Submitted:
29 March 2024
Posted:
29 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
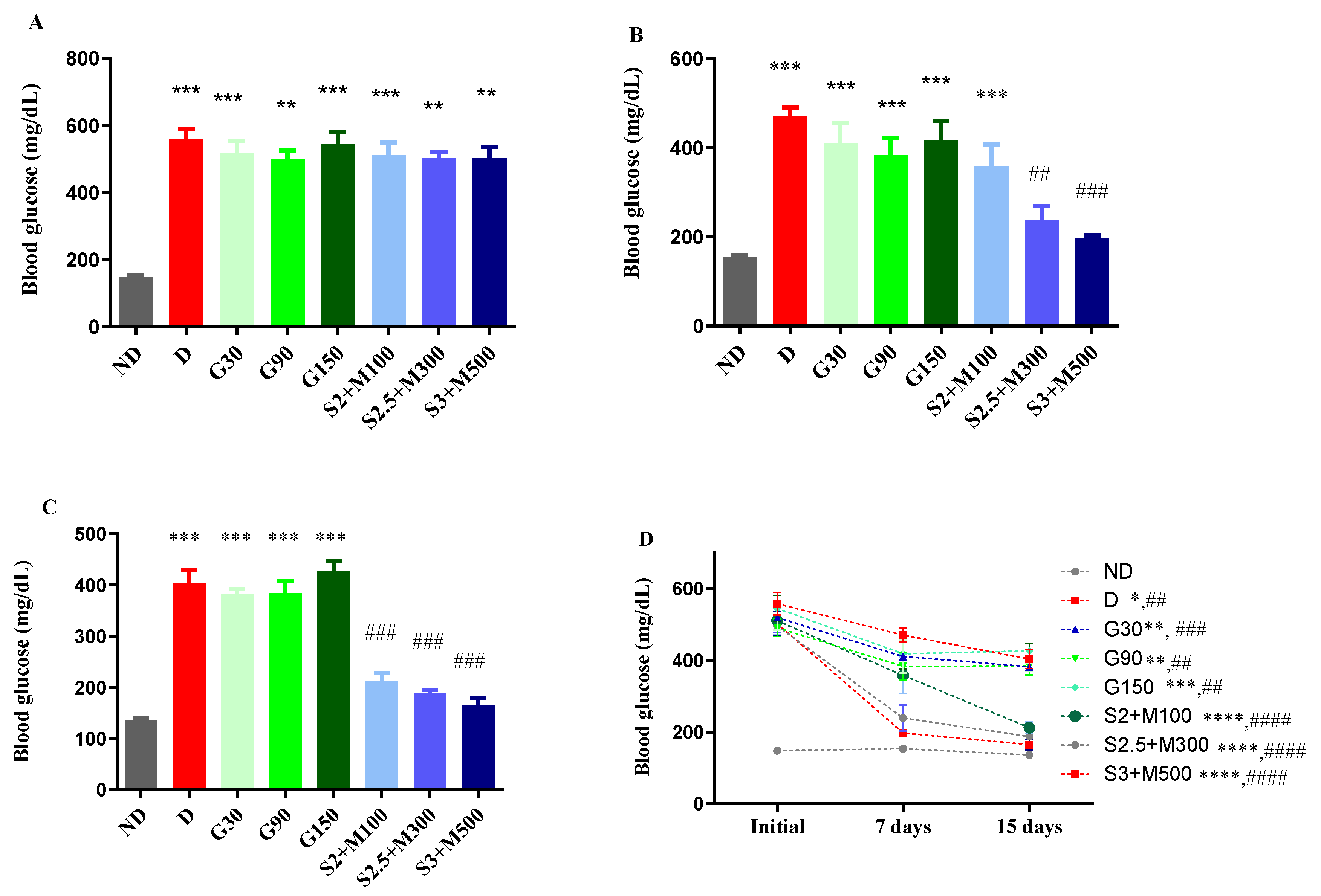
2.1. Blood Glucose Levels
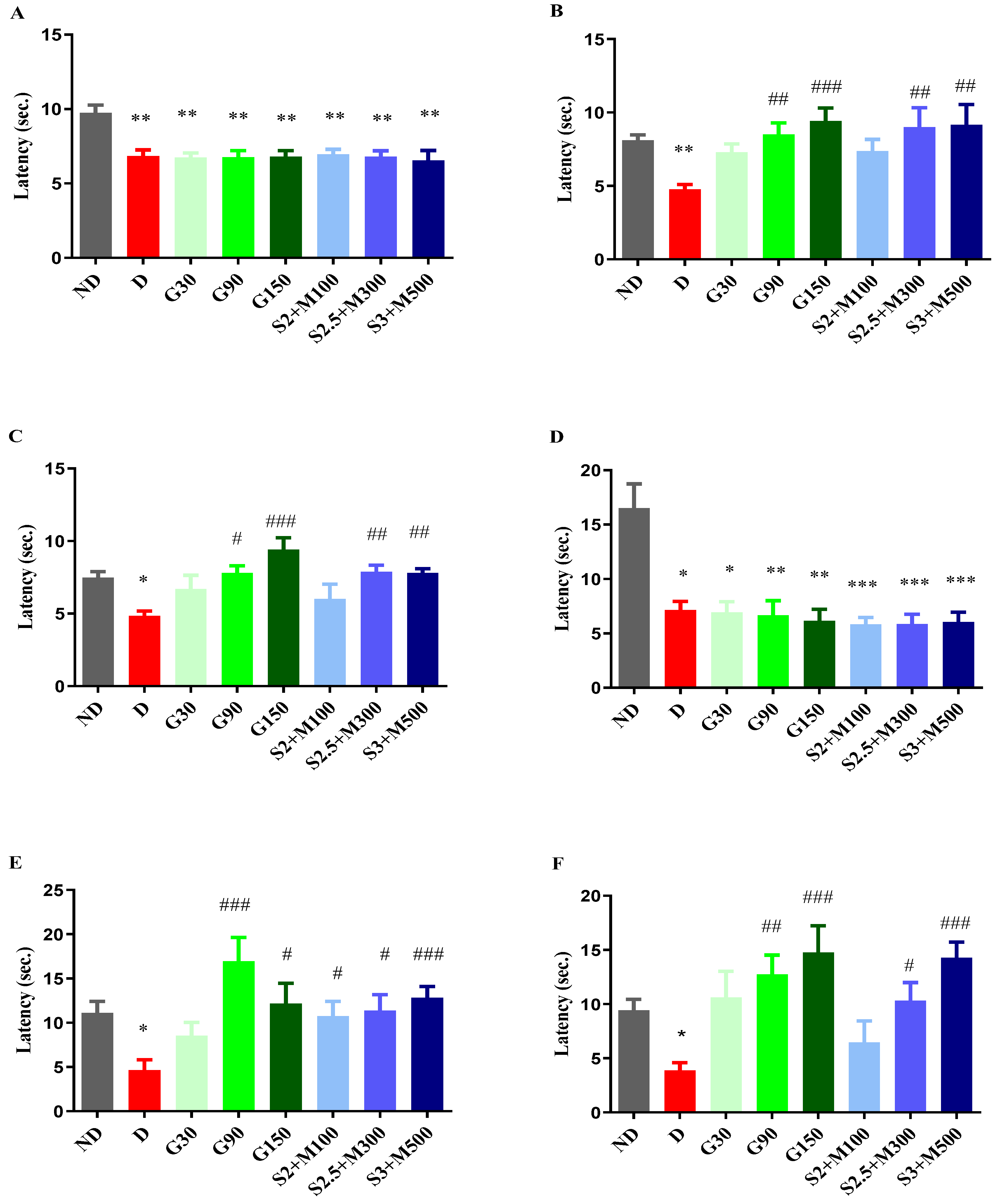
2.2. Tests for the Evaluations of antihyperalgesic Effect
2.2.1. Heat Hypersensitivity
2.2.2. Cold Hypersensitivity
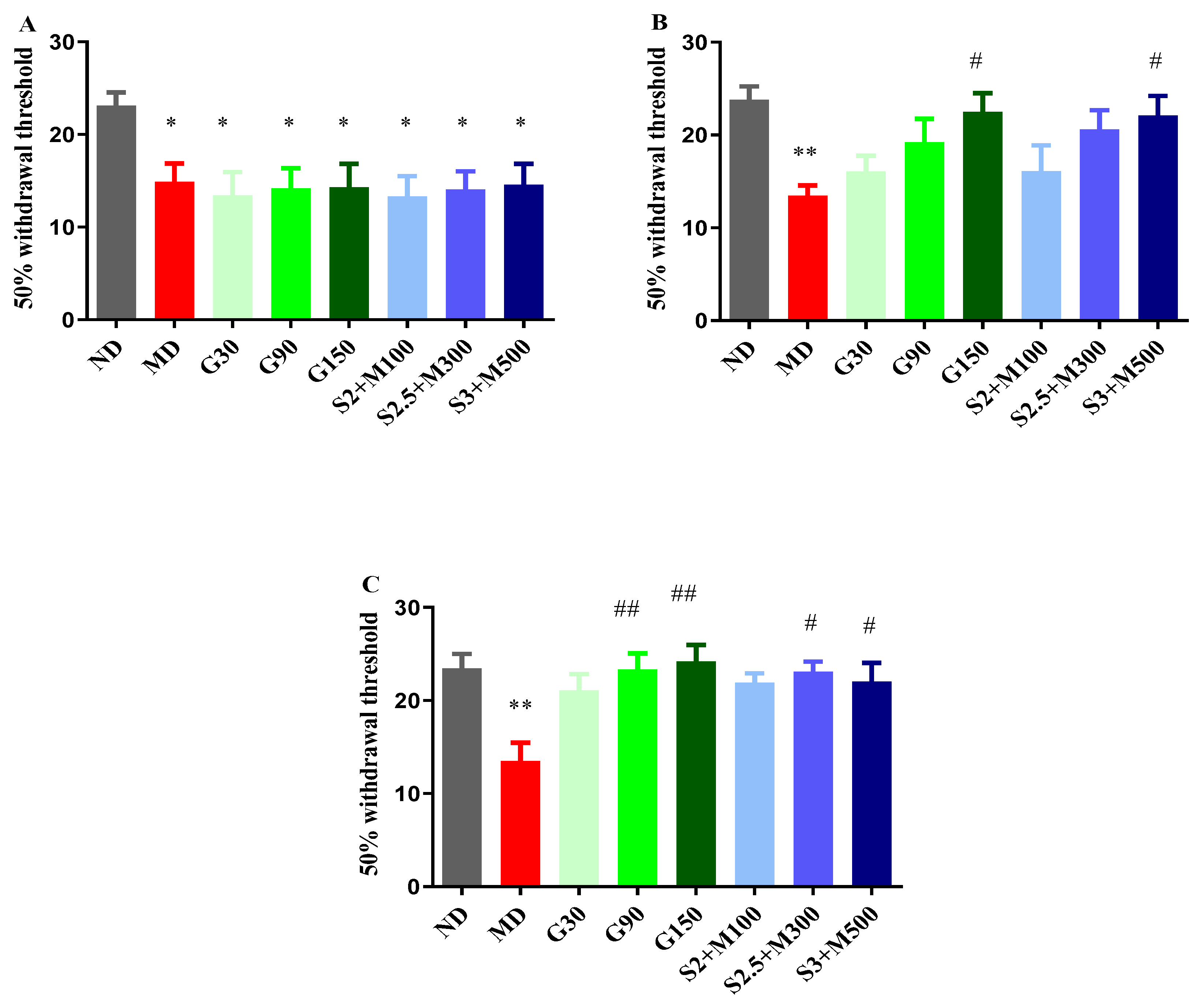
2.2.3. Tactile Hypersensitivity
2.3. Biochemical Assay of Rat Brain and Liver Homogenates
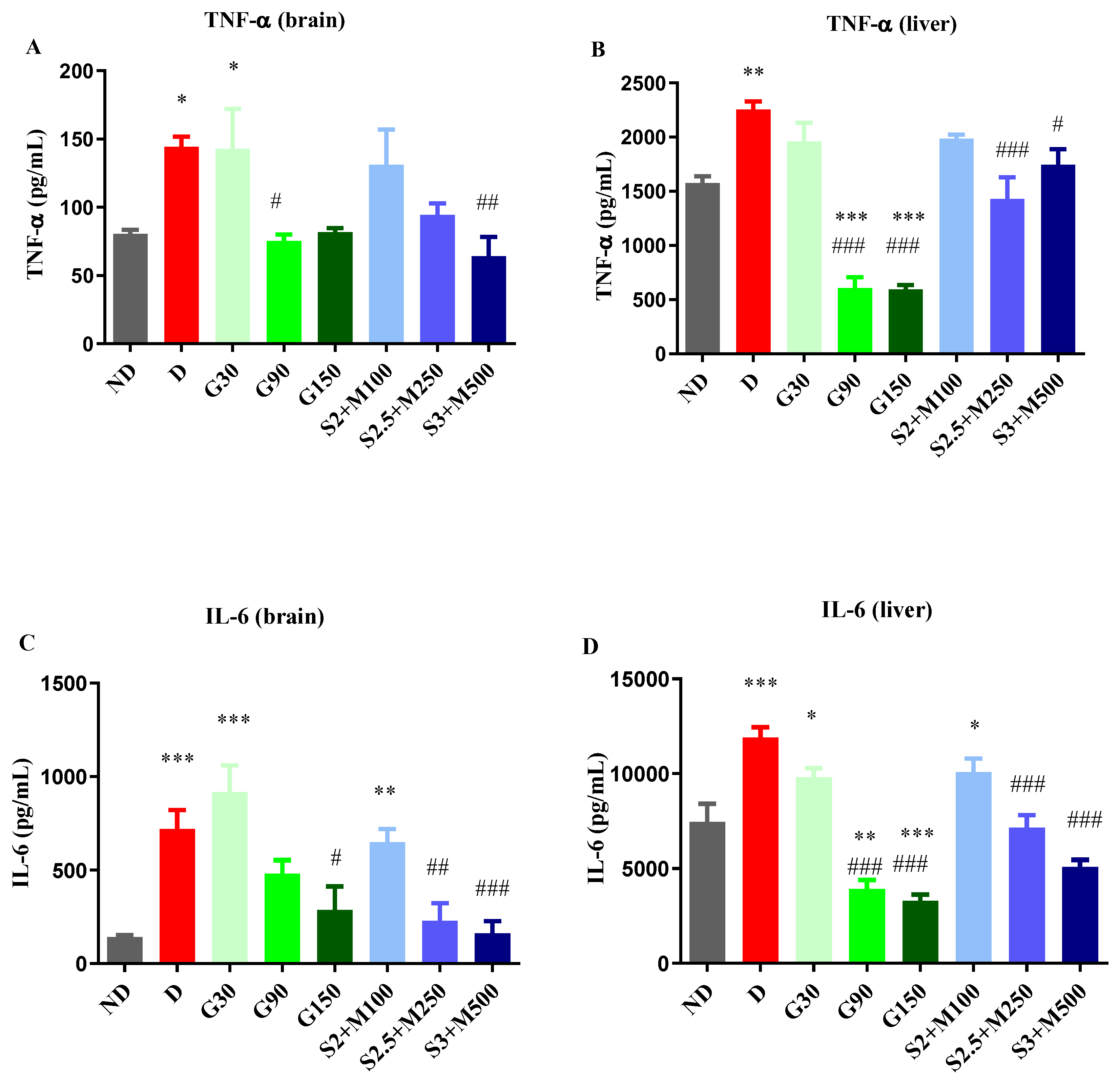
2.3.1. Assessment of TNF-α and Il-6
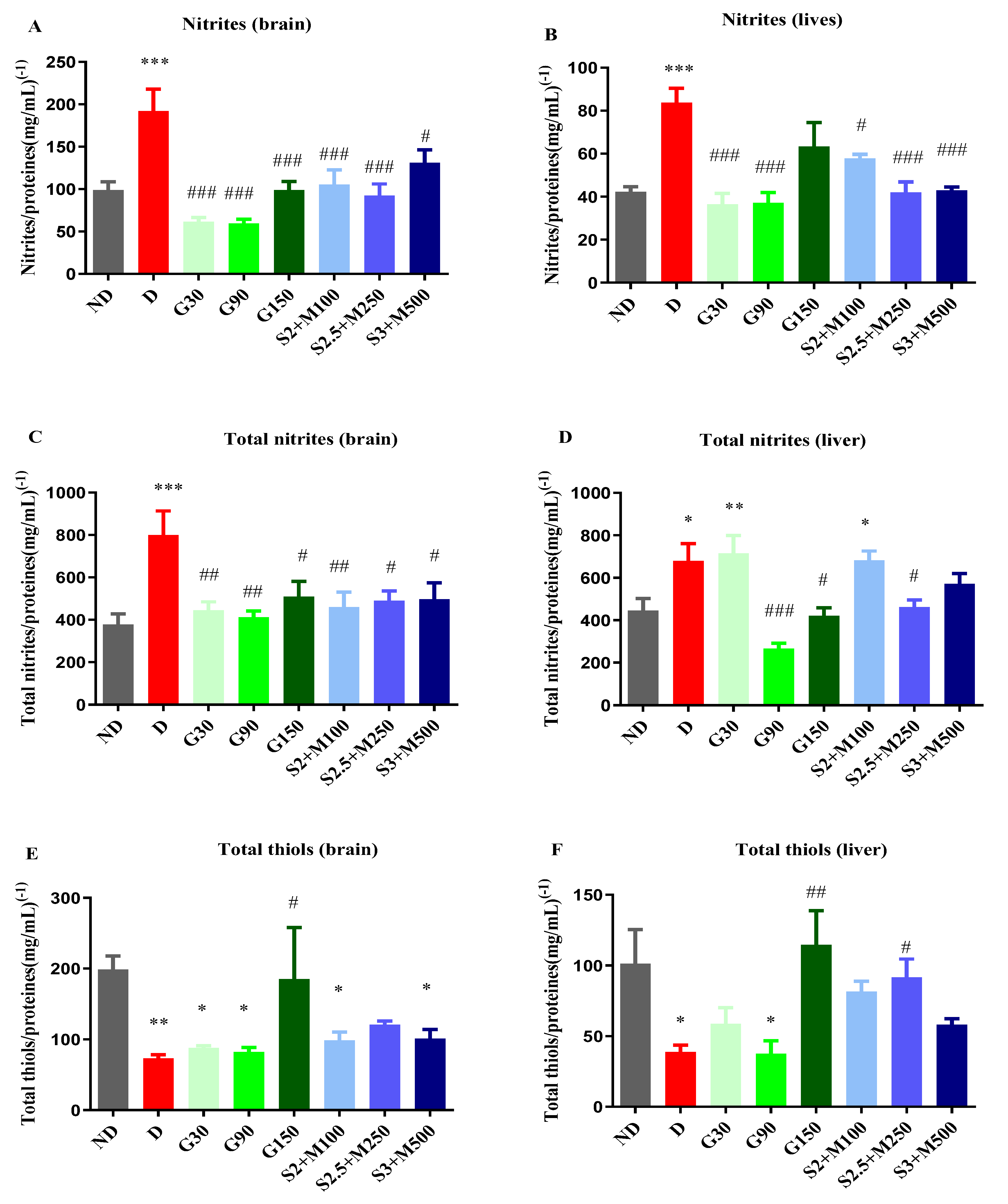
2.3.2. Assessment of NOS Activity
2.3.3. Assessment of Total Thiols
3. Discusions
4. Materials and Methods
4.1. Experimental Animals
4.2. Induction of Diabetes Mellitus and Treatments
4.3. Blood Glucose Levels
4.4. Tests for the Evaluation of Antihyperalgesic Effect
