Submitted:
28 March 2024
Posted:
29 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. General
1.2. Scope and Relevance
2. Methodology
3. Modeling and theory
3.1. General
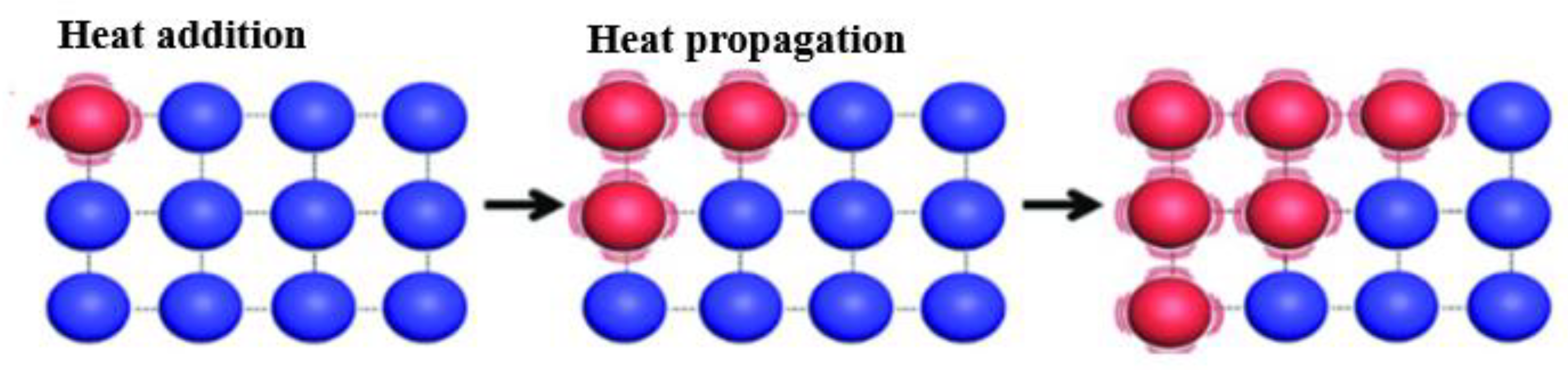
3.2. Concept of Thermal Conductivity
3.3. Impact of CNTs on Aqueous Nanofluid Thermal Conductivity
3.4. Proposed Model and Postulates
- (1)
- A CNT is a long nano-wide tube.
- (2)
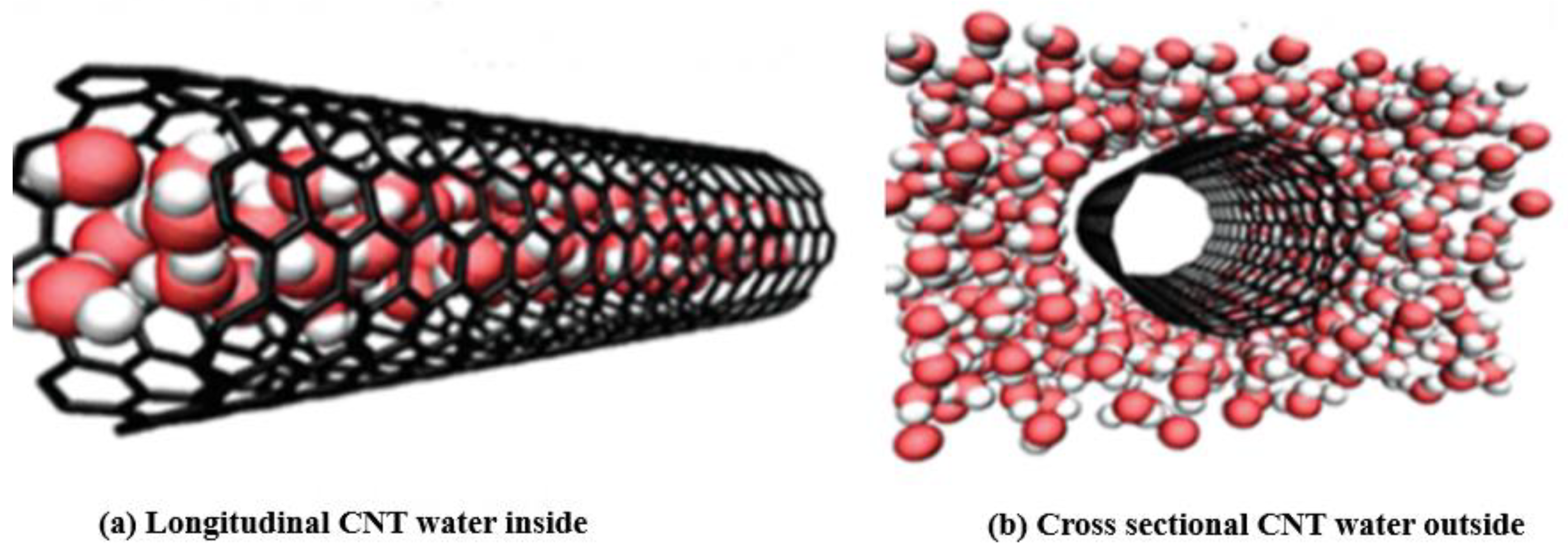
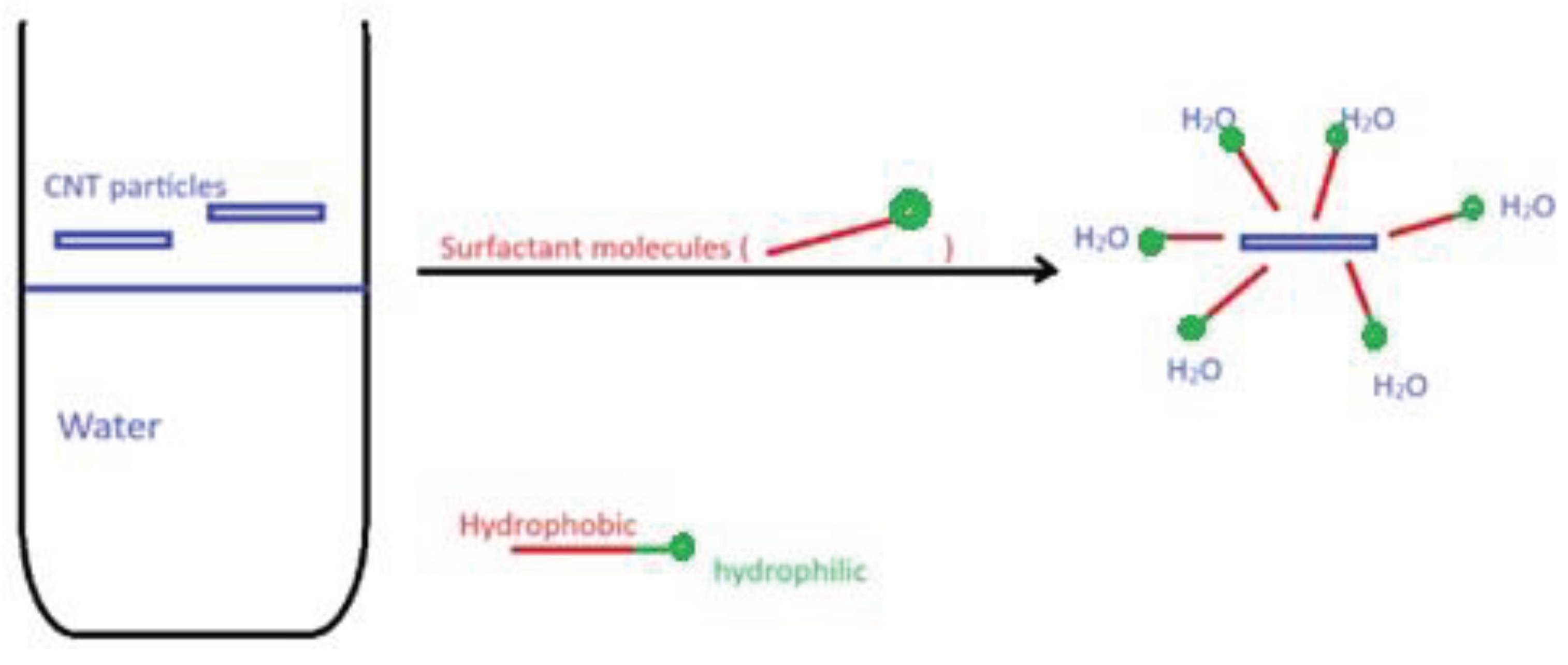
- When suspended in water, the CNT may interact with water molecules via weak interactions to keep the tubes suspended. The interactions vary depending on type and structure of the CNT. The interactions are further improved by functionalizing the tube with other polar groups such as -COOH or -SO3H.
