Introduction
Salt stress is a significant limiting factor in plant growth and production, with saline soil covering approximately 20% of the global arable land area, resulting in substantial annual losses estimated in the tens of billions of dollars globally (Morton et al., 2019). Although plants have different salt tolerance, the main adverse effects are osmotic stress, followed by ion toxicity. Salt stress promotes the accumulation of reactive oxygen species (ROS) in plants, leading to oxidative stress, disrupting ion balance, and altering osmotic pressure (Ashraf et al., 2018). Therefore, long-term exposure to salt stress can lead to dehydration reactions in plants under high osmotic stress, which together result in a significant decrease in crop yield and quality (Tyerman et al., 2019). In recent years, with rising temperatures, changes in irrigation methods, and soil degradation, crop production worldwide has faced the challenge of increasing soil salinization (Sahab et al., 2021). The increase in soil salinity can cause osmotic stress on terrestrial plants, thereby affecting their growth, development, and adaptability (Baena et al., 2024). Hence, the revelation of the molecular regulatory mechanisms of plant salt tolerance is crucial for the formulation of resistance breeding strategies and the creation of new crop germplasm, especially for the improvement of yield and quality of marginal land crops.
Alfalfa (Medicago sativa L.), known as the “king of forage”, due to its high yield, good nutritional quality, and wide adaptability, is widely planted worldwide (Radovic, Sokolovic & Markovic, 2009; Chen et al., 2020b). In addition to being an important forage resource for herbivores and providing essential nutrients for animals, alfalfa can also play a role as a soil conditioner, improving soil structure and enhancing soil fertility (Mokhtari et al., 2017). However, alfalfa’s growth and development are impeded by salt stress, leading to reduced yield and quality (He et al., 2022). Therefore, it is crucial to accelerate the cultivation of new salt tolerant alfalfa varieties through the revelation of salt tolerant molecular regulatory mechanisms for the establishment and yield improvement of alfalfa in saline alkali soil. At present, research on the salt tolerance of alfalfa mainly focuses on maintaining osmotic homeostasis and ion balance, hormone regulation, changes in antioxidant enzyme activity, and stress resistance gene response (Li et al., 2019; Gong, 2021), revealing the basic process of alfalfa response to salt stress. Among them, under salt stress, the ion homeostasis of alfalfa can be maintained by applying silicon (Meng et al., 2020), and the content of osmoregulatory substances can also be increased by enhancing the activity of vacuolar proton pump ATPase (Wang et al., 2016), thus maintaining homeostasis and enhancing its salt tolerance. Transgenic alfalfa can promote antioxidant enzyme activity by increasing the expression of ScABI3 and MsWRKY33 genes (Ma et al., 2023; Zhang et al., 2024). In addition, using genetic engineering technology to clone stress-resistant genes rstB and MsSPL8 into the alfalfa genome is also an effective method to improve the salt tolerance of alfalfa (Zhang & Wang, 2015; Gou et al., 2018), providing reference and inspiration for the creation of new salt-tolerant alfalfa germplasm.
In addition, exogenous plant growth regulators or plant hormones, especially melatonin, can enhance plant antioxidant capacity, reduce plant reactive oxygen species levels, and ultimately promote plant resistance to salt stress (Sarwar et al., 2018; Cen et al., 2020). Melatonin is a small molecule indoleamine hormone that serves as a growth regulator and potent antioxidant, with functions such as stimulating plant growth (Park & Back, 2012), promoting seed germination, and enhancing plant resistance to abiotic stress (Arnao & Hernández-Ruiz, 2015; Su et al., 2018). Research has shown that exogenous melatonin can activate antioxidant enzymes in maize seedlings, thereby reducing the accumulation of ROS caused by salt stress (Chen et al., 2018a). In addition, melatonin can also promote the growth parameters of sugar beet seedlings under salt stress conditions (Zhang et al., 2021). Melatonin enhances the tolerance of kidney beans to salt stress by enhancing ROS metabolism, promoting the expression of antioxidant defense-related genes, and enhancing photosynthetic capacity (ElSayed et al., 2021). Similarly, melatonin can also improve overall antioxidant capacity, increase key enzyme activity, and maintain a low level of ROS status, thereby affecting the photosynthetic efficiency of rice under salt stress conditions (Yan et al., 2021). Melatonin can not only enhance plant stress resistance by directly clearing excess ROS, but also interact with other plant hormones to regulate the expression of stress resistance genes, thereby improving plant salt tolerance (Gou et al., 2018). Among them, exogenous melatonin alleviates oxidative damage caused by salt stress by enhancing the expression of genes related to ABA and GA biosynthesis (Zhang et al., 2014). Melatonin can not only promote the biosynthesis of ethylene by increasing the level of ACC (Xu et al., 2017), but also promote the synthesis of indoleacetic acid by affecting the expression of endogenous indoleacetic acid-related genes (Wen et al., 2016). When plants are subjected to external stress, melatonin acts on the upstream signaling pathways of some defense genes to promote the synthesis of plant hormones such as jasmonic acid and salicylic acid, thereby assisting plants in resisting adverse external influences (Zhu & Lee, 2015; Arnao & Hernández-Ruiz, 2018). This indicate that melatonin can directly or indirectly participate in stress response by interfering with other plant hormones, regulating the expression of its upstream and downstream genes. However, although numerous studies have shown that exogenous application of melatonin can affect plant response to salt stress, the mechanism of melatonin mediated response of alfalfa to salt stress has not been fully explored (Li et al., 2012; Mukherjee et al., 2014; Li et al., 2017). In this study, we investigated the effect of exogenous application of a certain concentration of melatonin on the germination characteristics of alfalfa under salt stress. Based on physiological indicator detection and transcriptomic analysis, we further identified key differentially expressed genes and metabolic pathways in response to salt stress in alfalfa, providing potential research strategies for a deeper understanding of the molecular regulatory mechanism of salt tolerance in alfalfa and salt tolerance breeding.
Materials and Methods
Plant Material and Experimental Design
In this study, the experimental material utilized was alfalfa WL440HQ. Seeds of uniform size and full grains were selected, disinfected by soaking in 75% alcohol for 10 min, followed by rinsing with deionized water 3–4 times. Subsequently, petri dishes (ΦA=90mm) were prepared with 4 mL of various melatonin concentrations (0, 10, 50, 100, 200 μM) and 200 mM NaCl, with six replicates per treatment and approximately 30 seeds cultured per replicate. The petri dishes were then placed in a constant temperature incubator at 25 °C for 16 h during the day and 8 h at night. The germination rate was assessed every 24 h over a total period of 7 days, and the measurements of bud length and fresh weight were conducted at the end of the 7-day period. Following the measurements, the samples were stored in an ultra-low temperature refrigerator at -80 °C. Furthermore, the physiological and biochemical indexes of seedlings treated with 0 (WLCK), 200 mM NaCl (WLN), and 200 mM NaCl + 10 μM MT (WLNMT) were determined. Eventually, transcriptome sequencing was carried out to explore the underlying mechanisms related to the observed effects.
Measurement of Germination Rate, Shoot Length and Fresh Weight
The germination rate was calculated over a period of 7 consecutive days. On day 7, the vertical distance from the cotyledon node to the top bud of 10 alfalfa seedlings was measured using a calibrated ruler and recorded as the root length. At the same time, the fresh weight of the same 10 seedlings was weighed using an electronic balance.
Measurement of Physiological and Biochemical Indices
The peroxidase (POD) activity, superoxide dismutase (SOD) activity, and malondialdehyde (MDA) were measured using specific assay kits. The POD activity was determined by employing the POD activity assay kit (Abbkine, KTB1150, Wuhan, China), followed by the SOD activity determined with the SOD activity assay kit (Abbkine, KTB1030, Wuhan, China), and the MDA levels assessed with the malondialdehyde content assay kit (Abbkine, KTB1050, Wuhan, China). Furthermore, the levels of reduced glutathione (GSH) and superoxide anion (O2-) were measured using the GSH content assay kit (Solarbio, BC1175, Beijing, China) and the superoxide anion content assay kit (Solarbio, BC1295, Beijing, China), respectively. All measurements were conducted by the provided instructions accompanying each specific assay kit.
Transcriptomics Analysis
Collecting alfalfa sprout samples after 7 days of processing, followed by total RNA extraction using an RNA purification kit (QIAzolLysisReagent, Qiagen, Germany). The extracted RNA was then assessed for concentration and purity using the Nanodrop2000. Subsequently, the integrity of the RNA was confirmed through agarose gel electrophoresis. Further evaluation of RNA quality was conducted using a 5300 Bioanalyzer (Agilent) before quantification with ND-2000 (NanoDrop Technologies). Next, mRNA was isolated from the total RNA, spliced into small fragments of approximately 300 bp, and used to synthesize cDNA with the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen, CA) alongside random hexamer primers. The resulting cDNA fragments underwent purification, PCR amplification, and final library construction. These cDNA libraries underwent high-throughput sequencing on the Illumina NovaSeq 6000 platform (Illumina, USA). Following sequencing, the raw data were filtered using FASTp for high-quality readings. Subsequently, the sequencing data were aligned with the reference genome utilizing HISAT2 software in a targeted mode to obtain mapping data necessary for subsequent transcript assembly and expression calculation (Kim, Langmead & Salzberg, 2015; Chen et al., 2018b; Shen et al., 2020).
Functional Annotation and Enrichment Analysis of Differential Genes
After obtaining the Read Counts of the genes, the differential expression of genes between samples was analyzed using DESeq2 software to identify differentially expressed genes between samples (Love, Huber & Anders, 2014). The default screening criteria for significantly differentially expressed genes are FDR < 0.05 and |log
2FC|≥1. To further characterize the genes, the Gene Ontology (GO) of unigenes was accessed through the Blast2go program. Subsequently, GO annotation was performed on the obtained differentially expressed genes (DEGs), and GO enrichment analysis was carried out using Goatools software (
http://github.com/tanghaibao/GOatools) (Klopfenstein et al., 2018). The GO database facilitates the categorization of genes based on the biological processes they are involved in (
http://geneontolo gy.org/) (Ashburner et al., 2000; The Gene Ontology Consortium et al., 2023), the cellular components they compose, and the molecular functions they serve. Annotating differentially expressed genes with GO terms helps to elucidate their functional localization within the organism. Typically, the
p-value is automatically corrected, and a corrected
p-value (FDR) of <0.05 indicates a significant enrichment of the GO function.
