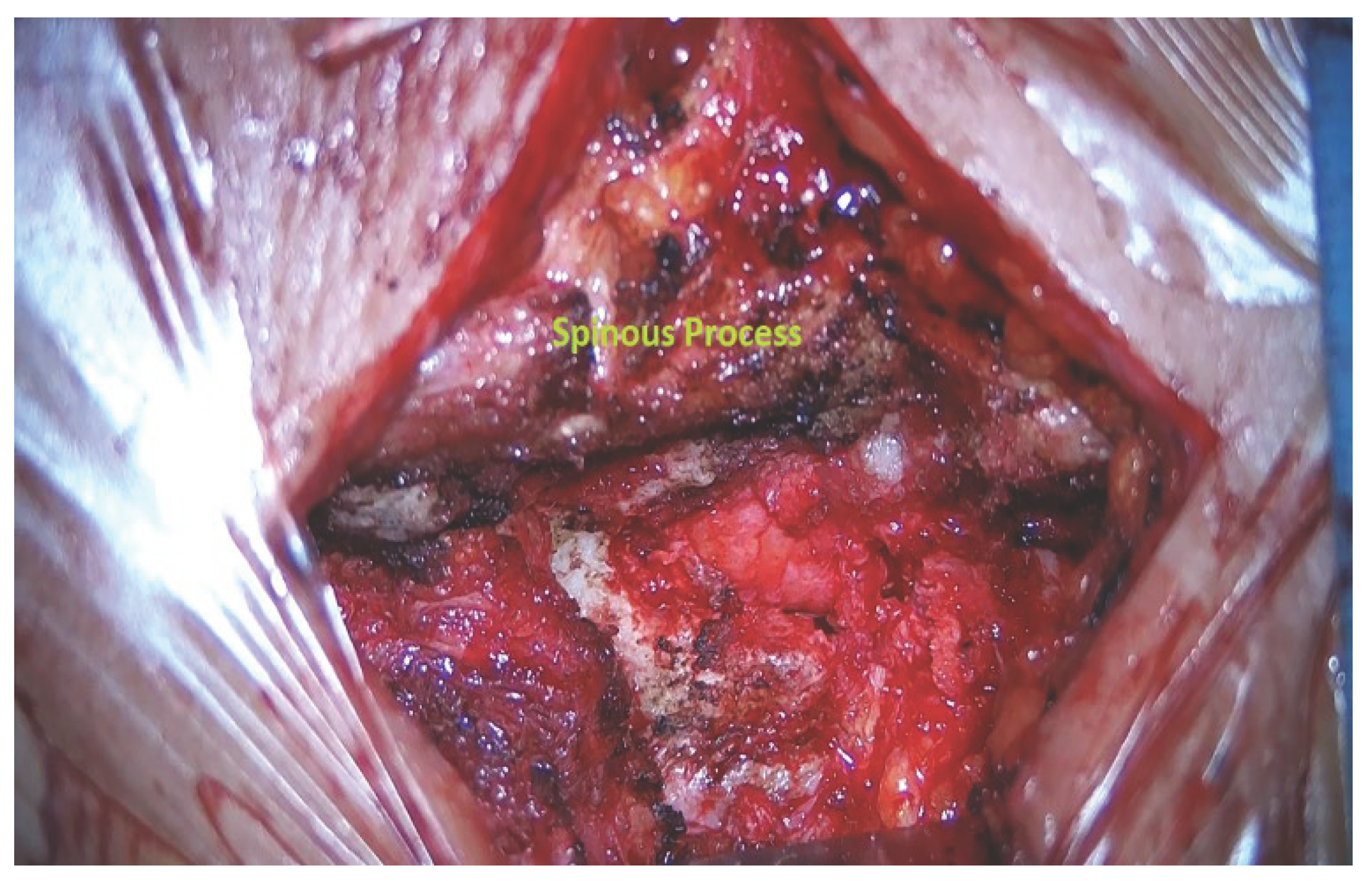
All surgeries were performed by an equipe of senior neurosurgeons using standard microsurgical technique. The patient was placed in the prone position under general anesthesia. A midline skin incision was centered on the radiograph marker positioned in the spinous process over the tumor. In case of total laminectomy (
Figure 1), dissection of the bilateral subperiosteal muscles was performed to levels above and below the tumor. In case of unilateral hemilaminectomy (
Figure 2), paravertebral muscles of the tumor side were dissected from the spinous process to the corresponding lamina subperiosteally. In every case, next a muscle-splitting dissection was carried out down to the lamina and sequential dilators were placed. Hemilaminectomy was carried out under intraoperative microscope using a high-speed pneumatic drill or piezoelectric device, while the soft tissues and ligamentum flavum were removed with a Kerrison rongeurs. In some patients, the exposure was completed with a partial facetectomy. Once exposed the dura mater, it was opened longitudinally at the midline in case of laminectomy approach and paramedially in case of hemilaminectomy. After opening the dura, the tumor was exposed and removed microscopically either as a whole or in pieces, depending on the consistency and the size of the lesion. The tumor was decompressed internally using the Cavitron ultrasonic aspirator and finally the capsula was dissected from the surrounding tissues. For meningiomas, the dura was partially removed or the attachment site was coagulated. For schwannomas, the tumor was excised by debulking of the mass with respect of the integrity nerve root. The dura mater was closed primarily with a running suture in a watertight fashion with 4-0 silk wire. Fibrin sealant and fat graft were applied to reinforce the closure. Muscularis fascia and skin were sutured in a standard fashion. Instrumentation of spinal instability was never required.
Statistical analysis: Values are presented as mean ± standard deviation (SD) for continuous variables and as numbers and percentages for categorical variables. Student’s t test, Chi-squared test, Mann-Whitney test and binary logistic regression analysis were used, as appropriate. Results were considered statistically significant for p value ≤0.05. Statistical analysis was performed by the software package SPSS, version 25.0 (Chicago, IL, USA).
3. Results
