Submitted:
28 March 2024
Posted:
29 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Induction of Prediabetes
2.3. Oral Glucose Tolerance Response
2.4. Mating
2.5. Male Pups Were Collected for the Study
2.6. Blood Collection and Tissue Harvesting
2.7. Biochemical Analysis
2.8. Glucocorticoid Receptor and Mineralocorticoid Receptor Gene Expression via Real-Time- PCR
2.9. Statistical Analysis
3. Results
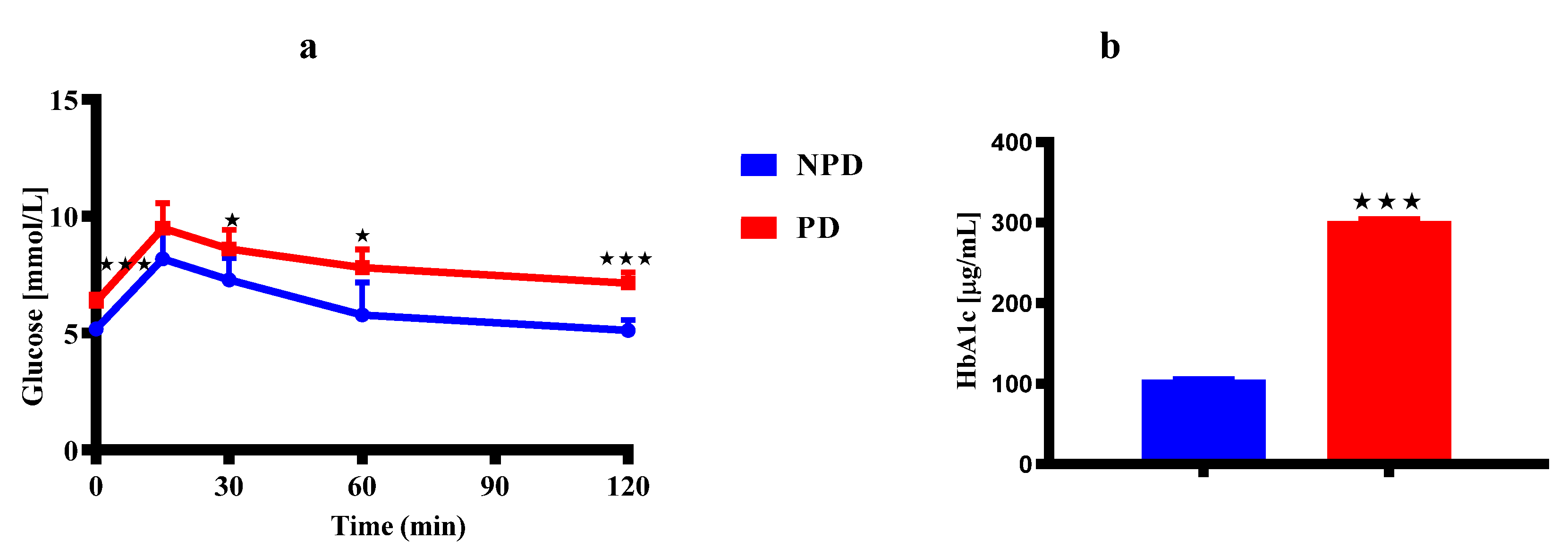
3.1. Dams Oral Glucose Tolerance (OGT) Response and Glycated Hemoglobin (HbA1c) Concentrations
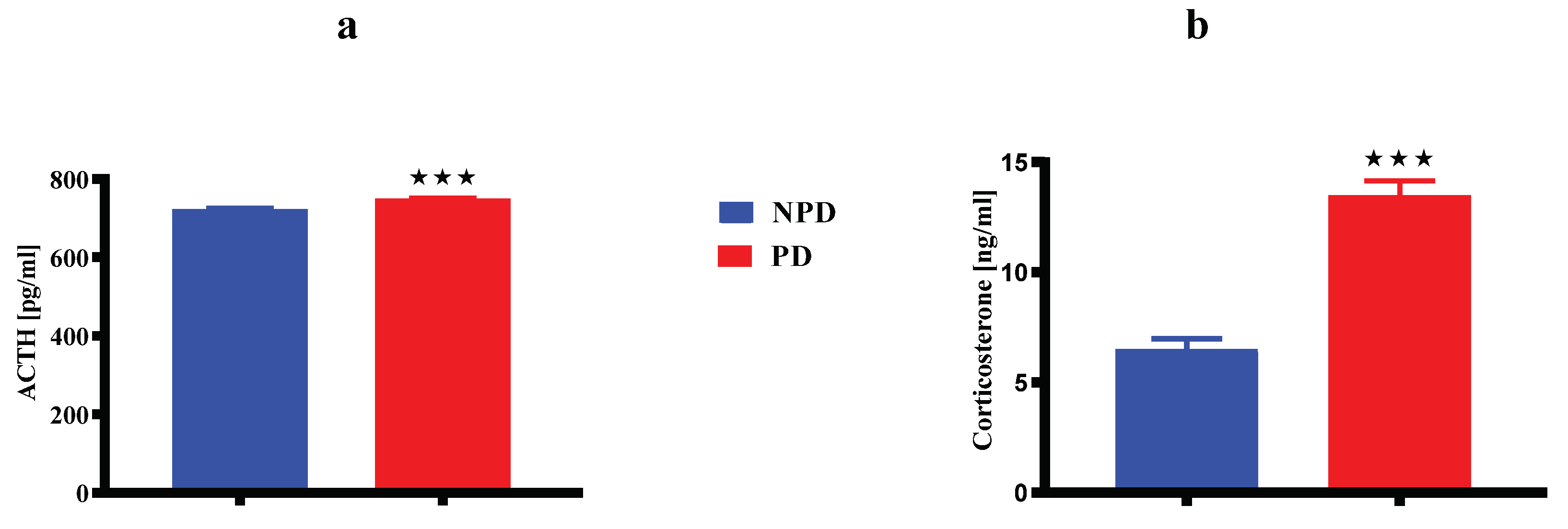
3.2. Dams Hypothalamic-Pituitary-Adrenal (HPA) Axis Components
3.3. Pups Hypothalamic-Pituitary-Adrenal (HPA) Axis Components
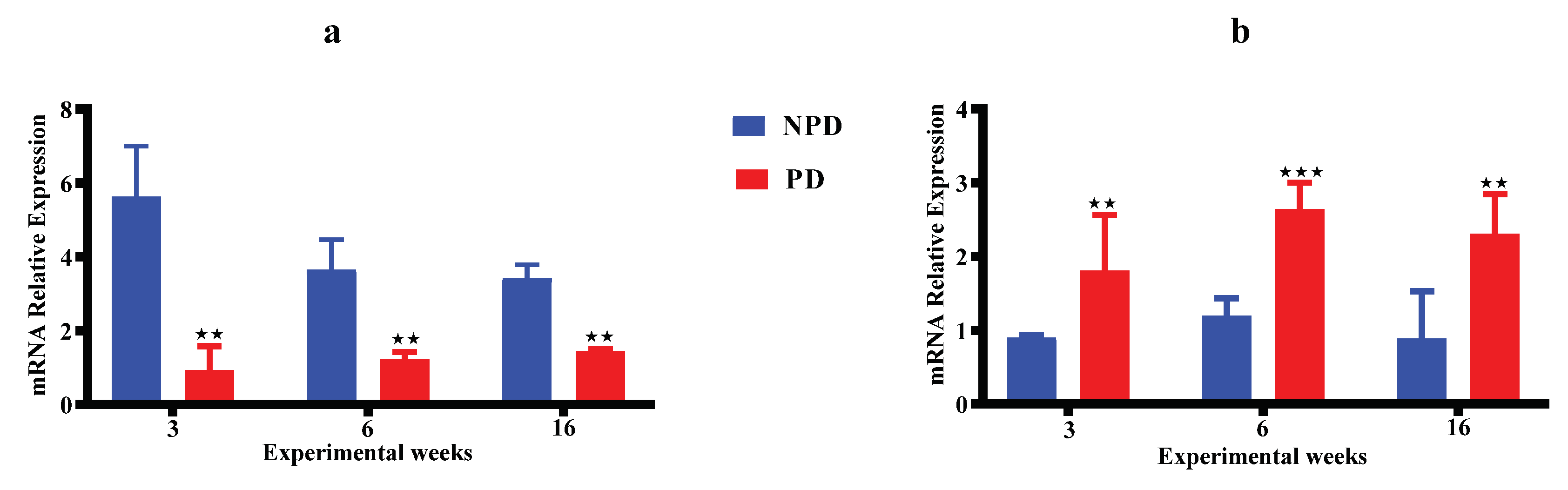
3.4. Pups Hippocampal Glucocorticoid Receptors (GR) & Mineralocorticoid Receptor (MR)
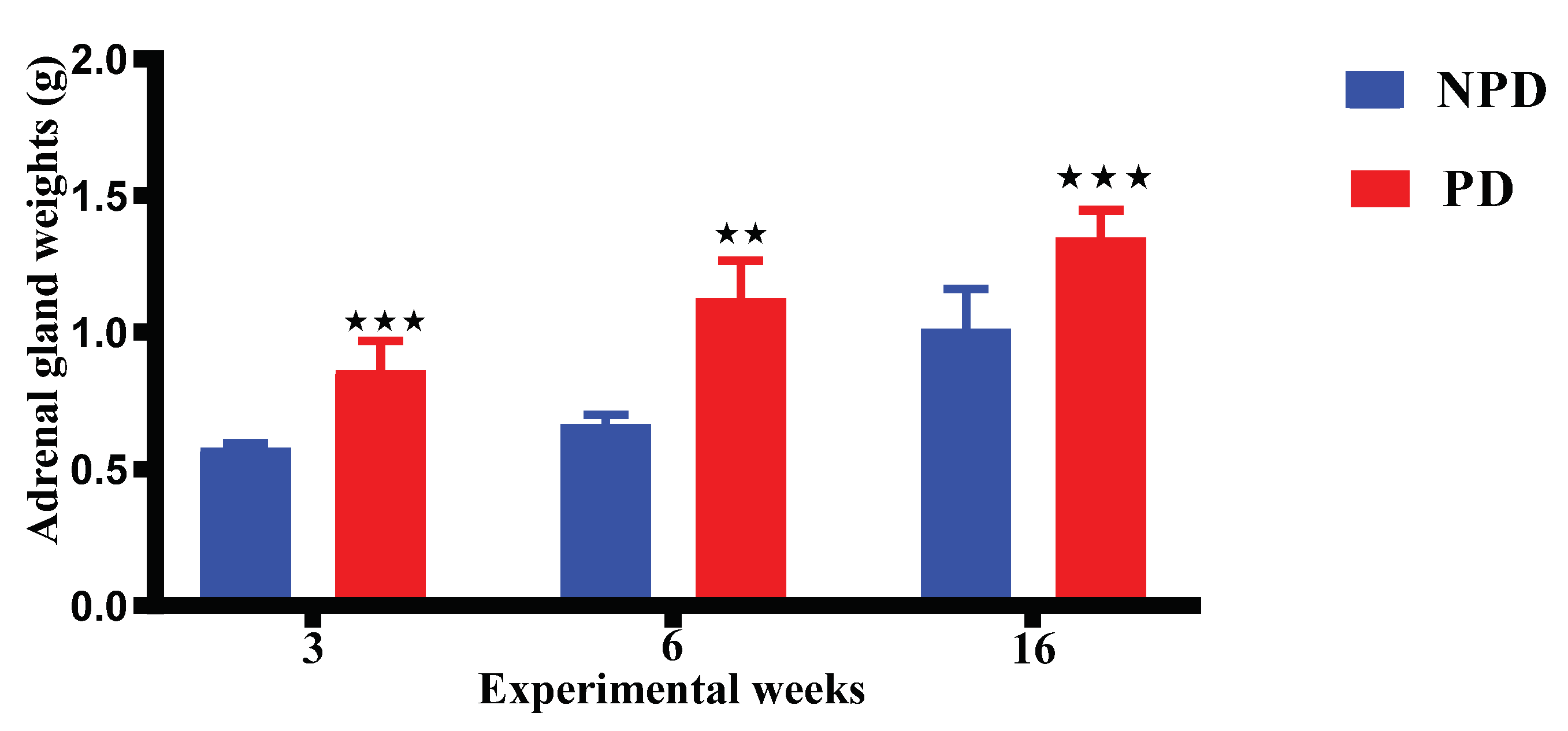
3.5. Pups Adrenal Gland Weight
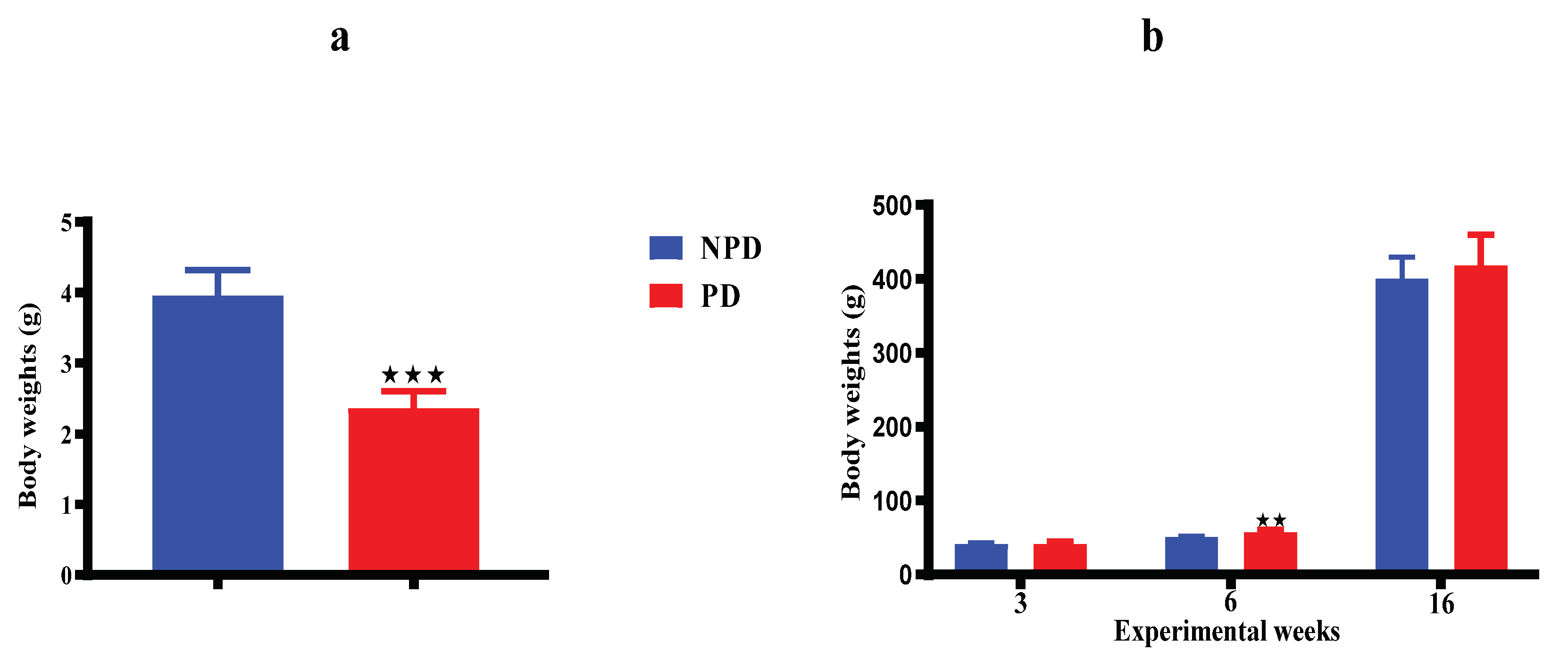
3.6. Pups Bodyweights
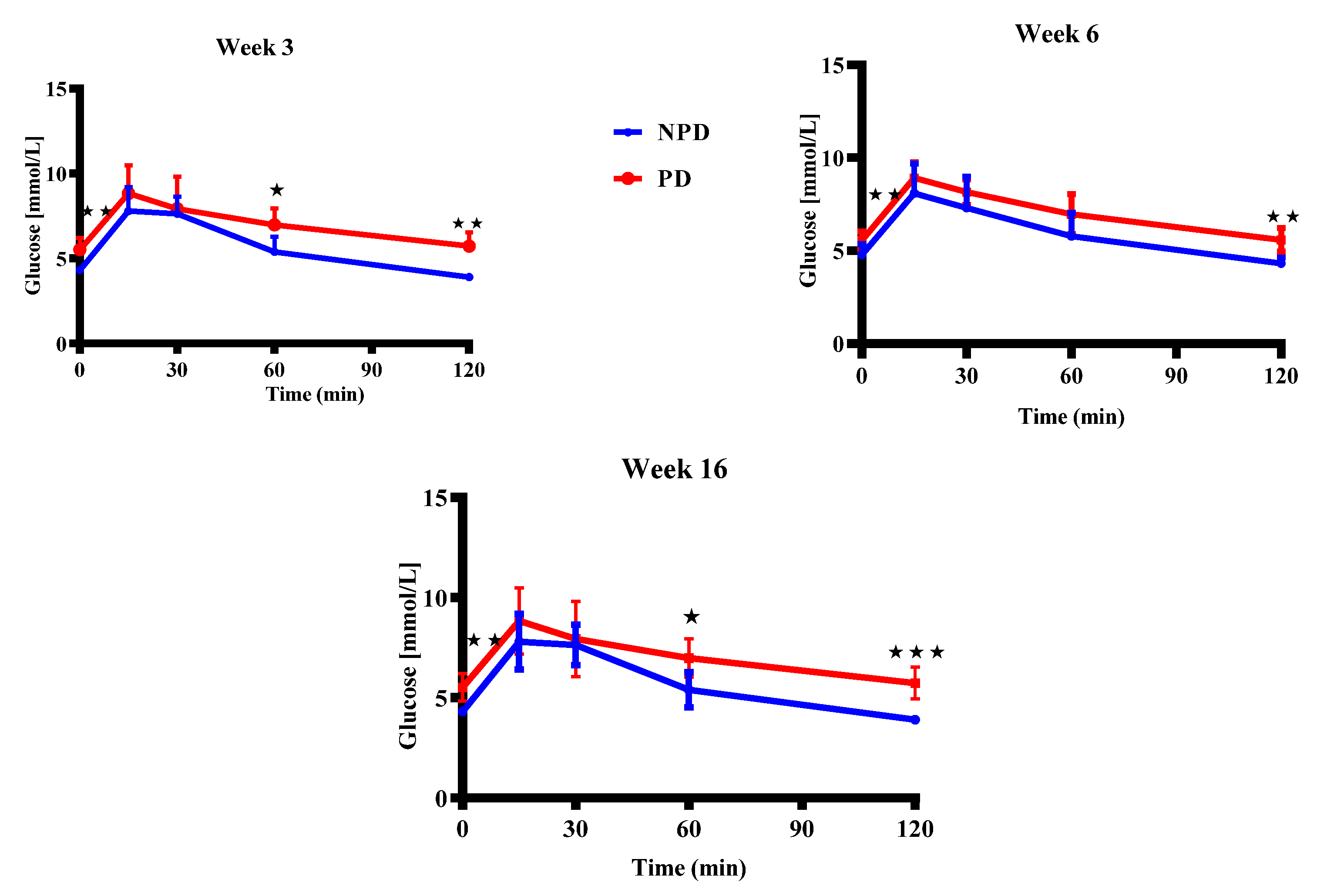
3.7. Pups Oral Glucose Tolerance (OGT) Response
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| ADA | American Diabetes Association |
| AREC | Animal Research Ethics Committee |
| BBB | Blood brain barrier |
| BRU | Biomedical Research Unit |