By annotating differentially expressed genes with KEGG, we can gain a deeper understanding of the pathway interactions and functional characteristics of these genes in organisms. Using the KEGG database (
https://www.genome.jp/kegg/) (Kanehisa, 2000; Kanehisa, 2019; Kanehisa et al., 2023), genes can be classified according to the pathway or function they are involved in. KEGG PATHWAY enrichment analysis is an analysis of genes in a gene set using a script written in R, with the principle of calculation being the same as that of GO function enrichment analysis. The KEGG pathway is considered significantly enriched by default when the
p-value is <0.05 (
P value_uncorrected). Consequently, a
p-value <0.05 indicates a significant enrichment of the KEGG pathway.
Weighted Gene Co-Expression Network Analysis
To identify closely related genes, the genes were classified into different modules based on the relevant growth and physiological indicators of the three different treatments. Utilizing the WGCNA R software package, a co-expression network was subsequently constructed to determine the modules harboring these related genes. Following this, the analysis was visualized using the Cytoscape (3.3.0) software in order to pinpoint the hub genes within the co-expression network.
qRT-PCR Analysis
To verify the accuracy of the transcriptomic data, we selected eight genes for qRT-PCR analysis. First, we used the cDNA synthesis kit PrimeScript RT Reagent kit with gDNA Eraser (Cat# RR047A, TaKaRa, Tokyo, Japan) to reverse transcribe the extracted mRNA. Subsequently, we used the qRT-PCR kit (Cat# RR420A; TaKaRa) for further analysis. All operations were performed according to the instructions of the kit, and each biological replicate included three technical replicates to ensure the reliability of the results. For internal control genes, we selected
Ms-ACTIN2 as a reference gene. For other primer sequences, please refer to
Supplementary Table S1 for details.
Results
Effect of MT on the Growth of NaCl-Stressed Alfalfa Plants
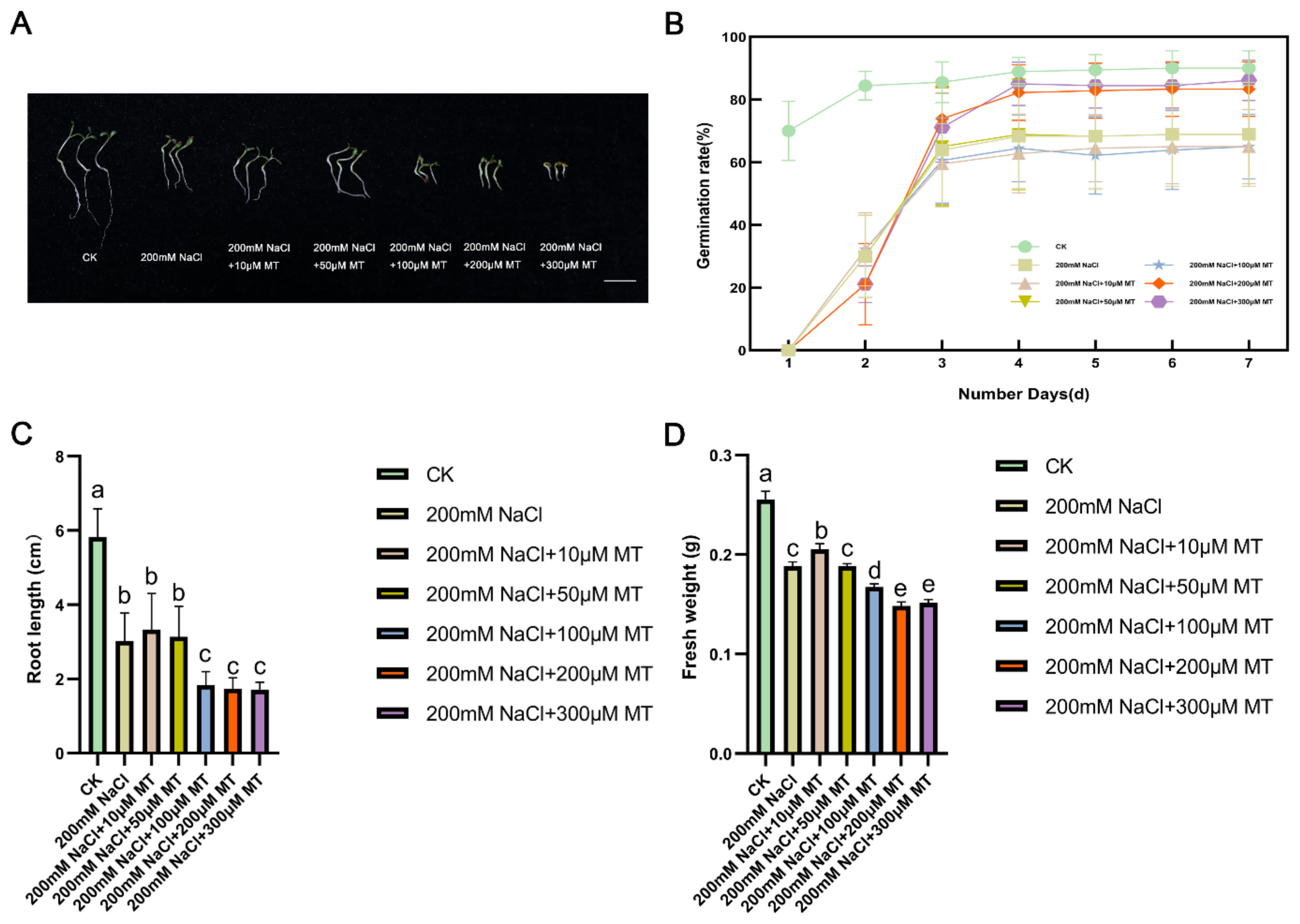
To study the seed germination and growth under salt stress, we counted the germination rate of alfalfa sprouts and measured their root length and fresh weight. Analysis of germination rates over seven days demonstrated differences among the treatments (
Figure 1A). On the seventh day, seeds treated with 200 μM and 300 μM MT exhibited higher germination rates compared to the salt stress group without MT. Conversely, seeds treated with 10 μM and 100 μM MT showed lower germination rates than those without MT. Notably, the 300 μM MT treatment resulted in germination rates closest to the CK group (
Figure 1B). In terms of fresh weight and root length, comparison between the salt stress groups treated with various concentrations of MT and the CK group revealed interesting results. The group treated with 10 μM MT displayed characteristics most similar to the CK group, with a fresh weight of 0.2053 g and a root length of 3.33 cm. In contrast, the salt stress group without MT displayed a fresh weight of 0.1884 g and a root length of 3.33 cm. Notably, the CK group had a fresh weight of 0.2553 g and a root length of 5.82 cm (
Figure 1C,D). These findings suggest the efficacy of 10 μM MT in ameliorating the growth of salt-stressed plants, as evidenced by superior fresh weight and root length outcomes compared to other MT concentrations.
Changes of Indicators in Oxidation System
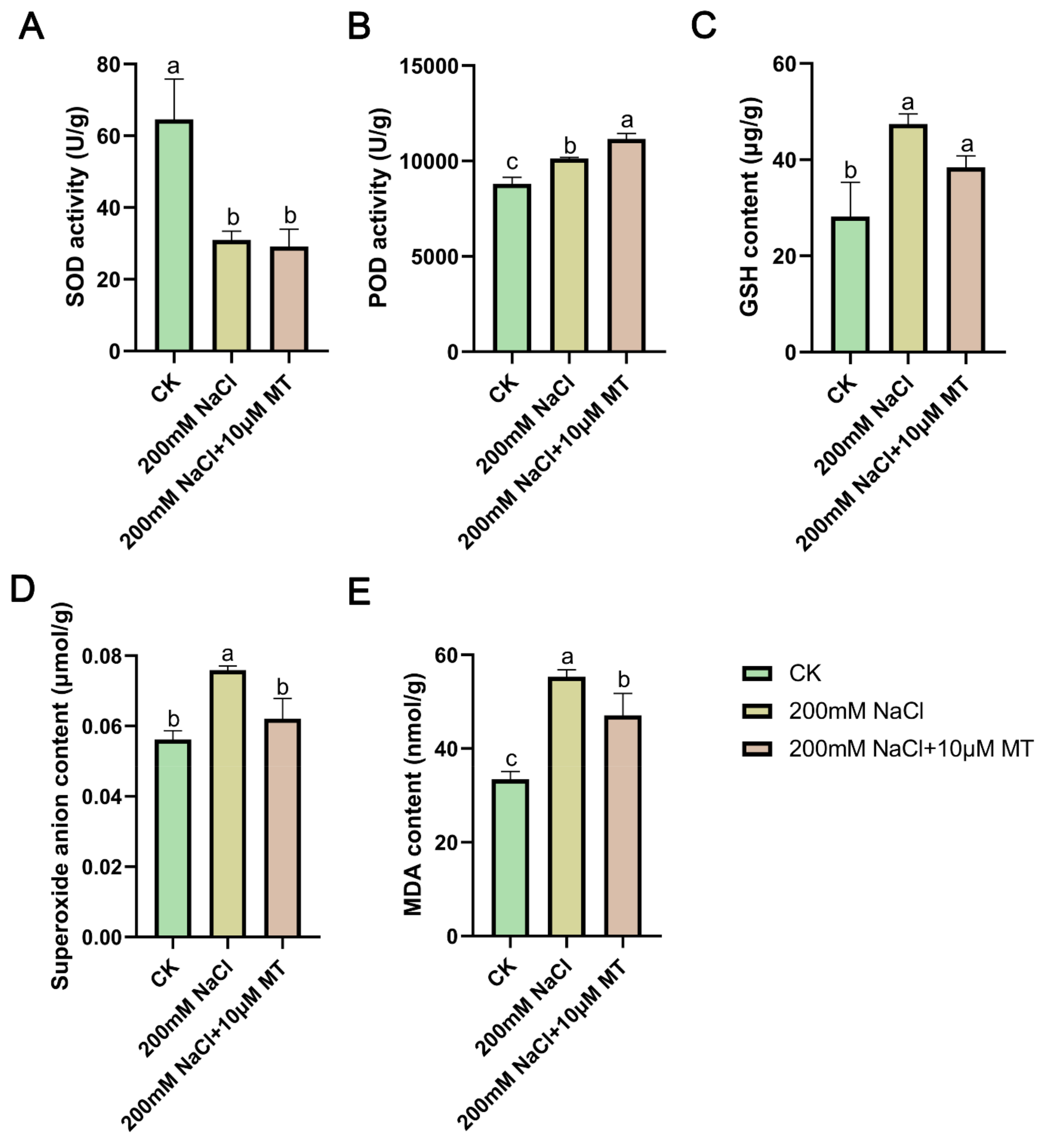
To thoroughly investigate the mechanism of melatonin (MT) on salt tolerance in alfalfa, we analyzed variations in oxidative system indexes under various treatments. Our examination of different concentrations of MT in alfalfa growth conditions under salt stress revealed that the group treated with 10 μM MT under salt stress exhibited the most favorable outcomes. Consequently, we assessed the physiological indexes in the control group (CK), the 200 mM NaCl treatment group, and the group treated with 10 μM MT under NaCl stress. It was found that SOD enzyme activity was reduced in the salt-stressed group, while POD (
Figure 2A,B), MDA, O
2- and GSH contents were increased in the salt-stressed group compared to the CK group. Interestingly, when 10 μM MT was added to the salt stress group, the activity of POD increased significantly by 10.17%, while the levels of MDA, O
2-, and GSH decreased by 14.91%, 18.22%, and 18.95% (
Figure 2C–E), respectively. Notably, there was minimal change in SOD enzyme activity with the addition of MT to the salt stress group.
