1. Introduction
A precisely tuned immune system is crucial to human health. It protects the organism against noxious factors, like pathogens and foreign substances. Nutrition is a major exogenous factor that modulates immune function [
1]. To ensure the proper function of immune cells and physical barriers, the immune system needs multiple specific nutrients, which play synergistic roles at every stage of the immune response in humans [
2,
3]. Several vitamins (D, A, C, E, folate, B6, B12) and minerals (zinc, iron, copper and selenium) were, thus, documented to be essential for an optimal immune balance [
4,
5,
6]. On the other hand, the mechanistic roles of micronutrients regarding the immune function have been well described recently. Some of the micronutrients like magnesium and Vitamin E have accepted European health claims with an immune-modulatory context [
7].
Since the Corona pandemic, some micronutrients have been researched more extensively. In particular, vitamin D, selenium, and zinc have been discussed with regard to their effects on the immune system [
8,
9,
10,
11,
12,
13]. Vitamin D acts as an immune system modulator, preventing excessive expression of inflammatory cytokines and increasing macrophages' 'oxidative burst' potential. Additionally, it stimulates the production of antimicrobial peptides, which are crucial for protecting the respiratory tract from infections [
14]. Observational studies have indicated a correlation between low blood concentrations of 25-hydroxyvitamin D3, the precursor of calcitriol, which is the active form of vitamin D, and viral respiratory tract infections, such as URI [
14,
15,
16]. The association between zinc deficiency and immune dysfunction was established already 50 years ago, and since then, extensive research has been conducted in this field [
17]. Zinc is involved in the regulation of the innate and adaptive immune response. It plays an essential role in lymphocyte formation, activation, and maturation, intercellular communication via cytokines, and the innate host defense [
4,
17,
18]. Insufficient zinc levels can result in thymus atrophy, lymphopenia, and impaired cellular and antibody-mediated immune responses [
17]. Selenium, which is also an essential micronutrient for human health, may contribute to a reduced immune function, some cancers, and viral diseases in case of a measurable deficiency [
19]. Animal models and epidemiological studies have provided evidence that low selenium levels can facilitate genetic mutations and increase the virulence of specific viruses, such as coxsackievirus, poliovirus, and murine influenza [
7].
Yet, the relationship between immunoactive micronutrient supplements and their effect on the occurrence and severity of respiratory infections remains inconclusive. Some studies suggest that standardized intake of micronutrients can reduce the occurrence of respiratory infections [
8,
9,
10,
11,
12,
13,
14], while others report no significant effect [
5,
14,
15,
16,
17,
18]. Especially studies with older people and/or those with micronutrient deficiencies demonstrated that a micronutrient supplementation can reduce the incidence and severity of URI [
11,
12,
13,
20]. However, for younger, otherwise healthy individuals, these benefits have not yet been consistently shown [
5]. Furthermore, no studies have explored the potential benefits of a personalized supplementation according to the individual micronutrient status.
Hence, the primary objective of our study was to investigate whether personalized supplementation based on individual blood levels of selenium, zinc, and vitamin D in healthy young volunteers could moderate the occurrence and severity of URI. To assess this, we used the Wisconsin Upper Respiratory Symptom Survey (WURSS-21), a standardized questionnaire for measuring URI symptoms [
21,
22].
2. Materials and Methods
Procedure for the intervention was followed in accordance with the ethical standards from the Helsinki Declaration and were approved by the ethics committee of the School of Medicine of the Technical University of Munich, Germany (75/21 S). All study participants provided online approval. The study was registered in the German Clinical Trials Register (DRKS): DRKS00023567.
