Preprint
Review
Nicotinamide Mononucleotide: Deciphering Metabolic Complexities for Improved Health Outcomes
Altmetrics
Downloads
380
Views
184
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Alerts
Abstract
Nicotinamide mononucleotide (NMN), a key NAD+ precursor, exhibits distinct metabolic characteristics and potential health benefits. Recent investigations underscore the pivotal role of gut bacteria and specialized uptake mechanisms in NMN metabolism. While human trials demonstrate NMN's safety and promising health outcomes, challenges persist. Variability in results is likely influenced by lifestyle, health conditions, and individual genetic and gut microbiota differences. Understanding how these factors affect NMN metabolism is crucial for personalized approaches and maximizing NMN's potential. Advances in delivery systems show promise for enhancing NMN bioavailability, but further understanding of optimal NAD+ levels and reliable measures of NMN's effects is necessary. This comprehensive approach is key to optimizing NMN's impact on human health and longevity.
Keywords:
Subject: Biology and Life Sciences - Aging
1. Introduction
NAD+, short for nicotinamide adenine dinucleotide, is a coenzyme found within every living cell with vital roles in various fundamental biological processes including, but not limited to, energy metabolism, DNA repair, cellular communication, and enzymatic activation. Originally, NAD+ was identified for its role in enhancing fermentation in yeast, where researchers found evidence of a coenzyme whose presence was crucial for alcoholic fermentation [1]. Though this early research predates our current understanding of NAD+, it prompted diverse biological investigations into its role in cellular redox reactions [2], electron transfer [3], glycolysis [4] the Krebs cycle [5], and fatty acid β-oxidation [6].
Beyond its metabolic functions, NAD+ acts as a coenzyme for vital proteins including sirtuins [7], polyadenosine diphosphate-ribose polymerases (PARPs) [8], and CD38 [9,10], participating in molecular deacetylation [11], ribosylation, glycohydrolase synthesis [9], and NAD+-dependent signal transduction. These actions contribute to metabolic homeostasis and cell signaling, highlighting the continuous demand for NAD+ within the body. Unsurprisingly, the mechanism of NAD+ production varies slightly across species but ultimately aims to maintain a delicate balance between production and recycling to ensure proper cellular function. Emerging research now suggests a close link between NAD+ levels, aging, and an increased risk of age-related diseases, such as neurodegenerative disorders [12], cellular senescence [13], and cardiovascular complications [14]. An analysis of human skin samples revealed an age-related accumulation of oxidative DNA damage, increased lipid peroxidation, and a significant (~70%) decline in NAD+ levels [15]. Data collected on human livers indicate a 30% loss between the ages of 45-60+, and two independent MRI studies [16,17] of the brain revealed a 10-25% decline in NAD+ levels from adolescence to old age. These findings align with observations in cell and rodent models, reinforcing the importance of understanding NAD+ metabolism in aging and prompting its progression to human clinical trials to evaluate whether the effects of aging can indeed be reduced, or even reversed.
Increasing NAD+ levels is a logical first step to remediating the natural age-related decrease of the coenzyme, and this can be achieved in a facile manner through exogenous intake, healthy diet, and exercise [18]. B vitamins and tryptophan from dietary intake contribute to a pool of NAD+ precursors, but the majority of NAD+ is biosynthesized from internally generated and recycled precursors. In this context, nicotinamide mononucleotide (NMN), emerges as a promising candidate for NAD+ boosting interventions. As a direct precursor to NAD+, NMN readily converts within cells, potentially mitigating the age-related NAD+ decline. This potential has generated significant interest in its role as a putative anti-aging strategy, requiring rigorous scientific exploration to fully understand the safety, efficacy, and long-term consequences of NMN supplementation and the subsequent effects of NAD+ boosting in humans.
2. NAD+ Biosynthesis Pathways
Three independent pathways, meticulously controlled by unique sets of enzymes and precursors, ensure sufficient NAD+ levels within its specific compartments: cytoplasm, mitochondria, and nucleus [19] (Figure 1). These pathways maintain an intricate balance of NAD+ synthesis, usage, recycling, and regeneration.
Serving as the predominant mechanism for maintaining cellular NAD+ homeostasis, the salvage pathway efficiently recycles NAD+ precursors, primarily utilizing nicotinamide (NAM) generated by the enzymatic breakdown of NAD+ in various cellular processes [20]. Dietary sources of nicotinamide riboside (NR) and NMN can also contribute to this pathway. Within this pathway, NMN serves as the key intermediate. To initiate NAD+ production, NMN is reportedly transported into cells via the recently discovered Slc12a8 transporter [21]. Alternatively, NR enters cells through equilibrative nucleoside transporters (ENTs) and is converted into NMN by NR kinases (NRKs) in one step [22]. NMN is then efficiently converted to NAD+ by the enzyme NMN adenyltransferase (NMNAT). The cycle continues with NAD+-consuming enzymes, such as CD38 and sirtuins, which release NAM in the reaction. NAM phorphorylribotransferase (NAMPT), the rate-limiting enzyme in this pathway, then catalyzes the conversion of NAM back to NMN, perpetuating the salvage loop. This process enables swift NAD+ production and continuous replenishment of precursors, independent of external resources.
The two remaining NAD+ biosynthetic pathways rely on dietary precursors, which can be advantageous when salvageable precursors are limited. The Preiss-Handler mechanism begins with nicotinic acid (NA) - a form of vitamin B3 or niacin found in fish, poultry, nuts, grains, and vitamin supplements. In three steps, NA is transformed into NA mononucleotide (NAMN), followed by NA adenine dinucleotide (NAAD), and finally to NAD+ by nicotinate phosphoribosyl transferase (NAPRT), NMNAT, and glutamine-dependent NAD+ synthetase (NADS) respectively [23,24]. Similar to the Preiss-Handler pathway, the de novo pathway relies on dietary tryptophan [25] which enters cells via specific amino acid transporters, LAT-1 and hPAT4 [26,27]. The enzymes indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO) then convert tryptophan to N-Formylkynurenine. A series of five subsequent enzymatic reactions lead to the formation of quinolinic acid (QA). Finally, the enzyme QPRT catalyzes the conversion of QA into NAMN, which can then feed into the Preiss-Handler for NAD+ production. The liver is the primary site for NAD+ synthesis from tryptophan, as most cells lack the enzymes required for the de novo pathway [28]. Consequently, the majority of cellular tryptophan is metabolized to NAM within the liver, released into circulation, and subsequently taken up by peripheral cells for conversion to NAD+ via the salvage pathway [29].
Boosting cellular NAD+ levels can be achieved through supplementation with NAD+ precursors that directly participate in NAD+ synthesis, without the need for lengthy conversion pathways. As a direct precursor, NR is one such candidate that is readily converted to NMN by NRKs, quickly integrated into the salvage pathway, and is well tolerated in moderate doses [30,31]. Preclinical studies show promise of its efficacy. NR is reported to have a short elimination half-life and the effects of long-term administration are still under investigation [32]. One step closer to NAD+ in the salvage pathway is NMN, which has been shown to increase NAD+ levels in as little as 30 minutes and is safe during one year of chronic dosing in mice [33]. NMN also appears to be more stable in water, and when taken orally, is rapidly absorbed, and metabolized, with many studies showing increased NAD+ biosynthesis and the improvement of several age-related ailments [34]. In murine models, NMN is studied extensively and has displayed several benefits such as improved mitochondrial metabolism, insulin secretion, and ischemia, to name a few [35,36]. While translating pre-clinical findings to humans is always a challenge, successfully applying data from animal models could pave the way for groundbreaking treatments for age-related conditions, potentially impacting the lives of the aging population. To that end, clinical trials evaluating the safety and efficacy of NMN in humans are underway and many echo the positive effects seen in animal models. This data is crucial to establish NMN as a viable NAD+ boosting precursor and determine to what extent previous clinical data is applicable.
3. Impact of Metabolism on Supplementation
Supplementation with NAD+ precursors offer a promising therapeutic approach to address declining NAD+ levels. However, a major challenge lies in the limited bioavailability of orally administered NAD+ precursors due to extensive metabolism in the gastrointestinal (GI) tract and liver.
3.1. Gastrointestinal Tract
Recent years have witnessed a surge in research exploring the impact of gut bacteria on NAD+ precursor metabolism and the complexities of cellular uptake mechanisms. Significant progress has been made, but interesting questions remain and warrant further investigation.
3.1.1. Interaction with Gut Microbiome
NAD+ precursors can be categorized into two chemical groups: amidated (NR, NMNAD+ precursors can be categorized into two chemical groups: amidated (NR, NMN, NAM) and deamidated (NA, NAMN, NAAD). Traditionally, these groups were thought to follow distinct biosynthetic routes. Amidated precursors were known to follow the salvage pathway (NR → NMN → NAD+), while deamidated precursors fueled the Preiss-Handler pathway (NA → NAMN → NAAD → NAD+). However, this paradigm shifted with the observation of increased NAAD (a deamidated intermediate) in blood cells after NR (an amidated precursor) supplementation [37]. This unexpected finding hinted at an interplay between the pathways, potentially involving previously unrecognized enzymatic activities in mammals for NAD+ production. Prior research identified a gut bacterial enzyme, PncC, capable of converting NMN into NAMN (a deamidated precursor) for NAD+ synthesis via the Preiss-Handler pathway [38]. This suggested a potential role for gut bacteria in NAD+ metabolism and could explain the rise in NAAD observed after NR supplementation.
Subsequent studies confirmed the crucial role of the gut microbiome in bridging the amidated and deamidated NAD+ pathways. In mice, orally administered NAM was deamidated by the microbial nicotinamidase PncA, generating NA, nicotinic acid riboside (NAR), and NAAD [39]. This microbial deamidation process was important for NAD+ synthesis in the colon, liver, and kidney. Similarly, another study showed that gut bacteria facilitated the deamidation of orally administered NMN, yielding the metabolites NAR and NAMN (in the gut and liver), and NAAD (in the liver) for incorporation into NAD+ via the de novo pathway [40]. In germ-free mice, NMN escaped microbial deamidation and instead fueled the salvage pathway, leading to elevated levels of amidated metabolites, NR and NMN.
Surprisingly, antibiotic treatment doubled gut NAD+ metabolite levels (including NMN, NR, NAD+, and NAM) in mice, even without NMN supplementation. This finding suggests potential competition by gut microbiota for both dietary and endogenous NAD+ sources. Further highlighting the complexity of NAD+ metabolism, Yaku et al. revealed a two-step process in NR utilization. Initially, direct NR uptake occurred in the small intestine for up to an hour, driving NAD+ synthesis through the classic salvage pathway even in the presence of gut bacteria [41]. Following this, the enzyme bone marrow stromal cell antigen 1 (BST1) hydrolyzed NR to NAM, which was then metabolized to NA by gut microbiota, fueling the Preiss-Handler pathway and becoming the major driver of NAD+ production. They also found that BST1 transformed NR into NAR through a base-exchange reaction using NA and NAM, providing another connection between amidated and deamidated precursors.
A key finding of these studies is that orally administered NR, NMN, and NAM predominantly undergo degradation in a process dependent on gut microbiota, resulting in the formation of NAM or NA and downstream deamidated metabolites. Only a minor portion of NAD+ precursors delivered orally integrate into tissues without significant alteration (Figure 2).
3.1.2. Uptake Mechanisms
Two primary pathways are currently recognized for NMN entry into enterocytes: an indirect route involving conversion to NR, and a direct route mediated by a specific NMN transporter. However, the relative importance and specific contributions of each pathway have not been determined. Initially, the prevailing model suggested NMN relies solely on an NR-mediated pathway. Here, the extracellular enzyme CD73 converts NMN to NR, which is then imported by equilibrative nucleoside transporters (ENTs) and phosphorylated back into NMN by NRK1/2 enzymes within the cell. Studies support this pathway, demonstrating the requirement of NRKs for NMN to boost NAD+ levels in muscle and liver cells [22,42]. However, the slow processing time of this pathway, exceeding several hours in some studies, cannot account for rapid NMN absorption within the gut (2-3 minutes) and tissue uptake (10-30 minutes) observed in mice [33]. Additionally, robust NMN uptake in cells despite inhibition of CD73/ENT or NRK1 further suggests an alternative route exists [22,43]. The discovery of Slc12a8, a highly specific NMN transporter abundantly expressed in the gut, pancreas, liver, and white adipose tissues, provides a compelling mechanism for rapid NMN absorption and distribution. Studies demonstrate that Slc12a8 deletion in these organs significantly reduces NMN uptake and NAD+ levels, supporting its role in direct NMN transport [21,44].
A published response to the initial study challenged the validity of the Slc12a8 findings, citing potential limitations in the methods used. However, Grozio et al. defended their work, providing evidence supporting their optimized analytical methods [21,45]. Furthermore, tracer studies on NMN metabolism yield inconsistent results as some suggest significant NMN conversion to NR in the gut, with limited direct absorption [40,46]. In contrast, another study detected NMN in the intestine within 10 minutes of oral intake, supporting the role of Slc12a8 in direct transport [33]. Using advanced quantification techniques, a later study found minimal NMN-to-NR conversion in the gut shortly after oral NMN administration with NAM emerging as the main degradation product [44]. The observed discrepancies in NMN transport findings may be influenced by the timing of measurements. Studies reporting no direct NMN transport employed later measurements (2-4 hours), potentially overlooking key metabolic events in the early minutes following ingestion.
The relative contributions of NRK1/2 and Slc12a8 to NMN uptake mechanisms likely vary depending on time, cell type, tissue, and physiological conditions. This dynamic interplay was illustrated in septic mice, where NRK1/2 enzyme levels decreased significantly, while Slc12a8 expression remained stable [47]. Notably, NMN supplementation still effectively increased NAD+ levels despite NRK1/2 pathway suppression, underscoring the critical role of alternative, NR-independent uptake mechanisms such as Slc12a8. Tissue-specific expression patterns further contribute to this complexity. Organs with inherently low NRK1 activity, such as the heart and white adipose tissue, might predominantly rely on Slc12a8-mediated uptake for NAD+ production [22,48,49]. This could be due to differences in metabolic demands, regulatory factors, or unique cellular processes within these tissues, warranting further investigation.
3.2. Portal Delivery
After absorption in the intestine, NAD+ precursors flow to the liver via the portal vein. Analysis of portal blood at three hours after oral NAM administration in mice revealed the presence of NAMN, NAR, NA, NMN, and NAM. While the major deamidated NMN metabolites, NAMN and NA, were detected, their concentrations were significantly lower (100-400-fold) compared to NAM, the predominant circulating precursor [29,39]. Four hours after NR gavage, elevated levels of NA and NAR appeared in portal blood, indicating bacterial contribution to NR metabolism. These metabolites were absent in germ-free mice, highlighting the essential role of gut microbiota. Interestingly, germ-free mice also exhibited a diminished increase in circulating NAM, further emphasizing the microbiota's influence on NAD+ precursor processing [39].
