Submitted:
28 March 2024
Posted:
29 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Culture of 3D Epidermal Equivalents and Stimulation with Cytokines and the JAK1/3 Inhibitor
2.4. Isolation of RNA and Analysis of mRNA by Real-Time RT-PCR
2.5. Western Blot Analysis
2.6. Histology, Morphometry and Immunofluorescence Analysis of HEEs
2.7. Lipid Extraction
2.8. GCMS Analysis
2.9. LC Separations
2.10. HRMS
2.11. Data Processing and Statistical Analysis
3. Results
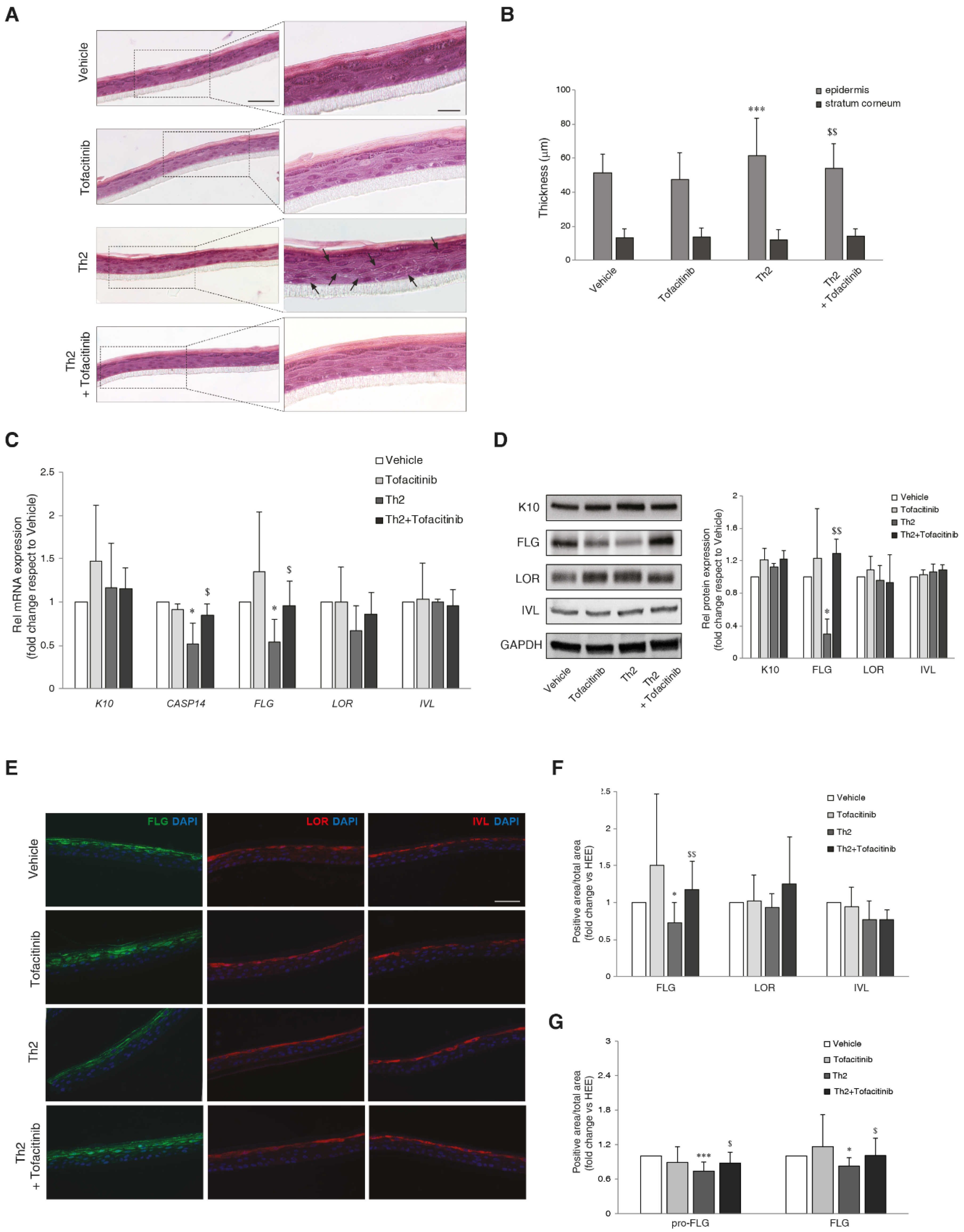
3.1. Effects of JAK/STAT Inhibition by Tofacitinib on Th2 Cytokine-Mediated Changes in Epidermal Morphology and Barrier Protein Expression
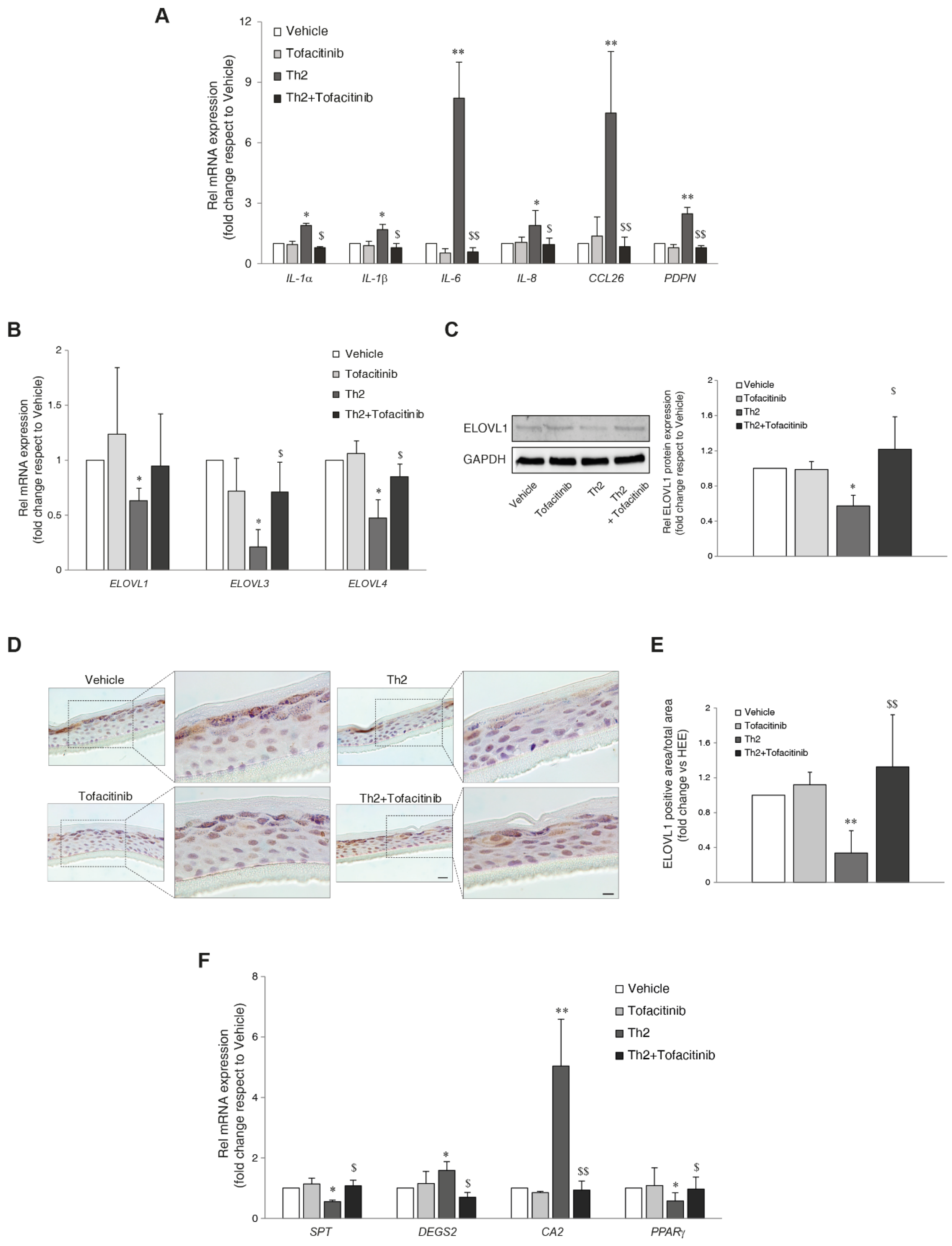
3.2. JAK/STAT Inhibition Counteracts the Induction of Inflammatory Genes in Th2-Treated Epidermal Equivalents