2.1. Study Participants
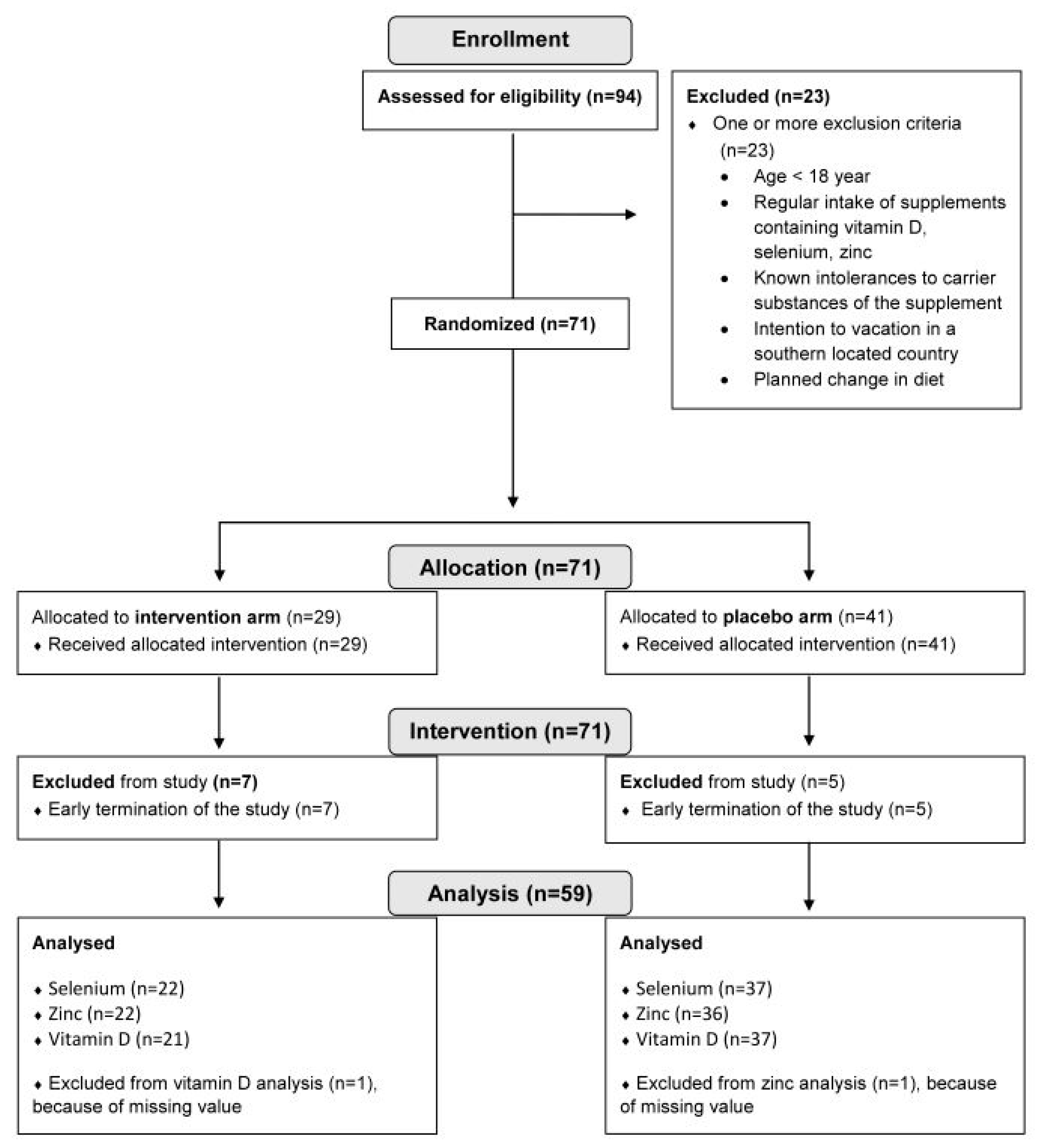
For the intervention study cohort, in total, 94 healthy adults aged 18–65 years were recruited via announcements on social media (LinkedIn, facebook, Instagram) and local newspaper advertisements. Power calculation was performed using G-power 3.1, assuming a Cohen effect size of 0.8, alpha error of 0.05, power of 0.9, and a dropout rate of 10%. A total of 62 study participants were determined (28 per arm + 10%).
Participant enrollment started on April 23rd, 2021, and continued until November 23rd, 2021. The participants’ eligibility was assessed with a detailed screening questionnaire. Exclusion criteria were age < 18 years, regular intake of supplements with vitamin D, selenium, zinc, and/or omega-3/omega-6 fatty acids, known intolerances to carrier substances of the supplement (guar gum, apple powder, potato starch), intention to go on vacation in southern climes (increased exposure to sunlight) and planned changes in dietary behavior (e.g. conversion to vegetarianism, veganism).