4.4.1. Heat Hypersensitivity
4.4.2. Cold Hypersensitivity
4.4.3. Tactile Hypersensitivity
4.5. Biochemical Assay of Rat Brain and Liver Homogenates
4.5.1. Assessment of TNF-α and Il-6
4.5.2. Assessment of NOS Activity
4.5.3. Assessment of Total Thiols
4.5.4. Protein Content
2.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oshitari T. Advanced Glycation End-Products and Diabetic Neuropathy of the Retina. Int J Mol Sci 2023; 24(3):2927. [CrossRef]
- Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Prim 2019 51 2019; 5(1):1–18.
- Pușcașu C, Zanfirescu A, Negreș S. Recent Progress in Gels for Neuropathic Pain. Gels 2023; 9(5):417. [CrossRef]
- Pușcașu C, Ungurianu A, Șeremet OC, Andrei C, Mihai DP, Negreș S. The Influence of Sildenafil–Metformin Combination on Hyperalgesia and Biochemical Markers in Diabetic Neuropathy in Mice. Medicina (B Aires) 2023; 59(8):1375. [CrossRef]
- Li C, Wang W, Ji Q, et al. Prevalence of painful diabetic peripheral neuropathy in type 2 diabetes mellitus and diabetic peripheral neuropathy: A nationwide cross-sectional study in mainland China. Diabetes Res Clin Pract 2023; 198:110602. [CrossRef]
- Tesfaye S, Chaturvedi N, Eaton SEM, et al. Vascular Risk Factors and Diabetic Neuropathy. N Engl J Med 2005; 352(4):341–350. [CrossRef]
- Yovera-Aldana M, Velásquez-Rimachi V, Huerta-Rosario A, et al. Prevalence and incidence of diabetic peripheral neuropathy in Latin America and the Caribbean: A systematic review and meta-analysis. Negida A, ed. PLoS One 2021; 16(5):e0251642. [CrossRef]
- Alleman CJM, Westerhout KY, Hensen M, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature. Diabetes Res Clin Pract 2015; 109(2):215–225. [CrossRef]
