Submitted:
28 March 2024
Posted:
29 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Source of Municipal Compost
2.2. List of Tested Polymers
2.3. Seed Types used in Germination Studies
2.4. Polymer Ash Content
2.5. Polymer Dry Solids Measurements
2.6. CHN Analysis of Tested Polymers
2.7. ASTM D5338 and ASTM D6400: Biodegradability Controls, Calculations, and Compostability Labeling Claims
2.8. Composting of Polymers: Thermophilic Respirometry
2.9. Differential Scanning Calorimetry (DSC)
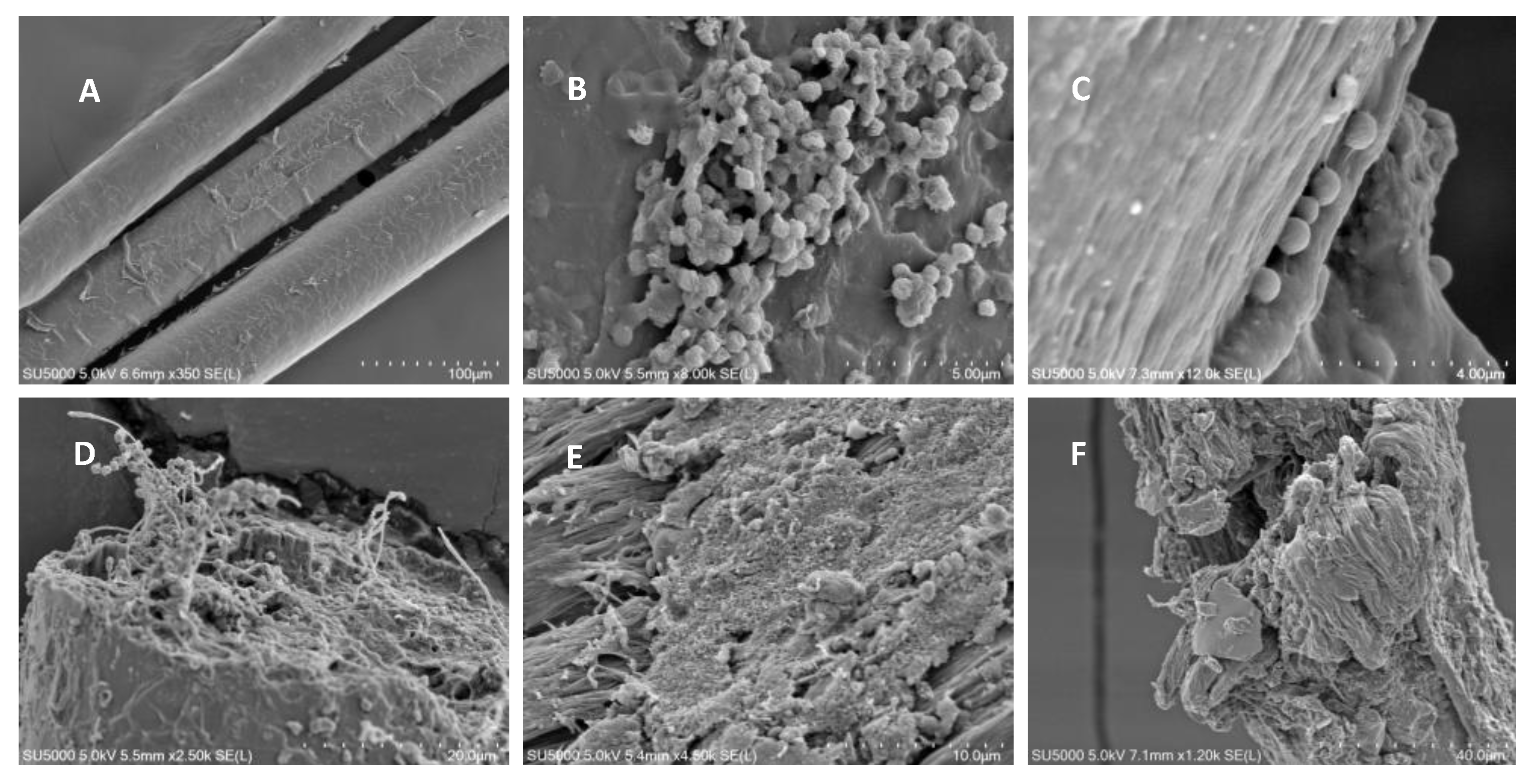
2.10. Scanning Electron Microscopy (SEM)
2.11. Compost Viability: Seed Germination Studies
3. Results and Discussion
3.1. Biodegradation of Common Personal Care Polymers
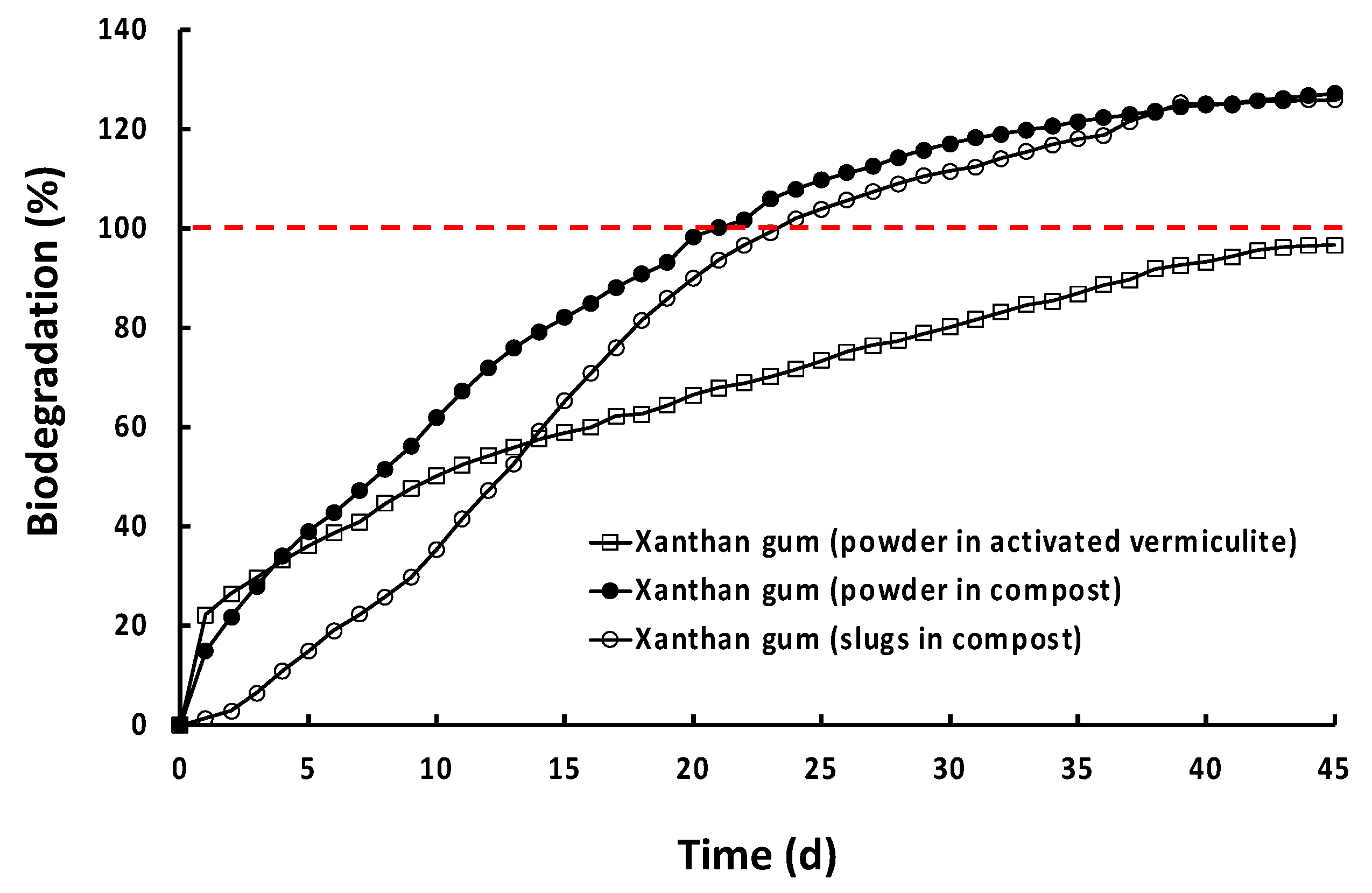
3.2. Biodegradation of Natural Polymers
3.3. Biodegradation of Proteins
3.4. Biodegradation of Synthetic Polymers
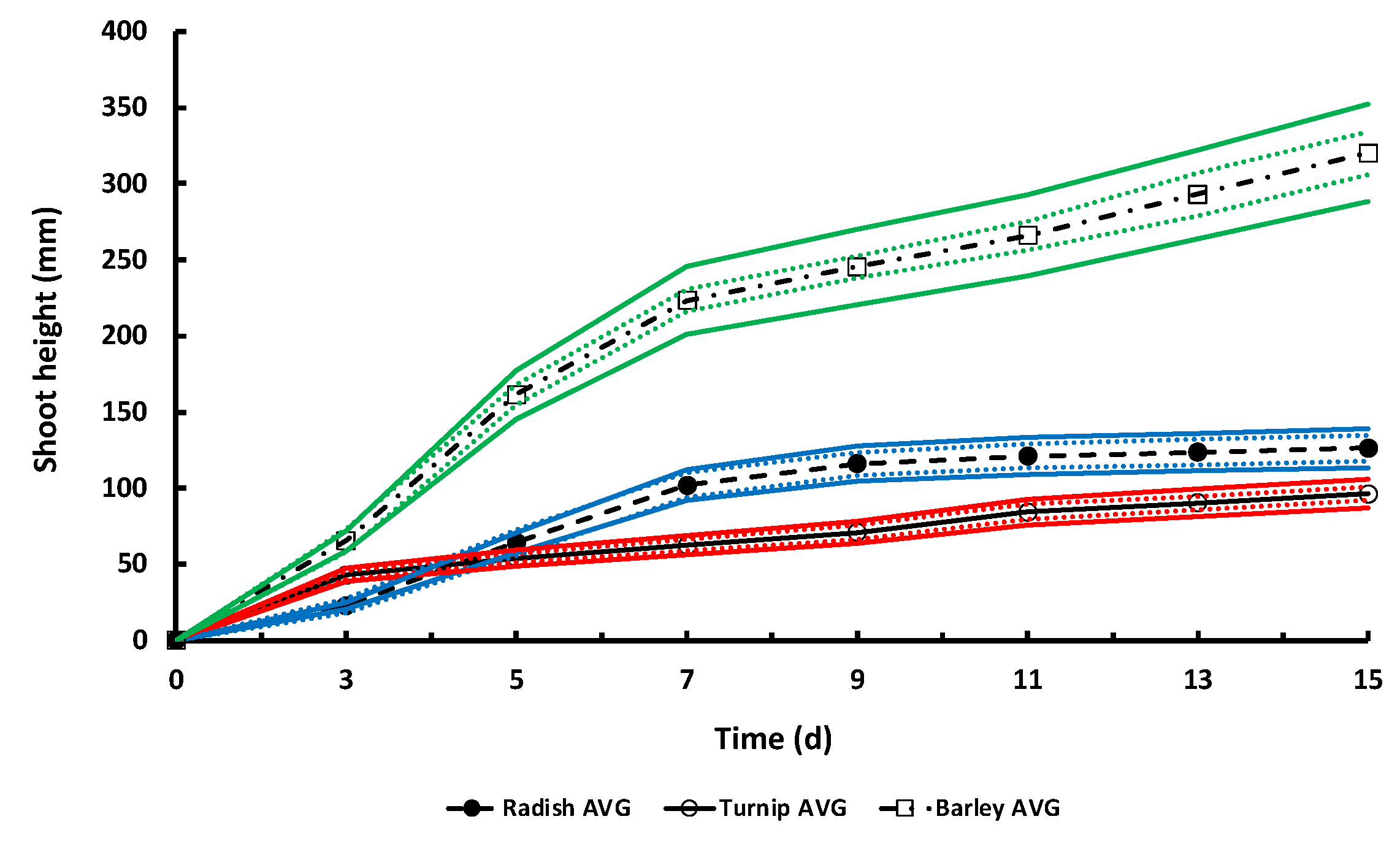
3.5. Seed Germination Studies
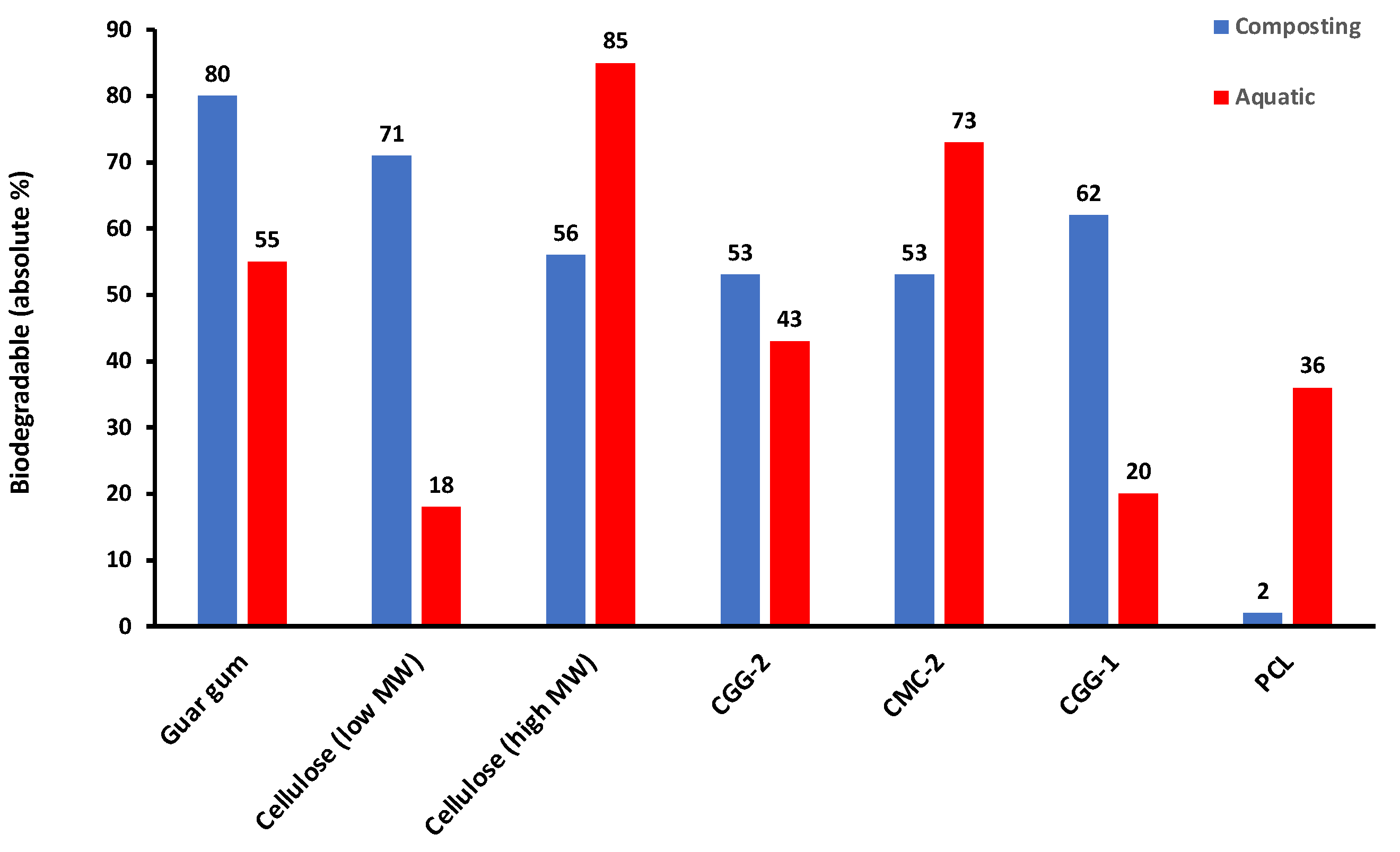
3.5. Comparing Aquatic and Composting Degradation Rates
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). (2020) Advancing sustainable materials management: 2018 fact sheet. Available online: https://www.epa.gov/sites/default/files/2021-01/documents/2018_ff_fact_sheet_dec_2020_fnl_508.pdf (accessed on 11 April 2022).