Randomization was performed online when participants registered for the study. Allocation to the study arms was carried out using Phyton Random Library. With a balancing feature to aim an equal group size.
Figure 1 depicts the participant flow chart of the intervention study.
2.2. Study Design
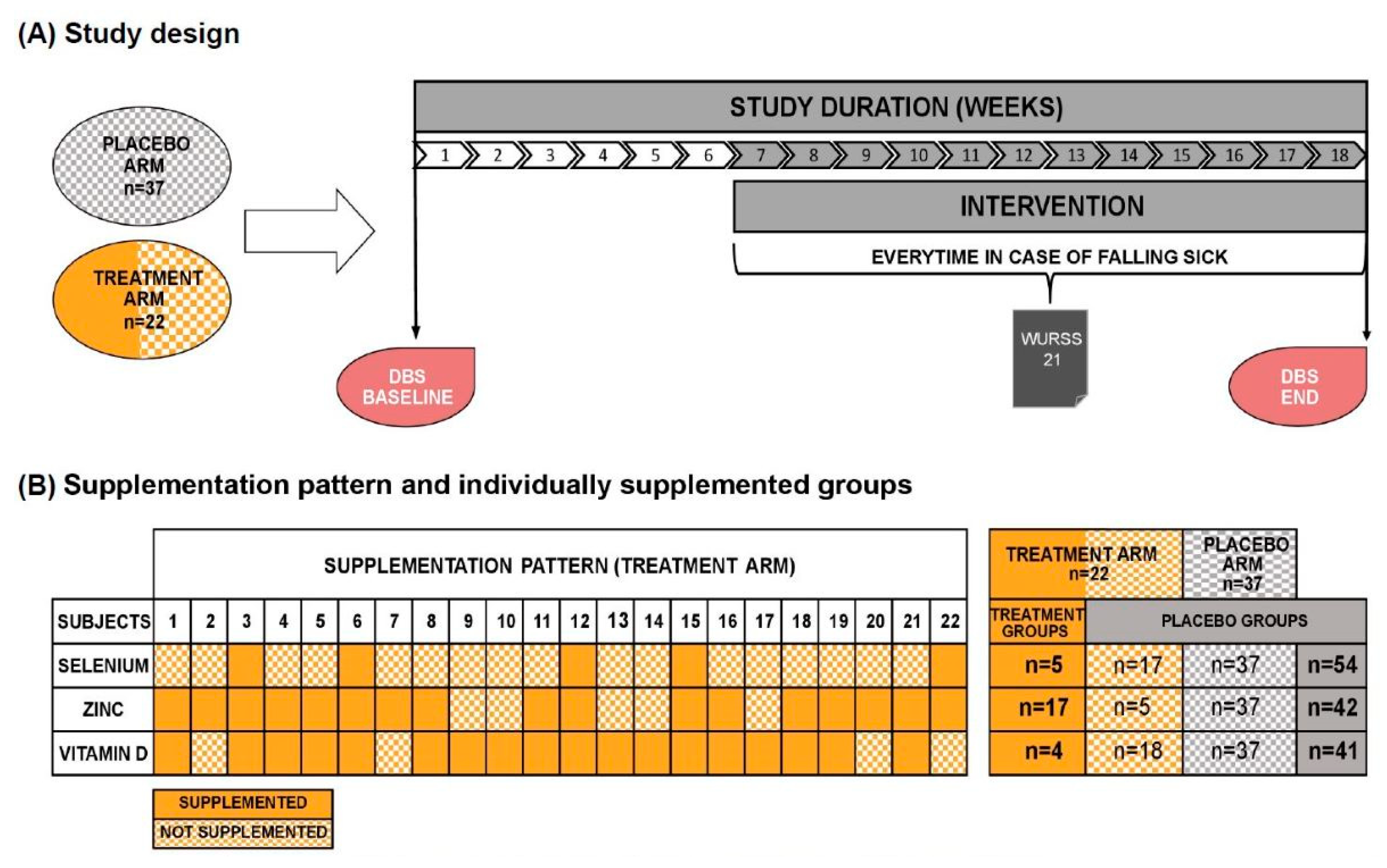
This was a randomized, double-blind, placebo-controlled study. At the beginning of the study all participants were asked to collect capillary blood from their fingertips with a home test kit for dried blood spots (DBS) on their own. Based on the DBS analysis, participants in the treatment arm received micronutrient supplementation with individual amounts of selenium, zinc and/or vitamin D. Threshold blood levels for initiating supplementation were determined according to an algorithm from LOEWI® and were 0.14 µg/mL for selenium, 7 µg/mL for zinc and 95 nmol/L for vitamin D. Accordingly, participants randomized to the treatment groups received either one, two, three or no micronutrients. The placebo arm received only carrier material as a supplement. However, participants from the treatment arm not receiving any micronutrient in the supplement due to blood levels were subsequently also analyzed as receiving “placebo” compared to the "treatment" group who received either one, two, or all three micronutrients.
