2. Materials and Methods
Reagents and antibodies. Anti-PD-L1 (10F.9G2), anti-CD45 (30-F11), anti-CD11b (M1/70), anti-Ly-6G (1A8), anti-Ly-6C (HK1.4), anti-F4/80 (BM8), anti-CD3 (17A2), anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-TCRγδ (UC7-13D5), anti-NK1.1 (S17016D), anti-IFN-γ (XMG1.2), anti-IL-17A (TC11-18H10.1), anti-IL-6 (MP5-20F3) and anti-TNF-α (MP6-XT22) were purchased from BioLegend (San Diego, CA, USA). Anti-IL-1β (EPR24895-116), anti-myeloperoxidase (MPO, EPR20257), anti-neutrophil erastase (NE, EPR7479) were purchased from Abcam (Cambridge, UK). Anti-Granzyme B (NGZB) and anti-perfolin (eBioOMAK-D), 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA), SYTOXTM Green and 7-amino-actinomycin D (7-AAD) were purchased from Thremo Fisher Scientific (Waltham, MA, USA). Anti-PD-L1 mAb (10F.9G2) and corresponding isotype antibody were purchased from BioXcell (Lebanon, NH, USA). phorbol-12-myristate-13-acetate (PMA), ionomycin, ALT activity assay kit were purchased from Sigma Aldrich (Laclede Avenue. St. Louis, MO, USA). Golgi StopTM was purchased from BD Bioscience (Franklin Lakes, NJ, USA). Percoll was purchased from GE health care (Chicago, IL, USA). Recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) was purchased from Peprotech (Cranbury, NJ, USA).
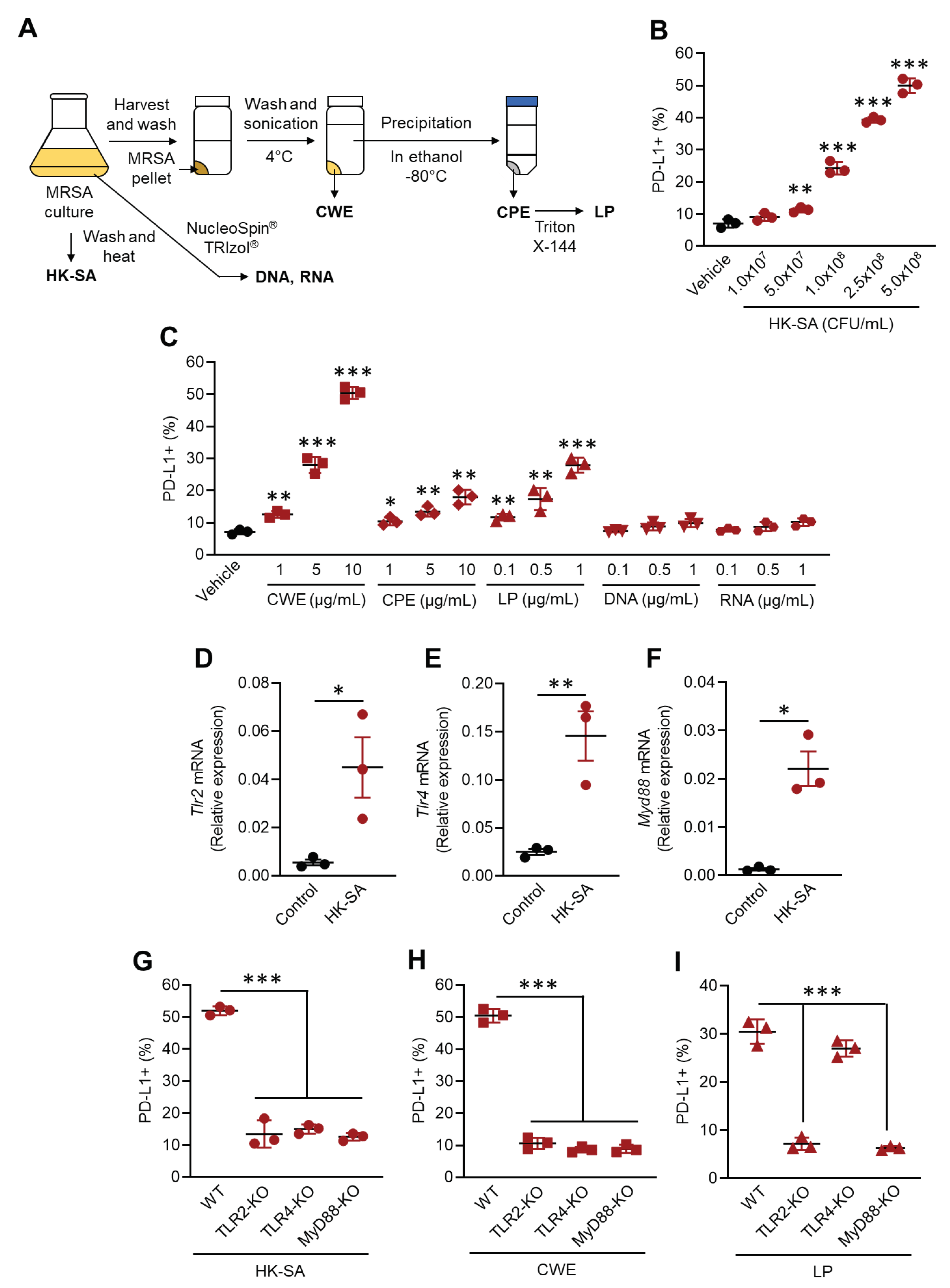
Methicillin-resistant Staphylococcus aureus culture and sample preparation. The frozen
S. aureus (MRSA; USA300) stock was thawed on ice, then transferred to a tryptic soy broth (TSB; BD Bioscience, Franklin Lakes, NJ, USA) The bacterial suspension was cultured at 37 °C for 18 h with shaking, then the grown bacteria were further cultured in TSB at 1:100 dilution at 37°C for 6-8 hours. The colony forming unit (CFU) was calculated by standard curve method in each culture. For Heat-killed
S. aureus (HK-SA) preparation, the bacterial suspension was heated at 95 °C for 15 min. The heated
S. aureus suspension was centrifuged at 10,000 rpm for 1 min to harvest the bacteria cells and washed with phosphate buffered saline (PBS), then the cell pellet was resuspended in PBS. Cell wall extract (CWE), crude protein extract (CPE), Lipoprotein (LP) and nucleic acids were isolated from
S. aureus by following a method described in previous reports with minor modifications [
13,
14]. Briefly, the cultured
S. aureus (10
8-9 CFU/mL) was harvested by centrifuging at 5,000