3.3. Modulation of Lipid Genes by Th2 Cytokines and Counteracting Effects of Tofacitinib
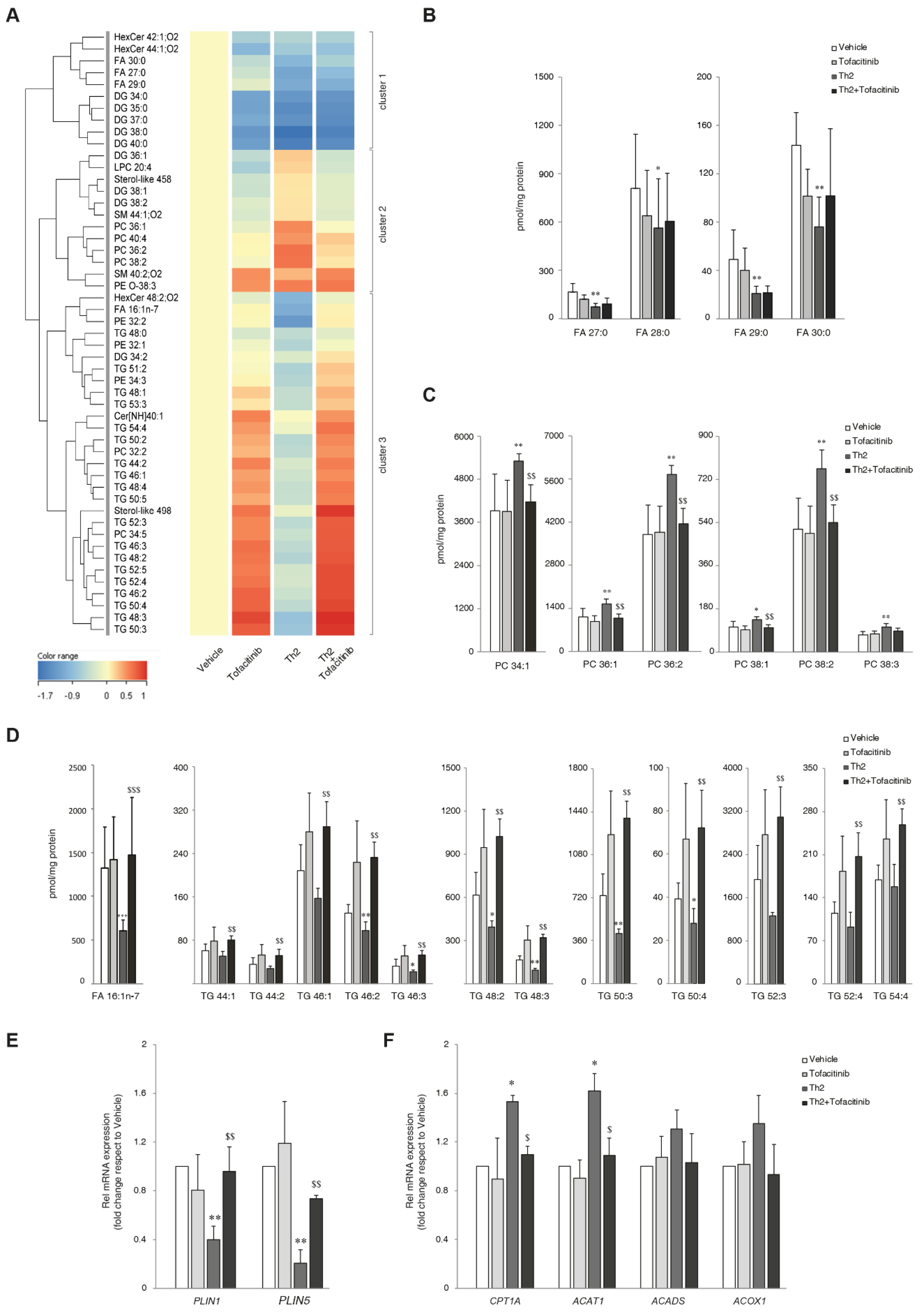
3.4. Alteration of Lipid Profiles Induced by Th2 Cytokines and Modulating Effects of Tofacitinib
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wolf, R.; Wolf, D. Abnormal epidermal barrier in the pathogenesis of atopic dermatitis. Clin Dermatol 2012, 30, 329-334.