- Polymers in liquid formulations—technical report: a landscape view of the global plfs market, Royal Society of Chemistry. Available online: https://www.rsc.org/globalassets/22-new-perspectives/sustainability/liquid-polymers/rsc-polymer-liquid-formulations-technical-report.pdf (accessed on 17 October 2022).
- Bashir, S.; Kimiko, S.; Mak, C.; Fang, J.; Gonçalves, D. Personal care and cosmetic products as a potential source of environmental contamination by microplastics in a densely populated Asian city. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Cousins, I.T.; Ng, C.A.; Wang, Z.; Scheringer, M. Why is high persistence alone a major cause of concern? Environ. Sci.: Processes Impacts 2019, 21, 781–792. [Google Scholar] [CrossRef]
- Rozman, U.; Kalčíková, G. The first comprehensive study evaluating the ecotoxicity and biodegradability of water-soluble polymers used in personal care products and cosmetics. Ecotoxicol. Environ. Saf. 2021, 228. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: a review and meta-analysis of current data. PloS one 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- ECHA Annex XV Restriction Report - Proposal for a Restriction:Intentionally added microplastics, Version 1.2. Available online: https://echa.europa.eu/documents/10162/05bd96e3-b969-0a7c-c6d0-441182893720 (accessed on 7 November 2019).