Differential Expression Analysis and Cluster Analysis of Genes in Alfalfa Seedlings
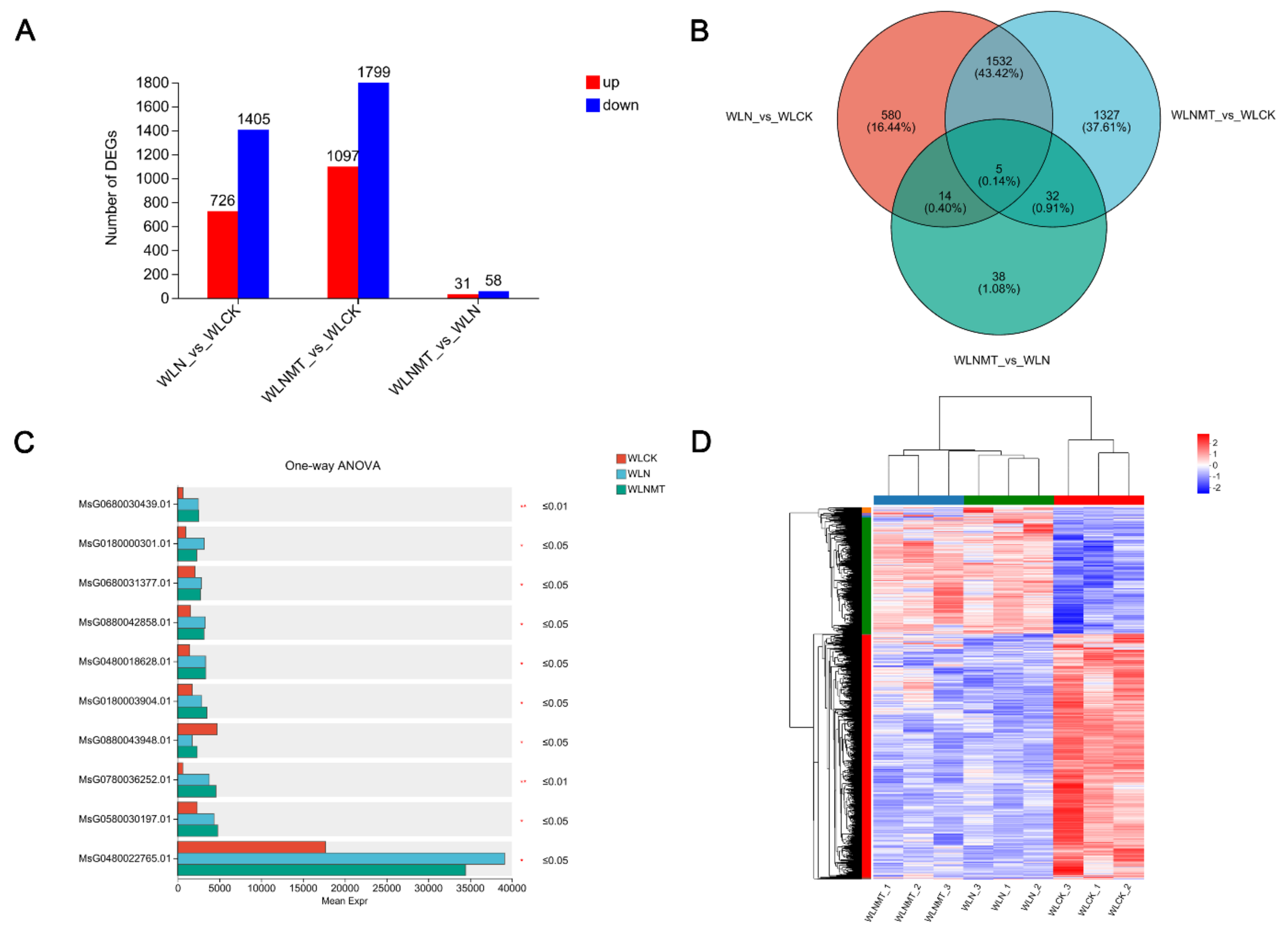
We first conducted transcriptomic analysis of WLCK, WLN, and WLNMT after evaluating changes in the oxidative system. By comparing the samples, we identified a total of 3,547 genes. Specifically, there were 726 up-regulated genes and 1,405 down-regulated genes in WLN compared to WLCK, 1,097 up-regulated genes and 1,799 down-regulated genes in WLNMT compared to WLCK, and 31 up-regulated genes and 58 down-regulated genes in WLNMT compared to WLN (
Figure 3A). Out of the 3,547 total genes, only 5 differentially expressed genes (DEGs) were common, representing merely 0.14% of the total genes. Notably, WLN shared 1,532 genes with WLCK and WLNMT (43.42% of total genes), WLN shared 14 genes with WLCK and WLNMT (0.40% of total genes), and WLNMT shared 32 genes with WLCK and WLN (0.32% of total genes). Additionally, there were 580 genes unique to WLCK (16.44% of total genes), 1,327 genes unique to WLNMT with WLCK (37.61% of total genes), and 38 genes unique to WLNMT with WLN (1.08% of total genes) (
Figure 3B;
Table S2). A one-way ANOVA with Tukey post-hoc multiple comparisons identified significant differences in several genes (
Figure 3C;
Table S2). Finally, to visually represent the data, a heat map was generated by clustering all identified DEGs (
Figure 3D;
Table S2).
GO Annotation Analysis and KEGG Enrichment Pathway Analysis of DEGs
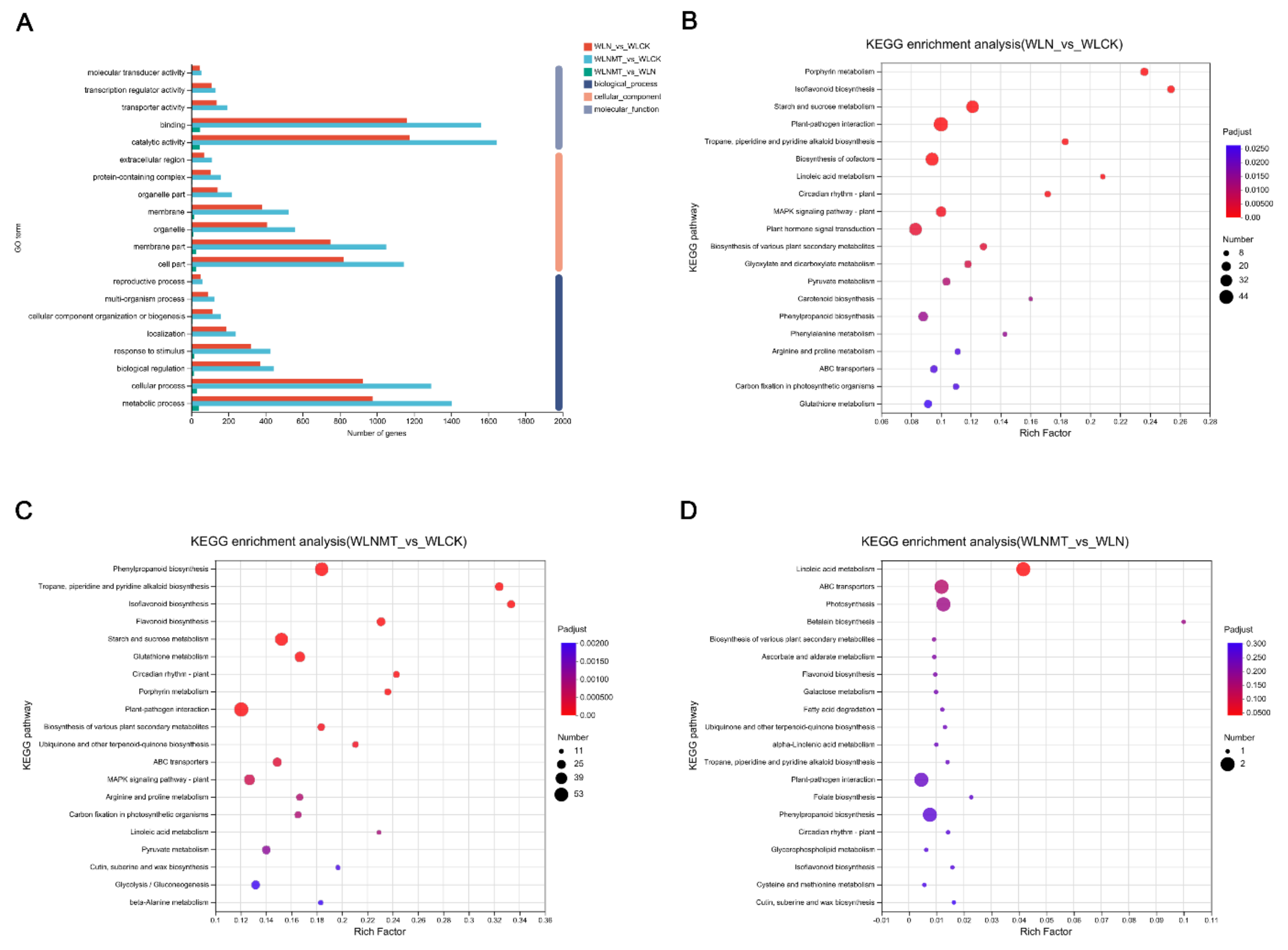
Analysis of gene ontology (GO) annotations revealed that differentially expressed genes (DEGs) were categorized into biological process (BP), cellular component (CC), and molecular function (MF). The DEGs from comparisons involving WLN versus WLCK, WLNMT versus WLCK, and WLNMT versus WLN, were primarily associated with metabolic processes, cellular processes, responses to stimuli, biological regulations, cellular component organization or biogenesis, and localization. Notably, the affected cellular components encompassed cell parts, membrane parts, organelles, protein-containing complexes, organelle parts, membranes, extracellular regions, and membrane-enclosed lumens. The primary molecular functions observed included binding, catalytic activity, structural molecule activity, transcription regulator activity, and molecular function regulation. In terms of biological processes, both metabolic and cellular processes were significantly enriched in WLN and WLCK as well as WLNMT and WLCK, with a stronger enrichment observed in WLNMT versus WLCK. Regarding cellular components, enrichment was observed in cellular parts and membrane parts in WLN versus WLCK and WLNMT versus WLCK, with greater significance in WLNMT versus WLCK. In the molecular function category, catalytic activity and binding activity were notably enriched in WLN versus WLCK and WLNMT versus WLCK, displaying a more pronounced enrichment in WLNMT versus WLCK (
Figure 4A;
Table S3).