g for 20 min, then the pellet was washed twice with Tris-HCl (20 mM, pH 8.0). The pellet was resuspended in Tris–HCl (20 mM, pH 8.0) and stored at -80℃ for overnight. The sample was centrifuged at 5,000
g for 20 min again, then bacterial cells were crushed with 0.3 mm stainless beads followed by sonication at 4℃ for 20 min. The treated sample was centrifuged at 5,000
g for 20 min, then the supernatant was harvested as containing proteins. The precipitated pellet was treated with protease (1 μg/mL) and lipoprotein lipase (1 μg/mL) at 37℃ for 1 h followed by heating at 95℃ for 15 min. The sample was washed with Tris–HCl (20 mM, pH 8.0) and centrifuged at 5,000
g for 20 min. The precipitated sample was used as CWE. The supernatant containing protein was mixed with equal volume of 100% of ethanol and kept at -80 °C for overnight. The sample was centrifuged at 12,000
g for 15 min, then the precipitated pellet was washed with 80% of ethanol and centrifuged again at 12,000
g for 15 min. The precipitated pellet was dissolved with 1 M urea/50 mM Tris-HCl, 50 mM ethylenediaminetetraacetic acid (EDTA) (pH 8.0) and treated with DNase (50 μg/mL) and RNase (50 μg/mL) at 37℃ for 1 h, then the sample was used as CPE. For LP isolation, Triton X-114 was added to the CPE (final 1%), then the suspension was incubated at 4 °C with gentle mixing. The sample was further incubated at 37 °C to form the micelle phase-containing LP. The micelle phase was collected, and LP was precipitated by following a method for CPE precipitation. The precipitated pellet was used as LP. DNA and RNA were isolated from bacteria using NucleoSpin
® and TRIzol
®, respectively.
Animal experiment. C57BL/6 mice were purchased from CLEA Japan (Tokyo, Japan) and The Jackson Laboratory (Bar Harbor, ME, USA). TLR2-KO mice (B6.129-Tlr2tm1Kir/J), TLR4-KO mice (B6(Cg)-Tlr4tm1.2Karp/J) and MyD88-KO mice (B6.129P2(SJL)-Myd88tm1.1Defr/J) were purchased from The Jackson Laboratory. All mice were bred in-house and maintained in a specific pathogen-free (SPF) facility with 12-hour light/dark cycles and allowed free access to food and water. Adult mice of both genders aged 8-20 weeks were used for each experiment. All animal experimental protocols were approved by the animal care and use committee of Jichi Medical University (20038-01), University of South China (202005053) and Shibata Gakuen University (2107).