- (3)
- Due to its directional structure, the CNT tube, together with bonded water molecules, forms a cluster that moves freely inside the nanofluid through the Brownian random motion.
- (4)
- A number of clusters may interact together to form a network of clusters that freely moves through the Brownian random motion.
- (5)
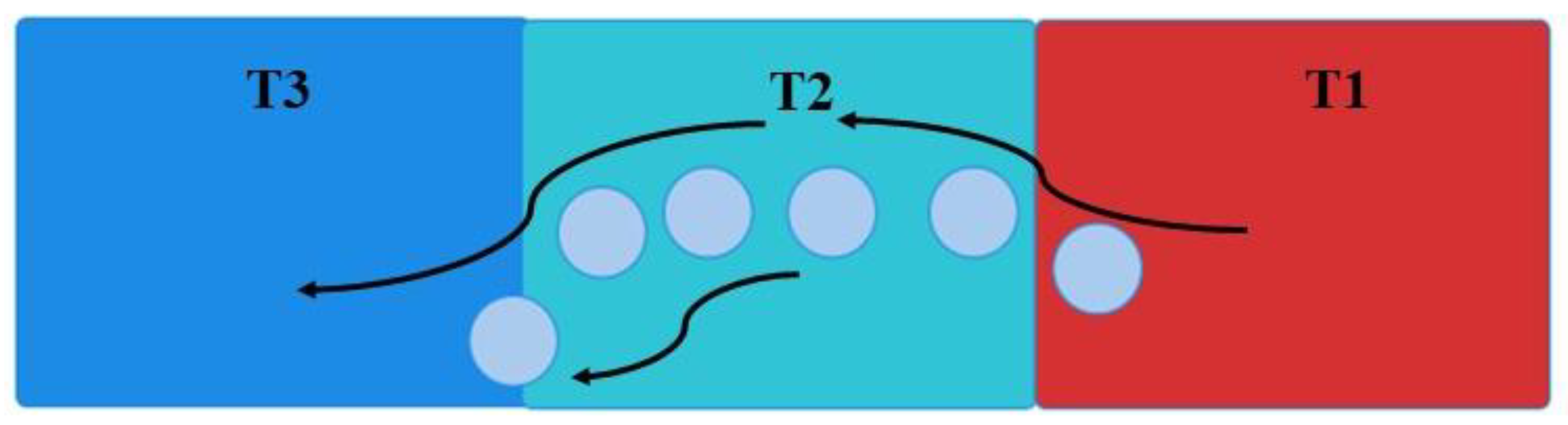
- Heat moves through a given cluster from one end (with the higher temperature) to another end (with lower the temperature) in a directional manner. The cluster behaves as a conducting channel.
- (6)
- The network also carries heat from the higher temperature side of fluid to the lower temperature side, through the Brownian motions. This process provides an additional path of heat transfer through convection.
