1. Introduction
Cancer refers to a group of diseases characterized by the uncontrolled growth and malignancy of cells in the body. Lung cancer is among the top five highly diagnosed cancers in the world and the second most diagnosed cancer in both men and women, and the most common cause of death due to cancer. The very high mortality rate also surpasses the death occurred due to breast, colorectal, and prostate cancers combined. The primary cause of this illness is cigarette smoking, which accounts for about 80% of instances of lung cancer [
1]. Lung cancer has a very high incidence rate with, an estimated 238,340 people (117,550 men and 120,790 women) will be diagnosed with lung cancer, and 127,070 people will die from the disease. In India, lung cancer accounts for 5.9% of all cancers and 8.1% of all cancer-related deaths [
2]. As per report in general population approximately 6.2% of men and 5.8% of women have propensity to acquire lung cancer in their lifetime, meaning that 1 in 16 men and 1 in 17 women are at risk to develop lung cancer in lifetime primarily the smokers/Ex smokers are at higher risk [
3]. Since most of the cases of lung cancer are diagnosed at later stages, making the treatment ineffective, and thus the disease become typically lethal. However, in the past twenty years, exciting gains in survival rate have resulted from the advancement in the screening techniques and their implementation, development of tailored treatments, and growing understanding of cancer biology. Primarily two types of lung cancer known are, the non-small cell lung cancer (NSCLC) that accounts for 80% of cases, and the small cell lung cancer (SCLC), which accounts for 15% of cases. NSCLC is further categorized as adenocarcinoma, squamous cell carcinoma and large cell carcinoma [
4]. Adenocarcinoma mainly originates in the glands that secrets mucus whereas squamous cell carcinoma is mainly seen in epithelium lining of lungs. In contrast to adenocarcinoma, squamous cell carcinoma is more aggressive and develops from cells lining of the airways in lungs. In comparison with the other two NSCLC subtypes, large cell carcinoma is more aggressive and can arise from any part of the lung [
5]. Small cell carcinoma, is the most prevalent subtype, followed by mixed small cell carcinoma. SCLC is typically more aggressive than NSCLC and is slightly more prevalent in women (14%) than in males (13%). As a result, at the time of diagnosis, the metastasis was observed more in SCLC patients than NSCLC patients that is the illness has migrated outside of the lungs (94% versus 70%) [
6].
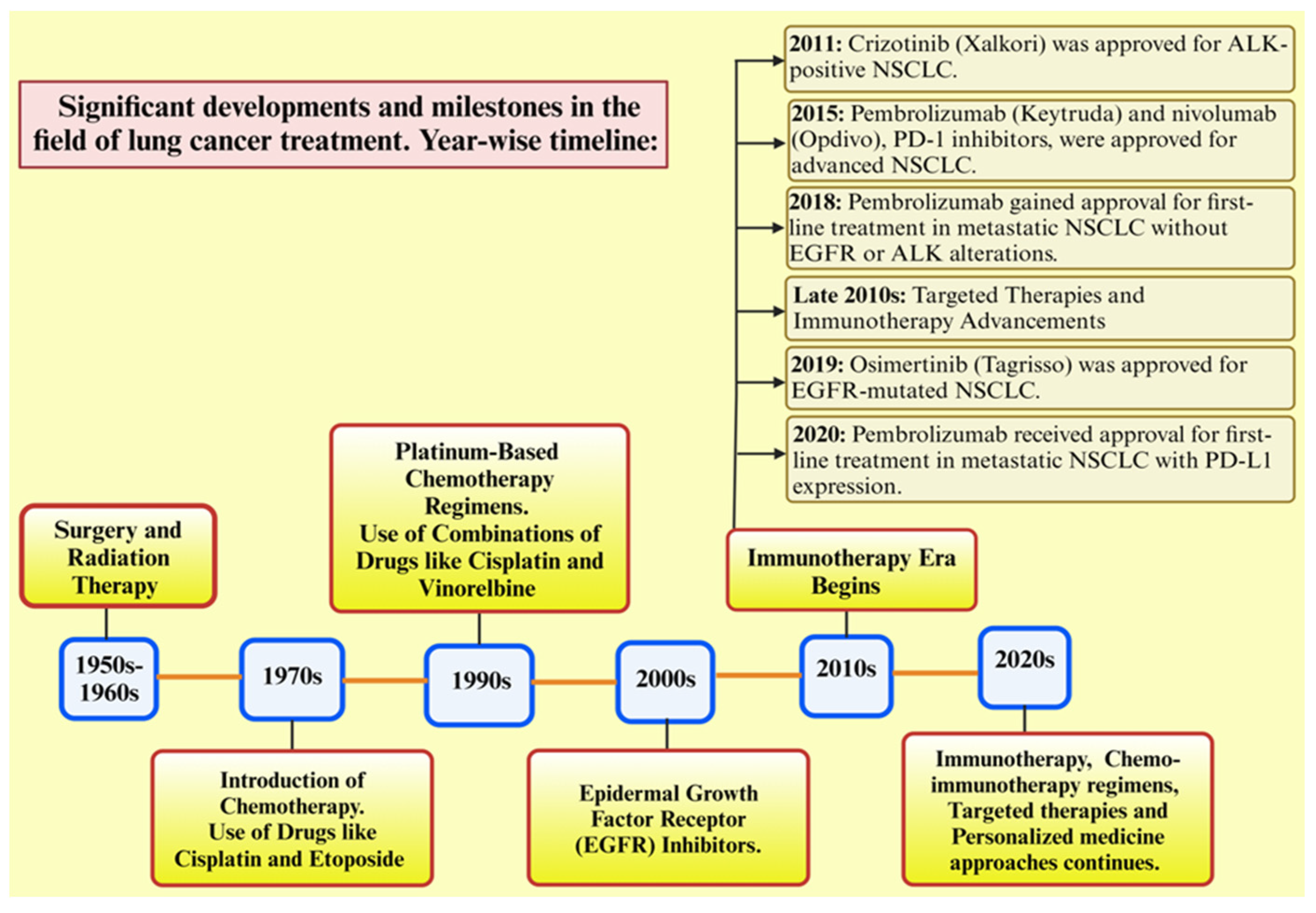
Figure 1, depicts the significant advancement in lung cancer. Despite these advances in therapeutic, resistance to drugs remains a major obstacle to the effectiveness of long-term treatment, eventually leading to therapeutic insensitivity, poor progression-free survival, and disease relapse [
7]. Drug resistance refers to the reduction in effectiveness of a medication, this phenomenon occurs when pathogens or cancers evolve and acquire resistance to the drugs used for treatment. Major treatment options for different types of lung cancer include surgery, chemotherapy, radiotherapy, immunotherapy, targeted therapy etc. Major issues related to drug resistance are therapeutic insensitivity, poor progression-free survival, and disease relapse. Genetic mutations, epigenetic modifications, uncontrolled drug efflux, tumour hypoxia, changes in the tumour microenvironment, and several other cellular and molecular changes are the causes of resistance mechanisms [
8].
Further, reprogramming of metabolic pathways is an essential characteristic of tumour cells. To meet the material, energy, and redox force requirements for fast multiplication, tumour cells remodel their metabolic pathways. Metabolic reprogramming through altering gene expression, cell state, and the tumour itself, causes certain metabolites to change in quantity or type both inside and outside of cells generating environment that is conducive for tumor growth and progression [
9]. The various metabolic changes take place in tumour cells are alteration in glucose metabolism, amino acid metabolism, and lipid metabolism. This reprogramming of tumour cells is beneficial for conferring drug resistance to many cancer cells [
10,
11,
12].
Consequently, this review aims to provide an overview of the currently availing therapeutic approaches to treat lung cancer, as well as issues pertaining to drug resistance that persist despite prolonged treatment. The respective review also delves drug resistance signaling pathways, and the role of tumour cell metabolic reprogramming in developing drug resistance in the tumour microenvironment. In addition, this work explores the junction of medication resistance and metabolic reprogramming to identify and create novel and effective therapeutic approaches for this persistent problem.
