1. Introduction
Several standard treatment choices following upper gastrointestinal tract interruption, like gastrostomy and jejunostomy have been documented in literature [
1]. However, they determine negative sequelae related to the elimination of saliva, malnutrition, aspiration pneumonia, and erosion of the skin around the stoma. This significantly impacts the patient's quality of life and ability to work. Individuals lacking esophageal reconstruction are unable to consume food orally or swallow saliva, necessitating a constant expulsion of approximately 0.6 liters of saliva daily, even during nighttime. [
2]
Reconstruction of the esophagus and restoration of the GI tract is therefore mandatory to re-establish an acceptable quality of life. Conventional surgical techniques such as the gastric pull up or locoregional flaps could represent valid alternatives in primary cases [
3]. However, their feasibility depends on tissue damage and defect dimensions, and they are contraindicated in secondary reconstructions.
Microsurgical reconstructive procedures represent the most effective solutions to date. Among these, intestinal flaps including the jejunum and colon free flaps [
4,
5], have historically been considered the standard for esophageal reconstruction [
6]. To avoid complications associated with intestinal flaps, encompassing restricted flap length, abdominal complications, prolonged swallowing (resulting from delayed bolus transit, dysmotility, and asynchronous peristalsis), as well as the potential for vascular disruption due to arteriosclerotic modifications and brief ischemic periods, safer and more accessible options are now available [
7].
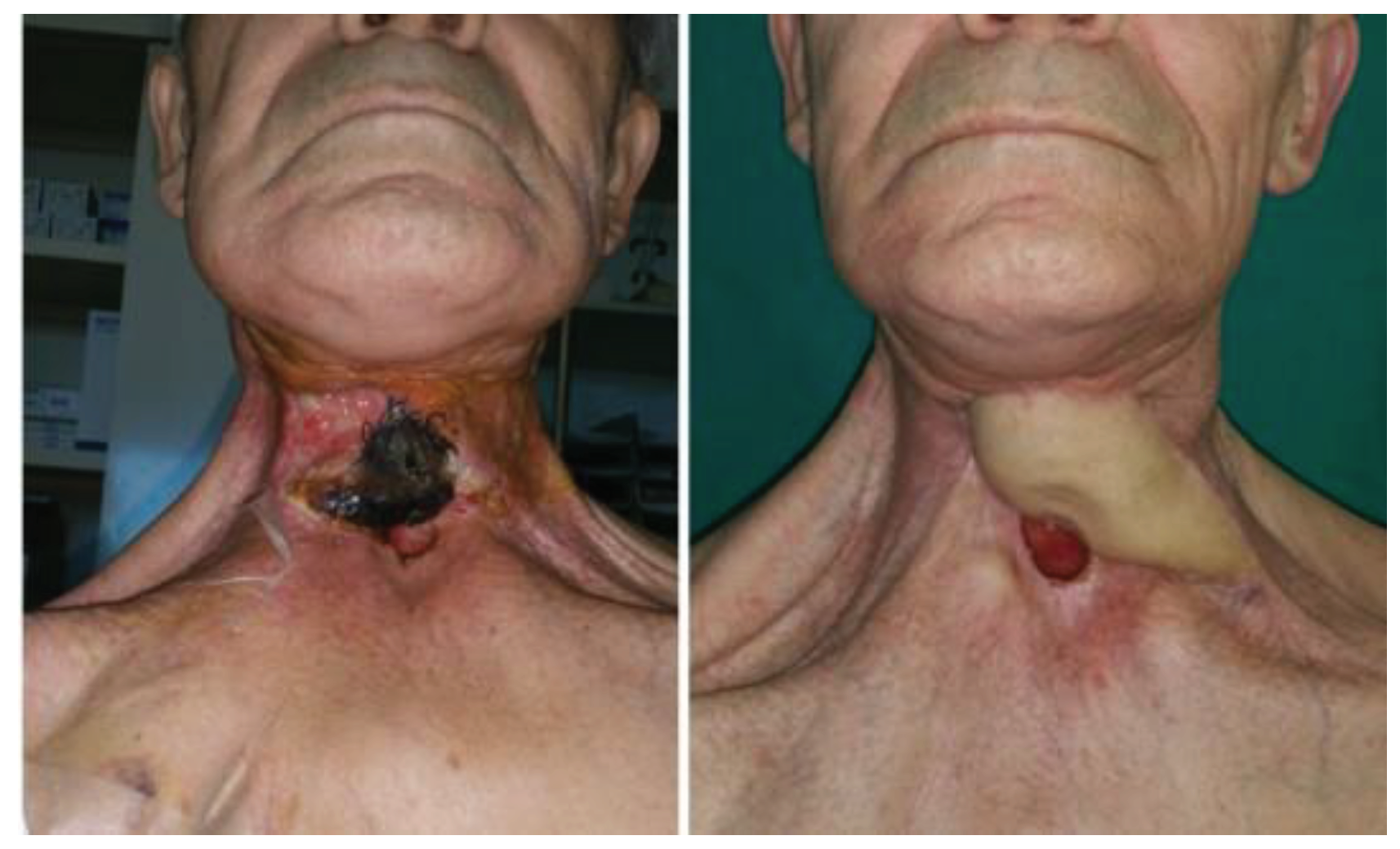
In regard to the donor site, fascio-cutaneous flaps may offer a more effective solution. Through a collaborative double-team approach, fascio-cutaneous flaps can be harvested simultaneously with the preparation of the recipient vessels. This allows them to be more easily shaped to precisely fit the soft tissue defect that requires coverage. Additionally, secondary cases, often marked by the failure of previous surgeries and typically associated with esophago-tracheal fistulas or complicated tracheostoma, present challenges in skin coverage (
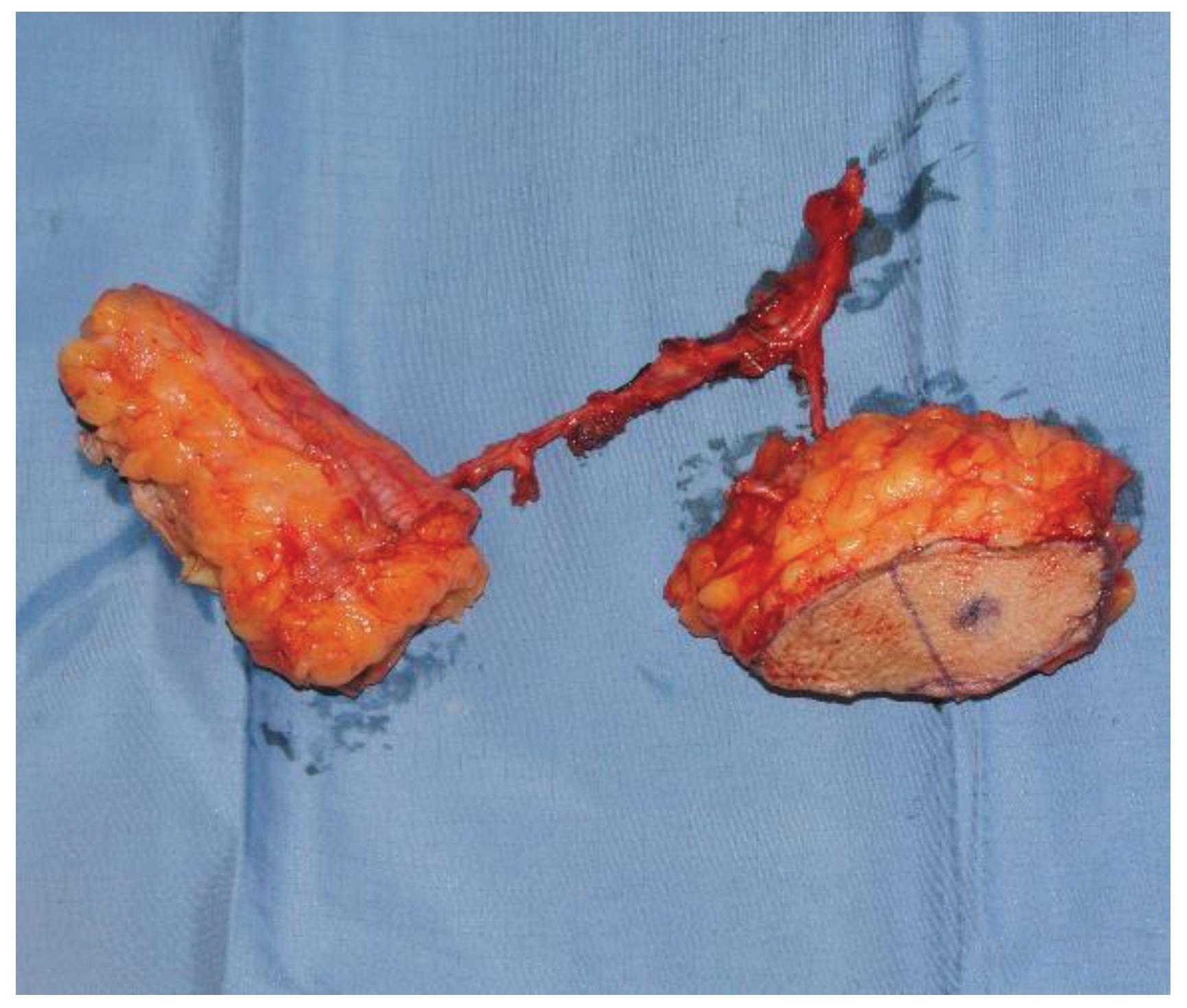
Figure 1). In this context, fascio-cutaneous flaps also allow the design of a double island flap based on several perforators, one for the reconstruction of the esophageal tract and the other for the neck coverage (
Figure 2). Finally, this procedure also allows the evaluation of the viability of the tubulized submerged flap [
8].