- (7)
- The cluster and network formation induce various effects on the base liquid physical properties, such as viscosity, surface tension, interface characteristics with the container walls, and others. Such variations may further affect thermal conductivity of the nanofluid.
3.5. Mechanisms of Thermal Conductivity Increase in Aqueous Nanofluids
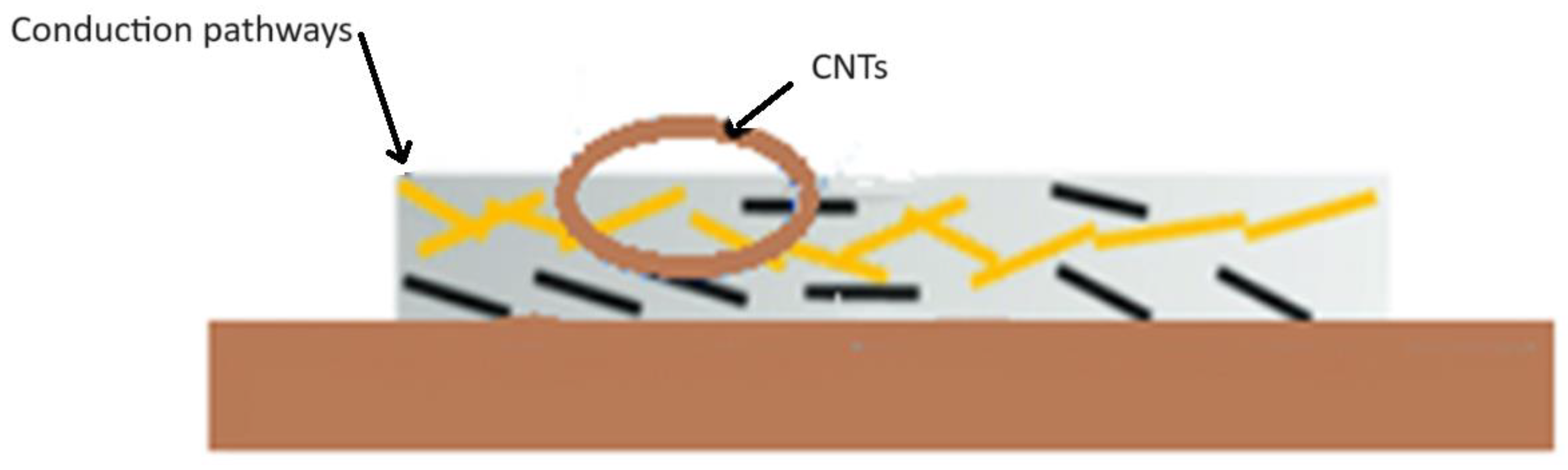
3.5.1. Conductive Network Formation
3.5.2. Boundary Layer Disruption
3.5.3. CNT Contact Resistance Reduction
3.5.4. Lowered Phonon Scattering
3.5.5. Increased Contact Points
3.5.6. Brownian Random Motion
3.5.7. Thermal Diffusivity
3.5.8. Surface Tension and Viscosity
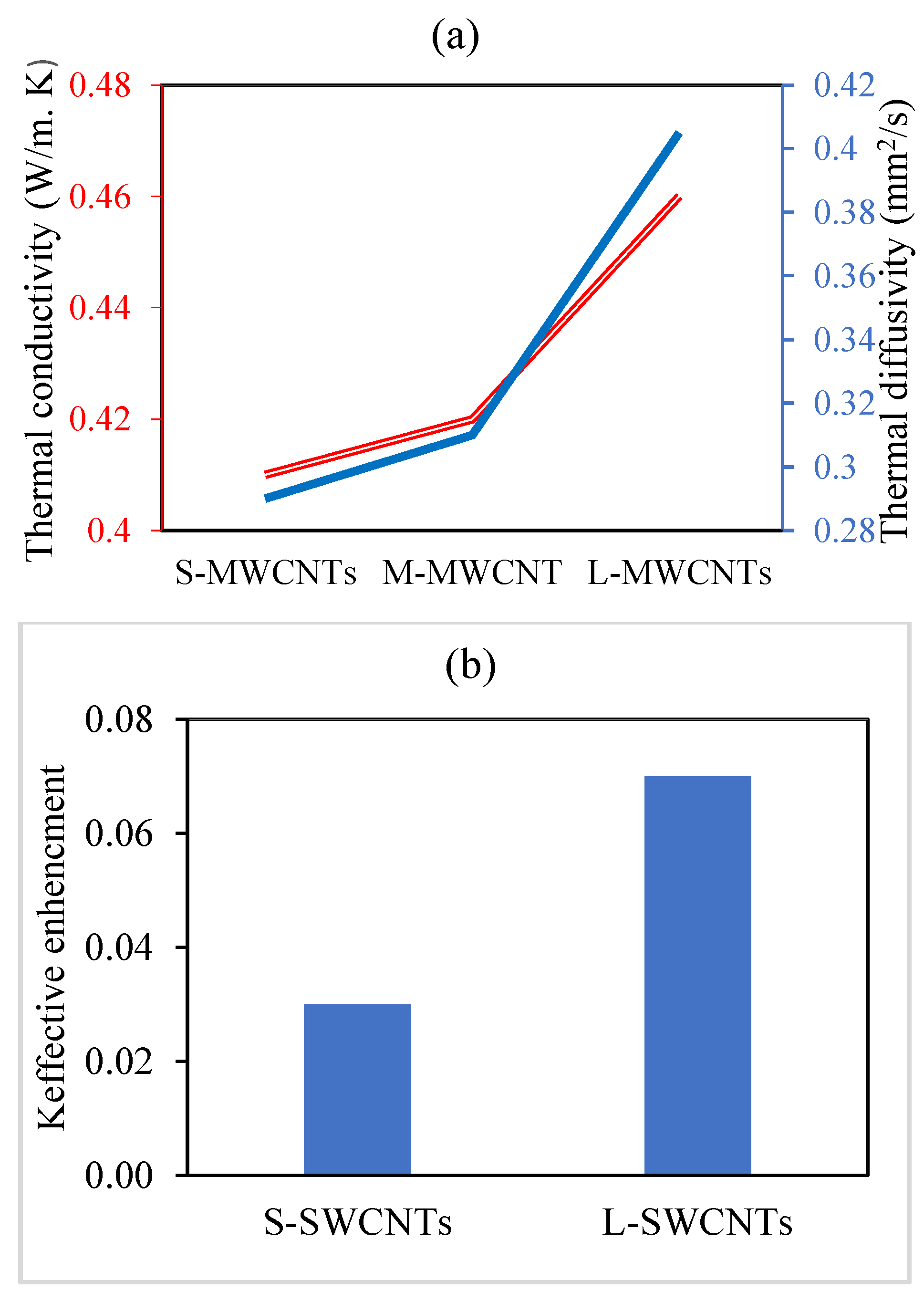
3.5.9. Type of Carbon Nanotube
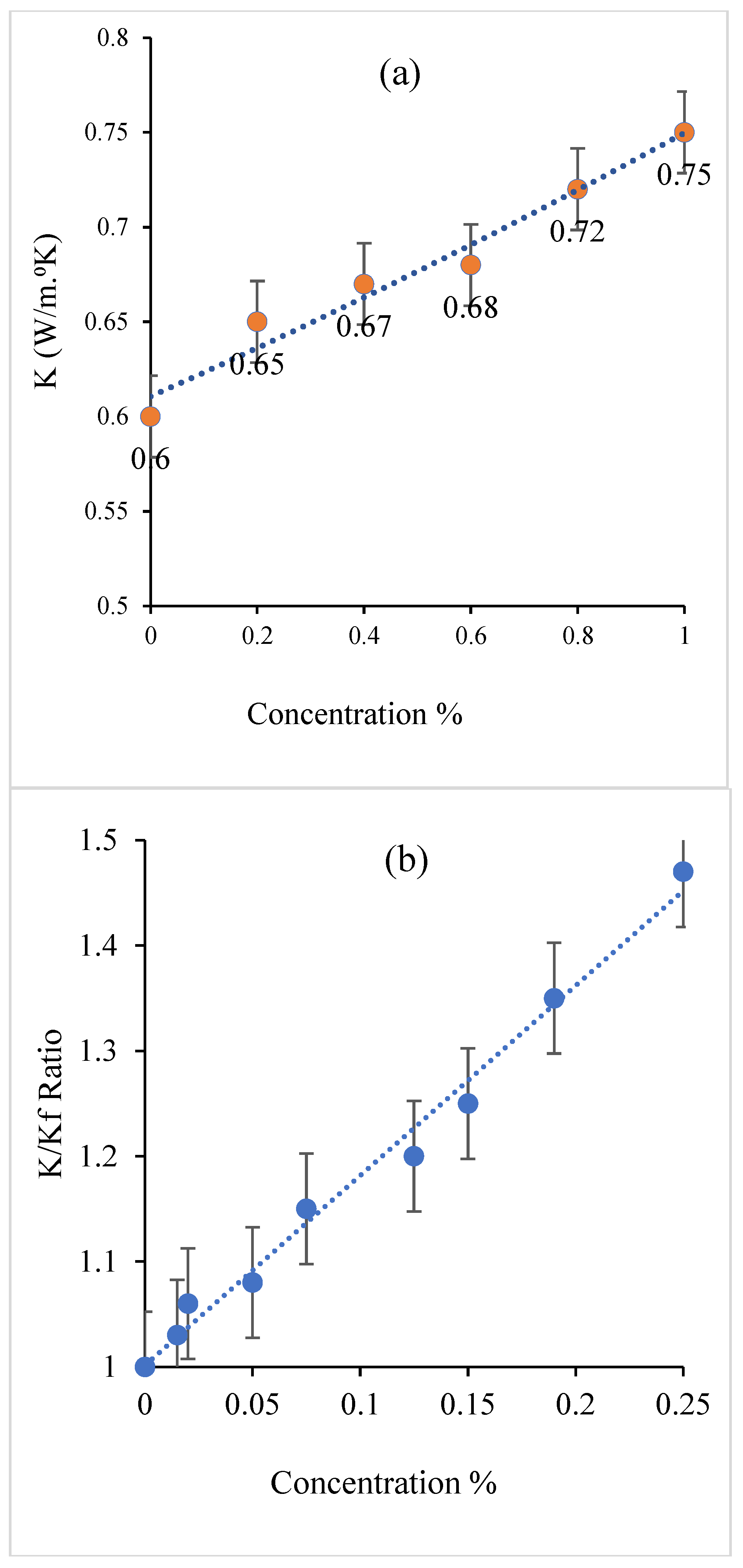