Under salt stress conditions, the differential gene expression analysis revealed distinct enrichment patterns in various metabolic pathways. Specifically, in the comparison between WLN and WLCK, the DEGs were predominantly enriched in porphyrin metabolism, isoflavone biosynthesis, and linoleic acid metabolism (
Figure 4B;
Table S4). Conversely, when comparing WLNMT with WLCK, the DEGs showed enrichment in isoflavone biosynthesis, tolkanes, piperidinium, and pyridine alkaloids biosynthesis (
Figure 4C;
Table S5). In the comparison between WLNMT and WLN, the DEGs exhibited enrichment in betulinic acid metabolism, ABC transporter proteins, and photosynthesis (
Figure 4D;
Table S6). These results suggest that biological processes, such as isoflavone biosynthesis, were significantly impacted by salt stress, as evidenced by the observed enrichment patterns across the various comparisons.
Gene Co-Expression Networks
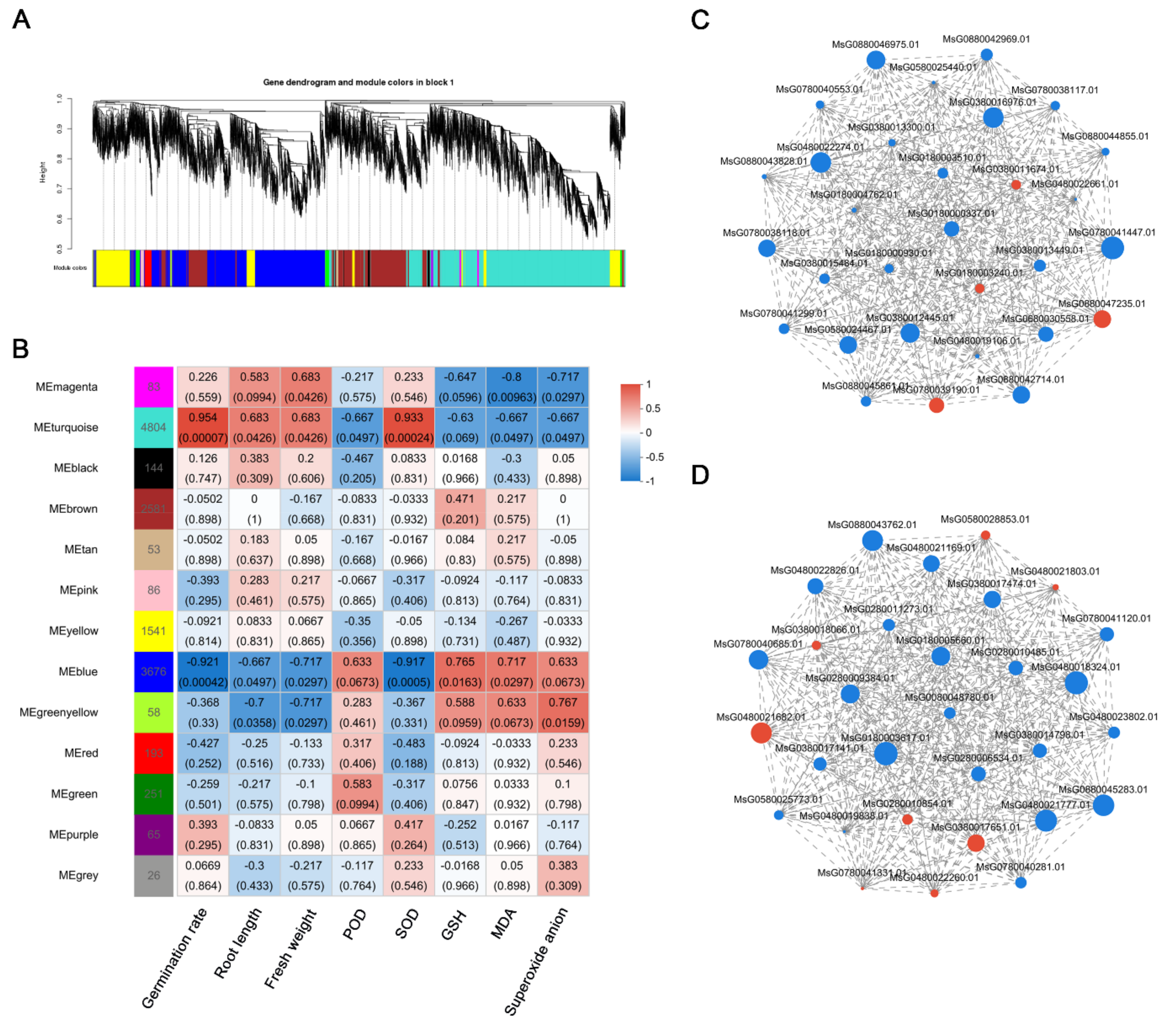
Thirteen co-expression modules were identified by the analysis conducted by Weighted Gene Co-expression Network Analysis (WGCNA) to investigate the correlation between Differentially Expressed Genes (DEGs) and physiological traits associated with salinity response (
Figure 5A;
Table S7). Module-trait analyses revealed that germination rate, root length, and fresh weight exhibited strong and positive correlations with gene transcript levels in the turquoise module, with correlation coefficients ranging from 0.683 to 0.954, indicating a potential linkage to enhanced growth under NaCl stress. Moreover, genes in the turquoise module demonstrated a significant positive correlation with SOD, suggesting their potential involvement in the augmented antioxidant capacity of plants under NaCl stress. Meanwhile, genes in the blue module exhibited strong and positive correlations with POD, GSH, MDA, and O
2- levels, with correlation coefficients ranging from 0.633 to 0.765, indicating their substantial accumulation under salt stress conditions. Furthermore, the turquoise module comprised a total of 4,804 DEGs, whereas the blue module contained 3,676 DEGs (
Figure 5B;
Table S7). By analyzing the top 30 genes in each module, the study revealed their close association with flavonoid biosynthesis, glutathione metabolism, phytohormone signaling, the MAPK signaling pathway, and ABC transporter proteins (
Figure 5C,D;
Tables S8 and S9).
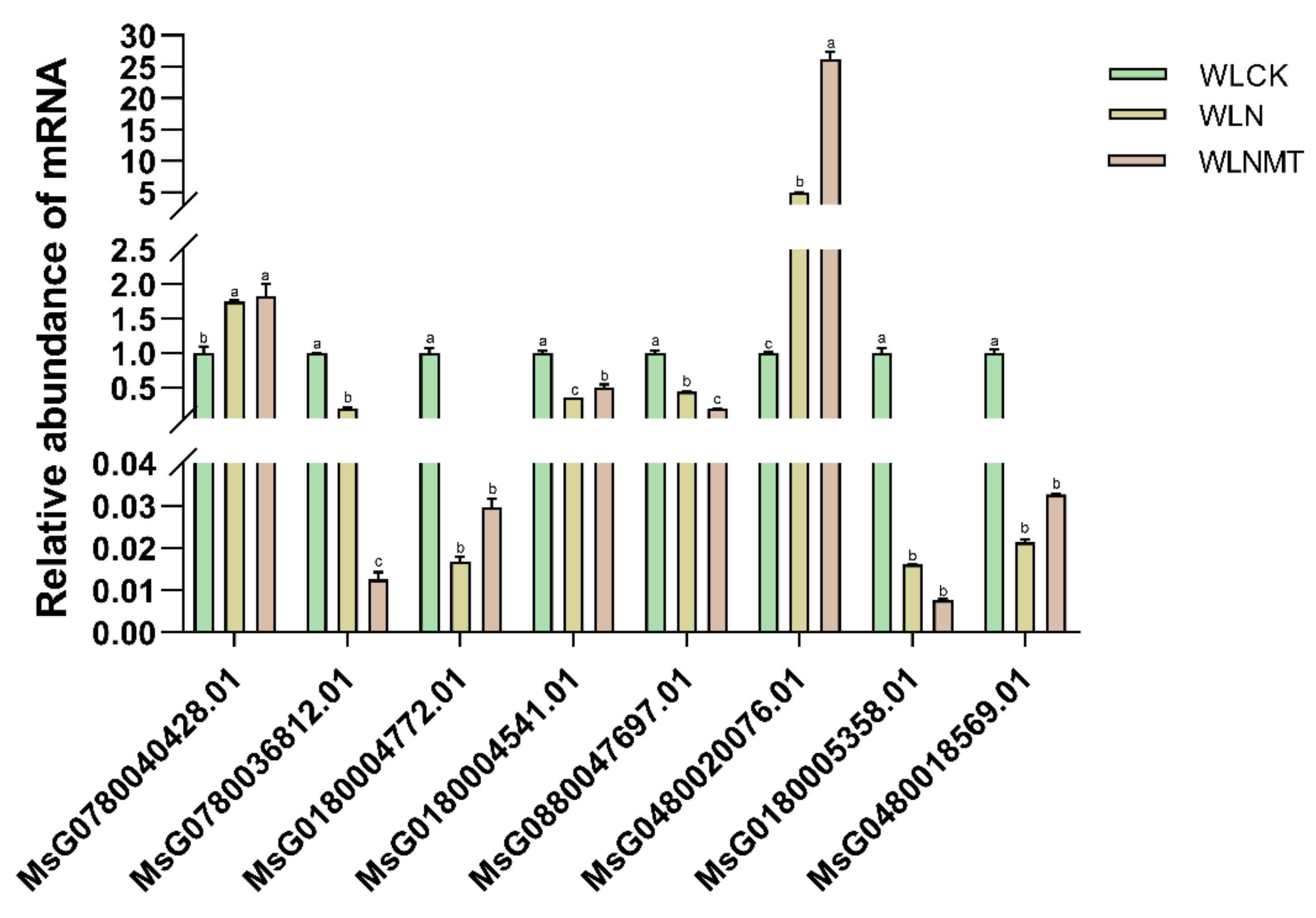
qRT-PCR Validation
We conducted qRT-PCR analysis on the eight selected genes, and the results showed that the measured mRNA relative abundance showed a consistent trend with the RNA-seq results (
Figure 6, raw data is shown in
Table S10), further validating the reliability of the transcriptome analysis results. The qRT-PCR results confirmed the accuracy of the RNA-Seq data, suggesting that these eight differentially expressed genes may play an important role in alfalfa’s response to salt stress.