2. Signalling Pathways Involves in Lung Cancer
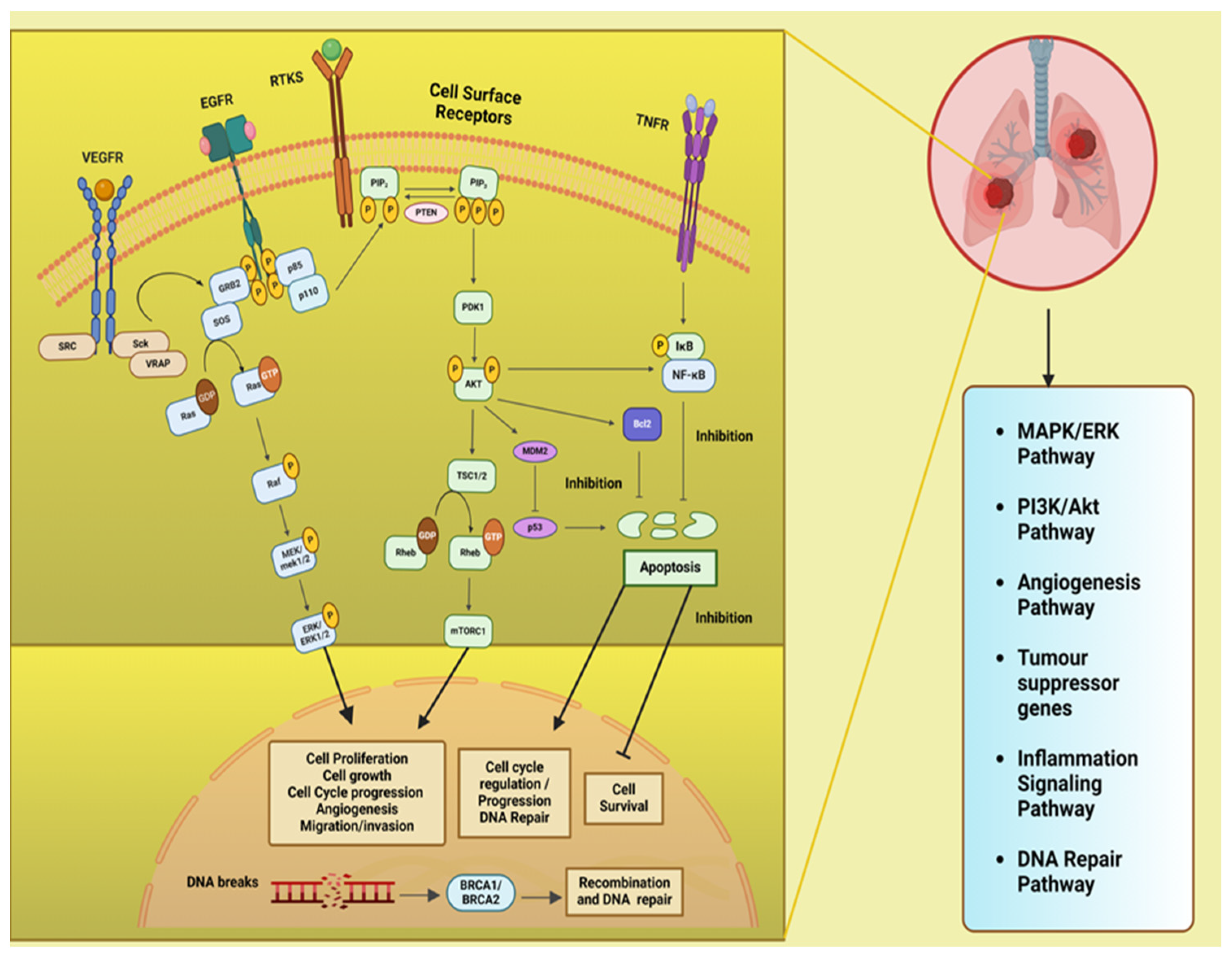
Figure 2, describe the different signalling pathway in lung which aberrations at different levels are involved in lung cancer development and progression. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is pivotal in regulating diverse cellular processes and is frequently dysregulated in cancers, playing a significant role in the initiation and advancement of tumors. The mitogen-activated protein kinases/extracellular signal-regulated kinase or the RAS/RAF/MEK/ERK (MAPK) are predominantly engage in the apoptosis, pathogenesis, progression survival and invasion. Besides there are other intracellular signaling in lung and which upon downregulation leads to cancer and are important targets for therapeutic treatments like the DNA repair pathways, inflammatory signaling pathway, ALK (Anaplastic Lymphoma Kinase) pathway, tumour suppressor genes. Additionally, these signalling pathways interact with one another; hence, enhancing one pathway may either strengthen or inhibit another [
13].
PI3K/AKT/mTOR signaling pathway and cross talk with Ras/Raf/MAPK (MEK)/ERK pathway and tumor supressor gene pathway: PI3K a family of lipid kinases and class IA PI3Ks comprises of a catalytic p110 subunit and a regulatory p85 subunit. When ligand bind to receptor tyrosine kinases (RTK) on cell membrane, such as epidermal growth factor receptors (EGFR), vascular endothelial growth factor receptors (VEGFR) and others, the PI3Ks activated, followed by the phosphorylation of PIP2 to PIP3 (phosphatidylinositol (4,5)–bisphosphate to phosphatidylinositol (3,4,5)–trisphosphate) which is catalyzed by the p110 subunit. The phosphatase and tensin homolog (PTEN) convert PIP3 to PIP2 and suppresses the PIP3-dependent processes and thus restricts cell survival, growth, and proliferation. Next, phosphoinositide-dependent protein kinase (PDK1) and mTORC1 (a serine/threonine kinase) mediate the phosphorylation and activation of Akt together. It is known that the activity of the kinase mTOR is negatively regulated by the TSC1/TSC2 complex (tuberous sclerosis protein 1/2) through inhibiting Rheb (Ras homolog enriched in brain) activation, a GTPase that activates mTOR. Further, the activated AKT kinases are capable of phosphorylating both TSC1 and TSC2. Akt also inhibits the pro-apoptotic Bcl-2 protein family members, p53 indirectly by up regulating MDM2 (mouse double minute 2 homolog), and NF-κB transcription factor, that causes an increased expression of cell survival and anti-apoptotic signals. The PI3K/Akt/mTOR pathway deregulation found to involve in tumorigenesis and disease progression in NSCLC. Various cellular processes such as survival, proliferation, migration, metastasis, angiogenesis, cellular metabolism, cellular senescence, genomic integrity, have been associated to the PI3K/Akt/mTOR signaling pathway in lung cancer [
13,
14,
15]. The VEGF/ VEGFR-2 dimers mediate tumor metastasis and angiogenesis and tumor survival, through two major signaling pathways branches, Ras/MAPK and PI3K/Akt. The VEGF on binding to VEGFR causes dimerization and phosphorylation of the intracellular tyrosine kinase domain that is followed by the activation of downstream proteins such as c-SRC (proto-oncogene tyrosine-protein kinase Src), Sck (Shc-like protein), and VRAP (VEGF receptor-associated protein). Activation of c-Src involves the adaptor molecule GRB2 (Growth factor receptor-bound protein 2)–associated binder 1. The adaptor proteins like GRB2 and SOS (Son of Sevenless), then recruit Ras (is a type of small GTP-binding protein) and phosphatidylinositol 3-kinase (PI3K), leading to the formation of signaling pathways branches, Ras/MAPK and PI3K/Akt [
16].
Mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK (MEK)/ERK) or the Ras/Raf/MAPK (MEK)/ERK pathway: After membrane receptor activation, adaptor proteins (like SOS) recruit Ras proteins to activate Raf (a serine/threonine protein kinase) that get phosporylated and activates MAPK kinase (MEK) followed by its phosphorylation and activation of ERK (extracellular signal-regulated kinase). ERK activates its cytoplasmic and/or nuclear targets. The PI3K/AKT pathway also interacts with the MAPK/ERK node under normal conditions and in the cancer cell [
16,
17].
Inflammatory signaling pathway: The TNF (Tumour necrosis factor) binds and activates the TNF receptors TNFR1 or TNFR2 that in turn activates the inflammatory signaling pathway. Both the cytokine and its receptors TNFR1 and TNFR2 are expressed in lung cancer. The TNF stimulate the activation of NF-κB (nuclear factor-κB), which is triggered by activation of inhibitor of NF-κB (IκB) kinase (IKK). The NF-κB translocates into the nucleus and promotes transcription of many NF-κB target genes. Many of which (like FLIP: cellular FLICE-inhibitory protein, BCL-XL: B-cell lymphoma-extra-large and others) have anti-apoptosis and pro-survival function [
18].
DNA repair pathway: The tumor suppressor proteins viz. breast cancer associated proteins BRCA1 and BRCA2 play an important role in the repair of DNA through a specific type of DNA repair pathway called double-strand break repair by homologous recombination (HR) repair. Many other genes known as BRCA-related genes also participate directly or indirectly. Mutations in the BRCA1/2 genes were observed in 5–10% of NSCLC cases [
19]. The BRCA1 and BRCA2 regulate HR repair through the assembly of the DNA recombinase-RAD51 onto broken DNA ends at the site of double strand breaks (DSBs) and held up replication forks [
20].
ALK (Anaplastic Lymphoma Kinase) pathway: Fusion of ALK with other genes leads to constitutive ALK activation, promoting cell proliferation and survival through downstream signaling pathways including PI3K/AKT and MAPK/ERK [
21].