3.5.10. CNT Concentration
4. Results and Discussion
4.1. CNT Type
4.2. CNT Concentration
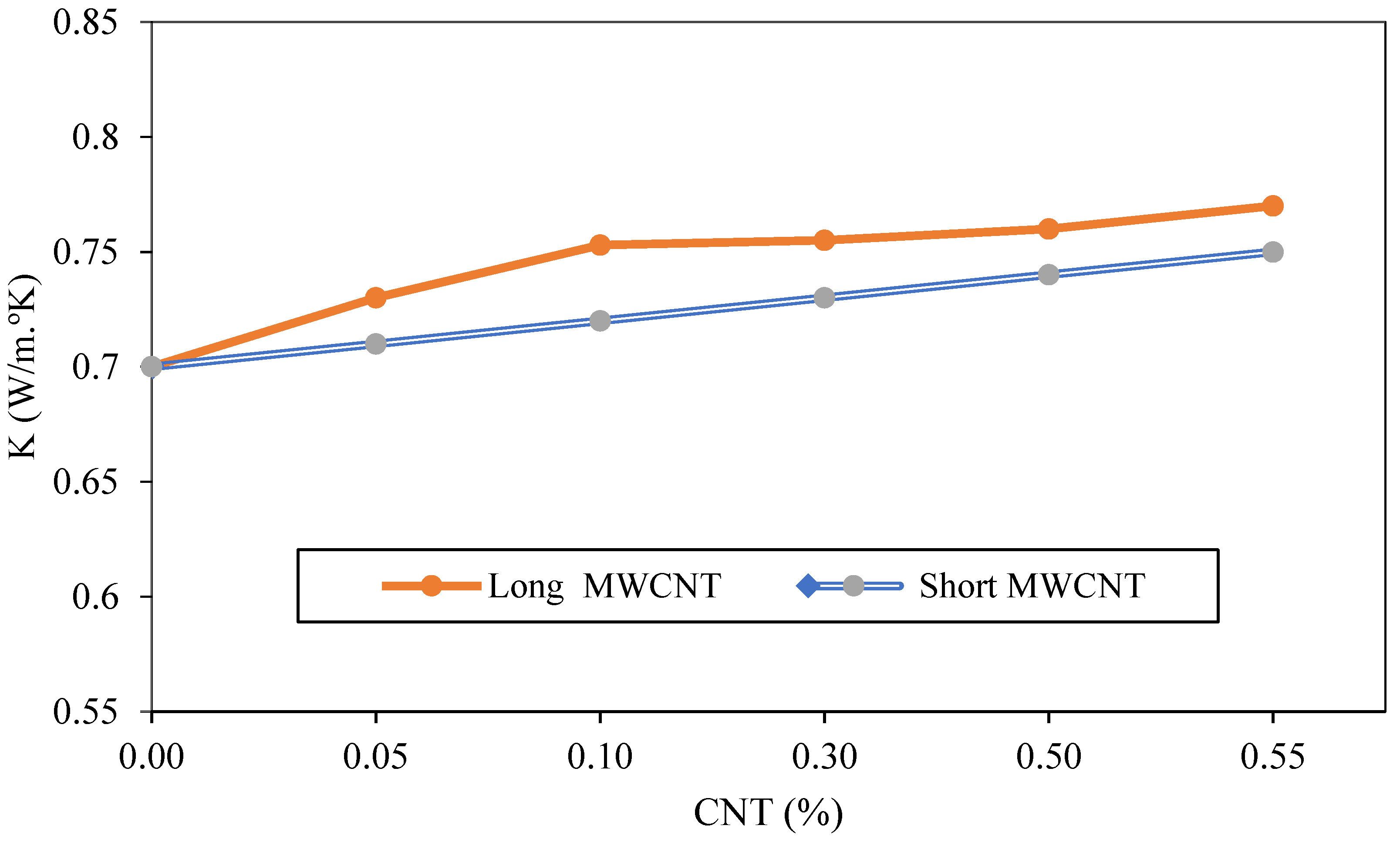
4.3. Aspect Ratio
4.4. Dispersion Quality
4.4.1. Functionalization
4.4.2. Sonication
4.4.3. Addition of Surfactant:
4.5. Temperature
5. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- N. Ali, A.M. Bahman, N.F. Aljuwayhel, S.A. Ebrahim, S. Mukherjee, A. Alsayegh, Nanomaterials, 11 (2021) 1628; [CrossRef]
- H. Xie, L. Chen, Physics Letters A, 373 (2009) 1861-1864; [CrossRef]
- S. Iijima, T. Ichihashi, Nature, 363 (1993) 603-605; [CrossRef]
- L.S. Salah, N. Ouslimani, D. Bousba, I. Huynen, Y. Danlée, H. Aksas, Journal of Nanomaterials, 2021 (2021) 1-31; [CrossRef]
- B. Kumanek, D. Janas, Journal of materials science, 54 (2019) 7397-7427; [CrossRef]
- V. Kumar, J. Madhukesh, A. Jyothi, B. Prasannakumara, M.I. Khan, Y.-M. Chu, Computational and Theoretical Chemistry, 1200 (2021) 113223; [CrossRef]
- P. Estellé, S. Halelfadl, M. Thierry, Journal of Thermal Engineering, 1 (2015) 381-390. 10.18186/jte.92293.
- M. Xing, J. Yu, R. Wang, International Journal of Heat and Mass Transfer, 88 (2015) 609-616; [CrossRef]
- M.J. Assael, C.-F. Chen, I. Metaxa, W.A. Wakeham, International Journal of Thermophysics, 25 (2004) 971-985; [CrossRef]
- L.S. Sundar, M.K. Singh, A.C. Sousa, International Communications in Heat and Mass Transfer, 52 (2014) 73-83; [CrossRef]
- science direct (2007-2023); https://www.sciencedirect.com/search?qs=cnt%20nano%20fluids&show=50&lastSelectedFacet=years&subjectAreas=1600&years=2023. (Accessed 4 /11/2023).