3.3. Hepatic First-Pass Metabolism
A major challenge for oral NMN supplementation is its extensive first-pass metabolism in the liver. Studies across various dosages (50 mg/kg to 500 mg/kg) consistently show that nearly all ingested NMN is converted into NAM within the liver [29,40,46]. This minimizes the amount of intact NMN available for direct NAD+ synthesis in the liver and prevents it from reaching peripheral tissues, significantly impacting overall bioavailability.
Bypassing gut metabolism through intraperitoneal (IP) injection offers a different approach. In mice injected with radiolabeled NMN (500 mg/kg), the NMN reaches the liver first via the portal vein. While some newly generated NAD+ in the kidneys and small intestines showed incorporation of labeled NR (suggesting localized NMN conversion to NR before NAD+ synthesis), most tissues relied on NMN-derived NAM for NAD+ production. Minimal intact NMN utilization was observed in the kidney and white adipose tissue [46]. It's important to note that this study only assessed NMN incorporation at two and four hours, potentially missing early metabolic events. For example, a separate study using IP injection of NMN (500 mg/kg) observed that mice displayed a rapid uptake, with liver NMN levels surging 15-fold within just 15 minutes after injection, followed by a return to baseline by 30 minutes. This rapid rise and subsequent decline suggest swift NMN metabolism. Notably, NAD+ levels continued to rise steadily for 60 minutes, highlighting the ongoing utilization of NMN or its metabolites for NAD+ synthesis [50].
When administered intravenously (50 mg/kg), NMN bypasses enteric and hepatic metabolism, enabling a small portion of intact NMN to directly participate in NAD+ synthesis within the liver and kidneys. While intact incorporation of NMN was still limited, the observed differences highlight the crucial role of administration methods in maximizing NMN bioavailability [29].
3.4. Bloodstream Dynamics and Tissue Distribution
Accurately measuring NMN in the blood remains a challenge for researchers. Limitations in current methods and the lack of a gold standard leads to conflicting reports. Some studies detect NMN in plasma minutes after oral intake [33], while others report undetectable levels [22,51]. This vast discrepancy (ranging from undetectable to 90 µM) creates significant gaps in our understanding of NMN's absorption, distribution, and fate within the bloodstream [33,52]. Advancements have been made in analytical methods, such as the development of dimeLC-MS/MS. This technique recently confirmed rapid NMN absorption (within 5 minutes) following oral and IP administration [44]. However, standardized methodologies and rigorous quality control measures are needed for a more comprehensive understanding of NMN's pharmacokinetics.
While NMN did not directly cross the blood-brain barrier in mice after IV administration, its impact on brain NAD+ levels has proven to be substantial [29]. NMN administration (500 mg/kg) via IP injection significantly increased hippocampal NAD+ levels by 34-39% within just 15 minutes [53], with similar effects observed in the hypothalamus [54]. Even a lower dose, NMN (62.5 mg/kg) sustained hippocampal mitochondrial NAD+ levels for 24 hours [55]. Intravenous NMN also showed minimal muscle uptake [29]. However, a clinical trial providing oral NMN to prediabetic women revealed elevated levels of proteins associated with insulin sensitivity in muscle tissues. Although muscle NAD+ levels remained unchanged, elevated NAD+ metabolites suggested a potential increase in muscle NAD+ turnover [56].
The chosen administration route significantly impacts NMN's fate. NMN (500 mg/kg) administered via IP injection in mice effectively boosted NAD+ levels in the liver, kidney, white adipose tissue, pancreas, and heart, with the liver showing the most prominent increase. Oral gavage (500 mg/kg) yielded similar effects in the liver but showed less efficacy in all other tissues [46]. In a pilot study, the administration of 300mg IV NMN in adults not only safely elevated NAD+ levels but also uniquely reduced triglycerides. A triglyceride-lowering effect has not been observed with oral NMN, indicating route-specific metabolic variations [57,58].
4. Optimizing Bioavailability: Delivery Strategies
Oral drug delivery revolutionized healthcare by offering a convenient and well-tolerated route for treatment [59]. However, challenges such as instability, poor water solubility, and physiological barriers hinder the efficacy of some compounds. Gastric acid, proteolytic enzymes, and first-pass metabolism in the liver further reduce bioavailability [60,61]. Similar challenges exist with other administration routes like intramuscular and intravenous injections, highlighting the need for alternative delivery strategies [62].
Liposomal delivery systems represent a promising strategy to address limitations associated with oral administration. These biocompatible carriers, comprised of natural lipids, offer protection for encapsulated therapeutics from degradation within the gastrointestinal tract and enhance therapeutic outcomes. [62]. Notably, liposomal structures exhibit structural similarities to cells lining the small intestine. Recent advancements have facilitated the engineering of liposomes capable of fusing with M cells located within the small intestine, [63,64] allowing entry into the lymphatic system – bypassing the hepatic processing altogether [65,66,67].
Since their introduction in the 1960’s [68] liposomal formulations have been extensively studied as a viable solution to delivering chemotherapeutics [69,70], antibiotics [71,72], vaccines [73], nutraceuticals [74,75,76] and more [77,78]. For instance, injection of liposomal NR significantly increased peak levels of NR in both plasma and brains of healthy mice, at 2.76 and 2.93 times higher than conventional NR, respectively [79]. A clinical trial by Davis et al. further showed a nearly 50% increase in circulating vitamin C with liposomal encapsulation [80]. Telange et al. demonstrated significantly higher apigenin delivery over 24 hours with liposomes compared to a pure suspension [81]. Similarly, Ye et al. used liposomes to deliver NADH to mouse models, achieving a 25% increase in the cellular NAD+/NADH ratio and improved therapeutic efficacy in sepsis [82]. These and numerous other studies [83,84,85] highlight the remarkable versatility and efficiency of liposomes as drug carriers. This versatility stems from their ability to encapsulate both water-soluble and fat-soluble molecules, enhancing stability and bioavailability [86].
The journey of NMN in the body highlights the necessity for improved delivery methods. Its extensive metabolism in the gut and liver reduces bioavailability. However, NMN's inherent lipophilicity, due to its aromatic ring and phosphate group, makes it well-suited for encapsulation in liposomes [62,87]. A study using a nano-drug delivery system for NMN in mice demonstrated improved absorption and higher NMN levels within tissues [88]. This approach is further supported by research showing effective cellular uptake of NMN encapsulated in liposomes [89]. Liposomal delivery offers a promising strategy to maximize NMN's therapeutic potential. By protecting NMN from breakdown during digestion, liposomes potentially deliver it intact to target tissues where its effects can be fully realized.
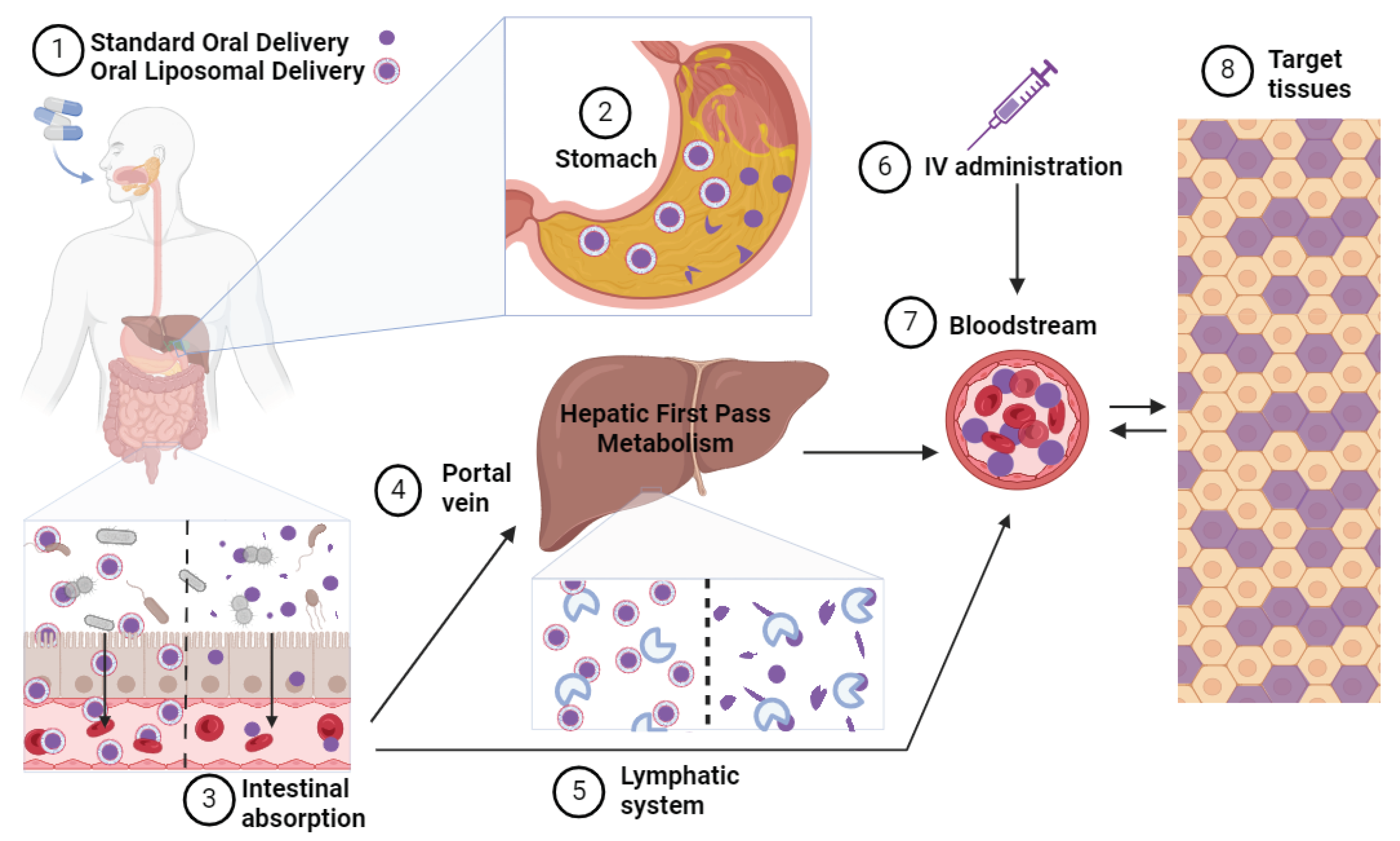
Figure 3.
NMN Administration Routes and Metabolic Processing. (1) Following ingestion of either a standard or liposomal formulation of NMN, (2) the capsule undergoes breakdown in the stomach, with liposomal formulations offering enhanced protection from degradation. (3) The standard NMN formulation undergoes extensive metabolism in the intestine by gut bacteria and intestinal enzymes before absorption into the enterocyte, while liposomes fuse with the intestinal lining, facilitating the absorption of encapsulated NMN. From the enterocyte, (4) standard NMN is absorbed into the portal vein and transported to the liver, where it undergoes first-pass metabolism and further enzymatic breakdown. (5) absorbed liposomes exit the enterocytes via the lymphatic system and enter the bloodstream, bypassing first-pass hepatic metabolism. (6) Intravenous administration of NMN directly introduces NMN into the bloodstream without undergoing processing in the intestine or liver. (7) Ultimately, NMN molecules enter the bloodstream for potential uptake into target tissues, albeit the amount reaching these tissues varies depending on the route of administration and the metabolic processing experienced en route.
Figure 3.
NMN Administration Routes and Metabolic Processing. (1) Following ingestion of either a standard or liposomal formulation of NMN, (2) the capsule undergoes breakdown in the stomach, with liposomal formulations offering enhanced protection from degradation. (3) The standard NMN formulation undergoes extensive metabolism in the intestine by gut bacteria and intestinal enzymes before absorption into the enterocyte, while liposomes fuse with the intestinal lining, facilitating the absorption of encapsulated NMN. From the enterocyte, (4) standard NMN is absorbed into the portal vein and transported to the liver, where it undergoes first-pass metabolism and further enzymatic breakdown. (5) absorbed liposomes exit the enterocytes via the lymphatic system and enter the bloodstream, bypassing first-pass hepatic metabolism. (6) Intravenous administration of NMN directly introduces NMN into the bloodstream without undergoing processing in the intestine or liver. (7) Ultimately, NMN molecules enter the bloodstream for potential uptake into target tissues, albeit the amount reaching these tissues varies depending on the route of administration and the metabolic processing experienced en route.
5. Functional Diversity of NAD+ Precursors
Despite sharing interconnected pathways, NAD+ precursors display diverse fates and functions, suggesting they are not simply interchangeable building blocks for NAD+ synthesis. Ongoing discoveries reveal their complexity, with each finding sparking new questions. Nevertheless, significant differences in precursor metabolism and bioavailability have been identified, highlighting their potential for tailored therapeutic interventions.
5.1. Efficacy and Therapeutic Applications of NAD+ Boosting Strategies
Although NAM is the principal contributor to basal NAD+ levels in mammals [29], its therapeutic efficacy is limited. Unlike NMN and NR, NAM can inhibit sirtuins, a class of proteins linked to NAD+ benefits. [90,91]. Additionally, NAM conversion to NAD+ has inherent limitations. The initial step requires NAMPT, an energy-dependent enzyme subject to feedback inhibition, limiting its ability to substantially elevate NAD+. Elevating dietary NAM also results in a corresponding increase in NAM methylation and excretion, thereby diminishing its NAD+-boosting efficacy [92].
Nicotinic acid (NA) stands apart from other NAD+ precursors due to its distinctive flushing effect, even at low doses of 50 mg [93]. This side effect arises from NA's direct activation of the GPR109A receptor, which is independent of processes associated with NAD+ biosynthesis [94,95]. Furthermore, NA exhibits lower efficacy in elevating NAD+ levels compared to NMN, NR, and NAM, rendering NA less enticing as an NAD+ booster [37].
NMN and NR are widely acknowledged as promising NAD+ boosters, raising NAD+ blood levels by 1.5 - 2.5-fold in clinical trials [57,96,97,98]. However, metabolic tracing studies reveal that NMN and NR are mostly metabolized to NA or NAM in the intestine [39,40]. While common logic suggests they would have similar effects and limitations as NA or NAM, they exhibit unique advantages despite their conversion. Notably, they lack the flushing side effects associated with nicotinic acid (NA) and do not appear to inhibit sirtuins, as seen with NAM [30,47,99,100,101]. Additionally, comparative studies showed that oral NR administration was more effective than NAM or NA at raising NAD+ levels in the liver of mice, and NMN was retained in the body longer than NAM [37,102]. Direct NMN transport to tissues could theoretically yield a more substantial increase in NAD+ production compared to NAM, as it bypasses the NAMPT-catalyzed reaction and is not subject to feedback inhibition.
