Submitted:
28 March 2024
Posted:
29 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Sampling of Groundwater and Testing of Physicochemical Variables
2.3. Drinking Water Suitability Assessment
2.3.1. Groundwater Quality Pollution index
2.3.2. Synthetic Pollution Index
2.4. Human Health Risk Assessment
2.4.1. Human Health Risks due to Fluoride and Nitrate Ingestion
2.4.2. Human Health Risks due to Nitrate Dermal Absorption
2.5. Spatial Mapping for Identification of Contamination Hotspots
2.6. Graphical and Chemometric Techniques for Contamination Source Identification
3. Results
3.1. General Groundwater Quality Characterization
3.1.1. Physical Parameters
3.1.2. Chemical Parameters
3.1.3. Prevalent Water Type
3.2. Drinking Water Suitability Assessment
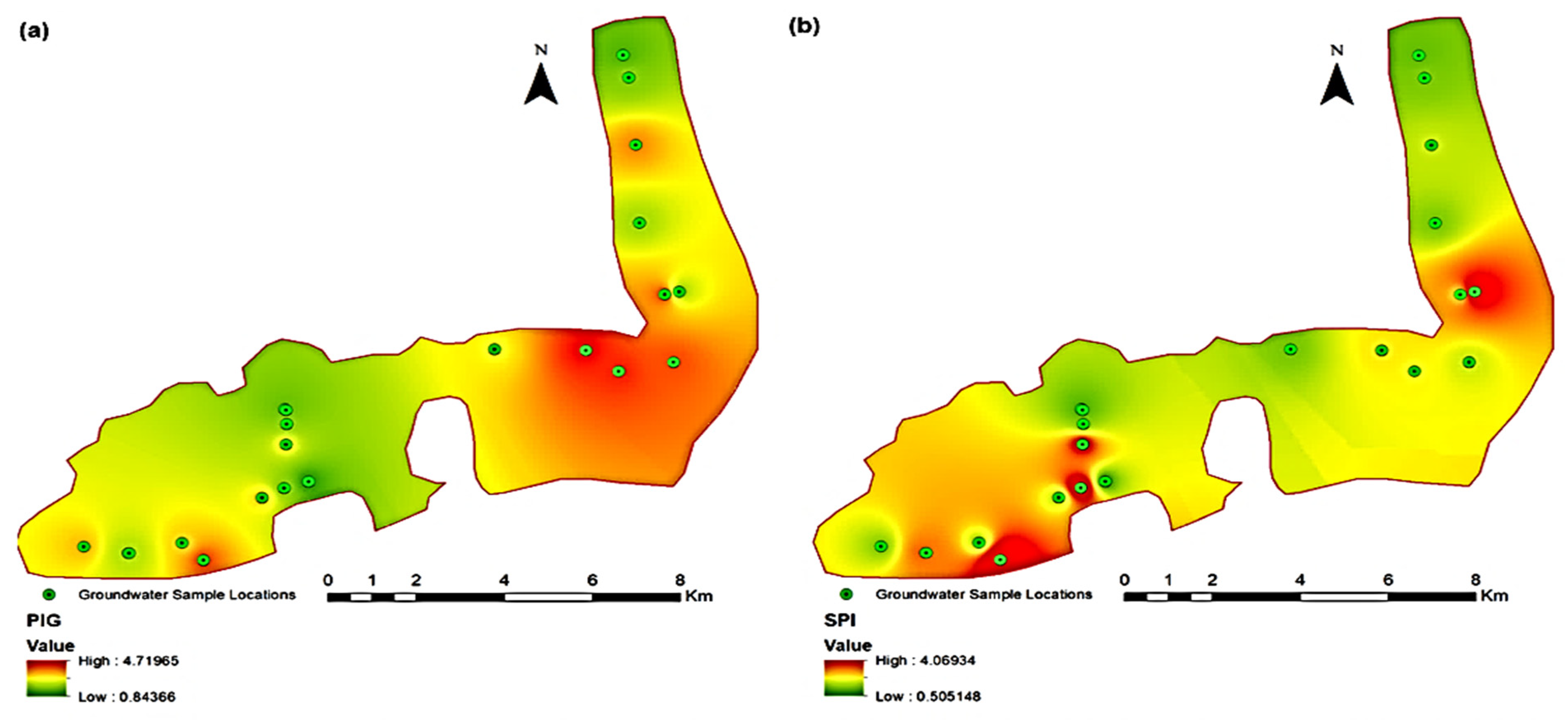
3.2.1. Pollution Index of Groundwater
3.2.2. Synthetic Pollution index
3.3. Human Health Risk Assessment
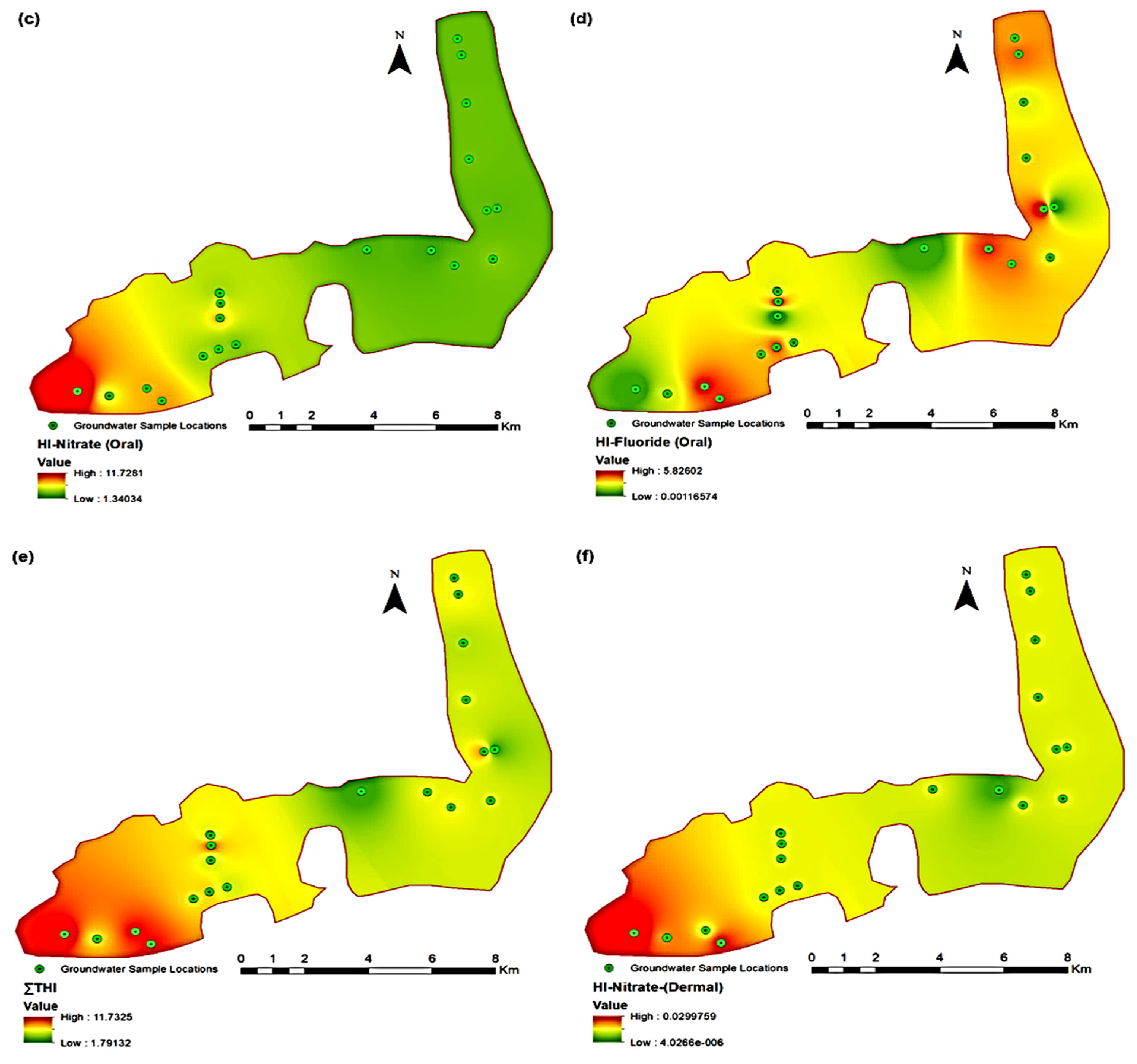
3.3.1. Nitrate Health Risk due to Ingestion
3.3.2. Fluoride Health Risk due to Ingestion
3.3.3. Total Hazard Index for Ingestion Pathway
3.3.4. Nitrate Health Risk due to Dermal Absorption
3.4. Spatial Mapping for Identification of Contamination Hotspots
3.5. Chemometric and Graphical Techniques for Contamination Source Identification
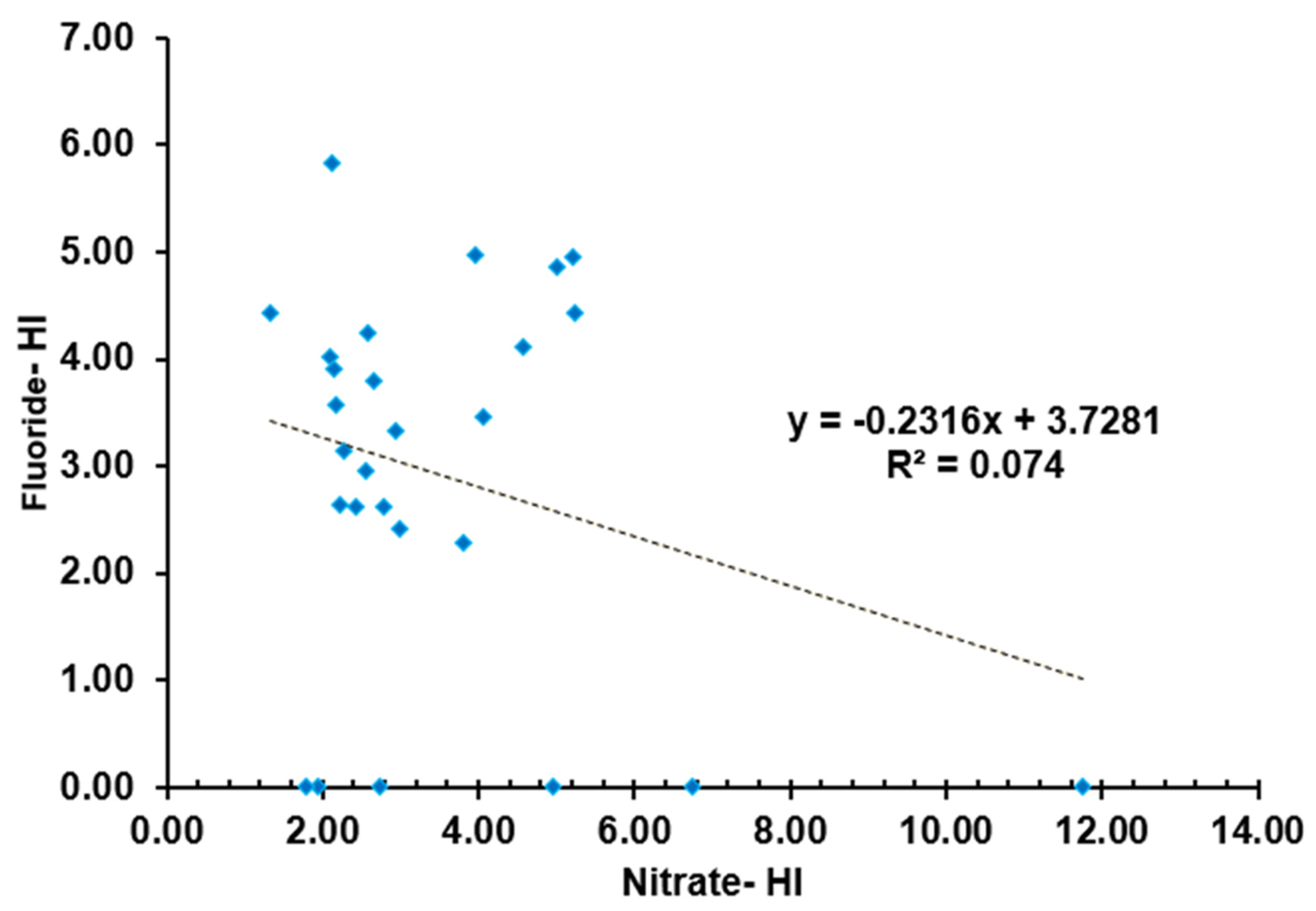
3.5.1. Correlation Analysis
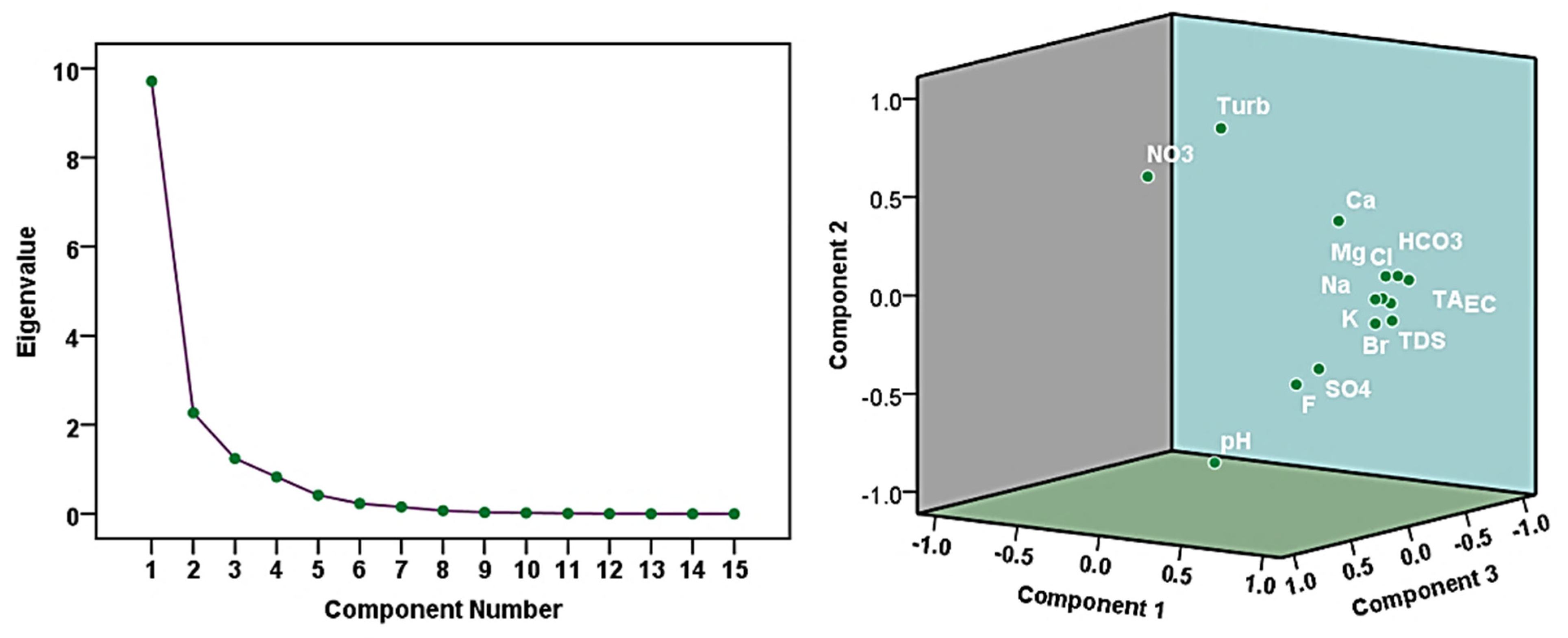
3.5.2. Principal Component Analysis
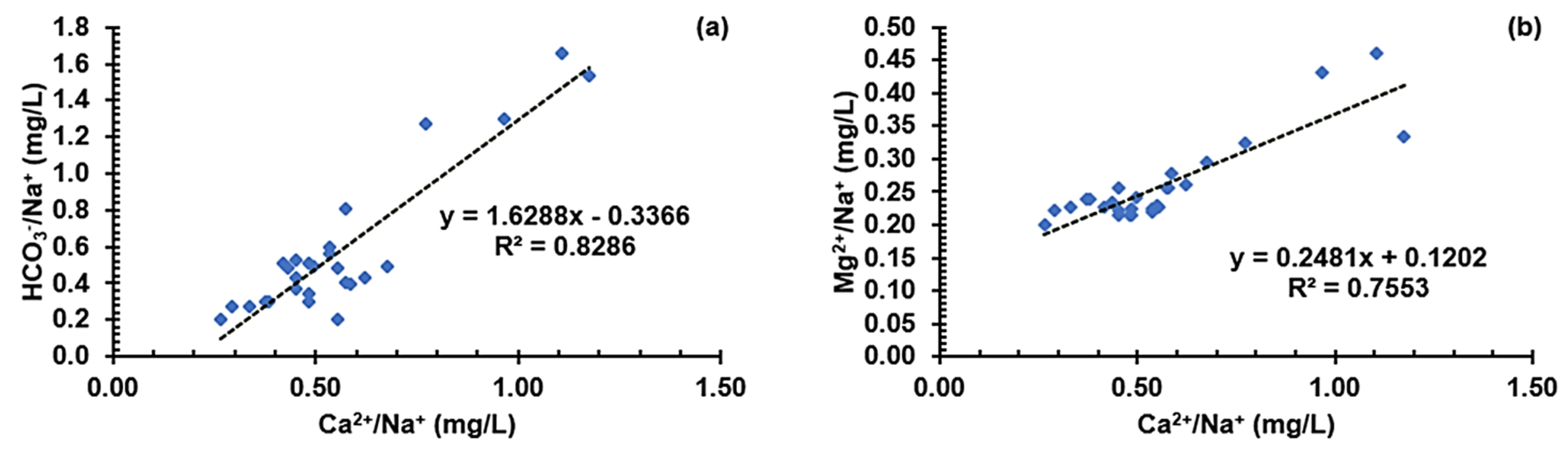
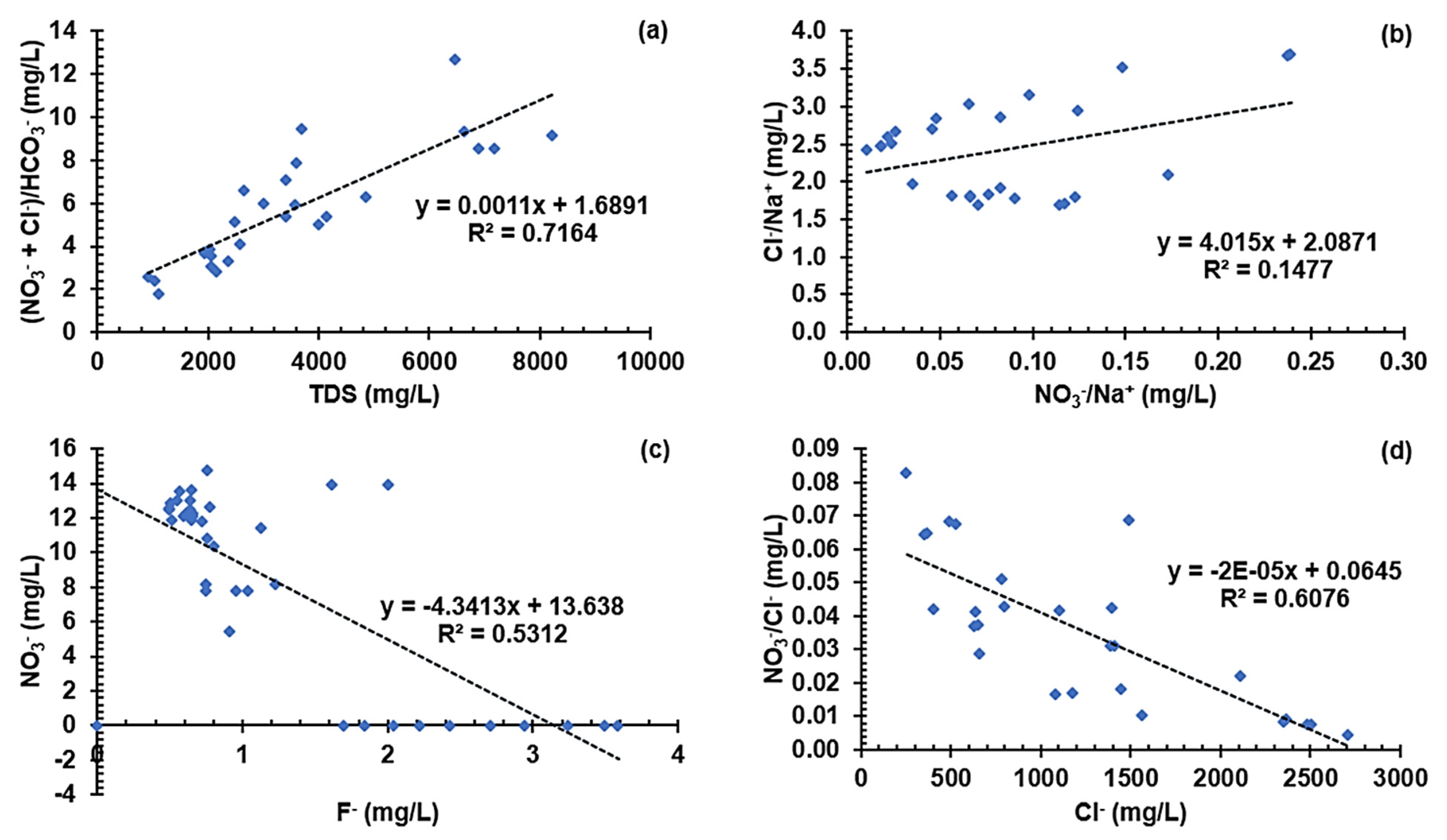
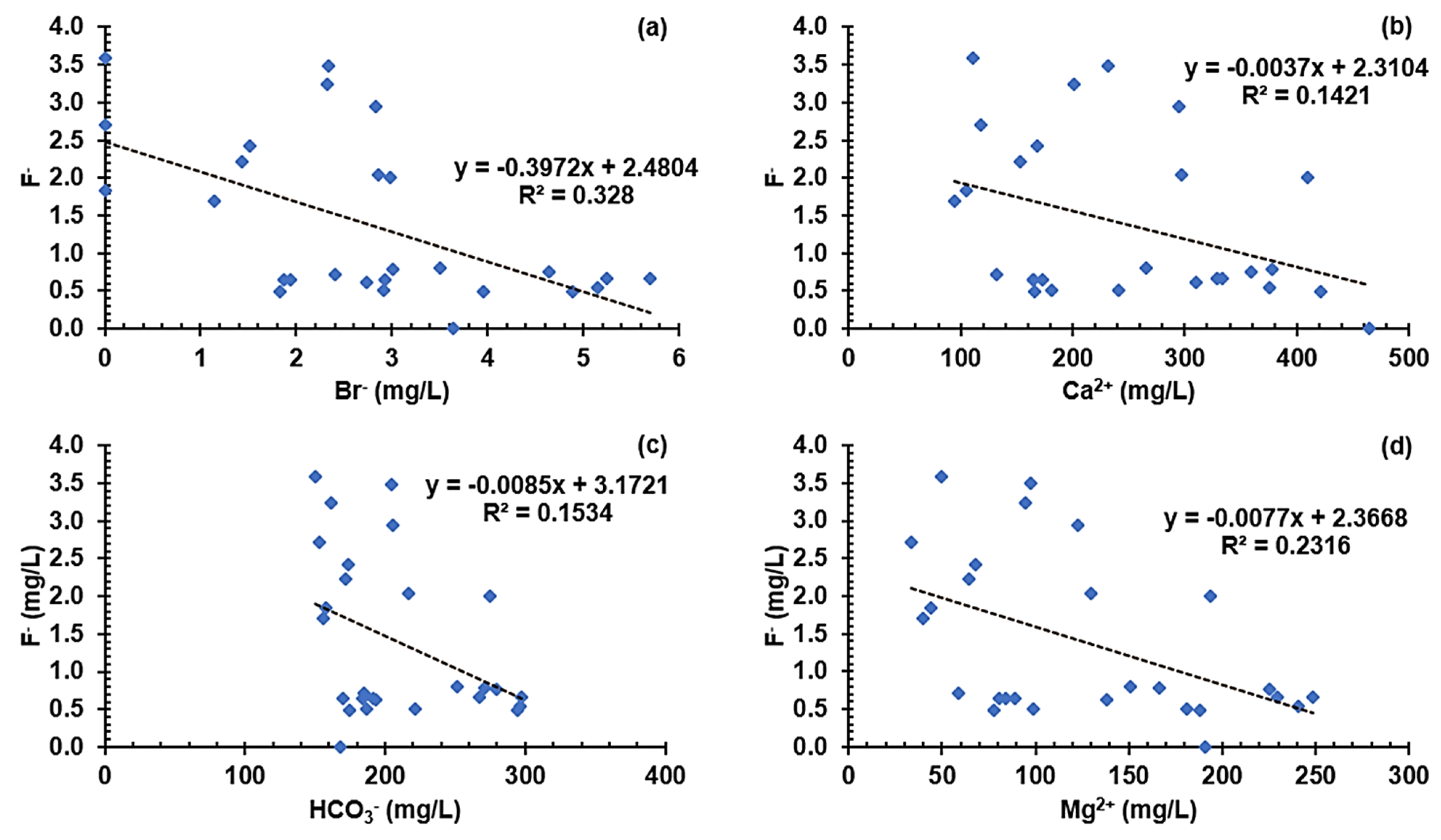
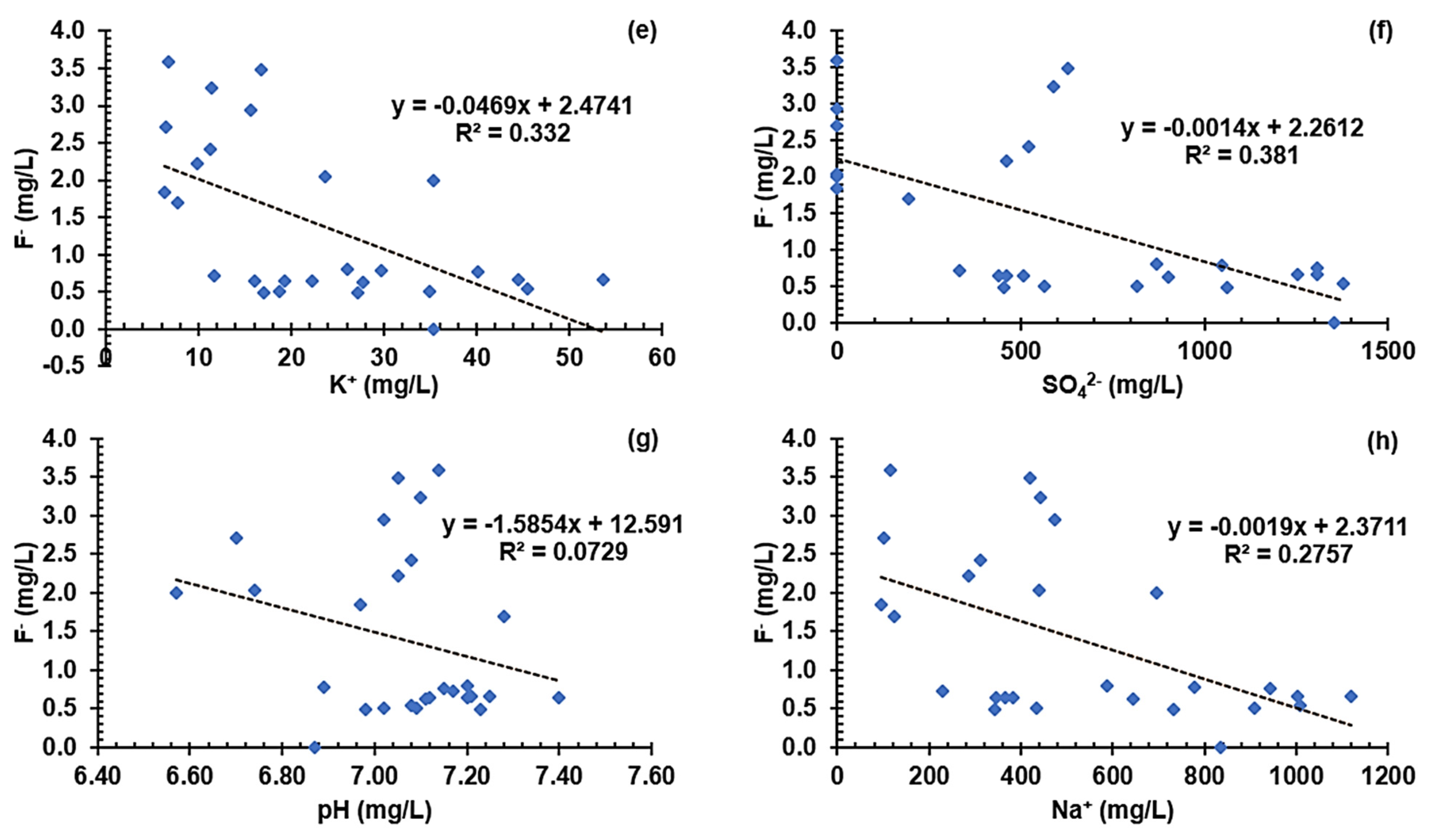
3.5.3. Graphical Characterization of Possible Contaminant Sources
4. Summary and Conclusions
References
- Li, P., He, X., Li, Y., & Xiang, G. (2018). Occurrence and Health Implication of Fluoride in Groundwater of Loess Aquifer in the Chinese Loess Plateau: A Case Study of Tongchuan, Northwest China. Exposure and Health, 11(2), 95–107. [CrossRef]
- Amiri, V., Sohrabi, N., Li, P., & Amiri, F. (2022). Groundwater Quality for Drinking and Non-Carcinogenic Risk of Nitrate in Urban and Rural Areas of Fereidan, Iran. Exposure and Health, 15(4), 807–823. [CrossRef]
- Ukah, B. U., Egbueri, J. C., Unigwe, C. O., & Ubido, O. E. (2019). Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. International Journal of Energy and Water Resources, 3(4), 291–303. [CrossRef]
- Yang, M., Zhao, A., Ke, H., & Chen, H. (2023). Geo-Environmental Factors’ Influence on the Prevalence and Distribution of Dental Fluorosis: Evidence from Dali County, Northwest China. Sustainability, 15(3), 1871. [CrossRef]
- Hagan, G. B., Minkah, R., Yiran, G. A. B., & Dankyi, E. (2022). Assessing groundwater quality in peri-urban Accra, Ghana: Implications for drinking and irrigation purposes. Groundwater for Sustainable Development, 17, 100761. [CrossRef]
- Yang, Q., Li, Z., Ma, H., Wang, L., & Martín, J. D. (2016). Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the Southeastern part of Ordos basin, China. Environmental Pollution, 218, 879–888. [CrossRef]