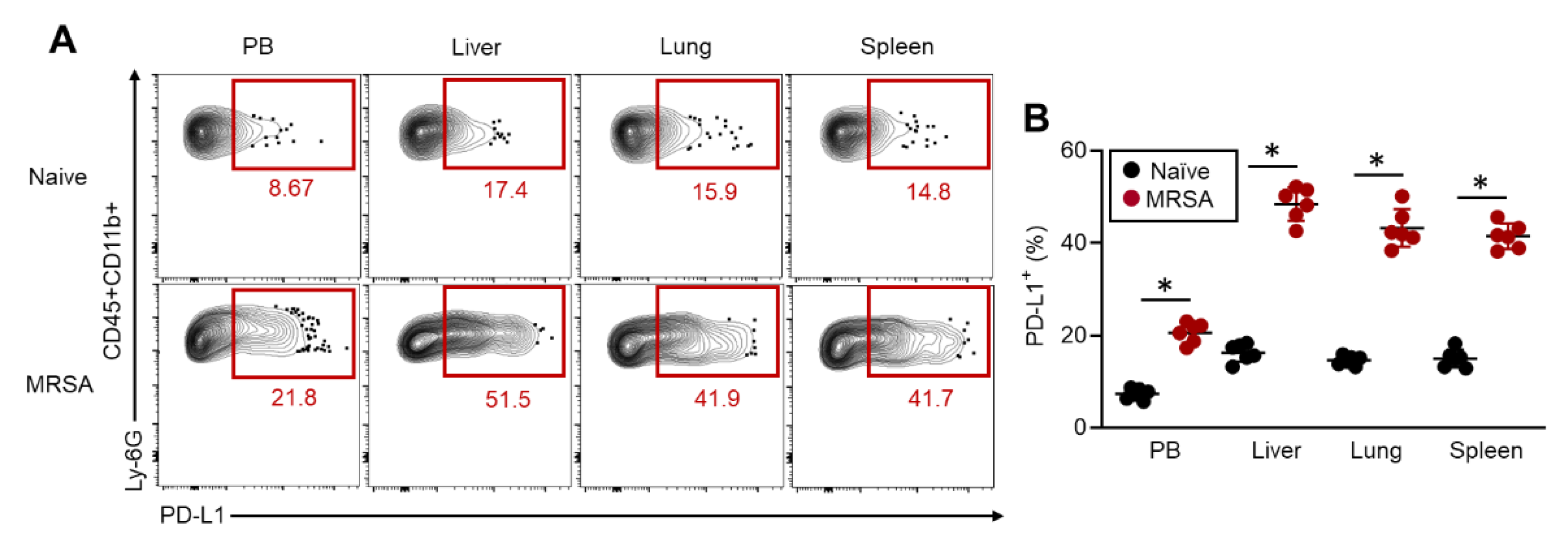
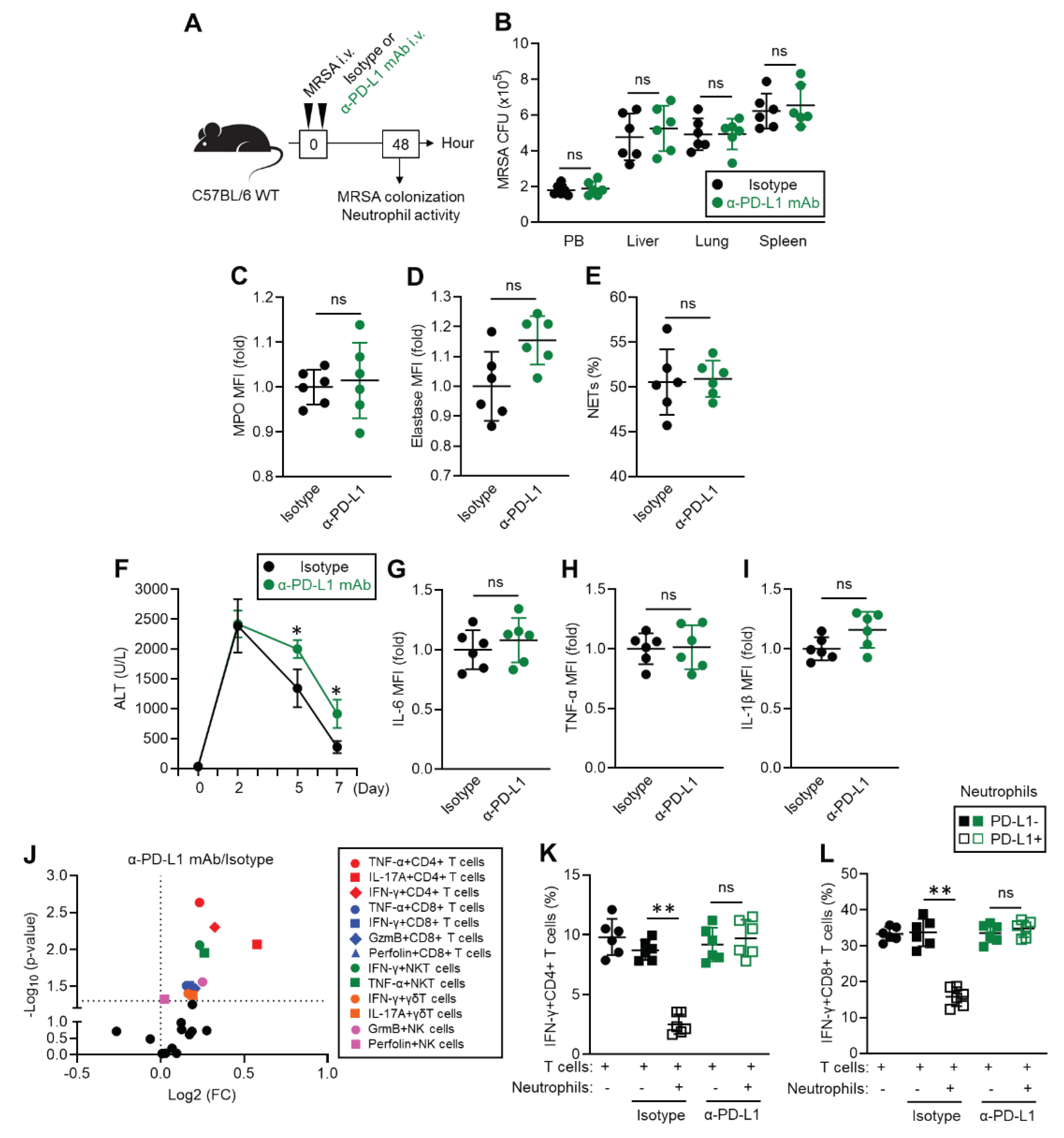
MRSA infection. MRSA infection was performed by following a method described in previous report with minor modification [
14]. Briefly, the MRSA was washed and resuspended in PBS at 1.0x10
9 CFU/mL concentration. The mice received an intravenous (i.v.) injection of MRSA (100 µl of suspension) through the retro orbital sinus. For in vivo PD-1/PD-L1 blockade, the MRSA-challenged mice were i.v. injected isotype antibody or anti-PD-L1 mAb (200 µg in 100 µl of saline). The blood samples and tissue samples (spleen, liver and lung) were collected at 48 h of post infection. Before organ extraction, the mice were perfused with PBS. The tissues were washed with PBS, then were chopped and homogenized in PBS. The processed tissue samples were filtered through a 70 μm strainer and centrifuged at 300 g for 5 min. The supernatants were collected and serially diluted with PBS prior to seeding on TSB plates. The plates were incubated at 37°C for overnight, then MRAS CFUs were determined in the samples.
Primary cell isolation. Peripheral blood was collected using ethylenediaminetetraacetic acid (EDTA)-2Na coated tube and treated with red blood cell (RBC) lysis buffer at room temperature (RT) for 10 min. The sample was washed with PBS and the leukocytes were collected by centrifugation at 300 g for 5 min. Spleen and Lung were mechanically crushed on a 70 µm cell strainer in RPMI complete medium, then the cells were collected by centrifugation at 300 g for 5 min. The cells were treated with RBC lysis buffer at RT for 10 min, then were washed with RPMI complete medium. After centrifugation at 300 g for 5 min, the precipitated cells were used as splenocytes and lung-isolated cells, respectively. Bone marrow (BM) cells were isolated from femurs and