Isotope labeling studies revealed an unexpected mechanism for NAD+ elevation. While NMN and NR are thought to directly elevate NAD+ as precursors, oral administration of labeled versions surprisingly led to an increase in unlabeled NAD+ metabolites [37,40]. This suggests indirect effects on endogenous NAD+ biosynthesis, potentially through activation of an unknown signaling pathway. Further investigation is needed to elucidate this novel mechanism and its contribution to the unique effects of NMN and NR.
NMN offers a potential advantage over NR due to its direct cellular uptake, independent of NRK1/2 enzymes. This tissue-specific requirement for NRK1/2 conversion could limit NR's efficacy in certain cell types, highlighting the potential therapeutic benefit of NMN in scenarios where NR might be less effective [22,48,49].
5.2. Physiological Context and Precursor Efficacy
The therapeutic efficacy of different precursors can diverge even within the same physiological context. For example, NR and NMN effectively promote hematopoiesis, but NA and NAM do not [103,104]. Oral supplementation with NR, but not NAM, restored NAD+ levels in the heart and improved cardiac function in a mouse model of cardiomyopathy [105]. While NMN supplementation has been shown to improve cardiac function in a model of Friedreich's ataxia cardiomyopathy [106], similar effects were not observed for NR [107].
Aging disrupts NAD+ balance, prompting the intestine to upregulate the NMN transporter Slc12a8. This regulatory mechanism forms a feedback loop, enabling NMN supplementation to effectively restore NAD+ levels [21]. Conversely, with age and disease, there can be a decline in NAMPT activity and expression, which plays a crucial role in the salvage pathway for NAD+ production by converting NAM to NMN [99,108,109]. This downstream bottleneck impedes the efficacy of NAM supplementation for NAD+ repletion under such conditions. In contrast, NMN bypasses the NAMPT step in the salvage pathway, demonstrating superior capability to elevate NAD+ levels and alleviate dysfunction associated with NAMPT deficiency [52,99,110].
NRK2, a key enzyme in the salvage pathway for NAD+ biosynthesis, exhibits stress-induced upregulation. This is observed in injured neurons, muscle subjected to injury or high-fat diets, and stressed cardiac tissue [111,112,113,114]. This suggests a protective mechanism, as NR or NMN supplementation effectively reduces disease severity in most conditions that exhibit stress-induced elevations in NRK2 [111,115,116].
6. Human Studies Discussion
Human trials exploring NMN supplementation have yielded promising results, consistently affirming its safety profile. However, the outcomes exhibit notable inconsistencies, underscoring areas for improvement and guiding future research endeavors towards resolving these discrepancies. Understanding the nuanced factors influencing NMN's efficacy—such as timing, dosage, and target populations—is essential to harness its full therapeutic potential.
6.1. Modulation of NAD+ Levels
Human trials measuring NMN's impact on serum NAD+ levels show mixed results, highlighting significant challenges in measuring this metabolite. While Yi et al. reported consistent dose-dependent increases in serum NAD+ and NMN, Katayoshi et al. found undetectable levels [117,118]. Further complicating the picture, Huang et al. observed a non-significant increase in serum NAD+/NADH, contradicting prior studies demonstrating efficacy at similar or lower doses [119].
Investigations into the impact of NMN supplementation on NAD+ concentrations reveal distinct NAD+ metabolic profiles within various blood cell fractions. Yamane et al. observed a significant increase in plasma NAD+ levels following daily NMN supplementation (250mg) for 3 months in healthy volunteers, peaking within the first month and remaining elevated throughout supplementation. Similarly, Okabe et al. noted a doubling of NAD+ levels after one month of NMN supplementation, with sustained elevation until supplementation cessation. Notably, Okabe et al. assessed NAD+ in whole blood, capturing both circulating and cellular NAD+, while Yamane et al. focused on plasma, crucial for systemic distribution [97,120]. This discrepancy elucidates markedly lower NAD+ concentrations in plasma reported by Yamane et al, approximately 100 times lower than in whole blood. Intriguingly, plasma NMN concentration was tenfold higher than in whole blood, highlighting the importance of sample type in NAD+ metabolism data interpretation.
Supplementation appears to influence NAD+ metabolism beyond simple conversion. Okabe et al. observed increases in NAMN, a deamidated metabolite of NMN, alongside elevated NAD+ [97]. Igarashi et al. also reported increased levels of NAD+ precursors (NR, NAR) after NMN supplementation, mirroring findings in animal studies [98].
NAD+ levels are dynamic, and influenced by diet, activity, and circadian rhythms. Studies suggest short-term fluctuations and a return to baseline within 2 hours after daytime NMN administration. Long-term monitoring indicates stable NAD+ levels except for NMN-induced increases and exercise-related elevations [121]. Intravenous NMN transiently elevates NAD+ and activates SIRT1/CD38 pathways [58].
Interestingly, NMN's effects on NAD+ levels appear tissue specific. Studies by Yoshino et al. and Pencina et al. observed increased NAD+ in PBMCs (blood cells) but not muscle tissue following NMN supplementation [56,122]. Furthermore, Qiu et al. showed reduced PBMC NAD+ in hypertensive patients, with NMN supplementation partially restoring these levels alongside lifestyle changes [123]. These findings highlight the complexity of NMN's influence on NAD+ metabolism and the need for further research on its tissue-specific effects and potential interactions with other interventions.
6.2. Sleep Regulation and Quality
Studies evaluating the effects of NMN on sleep using single doses (100-500mg) and short-term supplementation (250mg for 8 weeks) showed no significant changes in sleep measures [57,124]. However, a longer-term study (12 weeks) showed hints of potential benefit. Afternoon NMN intake (250mg) in this study reduced drowsiness in older adults, suggesting timing of the dose may play a role [125]. In a population with pre-existing sleep difficulties, 12 weeks of daily NMN supplementation (180mg) yielded statistically significant improvements in sleep quality, latency, and daytime function [126]. These findings suggest NMN's effect on sleep may depend on dose, duration, and potentially time of administration, with greater benefit observed in those with existing sleep issues.
6.3. Physical Performance
While some studies report improved gait speed, grip strength, and lower limb function [98,125], others show no significant effects on walking distance or in diabetic/obese populations [119,122,127]. Interestingly, NMN can improve muscle insulin sensitivity without impacting mitochondrial function or overall performance [56], suggesting a more nuanced effect on muscle health. Additionally, higher NMN doses (600-1200mg) might benefit trained athletes in terms of aerobic capacity [128]. Timing of administration (afternoon vs morning) may also influence outcomes [125]. Overall, the evidence for NMN's efficacy in enhancing physical performance remains inconclusive and warrants further investigation.
6.4. Cardiometabolic Health
Human trials investigating NMN's impact on cardiovascular health parameters yield mixed results. Several studies observed encouraging results, including reductions in weight, blood pressure, and cholesterol levels [118,122,123]. However, others have reported no significant changes in insulin sensitivity, lipid profiles, or vascular function [98,129]. Notably, positive effects on blood pressure and arterial stiffness were observed in hypertensive individuals and those with higher baseline glucose/BMI [118,123], suggesting potential benefits in specific subpopulations.
6.5. Glucose Metabolism and Regulation
In prediabetic women, 250mg/day NMN significantly enhanced muscle insulin sensitivity, but these effects were not observed systemically [56]. Additionally, a small trial in postmenopausal women receiving 300mg/day NMN for 8 weeks reported reduced HbA1c and increased adiponectin, suggesting potential anti-inflammatory and insulin-sensitizing properties [130]. Supplementation of NMN in healthy adults led to a significant increase in postprandial insulin levels after two months, suggesting enhanced glucose-stimulated insulin secretion [99]. However, the significance of this finding in healthy individuals remains uncertain [131].
6.6. Overall Well-Being and Quality of Life
A clinical trial showed significant improvements in subjective general health scores at day 60 for all NMN doses, with earlier improvements (day 30) in higher dose groups [117]. While another study did not reach statistical significance, it observed a trend towards improvement in the NMN group [129]. Postmenopausal women in a small NMN supplementation study (8 weeks) reported subjective improvements in allergies, joint pain, overall well-being, recovery, cognitive clarity, and hair quality [130].
6.7. Telomere Lengthening
A small-scale study demonstrated significant telomere lengthening in PBMCs of male volunteers after 30 days of NMN (300 mg/day) supplementation. Telomeres continued to elongate at 60 days and nearly doubled from baseline by 90 days of supplementation [132]. NMN's impact on telomeres may be linked to stabilizing telomeres and preventing tissue damage through its effect on NAD+ and the SIRT-1 pathway [133].
6.8. Side Effects and Safety Considerations
A growing body of human trials, currently encompassing 19 published studies, has investigated the safety of NMN supplementation. Early studies focused on single doses, with Irie et al.'s work demonstrating no adverse effects following ingestion of up to 500mg NMN [57]. Subsequent research has explored chronic administration, with studies reporting good tolerability for doses ≤500mg over at least a month. Higher doses (600-1250mg) administered for up to 6 weeks have also shown no significant side effects in Liao et al. and Fukamizu et al.'s trials [101,128]. Notably, Pencina et al. observed positive health outcomes alongside increased NAD+ levels in a study using 1000mg NMN twice daily for 2 weeks [122].
While existing evidence is encouraging, a definitive understanding of NMN's safety profile is still under development. Limitations in current research include short durations, small sample sizes, and a lack of diverse participants. Additionally, the potential for long-term effects, interactions with medications, and safety in individuals with pre-existing conditions require further exploration. To definitively establish a Tolerable Upper Intake Level (UL), long-term studies with age-specific considerations are crucial. Overall, NMN appears safe at moderate doses in healthy individuals, but ongoing research is essential to ensure its safe application in a wider population.
Table 2.
Clinical Trials Investigating NMN Supplementation.
| Group Treated | NMN Dose | Duration | Sample Type | NAD+ Metabolome Levels |
Functional Outcomes | Reference |
|---|---|---|---|---|---|---|
| Healthy Middle-Aged Japanese Men (40-60) | Placebo n = 13 |
8 weeks | PMBCs (pmol/mg) HPLC |
NAD: 26.83 |
↑ NAD+ in PBMCs Modest ↓ postprandialhyperinsulinemia |
[124] |
| 250 mg daily | NAD: 41.43 | |||||
| Healthy Adults (40-59) |
Placebo 2× Daily n = 18 |
12 Weeks | Blood Serum (ng/mL) HPLC Tandem MS |
NAM: 10.9 ± 4.8 NMN: <2 NAD: <5 |
↑ NAM in PBMCs ↓ CVD risk |
[118] |
| 125 mg NMN 2× Daily n = 18 |
NAM: 16.5 ± 6.3 NMN: <2 NAD: <5 |
|||||
| Overweight Adults (45+) |
Placebo 2× Daily n = 9 |
28 Days |
Whole Blood (ng/mL) HPLC Tandem MS |
NAM: 21.2 ± 7.77 NMN: 0.6 ± 0.08 NAD: 17.8 ± 2.77 1-MeNAM: 18.7 ± 7.42 2-PY: 394.7 ± 248 |
↑ Circulating NAD+ ↓ Weight, Cholesterol, BP |
[122] |
| 1g NMN 2× Daily n = 21 |
NAM: 19.7 ± 9.73 NMN: 0.7 ± 0.11 NAD: 19.4 ± 2.62 1-MeNAM: 17.9 ± 7.2 2-PY: 357.8 ± 151 |
|||||
| Patients Diagnosed with Mild Essential Hypertension (18-80) |
LM Group n = 10 |
6 Weeks | PMBCs (pmol/mg) HPLC-MS |
NMN: ~4.5 NAD: ~15 |
↑ NAD+ PBMCs (43%) ↑ ATP |
[123] |
| 800 mg NMN + LM n = 9 |
NMN: ~6 NAD: ~20 |
|||||
| Healthy Adults (20-80) |
Placebo n = 25 |
30 Days | Whole Blood (µM) Custom BRET NAD Sensor Assay and HPLC-MS |
NAD: 23.8 ± 5.5 | ↑ Blood NAD+ Dose-dependent ↑ 2/4-PY (esp. 1000mg NMN) No ↑ NMN (suggests NAD catabolism) |
[121] |
| 500 mg NMN Daily n = 25 |
NAD: 41.7 ± 13.0 | |||||
| 1000 mg NMN Daily n = 25 |
NAD: 58.8 ± 21.1 | |||||
| Healthy Adults (20-80) |
Placebo + Exercise n = 21 |
30 Days | Whole Blood (µM) Custom BRET NAD Sensor Assay and HPLC-MS |
NAD: 33.18 ± 7.2 | Exercise & NMN: ↑ NAD+ PBMCs (similar to 1000mg NMN) | [121] |
| 500 mg NMN + Exercise n = 21 |
NAD: 55.48 ± 21.4 | |||||
| Healthy Adults (20-65) |
250 mg NMN Daily n = 11 |
12 Weeks |
Blood Plasma (µM) HPLC-MS |
NMN Month 1: ~0.15 Month 4: ~0.5 NAD Month 1: ~0.055 Month 4: ~0.01 |
↑ NMN & NAD in plasma Transient ↑ insulin (2 mo) NMN ↑ (3 mo) NAD peak (1 mo) |
[120] |
| Males with Diabetes and Reduced Grip Strength of Walking Speed (65+) |
Placebo n = 8 |
24 Weeks | - | Not Measured | NMN: ↓ Frailty | [127] |
| 250 mg NMN Daily n = 8 | ||||||
| Healthy Adults (20-65) |
Placebo n = 15 |
4 Weeks | - | Not Measured | Safe & Well-Tolerated | [101] |
| 1250 mg NMN Daily n = 16 | ||||||
| Healthy Adults (40-65) BMI (18.5-35 kg/m2) |
Placebo n = 31 |
60 Days | Serum NAD/NADH (pmol/mL) Colorimetric Quantitation Kit |
NAD: 8.14 ± 4.86 | ↑ Serum NAD (11.3%) ↓ HOMA-IR ↑ Walking EnduranceImproved Well-being |
[119] |
| 300 mg NMN Daily n = 31 |
NAD: 9.07 ± 5.65 | |||||
| Healthy Men (65+) |
Placebo n = 21 |
12 Weeks | Whole Blood (µM) LC-Tandem MS |
NAM: 10.6 ± 1.6 NMN: 0.105 ± 0.013 NAD: 0.53 ± 0.12 NAMN: 0.05 ± 0.03 NR: 0.0308 ± 0.0088 NA: 0.00684 ± 0.00168 |
↑ NMN, NAD+, NR, NAMN Improved Gait, Walk, Grip (nominal) | [98] |
| 250 mg NMN Daily n = 21 |
NAM: 13.4 ± 2.4 NMN: 0.127 ± 0.019 NAD: 1.07 ± 0.16 NAMN: 3.51 ± 1.86 NR: 0.0549 ± 0.0241 NA: 0.00974 ± 0.00129 |
|||||
| Adults (65+) |
Placebo-AM n = 27 |
12 Weeks | - | Not Measured | Afternoon NMN: ↑ Limb Function ↓ Drowsiness (Older Adults) |
[125] |
| Placebo-PM n = 27 | ||||||
| 250 mg NMN-AM n = 27 | ||||||
| 250 mg NMN-PM n = 27 | ||||||