| CRH | Corticotropic releasing hormone |
| DNA | Deoxyribonucleic acid |
| DOHaD | Developmental origins of health and disease |
| ELISA | Enzyme linked immunosorbent assay. |
| GC | Glucocorticoids |
| GND | Gestational day |
| GR | Glucocorticoid receptor |
| HbA1c | Glycated haemoglobin A1c |
| HFHC | High fat high carbohydrate |
| HPA | Hypothalamic–adrenal–pituitary |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| IUGR | Intrauterine growth restriction |
| MR | Mineralocorticoid receptor |
| NPD | Non-prediabetes |
| OGTT | Oral glucose tolerance test |
| PD | Prediabetes |
| PCR | Polymerase chain reaction |
| RNA | Ribonucleic acid |
| T2DM | Type 2 diabetes mellitus |
| UKZN | University of KwaZulu-Natal |
References
- Lemley, C. , Fetal programming: maternal-fetal interactions and postnatal performance. Clinical Theriogenology 2020, 12, 252–267. [Google Scholar]
- Cerritelli, F.; Frasch, M.G.; Antonelli, M.C.; Viglione, C.; Vecchi, S.; Chiera, M.; Manzotti, A. A Review on the Vagus Nerve and Autonomic Nervous System During Fetal Development: Searching for Critical Windows. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, A.; Rico, L.C.; Stoehrmann, P.; Tillmann, K.E.; Weber-Stadlbauer, U.; Pollak, D.D. Interaction of the pre- and postnatal environment in the maternal immune activation model. Discov. Ment. Heal. 2023, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.J.; Tang, J.I.; Nyirenda, M.J. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin. Sci. 2007, 113, 219–232. [Google Scholar] [CrossRef]
- Braun, T.; Challis, J.R.; Newnham, J.P.; Sloboda, D.M. Early-Life Glucocorticoid Exposure: The Hypothalamic-Pituitary-Adrenal Axis, Placental Function, and Long-term Disease Risk. Endocr. Rev. 2013, 34, 885–916. [Google Scholar] [CrossRef] [PubMed]
- Solano, M.E.; Holmes, M.C.; Mittelstadt, P.R.; Chapman, K.E.; Tolosa, E. Antenatal endogenous and exogenous glucocorticoids and their impact on immune ontogeny and long-term immunity. Semin. Immunopathol. 2016, 38, 739–763. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.J.; De Oliveira, J.C.; Giachini, F.R.; Lima, V.V.; Tostes, R.C.; Bomfim, G.F. Programming of Vascular Dysfunction by Maternal Stress: Immune System Implications. Front. Physiol. 2022, 13, 787617. [Google Scholar] [CrossRef] [PubMed]
- Low, F.M., P. D. Gluckman, and M.A. Hanson, Epigenetic and developmental basis of risk of obesity and metabolic disease, in Cellular Endocrinology in Health and Disease. 2021, Elsevier. p. 289-313.