- W. Pabst, S. Hříbalová, International Journal of Heat and Mass Transfer, 206 (2023) 123941; [CrossRef]
- S. Kumar, A.D. Kothiyal, M.S. Bisht, A. Kumar, Case Studies in Thermal Engineering, 9 (2017) 108-121; [CrossRef]
- M.H. Ahmadi, A. Mirlohi, M.A. Nazari, R. Ghasempour, Journal of Molecular Liquids, 265 (2018) 181-188; [CrossRef]
- M. Devarajan, N. Parasumanna Krishnamurthy, M. Balasubramanian, B. Ramani, S. Wongwises, K. Abd El-Naby, R. Sathyamurthy, Micro & Nano Letters, 13 (2018) 617-621; [CrossRef]
- R. Sarviya, V. Fuskele, Materials Today: Proceedings, 4 (2017) 4022-4031; [CrossRef]
- E. Tugolukov, A.J. Ali, Journal of Thermal Engineering, 7 (2021) 66-90; [CrossRef]
- S. Wu, T. Yan, Z. Kuai, W. Pan, Energy Storage Materials, 25 (2020) 251-295; [CrossRef]
- Ali, E. Tugolukov, An Experimental Study on the Influence of Functionalized Carbon Nanotubes Cnt Taunit Series on the Thermal Conductivity Enhancement, in: IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2019, pp. 012001; [CrossRef]
- S. Özerinç, S. Kakaç, A.G. Yazıcıoğlu, Microfluidics and Nanofluidics, 8 (2010) 145-170; [CrossRef]
- W. Yu, D.M. France, J.L. Routbort, S.U. Choi, Heat transfer engineering, 29 (2008) 432-460; [CrossRef]
- Y. Zeng, A. Marconnet, Review of Scientific Instruments, 88 (2017); [CrossRef]
- N.B. Vargaftik, Handbook of Thermal Conductivity of Liquids and Gases, CRC-Press, 1994; [CrossRef]
- D. Bohne, S. Fischer, E. Obermeier, Berichte der Bunsengesellschaft für physikalische Chemie, 88 (1984) 739-742; [CrossRef]
- W. Huang, Z. Li, X. Liu, H. Zhao, S. Guo, Q. Jia, Annals of glaciology, 54 (2013) 189-195; [CrossRef]
- N. Charde, International Journal of Automotive and Mechanical Engineering, 7 (2013) 882-899; [CrossRef]
- S. Muhammad, G. Ali, Z. Shah, S. Islam, S.A. Hussain, Applied Sciences, 8 (2018) 482; [CrossRef]
- T.S. Gspann, S.M. Juckes, J.F. Niven, M.B. Johnson, J.A. Elliott, M.A. White, A.H. Windle, Carbon, 114 (2017) 160-168; [CrossRef]
- X. Yang, J. Cui, K. Xue, Y. Fu, H. Li, H. Yang, Nanotechnology Reviews, 10 (2021) 178-186; [CrossRef]
- A. Li, C. Zhang, Y.-F. Zhang, Polymers, 9 (2017) 437; [CrossRef]
- D.-K. Lee, J. Yoo, H. Kim, B.-H. Kang, S.-H. Park, Materials, 15 (2022) 1356; [CrossRef]
- D.A.G. Bruggeman, Annals of Physics, 416 (1935) 636-791;
- L. Kotrbová, W. Pabst, International Journal of Thermal Sciences, 197 (2024) 108805; [CrossRef]
- H. Wadell, The Journal of Geology, 43 (1935) 250-280; [CrossRef]
- J. Xu, B. Gao, F. Kang, Applied Thermal Engineering, 102 (2016) 972-979; [CrossRef]
- W. Yu, S. Choi, Journal of nanoparticle research, 5 (2003) 167-171; [CrossRef]
- H. Younes, G. Christensen, D. Li, H. Hong, A.A. Ghaferi, Journal of Nanofluids, 4 (2015) 107-132; [CrossRef]
- M. Navaei, A. Mahdavifar, J. Xu, J. Dimandja, G. McMurray, P. Hesketh, ECS Journal of Solid State Science and Technology, 4 (2015) S3011; [CrossRef]
- S.P. Jang, S.U. Choi, Applied physics letters, 84 (2004) 4316-4318; [CrossRef]
- E.V. Timofeeva, A.N. Gavrilov, J.M. McCloskey, Y.V. Tolmachev, S. Sprunt, L.M. Lopatina, J.V. Selinger, Physical Review E, 76 (2007) 061203; [CrossRef]
- K. Apmann, R. Fulmer, A. Soto, S. Vafaei, Materials, 14 (2021) 1291; [CrossRef]
- A. Zendehboudi, R. Saidur, I. Mahbubul, S. Hosseini, International Journal of Heat and Mass Transfer, 131 (2019) 1211-1231; [CrossRef]
- N. Sohrabi, N. Masoumi, A. Behzadmehr, S. Sarvari, Heat Transfer—Asian Research: Co-sponsored by the Society of Chemical Engineers of Japan and the Heat Transfer Division of ASME, 39 (2010) 141-150; [CrossRef]
- C. Kleinstreuer, Modern Fluid Dynamics, (2010);
- R. Prasher, P.E. Phelan, P. Bhattacharya, Nano letters, 6 (2006) 1529-1534; [CrossRef]
- S. Kaur, N. Raravikar, B.A. Helms, R. Prasher, D.F. Ogletree, Nature communications, 5 (2014) 3082; [CrossRef]
- M.M. Dahm, D.E. Evans, M.K. Schubauer-Berigan, M.E. Birch, J.E. Fernback, Annals of occupational hygiene, 56 (2012) 542-556; [CrossRef]
- H. Younes, M. Mao, S.S. Murshed, D. Lou, H. Hong, G. Peterson, Applied Thermal Engineering, 207 (2022) 118202; [CrossRef]
- G. Coccia, S. Tomassetti, G. Di Nicola, Renewable and Sustainable Energy Reviews, 151 (2021) 111573; [CrossRef]
- F. Schütt, S. Signetti, H. Krüger, S. Röder, D. Smazna, S. Kaps, S.N. Gorb, Y.K. Mishra, N.M. Pugno, R. Adelung, Nature communications, 8 (2017) 1215; [CrossRef]
- C. Gao, M. Guo, Y. Liu, D. Zhang, F. Gao, L. Sun, J. Li, X. Chen, M. Terrones, Y. Wang, Carbon, (2023) 118133; [CrossRef]
- K. Falk, F. Sedlmeier, L. Joly, R.R. Netz, L. Bocquet, Nano letters, 10 (2010) 4067-4073; https://pubs.acs.org/doi/10.1021/nl1021046.