| Healthy Individuals(20-70) |
Intravenous administration 300 mg NMN in saline (3mg/mL) Daily n = 10 |
5 Hours | Whole Blood NAD/NADH Assay Kit |
1.2-fold increase in Total NAD |
IV NMN: Safe, ↑ Blood NAD+ ↓ Triglycerides | [58] |
| Post-Menopausal Women (50-80) |
300 mg NMN Daily n = 16 |
8 Weeks | Whole Blood (ng/mL) HPLC-MS |
NAM: 164.7 ± 20 NMN: 1.28 ± 0.2 NAD: 13.7 ± 2 |
NAM ↑; NAD ↓; NMN ↔ | [130] |
| Healthy Adults (22-64) |
Placebo n = 15 |
12 Weeks | Whole Blood (µM) HPLC-MS |
NAM: 19 ± 3 NMN: 0.055 ± 0.01 NAD: 22 ± 2 NAMN (8 Wks): 0 NR: 0.04 ± 0.025 NA: 0.25 ± 0.06 |
Blood NAD+ ↑, NAMN ↑, (NMN, NA, NAR, NAAD, MNAM) ↔ | [97] |
| 125 mg NMN 2× Daily n = 15 |
NAM: 17 ± 8 NMN: 0.054 ± 0.016 NAD: 45 ± 20 NAD: 23 ± 8 NAMN: 2.0 ± 1.5 NR: 0.045 ± 0.005 NA: 0.26 ± 0.04 |
|||||
| Overweight Adults (55-80) |
Placebo 2× Daily n = 8 |
14 Days | Whole Blood (ng or µg /mL) HPLC Tandem-MS |
NMN: 0.0326 (µg) NAD: 1.36 (µg) NAM: 10.2 1-MeNAM: 7.57 2-PY: 103 NR: 0.406 |
↑ Blood NAD Metabolites ↓ Body Weight, Systolic BP, ↓ Diastolic BP |
[134] |
| 1000 mg NMN 1× Daily n = 12 |
NMN: 0.0882 (µg) NAD: 23.0 (µg) NAM: 65.2 1-MeNAM: 146 2-PY: 2150 NR: 1.30 |
|||||
| 1000 mg NMN 2× Daily n = 12 |
NMN: 0.148 (µg) NAD: 40.4 (µg) NAM: 140 1-MeNAM: 276 2-PY: 4230 NR: 1.48 |
|||||
| Healthy Adults (37-50) |
Placebo n = 20 |
60 Days | Blood Serum (nM) Colorimetric Quantitation Kit |
Day 0: 8.11 ± 5.16 Day 30: 9.83 ± 8.43 Day 60: 11.8 ± 9.4 |
Blood NAD: ↑ (all NMN groups, days 30 & 60) | [117] |
| 300 mg NMN n = 20 |
Day 0: 11.8 ± 11.7 Day 30: 29.8 ± 20.1 Day 60: 32.6 ±17.9 |
|||||
| 600 mg NMN n = 20 |
Day 0: 7.95 ± 3.29 Day 30: 39.0 ±12.6 Day 60: 45.3 ±11.8 |
|||||
| 900 mg NMN n = 20 |
Day 0: 10.5 ± 6.8 Day 30: 43.1 ± 14.3 Day 60: 48.5 ±19.8 |
|||||
| Young and Middle-Aged recreational Runners | Placebo n = 12 |
6 Weeks | - | Not Measured | Exercise Capacity ↑ (likely ↑ O2 utilization in skeletal muscle) |
[128] |
| 300 mg NMN n = 12 | ||||||
| 600 mg NMN n = 12 | ||||||
| 1200 mg NMN n = 12 | ||||||
| Overweight Post-Menopausal Women BMI (25.3-39.1 kg/m2) |
Placebo n = 12 |
10 Weeks | PMBCs (pmol/mg) | NAD: ~25 | ↑ 2-PY & 4-PY; ↑ PBMC NAD+ (43% vs placebo); ↑ Muscle NAD+ turnover & ↑ Muscle Insulin Sensitivity (25%) | [56] |
| 250 mg NMN n = 13 |
NAD: ~40 | |||||
| Healthy Men (40-60) |
100 mg NMN n = 10 |
12 Weeks | Blood Plasma (nM) HPLC Tandem MS |
2-PY: ~2000 4-PY: ~350 1-MeNAM: ~225 |
↑ Bilirubin (51.3%) ↓ Glucose (11.7%), Creatinine (5.1%), Chloride (2.3%) |
[57] |
| 250 mg NMN n = 10 |
2-PY: ~2500 4-PY: ~400 1-MeNAM: ~250 |
|||||
| 500 mg NMN n = 10 |
2-PY: ~4000 4-PY: ~750 1-MeNAM: ~300 |
7. Remaining Questions and Future Directions
As the field of NMN research continues to evolve, several key questions remain unanswered, pointing toward exciting future avenues of investigation. In this section, remaining uncertainties surrounding NMN and potential directions for future research are outlined, offering insights into the promising perspectives that lie ahead.
7.1. Reduced Precursors
While the prominent roles of NAD+/NADH and NADP+/NADPH in metabolic regulation are well established [135,136], recent research has identified novel reduced NAD+ precursors, including nicotinamide riboside hydride (NRH) and reduced nicotinamide mononucleotide hydride (NMNH) as promising candidates for raising cellular NAD+ levels, potentially exceeding the efficacy of established precursors like NR and NMN. NRH takes a different biochemical pathway than NR and studies show that it can effectively increase NAD+ up to 10-fold in various cell lines, with potency exceeding both NR and NMN [137]. Studies suggest NRH uptake via ENTs and conversion to NMNH through a kinase-mediated pathway independent of NAMPT and NMNAT [138,139]. Interestingly, NRH conversion is independent of NRK1/2 enzymes, unlike NR. Yang et al. further elucidated this pathway alleging that NRH is first converted to NMNH, which then contributes to NAD+ synthesis. NMNH also demonstrates potent NAD+ boosting, exceeding NMN in speed and magnitude. It offers additional benefits like promoting kidney cell healing after injury. Notably, increased NAD+ levels were detected in key organs like the kidney, liver, muscle, brain, brown adipose tissue, and heart. Interestingly, unlike NMN, both NRH and NMNH appear to utilize direct cellular uptake through ENTs [138,140], highlighting their distinct mechanisms of action.
7.2. Sex-Specific Differences in Response to Supplementation
Women tend to have lower whole blood NAD+ levels compared to men, as observed in clinical trials [121,141], suggesting sex-specific differences in NAD+ metabolism and its role in health. A study evaluating the influence of hormones on NAD+ levels revealed that a decrease in testosterone was strongly correlated with a downregulation of NAD+ production [142]. Preclinical models on NMN further support the significance of sex-related differences. In BESTO mice engineered for increased β-cell Sirt1, NMN supplementation restored the age-impaired protective effect of Sirt1 on insulin secretion and glucose tolerance only in females [143]. Similarly, NMN treatment reversed the significant decline in NAD+ levels and impaired glucose tolerance observed in female, but not male, NAMPT-heterozygous mice [52]. Interestingly, sex divergence extends beyond age-related changes. Even in baseline and stroke conditions, NAD+ levels differ between male and female mice [144]. This sex-specificity is further highlighted by the lifespan extension observed upon NAMPT overexpression, which was exclusive to female mice [145].
7.3. Individual Variability in Supplementation Effects
A recent study revealed significant heterogeneity in individual responses to NMN supplementation that may be attributed to underlying differences in gene expression patterns [121]. Individuals who exhibited a robust increase in NAD+ levels upon NMN supplementation ("responders") demonstrated higher expression of NAD+-synthesizing enzymes. Conversely, "non-responders" displayed a stronger expression of NAD+-consuming enzymes. This variability may explain some inconsistencies observed in human trials. This finding underscores the potential of a therapeutic approach that targets both NAD+ synthesis and degradation to achieve more robust and consistent elevations in NAD+ levels, especially for “non-responders.” Lifestyle interventions such as exercise and calorie restriction may offer benefits for individuals with high NAD+ consumption, potentially by rebalancing NAD+ homeostasis [121,146]. Trials targeting multiple aspects of NAD+ metabolism have shown benefits, but still exhibit individual variability in response [147]. A combined approach is likely optimal. Boosting NAD+ synthesis while controlling its breakdown through various methods might be the most effective way to increase NAD+ levels, particularly for individuals with high NAD+ consumption.
7.4. Precursor-Independent Regulatory Functions
Beyond its established role as an NAD+ precursor, NMN demonstrates emerging regulatory functions in mitochondrial [55,137,148] and neuronal [149,150,151] metabolic processes. Numerous preclinical studies demonstrate NMN's neuroprotective potential, enhancing cognition, reducing synaptic loss, improving brain mitochondrial function, and lowering inflammation [152,153,154,155,156,157,158]. However, a 2015 study raised concerns about NMN accumulation potentially causing axonal degeneration [159]. This effect appears to be mediated by SARM1, a NADase activated by NMN under low NMANT2 conditions (stress/injury) [109,160,161]. The key factor seems to be the NMN/NAD+ ratio, not absolute NMN levels [160]. NMN-mediated neuroprotection coincides with increased NAD+, suggesting a crucial balance [162,163]. While NMNAT2 levels may decrease with age (potentially increasing vulnerability), recent clinical trials suggest NAM, another NAD+ precursor, is safe and effective for glaucoma, a neurodegenerative disease [164,165]. Further research is needed on NMNAT2 levels, NMN/NR vulnerability, and long-term clinical trials.
NMN may influence cellular health by impacting mitochondrial DNA (mtDNA) replication. Studies suggest NMN sustains mitochondrial nucleotide levels, leading to increased mtDNA replication and NAD+ metabolite levels [166]. Additionally, HINT2, a protein with an NMN binding site, may improve mitochondrial NAD+ levels and cell survival [167,168]. However, research is limited to specific cell types, and the impact on enzymatic activity needs further exploration.
These models exemplify the need to carefully consider the metabolic roles of NAD+ precursors when designing and implementing NAD+ boosting strategies – particularly those that increase the permeability of the precursors [169,170] to mitigate undesirable and unintended effects as NAD+ metabolism isn’t as straightforward as once thought.
7.5. Influence of Gut Microbiota
Significant knowledge gaps remain regarding the intricate interplay between gut microbiota and NAD+ precursor metabolism. For example, a study in mice showed that dietary fiber supports bacterial NAD+ synthesis, but the impact of different fiber types and gut health on NAD+ synthesis pathways is not clear [171]. This study also uncovered a reciprocal exchange of precursors vital for NAD+ production, with potentially distinct metabolic outcomes in different segments. This regional variation is often overlooked in metabolic analyses. Multiple studies highlight the puzzling rise of unlabeled metabolites after labeled NMN supplementation, indicating an indirect effect on endogenous biosynthesis pathways. Potential explanations have been proposed, including altered enzyme kinetics, conversion of NMN to NR "reservoirs", or the reversal of the NMNAT reaction. While the mechanism has not been determined, the distinct metabolite profiles of germ-free vs. colonized mice strongly suggest gut bacteria play a key role in this process. Notably, the distinct metabolite profiles of germ-free vs. colonized mice strongly implicate gut bacteria in this process [40]. Tracing labeled metabolites in mouse fecal matter could shed light on the dynamics of this competition and elucidate the role of NMN in the microbiome. Furthermore, identifying signaling pathways affected by antibiotic treatment in mice could clarify the mechanisms underlying the observed differences in precursor elevations through amidated and deamidated pathways.
7.6. Refining Strategies for Clinical Applications
Traditionally, research on NAD+ precursors have heavily relied on measuring blood NAD+ levels as a marker of efficacy due to its ease and practicality. However, it is crucial to acknowledge its limitations. Blood NAD+ levels do not necessarily capture the full picture of a precursor's biological effects [106,107]. Studies show that even when NR and NMN achieve similar increases in blood NAD+, their impact on different tissues and organs can diverge [22,48,49]. Furthermore, the optimal dose for a specific benefit may not simply correlate with the highest NAD+ increase. For instance, various NR doses showed similar NAD+ elevation, but a lower dose was more effective in improving metabolic flexibility in obese mice [172].
The lack of a universally accepted definition for "optimal" NAD+ levels hinders our ability to draw clear conclusions from both individual and comparative studies. This is particularly challenging as significant variations in baseline whole blood NAD+ levels are influenced by factors such as age, ethnicity, gender, and lifestyle [97,134,173]. Consequently, supplementation may result in vastly different effects in NAD+-deficient individuals versus those with relatively high baseline levels. This variability in response makes it difficult to draw definitive conclusions on the effects of NAD+ supplementation in clinical trials. For instance, a post-hoc analysis of an NMN trial conducted by Yi et al. in 2023 [117] revealed a correlation between changes in NAD+ levels and improved walking test results, with an approximately 15 nmol/L increase in NAD+ [174]. The trial's low-dose group did not exhibit overall improvements in the walking test, possibly due to large variations in starting NAD+ levels, likely obscuring any potential benefits from the lower dosage. Establishing definitive levels for deficient, optimal, and high NAD+ could provide clearer guidance for designing effective interventions and interpreting study outcomes.
Current research highlights the importance of not just considering the effects of NMN and NR on various tissues, but also differences within cellular compartments. Distinct NAD+ pools exist in the cytosol, mitochondria, and nucleus, each serving specific functions [19]. The identification of separate transporters for NAD+ (SLC25A51) and NMN (Slc25a45) in mitochondria suggests a fascinating level of compartmentalized regulation within cells [43,175]. This implies that cells can precisely control the influx of these molecules based on specific needs, potentially offering a new avenue for understanding and influencing cellular health.
Sensitivity, specificity, and interference issues plague current approaches, impeding the interpretation of interventions and metabolic pathways. To address this, meticulous extraction protocols, stringent internal controls, and optimization of existing methods are crucial. Addressing these limitations while also investigating cutting-edge technologies ensures a path toward precise NMN detection.
8. Conclusions
NMN possesses distinct functionalities, but its effectiveness in humans remains limited by an incomplete understanding of its metabolism. Oral supplementation exhibits low bioavailability due to digestion and first-pass metabolism. Intravenous delivery offers somewhat higher bioavailability but raises safety and feasibility concerns. Emerging evidence highlights the complex interplay between NMN, gut microbiota, and individual differences in metabolism, further complicating the picture. Fortunately, ongoing research offers promising avenues. Advanced delivery systems like liposomes may bypass metabolic hurdles and improve NMN bioavailability. Additionally, reduced precursors like NMNH and NRH exhibit greater stability. However, these advancements raise new questions. Determining optimal NAD+ levels and understanding the complex effects of both deficiency and overabundance are crucial next steps.