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol 2017, 13, 425-437.
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int J Mol Sci 2020, 21, 2867. (accessed on Mar 25, 2024). [CrossRef]
- Karim, N.; Phinney, B.S.; Salemi, M.; Wu, P.; Naeem, M.; Rice, R.H. Human stratum corneum proteomics reveals cross-linking of a broad spectrum of proteins in cornified envelopes. Exp Dermatol 2019, 28, 618-622. (accessed on Jul 24, 2023). [CrossRef]
- Ishitsuka, Y.; Roop, D.R. Loricrin: Past, Present, and Future. International Journal of Molecular Sciences 2020, 21, 2271. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32218335. [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int J Mol Sci 2020, 21, 5382. (accessed on Jul 24, 2023). [CrossRef]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Annals of allergy, asthma, & immunology 2020, 124, 36-43. Available online:. https://doi.org/10.1016/j.anai.2019.10.008. [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 2008, 126, 332-337.
- He, A.; Chen, X.; Tan, M.; Chen, Y.; Lu, D.; Zhang, X.; Dean, J.M.; Razani, B.; Lodhi, I.J. Acetyl-CoA Derived from Hepatic Peroxisomal β-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation. Molecular cell 2020, 79, 30-42.e4. Available online:. https://doi.org/10.1016/j.molcel.2020.05.007. [CrossRef]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT 2013, 2, e24137.
- Gunduz, O. JAK/STAT pathway modulation: Does it work in dermatology? Dermatol Ther 2019, 32, e12903.
- Rodrigues, M.A.; Torres, T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatolog Treat 2020, 31, 33-40. (accessed on Aug 3, 2023). [CrossRef]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol 2021, 148, 927-940. (accessed on Aug 3, 2023). [CrossRef]
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging Topical and Systemic JAK Inhibitors in Dermatology. Front Immunol 2019, 10, 2847.
- O’Shea, J.J.; Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012, 36, 542-550.
- Bissonnette, R.; Papp, K.A.; Poulin, Y.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; Papacharalambous, J.; Ports, W.C. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. British journal of dermatology (1951) 2016, 175, 902-911. Available online: https://api.istex.fr/ark:/67375/WNG-2B42NBR1-3/fulltext.pdf. [CrossRef]
- Nakashima, C.; Yanagihara, S.; Otsuka, A. Innovation in the treatment of atopic dermatitis: Emerging topical and oral Janus kinase inhibitors. Allergology International 2022, 71, 40-46. Available online:. https://doi.org/10.1016/j.alit.2021.10.004. [CrossRef]
- Srivastava, A.; Stahle, M.; Pivarcsi, A.; Sonkoly, E. Tofacitinib Represses the Janus Kinase-Signal Transducer and Activators of Transcription Signalling Pathway in Keratinocytes. Acta Derm Venereol 2018, 98, 772-775.
- Hatano, Y.; Adachi, Y.; Elias, P.M.; Crumrine, D.; Sakai, T.; Kurahashi, R.; Katagiri, K.; Fujiwara, S. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol 2013, 22, 30-35.
- Ludovici, M.; Kozul, N.; Materazzi, S.; Risoluti, R.; Picardo, M.; Camera, E. Influence of the sebaceous gland density on the stratum corneum lipidome. Sci Rep 2018, 8, 11500-7.
- Camera, E.; Ludovici, M.; Galante, M.; Sinagra, J.L.; Picardo, M. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J Lipid Res 2010, 51, 3377-3388.
