1. Introduction
The healing of bone defects is a serious challenge worldwide. One branch of dentistry deals with bone defects. Bone defects can occur for pathological or physiological reasons. These defects can be simple or complex. In a complex bone defect, epithelial integrity is mostly impaired [
1,
2]. Bone defect healing involves complex mechanisms because it is related to the interaction between the immune and biological systems [
2,
3]. After bone damage has occurred, restoration progresses through different stages. These stages are (i) coagulation and homeostasis, (ii) inflammation, (iii) proliferation, and (iv) bone defect remodelling [
2,
3,
4].
Capsaicin (trans-8-methyl-N-vanilyl-6-nonenamide) is the main active ingredient of hot pepper, which is found in 0.1% to 1% in green and red peppers. It is known that capsaicin has anti-inflammatory, anti-oxidative and cholesterol-reducing effects [
5]. Capsaicin has a minimum level of inhibition of bone healing when applied at certain doses, and it has been suggested that it does this by activating TRPV-1, TNF-a, IL 6. [
2]. Capsaicin has different effects on the cell viability mechanism in different cell types. Studies have reported that cell cycle arrests and apatosis increase in cells exposed to capsaicin [
6]. The number of hepatic stellate cells responsible for the scar tissue formed in liver damage decreased in capsaicin treatment [
7].
Capsaicin administration has a minimum inhibitory concentration and activates Transient receptor potential vanilloid 1(TRPV-1), Tumor necrosis factor-alpha(TNF-α), and Interleukin-6(IL-6) [
8,
9].
Sympathetic and sensory nerves play an important role in bone metabolism. Recent studies have reported that the sympathetic nervous system increases bone resorption and decreases bone formation. In sensory nerve regulation, a physiological interaction between sympathetic and sensory nerves and osteoclastogenesis, based on the effects of the sensory neuropeptide calcitonin gene-related peptide (CGRP), takes place [
10,
11]. Capsaicin inhibits apoptosis by stimulating TRPV1 activity in respiration and increases the proliferation of muscle cells (ASMCs). It has been reported to increase proliferation in the gingival epithelium treated with capsaicin [
12,
13].
In a study, capsaicin was administered to newborn rats. When the rats became adults, tooth extraction was performed and a 21% decrease in alveolar bone resorption was observed in the capsaicin-treated group compared to the capsaicin-free group [
14]. In a rat study, capsaicin was administered and as a result, a 40% decrease in bone resorption was detected in adult rats. In the analysis, a decrease in the resorption surface and the number of osteoclasts was determined [
15]. Contrary to these studies, opposite situations were found in some studies. For example, Offley et al. In the study conducted by et al., it was reported that it decreased tarbecular integrity and increased osteoclase count in bone metabolism [
16]. It has been reported that the reason for this situation is that experiments were conducted on different bones in rats and different doses were used [
17].
Bone fragments required for bone augmentation can be supplied intraorally or extraorally if autogenous use is desired [
18]. The guided bone regeneration technique (GBR) has emerged in response to the inability to obtain sufficient bone from the intraoral region and high risks in the extraoral region. What is important in this technique is the cell aggregation potential. In addition, the suitability of the biomaterial and the selection of the correct surgical technique are important [
19,
20,
21]. The aim of this study is to evaluate the effects of systemic capsaicin administered at different doses on bone healing.
2. Materials and Methods
The study was conducted in Harran University Experimental Research Center after approval from the local ethics committee of animal experiments of Harran University. The rats required for the study were provided by the experimental research center of Fırat University. In the studies conducted, the experiments were carried out without causing any pain to the animals and all ethical rules were followed.
The present study aimed to investigate the effects of systemic capsaicin administration on bone healing. A total of 32 male wistar rats was used, their weight varying between 250 and 300 g. The rats were randomly divided into four groups of eight rats each.
First group (control group): A 7 mm defect was created in the rat calvarium, and no action was taken after washing with physiological saline.
