Submitted:
29 March 2024
Posted:
29 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Monitoring of Phenophases and Fruit Growth Measurements
2.3. BBCH Scale Characteristics
3. Results
3.1. Principal Growth Stage 0: Seed Germination
3.2. Principal Growth Stage 0: Bud Development
3.3. Principal Growth Stage 1: Leaf Development
3.4. Principal Growth Stage 3: Stem Elongation
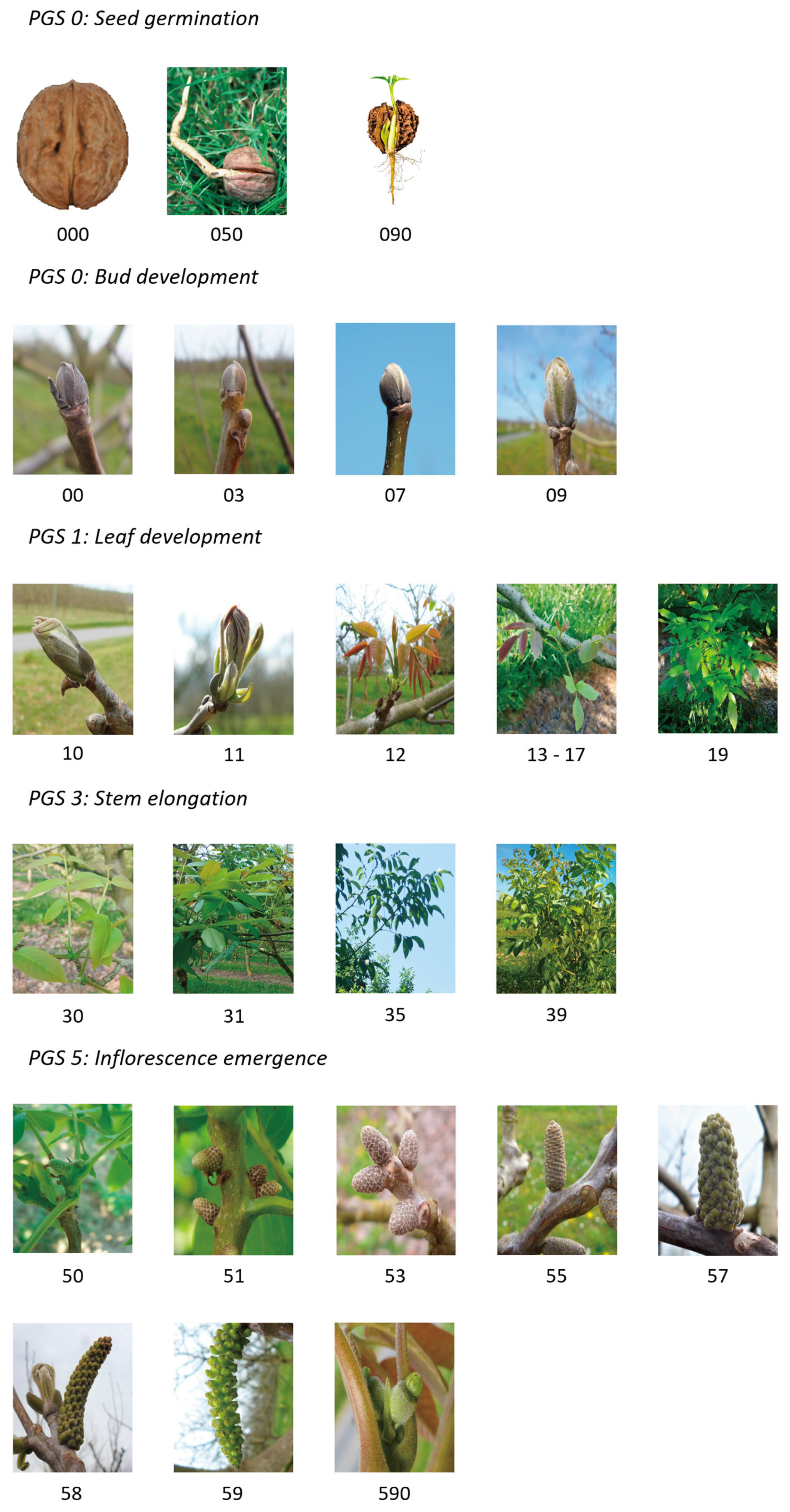
| BBCH codification | Germain et al., 1999 scale | IPGRI scale | Description |
|---|---|---|---|
| Principal growth stage 0: seed germination | |||
| 000 | Dry seed | ||
| 050 | The radicle emerges from the seed | ||
| 090 | Rootlet elongation, appearance of absorbent hairs and secondary root development, and soil emergence of the beetle | ||
| Principal growth stage 0: bud development | |||
| 00 | Af | Dormant buds: scale-covered bud | |
| 03 | Af2 | Fall of the hard scales of the first order. Bud still enveloped by other poorly differentiated semi-membranous scales | |
| 07 | Bf | The bud swells: the outer envelopes loosen, and the ends of the underlying bracts covered with a whitish down appear this is the so-called "woolly" or "white bud" stage. | |
| 09 | Cf | The bud elongates; the extremity of the terminal leaves of the outermost leaves can be distinguished; it's the bud burst | |
| Principal growth stage 1: leaf development | |||
| 10 | Cf2 | The scales and bracts move apart, the first leaves begin to separate | |
| 11 | Df | The bud is open, the first leaves separate, and their leaflets are well individualised | |
| 12 | Df2 | The first leaves are completely unfolded, first erect, then they take on a more or less oblique habit, revealing the female flowers in their centre. | |
| 13 | First leaf fully developed, loss of red foliage colour | ||
| 15 | More than two leaves are fully developed with a green foliage | ||
| 17 | All the leaves are fully expanded, are growing and turn dark green | ||
| 19 | All the leaves are mature and have their final length | ||
| Principal growth stage 3: stem elongation | |||
| 30 | Starting stem elongation | ||
| 31 | <10% of final stem length | ||