3. Mechanisms of Drug Resistance and Metabolic Reprogramming
3.1. Molecular Basis of Drug Resistance:
One of the significant issues that reduce the efficacy of chemotherapies employed to treat cancer is drug resistance. Prior to treatment, tumours may have an innate resistance to chemotherapy. However, tumours that are initially responsive to chemotherapy may potentially develop drug resistance while undergoing treatment. More than 90% of patients with metastatic cancer are thought to experience treatment failure because of resistance to chemotherapy. In addition, the resistant micro metastatic tumour cells may also lessen the efficacy of chemotherapy in the adjuvant setting [
22]. Analysis of some majorly mutated molecules in lung cancer at molecular level are KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; 25%), EGFR (epidermal growth factor receptor; 23%), ALK (anaplastic lymphoma kinase; 6%), PIK3CA (phosphoinositide-3-kinase, catalytic, alpha polypeptide; 3%), BRAF (v-Raf murine sarcoma viral oncogene homolog B1; 3%), and HER2 (human epidermal growth factor receptor 2; 1%) [
23].
3.2. Metabolic Pathways and their Alterations in Lung Cancer
Alteration in major metabolic pathways in signaling pathways involve in lung cancer progression are glycolysis, amino acid metabolic pathways (glutamine, serine, glycine, tryptophan), fatty acid synthesis pathways etc. Metabolic reprogramming or alteration is due to downregulation or upregulation of different metabolites levels in tumour cells as compared to normal cells [
24,
25]. It will be easier to implement and evaluate novel treatment strategies and targeted medicines for patients with lung cancer (LC) if these metabolic abnormalities are understood and the relevant metabolites that differ from the normal metabolites are identified. Changes in metabolomic pathways were verified by quantitative proteomics analysis of the main enzymes found in the disrupted pathways. This analysis identified 13 distinct biomarkers linked to metabolic disruption of NSCLC morbidity, implicated in four major pathways: aminoacyl-tRNA biosynthesis, glycine, serine, and threonine metabolism, tyrosine metabolism, and sphingolipid metabolism. Important enzymes involved in these pathways, including phosphoserine phosphatase, argininosuccinic acid catenase, tyrosinase, and 3-phosphoglycerate dehydrogenase, showed distinct variations in expression, according to the proteomics study [
26].
An increased in levels of glucose transporters (GLUT) in tumour cell is responsible for altered metabolism, GLUT 3 and GLUT 5 both are found to be higher in tumour cells. There are metabolic changes due to elevated glucose level inside cells [
27]. Warberg effect is evident in lung cancer cells, where glucose is metabolized via lactic acid fermentation rather than breakdown to pyruvate in normal cells. Tumour M2-Pyruvate Kinase (PKM2) is a glycolytic enzyme that is detected to be increased in LC and several other malignancies. It is necessary for the malignant transformation. [
28]. Lactate formed inside the cell is transported with help of transporter called monocarboxylate transporters (MCT), This lactate shuttle, mainly via MCT1 and MCT4, is an important way that cancer tissue keeps the balance of the connections between glycolytic and oxidative cells [
29].
Lung cancer cells show an increased uptake of glutamine. Once glutamine enters the cell, it is converted into glutamate which is further converted to α ketoglutarate with the help of the enzyme called glutaminase, this process is called glutaminolysis. Alanine-serine-cysteine-transporter-2 (ASCT2 or SLC1A5) mediates the uptake of these glutamines into the cancer cells, and it has been noted that LC patients express these transporters with greater frequency [
30]. L-type amino acid transporter 1 (LAT1), also known as SLC7A5/SLC3A2, is one of the various types of transporters found in LC patients. This is in the role of exchanging glutamine for vital amino acids like phenylalanine, valine, and methionine [
31]. Another transporter, SLC38A3, controls the transfer of glutamine and histidine, which in turn promotes the metastasis of NSCLC by modulating the PDK1/AKT signalling cascade, HIF-1α activates SLC38A1 and provides a variety of mechanisms to alter the glutamine level in the cell, whereas HIF-2α controls and upregulates the expression of SLC1A5 [
32,
33].
The serine synthesis pathway is a crucial pathway for energy production in cancer cells along with glycolysis. The precursors namely 3-phosphoglycerate and glutamate, which are generated by the glycolysis and glutaminolysis pathways, respectively, drive the manufacture of serine. Glycine that produced during the glycolysis process can be used as a source of carbon for the one-carbon metabolism [
34]. An enzyme known as phosphoserine aminotransferase (PSAT1) helps oxidise and catalyze the precursor 3-phosphoglycerate into 3-phosphoserine and α-KG (alpha- ketoglutarate), which is subsequently transformed to serine with the aid of another enzyme known as 1-3-phosphosrine phosphatase [
35]. It was found that NRF2 (nuclear factor erythroid 2–related factor 2) controls the expression of the critical serine synthesis enzymes PHGDH (phosphoglycerate dehydrogenase), PSAT1, and SHMT2 (Serine hydroxymethyltransferase-2) by activating ATF4 (Activating transcription factor 4) to promote glutathione and nucleotide formation. PSAT1 overexpression worsens the prognosis of cancer by encouraging cancer cell proliferation, metastasis, and chemoresistance [
36].
Glycine decarboxylase is necessary for cells that initiate tumours. When glycine decarboxylase is inhibited in cells with elevated serine hydroxy-methyltransferase 2 (SHMT2) levels, the accumulation of glycine is converted into toxic compounds such as methylglyoxal and aminoacetone, which results in cell growth arrest [
37].
Fatty acid synthesis pathways are also affected in lung cancer cells.The de novo production of fatty acids requires the enzyme FASN (fatty acid synthase), which has been shown to be highly expressed in NSCLC patients. Additionally, FASN was discovered to have a role in the metabolism of glucose by inhibiting the AKT/ERK pathway, which modified the LC cells [
38]. There are some other enzymes upregulated in different types of lung cancer cells like ATP citrate lyase enzyme (ACLY), AcCoA carboxylase (ACC1/2) [
39]. Cholesterol synthesis is also affected, as reported in an in vitro study that shows that the presence of 25-Hydroxycholesterol increased the LC cell growth and invasion. A vital regulator of lung development and morphogenesis, thyroid transcription factor 1 (TTF-1) specifically targets ATP-binding cassette transporter A1 (ABCA1) in lung cancer cells. TTF-1 may be utilised as a diagnostic marker for LC as it was discovered to be overexpressed in the LC samples [
40].
4. Current Therapeutic Approaches and Metabolic Considerations
4.1. Overview of Current Lung Cancer Therapies
There is some difference in the treatment approach of both types of lung cancer that is, NSCLC and SCLC.SCLC is more aggressive form of lung cancer which is diagnosed at a later stage and in most of the cases there are metastases, so in this case normally the treatment strategy used are chemotherapy, radiotherapy, targeted therapy, and immunotherapy. However, surgery is very limited in this case. In the case of NSCLC, chemotherapy, chemoradiotherapy, radiotherapy, targeted therapy, immunotherapy as well as surgery are used.
4.1.1. Chemotherapy
Chemotherapy is always being the first line of treatment for cancer. It is a standard treatment used in lung cancer. Chemotherapeutic drugs fall into four main categories: (1) alkylating agents (like platinum compounds like cisplatin and carboplatin), (2) microtubule-targeting drugs (like vinorelbine, paclitaxel, and docetaxel), (3) antimetabolites (like pemetrexed and gemcitabine), and (4) topoisomerase inhibitors (like etoposide) [
41]. In NSCLC, gemcitabine, taxanes, pemetrexed, and cisplatin and carboplatin are the main chemotherapy ingredients. Targeted therapy medications including EGFR inhibitors (erlotinib) and VEGFR inhibitors (bevacizumab) are also used. The mode of action of these drugs are different, cisplatin, carboplatin, and gemcitabine interfere with the DNA repair system, create DNA damage, and induce programmed cell death (apoptosis) in the cancer cell [
42], taxane based drugs disturbs microtubule dynamics, trigger cell cycle arrest, and induce apoptosis [
43]. Whereas, pemetrexed, methotrexate, areantifolate drugs, could cause cell cycle arrest in the S phase [
44]. For metastatic SLCL, the first line of treatment includes a combination of platinum and etoposide, nevertheless it shows a very poor survival rate as it relapses within one year [
45]. The only medication that is currently according to the accepted standard of care for second-line treatment for SCLC is topotecan [
46].
4.1.2. Radiotherapy
Radiotherapy is usually used in combination with chemotherapy especially in stage III unresectable NSCLC. The adverse effects of radiation therapy have decreased due to the development of intensity-modulated radiotherapy (IMRT), stereotactic body radiation (SBRT), and four-dimensional computed tomography (4DCT) [
47]. The mode of action is mostly caused by DNA damage, as the damaged DNA can trigger immune responses in lung cancer. As a result, combination of radiotherapy and immunotherapy could improve the recovery in lung cancer patients [
48]. If compared to conventional chemoradiotherapy, platinum-based chemotherapy, radiotherapy, and durvalumab (an immune checkpoint inhibitor of PD-L1), combinational therapy for lung cancer is more beneficial, according to a phase 3 trial report. This could significantly extend the overall survival (up to 4 years) for patients with stage III NSCLC [
49]. For SCLC patients, radiotherapy in combination with chemotherapy can be used in initial stage of treatment, but cannot be used in later metastases stage. Around 30% of newly diagnosed SCLC, the standard treatment with curative intent consists of four cycles of platinum-doublet chemotherapy combined with radiotherapy [
50].