Discussion
In recent years, advancements in multi-omics and data integration technologies have enabled a more comprehensive exploration of the growth and stress responses of alfalfa plants. These technologies, which include transcriptomics, proteomics, and metabolomics, have provided valuable insights into the underlying biological mechanisms of alfalfa plants (Song et al., 2016; Yang et al., 2021; Chen et al., 2021; Xu et al., 2021; Ma et al., 2021). Despite alfalfa’s ability to thrive moderately in saline soils, it is highly sensitive to elevated salt stress, with 50–200 mmol L−1 of NaCl significantly impeding its growth (Mokhtari et al., 2017b). For this study, alfalfa plants were chosen as research subjects, and root samples were obtained for transcriptome sequencing following exposure to NaCl stress. The subsequent detailed analysis of the transcriptome data uncovered significant insights. Specifically, the transcriptome analysis of alfalfa roots highlighted the enrichment of differentially expressed genes (DEGs) in key pathways such as isoflavone biosynthesis, flavonoid biosynthesis, ABC transporter proteins, MAPK signaling pathway, phytohormone signaling, and carotenoid biosynthesis in response to NaCl stress conditions. This focused investigation sheds light on the complex molecular processes underlying alfalfa’s response to salt stress, paving the way for a more nuanced understanding of its adaptive mechanisms.
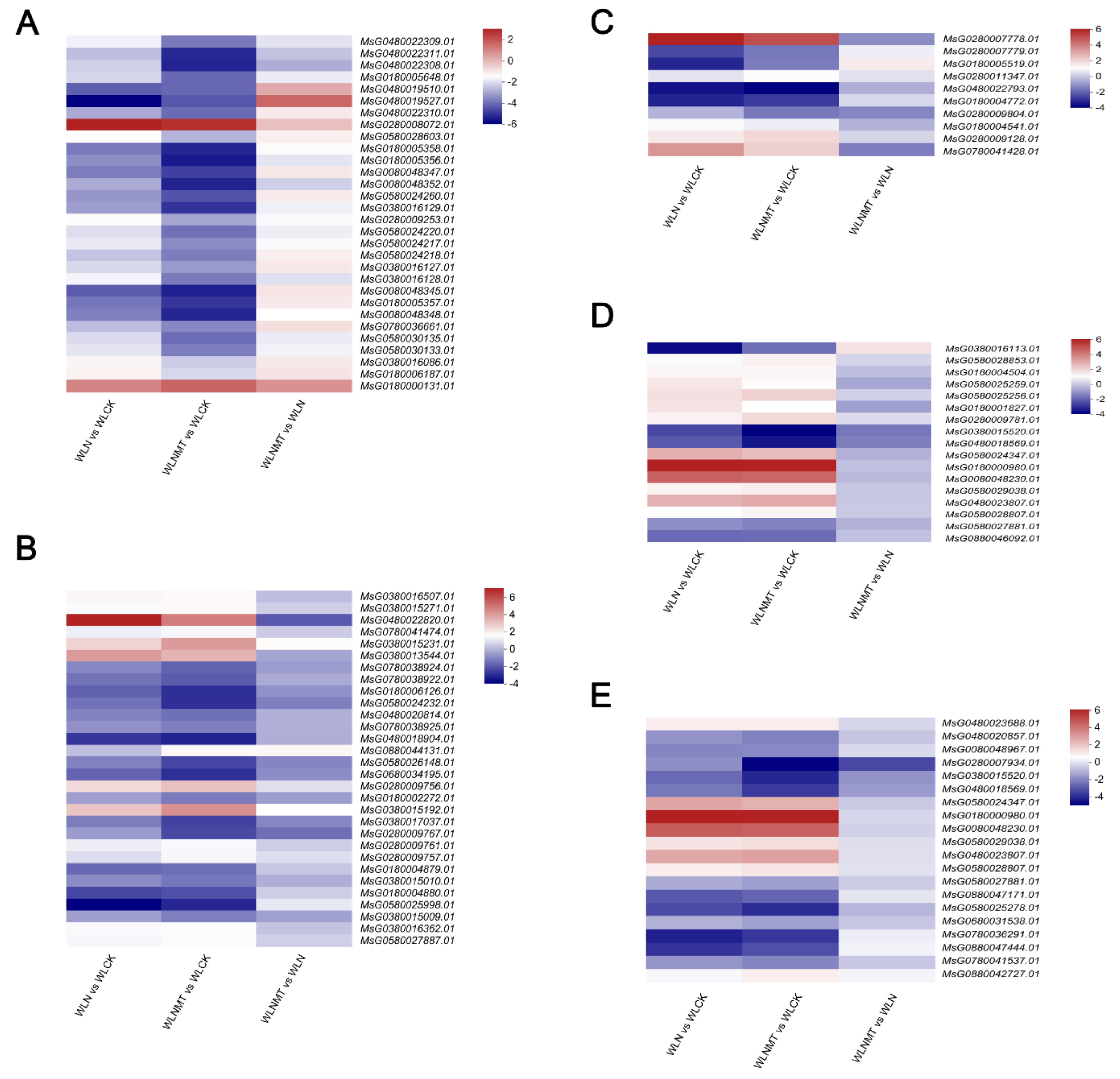
Biosynthesis of Flavonoid Compounds
Flavonoids, a major secondary metabolite in plants, play a crucial role in plant resistance. They can be classified into various types such as flavonols, flavanones, isoflavonoids, catechins, and anthocyanins, based on their molecular structure. During our transcriptomic analysis of young alfalfa shoots, we observed a significant up-regulation of genes
HCT,
DFR, and
CYP81E9 associated with flavonoid biosynthesis. This led to an increase in the synthesis of metabolites like naringenin chalcone, naringenin, white aspergillus, and white cornflower in WLN compared to WLCK, as well as in WLNMT compared to WLCK. Notably, genes involved in the phenylpropane biosynthetic pathway upstream of flavonoid biosynthesis were also up-regulated, resulting in enhanced production of flavonoid-initiating metabolites such as coumaroyl coenzyme A and caffeine coenzyme A, ultimately leading to increased flavonoid synthesis. The accumulation of flavonoids significantly boosts the antioxidant capacity of plants, thereby enhancing their salt tolerance. Furthermore, the up-regulation of
DFR stimulates the synthesis of anthocyanins, which has been shown to enhance the salt tolerance of kale-type oilseed rape (Kim et al., 2017). In our comparative analysis between WLNMT and WLCK, we observed a notable up-regulation of the aforementioned genes under the influence of melatonin compared to pure salt stress treatment. This finding suggests that melatonin may partially promote flavonoid synthesis in plants, thereby mitigating the detrimental effects of salt stress on alfalfa (
Figure 7A).
Plant Hormone Synthesis and Signal Transduction
Plant hormones display a crucial function in plant defense by regulating the response to stress. The intricate signal transduction pathways among different plant hormones work collaboratively in this regard. Specifically, gibberellins, abscisic acid, ethylene, jasmonic acid, and salicylic acid are key phytohormones involved in plant resistance (Waadt et al., 2022). Notably, our transcriptome analysis revealed up-regulation of the
DELLA gene, associated with the gibberellin signaling pathway, in alfalfa under salt stress conditions in WLN versus WLCK and WLNMT versus WLCK. Remarkably, the melatonin-treated group exhibited a more pronounced up-regulation of
DELLA. The
DELLAprotein, acting as a central negative regulator in the gibberellin signaling pathway, suppresses the expression of genes related to this pathway, thus impeding plant growth and facilitating rapid adaptation to the unfavorable environment of salt stress. In the context of salt stress, the content of gibberellin (GA) decreases in plants due to the down-regulation of
GA2ox, a pivotal gene in GA synthesis, as demonstrated (Zhu et al., 2019). Essentially, salt stress hampers GA synthesis in plants, resulting in decreased GA levels. Our transcriptome analysis further revealed a significant down-regulation of key genes involved in gibberellin synthesis,
KAO and
GA2ox, under salt stress conditions with melatonin treatment, aligning with prior research findings (
Figure 7C).
Abscisic acid (ABA) is a sesquiterpene phytohormone known to enhance salt tolerance in rice by regulating stomatal closure and improving water balance (Shahzad et al., 2017). It is considered a vital stress-responsive phytohormone crucial for the plant’s salt stress response. The levels of ABA in plants increase rapidly under salt stress conditions, leading to the activation of the ABA signaling pathway and subsequent stomatal closure along with other physiological plant responses (Chen et al., 2020a). In the current study,
NCED, a key gene for ABA synthesis, was significantly up-regulated after salt treatment.
CYP707A, a key gene in the ABA degradation pathway, was significantly down-regulated after salt treatment. The elevated ABA levels prompt its distribution within the plant, ultimately leading to stomatal closure (Poór et al., 2019). In the ABA pathway of action, ABA binds to the receptor protein
PYL, releasing the inhibition of
SnRK2 kinase activity by the phosphatase
PP2C, which then activates
SnRK2. This activation induces a plant stress response, inhibits growth, and prompts cells to enter dormancy to withstand external stresses. Notably, transcriptome analysis demonstrated a significant down-regulation in
SnRK2 kinase activity under WLNMT compared to WLCK conditions, suggesting that the melatonin application alleviated the inhibition of ABA signaling. This ultimately facilitated plant growth by promoting ABA inhibition and thus overcoming the plant’s stress response (
Figure 7D) (Poór et al., 2019).