| 33 | >10% and <50% of final stem length | ||
| 35 | >50% and <90% of final stem length | ||
| 39 | >90% of final stem length | ||
| Principal growth stage 5: inflorescence emergence | |||
| 50 | Amr | In early summer, the differentiated male catkin, globose in shape, has a pinkish hue while the buds remain green | |
| 51 | Amv | During the summer, the catkin grows slightly, becomes conical, reaches about 0.5 cm length, and takes on a green colour | |
| 53 | Amg | At the beginning of October, the catkin stops growing, it measures 0.5-0.8 cm and takes on a gray colour that it will keep all winter. | |
| 55 | Bm | About 3 weeks before the bud break, growth resumes, the catkin swells and lengthens to reach 1.3-2 cm length | |
| 57 | Cm | Catkin, stiff and oblique, reaches the size of a pencil and measures 3-4 cm. Its colour gradually changes from green-brown to light green; the flower clusters are distinct. | |
| 58 | Dm | Catkin loses its rigidity, becomes semi-drooping; the glomeruli separate | |
| 59 | Dm2 | The glomeruli space out and begin to open; the catkin hangs | |
| 590 | Ef | Appearance of female flowers | |
| Principal growth stage 6: flowering | |||
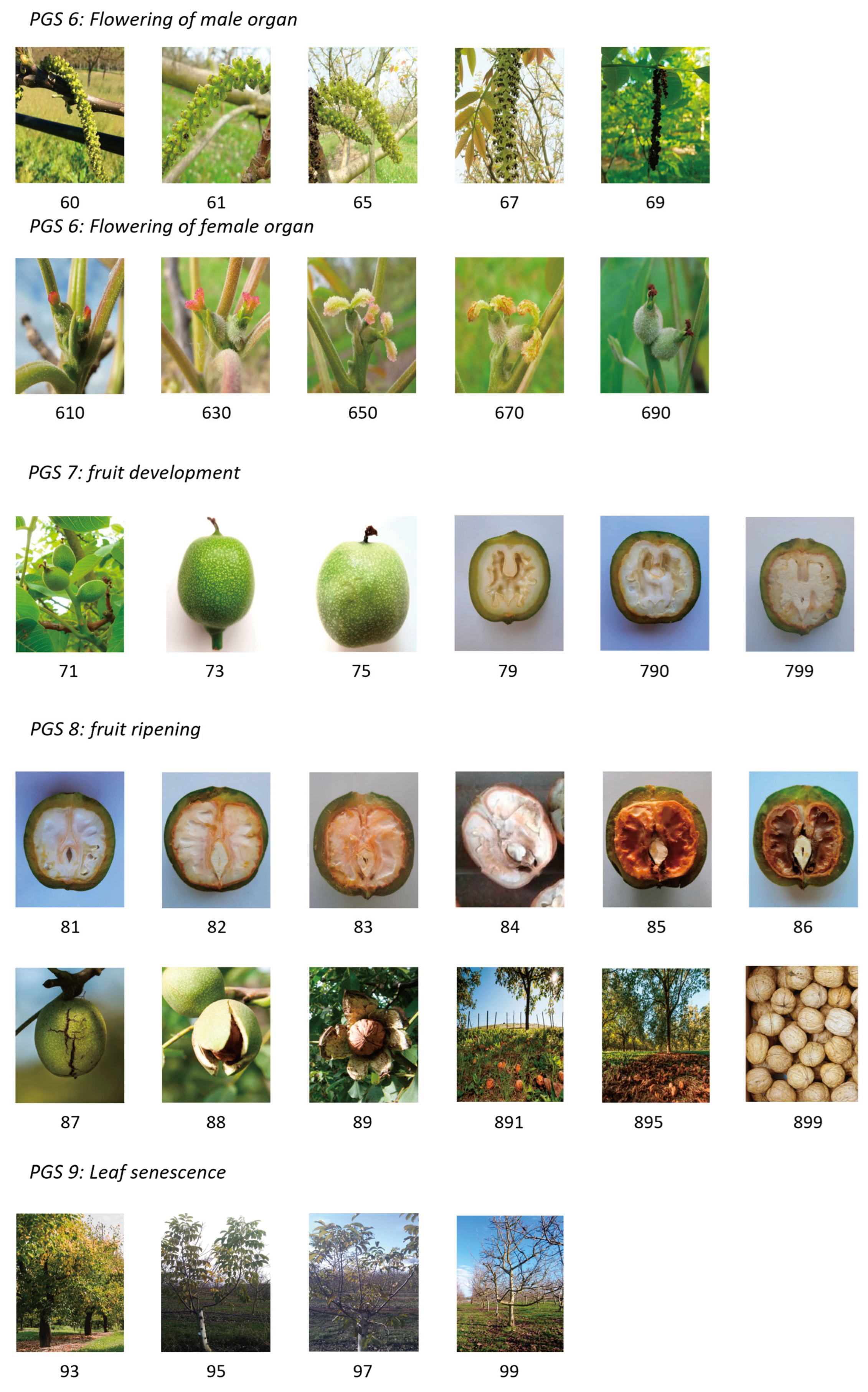
| 60 | Em | First male bloom date | Complete opening of the glomeruli and separation of the anthers which begin to turn yellow |
| 61 | Fm | Peak male bloom date | Beginning of anther dehiscence from base of catkin |
| 65 | Fm2 | Peak male bloom date | Complete anther dehiscence, full pollen emission |
| 67 | Gm | Last male bloom date | The anthers emptied of their pollen turn black |
| 69 | Hm | The catkin falls to the ground and dries up | |
| 610 | Ff | First female bloom date | Appearance of stigmata |
| 630 | Ff1 | Peak female bloom date | The orange-yellow stigmata are divergent. Their receptivity is optimal: it is the full female flowering. |
| 650 | FF2 | Peak female bloom date | Stigmata take on a pale green-yellow colour and are completely recurved |
| 670 | FF3 | Last female bloom date | The stigmata begin to become necrotic; these are streaked with fine brown threads. |