Figure 2 summarizes the study design, the originally randomized study arms and the spilt into individually supplemented groups. The treatment arm is colored two-tone, orange and orange-checkered. Full orange indicates supplementation with the respective micronutrient (treatment group), whereas volunteers appearing as orange checkered received no supplementation due to blood levels above the “critical supplementation level”. Consequently, we combined participants with an orange-checkered pattern from treatment arm with the volunteers from the placebo arm (grey-checkered) now referred as the placebo group (fully grey).
The average time period between DBS analysis and start of supplementation was six weeks to allow production of the personalized supplement. The intervention period was 12 weeks. The Wisconsin Upper Respiratory Symptom Survey-21 (WURSS-21) questionnaire was filled out in every case of falling sick during the study phase. Every WURSS-21 questionnaire was filled out on seven consecutive days. At the end of the intervention period, the participants provided another DBS sample.
2.3. Dried Blood Spot Analysis
After inclusion in the study, a home test kit with a detailed explanation for collecting DBS was send from the Core Facility Human Studies of the ZIEL- Institute for Food & Health, Freising, Germany to the participants. Participants independently collected DBS from their fingertips at the beginning of the study with a home test kit and after the intervention period. DBS were sent to Vitas AS, Norway for the analysis of selenium [µg/mL], zinc [µg/mL], vitamin D [nmol/L], iron [µg/mL], magnesium [µg/mL], omega-3 fatty acids and omega-6 fatty acids as % of fatty acid methyl ester (FAME). The personalized micronutrient supplement was produced according to the blood profiles of selenium, zinc, and vitamin D.
2.4. Study Product
The study product, provided by LOEWI®, was a powder consisting of carrier material guar gum, apple powder and potato starch. The product had to be taken daily during the complete intervention period (5 g). Whereas the placebo groups received the carrier material without micronutrients. The treatment groups received the powder with an individually calculated amount of selenium, zinc and vitamin D. Only nutrients below a defined threshold were added (see above).
Figure 2B shows the supplementation pattern and the individually supplemented groups. Selenium was supplemented in a range of 51.0 µg to 97.5 µg (mean=66.9 µg), zinc from 7.2 µg to 26.7 µg (mean=4.5 µg) and vitamin D from 22.5 µg to 97.5 µg (mean=55.0 µg) per day. Accordingly, the amount of supplemented micronutrients did not exceed the official national recommendations.
2.5. The Wisconsin Upper Respiratory Symptom Survey
We used the WURSS-21 [
21,
22] and the four additional symptoms dry cough, fever, olfactory or taste disorder, and limb pain to evaluate the number of episodes and the severity of URI during the intervention period. For each case of illness during the supplementation, participants were asked to fill out a WURSS-21 for seven consecutive days. Symptoms were ranked from 0 (no symptom) to 7 (severe symptom). Daily WURSS summary scores were calculated by summing the scores (0-7) from each of the fourteen symptoms. The day with the highest WURSS score was used to evaluate disease severity (WURSS
max). An URI was defined from a WURSS
max sum score of 7 or higher. The highest possible value for one day would be 105.3.
2.6. Data Analysis and Statistics
Data was analyzed in the R programming environment and GraphPad Prism 10. Results are presented as mean ± SD, unless otherwise indicated. P-values < 0.05 were regarded as statistically significant. Shapiro–Wilk test and quantil-quantil-plots were used to test normal distribution of data. According to distribution, either the t-test or Wilcoxon test was applied to assess differences in the cohorts. To assess differences within the placebo and treatment groups between baseline and the end of the intervention period the paired t-test was applied. One participant was excluded from the zinc and vitamin D analysis because of missing values due to low blood volume on the DBS filter paper.
