Submitted:
28 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
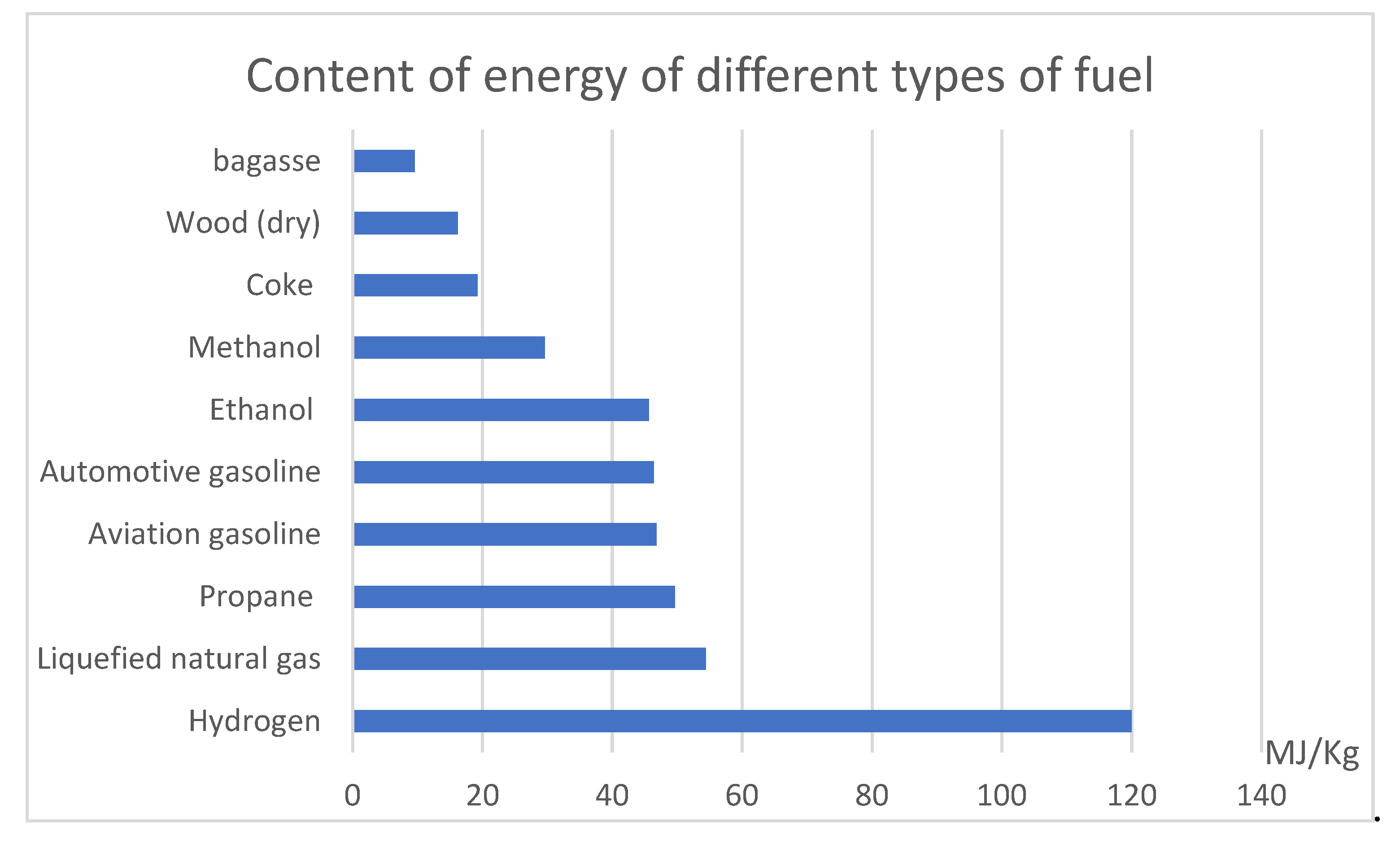
1.1. Why Hydrogen?
| Fuel | Energy Content (MJ/Kg) |
|---|---|
| Hydrogen | 120 |
| Liquefied natural gas | 54.4 |
| Propane | 49.6 |
| Aviation gasoline | 46.8 |
| Automotive gasoline | 46.4 |
| Ethanol | 45.6 |
| Methanol | 29.6 |
| Coke | 19.27 |
| Wood (dry) | 16.2 |
| Bagasse | 9.6 |
1.2. Hydrogen Production Methods
- Steam reforming of natural gas (SR): The method involves a catalytic conversion of hydrocarbon and steam to hydrogen and carbon oxides. It consists of the main steps of reforming or synthesis gas (syngas) generation, water-gas shift (WGS) and methanation or gas purification [11,12]. This method is the most common for producing hydrogen and has a TRL of 9 [111].
- Partial oxidation process (POX): The method involves the conversion of steam, oxygen and hydrocarbons to hydrogen and carbon oxides. The catalytic process occurs at 950 °C with feedstock from methane to naphtha. The non-catalytic process occurs at 1150–1315 °C with feedstock that includes methane, heavy oil and coal. After sulfur removal, pure O2 is used to partially oxidize hydrocarbon feedstock. The syngas produced is further treated in the same way as the product gas of the SR process [11,12].