- Xiao, J., Wang, L., Chai, N., Liu, T., Jin, Z., & Rinklebe, J. (2021). Groundwater hydrochemistry, source identification and pollution assessment in intensive industrial areas, eastern Chinese loess plateau. Environmental Pollution, 278, 116930. [CrossRef]
- Sheng, D., Meng, X., Wen, X., Wu, J., Yu, H., Wu, M., & Zhou, T. (2023). Hydrochemical characteristics, quality and health risk assessment of nitrate enriched coastal groundwater in northern China. Journal of Cleaner Production, 403, 136872. [CrossRef]
- Duraisamy, S., Govindhaswamy, V., Duraisamy, K., Krishinaraj, S., Balasubramanian, A., & Thirumalaisamy, S. (2018). Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environmental Geochemistry and Health, 41(2), 851–873. [CrossRef]
- Sajil Kumar, P. J., Elango, L., & James, E. J. (2013). Assessment of hydrochemistry and groundwater quality in the coastal area of South Chennai, India. Arabian Journal of Geosciences, 7(7), 2641–2653. [CrossRef]
- Appendix A-II: World Health Organization Guidelines. (1996). In Handbook of Drinking Water Quality (pp. 527–534). Wiley. [CrossRef]
- Abba, S. I., Egbueri, J. C., Benaafi, M., Usman, J., Usman, A. G., & Aljundi, I. H. (2023). Fluoride and nitrate enrichment in coastal aquifers of the Eastern Province, Saudi Arabia: The influencing factors, toxicity, and human health risks. Chemosphere, 336, 139083. [CrossRef]
- Qu, X., Zhai, P., Shi, L., Qu, X., Bilal, A., Han, J., & Yu, X. (2022). Distribution, enrichment mechanism and risk assessment for fluoride in groundwater: A case study of Mihe-Weihe River Basin, China. Frontiers of Environmental Science & Engineering, 17(6). [CrossRef]
- Ayejoto, D. A., & Egbueri, J. C. (2023). Human health risk assessment of nitrate and heavy metals in urban groundwater in Southeast Nigeria. Acta Ecologica Sinica. [CrossRef]
- Ayejoto, D. A., Agbasi, J. C., Egbueri, J. C., & Echefu, K. I. (2022). Assessment of oral and dermal health risk exposures associated with contaminated water resources: An update in Ojoto area, southeast Nigeria. International Journal of Environmental Analytical Chemistry, 1–21. [CrossRef]
- Babiker, I. (2004). Assessment of groundwater contamination by nitrate leaching from intensive vegetable cultivation using geographical information system. Environment International, 29(8), 1009–1017. [CrossRef]
- Rahmati, O., Samani, A. N., Mahmoodi, N., & Mahdavi, M. (2014). Assessment of the Contribution of N-Fertilizers to Nitrate Pollution of Groundwater in Western Iran (Case Study: Ghorveh–Dehgelan Aquifer). Water Quality, Exposure and Health, 7(2), 143–151. [CrossRef]
- Unigwe, C. O., Egbueri, J. C., & Omeka, M. E. (2022). Geospatial and statistical approaches to nitrate health risk and groundwater quality assessment of an alluvial aquifer in SE Nigeria for drinking and irrigation purposes. Journal of the Indian Chemical Society, 99(6), 100479. [CrossRef]
- Alum, O. L., Abugu, H. O., Onwujiogu, V. C., Ezugwu, A. L., Egbueri, J. C., Aralu, C. C., Ucheana, I. A., Okenwa, J. C., Ezeofor, C. C., Orjiocha, S. I., & Ihedioha, J. N. (2023). Characterization of the Hydrochemistry, Scaling and Corrosivity Tendencies, and Irrigation Suitability of the Water of the Rivers Karawa and Iyiaji. Sustainability, 15(12), 9366. [CrossRef]