Author Contributions
Conceptualization, R.C.; writing—original draft preparation, R.C. and C.B.; writing—review and editing, R.C. and C.B.
Funding
Article processing fees were funded by Renue By Science.
Conflicts of Interest
R.C. and C.B. are employees of Renue By Science.
References
- Harden, A.; Young, W.J. The Alcoholic Ferment of Yeast-Juice. Proc. R. Soc. Lond. B 1906, 77, 405–420. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Sig. 2018, 28, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T. Molecular Mechanism of Metabolic NAD(P)H-Dependent Electron-Transfer Systems: The Role of Redox Cofactors. BBA-Bioenergetics 2019, 1860, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, E.A.; Schury, M.P. Biochemistry, Anaerobic Glycolysis; StatPearls Publishing: Treasure Island, Florida, 2023. [Google Scholar]
- Leary, S.C.; Moyes, C.D. Chapter 15 - The Effects of Bioenergetic Stress and Redox Balance on the Expression of Genes Critical to Mitochondrial Function. In Cell and Molecular Response to Stress; Storey, K.B., Storey, J.M., Eds.; Environmental Stressors and Gene Responses; Elsevier, 2000; Vol. 1, pp. 209–229.
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Sig. Transduct. Target Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Paneni, F.; Stein, S.; Matter, C.M. Modulating Sirtuin Biology and Nicotinamide Adenine Diphosphate Metabolism in Cardiovascular Disease—From Bench to Bedside. Front. Physiol. 2021, 12, 755060. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Bagès, S.; Knobloch, G.; Ladurner, A.G.; Buschbeck, M. The Taming of PARP1 and Its Impact on NAD+ Metabolism. Mol. Metab. 2020, 38, 100950. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Chini, E.N. CD38 as a Regulator of Cellular NAD: A Novel Potential Pharmacological Target for Metabolic Conditions. Curr. Pharm. Design 2009, 15, 57–63. [Google Scholar] [CrossRef]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ Consumption by PARP1 in Response to DNA Damage Triggers Metabolic Shift Critical for Damaged Cell Survival. MBoC 2019, 30, 2584–2597. [Google Scholar] [CrossRef]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Nacarelli, T.; Lau, L.; Fukumoto, T.; Zundell, J.; Fatkhutdinov, N.; Wu, S.; Aird, K.M.; Iwasaki, O.; Kossenkov, A.V.; Schultz, D.; et al. NAD+ Metabolism Governs the Proinflammatory Senescence-Associated Secretome. Nat. Cell Biol. 2019, 21, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zuo, W.; Liu, Y.; Wu, K.; Liu, Q. NAD+ and Cardiovascular Diseases. Clin. Chim. Acta 2021, 515, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-Associated Changes In Oxidative Stress and NAD+ Metabolism In Human Tissue. PLOS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Bagga, P.; Hariharan, H.; Wilson, N.E.; Beer, J.C.; Shinohara, R.T.; Elliott, M.A.; Baur, J.A.; Marincola, F.M.; Witschey, W.R.; Haris, M.; et al. Single-Voxel 1H MR Spectroscopy of Cerebral Nicotinamide Adenine Dinucleotide (NAD+) in Humans at 7T Using a 32-Channel Volume Coil. Magn. Reson. Med. 2020, 83, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-H.; Lu, M.; Lee, B.-Y.; Ugurbil, K.; Chen, W. In Vivo NAD Assay Reveals the Intracellular NAD Contents and Redox State in Healthy Human Brain and Their Age Dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Kovač, V.; Milisav, I. Healthy Lifestyle Recommendations: Do the Beneficial Effects Originate from NAD+ Amount at the Cellular Level? Oxid. Med. Cell Longev. 2020, 2020, 8819627. [Google Scholar] [CrossRef]
- Cambronne, X.A.; Kraus, W.L. Location, Location, Location: Compartmentalization of NAD+ Synthesis and Functions in Mammalian Cells. Trends Biochem. Sci. 2020, 45, 858–873. [Google Scholar] [CrossRef]
- Chiarugi, A.; Dölle, C.; Felici, R.; Ziegler, M. The NAD Metabolome — a Key Determinant of Cancer Cell Biology. Nat. Rev. Cancer 2012, 12, 741–752. [Google Scholar] [CrossRef]
- Grozio, A.; Mills, K.F.; Yoshino, J.; Bruzzone, S.; Sociali, G.; Tokizane, K.; Lei, H.C.; Cunningham, R.; Sasaki, Y.; Migaud, M.E.; et al. Slc12a8 Is a Nicotinamide Mononucleotide Transporter. Nat. Metab. 2019, 1, 47–57. [Google Scholar] [CrossRef]
- Ratajczak, J.; Joffraud, M.; Trammell, S.A.J.; Ras, R.; Canela, N.; Boutant, M.; Kulkarni, S.S.; Rodrigues, M.; Redpath, P.; Migaud, M.E.; et al. NRK1 Controls Nicotinamide Mononucleotide and Nicotinamide Riboside Metabolism in Mammalian Cells. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Preiss, J.; Handler, P. Biosynthesis of Diphosphopyridine Nucleotide: I. IDENTIFICATION OF INTERMEDIATES. J. Biol. Chem. 1958, 233, 488–492. [Google Scholar] [CrossRef]
- Marletta, A.S.; Massarotti, A.; Orsomando, G.; Magni, G.; Rizzi, M.; Garavaglia, S. Crystal Structure of Human Nicotinic Acid Phosphoribosyltransferase. FEBS Open Bio 2015, 5, 419. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudlińska-Chylak, A.; Czeczotko, M.; Rejman, K. Food Products as Sources of Protein and Amino Acids—The Case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef]
- Scalise, M.; Galluccio, M.; Console, L.; Pochini, L.; Indiveri, C. The Human SLC7A5 (LAT1): The Intriguing Histidine/Large Neutral Amino Acid Transporter and Its Relevance to Human Health. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Pillai, S.M.; Meredith, D. SLC36A4 (hPAT4) Is a High Affinity Amino Acid Transporter When Expressed in Xenopus Laevis Oocytes. J. Biol. Chem. 2011, 286, 2455–2460. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Liu, L.; Su, X.; Quinn, W.J.; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E.; et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018, 27, 1067–1080.e5. [Google Scholar] [CrossRef]
- Berven, H.; Kverneng, S.; Sheard, E.; Søgnen, M.; Af Geijerstam, S.A.; Haugarvoll, K.; Skeie, G.-O.; Dölle, C.; Tzoulis, C. NR-SAFE: A Randomized, Double-Blind Safety Trial of High Dose Nicotinamide Riboside in Parkinson’s Disease. Nat. Commun. 2023, 14, 7793. [Google Scholar] [CrossRef]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic Nicotinamide Riboside Supplementation Is Well-Tolerated and Elevates NAD+ in Healthy Middle-Aged and Older Adults. Nat. Commun. 2018, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Mehmel, M.; Jovanović, N.; Spitz, U. Nicotinamide Riboside—The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Shade, C. The Science Behind NMN—A Stable, Reliable NAD+ Activator and Anti-Aging Molecule. Integr. Med. 2020, 19, 12–14. [Google Scholar]
- Caton, P.W.; Kieswich, J.; Yaqoob, M.M.; Holness, M.J.; Sugden, M.C. Nicotinamide Mononucleotide Protects against Pro-Inflammatory Cytokine-Mediated Impairment of Mouse Islet Function. Diabetologia 2011, 54, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide Mononucleotide, an Intermediate of NAD+ Synthesis, Protects the Heart from Ischemia and Reperfusion. PLoS One 2014, 9, e98972. [Google Scholar] [CrossRef]
- Trammell, S.A.J.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide Riboside Is Uniquely and Orally Bioavailable in Mice and Humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, L.; Bocci, P.; Amici, A.; Brunetti, L.; Ruggieri, S.; Romine, M.; Reed, S.; Osterman, A.L.; Rodionov, D.A.; Sorci, L.; et al. Identification of Nicotinamide Mononucleotide Deamidase of the Bacterial Pyridine Nucleotide Cycle Reveals a Novel Broadly Conserved Amidohydrolase Family. J. Biol. Chem. 2011, 286, 40365–40375. [Google Scholar] [CrossRef] [PubMed]
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020, 31, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.-J.; Chalmers, T.J.; Madawala, R.; Smith, G.C.; Li, C.; Das, A.; Poon, E.W.K.; Wang, J.; Tucker, S.P.; Sinclair, D.A.; et al. Host–Microbiome Interactions in Nicotinamide Mononucleotide (NMN) Deamidation. FEBS Lett. 2023, 597, 2196–2220. [Google Scholar] [CrossRef]
- Yaku, K.; Palikhe, S.; Izumi, H.; Yoshida, T.; Hikosaka, K.; Hayat, F.; Karim, M.; Iqbal, T.; Nitta, Y.; Sato, A.; et al. BST1 Regulates Nicotinamide Riboside Metabolism via Its Glycohydrolase and Base-Exchange Activities. Nat. Commun. 2021, 12, 6767. [Google Scholar] [CrossRef] [PubMed]
- Grozio, A.; Sociali, G.; Sturla, L.; Caffa, I.; Soncini, D.; Salis, A.; Raffaelli, N.; Flora, A.D.; Nencioni, A.; Bruzzone, S. CD73 Protein as a Source of Extracellular Precursors for Sustained NAD+ Biosynthesis in FK866-Treated Tumor Cells. J. Biol. Chem. 2013, 288, 25938. [Google Scholar] [CrossRef]
- Chen, L.; Wang, P.; Huang, G.; Cheng, W.; Liu, K.; Yu, Q. Quantitative Dynamics of Intracellular NMN by Genetically Encoded Biosensor 2023, 2023. 10.23.56 3573.
- Unno, J.; Mills, K.F.; Ogura, T.; Nishimura, M.; Imai, S. Absolute Quantification of Nicotinamide Mononucleotide in Biological Samples by Double Isotope-Mediated Liquid Chromatography-Tandem Mass Spectrometry (dimeLC-MS/MS). npj Aging 2024, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.S.; Brenner, C. Absence of Evidence That Slc12a8 Encodes a Nicotinamide Mononucleotide Transporter. Nat. Metab. 2019, 1, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Sauve, A.A.; Wang, Q.; Zhang, N.; Kang, S.; Rathmann, A.; Yang, Y. Triple-Isotope Tracing for Pathway Discernment of NMN-Induced NAD+ Biosynthesis in Whole Mice. Int. J. Mol. Sci. 2023, 24, 11114. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Ni, R.; Ding, W.; Ji, X.; Fan, G.-C.; Zhang, Z.; Peng, T. Nicotinamide Mononucleotide as a Therapeutic Agent to Alleviate Multi-Organ Failure in Sepsis. J.Transl. Med. 2023, 21, 883. [Google Scholar] [CrossRef] [PubMed]
- Karamanlidis, G.; Lee, C.F.; Garcia-Menendez, L.; Kolwicz, S.C.; Suthammarak, W.; Gong, G.; Sedensky, M.M.; Morgan, P.G.; Wang, W.; Tian, R. Mitochondrial Complex I Deficiency Increases Protein Acetylation and Accelerates Heart Failure. Cell Metab. 2013, 18, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Stromsdorfer, K.L.; Yamaguchi, S.; Yoon, M.J.; Moseley, A.C.; Franczyk, M.P.; Kelly, S.C.; Qi, N.; Imai, S.; Yoshino, J. NAMPT-Mediated NAD+ Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-Organ Insulin Sensitivity in Mice. Cell Rep. 2016, 16, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Yamada, K.; Shibata, T.; Osago, H.; Tsuchiya, M. Nicotinamide Phosphoribosyltransferase/Visfatin Does Not Catalyze Nicotinamide Mononucleotide Formation in Blood Plasma. PLoS One 2011, 6, e22781. [Google Scholar] [CrossRef]
- Revollo, J.R.; Körner, A.; Mills, K.F.; Satoh, A.; Wang, T.; Garten, A.; Dasgupta, B.; Sasaki, Y.; Wolberger, C.; Townsend, R.R.; et al. Nampt/PBEF/Visfatin Regulates Insulin Secretion in β Cells as a Systemic NAD Biosynthetic Enzyme. Cell Metab. 2007, 6, 363–375. [Google Scholar] [CrossRef]
- Stein, L.R.; Imai, S. Specific Ablation of Nampt in Adult Neural Stem Cells Recapitulates Their Functional Defects during Aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef]
- Yoon, M.J.; Yoshida, M.; Johnson, S.; Takikawa, A.; Usui, I.; Tobe, K.; Nakagawa, T.; Yoshino, J.; Imai, S. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab. 2015, 21, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Long, A.; Kristian, T. Nicotinamide Mononucleotide Alters Mitochondrial Dynamics by SIRT3-Dependent Mechanism in Male Mice. J. Neurosci. Res. 2019, 97, 975–990. [Google Scholar] [CrossRef]
- Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.-I.; et al. Nicotinamide Mononucleotide Increases Muscle Insulin Sensitivity in Prediabetic Women. Science 2021, 372, 1224–1229. [Google Scholar] [CrossRef]
- Irie, J.; Inagaki, E.; Fujita, M.; Nakaya, H.; Mitsuishi, M.; Yamaguchi, S.; Yamashita, K.; Shigaki, S.; Ono, T.; Yukioka, H.; et al. Effect of Oral Administration of Nicotinamide Mononucleotide on Clinical Parameters and Nicotinamide Metabolite Levels in Healthy Japanese Men. Endocr. J. 2020, 67, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Ichikawa, M.; Sugawara, S.; Katagiri, T.; Hirasawa, Y.; Ishikawa, T.; Matsunaga, W.; Gotoh, A. Nicotinamide Mononucleotide Is Safely Metabolized and Significantly Reduces Blood Triglyceride Levels in Healthy Individuals. Cureus 14. [CrossRef]
- Ingersoll, K.S.; Cohen, J. The Impact of Medication Regimen Factors on Adherence to Chronic Treatment: A Review of Literature. J. Behav. Med. 2008, 31, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Moroz, E.; Matoori, S.; Leroux, J.-C. Oral Delivery of Macromolecular Drugs: Where We Are after Almost 100 Years of Attempts. Adv. Drug Deliv. Rev. 2016, 101, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Musyaju, S.; Modi, H.R.; Okada-Rising, S.L.; Bailey, Z.S.; Scultetus, A.H.; Shear, D.A. Intranasal Delivery of Mitochondria Targeted Neuroprotective Compounds for Traumatic Brain Injury: Screening Based on Pharmacological and Physiological Properties. J. Trans. Med. 2024, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Des Rieux, A.; Pourcelle, V.; Cani, P.D.; Marchand-Brynaert, J.; Préat, V. Targeted Nanoparticles with Novel Non-Peptidic Ligands for Oral Delivery. Adv. Drug Deliv. Rev. 2013, 65, 833–844. [Google Scholar] [CrossRef]
- Wu, W.; Lu, Y.; Qi, J. Oral Delivery of Liposomes. Ther. Deliv. 2015, 6, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Darwis, Y.; Ali Khan, A.; Mudassir, J.; Mohtar, N. Advanced Drug Delivery to the Lymphatic System: Lipid-Based Nanoformulations. Int. J. Nanomed. 2013, 2733. [Google Scholar] [CrossRef] [PubMed]