| 690 | Gf | Drying and blackening of the stigmata | |
| Principal growth stage 7: fruit development | |||
| 71 | Beginning of fruit-husk growth | ||
| 75 | 50% of the final size in fruit husk | ||
| 79 | 100% of the final size in husk and beginning of lignification: beginning of resistance to the knife | ||
| 790 | 100% of the shell is lignified and the beginning of kernel filling | ||
| 799 | 100% lignified shell and kernel filling completed | ||
| Principal growth stage 8: fruit ripening | |||
| 81 | White septum in kernel | ||
| 82 | Beginning of browning with some brown pitting on the septum | ||
| 83 | Brown internal septum on 1/3 of its surface | ||
| 84 | Packing tissue brown date | Brown internal septum on 3/3 of its surface | |
| 85 | In allover brown septum but damp and matt | ||
| 86 | Shiny dry brown septum | ||
| 87 | Cracking of the husk | ||
| 88 | Opening of the husk: the nut remains trapped in the husk | ||
| 89 | Husk opens enough for the nut to freely fall to the ground | ||
| 891 | Harvest date | < 10% of the nuts have fallen to the ground | |
| 895 | Harvest date | > 50% of the nuts have fallen to the ground | |
| 899 | Harvest date | 100% of the nuts have fallen to the ground | |
| Principal growth stage 9: leaf senescence | |||
| 93 | Beginning of leaf colour change | ||
| 95 | 50% of the leaves have changed colour | ||
| 97 | All the leaves have changed colour and leaf fall begins | ||
| 99 | Defoliation date | All the leaves have fallen | |
3.5. Principal Growth Stage 5: Inflorescence Emergence
3.6. Principal Growth Stage 6: Flowering
3.6.1. Male Flowering
3.6.2. Female Flowering
3.7. Principal Growth Stage 7: Fruit Development
3.8. Principal Growth Stage 8: Fruit Ripening
 ), width (dash line
), width (dash line  ) and weight (dash-point line
) and weight (dash-point line  ) of ‘Lara’ cultivar associated with their standard deviation of mean.
) of ‘Lara’ cultivar associated with their standard deviation of mean.
 ), width (dash line
), width (dash line  ) and weight (dash-point line
) and weight (dash-point line  ) of ‘Lara’ cultivar associated with their standard deviation of mean.