Second group (graft group): A 7 mm defect was opened in the rat calvarium, and synthetic graft material was applied.
Third group (capsaicin 25 mg): A 7 mm defect was opened in the rat calvarium, and synthetic graft material was applied. Then, 25 mg/kg capsaicin was administered intraperitoneally.
Fourth group (capsaicin 50 mg): A 7 mm defect was opened in the rat calvarium, and synthetic graft material was applied. Then, 50 mg/kg of capsaicin was administered intraperitoneally [
22].
2.1. Surgical Procedure
Before the calvarial defects were opened, an intramuscular anesthetic was administered to the rats. This consisted of 90 mg/kg ketamine hydrochloride (Ketalar, Pfizer) and 10 mg/kg xylazine (Rompun-Bayer). Afterwards, asepsis was achieved with povidone iodine (Batticon-Adeka, Turkey) in the operation area. The operation area was covered with sterile drapes and local anaesthesia administered to the area, namely 0.5 cc of 4% articaine (Ultracain DS-Aventis, Istanbul, Turkey) containing 0.006 mg/ml epinephrine. A full thickness flap was opened with a no. 15 scalpel. The defect was created using a 7 mm trafen bur without damaging the brain. No material was placed in the defect in the control group. In the other groups, the defects were filled with synthetic grafts. For infection control, 50 mg/kg cefazolin sodium was administered for three days after the operation, and 1 mg/kg of tramadol hydrochloride was administered for pain control for three days after the operation. The operation area was sutured using a 3.0 silk suture.
During the experiment, four rats in total, one from each group, died. The surviving rats were observed on the 28th day, at the end of which they were sacrificed by means of a lethal dose of intraperitoneal ketamine hydrochloride (60 mg/kg). A full thickness flap was removed in the area using a size 15 scalpel. Then, using a piezo device (NSK, Tokyo, Japan), samples were taken from the region in healthy tissues without damaging the bone tissues. Likewise, the bone samples were disconnected from the brain under the guidance of physiological saline without creating heat in the tissues.
Samples obtained after sacrifice were preserved in formaldehyde and referred to pathology for histological analysis.
2.2. Histological Procedure
Samples kept in 10% neutral formalin were not softened in EDTA solution for decalcification. The softened samples were dehydrated, cleared with xylitol and embedded in paraffin blocks. Sections of 5-6 μm were obtained with a microtome. After staining with hematoxylon-eosin, bone healing was examined under a light microscope. The stained histological preparations were left for drying overnight, after which the sample surfaces were covered with a coverslip of methyl methacrylate. Digital images of all samples were taken at 4x magnification with a digital camera (Olympus® DP 70, Tokyo, Japan) connected to a light microscope (Olympus® BX50, Tokyo, Japan). Histological bone healing was assessed using the Image J Analysis Program (Image J version 1.44; National Institutes of Health, Bethesda, MD, USA).
2.3. Semi-Quantitative Scoring of Histopathologic Parameters
A semi-quantitative scoring was determined by examining the cells in the bone tissue such as osteoblast, osteocyte and osteoclast. In the evaluation of histological sections, fifteen different areas were scanned for each slide, and then the average value of ten randomly selected cells was determined. From these averages, ten points were obtained for each animal group, and then these values were analyzed statistically. The method we used has also been used in previous histochemical studies of bone tissue [
23,
24].
Inflammation: 0 = 13–15 İnflammatory cells per histological field, 1 = 10–13 İnflammatory cells per histological field, 2 = 7–10 İnflammatory cells per histological field, 3 = 4–7 İnflammatory cells per histological field, 4 = 1–4 İnflammatory cells per histological field [
25].