tibias. The cells were flushed from the bones using 10 mL syringe with 27G needle in RPMI complete medium. The cells were collected by centrifugation at 300 g for 5 min, then treated with RBC lysis buffer at RT for 10 min. After washing with PBS, the cells were collected by centrifugation at 300 g for 5 min. The precipitated cells were used as BM-isolated cells. Neutrophils were enriched from BM-isolated cells using Neutrophil Isolation kit, mouse (Miltenyi Biotec, Bergisch Gladbach, North Rhine-Westphalia, Germany). Hepatic leukocytes were isolated from whole liver. The liver was excised from PBS-perfused mouse and chopped and mechanically crashed on a 70 µm cell strainer in RPMI complete medium followed by digesting with collagenase (1 mg/mL) at 37℃ for 30 min. The samples were re-filtrated by passing through a 70 µm cell strainer and the cells were collected by centrifugation at 300 g for 5 min and the precipitated cells were re-suspended in 35% Percoll (diluted with PBS) for density gradient separation by centrifugation at 600 g for 20 min without acceleration and brake. After centrifugation, the precipitated cells were further treated with RBC lysis buffer at RT for 10 min. The samples were washed with RPMI complete medium and centrifuged at 300 g for 5 min. The precipitated cells were used as hepatic leukocytes. Splenic CD4+ and CD8+ T cells were isolated from splenocytes using CD4+ T cell isolation kit, mouse (Miltenyi Biotec, Bergisch Gladbach, North Rhine-Westphalia, Germany) and CD8a+ T cell isolation kit, mouse (Miltenyi Biotec), respectively. The isolation procedures were performed by following the product manuals. The purity of neutrophils, CD4+ or CD8+ T cells was assessed by flow cytometry and the sample with more than 90% of CD11b+Ly-6G+, CD4+ or CD8+ T cells was used for subsequent experiments.