- Rosenberger DC, Blechschmidt V, Timmerman H, Wolff A, Treede R-D. Challenges of neuropathic pain: focus on diabetic neuropathy. J Neural Transm 2020; 127(4):589–624. [CrossRef]
- Kaur M, Mishra S, Swarnkar P, et al. Understanding the role of hyperglycemia and the molecular mechanism associated with diabetic neuropathy and possible therapeutic strategies. Biochem Pharmacol 2023:115723. [CrossRef]
- Hills CE, Brunskill NJ. Cellular and physiological effects of C-peptide. Clin Sci 2009; 116(7):565–574. [CrossRef]
- Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev 2006; 22(4):257–273.
- Dröge W. Free Radicals in the Physiological Control of Cell Function. Physiol Rev 2002; 82(1):47–95. [CrossRef]
- Van Campenhout A, Van Campenhout C, Lagrou AR, et al. Impact of diabetes mellitus on the relationships between iron-, inflammatory- and oxidative stress status. Diabetes Metab Res Rev 2006; 22(6):444–454.
- Pușcașu C, Zanfirescu A, Negreș S, Șeremet OC. Exploring the Multifaceted Potential of Sildenafil in Medicine. Medicina (B Aires) 2023; 59(12):2190. [CrossRef]
- Huang LJ, Yoon MH, Choi J Il, Kim WM, Lee HG, Kim YO. Effect of Sildenafil on Neuropathic Pain and Hemodynamics in Rats. Yonsei Med J 2010; 51(1):82. [CrossRef]
- Wang L, Chopp M, Szalad A, et al. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience 2011; 193:399–410. [CrossRef]
- Deftu A-F, Chu Sin Chung P, Laedermann CJ, et al. The Antidiabetic Drug Metformin Regulates Voltage-Gated Sodium Channel Na V 1.7 via the Ubiquitin-Ligase NEDD4-2. eneuro 2022; 9(2):ENEURO.0409-21.2022.