- Senak, L.; McMullen, R.L. Dilute Solution Properties and Characterization of N-Vinyl Pyrrolidone Based Polymers. In Handbook of Pyrrolidone and Caprolactam Based Materials, Musa, O.M., Ed.; John Wiley & Sons: New York, USA, 2022; pp. 1313–1364. [Google Scholar]
- Halima, N.B. Poly(vinyl alcohol): review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.; Abu-Omar, M.; Scott, S.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Zeenat; Elahi, A.; Bukhari, D.; Shamim, S.; Rehman, A. Plastics degradation by microbes: a sustainable approach. J. King Saud Univ. Sci. 2021, 33. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: review. Springerplus 2013, 2. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Saucedo, J. Polymer biodegradation: mechanisms and estimation techniques—a review. Chemosphere, 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Bilal, H.; Raza, H.; Bibi, H.; Bibi, T. Plastic biodegradation through insects and microbes. J Biores. Manag. 2021, 8, 95–103. [Google Scholar]
- De Wilde, B.; Mortier, N.; Verstichel, S.; Briassoulis, D.; Babou, M.; Mistriotis, A.; Hiskakis, M. Report on current relevant biodegradation and ecotoxicity standards, kbbpps work package 6: biodegradability, deliverable 6.1 (2013). Available online: http://www.biobasedeconomy.eu/research-knowledge/kbbpps-knowledge-based-bio-based-products-pre-standardization (accessed on 8 April 2022).
