1. Introduction
The short term `motor control´ (e.g., Granit 1970) designates a wide range of research fields, in which researchers of various backgrounds have tried to figure out the mechanisms and constraints underlying animal and human movements. But what does `control´ mean? And what or who might exert it? Is movement controlled by volition or reflexes? Even modern experts don´t appear to be able to find a consensus about these questions: “... the issue has a rich history of philosophical and scientific debate; and, as this article demonstrates, present-day researchers still cannot reach a consensus on the meaning of the words and on whether it is possible to draw a scientific distinction between them” (Prochazka et al. 2000). The main underlying problem is that the issue plunges deeply into philosophy without physics. The present review will try to avoid these pitfalls.
Numerous neuroscientists have envisaged the motor system to be organized hierarchically (e.g., Cai et al. 2006; Haggie et al. 2023), top-down from the cerebral cortex (the realm of volition), via the basal ganglia (BG), cerebellum and brainstem to the spinal cord (the realm of reflexes). We will take the discussion through the opposite direction, from the spaces and time through which movements take place, the impact of gravity, the final common pathway from motoneurons (MNs) to muscles and their inputs including sensory feedback, the spinal cord upwards to the cerebral cortex, and touch upon internal models /Sect 9), neural networks (Sect 10) and sensory-motor learning (Sect 11). We will discuss them with particular emphasis on neurological conditions, which may disrupt normal motor functioning and provide further insights.
2. Movement in Space and Time
Per definitionem, movements proceed through space and time (
Figure 1). The central nervous system (CNS) must therefore construct representations of these four dimensions.
2.1. Divisions of Space
The space in which organisms move is not uniform. Functionally, interior and exterior spaces must be discerned, and the latter is further divided into peri-personal space (PPS) and far space (Cléry and Ben Hamed 2018; Di Pellegrino and Làdavas 2015).
2.1.1. Body Schemata
The body schema is a dynamic, internal 3D representation of the spatial and biomechanical properties of the body (Coslett 1998). For the control of the body´s posture and balance, navigation between objects and obstacles, limb movements in PPS, and grasping objects, the CNS needs information about the body configuration, size and spatial location (Cardinali et al. 2009; Jax and Colsett 2009; Rousseaux et al. 2014; Sattin et al. 2023). The body schema covers a variety of sensory-motor representations (de Vignemont 2010). A so-called postural body schema incorporates a representation of verticality, requiring sensory information on the earth vertical to which the body can be aligned (Sect 4.2.1). Probably, there is also a locomotor body schema that is used in navigation to estimate step length and walking distance (Dominici et al. 2009; Ivanenko et al. 2011).
The neural implementations of CNS body representations have not been completely revealed (Berlucchi and Aglioti 2010; de Vignemont 2010; Di Vita et al. 2016; Harris et al. 2015; Martel et al. 2016). In any case, the body schema appears to be a multiple, task-dependent, flexible, adaptable, online and real-time representation of the body in space, which is generated by sensory inputs, including cutaneous mechano-receptive, tactile, proprioceptive, vestibular, auditory and visual signals, and possibly inputs from small-fiber sensory afferents conveying nociception, itch, thermo-reception and pleasant touch (Berlucchi and Aglioti 2010; Jax and Colsett 2009; Sect 7.1). [It is mentioned in passing that there may be an additional map incorporating the body´s metric properties. The map is distorted and crudely resembles the motor homunculus. Yet subjects consciously perceive only the undistorted map (Proske and Gandevia 2012)].
2.1.2. Disturbance of the Body Schema
Large-fiber Sensory Polyneuropathy. The importance of large-fiber sensory nerve fibers (Sect 7.3.1) in establishing a somatosensory body schema is emphasized by the fact that their loss in the respective polyneuropathy compromises the sense of posture and movement, leaving the patient with a severe impairment to coordinate his/her movements in the sense of afferent ataxia (Blouin et al. 1993; Gallagherr and Cole 1995; Tuthill and Azim 2018). Active movements revealed that efference copies and internal models (Sect 9) appear to play a role in up-dating the initial hand position before reaches (Jax and Coslett 2009).
Hemispheric Brain Damage. Patients with left neglect (neglect is typically observed in patients with right hemispheric lesions) often fail to use and, in some instances, recognize the left side of the body. Patients with neglect, but not other subjects with brain lesions, identified pictures of the left (contra-lesional) hands significantly less reliably than pictures of the right hands. Neglect may thus be associated with a disruption of, or failure to attend to, the body schema (Coslett 1998). The body´s median sagittal axis (main zone around which movements are anchored) is distorted in patients with spatial neglect and combines ipsi-lesional translation and contra-lesional tilt. The distortion severity is related to lesions of the parietal, somatosensory and multi-modal association cortices. These effects suggest that neglect patients show a distortion of the body schema and the perceptive representations of the body (Rousseaux et al. 2014).
Chronic Pain may also change proprioceptive and other elements of sensory processing and distort the body schema (Tsay et al. 2015).
2.1.3. Far and Peri-Personal Space (PPS)
Peri-personal space (PPS) is usually defined as the space within arm´s, leg´s or mouth´s reach, where objects can be grasped and manipulated or where defensive and avoidance movements occur. These two functional spaces evidently require different neural organizations (Martel et al. 2016; de Vignemont and Iannetti 2015). Far space is sensed predominantly by vision and audition, while PPS involves primarily the tactile, auditory and visual senses, both representations requiring multi-sensory integration (Cardinali et al. 2009; Serino 2019; van der Stoep et al. 2015). PPS representation is modifiable and extensible, for example by the inclusion of tools (Arbib et al. 2009; Cléry and Ben Hamed 2018; Di Pellegrino and Làdavas 2015; Macaluso and Maravita 2010; Makin et al. 2008; Martel et al. 2016; Serino 2019). The PPS can be disturbed by diseases.
2.1.4. Disturbances of Peri-Personal Space (PPS)
In psycho-pathological conditions, including anxiety disorders such as phobias and trauma-related disorders, violations of PPS boundaries can elicit fear responses and/or altered states of bodily self-consciousness, even de-personalization, de-realization and out-of-body experiences. For example, it has been hypothesized that patients diagnosed with post-traumatic stress disorder (PTSD) and acute stress disorder (ASD) exhibit a larger PPS than do control persons as a self-protective function. PTSD patients with avoidance symptoms − the tendency of fleeing from trauma reminders (emotions, interactions, places) – likely require larger PPS boundaries in order to avoid inter-personal contact. In particular, for defensive purposes, patients with combat-related PTSD prefer significantly larger inter-personal distance as compared to controls (Rabellino et al. 2020).
Altered PPS has also been described for patients with schizophrenia (Ferroni et al. 2022; Lee et al. 2021; Noel et al. 2018) and autism (Mul et al. 2019; Noel et al. 2018). PPS was smaller in patients with schizophrenia than in matched controls (Lee et al. 2021). The same was found in patients with autism (Mul et al. 2019). Thus, in patients with schizophrenia or autism, the PPS alteration was opposite to that in PTSD patients.
2.2. Timing of Movements
For movement control, the CNS must also be able to represent time (Kornysheva 2016). Precise timing is an important aspect of motor control, in particular in two types of motor tasks: (i) sequential rhythmic movements or sustained movements of a definite duration (explicit timing); (ii) implicit use of temporal information, e.g., during coordination of movements in relation to moving objects or individuals within the external environment (implicit timing) (Avanzino et al. 2016).
During the performance of explicit timing tasks, explicit use of temporal information is made (e.g., estimates of the duration of different stimuli or of their inter-stimulus intervals) in order to represent precise temporal durations through a sustained or periodic motor act. The BG are activated during motor, but also perceptual, time processing tasks, involving both sub-second and supra-second temporal intervals. The cerebellum is more often activated (i) during motor than perceptual explicit timing tasks, (ii) when a synchronization to an external rhythm is required, and (iii) when processing involves sub-second rather than supra-second intervals (Avanzino et al. 2016).
Implicit timing occurs when temporal information can optimize performance on a non-temporal task. Time-dependent information on a movement is used to predict whether the movement outcome will agree with its goal, or will result in an execution error. In this case, cerebellar outflow pathways are primarily involved. Indeed, cerebellar networks optimize self-executed actions by recalibrating predictions, capturing the sensory consequences of the same actions. The cerebellum is engaged when temporal information is processed to predict the temporal outcome of a motor act (Avanzino et al. 2016; Sect 9.3).
Interestingly, it has been suggested that object behavior under the influence of gravity might also contribute to time estimation. The gravitational acceleration of falling objects can provide a time-stamp on events, because the motion duration of an object accelerated by gravity over a given path is fixed. Hence, the brain has mechanisms that exploit the presence of gravity to estimate the passage of time. “An internal model (Sect 9) of the effects of gravity is combined with multisensory signals to time the interception of falling objects, to time the passage through spatial landmarks during virtual navigation, to assess the duration of a gravitational motion, and to judge the naturalness of periodic motion under gravity” (Lacquaniti et al. 2015).
It might be assumed that to measure time would simply require a clock. Amazingly, although the CNS has clocks of different periodicity at almost all levels of organization, from individual cells to neuronal networks, there is no single universal clock but a number of distributed structures and mechanisms involved in timing (Kornysheva 2016).
In summary, modern theories about internal timing networks generally agree on the view that temporal data is processed in a distributed network with fluctuating involvement of individual components based on the specific task demands (Ashe and Bushara 2014; Balasubramaniam et al. 2021; Bareš et al. 2019; Boven and Cerminara 2023, Breska and Ivry 2016; Lawrenson et al. 2018; Merchant et al. 2015, Petter et al. 2016; Yamaguchi and Sakurai 2014). The neuronal networks involved in motor timing are supposed to comprise the lateral cerebellum, BG, as well as sensory-motor and prefrontal cortical areas. The BG and associated cortical areas may act as a hypothetical `internal clock´ when the movement is internally generated. When timing is used to make predictions on the outcome of a subjective or externally perceived motor act, cerebellar processing and outflow pathways appear to be primarily involved (Avanzino et al. 2016). Disturbances of timing processing occur in several movement disorders (Sect 8.2.6).
3. Kinematics
3.1. Expression and Functions
Kinematics´ is a term that describes the trajectories (position, velocity, acceleration) of objects in space and time, including those of organisms and their parts. Kinematics are widely used for clinical or experimental purposes. An organism´s movements result from external environmental influences (including gravity, inertia, resistances and dynamics in air, liquid or solid) and/or from self-generated driving forces, which are generated by muscular activities. In Newtonian mechanics, an object at rest or in constant straight motion does not change its motion state unless external forces change it.
3.2. Disruption of Kinematics
Movement kinematics can be deranged by (i) skeletal disorders (e.g., deformations, arthrosis etc.), (ii) muscular deficiencies (e.g., muscle fatigue, spasticity, wasting etc.), (iii) MN disorders (e.g., spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS), (iv) higher CNS diseases (e.g., spinal cord injury (SCI), stroke etc.), (v) external forces. In this review, we will concentrate on CNS diseases and external forces (below).
4. Kinetics (Dynamics)
Kinetics (or dynamics) describes the forces and torques driving kinematics. Posture and movement require forces to initiate and/or maintain and/or stop them against visco-elastic and inertial properties of the body and its parts suspended in a gravitational field.
4.1. Forward and Inverse Dynamics
To generate movements, the CNS must take account of several `component torques´ including the torques due to gravity, the dynamic interaction torques induced passively by the movement of the adjacent joint, and the torques produced by the muscles and passive tissue elements (Bastian et al. 1996). The dynamics of self-generated movements can be described under two aspects.
Forward Dynamics describes the sequence of events in the musculo-skeletal periphery (Otten 2003; Zajac and Gordon 1989), from muscle excitations as inputs and body motions as outputs.
Inverse Dynamics investigates how inputs to a system would have to be chosen so as to achieve a desired output. If the CNS used this approach, it would have to take account of the dynamics by inverting them. To compute inverse dynamics is very complex and often requires non-unique transformations. This has made this model not very popular among researchers.
4.2. Impairment of Kinetics
Like kinematics, kinetics can be influenced or disturbed by (i) gravity, (ii) other external forces impacting the body and its appendages, (iii) changes in body proportions due to skeletal deformations (not dealt with here), and (iv) many diseases affecting MN output.
4.2.1. Gravity
A variety of external forces can alter or oppose the effects of CNS-generated kinetics. But an ever-present persistent force is gravity that influences locomotion, reaching, and grasping of objects (
Figure 1), of which the CNS must take account when organizing movement. To this end, the CNS hosts an internal representation of gravity used to probe the environments, to interact with objects, and to plan and control movements (Hubbard 2020). Since the body does not have specialized sensory organs and CNS structures to measure the direction of gravity, information on the gravitational vector is provided by multiple sensory sources: the vestibular organs, the retina, skin, muscle, tendon, and visceral receptors (graviceptors) and integrated in a variety of interconnected brain areas, including the vestibular nuclei, cerebellum, thalamus, insula, retro-insula, parietal operculum, and temporo-parietal junction. Indeed, there is much evidence for the existence of a distributed multi-modal network representing gravity, which probably supports rapid but partial adaptation in a broad range of motor tasks. This internal model (Sect 9) requires the ability to feel and anticipate gravity´s actions for efficient control and accurate internal representations of the body configuration in space (Delle Monache et al. 2021; White et al. 2020). The timing of action and the interaction with moving objects would be determined by an internal model (Sect 9) of the laws of motion that necessarily depends on gravitational forces (White et al. 2020).
Gravity estimation and verticality percepts are constructed by the CNS such as the subjective haptic vertical (SHV), subjective postural vertical (SPV) and subjective visual vertical (SVV) (Dakin and Rosenberg 2018).
4.2.2. Distortions of Gravity Estimation
The complex construction of gravity perception can be disturbed by a number of factors including various diseases.
Peripheral Vestibular Disorders. Unilateral peripheral vestibular lesions not only lead to vertigo, nystagmus and imbalance, but also to a bias in the perception of verticality, which can be measured as tilt of the SVV (Glasauer et al. 2019). Patients with peripheral vestibular disorders showed misperception of the SVV in comparison with healthy controls. A greater misperception of SVV was seen in a subgroup of patients in the acute phase and after vestibular surgery (Obrero-Gaitán et al. 2021). Patients with acute unilateral vestibular disorders may show pronounced tilts of the SVV toward the side of the lesion (Bronstein 1999). It has been proposed that a SVV tilt might be caused by a vertical semicircular canal bias in the roll axis (Glasauer et al. 2019).
Cerebellar Degeneration. Under particular conditions, cerebellar damage may have an effect on verticality perception. A genetically determined and pure form of cerebellar degeneration (spino-cerebellar ataxia type 6) induced verticality perception errors but only under dynamic visual conditions (Dakin et al. 2018).
Stroke. Patients with a hemispheric stroke align their erect posture with an erroneous reference of verticality, tilted to the side opposite the lesion (Pérennou et al. 2014). Indeed, stroke patients may actively push away from the non-hemiparetic side (`pusher syndrome´), which leads to lateral postural imbalance and a tendency to fall toward the paralyzed side. Pusher patients experience their body as oriented `upright´ when it is tilted about 18 degrees to the non-hemi-paretic, ipsi-lesional side (Karnath et al. 2005). This mainly emerges in cases of damage to regions involved in processing body perception and graviceptive information, such as the posterior thalamus and parts of the insula, the superior temporal gyrus, and post-central gyrus. Lesions extending to temporo-parietal junction may be associated with feelings of disembodiment, i.e., the paradoxical, temporary sensation of being localized elsewhere with respect to one´s physical body. These out-of-body experiences are often accompanied by vestibular sensations such as feelings of flying or floating (Delle Monache et al. 2021). In patients with a brainstem stroke, latero-pulsion is usually ipsi-lesional, and may result from a pathological asymmetry of muscle tone, through vestibulo-spinal mechanisms (Pérennou et al. 2014). In an 80-year-old patient with acute hemi-nodular (cerebellar) infarction, the SVV deviated contra-lesionally by -21.1° when the patient was upright. After subtracting this offset, the perceived vertical closely matched the patient´s head orientation when the patient was roll-tilted. The abolished perception of the earth vertical in hemi-nodular stroke suggests a cerebellar contribution to spatial orientation (Tarnutzer et al. 2015). Many symptoms can be interpreted as failures to process or integrate information from multiple sensory sources and/or to reconcile these inputs with prior information resulting from previous experience with gravity (Delle Monache et al. 2021).