2.4. Statistical Analysis
Histological analysis of the rats in our study was performed with the SPSS (IBM® Ver 15.0 Windows, USA) program. Arithmetic mean values (M) and standard deviation (SD) values of histological analyzes (osteoblast, osteocytes, inflammation and osteoclast) were used. Normality tests of histological data were determined using the Kolmogorov-Smirnov test. Since the Kolmogorov-Smirnov test had a p value of < 0.05, it was determined that our study did not show a normal distribution. Comparisons of histological data between more than two experimental groups (group I, group II, group III and group IV) used the Kruskal-Wallis H test. The Mann Whitney U test was used to compare histological data between paired groups, with the result of the Kruskal-Wallis H test being significant (p<0.05). In all statistical tests, p value <0.05 was considered significant.
3. Results
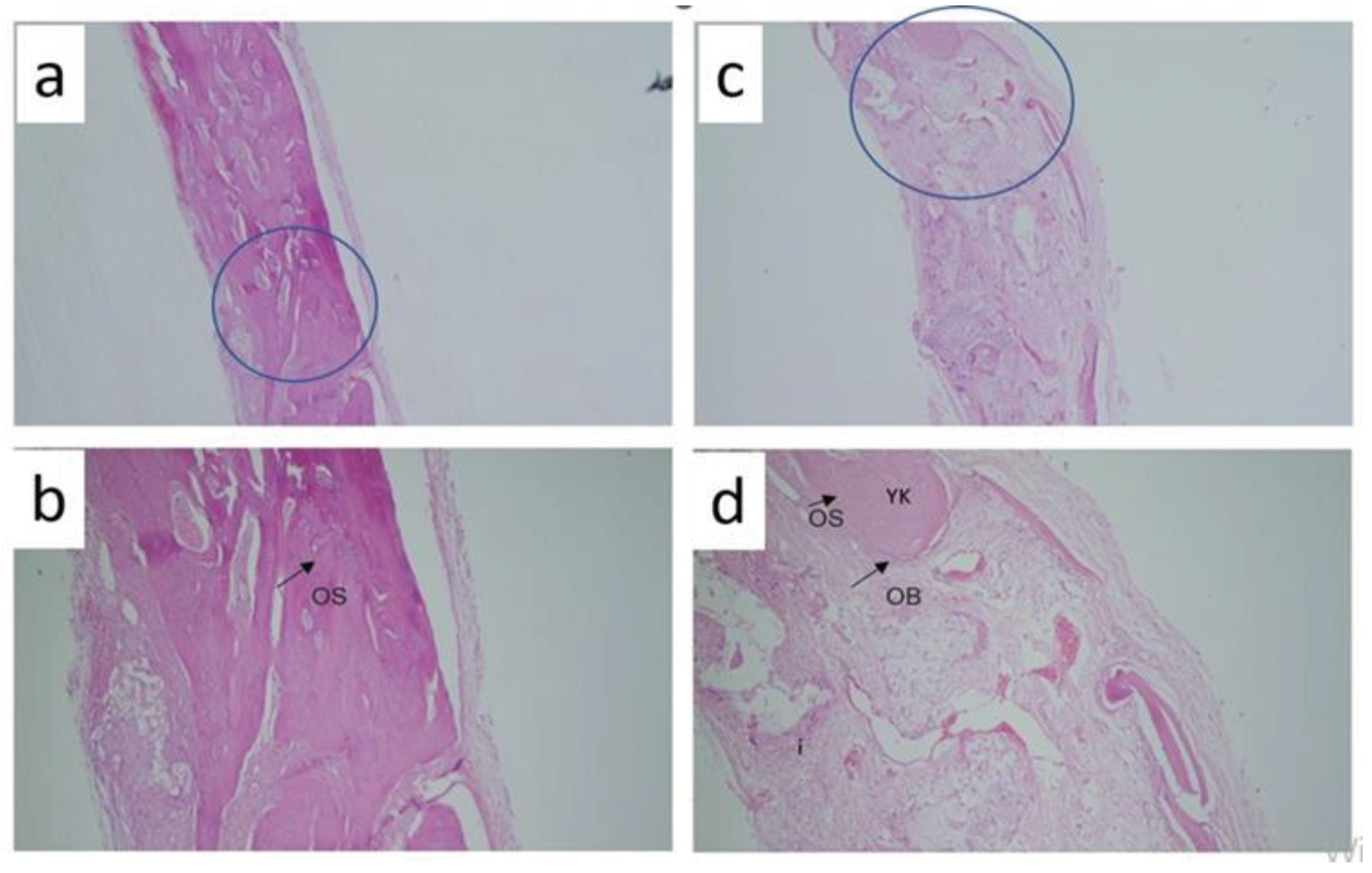
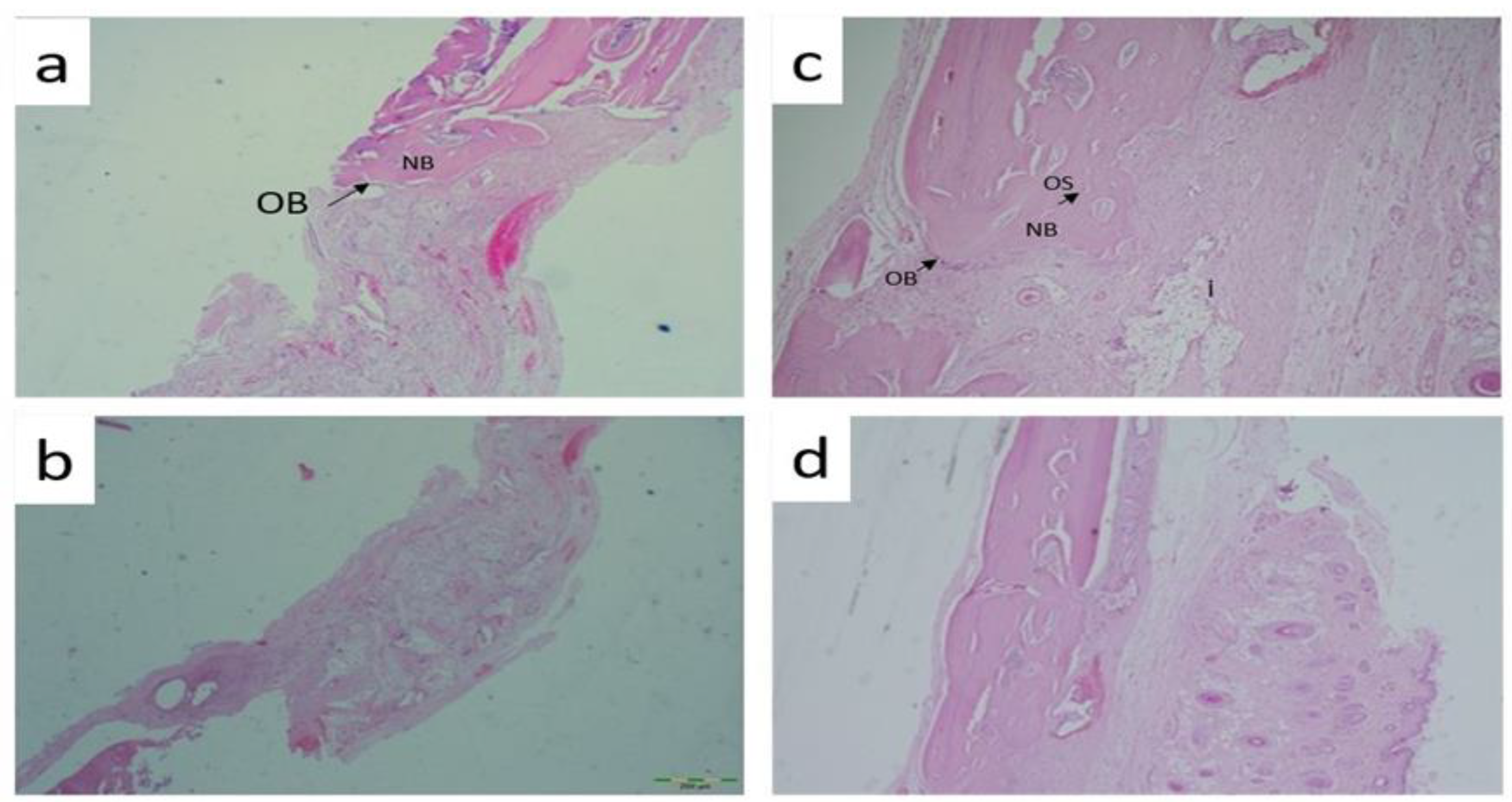
Histological analysis evaluated osteoblast numbers and while a value of 1.57±0.535 was obtained in the control group, a value of 2.14±0.69 was obtained in the graft group and a value of 2.57±0.53 was obtained in the capsaicin 25mg/kg group. Statistically significant differences were obtained between the control group and the groups administered gerft and 25 mg/kg. When looking at osteocyte numbers, the highest value was obtained in the capsaicin 50mg/kg applied group (3.43±0.53), while the lowest value was obtained in the control group (1.86±0.69) and statistically significant values were found between all groups (p=0.003). In addition, inflammation was evaluated and a significant difference was obtained only in the control group (3.00±0.58) and capsaicin 50mg/kg (2.00±0.58) group (p=0.010). The osteoclast counts revealed a significant difference between all the groups (p = 0.005) (
Figure 1 and
Figure 2).
4. Discussion
In the present study, vocacapsaicin, the active ingredient of which is capsaicin, was tested on rats. Bone healing was evaluated and no adverse events were found. An in-crease in bone healing was observed in rats given moderate levels of the active ingre-dient. Studies have been carried out on rabbits, which found no adverse effects on bone healing. Vocacapsaicin was applied before the operation in unilateral osteoma models. No adverse situation was found in its use as a local and systemic non-opioid agent in reducing pain [
26]. In our study, different doses of systemic capsaicin were adminis-tered to rats with a head defect, and a significant increase in bone healing was observed.
It has been reported that after the capsaicin active ingredient in vocacapsaicin is secreted, it affects trpv1 receptors in conjunction with c fibers [
27,
28,
29]. In a study, rats were evaluated radiographically in the administration of vocacapsaicin at a medium dose of 0.3 mg/kg. An increase in bone healing was observed compared to the control group. In studies, trpv 1 has been reported to be actively involved in bone healing for rats