We included 41 consecutive patients affected by spinal schwannoma and meningioma who underwent surgical resection with a follow up of maximum 10 years and minimum 1 year. Our study population consisted of 17 males and 24 females with a mean age of 62.5±14.6 years (range from 33 to 87 years) (
Table 1). The clinical onset and the type of the tumors are reported in
Table 1. In our surgical series of schwannomas, the average volume of schwannomas was 3,6 ±2,7 cm3, whereas the average volume of meningiomas was 1.1 ±0.5 cm3. We achieved the total excision in all patients. Compared with preoperative neurological dysfunction, after surgery there was a significant improvement in the neurological status (
Table 1). The total laminectomy was performed in 24 out of 41 patients (59%) while unilateral hemilaminectomy was performed in 17 out of 41 patients (41%) (
Table 1); hemilaminectomy was performed in 13 patients out of 24 affected by schwannoma and 4 patients out of 17 affected by meningiomas (
Table 2). In our surgical series, postoperative complications were observed in 9 (21.9%) patients out of 41 and included CSF leakage in 4 cases (9.7%) with the need of spinal drainage for 1 week, wound infection in 4 cases (9.7%) with healing after antibiotic therapy. We also reported epidural hematoma in one patients that required re-operation. No deaths were recorded in the series.
The operative time was longer for resection of schwannomas than for resection of meningiomas (209.6±79.0 vs 132.4±46.6 minutes, respectively; p=0.001), as well as the intraoperative blood loss was higher in the schwannoma group (1.6 vs 1.5 g/dl, respectively; p=0.000). We noticed no differences between the two groups of tumors regard to postoperative complications, hospital stay and postoperative length bed rest (
Table 2). In our surgical series, compared to total laminectomy, unilateral hemilaminectomy was associated with shorter length bed rest (77.8±33.3 vs 46.5±27.3 hours, respectively; p=0.003) and so with shorter hospital stay (11.9±4.8 vs 8.9±2.9 days, respectively; p=0.028). At 1month-follow up, the means NRS score was 4.29±1.9 in the group of laminectomy and 2.8±1.8 in the group of unilateral hemilaminectomy (p=0.02). Operative time was comparable between the total laminectomy and the unilateral hemilaminectomy groups (170.7±70.0 vs 187.3±87.4 minutes, respectively; p=0.503).
We found no differences between the two approaches groups in the intraoperative blood loss and incidence of postoperative complications (
Table 3).