5. Final Common Pathway: Motoneurons (MNs) to Muscles
The kinetics of active movements are delivered by muscle activities, which in turn are initiated and produced by motoneuron (MN) activities. The relationships between MN activities and movement are anything but simple because of the musculo-skeletal non-linearities and time-dependencies (Tsianos and Loeb 2017; Windhorst 2021a). MN activities are controlled by various inputs, of which there are at least three wide classes: (i) brainstem and spinal networks (e.g., locomotor CPGs), (ii) sensory inputs of various origins and (iii) various supraspinal structures. These inputs mostly interact at premotor levels, usually transmitted by interneurons, which constitute complex networks whose precise operations and functions are difficult to dissect (Windhorst 2021a).
Motor-output changes may affect muscle actions at a peripheral level, for example, muscle fatigue, spasticity, wasting etc. Furthermore, at a central level, MN diseases impact muscle actions, e.g., SMA and ALS (Windhorst and Dibaj 2023). Finally, pathological changes in MN inputs from CPGs, sensory afferents and descending tracts derange coordinated muscle actions.
6. Central Pattern Generators (CPGs)
6.1. Anatomy and Functions
Briefly, rhythmic body movements of vertebrates like walking, running, hopping, crawling, swimming, flying, scratching and respiration are generated by many interconnected neuron groups that organize the basic rhythmic brainstem or spinal outputs as well as the spatio-temporal patterns of muscle activities. The spatio-temporal patterns include flexion–extension alternation in intra-limb coordination, left–right coordination of bilateral limbs, and coordination of fore- and hindlimbs. These interneurons receive multiple inputs from descending tracts and sensory afferents, and in part project to MNs (reviews: Danner et al. 2017; Griener et al. 2013; Grillner and Kozlov 2021; McCrea and Rybak 2008; Zhong et al. 2012; Windhorst 2021a). The CPG activities must be adaptable to peripheral biomechanical conditions.
Besides sensory afferents, various tracts descending from supraspinal sources impinge on spinal CPGs, carrying commands and neuromodulatory signals. Whether, and if so how, pathological changes in these MN inputs can also affect the structures and operations of CPGs is not well known.
6.2. Impairment of Central Pattern Generators (CPGs)
CPG operation can be disturbed directly and/or indirectly by damage to their inputs. The former include rare spontaneous spinal cord infarctions (Stenimahitis et al. 2023). The latter encompass altered proprioceptive inputs from moving limbs (e.g., Akay et al. 2014) and SCIs interrupting signal transfer from supraspinal sources (e.g., Windhorst and Dibaj 2023).
6.2.1. Altered Proprioception
Proprioceptive feedback from muscle spindles and Golgi tendon organs (GTOs) (Sect 7.1) influences the generation of motor patterns during natural locomotion in mice. It is important for regulating the temporal parameters of walking and swimming, for the appropriate alternation in selected antagonist muscles at individual joints, and for the cross-joint coordination of limb muscle activities. Group Ia/II feedback from muscle spindles appears to predominantly influence the patterning the output of flexor muscles, whereas the joint and redundant activities of group Ia/II and group Ib afferents from GTOs determine the pattern of extensor muscle firing. All these functions are disturbed under conditions in which proprioceptive feedback from muscle spindles and GTOs is attenuated genetically and biomechanically (Akay et al. 2014). This suggests that changes in proprioceptive feedback under pathological conditions re-organize CGO network (see also below).
6.2.2. Altered Descending Influences
Similar to proprioceptive feedback, altered descending inputs may be assumed to re-organize CGO network under pathological conditions, such as in SCI. Various tracts descending from supraspinal sources impinge on spinal CPGs, carrying commands and neuromodulatory signals. One possibility for CPG dysfunctions is a change in neuromodulators, a number of them heavily influencing CPG activity and patterns. The removal of neuromodulators has indeed been suggested to perturb CPGs. Briefly mentioned here are serotonin (5-HT) and noradrenaline (NA). The modulatory 5-HT and NA pathways from the raphé magnus nucleus and locus coeruleus, respectively, are both unmyelinated and have low conduction velocities. Their role appears to be to fine-tune the properties of the spinal interneurons to be optimal for a stable operation of the spinal CPG (Grillner and El Manira 2020).
Depending on the location, SCI may damage or interrupt descending 5-HT projections. If so, this depletes 5-HT, dysregulates 5-HT transporters as well as elevates the expression, super-sensitivity and/or auto-activation of specific 5-HT receptors. These changes in the 5-HT system can produce varying degrees of locomotor dysfunction through to paralysis. Repletion of 5-HT restores limb coordination and improves locomotor function in experimental models of SCI (Ghosh and Pearse 2015; Windhorst and Dibaj 2023). In neonatal rats, a spinal-cord transection disorganizes the left-right hindlimb alternating pattern, which is restored after injecting a 5-HT2 receptor agonist (Gackière and Martinez 2014; Grillner and El Manira 2020).
In cats acutely spinalized at lower thoracic level, injection of either L-DOPA or NA α2-receptor-agonist (clonidine), elicited well-coordinated walking movements. This shows that a release of dopamine (DA) or NA at the spinal level can activate the locomotor circuits. NA is indeed released by stimulation of mesencephalic locomotor region (Grillner and El Manira 2020). It has been shown that, after partial SCI, the CPG network itself can undergo plastic changes (Martinez et al. 2011). How exactly this re-organization occurs and whether, if so, NA plays a role in the process is not yet known.
7. Sensory Inputs
7.1. Proprioception
Proprioceptors include muscle spindles, GTOs, joint and ligament mechano-receptors, cutaneous mechano-receptors, as well as GTO-like endings, Pacinian corpuscles, and Ruffini endings. Proprioceptors are involved in brainstem and spinal reflexes, help determine limb position and movement, contribute to the body schema (Sect 2.1.1) and probably also update internal models (Sect 9), which might underlie the control of posture and movement (Jayasinghe et al. 2021). Thus, some proprioceptive effects become conscious, while a bulk remains sub-conscious.
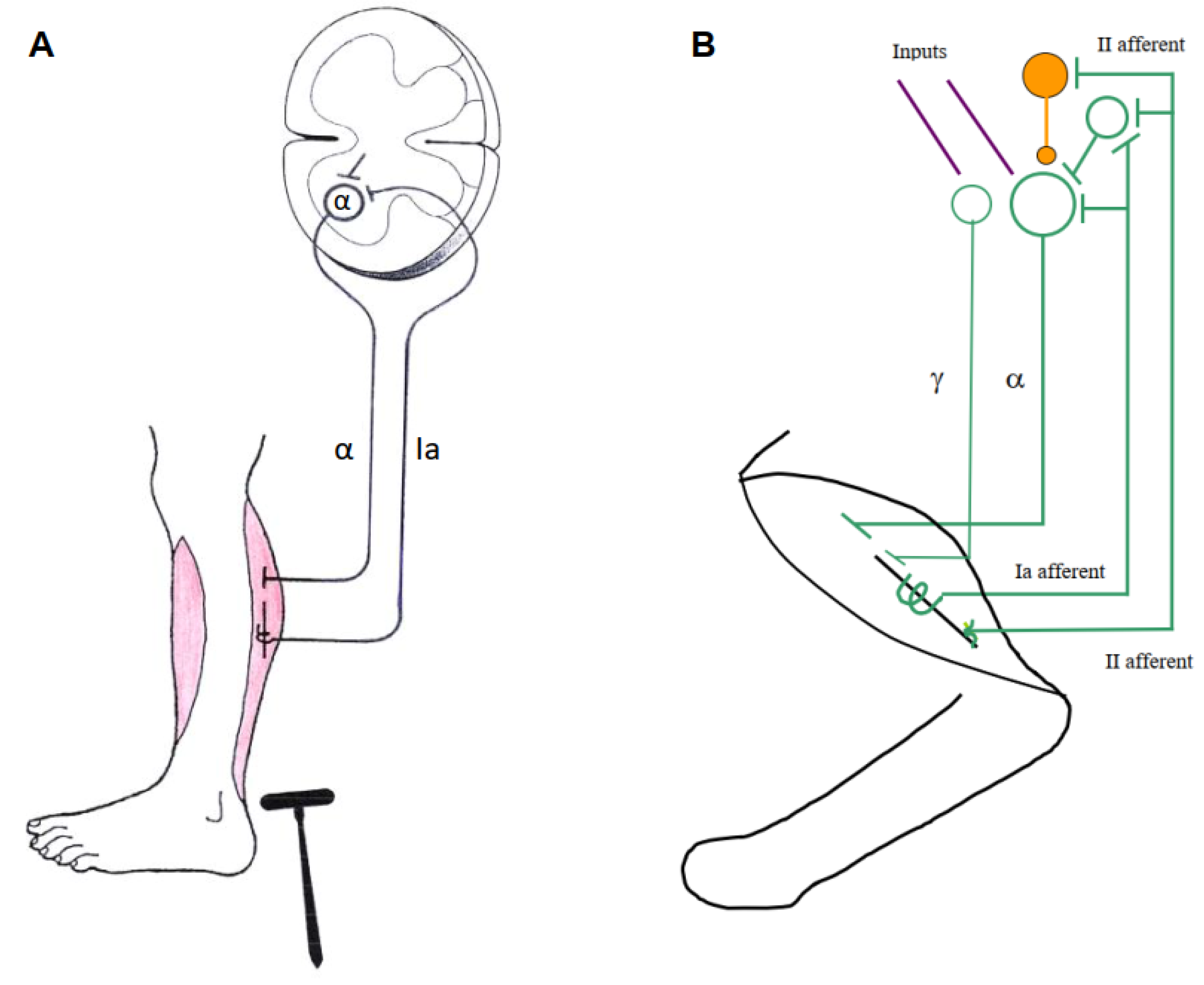
It is impossible to assign individual functions to the central actions of the above groups of afferents, for various reasons. First, muscle spindle group Ia and II afferents (supposed to be measure `internal muscle-length´) are the only ones with direct monosynaptic actions on skeleto-MNs (α-MNs and ß-MNs, innervating skeletal muscle fibers), but they also have indirect access to skeleto-MNs via interneurons. Moreover, they are under strong control of γ-motoneurons (γ-MNs, innervating intrafusal muscle fibers), which often change their discharge patterns (
Figure 2B). Second, group Ib afferents from GTOs (supposed to be measure `muscle-force´) exert excitatory and inhibitory actions on MNs via interneurons, which in part also receive convergent inputs from muscle spindle afferents. The influences of group Ia, II and Ib are strongly modulated by presynaptic inhibition. All other receptors listed above also first impinge on interneurons, which additionally receive convergent inputs from other sources. Thus, their effects on MNs are filtered by complex interneuronal networks whose operations have lost simple relationships to muscle length and force. They show a certain degree of random connectivity, but also differentiation as to their outputs to extensor and flexor, left and right, and forelimb and hindlimb MNs (Edgley 2001; Jankowska 1992; Schomburg 1990; Windhorst 2021a).
Proprioceptors yes or no? While undoubtedly group III (Aδ) and group IV (C) afferents from free nerve endings contribute to nociceptive reflexes (Schomburg et al. 2011), various supraspinal actions and pain and itch sensation, an open question is whether they should at least in part also be considered proprioceptors. Although they are mostly responsive to noxious, thermal and chemical stimuli, a substantial proportion of them responds to mechanical stimuli and influences central neurons at various levels (Laurin et al. 2015). At spinal level, chemically or metabolically activated group III/IV muscle afferents in cats have polysynaptic effects on α-MNs (Kniffki et al. 1981), change the discharge of γ-MNs (Johansson et al. 1993) and interneurons such as Renshaw cells (Windhorst et al. 1997), as they do in humans (Rossi et al. 2003). During acute and chronic muscle inflammation in cats, group III (Aδ) afferents help increase spinal reflex transmission (Schomburg et al. 2012, 2013, 2015). Importantly, during fatiguing muscle contractions (intra-muscularly releasing metabolites), presynaptic inhibition was increased and recurrent inhibition decreased, which could contribute in part to decrease the homonymous monosynaptic H-reflex (Kalezic et al. 2004). Signals elicited by group III and IV afferents also reach somatosensory and frontal cortical areas. Broadly confirmatory results were obtained in humans, as far as they are comparable to those cats. Group III and IV afferents may thus contribute to motor control, at spinal and possibly supraspinal levels (Laurin et al. 2015).
In summary, it has been proposed that, in both healthy and pathological populations, prolonged physical exercise, leading to muscle fatigue, would activate group III and IV muscle afferents, which would then act on central motor drive, modulate spinal reflexes from other sensory afferents, and thereby contribute to improve muscle performance by regulating peripheral fatigue development and by avoiding excessive muscle impairments. There are also indications that these afferents from a given active muscle could contribute to regulate the motor activity of the homonymous as well as surrounding skeletal muscles by acting at both spinal and supraspinal levels. Furthermore, following numerous neuromuscular traumata, the recovery of the sensory feedback may improve motor function (Laurin et al. 2015). It is not quite clear, though, which types of afferents might exert these effects.
7.2. Impairment of Sensory Inputs
Age. As people age, frailty and the tendency to fall do, with one reason being muscle weakness from sarcopenia. Muscle strength and proprioceptive acuity are correlated in the stability of standing, with acuity diminishing at high levels of muscle force. Thus, important reasons for the increase in the tendency to fall are a deterioration in proprioception and increased variability with age, in particular in the lower legs. The underlying reasons may be structural and functional changes in skeletal and intrafusal muscle fibers (Lord 2009; Proske and Gandevia 2012).
Exercise. After intense exercise, which causes muscle weakness, subjects are less sure about the placement of their fatigued limbs if they are not looking at them. Exercise can thus disturb proprioception, probably as a result of the accompanying fatigue. Eccentric exercise is special in that a component of the loss in force is due to muscle damage and muscle soreness. This damage could be due to a damage of muscle proprioceptors but the evidence is scarce. The soreness, too, can alter proprioception, presumably mediated by group III and IV muscle afferents. Many pathological conditions go along with abnormal perceptions of fatigue and effort. These include cortico-spinal tract (CST) lesions, SCI, and multiple sclerosis (MS; Sect 8.2.5) (Proske and Gandevia 2012).
Pain. The influence of joint pathology, particularly when associated with arthritis, can reduce voluntary strength and proprioception, perhaps as a result of the nociceptive input generated by joint movement and mediated by group III and IV afferents (Proske and Gandevia 2012).
Neurodegeneration of Proprioceptive Afferents. Dysfunction of proprioceptive afferents and other neurons has been implicated in neurodegenerative disease progression. Two examples are SMA and ALS, which are characterized by dysfunction and death of spinal MNs (Shadrach et al. 2021; Windhorst and Dibaj 2023). SMA is caused by the loss of the survival motoneuron (SMN) protein. Before MN cell death, SMA shows a reduction of proprioceptive afferent synapses on α-MNs which are vulnerable to synaptic pruning due to aberrantly up-regulated complement protein C1q. ALS pathogenesis has been associated with more than 20 genes in different molecular pathways. Degeneration of peripheral proprioceptive endings occurs in early pre-symptomatic stages, followed by a decrease in proprioceptive afferent central synapses on α-MNs. This suggest that proprioceptive afferent activity and synaptic excitation contribute to α-MN degeneration (Shadrach et al. 2021; Windhorst and Dibaj 2023).
Parkinson´s Disease (PD). It has been claimed that PD patients exhibit disturbed proprioception. If so, it is not due to malfunction of the peripheral sensory apparatus but results from problems with central processing of proprioceptive information. PD patients are impaired in detecting passive movements and show other changes, with one possible source for such abnormalities lying in the comparison between afferent feedback and the corollary discharge of the motor command (Proske and Gandevia 2012; Taylor 2009).