- Solangi, G. S., Siyal, A. A., Babar, M. M., & Siyal, P. (2019). Evaluation of drinking water quality using the water quality index (WQI), the synthetic pollution index (SPI) and geospatial tools in Thatta district, Pakistan. DESALINATION AND WATER TREATMENT, 160, 202–213. [CrossRef]
- Subba Rao, N. (2012). PIG: A numerical index for dissemination of groundwater contamination zones. Hydrological Processes, 26(22), 3344–3350. [CrossRef]
- Kom, K. P., Gurugnanam, B., & Bairavi, S. (2022). Non-carcinogenic health risk assessment of nitrate and fluoride contamination in the groundwater of Noyyal basin, India. Geodesy and Geodynamics, 13(6), 619–631. [CrossRef]
- Xiao, Y., Hao, Q., Zhang, Y., Zhu, Y., Yin, S., Qin, L., & Li, X. (2022). Investigating sources, driving forces and potential health risks of nitrate and fluoride in groundwater of a typical alluvial fan plain. Science of The Total Environment, 802, 149909. [CrossRef]
- Chakraborty, M., Tejankar, A., Coppola, G., & Chakraborty, S. (2022). Assessment of groundwater quality using statistical methods: A case study. Arabian Journal of Geosciences, 15(12). [CrossRef]
- Onjia, A., Huang, X., Trujillo González, J. M., & Egbueri, J. C. (2022). Editorial: Chemometric approach to distribution, source apportionment, ecological and health risk of trace pollutants. Frontiers in Environmental Science, 10. [CrossRef]
- Ben Ali, M., Hamdi, N., Rodriguez, M. A., Mahmoudi, K., & Srasra, E. (2018). Preparation and characterization of new ceramic membranes for ultrafiltration. Ceramics International, 44(2). [CrossRef]
- Dirks, H., Al Ajmi, H., Kienast, P., & Rausch, R. (2018). Hydrogeology of the Umm Er Radhuma Aquifer (Arabian peninsula). Grundwasser, 23(1), 5–15. [CrossRef]
- Al-Omran, A. M., Mousa, M. A., AlHarbi, M. M., & Nadeem, M. E. A. (2018). Hydrogeochemical characterization and groundwater quality assessment in Al-Hasa, Saudi Arabia. Arabian Journal of Geosciences, 11(4). [CrossRef]
- Alhawas, I., & A. Hassaballa, A. (2020). Representation of the spatial association between salinity and water chemical properties in Al-Hassa Oasis. International Journal of Agricultural and Biological Engineering, 13(2), 168–174. [CrossRef]
- Ismail, A. I. H., Hassaballa, A. A., Almadini, A. M., & Daffalla, S. (2022). Analyzing the Spatial Correspondence between Different Date Fruit Cultivars and Farms’ Cultivated Areas, Case Study: Al-Ahsa Oasis, Kingdom of Saudi Arabia. Applied Sciences, 12(11), 5728. [CrossRef]
- Wang, G., Su, W., Hu, B., AL-Huqail, A., Majdi, H. S., Algethami, J. S., Jiang, Y., & Ali, H. E. (2022). Assessment in carbon-based layered double hydroxides for water and wastewater: Application of artificial intelligence and recent progress. Chemosphere, 308(P3), 136303. [CrossRef]
- Al Tokhais, A., & Rausch, R. (2008). The Hydrogeology of Al Hassa Springs.
- APHA. (2012). Standard Methods for the Examination of Water and Wastewater. 1496.