Flow cytometry and cell sorting. Flow cytometry analysis was performed by using LSRII (BD Bioscience, Franklin Lakes, NJ, USA) and cell sorting was performed by using FACS Aria II (BD Bioscience). For extracellular marker staining, the samples were stained with fluorochrome-conjugated mAb or tetramer in the presence of anti-CD16/CD32 mAb for blocking of Fc gamma Receptor (FcγR) II/III at 4°C for 30 min. Intracellular staining was performed by using the BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit (BD Bioscience) kit For intracellular staining, the extracellular-stained cells were fixed at 4℃ for 20 min followed by staining of intracellular targets at 4°C for 30 min. The samples studied for IL-1β production in netrophils were treated with GolgiStopTM (1 μg/mL) during the in vitro reaction. The samples studied for cytokine production in T cells were stimulated with PMA (100 ng/mL) and ionomycin (250 ng/mL) in the presence of GolgiStopTM (1 μg/mL) at 37°C for 5 h. ROS production was measured by staininig with H2DCFDA (5 μM) at 37°C for 30 min. The data was analyzed by FlowJo 10 (BD Bioscience).
Real-Time Polymerase Chain Reaction (Real-time PCR). The total RNA was isolated from neutrophils using TRizol (Thermo Fisher Scientific). The concentration and purity or RNA were measured by NanoDrop 2000c (Thermo Fisher Scientific). Total RNA (250-500 ng) was used for reverse transcription for making complementary DNA (cDNA) using a PrimeScript™ RT-PCR Kit (Takara, Tokyo, Japan). The cDNA was used for quantitative PCR performed using a Thermal Cycler Dice
® Real Time System III (Takara, Tokyou, Japan). The mRNA expressions were quantified by ΔCt method. The primer sequences used in the assay were represented in
Supplemental Table S1.
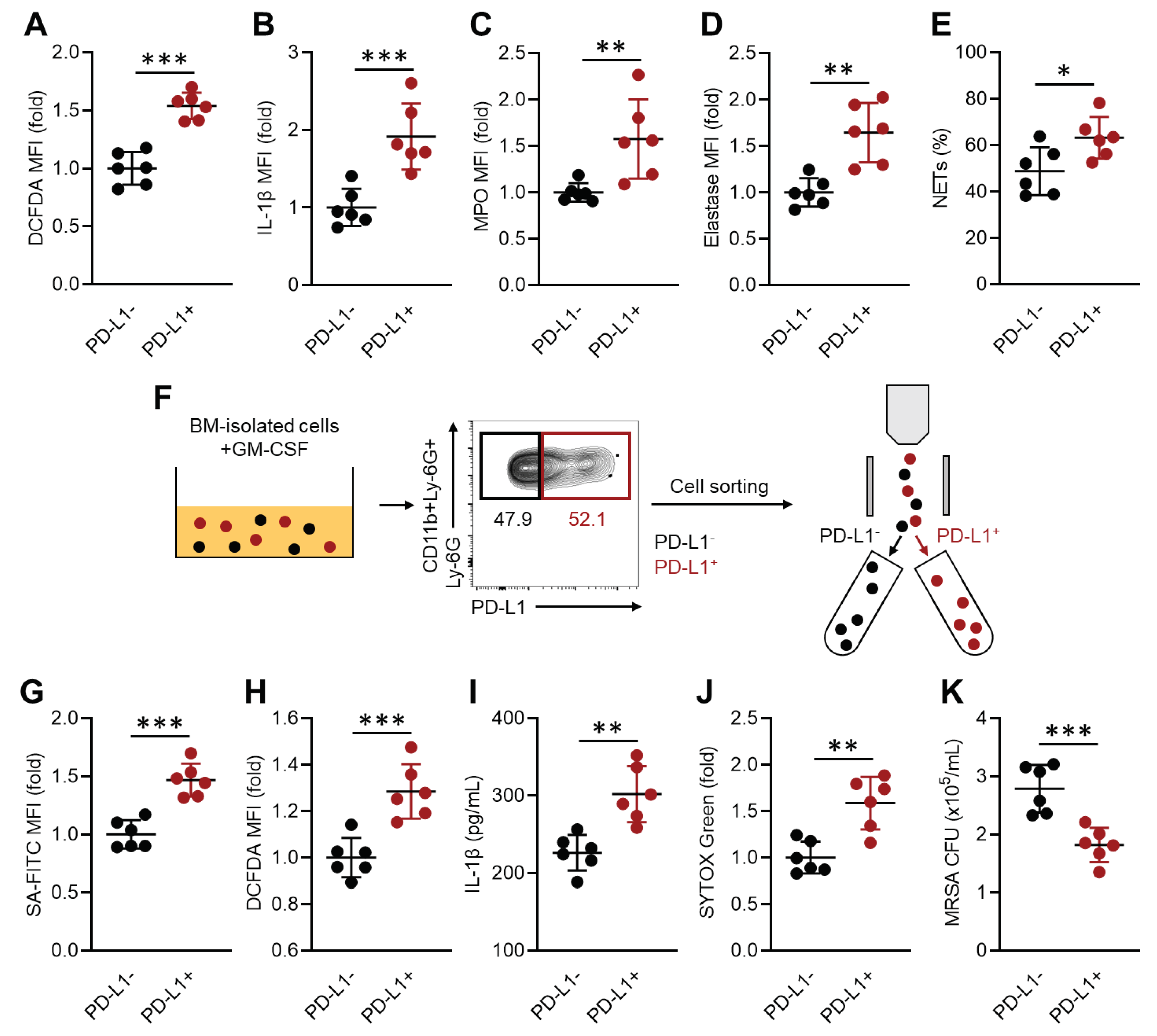
Culture for generating PD-L1+ neutrophils. BM-isolated cells (3.0-5.0x106/mL) were culture with RPMI complete medium supplemented with GM-CSF (100 ng/mL) at 37°C for 24 h. The PD-L1- and PD-L1+ cells were isolated from CD11b+Ly-6G+ population in the pre-cultured BM cells by cell sorter.
Phagocytosis assay. BM-isolated neutrophils (1.0x107/mL) were incubated FITC labeled S. aureus (SA-FITC; 25 µg/mL) in RPMI complete medium at 37°C for 2 h. The cells were washed with PBS and were analyzed by flow cytometry. The fluorescence signal (mean fluorescence intensity; MFI) originated from intracellular incorporated bacteria was used for assessing phagocytosis activity in the neutrophils.
Neutrophil stimulation. BM-isolated neutrophils (1.0x107/mL) were seeded in 96 well plats with RPMI complete medium. For PD-L1 upregulation studies, the cells were treated with vehicle (PBS), HK-SA, CWE, CPE, LP, DNA or RNA at indicated concentrations at 37°C for 6 h. After incubation, the cells were subjected to flow cytometry. Alternatively, the neutrophils were cultured at 37℃ for 3 h in total RNA isolation. For functional studies, the neutrophils were treated with PBS or HK-SA (1.0x108 CFU/mL, MOI=1:10) at 37°C for 6 h, then the cells were subjected to flow cytometry assay. For IL-1β production assay, the neutrophils were cultured with PBS or HK-SA (same MOI to functional studies) at 37°C for 18 h, then the plate was immediately frozen at -80℃. The plate was centrifuged at 300 g for 5 min, and supernatant was subjected to IL-1β ELISA.
In vitro MRSA killing. Neutrophils (1.0x107/mL) were mixed with MRSA (1.0x108 CFU/mL, MOI=1:10) in RPMI complete medium. The samples were incubated at 37°C for 5 h. The samples were centrifuged at 300 g for 5 min, then the supernatants were discarded, and the precipitated cells were washed with PBS. The cells were again collected by centrifugation at 300 g for 5 min, then treated with PBS/0.1% Triton X-100 at RT for 15 min for lysing the cells. The supernatant samples were used for determination of MRSA CFUs by seeding on a TSB agar plate. The plates were incubated at 37°C for overnight, then MRAS CFUs were determined in each sample.
Neutrophil and T cell coculture. The neutrophils (2.5x106/mL) and CD4+ or CD8+ T cells (2.5x106/mL) (at a ratio=1:1) were co-cultured in the presence of anti-CD3 mAb (10 µg/mL) and anti-CD28 mAb (2 µg/mL) at 37°C for 24 h. For PD-1/PD-L1 blockade, the co-cultures were further treated with anti-PD-L1 mAb (10 µg/mL) or isotype antibody (10 µg/mL). At the last 5 h, the cells were stimulated with PMA (100 ng/mL) and ionomycin (250 ng/mL) in the presence of GolgiStopTM (1 µg/mL). The IFN-γ production in CD4+ and CD8+ T cells were analyzed by flow cytometry.