- Ge A, Wang S, Miao B, Yan M. Effects of metformin on the expression of AMPK and STAT3 in the spinal dorsal horn of rats with neuropathic pain. Mol Med Rep 2018; 17(4):5229–5237. [CrossRef]
- Pușcașu C, Mihai P, Zbârcea CE, et al. Investigation of antihyperalgesic effects of different doses of sildenafil and metformin in alloxan-induced diabetic neuropathy in mice. Farmacia 2023; 71:3.
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. [CrossRef]
- Finnerup NB, Haroutounian S, Baron R, et al. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain 2018; 159(11):2339–2346. [CrossRef]
- Pușcașu C, Văleanu A, Ștefănescu E, Mirela Blebea N, Negreș S. Evaluating the synergism between sildenafil and metformin in an animal model of diabetic neuropathy induced by alloxan. Farmacia 2023; 71:4.
- Moisset X, Bouhassira D, Avez Couturier J, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev Neurol (Paris) 2020; 176(5):325–352. [CrossRef]
- Oliveira RAA de, Baptista AF, Sá KN, et al. Pharmacological treatment of central neuropathic pain: consensus of the Brazilian Academy of Neurology. Arq Neuropsiquiatr 2020; 78(11):741–752.
- Holbech JV, Jung A, Jonsson T, Wanning M, Bredahl C, Bach F. Combination treatment of neuropathic pain: Danish expert recommendations based on a Delphi process. J Pain Res 2017; Volume 10:1467–1475. [CrossRef]
- Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol 2013; 12(11):1084–1095. [CrossRef]
- Eisenberg E, Suzan E. Drug Combinations in the Treatment of Neuropathic Pain. Curr Pain Headache Rep 2014; 18(12):463. [CrossRef]
- Saleem Mir M, Maqbool Darzi M, Musadiq Khan H, Ahmad Kamil S, Hassan Sofi A, Ahmad Wani S. Pathomorphological effects of Alloxan induced acute hypoglycaemia in rabbits. Alexandria J Med 2013; 49(4):343–353.
- Olivenza R, Moro MA, Lizasoain I, et al. Chronic Stress Induces the Expression of Inducible Nitric Oxide Synthase in Rat Brain Cortex. J Neurochem 2001; 74(2):785–791. [CrossRef]
- Kouidrat Y, Pizzol D, Cosco T, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med 2017; 34(9):1185–1192. [CrossRef]
- Escobar-Jiménez F. Eficacia y seguridad del sildenafilo en varones con diabetes mellitus tipo 2 y disfunción eréctil. Med Clin (Barc) 2002; 119(4):121–124. [CrossRef]
- Phé V, Rouprêt M. Erectile dysfunction and diabetes: A review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab 2012; 38(1):1–13. [CrossRef]
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43(2):487–493. [CrossRef]
- Sun WT, Lei CL, Bi CC, Chen ZL, Zhang L. Effect of alloxan time administerDrug on establishing diabetic rabbit model. Int J Ophthalmol 2010; 3(3):200. [CrossRef]
- Raafat K, Aboul-Ela M, El-Lakany A. Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. Arch Pharm Res 2021; 44(8):1–10. [CrossRef]
- Bhalla V, Oyster NM, Fitch AC, et al. AMP-activated Kinase Inhibits the Epithelial Na+ Channel through Functional Regulation of the Ubiquitin Ligase Nedd4-2. J Biol Chem 2006; 281(36):26159–26169. [CrossRef]
- Mia S, Munoz C, Pakladok T, et al. Downregulation of Kv1.5 K + Channels by the AMP-Activated Protein Kinase. Cell Physiol Biochem 2012; 30(4):1039–1050.
- Wei J, Wei Y, Huang M, Wang P, Jia S. Is metformin a possible treatment for diabetic neuropathy? J Diabetes 2022; 14(10):658–669.
- A. Shamsaldeen Y, S. Mackenzie L, A. Lione L, D. Benham C. Methylglyoxal, A Metabolite Increased in Diabetes is Associated with Insulin Resistance, Vascular Dysfunction and Neuropathies. Curr Drug Metab 2016; 17(4):359–367.
- Afshari K, Dehdashtian A, Haddadi N-S, et al. Anti-inflammatory effects of Metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord 2018; 56(11):1032–1041. [CrossRef]
- Byrne FM, Cheetham S, Vickers S, Chapman V. Characterisation of Pain Responses in the High Fat Diet/Streptozotocin Model of Diabetes and the Analgesic Effects of Antidiabetic Treatments. J Diabetes Res 2015; 2015:1–13. [CrossRef]
- Melemedjian OK, Asiedu MN, Tillu D V., et al. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain 2011; 7. [CrossRef]
- Mao-Ying QL, Kavelaars A, Krukowski K, et al. The Anti-Diabetic Drug Metformin Protects against Chemotherapy-Induced Peripheral Neuropathy in a Mouse Model. PLoS One 2014; 9(6):e100701. [CrossRef]
- Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience 1992; 50(1):7–10. [CrossRef]
- Patil CS, Padi S V., Singh VP, Kulkarni SK. Sildenafil induces hyperalgesia via activation of the NO-cGMP pathway in the rat neuropathic pain model. Inflammopharmacology 2006; 14(1–2):22–27.