- Oyedele, A. A., Ayodele, O. S., & Olabode, O. F. (2019). Groundwater quality assessment and characterization of shallow basement aquifers in parts of ado ekiti metropolis, Southwestern Nigeria. SN Applied Sciences, 1(7). [CrossRef]
- Rao, N. S., Sunitha, B., Rambabu, R., Rao, P. V. N., Rao, P. S., Spandana, B. D., Sravanthi, M., & Marghade, D. (2018). Quality and degree of pollution of groundwater, using PIG from a rural part of Telangana State, India. Applied Water Science, 8(8). [CrossRef]
- Egbueri, J. C., & Unigwe, C. O. (2019). An integrated indexical investigation of selected heavy metals in drinking water resources from a coastal plain aquifer in Nigeria. SN Applied Sciences, 1(11). [CrossRef]
- Singh, P. S. (2017). Small-Angle Scattering Techniques (SAXS/SANS). In Membrane Characterization. Elsevier B.V. [CrossRef]
- Raja, V., & Neelakantan, M. A. (2021). Evaluation of groundwater quality with health risk assessment of fluoride and nitrate in Virudhunagar district, Tamil Nadu, India. Arabian Journal of Geosciences, 14(1). [CrossRef]
- Naderi, M., Jahanshahi, R., & Dehbandi, R. (2020). Two distinct mechanisms of fluoride enrichment and associated health risk in springs’ water near an inactive volcano, southeast Iran. Ecotoxicology and Environmental Safety, 195, 110503. [CrossRef]
- Iqbal, J., Su, C., Wang, M., Abbas, H., Baloch, M. Y. J., Ghani, J., Ullah, Z., & Huq, M. E. (2023). Groundwater fluoride and nitrate contamination and associated human health risk assessment in South Punjab, Pakistan. Environmental Science and Pollution Research, 30(22), 61606–61625. [CrossRef]
- Rao, N. S., Dinakar, A., & Kumari, B. K. (2021). Appraisal of vulnerable zones of non-cancer-causing health risks associated with exposure of nitrate and fluoride in groundwater from a rural part of India. Environmental Research, 202, 111674. [CrossRef]
- Egbueri, J. C., Agbasi, J. C., Ikwuka, C. F., Chiaghanam, O. I., Khan, M. I., Khan, M. Y. A., Khan, N., & Uwajingba, H. C. (2023). Nitrate health risk and geochemical characteristics of water in a semi-urban: Implications from graphical plots and statistical computing. International Journal of Environmental Analytical Chemistry, 1–21. [CrossRef]
- Egbueri, J. C., Agbasi, J. C., Ayejoto, D. A., Khan, M. I., & Khan, M. Y. A. (2023a). Extent of anthropogenic influence on groundwater quality and human health-related risks: An integrated assessment based on selected physicochemical characteristics. Geocarto International, 38(1). [CrossRef]
- Li, P., He, X., Li, Y., & Xiang, G. (2018). Occurrence and Health Implication of Fluoride in Groundwater of Loess Aquifer in the Chinese Loess Plateau: A Case Study of Tongchuan, Northwest China. Exposure and Health, 11(2), 95–107. [CrossRef]
- Luo, M., Zhang, Y., Li, H., Hu, W., Xiao, K., Yu, S., Zheng, C., & Wang, X. (2022). Pollution assessment and sources of dissolved heavy metals in coastal water of a highly urbanized coastal area: The role of groundwater discharge. Science of The Total Environment, 807, 151070. [CrossRef]
- Shaibur, M. R., Ahmmed, I., Sarwar, S., Karim, R., Hossain, M. M., Islam, M. S., Shah, M. S., Khan, A. S., Akhtar, F., Uddin, M. G., Rahman, M. M., Salam, M. A., & Ambade, B. (2023). Groundwater Quality of Some Parts of Coastal Bhola District, Bangladesh: Exceptional Evidence. Urban Science, 7(3), 71. [CrossRef]
- Oiro, S., & Comte, J.-C. (2019). Drivers, patterns and velocity of saltwater intrusion in a stressed aquifer of the East African coast: Joint analysis of groundwater and geophysical data in southern Kenya. Journal of African Earth Sciences, 149, 334–347. [CrossRef]
- Mokoena, P., Manyama, K., van Bever Donker, J., & Kanyerere, T. (2021). Investigation of groundwater salinity using geophysical and geochemical approaches: Heuningnes catchment coastal aquifer. Western Cape Province, South Africa. Environmental Earth Sciences, 80(5). [CrossRef]
- Gopinath, S., & Srinivasamoorthy, K. (2015). Application of Geophysical and Hydrogeochemical Tracers to Investigate Salinisation Sources in Nagapatinam and Karaikal Coastal Aquifers, South India. Aquatic Procedia, 4, 65–71. [CrossRef]
- Himi, M., Tapias, J., Benabdelouahab, S., Salhi, A., Rivero, L., Elgettafi, M., El Mandour, A., Stitou, J., & Casas, A. (2017). Geophysical characterization of saltwater intrusion in a coastal aquifer: The case of Martil-Alila plain (North Morocco). Journal of African Earth Sciences, 126, 136–147. [CrossRef]
- Alqarawy, A., El Osta, M., Masoud, M., Elsayed, S., & Gad, M. (2022). Use of Hyperspectral Reflectance and Water Quality Indices to Assess Groundwater Quality for Drinking in Arid Regions, Saudi Arabia. Water, 14(15), 2311. [CrossRef]
- Alshehri, F., & Abdelrahman, K. (2023). Integrated approach for the investigation of groundwater quality using hydrochemical and geostatistical analyses in Wadi Fatimah, western Saudi Arabia. Frontiers in Earth Science, 11. [CrossRef]
- Hem, J. D. (1985). Study and Interpretation the Chemical of Natural of Characteristics Water. USGS Science for a Changing World, 272. http://pubs.usgs.gov/wsp/wsp2254/pdf/wsp2254a.pdf.
- Karmakar, B., Singh, M. K., Choudhary, B. K., Singh, S. K., Egbueri, J. C., Gautam, S. K., & Rawat, K. S. (2021). Investigation of the hydrogeochemistry, groundwater quality, and associated health risks in industrialized regions of Tripura, northeast India. Environmental Forensics, 24(5–6), 285–306. [CrossRef]
- Lakshmanan, E., Kannan, R., & Senthil Kumar, M. (2003). Major ion chemistry and identification of hydrogeochemical processes of ground water in a part of Kancheepuram district, Tamil Nadu, India. Environmental Geosciences, 10(4), 157–166. [CrossRef]
- Ashrafuzzaman, M., Gomes, C., & Guerra, J. (2023). The Changing Climate Is Changing Safe Drinking Water, Impacting Health: A Case in the Southwestern Coastal Region of Bangladesh (SWCRB). Climate, 11(7), 146. [CrossRef]
- Hui, T., Du, J., Sun, Q., Liu, Q., Kang, Z., & Jin, H. (2020). Using the Water Quality Index (WQI), and the Synthetic Pollution Index (SPI) to Evaluate the Groundwater Quality for Drinking Purpose in Hailun, China. Sains Malaysiana, 49(10), 2383–2401. [CrossRef]
- Agbasi, J. C., & Egbueri, J. C. (2022). Assessment of PTEs in water resources by integrating HHRISK code, water quality indices, multivariate statistics, and ANNs. Geocarto International, 37(25), 10407–10433. [CrossRef]
- Grema, H. M., Hamidu, H., Suleiman, A., Kankara, A. I., Umaru, A. O., & Abdulmalik, N. F. (2022). Cadmium geochemistry and groundwater pollution status evaluation using indexing and spatial analysis for Keffe community and Environs Sokoto Basin, North Western Nigeria. Nigerian Journal of Basic and Applied Sciences, 30(1), 5–23. [CrossRef]
- Jamali, M. Z., Solangi, G. S., Keerio, M. A., Keerio, J. A., & Bheel, N. (2022). Assessing and mapping the groundwater quality of Taluka Larkana, Sindh, Pakistan, using water quality indices and geospatial tools. International Journal of Environmental Science and Technology, 20(8), 8849–8862. [CrossRef]