and rabbits. In a study, a unilateral femur fracture model was created, and a delay in bone healing was observed in the TRPV1-deficient group. In addition, a negative situation was observed in the balance of osteoblasts and osteoclasts [
30]. A similar study was conducted on wild rats, which found an increase in TRPV1 immunofluorescence [
31]. Other studies ad-ministered TRPV1 antagonist and reported that osteoclast activity was inhibited [
32,
33]. The effect of the use of vocacapsaicin on bone healing has not been fully clarified. However, it is currently believed that TRPV1 receptor family is involved in bone healing mechanism [
34].
In our study, systemic capsaicin was administered at doses of 0.25 mg/kg and 0.50 mg/kg. The results were evaluated histologically. An increase in osteoblast activity was observed. We think that this is due to the effect of capsaicin on TRPV1 receptors.
It has been reported that capsaicin causes an increase in CGRP release from cardiac C-fibre nerve endings in the heart [
35]. In particular, used Calcitonin Receptor Like Receptor(CLR/RAMP1) receptors, not only did vascular relaxation emerge from CGRP signalling but it also reportedly modulated inflammation by regulating proinflamma-tory cytokine production in dendritic cells [
36]. Histological evaluations revealed a sig-nificant decrease in inflammation in the capsaicin-applied groups. We think that the effect of capsaicin on CGRP release, which regulates proin-flammatory cytokine production in dendritic cells.