- Water Electrolysis: The method uses electrical current in order to separate water into oxygen and hydrogen. This way green hydrogen is produced without any direct emissions of carbon dioxide. The reaction is very endothermic. Thus, renewable energy sources can provide the required energy input [12,13,14,15,16]
2. Alternative Processes from Biomass
2.1. Biological Treatment
2.1.1. Dark Fermentation
2.1.2. Photo Fermentation
2.1.3. Biocatalyzed Electrolysis
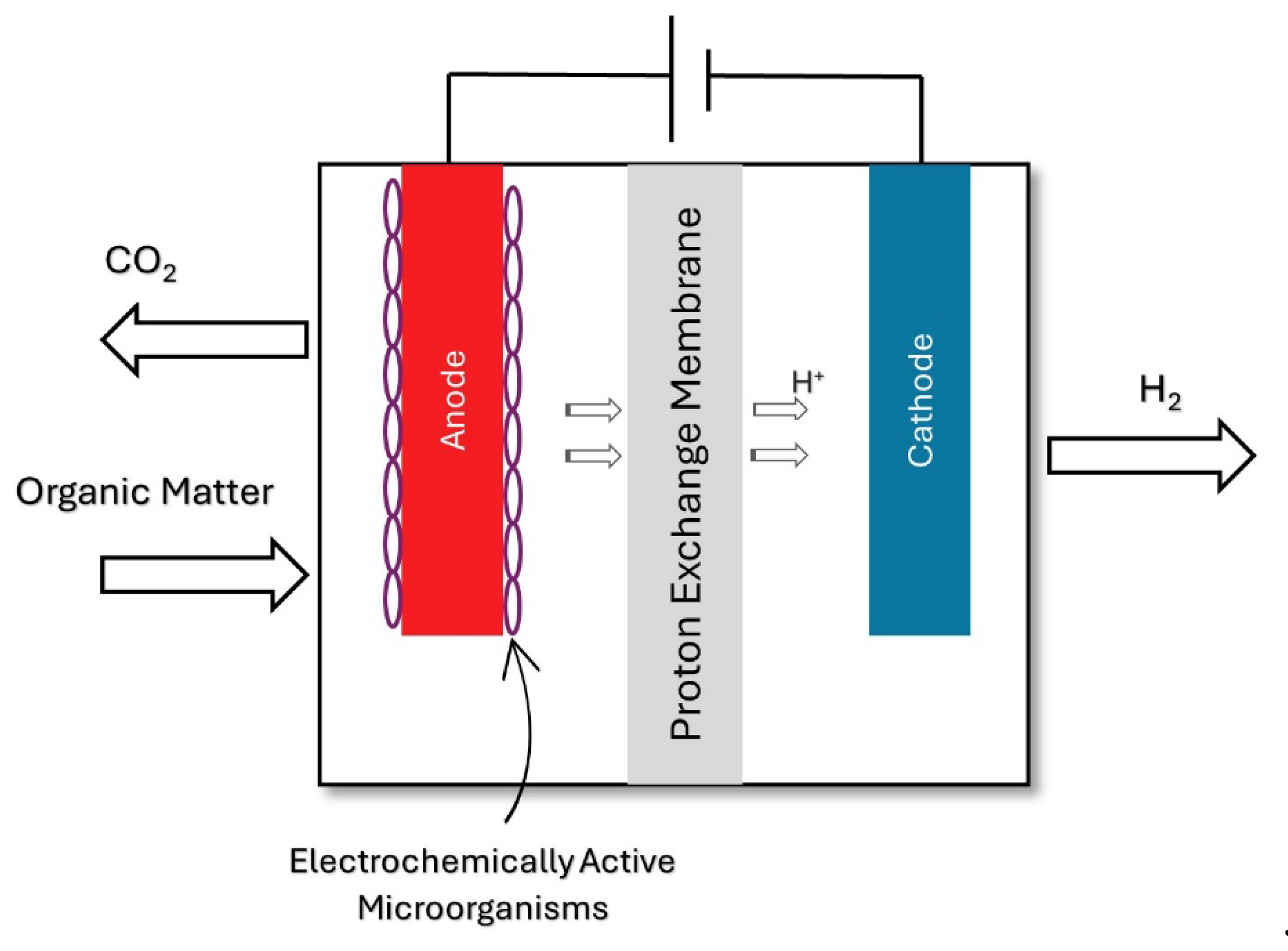
2.2. Electrochemical Treatment
2.2.1. Electrooxidation
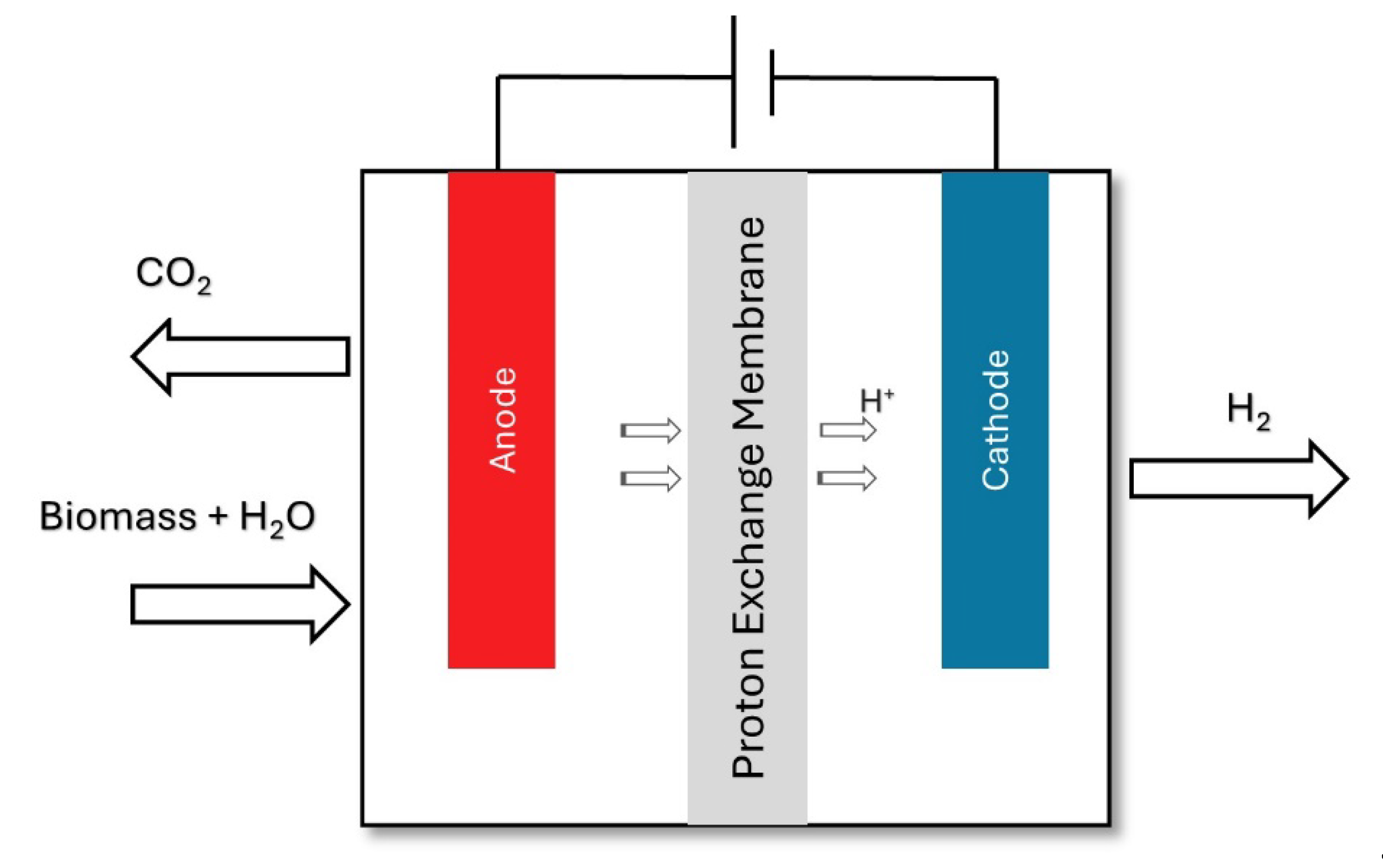
2.3. Thermochemical Treatment
2.3.1. Gasification
2.3.2. Pyrolysis
2.3.3. Biogas Reforming
| Composite | Percentage |
|---|---|
| CH4 | 55-70 (vol%) |
| CO2 | 30-45 (vol%) |
| H2S | 500-4000 (ppm) |
| NH 3 | 100-800 (ppm) |
| H2 | <1 (vol%) |
| N2 | <1 (vol%) |
| O2 | <1 (vol%) |
| H2O | <1 (vol%) |
| Composite | Landfill | Anaerobic digester |
|---|---|---|
| CH4 | 44.2 (mol%) | 58.1 (mol%) |
| CO2 | 34.0 (mol%) | 33.9 (mol%) |
| LHV | 12.7 (MJ/kg) | 17.8 (MJ/kg) |
3. Prospects of Hydrogen Production from Biomass in Scale
4. Conclusions
Acknowledgments
References
- Veluru Sridevi, Dadi Venkata Surya, Busigari Rajasekhar Reddy, Manan Shah, Ribhu Gautam, Tanneru Hemanth Kumar, Harish Puppala, Kocherlakota Satya Pritam, Tanmay Basak,Challenges and opportunities in the production of sustainable hydrogen from lignocellulosic biomass using microwave-assisted pyrolysis: A review,International Journal of Hydrogen Energy,Volume 52, Part A,2024,Pages 507-531,ISSN 0360-3199. (https://www.sciencedirect.com/science/article/pii/S0360319923030902). [CrossRef]
- Van Giao Nguyen, Thanh Xuan Nguyen-Thi, Phuoc Quy Phong Nguyen, Viet Dung Tran, Ümit Ağbulut, Lan Huong Nguyen, Dhinesh Balasubramanian, Wieslaw Tarelko, Suhaib A. Bandh, Nguyen Dang Khoa Pham, Recent advances in hydrogen production from biomass waste with a focus on pyrolysis and gasification, International Journal of Hydrogen Energy, Volume 54, 2024, Pages 127-160, ISSN 0360-3199. [CrossRef]