- Béguin, P. Aubert, J-P. The biological degradation of cellulose. Microbiol. Rev. 1994, 13, 25–58. [Google Scholar]
- Dimarogona, M.; Topakas, E.; Christakopoulos, P. Cellulose degradation by oxidative enzymes. Comput. Struct. Biotechnol. J. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Rishi, V.; Soni, S.K.; Rishi, P. A novel multi-enzyme preparation produced from Aspergillus niger using biodegradable waste: a possible option to combat heterogeneous biofilms. AMB Express 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K. Chapter 15: Chitosan Based Nanoparticulate System for Controlled Delivery of Biological Macromolecules. In Nanomaterials for Drug Delivery and Therapy, Grumezescu, A.M. Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 435–459. [Google Scholar]
- Huang, C. Li, X.; Du, Y. Biodegradation of xanthan by newly isolated Cellulomonas sp. lx, releasing elicitor-active xantho-oligosaccharides-induced phytoalexin synthesis in soybean cotyledons. Process Biochem. 2005, 40, 3701–3706. [Google Scholar]
- Sarian, F.D. van der Kaaij, R.M.; Kralj, S.; Wijbenga, D.J.; Binnema, D.J.; van der Maarel, M.J.; Dijkhuizen, L. Enzymatic degradation of granular potato starch by Microbacterium aurum strain B8.A. Appl. Microbiol. Biotechnol. 2012, 93, 645–654. [Google Scholar] [CrossRef]
- OECD (1992); Test No. 301: Ready Biodegradability, OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing: Paris, France. [CrossRef]
- OECD (1992); Test No. 306: Biodegradability in Seawater, OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing: Paris, France. [CrossRef]
- OECD (1981); Test No. 302A: Inherent Biodegradability: Modified SCAS Test, OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing: Paris, France. [CrossRef]
- OECD (1992); Test No. 302B: Inherent Biodegradability: Zahn-Wellens/ EVPA Test, OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing: Paris, France. [CrossRef]
- OECD (2009); Test No. 302C: Inherent Biodegradability: Modified MITI Test (II), OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing: Paris, France. [CrossRef]
- OECD (2014); Test No. 310: Ready Biodegradability—CO2 in sealed vessels (Headspace Test), OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing: Paris, France. [CrossRef]
- Shchegolkova, N.M.; Krasnov, G.S.; Belova, A.A.; Dmitriev, A.A.; Kharitonov, S.L.; Klimina, K.M.; Melnikova, N.V.; Kudryavtseva, A.V. Microbial community structure of activated sludge in treatment plants with different wastewater compositions. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau. (1990); Detailed Housing Characteristics, Structural Characteristics: 1990 (Table 12). Available online: https://www2.census.gov/library/publications/dicennial/1990/ch-2/ch-2-1.pdf (accessed on 2 April 2022).
- Rolsky, C.; Kelkar, V. Degradation of polyvinyl alcohol in us wastewater treatment plants and subsequent nationwide emission estimate. Int. J. Environ. Res. Public Health. 2021, 18. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Done, H.Y.; Halden, R.U. United states national sewage sludge repository at Arizona State University—a new resource and research tool for environmental scientists, engineers, and epidemiologists. Environ. Sci. Pollut. Res. Int. 2015, 22, 1577–1586. [Google Scholar] [CrossRef]
- Environmental Protection Agency, Basic information about biosolids. Available online: https://www.epa.gov/biosolids/basic-information-about-biosolids (accessed on 18 April 2022).
- Hubbe, M.A.; Nousa, M. Sánchez, C. Composting as a way to convert cellulosic biomass and organic waste into high value soil amendments: a review. Bioresources, 2010, 5, 2808–2854. [Google Scholar] [CrossRef]
- van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in Eastern Spanish agricultural soils. Environ. Pollut. 2020, 261. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; English, M.E.; Schaeffer, S.M.; Miles, C.A.; Zelenyuk, A.; Suski, K.; Flury, M. Release of micro- and nanoparticles from biodegradable plastic during in situ composting. Sci. Total Environ. 2019, 675, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yang, S.; Zhu, L.; Liu, C.; Zhang, Y.; Zhang, Y. Effect of microplastics in sludge impacts on the vermicomposting. Bioresour. Technol. 2021, 326. [Google Scholar] [CrossRef] [PubMed]
- Wilske, B.; Bai, M.; Lindenstruth, B.; Bach, M.; Rezaie, Z.; Frede, H.G.; Breuer, L. Biodegradability of a polyacrylate superabsorbent in agricultural soil. Environ. Sci. Pollut. 2014, 21, 9453–9460. [Google Scholar] [CrossRef] [PubMed]
- Gaytán, I.; Burelo, M.; Loza-Tavera, H. Current status on the biodegradability of acrylic polymers: microorganisms, enzymes and metabolic pathways involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Savage, G.M.; Golueke, C.G. Ch. 12: Composting of Municipal Solid Wastes. In Integrated Solid Waste Management: Engineering Principles and Management Issues, 2nd ed.; Tchobanoglous, G., Kreith, F., Eds.; McGraw-Hill: New York, USA, 2002; pp. 1–70. [Google Scholar]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and enzymatic degradation of synthetic plastics. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Grima, S.; Bellon-Maurel, V.; Feuilloley, P.; Silvestre, F. Aerobic biodegradation of polymers in solid-state conditions: a review of physiochemical parameter settings in laboratory simulations. J. Polym. Environ. 2000, 8, 183–195. [Google Scholar] [CrossRef]
- ASTM Standard D5338-15 (2015); Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions, Incorporating Thermophilic Temperatures. ASTM International: West Conshohocken, PA, USA.
- ISO 14855-1 (2012); “Determination of the Ultimate Aerobic Biodegradability and Disintegration of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide,” Organization for Standardization.
- ISO 14855-2 (2018); “Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide, Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory-Scale Test,” Organization for Standardization.
- EN 13432 (2012); “Packaging—Requirements for Packaging Recoverable Through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging,” European Committee for Standardization.
- ASTM Standard D6400-12 (2015); Standard Specification for Labeling Plastic Designed to be Aerobically Composted in Municipal or Industrial Facilities. ASTM International: West Conshohocken, PA, USA.
- ASTM D5988-03 (2003); Standard Test Method for Determining Aerobic Biodegradation in Soil of Plastic Materials or Residual Plastic Materials after Composting. ASTM International: Philadelphia, PA, USA.
- Standard Method 2540 D (2017); Total Suspended Solids Dried at 103–105°C, in Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: New York, USA.
- OECD (2006); Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test, in OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing: Paris, France.