- Sahatsapan, N.; Pamornpathomkul, B.; Rojanarata, T.; Ngawhirunpat, T.; Poonkhum, R.; Opanasopit, P.; Patrojanasophon, P. Feasibility of Mucoadhesive Chitosan Maleimide-Coated Liposomes for Improved Buccal Delivery of a Protein Drug. J. Drug Deliv. Sci. Tec. 2022, 69, 103173. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Bhagya, N.; Chandrashekar, K.R. Liposome Encapsulated Anticancer Drugs on Autophagy in Cancer Cells – Current and Future Perspective. Int. J. Pharm. 2023, 642, 123105. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the Treatment of Brain Cancer—A Review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as Vaccine Delivery Systems: A Review of the Recent Advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Akram, N.; Afzaal, M.; Saeed, F.; Shah, Y.A.; Faisal, Z.; Asghar, A.; Ateeq, H.; Nayik, G.A.; Wani, S.H.; Hussain, M.; et al. Liposomes: A Promising Delivery System for Active Ingredients in Food and Nutrition. Int. J. Food Prop. 2023, 26, 2476–2492. [Google Scholar] [CrossRef]
- Latrobdiba, Z.M.; Fulyani, F.; Anjani, G. Liposome Optimisation for Oral Delivery of Nutraceuticals in Food: A Review. Food Res. 2023, 7, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. 2016, 15. [Google Scholar]
- Balazs, D.A.; Godbey, WT. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S.H. Liposomes for Oral Delivery of Protein and Peptide-Based Therapeutics: Challenges, Formulation Strategies, and Advances. J. Mater. Chem. B 2021, 9, 4773–4792. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kong, Q.; Chen, Y.; Yang, Z.; Wu, Z.; Xiao, Y.; Chen, Y.; Yu, Z.; Luo, X.; Qu, W. Liposome-Based Loading Enhances the Distribution of Nicotinamide Riboside Chloride into the Brain and Its Neuroprotective Effects in Cerebral Ischemic Mice. Journal of Neurorestoratology 2024, 100111. [Google Scholar] [CrossRef]
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-Encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr. Metab. Insights 2016, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in Vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef]
- Ye, M.; Zhao, Y.; Wang, Y.; Xie, R.; Tong, Y.; Sauer, J.-D.; Gong, S. NAD(H)-Loaded Nanoparticles for Efficient Sepsis Therapy via Modulating Immune and Vascular Homeostasis. Nat. Nanotechnol. 2022, 17, 880–890. [Google Scholar] [CrossRef]
- Khot, K.B.; Gopan, G.; Bandiwadekar, A.; Jose, J. Current Advancements Related to Phytobioactive Compounds Based Liposomal Delivery for Neurodegenerative Diseases. Ageing Res. Rev. 2023, 83, 101806. [Google Scholar] [CrossRef]
- Nsairat, H.; Alshaer, W.; Odeh, F.; Esawi, E.; Khater, D.; Bawab, A.A.; El-Tanani, M.; Awidi, A.; Mubarak, M.S. Recent Advances in Using Liposomes for Delivery of Nucleic Acid-Based Therapeutics. OpenNano 2023, 11, 100132. [Google Scholar] [CrossRef]
- Swain, B.; Koilpillai, J.; Narayanasamy, D. Systematic Review on Recent Advancements and Liposomal Technologies to Develop Stable Liposome. Curr. Trends Biotechnol. Pharm. 2023, 17, 735–748. [Google Scholar] [CrossRef]
- Maurer, N.; Fenske, D.B.; Cullis, P.R. Developments in Liposomal Drug Delivery Systems. Expert Opin. Biol. Ther. 2001, 1, 923–947. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.D.; Jones, D.S.; Andrews, G.P.; Li, S. Understanding the Physicochemical Properties and Degradation Kinetics of Nicotinamide Riboside, a Promising Vitamin B3 Nutritional Supplement. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Zhang, D.; Yau, L.-F.; Bai, L.-B.; Tong, T.-T.; Cao, K.-Y.; Yan, T.-M.; Zeng, L.; Jiang, Z.-H. Hydroxyapatite-Based Nano-Drug Delivery System for Nicotinamide Mononucleotide (NMN): Significantly Enhancing NMN Bioavailability and Replenishing in Vivo Nicotinamide Adenine Dinucleotide (NAD+) Levels. J. Pharm. Pharmacol. 2023, 75, 1569–1580. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H.; Ye, P.; Zhu, L.; Ren, Y.; Lei, J. Controlled Production of Liposomes with Novel Microfluidic Membrane Emulsification for Application of Entrapping Hydrophilic and Lipophilic Drugs. J. Ind. Eng. Chem. 2024, 131, 470–480. [Google Scholar] [CrossRef]
- Rymarchyk, S.; Kang, W.; Cen, Y. Substrate-Dependent Sensitivity of SIRT1 to Nicotinamide Inhibition. Biomolecules 2021, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Bitterman, K.J.; Anderson, R.M.; Cohen, H.Y.; Latorre-Esteves, M.; Sinclair, D.A. Inhibition of Silencing and Accelerated Aging by Nicotinamide, a Putative Negative Regulator of Yeast Sir2 and Human SIRT1. J. Biol. Chem. 2002, 277, 45099–45107. [Google Scholar] [CrossRef] [PubMed]
- Kang-Lee, Y.A.; McKee, R.W.; Wright, S.M.; Swendseid, M.E.; Jenden, D.J.; Jope, R.S. Metabolic Effects of Nicotinamide Administration in Rats. J. Nutr. 1983, 113, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Minto, C.; Vecchio, M.G.; Lamprecht, M.; Gregori, D. Definition of a Tolerable Upper Intake Level of Niacin: A Systematic Review and Meta-Analysis of the Dose-Dependent Effects of Nicotinamide and Nicotinic Acid Supplementation. Nutr. Rev. 2017, 75, 471–490. [Google Scholar] [CrossRef]
- Tunaru, S.; Kero, J.; Schaub, A.; Wufka, C.; Blaukat, A.; Pfeffer, K.; Offermanns, S. PUMA-G and HM74 Are Receptors for Nicotinic Acid and Mediate Its Anti-Lipolytic Effect. Nat. Med. 2003, 9, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H. Niacin Use and Cutaneous Flushing: Mechanisms and Strategies for Prevention. Am. J. Cardiol. 2008, 101, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An Open-Label, Non-Randomized Study of the Pharmacokinetics of the Nutritional Supplement Nicotinamide Riboside (NR) and Its Effects on Blood NAD+ Levels in Healthy Volunteers. PLoS One 2017, 12, e0186459. [Google Scholar] [CrossRef]
- Okabe, K.; Yaku, K.; Uchida, Y.; Fukamizu, Y.; Sato, T.; Sakurai, T.; Tobe, K.; Nakagawa, T. Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Front. Nutr. 2022, 9, 868640. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nakagawa-Nagahama, Y.; Miura, M.; Kashiwabara, K.; Yaku, K.; Sawada, M.; Sekine, R.; Fukamizu, Y.; Sato, T.; Sakurai, T.; et al. Chronic Nicotinamide Mononucleotide Supplementation Elevates Blood Nicotinamide Adenine Dinucleotide Levels and Alters Muscle Function in Healthy Older Men. npj Aging 2022, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, S.-R.; Huang, X.-Z.; Xie, Q.; Xu, Y.-Y.; Shang, D.; Hao, C.-M. Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1–Dependent Manner. J. Am. Soc. Nephrol. 2017, 28, 2337–2352. [Google Scholar] [CrossRef] [PubMed]
- Fukamizu, Y.; Uchida, Y.; Shigekawa, A.; Sato, T.; Kosaka, H.; Sakurai, T. Safety Evaluation of β-Nicotinamide Mononucleotide Oral Administration in Healthy Adult Men and Women. Sci. Rep. 2022, 12, 14442. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Mori, N.; Shibata, K. β-Nicotinamide Mononucleotide, an Anti-Aging Candidate Compound, Is Retained in the Body for Longer than Nicotinamide in Rats. J. Nutr. Sci. Vitaminol. 2016, 62, 272–276. [Google Scholar] [CrossRef]
- Vannini, N.; Campos, V.; Girotra, M.; Trachsel, V.; Rojas-Sutterlin, S.; Tratwal, J.; Ragusa, S.; Stefanidis, E.; Ryu, D.; Rainer, P.Y.; et al. The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance. Cell Stem Cell 2019, 24, 405–418.e7. [Google Scholar] [CrossRef]
- Konieczna, I.M.; Panuganti, S.; DeLuca, T.A.; Papoutsakis, E.T.; Eklund, E.A.; Miller, W.M. Administration of Nicotinamide Does Not Increase Platelet Levels in Mice. Blood Cells Mol. Dis. 2013, 50, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Dollerup, O.L.; Chubanava, S.; Agerholm, M.; Søndergård, S.D.; Altıntaş, A.; Møller, A.B.; Høyer, K.F.; Ringgaard, S.; Stødkilde-Jørgensen, H.; Lavery, G.G.; et al. Nicotinamide Riboside Does Not Alter Mitochondrial Respiration, Content or Morphology in Skeletal Muscle from Obese and Insulin-Resistant Men. J. Physiol. 2020, 598, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.S.; Abraham, D.M.; Hershberger, K.A.; Bhatt, D.P.; Mao, L.; Cui, H.; Liu, J.; Liu, X.; Muehlbauer, M.J.; Grimsrud, P.A.; et al. Nicotinamide Mononucleotide Requires SIRT3 to Improve Cardiac Function and Bioenergetics in a Friedreich’s Ataxia Cardiomyopathy Model. JCI Insight 2, e93885. [CrossRef]
- Stram, A.R.; Pride, P.M.; Payne, R.M. NAD+ Replacement Therapy with Nicotinamide Riboside Does Not Improve Cardiac Function in a Model of Mitochondrial Heart Disease. FASEB J. 2017, 31, 602.15. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3–Dependent Mitochondrial Dynamic Improvements Protect against Acute Kidney Injury. J Clin. Invest. 2015, 125, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD Biosynthesis Pathway Mediated by Nicotinamide Phosphoribosyltransferase Regulates Sir2 Activity in Mammalian Cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.B.; Kubota, S.; Ban, N.; Yoshida, M.; Santeford, A.; Sene, A.; Nakamura, R.; Zapata, N.; Kubota, M.; Tsubota, K.; et al. NAMPT-Mediated NAD+ Biosynthesis Is Essential for Vision in Mice. Cell Rep. 2016, 17, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Araki, T.; Milbrandt, J. Stimulation of Nicotinamide Adenine Dinucleotide Biosynthetic Pathways Delays Axonal Degeneration after Axotomy. J. Neurosci. 2006, 26, 8484–8491. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.A.; Shcherbina, A.; Ricke, D.O.; Pop, R.; Carrigan, C.T.; Gifford, C.A.; Urso, M.L.; Kottke, M.A.; Meissner, A. In Vivo Monitoring of Transcriptional Dynamics After Lower-Limb Muscle Injury Enables Quantitative Classification of Healing. Sci. Rep. 2015, 5, 13885. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.E.; Farquharson, A.J.; Horgan, G.W.; Williams, L.M. Tissue-Specific Regulation of Sirtuin and Nicotinamide Adenine Dinucleotide Biosynthetic Pathways Identified in C57Bl/6 Mice in Response to High-Fat Feeding. J. Nutr. Biochem. 2016, 37, 20–29. [Google Scholar] [CrossRef]
- Ahmad, F.; Tomar, D.; Aryal A C, S.; Elmoselhi, A.B.; Thomas, M.; Elrod, J.W.; Tilley, D.G.; Force, T. Nicotinamide Riboside Kinase-2 Alleviates Ischemia-Induced Heart Failure through P38 Signaling. BBA-Mol. Basis Dis. 2020, 1866, 165609. [Google Scholar] [CrossRef]
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Diguet, N.; Trammell, S.A.J.; Tannous, C.; Deloux, R.; Piquereau, J.; Mougenot, N.; Gouge, A.; Gressette, M.; Manoury, B.; Blanc, J.; et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 2018, 137, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Maier, A.B.; Tao, R.; Lin, Z.; Vaidya, A.; Pendse, S.; Thasma, S.; Andhalkar, N.; Avhad, G.; Kumbhar, V. The Efficacy and Safety of β-Nicotinamide Mononucleotide (NMN) Supplementation in Healthy Middle-Aged Adults: A Randomized, Multicenter, Double-Blind, Placebo-Controlled, Parallel-Group, Dose-Dependent Clinical Trial. GeroScience 2022, 45, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Katayoshi, T.; Uehata, S.; Nakashima, N.; Nakajo, T.; Kitajima, N.; Kageyama, M.; Tsuji-Naito, K. Nicotinamide Adenine Dinucleotide Metabolism and Arterial Stiffness after Long-Term Nicotinamide Mononucleotide Supplementation: A Randomized, Double-Blind, Placebo-Controlled Trial. Sci. Rep. 2023, 13, 2786. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, X.; Wang, S.; Wu, Z.; Zhou, Z.; Shao, G.; Ren, C.; Kuang, M.; Zhou, Y.; Jiang, A.; et al. Treatment of Inflammatory Bowel Disease: Potential Effect of NMN on Intestinal Barrier and Gut Microbiota. Curr. Res. Food Sci. 2022, 5, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Imai, M.; Bamba, T.; Uchiyama, S. Nicotinamide Mononucleotide (NMN) Intake Increases Plasma NMN and Insulin Levels in Healthy Subjects. Clin. Nutr. 2023, 56, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, M.; Hou, Y.; Luan, J.; Liu, R.; Chen, L.; Hu, M.; Yu, Q. Fingerstick Blood Assay Maps Real-world NAD + Disparity across Gender and Age. Aging Cell 2023, 22, e13965. [Google Scholar] [CrossRef]
- Pencina, K.M.; Valderrabano, R.; Wipper, B.; Orkaby, A.R.; Reid, K.F.; Storer, T.; Lin, A.P.; Merugumala, S.; Wilson, L.; Latham, N.; et al. Nicotinamide Adenine Dinucleotide Augmentation in Overweight or Obese Middle-Aged and Older Adults: A Physiologic Study. J. Clin. Endocrinol. Metab. 2023, 108, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, S.; Chen, X.; Wu, X.; Zhou, Z.; Zhang, J.; Tu, Q.; Dong, B.; Liu, Z.; He, J.; et al. NAD+ Exhaustion by CD38 Upregulation Contributes to Blood Pressure Elevation and Vascular Damage in Hypertension. Sig. Transduct. Target Ther. 2023, 8, 1–14. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Irie, J.; Mitsuishi, M.; Uchino, Y.; Nakaya, H.; Takemura, R.; Inagaki, E.; Kosugi, S.; Okano, H.; Yasui, M.; et al. Safety and Efficacy of Long-Term Nicotinamide Mononucleotide Supplementation on Metabolism, Sleep, and Nicotinamide Adenine Dinucleotide Biosynthesis in Healthy, Middle-Aged Japanese Men. Endocr. J. 2024, 71, 153–169. [Google Scholar] [CrossRef]
- Kim, M.; Seol, J.; Sato, T.; Fukamizu, Y.; Sakurai, T.; Okura, T. Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2022, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, C.; Qiang, L.; Liu, J.; Qiu, Z.; Zhang, Z.; Zhang, J.; Li, Y.; Zhang, M. Clinical Observation of the Effect of Nicotinamide Mononucleotide on the Improvement of Insomnia in Middle-Aged and Old Adults. Am. J. Trans. Med. 2022, 6, 167–176. [Google Scholar]
- Akasaka, H.; Nakagami, H.; Sugimoto, K.; Yasunobe, Y.; Minami, T.; Fujimoto, T.; Yamamoto, K.; Hara, C.; Shiraki, A.; Nishida, K.; et al. Effects of Nicotinamide Mononucleotide on Older Patients with Diabetes and Impaired Physical Performance: A Prospective, Placebo-Controlled, Double-Blind Study. Geriatr. Gerontol. Int. 2023, 23, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhao, Y.; Wang, D.; Zhang, X.; Hao, X.; Hu, M. Nicotinamide Mononucleotide Supplementation Enhances Aerobic Capacity in Amateur Runners: A Randomized, Double-Blind Study. J. Int. Soc. Sport Nutr. 2021, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. A Multicentre, Randomised, Double Blind, Parallel Design, Placebo Controlled Study to Evaluate the Efficacy and Safety of Uthever (NMN Supplement), an Orally Administered Supplementation in Middle Aged and Older Adults. Front. Aging 2022, 3, 851698. [Google Scholar] [CrossRef]