- K.M. Akkoli, N.R. Banapurmath, M.M. Shivashimpi, Manzoore Elahi M. Soudagar, Irfan Anjum Badruddin, Mashhour A. Alazwari, V.S. Yaliwal, M.A. Mujtaba, Naveed Akram, Marjan Goodarzi, Mohammad Reza Safaei, Harish Venu, Effect of injection parameters and producer gas derived from redgram stalk on the performance and emission characteristics of a diesel engine, Alexandria Engineering Journal, Volume 60, Issue 3, 2021, Pages 3133-3142, ISSN 1110-0168. [CrossRef]
- Nagarajan J, Balasubramanian D, Khalife E, Usman KM. Optimization of compression ignition engine fuelled with Cotton seed biodiesel using Diglyme and injection pressure. J Technol Innov 2022;2:52e61. [CrossRef]
- Shayan Sharafi laleh, Mohsen Zeinali, S.M.S. Mahmoudi, Saeed Soltani, Marc A. Rosen, Biomass co-fired combined cycle with hydrogen production via proton exchange membrane electrolysis and waste heat recovery: Thermodynamic assessment, International Journal of Hydrogen Energy, Volume 48, Issue 87, 2023, Pages 33795-33809, ISSN 0360-3199. [CrossRef]
- Liu W, Qi Y, Zhang R, Zhang Q, Wang Z. Hydrogen production from ammonia-rich combustion for fuel reforming under high temperature and high pressure conditions. Fuel 2022;327:124830. [CrossRef]
- Rosa L, Mazzotti M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew Sustain Energy Rev 2022;157:112123. [CrossRef]
- Nemmour A et al., Green hydrogen-based E-fuels (E-methane, E-methanol, E-ammonia) to support clean energy transition: A literature review, International Journal of Hydrogen Energy. [CrossRef]
- Johan M Ahlström, Renewable Hydrogen Production from Biomass https://www.etipbioenergy.eu/images/Renewable_Hydrogen_Production_from_Biomass.pdf.
- Lepage T, Kammoun M, Schmetz Q, Richel A. Biomass-to-hydrogen: a review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021;144:105920. [CrossRef]
- Engin GÜRTEKİN, Faculty of Engineering, Department of Environmental Engineering Firat University, Turkey, Biological Hydrogen Production Methods, https://i-sem.info/PastConferences/ISEM2014/ISEM2014/papers/A10-ISEM2014ID80.pdf.