4.1.3. Targeted Therapy
In lung cancer, targeted therapy refers to the application of medications or other compounds that specifically target and obstruct the growth and metastasis of cancer cells. These treatments function by either focusing on certain chemicals implicated in the initiation and spread of cancer or by obstructing the signals that propel the growth of cancer cells. Anaplastic Lymphoma Kinase (ALK), Epidermal Growth Factor Receptor (EGFR), ROS1 (ROS proto-oncogene 1), serine/threonine-protein kinase B-Raf (BRAF) inhibitors are now the mainstays of lung cancer therapy. However, a new class of medications has emerged recently that can target the formerly "un-druggable" KRAS mutation [
51,
52]. Certain drugs approved by FDA include, gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib, which are used as EGFR inhibitors. ALK inhibitors are crizotinib, alectinib, brigatinib, ceritinib, and lorlatinib. ROS 1 inhibitors are crizotinib, lorlatinib, entrectinib and brigatinib. RET (rearranged during transfection) inhibitors are pralsetinib and selpercatinib [
53,
54]. The FDA-approved medications improved patients' overall survival (OS) by a large amount. The median prolong free survival (mPFS) was improved by approximately 10.8 months with gefitinib, approximately 14 months with erlotinib, approximately 48 months with afatinib, and up to 14.7 months with dacomitinib [
55,
56]. There are certain drugs under clinical trial like pyrotinib for advanced NSCLC with HER2 mutation was proved to prolong the PFS of patients for 6.9 months and the median OS for 14.4 months in clinical trial [
57]. There are some combinational therapies uses for lung cancer like erlotinib an EGFR inhibitor and bevacizumab a monoclonal antibody targeting VEGF could prolong the PFS of NSCLC patients..In advanced NSCLC with an EGFR mutation, apatinib a VEGFR inhibitor plus gefitinib, a first-generation EGFR TKI (tyrosine kinase inhibitor) may extend the mPFS for 19.2 months. However, this combination therapy has some adverse effects and lowers quality of life [
58].
4.1.4. Immunotherapy
Immunotherapy is a newer therapeutic approach for cancer treatment, and is appealing to researchers because of its promising result in dealing the disease. It entails boosting the immune system's capacity to identify and combat cancerous cells. Even while cancer cells frequently find ways to avoid detection, the immune system can identify and eliminate aberrant cells, including cancer cells. The goal of immunotherapy is to get past these defence mechanisms and improve the immune system's capacity to recognise and destroy cancer cells [
59]. This therapy mainly focuses on inhibition of immune checkpoints cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), programmed death 1 (PD-1), programmed death-ligand 1(PD-L1). These antibodies appear to have the potential to restore antitumor immunity by focusing on the host immune system to force it to combat cancer cells [
60]. Currently FDA approved immunotherapies in use are adoptive T-cell therapy, cancer vaccine, immune checkpoint inhibitors (ICIs), and cytokine modulators. Some of the monoclonal antibodies used for treating lung cancer are anti-CTLA-4 antibody ipilimumab, anti-PD-1 antibodies pembrolizumab, and nivolumab, and anti-PD-L1 antibodies atezolizumab, durvalumab, and avelumab [
61]. The overall survival and effect of these drugs are different in patients according to their mutation. It was shown that in metastatic NSCLC patients with high PD-L1 expression (≥50%) and without EGFR or ALK mutation drugs like pembrolizumab and atezolizumab could improve PFS for 10.3 months and median OS for 15.5 months [
62]. In a phase III trial, durvalumab was shown to improve PFS up to 907 days and OS up to 1,420 days in patients with unresectable stage III NSCLC when chemotherapy and radiation failed [
63]. Immunotherapy is also used in combinational therapy along with chemotherapy and radiotherapy. There are various trials going on that suggest improvement in survival rate, when immunotherapy is used in combination with other monoclonal antibodies, chemotherapy, or radiotherapy, like durvalumab, an anti-PD-L1 antibody, has better results than when used alone. The most popular combination consists of radiation or chemotherapy along with anti-CTLA-4 antibody called tremelimumab and durvalumab [
64]. A phase 3 trial shows when pembrolizumab an anti-PD-1 antibody used with radiotherapy, could improve the overall survival of metastatic NSCLC patients [
65]. Immunotherapy is used as first line treatment for SCLC in combination with chemotherapy also shows good result in SCLC.
4.1.5. Antiangiogenic Therapy
Angiogenesis is a physiological process by which new blood vessels are formed. In cancer cells there is a significant increase in angiogenesis. In tumour microenvironment, as the cell proliferates it requires more blood vessels to supply blood so there is a increase in this process. The major angiogenic factors are, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), tumour necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), and the angiopoietins (Ang) [
66]. In the tumour microenvironment, VEGF may suppress the immune cells response, consequently VEGF inhibitors may also boost immune cell capability, and therefore antiangiogenic therapy could combine with immunotherapy to benefit cancer patients [
67]. The FDA has approved bevacizumab, a monoclonal antibody that targets VEGF, and it may be used in the treatment of non-small cell lung cancer. The bevacizumab in combination with monoclonal antibodies like atezolizumab are confirmed to be a potential therapy for the non-squamous NSCLC with higher PD-L1 expression (≥50%) but without EGFR/ALK/ROS1 mutations. In patients of NSCLC with KRAS and STK11 mutations, as well as STK11 (Serine/Threonine Kinase 11), KEAP1 (Kelch-like ECH-associated protein 1), TP53 (tumor protein p53), and/or strong PD-L1 expression (≥50%), bevacizumab in combination with atezolizumab and chemotherapy (carboplatin and paclitaxel) could serve as the first-line treatment [
68].
4.2. Impact on Metabolic Pathways
Lung cancer therapeutic approaches have a significant effect on metabolic pathways of cells. Metabolism refers to the complex set of chemical processes that occur within living organisms to maintain life. In recent time various metabolomics studies shows the difference in metabolic pathways in patients undergoing treatment. Study are there for the Proteomics, and genomics effect of chemotherapeutic drug anlotinib, a new oral tyrosine kinase inhibitor that targets platelet-derived growth factor receptor-α, c-Kit, fibroblast growth factor receptors 1, 2, and 3 (FGFR), vascular endothelial growth factor receptors 1, 2, and 3 (VEGF), and suppresses the proliferation of cancer cells [
69]. Anlotinib regulates some essential cancer cell metabolism like amino acids metabolism of tryptophan, threonine, glycine, serine, phenylalanine, and amino acid biosynthesis of valine, leucine, isoleucine pathways, and glyoxylate and decarboxylate pathways [
70]. There are some metabolites which act as potential biomarkers to check anlotinib efficacy like glycine-associated glycocholic and glycodeoxycholic acid [
71]. Some chemotherapeutic drugs show alteration in metabolism as it leads to a reduction in valine and lactate, ultimately showing relieved glycolysis. Drugs show their effect by reversing Warburg effect, also affects the high serum level of lipids, choline, and isobutyrate etc. A549 cells (adenocarcinomic) treated with cisplatin had increased lipid levels but decreased niacinamide levels [
72]. Chemotherapy causes the integrity of the cell membrane to be disrupted, which leads to cell membrane rupture and degradation. Lipids and glycoproteins are consequently more concentrated in the blood. Additionally, it has been proposed that chemotherapy raises the levels of the 3-hydroxybutyrate metabolite in sera, which in turn enhances lipolysis [
73]. Chemotherapy is necessary for many biological functions. It alters the energy metabolism, disrupts the production of phosphatidylcholine, disrupts the metabolites in lung cancer serum, and has an impact on DNA replication and microtubule function. Therefore, any chemotherapy treatment may disrupt metabolic pathways and result in the production of metabolites [
74]. Patients with lung cancer are expected to have altered metabolite profiles following surgery. Metabolomic studies could offer a deeper understanding of these operation-related modifications, as a study showed higher levels of sphingolipids such as ceramide and sphingomyelin in lung cancer patients compared to controls in both pre- and post-surgery patients [
75].