3. Results
3.1. Participant Characteristics
In total, 94 volunteers were screened for our inclusion and exclusion criteria. Participant flow chart is shown in
Figure 1. For analysis, the study cohort finally encompassed 59 participants with 37 subjects in the placebo arm and 22 subjects in the treatment arm. As shown in
Figure 2B and
Table 1, the individually supplemented groups for selenium, zinc and vitamin D were as follows: selenium (treatment group n=5, placebo group n=54), zinc (treatment group n=17, placebo group n=42), vitamin D (treatment group n=18, placebo group n=41). The placebo groups encompassed the placebo arm (randomized) plus the participants from the treatment arm without a deficit of the respective micronutrient. The treatment groups encompassed all participants with deficiencies for the respective micronutrient from the treatment arm.
Table 1 depicts the study participants´ baseline characteristics and the division of the study arms into groups receiving the respective supplements.
3.2. Effect of Personalized Supplementation on the Micronutrient Status
In the following, volunteers in the intervention arm who did not receive any micronutrient treatment according to their baseline blood levels are analyzed together with the originally randomized placebo arm as one group.
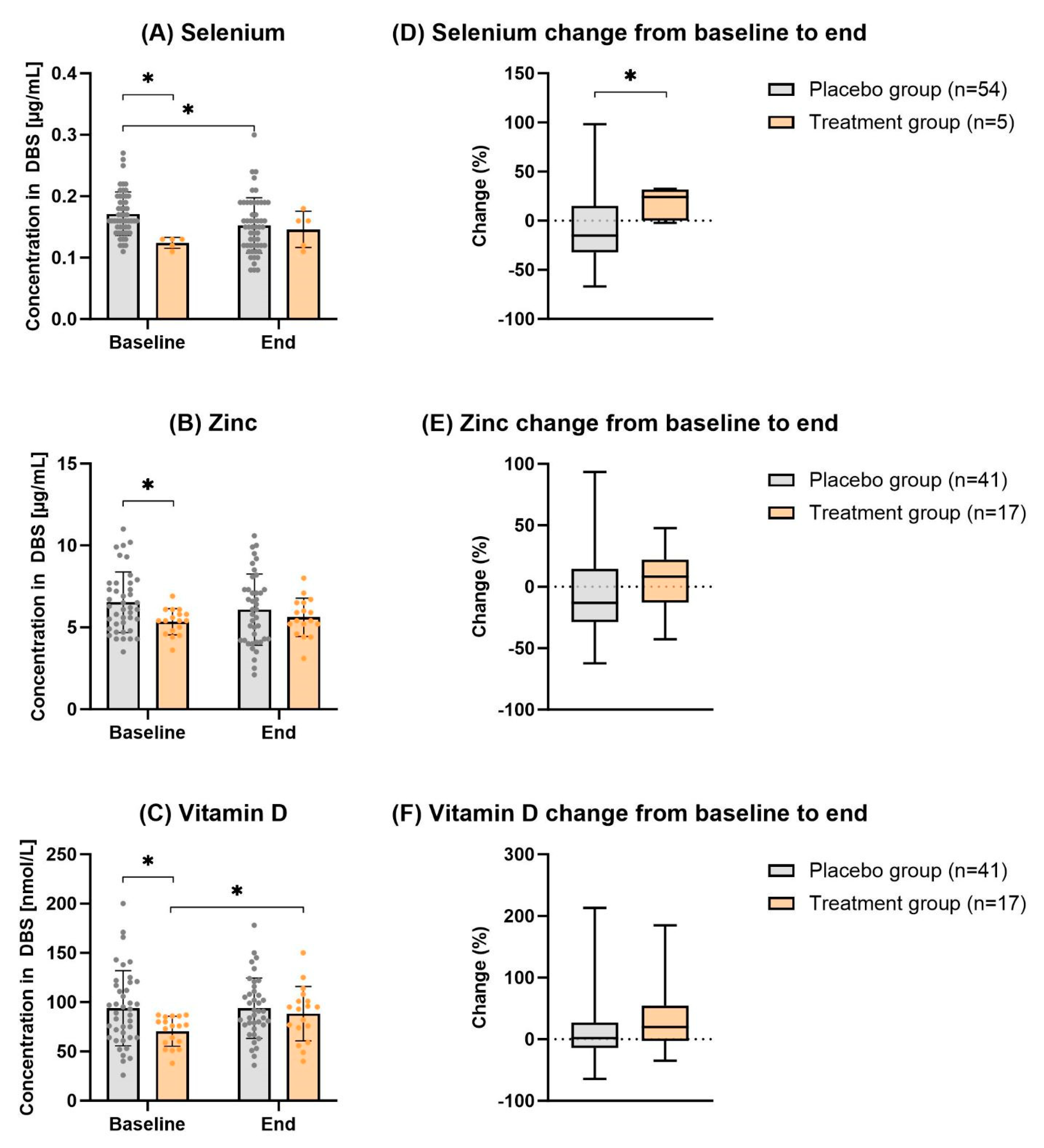
Figure 3 and
Table 2 how the effect of individual supplementation with selenium, zinc and vitamin D. At baseline, the placebo and treatment groups significantly differed in terms of the status of all three micronutrients. As expected, the placebo group showed significantly higher levels than the treatment group (
p-value
selenium=0.002,
p-value
zinc=0.024,
p -value
vitamin D =0.017). After the supplementation period of 12 weeks, the differences between the placebo and treatment group were no longer significant (
p-value
selenium=0.660,
p-value
zinc=0.946,
p-value
vitamin D=0.628). Thus, at the end of the study duration, the values of the different treatment groups adjusted to those of the untreated placebo groups (and
Table 2). Although all blood levels of single micronutrients in the supplemented group seem to increase over time, only vitamin D reached statistical significance after 12 weeks compared to the baseline levels (
p-value=0.017).
3.3. Individual Supplementation with Micronutrients does not Reduce Frequency or Moderate Severity of URI