To date, there is a lack of recent studies in the literature that offer a thorough examination of the clinical attributes of different treatment options, coupled with actionable insights to aid healthcare practitioners in their decision-making process.
The aim of this study was describing a cohort of patients who underwent secondary repair of esophageal defects due to the failure of immediate reconstruction following ablation of cervical esophageal cancer, while also furnishing pragmatic insights for the effective handling and therapeutic approaches in such scenarios, drawing from both the authors' expertise and comprehensive literature analysis.
2. Patients and Methods
We retrospectively reviewed the electronic medical records of the Plastic Surgery Clinic at the University of Trieste to identify cases of patients who underwent secondary esophageal microsurgical reconstructions following oncological surgery between January 2011 and September 2022. The study protocol was approved by the University of Trieste Ethics Committee on Clinical Investigation in compliance with the Helsinki Declaration. All patients signed an informed consent form.
The study included all consecutive patients that met the following inclusion criteria: a) age ≥18 years; b) previous surgery for cervical esophageal carcinoma; c) secondary esophageal microsurgical reconstructions; d) minimum follow-up of 9 months.
Based on our institutional policy, the following parameters were considered to guide the reconstructive choice: a) general status and comorbidities; b) previous or programmed radiotherapy of the cervical region or neck; d) CT angiography evaluation of defect dimensions, available space at the cervical level and quality of remaining neck vessels after oncologic surgery; e) flap availability; f) nutritional status; g) skin pliability for fascio-cutaneous flaps. Following this evaluation, our options usually included three types of flaps: the Antero-Lateral Thigh (ALT) flap, the Radial Forearm Flap (RFF), and the parascapular flap. The first two are the primary choices in our institution, while the parascapular flap is selected as a backup option in case the first two were not feasible.
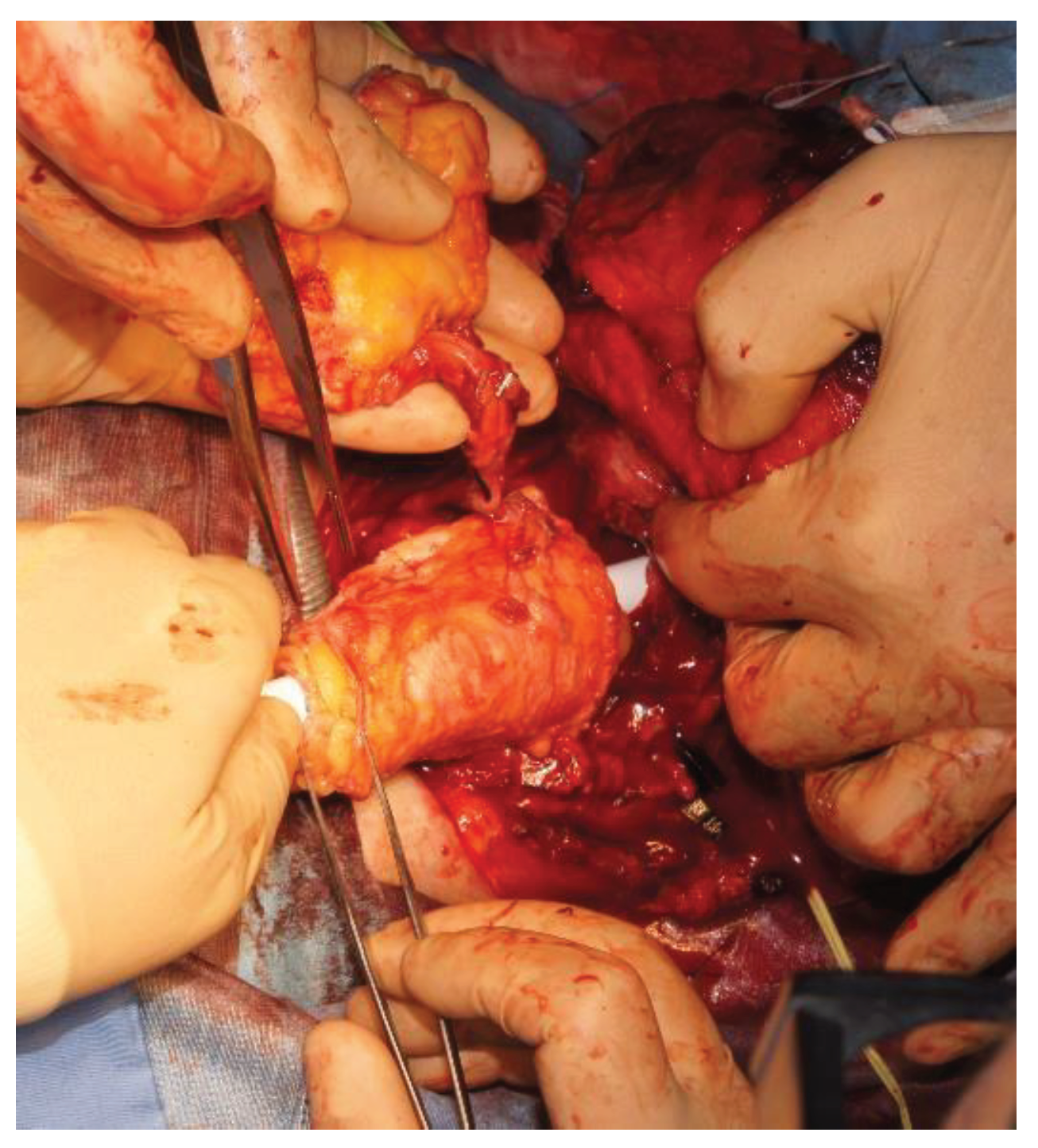
At our institution, all patients eligible for secondary esophageal reconstruction with microvascular flaps receive prophylactic antibiotic, antithrombotic, and anti-reflux treatment during the perioperative period. Furthermore, they are planned to have a nasogastric tube and a self-expandable plastic stent (SEPS) positioned within the tubularized flap to allow placement at the cervical level, in order to prevent wrinkling and potential kinking (
Figure 3). The device was to be kept in place for the first 5 days. All flaps are monitored every hour in the first 48 hours after surgery, and then every 3 hours till the end of the first week. In all flaps with an external skin island, the following parameters are evaluated: color, temperature, capillary refill, texture, and arterial flow assessment via doppler probe. Fully buried flaps are monitored via Licox® PtO2 mini-invasive monitoring system or via Synovis Flow Coupler® doppler device placed on the venous anastomosis when available.