- Abed, M. F., Zarraq, G. A., & Ahmed, S. H. (2022). Assessment of Groundwater Pollution using Aqueous Geo-Environmental Indices, Baiji Province, Salah Al-Din, Iraq. Iraqi Geological Journal, 55(1B), 94–104. [CrossRef]
- Choudhary, M., Muduli, M., & Ray, S. (2022). A comprehensive review on nitrate pollution and its remediation: Conventional and recent approaches. Sustainable Water Resources Management, 8(4). [CrossRef]
- Alharbi, T., Abdelrahman, K., El-Sorogy, A. S., & Ibrahim, E. (2023). Contamination and health risk assessment of groundwater along the Red Sea coast, Northwest Saudi Arabia. Marine Pollution Bulletin, 192, 115080. [CrossRef]
- Biswas, T., Pal, S. C., Chowdhuri, I., Ruidas, D., Saha, A., Islam, A. R. M. T., & Shit, M. (2023). Effects of elevated arsenic and nitrate concentrations on groundwater resources in deltaic region of Sundarban Ramsar site, Indo-Bangladesh region. Marine Pollution Bulletin, 188, 114618. [CrossRef]
- Qasemi, M., Darvishian, M., Nadimi, H., Gholamzadeh, M., Afsharnia, M., Farhang, M., Allahdadi, M., Darvishian, M., & Zarei, A. (2023). Characteristics, water quality index and human health risk from nitrate and fluoride in Kakhk city and its rural areas, Iran. Journal of Food Composition and Analysis, 115, 104870. [CrossRef]
- B., M. R., & V., S. (2020). Geochemical and health risk assessment of fluoride and nitrate toxicity in semi-arid region of Anantapur District, South India. Environmental Chemistry and Ecotoxicology, 2, 150–161. [CrossRef]
- Giri, S., Mahato, M. K., Singh, P. K., & Singh, A. K. (2021). Non-carcinogenic health risk assessment for fluoride and nitrate in the groundwater of the mica belt of Jharkhand, India. Human and Ecological Risk Assessment: An International Journal, 27(7), 1939–1953. [CrossRef]
- Hossain, M., & Patra, P. K. (2020). Hydrogeochemical characterisation and health hazards of fluoride enriched groundwater in diverse aquifer types. Environmental Pollution, 258, 113646. [CrossRef]
- Fejerskov, O., Larsen, M. J., Richards, A., & Baelum, V. (1994). Dental Tissue Effects of Fluoride. Advances in Dental Research, 8(1), 15–31. [CrossRef]
- Boyle, D. R., & Chagnon, M. (1995). An incidence of skeletal fluorosis associated with groundwaters of the maritime carboniferous basin, Gasp region, Quebec, Canada. Environmental Geochemistry and Health, 17(1). [CrossRef]
- Chen, Q., Lu, Q., Song, Z., Chen, P., Cui, Y., Zhang, R., Li, X., & Liu, J. (2013). The levels of fluorine in the sediments of the aquifer and their significance for fluorosis in coastal region of Laizhou Bay, China. Environmental Earth Sciences, 71(10), 4513–4522. [CrossRef]
- Yang, J., & Jiang, G. (2003). Experimental study on properties of pervious concrete pavement materials. Cement and Concrete Research, 33(3), 381–386. [CrossRef]
- Tajwar, M., Uddin, A., Lee, M.-K., Nelson, J., Zahid, A., & Sakib, N. (2023). Hydrochemical Characterization and Quality Assessment of Groundwater in Hatiya Island, Southeastern Coastal Region of Bangladesh. Water, 15(5), 905. [CrossRef]
- Li, S., & Zhang, Q. (2008). Geochemistry of the upper Han River basin, China, 1: Spatial distribution of major ion compositions and their controlling factors. Applied Geochemistry, 23(12), 3535–3544. [CrossRef]
- Zhang, Y. P., Li, P. P., Liu, P. F., Zhang, W. Q., Wang, J. C., Cui, C. X., Li, X. J., & Qu, L. B. (2019). Fast and simple fabrication of superhydrophobic coating by polymer induced phase separation. Nanomaterials. [CrossRef]
- Yu, G., Wang, J., Liu, L., Li, Y., Zhang, Y., & Wang, S. (2020). The analysis of groundwater nitrate pollution and health risk assessment in rural areas of Yantai, China. BMC Public Health, 20(1), 437. [CrossRef]
- Egbueri, J. C., & Agbasi, J. C. (2022). Data-driven soft computing modeling of groundwater quality parameters in southeast Nigeria: Comparing the performances of different algorithms. Environmental Science and Pollution Research, 29(25), 38346–38373. [CrossRef]
- Rafique, T., Naseem, S., Usmani, T. H., Bashir, E., Khan, F. A., & Bhanger, M. I. (2009). Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. Journal of Hazardous Materials, 171(1–3), 424–430. [CrossRef]
- Rasool, A., Farooqi, A., Xiao, T., Ali, W., Noor, S., Abiola, O., Ali, S., & Nasim, W. (2017). A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environmental Geochemistry and Health, 40(4), 1265–1281. [CrossRef]
- Yadav, K. K., Kumar, S., Pham, Q. B., Gupta, N., Rezania, S., Kamyab, H., Yadav, S., Vymazal, J., Kumar, V., Tri, D. Q., Talaiekhozani, A., Prasad, S., Reece, L. M., Singh, N., Maurya, P. K., & Cho, J. (2019). Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicology and Environmental Safety, 182, 109362. [CrossRef]
- Liu, J., Peng, Y., Li, C., Gao, Z., & Chen, S. (2021). A characterization of groundwater fluoride, influencing factors and risk to human health in the southwest plain of Shandong Province, North China. Ecotoxicology and Environmental Safety, 207, 111512. [CrossRef]
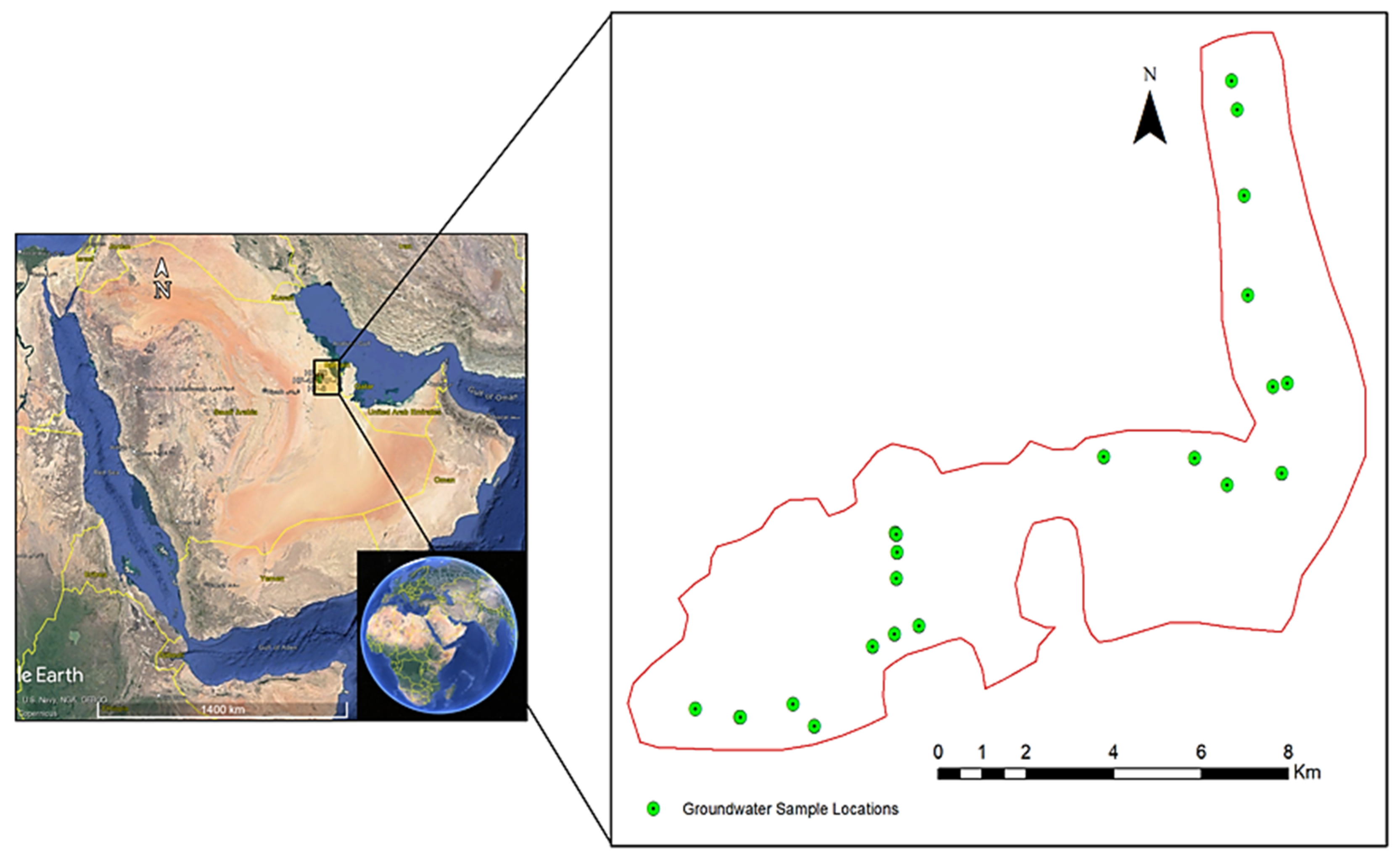
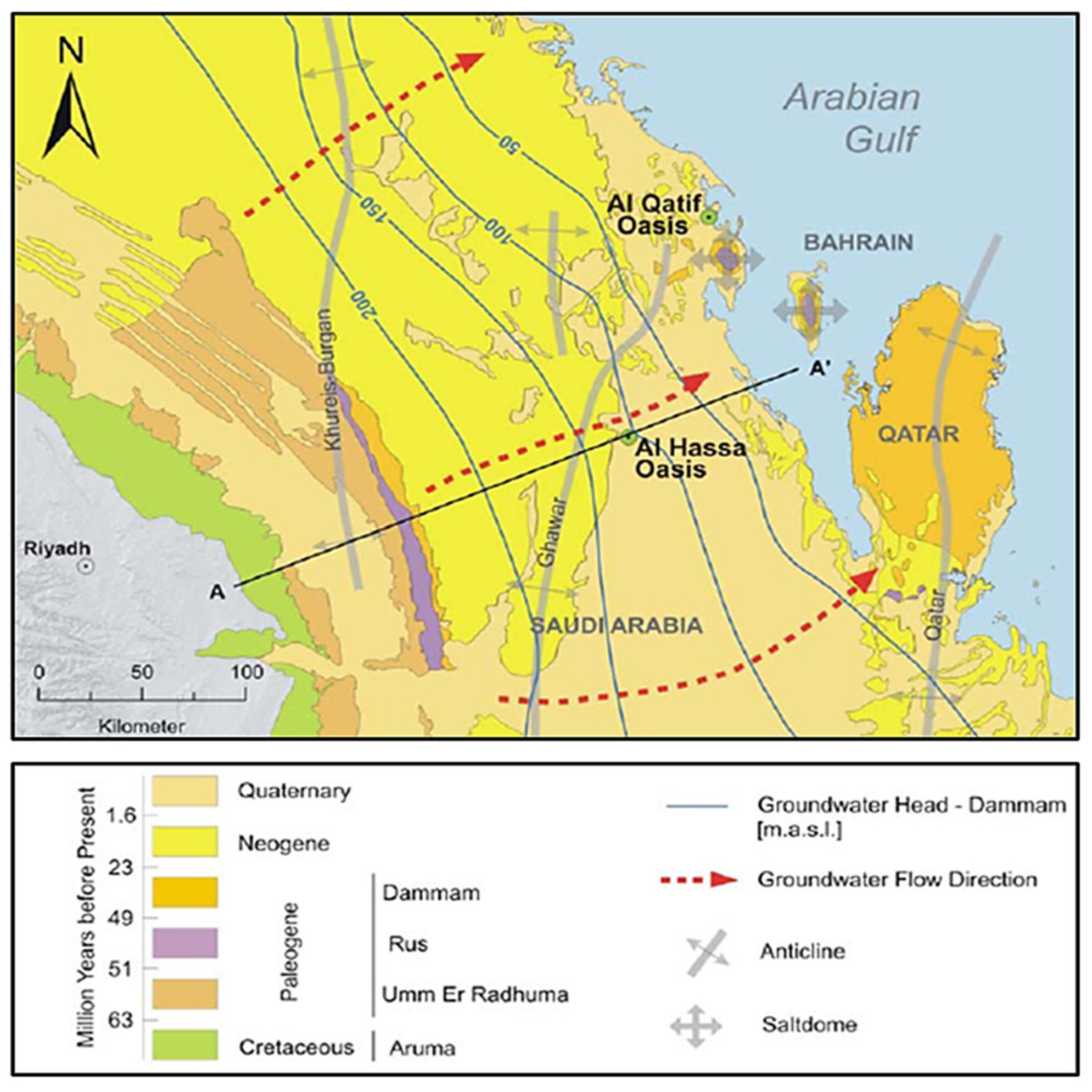
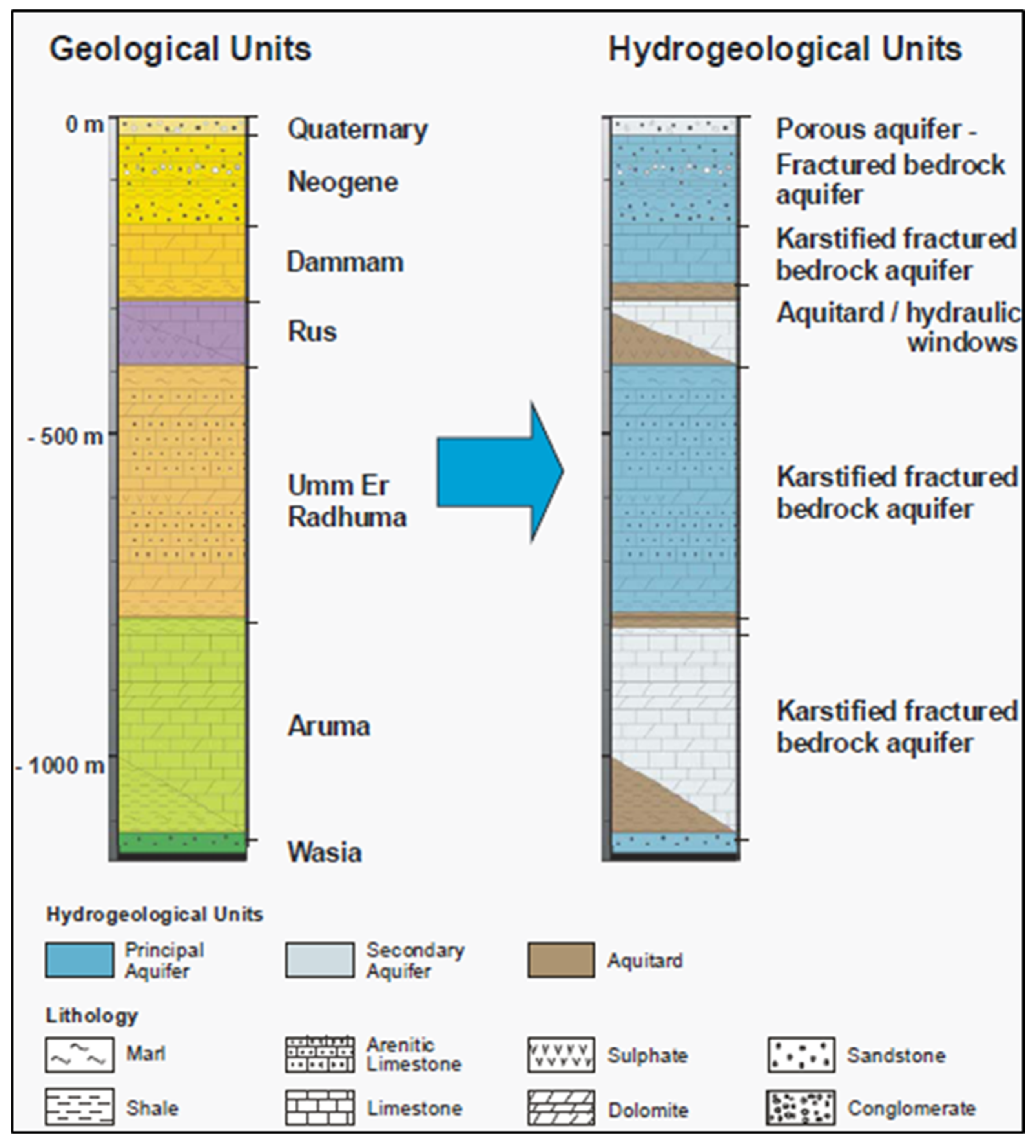
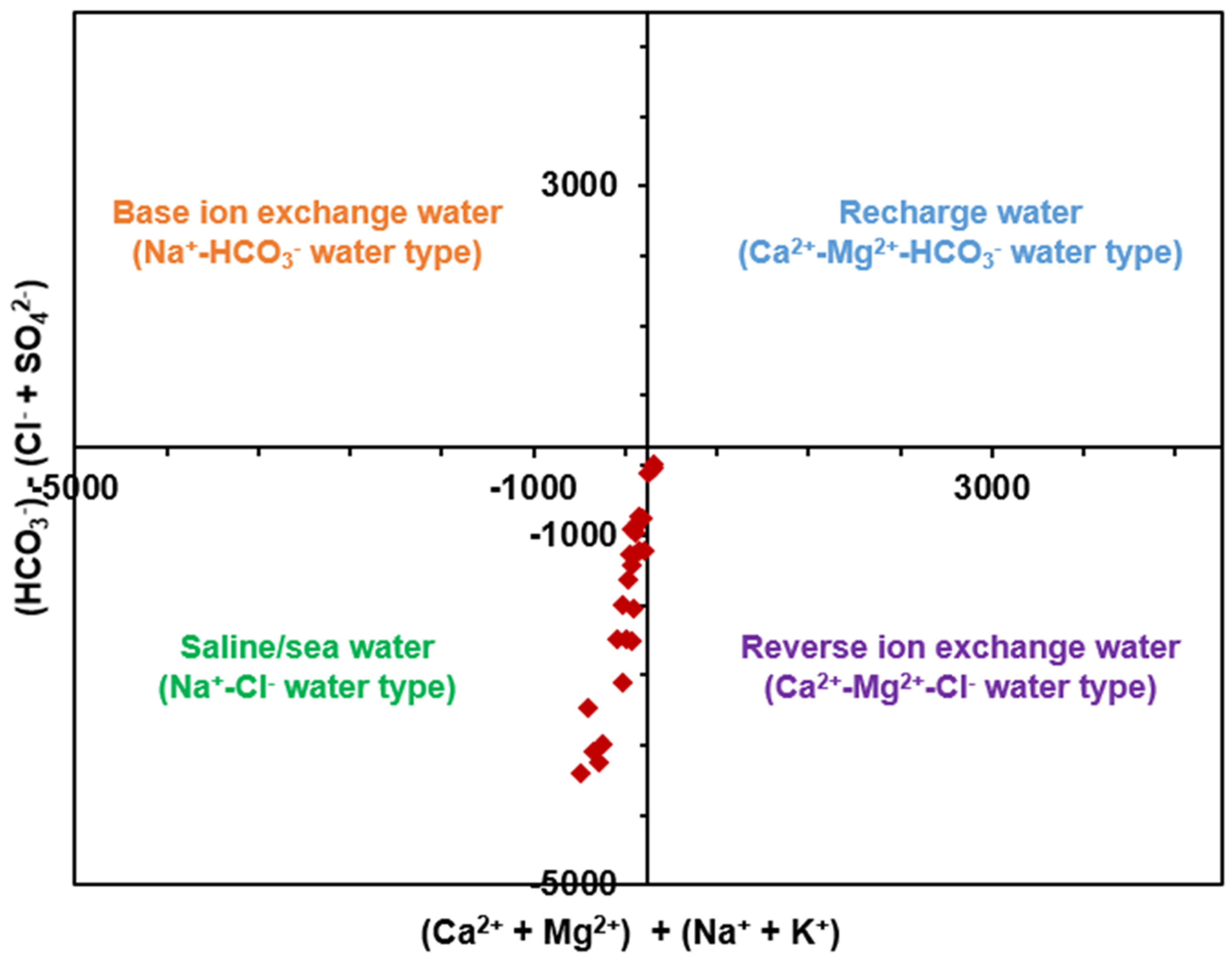
| S/No. | Parameter | WHO (2022, 2017) standard | Unit | Rw | Wp |
|---|---|---|---|---|---|
| 1 | pH | 6.5-8.5 | - | 3 | 0.0555 |
| 2 | EC | 1000 | uS/cm | 4 | 0.0740 |
| 3 | TDS | 1000 | mg/L | 4 | 0.0740 |
| 4 | Turbidity | 4 | NTU | 3 | 0.0555 |
| 5 | HCO3- | 250 | mg/L | 4 | 0.0740 |
| 6 | Na+ | 200 | mg/L | 4 | 0.0740 |
| 7 | K+ | 12 | mg/L | 4 | 0.0740 |
| 8 | Mg2+ | 50 | mg/L | 4 | 0.0740 |
| 9 | Ca2+ | 75 | mg/L | 4 | 0.0740 |
| 10 | SO42- | 250 | mg/L | 4 | 0.0740 |
| 11 | Cl- | 250 | mg/L | 4 | 0.0740 |
| 12 | NO3- | 50 | mg/L | 4 | 0.0740 |
| 13 | F- | 1.5 | mg/L | 4 | 0.0740 |
| 14 | Br- | 2 | mg/L | 4 | 0.0740 |
| ∑Rw = 54 | ∑Wp = 1.00 |
| (a) Parameters for NO3- and F- ingestion health risk assessment [12,22,23,42] | ||||
| Parameter | Unit | Infants (< 2 years) | Children (2–16 years) | Adults (> 16 years) |
| Chronic daily intake (CDI) | mg/kg/day | – | – | – |
| Concentration in groundwater (C) | mg/L | – | – | – |
| Ingestion rate of water (IR) | L/day | 0.65 | 1.50 | 2.00 |
| Exposure frequency (EF) | Days/year | 365 | 365 | 365 |
| Exposure duration (ED) | year | 0.50 | 6.00 | 30.00 |
| Average body weight (BW) | kg | 6.94 | 25.90 | 64.70 |
| Average time (AT) | day | 182.50 | 2190.00 | 10950.00 |
| Reference dose (RfD) (NO3-) | mg/kg/day | 1.6 | 1.6 | 1.6 |
| Reference dose (RfD) (F-) | mg/kg/day | 0.04 | 0.04 | 0.04 |
| (b) Parameters for NO3- dermal health risk assessment [14,22,23,42,43,44] | ||||
| Parameter | Unit | Children | Women | Men |
| Dermally absorbed dose (DAD) | mg/kg/day | – | – | – |
| Concentration in groundwater (C) | mg/L | – | – | – |
| Duration of the contact (TC) | hr/day | 0.4 | 0.4 | 0.4 |
| Rate of bathing (EV) | time/day | 1 | 1 | 1 |
| Dermal adsorption parameter (Ki) | cm/h | 0.001 | 0.001 | 0.001 |