4.3. Considerations for Overcoming Drug Resistance
Drug resistance in lung cancer is a significant challenge in the management of the disease. It refers to the ability of cancer cells to survive and continue growing despite the presence of drugs designed to eliminate or control them. There are various mechanisms through which lung cancer cells can develop resistance to different types of treatment, including chemotherapy, targeted therapy, and immunotherapy. There are some key mechanisms by which lung cancer shows drug resistance that includes multidrug resistance (MDR), DNA repair mechanism, activation of alternative pathways, changes in tumour microenvironment, intertumoral heterogeneity etc. [
76]. Targeted and multifunctional nanoscale drug constructions have been developed as an outcome of nanotechnology advancements. Important biological barriers can be overcome with the use of promising methods including the encapsulation of different active treatments, surface functionalization of nanomedicine components, and potential regulation of these components. These characteristics improve the transport of various therapeutic drugs directly to the tumour microenvironment (TME), which reverses the anticancer treatment-resistant state of LC [
77]. Some of the newer nanoparticle-based approach includes applying magnetic nanoparticles (NPs), namely the commercially available ferrofluids, as intravenous medication delivery systems. After injection, the tumour site was exposed to an external magnetic field, which caused the NPs to accumulate there and decreased the drug's systemic toxicity. Moreover, NPs have the potential to serve as carriers of several anticancer agents, such as radionuclides, cancer-specific antibodies, and genes. On the other hand, within the past 10 years, photodynamic therapy (PDT) has become a viable therapeutic strategy for the treatment of cancer. As most photosensitizers exhibit hydrophobic properties, so suitable delivery mechanisms are necessary [
78]. Delivering siRNAs via NPs is a further novel approach to stop DR. Different siRNAs could be delivered in tandem to silence many genes, including the DR-related genes. The physicochemical characteristics of siRNAs hinder their cellular uptake due to their difficulty in navigating phospholipid membranes. Thus, the creation of novel RNAi technologies and suitable carriers is necessary. The technology of genome editing could offer a foundation for the creation of fresher, more effective strategies, another recent approach is the use of biocompatible compounds use of biopolymers, such as tamarind seed polysaccharide PST, to prepare PTX-loaded (Paclitaxel) NPs through epichlorohydrin crosslinking [
79]. Using nanoparticles to deliver therapy to cure drug resistant lung cancer is a growing area of research to deal with drug resistance issue.
5. Emerging Therapeutic Strategies Targeting Metabolism
Besides the present therapeutic approaches there are many new emerging treatment options under development, which mainly targets the metabolic changes occur due to cancer progression. Targeting metabolism has been an area of interest in the development of therapeutic strategies for lung cancer. Some major metabolic processes that can be targeted are, glucose metabolism inhibition, mitochondria, fatty acid, amino acid metabolism, targeting oncogenic pathways, and immunometabolism [
80]. Exciting developments in molecular targeted therapy and immunotherapy in combination with new surgical, pathological, radiographical, and radiation techniques have resulted in spectacular improvement in patients’ survival. KRAS is nearly exclusively seen in adenocarcinomas, while TP53 is the most frequently mutated gene in non-small cell lung cancers (NSCLCs). RAF-MEK-MAPK and PI3K-Akt-mTOR are the two signalling pathways that are most frequently altered in lung cancer [
81]. TP53 is mutated in 46% of NSCLC tumours. There is less apoptosis due to TP53-induced glycolysis and apoptosis regulator (TIGAR) suppression and TP53-induced glycolysis. Further, there is more glycolysis and glucose transport due to the production of phosphoglucomutase (PGM)). Additionally, TP53 is no longer able to prevent glucose 6 phosphate dehydrogenase G6PD activity [
82]. In addition to controlling glucose levels, the PI3K-Akt-mTOR signal transduction pathway triggers cancerous mutations that have a variety of negative side effects, including angiogenesis, proliferation, and differentiation. As a result, it establishes a clear connection between metabolic targeting, treatment response, and carcinogenesis [
80]. Most of the cancer signalling pathways mutated in lung cancer like RAS-RAF-MEK-MAPK pathway is mutated in 58% of all NSCLC tumour’s and 76% of lung adenocarcinomas [
83], KRAS and EGFR pathways, KEAP1/NRF2 pathway etc have a great impact on major metabolic pathways like glucose metabolism, amino acid metabolism pathways, reactive oxygen species (ROS) regulation [
84]. Therefore, proper targeting of these metabolic pathways gives us better approach to develop new therapeutic options. There are various drugs under development that targets glucose metabolism. Glucose transporter (GLUT) inhibitors like fasentin, WZB117 (2-fluoro-6-(m-hydroxybenzoyloxy) phenyl m-hydroxybenzoate), DRB18 (a pan-GLUT inhibitor), STF31 (selective glucose transporter GLUT1 inhibitor) etc are under pre-clinical trials. These drugs are mainly targeting the transport of glucose. The WZB117 and DRB18 demonstrated inhibition under in vitro and in vivo conditions in NSCLC cancer models [
85]. Shikonin, a drug use for late-stage lung cancer treatment is a pyruvate kinase M2 (PKM2) inhibitor is given to patients not undergoing surgery, radiotherapy, or chemotherapy. Activation of pyruvate kinase M2 (PKM2) appears to be inactivated in cancer and raises the ratio of glycolysis to glucose oxidation [
86]. Some other drugs for lung cancer therapy under trial includes dichloroacetate (DCA), which is a pyruvate dehydrogenase kinase 1 (PDK1) inhibitors. Also, PSTMB targets lactate dehydrogenase A (LDHA) [
87].
Major targets for mutation in lung cancer includes EGFR mutations, ALK rearrangements, ROS1 rearrangements, RET rearrangements, NTRK (neurotrophic tyrosine receptor kinase) fusions, MET (mesenchymal epithelial transition factor) exon 14 skipping mutation, KRAS G12C mutation, BRAF V600E mutation, and ERBB2 (HER2) mutation. There are several drugs approved by FDA for targeting these mutations, still further translational research are exploring novel driver mutations, turning traditionally undruggable mutations into therapeutic targets [
88]. There are various drugs under human phase1/2 clinical trials which are having some different mechanism of action than existing drugs.For example, Patritumabderuxtecan is a monotherapy drug under phase 1 clinical trial which is an anti-HER3 antibody-drug conjugate targeting mutated EGFR and NSCLC progressed after tyrosine kinase inhibitors (TKI) showing an objective response rate (ORR) of 39%. Some other monotherapy drugs under trial are Pemigatinib (aFGFR1-3 inhibitor), Telisotuzumabvedotin (anti-MET antibody-drug conjugate), Taletrectinib (ROS1 inhibitor), and Trastuzumabderuxtecan (anti-HER2 antibody-drug conjugate) etc. [
89,
90].
Combinational therapy is a recent approach which targets several pathways associated with cancer development like PI3K/AKT/mTOR and RAF/MEK/ERK, where, tumour cell receptors are targeted. There are certain drugs under different phases of clinical trials like Anlotinib (multi targeting TKI) and used in combination with Osimertinibis as a first line of therapy for mutated EGFR and NSCLC [
91]. Other drugs under clinical trials are Alisertib (Aurora kinase inhibitor), Sapanisertib (m TOR inhibitor), Repotrectinib (ROS1/TRK/ALK inhibitor), all these are used in combination with Osimertinib [
92,
93]. Some of the current therapeutic approaches which are under development or clinical trails are listed in the
Table 1 below.
Table 1.
Current therapeutic approaches for the Lung Cancer Treatment.
Table 1.
Current therapeutic approaches for the Lung Cancer Treatment.