- L. De Bellis, P.E. Phelan, R.S. Prasher, Journal of thermophysics and heat transfer, 14 (2000) 144-150; [CrossRef]
- X. Cui, J. Wang, G. Xia, Nanoscale, 14 (2022) 99-107; [CrossRef]
- X. Qian, J. Zhou, G. Chen, Nature Materials, 20 (2021) 1188-1202; [CrossRef]
- P.-C. Ma, N.A. Siddiqui, G. Marom, J.-K. Kim, Composites Part A: Applied Science and Manufacturing, 41 (2010) 1345-1367; [CrossRef]
- I. Francis, S.C. Saha, Heliyon, 8 (2022); [CrossRef]
- D.H. Robert Resnick, Kenneth S. Krane, Physics, Volume 1, Califirnia, 2002.
- D. Halliday, R. Resnick, K.S. Krane, Physics, Volume 2, John Wiley & Sons, 2010.
- R. Dubey, D. Dutta, A. Sarkar, P. Chattopadhyay, Nanoscale Advances, 3 (2021) 5722-5744; [CrossRef]
- N. Berrada, S. Hamze, A. Desforges, J. Ghanbaja, J. Gleize, T. Mare, B. Vigolo, P. Estellé, Journal of Molecular Liquids, 293 (2019) 111473; [CrossRef]
- A. Rehman, Z. Salleh, T. Gul, Cogent Engineering, 7 (2020) 1772945; [CrossRef]
- A.M. Hussein, K. Sharma, R. Bakar, K. Kadirgama, Renewable and Sustainable Energy Reviews, 29 (2014) 734-743; [CrossRef]
- N.A.C. Sidik, R. Mamat, International Communications in Heat and Mass Transfer, 66 (2015) 11-22; [CrossRef]
- Y. Li, G. Georges, ACS Nano, 17 (2023) 19471-19473; [CrossRef]
- O.A. Orole Mr, Electronic Thesis and Dissertation Repository, (2023); https://ir.lib.uwo.ca/etd/9784.
- R. Lenin, P.A. Joy, C. Bera, Journal of Molecular Liquids, 338 (2021) 116929; [CrossRef]
- N. Jha, S. Ramaprabhu, Journal of applied physics, 106 (2009); [CrossRef]
- R. Walvekar, I.A. Faris, M. Khalid, Heat Transfer—Asian Research, 41 (2012) 145-163; [CrossRef]
- W. Tian, Y. Bao, G. Qin, L. Liu, X. Zheng, Journal of Molecular Liquids, 392 (2023) 123433; [CrossRef]
- J. Hassan, G. Diamantopoulos, D. Homouz, G. Papavassiliou, Nanotechnology Reviews, 5 (2016) 341-354; [CrossRef]
- A. Nasiri, M. Shariaty-Niasar, A.M. Rashidi, R. Khodafarin, International Journal of heat and Mass transfer, 55 (2012) 1529-1535; [CrossRef]
- S. Choi, Z.G. Zhang, W. Yu, F. Lockwood, E. Grulke, Applied physics letters, 79 (2001) 2252-2254; [CrossRef]
- H. Xie, H. Lee, W. Youn, M. Choi, Journal of Applied physics, 94 (2003) 4967-4971; [CrossRef]
- D. Wen, Y. Ding, Journal of thermophysics and heat transfer, 18 (2004) 481-485; [CrossRef]
- Y.-j. Hwang, J. Lee, C. Lee, Y. Jung, S. Cheong, C. Lee, B. Ku, S. Jang, Thermochimica Acta, 455 (2007) 70-74; [CrossRef]
- L. Chen, H. Xie, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 352 (2009) 136-140; [CrossRef]
- M.T. Chaichan, H.A. Kazem, M.K. Al-Ghezi, A.H. Al-Waeli, A.J. Ali, K. Sopian, A.A.H. Kadhum, W.N.R. Wan Isahak, M.S. Takriff, A.A. Al-Amiery, ACS omega, 8 (2023) 29910-29925; [CrossRef]
- S. Lotfizadeh, T. Matsoukas, Journal of Nanomaterials, 2015 (2015) 8-8; [CrossRef]
- P. Ebin, J.S. Babu, Applied Nanoscience, (2023) 1-13; [CrossRef]
- M. Liu, M.C. Lin, C. Wang, Nanoscale research letters, 6 (2011) 1-13; [CrossRef]
- E. Batiston, P.J.P. Gleize, P. Mezzomo, F. Pelisser, P.R.d. Matos, Revista IBRACON de Estruturas e Materiais, 14 (2021) e14510; [CrossRef]
- M. Barrejón, M. Prato, Advanced Materials Interfaces, 9 (2022) 2101260; [CrossRef]
- M. Farbod, A. Ahangarpour, S.G. Etemad, Particuology, 22 (2015) 59-65; [CrossRef]
- J. Nanda, C. Maranville, S.C. Bollin, D. Sawall, H. Ohtani, J.T. Remillard, J. Ginder, The Journal of Physical Chemistry C, 112 (2008) 654-658; [CrossRef]
- A. Asadi, I.M. Alarifi, V. Ali, H.M. Nguyen, Ultrasonics sonochemistry, 58 (2019) 104639; [CrossRef]
- A. Rehman, S. Yaqub, M. Ali, H. Nazir, N. Shahzad, S. Shakir, R. Liaquat, Z. Said, Journal of Molecular Liquids, 391 (2023) 123350; [CrossRef]
- H. Xie, W. Yu, Y. Li, L. Chen, Nanoscale research letters, 6 (2011) 1-12; [CrossRef]