Enzyme-linked immunosorbent assay (ELISA). Mouse IL-1 beta/IL-1F2 DuoSet ELISA kit was purchased from R&D Systems (Minneapolis, MN, USA). All procedures were followed by manufacture’s instruction.
Statistics. Student t-test and one-way analysis of variance (ANOVA) were used to analyze the data for significant differences. Values of p < 0.05, p < 0.01, and p < 0.001 were regarded as significant.
4. Discussion
The functional suppression based on PD-1/PD-L1 interaction was originally found in anti-tumor immunity, particularly between PD-L1 expressing tumor cells and PD-1 upregulated CD8+ T cells, which impairs the function of CD8+ T cells [
16,
18]. Recent studies have revealed that PD-L1 expression is widely conserved in myeloid lineage cells, and the protein also interacts with PD-1 expressed on T cells to suppress their activity. This finding results in targeting dendritic cells (DCs) and macrophages for PD-L1 blockade to recover anti-tumor immunity [
19,
20]. While the study of PD-L1+ neutrophils is still a minor field and has far less accumulated evidence than other myeloid cells. Additionally, there are some possibilities that PD-L1 may has unknown roles distinct from general immunosuppressive role in cancer. In fact, a previous report documented another function of PD-L1 as stabilizing neutrophil extracellular traps (NETs) formation [
21]. Given that neutrophils are abundantly circulating in our bloodstream and frequently involved in diverse immune responses, we decided to investigate the role of PD-L1+ neutrophils in infectious diseases.
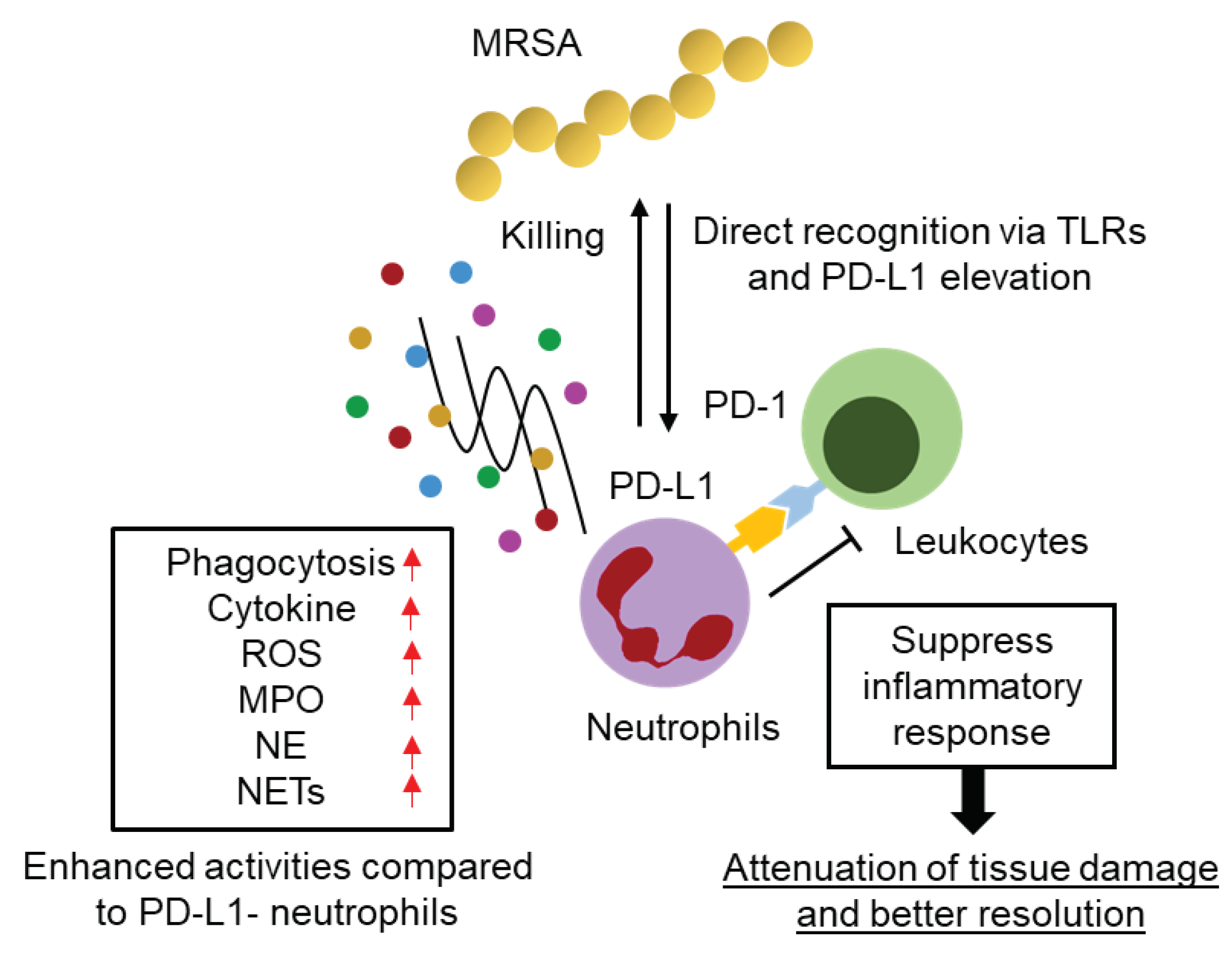
An interesting finding in our results is the functional difference between PD-L1- and PD-L1+ neutrophil in MRSA infection. It was clear that PD-L1+ neutrophil possesses significantly enhanced anti-bacterial activity compared to PD-L1- neutrophil. The increased population of PD-L1+ neutrophil in MRSA-infected mice suggests that this population may predominantly combat bacteria during infection. This evidence supports the concept that neutrophil population is composed by heterogeneous manner, and each population has specific role in various conditions including infection [
22]. However, we have not elucidated the mechanism by which neutrophil control PD-L1 expression in their population. We found that MRSA cell wall extract (CWE) and lipoproteins (LP) trigger PD-L1 expression through Toll-like receptors (TLRs); however, this response spontaneously occurs in the neutrophils of MRSA-challenged mice. Therefore, this finding cannot explain the selective upregulation of PD-L1 expression in neutrophils. Indeed, it is challenging to elucidate the PD-L1 upregulation mechanism under physiological conditions, as various factors may influence neutrophil characteristics. Additionally, we have not explored mechanistic aspects of how PD-L1 expression enhances neutrophil function against MRSA invasion. A previous report revealed that PD-L1 expression prevents apoptosis and extends their survival in human neutrophils [