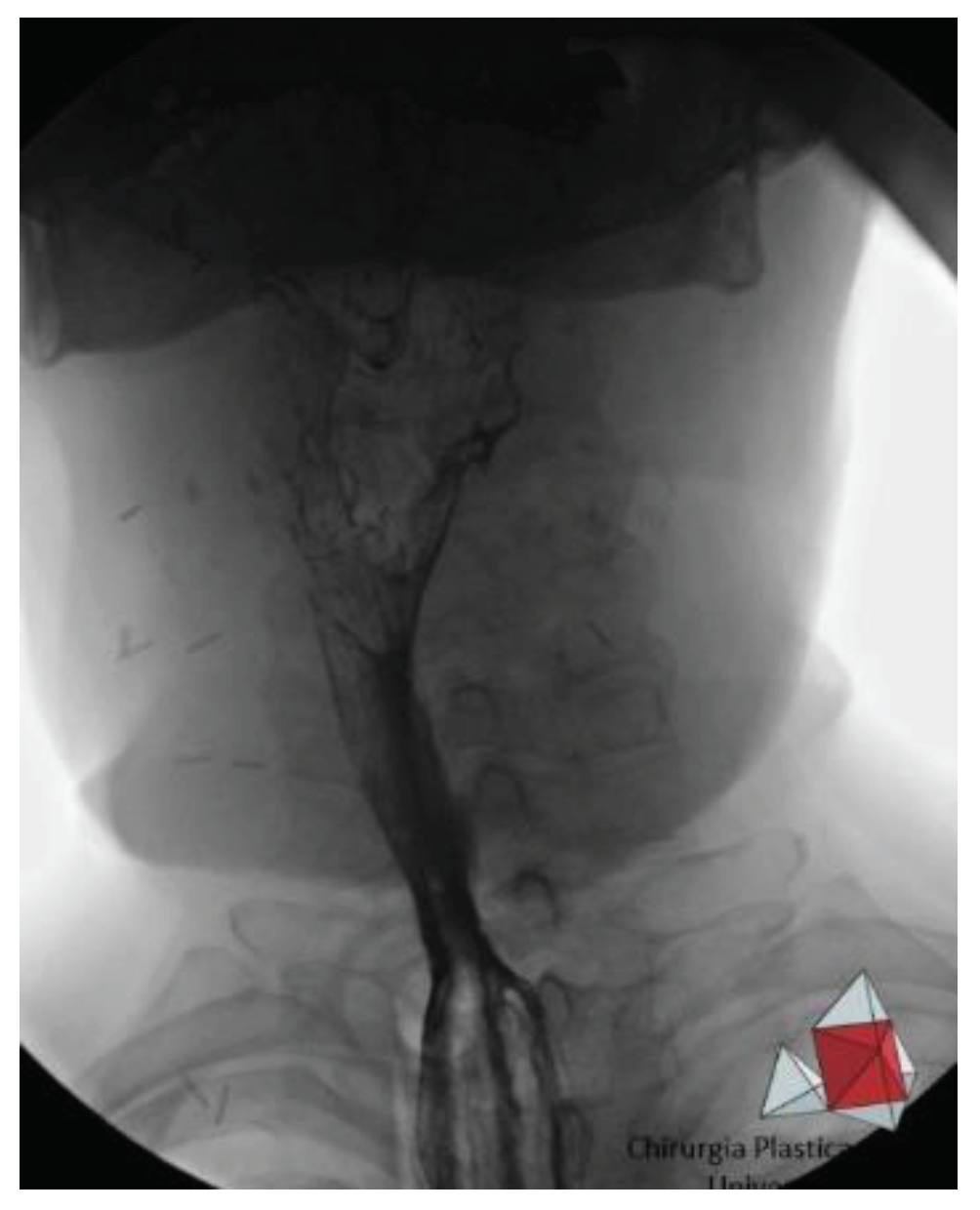
Patient are assessed for the presence of fistulas, esophageal strictures, and transit function via barium meal 30 days after surgery (
Figure 4).
3. Results
From January 1st, 2011, to September 20th, 2022, 13 patients underwent secondary esophageal reconstructions due to the failure of immediate reconstruction following ablation of cervical esophageal cancer (median [IQR] age at surgery, 58 (51–71) years; 10 [76.9%] males) (Table 1).
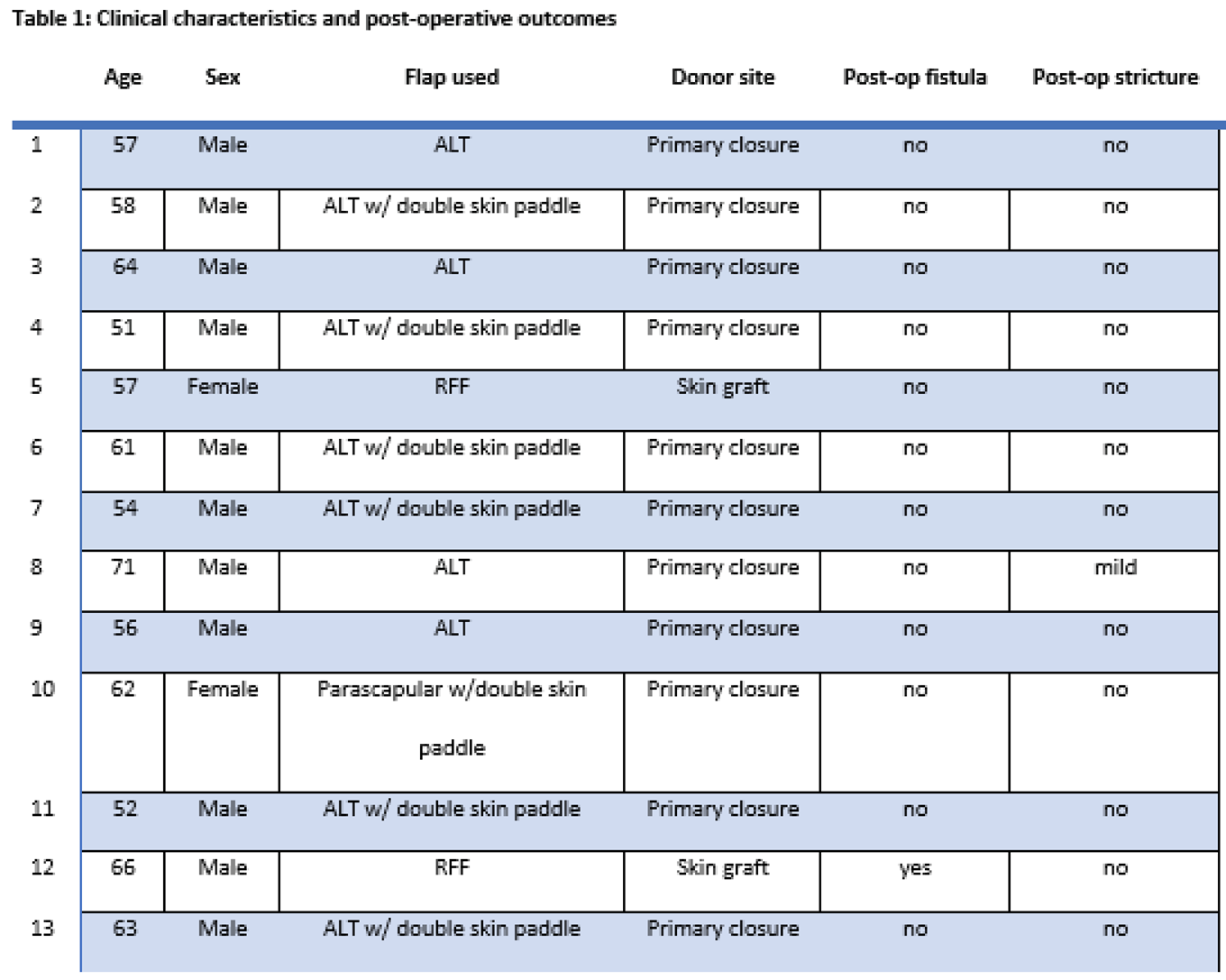
Seven patients exhibited pharyngo-cutaneous fistulas, five experienced dysphagia due to pharyngoesophageal strictures, and one patient had recurrent disease. All 13 patients had a history of prior radiotherapy and 7 had undergone completion lymph node dissection. Eleven patients had history of alcohol consumption and 9 were former smokers.
We chose an ALT flap in 10 cases, of which 6 with dual-paddle, one tubulized for GI tract reconstruction and the other for neck skin coverage. RFF was used in 2 cases, while 1 patient was treated with a chimeric parascapular flap with double skin paddle. All ALT flap donor sites and the parascapular flap donor site were repaired via primary closure, while RFF donor sites were covered via split-thickness skin graft.