) of ‘Lara’ cultivar associated with their standard deviation of mean.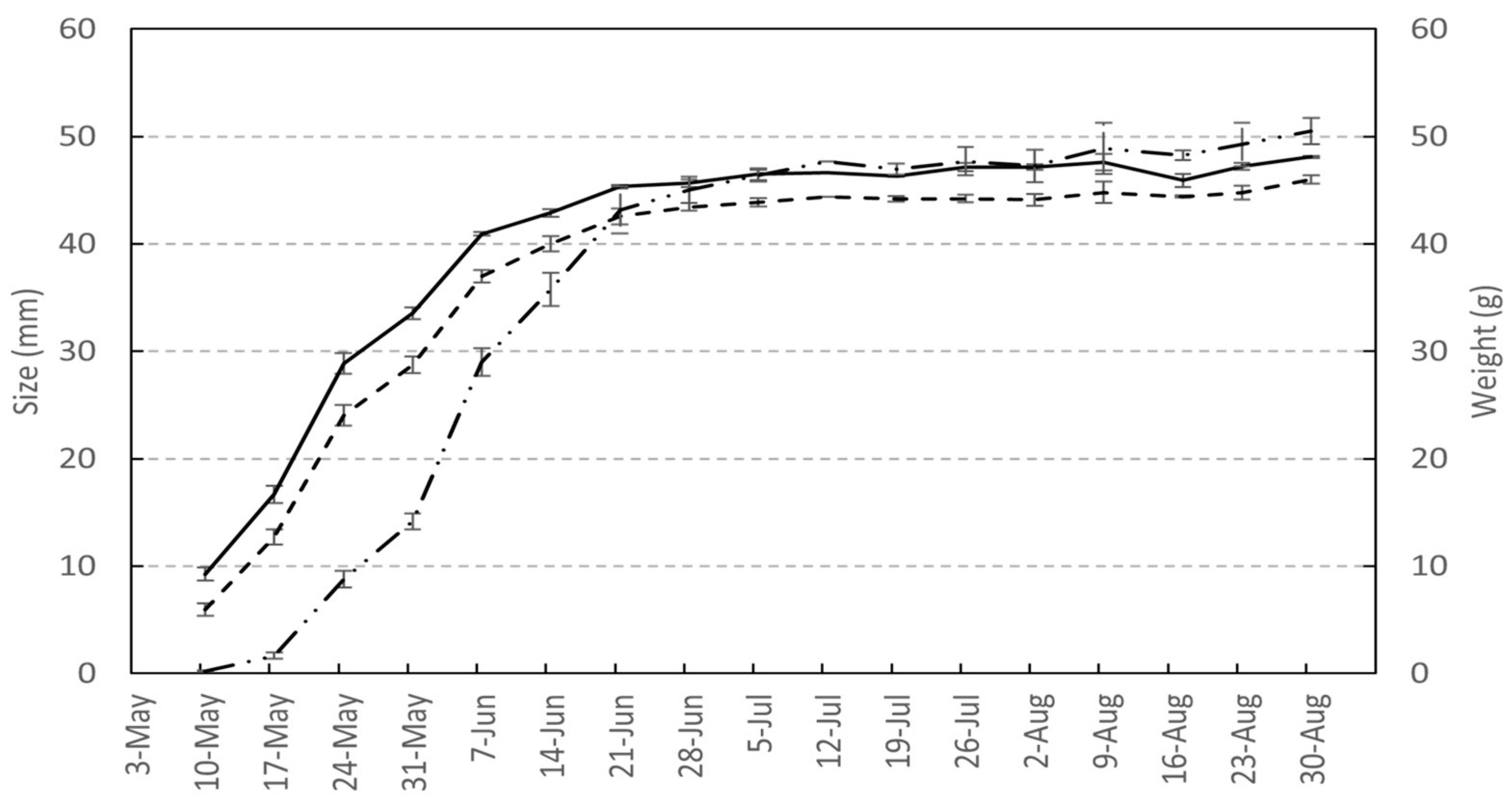
3.9. Principal Growth Stage 9: Leaf Senescence
4. Discussion
4.1. Flowering Phenophases
4.2. Fruit Development
4.3. Orchard and Research Implications of BBCH Codification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehder, A. Manual of Cultivated Trees and Shrubs. Man. Cultiv. Trees Shrubs 1949. [Google Scholar]
- Germain, É.; Prunet, J.-P.; Garcin, A. Le noyer; Monographie; CTIFL: Paris, 1999; ISBN 978-2-87911-104-9. [Google Scholar]
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and Future of Genetic Improvement. Tree Genet. Genomes 2018, 14, 1. [Google Scholar] [CrossRef]
- Woodworth, R.H. Meiosis of microsporogenesis in the Juglandaceae. Am. J. Bot. 1930, 17, 863–869. [Google Scholar] [CrossRef]
- Manning, W.E. The Classification Within the Juglandaceae. Ann. Mo. Bot. Gard. 1978, 65, 1058. [Google Scholar] [CrossRef]
- Stanford, A.M.; Harden, R.; Parks, C.R. Phylogeny and Biogeography of Juglans (Juglandaceae) Based on matK and ITS Sequence Data. Am. J. Bot. 2000, 87, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Zeven, A.C.; de Wet, J.M. Dictionary of Cultivated Plants and Their Regions of Diversity: Excluding Most Ornamentals, Forest Trees and Lower Plants, 2nd ed.; rev.; Centre for Agricultural Publishing and Documentation: Wageningen, 1982; ISBN 978-90-220-0785-3. [Google Scholar]
- Leslie, C.; McGranahan, G. Native Populations of Juglans Regia—A Draft. 1988, 19, 111–124. [Google Scholar]
- Carrion, J.S.; Sanchez-Gomez, P. Palynological Data in Support of the Survival of Walnut (Juglans regia L.) in the Western Mediterranean Area During Last Glacial Times. J. Biogeogr. 1992, 19, 623. [Google Scholar] [CrossRef]
- Pollegioni, P.; Woeste, K.; Chiocchini, F.; Del Lungo, S.; Ciolfi, M.; Olimpieri, I.; Tortolano, V.; Clark, J.; Hemery, G.E.; Mapelli, S.; et al. Rethinking the History of Common Walnut (Juglans regia L.) in Europe: Its Origins and Human Interactions. PLoS ONE 2017, 12, e0172541. [Google Scholar] [CrossRef] [PubMed]