| Target |
Signaling Pathway/ Metabolic Process |
Drug Name/ Type |
Action/ Effect |
Development Stage |
References |
| Glucose Transport |
Glycolysis |
Fasentin, WZB117, DRB18, STF31 |
Inhibit glucose transporter (GLUT), Inhibits LDHA, disrupting lactate production and glycolysis |
Pre-clinical trials |
[85] |
| Pyruvate Kinase M2 (PKM2) |
Glycolysis/ Warburg effect |
Shikonin |
PKM2 inhibitor, encourages metabolic shift from glycolysis to glucose oxidation |
Use in later-stage treatment |
[86] |
| Pyruvate Dehydrogenase Kinase 1 (PDK1) |
Glycolysis and mitochondrial metabolism |
Dichloroacetate (DCA) |
Inhibits PDK1, promoting oxidative phosphorylation over glycolysis |
Under trial |
[87] |
| Lactate Dehydrogenase A (LDHA) |
Glycolysis |
PSTMB |
Inhibits LDHA, reducing lactate production and potentially disrupting cancer cell metabolism |
Under trial |
[87] |
| EGFR mutations, ALK rearrangements, and others |
EGFR/ ALKsignaling pathway |
Erlotinib, Afatinib, Osimertinib, Gefitinib, Crizotinib, Ceritinib, Alectinib, Brigatinib and others |
Target specific genetic mutations in lung cancer |
FDA-approved for specific mutations |
[88] |
| Mutated EGFR, NSCLC |
EGFR pathway |
Patritumabderuxtecan |
Anti-HER3 antibody-drug conjugate |
Phase 1 clinical trial |
[77] |
| FGFR1-3, MET, ROS1, HER2 |
Various pathways |
Pemigatinib, Telisotuzumabvedotin, Taletrectinib, Trastuzumabderuxtecan |
Various monotherapy drugs targeting different pathways |
Human phase 1/2 clinical trials |
[89,90] |
| EGFR mutated NSCLC |
PI3K/AKT/mTOR, RAF/MEK/ERK |
Anlotinib + Osimertinib |
Multi-targeting TKI in combination with EGFR inhibitor |
Clinical trials |
[91] |
| Various targets |
PI3K/AKT/mTOR, RAF/MEK/ERK |
Alisertib, Sapanisertib, Repotrectinib + Osimertinib |
Targeting tumor cell receptors with Osimertinib |
Clinical trials |
[92,93] |
6. Future Perspectives: Navigating the Integrated Landscape
After many advancements in biomedical research and newer developed technology in field of diagnosis, and treatment unfortunately we are not able to control the high mortality rate of lung cancer. So, this is hightime to adopt better analysis method to get better result. One such newer approach is system biology, the goal of systems biology analysis is to comprehend the characteristics of a particular system, which in the context of cancer may include xenografts, tumour cell lines, genetically altered mice, or primary cells taken from patients either before or after treatment. Many components generated through interaction between the systems, and experiments in consecutive repeating cycles are integrated with theory, simulation, and mathematical modelling [
94]. This system biology approach in lung cancer is use for studying novel biomarkers, therapeutic targets etc. True progress in understanding lung cancer biology has been made using a systems biology approach that integrates data generated from siRNA library screens with the data obtained from clinically significant LC samples or mice models using gene expression data, DNA sequencing, comparative genomic hybridization (CGH) analysis, miRNA profiling, protein, or protein phosphorylation signalling network assessments. In fact, this has led to better NSCLC prognoses and therapy prediction techniques [
95]. Key steps involved in system biology approach include comprehensive measurement and quantification of changes at the protein, DNA, RNA, or miRNA and signalling levels. Further, combining the data from measurements to create a comprehensive picture of the system in issue; along with the evaluation of its dynamic changes, which, in the context of tumours, refers to the development of a more malignant phenotype, as well as its reactions to targeted agent, CT, and radiation therapies; and modelling the system with the help of the integrated data [
96]. Recent studies show how we can narrow down our study related to genes involved in lung cancer using system biology approach. Cluster ONE plugin a cytoscape software was used to analyze and cluster networks this network analysis showed seven genes (BRCA1-TP53-CASP3-PLK1-VEGFA-MDM2-CCNB1 and PLK1), and two genes (PLK1 and TYMS; Polo-like kinase 1 and thymidylate synthetase) mainly involved in diseases development this is a comprehensive gene information approach [
97]. For developing effective new markers which help to detect NSCLC in early stage, a study generated a very accurate model with the least absolute shrinkage and selection operator (LASSO) for predicting non-small cell lung cancer (NSCLC) using gene expression data and interaction networks. Using TCGA and GTEx data (The Cancer Genome Atlas Program and Genotype-Tissue Expression), the differentially expressed genes (DEGs) in NSCLC in comparison with normal tissues were discovered [
98]. The above-mentioned studies show the wide application of system biology and other integrated approaches to deal with lung cancer.
Along with the application of system biology approach, we need to use various cutting-edge technologies to better handle the diseases. For superior diagnosis and treatment options artificial intelligence (AI) and machine learning (ML) approach for precision medicine, nanotechnology-based drug delivery system, immunotherapy, combinational therapy, and some other new approaches can be used [
99]. An operational flow based on AI models and medical management platforms with high-performance computing needs to be set up for precision cancer genomics in clinical practice to comprehend massive biodata in cancer genomics. Fundamental approach of using AI tools in cancer includes AI model for mutational analysis, single-cell genomics and computational biology, text mining for cancer gene target identification, computational prediction of pathogenic variants of cancer susceptibility genes, and NVIDIA graphics processing units [
100]. Nanotechnology based drug delivery system always increases the availability of therapeutic drug on tumour site with reduced toxicity. Treatment for lung cancer involves the use of certain nanomedicine materials, such as lipid, polymer, magnetic, and hafnium oxide nanoparticles [
101]. Some other recently developed treatment options are newer radiotherapy approach like use of Boron Neutron Capture Therapy (BNCT), Proton therapy, Brachytherapy, Stereotactic Body Radiation Therapy (SBRT) [
102,
103]. There are some other emerging therapies for lung cancer like cryoablation, photodynamic therapy, hyperthermia etc. Cryoablation is a therapeutic approach where tumours are destroyed by lowering the temperature. This procedure entails attaching cryoprobes to pressurised argon, which causes the probe to rapidly cool after expanding to as low as -160°C. As a result, an ice ball forms at the tip of the cryoprobe, and the freezing and thawing process damages the cell membrane and causes microvascular damage, which in turn causes hypotonic stress and ultimately results in cell necrosis [
104]. In photodynamic therapy the use of non-invasive photosensitive compounds and light activation that destroy the tumour region. This technique has shown good efficacy in enhancing the rate of survival of lung cancer patients [
105]. Hyperthermia therapy causes an elevation in the body tissue temperature. For this heat is applied locally to raise the temperature to between 42 and 45°C using external sources such microwaves, radio waves, lasers, ultrasound, etc. The goal of this procedure is to eradicate cancer cells or stop their proliferation without endangering healthy tissues [
106].
Although there are many emerging therapeutic approaches, nevertheless all the above-mentioned challenges and limitations need to be addressed to develop complete cure to minimize side effect and enhance efficacy of treatment in lung cancer. One of the major challenges is to making it available cost-effective treatment to all patients, to reduce decrease the cancer related mortality.
7. Conclusions
The fight against lung cancer, a leading global health challenge, necessitates a multifaceted approach due to the complex interplay of drug resistance, metabolic reprogramming, and the genetic landscape of the disease. This review has highlighted the critical role of targeting pathways, mutations, and genetic alterations, alongside the importance of understanding the cancer metabolism for effective therapeutic strategies. The advancements in targeted therapies, immunotherapies, and combinational treatments, complemented by emerging options like metabolic inhibitors and nanocarriers, underscore the potential to significantly improve patient outcomes. Moreover, the integration of mathematical modelling and systems biology approaches offers promising avenues for enhanced detection and personalized treatment strategies. The adoption of AI-ML technologies and innovative techniques such as hyperthermia, cryoablation, and photodynamic therapy further expands our arsenal against lung cancer. Through a comprehensive understanding of the disease's underlying mechanisms and leveraging interdisciplinary approaches and cutting-edge technologies, we can pave the way for more effective treatments and ultimately, improve the survival rates and quality of life for lung cancer patients.
Author Contributions
Conceptualization, H.K.V. and B.L.; methodology, P.M., R.A. and B.P., validation; H.K.V. and B.L.; resources and data curation, P.M., R.A. and B.P. writing—original draft preparation review and editing; P.M., B.P. and H.K.V. supervision H.K.V. All authors read and approve the final version of manuscript for the submission.
Funding
No Funding Received.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Islami, F.; et al., Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin, 2018. 68(1): P. 31-54. [CrossRef]
- Singh, N.; et al., Lung Cancer in India. J Thorac Oncol, 2021. 16(8): P. 1250-1266. [CrossRef]
- Thun, M.J.; et al., 50-year trends in smoking-related mortality in the United States. N Engl J Med, 2013. 368(4): P. 351-64. [CrossRef]
- Lemjabbar-Alaoui, H.; et al., Lung cancer: Biology and treatment options. Biochim Biophys Acta, 2015. 1856(2): P. 189-210. [CrossRef]
- Anusewicz, D., M. Orzechowska, and A.K. Bednarek, Lung squamous cell carcinoma and lung adenocarcinoma differential gene expression regulation through pathways of Notch, Hedgehog, Wnt, and ErbB signalling. Scientific Reports, 2020. 10(1): P. 21128. [CrossRef]
- Rudin, C.M.; et al., Small-cell lung cancer. Nat Rev Dis Primers, 2021. 7(1): P. 3. [CrossRef]
- Ashrafi, A.; et al., Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. 2022. 14(19): P. 4562. [CrossRef]
- Vasan, N., J. Baselga, and D.M.J.N. Hyman, A view on drug resistance in cancer. 2019. 575(7782): P. 299-309. [CrossRef]
- Li, X.; et al., Tumor metabolic reprogramming in lung cancer progression. Oncol Lett, 2022. 24(2): P. 287. [CrossRef]
- Kalyanaraman, B., Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol, 2017. 12: P. 833-842. [CrossRef]
- Verma, H.K.; et al., A Retrospective Look at Anti-EGFR Agents in Pancreatic Cancer Therapy. Current Drug Metabolism, 2019. 20(12): P. 958-966. [CrossRef]
- Verma, H.K., G. Falco, and L.V.K.S. Bhaskar, Molecular Signaling Pathways Involved in Gastric Cancer Chemoresistance, in Theranostics Approaches to Gastric and Colon Cancer, G.S.R. Raju and L.V.K.S. Bhaskar, Editors. 2020, Springer Singapore: Singapore. p. 117-134.