4. Discussion
Surgery is the first choice treatment for spinal schwannoma and meningioma. The surgical goals are gross total tumor resection and the maintenance or restore neurological functions. Laminectomy offers a large exposure of the dorsal surface of the spinal cord and nerve roots with wide working area. However, total laminectomy is associated with tissue trauma and high intraoperative blood loss, prolonged postoperative bed rest and hospitalization and furthermore, this conventional approach is a very invasive and extensive procedure that may result in gradually increasing spinal instability or deformity [
16,
21,
22,
23]. According to the “three column” concept of the spine proposed by Denis in 1983 [
24], the preservation of the integrity of the posterior column muscles and ligaments is of great importance for the spinal stability and the sagittal balance [
23]. As described by Ogden et al., there is a strong correlation between the overall extent of removal of the posterior elements and the mobility and instability of the vertebrae during axial loading [
23]. The rationale for unilateral hemilaminectomy, which is less invasive, is to preserve the supraspinous and interspinous ligaments, the paravertebral muscle of one side and posterior bony elements as much as possible [
25].
In this approach, in fact, the aim is to remove just a sufficient amount of bone, which could even mean removing the lower part of the upper lamina and the upper part of the lower lamina to achieve the dural exposure targeted.
For this reason, unilateral hemilaminectomy has more benefits with regard to postoperative spinal stability comparing with total laminectomy [
18,
26,
27]. Nowadays hemilaminectomy is widely used in spinal degenerative diseases but Yasargil et al., with their experience in microneurosurgery, recommended hemilaminectomy for nearly any type of intradural tumors [
29]. Literature documents that this technique is associated with reduced postoperative pain, low intraoperative blood loss, earlier mobilization and shorter hospital stays [
17,
25,
26,
28]. Furthermore several studies [
15,
16,
17,
18,
26,
27,
28] reported that hemilaminectomy could be applied to spinal tumors without significant adverse effects and that it was even considered superior to the laminectomy in intradural extramedullary tumors. Some Authors have demonstrated that hemilaminectomy could also performed for the resection of partial intramedullary tumors with favourable results [
29,
30,
31]. Sun et al. described a potential application of the hemilaminectomy in all lateral intradural lesions, whether located ventrally or laterally [
32].
In our retrospective analysis, 24 spinal schwannomas with average tumor volume of 3,6 ±2,7 cm3 and 4 meningiomas with average tumor volume of 1,1 ±0,5 cm3 were totally resected by unilateral hemilaminectomy with favourable outcomes. Compared to total laminectomy, we documented that unilateral hemilaminectomy had shorter hospital stays, reduced length of postoperative bed rest and less postoperative pain with statistically significance. Our results, in line with the results of previous studies [
16,
17,
18,
25,
26,
27,
28,
31,
32,
33,
34], demonstrated that this approach is useful and safe also for the resection of large spinal meningioma and schwannoma. In our study we identified unilateral hemilaminectomy as safe and efficacy approach for also large tumors, suggesting that the size of the tumor is not a contraindication for this approach, as reported by Yeo et al. [
16]. According to Yeo et al. [
16], unilateral hemilaminectomy combined with microsurgical technique provides sufficient space for the resection of benign spinal cord tumors in various sizes. We analysed clinical and operative features of our surgical series of 24 spinal schwannomas and 17 meningiomas. Based on our findings, females were affected more often by meningiomas and these were located mostly frequent in thoracic spine, in accordance with the literature [
1,
2]. In our surgical series, schwannomas have greater volume at diagnosis. This was due to the location of all meningiomas in the dorsal region where the diameter of the spinal canal is narrow respect to the lumbar spine. For this reason, meningiomas were diagnosed early respect to schwannomas, that was localized more frequently in the lumbar spine in a wide spinal canal. One possible disadvantage of unilateral hemilaminectomy for large tumors is the narrow surgical corridor formed by the spinous processes and ipsilateral facet joint with risks of dural and nerve root damage or incomplete tumor removal. However, in our experience and in accordance with some Authors [
16,
18,
26,
27,
28,
29,
33,
34], partial facetectomy, undercutt of the spinous process base, oblique tilting of the operating table and ipsilateral dural flap fixed to the muscle or fascia near the facet joint allow to visualize controlateral side of the spinal cord and to remove completely the tumor.