- Lieth, H. Phenology and Seasonality Modeling. Springer Science & Business Media, 2013; Volume 8, ISBN 3-642-51863-X. [Google Scholar] [CrossRef]
- Chmielewski, F.-M.; Rötzer, T. Response of Tree Phenology to Climate Change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Telling Time with Trees. Nat. Clim. Change 2022, 12, 299–299. [CrossRef]
- Charrier, G.; Bonhomme, M.; Lacointe, A.; Améglio, T. Are Budburst Dates, Dormancy and Cold Acclimation in Walnut Trees (Juglans regia L.) under Mainly Genotypic or Environmental Control? Int. J. Biometeorol. 2011, 55, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European Phenological Response to Climate Change Matches the Warming Pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Chuine, I.; Morin, X.; Bugmann, H. Warming, Photoperiods, and Tree Phenology. Science 2010, 329, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Piao, S.; Vitasse, Y.; Zhao, H.; De Boeck, H.J.; Liu, Q.; Yang, H.; Weber, U.; Hänninen, H.; Janssens, I.A. Increased Heat Requirement for Leaf Flushing in Temperate Woody Species over 1980–2012: Effects of Chilling, Precipitation and Insolation. Glob. Change Biol. 2015, 21, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Peñuelas, J.; et al. Declining Global Warming Effects on the Phenology of Spring Leaf Unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Piao, S.; Delpierre, N.; Hao, F.; Hänninen, H.; Liu, Y.; Sun, W.; Janssens, I.A.; Campioli, M. Larger Temperature Response of Autumn Leaf Senescence than Spring Leaf-out Phenology. Glob. Change Biol. 2018, 24, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.; Vitasse, Y.; Bugmann, H.; Bigler, C. Phenological Shifts Induced by Climate Change Amplify Drought for Broad-Leaved Trees at Low Elevations in Switzerland. Agric. For. Meteorol. 2021, 307, 108485. [Google Scholar] [CrossRef]
- Črepinšek, Z.; Solar, M.; Štampar, F.; Solar, A. Shifts in Walnut (Juglans regia L.) Phenology Due to Increasing Temperatures in Slovenia. J. Hortic. Sci. Biotechnol. 2009, 84, 59–64. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Baciu, A.; Botu, M.; Achim, Gh. Environmental factors' influence on walnut flowering. Acta Hortic. 2010, 83–88. [Google Scholar] [CrossRef]