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Zhang, L. Role of the hypothalamic–pituitary–adrenal axis in developmental programming of health and disease. Front. Neuroendocr. 2012, 34, 27–46. [Google Scholar] [CrossRef]
- Seckl, J.R. , Glucocorticoids, developmental ‘programming’and the risk of affective dysfunction. Progress in brain research 2007, 167, 17–34. [Google Scholar]
- Lesage, J.; Sebaai, N.; Leonhardt, M.; Dutriez-Casteloot, I.; Breton, C.; Deloof, S.; Vieau, D. Perinatal maternal undernutrition programs the offspring hypothalamo–pituitary–adrenal (HPA) axis. Stress 2006, 9, 183–198. [Google Scholar] [CrossRef]
- Kapoor, A.; Petropoulos, S.; Matthews, S.G. Fetal programming of hypothalamic–pituitary–adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res. Rev. 2008, 57, 586–595. [Google Scholar] [CrossRef]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2016, 178, 43–65. [Google Scholar] [CrossRef]
- Kalantaridou, S.; Zoumakis, E.; Makrigiannakis, A.; Lavasidis, L.; Vrekoussis, T.; Chrousos, G. Corticotropin-releasing hormone, stress and human reproduction: an update. J. Reprod. Immunol. 2010, 85, 33–39. [Google Scholar] [CrossRef]
- Sirianni, R.; Rehman, K.S.; Carr, B.R.; Parker, C.R.; Rainey, W.E. Corticotropin-Releasing Hormone Directly Stimulates Cortisol and the Cortisol Biosynthetic Pathway in Human Fetal Adrenal Cells. J. Clin. Endocrinol. Metab. 2005, 90, 279–285. [Google Scholar] [CrossRef]
- Iliodromiti, Z.; Antonakopoulos, N.; Sifakis, S.; Tsikouras, P.; Daniilidis, A.; Dafopoulos, K.; Botsis, D.; Vrachnis, N. Endocrine, paracrine, and autocrine placental mediators in labor. Hormones 2012, 11, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Stocker, C.J.; Arch, J.R.S.; Cawthorne, M.A. Fetal origins of insulin resistance and obesity. Proc. Nutr. Soc. 2005, 64, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Ilias, I. Maternal and Fetal Hypothalamic-Pituitary-Adrenal Axes During Pregnancy and Postpartum. Ann. New York Acad. Sci. 2003, 997, 136–149. [Google Scholar] [CrossRef] [PubMed]
- St-Jean, M., I. Bourdeau, and A. Lacroix, Adrenal cortex and medulla physiology during pregnancy, labor, and puerperium, in Maternal-Fetal and Neonatal Endocrinology. 2020, Elsevier. p. 101-116.
- Lee, J.H. and D.J. Torpy, Adrenal insufficiency in pregnancy: Physiology, diagnosis, management and areas for future research. Reviews in Endocrine and Metabolic Disorders. 2023, 24, 57–69.
- Duthie, L.; Reynolds, R.M. Changes in the Maternal Hypothalamic-Pituitary-Adrenal Axis in Pregnancy and Postpartum: Influences on Maternal and Fetal Outcomes. Neuroendocrinology 2013, 98, 106–115. [Google Scholar] [CrossRef]
- Ruffaner-Hanson, C.; Noor, S.; Sun, M.S.; Solomon, E.; Marquez, L.E.; Rodriguez, D.E.; Allan, A.M.; Caldwell, K.K.; Bakhireva, L.N.; Milligan, E.D. The maternal-placental-fetal interface: Adaptations of the HPA axis and immune mediators following maternal stress and prenatal alcohol exposure. Exp. Neurol. 2022, 355, 114121–114121. [Google Scholar] [CrossRef]
- Valsamakis, G.; Chrousos, G.; Mastorakos, G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology 2018, 100, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behav. Immun. 2005, 19, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Sze, Y. and P.J. Brunton, How is prenatal stress transmitted from the mother to the fetus? Journal of Experimental Biology 2024, 227(Suppl_1).
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. New York Acad. Sci. 2016, 1391, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I., H. Randeva, and C. Tsigos, Stress, insulin resistance, and type 2 diabetes. Stress: Neuroendocrinology and neurobiology, handbook of stress 2017, 2, 351–358. [Google Scholar]
- Nugent, J.L. , Effects of Glucocorticoids on Placental Development and Function: Implications for Fetal Growth Restriction. 2012: The University of Manchester (United Kingdom).