With this approach we create a wide working corridor for the total excision of the tumor, even large ones, with the assistence of the microscope.
Recently, Alvarez-Crespo et al. reported that the average operative time for resection of spinal schwannomas was 293 minutes with the average intraoperative blood loss of 451.88 ml [
35]. In our surgical series, the operative time was 209.6±79 minutes with less intraoperative blood loss of 160 ml. These findings suggest that the surgical management of spinal schwannoma could be challenging because of its extensive vascularity causing significant intraoperative blood loss. Therefore, we suggest that unilateral hemilaminectomy should be used as a suitable option in spinal schwannomas removal, since one of the major benefit of this approach is reducing intraoperative blood less.
In this study we found that unilateral hemilaminectomy approach had at least equivalent or longer operative time compared to total laminectomy but may depends on neurosurgeons’ skill. Therefore, we suggest that unilateral hemilaminectomy should be used as a suitable option in spinal meningiomas and schwannomas removal, since one of the major benefit of this approach is the control of intraoperative blood loss that in case of schwannomas is mandatory.
The limitations of this study is the retrospective analysis and the small number of patients with the need of further studies.
However, our study confirms that unilateral hemilaminectomy approach for resection of spinal schwannoma and meningioma offers several advantages compared to standard total laminectomy, including shorter postoperative bed rest, shorter hospital stays and reduced postoperative pain. Furthermore in case of infection of the surgical site the use of the VAC therapy allowed the healing in a shorter time [
36]. It is mandatory that neurosurgeon should have adequate experience with unilateral hemilaminectomy approach prior to attempting resection of spinal tumors in order to remove these lesions totally without spinal cord and nerve roots injury. More extensive randomized and prospective trials are necessary to elucidate the role of unilateral hemilaminectomy in the surgical management of spinal tumors.
5. Conclusions
Our study documents that unilateral hemilaminectomy approach for the resection of spinal schwannoma and meningioma offers several advantages compared to standard total laminectomy, including shorter postoperative bed rest, shorter hospital stays and less postoperative pain, allowing adequate vision of the surgical area and control of intra-operative bleeding. It is mandatory that neurosurgeon have adequate experience with unilateral hemilaminectomy approach in order to totally remove these tumoral lesions without injury of the spinal cord and nerve roots. More extensive randomized and prospective trials are necessary to elucidate the role of unilateral hemilaminectomy in the surgical management of spinal tumor.
The study was conducted in accordance with the Declaration of Helsinki and Ethical approval was waived by our local ethics committee in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Author Contributions
Conceptualization, Serena Vittoria Lisi and Mauro Dobran and Fabiola Cappella; methodology, Fabiola Cappella; software, Andrea Mattioli; validation, Serena Vittoria Lisi, Mauro Dobran; formal analysis, Mario Chiapponi; investigation, Elena Bianchi; resources, Mario Chiapponi; data curation, Denis Aiudi; writing—original draft preparation, Alessandro Di Rienzo; writing—review and editing, Serena Vittoria Lisi; visualization, Denis Aiudi; supervision, Mauro Dobran; project administration, Mauro Dobran; funding acquisition, Fabiola Cappella. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent for this publication was not due because it is a retrospective study with anonymous patients.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abul-Kasim, K.; Thurnher, M.M.; McKeever, P.; Sundgren, P.C. Intradural spinal tumors: current classification and MRI features. Neuroradiology 2007, 50, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Ottenhausen, M.; Ntoulias, G.; Bodhinayake, I.; Ruppert, F.-H.; Schreiber, S.; Förschler, A.; Boockvar, J.A.; Jödicke, A. Intradural spinal tumors in adults—update on management and outcome. Neurosurg. Rev. 2018, 42, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Tonn JC, Grossman SA, Rutka JT, Westphal M. Neuro-oncology of CNS tumors. Springer, Berlin Heidelberg City 2006.
- Setzer M, Vatter H, Marquardt G, Seifert V, Vrionis FD. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus 2007, 23, E14.
- Gottfried, O.N.; Gluf, W.; Quinones-Hinojosa, A.; Kan, P.; Schmidt, M.H. Spinal meningiomas: surgical management and outcome. Neurosurg. Focus 2003, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Lenzi J, Anichini G, Landi A et al. Spinal nerves schwannomas: experience on 376 cases-historic overview on how clinical, radiological and surgical practices have changed over a course of 60 years. Neurol Res Int. 2017 (1):3568359-3568312.