MAPK Signalling Pathway
The MAPK (Mitogen-Activated Protein Kinase) cascade response pathway is a crucial mechanism in plants’ response to adversity stress and represents a significant pathway for their adaptation to harsh environments. MAPK, a type of protein kinase, functions as a key regulator within plant cells. When plants encounter adversity stressors such as drought, high temperature, low temperature, or salt stress, the MAPK pathway is activated, initiating a cascade response. This process involves the phosphorylation and activation of multiple protein kinases, ultimately regulating the expression of downstream responsive genes (Kumar, Raina & Sultan, 2020). Through research on Arabidopsis thaliana, a MAPK signaling pathway consisting of
MKKK1,
MKK2, and
MPK4/
MPK6 was discovered. The results showed that plants overexpressing
MKK2 had stronger salt tolerance (Teige et al., 2004). In rice, salt tolerance is closely related to specific MAPK genes, including
OsMPK44,
OsMPK5, and
OsMPK4, which are known to be involved in salt stress tolerance. By increasing the expression of
OsMPK5 and
OsMPK44 genes, it was observed that the resistance of rice to salt stress was enhanced (Moustafa et al., 2014). The above results indicate that the MAPK signaling pathway plays an important role in plants’ response to salt stress. In our current study, transcriptome analysis unveiled that under salt stress, the expression of
PYL, a critical gene in the ABA action pathway of the MAPK signaling approach, is down-regulated. This downregulation alleviates the inhibitory impact of
PP2C on the
SnRK2 gene. This further led to an increase in the expression of MAPK downstream gene
MKK3, which helped alfalfa adapt to salt stress conditions. At the same time, the expression of
CALM (
MsG0880047171.01, MsG0580025278.01, MsG0780036291.01, MsG0880047444.01) also showed a downward trend, resulting in a decrease in the synthesis of calcium-binding proteins and a decrease in the expression of
MPK8 gene, in order to maintain the balance of reactive oxygen species in alfalfa and thus enhance its tolerance to salt stress (
Figure 7E).
ABC Transporters
ABC transporter proteins, as one of the large-scale protein families, play an essential role in organ growth, plant nutrient uptake, plant development regulation, abiotic stress response, and pathogen resistance (Kang et al., 2011). In this study, KEGG enrichment analysis under WLNMT vs. WLN indicated that under salt stress, the expression of ABCC2 (MsG0680031988.01) was up-regulated. Moreover, the genes encoding ABCG proteins, specifically ABCG2 (MsG0480023470.01, MsG0480021815.01, MsG0480021814.01, MsG0180003146.01, MsG0680034419.01), were also up-regulated. ABCC transporter proteins have shown involvement in detoxification processes of abscisic acid and glucosyl esters, vesicular transport, organic anion transport, regulation of chlorophyll degradation, and phytic acid content of plant seeds in prior studies (Andolfo et al., 2015). While ABCG transporter proteins are the largest subgroup within the plant ABC transporter protein family, they have been associated with various essential functions. Many proteins within this family are implicated in plant resistance mechanisms against pathogens, antifungal compounds, and herbicides. Additionally, they play crucial roles in the transmission of signaling molecules and secretion of volatile compounds (Stein et al., 2006; Kim et al., 2007; Ruocco et al., 2011). The transporter substrates of ABCG transporter proteins include terpenoids, flavonoids, phytohormones, and heavy metals (Wang et al., 2024). This suggests that ABCG transporters can enhance the antioxidant capacity of plants by facilitating the transport of flavonoids and certain phytohormones. Ultimately, this process improves the salt tolerance of plants.
Biosynthesis of Physiological Regulatory Substances
Under salt stress conditions, plants adjust their phytohormone levels and synthesize organic osmotic solutes in response to osmotic stress, thereby increasing their tolerance to salt stress (Yang et al., 2020; Singh et al., 2022). A variety of osmoprotectants derived from amino acids, such as proline, arginine, and alanine (Suprasanna et al., 2014), accumulate in cells during salt stress, reducing the osmotic potential of the cells and scavenging reactive oxygen species (Ashraf & Foolad, 2007; Hayat et al., 2012). Among these osmoprotectants, proline accumulates significantly, playing a crucial role in plant response to salt stress, while alanine is also actively involved in plant response to abiotic stresses (Singh et al., 2022). In this study, using KEGG enrichment pathway analysis, we investigated the pathway of proline synthesis from glutamate. The expression of key genes involved in proline biosynthesis, namely Δ1-pyrroline-5-carboxylate synthetase (P5CS) and proC, was significantly up-regulated, whereas PRODH, linked to proline degradation, was down-regulated when comparing different treatment conditions. Specifically, P5CS, a key enzyme for proline biosynthesis, showed increased expression, indicating enhanced proline synthesis under salt stress conditions. This upregulation was further promoted by the addition of MT, suggesting that MT effectively assists plants in resisting the adverse effects of salt stress by enhancing proline synthesis. Consequently, the findings of this study offer a new perspective on the role of MT in plant response to salt stress, emphasizing its potential to enhance plant resistance mechanisms.
In this study, we conducted transcriptome analysis of tryptophan metabolic processes to explore the effect of melatonin synthesis on the response of plants to salt stress (Back, 2021). It was noted that the expression of key enzymes involved in melatonin synthesis, including N-acetylserotonin methyltransferase (ASMT, MsG0480020076.01, MsG0480020072.01), experienced significant up-regulation in WLN compared to WLCK, and in WLNMT compared to WLCK. ASMT is known to play a critical role in melatonin synthesis. Additionally, the up-regulation of Tyrosine decarboxylase (TDC, MsG0880043065.01) was also observed when melatonin was introduced to the treatment group, confirming the increased melatonin synthesis in plants. Melatonin, known to play a crucial role in abiotic stresses, particularly in salt stress conditions, appears to be an essential factor in plant responses. Based on these findings, the hypothesis was formulated that plants combat the negative effects of salt stress by boosting the levels of endogenous melatonin. This hypothesis was substantiated through experiments showing that the application of exogenous melatonin effectively mitigated the impact of salt stress on plants. Consequently, these results offer novel insights into the mechanisms involved in plant responses to salt stress.
Conclusions
Transcriptomics was employed in this study to analyze the impact of NaCl stress on gene transcription levels in alfalfa plants. It was observed that flavonoids, glutathione, and various phytohormones are crucial for plant adaptation under salt stress. Under salt stress conditions, genes related to oxidoreductase activity and secondary metabolism demonstrated decreased expression. However, supplementation with melatonin was found to enhance the expression levels of genes inhibited by salt stress, which ultimately led to an increase in the synthesis and metabolism of flavonoids, glutathione, and phytohormones, as well as the activation of ABC transporter proteins and the MAPK signaling pathway, thereby mitigating the negative effects of salt stress on gene expression in alfalfa plants. Consequently, the ability of plants to handle salt stress improved. The study elucidated the potential molecular mechanisms of salt stress in alfalfa plants and identified several key genes, paving the way for a more comprehensive examination of salt tolerance in alfalfa plants.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplemental information for this article can be found online.
Author Contributions
Wenxuan Zhu performed the experiments, analyzed the data, prepared figures and/or tables, authored drafts of the article, and approved the final draft. Xiangling Ren conceived and designed the experiments, and approved the final draft. Zirui Liu performed the experiments, analyzed the data, and approved the final draft. Guomin Li performed the experiments, analyzed the data, and approved the final draft. Yingao Li performed the experiments, analyzed the data, and approved the final draft. Jingzhuo Wang performed the experiments, analyzed the data, and approved the final draft. Defeng Li conceived and designed the experiments, and approved the final draft. Yinghua Shi conceived and designed the experiments, and approved the final draft. Chengzhang Wang conceived and designed the experiments, and approved the final draft. Hao Sun conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft. Xiaoyan Zhu conceived and designed the experiments, and approved the final draft.
Funding
This work was supported by the earmarked fund for the National Science Foundation of China (No. 32001393), the China Postdoctoral Science Foundation (2022M721043), the Science and Technology Innovation 2030-Major Project (2022ZD04011), the Henan Science and Technology Research Projects (222103810006), President fund project of Guangdong Academy of Agricultural Sciences (202111), the China Agriculture Research System (CARS-34). The funding body played no role in the design of the study, the collection, analysis and interpretation of the data or the writing of the manuscript.
Data Availability Statement
The following information was supplied regarding data availability: The raw data are available in the
Supplemental Files.
Conflicts of Interest
The authors declare there are no competing interests.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences: Sequences are available at NCBI GenBank: PRJNA1099414.