- Pavlos Nikolaidis, Andreas Poullikkas, A comparative overview of hydrogen production processes, Renewable and Sustainable Energy Reviews, Volume 67, 2017, Pages 597-611, ISSN 1364-0321. [CrossRef]
- Richa Kothari, D. Buddhi, R.L. Sawhney,Comparison of environmental and economic aspects of various hydrogen production methods,Renewable and Sustainable Energy Reviews,Volume 12, Issue 2,2008,Pages 553-563,ISSN 1364-0321. (https://www.sciencedirect.com/science/article/pii/S1364032106001158). [CrossRef]
- Havva Balat, Elif Kırtay,Hydrogen from biomass – Present scenario and future prospects, International Journal of Hydrogen Energy,Volume 35, Issue 14,2010,Pages 7416-7426,ISSN 0360-3199. (https://www.sciencedirect.com/science/article/pii/S0360319910008396). [CrossRef]
- Ibrahim Dincer, Canan Acar, Review and evaluation of hydrogen production methods for better sustainability, International Journal of Hydrogen Energy, Volume 40, Issue 34, 2015, Pages 11094-11111, ISSN 0360-3199. (https://www.sciencedirect.com/science/article/pii/S0360319914034119). [CrossRef]
- Santanu Kumar Dash , Suprava Chakraborty and Devaraj Elangovan, A Brief Review of Hydrogen Production Methods and Their Challenges, Energies 2023, 16(3), 1141. [CrossRef]
- Demirbas, A. (2001) Biomass Resource Facilities and Biomass Conversion Processing for Fuels and Chemicals. Energy Conversion and Management, 42, 1357-1378. [CrossRef]
- Peter McKendry, Energy production from biomass (part 1): overview of biomass, Bioresource Technology, Volume 83, Issue 1, 2002, Pages 37-46, ISSN 0960-8524. (https://www.sciencedirect.com/science/article/pii/S0960852401001183). [CrossRef]
- Flamos a, Georgallis PG, Doukas H, Psarras J. Using biomass to achieve European Union energy targets—a review of biomass status, potential, and supporting policies. Int J Green Energy 2011;8(4):411–28. [CrossRef]
- Mostafa El-Shafie*, Shinji Kambara, Yukio Hayakawa, Environmental and Renewable Energy Systems Division, Gifu University, Gifu City, Japan Hydrogen Production Technologies Overview, https://www.scirp.org/pdf/JPEE_2019012515395175.pdf.