- Ozawa H, Kokubun S, Aizawa T, Hoshikawa T, Kawahara C. Spinal dumb-bell tumors: an analysis of a series of 118 cases. J Neurosurg Spine 2007, 7, 587–59.
- Vecil, G.G.; McCutcheon, I.E.; Mendel, E. Extended lateral parascapular approach for resection of a giant multi-compartment thoracic schwannoma. Acta Neurochir. 2008, 150, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Bresnahan L, Ogden AT, Natarajan RN, Fessler RG. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine 2009, 34, 17–23.
- Papagelopoulos, P.J.; Peterson, H.A.; Ebersold, M.J.; Emmanuel, R.P.; Choudhury, S.N.; Quast, L.M.R. Spinal Column Deformity and Instability After Lumbar or Thoracolumbar Laminectomy for Intraspinal Tumors in Children and Young Adults. Spine 1997, 22, 442–451. [Google Scholar] [CrossRef]
- Iacoangeli, M.; Nasi, D.; Colasanti, R.; Pan, B.; Re, M.; Di Rienzo, A.; di Somma, L.; Dobran, M.; Specchia, N.; Scerrati, M. Endoscopic Endonasal Odontoidectomy with Anterior C1 Arch Preservation in Rheumatoid Arthritis: Long-Term Follow-Up and Further Technical Improvement by Anterior Endoscopic C1-C2 Screw Fixation and Fusion. World Neurosurg. 2017, 107, 820–829. [Google Scholar] [CrossRef]
- Fessler, R.G.; Khoo, L.T. Minimally Invasive Cervical Microendoscopic Foraminotomy: An Initial Clinical Experience. Neurosurgery 2002, 51, S2–37. [Google Scholar] [CrossRef]
- Feng C, Ting Z, Gang G, Shengqiang D, Yunxing S, Lijun L, Genle Z, Bin C, Xiaojian W, Chen Yu. Comparison of the minimally invasive and conventional open surgery approach in the treatment of lumbar stenosis: a systematic review and a meta-analysis. Ann Acad Med Singap 2017, 46, 124–137.
- Phan K and Mobbs, RJ. Minimally invasive versus open laminectomy for lumbar stenosis: a systematic review and meta-analysis. Spine 2016, 41, E91–100. [Google Scholar] [CrossRef]
- Goodarzi A, Clouse J, Capizzano T, Kim KD, Panchal R. The optimal surgical approach to intradural spinal tumors: laminectomy or hemilaminectomy? Cureus 2020, 12, e7084.
- Yeo, D.K.; Bin Im, S.; Park, K.W.; Shin, D.S.; Kim, B.T.; Shin, W.H. Profiles of Spinal Cord Tumors Removed through a Unilateral Hemilaminectomy. J. Korean Neurosurg. Soc. 2011, 50, 195–200. [Google Scholar] [CrossRef]
- Pompili, A.; Caroli, F.; Crispo, F.; Giovannetti, M.; Raus, L.; Vidiri, A.; Telera, S. Unilateral Laminectomy Approach for the Removal of Spinal Meningiomas and Schwannomas: Impact on Pain, Spinal Stability, and Neurologic Results. World Neurosurg. 2015, 85, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Zhou, Y.; Yao, D.; Zhang, F.; Wang, X.; Jiang, X.; Xiong, N.; Zhao, H. Efficacy of unilateral hemilaminectomy for intraspinal tumor resection: a systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 984–999. [Google Scholar] [CrossRef]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2016, 475, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement Properties of Visual Analogue Scale, Numeric Rating Scale and Pain Severity Subscale of the Brief Pain Inventory in patients with low back pain: a systematic review. The Journal of Pain 2019, 3, 245–263.