| Skin surface area (SSA) | cm2 | 12,000 | 16,600 | 16,600 |
| Exposure duration (ED) | year | 12 | 67 | 64 |
| Exposure frequency (EF) | days/year | 365 | 365 | 365 |
| Unit conversion factors (CF) | – | 0.001 | 0.001 | 0.001 |
| Average body weight (BW) | kg | 15 | 55 | 65 |
| Average time (AT) | day | 4380 | 24,455 | 23,360 |
| Reference dose (RfD) | mg/kg/day | 1.6 | 1.6 | 1.6 |
| Sample ID | pH | EC (uS) | TDS (mg/L) | TA (ppm) | Turb. (NTU) | HCO3- (mg/L) | Na+ (mg/L) | K+ (mg/L) | Mg2+ (mg/L) | Ca2+ (mg/L) | SO42- (mg/L) | Cl- (mg/L) | NO3- (mg/L) | F- (mg/L) | Br- (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AHF-1 | 6.7 | 2135.82 | 928 | 153.41 | 80.9 | 153.41 | 100.31 | 6.46 | 33.59 | 117.96 | 0.00 | 371.33 | 24.03 | 0.00 | 0.00 |
| AHF-2 | 6.57 | 8203.25 | 3592 | 274.68 | 83.03 | 274.68 | 695.85 | 35.31 | 194.11 | 409.68 | 0.00 | 2111.03 | 46.02 | 0.97 | 2.98 |
| AHF-3 | 7.14 | 2271.11 | 2156 | 150 | 84.1 | 150 | 115.03 | 6.75 | 49.66 | 111.37 | 0.00 | 405.18 | 17.05 | 0.00 | 0.00 |
| AHF-4 | 6.74 | 5892.84 | 2636 | 216.43 | 56.2 | 216.43 | 440.70 | 23.66 | 129.73 | 297.53 | 0.00 | 1388.57 | 43.35 | 0.00 | 2.86 |
| AHF-5 | 6.97 | 2062.97 | 1020 | 157.51 | 84 | 157.51 | 95.03 | 6.30 | 43.89 | 105.11 | 0.00 | 350.27 | 22.58 | 0.93 | 0.00 |
| AHF-6 | 7.02 | 7422.71 | 3413 | 205.56 | 85.07 | 205.56 | 473.98 | 15.58 | 122.52 | 295.20 | 0.00 | 1394.07 | 59.19 | 0.00 | 2.83 |
| AHF-10 | 6.87 | 7703.7 | 3675 | 168.23 | 1.11 | 168.23 | 836.50 | 35.39 | 191.21 | 464.05 | 1353.35 | 1493.93 | 102.93 | 0.00 | 3.65 |
| AHF-11 | 7.28 | 2187.85 | 1114 | 155.71 | 0.95 | 155.71 | 122.81 | 7.73 | 39.69 | 94.83 | 195.18 | 256.21 | 21.27 | 0.58 | 1.15 |
| AHF-12 | 7.08 | 4727.23 | 2345 | 173.64 | 0.66 | 173.64 | 312.86 | 11.25 | 68.30 | 168.10 | 522.09 | 529.41 | 35.71 | 0.76 | 1.52 |
| AHF-13 | 7.05 | 4154.83 | 2060 | 171.64 | 0.74 | 171.64 | 285.94 | 9.85 | 64.16 | 153.36 | 459.39 | 489.32 | 33.48 | 0.50 | 1.44 |
| AHF-14 | 7.1 | 5216.37 | 2480 | 161.73 | 0.97 | 161.73 | 441.86 | 11.47 | 95.05 | 200.75 | 589.31 | 785.21 | 40.13 | 0.90 | 2.32 |
| AHF-15 | 7.05 | 5570.21 | 2562 | 204.35 | 1.38 | 204.35 | 420.44 | 16.73 | 97.25 | 232.01 | 627.46 | 803.20 | 34.70 | 1.09 | 2.34 |
| AHF-16 | 7.23 | 4508.67 | 1931 | 174.63 | 0.88 | 174.63 | 344.06 | 17.10 | 77.57 | 166.53 | 453.47 | 633.82 | 26.24 | 0.53 | 1.83 |
| AHF-17 | 7.2 | 4623.15 | 2054 | 191.81 | 0.84 | 191.81 | 364.49 | 19.24 | 80.84 | 165.37 | 461.09 | 655.51 | 24.47 | 0.58 | 1.87 |
| AHF-18 | 6.89 | 9129.5 | 4130 | 270.84 | 0.27 | 270.84 | 777.95 | 29.72 | 166.20 | 377.53 | 1046.89 | 1410.1 | 43.98 | 1.07 | 3.01 |
| AHF-19 | 6.98 | 9181.53 | 4014 | 294.37 | 0.82 | 294.37 | 732.96 | 27.21 | 188.20 | 421.37 | 1060.7 | 1448.49 | 25.80 | 0.73 | 3.96 |
| AHF-20 | 7.4 | 4539.89 | 2032 | 170.12 | 0.98 | 170.12 | 347.04 | 22.29 | 84.00 | 172.98 | 505.50 | 630.22 | 23.26 | 0.83 | 1.94 |
| AHF-21 | 7.11 | 7568.41 | 3577 | 193.35 | 0.85 | 193.35 | 644.35 | 27.73 | 138.09 | 310.06 | 902.84 | 1099.45 | 45.60 | 1.09 | 2.73 |
| AHF-22 | 7.09 | 11179.73 | 6462.92 | 187.07 | 1.63 | 187.07 | 908.38 | 34.88 | 181.18 | 241.21 | 817.18 | 1856.8 | 19.48 | 0.58 | 4.89 |
| AHF-23 | 7.12 | 5278.81 | 2988.71 | 184.44 | 1.17 | 184.44 | 453.30 | 16.00 | 88.95 | 165.35 | 437.78 | 858.50 | 18.39 | 0.88 | 2.93 |
| AHF-24 | 7.17 | 3436.72 | 1924.95 | 184.9 | 1.24 | 184.9 | 356.30 | 11.64 | 58.91 | 132.22 | 333.32 | 659.01 | 18.93 | 0.78 | 2.40 |
| AHF-25 | 7.02 | 6038.54 | 3430.38 | 221.61 | 1.98 | 221.61 | 546.30 | 18.75 | 99.21 | 181.26 | 563.70 | 950.60 | 20.01 | 0.69 | 2.91 |
| AHF-26 | 7.21 | 11450.32 | 6624.58 | 267.36 | 1.12 | 267.36 | 1001.72 | 44.56 | 229.26 | 333.65 | 1253.69 | 1986.3 | 18.65 | 1.28 | 5.25 |
| AHF-27 | 7.15 | 11887.42 | 6886.15 | 279.14 | 0.46 | 279.14 | 986.60 | 40.17 | 225.44 | 359.21 | 1305.53 | 1869.7 | 22.40 | 0.65 | 4.65 |
| AHF-28 | 7.08 | 12366.16 | 7173.24 | 296.68 | 3.15 | 296.68 | 1008.78 | 45.49 | 241.17 | 375.94 | 1379.52 | 1956.8 | 18.81 | 0.86 | 5.15 |
| AHF-29 | 7.25 | 14114.58 | 8226.97 | 297.15 | 1.11 | 297.15 | 1120.09 | 53.68 | 248.40 | 328.73 | 1308.41 | 2169.6 | 11.74 | 0.97 | 5.70 |
| AHF-30 | 7.2 | 8473.84 | 4857.47 | 251.83 | 1.91 | 251.83 | 652.90 | 26.10 | 150.59 | 265.08 | 871.22 | 1245.3 | 15.67 | 0.00 | 3.50 |
| Sample ID | Ow (pH) |
Ow (EC) |
Ow (TDS) | Ow (Turb.) | Ow (HCO3-) | Ow (Na+) |
Ow (K+) |
Ow (Mg2+) |
Ow (Ca2+) |
Ow (SO42-) |
Ow (Cl-) |
Ow (NO3-) |
Ow (F-) |
Ow (Br-) |
PIG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AHF-1 | 0.0531 | 0.1581 | 0.0687 | 1.1225 | 0.0454 | 0.0371 | 0.0398 | 0.0497 | 0.1164 | 0.0000 | 0.1099 | 0.0356 | 0.0000 | 0.0000 | 1.8363 |
| AHF-2 | 0.0521 | 0.6070 | 0.2658 | 1.1520 | 0.0813 | 0.2575 | 0.2177 | 0.2873 | 0.4042 | 0.0000 | 0.6249 | 0.0681 | 0.0480 | 0.1104 | 4.1763 |
| AHF-3 | 0.0566 | 0.1681 | 0.1595 | 1.1669 | 0.0444 | 0.0426 | 0.0416 | 0.0735 | 0.1099 | 0.0000 | 0.1199 | 0.0252 | 0.0000 | 0.0000 | 2.0082 |
| AHF-4 | 0.0534 | 0.4361 | 0.1951 | 0.7798 | 0.0641 | 0.1631 | 0.1459 | 0.1920 | 0.2936 | 0.0000 | 0.4110 | 0.0642 | 0.0000 | 0.1058 | 2.9039 |
| AHF-5 | 0.0553 | 0.1527 | 0.0755 | 1.1655 | 0.0466 | 0.0352 | 0.0389 | 0.0649 | 0.1037 | 0.0000 | 0.1037 | 0.0334 | 0.0460 | 0.0000 | 1.9213 |
| AHF-6 | 0.0557 | 0.5493 | 0.2526 | 1.1803 | 0.0608 | 0.1754 | 0.0961 | 0.1813 | 0.2913 | 0.0000 | 0.4126 | 0.0876 | 0.0000 | 0.1046 | 3.4476 |
| AHF-10 | 0.0545 | 0.5701 | 0.2720 | 0.0154 | 0.0498 | 0.3095 | 0.2183 | 0.2830 | 0.4579 | 0.4006 | 0.4422 | 0.1523 | 0.0000 | 0.1349 | 3.3603 |
| AHF-11 | 0.0577 | 0.1619 | 0.0824 | 0.0132 | 0.0461 | 0.0454 | 0.0477 | 0.0587 | 0.0936 | 0.0578 | 0.0758 | 0.0315 | 0.0284 | 0.0424 | 0.8426 |
| AHF-12 | 0.0561 | 0.3498 | 0.1735 | 0.0092 | 0.0514 | 0.1158 | 0.0694 | 0.1011 | 0.1659 | 0.1545 | 0.1567 | 0.0529 | 0.0373 | 0.0562 | 1.5497 |
| AHF-13 | 0.0559 | 0.3075 | 0.1524 | 0.0103 | 0.0508 | 0.1058 | 0.0607 | 0.0950 | 0.1513 | 0.1360 | 0.1448 | 0.0495 | 0.0247 | 0.0531 | 1.3978 |
| AHF-14 | 0.0563 | 0.3860 | 0.1835 | 0.0135 | 0.0479 | 0.1635 | 0.0707 | 0.1407 | 0.1981 | 0.1744 | 0.2324 | 0.0594 | 0.0445 | 0.0860 | 1.8568 |
| AHF-15 | 0.0559 | 0.4122 | 0.1896 | 0.0191 | 0.0605 | 0.1556 | 0.1032 | 0.1439 | 0.2289 | 0.1857 | 0.2377 | 0.0513 | 0.0538 | 0.0865 | 1.9840 |
| AHF-16 | 0.0573 | 0.3336 | 0.1429 | 0.0122 | 0.0517 | 0.1273 | 0.1054 | 0.1148 | 0.1643 | 0.1342 | 0.1876 | 0.0388 | 0.0262 | 0.0677 | 1.5642 |
| AHF-17 | 0.0571 | 0.3421 | 0.1520 | 0.0117 | 0.0568 | 0.1349 | 0.1187 | 0.1196 | 0.1632 | 0.1365 | 0.1940 | 0.0362 | 0.0284 | 0.0690 | 1.6201 |
| AHF-18 | 0.0546 | 0.6756 | 0.3056 | 0.0037 | 0.0802 | 0.2878 | 0.1833 | 0.2460 | 0.3725 | 0.3099 | 0.4174 | 0.0651 | 0.0526 | 0.1113 | 3.1656 |
| AHF-19 | 0.0553 | 0.6794 | 0.2970 | 0.0114 | 0.0871 | 0.2712 | 0.1678 | 0.2785 | 0.4158 | 0.3140 | 0.4288 | 0.0382 | 0.0361 | 0.1466 | 3.2271 |
| AHF-20 | 0.0587 | 0.3360 | 0.1504 | 0.0136 | 0.0504 | 0.1284 | 0.1375 | 0.1243 | 0.1707 | 0.1496 | 0.1865 | 0.0344 | 0.0410 | 0.0719 | 1.6533 |