During a median follow-up of 50 months, no flap failures were observed. One patient developed a post-operative stricture after ALT flap reconstruction, but he still retained the ability to swallow semi-liquid foods. Additionally, there was a singular occurrence of tracheoesophageal fistula, attributed to the tracheal tube, in a former smoker who underwent RFF reconstruction and had a history of previous radiotherapy and completion lymph node dissection.
4. Discussion
Secondary cases of cervical esophageal reconstruction can be challenging due to the extensive damage to soft tissues producing large defect size after debridement and to the poor conditions of recipient vessels due to previous surgery and radiotherapy [
9]. In this context, locoregional flaps are usually insufficient, while intestinal flaps bring a high risk of complications to a patient already burdened by a compromised status [
10]. Therefore, these patients require a thoughtful approach, preferring safe techniques and employing an extensive preoperative examination.
CT-angiography guides the choice of the recipient vessels for anastomosis, especially in patients with previous cervical lymph node dissection and/or radiation. Recipient vessels in esophageal reconstruction with free flaps usually include facial artery or superior thyroid artery, but in secondary cases those vessels could be damaged or unsuitable. It is a great advantage to have a long pedicle length available, allowing for distant anastomosis, even on extra-regional vessels such as – for instance – thyrocervical trunk, transverse cervical artery or thoracoacromial artery, which represent a good choice in case of post-radiation cervical damage.