- Wang L, Chopp M, Szalad A, et al. Sildenafil ameliorates long term peripheral neuropathy in type II diabetic mice. PLoS One 2015; 10(2). [CrossRef]
- Ebenezer GJ, O’Donnell R, Hauer P, Cimino NP, McArthur JC, Polydefkis M. Impaired neurovascular repair in subjects with diabetes following experimental intracutaneous axotomy. Brain 2011; 134(6):1853–1863. [CrossRef]
- Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil-induced peripheral analgesia and activation of the nitric oxide–cyclic GMP pathway. Brain Res 2001; 909(1–2):170–178. [CrossRef]
- Bezerra MM, Lima V, Girão VCC, Teixeira RC, Graça JR V. Antinociceptive activity of sildenafil and adrenergic agents in the writhing test in mice. Pharmacol Rep 2008; 60(3):339–344.
- Augusto PSA, Braga A V., Rodrigues FF, et al. Metformin antinociceptive effect in models of nociceptive and neuropathic pain is partially mediated by activation of opioidergic mechanisms. Eur J Pharmacol 2019; 858:172497. [CrossRef]
- Lós DB, Oliveira WH de, Duarte-Silva E, et al. Preventive role of metformin on peripheral neuropathy induced by diabetes. Int Immunopharmacol 2019; 74:105672. [CrossRef]
- Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res 2013; 36(3):237–251. [CrossRef]
- Back SK, Won SY, Hong SK, Na HS. Gabapentin relieves mechanical, warm and cold allodynia in a rat model of peripheral neuropathy. Neurosci Lett 2004; 368(3):341–344. [CrossRef]
- LaBuda CJ, Fuchs PN. Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain. Neurosci Lett 2000; 290(2):137–140. [CrossRef]
- Zheng H, Sun W, Zhang Q, et al. Proinflammatory cytokines predict the incidence of diabetic peripheral neuropathy over 5 years in Chinese type 2 diabetes patients: A prospective cohort study. EClinicalMedicine 2021; 31:100649. [CrossRef]
- Fischer R, Maier O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxid Med Cell Longev 2015; 2015:1–18. [CrossRef]
- Gonzalez-Clemente JM, Mauricio D, Richart C, et al. Diabetic neuropathy is associated with activation of the TNF-alpha system in subjects with type 1 diabetes mellitus. Clin Endocrinol (Oxf) 2005; 63(5):525–529. [CrossRef]
- Andrews M, Soto N, Arredondo M. Efecto de metformina sobre la expresión del factor de necrosis tumoral-α, los receptores Toll-like 2/4 y la PCR ultra sensible en sujetos obesos con diabetes tipo 2. Rev Med Chil 2012; 140(11):1377–1382.
- Hyun B, Shin S, Lee A, et al. Metformin Down-regulates TNF-α Secretion via Suppression of Scavenger Receptors in Macrophages. Immune Netw 2013; 13(4):123. [CrossRef]
- Pan Y, Sun X, Jiang L, et al. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflammation 2016; 13(1):294. [CrossRef]
- Zhao S, Zhang L, Lian G, et al. Sildenafil attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia. Int Immunopharmacol 2011; 11(4):468–474.
- Karakoyun B, Uslu U, Ercan F, et al. The effect of phosphodiesterase-5 inhibition by sildenafil citrate on inflammation and apoptosis in rat experimental colitis. Life Sci 2011; 89(11–12):402–407. [CrossRef]
- Hube F, Lee Y-M, Röhrig K, Hauner H. The Phosphodiesterase Inhibitor IBMX Suppresses TNF-α Expression in Human Adipocyte Precursor Cells: A Possible Explanation for its Adipogenic Effect. Horm Metab Res 1999; 31(06):359–362. [CrossRef]
- Lee B-S, Jun I-G, Kim S-H, Park JY. Intrathecal Gabapentin Increases Interleukin-10 Expression and Inhibits Pro-Inflammatory Cytokine in a Rat Model of Neuropathic Pain. J Korean Med Sci 2013; 28(2):308. [CrossRef]
- Herder C, Bongaerts BWC, Rathmann W, et al. Association of Subclinical Inflammation With Polyneuropathy in the Older Population. Diabetes Care 2013; 36(11):3663–3670. [CrossRef]
- Zhou J, Zhou S. Inflammation: Therapeutic Targets for Diabetic Neuropathy. Mol Neurobiol 2014; 49(1):536–546. [CrossRef]
- Cameron N, Cotter M. The neurocytokine, interleukin-6, corrects nerve dysfunction in experimental diabetes. Exp Neurol 2007; 207(1):23–29. [CrossRef]
- Magrinelli F, Briani C, Romano M, et al. The Association between Serum Cytokines and Damage to Large and Small Nerve Fibers in Diabetic Peripheral Neuropathy. J Diabetes Res 2015; 2015:1–7. [CrossRef]
- Di Luigi L, Sgrò P, Duranti G, et al. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int J Mol Sci 2020; 21(9):3161.