- Bellia, G.; Tosin, M.; Degli-Innocenti, F. Test method of composting in vermiculite is unaffected by the priming effect. Polym. Degrad. Stabil. 2000, 69, 113–120. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: a question of microbial competition? Soil Biol. and Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Bernard, L.; Basile-Doelsch, I.; Derrien, D.; Fanin, N.; Fontaine, S.; Guenet, B.; Karimi, B.; Marsden, C.; Maron, P.-A. Advancing the mechanistic understanding of the priming effect on soil matter mineralization. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Gaenssle, A.; Satyawan, C.A.; Xiang, G.; van der Maarel, M.; Jurak, E. Long chains and crystallinity govern the enzymatic degradability of gelatinized starches from conventional and new sources. Carbohydr. Polym. 2021, 260. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Shin, G.; Lee, M.; Koo, J.M.; Jeon, H.; Ok, Y.S.; Hwang, D.S.; Hwang, S.Y.; Oh, D.X.; Park, J. Biodegradable chito-beads replacing non-biodegradable microplastics for cosmetics. Green Chem. 2021, 23, 6953–6965. [Google Scholar] [CrossRef]
- Kothari, N.; Bhagia, S.; Zaher, M.; Pu, Y.; Mittal, A.; Yoo, C.G.; Himmel, M.; Ragauskas, A.J.; Kumar, R.; Wyman, C.E. Cellulose hydrolysis by Clostridium thermocellulum is agnostic to substrate structural properties in contrast to fungal cellulases. Green Chem. 2019, 21, 2810–2822. [Google Scholar] [CrossRef]
- Mattonai, M.; Pawcenis, D.; del Seppia, S.; Łojewska, J.; Ribechini, E. Effect of ball-milling on crystallinity index, degree of polymerization and thermal stability of cellulose. Bioresour. Technol. 2018, 270, 270–277. [Google Scholar] [CrossRef]
- Kanmani, P.; Dhivya, E.; Aravind, J.; Kumaresan, K. Extraction and analysis of pectin from citrus peels: augmenting the yield from Citrus limon using statistical experimental design. Iranica J. Energy Environ. 2014, 5, 303–312. [Google Scholar] [CrossRef]
- Khattab, A.M. The Microbial Degradation for Pectin, Pectins—The New-Old Polysaccharides. IntechOpen. 2022. [Google Scholar] [CrossRef]
- Gillece, T.; Senak, L.; McMullen, R.L. Characterization of bleached hair: vibrational spectroscopy, thermal analysis, and determination of equivalent damage factor. J. Cosmet. Sci. 2021, 72, 519–546. [Google Scholar]