- Luedeling, E. Climate Change Impacts on Winter Chill for Temperate Fruit and Nut Production: A Review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Cook, B.I.; Wolkovich, E.M.; Davies, T.J.; Ault, T.R.; Betancourt, J.L.; Allen, J.M.; Bolmgren, K.; Cleland, E.E.; Crimmins, T.M.; Kraft, N.J.B.; et al. Sensitivity of Spring Phenology to Warming Across Temporal and Spatial Climate Gradients in Two Independent Databases. Ecosystems 2012, 15, 1283–1294. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.K.; Chamberlain, C.J.; Morales-Castilla, I.; Buonaiuto, D.M.; Flynn, D.F.B.; Savas, T.; Samaha, J.A.; Wolkovich, E.M. Winter Temperatures Predominate in Spring Phenological Responses to Warming. Nat. Clim. Change 2020, 10, 1137–1142. [Google Scholar] [CrossRef]
- Luedeling, E.; Zhang, M.; McGranahan, G.; Leslie, C. Validation of Winter Chill Models Using Historic Records of Walnut Phenology. Agric. For. Meteorol. 2009, 149, 1854–1864. [Google Scholar] [CrossRef]
- Arora, R.; Rowland, L.J.; Tanino, K. Induction and Release of Bud Dormancy in Woody Perennials: A Science Comes of Age. HortScience 2003, 38, 911–921. [Google Scholar] [CrossRef]
- Petri, J.L.; Leite, G.B. Consequences of insufficient winter chilling on apple tree bud-break. Acta Hortic. 2004, 53–60. [Google Scholar] [CrossRef]
- Man, R.; Lu, P.; Dang, Q.-L. Insufficient Chilling Effects Vary among Boreal Tree Species and Chilling Duration. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Man, R.; Lu, P.; Dang, Q.-L. Effects of Insufficient Chilling on Budburst and Growth of Six Temperate Forest Tree Species in Ontario. New For. 2021, 52, 303–315. [Google Scholar] [CrossRef]
- Bernard, A.; Marrano, A.; Donkpegan, A.; Brown, P.J.; Leslie, C.A.; Neale, D.B.; Lheureux, F.; Dirlewanger, E. Association and Linkage Mapping to Unravel Genetic Architecture of Phenological Traits and Lateral Bearing in Persian Walnut (Juglans regia L.). BMC Genomics 2020, 21, 203. [Google Scholar] [CrossRef]