23]. If this functional modification is also occurred in MRSA infection, it might be a possible mechanistic change that prolongs the survival of neutrophils and maintains their effective function against pathogens. Recent finding suggests that PD-L1 can act as a transcription factor [
24], raising the possibility that neutrophils also possess a PD-L1-mediated transcriptional regulation mechanism in immune activity. Further investigation is required to elucidate whether increased PD-L1 expression is merely a marker or serves a specific function in neutrophil.
As another new insight into the role of PD-L1+ neutrophils in MRSA infection, we propose a supportive role in inflammatory resolution by suppressing inflammatory activity of leukocytes via PD-1/PD-L1 interaction. Our data suggest that inflammatory leukocytes were increased in the liver of MRSA-challenged mice upon administration of anti-PD-L1 mAb. PD-L1 expression is predominantly elevated in neutrophils in the liver of MRSA-infected mice compared to the naïve condition; therefore, we suspect that these cells are playing a suppressive role against inflammatory leukocytes. We also provide strong evidence of PD-L1+ neutrophil-mediated suppressive effects through
in vitro neutrophil and T cell co-culture. In summary, blocking PD-L1 on neutrophils impairs indispensable negative regulation of leukocyte inflammatory responses in MRSA-infected livers that consequently delays inflammatory resolution. This represents a new understanding of the role of PD-L1+ neutrophils in regulating excessive inflammation during infectious diseases. Although our results strongly support the suppressive role of PD-L1+ neutrophils against leukocytes, our study has some limitations. We have not performed neutrophil depletion to prove the importance of their suppressive role against leukocytes. Since neutrophil depletion simply accelerates MRSA colonization during the acute phase, we were unable to take this approach. One possibility is to use neutrophil-specific PD-L1 knockout (KO) mice, which can be created by crossing
Pdl1-floxed mice and S100 calcium-binding protein A8 (calgranulin A)-Cre mice [
25,
26]. Additionally, PD-L1 expression in other cells may need to be characterized more deeply under MRSA infection. For instance, other myeloid cells, such as DCs and macrophages, and hepatocytes might express PD-L1 in MRSA-infected mice by excessive inflammation. Since neutrophils frequently circulate in the bloodstream and reside in tissues, PD-L1+ neutrophils may have a higher frequency of contact with leukocytes than other PD-L1+ cells. However, the background effect of elevated PD-L1 expressions in other cells must be considered.
Recent studies have revealed novel roles and functions of neutrophils that far exceed traditional understanding. This report also provides new evidence in neutrophil biology. Since the evidence is still accumulating and some findings are contradictory, immunotherapy targeting neutrophils has not yet been applied clinically. However, summarizing solid findings may support the creation of new strategies for neutrophil-based therapy, similar to targeting T cells, in the future.