References
- Andolfo G, Ruocco M, Di Donato A, Frusciante L, Lorito M, Scala F, Ercolano MR. Genetic variability and evolutionary diversification of membrane ABC transporters in plants. BMC Plant Biology 2015, 15, 51. [CrossRef]
- Arnao MB, Hernández-Ruiz J. Functions of melatonin in plants: A review. Journal of Pineal Research 2015, 59, 133–150. [CrossRef]
- Arnao MB, Hernández-Ruiz J. Melatonin and its relationship to plant hormones. Annals of Botany 2018, 121, 195–207. [CrossRef]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene Ontology: Tool for the unification of biology. Nature Genetics 2000, 25, 25–29. [CrossRef]
- Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiology and Biochemistry 2018, 123, 268–280. [CrossRef]
- Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany 2007, 59, 206–216. [CrossRef]
- Back K. Melatonin metabolism, signaling and possible roles in plants. The Plant Journal 2021, 105, 376–391. [CrossRef]
- Baena G, Xia L, Waghmare S, Yu Z, Guo Y, Blatt MR, Zhang B, Karnik R. Arabidopsis SNARE SYP132 impacts on PIP2;1 trafficking and function in salinity stress. The Plant Journal 2024, tpj.16649. [CrossRef]
- Cen H, Wang T, Liu H, Tian D, Zhang Y. Melatonin Application Improves Salt Tolerance of Alfalfa (Medicago sativa L.) by Enhancing Antioxidant Capacity. Plants 2020, 9, 220. [CrossRef]
- Chen K, Li G, Bressan RA, Song C, Zhu J, Zhao Y. Abscisic acid dynamics, signaling, and functions in plants. Journal of Integrative Plant Biology 2020, 62, 25–54. [CrossRef]
- Chen Y, Mao J, Sun L, Huang B, Ding C, Gu Y, Liao J, Hu C, Zhang Z, Yuan S, Yuan M. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiologia Plantarum 2018, 164, 349–363. [CrossRef]
- Chen L, Xia F, Wang M, Mao P. Physiological and proteomic analysis reveals the impact of boron deficiency and surplus on alfalfa (Medicago sativa L.) reproductive organs. Ecotoxicology and Environmental Safety 2021, 214, 112083. [CrossRef]
- Chen H, Zeng Y, Yang Y, Huang L, Tang B, Zhang H, Hao F, Liu W, Li Y, Liu Y, Zhang X, Zhang R, Zhang Y, Li Y, Wang K, He H, Wang Z, Fan G, Yang H, Bao A, Shang Z, Chen J, Wang W, Qiu Q. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nature Communications 2020, 11, 2494. [CrossRef]
- Chen Y, Zhang S, Du S, Zhang X, Wang G, Huang J, Jiang J. Effects of Exogenous Potassium (K+) Application on the Antioxidant Enzymes Activities in Leaves of Tamarix ramosissima under NaCl Stress. Genes 2022, 13, 1507. [CrossRef] [PubMed]
- Chen S, Zhou Y, Chen Y, Gu J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [CrossRef]
- ElSayed AI, Rafudeen MS, Gomaa AM, Hasanuzzaman M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiologia Plantarum 2021, 173, 1369–1381. [CrossRef] [PubMed]
- Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I, Pereira E, Tuteja N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiology and Biochemistry 2013, 70, 204–212. [CrossRef]
- Gong Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. Journal of Integrative Plant Biology 2021, 63, 429–430. [CrossRef]
- Gou J, Debnath S, Sun L, Flanagan A, Tang Y, Jiang Q, Wen J, Wang Z. From model to crop: Functional characterization of SPL 8 in M. truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa. Plant Biotechnology Journal 2018, 16, 951–962. [CrossRef]
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: A review. Plant Signaling & Behavior 2012, 7, 1456–1466. [CrossRef]
- He F, Wei C, Zhang Y, Long R, Li M, Wang Z, Yang Q, Kang J, Chen L. Genome-Wide Association Analysis Coupled With Transcriptome Analysis Reveals Candidate Genes Related to Salt Stress in Alfalfa (Medicago sativa L.). Frontiers in Plant Science 2022, 12, 826584. [CrossRef]
- Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 2000, 28, 27–30. [CrossRef]
- Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Science 2019, 28, 1947–1951. [CrossRef]
- Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Research 2023, 51, D587–D592. [CrossRef]
- Kang J, Park J, Choi H, Burla B, Kretzschmar T, Lee Y, Martinoia E. Plant ABC Transporters. The Arabidopsis Book 2011, 9, e0153. [CrossRef] [PubMed]
- Kim D, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. The Plant Journal 2007, 50, 207–218. [CrossRef]
- Kim D, Langmead B, Salzberg SL. HISAT: A fast spliced aligner with low memory requirements. Nature Methods 2015, 12, 357–360. [CrossRef]
- Kim J, Lee WJ, Vu TT, Jeong CY, Hong S-W, Lee H. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Reports 2017, 36, 1215–1224. [CrossRef]
- Klopfenstein DV, Zhang L, Pedersen BS, Ramírez F, Warwick Vesztrocy A, Naldi A, Mungall CJ, Yunes JM, Botvinnik O, Weigel M, Dampier W, Dessimoz C, Flick P, Tang H. GOATOOLS: A Python library for Gene Ontology analyses. Scientific Reports 2018, 8, 10872. [CrossRef]
- Kumar K, Raina SK, Sultan SM. Arabidopsis MAPK signaling pathways and their cross talks in abiotic stress response. Journal of Plant Biochemistry and Biotechnology 2020, 29, 700–714. [CrossRef]
- Li H, Chang J, Chen H, Wang Z, Gu X, Wei C, Zhang Y, Ma J, Yang J, Zhang X. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Frontiers in Plant Science 2017, 8. [CrossRef]
- Li J, Liu J, Zhu T, Zhao C, Li L, Chen M. The Role of Melatonin in Salt Stress Responses. International Journal of Molecular Sciences 2019, 20, 1735. [CrossRef]
- Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, Jia D, Fu M, Ma F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. Journal of Pineal Research 2012, 53, 298–306. [CrossRef]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 2014, 15, 550. [CrossRef]
- Ma L, Li X, Zhang J, Yi D, Li F, Wen H, Liu W, Wang X. MsWRKY33 increases alfalfa (Medicago sativa L.) salt stress tolerance through altering the ROS scavenger via activating MsERF5 transcription. Plant Cell Environment 2023, 46, 3887–3901. [CrossRef]
- Ma Q, Xu X, Wang W, Zhao L, Ma D, Xie Y. Comparative analysis of alfalfa (Medicago sativa L.) seedling transcriptomes reveals genotype-specific drought tolerance mechanisms. Plant Physiology and Biochemistry 2021, 166, 203–214. [CrossRef]
- Meng Y, Yin Q, Yan Z, Wang Y, Niu J, Zhang J, Fan K. Exogenous Silicon Enhanced Salt Resistance by Maintaining K+/Na+ Homeostasis and Antioxidant Performance in Alfalfa Leaves. Frontiers in Plant Science 2020, 11, 1183. [CrossRef] [PubMed]
- Mokhtari F, Rafiei F, Shabani L, Shiran B. Differential expression pattern of transcription factors across annual Medicago genotypes in response to salinity stress. Biologia plantarum 2017, 61, 227–234. [CrossRef]
- Mokhtari F, Rafiei F, Shabani L, Shiran B. Differential expression pattern of transcription factors across annual Medicago genotypes in response to salinity stress. Biologia plantarum 2017, 61, 227–234. [CrossRef]
- Morton MJL, Awlia M, Al-Tamimi N, Saade S, Pailles Y, Negrão S, Tester M. Salt stress under the scalpel—dissecting the genetics of salt tolerance. The Plant Journal 2019, 97, 148–163. [CrossRef]
- Moustafa K, AbuQamar S, Jarrar M, Al-Rajab AJ, Trémouillaux-Guiller J. MAPK cascades and major abiotic stresses. Plant Cell Reports 2014, 33, 1217–1225. [CrossRef] [PubMed]
- Mukherjee S, David A, Yadav S, Baluška F, Bhatla SC. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiologia Plantarum 2014, 152, 714–728. [CrossRef] [PubMed]
- Park S, Back K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. Journal of Pineal Research 2012, 53, 385–389. [CrossRef] [PubMed]
- Poór P, Borbély P, Czékus Z, Takács Z, Ördög A, Popović B, Tari I. Comparison of changes in water status and photosynthetic parameters in wild type and abscisic acid-deficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. Journal of Plant Physiology 2019, 232, 130–140. [CrossRef] [PubMed]
- Radovic J, Sokolovic D, Markovic J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnology in Animal Husbandry 2009, 25, 465–475. [CrossRef]
- Reinemer P, Dirr HW, Ladenstein R, Huber R, Lo Bello M, Federici G, Parker MW. Three-dimensional structure of class π glutathione S-transferase from human placenta in complex with S-hexylglutathione at 2.8 Å resolution. Journal of Molecular Biology 1992, 227, 214–226. [CrossRef]
- Ruocco M, Ambrosino P, Lanzuise S, Woo SL, Lorito M, Scala F. Four potato (Solanum tuberosum) ABCG transporters and their expression in response to abiotic factors and Phytophthora infestans infection. Journal of Plant Physiology 2011, 168, 2225–2233. [CrossRef]
- Sahab S, Suhani I, Srivastava V, Chauhan PS, Singh RP, Prasad V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Science of The Total Environment 2021, 764, 144164. [CrossRef]
- Sarwar M, Saleem MF, Ullah N, Rizwan M, Ali S, Shahid MR, Alamri SA, Alyemeni MN, Ahmad P. Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Scientific Reports 2018, 8, 17086. [CrossRef]
- Shahzad R, Khan AL, Bilal S, Waqas M, Kang S-M, Lee I-J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environmental and Experimental Botany 2017, 136, 68–77. [CrossRef]
- Shen C, Du H, Chen Z, Lu H, Zhu F, Chen H, Meng X, Liu Q, Liu P, Zheng L, Li X, Dong J, Liang C, Wang T. The Chromosome-Level Genome Sequence of the Autotetraploid Alfalfa and Resequencing of Core Germplasms Provide Genomic Resources for Alfalfa Research. Molecular Plant 2020, 13, 1250–1261. [CrossRef]
- Singh P, Choudhary KK, Chaudhary N, Gupta S, Sahu M, Tejaswini B, Sarkar S. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Frontiers in Plant Science 2022, 13, 1006617. [CrossRef] [PubMed]
- Song L, Jiang L, Chen Y, Shu Y, Bai Y, Guo C. Deep-sequencing transcriptome analysis of field-grown Medicago sativa L. crown buds acclimated to freezing stress. Functional Integrative Genomics 2016, 16, 495–511. [CrossRef]
- Stein M, Dittgen J, Sánchez-Rodríguez C, Hou B-H, Molina A, Schulze-Lefert P, Lipka V, Somerville S. Arabidopsis PEN3/PDR8, an ATP Binding Cassette Transporter, Contributes to Nonhost Resistance to Inappropriate Pathogens That Enter by Direct Penetration. The Plant Cell 2006, 18, 731–746. [CrossRef] [PubMed]
- Su X, Xin L, Li Z, Zheng H, Mao J, Yang Q. Physiology and transcriptome analyses reveal a protective effect of the radical scavenger melatonin in aging maize seeds. Free Radical Research 2018, 52, 1094–1109. [CrossRef]
- Suprasanna P, Rai AN, Kumari PH, Kumar SA, Kishor PBK. 2014. Modulation of proline: Implications in plant stress tolerance and development. In: Anjum NA, Gill SS, Gill R eds. Plant adaptation to environmental change: Significance of amino acids and their derivatives. UK: CABI, 68–96. [CrossRef]
- Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H. The MKK2 Pathway Mediates Cold and Salt Stress Signaling in Arabidopsis. Molecular Cell 2004, 15, 141–152. [CrossRef] [PubMed]
- The Gene Ontology Consortium, Aleksander SA, Balhoff J, Carbon S, Cherry JM, Drabkin HJ, Ebert D, Feuermann M, Gaudet P, Harris NL, Hill DP, Lee R. The Gene Ontology knowledgebase in 2023. GENETICS 2023, 224, iyad031. [CrossRef] [PubMed]