- He, H.; et al., Targeting Signaling Pathway Networks in Several Malignant Tumors: Progresses and Challenges. Front Pharmacol, 2021. 12(675675). [CrossRef]
- Cheng, H.; et al., Targeting the PI3K/AKT/mTOR pathway: Potential for lung cancer treatment. Lung Cancer Manag, 2014. 3(1): P. 67-75. [CrossRef]
- Sanaei, M.J.; et al., The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Transl Oncol, 2022. 18: P. 101364. [CrossRef]
- He, Y.; et al., Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther, 2021. 6(1): P. 021-00828. [CrossRef]
- Pradhan, R.; et al., MAPK pathway: A potential target for the treatment of non-small-cell lung carcinoma. Future Med Chem, 2019. 11(8): P. 793-795. [CrossRef]
- Gong, K.; et al., Tumor necrosis factor in lung cancer: Complex roles in biology and resistance to treatment. Neoplasia, 2021. 23(2): P. 189-196. [CrossRef]
- Venugopala, K.N., Targeting the DNA Damage Response Machinery for Lung Cancer Treatment. Pharmaceuticals (Basel), 2022. 15(12). [CrossRef]
- Mamdani, H. and S.I. Jalal, DNA repair in lung cancer: Potential not yet reached. Lung Cancer Manag, 2016. 5(1): P. 5-8. [CrossRef]
- Ou, S.I. and K. Shirai, Anaplastic Lymphoma Kinase (ALK) Signaling in Lung Cancer. Adv Exp Med Biol, 2016. 893: P. 179-187. [CrossRef]
- Longley, D. and P. Johnston, Molecular mechanisms of drug resistance. 2005. 205(2): P. 275-292. [CrossRef]
- Zappa, C. and S.A. Mousa, Non-small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res, 2016. 5(3): P. 288-300. [CrossRef]
- Kannampuzha, S.; et al., A Systematic Role of Metabolomics, Metabolic Pathways, and Chemical Metabolism in Lung Cancer. Vaccines (Basel), 2023. 11(2). [CrossRef]
- Ratre, Y.K.; et al., Therapeutic Targeting of Glutamine Metabolism in Colorectal Cancer, in Colon Cancer Diagnosis and Therapy: Volume 2, N.K. Vishvakarma, G.P. Nagaraju, and D. Shukla, Editors. 2021, Springer International Publishing: Cham. p. 333-356.
- Xu, H.D.; et al., Discovery of potential therapeutic targets for non-small cell lung cancer using high-throughput metabolomics analysis based on liquid chromatography coupled with tandem mass spectrometry. RSC Adv, 2019. 9(19): P. 10905-10913. [CrossRef]
- Pezzuto, A.; et al., Expression and role of p16 and GLUT1 in malignant diseases and lung cancer: A review. Thorac Cancer, 2020. 11(11): P. 3060-3070. [CrossRef]
- Zahra, K.; et al., Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front Oncol, 2020. 10: P. 159. [CrossRef]
- Mohamed, A.; et al., Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer, 2014. 15(1): P. 7-15. [CrossRef]
- Hassanein, M.; et al., SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res, 2013. 19(3): P. 560-70. [CrossRef]
- Miller, D.M.; et al., c-Myc and cancer metabolism. Clin Cancer Res, 2012. 18(20): P. 5546-53. [CrossRef]
- Morotti, M.; et al., Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc Natl Acad Sci U S A, 2019. 116(25): P. 12452-12461. [CrossRef]
- Yoo, H.C.; et al., A Variant of SLC1A5 Is a Mitochondrial Glutamine Transporter for Metabolic Reprogramming in Cancer Cells. Cell Metab, 2020. 31(2): P. 267-283.e12. [CrossRef]
- Sowers, M.L.; et al., Analysis of glucose-derived amino acids involved in one-carbon and cancer metabolism by stable-isotope tracing gas chromatography mass spectrometry. Anal Biochem, 2019. 566: P. 1-9. [CrossRef]
- Yang, M. and K.H. Vousden, Serine and one-carbon metabolism in cancer. Nat Rev Cancer, 2016. 16(10): P. 650-62. [CrossRef]
- Yang, S.; et al., Fatty acid-binding protein 5 controls lung tumor metastasis by regulating the maturation of natural killer cells in the lung. FEBS Lett, 2021. 595(13): P. 1797-1805. [CrossRef]
- Schwarcz, R. and T.W. Stone, The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology, 2017. 112(Pt B): P. 237-247. [CrossRef]
- Chang, L.; et al., Inhibition of FASN suppresses the malignant biological behavior of non-small cell lung cancer cells via deregulating glucose metabolism and AKT/ERK pathway. Lipids Health Dis, 2019. 18(1): P. 118. [CrossRef]
- Li, E.Q.; et al., Synthesis and anti-cancer activity of ND-646 and its derivatives as acetyl-CoA carboxylase 1 inhibitors. Eur J Pharm Sci, 2019. 137: P. 105010. [CrossRef]
- Lai, S.C.; et al., Thyroid transcription factor 1 enhances cellular statin sensitivity via perturbing cholesterol metabolism. Oncogene, 2018. 37(24): P. 3290-3300. [CrossRef]
- Olaussen, K. and S.J.A.o.O. Postel-Vinay, Predictors of chemotherapy efficacy in non-small-cell lung cancer: A challenging landscape. 2016. 27(11): P. 2004-2016. [CrossRef]
- Dasari, S. and P.B. Tchounwou, Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol, 2014. 740: P. 364-78. [CrossRef]
- Baxevanos, P. and G.J.A.o.T.M. Mountzios, Novel chemotherapy regimens for advanced lung cancer: Have we reached a plateau? 2018, 2018. 6(8): P. 139. [CrossRef]
- Visentin, M., R. Zhao, and I.D. Goldman, The antifolates. Hematol Oncol Clin North Am, 2012. 26(3): P. 629-48, ix. [CrossRef]
- Hiddinga, B.I.; et al., Recent developments in the treatment of small cell lung cancer. 2021. 30(161): P. 210079. [CrossRef]
- Horita, N.; et al., Topotecan for Relapsed Small-cell Lung Cancer: Systematic Review and Meta-Analysis of 1347 Patients. Scientific Reports, 2015. 5(1): P. 15437. [CrossRef]
- Vinod, S.K. and E. Hau, Radiotherapy treatment for lung cancer: Current status and future directions. Respirology, 2020. 25 Suppl 2: P. 61-71. [CrossRef]
- Miyasaka, Y.; et al., A Promising Treatment Strategy for Lung Cancer: A Combination of Radiotherapy and Immunotherapy. Cancers (Basel), 2021. 14(1). [CrossRef]
- Antonia, S.J.; et al., Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med, 2018. 379(24): P. 2342-2350. [CrossRef]
- Corso, C.D.; et al., Role of chemoradiotherapy in elderly patients with limited-stage small-cell lung cancer. 2015. 33(36): P. 4240. [CrossRef]
- Drosten, M. and M. Barbacid, Targeting KRAS mutant lung cancer: Light at the end of the tunnel. Mol Oncol, 2022. 16(5): P. 1057-1071. [CrossRef]
- Díaz-Serrano, A.; et al., Targeting EGFR in Lung Cancer: Current Standards and Developments. Drugs, 2018. 78(9): P. 893-911. [CrossRef]
- Chevallier, M.; et al., Oncogenic driver mutations in non-small cell lung cancer: Past, present and future. World J Clin Oncol, 2021. 12(4): P. 217-237. [CrossRef]
- Cheng, Y., T. Zhang, and Q. Xu, Therapeutic advances in non-small cell lung cancer: Focus on clinical development of targeted therapy and immunotherapy. MedComm (2020), 2021. 2(4): P. 692-729. [CrossRef]
- Nagano, T., M. Tachihara, and Y. Nishimura, Dacomitinib, a second-generation irreversible epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) to treat non-small cell lung cancer. Drugs Today (Barc), 2019. 55(4): P. 231-236. [CrossRef]
- Maemondo, M.; et al., Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med, 2010. 362(25): P. 2380-8. [CrossRef]
- Villalobos, P. and Wistuba, II, Lung Cancer Biomarkers. Hematol Oncol Clin North Am, 2017. 31(1): P. 13-29. [CrossRef]
- Zhao, H.; et al., Apatinib Plus Gefitinib as First-Line Treatment in Advanced EGFR-Mutant NSCLC: The Phase III ACTIVE Study (CTONG1706). J Thorac Oncol, 2021. 16(9): P. 1533-1546. [CrossRef]
- Zhang, H. and J. Chen, Current status and future directions of cancer immunotherapy. J Cancer, 2018. 9(10): P. 1773-1781. [CrossRef]
- Buchbinder, E.I. and A. Desai, CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol, 2016. 39(1): P. 98-106. [CrossRef]
- Nasser, N.J., M. Gorenberg, and A. Agbarya, First line Immunotherapy for Non-Small Cell Lung Cancer. Pharmaceuticals (Basel), 2020. 13(11). [CrossRef]
- Santini, F.C. and C.M. Rudin, Atezolizumab for the treatment of non-small cell lung cancer. Expert Rev Clin Pharmacol, 2017. 10(9): P. 935-945. [CrossRef]
- Heymach, J.V.; et al., Perioperative Durvalumab for Resectable Non–Small-Cell Lung Cancer. 2023. 389(18): P. 1672-1684. [CrossRef]
- Johnson, M.L.; et al., Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol, 2023. 41(6): P. 1213-1227. [CrossRef]
- Theelen, W.; et al., Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir Med, 2021. 9(5): P. 467-475. [CrossRef]
- Ucuzian, A.A.; et al., Molecular mediators of angiogenesis. J Burn Care Res, 2010. 31(1): P. 158-75. [CrossRef]
- Jayson, G.C.; et al., Antiangiogenic therapy in oncology: Current status and future directions. Lancet, 2016. 388(10043): P. 518-29. [CrossRef]
- West, H.J.; et al., Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: Subgroup results from the phase III IMpower150 trial. J Immunother Cancer, 2022. 10(2).