- Marrano, A.; Sideli, G.M.; Leslie, C.A.; Cheng, H.; Neale, D.B. Deciphering of the Genetic Control of Phenology, Yield, and Pellicle Color in Persian Walnut (Juglans regia L.). Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Bleiholder, H.; Van Den Boom, J.; Langelüddeke, P.; Stauss, R. Einkeitliche Codierung Der Phänologischen Stadien Bei Kultur-Und Schadpflanzen. Gesunde Pflanz. 1989, 41, 381–384. [Google Scholar]
- Hack, H.; Bleiholder, H.; Buhr, L.; Meier, U.; Schnock-Fricke, U.; Weber, E.; Witzenberger, A. Einheitliche Codierung der phänologischen Entwicklungsstadien mono-und dikotyler Pflanzen—Erweiterte BBCH-Skala, Allgemein. Nachrichtenblatt Dtsch. Pflanzenschutzdienstes 1992, 44, 265–270. [Google Scholar]
- Sakar, E.H.; El Yamani, M.; Boussakouran, A.; Rharrabti, Y. Codification and Description of Almond (Prunus dulcis) Vegetative and Reproductive Phenology According to the Extended BBCH Scale. Sci. Hortic. 2019, 247, 224–234. [Google Scholar] [CrossRef]
- Adiga, J.D.; Muralidhara, B.M.; Preethi, P.; Savadi, S. Phenological Growth Stages of the Cashew Tree (Anacardium occidentale L.) According to the Extended BBCH Scale. Ann. Appl. Biol. 2019, 175, 246–252. [Google Scholar] [CrossRef]
- Larue, C.; Barreneche, T.; Petit, R.J. Efficient Monitoring of Phenology in Chestnuts. Sci. Hortic. 2021, 281, 109958. [Google Scholar] [CrossRef]
- Paradinas, A.; Ramade, L.; Mulot-Greffeuille, C.; Hamidi, R.; Thomas, M.; Toillon, J. Phenological Growth Stages of ‘Barcelona’ Hazelnut (Corylus Avellana L.) Described Using an Extended BBCH Scale. Sci. Hortic. 2022, 296, 110902. [Google Scholar] [CrossRef]
- Toillon, J.; Hamidi, R.; Paradinas, A.; Ramade, L.; Thomas, M.; Suarez Huerta, E. Consolidated BBCH Scale for Hazelnut Phenotyping. Acta Hortic. 2023, 159–168. [Google Scholar] [CrossRef]
- Han, M.; Peng, F.; Marshall, P. Pecan Phenology in Southeastern China: Pecan Phenology in Southeastern China. Ann. Appl. Biol. 2018, 172, 160–169. [Google Scholar] [CrossRef]
- Papillon, S.; Robin, J.; Tranchand, E.; Hebrard, M.-N.; Philibert, J.; Barbedette, M.; Lheureux, F.; Toillon, J. Application of BBCH Codification to Walnut (Juglans regia L.) Phenophase. Presented at the IX International Symposium on Walnut & Pecan, Grenoble, France, 2023. [CrossRef]