During the entire study duration of seven months, a total of 16 URI occurred in the study cohort; seven in the placebo arm and nine in the treatment arm. A summary of URI cases in the individual supplemented groups is provided in the
Table 3.
Interestingly, WURSSmax did not significantly differ in the single supplemented treatment and placebo groups but, noteworthy, the treatment and placebo arms, analyzed according to the initial intention to treat, show significant differences in the severity score (p-value=0.045).
4. Discussion
Despite various attempts in the past to supplement micronutrients as an adjuvant treatment for infections, the data remains controversial, and there is still limited evidence that individual micronutrients can be used to prevent or ameliorate URI symptoms [
5,
8,
14,
15,
16,
17]. Therefore, we aimed to investigate if a combination of selected immunoactive micronutrients (selenium, zinc, and vitamin D) with a personalized supplementation strategy based on individual blood levels could be effective to reduce the frequency and severity of URI. To our knowledge, this is the first study with such an approach.
First, we could see that supplementation over 12 weeks, in line with existing national recommendations [
23,
24,
25], was able to alter relatively low blood levels of the selected micronutrients. The blood values became similar between the groups and the previously observed significance for selenium, zinc and vitamin D disappeared between the groups after 12 weeks. The fact that the blood levels after supplementation within the treatment group did not reach statistical significance between baseline and the end of the study may be due to a small number of individuals being effectively treated. This could be related to a selection bias, where participants are more likely to join the study if they have some interest in healthy eating and are less likely to be nutrient deficient due to a balanced diet compared to the average population. In addition, we used only moderate doses to correct deficiencies, which may require more time to be reflected in the results. It should also be noted that our intervention study was conducted in otherwise healthy individuals who did not have clinically relevant deficiencies for the three micronutrients according to EFSA reference values [
23,
24,
25]. Furthermore, the blood levels at which our participants received the respective supplementation were interpreted by an algorithm from LOEWI®, which is a continuum, and, therefore, the subjects received individual doses that did not follow a yes-no approach related to fixed thresholds.