Bone marrow mesenchymal stem cells (BMSCs) play a role in the mechanism of bone formation and destruction and in maintaining this balance [
37,
38]. The differentiation of stem cells into osteoblasts is known as osteogenic differentiation [
39]. Osteoporosis occurs as a result of decreased proliferative ability and abnormal differentiation in bone mesenchymal stem cells. Sirtuin 6 (SIRT6), a member of the sirtuin deacetylase family, plays a role in osteogenic differentiation [
40,
41]. Overexpression of SIRT6 in human BMSCs disrupts the TRPV1 mechanism, resulting in inhibition of the release of CGRP. Subsequently, osteogenic differentiation is inhibited. As a result of the capsaicin treatment, TRPV1 is activated, the expression of CGRP increases, and the genes involved in osteogenic differentiation, namely osteocalcin (OCN), bone sialoprotein (BSP) and osteopontin (OPT), increase [
42]. The rise in alkaline phosphatase activity indicates that capsaicin supports osteogenic activity [
43].
Similarly, in our study, a significant increase in the amount of osteocytes was observed in the capsaicin-applied groups. As emphasized ın previous studies, we think that this is due to the positive effect of capsaicin on the osteogenic activity occurring between TRPV1, CGRP and SIRT 6. Capsaicin can be consumed with daily foods. This consumption is excessıve in some societies. Therefore, the positive or negative effects of the effect of capsaicin consumption rate on bone methodology has been evaluated. As a result, positive effects were observed in the doses we use. When we researched, the effects of this sıiuation on various factors were determined. Due to its positive effects on these factors, we determined their positive effects on bone metabolism in the doses used.
5. Conclusions
The foods we frequently use in our daily lives also contain capsaicin (hot peppers and their derivatives). That's why we used capsaicin in our study. In our study, previous studies were evaluated and it was determined that the effect of the dose to be used on bone healing was very important. so two different doses were used. The effects of the doses used on metabolism were investigated. Its effects on bone metabolism were evaluated. In our study, the systemic effect of capsaicin in rats with a head defect was evaluated. As a result of the analyses, positive effects on bone healing were observed when capsaicin 0.25 mg/kg and 0.50 mg/kg was administered intraperitoneally. Since capsaicin is found in foods and is a herbal product, we think that it can be used clinically after adjusting the doses. There are various limitations in our study, especially the number of subjects kept limited to avoid sacrificing too many animals. The more studies are needed for more accurate information.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure 1: Histological analysis A) Hematoxylin-Eosin Staining Control group 40x B) Hema-toxy-lin-Eosin Staining Control group 100x C) Hematoxylin-Eosin Staining Graft group 40x D) He-matoxylin-Eosin Staining Graft group 100x; Figure 2: Histological analysis A) Hematoxylin-Eosin Staining Capsaisin 25 mg 40x B) Hematoxy-lin-Eosin Staining Capsaisin 25 mg 100x C) Hematoxylin-Eosin Staining Capsaicin 50 mg (40x) D) Hematoxylin-Eosin Staining Capsaicin 50 mg (40x).Table : Histological analysis results between groups.
Author Contributions
Conceptualization, M.G. and M.B.B., M.E.P.; methodology, M.G. and M.B.B.; software, M.G. and M.B.B. and S.D. validation, M.G. and M.B.B. and S.D.; formal analysis, M.G., M.B.B. and A.T. investiga-tion, M.G., M.B.B. and A.T., resources, M.G., M.B.B. and A.T.; data curation, M.G., M.B.B. and A.T.; writing—original draft preparation, M.G., M.B.B.; writing—review and editing, M.G., M.B.B., A.T., S.D., G.A.; visualization, M.G., M.B.B., A.T., S.D., G.A; supervision, M.G., M.B.B., A.T., S.D., G.A; project administration, M.G., M.B.B., A.T., S.D., G.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declared that this study has received no financial support.
Acknowledgments
Author Contributions Ethical Approval: Ethics committee approval was received for this study from Harran University, Animal Experiments Local Ethics Committeein accordance with the World Medical Association Declaration of Helsinki, with the ap-proval number 2019-006-03.