- Germain, E.; Jalinat, J.; Marchou, M. Divers Aspects de La Biologie Florale Du Noyer. 1975. [Google Scholar]
- International Plant Genetic Resources Institute. Descriptors for Walnut (Juglans spp.); Bioversity International, 1994; ISBN 92-9043-211-X. [Google Scholar]
- Mahmoodi, R.; Hassani, D.; Amiri, M.E.; Jaffaraghaei, M. Phenological and Pomological Characteristics of Five Promised Walnut Genotypes in Karaj, Iran. J. Nuts 2016, 7. [Google Scholar] [CrossRef]
- Soleimani, A.; Rabiei, V.; Hassani, D.; Mozaffari, M.R. Effect of Genetic-Environment Interaction on Phenology of Some Persian Walnut (Juglans regia L.) Genotypes. Crop Breed. J. 2020. [Google Scholar] [CrossRef]
- Shah, R.A.; Bakshi, P.; Sharma, N.; Jasrotia, A.; Itoo, H.; Gupta, R.; Singh, A. Diversity Assessment and Selection of Superior Persian Walnut (Juglans regia L.) Trees of Seedling Origin from North-Western Himalayan Region. Resour. Environ. Sustain. 2021, 3, 100015. [Google Scholar] [CrossRef]
- McGranahan, G.; Leslie, C. ‘Robert Livermore’, a Persian Walnut Cultivar with a Red Seedcoat. HortScience 2004, 39, 1772. [Google Scholar] [CrossRef]
- McGranahan, G.; Leslie, C. Walnut Tree Named ‘Forde’ 2006. USPP16495P3. https://patents.google.com/patent/USPP16495P3/en.
- McGranahan, G.; Leslie, C. Walnut Tree Named ‘Gillet’ 2006. USPP17135P3. https://patents.google.com/patent/USPP17135P3/en.
- McGranahan, G.; Leslie, C. Walnut Variety Named “DURHAM” 2017. US20170215313P1. https://patents.google.com/patent/US20170215313P1/en.
- McGranahan, G.H.; Forde, H.I.; Snyder, R.G.; Sibbett, G.S.; Reil, W.; Hasey, J.; Ramos, D.E. ‘Tulare’ Persian Walnut. HortScience 1992, 27, 186–187. [Google Scholar] [CrossRef]
- Leslie, C.A.; McGranahan, G.H. The Origin of the Walnut. Walnut Prod. Man. 1998, 3–7. [Google Scholar]
- Germain, E.; Jalinat, J.; Marchou, M. Divers Aspects de La Biologie Florale Du Noyer. In Le noyer; Invuflec, 1975; p. 187. [Google Scholar]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic Resources, Chemistry, by-Products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