- Byrne, D.; Boeije, G.; Croft, I.; Hüttmann, G.; Luijkx, G.; Meier, F.; Parulekar, Y.; Stijntjes, G. Biodegradability of polyvinyl alcohol based film used for liquid detergent capsules. Tenside Surfactants Deterg. 2021, 58, 88–96. [Google Scholar] [CrossRef]

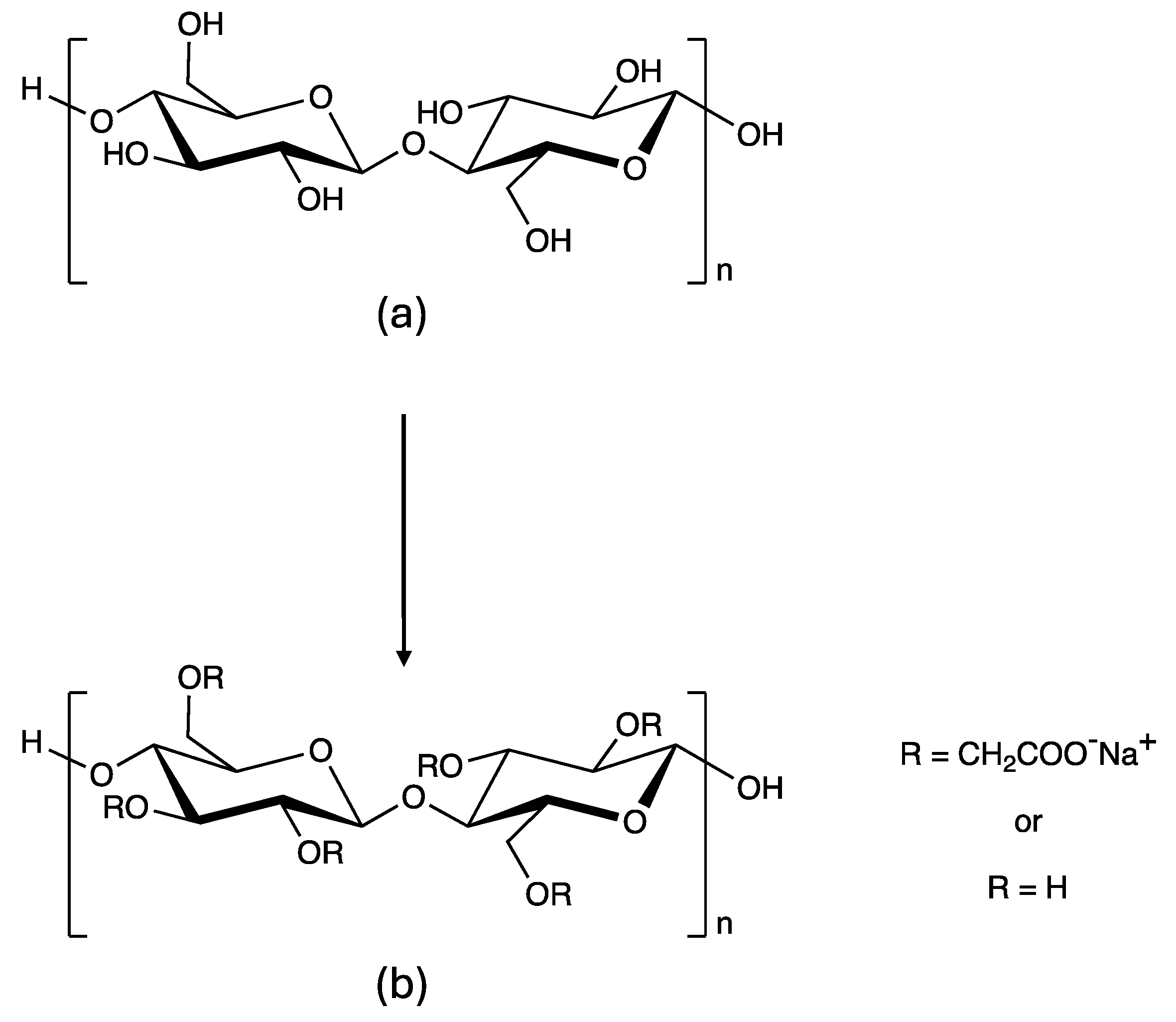
| Chemical / Trade Name | INCI/Common Name | Moisture | Ash | CO2 (g)* |
| Aqualon 7L2Fa | Cellulose Gum^ | 7.5 | 36.4 | 139 |
| Aqualon 7H4F a | Cellulose Gum^ | 6.9 | 36.0 | 144 |
| Aquasorb A380a | Cellulose Gum^ | 5.1 | 35.3 | 142 |
| Aquasorb A500a | Cellulose Gum^ | 6.0 | 35.5 | 142 |
| Avicel PH101b | Microcrystalline Cellulose | 5.4 | 0.2 | 154 |
| Avicel PH102b | Microcrystalline Cellulose | 6.0 | 0.1 | 165 |
| Bovine gelatinc | Gelatin | 11.0 | 1.0 | 164 |
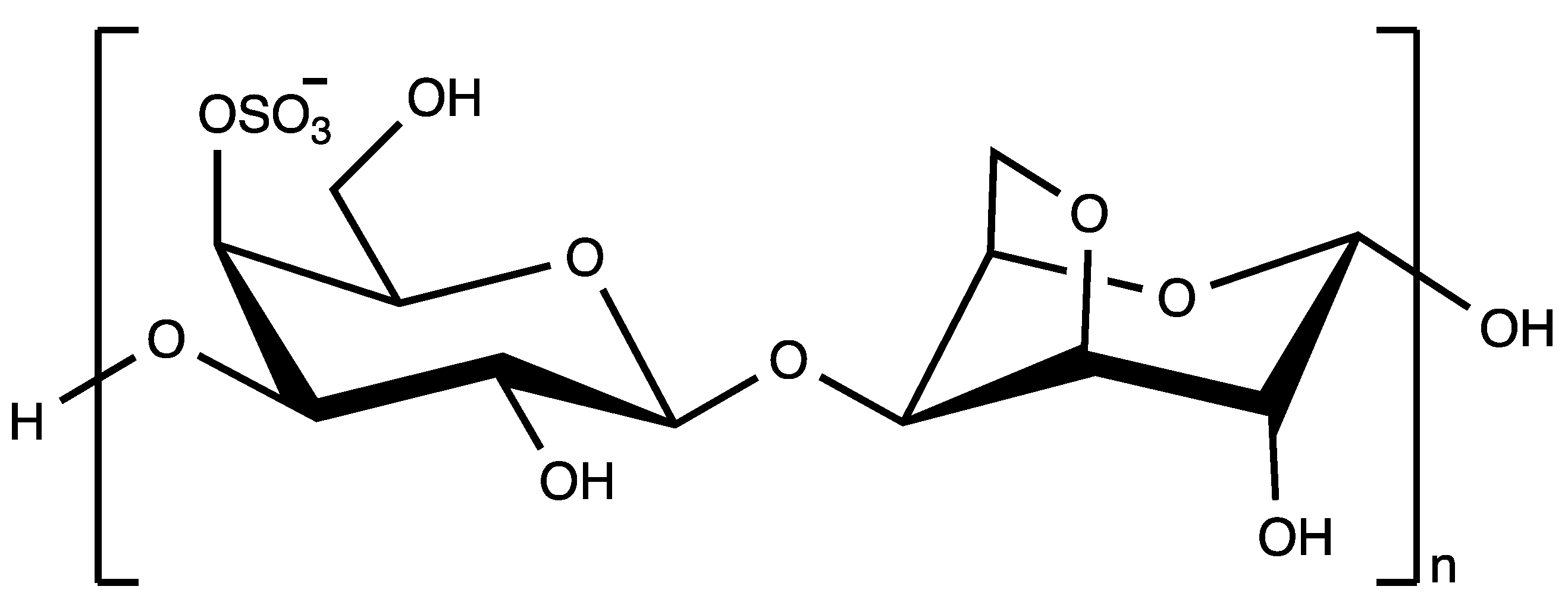
| κ-Carrageenanc | Carrageenan | 6.9 | 33.9 | 84 |
| Cassia guma | Cassia Gum | 10.8 | 2.0 | 145 |
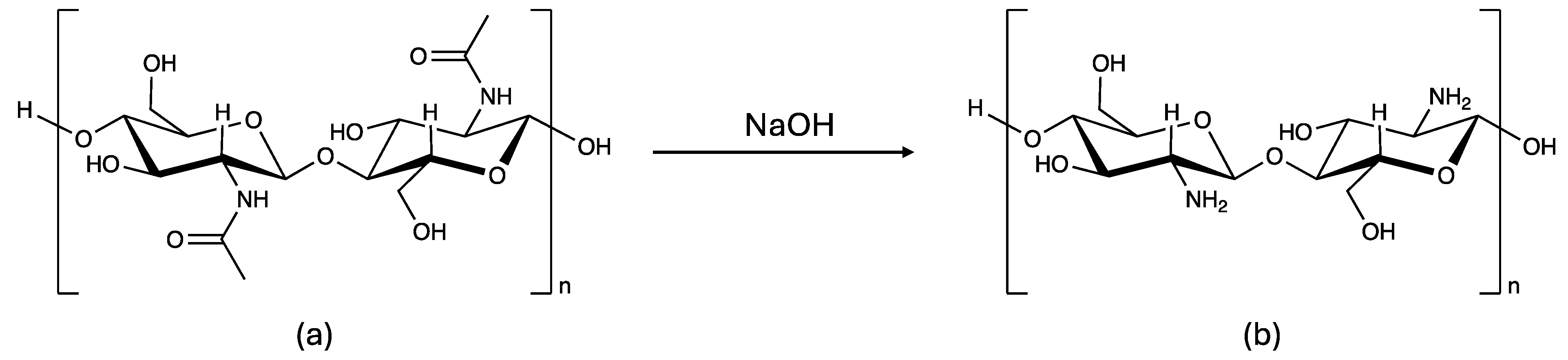
| Chitinc | Chitin | 5.4 | 1.1 | 164 |
| Chitosanc | Chitosan, 50-190 KDa, 76% deacetylation | 7.1 | 0.3 | 153 |
| Citrus peel pectinc | Pectin | 7.2 | 15.1 | 146 |
| Crosslinked pAAd | Carbomer | 8.6 | 0.1 | 174 |
| Guar gumc | Guar Gum | 11.3 | 1.1 | 171 |
| Keratin (bleached hair)e | Bleached European dark brown hair | 12.7 | 6.5 | 153 |
| Keratin (virgin hair)e | Virgin European dark brown hair | 11.7 | 1.7 | 163 |
| Locust bean gumc | Ceratonia Siliqua Gum | 12.5 | 1.0 | 147 |
| Maize amylopectinc | Amylopectin | 11.9 | 0.6 | 147 |
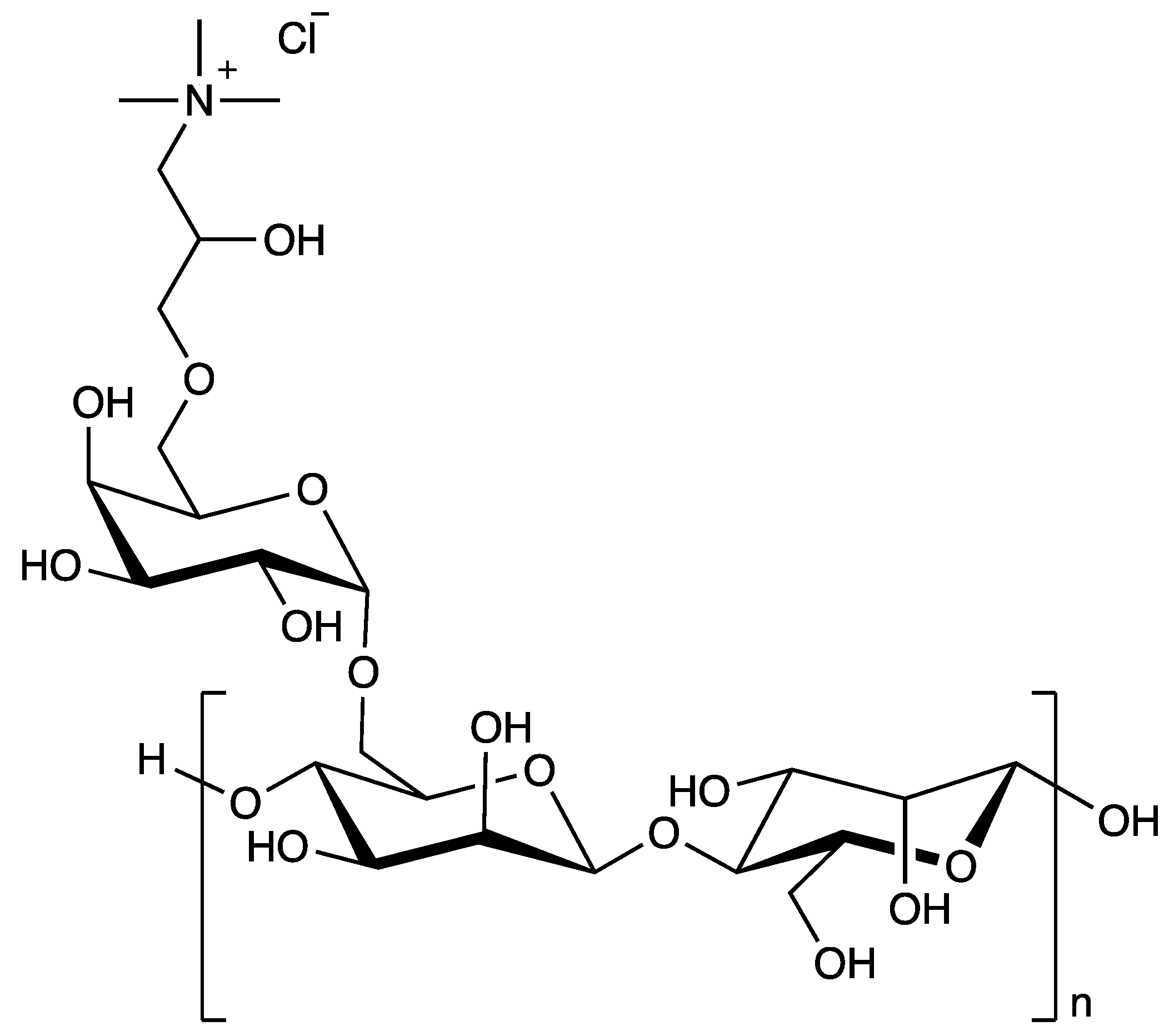
| N-Hance 3196a | Guar Hydroxypropyltrimonium Chloride | 9.5 | 1.0 | 151 |
| poly(OAA/Acrylates/BAEM)f | OAA/Acrylates/BAEM Copolymer | 3.6 | 2.4 | 226 |
| Polycaprolactamc | Nylon 6 | 0.5 | 0.1 | 212 |
| Polycaprolactone (80 kDa)c | Polycaprolactone | 1.2 | 0.1 | 234 |
| Polyethylenec | Polyethylene | 0.0 | 0.3 | 313 |
| Polylactic acid (60 kDa)c | Polylactic Acid | 0.6 | 0.1 | 186 |
| Polystyrenec | Polystyrene | 0.1 | 0.1 | 335 |
| Potato starchc | Potato Starch | 8.1 | 0.6 | 151 |
| PVA, ≥ 85% hyd.c | Polyvinyl Alcohol, 30 kDa, ≥ 85% hydrolyzed | 3.4 | 0.8 | 197 |
| PVA, ≥ 98% hyd.c | Polyvinyl Alcohol, <50 kDa, ≥ 98% hydrolyzed | 3.4 | 0.2 | 194 |
| SigmaCell Type 20 cellulosec | Cellulose (cotton linters) | 5.0 | 0.1 | 156 |
| Sodium alginic acidc | Alginic Acid, Sodium Salt | 13.2 | 23.2 | 114 |
| Soluble starch (potato)c | Soluble Starch | 17.0 | 0.9 | 138 |
| Soy protein acid hydrolysatec | Hydrolyzed Soy Protein | 11.7 | 18.1 | 146 |