| AHF-21 | 0.0564 | 0.5601 | 0.2647 | 0.0118 | 0.0572 | 0.2384 | 0.1710 | 0.2044 | 0.3059 | 0.2672 | 0.3254 | 0.0675 | 0.0536 | 0.1011 | 2.6848 |
| AHF-22 | 0.0562 | 0.8273 | 0.4783 | 0.0226 | 0.0554 | 0.3361 | 0.2151 | 0.2681 | 0.2380 | 0.2419 | 0.6952 | 0.0288 | 0.0285 | 0.1810 | 3.6724 |
| AHF-23 | 0.0565 | 0.3906 | 0.2212 | 0.0162 | 0.0546 | 0.1412 | 0.0987 | 0.1316 | 0.1631 | 0.1296 | 0.3216 | 0.0272 | 0.0435 | 0.1084 | 1.9041 |
| AHF-24 | 0.0568 | 0.2543 | 0.1424 | 0.0172 | 0.0547 | 0.0849 | 0.0718 | 0.0872 | 0.1305 | 0.0987 | 0.1951 | 0.0280 | 0.0385 | 0.0889 | 1.3490 |
| AHF-25 | 0.0557 | 0.4469 | 0.2538 | 0.0275 | 0.0656 | 0.1605 | 0.1156 | 0.1468 | 0.1788 | 0.1669 | 0.3472 | 0.0296 | 0.0340 | 0.1077 | 2.1366 |
| AHF-26 | 0.0572 | 0.8473 | 0.4902 | 0.0155 | 0.0791 | 0.3706 | 0.2748 | 0.3393 | 0.3292 | 0.3711 | 0.7343 | 0.0276 | 0.0631 | 0.1941 | 4.1934 |
| AHF-27 | 0.0567 | 0.8797 | 0.5096 | 0.0064 | 0.0826 | 0.3487 | 0.2477 | 0.3337 | 0.3544 | 0.3864 | 0.7004 | 0.0332 | 0.0320 | 0.1720 | 4.1433 |
| AHF-28 | 0.0561 | 0.9151 | 0.5308 | 0.0437 | 0.0878 | 0.3732 | 0.2805 | 0.3569 | 0.3709 | 0.4083 | 0.7420 | 0.0278 | 0.0422 | 0.1905 | 4.4260 |
| AHF-29 | 0.0575 | 1.0445 | 0.6088 | 0.0154 | 0.0880 | 0.4144 | 0.3310 | 0.3676 | 0.3243 | 0.3873 | 0.8021 | 0.0174 | 0.0480 | 0.2109 | 4.7172 |
| AHF-30 | 0.0571 | 0.6271 | 0.3595 | 0.0265 | 0.0745 | 0.2172 | 0.1609 | 0.2229 | 0.2615 | 0.2579 | 0.4650 | 0.0232 | 0.0000 | 0.1294 | 2.8827 |
| Minimum | 0.0521 | 0.1527 | 0.0687 | 0.0037 | 0.0444 | 0.0352 | 0.0389 | 0.0497 | 0.0936 | 0.0000 | 0.0758 | 0.0174 | 0.0000 | 0.0000 | 0.8426 |
| Maximum | 0.0587 | 1.0445 | 0.6088 | 1.1803 | 0.0880 | 0.4144 | 0.3310 | 0.3676 | 0.4579 | 0.4083 | 0.8021 | 0.1523 | 0.0631 | 0.2109 | 4.7172 |
| Mean | 0.0560 | 0.4970 | 0.2584 | 0.2557 | 0.0620 | 0.1941 | 0.1418 | 0.1857 | 0.2429 | 0.1803 | 0.3635 | 0.0457 | 0.0315 | 0.1011 | 2.6157 |
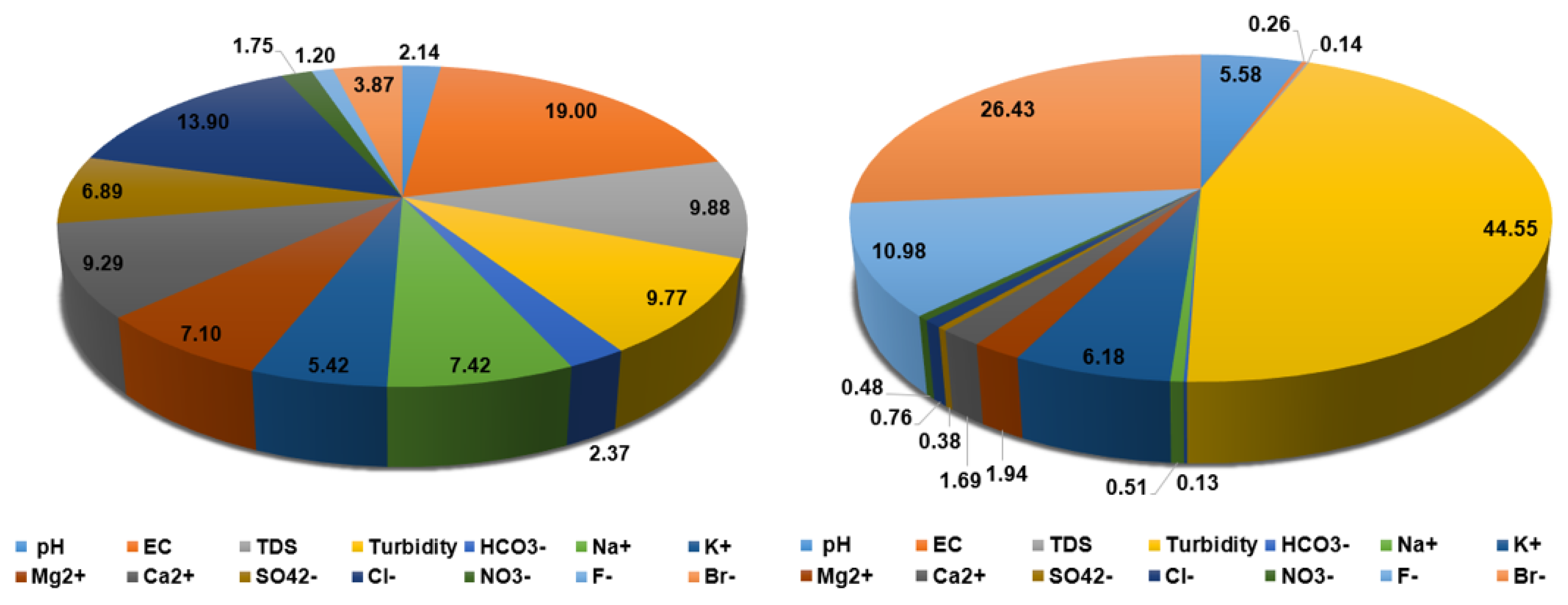
| Contribution (%) | 2.1405 | 18.9992 | 9.8801 | 9.7743 | 2.3714 | 7.4212 | 5.4225 | 7.0981 | 9.2854 | 6.8935 | 13.8966 | 1.7474 | 1.2040 | 3.8658 |
| Sample ID | SPI (pH) |
SPI (EC) | SPI (TDS) | SPI (Turb.) | SPI (HCO3-) | SPI (Na+) |
SPI (K+) |
SPI (Mg2+) |
SPI (Ca2+) |
SPI (SO2+) |
SPI (Cl-) |
SPI (NO3-) |
SPI (F-) |
SPI (Br-) |
Final SPI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AHF-1 | 0.0797 | 0.0012 | 0.0005 | 2.9479 | 0.0014 | 0.0015 | 0.0262 | 0.0078 | 0.0122 | 0.0000 | 0.0035 | 0.0056 | 0.0000 | 0.0000 | 3.0876 |
| AHF-2 | 0.0782 | 0.0048 | 0.0021 | 3.0255 | 0.0026 | 0.0101 | 0.1429 | 0.0453 | 0.0425 | 0.0000 | 0.0197 | 0.0107 | 0.2521 | 0.4349 | 4.0715 |
| AHF-3 | 0.0850 | 0.0013 | 0.0013 | 3.0645 | 0.0014 | 0.0017 | 0.0273 | 0.0116 | 0.0115 | 0.0000 | 0.0038 | 0.0040 | 0.0000 | 0.0000 | 3.2133 |
| AHF-4 | 0.0802 | 0.0034 | 0.0015 | 2.0479 | 0.0020 | 0.0064 | 0.0958 | 0.0303 | 0.0308 | 0.0000 | 0.0130 | 0.0101 | 0.0000 | 0.4167 | 2.7381 |
| AHF-5 | 0.0829 | 0.0012 | 0.0006 | 3.0609 | 0.0015 | 0.0014 | 0.0255 | 0.0102 | 0.0109 | 0.0000 | 0.0033 | 0.0053 | 0.2415 | 0.0000 | 3.4451 |
| AHF-6 | 0.0835 | 0.0043 | 0.0020 | 3.0999 | 0.0019 | 0.0069 | 0.0631 | 0.0286 | 0.0306 | 0.0000 | 0.0130 | 0.0138 | 0.0000 | 0.4122 | 3.7598 |
| AHF-10 | 0.0817 | 0.0045 | 0.0021 | 0.0404 | 0.0016 | 0.0122 | 0.1433 | 0.0446 | 0.0481 | 0.0126 | 0.0139 | 0.0240 | 0.0000 | 0.5314 | 0.9606 |
| AHF-11 | 0.0866 | 0.0013 | 0.0006 | 0.0346 | 0.0015 | 0.0018 | 0.0313 | 0.0093 | 0.0098 | 0.0018 | 0.0024 | 0.0050 | 0.1493 | 0.1669 | 0.5021 |
| AHF-12 | 0.0842 | 0.0028 | 0.0014 | 0.0240 | 0.0016 | 0.0046 | 0.0456 | 0.0159 | 0.0174 | 0.0049 | 0.0049 | 0.0083 | 0.1962 | 0.2213 | 0.6331 |
| AHF-13 | 0.0839 | 0.0024 | 0.0012 | 0.0270 | 0.0016 | 0.0042 | 0.0399 | 0.0150 | 0.0159 | 0.0043 | 0.0046 | 0.0078 | 0.1296 | 0.2092 | 0.5464 |
| AHF-14 | 0.0845 | 0.0030 | 0.0014 | 0.0353 | 0.0015 | 0.0064 | 0.0464 | 0.0222 | 0.0208 | 0.0055 | 0.0073 | 0.0094 | 0.2337 | 0.3386 | 0.8162 |
| AHF-15 | 0.0839 | 0.0032 | 0.0015 | 0.0503 | 0.0019 | 0.0061 | 0.0677 | 0.0227 | 0.0240 | 0.0059 | 0.0075 | 0.0081 | 0.2827 | 0.3406 | 0.9062 |
| AHF-16 | 0.0860 | 0.0026 | 0.0011 | 0.0321 | 0.0016 | 0.0050 | 0.0692 | 0.0181 | 0.0173 | 0.0042 | 0.0059 | 0.0061 | 0.1376 | 0.2669 | 0.6538 |
| AHF-17 | 0.0857 | 0.0027 | 0.0012 | 0.0306 | 0.0018 | 0.0053 | 0.0779 | 0.0189 | 0.0171 | 0.0043 | 0.0061 | 0.0057 | 0.1490 | 0.2720 | 0.6783 |
| AHF-18 | 0.0820 | 0.0053 | 0.0024 | 0.0098 | 0.0025 | 0.0113 | 0.1203 | 0.0388 | 0.0391 | 0.0098 | 0.0132 | 0.0103 | 0.2765 | 0.4383 | 1.0596 |
| AHF-19 | 0.0831 | 0.0054 | 0.0023 | 0.0299 | 0.0027 | 0.0107 | 0.1102 | 0.0439 | 0.0437 | 0.0099 | 0.0135 | 0.0060 | 0.1894 | 0.5773 | 1.1280 |
| AHF-20 | 0.0880 | 0.0026 | 0.0012 | 0.0357 | 0.0016 | 0.0051 | 0.0903 | 0.0196 | 0.0179 | 0.0047 | 0.0059 | 0.0054 | 0.2153 | 0.2832 | 0.7766 |
| AHF-21 | 0.0846 | 0.0044 | 0.0021 | 0.0310 | 0.0018 | 0.0094 | 0.1123 | 0.0322 | 0.0321 | 0.0084 | 0.0103 | 0.0106 | 0.2817 | 0.3984 | 1.0192 |
| AHF-22 | 0.0844 | 0.0065 | 0.0038 | 0.0594 | 0.0017 | 0.0132 | 0.1412 | 0.0423 | 0.0250 | 0.0076 | 0.0219 | 0.0045 | 0.1495 | 0.7129 | 1.2740 |
| AHF-23 | 0.0847 | 0.0031 | 0.0017 | 0.0426 | 0.0017 | 0.0056 | 0.0648 | 0.0207 | 0.0171 | 0.0041 | 0.0101 | 0.0043 | 0.2285 | 0.4271 | 0.9162 |
| AHF-24 | 0.0853 | 0.0020 | 0.0011 | 0.0452 | 0.0017 | 0.0033 | 0.0471 | 0.0137 | 0.0137 | 0.0031 | 0.0061 | 0.0044 | 0.2024 | 0.3501 | 0.7794 |
| AHF-25 | 0.0835 | 0.0035 | 0.0020 | 0.0721 | 0.0021 | 0.0063 | 0.0759 | 0.0231 | 0.0188 | 0.0053 | 0.0109 | 0.0047 | 0.1785 | 0.4243 | 0.9111 |
| AHF-26 | 0.0858 | 0.0067 | 0.0039 | 0.0408 | 0.0025 | 0.0146 | 0.1804 | 0.0535 | 0.0346 | 0.0117 | 0.0231 | 0.0043 | 0.3314 | 0.7645 | 1.5578 |
| AHF-27 | 0.0851 | 0.0069 | 0.0040 | 0.0168 | 0.0026 | 0.0137 | 0.1626 | 0.0526 | 0.0372 | 0.0122 | 0.0221 | 0.0052 | 0.1679 | 0.6775 | 1.2664 |
| AHF-28 | 0.0842 | 0.0072 | 0.0042 | 0.1148 | 0.0028 | 0.0147 | 0.1842 | 0.0562 | 0.0390 | 0.0129 | 0.0234 | 0.0044 | 0.2215 | 0.7504 | 1.5198 |
| AHF-29 | 0.0863 | 0.0082 | 0.0048 | 0.0404 | 0.0028 | 0.0163 | 0.2173 | 0.0579 | 0.0341 | 0.0122 | 0.0253 | 0.0027 | 0.2519 | 0.8310 | 1.5912 |
| AHF-30 | 0.0857 | 0.0049 | 0.0028 | 0.0696 | 0.0023 | 0.0086 | 0.1057 | 0.0351 | 0.0275 | 0.0081 | 0.0147 | 0.0037 | 0.0000 | 0.5097 | 0.8784 |
| Min. | 0.0782 | 0.0012 | 0.0005 | 0.0098 | 0.0014 | 0.0014 | 0.0255 | 0.0078 | 0.0098 | 0.0000 | 0.0024 | 0.0027 | 0.0000 | 0.0000 | 0.5021 |
| Max. | 0.0880 | 0.0082 | 0.0048 | 3.0999 | 0.0028 | 0.0163 | 0.2173 | 0.0579 | 0.0481 | 0.0129 | 0.0253 | 0.0240 | 0.3314 | 0.8310 | 4.0715 |
| Mean | 0.0840 | 0.0039 | 0.0020 | 0.6715 | 0.0020 | 0.0076 | 0.0931 | 0.0293 | 0.0255 | 0.0057 | 0.0115 | 0.0072 | 0.1654 | 0.3983 | 1.5070 |
| Cont. (%) | 5.5756 | 0.2598 | 0.1351 | 44.5548 | 0.1297 | 0.5074 | 6.1794 | 1.9413 | 1.6930 | 0.3771 | 0.7601 | 0.4779 | 10.9763 | 26.4323 | - |