- Satyapal, S., Petrovic, J., Read, C., Thomas, G. and Ordaz, G. (2007) The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards Meeting Hydrogen-Powered Vehicle Requirements. Catalysis Today, 120, 246-256. [CrossRef]
- Kapdan IK, Kargi F. Bio-hydrogen production from waste materials, Enzym Microb Technol 2006;38(5):569–82. [CrossRef]
- Debabrata Das, T. Nejat Veziroglu, Advances in biological hydrogen production processes, International Journal of Hydrogen Energy, Volume 33, Issue 21, 2008, Pages 6046-6057, ISSN 0360-3199. [CrossRef]
- B. Parkinson, P. Balcombe, J. F. Speirs, A. D. Hawkes and K. Hellgardt, Levelized cost of CO2 mitigation from hydrogen production routes, Energy Environ. Sci., 2019, 12, 19-40. [CrossRef]
- Levin DB, Pitt L, Love M. Biohydrogen production: prospects and limitations to practical application. Int. J. Hydrogen Energy. 2004; 29: 173-85. [CrossRef]
- Zuttel A. Materials for hydrogen storage. Mater Today 2003;6(9):24–33. [CrossRef]
- Sakintuna B, Lamari-Darkrim F, Hirscher M. Metal hydride materials for solid hydrogen storage: a review. Int J Hydrog Energy 2007;32(9):1121–40. [CrossRef]
- Ni M, Leung DYC, Leung MKH, Sumathy K. An overview of hydrogen production from biomass. Fuel Process Technol 2006;87(5):461–72. [CrossRef]
- Holladay JD, Hu J, King DL, Wang Y. An overview of hydrogen production technologies. Catal Today 2009;139(4):244–60. [CrossRef]
- T. Lepage, M. Kammoun, Q. Schmetz, and A. Richel, “Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment,” Biomass and Bioenergy, vol. 144. Elsevier Ltd, p. 105920, Jan. 01, 2021. [CrossRef]
- Isabela C. Moia, Aikaterini Kanaropoulou, Demetrios F. Ghanotakis, Pietro Carlozzi, Eleftherios Touloupakis, Photofermentative hydrogen production by immobilized Rhodopseudomonas sp. S16-VOGS3 cells in photobioreactors, Energy Reviews, Volume 3, Issue 1, 2024, 100055, ISSN 2772-9702. [CrossRef]
- Federov AS, Tsygankov AA, Rao KK, Hall DO. Hydrogen photoproduction by Rhodobacter sphaeroides immobilised on polyurethane foam. Biotechnol. Lett. 2008; 20: 1007-09. [CrossRef]
- Tsygankov AA, Federov AS, Laurinavichene TV, Gogotov IN, Rao KK, Hall DO. Actual and potential rates of hydrogen photoproduction by continuous culture of the purple nonsulphur bacteria Rhodobacter capsulatus. Appl. Environ. Microbiol. 1998; 49: 102-07.
- Zurrer H, Bachofen R. Hydrogen production by the photosynthetic bacterium, Rhhodospirillum rubrum. Appl. Environ. Microbiol. 1979; 37: 789-93. [CrossRef]
- Fascetti, Eugenio and Oreste Todini. “Rhodobacter sphaeroides RV cultivation and hydrogen production in a one- and two-stage chemostat.” Applied Microbiology and Biotechnology 44 (1995): 300-305.Rozendal, R.A., 2007. Hydrogen production through biocatalyzed electrolysis. PhD thesis Wageningen University, Wageningen, the Netherlands – with references – with summary in Dutch. ISBN 978-90-8504-731-5. [CrossRef]
- R. Moreno, A. Escapa, J. Cara, B. Carracedo, X. Gómez, A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis, International Journal of Hydrogen Energy, Volume 40, Issue 1, 2015, Pages 168-175, ISSN 0360-3199. (https://www.sciencedirect.com/science/article/pii/S0360319914029966 ). [CrossRef]
- S. Cheng, B.E. Logan, Sustainable and efficient biohydrogen production via electrohydrogenesis Proc Natl Acad Sci U S A, 104 (2007), pp. 18871-18873. [CrossRef]
- V. Stenberg, M. Rydén, T. Mattisson, A. Lyngfelt , Exploring novel hydrogen production processes by integration of steam methane reforming with chemical-looping combustion (CLC-SMR) and oxygen carrier aided combustion (OCAC-SMR) Int. J. Greenh. Gas Control., 74 (2018), pp. 28-39. [CrossRef]
- G.W. Huber, S. Iborra, A. Corma, Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering Chem. Rev., 106 (2006), pp. 4044-4098, 10.1021/cr068360d. [CrossRef]
- Molino, S. Chianese, and D. Musmarra, “Biomass gasification technology: The state of the art overview,” J. Energy Chem., vol. 25, no. 1, pp. 10–25, Jan. 2016. [CrossRef]
- Xu, S. Chen, A. Soomro, Z. Sun, W. Xiang Hydrogen rich syngas production from biomass gasification using synthesized Fe/CaO active catalysts, J. Energy Inst., 91 (2018), pp. 805-816. [CrossRef]