- Tyerman SD, Munns R, Fricke W, Arsova B, Barkla BJ, Bose J, Bramley H, Byrt C, Chen Z, Colmer TD, Cuin T, Day DA, Foster KJ, Gilliham M, Henderson SW, Horie T, Jenkins CLD, Kaiser BN, Katsuhara M, Plett D, Miklavcic SJ, Roy SJ, Rubio F, Shabala S, Shelden M, Soole K, Taylor NL, Tester M, Watt M, Wege S, Wegner LH, Wen Z. Energy costs of salinity tolerance in crop plants. New Phytologist 2019, 221, 25–29. [CrossRef]
- Waadt R, Seller CA, Hsu P-K, Takahashi Y, Munemasa S, Schroeder JI. Plant hormone regulation of abiotic stress responses. Nature Reviews Molecular Cell Biology 2022, 23, 680–694. [CrossRef]
- Wang F, Wang C, Sun Y, Wang N, Li X, Dong Y, Yao N, Liu X, Chen H, Chen X, Wang Z, Li H. Overexpression of vacuolar proton pump ATPase (V-H+-ATPase) subunits B, C and H confers tolerance to salt and saline-alkali stresses in transgenic alfalfa (Medicago sativa L.). Journal of Integrative Agriculture 2016, 15, 2279–2289. [CrossRef]
- Wang Y, Zhang X, Yan Y, Niu T, Zhang M, Fan C, Liang W, Shu Y, Guo C, Guo D, Bi Y. GmABCG5, an ATP-binding cassette G transporter gene, is involved in the iron deficiency response in soybean. Frontiers in Plant Science 2024, 14, 1289801. [CrossRef] [PubMed]
- Wen D, Gong B, Sun S, Liu S, Wang X, Wei M, Yang F, Li Y, Shi Q. Promoting Roles of Melatonin in Adventitious Root Development of Solanum lycopersicum L. by Regulating Auxin and Nitric Oxide Signaling. Frontiers in Plant Science 2016, 7. [CrossRef]
- Xu Z, Chen X, Lu X, Zhao B, Yang Y, Liu J. Integrative analysis of transcriptome and metabolome reveal mechanism of tolerance to salt stress in oat (Avena sativa L.). Plant Physiology and Biochemistry 2021, 160, 315–328. [CrossRef]
- Xu L, Yue Q, Bian F, Sun H, Zhai H, Yao Y. Melatonin Enhances Phenolics Accumulation Partially via Ethylene Signaling and Resulted in High Antioxidant Capacity in Grape Berries. Frontiers in Plant Science 2017, 8, 1426. [CrossRef]
- Yang Z, Li J-L, Liu L-N, Xie Q, Sui N. Photosynthetic Regulation Under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Frontiers in Plant Science 2020, 10, 1722. [CrossRef]
- Yang X, Zhang Y, Lai J, Luo X, Han M, Zhao S, Zhu Y. Analysis of the biodegradation and phytotoxicity mechanism of TNT, RDX, HMX in alfalfa (Medicago sativa). Chemosphere 2021, 281, 130842. [CrossRef]
- Zhang P, Liu L, Wang X, Wang Z, Zhang H, Chen J, Liu X, Wang Y, Li C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886. [CrossRef] [PubMed]
- Zhang W-J, Wang T. Enhanced salt tolerance of alfalfa (Medicago sativa) by rstB gene transformation. Plant Science 2015, 234, 110–118. [CrossRef]
- Zhang Y, Zhang Y, Wang C, Xiao J, Huang M, Zhuo L, Zhang D. Enhancement of salt tolerance of alfalfa: Physiological and molecular responses of transgenic alfalfa plants expressing Syntrichia caninervis-derived ScABI3. Plant Physiology and Biochemistry 2024, 207, 108335. [CrossRef]
- Zhang H, Zhang N, Yang R, Wang L, Sun Q, Li D, Cao Y, Weeda S, Zhao B, Ren S, Guo Y. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). Journal of Pineal Research 2014, 57, 269–279. [CrossRef] [PubMed]
- Zhu Z, Lee B. Friends or Foes: New Insights in Jasmonate and Ethylene Co-Actions. Plant and Cell Physiology 2015, 56, 414–420. [CrossRef] [PubMed]
- Zhu T, Lin J, Zhang M, Li L, Zhao C, Chen M. Phytohormone involved in salt tolerance regulation of Elaeagnus angustifolia L. seedlings. Journal of Forest Research 2019, 24, 235–242. [CrossRef]
Figure 1.
Effect of melatonin (MT) application on seed germination and young shoot growth. (A) Alfalfa seedling phenotypes. Bar = 2 cm. (B) Seed germination was evaluated after a 7-day treatment. The line graph displays the average germination rate of six biological replicates, each consisting of 30 seeds, along with the standard deviation. (C) The shoot lengths of alfalfa seedlings were measured after 7 days of treatment. The values in each column represent the mean of three biological replicates, with different lowercase letters indicating significant differences between treatments (p < 0.05). (D) The fresh weight of alfalfa seedlings was measured after 7 days of treatment. The values in each column represent the mean of three biological replicates. Different lowercase letters indicate significant differences as determined by Duncan’s test. CK serves as the control group.
Figure 1.
Effect of melatonin (MT) application on seed germination and young shoot growth. (A) Alfalfa seedling phenotypes. Bar = 2 cm. (B) Seed germination was evaluated after a 7-day treatment. The line graph displays the average germination rate of six biological replicates, each consisting of 30 seeds, along with the standard deviation. (C) The shoot lengths of alfalfa seedlings were measured after 7 days of treatment. The values in each column represent the mean of three biological replicates, with different lowercase letters indicating significant differences between treatments (p < 0.05). (D) The fresh weight of alfalfa seedlings was measured after 7 days of treatment. The values in each column represent the mean of three biological replicates. Different lowercase letters indicate significant differences as determined by Duncan’s test. CK serves as the control group.
Figure 2.
Melatonin from external sources reduces oxidative stress caused by NaCl treatment in alfalfa seedlings. (A) SOD activity. (B) POD activity. (C) MDA content. (D) O2- content. (E) GSH content. Each column presents the mean value and standard deviation of three biological replicates, with each replicate comprising three plants. Significant differences (p < 0.05) are denoted by distinct lowercase letters based on the results of Duncan’s test.
Figure 2.
Melatonin from external sources reduces oxidative stress caused by NaCl treatment in alfalfa seedlings. (A) SOD activity. (B) POD activity. (C) MDA content. (D) O2- content. (E) GSH content. Each column presents the mean value and standard deviation of three biological replicates, with each replicate comprising three plants. Significant differences (p < 0.05) are denoted by distinct lowercase letters based on the results of Duncan’s test.
Figure 3.
Control differentially expressed genes (DEGs), CK (WLCK), NaCl (WLN) and MT with NaCl (WLNMT) were visualized in combination. (A) Variations in gene expression indicate the quantity of genes that are upregulated and downregulated among different treatment groups. (B) The Venn diagram illustrates differentially expressed genes (DEGs) that are unique and shared. (C) Analysis of variance for multiple groups. * p < 0.05, ** p < 0.01. (D) Heatmap displaying log2FC values of differentially expressed genes in response to salt treatment compared to the control group.
Figure 3.
Control differentially expressed genes (DEGs), CK (WLCK), NaCl (WLN) and MT with NaCl (WLNMT) were visualized in combination. (A) Variations in gene expression indicate the quantity of genes that are upregulated and downregulated among different treatment groups. (B) The Venn diagram illustrates differentially expressed genes (DEGs) that are unique and shared. (C) Analysis of variance for multiple groups. * p < 0.05, ** p < 0.01. (D) Heatmap displaying log2FC values of differentially expressed genes in response to salt treatment compared to the control group.
Figure 4.
Functional annotation and KEGG enrichment analysis of differential gene expression. (A) Based on the Gene Ontology annotation analysis of Differentially Expressed Genes (DEGs), the DEGs were categorized into biological processes, cellular components, and molecular functions. These categories are represented by colors from top to bottom corresponding to the WLN_vs_WLCK, WLNMT_vs_WLCK, and WLNMT_vs_WLN comparisons. (B-D) Bubble plots representing KEGG pathway enrichment analyses. (B) KEGG functional classification of DEGs in WLN_vs_WLCK. (C) KEGG functional classification of DEGs in WLNMT_vs_WLCK. (D) KEGG functional classification of DEGs in WLNMT_vs_WLN.
Figure 4.
Functional annotation and KEGG enrichment analysis of differential gene expression. (A) Based on the Gene Ontology annotation analysis of Differentially Expressed Genes (DEGs), the DEGs were categorized into biological processes, cellular components, and molecular functions. These categories are represented by colors from top to bottom corresponding to the WLN_vs_WLCK, WLNMT_vs_WLCK, and WLNMT_vs_WLN comparisons. (B-D) Bubble plots representing KEGG pathway enrichment analyses. (B) KEGG functional classification of DEGs in WLN_vs_WLCK. (C) KEGG functional classification of DEGs in WLNMT_vs_WLCK. (D) KEGG functional classification of DEGs in WLNMT_vs_WLN.
Figure 5.
WGCNA identified correlations between melatonin-regulated salt stress-related physiological indicators and differentially expressed genes. (A) A module classification tree uses colors to represent different modules, with grey indicating genes that have not been assigned to a specific module. (B) Module-phenotype correlations heatmap depicting relationships between modules and physiological parameters. (C) Co-expression network of DEGs in MEturquoise. (D) Co-expression network of DEGs in MEblue.
Figure 5.
WGCNA identified correlations between melatonin-regulated salt stress-related physiological indicators and differentially expressed genes. (A) A module classification tree uses colors to represent different modules, with grey indicating genes that have not been assigned to a specific module. (B) Module-phenotype correlations heatmap depicting relationships between modules and physiological parameters. (C) Co-expression network of DEGs in MEturquoise. (D) Co-expression network of DEGs in MEblue.
Figure 6.
The mRNA relative abundance of WLN, WLN and WLNMT was calculated by qRT-PCR quantitative analysis. The bars with the same superscript letter indicated that the relative expression of the gene was not significantly different (p < 0.01). The data were expressed as 3 replicates ±SE.
Figure 6.
The mRNA relative abundance of WLN, WLN and WLNMT was calculated by qRT-PCR quantitative analysis. The bars with the same superscript letter indicated that the relative expression of the gene was not significantly different (p < 0.01). The data were expressed as 3 replicates ±SE.
Figure 7.
A clustered heat map was created using the log2FC values of key DEGs under three distinct treatments. Italics denote the gene ID of specific enzymes or transcription factors, including essential genes related to flavonoid biosynthesis (A), glutathione biosynthesis (B), gibberellins (C), abscisic acid synthesis and signaling (D), and the MAPK signaling pathway (E).
Figure 7.
A clustered heat map was created using the log2FC values of key DEGs under three distinct treatments. Italics denote the gene ID of specific enzymes or transcription factors, including essential genes related to flavonoid biosynthesis (A), glutathione biosynthesis (B), gibberellins (C), abscisic acid synthesis and signaling (D), and the MAPK signaling pathway (E).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).