- Krontira, A.C.; Cruceanu, C.; Binder, E.B. Glucocorticoids as Mediators of Adverse Outcomes of Prenatal Stress. Trends Neurosci. 2020, 43, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Verma, A.; Garg, R.; Singh, J.; Verma, H. Cardiometabolic Risk Factors Associated With Type 2 Diabetes Mellitus: A Mechanistic Insight. Clin. Med. Insights: Endocrinol. Diabetes 2023, 16. [Google Scholar] [CrossRef]
- Lima, J.E.; Moreira, N.C.; Sakamoto-Hojo, E.T. Mechanisms underlying the pathophysiology of type 2 diabetes: From risk factors to oxidative stress, metabolic dysfunction, and hyperglycemia. Mutat. Res. Toxicol. Environ. Mutagen. 2021, 874-875, 503437. [Google Scholar] [CrossRef] [PubMed]
- Boekelheide, K.; Blumberg, B.; Chapin, R.E.; Cote, I.; Graziano, J.H.; Janesick, A.; Lane, R.; Lillycrop, K.; Myatt, L.; States, J.C.; et al. Predicting Later-Life Outcomes of Early-Life Exposures. Environ. Heal. Perspect. 2012, 120, 1353–1361. [Google Scholar] [CrossRef]
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Early life programming of health and disease: The long-term consequences of obesity in pregnancy. J. Hum. Nutr. Diet. 2022, 35, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, W.; Zuo, R.; Sun, K. Mechanisms for establishment of the placental glucocorticoid barrier, a guard for life. Cell. Mol. Life Sci. 2018, 76, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R. , Fetal growth in diabetic pregnancy. Clinical obstetrics and gynecology 1997, 40, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U.; Cervar, M.; Puerstner, P.; Schmut, O.; Haas, J.; Mauschitz, R.; Arikan, G.; Desoye, G. Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia 2001, 44, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U.; Cervar, M.; Puerstner, P.; Schmut, O.; Haas, J.; Mauschitz, R.; Arikan, G.; Desoye, G. Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia 2001, 44, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol. 2014, 10, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Howland, M.A.; Sandman, C.A.; Glynn, L.M. Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Rev. Endocrinol. Metab. 2017, 12, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Grace, C.E.; Kim, S.-J.; Rogers, J.M. Maternal influences on epigenetic programming of the developing hypothalamic-pituitary-adrenal axis. Birth Defects Res. Part A: Clin. Mol. Teratol. 2011, 91, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Selvam, N.; K, J.; Mithra, P. Mediation effect of cord blood cortisol levels between maternal prepregnancy body mass index and birth weight: a hospital-based cross-sectional study. Clin. Exp. Pediatr. 2022, 65, 500–506. [Google Scholar] [CrossRef]
- de Mendonça, E.L.S.S., et al., Premature birth, low birth weight, small for gestational age and chronic non-communicable diseases in adult life: A systematic review with meta-analysis. Early human development. 2020, 149, 105154.
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Starikov, R.; Has, P.; Wu, R.; Nelson, D.M.; He, M. Small-for-gestational age placentas associate with an increased risk of adverse outcomes in pregnancies complicated by either type I or type II pre-gestational diabetes mellitus. J. Matern. Neonatal Med. 2020, 35, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.; Jacob, C.; Barker, M.; Fall, C.H.D.; Hanson, M.; Harvey, N.C.; Inskip, H.M.; Kumaran, K.; Cooper, C. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.; Carpenter, D.O.; Callaway, L.; Sly, P.D. Early-life risk factors for chronic nonrespiratory diseases. Eur. Respir. J. 2014, 45, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D. BIRTH WEIGHT AND ADULTHOOD DISEASE AND THE CONTROVERSIES. Fetal Matern. Med. Rev. 2006, 17, 205–227. [Google Scholar] [CrossRef]
- Tian, M.; Reichetzeder, C.; Li, J.; Hocher, B. Low birth weight, a risk factor for diseases in later life, is a surrogate of insulin resistance at birth. J. Hypertens. 2019, 37, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Grella, P. , Low birth weight and early life origins of adult disease: insulin resistance and type 2 diabetes. Clinical and Experimental Obstetrics & Gynecology 2007, 34, 9–13. [Google Scholar]
- Buysschaert, M.; Medina, J.L.; Bergman, M.; Shah, A.; Lonier, J. Prediabetes and associated disorders. Endocrine 2014, 48, 371–393. [Google Scholar] [CrossRef]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef]
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus — implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020, 16, 321–331. [Google Scholar] [CrossRef]
- Kaur, G.; Lakshmi, P.V.M.; Rastogi, A.; Bhansali, A.; Jain, S.; Teerawattananon, Y.; Bano, H.; Prinja, S. Diagnostic accuracy of tests for type 2 diabetes and prediabetes: A systematic review and meta-analysis. PLOS ONE 2020, 15, e0242415. [Google Scholar] [CrossRef]
- Colagiuri, S. Definition and Classification of Diabetes and Prediabetes and Emerging Data on Phenotypes. Endocrinol. Metab. Clin. North Am. 2021, 50, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Hostalek, U. Global epidemiology of prediabetes - present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Li, J.; Yan, N.; Li, N.; Liu, X.; Li, X.; Zhang, J.; Wang, Q.; Yang, C.; Qiu, J.; et al. Increased TG Levels and HOMA-IR Score Are Associated With a High Risk of Prediabetes: A Prospective Study. Asia Pac. J. Public Heal. 2023, 35, 413–419. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: a high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Khathi, A.; Luvuno, M.; Mabandla, M. VOLUNTARY INGESTION OF A HIGH-FAT HIGH-CARBOHYDRATE DIET: A MODEL FOR PREDIABETES. 2018, 74. 74. [CrossRef]
- Mzimela, N.C.; Ngubane, P.S.; Khathi, A. The changes in immune cell concentration during the progression of pre-diabetes to type 2 diabetes in a high-fat high-carbohydrate diet-induced pre-diabetic rat model. Autoimmunity 2019, 52, 27–36. [Google Scholar] [CrossRef]
- Naidoo, K.; Ngubane, P.S.; Khathi, A. Investigating the Effects of Diet-Induced Pre-Diabetes on the Functioning of Calcium-Regulating Organs in Male Sprague Dawley Rats: Effects on Selected Markers. Front. Endocrinol. 2022, 13, 914189. [Google Scholar] [CrossRef]
- Mosili, P.; Mkhize, B.C.; Ngubane, P.; Sibiya, N.; Khathi, A. The dysregulation of the hypothalamic–pituitary–adrenal axis in diet-induced prediabetic male Sprague Dawley rats. Nutr. Metab. 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Mapanga, R.F.; Tufts, M.A.; Shode, F.O.; Musabayane, C.T. Renal Effects of Plant-Derived Oleanolic Acid in Streptozotocin-Induced Diabetic Rats. Ren. Fail. 2009, 31, 481–491. [Google Scholar] [CrossRef]
- S, P.; R, R.; R, K. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Qulu, L.; Daniels, W.; Mabandla, M.V. Exposure to prenatal stress has deleterious effects on hippocampal function in a febrile seizure rat model. Brain Res. 2015, 1624, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, N.V.P.; Qulu, L.; Mabandla, M.V. The Effect of Quercetin on Pro- and Anti-Inflammatory Cytokines in a Prenatally Stressed Rat Model of Febrile Seizures. J. Exp. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.S., et al., Development and maturation of the male reproductive system. Birth Defects Research Part B Developmental and Reproductive Toxicology. 2003, 68, 125–136.
- Ghasemi, A., S. Jeddi, and K. Kashfi, The laboratory rat: Age and body weight matter. EXCLI journal. 2021, 20, 1431.
- Arellano, J.I., A. Duque, and P. Rakic, A coming-of-age story: adult neurogenesis or adolescent neurogenesis in rodents? Frontiers in Neuroscience. 2024, 18, 1383728.
- Alberts, J.R. , Olfactory contributions to behavioral development in rodents. Mammalian olfaction, reproductive processes, and behavior, 1976, 67-94.