Although we achieved comparable levels at the end of the trial with the individually composed nutritional supplement compared to the control group with normal blood values, we did not see an improvement in the occurrence or severity of URI in the treated participants. Our results show that neither the occurrence nor the severity of URI during the study period differed between the intervention and placebo groups. The relatively low frequency of infection events is due, among other things, to the fact that the applicable contact restrictions of the Corona pandemic prevented more URIs during the study period. Interestingly, when comparing the overall treatment group with the placebo group and analyzing the severity of illness according to intention to treat, the treatment group had significantly higher WURSS
max scores. This result may seem surprising at first glance, as there are several studies suggesting a beneficial effect of immunoactive micronutrient supplementation on URI and Covid-19, especially vitamin D [
8,
26,
27] and zinc [
10,
14]. For URI, however, a positive effect was only shown in severe deficiency states [
19,
28]. Affirmingly, several other studies were also not able to show any significant amelioration in the frequency and severity of URIs when supplementing micronutrients [
12,
14,
15,
17,
18]. For instance, a recent meta-analysis by Vlieg-Boerstra et al. found no association between the supplementation of proposed immunoactive nutrients, for example, vitamin D supplementation, and improved immune system response in the general European population [
5]. Furthermore, the beneficial effects of supplementation reported in the literature have mainly been observed in studies of (older) individuals with low blood levels of micronutrients and in longer-term supplementation studies [
11,
12,
28,
29]. In our study, all our participants were younger and did not show signs of deficiency in the selected micronutrients, and it must be noted that we only used short-term supplementation without pharmacological doses. Regarding the higher severity scores of the participants in the treatment arm, it is of course possible that the small number of participants may have biased our results. However, the findings show that even with adequate micronutrient status and supplementation with immunomodulatory micronutrients, people are not resistant to URIs and that other factors may contribute to the incidence and severity of URIs.
5. Conclusions
In conclusion, our results suggest that a strong immune defense does not depend solely on acute supplementation with individual micronutrients or a combination of vitamin D, zinc and selenium. Rather, it appears to be a more complex interplay beyond the classical immunoactive micronutrients, pointing to the importance of a balanced diet providing all critical bioctives. Therefore, it is advisable to maintain a healthy, balanced diet that meets the recommended daily values of these nutrients to ensure optimal functioning of physical barriers and immune cells. In addition, we believe it is important to consider other factors such as age, sex, comorbidity and lifestyle. For future studies, we propose a more global approach that considers multiple factors, such as diet, when addressing immune system integrity
Author Contributions
Conceptualization, Melanie Haas, Laura Schinhammer and Thomas Skurk; Data curation, Laura Schinhammer; Funding acquisition, Thomas Skurk; Investigation, Melanie Haas and Laura Schinhammer; Project administration, Laura Schinhammer and Thomas Skurk; Resources, Thomas Skurk; Supervision, Thomas Skurk; Visualization, Melanie Haas; Writing – original draft, Melanie Haas; Writing – review & editing, Beate Brandl, Laura Schinhammer and Thomas Skurk.
Funding
Thomas Skurk reports financial support was provided by European Institute of Innovation and Technology (EIT).
Institutional Review Board Statement
Procedure for the intervention was followed in accordance with the ethical standards from the Helsinki Declaration and were approved by the ethics committee of the School of Medicine of the Technical University of Munich, Germany (75/21 S, February 25, 2021). All study participants provided online approval. The study was registered in the Ger-man Clinical Trials Register (DRKS): DRKS00023567.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data described in the manuscript will be made available upon request.
Acknowledgments
We would like to thank all the participants for their dedicated contribution in the study. We thank Margot Maier for outstanding support with data collection, monitoring data, and her technical assistance. Additionally, we are thankful to Silvia Kolossa, Calvin Devereux and Thorsten Schiefer from LOEWI© for providing us with the personalized supplements. We would like to thank Stephan Haug for his support in the statistical analysis.
Conflicts of Interest
The corresponding author declares that the other authors have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Albers, R.; Bourdet-Sicard, R.; Braun, D.; Calder, P.C.; Herz, U.; Lambert, C.; Lenoir-Wijnkoop, I.; Méheust, A.; Ouwehand, A.; Phothirath, P.; et al. Monitoring immune modulation by nutrition in the general population: Identifying and substantiating effects on human health. Br. J. Nutr. 2013, 110, S1–S30. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef]
- Boerstra, B.V.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Elberink, H.O.; Schöll, I.P.; et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy 2021, 77, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health: Regulation (EU) No 432/2012.
- Laird. Vitamin D and Inflammation: Potential Implications for Severity of Covid-19.