- Srinivasakannan, N. Balasubramanian , Variations in the design of dual fluidized bed gasifiers and the quality of syngas from biomass, Energy Sources, Part A Recover Util. Environ. Eff., 33 (2011), pp. 349-359. [CrossRef]
- J. M. Ahlström, A. Alamia, A. Larsson, C. Breitholtz, S. Harvey, and H. Thunman, “Bark as feedstock for dual fluidized bed gasifiers—Operability, efficiency, and economics,” Int. J. Energy Res., vol. 43, no. 3, pp. 1171–1190, Mar. 2019. [CrossRef]
- H. De Lasa, E. Salaices, J. Mazumder, R. Lucky, Catalytic steam gasification of biomass: catalysts, thermodynamics and kinetics Chem. Rev., 111 (2011), pp. 5404-5433. [CrossRef]
- M. Cortazar a, L. Santamaria a, G. Lopez a b, J. Alvarez c, L. Zhang d, R. Wang d, X. Bi d, Olazar a, A comprehensive review of primary strategies for tar removal in biomass gasification. [CrossRef]
- Hannula and E. Kurkela, “A semi-empirical model for pressurised air-blown fluidised-bed gasification of biomass,” Bioresour. Technol., vol. 101, no. 12, pp. 4608–4615, Jun. 2010. [CrossRef]
- F. Weiland, H. Hedman, M. Marklund, H. Wiinikka, O. Öhrman, and R. Gebart, “Pressurized oxygen blown entrained-flow gasification of wood powder,” Energy and Fuels, vol. 27, no. 2, pp. 932–941, Feb. 2013. [CrossRef]
- D. Mohan, C. U. Pittman, and P. H. Steele, “Pyrolysis of wood/biomass for bio-oil: A critical review,” Energy and Fuels, vol. 20, no. 3. American Chemical Society , pp. 848–889, May 2006. [CrossRef]
- M. Ni, D.Y.C. Leung, M.K.H. Leung, K. Sumathy, An overview of hydrogen production from biomass, Fuel Process, Technol., 87 (2006). [CrossRef]
- Demirbaş a. Yields of hydrogen-rich gaseous products via pyrolysis from selected biomass samples. Fuel 2001;80(13):1885–91. [CrossRef]
- DUMAN, Gozde; UDDIN, Md Azhar; YANIK, Jale. Hydrogen production from algal biomass via steam gasification. Bioresource technology, 2014, 166: 24-30. [CrossRef]
- P. Bi, J. Wang, Y. Zhang, P. Jiang, X. Wu, J. Liu, et al. From lignin to cycloparaffins and aromatics: directional synthesis of jet and diesel fuel range biofuels using biomass Bioresour Technol, 183 (2015), pp. 10-17. [CrossRef]
- Yongnan Zhang a, Yunyi Liang a, Suiyi Li a, Yan Yuan b, Daihui Zhang c, Yingji Wu a, Huan Xie a, Kathirvel Brindhadevi d, Arivalagan Pugazhendhi e f, Changlei Xia a, A review of biomass pyrolysis gas: Forming mechanisms, influencing parameters, and product application upgrades. [CrossRef]
- P.S. Rezaei, H. Shafaghat, W.M.A.W. Daud, Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review, Appl Catal A, 469 (2014), pp. 490-511. [CrossRef]
- Yaovi Holade, Nazym Tuleushova, Sophie Tingry, Karine Servat, Teko Napporn, et al.. Recent advances in the electrooxidation of biomass-based organic molecules for energy, chemicals and hydrogen production. Catalysis Science & Technology, 2020, 10 (10), pp.3071-3112. ff10.1039/C9CY02446Hff. ffhal-02869726f, HAL Id: hal-02869726 https://hal.science/hal-02869726.
- Hui Luo, Jesús Barrio, Nixon Sunny, Alain Li, Ludmilla Steier, Nilay Shah, Ifan E. L. Stephens, Maria-Magdalena Titirici, Progress and Perspectives in Photo- and Electrochemical-Oxidation of Biomass for Sustainable Chemicals and Hydrogen Production. [CrossRef]
- Youngkook Kwon, Klaas Jan P. Schouten, Prof. Marc T. M. Koper, Mechanism of the Catalytic Oxidation of Glycerol on Polycrystalline Gold and Platinum Electrodes. [CrossRef]
- Valentina Bambagioni Dr., Manuela Bevilacqua Dr., Claudio Bianchini Dr., Jonathan Filippi Dr., Alessandro Lavacchi Dr., Andrea Marchionni Dr., Francesco Vizza Dr., Pei Kang Shen Prof., Self-Sustainable Production of Hydrogen, Chemicals, and Energy from Renewable Alcohols by Electrocatalysis. [CrossRef]
- Gomes, J.F., Tremiliosi-Filho, G. Spectroscopic Studies of the Glycerol Electro-Oxidation on Polycrystalline Au and Pt Surfaces in Acidic and Alkaline Media. Electrocatal 2, 96–105 (2011). [CrossRef]
- IRENA, Hydrogen: A Renewable Energy Perspective—Report Prepared for the 2nd Hydrogen Energy Ministerial Meeting in Tokyo, Japan, IRENA, Abu Dhabi 2019. Google Scholar, ISBN : 978-92-9260-151-5.