| Sample ID | (a) NO3- ingestion health risk | (b) F- ingestion health risk | (c) THI due to NO3- and F- ingestion | (d) NO3- dermal health risk | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HQ (< 2 yrs.) | HQ (2-16 yrs.) | HQ (> 16 yrs.) | HI (∑HQ) | HQ (< 2 yrs.) | HQ (2-16 yrs.) | HQ (> 16 yrs.) | HI (∑HQ) | THI (< 2 yrs.) | THI (2-16 yrs.) | THI (> 16 yrs.) | ∑THI | HQ (children) | HQ (women) | HQ (men) | HI (∑HQ) | ||||
| AHF-1 | 1.41 | 0.87 | 0.46 | 2.74 | 0.00 | 0.00 | 0.00 | 0.00 | 1.41 | 0.87 | 0.46 | 2.74 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-2 | 2.69 | 1.67 | 0.89 | 5.25 | 2.28 | 1.41 | 0.75 | 4.44 | 4.97 | 3.07 | 1.64 | 9.69 | 0.01 | 0.00 | 0.00 | 0.02 | |||
| AHF-3 | 1.00 | 0.62 | 0.33 | 1.94 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.62 | 0.33 | 1.94 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-4 | 2.54 | 1.57 | 0.84 | 4.94 | 0.00 | 0.00 | 0.00 | 0.00 | 2.54 | 1.57 | 0.84 | 4.94 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-5 | 1.32 | 0.82 | 0.44 | 2.58 | 2.18 | 1.35 | 0.72 | 4.25 | 3.50 | 2.17 | 1.16 | 6.83 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-6 | 3.46 | 2.14 | 1.14 | 6.75 | 0.00 | 0.00 | 0.00 | 0.00 | 3.46 | 2.14 | 1.14 | 6.75 | 0.01 | 0.00 | 0.00 | 0.02 | |||
| AHF-10 | 6.03 | 3.73 | 1.99 | 11.74 | 0.00 | 0.00 | 0.00 | 0.00 | 6.03 | 3.73 | 1.99 | 11.74 | 0.02 | 0.01 | 0.01 | 0.03 | |||
| AHF-11 | 1.25 | 0.77 | 0.41 | 2.43 | 1.35 | 0.83 | 0.45 | 2.63 | 2.59 | 1.60 | 0.86 | 5.05 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-12 | 2.09 | 1.29 | 0.69 | 4.07 | 1.77 | 1.10 | 0.59 | 3.45 | 3.86 | 2.39 | 1.27 | 7.53 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-13 | 1.96 | 1.21 | 0.65 | 3.82 | 1.17 | 0.72 | 0.39 | 2.28 | 3.13 | 1.94 | 1.03 | 6.10 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-14 | 2.35 | 1.45 | 0.78 | 4.58 | 2.11 | 1.31 | 0.70 | 4.12 | 4.46 | 2.76 | 1.47 | 8.69 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-15 | 2.03 | 1.26 | 0.67 | 3.96 | 2.55 | 1.58 | 0.84 | 4.98 | 4.59 | 2.84 | 1.51 | 8.93 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-16 | 1.54 | 0.95 | 0.51 | 2.99 | 1.24 | 0.77 | 0.41 | 2.42 | 2.78 | 1.72 | 0.92 | 5.42 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-17 | 1.43 | 0.89 | 0.47 | 2.79 | 1.35 | 0.83 | 0.44 | 2.62 | 2.78 | 1.72 | 0.92 | 5.41 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-18 | 2.57 | 1.59 | 0.85 | 5.02 | 2.50 | 1.54 | 0.82 | 4.87 | 5.07 | 3.14 | 1.67 | 9.88 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-19 | 1.51 | 0.93 | 0.50 | 2.94 | 1.71 | 1.06 | 0.56 | 3.33 | 3.22 | 1.99 | 1.06 | 6.28 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| AHF-20 | 1.36 | 0.84 | 0.45 | 2.65 | 1.95 | 1.20 | 0.64 | 3.79 | 3.31 | 2.05 | 1.09 | 6.44 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-21 | 2.67 | 1.65 | 0.88 | 5.20 | 2.55 | 1.57 | 0.84 | 4.96 | 5.21 | 3.22 | 1.72 | 10.16 | 0.01 | 0.00 | 0.00 | 0.02 | |||
| AHF-22 | 1.14 | 0.71 | 0.38 | 2.22 | 1.35 | 0.84 | 0.45 | 2.63 | 2.49 | 1.54 | 0.82 | 4.85 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-23 | 1.08 | 0.67 | 0.36 | 2.10 | 2.07 | 1.28 | 0.68 | 4.02 | 3.14 | 1.94 | 1.04 | 6.12 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-24 | 1.11 | 0.69 | 0.37 | 2.16 | 1.83 | 1.13 | 0.60 | 3.56 | 2.94 | 1.82 | 0.97 | 5.72 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-25 | 1.17 | 0.72 | 0.39 | 2.28 | 1.61 | 1.00 | 0.53 | 3.14 | 2.78 | 1.72 | 0.92 | 5.43 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-26 | 1.09 | 0.68 | 0.36 | 2.13 | 2.99 | 1.85 | 0.99 | 5.84 | 4.09 | 2.53 | 1.35 | 7.96 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-27 | 1.31 | 0.81 | 0.43 | 2.56 | 1.52 | 0.94 | 0.50 | 2.96 | 2.83 | 1.75 | 0.93 | 5.51 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-28 | 1.10 | 0.68 | 0.36 | 2.15 | 2.00 | 1.24 | 0.66 | 3.90 | 3.10 | 1.92 | 1.02 | 6.05 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| AHF-29 | 0.69 | 0.43 | 0.23 | 1.34 | 2.28 | 1.41 | 0.75 | 4.43 | 2.96 | 1.83 | 0.98 | 5.77 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| AHF-30 | 0.92 | 0.57 | 0.30 | 1.79 | 0.00 | 0.00 | 0.00 | 0.00 | 0.92 | 0.57 | 0.30 | 1.79 | 0.00 | 0.00 | 0.00 | 0.01 | |||
| Min. | 0.69 | 0.43 | 0.23 | 1.34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.92 | 0.57 | 0.30 | 1.79 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Max. | 6.03 | 3.73 | 1.99 | 11.74 | 2.99 | 1.85 | 0.99 | 5.84 | 6.03 | 3.73 | 1.99 | 11.74 | 0.02 | 0.01 | 0.01 | 0.03 | |||
| Average | 1.81 | 1.12 | 0.60 | 3.52 | 1.49 | 0.92 | 0.49 | 2.91 | 3.30 | 2.04 | 1.09 | 6.43 | 0.01 | 0.00 | 0.00 | 0.01 | |||
| Parameter | pH | EC | TDS | TA | Turbidity | HCO3- | Na+ | K+ | Mg2+ | Ca2+ | SO4- | Cl- | NO3- | F- | Br- |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.000 | ||||||||||||||
| EC | 0.040 | 1.000 | |||||||||||||
| TDS | 0.145 | 0.971** | 1.000 | ||||||||||||
| TA | -0.109 | 0.840** | 0.784** | 1.000 | |||||||||||
| Turbidity | -0.574** | -0.331 | -0.321 | -0.190 | 1.000 | ||||||||||
| HCO3- | -0.109 | 0.840** | 0.784** | 1.000** | -0.190 | 1.000 | |||||||||
| Na+ | 0.000 | 0.981** | 0.938** | 0.792** | -0.369 | 0.792** | 1.000 | ||||||||
| K+ | 0.021 | 0.945** | 0.916** | 0.799** | -0.312 | 0.799** | 0.965** | 1.000 | |||||||
| Mg2+ | -0.081 | 0.968** | 0.923** | 0.848** | -0.250 | 0.848** | 0.981** | 0.966** | 1.000 | ||||||
| Ca2+ | -0.345 | 0.791** | 0.666** | 0.749** | -0.134 | 0.749** | 0.844** | 0.801** | 0.887** | 1.000 | |||||
| SO42- | 0.286 | 0.790** | 0.778** | 0.600** | -0.697** | 0.600** | 0.834** | 0.776** | 0.771** | 0.646** | 1.000 | ||||
| Cl- | -0.078 | 0.961** | 0.951** | 0.816** | -0.169 | 0.816** | 0.944** | 0.939** | 0.960** | 0.767** | 0.659** | 1.000 | |||
| NO3- | -0.470* | -0.005 | -0.142 | -0.159 | 0.116 | -0.159 | 0.118 | 0.045 | 0.123 | 0.480* | 0.060 | -0.014 | 1.000 | ||
| F- | 0.221 | 0.285 | 0.251 | 0.331 | -0.413* | 0.331 | 0.306 | 0.301 | 0.251 | 0.120 | 0.345 | 0.219 | -0.272 | 1.000 | |
| Br- | 0.122 | 0.951** | 0.926** | 0.778** | -0.466* | 0.778** | 0.945** | 0.911** | 0.925** | 0.742** | 0.787** | 0.931** | -0.004 | 0.291 | 1.000 |
| Parameter | Communality | Principal Components (PCs) | ||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | ||
| pH | 0.788 | 0.023 | -0.877 | 0.137 |
| EC | 0.969 | 0.984 | 0.007 | -0.028 |
| TDS | 0.912 | 0.945 | -0.095 | -0.095 |
| TA | 0.888 | 0.877 | 0.070 | -0.338 |
| Turbidity | 0.868 | -0.381 | 0.691 | -0.496 |
| HCO3- | 0.888 | 0.877 | 0.070 | -0.338 |
| Na+ | 0.981 | 0.984 | 0.046 | 0.107 |
| K+ | 0.928 | 0.963 | 0.035 | 0.015 |
| Mg2+ | 0.988 | 0.982 | 0.150 | 0.014 |
| Ca2+ | 0.937 | 0.833 | 0.445 | 0.211 |
| SO42- | 0.904 | 0.826 | -0.285 | 0.374 |
| Cl- | 0.935 | 0.948 | 0.126 | -0.140 |
| NO3- | 0.942 | 0.017 | 0.660 | 0.711 |
| F- | 0.367 | 0.340 | -0.486 | -0.124 |
| Br- | 0.925 | 0.955 | -0.086 | 0.066 |
| Eigenvalue | – | 9.711 | 2.266 | 1.244 |
| Variability (%) | – | 64.740 | 15.107 | 8.291 |
| Cumulative (%) | – | 64.740 | 79.847 | 88.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