- Girodon, F.; Galan, P.; Monget, A.L.; Boutron-Ruault, M.C.; Brunet-Lecomte, P.; Preziosi, P.; Arnaud, J.; Manuguerra, J.C.; Hercberg, S. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: A randomized controlled trial. MIN. VIT. AOX. geriatric network. Arch. Intern. Med. 1999, 159, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Veverka, D.V.; Wilson, C.; Martinez, M.A.; Wenger, R.; Tamosuinas, A. Use of zinc supplements to reduce upper respiratory infections in United States Air Force Academy Cadets. Complement. Ther. Clin. Prac. 2009, 15, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ivory, K.; Prieto, E.; Spinks, C.; Armah, C.N.; Goldson, A.J.; Dainty, J.R.; Nicoletti, C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin. Nutr. 2015, 36, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Broome. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. 2004.
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Hunter, J.; Arentz, S.; Goldenberg, J.; Yang, G.; Beardsley, J.; Myers, S.P.; Mertz, D.; Leeder, S. Zinc for the prevention or treatment of acute viral respiratory tract infections in adults: A rapid systematic review and meta-analysis of randomised controlled trials. BMJ Open 2021, 11, e047474. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, L.C.; King, T.S.; Cardet, J.C.; Craig, T.; Holguin, F.; Jackson, D.J.; Kraft, M.; Peters, S.P.; Ross, K.; Sumino, K.; et al. Vitamin D Supplementation and the Risk of Colds in Patients with Asthma. Am. J. Respir. Crit. Care Med. 2016, 193, 634–641. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Hemilä, H.; Cohen, A.H.; Rinat, B.; Choleva, L.; Constantini, N.W. Vitamin D Supplementation and Upper Respiratory Tract Infections in Adolescent Swimmers: A Randomized Controlled Trial. Pediatr. Exerc. Sci. 2015, 27, 113–119. [Google Scholar] [CrossRef]
- Li-Ng, M.; Aloia, J.F.; Pollack, S.; Cunha, B.A.; Mikhail, M.; Yeh, J.; Berbari, N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiology Infect. 2009, 137, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Slow, S.; Chambers, S.T.; Jennings, L.C.; Stewart, A.W.; Priest, P.C.; Florkowski, C.M.; Livesey, J.H.; Camargo, C.A.; Scragg, R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: The VIDARIS randomized controlled trial. JAMA 2012, 308, 1333–1339. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M.; Bhadada, S.K.; Shetty, A.J.; Singh, B.; Vyas, A. Vitamin D supplementation and clinical outcomes in COVID-19: A systematic review and meta-analysis. J. Endocrinol. Investig. 2021, 45, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Pae, M.; Wu, D. Nutritional modulation of age-related changes in the immune system and risk of infection. Nutr. Res. 2017, 41, 14–35. [Google Scholar] [CrossRef]
- Barrett, B.; Brown, R.; Mundt, M.; Safdar, N.; Dye, L.; Maberry, R.; Alt, J. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J. Clin. Epidemiology 2005, 58, 609–617. [Google Scholar] [CrossRef]
- Barrett, B.; Brown, R.L.; Mundt, M.P.; Thomas, G.R.; Barlow, S.K.; Highstrom, A.D.; Bahrainian, M. Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS-21). Health Qual. Life Outcomes 2009, 7, 76. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Griffiths, C.J.; Martineau, A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef]
- BourBour, F.; Dahka, S.M.; Gholamalizadeh, M.; Akbari, M.E.; Shadnoush, M.; Haghighi, M.; Taghvaye-Masoumi, H.; Ashoori, N.; Doaei, S. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch. Physiol. Biochem. 2020, 129, 16–25. [Google Scholar] [CrossRef]
- Hosseini, B.; El Abd, A.; Ducharme, F.M. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2134. [Google Scholar] [CrossRef]
- Jolliffe, D.; Camargo, C.; Sluyter, J.; Martineau, A. S100 Vitamin D supplementation to prevent acute respiratory infections: Systematic review and meta-analysis of aggregate data from randomised controlled trials. British Thoracic Society Winter Meeting, Wednesday 17 to Friday 19 February 2021, Programme and Abstracts. LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE; pp. 276–292.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).