- F. Grzegorzewski, A. Benhaim, Y. Itzhaik Alkotzer, E. Zelinger, N. Yaakov, G. Mechrez, Polymers, 11 (2019) 1480; [CrossRef]
- S. Witharana, I. Palabiyik, Z. Musina, Y. Ding, Powder technology, 239 (2013) 72-77; [CrossRef]
- I. Sharmin, M.A. Gafur, N.R. Dhar, SN Applied Sciences, 2 (2020) 1-18; [CrossRef]
- M. Yeganeh, N. Shahtahmasebi, A. Kompany, E. Goharshadi, A. Youssefi, L. Šiller, International Journal of Heat and Mass Transfer, 53 (2010) 3186-3192; [CrossRef]
- Rashmi Walvekar, Ismail Ahmad Faris, and Mohammad Khalid, Thermal Conductivity of Carbon NanotubeNanofluid—Experimental and Theoretical Study, Heat Transfer Engineering, 41 (2012)145-163. [CrossRef]
- W. Yu, H. Xie, W. Chen, Journal of Applied Physics, 107 (2010); [CrossRef]
- A. Navaei, H. Mohammed, K. Munisamy, H. Yarmand, S. Gharehkhani, Powder technology, 286 (2015) 332-341; [CrossRef]
- W. Cui, Y. Yuan, L. Sun, X. Cao, X. Yang, Renewable Energy, 99 (2016) 1029-1037; [CrossRef]

| Phase | Material | Thermal Conductivity [W/m·K] | Ref. |
|---|---|---|---|
| Liquid | Water | 0.5918-0.609 | [23] |
| Ethylene glycol | 0.246 | [24] | |
| Engine oil | 0.145 | [24] | |
| Water | 0.51 | [23] | |
| Castor oil | 0.18 | [23] | |
| Ethanol | 0.171 | [23] | |
| Acetic acid | 0.193 | [23] | |
| Phenol | 0.19 | [23] | |
| Solid | Snow (dry) | 0.050–0.250 | [23] |
| Aluminum | 237 | [23] | |
| Ductile steel | 80 | [23] | |
| Stainless steel 304 | 16.2 | [25] | |
| Carbon steel | 54 | [26] | |
| Gold | 320 | [23] | |
| Aluminum nitride | 321 | [23] | |
| Beryllium | 209–330 | [23] | |
| Bismuth | 7.97 | [23] | |
| Boron arsenide | 1300 | [23] | |
| Copper (pure) | 401 | [23] | |
| Diamond | 1000-2300 | [23] | |
| Germanium | 60.2 | [23] | |
| Polyurethane foam | 0.03 | [23] | |
| Expanded polystyrene | 0.033–0.046 | [23] | |
| Manganese | 7.810 | [23] | |
| Ice | 2.22 | [25] | |
| Silica aerogel | 0.02 | [23] | |
| Silicon nitride | 90-177 | [23] | |
| Silver | 406 | [23] | |
| SWCNT | <6000 | [27] | |
| MWCNT | <3000 | [27] |
| No. | Sonication Time | Particle | Stability | Nano Fluid | Ref. |
|---|---|---|---|---|---|
| 1 | 60 min | MWCNT 5-15 μm functionalized | 80 days | CNT Water | [84] |
| 2 | 60 min | MWCNT | 30 days | CNT Water | [86] |
| 3 | 45 min | Various types | 117 days | CNT-water | [72] |
| Surfactant | Surfactant |
|---|---|
| Cetyltrimethylammonium bromide (CTAB). | Sodium dodecyl benzene sulfonate (SDBS), |
| Anionic (SDS)( Sodium Dodecyl Sulfate | sodium dodecyl sulfate (SDS) |
| Cationic (CTAB) Cetyltrimethylammonium bromide | Tween 80 polysorbate 80 |
| Nonionic (LAE-7) Shazand Petrochemical complex | Tergitol NP-10 |
| Amphoteric (CHAPS) Nonionic surfactants | Poly-Vinyl-Pyrrolidone (PVP) |
| Hydropalat 5040 Sodium polyacrylate in aqueous solution | Gum Arabic (GA) |
| Aerosol OT-70 PG | Hexadecyl-Trimethyl-Ammonium-bromide (CTAB) |
| Oleic Acid | Antiterra 250 |
| Laurate salt | Disperbyk 190 |
| Sodium dodecyl sulfate (SDS) | Hypermer LP1 |
| Disponil A 1580 | Aerosol TR-70 |
| Aerosol TR-70 HG |
| No. | Temperature Range ºC | Thermal Conductivity Enhancement% | Ref. | |
|---|---|---|---|---|
| Lower Temperature | Higher Temperature | |||
| 1 | 60-80 | 15.0 | 35.0 | [91] |
| 2 | 30 - 50 | 7.2 | 9.8 | [92] |
| 3 | 60 - 80 | 25.0 | 45.0 | [91] |
| Working Temperature (ºC) | Thermal Conductivity (W/m.K) for Various CNT Concentrations (mass%) | |||||
|---|---|---|---|---|---|---|
| (0.00) Pure Water | 0.01 | 0.02 | 0.04 | 0.08 | 0.1 | |
| 20 | 0.6 | 0.6 | 0.64 | 0.65 | 0.66 | 0.7 |
| 30 | 0.6 | 0.7 | 0.66 | 0.7 | 0.75 | 0.8 |
| 40 | 0.6 | 0.9 | 1.2 | 1.5 | 1.7 | 1.9 |
| 50 | 0.6 | 1.25 | 1.5 | 1.7 | 1.9 | 2.2 |
| 60 | 0.6 | 1.5 | 1.7 | 1.9 | 2.1 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