- Morita, Y.; Izawa, H.; Hirano, A.; Mayumi, E.; Isozaki, S.; Yonei, Y. Clinical Evaluation of Changes in Biomarkers by Oral Intake of NMN. Glycative Stress Res. 2022, 9, 33–41. [Google Scholar] [CrossRef]
- Thota, R.N.; Moughan, P.J.; Singh, H.; Garg, M.L. Significance of Postprandial Insulin and Triglycerides to Evaluate the Metabolic Response of Composite Meals Differing in Nutrient Composition – A Randomized Cross-Over Trial. Front. Nutr. 2022, 9, 816755. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.-M.; Bao, T.; Gao, L.; Ru, M.; Li, Y.; Jiang, L.; Ye, C.; Wang, S.; Wu, X. The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Amano, H.; Chaudhury, A.; Rodriguez-Aguayo, C.; Lu, L.; Akhanov, V.; Catic, A.; Popov, Y.V.; Verdin, E.; Johnson, H.; Stossi, F.; et al. Telomere Dysfunction Induces Sirtuin Repression That Drives Telomere-Dependent Disease. Cell Metab. 2019, 29, 1274–1290.e9. [Google Scholar] [CrossRef]
- Pencina, K.M.; Lavu, S.; Dos Santos, M.; Beleva, Y.M.; Cheng, M.; Livingston, D.; Bhasin, S. MIB-626, an Oral Formulation of a Microcrystalline Unique Polymorph of β-Nicotinamide Mononucleotide, Increases Circulating Nicotinamide Adenine Dinucleotide and Its Metabolome in Middle-Aged and Older Adults. The Journals of Gerontology: Series A 2023, 78, 90–96. [Google Scholar] [CrossRef]
- Yang, Y.; Sauve, A.A. NAD+ Metabolism: Bioenergetics, Signaling and Manipulation for Therapy. Biochim. Biophys. Acta Proteom. 2016, 1864, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Ying, W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Sig. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lin, X.; Tang, H.; Fan, Y.; Zeng, S.; Jia, L.; Li, Y.; Shi, Y.; He, S.; Wang, H.; et al. Mitochondrial DNA Mutation Exacerbates Female Reproductive Aging via Impairment of the NADH/NAD+ Redox. Aging Cell 2020, 19, e13206. [Google Scholar] [CrossRef] [PubMed]
- Giroud-Gerbetant, J.; Joffraud, M.; Giner, M.P.; Cercillieux, A.; Bartova, S.; Makarov, M.V.; Zapata-Pérez, R.; Sánchez-García, J.L.; Houtkooper, R.H.; Migaud, M.E.; et al. A Reduced Form of Nicotinamide Riboside Defines a New Path for NAD+ Biosynthesis and Acts as an Orally Bioavailable NAD+ Precursor. Mol. Metab. 2019, 30, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mohammed, F.S.; Zhang, N.; Sauve, A.A. Dihydronicotinamide Riboside Is a Potent NAD+ Concentration Enhancer in Vitro and in Vivo. J. Biol. Chem. 2019, 294, 9295–9307. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Pérez, R.; Tammaro, A.; Schomakers, B.V.; Scantlebery, A.M.L.; Denis, S.; Elfrink, H.L.; Giroud-Gerbetant, J.; Cantó, C.; López-Leonardo, C.; McIntyre, R.L.; et al. Reduced Nicotinamide Mononucleotide Is a New and Potent NAD+ Precursor in Mammalian Cells and Mice. FASEB J. 2021, 35, e21456. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shen, J.; Liu, C.; Kuang, Z.; Tang, Y.; Qian, Z.; Guan, M.; Yang, Y.; Zhan, Y.; Li, N.; et al. Nicotine Rebalances NAD+ Homeostasis and Improves Aging-Related Symptoms in Male Mice by Enhancing NAMPT Activity. Nat. Commun. 2023, 14, 900. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Kang, Y.; Ji, X.; Li, S.; Li, Y.; Zhang, G.; Cui, H.; Shi, G. Testosterone Upregulates the Expression of Mitochondrial ND1 and ND4 and Alleviates the Oxidative Damage to the Nigrostriatal Dopaminergic System in Orchiectomized Rats. Oxid. Med. Cell Longev. 2017, 2017, 1202459. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.M.; Mills, K.F.; Satoh, A.; Imai, S. Age-Associated Loss of Sirt1-Mediated Enhancement of Glucose-Stimulated Insulin Secretion in Beta Cell-Specific Sirt1-Overexpressing (BESTO) Mice. Aging Cell 2008, 7, 78–88. [Google Scholar] [CrossRef]
- Siegel, C.S.; McCullough, L.D. Nicotinamide Adenine Dinucleotide (NAD+) and Nicotinamide: Sex Differences in Cerebral Ischemia. Neuroscience 2013, 237, 223–231. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019, 30, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Das, S.K.; Pieper, C.F.; Lewis, M.R.; Klein, S.; Dixit, V.D.; Gupta, A.K.; Villareal, D.T.; Bhapkar, M.; Huang, M.; et al. Long-Term Moderate Calorie Restriction Inhibits Inflammation without Impairing Cell-Mediated Immunity: A Randomized Controlled Trial in Non-Obese Humans. Aging 2016, 8, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.D.; Quigley, S.N.Z.; Chachra, S.S.; Conlon, N.; Ford, D. The Use of a Systems Approach to Increase NAD+ in Human Participants. npj Aging 2024, 10, 1–12. [Google Scholar] [CrossRef]
- Uddin, G.M.; Youngson, N.A.; Sinclair, D.A.; Morris, M.J. Head to Head Comparison of Short-Term Treatment with the NAD+ Precursor Nicotinamide Mononucleotide (NMN) and 6 Weeks of Exercise in Obese Female Mice. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Llobet Rosell, A.; Paglione, M.; Gilley, J.; Kocia, M.; Perillo, G.; Gasparrini, M.; Cialabrini, L.; Raffaelli, N.; Angeletti, C.; Orsomando, G.; et al. The NAD+ Precursor NMN Activates dSarm to Trigger Axon Degeneration in Drosophila. eLife 2022, 11, e80245. [Google Scholar] [CrossRef] [PubMed]
- Sambashivan, S.; Freeman, M.R. SARM1 Signaling Mechanisms in the Injured Nervous System. Curr. Opin. Neurobiol. 2021, 69, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Zhu, J.; Shi, Y.; Gu, W.; Kobe, B.; Ve, T.; DiAntonio, A.; Milbrandt, J. Nicotinic Acid Mononucleotide Is an Allosteric SARM1 Inhibitor Promoting Axonal Protection. Exp. Neurol. 2021, 345, 113842. [Google Scholar] [CrossRef]
- Kiss, T.; Nyúl-Tóth, Á.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.D.; Garman, L.; et al. Nicotinamide Mononucleotide (NMN) Supplementation Promotes Neurovascular Rejuvenation in Aged Mice: Transcriptional Footprint of SIRT1 Activation, Mitochondrial Protection, Anti-Inflammatory, and Anti-Apoptotic Effects. GeroScience 2020, 42, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, L.; Farokhi-Sisakht, F.; Badalzadeh, R.; Khabbaz, A.; Mahmoudi, J.; Sadigh-Eteghad, S. Nicotinamide Mononucleotide and Melatonin Alleviate Aging-Induced Cognitive Impairment via Modulation of Mitochondrial Function and Apoptosis in the Prefrontal Cortex and Hippocampus. Neuroscience 2019, 423, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Valcarcel-Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Tucsek, Z.; Kiss, T.; Hertelendy, P.; Kinter, M.; Ballabh, P.; Süle, Z.; et al. Nicotinamide Mononucleotide (NMN) Supplementation Rescues Cerebromicrovascular Endothelial Function and Neurovascular Coupling Responses and Improves Cognitive Function in Aged Mice. Redox Biol. 2019, 24, 101192. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, X.; Xu, K.; Liu, S.; Zhu, X.; Yang, J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: An Update. Adv. Nutr. 2023, 14, 1416–1435. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-C.; Kong, Y.-Y.; Li, G.-Q.; Guan, Y.-F.; Wang, P.; Miao, C.-Y. Nicotinamide Mononucleotide Attenuates Brain Injury after Intracerebral Hemorrhage by Activating Nrf2/HO-1 Signaling Pathway. Sci. Rep. 2017, 7, 717. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-C.; Kong, Y.-Y.; Hua, X.; Li, G.-Q.; Zheng, S.-L.; Cheng, M.-H.; Wang, P.; Miao, C.-Y. NAD Replenishment with Nicotinamide Mononucleotide Protects Blood–Brain Barrier Integrity and Attenuates Delayed Tissue Plasminogen Activator-Induced Haemorrhagic Transformation after Cerebral Ischaemia. Br. J. Pharmacol 2017, 174, 3823–3836. [Google Scholar] [CrossRef]
- Park, J.H.; Long, A.; Owens, K.; Kristian, T. Nicotinamide Mononucleotide Inhibits Post-Ischemic NAD+ Degradation and Dramatically Ameliorates Brain Damage Following Global Cerebral Ischemia. Neurobiol. of Dis. 2016, 95, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Nascimento-Ferreira, I.; Orsomando, G.; Mori, V.; Gilley, J.; Brown, R.; Janeckova, L.; Vargas, M.E.; Worrell, L.A.; Loreto, A.; et al. A Rise in NAD Precursor Nicotinamide Mononucleotide (NMN) after Injury Promotes Axon Degeneration. Cell Death Differ. 2015, 22, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Figley, M.D.; Gu, W.; Nanson, J.D.; Shi, Y.; Sasaki, Y.; Cunnea, K.; Malde, A.K.; Jia, X.; Luo, Z.; Saikot, F.K.; et al. SARM1 Is a Metabolic Sensor Activated by an Increased NMN/NAD+ Ratio to Trigger Axon Degeneration. Neuron 2021, 109, 1118–1136. [Google Scholar] [CrossRef] [PubMed]
- Essuman, K.; Summers, D.W.; Sasaki, Y.; Mao, X.; DiAntonio, A.; Milbrandt, J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity That Promotes Pathological Axonal Degeneration. Neuron 2017, 93, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.O.; Allen, H.M.; Yu, L.; Li-Kroeger, D.; Bakhshizadehmahmoudi, D.; Hatcher, A.; McCabe, C.; Xu, J.; Bjorklund, N.; Taglialatela, G.; et al. NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies. PLOS Biol. 2016, 14, e1002472. [Google Scholar] [CrossRef] [PubMed]
- Milde, S.; Adalbert, R.; Elaman, M.H.; Coleman, M.P. Axonal Transport Declines with Age in Two Distinct Phases Separated by a Period of Relative Stability. Neurobiol. Aging 2015, 36, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in Inner Retinal Function in Glaucoma with Nicotinamide (Vitamin B3) Supplementation: A Crossover Randomized Clinical Trial. Clin. Experiment. Opthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- De Moraes, C.G.; John, S.W.M.; Williams, P.A.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M. Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glaucoma: A Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 11–18. [Google Scholar] [CrossRef]
- Nomiyama, T.; Setoyama, D.; Yasukawa, T.; Kang, D. Mitochondria Metabolomics Reveals a Role of β-Nicotinamide Mononucleotide Metabolism in Mitochondrial DNA Replication. J. Biochem. 2022, 171, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Chen, Z.; Huang, Y.; Xia, Y.; Chen, A.; Lu, D.; Wu, Y.; Zhang, N.; Zhang, P.; Li, S.; et al. Overexpression of the Histidine Triad Nucleotide-Binding Protein 2 Protects Cardiac Function in the Adult Mice after Acute Myocardial Infarction. Acta Physiol. 2020, 228, e13439. [Google Scholar] [CrossRef] [PubMed]
- Mengkang, F.; Huang, Y.I.N.; Qian, J. Histidine Triad Nucleotide-Binding Protein 2 Protects Cardiomyocytes via Maintaining NAD Homeostasis in Virto and in Vivo. Eur. Heart J. 2020, 41, ehaa946–0843. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Xie, X.J.; Li, W.H.; Liu, J.; Chen, Z.; Zhang, B.; Li, T.; Li, S.L.; Lu, J.G.; Zhang, L.; et al. A Cell-Permeant Mimetic of NMN Activates SARM1 to Produce Cyclic ADP-Ribose and Induce Non-Apoptotic Cell Death. iScience 2019, 15, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Loreto, A.; Angeletti, C.; Gu, W.; Osborne, A.; Nieuwenhuis, B.; Gilley, J.; Merlini, E.; Arthur-Farraj, P.; Amici, A.; Luo, Z.; et al. Neurotoxin-Mediated Potent Activation of the Axon Degeneration Regulator SARM1. eLife 2021, 10, e72823. [Google Scholar] [CrossRef]
- Chellappa, K.; McReynolds, M.R.; Lu, W.; Zeng, X.; Makarov, M.; Hayat, F.; Mukherjee, S.; Bhat, Y.R.; Lingala, S.R.; Shima, R.T.; et al. NAD Precursors Cycle between Host Tissues and the Gut Microbiome. Cell Metab. 2022, 34, 1947–1959. [Google Scholar] [CrossRef]
- Shi, W.; Hegeman, M.A.; van Dartel, D.A.M.; Tang, J.; Suarez, M.; Swarts, H.; van der Hee, B.; Arola, L.; Keijer, J. Effects of a Wide Range of Dietary Nicotinamide Riboside (NR) Concentrations on Metabolic Flexibility and White Adipose Tissue (WAT) of Mice Fed a Mildly Obesogenic Diet. Mol. Nutr. Food Res. 2017, 61, 1600878. [Google Scholar] [CrossRef] [PubMed]
- Breton, M.; Costemale-Lacoste, J.-F.; Li, Z.; Lafuente-Lafuente, C.; Belmin, J.; Mericskay, M. Blood NAD Levels Are Reduced in Very Old Patients Hospitalized for Heart Failure. Experimental Gerontology 2020, 139, 111051. [Google Scholar] [CrossRef]
- Kuerec, A.H.; Wang, W.; Yi, L.; Tao, R.; Lin, Z.; Vaidya, A.; Pendse, S.; Thasma, S.; Andhalkar, N.; Avhad, G.; et al. Towards Personalized Nicotinamide Mononucleotide (NMN) Supplementation: Nicotinamide Adenine Dinucleotide (NAD) Concentration. Mechanisms of Ageing and Development 2024, 218, 111917. [Google Scholar] [CrossRef]
- Luongo, T.S.; Eller, J.M.; Lu, M.-J.; Niere, M.; Raith, F.; Perry, C.; Bornstein, M.R.; Oliphint, P.; Wang, L.; McReynolds, M.R.; et al. SLC25A51 Is a Mammalian Mitochondrial NAD+ Transporter. Nature 2020, 588, 174–179. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Biosynthetic pathways of NAD+ synthesis in mammalian cells. The salvage pathway is the body’s main and most efficient source of NAD+. Abbreviations: 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxy anthranilic acid; ACMS, 2-amino-3-carboxymuconic semialdehyde; QPRT, quinolinate phosphoribosyltransferase; NA, nicotinic acid; NAPRT, nicotinic acid phosphoribosyltransferase; NAMN, nicotinic acid mononucleotide; NMNAT, nicotinamide mononucleotide adenylyltransferase; NAAD, nicotinic acid adenine dinucleotide; NADS, NAD+ synthetase; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide; NRK, nicotinamide riboside kinase; NAD+/NADH, nicotinamide adenine dinucleotide; NAM, nicotinamide; NAMPT, nicotinamide phosphoribosyltransferase; 3-HAAO, 3-Hydroxyanthranilate 3,4-Dioxygenase; NADP/NADPH, Nicotinamide adenine dinucleotide phosphate; TDO2, Tryptophan 2,3-dioxygenase; KYNU, kynureninase KFase, kynurenine formidase, KMO, Kynurenine 3-Monooxygenase.