Evaluation of the affected area is necessary to design a tissue flap that can accurately match the extension of the defect: given the anatomical complexity of the region and aiming for a functional reconstructed tract, there shouldn’t be any excess tissue both in the neck skin externally and internally at the level of the two circular sutures positioned distally at esophageal stricture and proximally at the tongue base. Excess tissue near the anastomosis between the flap and the distal portion of the esophagus can ripple creating saliva stagnation and subsequent fistulas and suture dehiscence. Moreover, the reconstructed cervical esophagus is prone to excoriation by the tracheal cannula curvature, especially when rigid cannulas are used, therefore promoting fistulization [
11]
The most suitable flap must then be chosen according to its thickness, its structure, and the available space at the cervical level. Our first choice for secondary esophageal reconstruction is the ALT flap, followed by the RFF and the parascapular flap.
Anterolateral Thigh Flap
ALT flap is widely described for reconstruction of head and neck defects [
12] and it is our first choice when its thickness is less than 2 cm. ALT allows a long pedicle to reach the recipient vessels even in unconventional sites, and it can be designed as a chimeric flap including a portion of vastus lateralis muscle for protection of the great vessels, or with a second skin island for skin coverage of the neck. It also offers the possibility of harvesting a large portion of fascia which provides greater protection of the sutures and leakage prevention [
13].
The ALT flap can be harvested simultaneously while the second team is working on the cervical district. Its pedicle enters laterally once the flap is inset in the cervical region and it can be easily positioned where needed to reach distant recipient vessels. In case of tubulized ALT, the flap insetting and suturing of the posterior area can be challenging because of the firm texture of this flap; a modified technique is to make the proximal and distal circumferential sutures first, and then to tubulize the flap and to suture its long axis. We used this modified technique in 4 cases.
Radial Forearm Flap
The radial forearm flap (RFF) is our second choice for secondary esophageal reconstruction. Therefore, we used it when the ALT flap turned out to be too thick, making it unsuitable for an optimal tubulization. However, because of its thinner dermis, the RFF does not provide the same sturdy shape once tubulized as the ALT flap, which is why greater precision must be applied to design and harvest the flap. In fact, it is mandatory to obtain exact shape and dimensions, not exceeding the tissue defect length, to avoid kneeling or creation of pouches that can collect saliva and then decubitate and fistulize. For this reason, fistulization rates are higher in RFF than ALT flap in literature (RFF 17-67% VS ALT 0-13%) [
14]. The pedicle of the RFF is very long and sturdy with two available venous drainage systems. However, because the pedicle enters axial to the flap, it can only be placed superiorly or inferiorly when the flap is placed to cover the defect and this requires a thoughtful programming as it may not fall in the preferred location compared to the ALT perforator flap.
Parascapular Flap
The parascapular flap is a safe flap, and it can be designed with a double skin paddle, or even chimeric with a portion of latissimus dorsi muscle [
15]. However, its application is limited because its dermis is usually very thick and rigid, making it difficult to be tubulized in place and making this flap inadequate in case of insufficient room in the recipient site. The surgery is also complicated by patient positioning, requiring its rotation on the table to harvest, and then inset the flap.
For this reason, we kept it as a salvage flap in one single case when the other flaps were unusable as they had already been used for other reconstructions and subsequent failures.
Surgical Tips
All these flaps should be tubulized with the skin facing the internal lumen. For this reason, the flap must be designed slightly larger, since the "reverse" rotation is somewhat spoiled by the dermis, resulting in a more difficult rotation in this direction.
It must be noted how the shape of the cervical esophagus design is trapezoidal instead of cylindrical: mean diameter at the tongue base is usually about 4-5 cm, while it is smaller inferiorly, about 3cm, with a structure colliding on itself in a virtual esophageal lumen.
In our experience, we prefer not to place the longitudinal suture of the flap posteriorly, as the posterior area is most prone to collections and possible fistulization; however, some authors have different opinion, and prefer to place the vertical suture posteriorly, facing the prevertebral fascia [
16].
Multiple layers of suture should be done, at least three: a cutaneous suture positioned on the lumen side, a subcutaneous suture on the dermis and then one on the fascia that is harvested with a larger area than the cutaneous island, precisely to create an outer layer of tissue protecting the first two.
During tubulization, the vertical suture line can be overlapped with de-epithelialized flap skin edges along with invagination of the esophagostoma into the skin tube to cover the end-to-end anastomosis with soft tissue
A vertical incision of the proximal esophagus allows the ‘key’ of the skin flap to expand the caliber of the anastomosis and break up the circumferential suture line in an attempt to prevent contraction and stenosis [
17]
The pedicle entry site must also be carefully chosen so that it is placed on one of the two sides of the flap, avoiding the posterior and superior areas that are prone to crushing, and chosen side must clearly coincide with the side of the receiving vessels.