- Maiellaro, M.; Bottillo, G.; Cavallo, A.; Camera, E. Comparison between ammonium formate and ammonium fluoride in the analysis of stratum corneum lipids by reversed phase chromatography coupled with high resolution mass spectrometry. Sci Rep 2024, 14, 40. (accessed on Jan 8, 2024). [CrossRef]
- Amano, W.; Nakajima, S.; Kunugi, H.; Numata, Y.; Kitoh, A.; Egawa, G.; Dainichi, T.; Honda, T.; Otsuka, A.; Kimoto, Y.; Yamamoto, Y.; Tanimoto, A.; Matsushita, M.; Miyachi, Y.; Kabashima, K. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J Allergy Clin Immunol 2015, 136, 667-677.e7.
- Clarysse, K.; Pfaff, C.M.; Marquardt, Y.; Huth, L.; Kortekaas Krohn, I.; Kluwig, D.; Lüscher, B.; Gutermuth, J.; Baron, J. JAK1/3 inhibition preserves epidermal morphology in full-thickness 3D skin models of atopic dermatitis and psoriasis. J Eur Acad Dermatol Venereol 2019, 33, 367. [CrossRef]
- Hoste, E.; Kemperman, P.; Devos, M.; Denecker, G.; Kezic, S.; Yau, N.; Gilbert, B.; Lippens, S.; De Groote, P.; Roelandt, R.; Van Damme, P.; Gevaert, K.; Presland, R.B.; Takahara, H.; Puppels, G.; Caspers, P.; Vandenabeele, P.; Declercq, W. Caspase-14 Is Required for Filaggrin Degradation to Natural Moisturizing Factors in the Skin. J Invest Dermatol 2011, 131, 2233-2241.
- Honma, M.; Minami-Hori, M.; Takahashi, H.; Iizuka, H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: regulation by TGF-beta and STAT-3 activating cytokines, IFN-gamma, IL-6, and IL-22. J Dermatol Sci 2012, 65, 134-140.
- Jiang, Y.J.; Lu, B.; Kim, P.; Paragh, G.; Schmitz, G.; Elias, P.M.; Feingold, K.R. PPAR and LXR Activators Regulate ABCA12 Expression in Human Keratinocytes. Journal of Investigative Dermatology 2008, 128, 104-109. Available online:. https://doi.org/10.1038/sj.jid.5700944. [CrossRef]
- Iwabuchi, K.; Nakayama, H.; Oizumi, A.; Suga, Y.; Ogawa, H.; Takamori, K. Role of Ceramide from Glycosphingolipids and Its Metabolites in Immunological and Inflammatory Responses in Humans. Mediators Inflamm 2015, 2015, 120748.
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; Merrill, A.H.; Murphy, R.C.; O’Donnell, V.B.; Oskolkova, O.; Subramaniam, S.; Wakelam, M.J.O.; Spener, F. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J Lipid Res 2020, 61, 1539-1555. (accessed on Jul 25, 2023). [CrossRef]
- Dahlhoff, M.; Camera, E.; Picardo, M.; Zouboulis, C.C.; Chan, L.; Chang, B.H.; Schneider, M.R. PLIN2, the major perilipin regulated during sebocyte differentiation, controls sebaceous lipid accumulation in vitro and sebaceous gland size in vivo. Biochim Biophys Acta 2013, 1830, 4642-4649.
- Zhang, C.; Liu, P. The New Face of the Lipid Droplet: Lipid Droplet Proteins. Proteomics 2019, 19, e1700223.
- Jakobs, B.S.; Wanders, R.J. Fatty acid beta-oxidation in peroxisomes and mitochondria: the first, unequivocal evidence for the involvement of carnitine in shuttling propionyl-CoA from peroxisomes to mitochondria. Biochem Biophys Res Commun 1995, 213, 1035-1041. (accessed on Jul 24, 2023). [CrossRef]
- Pavel, P.; Leman, G.; Hermann, M.; Ploner, C.; Eichmann, T.O.; Minzaghi, D.; Radner, F.P.W.; Del Frari, B.; Gruber, R.; Dubrac, S. Peroxisomal Fatty Acid Oxidation and Glycolysis Are Triggered in Mouse Models of Lesional Atopic Dermatitis. JID Innov 2021, 1, 100033. (accessed on Sep 15, 2023). [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J.A. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annual review of physiology 2016, 78, 23-44. Available online:. https://doi.org/10.1146/annurev-physiol-021115-105045. [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018, 10, 1502. (accessed on Aug 3, 2023). [CrossRef]
- Vamecq, J.; Andreoletti, P.; El Kebbaj, R.; Saih, F.; Latruffe, N.; El Kebbaj, M.H.S.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Peroxisomal Acyl-CoA Oxidase Type 1: Anti-Inflammatory and Anti-Aging Properties with a Special Emphasis on Studies with LPS and Argan Oil as a Model Transposable to Aging. Oxidative Medicine and Cellular Longevity 2018, 2018, 6986984-13. Available online:. https://doi.org/10.1155/2018/6986984. [CrossRef]
- Leman, G.; Pavel, P.; Hermann, M.; Crumrine, D.; Elias, P.M.; Minzaghi, D.; Goudounèche, D.; Roshardt Prieto, N.M.; Cavinato,M., Wanner,A.; Blunder, S.; Gruber, R.; Jansen-Dürr, P.; Dubrac, S. Mitochondrial Activity Is Upregulated in Nonlesional Atopic Dermatitis and Amenable to Therapeutic Intervention. J Invest Dermatol 2022, 142, 2623-2634.e12.
- Ge, F.; Sun, K.; Hu, Z.; Dong, X. Role of Omega-Hydroxy Ceramides in Epidermis: Biosynthesis, Barrier Integrity and Analyzing Method. Int J Mol Sci 2023, 24, 5035. [CrossRef]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.J.M.; van den Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp Dermatol 2018, 27, 501-511.
- Smits, J.P.H.; Niehues, H.; Rikken, G.; van Vlijmen-Willems, I.M.J.J.; van de Zande, G. W. H. J. F.; Zeeuwen, P.L.J.M.; Schalkwijk, J.; van den Bogaard, E.H. Immortalized N/TERT keratinocytes as an alternative cell source in 3D human epidermal models. Sci Rep 2017, 7, 11838-y.
- Rikken, G.; Niehues, H.; van den Bogaard, E.H. Organotypic 3D Skin Models: Human Epidermal Equivalent Cultures from Primary Keratinocytes and Immortalized Keratinocyte Cell Lines. Methods Mol Biol 2020, 2154, 45-61.
- Rabionet, M.; Gorgas, K.; Sandhoff, R. Ceramide synthesis in the epidermis. Biochim Biophys Acta 2014, 1841, 422-434. (accessed on Mar 21, 2024). [CrossRef]
- Janssens, M.; van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; Lavrijsen, A.P.; Bouwstra, J.A. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res 2012, 53, 2755-2766. (accessed on Dec 1, 2023). [CrossRef]
- Goleva, E.; Berdyshev, E.; Leung, D.Y. Epithelial barrier repair and prevention of allergy. J Clin Invest 2019, 129, 1463-1474. (accessed on Dec 1, 2023). [CrossRef]
- Leung, D.Y.M.; Berdyshev, E.; Goleva, E. Cutaneous barrier dysfunction in allergic diseases. J Allergy Clin Immunol 2020, 145, 1485-1497. (accessed on Dec 1, 2023). [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345-360. (accessed on Dec 1, 2023). [CrossRef]
- Boer, D.E.C.; van Smeden, J.; Al-Khakany, H.; Melnik, E.; van Dijk, R.; Absalah, S.; Vreeken, R.J.; Haenen, C.C.P.; Lavrijsen, A.P.M.; Overkleeft, H.S.; Aerts, J.M.F.G.; Bouwstra, J.A. Skin of atopic dermatitis patients shows disturbed beta-glucocerebrosidase and acid sphingomyelinase activity that relates to changes in stratum corneum lipid composition. Biochim Biophys Acta Mol Cell Biol Lipids 2020, 1865, 158673.
- Sawada, E.; Yoshida, N.; Sugiura, A.; Imokawa, G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: an implication for the disrupted barrier mechanism in atopic dermatitis. J Dermatol Sci 2012, 68, 25-35.
- Cha, H.J.; He, C.; Zhao, H.; Dong, Y.; An, I.; An, S. Intercellular and intracellular functions of ceramides and their metabolites in skin (Review). Int J Mol Med 2016, 38, 16-22. (accessed on Mar 21, 2024). [CrossRef]
- Turpin-Nolan, S.M.; Brüning, J.C. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol 2020, 16, 224-233. (accessed on Mar 21, 2024). [CrossRef]
- Blaess, M.; Deigner, H. Derailed Ceramide Metabolism in Atopic Dermatitis (AD): A Causal Starting Point for a Personalized (Basic) Therapy. Int J Mol Sci 2019, 20, 3967. (accessed on Mar 21, 2024). [CrossRef]
- Tsurumaki, H.; Katano, H.; Sato, K.; Imai, R.; Niino, S.; Hirabayashi, Y.; Ichikawa, S. WP1066, a small molecule inhibitor of the JAK/STAT3 pathway, inhibits ceramide glucosyltransferase activity. Biochem Biophys Res Commun 2017, 491, 265-270.
- Murakami, C.; Sakane, F. Sphingomyelin synthase-related protein generates diacylglycerol via the hydrolysis of glycerophospholipids in the absence of ceramide. J Biol Chem 2021, 296, 100454. (accessed on Mar 21, 2024). [CrossRef]
- Günenc, A.N.; Graf, B.; Stark, H.; Chari, A. Fatty Acid Synthase: Structure, Function, and Regulation. Subcell Biochem 2022, 99, 1-33. (accessed on Dec 1, 2023). [CrossRef]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta 2014, 1841, 295-313. (accessed on Dec 1, 2023). [CrossRef]
- Li, S.; Villarreal, M.; Stewart, S.; Choi, J.; Ganguli-Indra, G.; Babineau, D.C.; Philpot, C.; David, G.; Yoshida, T.; Boguniewicz, M.; Hanifin, J.M.; Beck, L.A.; Leung, D.Y.; Simpson, E.L.; Indra, A.K. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br J Dermatol 2017, 177, e125-e127.
- Alessia Cavallo, Emanuela Camera, Grazia Bottillo, Miriam Maiellaro, Mauro Truglio, Federico Marini, Marlène Chavagnac-Bonneville, Aurélie Fauger, Eric Perrier, Flavia Pigliacelli, Mauro Picardo, Antonio Cristaudo, Maria Mariano Biosignatures of defective sebaceous gland activity in sebum-rich and sebum-poor skin areas in adult atopic dermatitis. Experimental Dermatology. [CrossRef]
- Kawabata, K.; Karahashi, M.; Sakamoto, T.; Tsuji, Y.; Yamazaki, T.; Okazaki, M.; Mitsumoto, A.; Kudo, N.; Kawashima, Y. Fatty Acid β-Oxidation Plays a Key Role in Regulating cis-Palmitoleic Acid Levels in the Liver. Biol Pharm Bull 2016, 39, 1995-2008. (accessed on Dec 4, 2023). [CrossRef]
- Sampath, H.; Flowers, M.T.; Liu, X.; Paton, C.M.; Sullivan, R.; Chu, K.; Zhao, M.; Ntambi, J.M. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem 2009, 284, 19961-19973.
- Zwara, A.; Wertheim-Tysarowska, K.; Mika, A. Alterations of Ultra Long-Chain Fatty Acids in Hereditary Skin Diseases-Review Article. Front Med (Lausanne) 2021, 8, 730855.
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; Norquest, K.A.; Zheng, T.; Seibold, M.A.; Leung, D.Y. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3, 10.1172/jci.insight.98006. eCollection 2018 Feb 22.
- Pavel, P.; Blunder, S.; Moosbrugger-Martinz, V.; Elias, P.M.; Dubrac, S. Atopic Dermatitis: The Fate of the Fat. Int J Mol Sci 2022, 23, 2121. (accessed on Sep 15, 2023). [CrossRef]
- Zhang, L.; Wen, X.; Hou, Y.; Yang, Y.; Song, W.; Zeng, Y.; Sun, J. Integrated metabolomics and lipidomics study of patients with atopic dermatitis in response to dupilumab. Front Immunol 2022, 13, 1002536. (accessed on Sep 15, 2023). [CrossRef]
- Berdyshev, E.; Goleva, E.; Bissonnette, R.; Bronova, I.; Bronoff, A.S.; Richers, B.N.; Garcia, S.; Ramirez-Gama, M.; Taylor, P.; Praestgaard, A.; Agueusop, I.; Jurvilliers, P.; Boguniewicz, M.; Levit, N.A.; Rossi, A.B.; Zhang, A.; Leung, D.Y.M. Dupilumab significantly improves skin barrier function in patients with moderate-to-severe atopic dermatitis. Allergy 2022, 77, 3388-3397. (accessed on Sep 15, 2023). [CrossRef]
- Khanna, D.; Padilla, C.; Tsoi, L.C.; Nagaraja, V.; Khanna, P.P.; Tabib, T.; Kahlenberg, J.M.; Young, A.; Huang, S.; Gudjonsson, J.E.; Fox, D.A.; Lafyatis, R. Tofacitinib blocks IFN-regulated biomarker genes in skin fibroblasts and keratinocytes in a systemic sclerosis trial. JCI Insight 2022, 7, e159566. [CrossRef]
- Bhattacharya, N.; Sato, W.J.; Kelly, A.; Ganguli-Indra, G.; Indra, A.K. Epidermal Lipids: Key Mediators of Atopic Dermatitis Pathogenesis. Trends Mol Med 2019, 25, 551-562.
- Zhang, C.; Chinnappan, M.; Prestwood, C.A.; Edwards, M.; Artami, M.; Thompson, B.M.; Eckert, K.M.; Vale, G.; Zouboulis, C.C.; McDonald, J.G.; Harris-Tryon, T.A. Interleukins 4 and 13 drive lipid abnormalities in skin cells through regulation of sex steroid hormone synthesis. Proc Natl Acad Sci U S A 2021, 118, 10.1073/pnas.2100749118.
- Dodington, D.W.; Desai, H.R.; Woo, M. JAK/STAT - Emerging Players in Metabolism. Trends Endocrinol Metab 2018, 29, 55-65.
- Williams, M.L.; Rutherford, S.L.; Feingold, K.R. Effects of cholesterol sulfate on lipid metabolism in cultured human keratinocytes and fibroblasts. J Lipid Res 1987, 28, 955-967.
- Ponec, M.; Kempenaar, J.; Weerheim, A.; de Lannoy, L.; Kalkman, I.; Jansen, H. Triglyceride metabolism in human keratinocytes cultured at the air-liquid interface. Arch Dermatol Res 1995, 287, 723-730. (accessed on Jul 24, 2023). [CrossRef]
- Schneider, M.R.; Zhang, S.; Li, P. Lipid droplets and associated proteins in the skin: basic research and clinical perspectives. Arch Dermatol Res 2016, 308, 1-6. Available online: https://link.springer.com/article/10.1007/s00403-015-1599-2. [CrossRef]
- Schurer, N.Y.; Monger, D.J.; Hincenbergs, M.; Williams, M.L. Fatty acid metabolism in human keratinocytes cultivated at an air-medium interface. J Invest Dermatol 1989, 92, 196-202.
- Radner, F.P.W.; Fischer, J. The important role of epidermal triacylglycerol metabolism for maintenance of the skin permeability barrier function. Biochim Biophys Acta 2014, 1841, 409-415.
- Moessinger, C.; Klizaite, K.; Steinhagen, A.; Philippou-Massier, J.; Shevchenko, A.; Hoch, M.; Ejsing, C.S.; Thiele, C. Two different pathways of phosphatidylcholine synthesis, the Kennedy Pathway and the Lands Cycle, differentially regulate cellular triacylglycerol storage. BMC Cell Biol 2014, 15, 43. (accessed on Dec 4, 2023). [CrossRef]
- Schäfer, L.; Kragballe, K. Abnormalities in Epidermal Lipid Metabolism in Patients with Atopic Dermatitis. Journal of Investigative Dermatology 1991, 96, 10-15. Available online:. https://doi.org/10.1111/1523-1747.ep12514648. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