Human Genetics. Human patients with null mutations in mechano-sensitive piezo2 channels exhibited diminished sensitivity to light touch, vibration, and two-point touch discrimination in the glabrous skin (Chesler et al. 2016), as well as a lack of proprioception, impairing motor coordination, and various degrees of joint malformation (Moon et al. 2021).
Mutant Mouse Models. Motor coordination is disturbed in mouse mutants that lack the mechano-sensitive piezo2 channels. Conditional knockout of piezo2 in the dorsal-root ganglion parvalbumin neurons ablated the proprioceptive function. Mutants showed atypical limb coordination in tail-suspended posture, as well as abnormal and less-fluent walking. This also occurred in the HoxB8-Cre-dependent conditional knockout of piezo2. Parvalbumin neuron-specific knockout of piezo2 also elicited behavioral deficits in several balance and movement tests (Moon et al. 2021). – Mutation of the early growth response 3 (Egr3) gene disrupts proprioceptive feedback from group Ia/II afferents. Egr3-deficient mice exhibit ataxic gait, demonstrating that locomotion is affected by the absence of sensory feedback. Kinematic and EMG analysis of Egr3-deficient mice demonstrated that feedback from muscle spindles controls the timing of ankle flexion for proper foot placement and enables the correct matching of extensor muscle activity to different locomotive speeds. Locomotor patterns were more strongly impaired both in Egr3-deficient mice during swimming, which does not require significant GTO activation, and in mouse models that lack all proprioceptive afferents, suggesting that group Ib afferents that are unaffected in Egr3-deficient mice may compensate for the loss of muscle-spindle feedback (Shadrach et al. 2021).
Absence of Spinal Cord Interneurons. Genetically modified mice without RORα interneurons are impaired in sensing light touch on the foot sole, which does not lead to severe deficits in gross locomotion, but to deficits in precise foot placement (Bourane et al. 2015; Bui et al. 2015). Absence of dI3 interneurons in genetically modified mice entails a reduction of grip force in the face of increasing loads and other deficits (Bui et al. 2015).
7.3. Sensory Polyneuropathies
The heterogeneous group of sensory polyneuropathies are caused by various pathophysiological conditions that lead to dysfunction of peripheral sensory nerve fibers, including metabolic, toxic, inflammatory and genetic diseases. Symptoms mainly depend on the type of fibers affected. Damage to large myelinated sensory fibers leads to proprioceptive deficits and afferent ataxia (`ataxia-predominant” pathology) while damage to small thinly myelinated and unmyelinated sensory fibers leads to neuropathic pain (`pain-predominant´ pathology). Although ataxia and proprioceptive deficits are typical for large-fiber polyneuropathies, damage to relatively small fibers often occurs previously, simultaneously or subsequently, resulting in mixed-fiber polyneuropathies (Gwathmey and Pearson 2019).
7.3.1. Large-Fiber Polyneuropathies
Large-fiber neuropathies affect group I and II afferents from cutaneous mechano-receptors, muscle spindles, and GTOs.
Etiology and Symptoms. Sensory ataxia occurs in conditions affecting proprioceptive fibers in the peripheral nervous system (PNS) and CNS (spinal cord disorder). The peripheral neuropathies are also referred to as `ataxic neuropathies´, which represent a wide and heterogeneous range of disorders that may affect dorsal-root nerves, dorsal-root ganglia, nerve trunks, distal nerve endings or all of them (Mathis et al. 2021). The degradation of proprioception entails the loss of movement acuity, which is worsened by the deprivation of complementary sensory modality, and poor novel motor learning (Moon et al. 2021). Rare forms of a substantial, yet specific loss of the large-afferent fibers from GTOs and muscle spindles resulted in a substantial loss of position and movement sensation, regardless of intact small fibers that innervate joint articular tissues and skin. Vision can of course provide information about limb configuration and hand position, which may explain why de-afferented individuals can adapt to a novel force field, and up-date internal models (Sect 9) used to predict the effects of limb dynamics using vision alone (Jayasinghe et al. 2021). But long-lasting neuropathy in diabetic patients may be accompanied by vestibular and visual impairments (Felicetti et al. 2021).
Stretch Reflexes during Walking. When, during upright stance or the stance phase of walking, the supporting foot is suddenly rotated upwards, which stretches the calf muscles, EMG recorded from the soleus muscle shows two-three reflex bursts of different latencies: a short-latency reflex (SLR), medium-latency reflex (MLR) and maybe a long-latency reflex (LLR). There is evidence confirming that, during the stance phase of human walking, the soleus SLR is due to group Ia afferent excitation (Grey et al. 2004), and that group II afferents contribute to the MLR (
Figure 2B) (Grey et al. 2001). Changes were seen in patients with de-myelination of large sensory fibers (CMT1A and Anti-myelin-associated-glycoprotein neuropathy). – In another experiment, small-amplitude and slow-velocity ankle dorsi-flexion enhancements and reductions were applied during the locomotor stance phase. The patients exhibited absent light-touch sense in the toes and feet and absent quadriceps and Achilles tendon reflexes (
Figure 2A). Their soleus stretch reflex showed only a single EMG burst with delayed onset and longer duration than the SLR and MLR of healthy subjects. In the patients, the soleus EMG increased during the dorsi-flexion enhancements, but the velocity sensitivity of this response was decreased compared with the healthy volunteers. This indicates that the enhancement of the soleus EMG is mainly sensitive to feedback from group Ia and II muscle spindle afferents (Mazzaro et al. 2005).
Inter-joint Coordination. Patients de-afferented by large-fiber sensory neuropathy and healthy controls were compared in regard to the control of hand path in a planar movement-reversal task. In this task, subjects had to move their hand out and back along a series of straight-line segments in the horizontal plane without visual feedback. In controls, hand paths were straight with sharp bends at the outermost point. By contrast, patients showed errors at movement reversals, consisting of widened hand paths resulting from de-synchronization in the reversals of elbow and shoulder motions. These errors reflected an inability to program elbow muscle contractions in accord with interaction torques produced at the elbow by variations in acceleration of the shoulder. The reversal errors were substantially reduced after patients had practiced for a few trials while visually monitoring movements of their arm. The improvement was not limited to the direction where they had practiced with vision, but also extended to other directions in which the elbow torques were different. This suggests that practice with vision of the arm served to improve the general rules that subjects used to plan movement, rather than simply improving the performance of a specific response. This has been interpreted to mean that (i) both the planning and the learning of movement required an internal model (Sect 9) of the dynamic properties of the limb that takes account of interaction torques acting at different joints; (ii) this internal model is normally established and updated using proprioceptive information; but (iii) when without proprioception, vision of the limb in motion partially substitutes for proprioception (Ghez and Sainburg 1995; Jayasinghe et al. 2021). Such deficits are different for the left and right hand. Without vision of hand position, a de-afferented subject exhibited deficits in reaching trajectory and final posture. The patient´s non-dominant left hand produced large initial direction errors as well as oscillations at the target. Her dominant right hand produced less initial direction errors, but, at the end of movement, showed large drifts away from the target (Jayasinghe et al. 2021).
Pyridoxine Intoxication in Cats. Experimental evidence for the importance of cutaneous and proprioceptive sensory afferents in postural control is that pyridoxine (vitamin B6) in high doses produces selective large-fiber sensory loss accompanied by disturbances of quiet upright stance. After being trained to stand on a movable platform under control conditions, cats given toxic doses of pyridoxine displayed ataxia. Excursions of the body center of mass (COM) in the direction opposite to that of platform translation were also exaggerated, and the time at which the COM subsequently reversed direction was delayed (Stapley et al. 2002). – Task-level goals such as maintaining standing balance are achieved through coordinated muscle activities. The structure of muscle synergies can change with motor training, neurological disorders, and rehabilitation. The changes in the structure of synergies for reactive balance recovery following pyridoxine-induced large-fiber peripheral somatosensory neuropathy were evaluated in four adult cats. Reactive balance recovery was assessed using multi-directional translational support-surface perturbations over days to weeks throughout initial impairment and subsequent recovery of balance ability. All cats showed changes in the structure of synergies for reactive balance recovery after somatosensory loss, indicating that somatosensory mechanisms contribute to synergy structure, and therefore may contribute to some of the pathological changes in synergy structure in neurological disorders (Payne et al. 2020).
7.3.2. Mixed-Fiber Polyneuropathy: Diabetes Mellitus
Etiology and symptoms. Diabetic peripheral neuropathy (DPN) is the most common neuropathy. The vast majority of diabetic polyneuropathies are mixed-fiber neuropathies (Galosi et al. 2020; Itani et al. 2021). As already mentioned, both large myelinated as well as small thinly myelinated and unmyelinated fibers are affected. The most common presentation of DPN is a distal symmetric polyneuropathy with predominantly sensory and autonomic manifestations. Patients suffer from pain, hyperalgesia, tingling and burning sensation, numbness, trophic changes in the feet (foot ulcera), and/or weakness that begin in the feet and spread proximally over time and autonomic disturbances (Said 2007; Sanaye and Kavishwar 2023). Sensory symptoms are more prominent than motor involvement. The sensitivity to mechanical stimuli in the plantar skin declines with age and neuropathy. Neuropathy disrupts sensory inputs from both skin and muscle (Felicetti et al. 2021). Motor systems in the brain and spinal cord are also affected, mainly the cortico-muscular pathways (CST and spinal MNs), impairing the transmission of motor commands from the brain to the muscles. In the CST and MNs with long axons, axonal damage of the proximo-distal phenotype occurs, leading to pronounced weakness at the ankle and knee in type 1 and type 2 diabetic patients. Muscle weakness is paralleled by muscular atrophy within the feet and lower legs; even with preserved muscle strength, diabetes per se causes lower strength per unit striated muscle and slower movements of the feet and legs, unstable gait, and more frequent falls (Andersen 2014; Muramatsu 2020).
Motor Effects in Diabetic Polyneuropathy and CMT1A. Most patients with CMT1A and patients with diabetic neuropathy (DNP) had reduced or absent tendon-tap reflexes (
Figure 2A). The strength of foot dorsi-flexor muscles and conduction velocity of leg nerves were more impaired in CMT1A than DNP, whereas joint-position sense was more affected in DNP. Body sway during upright stance was larger in DNP than in CMT1A and controls. During gait, the distribution of foot-sole contact pressure was abnormal in CMT1A but not in DNP. Velocity and step length were decreased, and foot-yaw angle at foot flat increased, in DNP with respect to CMT1A and controls. Gait velocity and step length were decreased also in CMT1A, but to a smaller extent than in DNP, so that the difference between patient groups was significant. The duration of the double-support phase was protracted in DNP compared to CMT1A and controls. Hence, the changes in body sway and gait stance phase were larger in DNP than CMT1A, indicating more impaired static and dynamic control of balance when neuropathy affected the small in addition to the large afferent fibers. Diminished somatosensory input from the smaller fibers rather than muscle weakness or foot deformity played a role in the modulation of the support phase of gait (Nardone et al. 2014).
γ-MN Impairment. Reduced or absent stretch reflexes could also result from altered γ-MNs. Intrafusal muscle fibers of diabetic humans exhibited severe atrophy, and there were only a few fine γ-MN axons and their terminations in the intrafusal muscle fibers. Muscle spindle sensitivity to vibratory stimuli was reduced in muscles around the ankle joint in diabetic humans. In diabetic rats, morphological alterations of MNs suggested that γ-MNs are more likely to be affected by diabetes than α-MNs. The distribution of average soma diameters in retrogradely labelled medial gastrocnemius MNs of control animals was bimodal, with larger groups corresponding to α-MNs and smaller groups, to γ-MNs, while in diabetic rats, the number of smaller medial gastrocnemius MNs was reduced after 12-weeks and virtually abolished later, so that the size distribution became unimodal. In parallel to the loss of smaller MNs, muscle spindles showed motor denervation atrophy. Generally, the brain and spinal cord are also affected (Muramatsu 2020; Sect 8.2.4).
7.3.3. Small-Fiber Polyneuropathies
Etiology and symptoms. Small-fiber (poly-)neuropathies involve preferential damage to the small-diameter somatic fibers [Aδ (III) and unmyelinated C (IV) fibers], which transmit noxious, itch and thermal signals and regulate preganglionic sympathetic and parasympathetic function [Aδ (III) fibers], as well as postganglionic autonomic function (C fibers) (Oaklander and Nolano 2019). Examples include diabetic, toxic, inflammatory and genetic causes. The typical clinical presentation is that of a symmetrical, length-dependent polyneuropathy with sensory and/or autonomic symptoms, including numbness, neuropathic pain, painful dysesthesias, loss of temperature sensation, autonomic dysfunction, or a combination, as well as orthostatic dizziness (Terkelsen et al. 2017). They have been associated with a broader disease spectrum, including metabolic diseases (diabetes mellitus, glucose intolerance), dys-immunity syndromes (Sjögren's syndrome, sarcoidosis, monoclonal gammopathy), and genetic abnormalities (familial amyloidosis, Fabry disease, sodium-channel mutations), fibromyalgia and an autoimmune disease targeting voltage-gated potassium channels (Gwathmey and Pearson 2019; Sène 2018; Sopacua et al. 2019). Autonomic dysfunction could also impact proprioception because muscle spindles receive sympathetic innervation (Felicetti et al. 2021).
Differential Effects of Group III and Group IV Fibers on Reflexes. In high spinal cats, the contributions of nociceptive group III and group IV fibers originating from the central pad of the foot to nociceptive spinal flexor reflex pathways and to nociceptive excitatory reflex pathways to foot extensors was investigated. Persisting effects after complete block of group III fibers by tetrodotoxin (TTX) application were thus attributed to nociceptive group IV fibers. Both group III and group IV fibers contributed to nociceptive reflexes. The effects of group III fibers were evoked with a distinctly shorter delay than those of group IV fibers. Group III fibers partly exerted a significant inhibitory influence on the group IV fiber action. Treatment with different opioid receptor agonists (DAMGO and DSLET) and subsequently with naloxone revealed that a distinct part of the opioid action on nociceptive reflex pathways was evidently exerted via group III fibers (Schomburg et al. 2011).
A Little Enigma. In view of the fact that, in cats, activation of group III/IV afferents exert effects on α-MNs and γ-MNs, it appears surprising that motor symptoms have rarely been reported in human small-fiber neuropathy. An exception are, in patients with diabetic symmetrical polyneuropathy (DSPN), increases in the thresholds of perception and nociceptive withdrawal reflex, elicited by electrical stimulations on the plantar foot site, indicating that “...patients with type 1 diabetes and DSPN have significantly changed spinal and supraspinal processing of the somatosensory input” (Nedergaard et al. 2021). This might have been expected because of the prominent significance of the withdrawal reflex for survival. It should however be emphasized again that group III/IV afferents contact and modulate many spinal interneurons involved in motor control and may thus exert less conspicuous effects in the background. Small-fiber neuropathies would then be expected to indirectly disturb many actions and movements. In humans, the differences between CMT1A and DNP patients suggest that they are due to the existence of small-fiber neuropathy in DNP. In cats, nociceptive group III /IV fibers have differentiated spinal reflex effects. Another example is peripheral muscle fatigue during and after exercise that activates group III/IV afferents, which in turn contribute to improve muscle performance by regulating the peripheral fatigue development and by avoiding excessive muscle impairments (Decherchi and Dousset 2003; Gandevia 2001; Laurin et al. 2015; Monjo et al. 2015).
8. Supraspinal Motor Commands to Motoneurons (MNs)
8.1. Overview
Similar to proprioceptive afferents, the higher brain echelons send their motor commands to MNs usually through a network of a variety of descending tracts and intermediate nuclei, which amounts to complex transformations. Only minorities of nerve fibers descending from supraspinal structures contact MNs monosynaptically, most effects are mediated via interneurons which commonly receive inputs also from sensory fibers (Jankowska 1992; Schomburg 1990; Windhorst 2021a).
The structures closest to MN output that can be deranged are ascending and descending tracts that can be damaged by SCI (Windhorst and Dibaj 2023). We will here concentrate on a few movement disorders caused by diseases of supraspinal structures.