- Kim YD, Kim YH, Cho YM, et al. Metformin ameliorates IL-6-induced hepatic insulin resistance via induction of orphan nuclear receptor small heterodimer partner (SHP) in mouse models. Diabetologia 2012; 55(5):1482–1494. [CrossRef]
- de Brito TV, Júnior GJD, da Cruz Júnior JS, et al. Gabapentin attenuates intestinal inflammation: Role of PPAR-gamma receptor. Eur J Pharmacol 2020; 873:172974.
- Phaniendra A, Jestadi DB, Periyasamy L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J Clin Biochem 2015; 30(1):11–26. [CrossRef]
- Zhao K, Huang Z, Lu H, Zhou J, Wei T. Induction of inducible nitric oxide synthase increases the production of reactive oxygen species in RAW264.7 macrophages. Biosci Rep 2010; 30(4):233–241. [CrossRef]
- Lubos E. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci 2008; Volume(13):5323. [CrossRef]
- Zochodne DW, Verge VMK, Cheng C, et al. Nitric Oxide Synthase Activity and Expression in Experimental Diabetic Neuropathy. J Neuropathol Exp Neurol 2000; 59(9):798–807. [CrossRef]
- Forouzanfar F, Tanha NK, Pourbagher-Shahri AM, Mahdianpour S, Esmaeili M, Ghazavi H. Synergistic effect of ellagic acid and gabapentin in a rat model of neuropathic pain. Metab Brain Dis 2023; 38(4):1421–1432. [CrossRef]
- Rossi R, Giustarini D, Milzani A, Dalle-Donne I. Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J Cell Mol Med 2009; 13(9B):3131–3140. [CrossRef]
- Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 1999; 13(10):1169–1183. [CrossRef]
- Ewis SA, Abdel-Rahman MS. Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. J Appl Toxicol 1995; 15(5):387–390. [CrossRef]
- Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact 2011; 192(3):233–242. [CrossRef]
- Abdollahi M, Fooladian F, Emami B, Zafari K, Bahreini-Moghadam A. Protection by sildenafil and theophylline of lead acetate-induced oxidative stress in rat submandibular gland and saliva. Hum Exp Toxicol 2003; 22(11):587–592. [CrossRef]
- Hüsamettin Baran A, Berk A, Bahadır Kaymaz M, Aktay G, Corresponding °, Baran AH. Antioxidant Effect of Sildenafil on Cadmium-Induced Liver, Lung and Kidney Injury. J Pharm Sci 2020; 45:37–44.
- Negreş S, Chiriţă C, Moroşan E, Arsene AL. Experimental pharmacological model of diabetes induction with aloxan in rat. Farmacia 2013; 61(2).
- Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future directions. Expert Rev Neurother 2005; 5(6):823–830. [CrossRef]
- New, RAT. Screening Methods in Pharmacology. Yale J. Biol. Med. 1965, 38, 309. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2591164 (accessed on 7 February 2023).
- Tsagareli MG, Tsiklauri N, Zanotto KL, et al. Behavioral evidence of thermal hyperalgesia and mechanical allodynia induced by intradermal cinnamaldehyde in rats. Neurosci Lett 2010; 473(3):233–236. [CrossRef]
- Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci Lett 2010; 484(1):62–65. [CrossRef]
- Dixon WJ. The Up-and-Down Method for Small Samples. J Am Stat Assoc 1965; 60(312):967–978.
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53(1):55–63.
- Gradinaru D, Margina D, Borsa C, et al. Adiponectin: possible link between metabolic stress and oxidative stress in the elderly. Aging Clin Exp Res 2017; 29(4):621–629. [CrossRef]
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric oxide Biol Chem 2001; 5(1):62–71. [CrossRef]
- Nitulescu G, Mihai DP, Nicorescu IM, et al. Discovery of natural naphthoquinones as sortase A inhibitors and potential anti-infective solutions against Staphylococcus aureus. Drug Dev Res 2019; 80(8):1136–1145. [CrossRef]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193(1):265–275.
- Mihai DP, Ungurianu A, Ciotu CI, et al. Effects of venlafaxine, risperidone and febuxostat on cuprizone-induced demyelination, behavioral deficits and oxidative stress. Int J Mol Sci 2021; 22(13):7183. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