- M. A. Salam, K. Ahmed, N. Akter, T. Hossain, and B. Abdullah, “A review of hydrogen production via biomass gasification and its prospect in Bangladesh,” International Journal of Hydrogen Energy, vol. 43, no. 32. Elsevier Ltd, pp. 14944–14973, Aug. 09, 2018. [CrossRef]
- M. Wang, G. Wang, Z. Sun, Y. Zhang, and D. Xu, “Review of renewable energy-based hydrogen production processes for sustainable energy innovation,” Glob. Energy Interconnect., vol. 2, no. 5, pp. 436–443, Oct. 2019. [CrossRef]
- M. Y. Azwar, M. A. Hussain, and A. K. Abdul-Wahab, “Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review,” Renewable and Sustainable Energy Reviews, vol. 31. Pergamon, pp. 158–173, Mar. 01, 2014. [CrossRef]
- van Wyk, J. P. H. Biotechnology and the utilization of biowaste as a resource for bioproduct development. Trends Biotechnol. 2001, 19, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L. T.; Karim, K.; Al-Dahhan, M. H.; Wrenn, B. A.; Domiguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- P. Parthasarathy, K.S. Narayanan, Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield - a review, Renew. Energy, 66 (2014), pp. 570-579. [CrossRef]
- J. Yao, M. Kraussler, F. Benedikt, H. Hofbauer, Techno-economic assessment of hydrogen production based on dual fluidized bed biomass steam gasification, biogas steam reforming, and alkaline water electrolysis processes, Energy Convers. Manag., 145 (2017), pp. 278-292. [CrossRef]
- M. Kumar, A.O. Oyedun, A. Kumar, A comparative analysis of hydrogen production from the thermochemical conversion of algal biomass, Int. J. Hydrogen Energy, 44 (2019), pp. 10384-10397. [CrossRef]
- V. Martinez-Merino, M.J. Gil, A. Cornejo, Biomass sources for hydrogen production, Renew. Hydrog. Technol. Prod., Purif., Storage, Appl. Saf., Elsevier B.V. (2013), pp. 87-110. [CrossRef]
- Y. Zhang, T.R. Brown, G. Hu, R.C. Brown, Techno-economic analysis of monosaccharide production via fast pyrolysis of lignocellulose, Bioresour. Technol., 127 (2013), pp. 358-365. [CrossRef]
- Ghimire, L. Frunzo, F. Pirozzi, E. Trably, R. Escudie, P.N.L. Lens, G. Esposito, A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products, Appl. Energy, 144 (2015), pp. 73-95. [CrossRef]
- D.C. Aiken, T.P. Curtis, E.S. Heidrich, Avenues to the financial viability of microbial electrolysis cells [MEC] for domestic wastewater treatment and hydrogen production, Int. J. Hydrogen Energy, 44 (2019), pp. 2426-2434. [CrossRef]
- M. Kammoun, H. Ayeb, T. Bettaieb, A. Richel, Chemical characterisation and technical assessment of agri-food residues, marine matrices, and wild grasses in the South Mediterranean area: a considerable inflow for biorefineries, Waste Manag., 118 (2020), pp. 247-257. [CrossRef]
- L. Caizán-Juanarena, I. Servin-Balderas, X. Chen, C.J. Buisman, A. Heijne, Electrochemical and microbiological characterization of single carbon granules in a multi-anode microbial fuel cell, J. Power Sources, 435 (2019), p. 126514. [CrossRef]
- P. Kalyani, A. Anitha, Biomass carbon & its prospects in electrochemical energy systems, International Journal of Hydrogen Energy, Volume 38, Issue 10, 2013, Pages 4034-4045, ISSN 0360-3199. (https://www.sciencedirect.com/science/article/pii/S036031991300133X). [CrossRef]
- Leichang Cao, Iris K.M. Yu, Xinni Xiong, Daniel C.W. Tsang, Shicheng Zhang, James H. Clark, Changwei Hu, Yun Hau Ng, Jin Shang, Yong Sik Ok, Biorenewable hydrogen production through biomass gasification: A review and future prospects, Environmental Research, Volume 186, 2020, 109547, ISSN 0013-9351. (https://www.sciencedirect.com/science/article/pii/S0013935120304400). [CrossRef]
- Wei Liu, Yong Cui, Xu Du, Zhe Zhang, Zisheng Chaob, Yulin Deng, High efficiency hydrogen evolution from native biomass electrolysis, Energy Environ. Sci., 2016, 9, 467. [CrossRef]
- Liu, Hong, Stephen Grot, and Bruce E. Logan. "Electrochemically assisted microbial production of hydrogen from acetate." Environmental science & technology 39.11 (2005): 4317-4320. [CrossRef]
- Ditzig, Jenna, Hong Liu, and Bruce E. Logan. "Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR)." International Journal of Hydrogen Energy 32.13 (2007): 2296-2304. [CrossRef]