8.2. Movement Disorders
The etiology, particularly the pathologically affected area of the CNS, was formerly used to classify movement disorders (Baumann 1963). Cerebellar syndromes and lesions of the CST (along with lesions of associated descending inhibitory pathways) result in the movement derangements ataxia and spasticity, respectively (see also the review by Windhorst and Dibaj 2023). However, the term `movement disorders´ often refers primarily to the group of BG disorders, which are divided into two broad groups: hypokinetic and hyperkinetic movement disorders (Camargo and Teive 2019; Fahn 2011; Sian et al. 1999). Strictly speaking, the emphasis on phenomenology as the key element in differentiating various movement disorders (Fahn 2011; Jankovic et al. 2015) makes the correlation between `movement disorders´ and BG disorders obsolete. In this sense, the anatomical etiology of the movement disorders is not taken into account in the classification and various sites of the nervous system other than the BG such as cortex, cerebellum, cranial nerves, spinal cord, and even peripheral nerves are all included as sources in selected movement disorders (Fahn 2011).
In movement disorders, various data on time processing and motor control suggest a dysfunction of the BG and cerebellum. In certain cases, time-processing deficits could directly contribute to prominent symptoms, e.g., bradykinesia in PD (Avanzino et al. 2016).
8.2.1. Cerebellar Disorders
Etiology and Symptoms. Cerebellar disorders may have many causes: degenerations [in particular multiple system atrophy, recessive ataxias (e.g., Friedreich ataxia and autosomal dominant spino-cerebellar ataxias)], immune-mediated diseases (especially MS), primary or metastatic diseases, stroke, infections (abscess, cerebellitis) or post-traumatic origin, hypoglycemia, hypoxia, leukodystrophy, lipidoses, toxicity (Manto et al. 2023). Patients may exhibit pure cerebellar signs or combinations of cerebellar and extra-cerebellar deficits. Lesions of the vestibulo-cerebellar, vestibulo-spinal, or cerebellar oculomotor systems lead to vertigo, dizziness, and imbalance. Cerebellar damage almost always leads to oculomotor deficits, such as abnormal smooth pursuit, dysmetric saccades, misalignment of the eyes, and nystagmus during gaze holding. Cerebellar disorders may show, to varying degrees, limb hypotonia, a- or dysdiadochokinesia, dysmetria, grasping deficits and various tremor phenomenologies. Gait is staggering with a wide base, and tandem gait is frequently impaired. Moreover, the cerebellum´s non-motor functions (beyond the present scope) may be disturbed (Bodranghien et al. 2016; Manto et al. 2023).
8.2.2. Parkinson´s Syndromes
Parkinson´s Disease (PD) is the most common cause of paucity of movement (Fahn 2011; Sian et al. 1999). In contrast, the phenomenology of hyperkinetic movement disorders is diverse (Camargo and Teive 2019; Fahn 2011). The group includes, among others, the following types of movement disorders: tremor, dystonia, myoclonus, tics, and chorea.
Etiology and Symptoms. Patients with Parkinson’s syndromes face several challenges in activities of daily living due to hypokinesia (reduced amplitude of movements), bradykinesia (reduced speed) and akinesia (increased latency of onset) as well as due to increased muscle tone (rigor) and tremor (typically at rest). Activities of the lower and/or upper limbs and trunk are affected, such as upright posture and stance, e.g., reacting to prevent a fall following an unexpected postural perturbation, as well as walking, reaching, and grasping (Fahn 2011; Fasano et al. 2022). Thereby, coordinative along with intensive troubling aspects of limb movements and trunk posture shed lights on a progressive maladaptation to an altered sensory-motor apparatus. The phenomenon of the limb movement derangement can become complex as has been described for alien limb syndrome (Biran and Chatterjee 2004), which is observed, for example, in patients with corticobasal degeneration, an atypical Parkinson’s syndrome. Abnormal movement control, abnormal posture, and involuntary motor activity have been described in the affected limbs of the patients with corticobasal degeneration. The most common presenting symptoms of ischemic stroke are speech disturbance and weakness on one-half of the body (hemiparesis) (Campbell et al. 2019; Ekkert et al. 2021; Mansfield et al. 2018; Murala et al. 2022; Yew and Cheng 2015).
8.2.3. Spasticity after Stroke
Strokes can occur in various sizes at various CNS loci and generate a spectrum of damages and symptoms. Stroke can be categorized as ischemic stroke, intra-cerebral hemorrhage, or subarachnoid hemorrhage. The majority of strokes are ischemic caused by arterial occlusion. The etiology of ischemic stroke is multi-factorial. It can be due to an in-situ thrombus or a distant embolus. Genetic causes make a significant contribution to ischemic stroke genesis. Albeit rare, stroke can also be caused in infections.
Spasticity. Major causes of spasticity, for example after ischemic lesions of the CST, are decreased depressive activity at the synapses of MNs, changes in MN properties, and changes in muscle properties. Changes in various spinal circuits are responsible for spasticity, including presynaptic, recurrent, and reciprocal inhibition of MNs (Windhorst and Dibaj 2023).
Sensory Feedback During Spastic Walk. During treadmill walking, patients with hemiparetic spastic stroke and age-matched healthy volunteers were tested with three types of ankle perturbations applied by a robotic actuator attached to the foot and leg. Fast dorsi-flexion perturbations were used to elicit stretch reflexes in the soleus muscle. Compared with the healthy volunteers, the soleus SLR was facilitated in the patients. Fast plantar-flexion perturbations were applied during the stance phase to unload the plantar flexor muscles, thus removing the afferent input from these muscles to the soleus MNs. These perturbations produced a decrease in soleus activity that was significantly smaller in the patients compared with the control subjects. Slow-velocity, small-amplitude ankle trajectory modifications mimicking small deviations in the walking surface were applied to evaluate the afferent-mediated amplitude modulation of the locomotor soleus EMG. In the healthy volunteers, these perturbations caused gradual increments and decrements on the soleus EMG, while in the patients, the soleus EMG modulation was significantly depressed. This indicated that, although the stretch reflex response was facilitated during spastic gait, the contribution of afferent feedback to the ongoing locomotor soleus activity was depressed in patients with spastic stroke (Mazzaro et al. 2007).
8.2.4. Diabetic CNS Effects
Diabetic patients show several motor dysfunctions, including an increased risk of falling, increased body sway, altered gait and balance, and a significant increase in the risk of physical disability. Diabetes also affects CNS neurons and glial cells, leading to dysfunction and cell death. Affected structures include the motor and sensory cortices, BG, cerebellum, brainstem, and spinal cord (Muramatsu 2020).
Cerebral Cortex and CST. Diabetic patients showed a widespread reduction in gray matter and white matter volumes and densities, and in humans with both type 1 and type 2 diabetes, the volume of CST was decreased. Middle-aged humans with type 1 diabetes showed frontal gray matter atrophy, including the M1. Humans with type 2 diabetes, exhibited a decrease in the cortical surface area of the paracentral lobe corresponding to the M1 and primary somatosensory cortex of the lower extremity. Diabetic rats showed various morphological changes in neurons and glia cells and reductions in the forelimb area of M1 and exhibited size reductions in the hindlimb area at 4 weeks and in the trunk and forelimb areas after 13 weeks, with the hindlimb and trunk-area reductions being the most severe after 23 weeks. The conduction velocity of motor descending pathways in both the CST and the rubro-spinal tract (RuST) was reduced. In rats with type 1 diabetes, motor dysfunctions were correlated with reductions in presynaptic terminals around the MNs (Muramatsu 2020).
8.2.5. Multiple Sclerosis (MS)
Etiology and Symptoms. MS is a potentially progressive, autoimmune CNS disorder, resulting from an autoimmune attack on CNS white matter (Cotsapas et al. 2018). Although the etiology of multiple sclerosis is still unknown, there is now a better understanding of the underlying genetic and environmental factors, including low vitamin D level, cigarette smoking, obesity, the influence of gut microbiota, and Epstein-Barr virus infection (Baecher-Allan et al. 2018; Bjornevik et al. 2022; Correale et al. 2022; Thompson et al. 2018). Typically, multiple sclerosis occurs in young adults aged 20 to 30 years with unilateral optic neuritis, sensory impairment, myelitis, or brainstem syndromes such as internuclear ophthalmoplegia, each developing over several days (McGinley et al. 2021). The ability to maintain position is decreased, movement limited and slowed towards limits of stability, and responses to postural displacements and perturbations are delayed. Walking alterations include reduced gait speed, impaired walking balance, and reduced walking-related physical activity. Falls are associated with injuries, reduced participation, and increased fear of falling. Symptoms also include weakness, spasticity and fatigability, as well as changes in sensation, vision, cognition and bladder function (Cameron and Nilsagard 2018).
Long-latency Soleus Stretch Reflexes During Spastic Walk. In eight healthy subjects walking at normal speed and nine spastic MS patients and ten age-matched healthy subjects walking slowly, soleus (MLR, peak latency of approximately 85 ms) and long-latency reflexes (LLR, peak latency of approximately 115 ms) were elicited by applying an eight-degrees stretch to the ankle extensors of the left leg. When present in walking healthy subjects, MLR and LLR were modulated in a similar way and with the same amplitudes as previously described for the soleus SLR, while the spastic patients´ SLR was significantly less modulated during walking. All patients´ LLR responses were absent or much suppressed during walking. It was argued that, in healthy subjects, part of the LLR was mediated by a transcortical route (Sinkjaer et al. 1999).
8.2.6. Timing Derangements in Movement Disorders
Parkinsonian patients show a reduced accuracy for sub-second and supra-second intervals, but inconsistent changes in performance variability. The underlying mechanisms are unclear, but PD patients might activate an alternative timing network relying more on cerebellar activation than healthy controls and motor timing networks are modulated by DA stimulation (Avanzino et al. 2016).
Huntington´s Disease (HD). Explicit timing abilities progressively deteriorate in mutation carriers as they approach clinical disease onset. During progression of the disease, the cortico-BG-sensory-motor and associative loops get progressively involved in parallel to a similarly progressive timing deficit. HD patients exhibit a reduced accuracy and increased performance variability (Avanzino et al. 2016).
Dystonia. Patients exhibit selective implicit timing-task abnormalities, which indicates that dystonia is a broader network disorder, in which the crucial nodes are located not only in the BG and the sensory-motor cortex, but also in the cerebellum. In musician´s dystonia, the accuracy of the affected hand is reduced, and finger-tapping performance is variable, depending on the finger affected. In writer´s cramp and cervical dystonia, the accuracy on temporal prediction of hand motion is reduced, but not of inanimate object motion (Avanzino et al. 2016).
9. Internal Models
The notion of internal models, borrowed from engineering concepts, has become popular in neuroscience, particularly in motor control. For example, the ability to make smooth and accurate reaching movements is considered to be based on adaptable internal models of limbs and objects in the CNS, which relate motor commands to changes in limb and/or object states (i.e., position and velocity) to manipulate them in an accurate, predictable manner. Thus, an internal model of the forearm might use estimates of forearm inertia, damping and other dynamic properties to specify how forces applied at the elbow joint would move the forearm (Bhanpuri et al. 2014).
Many times, the notion of internal models remains abstract because an internal model often cannot be pinpointed in terms of neurons or neuronal networks constituting them. The identities, anatomies, structures and/or locations of internal models are not necessarily defined or known. Eye movement control uses internal models (Lisberger 2009; Loeb 2021). Forward internal models (below) may also be used to distinguish sensory signals generated by externally imposed movements (ex-afference) from internal signals generated by self-motion (re-afference) (Cullen and Brooks 2015). It has also been suggested that the spinal cord uses a feedforward model for motor learning (Brownstone et al. 2015; Windhorst 2021a). There could be dedicated internal models of gravity (Sect 4.1; Hubbard 2020; Lacquaniti et al. 2015), verticality (Barra et al. 2010), laws of object motion (La Scaleia et al. 2015; White et al. 2020), spatial orientation (Ivanenko and Gurfinkel 2018), body geometry (Lacquaniti et al. 1992), and postural control (Dakin and Bolton 2018), locomotion and self-motion (Cullen and Brooks 2015).
Internal models have been roughly divided into forward and inverse internal models (Lawrenson et al. 2018; Loeb 2021; McNamee and Wolpert 2019).
9.1. Inverse Internal Models
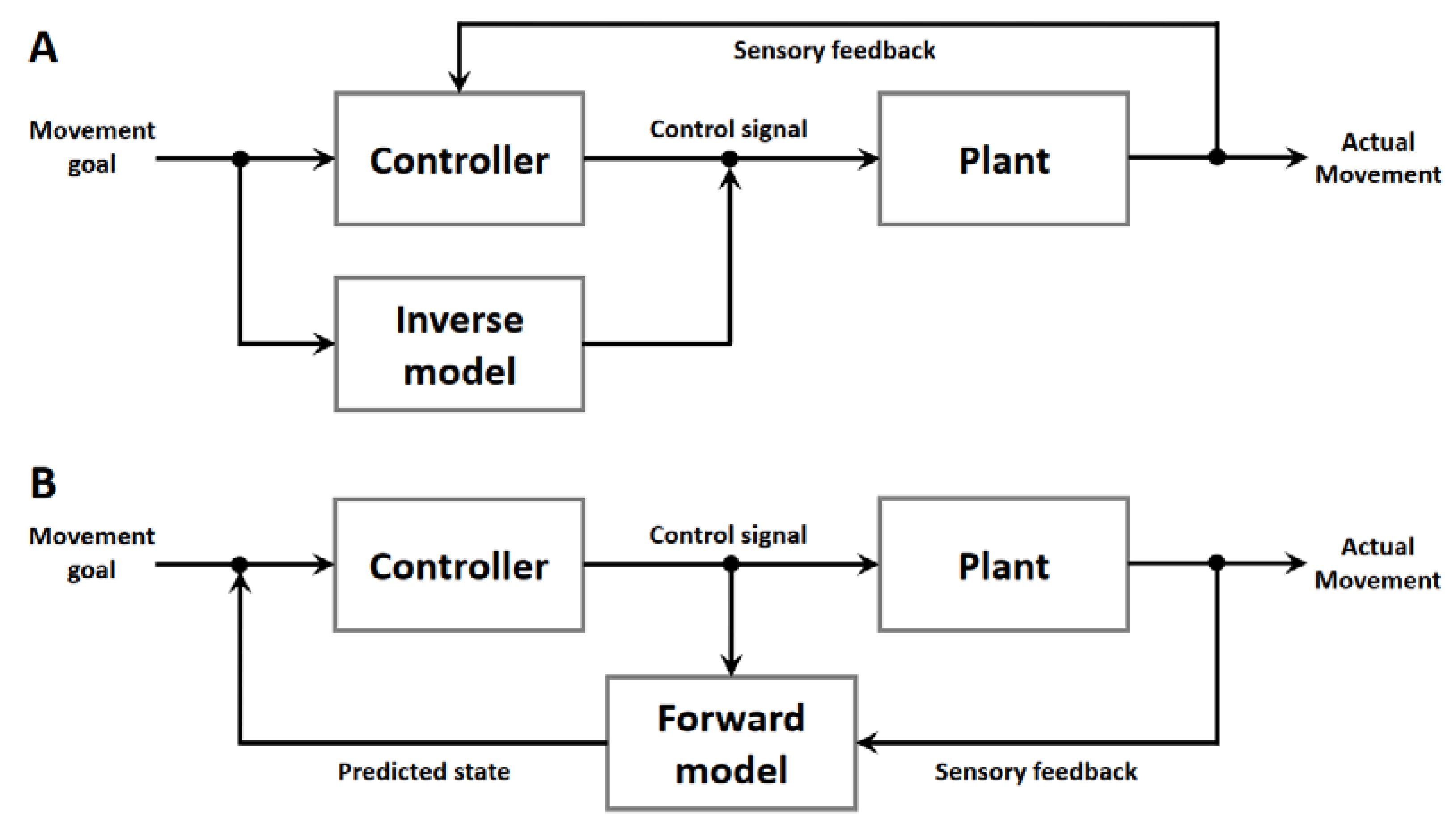
Briefly, an inverse internal model denotes a control mechanism that transforms a desired state into actions of an effector. For example, the motor cortex could define and send motor commands to all those structures involved in the execution of the desired movement, such as reaching, grasping etc. (
Figure 3A). However, the relationships between motor cortex activity and skeleto-muscular output is anything but simple.