| Styleze ES-1a | Guar Hydroxypropyltrimonium Chloride | 9.3 | 2.8 | 165 |
| Styleze ES-Duraa | Guar Hydroxypropyltrimonium Chloride | 7.7 | 3.0 | 153 |
| Xanthan gumc | Xanthan Gum | 15.0 | 0.6 | 131 |
| Zein proteinc | Zein | 6.8 | 1.8 | 220 |
| α-Cellulosec | Microcrystalline Cellulose | 7.5 | 0.4 | 153 |
| Polymer ID | Commercial Trade Name |
| CMC-1 | Aqualon 7L2F |
| CMC-2 | Aqualon 7H4F |
| CMC-3 | Aquasorb A380 |
| CMC-4 | Aquasorb A500 |
| MCC-1 | Avicel PH101 |
| MCC-2 | Avicel PH102 |
| CGG-1 | N-Hance 3196 |
| CGG-2 | Styleze ES-1 |
| CGG-3 | Styleze ES-Dura |
| Drying Method | Sample Size (g) | Temperature (°C) | Time (h) | Dry Solids (%) |
| Sartorius MA 30 | 4-6 | 140 | 0.25 | 47.9 ± 1.1 (n=10) |
| TGA Q5000 isotherm | 0.02 | 175 | 1.0 | 46.2 ± 1.3 (n=3) |
| Forced-air oven (2540D) | 25-35 | 105 | 24 | 48.3 ± 1.1 (n=5) |
| Vacuum oven (2540D) | 25-35 | 105 | 24 | 48.1 ± 0.3 (n=5) |
| DVS isotherm* | 0.02 | 25 | 24 | 48.8 ± 1.5 (n=4) |
| Polymer ID | Relative Biodegradability (%) | Absolute Biodegradability (%) | |||||
| 5 | 10 | 20 | 30 | 60 | 90 d | ||
| Sodium alginic acid | 100 | 50 | 96 | 122 | 175 | 177 | 179 |
| Soluble starch (potato) | 100 | 57 | 89 | 109 | 119 | 134 | 144 |
| Potato starch | 100 | 87 | 96 | 99 | 101 | 128 | 140 |
| Xanthan gum | 100 | 39 | 67 | 98 | 102 | 121 | 122 |
| Citrus peel pectin | 100 | 51 | 63 | 74 | 78 | 100 | 120 |
| Guar gum | 100 | 35 | 60 | 89 | 100 | 101 | 102 |
| MCC-2 | 100 | 16 | 33 | 57 | 83 | 94 | 99 |
| MCC-1 | 100 | 19 | 32 | 58 | 81 | 90 | 97 |
| Cassia gum | 100 | 77 | 89 | 92 | 95 | 95 | 97 |
| Locust bean gum | 100 | 71 | 80 | 84 | 86 | 89 | 94 |
| Amylopectin (maize) | 100 | 69 | 80 | 84 | 86 | 92 | 93 |
| α-cellulose | 100 | 6 | 13 | 28 | 48 | 82 | 88 |
| CMC-4 (powder) | 100 | 5 | 10 | 38 | 65 | 85 | 87 |
| Chitin | 100 | 17 | 39 | 55 | 63 | 75 | 85 |
| CMC-1 | 100 | 5 | 12 | 33 | 55 | 72 | 84 |
| SigmaCell Type 20 cellulose | 100 | 58 | 73 | 79 | 81 | 81 | 81 |
| CGG-2 | 98 | 12 | 43 | 58 | 66 | 74 | 79 |
| CGG-1 | 95 | 19 | 33 | 51 | 63 | 70 | 77 |
| CMC-4 (film shards) | 93 | 14 | 21 | 46 | 57 | 64 | 75 |
| κ-Carrageenan | 90 | 3 | 13 | 36 | 49 | 67 | 75 |
| CMC-3 | 90 | 39 | 59 | 68 | 73 | 73 | 73 |
| CGG-3 | 90 | 37 | 42 | 49 | 56 | 63 | 72 |
| Chitosan (pH = 6.0) | 22 | 2 | 4 | 8 | 10 | 12 | 18 |