- Panjabi, M.M. Clinical spinal instability and low back pain. J. Electromyogr. Kinesiol. 2003, 13, 371–379. [Google Scholar] [CrossRef]
- Yasuoka, S.; Peterson, H.A.; MacCarty, C.S. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J. Neurosurg. 1982, 57, 441–445. [Google Scholar] [CrossRef]
- Ogden, A.T.; Bresnahan, L.; Smith, J.S.; Natarajan, R.; Fessler, R.G. Biomechanical comparison of traditional and minimally invasive intradural exposures using finite element analysis. Clin. Biomech. 2009, 24, 143–147. [Google Scholar] [CrossRef]
- Denis, F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine 1983, 8, 817–831. [Google Scholar] [CrossRef]
- Koch Wiewrodt D, Wagner W, Perneczky A. Unilateral multilevel interlaminar fenestration instead of laminectomy or hemilaminectomy: an alternative surgical approach to instraspinal space occupying lesions. J Neurosurg Spine. 2007, 6, 485–492.
- Mannion, R.J.; Nowitzke, A.M.; Efendy, J.; Wood, M.J. Safety and Efficacy of Intradural Extramedullary Spinal Tumor Removal Using a Minimally Invasive Approach. Neurosurg. 2011, 68, 208–216. [Google Scholar] [CrossRef]
- Turel, M.K.; D’souza, W.P.; Rajshekhar, V. Hemilaminectomy approach for intradural extramedullary spinal tumors: an analysis of 164 patients. Neurosurg. Focus 2015, 39, E9. [Google Scholar] [CrossRef]
- Sim, J.-E.; Noh, S.-J.; Song, Y.-J.; Kim, H.-D. Removal of Intradural-Extramedullary Spinal Cord Tumors with Unilateral Limited Laminectomy. J. Korean Neurosurg. Soc. 2008, 43, 232–236. [Google Scholar] [CrossRef]
- Yasargil MG, Tranmer BI, Adamson TE, Roth P. Unilateral partial hemi-laminectomy for the removal of extra- and intramedullary tumors and AVMs. Adv Tech Stand Neurosurg 1991, 18, 113–132.
- Chiou SM, Eggert HR, Laborde G, Seeger W. Microsurgical unilateral approaches for spinal tumor surgery: eight years’ experience in 256 primary operated patients. ACta Neurochir 1989, 100, 127–133.
- Balak, N. Unilateral partial hemilaminectomy in the removal of a large spinal ependymoma. Spine J. 2008, 8, 1030–1036. [Google Scholar] [CrossRef]
- Sun, C.-X.; Meng, X.-L.; Xie, S.-N.; Yu, Y.; Yang, H.-J.; Wu, B. Unilateral hemilaminectomy for patients with intradural extramedullary tumors. J. Zhejiang Univ. Sci. B 2011, 12, 575–581. [Google Scholar] [CrossRef]
- Iacoangeli, M.; Gladi, M.; Rienzo, D.; Dobran, M.; Alvaro, L.; Nocchi, N.; Di Somma, L.G.M.; Colasanti, R.; Scerrati, M. Minimally invasive surgery for benign intradural extramedullary spinal meningiomas: experience of a single institution in a cohort of elderly patients and review of the literature. Clin. Interv. Aging 2012, 7, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Dobran, M.; Paracino, R.; Nasi, D.; Aiudi, D.; Capece, M.; Carrassi, E.; Lattanzi, S.; DI Rienzo, A.; Iacoangeli, M. Laminectomy versus Unilateral Hemilaminectomy for the Removal of Intraspinal Schwannoma: Experience of a Single Institution and Review of Literature. J. Neurol. Surg. Part A: Central Eur. Neurosurg. 2021, 82, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Crespo, D.J.; Conlon, M.; Kazim, S.F.; Skandalakis, G.P.; Bowers, C.A.; Chhabra, K.; Tarawneh, O.; Arbuiso, S.; Cole, K.L.; Dominguez, J.; et al. Clinical Characteristics and Surgical Outcomes of 2542 Patients with Spinal Schwannomas: A Systematic Review and Meta-Analysis. World Neurosurg. 2024, 182, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Dobran, M.; Mancini, F.; Nasi, D.; Scerrati, M. A case of deep infection after instrumentation in dorsal spinal surgery: the management with antibiotics and negative wound pressure without removal of fixation. BMJ Case Rep. Jul 28;2017:bcr2017220792. doi: 10.1136/bcr-2017-220792. PMID: 28756380; PMCID: PMC5623226. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).