9.2. Forward Internal Models
Forward internal models (
Figure 3B) are structures predicting the consequences of actions and can be used to overcome time delays associated with feedback control. Forward models are typically adaptive, updated by experience.
In sensory-motor systems, a forward internal model is assumed to predict the sensory consequences of motor commands by integrating efference copies of motor signals with current sensory signals (ex-afferences). An extension of this model is its plasticity in response to output errors. Forward internal models may also serve other functions, such as filtering sensory signals, enhancing or attenuating information for the control of movements, and cancelling the sensory effects of self-generated movements to enhance more important sensory inputs (Wolpert et al. 1998).
9.3. Master Model Cerebellum?
Internal models have been suggested to be situated throughout the nervous system. A prominent place is the cerebellum, which in sensory-motor learning has been called “A Critical Subcortical Node in Sensorimotor Learning” (Kim et al. 2021).
9.3.1. Forward Internal Model in the Cerebellum
The least controversial model version is the forward internal model, by which the cerebellum estimates the sensory consequences of motor commands (
Figure 3B) that enables predictive control and requires updating to adjust for persistent sensory errors that result from perturbations (Imamizu and Kawato 2012; Ishikawa et al. 2016; Krakauer and Mazzoni 2011; Nowak et al. 2007; Popa and Ebner 2019; Soetedjo and Horwitz 2023; Streng et al. 2022; Tanaka et al. 2020; Therrien and Bastian 2019).
9.3.2. Inverse Internal Model(s) in the Cerebellum
More controversial has been the hypothesis that the cerebellar circuitry provides inverse models (Ioffe et al. 2007; Lisberger 2009; Spampinato and Celnik 2021; Yavari et al. 2016), possibly because of different experimental conditions. However, in the relatively simple case of ocular following responses, the complex temporal pattern of the Purkinje-cell firing rate elicited by movements of a large visual scene can be reconstructed by an inverse-dynamics representation, which uses the position, velocity and acceleration of eye movements. It has been concluded that these Purkinje cells primarily contribute dynamic command signals (Lisberger 2009; Shidara et al. 1993; see also: Wolpert et al. 1998).
Finally, various combinations of the two model types in learning have been suggested (Honda et al. 2018; Passot et al. 2013; Wolpert et al. 1998). For example, a computational model of the ocular following response included multiple paired forward and inverse models, this arrangement being advantageous for motor learning and control (Wolpert et al. 1998). Further, also in cerebellar eye movement control, saccades have been proposed to depend on internal models, the ipsilateral oculomotor vermis being part of a forward model that predicts eye displacement, whereas the contralateral oculomotor vermis being part of an inverse model that creates the force required to move the eyes accurately (Soetedjo and Horwitz 2023).
9.3.3. Diseased Internal Model(s) in the Cerebellum?
Obviously, cerebellar damage also impairs the operation of the internal models. It is not well understood, though, how a damaged internal model could lead to patient-specific movement characteristics. This is, in part, because cerebellar subjects may show different behaviors; some tend to overshoot (hypermetria, most common), while others undershoot (hypometria), when reaching to targets (Bhanpuri et al. 2014; Therrien and Bastian 2015).
Dysmetria. The comparison of kinematic and EMG recordings during goal-oriented arm movements in normal subjects and cerebellar patients led Cabaraux et al. (2020) to argue that impairment in the predictive computation for voluntary movements would explain several characteristics accompanied by dysmetria. In control subjects, a component of movement kinematics was predictive for target motions, while in cerebellar patients, the predictive component lagged behind the target motion and was compensated for by a feedback component. Dysmetria would thus result from deficits in the predictive computation of the cerebellar internal forward model (Cabaraux et al. 2020; also: Therrien and Bastian 2019).
Underestimation of Inertia would cause an overshoot and overestimation of inertia an undershoot. Bhanpuri et al. (2014) constructed a computer model of dysmetric movements with mis-estimates of arm inverse dynamics. Similar movement patterns could be modelled using a biased inverse model or a biased forward model. The authors concluded that changes in specific internal dynamics could explain differences in patient behavior and that, normally, the cerebellum appears critical to upholding unbiased internal dynamic models of the arm.
Deficiency in Prediction of Inter-segmental Interaction Torques. Another factor contributing to dysmetria may relate to the missing prediction and compensation for inter-segmental joint torques. When cerebellar patients made slow and accurate reaching movements in the sagittal plane to a target directly in front of them, they produced abnormally curved wrist paths with target undershoot and tended to move one joint at a time (de-composition), while they overshot the target during fast and accurate reaches. Compared with control subjects, cerebellar patients also produced very different torque profiles. In slow and accurate reaches, they produced abnormal elbow muscle torques that prevented the normal elbow extension early in the reaches. This was interpreted as resulting from an inability to produce muscle torques that appropriately predict, accommodate and compensate for the dynamic interaction torques (Bastian et al. 1996, 2000).
Deficiency in Prediction of Grip Force. Compared to controls, patients with unilateral or bilateral cerebellar damage who exerted feedforward control of grip force with a hand-held object during cyclic vertical arm movements, anticipated speed-related changes in load magnitudes by adjusting the grip force. Hence, cerebellar lesions affect the processing of predictive grip-force modulation by and internal feedforward model in anticipation of inertial loads (Rost et al. 2005; also: Therrien and Bastian 2019).
Intention Tremor. This low-frequency oscillation when approaching a target has been interpreted as arising from deficient predictive sensory state estimation and its replacement with actual sensory feedback which would be delayed, which in turn would cause the oscillations (Therrien and Bastian 2019).
Deficiency in Prediction of Proprioception. Movement control requires proprioception to estimate the actual body and limb state for comparison with the prediction of limb position, which is thought to be contributed by the cerebellum, particularly when a person actively moves the limb. In fact, cerebellar patients have proprioceptive deficits compared with controls during active movement, but not when the arm is moved passively. Predictability enabled by an internal model of the body and environment has been suggested to be important for active movement to benefit proprioception (Bhanpuri et al. 2013; see also: Therrien and Bastian 2019).
The cerebellum has been implicated in the control of the sensitivity of muscle spindle stretch receptors via the fusimotor system. The question therefore arises whether the proprioceptive deficits result from changes in cerebellar influences on muscle spindles. In freely moving cats, recordings were made from nine muscle spindle afferents before and during reversible inactivation of cerebellar interpositus and dentate nuclei. In normal cats, fusimotor action greatly altered spindle stretch sensitivity but varied with motor task. The whole spectrum of spindle stretch sensitivity persisted during ataxia. Hence, the cerebellar nuclei studied are not primarily responsible for fusimotor control, nor is the ataxia primarily caused by disordered proprioceptive sensitivity (Gorassini et al. 1993).
Model Predictions of Muscle Fatigue. Peripheral muscle fatigue is reported by metabolite- sensitive group III and IV afferents with various widespread actions in the CNS (Gandevia 2001). Cerebellar internal models have been proposed to deal with this internal perturbation by predicting upcoming fatigue by use of a forward internal model. For example, in a strong voluntary contraction expected to cause fatigue, the model might receive an efference copy of the motor command and predict the fatigue-induced sensory feedback which would be compared in the CNS with the actual feedback, and the difference would be used to adapt the motor commands so as to make the best of the situation (Monjo et al. 2015)
10. Neural Networks
Using a multitude of methods, neuroscientists have made numerous attempts at dissecting and assigning functions to more or less great portions of the CNS and their constituent neurons. This has turned out to be difficult. A resort has been the notion of neural networks.
10.1. Artificial Neural Networks (ANNs)
According to Wikipedia, “artificial neural networks (ANNs, also shortened to neural networks (NNs) or neural nets) are a branch of machine learning models that are built using principles of neuronal organization discovered by connectionism in the biological neural networks constituting animal brains”.
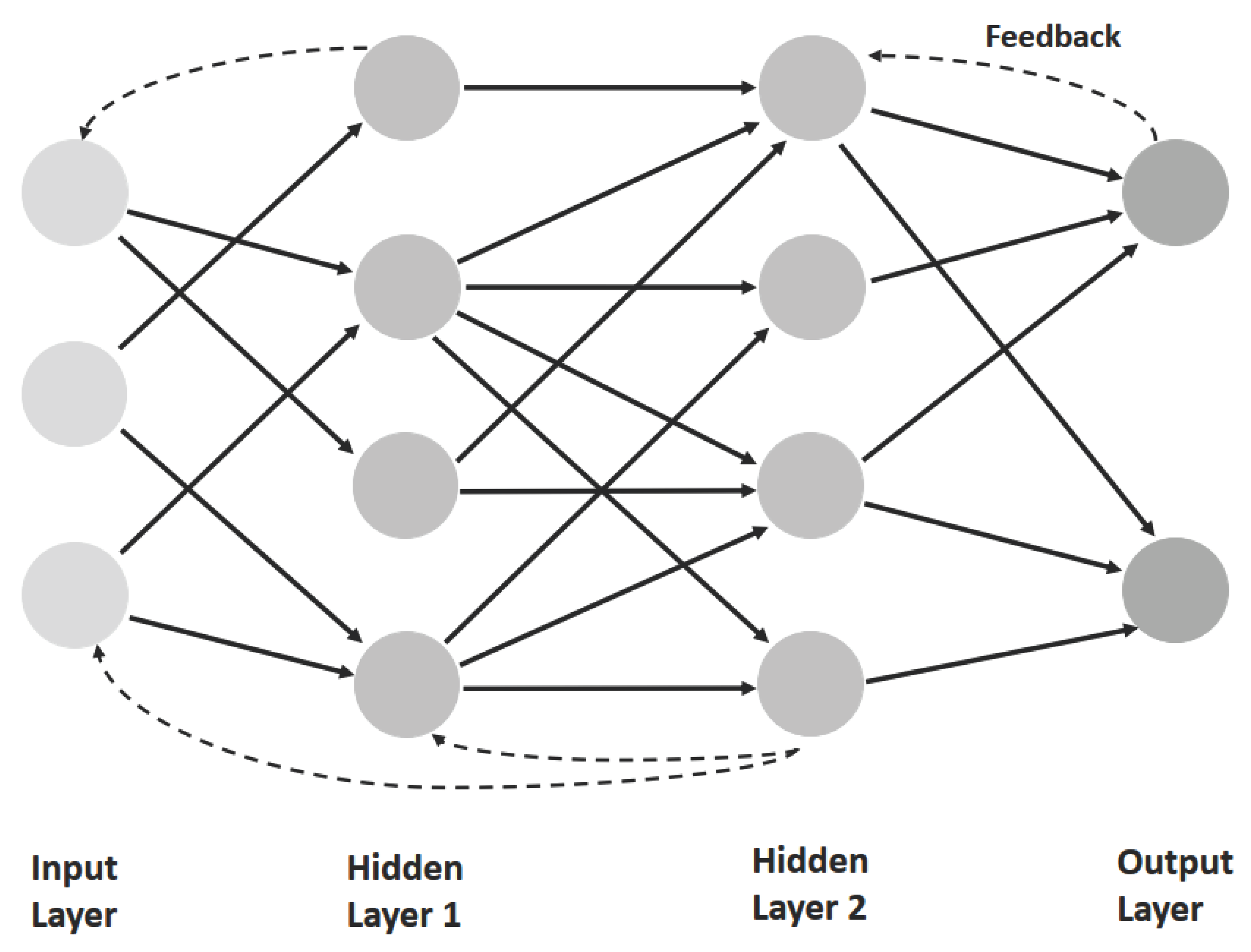
Neural networks are parallel, distributed information processing structures consisting of artifial `processing elements´ (
Figure 4). Each processing unit of number
i bears some similarity to a real neuron in that it integrates (sums) many inputs
aip, which are weighted by factors
wij. Elementary units of this sort are connected into nets, which may consist of several layers, including an input and an output layer and intermediate `hidden´ units connected by forward and/or recurrent links. The essential feature of these networks is that the strength of their `synaptic´ connections (as expressed in
wij) are modifiable by certain learning rules.
In supervised learning, a desired target is specified by an external teacher, by higher-level goals, or externally by the environment, e.g., during imitation learning. The discrepancy between desired output and actual output is used as an error signal that in turn is used to instruct the learning process.
At the other extreme, learning occurs without guidance, the network then self-organizing itself. In this case, unsupervised learning (in sensory-motor learning), the environment provides neither a desired target nor reward or punishment. An example is Hebbian learning.
10.2. Modeling of Spinal Neuronal Networks
With the advent of powerful computers, it has become popular to simulate natural neuronal networks by more or less similarly structured ANNs that are able to learn. Two examples of the operation of spinal-cord networks are briefly presented here.
The first study (Enander et al. 2022a). explored how the selective formation of the monosynaptic projections from group Ia muscle spindle afferents to homonymous skeleto-MNs could be explained by circuit formation based on learning. The initially randomized gains in the neural network were adjusted according to a Hebbian plasticity rule, and the model system was exercised with spontaneous muscle-activity patterns similar to those occurring during early fetal development. Stronger and more coordinated muscle activity patterns observed later during neonatal locomotion impaired projection selectivity. Hence, important aspects of the skeleto-muscular system´s mechanical dynamics were imprinted onto a neural network (Enander et al. 2022a).
The second study (Enander et al. 2022b) ventured into the complex spinal interneuronal networks. The muscle-specific details of observed connectivity patterns could, in part, result from Hebbian adaptation during early development. The authors constructed a simplified model of the skeleto-muscular system with realistic muscles and sensors and connected it to a recurrent, random neuronal network consisting of both excitatory and inhibitory interneurons endowed with Hebbian learning rules. A variety of randomized muscle twitches typical of those occurring during fetal development was used to allow the network to learn. This consistently resulted in diverse and stable patterns of connectivity and activity that included subsets of the interneurons. Hebbian learning led to diverse interneurons whose connectivity reflected the mechanical properties of the system (Enander et al. 2022b).
It should be mentioned that Hebbian learning mechanisms are not the only ones in the spinal cord, as illustrated by plastic processes occurring for example during SCI (Windhorst and Dibaj 2023).
11. Sensory-Motor Learning
It seems evident that the nervous system must have, and has, the ability to learn from past experiences, to adapt to novel conditions, including diseases, and to develop motor skill and motor learning in order to improve performance. Thus, nervous system structures and operations need to be plastic and adaptable, on a short-term to long-term basis (Butz et al. 2009; Grau 2014; Krakauer et al. 2019; Pascual-Leone et al. 2005; Schouenborg 2004; Windhorst and Dibaj 2023; Wolpert et al. 2001). This also applies to internal models and neuronal networks.
Sensory-motor learning does not involve a single, but rather various processes, ranging from relatively low-level mechanisms for maintaining movement calibration, to making high-level cognitive decisions about how to act in a novel situation. Learning, even in simple perturbation studies (below), can occur at multiple levels: cerebellum-based adaptation of internal models can improve action execution, while prefrontal cortex (PFC) and BG-based processes may improve action selection (Krakauer et al. 2019; Spampinato and Celnik 2021; Taylor and Ivry 2014).
11.1. Motor Adaptation and Learning
Motor adaptation occurs after perturbations such as unexpected external forces that interfere with movement. Implicit adaptation uses a sensory-prediction-error-driven mechanism dependent on the cerebellum (Krakauer et al. 2019).
Motor-skill learning elevates performance above baseline levels by repeated training (Shmuelof and Krakauer 2011). Practice-induced improvement of motor acuity is accompanied by changes in M1 (MI), premotor cortex (PM) and the cerebellum (Krakauer et al. 2019).
Learning of new skills requires building de-novo controllers (Krakauer et al. 2019). It has been suggested that humans are capable of acquiring new motor patterns via the formation of internal model representations of the movement dynamics and through positive reinforcement (Spampinato and Celnik 2021).
Explicit processes contribute to motor learning (Krakauer et al. 2019). Cognitive strategies and heuristics may enable humans to rapidly explore and evaluate novel solutions to enable flexible, goal-oriented behavior (McDougle et al. 2016; Spampinato and Celnik 2021). These processes involve PFC and hippocampus (Krakauer et al. 2019).
11.2. Motor Variability and Learning
Trial-to-trial variability in repeated movements and motor skills is ubiquitous and usually considered an unwanted consequence of a noisy nervous system. But motor variability could also be an indication of how sensory-motor systems operate and learn. In this view, motor variability is considered a purposeful exploration of motor space that, when coupled with reinforcement, can drive motor learning (Dhawale et al. 2017; Hossner and Zahno 2022).
11.3. Learning of Internal Models
It has been argued that the predictive computation of the cerebellar forward internal model affords error-based motor learning, coordination of multiple degrees of freedom, and appropriate timing of muscle activities (Cabaraux et al. 2020).
Hand kinematics during reaching have been suggested to be learned from errors in extent and direction in an extrinsic coordinate system, while dynamics from proprioceptive errors in an intrinsic coordinate system. Learning and consolidation of a rotated spatial reference frame and altered inter-segmental dynamics did not interfere with each other and consolidated in parallel. This has been interpreted to suggest that separate kinematic and dynamic models were constructed based on errors computed in different coordinate frames, and possibly, in different sensory modalities (Krakauer et al. 1999).
11.4. Structures and Mechanisms Underlying Learning
Motor adaptation, skill acquisition and motor-sequence learning recruit multiple CNS systems, including the cerebellum, BG and cerebral cortex, and even the spinal cord, with the cerebellum having a prominent role (Christiansen et al. 2017; D´Angelo 2018; De Zeeuw and ten Brinke 2015; Grau 2014; Hull 2020; Windhorst 2021b; Windhorst and Dibaj 2023; Wolpaw 2006; Yang and Lisberger 2014).
In rodents, task-learning goes along with target-specific routing of sensory information to specific downstream cortical regions, with higher-order cortical regions such as the posterior parietal cortex (PPC), medial PM, and hippocampus appearing to play important roles in learning- and context-dependent processing of sensory input (Crochet et al. 2019). Many further processes and mechanisms at circuit, synaptic and molecular levels contribute to adaptation and learning. Motor learning is co-determined by neuromodulators. For example, DA plays multi-faceted roles in synaptic plasticity and appears to be necessary for some types of motor learning, either by functioning as a reward prediction error, through passive facilitating of normal BG activity, or through other mechanisms (Wood 2021). DA modulates long-term potentiation (LTP) and long-term depression (LTD) in several cortical and sub-cortical areas (Speranza et al. 2021).
11.5. Disruption of Sensory-motor Learning
If the different forms of learning and their neuronal substrates remain intact after neurological damage, they could be used to facilitate motor relearning. For example, patients with cerebral stroke are still able to adapt to environmental changes, likely through error-based learning, and to show after-effects from adaptation leading to improved gait step-symmetry. Similarly, providing success- and failure-based reinforcement feedback to stroke patients enhances the rate of motor learning. Depending on the polarity applied to the cerebellar cortex, transcranial direct current stimulation changes the rate of motor adaptation in locomotor, visuo-motor and force-related perturbations. Targeting the cerebellum with brain stimulation improved gait and balance control in both stroke and ataxic patients (Spampinato and Celnik 2021).
Impaired Learning in Sensory Large-fiber Loss. A Case Study. A subject (IW) with loss of large-diameter sensory fibers below the neck was impaired in sensing the static position of his upper limbs. In a force-field perturbation, his ability to discriminate in which reaching direction the trajectory had been diverted was unimpaired. IW was still able to adapt to force fields when visual feedback was present, and even when visual feedback about the lateral perturbation of the hand was withdrawn. In this case, however, he did not show use-dependent learning, as evident in the control subjects as a drift of the intended reaching direction in the perturbed direction. This suggests that this form of learning may depend on static position sense at the end of the movement, and indicates that dynamic and static proprioception play dissociable roles (Yousif et al. 2015).
Deficits of Adaptation in Cerebellar Patients. Cerebellar dysfunction impairs implicit motor adaptation, which has been attributed to an impairment in error-based learning, specifically, from a deficit in using sensory prediction errors to update an internal model. Patients with spino-cerebellar ataxia displayed impairments in both implicit adaptation to a visuo-motor rotation and aiming. The patients had difficulties in developing and/or maintaining an aiming solution in response to a visuo-motor perturbation (Butcher et al. 2017). More generally, patients with cerebellar degeneration exhibit deficits in force-field adaptation, locomotor adaptation, saccadic adaptation, visuo-motor adaptation, and speech adaptation (Krakauer et al. 2019; Rabe et al. 2009; Therrien and Bastian 2015). In cerebellar patients with atrophy of the intermediate and lateral zones of the anterior lobe may show correlations with deficits in adaptation to altered force fields. Atrophy of the intermediate zone of the posterior lobe on the other hand correlates with impaired adaptation to altered visuo-motor associations (Rabe et al. 2009). Brain imaging of humans with cerebellar disorders also indicates that, within the anterior cerebellar arm area, a more anterior part including lobules IV and V is related to force-field adaptation. A more posterior part of lobule VI, extending into lobule V, is associated with visuo-motor adaptation, and the postero-lateral cerebellum may contribute to both tasks (Donchin et al. 2012).
12. Conclusions and Comments
Voluntary goal-directed movements, potentially superimposed on locomotion or similarly complex movements (
Figure 1), involve a large number of interacting structures and processes. Skeletal muscles and the skeletal and tendinous elements to which they are attached present their own complex problems (non-linearities, inter-segmental interaction torques etc.), which have to be dealt with by the nervous system, involving sensory inputs and associated reflexes and possibly internal models.
Internal Models. While the concept of internal models has found support as a representation of the oculomotor system controlling eye movements (Lisberger 2009), its applicability to much more complicated limb control is still being debated (Loeb 2021). Nonetheless, this concept has been widely used, in particular in regard to cerebellar functions, although little data are available showing how they could be instantiated anatomically and physiologically (Cabaraux et al. 2020). How, for example, would the spinal interneuronal circuits in all their intricacies (Windhorst 2021a) fit into a scheme of internal model transforming cortical commands into muscle forces and movements? “The addition of intermediary circuitry between command and motor output plus the integration of sensory feedback in that intermediary circuitry result in two important effects on the motor control problem. First, this essentially precludes the development of an internal model of the plant, much less an inversion of this model by which to compute optimal command signals. If the brain had internal models of the plant that it was trying to control, those models would have to include all spinal interneurons and their connectivity to the motor pools and each other as well as the mechanical dynamics of the musculoskeletal system itself” (Loeb 2021).
Neural-network Concepts. Ultimately, then, one appears to be left with the neural-network concept that the operative neuronal circuits are forced to learn their tasks with some support from inherited substrates and functions (such as the CPG) (Loeb 2021). While these networks show much random connectivity, this does not imply that they could not contain some prominent routes, such as, at spinal level, muscle spindle afferent to MNs connections, reciprocal Ia inhibition between antagonist MN pools, or the connections linking left and right CPGs (Maxwell and Soteropoulos 2020). It should be noted that internal models are also neuronal networks, so there is no excluding dichotomy between the two concepts. Finally, at brainstem level, the oculomotor neural integrator can be modelled as a neural network (Arnold and Robinson 1997).
Variability and Adaptation. It has been argued in Sect 11.2 that motor variability may be advantageous for exploring opportunities in motor space. It has been proposed (Cai et al. 2006) that changes in the physiological states underlie variability in the limb kinematics during, for example, stepping under normal conditions, resulting from “...rapid, and sometimes persistent, changes in functional connectivity between a given combination of spatially and temporally linked sensory and motor circuits that are involved in the generation of posture and locomotion”. This is even more important during and after the `re-organization´ of the spinal and other circuitries following CNS lesions like SCI (Cai et al. 2006; Windhorst and Dibaj 2023).
Diseases as Probes. The complexity of the neuromuscular system makes it prone to diseases, which come in a large variety (genetics, autoimmune processes, degenerations, infections, lesions) and emphasize its vulnerability. We have here tried to order them according to some main notions often used in motor control in order to show their loci of aggression, which may give rise to diverse symptoms and, again, reveal system complexity. Very much like muscle fatigue may be used as an investigative tool in motor control (Monjo et al. 2015), so diseases and their effects could be seen as probes into normal functioning. This approach has been quite fruitful and has opened insights into the versatile and intricate functions of diverse neuronal networks involved in motor control. But, what then is `motor control´? What controls what? The more details we know, the less a hierarchical scheme with a top controller and downstream slaves appears likely. “Ignoramus et ignorabimus” (Du Bois-Reymond 1872).
Author Contributions
Both authors wrote parts of the text, prepared the figures, searched and provided literature, revised and edited the manuscript, and approved the final manuscript.
Funding
This research received no external funding.
Acknowledgements
UW is grateful to his wife for her indulgence and patience.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5-HT |
5-hydroxy-tryptophan, serotonin |
| ACC |
anterior cingulate cortex |
| ALS |
amyotrophic lateral sclerosis |
| α-MN |
α-motoneuron (innervating skeletal muscle fibers) |
| ANN |
artificial neural network |
| ASD |
acute stress disorder |
| ß-MN |
ß-motoneuron (innervating both skeletal and intrafusal muscle fibers) |
| BG |
basal ganglia |
| CMT1A |
Charcot-Marie-Tooth type 1A |
| CNS |
central nervous system |
| COM |
center of mass |
| CPG |
central pattern generator |
| CST |
cortico-spinal tract |
| DA |
dopamine |
| DNP |
diabetic neuropathy |
| DSPN |
diabetic symmetrical polyneuropathy |
| EMG |
electromyographic, electromyography |
| γ-MN |
γ-motoneuron (innervating intrafusal muscle fibers of muscle spindles) |
| GTO |
Golgi tendon organ |
| HD |
Huntington´s disease |
| LLR |
long-latency reflex |
| LTD |
long-term depression |
| LTP |
long-term potentiation |
| MI |
primary motor cortex |
| MLR |
medium-latency reflex |
| MN |
motoneuron |
| MS |
multiple sclerosis |
| NA |
noradrenaline |
| PD |
Parkinson´s disease |
| PFC |
prefrontal cortex |
| PM |
premotor cortex |
| PNS |
peripheral nervous system |
| PPC |
posterior parietal cortex |
| PPS |
peri-personal space |
| PTSD |
post-traumatic stress disorder |
| RuST |
rubro-spinal tract |
| SCI |
spinal cord injury |
| SHV |
subjective haptic vertical |
| SLR |
short-latency reflex |
| SMA |
spinal muscular atrophy |
| SMN |
survival motoneuron |
| SPV |
subjective postural vertical |
| SVV |
subjective visual vertical |
References
- Akay, T.; Tourtellotte, W.G.; Arber, S.; Jessell, T.M. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci USA 2014, 111, 16877–16882. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H. Motor neuropathy. Handb Clin Neurol 2014, 126, 81–95. [Google Scholar] [PubMed]
- Arbib, M.A.; Bonaiuto, J.B.; Jacobs, S.; Frey, S.H. Tool use and the distalization of the end-effector. Psychol Res 2009, 73, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.B.; Robinson, D.A. The oculomotor integrator: testing of a neural network model. Exp Brain Res 1997, 113, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Ashe, J.; Bushara, K. The olivo-cerebellar system as a neural clock. Adv Exp Med Biol 2014, 829, 155–165. [Google Scholar] [PubMed]
- Avanzino, L.; Pelosin, E.; Vicario, C.M.; Lagravinese, G.; Abbruzzese, G.; Martino, D. Time processing and motor control in movement disorders. Front Hum Neurosci 2016, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: mechanisms and immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, R.; Haegens, S.; Jazayeri, M.; Merchant, H.; Sternad, D.; Song, J.-H. Neural encoding and representation of time for sensorimotor control and learning. J Neurosci 2021, 41, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Bareš, M.; Apps, R.; Avanzino, L.; Breska, A.; D'Angelo, E.; Filip, P.; Gerwig, M.; Ivry, R.B.; Lawrenson, C.L.; Louis, E.D.; et al. Consensus paper: Decoding the contributions of the cerebellum as a time machine. From neurons to clinical applications. Cerebellum 2019, 18, 266–286. [Google Scholar] [CrossRef]
- Barra, J.; Marquer, A.; Joassin, R.; Reymond, C.; Metge, L.; Chauvineau, V.; Pérennou, D. Humans use internal models to construct and update a sense of verticality. Brain 2010, 133 Pt 12, 3552–3563. [Google Scholar] [CrossRef]
- Bastian, A.J.; Martin, T.A.; Keating, J.G.; Thach, W.T. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 1996, 76, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.J.; Zackowski, K.M.; Thach, W.T. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol 2000, 83, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Baumann, J. The classification of the diseases of the extrapyramidal system. Acta Neurol Scand Suppl 1963, 39, 102–107. [Google Scholar] [CrossRef]
- Berlucchi, G.; Aglioti, S. The body in the brain revisited. Exp Brain Res 2010, 200, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Okamura, A.M.; Bastian, A.J. Predictive modeling by the cerebellum improves proprioception. J Neurosci 2013, 33, 14301–14306. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Okamura, A.M.; Bastian, A.J. Predicting and correcting ataxia using a model of cerebellar function. Brain 2014, 137, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Biran, I.; Chatterjee, A. Alien hand syndrome. Arch Neurol 2004, 61, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Blouin, J.; Bard, C.; Teasdale, N.; Paillard, J.; Fleury, M.; Forget, R.; Lamarre, Y. Reference systems for coding spatial information in normal subjects and a deafferented patient. Exp Brain Res 1993, 93, 324–331. [Google Scholar] [CrossRef]
- Bodranghien, F.; Bastian, A.; Casali, C.; Hallett, M.; Louis, E.D.; Manto, M.; Mariën, P.; Nowak, D.A.; Schmahmann, J.D.; Serrao, M.; et al. Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cebellum 2016, 15, 369–391. [Google Scholar] [CrossRef]
- Bourane, S.; Grossmann, K.S.; Britz, O.; Dalet, A.; Del Barrio, M.G.; Stam, F.J.; Garcia-Campmany, L.; Koch, S.; Goulding, M. Identification of a spinal circuit for light touch and fine motor control. Cell 2015, 160, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Boven, E.; Cerminara, N. Cerebellar contributions across behavioural timescales: a review from the perspective of cerebro-cerebellar interactions. Front Syst Neurosci 2023, 17, 1211530. [Google Scholar] [CrossRef] [PubMed]