- Xie, C.; et al., Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. 2018. 109(4): P. 1207-1219. [CrossRef]
- Pan, X.; et al., A Serum Metabolomic Study Reveals Changes in Metabolites During the Treatment of Lung Cancer-Bearing Mice with Anlotinib. Cancer Manag Res, 2021. 13: P. 6055-6063. [CrossRef]
- Hu, T.; et al., Longitudinal Pharmacometabonomics for Predicting Malignant Tumor Patient Responses to Anlotinib Therapy: Phenotype, Efficacy, and Toxicity. Front Oncol, 2020. 10: P. 548300. [CrossRef]
- Duarte, I.F.; et al., Potential Markers of Cisplatin Treatment Response Unveiled by NMR Metabolomics of Human Lung Cells. Molecular Pharmaceutics, 2013. 10(11): P. 4242-4251. [CrossRef]
- Christofk, H.R.; et al., The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature, 2008. 452(7184): P. 230-3. [CrossRef]
- Koussounadis, A.; et al., Chemotherapy-induced dynamic gene expression changes in vivo are prognostic in ovarian cancer. British Journal of Cancer, 2014. 110(12): P. 2975-2984. [CrossRef]
- Yang, D.; et al., Clinical significance of circulating tumor cells and metabolic signatures in lung cancer after surgical removal. Journal of Translational Medicine, 2020. 18(1): P. 243. [CrossRef]
- Haider, M.; et al., Nanomedicine Strategies for Management of Drug Resistance in Lung Cancer. 2022. 23(3): P. 1853. [CrossRef]
- Alexiou, C.; et al., Targeting cancer cells: Magnetic nanoparticles as drug carriers. Eur Biophys J, 2006. 35(5): P. 446-50. [CrossRef]
- Deng, X.; et al., Tumour microenvironment-responsive nanoplatform based on biodegradable liposome-coated hollow MnO2 for synergistically enhanced chemotherapy and photodynamic therapy. 2022. 30(3): P. 334-347. [CrossRef]
- Reshma, P.; et al., Overcoming drug-resistance in lung cancer cells by paclitaxel loaded galactoxyloglucan nanoparticles. 2019. 136: P. 266-274. [CrossRef]
- Vanhove, K.; et al., The Metabolic Landscape of Lung Cancer: New Insights in a Disturbed Glucose Metabolism. Front Oncol, 2019. 9: P. 1215. [CrossRef]
- Dhawan, A.; et al., Metabolic targeting, immunotherapy and radiation in locally advanced non-small cell lung cancer: Where do we go from here? 2022. 12. [CrossRef]
- Jiang, P.; et al., p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol, 2011. 13(3): P. 310-6. [CrossRef]
- Kim, D.; et al., Activation of mitochondrial TUFM ameliorates metabolic dysregulation through coordinating autophagy induction. Commun Biol, 2021. 4(1): P. 1. [CrossRef]
- Itoh, K.; et al., Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J Clin Biochem Nutr, 2015. 56(2): P. 91-7. [CrossRef]
- Shriwas, P.; et al., A small-molecule pan-class I glucose transporter inhibitor reduces cancer cell proliferation in vitro and tumor growth in vivo by targeting glucose-based metabolism. Cancer Metab, 2021. 9(1): P. 14. [CrossRef]
- Chen, J.; et al., Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene, 2011. 30(42): P. 4297-306. [CrossRef]
- Kim, E.Y.; et al., A Novel Lactate Dehydrogenase Inhibitor, 1-(Phenylseleno)-4-(Trifluoromethyl) Benzene, Suppresses Tumor Growth through Apoptotic Cell Death. Sci Rep, 2019. 9(1): P. 3969. [CrossRef]
- Abughanimeh, O.; et al., Novel targeted therapies for advanced non-small lung cancer. Semin Oncol, 2022. [CrossRef]
- Jänne, P.A.; et al., Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor-Resistant, EGFR-Mutated Non-Small Cell Lung Cancer. Cancer Discov, 2022. 12(1): P. 74-89. [CrossRef]
- Li, S.; et al., Emerging Targeted Therapies in Advanced Non-Small-Cell Lung Cancer. Cancers (Basel), 2023. 15(11). [CrossRef]
- Han, B.; et al., Phase Ib/IIa study evaluating the safety and clinical activity of osimeritinib combined with anlotinib in EGFRm, treatment-naive advanced NSCLC patients (AUTOMAN), 2022, American Society of Clinical Oncology. [CrossRef]
- Elamin, Y.Y.; et al., Results of a phase 1b study of osimertinib plus sapanisertib or alisertib for osimertinib-resistant, EGFR-mutant non–small cell lung cancer (NSCLC), 2022, American Society of Clinical Oncology. [CrossRef]
- Cho, B.C.; et al., MA11. 07 phase 1/2 TRIDENT-1 study of repotrectinib in patients with ROS1+ or NTRK+ advanced solid tumors. 2021. 16(3): P. S174-S175. [CrossRef]
- Viktorsson, K., R. Lewensohn, and B. Zhivotovsky, Systems biology approaches to develop innovative strategies for lung cancer therapy. Cell Death & Disease, 2014. 5(5): P. e1260-e1260. [CrossRef]
- Ocak, S.; et al., High-throughput molecular analysis in lung cancer: Insights into biology and potential clinical applications. Eur Respir J, 2009. 34(2): P. 489-506. [CrossRef]
- Feng, L.; et al., Systems biology analysis of the lung cancer-related secretome. Oncol Rep, 2018. 40(2): P. 1103-1118. [CrossRef]
- Parvin, S.; et al., Prediction of Genes Involved in Lung Cancer with a Systems Biology Approach Based on Comprehensive Gene Information. Biochem Genet, 2022. 60(4): P. 1253-1273. [CrossRef]
- Ahmed, F.; et al., A Systems Biology and LASSO-Based Approach to Decipher the Transcriptome-Interactome Signature for Predicting Non-Small Cell Lung Cancer. Biology (Basel), 2022. 11(12). [CrossRef]
- La’ah, A.S. and S.-H. Chiou, Cutting-Edge Therapies for Lung Cancer2024. [CrossRef]
- Lin, P.C.; et al., Cutting-Edge AI Technologies Meet Precision Medicine to Improve Cancer Care. Biomolecules, 2022. 12(8). [CrossRef]
- Agrawal, A. and S. Bhattacharya, Cutting-edge Nanotechnological Approaches for Lung Cancer Therapy. Curr Drug Res Rev, 2022. 14(3): P. 171-187. [CrossRef]
- Wang, S.; et al., Boron Neutron Capture Therapy: Current Status and Challenges. Front Oncol, 2022. 12: P. 788770. [CrossRef]
- Han, Y., Current status of proton therapy techniques for lung cancer. Radiat Oncol J, 2019. 37(4): P. 232-248. [CrossRef]
- Niu, L., K. Xu, and F. Mu, Cryosurgery for lung cancer. J Thorac Dis, 2012. 4(4): P. 408-19. [CrossRef]
- Ma, Q.L.; et al., Chlorin e6 mediated photodynamic therapy triggers resistance through ATM-related DNA damage response in lung cancer cells. Photodiagnosis Photodyn Ther, 2022. 37: P. 102645. [CrossRef]
- Dunne, M., M. Regenold, and C. Allen, Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv Drug Deliv Rev, 2020. 163-164: P. 98-124. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).