- Rozendal, René A., et al. "Principle and perspectives of hydrogen production through biocatalyzed electrolysis." International journal of hydrogen energy 31.12 (2006): 1632-1640. [CrossRef]
- Lalaurette, Elodie, et al. "Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis." international journal of hydrogen energy 34.15 (2009): 6201-6210. [CrossRef]
- Cheng, Shaoan, and Bruce E. Logan. "Sustainable and efficient biohydrogen production via electrohydrogenesis." Proceedings of the National Academy of Sciences 104.47 (2007): 18871-18873. [CrossRef]
- Lu, Lu, et al. "Hydrogen production from proteins via electrohydrogenesis in microbial electrolysis cells." Biosensors and Bioelectronics 25.12 (2010): 2690-2695. [CrossRef]
- Abudukeremu Kadier, Mohd Sahaid Kalil, Peyman Abdeshahian, K. Chandrasekhar, Azah Mohamed, Nadia Farhana Azman, Washington Logroño, Yibadatihan Simayi, Aidil Abdul Hamid, Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals, Renewable and Sustainable Energy Reviews, Volume 61, 2016, Pages 501-525, ISSN 1364-0321. (https://www.sciencedirect.com/science/article/pii/S136403211630034X). [CrossRef]
- Cheng, Shaoan, and Bruce E. Logan. "High hydrogen production rate of microbial electrolysis cell (MEC) with reduced electrode spacing." Bioresource technology 102.3 (2011): 3571-3574. [CrossRef]
- a Flamos, P.G. Georgallis, H. Doukas, J. Psarras, Using biomass to achieve European Union energy targets—a review of biomass status, potential, and supporting policies, " International Journal of Green Energy 8.4 (2011): 411-428. [CrossRef]
- Ravindra Kumar, Anil Kumar, Amit Pal, Overview of hydrogen production from biogas reforming: Technological advancement, International Journal of Hydrogen Energy, Volume 47, Issue 82, 2022, Pages 34831-34855, ISSN 0360-3199. [CrossRef]
- Helton José Alves, Cícero Bley Junior, Rafael Rick Niklevicz, Elisandro Pires Frigo, Michelle Sato Frigo, Carlos Henrique Coimbra-Araújo, Overview of hydrogen production technologies from biogas and the applications in fuel cells, International Journal of Hydrogen Energy, Volume 38, Issue 13, 2013, Pages 5215-5225, ISSN 0360-3199. [CrossRef]
- C.S. Lau, A. Tsolakis, M.L. Wyszynski, Biogas upgrade to syn-gas (H2–CO) via dry and oxidative reforming, International Journal of Hydrogen Energy, Volume 36, Issue 1, 2011, Pages 397-404, ISSN 0360-3199. [CrossRef]
- Effendi, K. Hellgardt, Z.-G. Zhang, T. Yoshida, Optimising H2 production from model biogas via combined steam reforming and CO shift reactions, Fuel, Volume 84, Issues 7–8, 2005, Pages 869-874, ISSN 0016-2361. [CrossRef]
- Philipp Kolbitsch, Christoph Pfeifer, Hermann Hofbauer, Catalytic steam reforming of model biogas, Fuel, Volume 87, Issue 6, 2008, Pages 701-706, ISSN 0016-2361. [CrossRef]
- Gioele Di Marcoberardino, Stefano Foresti, Marco Binotti, Giampaolo Manzolini, Potentiality of a biogas membrane reformer for decentralized hydrogen production, Chemical Engineering and Processing - Process Intensification, Volume 129, 2018, Pages 131-141, ISSN 0255-2701. [CrossRef]
- Helton José Alves, Cícero Bley Junior, Rafael Rick Niklevicz, Elisandro Pires Frigo, Michelle Sato Frigo, Carlos Henrique Coimbra-Araújo, Overview of hydrogen production technologies from biogas and the applications in fuel cells, International Journal of Hydrogen Energy, Volume 38, Issue 13, 2013, Pages 5215-5225, ISSN 0360-3199. [CrossRef]
- J.D. Holladay, J. Hu, D.L. King, Y. Wang, An overview of hydrogen production technologies, Catalysis Today, Volume 139, Issue 4, 2009, Pages 244-260, ISSN 0920-5861. [CrossRef]
- Kristina Göransson, Ulf Söderlind, Jie He, Wennan Zhang, Review of syngas production via biomass DFBGs, Renewable and Sustainable Energy Reviews, Volume 15, Issue 1, 2011, Pages 482-492, ISSN 1364-0321. [CrossRef]
- P. Ugarte, P. Durán, J. Lasobras, J. Soler, M. Menéndez, J. Herguido, Dry reforming of biogas in fluidized bed: Process intensification, International Journal of Hydrogen Energy, Volume 42, Issue 19, 2017, Pages 13589-13597, ISSN 0360-3199. [CrossRef]
- S. N. Paglieri steve.paglieri@lanl.gov & J. D. Way (2002) INNOVATIONS IN PALLADIUM MEMBRANE RESEARCH, Separation and Purification Methods, 31:1, 1-169. [CrossRef]
- