Submitted:
28 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
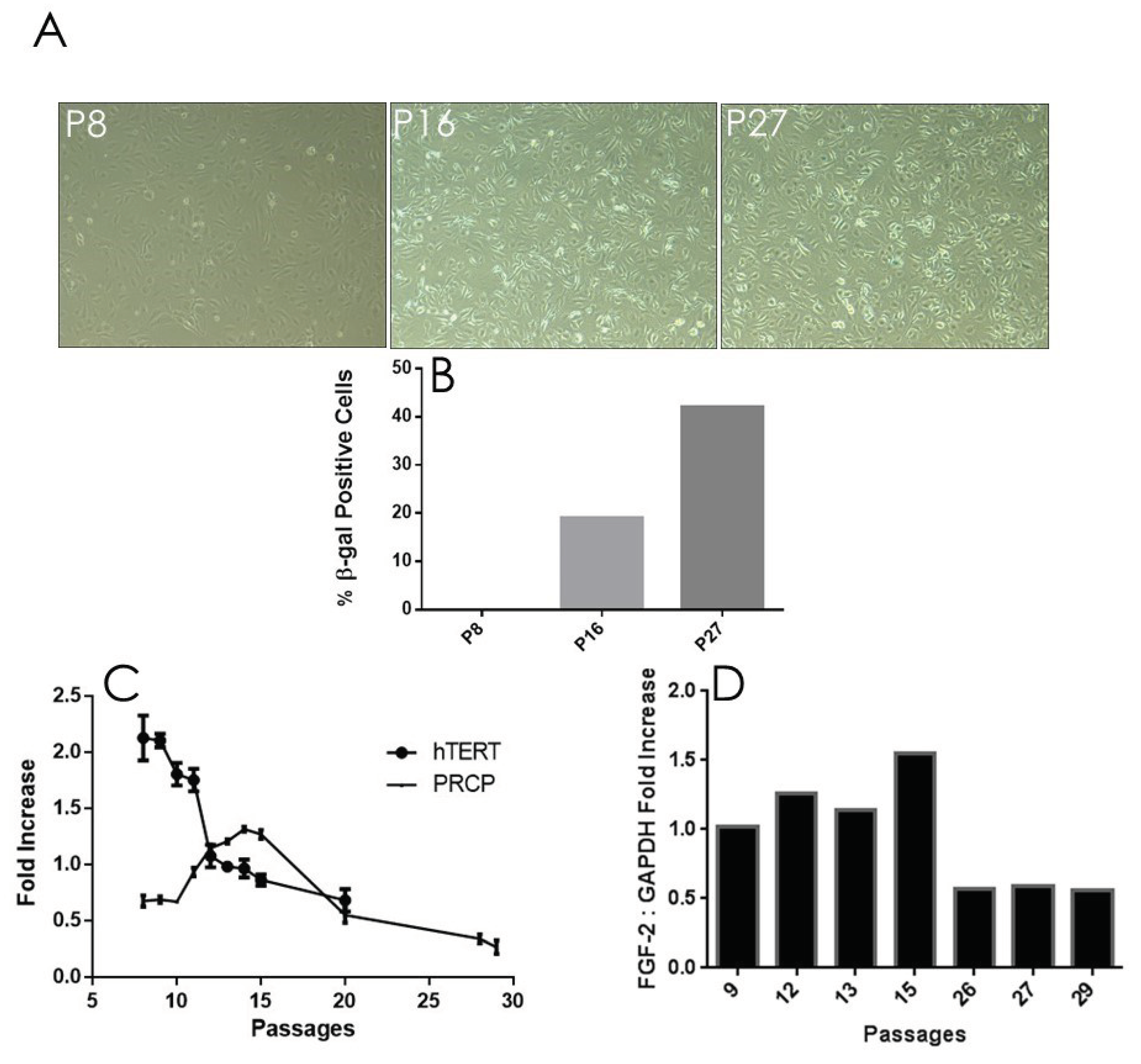
2.1. Effect of Aging on Kallikrein Activity in HPAEC Line
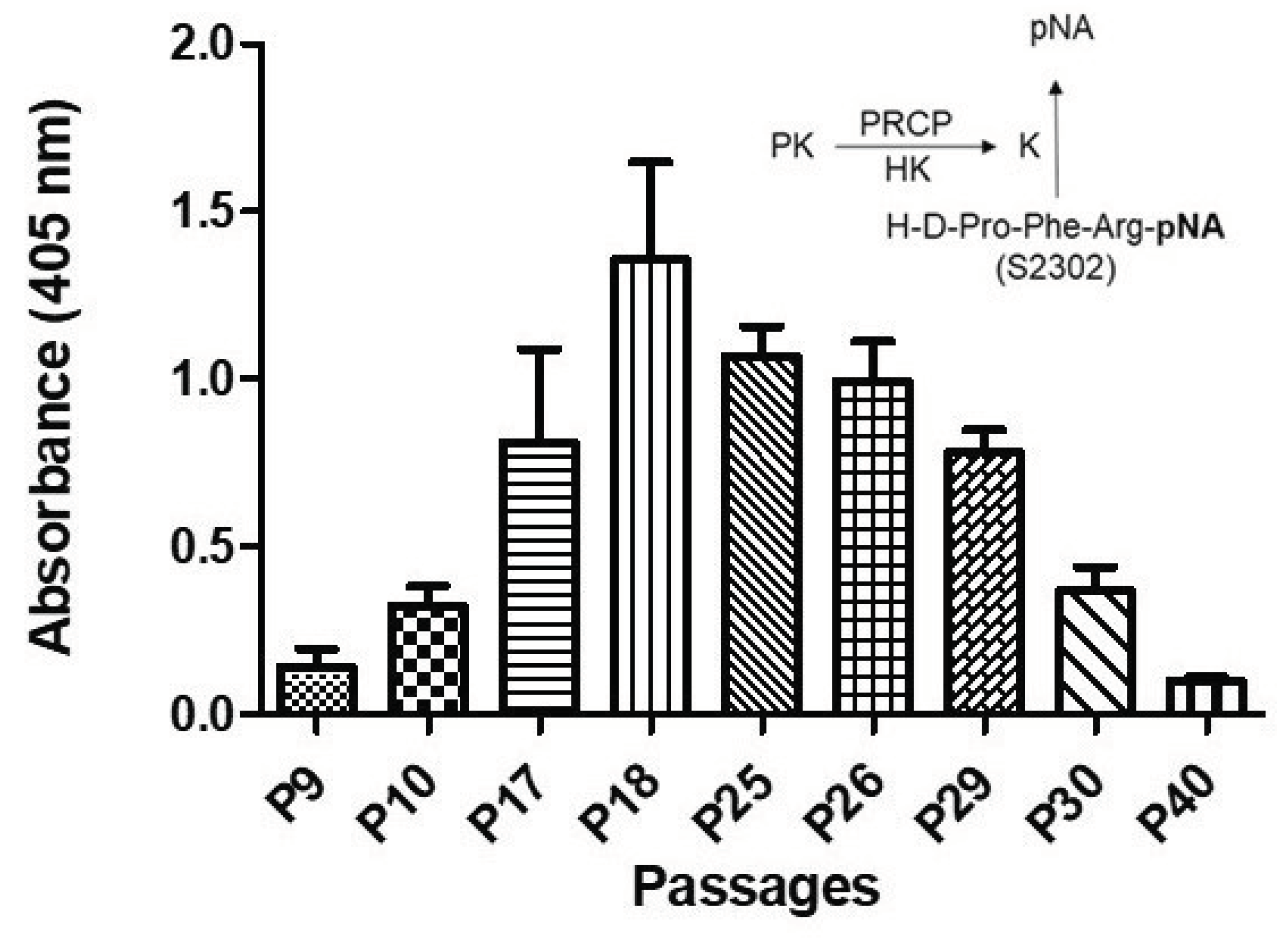
2.2. Correlation between PRCP Expression and Kallikrein Activity in HPAECs at Various Passages
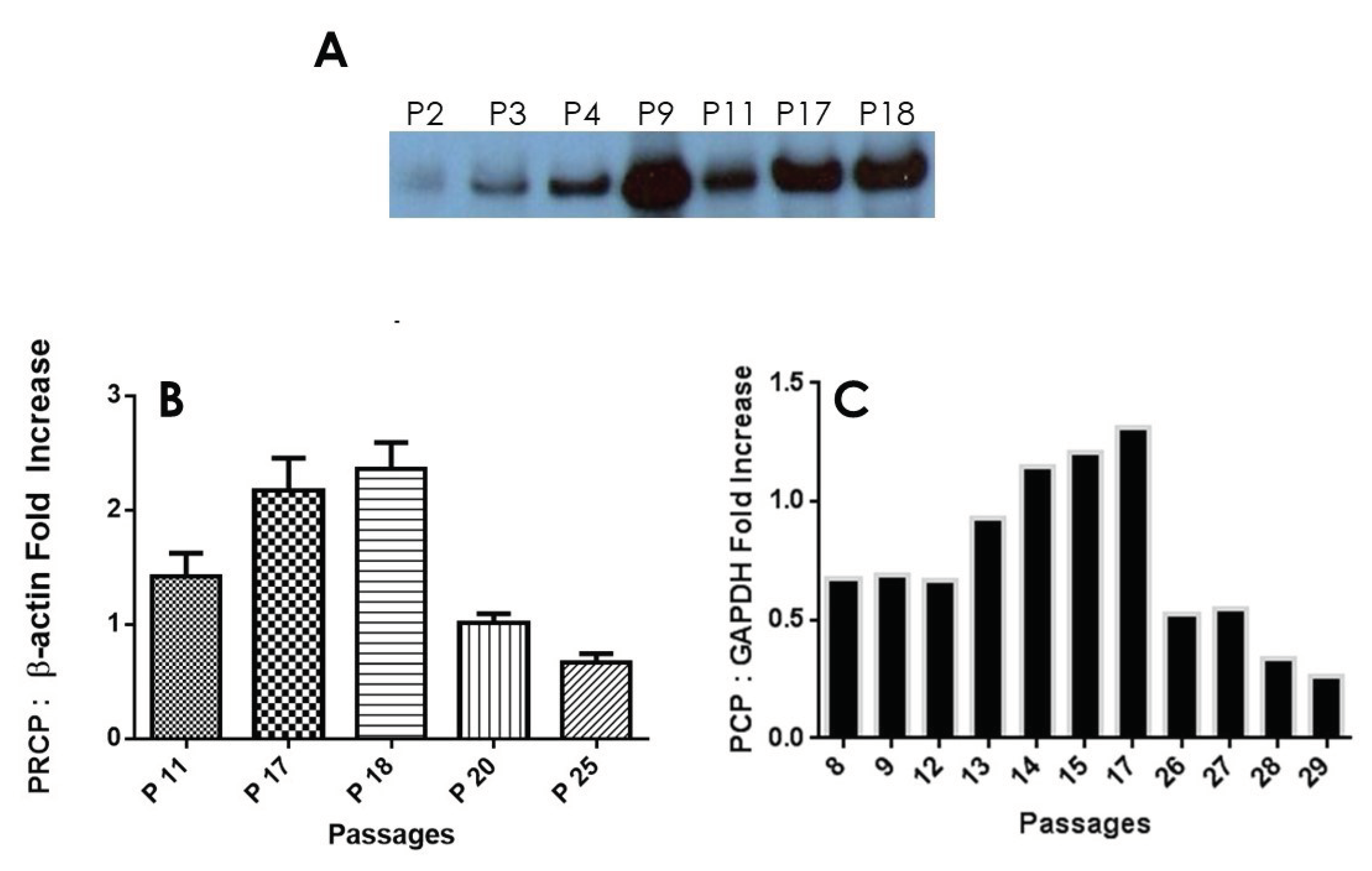
2.3. PRCP Delays Cellular Senescence through an NO-Dependent Mechansim
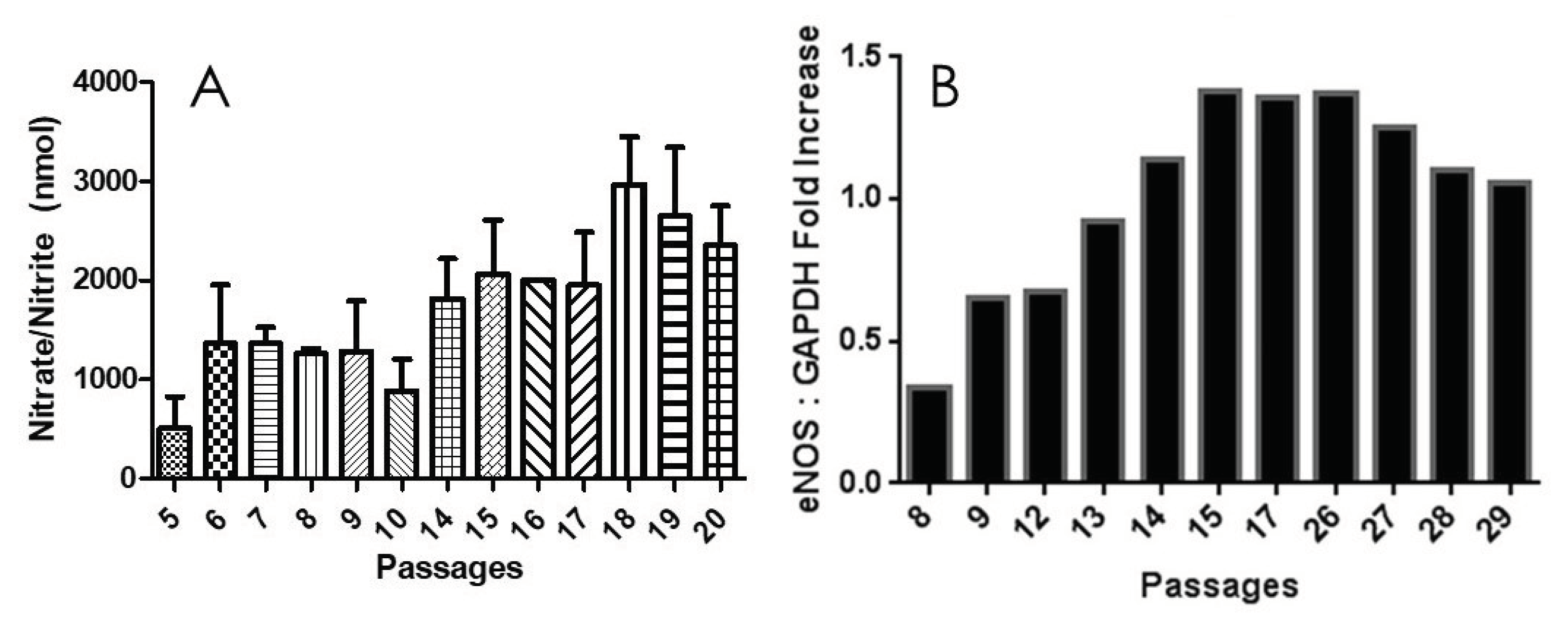
2.4. β-Galactosidase Activity Increases with Age
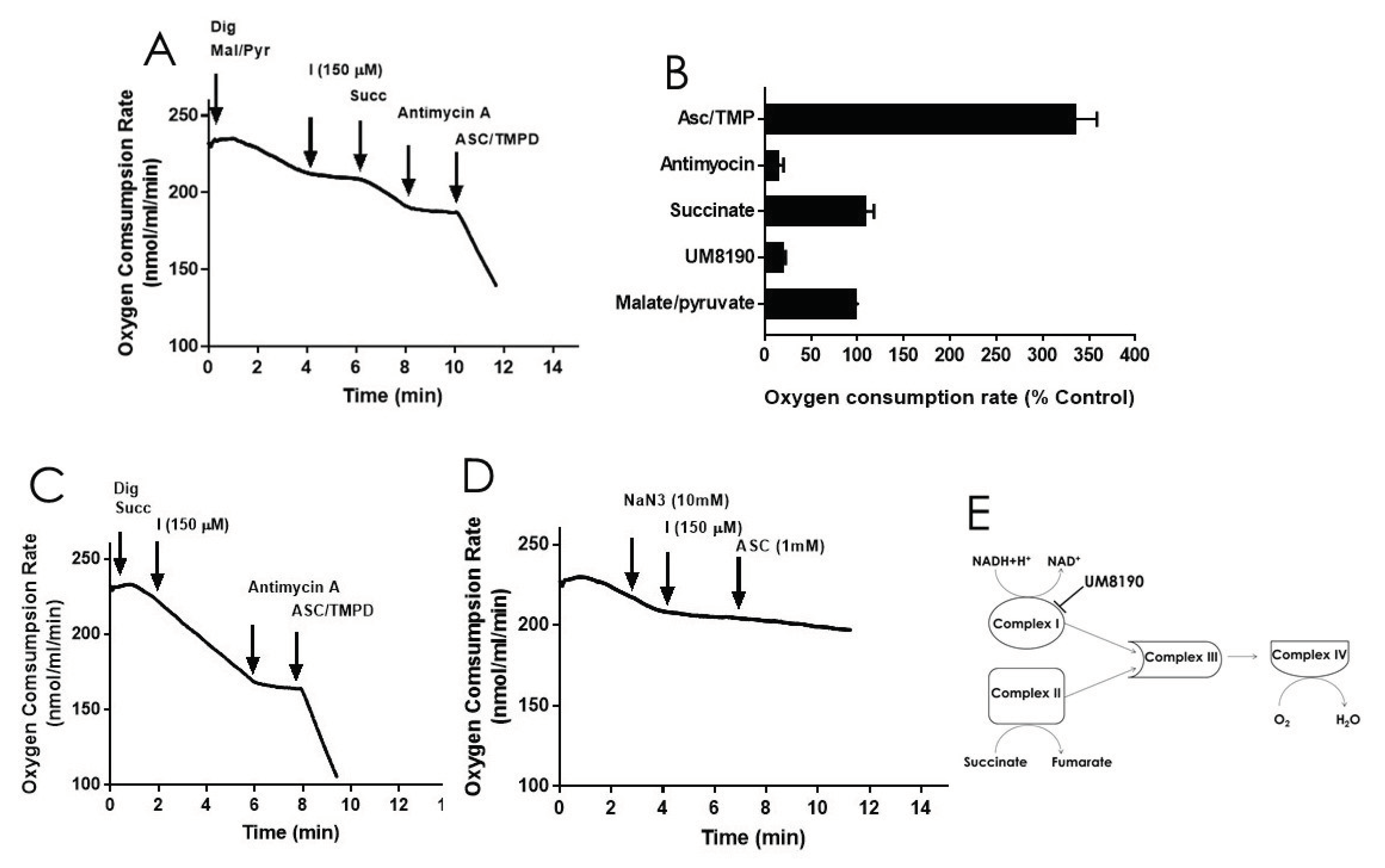
2.5. Effect of UM8190-Induced PRCP Inhibition on Mitochondrial Functions
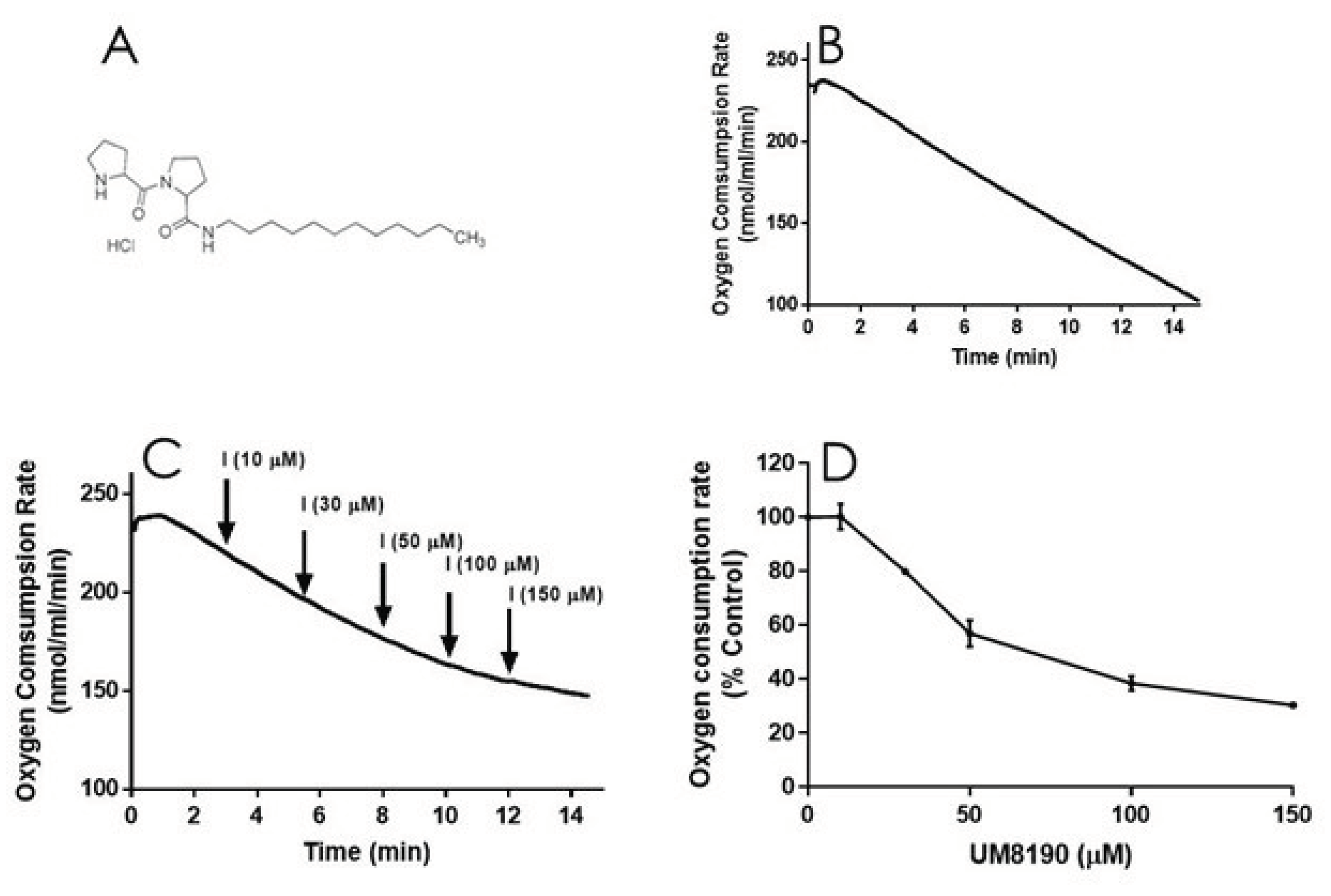
2.6. Effect of UM8190 on Mitochondrial Generation of Reactive Oxygen Species (ROS)
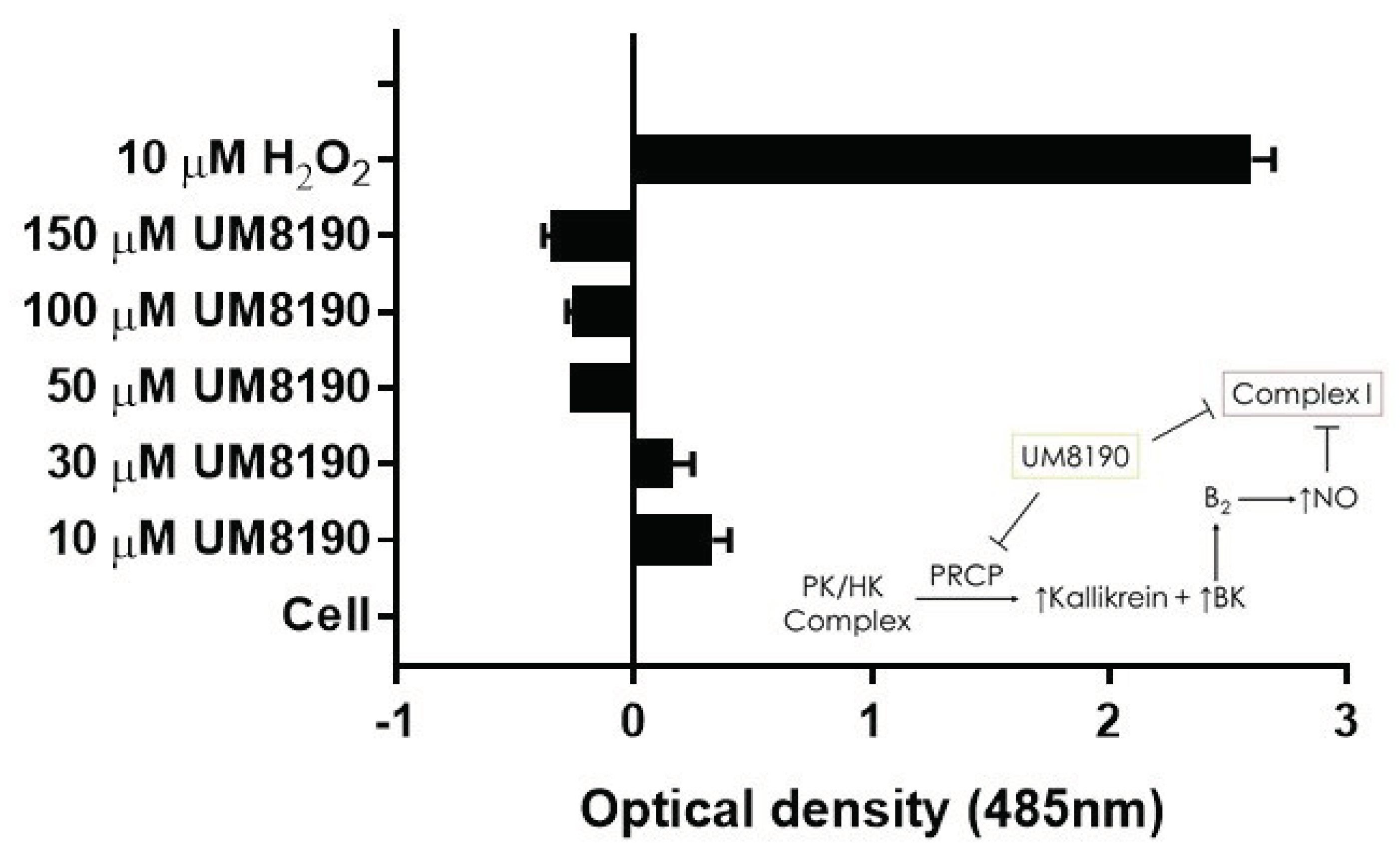
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Endothelial Cell Culture
4.3. HK/PK Activity
4.4. Western Blot
4.5. Nitrite/Nitrate Assay
4.6. Cellular Senescence Assay Kit
4.7. Reverse-Transcriptase Polymerase Chain reaction and Agarose Gel
4.8. Oxygen Consumption Rate Measurements on HPAE Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ang III | Angiotensin III |
| Ang 2-7 | Angiotensin 2-7 |
| ATP | Adenosine triphosphate |
| β-Gal | β-galactosidase |
| BKB2 | Bradykinin B2 receptor |
| BK | Bradykinin |
| CVD | Cardiovascular diseases |
| EGFR | Epidermal Growth Factor Receptor |
| eNOS | Endothelial nitric oxide synthase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| H2O2 | Hydrogen Peroxide |
| HK | High molecular weight |
| HPAEC | Human pulmonary artery endothelial cells |
| hTERT | Human telomerase reverse transcriptase |
| IgAN | IgA nephropathy |
| KKS | Kallikrein-kinin system |
| mDNA | Mitochondrial DNA |
| Mal/Pyr | Malate/ pyruvate |
| NAD | Nicotinamide Adenine Dinucleotide |
| NADH | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NaN3 | Sodium azide |
| NO | Nitric oxide |
| PAR | Protease-activated receptor |
| PK | Prekallikrein |
| PRCP | Prolylcarboxypeptidase |
| PTCA | percutaneous transluminal coronary angioplasty |
| RAS | Renin-angiotensin system |
| ROS | Reactive oxygen species |
| SHRs | Spontaneously hypertensive rats |
| T2DM | Type 2 diabetes mellitus |
| TMPD | N,N,N’,N’-tetramethyl-p-phenylenediamine |
References
- Hayflick, L., The Cell Biology of Aging. Journal of Investigative Dermatology 1979, 73, (1), 8-14. [CrossRef]
- Shay, J. W.; Wright, W. E., Hayflick, his limit, and cellular ageing. Nature reviews. Molecular cell biology 2000, 1, (1), 72-6. [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A. J.; Galvan, V.; Csiszar, A., Mechanisms of Vascular Aging. Circulation Research 2018, 123, (7), 849-867. [CrossRef]
- Saretzki, G.; Wan, T. Telomerase in Brain: The New Kid on the Block and Its Role in Neurodegenerative Diseases. Biomedicines 2021, 9, 490. [CrossRef]
- Brandt, M.; Dörschmann, H.; Khraisat, S.; Knopp, T.; Ringen, J.; Kalinovic, S.; Garlapati, V.; Siemer, S.; Molitor, M.; Göbel, S.; et al. Telomere Shortening in Hypertensive Heart Disease Depends on Oxidative DNA Damage and Predicts Impaired Recovery of Cardiac Function in Heart Failure. Hypertension 2022, 79, 2173–2184. [CrossRef]
- Levstek, T.; Podkrajšek, K.T. Telomere Attrition in Chronic Kidney Diseases. Antioxidants 2023, 12, 579. [CrossRef]
- Ahmed, W.; Lingner, J. PRDX1 Counteracts Catastrophic Telomeric Cleavage Events That Are Triggered by DNA Repair Activities Post Oxidative Damage. Cell Rep. 2020, 33, 108347. [CrossRef]
- Hayashi, T.; Kotani, H.; Yamaguchi, T.; Taguchi, K.; Iida, M.; Ina, K.; Maeda, M.; Kuzuya, M.; Hattori, Y.; Ignarro, L.J. Endothelial cellular senescence is inhibited by liver X receptor activation with an additional mechanism for its atheroprotection in diabetes. Proc. Natl. Acad. Sci. 2014, 111, 1168–1173. [CrossRef]
- Ferroni, P.; Basili, S.; Paoletti, V.; Davì, G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 222–233. [CrossRef]
- Arabshomali, A.; Bazzazzadehgan, S.; Mahdi, F.; Shariat-Madar, Z. Potential Benefits of Antioxidant Phytochemicals in Type 2 Diabetes. Molecules 2023, 28, 7209. [CrossRef]
- Aubert, G.; Lansdorp, P. M., Telomeres and Aging. Physiological reviews 2008, 88, (2), 557-579.
- Brown, N.J.; E Vaughan, D. Prothrombotic effects of angiotensin.. 2000, 45, 419–29.
- Xu, S.; Lind, L.; Zhao, L.; Lindahl, B.; Venge, P. Plasma Prolylcarboxypeptidase (Angiotensinase C) Is Increased in Obesity and Diabetes Mellitus and Related to Cardiovascular Dysfunction. Clin. Chem. 2012, 58, 1110–1115. [CrossRef]
- Gittleman, H.R.; Merkulova, A.; Alhalabi, O.; Stavrou, E.X.; Veigl, M.L.; Barnholtz-Sloan, J.S.; Schmaier, A.H. A Cross-sectional Study of KLKB1 and PRCP Polymorphisms in Patient Samples with Cardiovascular Disease. Front. Med. 2016, 3, 17–17. [CrossRef]
- Skidgel, R.A.; Erdös, E.G. Cellular carboxypeptidases. Immunol. Rev. 1998, 161, 129–141. [CrossRef]
- Mallela, J.; Perkins, R.; Yang, J.; Pedigo, S.; Rimoldi, J.; Shariat-Madar, Z. The functional importance of the N-terminal region of human prolylcarboxypeptidase. Biochem. Biophys. Res. Commun. 2008, 374, 635–640. [CrossRef]
- Maier, C.; Schadock, I.; Haber, P.K.; Wysocki, J.; Ye, M.; Kanwar, Y.; Flask, C.A.; Yu, X.; Hoit, B.D.; Adams, G.N.; et al. Prolylcarboxypeptidase deficiency is associated with increased blood pressure, glomerular lesions, and cardiac dysfunction independent of altered circulating and cardiac angiotensin II. J. Mol. Med. 2017, 95, 473–486. [CrossRef]
- Nguyen, B.Y.; Zhou, F.; Binder, P.; Liu, W.; Hille, S.S.; Luo, X.; Zi, M.; Zhang, H.; Adamson, A.; Ahmed, F.Z.; et al. Prolylcarboxypeptidase Alleviates Hypertensive Cardiac Remodeling by Regulating Myocardial Tissue Angiotensin II. J. Am. Hear. Assoc. 2023, 12, e028298. [CrossRef]
- Merkulova, A.A.; Abdalian, S.; Silbak, S.; Pinheiro, A.; Schmaier, A.H. C1 inhibitor and prolylcarboxypeptidase modulate prekallikrein activation on endothelial cells. J. Allergy Clin. Immunol. 2023, 152, 961–971.e7. [CrossRef]
- Wang, J.; Matafonov, A.; Madkhali, H.; Mahdi, F.; Watson, D.; Schmaier, A.; Gailani, D.; Shariat-Madar, Z. Prolylcarboxypeptidase Independently Activates Plasma Prekallikrein (Fletcher Factor). Curr. Mol. Med. 2014, 14, 1173–1185. [CrossRef]
- Zhang, M.; Lin, D.; Luo, C.; Wei, P.; Cui, K.; Chen, Z. Tissue Kallikrein Protects Rat Prostate against the Inflammatory Damage in a Chronic Autoimmune Prostatitis Model via Restoring Endothelial Function in a Bradykinin Receptor B2-Dependent Way. Oxidative Med. Cell. Longev. 2022, 2022, 1–16. [CrossRef]
- Molaei, A.; Molaei, E.; Hayes, A.W.; Karimi, G. Mas receptor: a potential strategy in the management of ischemic cardiovascular diseases. Cell Cycle 2023, 22, 1654–1674. [CrossRef]
- Hayashi, T.; Matsui-Hirai, H.; Miyazaki-Akita, A.; Fukatsu, A.; Funami, J.; Ding, Q.-F.; Kamalanathan, S.; Hattori, Y.; Ignarro, L.J.; Iguchi, A. Endothelial cellular senescence is inhibited by nitric oxide: Implications in atherosclerosis associated with menopause and diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 17018–17023. [CrossRef]
- Gaidarov, I.; Adams, J.; Frazer, J.; Anthony, T.; Chen, X.; Gatlin, J.; Semple, G.; Unett, D.J. Angiotensin (1–7) does not interact directly with MAS1, but can potently antagonize signaling from the AT1 receptor. Cell. Signal. 2018, 50, 9–24. [CrossRef]
- Kovarik, J.J.; Kopecky, C.; Antlanger, M.; Domenig, O.; Kaltenecker, C.C.; Werzowa, J.; Hecking, M.; Mahr, S.; Grömmer, M.; Wallner, C.; et al. Effects of angiotensin-converting-enzyme inhibitor therapy on the regulation of the plasma and cardiac tissue renin-angiotensin system in heart transplant patients. J. Hear. Lung Transplant. 2017, 36, 355–365. [CrossRef]
- Wu, Y.; Yang, H.; Yang, B.; Yang, K.; Xiao, C. Association of polymorphisms in prolylcarboxypeptidase and chymase genes with essential hypertension in the Chinese Han population. J. Renin-Angiotensin-Aldosterone Syst. 2012, 14, 263–270. [CrossRef]
- Liu, J.; Hakucho, A.; Fujimiya, T. Angiotensinase C mRNA and Protein Downregulations Are Involved in Ethanol-Deteriorated Left Ventricular Systolic Dysfunction in Spontaneously Hypertensive Rats. BioMed Res. Int. 2015, 2015, 1–10. [CrossRef]
- Wang, L.; Feng, Y.; Zhang, Y.; Zhou, H.; Jiang, S.; Niu, T.; Wei, L.-J.; Xu, X.; Xu, X.; Wang, X. Prolylcarboxypeptidase gene, chronic hypertension, and risk of preeclampsia. Am. J. Obstet. Gynecol. 2006, 195, 162–171. [CrossRef]
- Adams, G.N.; Stavrou, E.X.; Fang, C.; Merkulova, A.; Alaiti, M.A.; Nakajima, K.; Morooka, T.; Merkulov, S.; LaRusch, G.A.; I Simon, D.; et al. Prolylcarboxypeptidase promotes angiogenesis and vascular repair. Blood 2013, 122, 1522–1531. [CrossRef]
- Kolte, D.; Bryant, J.; Holsworth, D.; Wang, J.; Akbari, P.; Gibson, G.; Shariat-Madar, Z. Biochemical characterization of a novel high-affinity and specific plasma kallikrein inhibitor. Br. J. Pharmacol. 2010, 162, 1639–1649. [CrossRef]
- Zhao, Y.; Qiu, Q.; Mahdi, F.; Shariat-Madar, Z.; Røjkjær, R.; Schmaier, A.H. Assembly and activation of HK-PK complex on endothelial cells results in bradykinin liberation and NO formation. Am. J. Physiol. Circ. Physiol. 2001, 280, H1821–H1829. [CrossRef]
- Schmaier, A.H. Physiologic activities of the Contact Activation System. Thromb. Res. 2014, 133, S41–S44. [CrossRef]
- Ngo, M.-L.; Mahdi, F.; Kolte, D.; Shariat-Madar, Z. Upregulation of prolylcarboxypeptidase (PRCP) in lipopolysaccharide (LPS) treated endothelium promotes inflammation. J. Inflamm. 2009, 6, 3–3. [CrossRef]
- Kichuk, M.R.; Seyedi, N.; Zhang, X.; Marboe, C.C.; Michler, R.E.; Addonizio, L.J.; Kaley, G.; Nasjletti, A.; Hintze, T.H.; D, G.; et al. Regulation of Nitric Oxide Production in Human Coronary Microvessels and the Contribution of Local Kinin Formation. Circ. 1996, 94, 44–51. [CrossRef]
- Förstermann, U.; Münzel, T., Endothelial Nitric Oxide Synthase in Vascular Disease. Circulation 2006, 113, (13), 1708-1714.
- Wada, Y.; Umeno, R.; Nagasu, H.; Kondo, M.; Tokuyama, A.; Kadoya, H.; Kidokoro, K.; Taniguchi, S.; Takahashi, M.; Sasaki, T.; et al. Endothelial Dysfunction Accelerates Impairment of Mitochondrial Function in Ageing Kidneys via Inflammasome Activation. Int. J. Mol. Sci. 2021, 22, 9269. [CrossRef]
- Vasa, M.; Breitschopf, K.; Zeiher, A.M.; Dimmeler, S. Nitric Oxide Activates Telomerase and Delays Endothelial Cell Senescence. Circ. Res. 2000, 87, 540–542. [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [CrossRef]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [CrossRef]
- Jun, J.-I.; Lau, L.F. Cellular senescence controls fibrosis in wound healing. Aging 2010, 2, 627–631. [CrossRef]
- Aon-Im, P.; Monthakantirat, O.; Daodee, S.; Chulikhit, Y.; Sriya, N.; Boonyarat, C.; Chumwangwapee, T.; Khamphukdee, C.; Kijjoa, A., Evaluation of the Impact of Alternanthera philoxeroides (Mart.) Griseb. Extract on Memory Impairment in D-Galactose-Induced Brain Aging in Mice through Its Effects on Antioxidant Enzymes, Neuroinflammation, and Telomere Shortening. Molecules 2024, 29, (2).
- Quiles, J.; Cabrera, M.; Jones, J.; Tsapekos, M.; Caturla, N. In Vitro Determination of the Skin Anti-Aging Potential of Four-Component Plant-Based Ingredient. Molecules 2022, 27, 8101. [CrossRef]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast Growth Factor 2—A Review of Stabilisation Approaches for Clinical Applications. Pharmaceutics 2020, 12, 508. [CrossRef]
- Chen, P. Y.; Qin, L.; Li, G.; Tellides, G.; Simons, M., Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFbeta)-dependent smooth muscle cell phenotype modulation. Sci Rep 2016, 6, 33407.
- Dranka, B.P.; Hill, B.G.; Darley-Usmar, V.M. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010, 48, 905–914. [CrossRef]
- Pandya, J. D.; Sullivan, P. G.; Leung, L. Y.; Tortella, F. C.; Shear, D. A.; Deng-Bryant, Y., Advanced and High-Throughput Method for Mitochondrial Bioenergetics Evaluation in Neurotrauma. Methods Mol Biol 2016, 1462, 597-610.
- Finlin, B.S.; Memetimin, H.; Confides, A.L.; Kasza, I.; Zhu, B.; Vekaria, H.J.; Harfmann, B.; Jones, K.A.; Johnson, Z.R.; Westgate, P.M.; et al. Human adipose beiging in response to cold and mirabegron. J. Clin. Investig. 2018, 3. [CrossRef]
- Stuehr, D.J.; Nathan, C.F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells.. J. Exp. Med. 1989, 169, 1543–1555. [CrossRef]
- Brown, G.C.; Borutaite, V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2004, 1658, 44–49. [CrossRef]
- Rabey, F.M.; Gadepalli, R.S.; Diano, S.; Cheng, Q.; Tabrizian, T.; Gailani, D.; Rimoldi, J.M.; Shariat-Madar, Z. Influence of a novel inhibitor (UM8190) of prolylcarboxypeptidase (PRCP) on appetite and thrombosis.. Curr. Med. Chem. 2012, 19, 4194–206. [CrossRef]
- Reversible Membrane Permeabilization of Mammalian Cells Treated with Digitonin and Its Use for Inducing Nuclear Reprogramming by Xenopus Egg Extracts. Cloning and Stem Cells 2008, 10, (4), 535-542.
- Liu, J.; Xiao, N.; DeFranco, D. B., Use of digitonin-permeabilized cells in studies of steroid receptor subnuclear trafficking. Methods 1999, 19, (3), 403-9.
- Iverson, T. M.; Singh, P. K.; Cecchini, G., An evolving view of complex II-noncanonical complexes, megacomplexes, respiration, signaling, and beyond. J Biol Chem 2023, 299, (6), 104761.
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial Complex II Can Generate Reactive Oxygen Species at High Rates in Both the Forward and Reverse Reactions. J. Biol. Chem. 2012, 287, 27255–27264. [CrossRef]
- Fink, B.; Laude, K.; McCann, L.; Doughan, A.; Harrison, D.G.; Dikalov, S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am. J. Physiol. Physiol. 2004, 287, C895–C902. [CrossRef]
- Zielonka, J.; Kalyanaraman, B., Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med 2010, 48, (8), 983-1001.
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [CrossRef]
- Qin, Y.-F.; Tian, H.-H.; Sun, F.; Qin, X.-P. [Effect of losartan on the protection of the kidney and PRCP-kallikrein axis of the two-kidney, one-clipped renovascular hypertensive rats].. 2013, 48, 59–65.
- Krochmal, M.; Cisek, K.; Filip, S.; Markoska, K.; Orange, C.; Zoidakis, J.; Gakiopoulou, C.; Spasovski, G.; Mischak, H.; Delles, C.; et al. Identification of novel molecular signatures of IgA nephropathy through an integrative -omics analysis. Sci. Rep. 2017, 7, 1–13. [CrossRef]
- Herrera, M.D.; Mingorance, C.; Rodriguez-Rodriguez, R.; Alvarez de Sotomayor, M. Endothelial dysfunction and aging: An update. Ageing Res. Rev. 2010, 9, 142–152. [CrossRef]
- Vita, J. A., Endothelial Function. Circulation 2011, 124, (25), e906-e912.
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [CrossRef]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [CrossRef]
- Han, Y.; Kim, S.Y. Endothelial senescence in vascular diseases: current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 2023, 55, 1–12. [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [CrossRef]
- Duan, L.; Motchoulski, N.; Danzer, B.; Davidovich, I.; Shariat-Madar, Z.; Levenson, V.V. Prolylcarboxypeptidase Regulates Proliferation, Autophagy, and Resistance to 4-Hydroxytamoxifen-induced Cytotoxicity in Estrogen Receptor-positive Breast Cancer Cells. J. Biol. Chem. 2011, 286, 2864–2876. [CrossRef]
- Guevara-Lora, I.; Sordyl, M.; Niewiarowska-Sendo, A.; Bras, G.; Korbut, E.; Goralska, J.; Malczewska-Malec, M.; Solnica, B.; Kozik, A. The effect of bradykinin on the pro-inflammatory response of human adipocytes. Acta Biochim. Pol. 2022, 69, 495–505. [CrossRef]
- Dai, D.-F.; Rabinovitch, P.S.; Ungvari, Z. Mitochondria and Cardiovascular Aging. Circ. Res. 2012, 110, 1109–1124. [CrossRef]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 1–15. [CrossRef]
- Owen, M. R.; Doran, E.; Halestrap, A. P., Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000, 348 Pt 3, (Pt 3), 607-14.
- Abrosimov, R.; Baeken, M. W.; Hauf, S.; Wittig, I.; Hajieva, P.; Perrone, C. E.; Moosmann, B., Mitochondrial complex I inhibition triggers NAD(+)-independent glucose oxidation via successive NADPH formation, "futile" fatty acid cycling, and FADH(2) oxidation. Geroscience 2024.
- Degli Esposti, M., Inhibitors of NADH–ubiquinone reductase: an overview. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1998, 1364, (2), 222-235.
- Kita, T.; Clermont, A.C.; Murugesan, N.; Zhou, Q.; Fujisawa, K.; Ishibashi, T.; Aiello, L.P.; Feener, E.P. Plasma Kallikrein-Kinin System as a VEGF-Independent Mediator of Diabetic Macular Edema. Diabetes 2015, 64, 3588–3599. [CrossRef]
- Bergsma, D.; Chen, S.; Buchweitz, J.; Gerszten, R.; Haab, B.B. Antibody-Array Interaction Mapping, a New Method to Detect Protein Complexes Applied to the Discovery and Study of Serum Amyloid P Interactions with Kininogen in Human Plasma. Mol. Cell. Proteom. 2010, 9, 446–456. [CrossRef]
- Pinheiro, A.S.; Silbak, S.; Schmaier, A.H. Bradykinin – An elusive peptide in measuring and understanding. Res. Pr. Thromb. Haemost. 2022, 6, e12673. [CrossRef]
- Senchenkova, E. Y.; Russell, J.; Almeida-Paula, L. D.; Harding, J. W.; Granger, D. N., Angiotensin II-mediated microvascular thrombosis. Hypertension 2010, 56, (6), 1089-95.
- Dielis, A.W.; Smid, M.; Spronk, H.M.; Hamulyak, K.; Kroon, A.A.; Cate, H.T.; de Leeuw, P.W. The Prothrombotic Paradox of Hypertension. Hypertension 2005, 46, 1236–1242. [CrossRef]
- Bird, J. E.; Smith, P. L.; Wang, X.; Schumacher, W. A.; Barbera, F.; Revelli, J. P.; Seiffert, D., Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb Haemost 2012, 107, (6), 1141-50.
- Wang, J.-K.; Li, Y.; Zhao, X.-L.; Liu, Y.-B.; Tan, J.; Xing, Y.-Y.; Adi, D.; Wang, Y.-T.; Fu, Z.-Y.; Ma, Y.-T.; et al. Ablation of Plasma Prekallikrein Decreases Low-Density Lipoprotein Cholesterol by Stabilizing Low-Density Lipoprotein Receptor and Protects Against Atherosclerosis. Circ. 2022, 145, 675–687. [CrossRef]
- Shariat-Madar, Z.; Mahdi, F.; Warnock, M.; Homeister, J.W.; Srikanth, S.; Krijanovski, Y.; Murphey, L.J.; Jaffa, A.A.; Schmaier, A.H. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood 2006, 108, 192–199. [CrossRef]
- Simão, F.; Ustunkaya, T.; Clermont, A.C.; Feener, E.P. Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke. Blood 2017, 129, 2280–2290. [CrossRef]
- Czokało-Plichta, M.; Skibinska, E.; Kosiorek, P.; Musiał, W.J. Kallikrein-kinin system activation and its interactions with other plasma haemostatic components in the coronary artery disease.. 2001, 46, 209–24.
- Aspelin, T.; Eriksen, M.; Ilebekk, A.; Björkman, J.-A.; Lyberg, T. Different cardiac tissue plasminogen activator release patterns by local stimulation of the endothelium and sympathetic nerves in pigs. Blood Coagul. Fibrinolysis 2012, 23, 714–722. [CrossRef]
- Hou, W.; Yin, J.; Alimujiang, M.; Yu, X.; Ai, L.; Bao, Y.; Liu, F.; Jia, W. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J. Cell. Mol. Med. 2017, 22, 1316–1328. [CrossRef]
- Chang, A. M.; Halter, J. B., Aging and insulin secretion. American Journal of Physiology-Endocrinology and Metabolism 2003, 284, (1), E7-E12.
- Harris, A.; Wirostko; Ehrlich, R.; Kheradiya, N.S.; Winston, D.M.; A Ciulla, T.; Wirostko, B. Age-related macular degeneration and the aging eye. Clin. Interv. Aging 2008, ume 3, 473–482. [CrossRef]
- Cucu, I. Signaling Pathways in Inflammation and Cardiovascular Diseases: An Update of Therapeutic Strategies. Immuno 2022, 2, 630–650. [CrossRef]
- Liu, Y.; Sun, Y.; Guo, Y.; Shi, X.; Chen, X.; Feng, W.; Wu, L.-L.; Zhang, J.; Yu, S.; Wang, Y.; et al. An Overview: The Diversified Role of Mitochondria in Cancer Metabolism. Int. J. Biol. Sci. 2023, 19, 897–915. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