Figure 1.
Biosynthetic pathways of NAD+ synthesis in mammalian cells. The salvage pathway is the body’s main and most efficient source of NAD+. Abbreviations: 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxy anthranilic acid; ACMS, 2-amino-3-carboxymuconic semialdehyde; QPRT, quinolinate phosphoribosyltransferase; NA, nicotinic acid; NAPRT, nicotinic acid phosphoribosyltransferase; NAMN, nicotinic acid mononucleotide; NMNAT, nicotinamide mononucleotide adenylyltransferase; NAAD, nicotinic acid adenine dinucleotide; NADS, NAD+ synthetase; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide; NRK, nicotinamide riboside kinase; NAD+/NADH, nicotinamide adenine dinucleotide; NAM, nicotinamide; NAMPT, nicotinamide phosphoribosyltransferase; 3-HAAO, 3-Hydroxyanthranilate 3,4-Dioxygenase; NADP/NADPH, Nicotinamide adenine dinucleotide phosphate; TDO2, Tryptophan 2,3-dioxygenase; KYNU, kynureninase KFase, kynurenine formidase, KMO, Kynurenine 3-Monooxygenase.
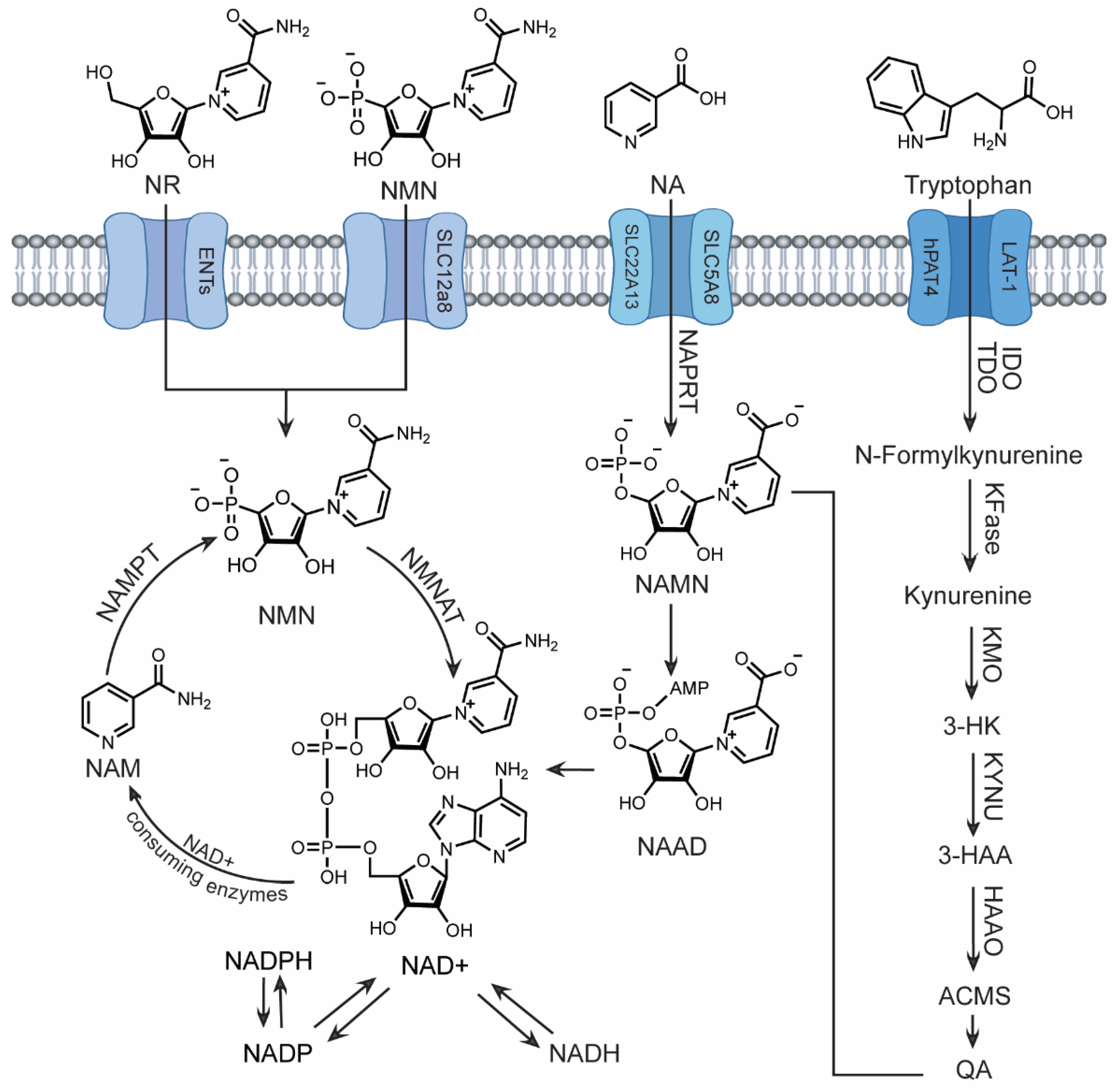
Figure 2.
Metabolic fates of orally administered NAD+ Precursors. 1. NMN can be deaminated by the bacterial enzyme pncC to form NAMN. Alternatively, NMN may be broken down by other gut enzymes to produce NR or NAM, which is then metabolized by pncA to form NA and follow the Preiss Handler pathway. This deamidated route results in increased levels of NAR and NAMN in the gut and liver, and elevated NAAD specifically in the liver. A portion of oral NMN may follow the salvage route after direct entry through SLC12a8 or indirect entry through ENTs via conversion to NR. 2. During the early phase of metabolism, oral NR can directly enter cells and undergo the canonical salvage pathway. In the later phase, NR is converted into NAM by BST1, resulting in increased NA in the gut, elevated NA and NAR in the portal blood, and an increase in both amidated (NA, NAR, NAMN, and NAAD) and deamidated (NAM and NMN) metabolites in the liver. Additionally, NR may undergo a BST-1 catalyzed base-exchange reaction using NAM and NR, converting it to NAR. 3. NAM is rapidly converted to NA by the bacterial enzyme pncA in the colon, leading to elevation of deamidated precursors (NAMN, NAR, and NAAD) as well as amidated precursors (NAM and NMN) in the gut cell, portal vein, and liver.
Figure 2.
Metabolic fates of orally administered NAD+ Precursors. 1. NMN can be deaminated by the bacterial enzyme pncC to form NAMN. Alternatively, NMN may be broken down by other gut enzymes to produce NR or NAM, which is then metabolized by pncA to form NA and follow the Preiss Handler pathway. This deamidated route results in increased levels of NAR and NAMN in the gut and liver, and elevated NAAD specifically in the liver. A portion of oral NMN may follow the salvage route after direct entry through SLC12a8 or indirect entry through ENTs via conversion to NR. 2. During the early phase of metabolism, oral NR can directly enter cells and undergo the canonical salvage pathway. In the later phase, NR is converted into NAM by BST1, resulting in increased NA in the gut, elevated NA and NAR in the portal blood, and an increase in both amidated (NA, NAR, NAMN, and NAAD) and deamidated (NAM and NMN) metabolites in the liver. Additionally, NR may undergo a BST-1 catalyzed base-exchange reaction using NAM and NR, converting it to NAR. 3. NAM is rapidly converted to NA by the bacterial enzyme pncA in the colon, leading to elevation of deamidated precursors (NAMN, NAR, and NAAD) as well as amidated precursors (NAM and NMN) in the gut cell, portal vein, and liver.
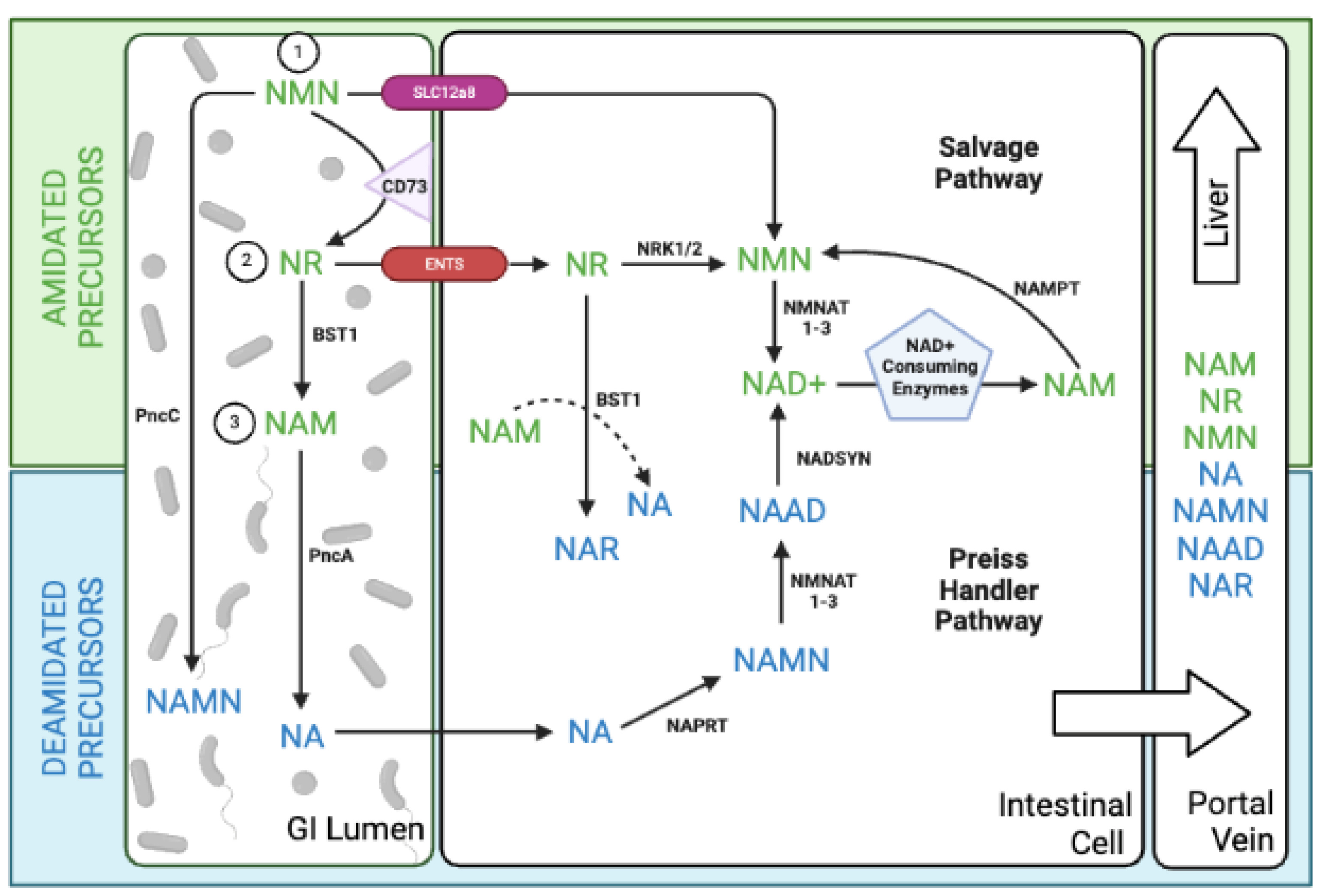
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