Conflicts of Interest
No conflict of interest was declared by the authors.
References
- Zarrabi M, Afzal E, Asghari MH, et al. Inhibition of MEK/ERK signalling pathway promotes erythroid differentiation and reduces HSCs engraftment in ex vivo expanded haematopoietic stem cells. J Cell Mol Med. 2018;22:1464-74. [CrossRef]
- Setiawan F, Yudianto A, Wahjuningrum DA, et al. Increase of CD 34 in bone defect healing lesions of periodontitis by capsaicin administration. J Int Dent Med Res. 2021;14:855-9.
- Björnfot HS, Clark R, Zwicker S, et al. Gingival tissue inflammation promotes increased matrix metalloproteinase-12 production by CD200Rlow monocyte-derived cells in periodontitis. J Immunol. 2017;199:4023-35. [CrossRef]
- Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol. 2017;20:189-93. [CrossRef]
- Zhang S, Wang D, Huang J, et al. Application of capsaicin as a potential new therapeutic drug in human cancers. J Clin Pharm Ther. 2020;45:16-28. [CrossRef]
- Ibrahim M, Jang M, Park M, et al. Capsaicin inhibits the adipogenic differentiation of bone marrow mesenchymal stem cells by regulating cell proliferation, apoptosis, oxidative and nitrosative stress. Food Funct. 2015;6:2165-78. [CrossRef]
- Bitencourt S, Mesquita F, Basso B, et al. Capsaicin modulates proliferation, migration, and activation of hepatic stellate cells. Cell Biochem Biophys. 2014;68:387-96.
- Setiawan F, Sunariani J, Yudianto A, et al. Mooduto L, Radhianto E. 2020. Capsaicin’s Inhibition Effects on Biofilm Aerococcus viridans. Ind J For Med & Tox. 2020;14:1906-12.
- Sunariani J, Suhartini T, Mooduto L, et al. Transient receptor potential cation channel subfamily V member-1 and matrix metalloproteinase-8 expression of capsaicin administration in periodontitis. Sys Rev Pharm 2020;1:997-1000.
- Togari A, Arai M. Pharmacological topics of bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci. 2008;106:542-6. [CrossRef]
- Togari A, Arai M, Kondo H, et al. The neuro-osteogenic network: the sympathetic regulation of bone resorption. Jpn. Dent. Sci. Rev. 2012;48:61 – 70. [CrossRef]
- Zhao LM, Kuang HY, Zhang LX, et al. Effect of TRPV1 channel on proliferation and apoptosis of airway smooth muscle cells of rats. J Huazhong Univ Sci Technolog Med Sci. 2014;34:504-9. [CrossRef]
- Takahashi N, Matsuda Y, Yamada H, et al. Epithelial TRPV1 signaling accelerates gingival epithelial cell proliferation. J Dent Res. 2014;93:1141-7. [CrossRef]
- Hill EL, Turner R, Elde R. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience, 1991;44:747-55. [CrossRef]
- Adam C, Llorens A, Baroukh B, et al. Effects of capsaicin-induced sensory denervation on osteoclastic resorption in adult rats. Exp Physiol. 2000;85:61-6.
- Offley SC, Guo TZ, Wei T, et al. Capsaicin-sensitive sensory neurons contribute to the caintenance of trabecular bone integrity. J Bone Miner Res. 2005;20:257-67.
- Ding Y, Arai M, Kondo H, et al. Effects of capsaicin-induced sensory denervation on bone metabolism in adult rats. Bone. 2010;46:1591-96. [CrossRef]
- Khoury F, Hanser T. Three-dimensional vertical alveolar ridge augmentation in the posterior maxilla: A 10-year clinical study. Int J Oral Maxillofac Implants. 2019;34:471-80. [CrossRef]
- Hämmerle CH, Jung RE, Feloutzis A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J Clin Periodontol. 2002;29:226-31.
- Dahlin C, Sennerby L, Lekholm U, et al. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989;4:19-25.
- Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review. Clin Oral Implants Res. 2009;20:113-23. [CrossRef]
- Moran C, Morales L, Razo SR, et. al. Effect of sensorial denervation induced by capsaicin injection at birth or on day three of life, on puberty, induced ovulation and pregnancy. Life Sci 2003; 73: 2113-25.
- Lee MK, DeConde AS, Lee M, et al. Biomimetic scaffolds facilitate healing of critical-sized segmental mandibular defects. Am J Otolaryngol. 2015;36:1-6.
- Erdmann N, Bondarenko A, Hewicker-Trautwein M, et al. Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: a comparative study in rabbits. Biomed Eng Online. 2010;9:63. [CrossRef]
- Gül M, Günay A. Effect of caffeic acid phenethyl ester and Ankaferd Blood Stopper® on palatal wound healing in the diabetic rats. SRM J Res Dent Sci.2020;11:172-77. [CrossRef]
- Knotts T, Mease K, Sangameswaran L, et al. Pharmacokinetics and local tissue response to local instillation of vocacapsaicin, a novel capsaicin prodrug, in rat and rabbit osteotomy models. J Orthop Res. 2022;40:2281-93. [CrossRef]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487-517. [CrossRef]
- Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816-24. [CrossRef]
- Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531-43. [CrossRef]
- He LH, Liu M, He Y, et al. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci Rep. 2017;22:42385. [CrossRef]
- Davidson EM, Haroutounian S, Kagan L, et al. A novel proliposomal ropivacaine oil: pharmacokinetic-pharmacodynamic studies after subcutaneous administration in pigs. Anesth Analg. 2016;122:1663-72. [CrossRef]
- Idris AI, Landao-Bassonga E, Ralston SH. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone. 2010;46:1089-99. [CrossRef]
- Rossi F, Bellini G, Torella M, et al. The genetic ablation or pharmacological inhibition of TRPV1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice. Br J Pharmacol. 2014;171:2621-30. [CrossRef]
- Ambrosio L, Raucci MG, Vadalà G, et al. Innovative Biomaterials for the Treatment of Bone Cancer. Int J Mol Sci. 2021;22: 8214. [CrossRef]
- Imamura M, Smith NC, Garbarg M, et al. Histamine H3-receptor-mediated inhibition of calcitonin gene-related peptide release from cardiac C fibers. A regulatory negative-feedback loop. Circ Res. 1996;78:863-9. [CrossRef]
- Tsujikawa K, Yayama K, Hayashi T, et al. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc Natl Acad Sci USA. 2007;104:16702-7. [CrossRef]
- Bao M, Zhang K, Wei Y, et al. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2020;53:12735. [CrossRef]
- Fan JZ, Yang L, Meng GL, et al. Estrogen improves the proliferation and differentiation of hBMSCs derived from postmenopausal osteoporosis through notch signaling pathway. Mol Cell Biochem. 2014;392:85-93. [CrossRef]
- Guo L, Wang R, Zhang K, et al. A PINCH-1-Smurf1 signaling axis mediates mechano-regulation of BMPR2 and stem cell differentiation. J Cell Biol. 2019;218:3773-94. [CrossRef]
- Jain AK, Xi Y, McCarthy R, et al. LncPRESS1 Is a p53-Regulated LncRNA that Safeguards Pluripotency by Disrupting SIRT6-Mediated De-acetylation of Histone H3K56. Mol Cell. 2016;64:967-81. [CrossRef]
- Lefort K, Brooks Y, Ostano P, et al. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013;32:2248-63.
- Xiao F, Zhou Y, Liu Y, et al. Inhibitory effect of Sirtuin6 (SIRT6) on osteogenic differentiation of bone marrow mesenchymal stem cells. Med Sci Monit. 2019;25:8412-21. [CrossRef]
- Yuan M, Zhao L, Li Y, et al. Capsaicin on stem cell proliferation and fate determination - a novel perspective. Pharmacol Res. 2021;167:105566. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).