- Breska, A.; Ivry, R.B. Taxonomies of timing: Where does the cerebellum fit in? Curr Opin Behav Sci 2016, 8, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, A.M. The interaction of otolith and proprioceptive information in the perception of verticality. The effects of labyrinthine and CNS disease. Ann NY Acad Sci 1999, 871, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Brownstone, R.M.; Bui, T.V.; Stifani, N. Spinal circuits for motor learning. Curr Opin Neurobiol 2015, 33, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.V.; Stifani, N.; Panek, I.; Farah, C. Genetically identified spinal interneurons integrating tactile afferents for motor control. J Neurophysiol 2015, 114, 3050–3063. [Google Scholar] [CrossRef] [PubMed]
- Butcher, P.A.; Ivry, R.B.; Kuo, S.-H.; Rydz, D.; Krakauer, J.W.; Taylor, J.A. The cerebellum does more than sensory prediction error-based learning in sensorimotor adaptation tasks. J Neurophysiol 2017, 118, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Butz, M.; Wörgötter, F.; van Ooyen, A. Activity-dependent structural plasticity. Brain Res Rev 2009, 60, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Cabaraux, P.; Gandini, J.; Kakei, S.; Manto, M.; Mitoma, H.; Tanaka, H. Dysmetria and errors in predictions: The role of internal forward model. Int J Mol Sci 2020, 21, 6900. [Google Scholar] [CrossRef]
- Cai, L.L.; Courtine, G.; Fong, A.J.; Burdick, J.W.; Roy, R.R.; Edgerton, V.R. Plasticity of functional connectivity in the adult spinal cord. Philos Trans R Soc Lond B Biol Sci 2006, 361, 1635–1646. [Google Scholar] [CrossRef]
- Camargo, C.H.F.; Teive, H.A.G. Use of botulinum toxin for movement disorders. Drugs Context 2019, 8, 212586. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. Handb Clin Neurol 2018, 159, 237–250. [Google Scholar] [PubMed]
- Cardinali, L.; Brozzoli, C.; Farnè, A. Peripersonal space and body schema: two labels for the same concept? Brain Topogr 2009, 21, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Chesler, A.T.; Szczot, M.; Bharucha-Goebel, D.; Čeko, M.; Donkervoort, S.; Laubacher, C.; Hayes, L.H.; Alter, K.; Zampieri, C.; Stanley, C.; et al. The role of PIEZO2 in human mechanosensation. N Engl J Med 2016, 375, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.; Lundbye-Jensen, J.; Perez, M.A.; Nielsen, J.B. How plastic are human spinal cord motor circuitries? Exp Brain Res 2017, 235, 3243–3249. [Google Scholar] [CrossRef] [PubMed]
- Cléry, J.; Ben Hamed, S. Frontier of self and impact prediction. Front Psychol 2018, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Hohlfeld, R.; Baranzini, S.E. The role of gut microbiota in multiple sclerosis. Nat Rev Neurol 2022, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Coslett, H.B. Evidence for a disturbance of the body schema in neglect. Brain Cogn 1998, 37, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Cotsapas, C.; Mitrovic, M.; Hafler, D. Multiple sclerosis. Handb Clin Neurol 2018, 148, 723–730. [Google Scholar]
- Crochet, S.; Lee, S.-H.; Petersen, C.C.H. Neural circuits for goal-directed sensorimotor transformations. Trends Neurosci 2019, 42, 66–77. [Google Scholar] [CrossRef]
- Cullen, K.E.; Brooks, J.X. Neural correlates of sensory prediction errors in monkeys: Evidence for internal models of voluntary self-motion in the cerebellum. Cerebellum 2015, 14, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Dakin, C.J.; Bolton, D.A.E. Forecast or fall: prediction´s importance to postural control. Front Neurol 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Dakin, C.J.; Peters, A.; Giunti, P.; Day, B.L. Cerebellar dgeneration increases visual influence on dynamic estimates of verticality. Curr Biol 2018, 28, 3589–3598. [Google Scholar] [CrossRef] [PubMed]
- Dakin, C.J.; Rosenberg, A. Gravity estimation and verticality perception. Handb Clin Neurol 2018, 159, 43–59. [Google Scholar] [PubMed]
- D´Angelo, E. Physiology of the cerebellum. Handb Clin Neurol 2018, 154, 85–108. [Google Scholar] [PubMed]
- Danner, S.M.; Shevtsova, N.A.; Frigon, A.; Rybak, I.A. Computational modeling of spinal circuits controlling limb coordination and gaits in quadrupeds. Elife. 2017, 6, e31050. [Google Scholar] [CrossRef] [PubMed]
- Decherchi, P.; Dousset, E. Role of metabosensitive afferent fibers in neuromuscular adaptive mechanisms. Can J Neurol Sci 2003, 30, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Indovina, I.; Zago, M.; Daprati, E.; Lacquaniti, F.; Bosco, G. Watching the effects of gravity. Vestibular cortex and the neural representation of “visual” gravity. Front Integr Neurosci 2021, 15, 793634. [Google Scholar] [CrossRef] [PubMed]
- De Vignemont, F. Body schema and body image--pros and cons. Neuropsychologia 2010, 48, 669–680. [Google Scholar] [CrossRef]
- De Vignemont, F.; Iannetti, G.D. How many peripersonal spaces? Neuropsychologia 2015, 70, 327–334. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Ten Brinke, M.M. Motor learning and the cerebellum. Cold Spring Harb Perspect Biol. 2015, 7, a021683. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, A.K.; Smith, M.A.; Ölveczky, B.P. The role of variability in motor learning. Annu Rev Neurosci 2017, 40, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Di Pellegrino, G.; Làdavas, E. Peripersonal space in the brain. Neuropsychologia 2015, 66C, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Di Vita, A.; Boccia, M.; Palermo, L.; Guariglia, C. To move or not to move, that is the question! Body schema and non-action oriented body representations: An fMRI meta-analytic study. Neurosci Biobehav Rev 2016, 68, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Dominici, N.; Daprati, E.; Nico, D.; Cappellini, G.; Ivanenko, Y.P.; Lacquaniti, F. Changes in the limb kinematics and walking-distance estimation after shank elongation: evidence for a locomotor body schema? J Neurophysiol 2009, 101, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Donchin, O.; Rabe, K.; Diedrichsen, J.; Lally, N.; Schoch, B.; Gizewski, E.R.; Timmann, D. Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol 2012, 107, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Du Bois-Reymond, E.M. Über die Grenzen des Naturerkennens“ (lecture held at a meeting of the Gesellschaft Deutscher Naturforscher und Ärzte (GDNA), Leipzig 1872.
- Edgley, S.A. Organisation of inputs to spinal interneurone populations. J Physiol (Lond) 2001, 533, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ekkert, A.; Šliachtenko, A.; Grigaité, J.; Burnyté, B.; Utkus, A.; Jatužis, D. Ischemic stroke genetics: What is new and how to apply it in clinical practice? Genes (Basel) 2021, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Enander, J.M.D.; Jones, A.M.; Kirkland, M.; Hurless, M.; Jörntell, H.; Loeb, G.E. A model for self-organization of sensorimotor function: the spinal monosynaptic loop. J Neurophysiol 2022, 127, 1460–1477. [Google Scholar] [CrossRef]
- Enander, J.M.D.; Loeb, G.E.; Jörntell, H. A model for self-organization of sensorimotor function: spinal interneuronal integration. J Neurophysiol 2022, 127, 1478–1495. [Google Scholar] [CrossRef]
- Fahn, S. Classification of movement disorders. Mov Disord 2011, 26, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Mazzoni, A.; Falotico, E. Reaching and grasping movements in Parkinson’s disease: a review. J Parkinsons Dis 2022, 12, 1083–1113. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, G.; Thoumie, P.; Do, -C. ; Schieppati, M. Cutaneous and muscular afferents from the foot and sensory fusion processing: Physiology and pathology in neuropathies. J Peripher Nerv Syst 2021, 26, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, F.; Ardizzi, M.; Magnani, F.; Ferri, F.; Langiulli, N.; Rastelli, F.; Lucarini, V.; Giustozzi, F.; Volpe, R.; Marchesi, C.; et al. Tool-use extends peripersonal space boundaries in schizophrenic patients. Schizophr Bull 2022, 48, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Gackière, F.; Vinay, L. Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord. Front Neural Circuits 2014, 8, 102. [Google Scholar] [PubMed]
- Gallagher, S.; Cole, J. Body image and body schema in a deafferented subject. J Mind Behav 1995, 16, 369–389. [Google Scholar]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Ghez, C.; Sainburg, R. Proprioceptive control of interjoint coordination. Can J Physiol Pharmacol 1995, 73, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Pearse, D.D. The role of the serotonergic system in locomotor recovery after spinal cord injury. Front Neural Circuits 2015, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.; Dieterich, M.; Brandt, T. Computational neurology of gravity perception involving semicircular canal dysfunction in unilateral vestibular lesions. Prog Brain Res 2019, 248, 303–317. [Google Scholar]
- Gorassini, M.; Prochazka, A.; Taylor, J.L. Cerebellar ataxia and muscle spindle sensitivity. J Neurophysiol 1993, 70, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Granit, R. The basis of motor control. Academic Press, London New York, 1970.
- Grau, J.W. Learning from the spinal cord: How the study of spinal cord plasticity informs our view of learning. Neurobiol Learning Memory 2014, 108, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.J.; Ladouceur, M.; Andersen, J.B.; Nielsen, J.B.; Sinkjaer, T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol 2001, 534 Pt 3, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.J.; Mazzaro, N.; Nielsen, J.B.; Sinkjaer, T. Ankle extensor proprioceptors contribute to the enhancement of the soleus EMG during the stance phase of human walking. Can J Physiol Pharmacol 2004, 82, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Griener, A.; Dyck, J.; Gosgnach, S. Regional distribution of putative rhythm-generating and pattern-forming components of the mammalian locomotor CPG. Neuroscience 2013, 250, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; El Manira, A. Current principles of motor control, with special reference to vertebrate locomotion. Physiol Rev 2020, 100, 271–320. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Kozlov, A. The CPGs for limbed locomotion - facts and fiction. Int J Mol Sci 2021, 22, 5882. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, K.G.; Pearson, K.T. Diagnosis and management of sensory polyneuropathy. BMJ 2019, 365, I1108. [Google Scholar] [CrossRef] [PubMed]
- Haggie, L.; Schmid, L.; Röhrle, O.; Besier, T.; McMorland, A.; Saini, H. Linking cortex and contraction - Integrating models along the corticomuscular pathway. Front Physiol 2023, 14, 1095260. [Google Scholar] [CrossRef]
- Harris, L.R.; Carnevale, M.J.; D´Amour, S.; Fraser, L.E.; Harrar, V.; Hoover, A.E.N.; Mander, C.; Pritchett, L.M. How our body influences our perception of the world. Front Psychol 2015, 6, 819. [Google Scholar] [CrossRef]
- Honda, T.; Nagao, S.; Hashimoto, Y.; Ishikawa, K.; Yokota, T.; Mizusawa, H.; Ito, M. Tandem internal models execute motor learning in the cerebellum. Proc Natl Acad Sci U S A 2018, 115, 7428–7433. [Google Scholar] [CrossRef] [PubMed]
- Hossner, E.-J.; Zahno, S. Beyond task-space exploration: On the role of variance for motor control and learning. Front Psychol 2022, 13, 935273. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.L. Representational gravity: Empirical findings and theoretical implications. Psychon Bull Rev 2020, 27, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Hull, C. Prediction signals in the cerebellum: beyond supervised motor learning. Elife 2020, 9, e54073. [Google Scholar] [CrossRef] [PubMed]
- Ioffe, M.E.; Chernikova, L.A.; Ustinova, K.I. Role of cerebellum in learning postural tasks. Cerebellum 2007, 6, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Imamizu, H.; Kawato, M. Cerebellar internal models: implications for the dexterous use of tools. Cerebellum 2012, 11, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Tomatsu, S.; Izawa, J.; Kakei, S. The cerebro-cerebellum: Could it be the loci of forward models? Neurosci Res 2016, 104, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Dominici, N.; Daprati, E.; Nico, D.; Cappellini, G.; Lacquaniti, F. Locomotor body scheme. Hum Mov Sci 2011, 30, 341–451. [Google Scholar] [CrossRef] [PubMed]
- Ivaneko, Y.; Gurfinkel, V.S. Human postural control. Front Neurosci 2018, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Bressman, S.; Dauer, W.; Kang, U.J. Clinical and scientific perspectives on movement disorders: Stanley Fahn’s contributions. Mov Disord 2015, 30, 1862–1869. [Google Scholar] [CrossRef]
- Jankowska, E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 1992, 38, 335–378. [Google Scholar] [CrossRef] [PubMed]
- Jax, S.A.; Coslett, H.B. Disorders of the perceptual-motor system. Adv Exp Med Biol 2009, 629, 377–391. [Google Scholar] [PubMed]
- Jayasinghe, S.A.L.; Sarlegna, F.R.; Scheidt, R.A.; Sainburg, R.L. Somatosensory deafferentation reveals lateralized roles of proprioception in feedback and adaptive feedforward control of movement and posture. Curr Opin Physiol 2021, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Djupsjöbacka, M. Sjölander Influences on the gamma-muscle spindle system from muscle afferents stimulated by KCl and lactic acid. Neurosci Res 1993, 16, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kalezic, I.; Bugaychenko, L.A.; Kostyukov, A.I.; Pilyavskii, A.I.; Ljubisavljevid, M.; Windhorst, U.; Johansson, H. Fatigue-related depression of the feline monosynaptic gastrocnemius-soleus reflex. J Physiol 2004, 556 Pt 1, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Karnath, H.O.; Johanssen, L.; Broetz, D.; Küker, W. Posterior thalamic hemorrhage induces "pusher syndrome". Neurology 2005, 64, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Avraham, G.; Ivry, R.B. The psychology of reaching: action selection, movement implementation, and sensorimotor learning. Annu Rev Psychol 2021, 72, 61–95. [Google Scholar] [CrossRef]
- Kniffki, K.D.; Schomburg, E.D.; Steffens, H. Synaptic effects from chemically activated fine muscle afferents upon alpha-motoneurones in decerebrate and spinal cats. Brain Res 1981, 206, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kornysheva, K. Encoding temporal features of skilled movements - what, whether and how? Adv Exp Med Biol 2016, 957, 35–54. [Google Scholar]
- Krakauer, J.W.; Ghilardi, M.F.; Ghez, C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 1999, 2, 1026–1031. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Hadjiosif, A.M.; Xu, J.; Wong, A.L.; Haith, A.M. Motor learning. Compr Physiol 2019, 9, 613–663. [Google Scholar] [PubMed]
- Krakauer, J.W.; Mazzoni, P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 2011, 21, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, F.; Borghese, N.A.; Carrozzo, M. Internal models of limb geometry in the control of hand compliance. J Neurosci 1992, 12, 1750–1762. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, F.; Bosco, G.; Gravano, S.; Indovina, I.; La Scaleia, B.; Maffei, V.; Zago, M. Gravity in the brain as a reference for space and time perception. Multisens Res 2015, 28, 397–426. [Google Scholar] [CrossRef] [PubMed]
- La Scaleia, B.; Zago, M.; Lacquaniti, F. Hand interception of occluded motion in humans: a test of model-based vs. on-line control. J Neurophysiol 2015, 114, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Laurin, J.; Pertici, V.; Doucet, E.; Marqueste, T.; Decherchi, P. Group III and IV muscle afferents: role on central motor drive and clinical implications. Neuroscience 2015, 290, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lawrenson, C.; Bares, M.; Kamondi, A.; Kovács, A.; Lumb, B.; Apps, R.; Filip, P.; Manto, M. The mystery of the cerebellum: clues from experimental and clinical observations. Cerebellar Ataxias 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Hong, S.J.; Baxter, T.; Scott, J.; Shenoy, S.; Buck, L.; Bodenheimer, B.; Park, S. Altered peripersonal space and the bodily self in schizophrenia: a virtual reality study. Schizophr Bull 2021, 47, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Lisberger, S.G. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience 2009, 162, 763–776. [Google Scholar] [CrossRef]
- Loeb, G.E. Learning to use muscles. J Hum Kinet 2021, 76, 9–33. [Google Scholar] [CrossRef]
- Lord, S.R. Proprioception: effect of aging. In: Binder MD, Hirokawa N, Windhorst U (eds) Encyclopedia of neuroscience. 2009. [Google Scholar]
- Macaluso, E.; Maravita, A. The representation of space near the body through touch and vision. Neuropsychologia 2010, 48, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Makin, T.R.; Holmes, N.P.; Ehrsson, H.H. On the other hand: Dummy hands and peripersonal space. Behav Brain Res 2008, 191, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Inness, E.L.; Mcilroy, W.E. Stroke. Handb Clin Neurol 2018, 159, 205–228. [Google Scholar] [PubMed]
- Manto, M.; Serrao, M.; Castiglia, S.F.; Timmann, D.; Tzvi-Tinker, E.; Pan, M.-K.; Kuo, S.-H.; Ugawa, Y. Neurophysiology of cerebellar ataxias and gait disorders. Clin Neurophysiol Pract 2023, 8, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.; Cardinali, L.; Roy, A.C.; Farnè, A. Tool-use: An open window into body representation and its plasticity. Cogn Neuropsychol 2016, 33, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Delivet-Mongrain, H.; Leblond, H.; Rossignol, S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J Neurophysiol 2011, 106, 1969–1984. [Google Scholar] [CrossRef]
- Mathis, S.; Duval, F.; Soulages, A.; Solé, G.; Le Masson, G. The ataxic neuropathies. J Neurol 2021, 268, 3675–3689. [Google Scholar] [CrossRef]
- Maxwell, D.J.; Soteropoulos, D.S. The mammalian spinal commissural system: properties and functions. J Neurophysiol 2020, 123, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, N.; Grey, M.J.; Sinkjaer, T.; Andersen, J.B.; Pareyson, D.; Schieppati, M. Lack of on-going adaptations in the soleus muscle activity during walking in patients affected by large-fiber neuropathy. J Neurophysiol 2005, 93, 3075–3085. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, N.; Nielsen, J.F.; Grey, M.J.; Sinkjaer, T. Decreased contribution from afferent feedback to the soleus muscle during walking in patients with spastic stroke. J Stroke Cerebrovasc Dis 2007, 16, 135–144. [Google Scholar] [CrossRef]
- McCrea, D.A.; Rybak, I.A. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 2008, 57, 134–146. [Google Scholar] [CrossRef] [PubMed]
- McDougle, S.D.; Ivry, R.B.; Taylor, J.A. Taking aim at the cognitive side of learning in sensorimotor adaptation atsks. Trends Cogn Sci 2016, 20, 535–544. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and treatment of multiple sclerosis: A review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- McNamee, D.; Wolpert, D.M. Internal models in biological control. Annu Rev Control Robot Auton Syst 2019, 2, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Merchant, H.; Grahn, J.; Trainor, L.; Rohrmeier, M.; Fitch, W.T. Finding the beat: a neural perspective across humans and non-human primates. Philos Trans R Soc Lond B Biol Sci 2015, 370, 20140093. [Google Scholar] [CrossRef] [PubMed]
- Monjo, F.; Terrier, R.; Forestier, N. Muscle fatigue as an investigative tool in motor control: A review with new insights on internal models and posture-movement coordination. Hum Mov Sci 2015, 44, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kim, J.; Seong, Y.; Suh, B.-C.; Kang, K.J.; Choe, H.K.; Kim, K. Proprioception, the regulator of motor function. BMB Rep 2021, 54, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mul, C.L.; Cardini, F.; Stagg, S.D.; Sadeghi Esfahlani, S.; Kiourtsoglou, D.; Cardellicchio, P.; Aspell, J.E. Altered bodily self-consciousness and peripersonal space in autism. Autism 2019, 23, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Murala, S.; Nagarajan, E.; Bolln, P.C. Infectious causes of ctroke. J Stroke Cerebrovasc Dis 2022, 31, 106274. [Google Scholar] [CrossRef]
- Muramatsu, K. Diabetes mellitus-related dysfunction of the motor system. Int J Mol Sci 2020, 21, 7485. [Google Scholar] [CrossRef]
- Nardone, A.; Corna, S.; Turcato, A.M.; Schieppati, M. Afferent control of walking: are there distinct deficits associated to loss of fibres of different diameter? Clin Neurophysiol 2014, 125, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, R.B.; Nissen, T.D.; Mørch, C.D.; Meldgaard, T.; Juhl, A.H.; Jakobsen, P.E.; Karmisholt, J.; Brock, B.; Drewes, A.M.; Brock, C. Diabetic neuropathy influences control of spinal mechanisms. J Clin Neurophysiol 2021, 38, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Noel, J.P.; Blanke, O.; Serino, A. From multisensory integration in peripersonal space to bodily self-consciousness: from statistical regularities to statistical inference. Ann N Y Acad Sci 2018, 1426, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.A.; Topka, H.; Timmann, D.; Boecker, H.; Hermsdörfer, J. The role of the cerebellum for predictive control of grasping. Cerebellum 2007, 6, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Obrero-Gaitán, E.; Molina, F.; Montilla-Ibañez, M.-d.e.-A.; Del-Pino-Casado, R.; Rodriguez-Almagro, D.; Lomas-Vega, R. Misperception of visual vertical in peripheral vestibular disorders. A systematic review with meta-analysis. Laryngoscope 2021, 131, 1110–1121. [Google Scholar] [PubMed]
- Oaklander, A.L.; Nolano, M. Scientific advances in and clinical approaches to small-fiber Polyneuropathy, a review. JAMA Neurol 2019, 76, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Otten, E. Inverse and forward dynamics: models of multi-body systems. Philos Trans R Soc Lond B Biol Sci 2003, 358, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The plastic human brain cortex. Annu Rev Neurosci 2005, 28, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Passot, J.-B.; Luque, N.R.; Arleo, A. Coupling internal cerebellar models enhances online adaptation and supports offline consolidation in sensorimotor tasks. Front Comput Neurosci 2013, 7, 95. [Google Scholar] [CrossRef]
- Payne, A.M.; Sawers, A.; Allen, J.L.; Stapley, P.J.; Mcpherson, J.M.; Ting, L.H. Reorganization of motor modules for standing reactive balance recovery following pyridoxine-induced large-fiber peripheral sensory neuropathy in cats. J Neurophysiol 2020, 124, 868–882. [Google Scholar] [CrossRef]
- Pérennou, D.; Piscicelli, C.; Barbieri, G.; Jaeger, M.; Marquer, A.; Barra, J. Measuring verticality perception after stroke: why and how? Neurophysiol Clin 2014, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Petter, E.A.; Lusk, N.A.; Hesslow, G.; Meck, W.H. Interactive roles of the cerebellum and striatum in sub-second and supra-second timing: Support for an initiation, continuation, adjustment, and termination (ICAT) model of temporal processing. Neurosci Biobehav Rev 2016, 71, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.S.; Ebner, T.J. Cerebellum, predictions and errors. Front Cell Neurosci 2019, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, A.; Clarac, F.; Loeb, G.E.; Rothwell, J.C.; Wolpaw, J.R. What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res 2000, 130, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 2012, 92, 1651–1697. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Livne, O.; Gizewski, E.R.; Aurich, V.; Beck, A.; Timmann, D.; Donchin, O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 2009, 101, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Rabellino, D.; Frewen, P.A.; McKinnon, M.C.; Lanius, R.A. Peripersonal Space and Bodily Self-Consciousness: Implications for Psychological Trauma-Related Disorders. Front Neurosci 2020, 14, 586605. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Mazzocchio, R.; Decchi, B. Effect of chemically activated fine muscle afferents on spinal recurrent inhibition in humans. Clin Neurophysiol 2003, 114, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Rost, K.; Nowak, D.A.; Timmann, D.; Hermsdörfer, J. Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clin Neurophysiol 2005, 116, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, M.; Honoré, J.; Saj, A. Body representations and brain damage. Neurophysiol Clin 2014, 44, 59–67. [Google Scholar] [CrossRef]
- Said, G. Diabetic neuropathy--a review. Nat Clin Pract Neurol 2007, 3, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Sanaye, M.M.; Kavishwar, S.A. Diabetic neuropathy: review on molecular mechanisms. Curr Mol Med 2023, 23, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Sattin, D.; Parma, C.; Zulueta, A.; Lanzone, J.; Giani, L.; Vassalo, M.; Picozzi, M.; Parati, E.A. An overview of the body schema and body image: Theoretical models, methodological settings and pitfalls for rehabilitation of persons with neurological disorders. Brain Sci 2023, 13, 1410. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, E.D. Spinal sensorimotor systems and their supraspinal control. Neurosci Res 1990, 7, 265–340. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, E.D.; Dibaj, P.; Steffens, H. Differentiation between Aδ and C fibre evoked nociceptive reflexes by TTX resistance and opioid sensitivity in the cat. Neurosci Res 2011, 69, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, E.D.; Kalezic, I.; Dibaj, P.; Steffens, H. Reflex transmission to lumbar α-motoneurones in the mouse similar and different to those in the cat. Neurosci Res 2013, 76, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, E.D.; Steffens, H.; Dibaj, P.; Sears, T.A. Major contribution of Aδ-fibres to increased reflex transmission in the feline spinal cord during acute muscle inflammation. Neurosci Res 2012, 72, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, E.D.; Steffens, H.; Pilyavskii, A.I.; Maisky, V.A.; Brück, W.; Dibaj, P.; Sears, T.A. Long lasting activity of nociceptive muscular afferents facilitates bilateral flexion pattern in the feline spinal cord. Neurosci Res 2015, 95, 95–51. [Google Scholar] [CrossRef] [PubMed]
- Schouenborg, J. Learning in sensorimotor circuits. Curr Opin Neurobiol 2004, 14, 693–697. [Google Scholar] [CrossRef]
- Sène, D. Small fiber neuropathy: Diagnosis, causes, and treatment. Joint Bone Spine 2018, 85, 553–559. [Google Scholar] [CrossRef]
- Serino, A. Peripersonal space (PPS) as a multisensory interface between the individual and the environment, defining the space of the self. Neurosci Biobehav Rev 2019, 99, 138–159. [Google Scholar] [CrossRef] [PubMed]
- Shadrach, J.L.; Gomez-Frittelli, J.; Kaltschmidt, J.A. Proprioception revisited: where do we stand? Curr Opin Physiol 2021, 21, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Shidara, M.; Kawano, K.; Gomi, H.; Kawato, M. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature 1993, 365, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Shmuelof, L.; Krakauer, J.W. Are we ready for a natural history of motor learning? Neuron 2011, 72, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Sian, J.; Gerlach, M.; Youdim, M.B.; Riederer, P. Parkinson’s disease: a major hypokinetic basal ganglia disorder. J Neural Transm (Vienna) 1999, 106, 443–476. [Google Scholar] [CrossRef] [PubMed]
- Sinkjaer, T.; Andersen, J.B.; Nielsen, J.F.; Hansen, H.J. Soleus long-latency stretch reflexes during walking in healthy and spastic humans. Clin Neurophysiol 1999, 110, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Soetedjo, R.; Horwitz, G. Closed-loop optogenetic perturbation of macaque oculomotor cerebellum: evidence for an internal saccade model. J Neurosci 2023, 44, e1317232023. [Google Scholar] [CrossRef] [PubMed]
- Sopacua, M.; Hoeijmakers, J.G.J.; Merkies, I.S.J.; Lauria, G.; Waxman, S.G.; Faber, C.G. Small-fiber neuropathy: Expanding the clinical pain universe. J Peripher Nerv Syst 2019, 24, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.; Celnik, P. Multiple motor learning processes in humans: Defining their neurophysiological bases. Neuroscientist 2021, 27, 246–267. [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The neuromodulator of long-term synaptic plasticity, reward and movement bontrol. Cells 2021, 0, 735. [Google Scholar] [CrossRef]
- Stapley, P.J.; Ting, L.H.; Hulliger, M.; Macpherson, J.M. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci 2002, 22, 5803–5807. [Google Scholar] [CrossRef] [PubMed]
- Stenimahitis, V.; Fletcher-Sandersjöö, A.; El-Hajj, V.G.; Hultling, C.; Andersson, M.; Sveinsson, O.; Elmi-Terander, A.; Edström, E. Long-term outcomes after periprocedural and spontaneous spinal cord infarctions: A population-based cohort study. Neurology 2023, 101, e114–e124. [Google Scholar] [CrossRef] [PubMed]
- Streng, M.L.; Popa, L.S.; Ebner, T.J. Cerebellar representations of errors and internal models. Cerebellum 2022, 21, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ishikawa, T.; Lee, J.; Kakei, S. The cerebro-cerebellum as a locus of forward model: A review. Front Syst Neurosic 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Tarnutzer, A.A.; Wichmann, W.; Straumann, D.; Bockisch, C.J. The cerebellar nodulus: perceptual and ocular processing of graviceptive input. Ann Neurol 2015, 77, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Ivry, R.B. Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog Brain Res 2014, 210, 217–253. [Google Scholar] [PubMed]
- Taylor, J.L. Movement sense. In: Binder MD, Hirokawa N, Windhorst U (eds) Encyclopedia of neuroscience. 2009. [Google Scholar]
- Terkelsen, A.J.; Karlsson, P.; Lauria, G.; Freeman, R.; Finnerup, N.B.; Jensen, T.S. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 2017, 16, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.S.; Bastian, A.J. Cerebellar damage impairs internal predictions for sensory and motor function. Curr Opin Neurobiol 2015, 33, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.S.; Bastian, A.J. The cerebellum as a movement sensor. Neurosci Lett 2019, 688, 37–40. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Tsay, A.; Allen, T.J.; Proske, U.; Giummarra, M.J. Sensing the body in chronic pain: A review of psychophysical studies implicating altered body representation. Neurosci Biobehav Rev 2015, 52, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Tsianos, G.A.; Loeb, G.E. Muscle and limb mechanics. Compr Physiol 2017, 7, 429–462. [Google Scholar] [PubMed]
- Tuthill, J.C.; Azim, E. Proprioception. Curr Biol 2018, 28, R194–R203. [Google Scholar] [CrossRef] [PubMed]
- Van der Stoep, N.; Nijboer, T.C.W.; van der Stigchel, S.; Spence, C. Multisensory interactions in the depth plane in front and rear space: a review. Neuropsychologia 2015, 70, 335–349. [Google Scholar] [CrossRef] [PubMed]
- White, O.; Graveau, J.; Bringoux, L.; Crevecoeur, F. The gravitational imprint on sensorimotor planning and control. J Neurophysiol 2020, 124, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Windhorst, U. Spinal cord circuits: models and reality. Neurophysiology 2021, 53, 142–222. [Google Scholar] [CrossRef]
- Windhorst, U. Sensomotion – Sensory-motor systems. Future Worlds Center, Nicosia, Cyprus, 2021.
- Windhorst, U. Dibaj P Plastic spinal motor circuits in health and disease. J Integr Neurosci 2023, 22, 22–167. [Google Scholar] [CrossRef] [PubMed]
- Windhorst, U.; Meyer-Lohmann, J.; Kirmayer, D.; Zochodne, D. Renshaw cell responses to intra-arterial injection of muscle metabolites into cat calf muscles. Neurosci Res 1997, 27, 27–235. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R. The education and re-education of the spinal cord. Prog Brain Res 2006, 157, 157–261. [Google Scholar]
- Wolpert, D.M.; Ghahramani, Z.; Flanagan, J.R. Perspectives and problems in motor learning. Trends Cogn Sci 2001, 5, 487–494. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Miall, R.C.; Kawato, M. Internal models in the cerebellum. Trends Cogn Sci 1998, 2, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.N. New roles for dopamine in motor skill acquisition: lessons from primates, rodents, and songbirds. J Neurophysiol 2021, 125, 2361–2374. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Sakurai, Y. Spike-coding mechanisms of cerebellar temporal processing in classical conditioning and voluntary movements. Cerebellum 2014, 13, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lisberger, S.G. Role of plasticity at different sites across the time course of cerebellar motor learning. J Neurosci 2014, 4, 7077–7090. [Google Scholar] [CrossRef]
- Yavari, F.; Mahdavi, S.; Towhidkhah, F.; Ahmadi-Pajouh, M.-A.; Ekhtiari, H.; Darainy, M. Cerebellum as a forward but not inverse model in visuomotor adaptation task: a tDCS-based and modeling study. Exp Brain Res 2016, 234, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Yew, K.S.; Cheng, E.M. Diagnosis of acute stroke. Am Fam Physician 2015, 91, 528–536. [Google Scholar] [PubMed]
- Yousif, N.; Cole, J.; Rothwell, J.; Diedrichsen, J. Proprioception in motor learning: lessons from a deafferented subject. Exp Brain Res 2015, 233, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Zajac, F.E.; Gordon, M.E. Determining muscle´s force and action in multi-articular movement. Exerc Sport Sci Rev 1989, 17, 187–230. [Google Scholar] [CrossRef]
- Zhong, G.; Shevtsova, N.A.; Rybak, I.A.; Harris-Warrick, R.M. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol (Lond) 2012, 590, 4735–4759. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).