5. Conclusions
Secondary repair of esophageal defects due to the failure of immediate reconstruction following ablation of cervical esophageal cancer represent a reconstructive challenge due to the extended tissue damage and poor recipient vessels. In this context, safer flaps with long and sturdy pedicles like ALT or RFF represent a good choice in our experience, with low complication rates in terms of flap failure, fistula and neo-esophagus stricture, and good functional outcomes.
Author Contributions
VR: AF, GP wrote the article and contributed to the design the study, VR, FCN, SB performed the surgeries, GM-AR reviewed the literature, VR, AF, GP contributed to the analysis of the results and to the writing of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee) of the University of Trieste.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy issues.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gauderer, M.W.; Ponsky, J.L.; Lzant, R.J., Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg 1980, 15, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Sørensen, C.; Proctor, G.; Carpenter, G. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018, 24, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Bich, T.A.; Vuong, N.L.; Tu, N.C.H.T.N.C.; Truong, T.M.; Trung, L.V. Long-Term Survival of Patients After Total Pharyngolaryngoesophagectomy With Gastric Pull-Up Reconstruction for Hypopharyngeal or Laryngeal Cancer Invading Cervical Esophagus. Ann. Otol. Rhinol. Laryngol. 2022, 132, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T.; Harii, K.; Ebihara, S.; et al. Free colon transfer: a versatile method for reconstruction of pharyngoesophageal defects with a large pharyngostoma. Ann Plast Surg 1996, 37, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.W.; Temple, J.R.; Codner, M.A. Reconstruction of the pharynx and overlying soft tissue by a partitioned free jejunal flap. Plast Reconstr Surg 1996, 97, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Tang, Y.B. Microsurgical reconstruction of the esophagus. Semin Surg Oncol. 2000, 19, 235–45. [Google Scholar] [CrossRef] [PubMed]
- Bozikov, K.; Arnez, Z. Factors predicting free flap complications in head and neck reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.; Hung, K.; Tsai, C.; Lee, Y. Externalized double monitoring skin paddles for buried anterolateral thigh flap in pharyngoesophageal reconstruction. Microsurgery 2018, 39, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Hanasono, M.M.; Skoracki, R.J.; Baumann, D.P.; Lewin, J.S.; Weber, R.S.; Robb, G.L. Pharyngoesophageal reconstruction with the anterolateral thigh flap after total laryngopharyngectomy. Cancer 2010, 116, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- De Frémicourt, M.; Temam, S.; Janot, F.; Kolb, F.; Qassemyar, Q. The anterolateral thigh perforator flap in pharyngo-esophageal reconstruction. Annales de Chirurgie Plastique Esthétique 2018, 63, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Couraud, L.; Ballester, M.J.; Delaisement, C. Acquired tracheoesophageal fistula and its management. Semin Thorac Cardiovasc Surg. 1996, 8, 392–399. [Google Scholar] [PubMed]
- Koshima, I.; Fukuda, H.; Yamamoto, H.; et al. Free anterolateral thigh flaps for reconstruction of head and neck defects. Plast Reconstr Surg 1993, 92, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Myers, H.; Grinsell, D. Single-stage reconstruction of combined hypopharyngeal and anterior neck skin defects with the dual-paddle anterolateral thigh flap. Eur. J. Plast. Surg. 2017, 41, 299–306. [Google Scholar] [CrossRef]
- Yu, P.; Lewin, J.S.; Reece, G.P.; Robb, G.L. Comparison of Clinical and Functional Outcomes and Hospital Costs following Pharyngoesophageal Reconstruction with the Anterolateral Thigh Free Flap versus the Jejunal Flap. Plast. Reconstr. Surg. 2006, 117, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Antohi, N.; Tibirna, G. The Combined Latissimus Dorsi and Scapular Free Flap for the Complex Anterior Neck Defect after Enlarged Total Laryngectomy. Ann. Plast. Surg. 1994, 33, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Houghton, L.; Gillmartin, E.; Jackson, S.; Lancaster, J.; Jones, T.; Blackburn, T.; Homer, J.; Loughran, S.; Ascott, F.; et al. Outcomes following pharyngolaryngectomy reconstruction with the anterolateral thigh (ALT) free flap. Br. J. Oral Maxillofac. Surg. 2012, 50, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Nagel, T.H.; Hayden, R.E. Advantages and limitations of free and pedicled flaps in reconstruction of pharyngoesophageal defects. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 407–413. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).