- Peijs, G.L.A.M. , Development of social behaviour in the rat. 1977, [Sl: sn].
- Makowska, I.J. , Understanding the welfare of rats living in standard versus semi-naturalistic laboratory environments. 2016, University of British Columbia.
- Foster, R.G. Sleep, circadian rhythms and health. Interface Focus 2020, 10, 20190098. [Google Scholar] [CrossRef] [PubMed]
- González, M.M.C. Dim Light at Night and Constant Darkness: Two Frequently Used Lighting Conditions That Jeopardize the Health and Well-being of Laboratory Rodents. Front. Neurol. 2018, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.B. and K.M. Hope, Circadian rhythm: Light-dark cycles. Integrative and Functional Medical Nutrition Therapy: Principles and Practices, 2020, 577-594.
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human's. 2013, 4, 624–630.
- Sheard, P.; McCaig, C.; Harris, A. Critical periods in rat motoneuron development. Dev. Biol. 1984, 102, 21–31. [Google Scholar] [CrossRef]
- Picut, C.A.; Ziejewski, M.K.; Stanislaus, D. Comparative Aspects of Pre- and Postnatal Development of the Male Reproductive System. Birth Defects Res. 2017, 110, 190–227. [Google Scholar] [CrossRef]
- Beckman, D.A. and M. Feuston, Landmarks in the development of the female reproductive system. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2003, 68, 137–143.
- Bell, M.R. Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology 2018, 159, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.P.; Shetty, A.K. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem. 2004, 89, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R. , Comparing rat’s to human’s age: how old is my rat in people years? Nutrition 2005, 21, 775. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S. and P. Sengupta, Rabbits and men: relating their ages. Journal of basic and clinical physiology and pharmacology. 2018, 29, 427–435.
- Wang, C.; Liu, Y.; Wang, H.; Gao, F.; Guan, X.; Shi, B. Maternal Exposure to Oxidized Soybean Oil Impairs Placental Development by Modulating Nutrient Transporters in a Rat Model. Mol. Nutr. Food Res. 2021, 65, 2100301. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, X.; Fang, M.; Chen, G.; Cao, J.; Qu, H.; Wang, H. Maternally derived low glucocorticoid mediates adrenal developmental programming alteration in offspring induced by dexamethasone. Sci. Total. Environ. 2021, 797, 149084. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: a lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef] [PubMed]
- ztürk, H.N.O. and P.F. Türker, Fetal programming: could intrauterin life affect health status in adulthood? Obstetrics & Gynecology Science. 2021, 64, 473.
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 1: outcomes. Nat. Rev. Endocrinol. 2014, 10, 391–402. [Google Scholar] [CrossRef]
- van Bodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Pofi, R.; Tomlinson, J.W. Glucocorticoids in pregnancy. Obstet. Med. 2019, 13, 62–69. [Google Scholar] [CrossRef]
- Grilo, L.F.; Tocantins, C.; Diniz, M.S.; Gomes, R.M.; Oliveira, P.J.; Matafome, P.; Pereira, S.P. Metabolic Disease Programming: From Mitochondria to Epigenetics, Glucocorticoid Signalling and Beyond. Eur. J. Clin. Investig. 2021, 51, e13625. [Google Scholar] [CrossRef] [PubMed]
- Nakanga, W.P. , Accuracy and utility of fasting and stimulated glucose for diagnosis of diabetes in Sub-Saharan Africa. 2022: University of Exeter (United Kingdom).
- Newsholme, P.; Cruzat, V.; Arfuso, F.; Keane, K. Nutrient regulation of insulin secretion and action. J. Endocrinol. 2014, 221, R105–R120. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Hollander, and C. Spellman, Controversies in prediabetes: do we have a diagnosis? Postgraduate medicine. 2012, 124, 109–118.
- Kashyap, S.R.; Desouza, C.; Aroda, V.R.; Kim, S.H.; Neff, L.M.; Wu, S.S.; Raskin, P.; Pratley, R. Glycemic and metabolic sub-classification of prediabetes and risk factors for cardiovascular disease in the D2d cohort. Am. J. Prev. Cardiol. 2023, 15, 100525. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C. , et al., Pathogenesis of type 2 diabetes mellitus. 2021. [Google Scholar]
- Chia, C.W.; Egan, J.M.; Ferrucci, L.; Lleva, R.R.; Inzucchi, S.E.; Egan, B.M.; Hennes, M.M.; Stepniakowski, K.T.; O’shaughnessy, I.M.; Kissebah, A.H.; et al. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Mavropulo-Stolyarenko, G.; Karonova, T.; Thieme, D.; Hoehenwarter, W.; Ihling, C.; Stefanov, V.; Grishina, T.; Frolov, A. Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 2329. [Google Scholar] [CrossRef]
- Awasthi, S.; Saraswathi, N.T. Non-enzymatic glycation mediated structure–function changes in proteins: case of serum albumin. RSC Adv. 2016, 6, 90739–90753. [Google Scholar] [CrossRef]
- Díaz, A.; López-Grueso, R.; Gambini, J.; Monleón, D.; Mas-Bargues, C.; Abdelaziz, K.M.; Viña, J.; Borrás, C. Sex Differences in Age-Associated Type 2 Diabetes in Rats—Role of Estrogens and Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sandman, C.A.; Glynn, L.; Schetter, C.D.; Wadhwa, P.; Garite, T.; Chicz-DeMet, A.; Hobel, C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides 2006, 27, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Bleker, L.S.; van Dammen, L.; Leeflang, M.M.; Limpens, J.; Roseboom, T.J.; de Rooij, S.R. Hypothalamic-pituitary-adrenal axis and autonomic nervous system reactivity in children prenatally exposed to maternal depression: A systematic review of prospective studies. Neurosci. Biobehav. Rev. 2018, 117, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulou, I., A. Diakoumi, and P. Moutsatsou, Glucocorticoid receptor signaling in diabetes. International journal of molecular sciences. 2021, 22, 11173.
- Bruehl, H.; Rueger, M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Javier, E.; Arentoft, A.; Wolf, O.T.; Convit, A. Hypothalamic-Pituitary-Adrenal Axis Dysregulation and Memory Impairments in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 2439–2445. [Google Scholar] [CrossRef]
- Johnstone, H.A.; Wigger, A.; Douglas, A.J.; Neumann, I.D.; Landgraf, R.; Seckl, J.R.; Russell, J.A. Attenuation of Hypothalamic-Pituitary-Adrenal Axis Stress Responses in Late Pregnancy: Changes in Feedforward and Feedback Mechanisms. J. Neuroendocr. 2000, 12, 811–822. [Google Scholar] [CrossRef]
- Neumann, I.D. INCREASED BASAL ACTIVITY OF THE HYPOTHALAMO–PITUITARY–ADRENAL AXIS DURING PREGNANCY IN RATS BRED FOR HIGH ANXIETY-RELATED BEHAVIOUR. Psychoneuroendocrinology 1998, 23, 449–463. [Google Scholar] [CrossRef]
- Brunton, P.J. Resetting the Dynamic Range of Hypothalamic-Pituitary-Adrenal Axis Stress Responses Through Pregnancy. J. Neuroendocr. 2010, 22, 1198–1213. [Google Scholar] [CrossRef]
- Morsi, A.; DeFranco, D.; Witchel, S.F. The Hypothalamic-Pituitary-Adrenal Axis and the Fetus. Horm. Res. Paediatr. 2018, 89, 380–387. [Google Scholar] [CrossRef]
- Berens, A.E. and C.A. Nelson, Neurobiology of fetal and infant development. Handbook of infant mental health, 2019, 41-62.
- Bale, T.L.; Baram, T.Z.; Brown, A.S.; Goldstein, J.M.; Insel, T.R.; McCarthy, M.M.; Nemeroff, C.B.; Reyes, T.M.; Simerly, R.B.; Susser, E.S.; et al. Early Life Programming and Neurodevelopmental Disorders. Biol. Psychiatry 2010, 68, 314–319. [Google Scholar] [CrossRef]
- O’Leary, N. , An investigation of the role of antenatal depression, maternal cortisol and postnatal interactive behaviour on infant neurodevelopment in the first year of life. 2019, Trinity College.
- Rajendram, R., V. R. Preedy, and V.B. Patel, Diet, nutrition, and fetal programming. 2017, Springer.
- Castellano, J.M. and M. Tena-Sempere, Animal modeling of early programming and disruption of pubertal maturation. Puberty from Bench to Clinic. 2016, 29, 87–121.
- Miranda, A.; Sousa, N. Maternal hormonal milieu influence on fetal brain development. Brain Behav. 2018, 8, e00920. [Google Scholar] [CrossRef] [PubMed]
- Rufaka, A. , Does maternal stress, depression and anxiety affect fetal neurobehavioral development? a review. 2021, Brac University.
- Welberg, L.A. and J.R. Seckl, Prenatal stress, glucocorticoids and the programming of the brain. Journal of neuroendocrinology. 2001, 13, 113–128.
- Galeeva, A.; Ordyan, N.; Pivina, S.; Pelto-Huikko, M. Expression of glucocorticoid receptors in the hippocampal region of the rat brain during postnatal development. J. Chem. Neuroanat. 2006, 31, 216–225. [Google Scholar] [CrossRef]
- Pryce, C.R.; Aubert, Y.; Maier, C.; Pearce, P.C.; Fuchs, E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacol. 2010, 214, 33–53. [Google Scholar] [CrossRef]
- Coe, C.L.; Kramer, M.; Czéh, B.; Gould, E.; Reeves, A.J.; Kirschbaum, C.; Fuchs, E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile Rhesus monkeys. Biol. Psychiatry 2003, 54, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2014, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.G.; Owen, D.; Kalabis, G.; Banjanin, S.; Setiawan, E.B.; Dunn, E.A.; Andrews, M.H. Fetal Glucocorticoid Exposure and Hypothalamo-Pituitary-Adrenal (HPA) Function After Birth. Endocr. Res. 2004, 30, 827–836. [Google Scholar] [CrossRef]
- FowdenA. L.; ForheadA.J. Endocrine mechanisms of intrauterine programming. Reproduction 2004, 127, 515–526. [Google Scholar] [CrossRef]
- Fowden, A.L.; Forhead, A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [CrossRef]
- Katugampola, H., E. F. Gevers, and M.T. Dattani, Fetal Endocrinology. Brook’s Clinical Pediatric Endocrinology, 2019, 47-104.
- Fisher, D.A. , Endocrinology of fetal development. Williams textbook of endocrinology, 1998, 811-841.
- Fowden, A.; Valenzuela, O.; Vaughan, O.; Jellyman, J.; Forhead, A. Glucocorticoid programming of intrauterine development. Domest. Anim. Endocrinol. 2016, 56, S121–S132. [Google Scholar] [CrossRef] [PubMed]
- Suhag, A.; Berghella, V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Curr. Obstet. Gynecol. Rep. 2013, 2, 102–111. [Google Scholar] [CrossRef]
- Dinu, M.; Stancioi-Cismaru, A.F.; Gheonea, M.; Luciu, E.D.; Aron, R.M.; Pana, R.C.; Marinas, C.M.; Degeratu, S.; Sorop-Florea, M.; Carp-Veliscu, A.; et al. Intrauterine Growth Restriction—Prediction and Peripartum Data on Hospital Care. Medicina 2023, 59, 773. [Google Scholar] [CrossRef] [PubMed]
- AlKhalifa, M.A.; Hsu, S.; Raza, G.; Ismail, M.S. Diabetes in pregnancy: A comparison of guidelines. 2021, 7. 7. [CrossRef]
- Phillips, D.I.W.; Walker, B.R.; Reynolds, R.M.; Flanagan, D.E.H.; Wood, P.J.; Osmond, C.; Barker, D.J.P.; Whorwood, C.B. Low Birth Weight Predicts Elevated Plasma Cortisol Concentrations in Adults From 3 Populations. Hypertension 2000, 35, 1301–1306. [Google Scholar] [CrossRef]
- Levitt, N.S., et al., Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: early programming of cortisol axis. The Journal of Clinical Endocrinology & Metabolism 2000, 85, 4611–4618. 2000, 85, 4611–4618.
- Katugampola, H., M. Cerbone, and M.T. Dattani, Normal hypothalamic and Pituitary Development and Physiology in the Fetus and Neonate, in Maternal-Fetal and Neonatal Endocrinology. 2020, Elsevier. p. 527-545.
- Antonini, S.R., M. F. Stecchini, and F.S. Ramalho, Development and function of the adrenal cortex and medulla in the fetus and neonate, in Maternal-Fetal and Neonatal Endocrinology. 2020, Elsevier. p. 611-623.
- Androulakis, I.P. Circadian rhythms and the HPA axis: A systems view. Wiley Interdiscip. Rev. Syst. Biol. Med. 2021, 13, e1518–e1518. [Google Scholar] [CrossRef]
- Kamgang, V.W., M. Murkwe, and M. Wankeu-Nya, Biological Effects of Cortisol. 2023.
- Poore, K. and A. Fowden, The effect of birth weight on hypothalamo-pituitary-adrenal axis function in juvenile and adult pigs. The Journal of physiology. 2003, 547, 107–116.
- Phillips, D., et al., Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? The Journal of Clinical Endocrinology & Metabolism. 1998, 83, 757–760.