| Chitosan (as received) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polymer ID | Relative Biodegradability (%) | Absolute Biodegradability (%) | |||||
| 5 | 10 | 20 | 30 | 60 | 90 d | ||
| Soy acid hydrolysate | 100 | 78 | 99 | 118 | 135 | 147 | 151 |
| Zein | 100 | 78 | 85 | 95 | 98 | 98 | 98 |
| Bovine gelatin | 100 | 82 | 90 | 91 | 91 | 91 | 91 |
| Keratin (bleached hair) | 100 | 45 | 76 | 87 | 87 | 87 | 87 |
| Keratin (virgin hair) | 11 | 1 | 5 | 8 | 9 | 9 | 9 |
| Polymer ID | Relative Biodegradability (%) |
Absolute Biodegradability (%) | |||||
| 5 | 10 | 20 | 30 | 60 | 90 d | ||
| PCL (80 kDa), 30g | 100 | 5 | 13 | 39 | 70 | 99 | 102 |
| PLA (60 kDa) | 100 | 10 | 11 | 31 | 35 | 61 | 90 |
| PCL (80 kDa), 100g | 100 | 6 | 13 | 27 | 30 | 32 | 32 |
| poly(OAA/Acrylates/BAEM) | 15 | 10 | 12 | 12 | 12 | 12 | 12 |
| Polycaprolactam | 12 | 8 | 10 | 10 | 10 | 10 | 10 |
| Polystyrene | 6 | 5 | 5 | 5 | 5 | 5 | 5 |
| PVA, ≥ 31kDa, ≥ 98% hyd. | 4 | 3 | 3 | 3 | 3 | 3 | 3 |
| Crosslinked pAA | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| PVA, 30 kDa, ≥ 85% hyd. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Polyethylene | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polymer ID | % Germinated (Radish) |
% Germinated (Turnip) |
% Germinated (Barley) |
Pass/Fail |
| Amylopectin | 94 | 89 | 94 | Pass |
| CMC-1 | 83 | 83 | 89 | Pass |
| CMC-3 | 89 | 89 | 100 | Pass |
| CMC-4 | 83 | 83 | 83 | Pass |
| MCC-1 | 94 | 89 | 89 | Pass |
| MCC-2 | 89 | 89 | 89 | Pass |
| κ-Carrageenan | 89 | 89 | 94 | Pass |
| Cassia gum | 89 | 89 | 94 | Pass |
| Chitin | 94 | 94 | 83 | Pass |
| Compost | 89 | 83 | 89 | Pass |
| Gelatin | 94 | 89 | 89 | Pass |
| Guar gum | 100 | 89 | 94 | Pass |
| Keratin (bleached hair) | 94 | 94 | 100 | Pass |
| Locust bean gum | 94 | 94 | 89 | Pass |
| CGG-1 | 100 | 89 | 94 | Pass |
| PCL (30 g) | 94 | 89 | 89 | Pass |
| Pectin | 89 | 89 | 94 | Pass |
| PLA (30 g) | 94 | 94 | 94 | Pass |
| Potato starch | 94 | 100 | 100 | Pass |
| SigmaCell Type 20 | 94 | 94 | 89 | Pass |
| Sodium alginate | 89 | 83 | 94 | Pass |
| Soluble starch | 83 | 89 | 83 | Pass |
| Soy protein acid hydrolysate | 94 | 94 | 89 | Pass |
| CGG-2 | 94 | 100 | 89 | Pass |
| CGG-3 | 94 | 89 | 100 | Pass |
| Xanthan gum | 94 | 83 | 83 | Pass |
| Zein | 94 | 94 | 83 | Pass |
| α-cellulose | 100 | 94 | 94 | Pass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