- Campana, G.; Loizzo, S.; Fortuna, A.; Rimondini, R.; Maroccia, Z.; Scillitani, A.; Falchetti, A.; Spampinato, S.M.; Persani, L.; Chiodini, I. Early post-natal life stress induces permanent adrenocorticotropin-dependent hypercortisolism in male mice. Endocrine 2021, 73, 186–195. [Google Scholar] [CrossRef]
- Fowden, A.L.; Vaughan, O.R.; Murray, A.J.; Forhead, A.J. Metabolic Consequences of Glucocorticoid Exposure before Birth. Nutrients 2022, 14, 2304. [Google Scholar] [CrossRef]
- Reynolds, R.M. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis – 2012 Curt Richter Award Winner. Psychoneuroendocrinology 2013, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.R.; Spencer-Segal, J.L. Glucocorticoids and the Brain after Critical Illness. Endocrinology 2021, 162. [Google Scholar] [CrossRef] [PubMed]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Harris, A.; Holmes, M.; de Kloet, E.; Chapman, K.; Seckl, J. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology 2012, 38, 648–658. [Google Scholar] [CrossRef]
- Ladd, C.; Huot, R.L.; Thrivikraman, K.; Nemeroff, C.B.; Plotsky, P.M. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mrna and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol. Psychiatry 2004, 55, 367–375. [Google Scholar] [CrossRef]
- Castro, M. , et al., Physiology and pathophysiology of the HPA axis. Cushing’s Syndrome: Pathophysiology, Diagnosis and Treatment, 2011, 1-20.
- de Kloet, E.R.; van Acker, S.A.; Sibug, R.M.; Oitzl, M.S.; Meijer, O.C.; Rahmouni, K.; de Jong, W. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int. 2000, 57, 1329–1336. [Google Scholar] [CrossRef]
- Gheorghe, C.P.; Goyal, R.; Mittal, A.; Longo, L.D. Gene expression in the placenta: maternal stress and epigenetic responses. Int. J. Dev. Biol. 2010, 54, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J.R. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 2004, 151, U49–U62. [Google Scholar] [CrossRef]
- Harvey, P.W.; Sutcliffe, C. Adrenocortical hypertrophy: establishing cause and toxicological significance. J. Appl. Toxicol. 2010, 30, 617–626. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Figueiredo, H.F.; Ostrander, M.M.; Choi, D.C.; Engeland, W.C.; Herman, J.P. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am. J. Physiol. Metab. 2006, 291, E965–E973. [Google Scholar] [CrossRef]
- A Coffman, J. Chronic stress, physiological adaptation and developmental programming of the neuroendocrine stress system. Futur. Neurol. 2020, 15, FNL39. [Google Scholar] [CrossRef]
- Bourque, S.L.; Davidge, S.T. Developmental programming of cardiovascular function: a translational perspective. Clin. Sci. 2020, 134, 3023–3046. [Google Scholar] [CrossRef]
- Matthews, S.G. , Early programming of the hypothalamo–pituitary–adrenal axis. Trends in Endocrinology & Metabolism 2002, 13, 373–380. [Google Scholar]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J. Neuroendocr. 2018, 30, e12590. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.M.V.; Syddall, H.E.; Wood, P.J.; Chrousos, G.P.; Phillips, D.I.W. Fetal Programming of the Hypothalamic-Pituitary-Adrenal (HPA) Axis: Low Birth Weight and Central HPA Regulation. J. Clin. Endocrinol. Metab. 2004, 89, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Tegethoff, M.; Pryce, C.; Meinlschmidt, G. Effects of Intrauterine Exposure to Synthetic Glucocorticoids on Fetal, Newborn, and Infant Hypothalamic-Pituitary-Adrenal Axis Function in Humans: A Systematic Review. Endocr. Rev. 2009, 30, 753–789. [Google Scholar] [CrossRef] [PubMed]
- Möllers, L.S.; Yousuf, E.I.; Hamatschek, C.; Morrison, K.M.; Hermanussen, M.; Fusch, C.; Rochow, N. Metabolic-endocrine disruption due to preterm birth impacts growth, body composition, and neonatal outcome. Pediatr. Res. 2021, 91, 1350–1360. [Google Scholar] [CrossRef]
- Singhal, A. , Should we promote catch-up growth or growth acceleration in low-birthweight infants? Low-birthweight baby: born too soon or too small 2015, 81, 51–60. [Google Scholar]
- Kosinska, M., B. Stoinska, and J. Gadzinowski, Catch-up growth among low birth weight infants: Estimation of the time of occurrence of compensatory events. Anthropological Review. 2004, 67, 87–95.
- Dulloo, A.G.; Jacquet, J.; Seydoux, J.; Montani, J.-P. The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int. J. Obes. 2006, 30, S23–S35. [Google Scholar] [CrossRef]
- Dulloo, A.G. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pr. Res. Clin. Endocrinol. Metab. 2008, 22, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Haghdoost, A.A.; Jamshidi, F.; Aliramezany, M.; Moosazadeh, M. Low birthweight or rapid catch-up growth: which is more associated with cardiovascular disease and its risk factors in later life? A systematic review and cryptanalysis. Ann. Trop. Paediatr. 2014, 35, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.N.; Fu, Q.; Callaway, C.W.; McKnight, R.A.; McMillen, I.C.; Ross, M.G.; Lane, R.H.; Desai, M. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. Am. J. Physiol. Liver Physiol. 2010, 299, G1023–G1029. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Zabielski, R.; Hammon, H.M.; Metges, C.C. Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. 2009, 17–35. [Google Scholar]
- Isganaitis, E. Developmental Programming of Body Composition: Update on Evidence and Mechanisms. Curr. Diabetes Rep. 2019, 19, 60. [Google Scholar] [CrossRef]
- Warner, M.J.; Ozanne, S.E. Mechanisms involved in the developmental programming of adulthood disease. Biochem. J. 2010, 427, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Correia-Branco, A.; Keating, E.; Martel, F. Maternal Undernutrition and Fetal Developmental Programming of Obesity: The Glucocorticoid Connection. Reprod. Sci. 2014, 22, 138–145. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Dabelea, D.; Roumain, J.; Hanson, R.L.; Bennett, P.H.; Knowler, W.C. Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes 2000, 49, 445–449. [Google Scholar] [CrossRef]
- Rose, A.J.; Vegiopoulos, A.; Herzig, S. Role of glucocorticoids and the glucocorticoid receptor in metabolism: Insights from genetic manipulations. J. Steroid Biochem. Mol. Biol. 2010, 122, 10–20. [Google Scholar] [CrossRef]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]

| Developmental Stages in Rat/Human | Rat (Male) | Human |
|---|---|---|
| Neonatal/newborn | 0-7 days | 0-28 days |
| Infantile/infant | 8-20 days (1-2 weeks) | 1-23 months |
| Juvenile/child | 21-32 days (3-5 weeks) | 2-12 years |
| Peripubertal/adolescent | 33-55 days (5-8 weeks) | 12-16 years |
| Late puberty/adolescent | 56-70 days (8-10 weeks) | |
| Emerging adulthood | 70-150 days (10-21 weeks) | 18-25 years |
| Sequence Name | Sequence |
|---|---|
| Glucocorticoid receptor (Nr3c1 gene) | Forward: 5′-ACCTCGATGACCAAATGACC-3′ Reverse: 5′-AGCAAAGCAGAGCAGGTTTC-3′ |
| Mineralocorticoid receptor (Nr3c2 gene) | Forward: 5′-AAAGGGTAGTGTGTGCAGGG-3′ Reverse 5′-GTTCTCCTAGTTCCCGGAGG-3′ |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Forward: 5′-AGTGCCAGCCTCGTCTCATA-3′ Reverse 5′-GATGGTGATGGGTTTCCCGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





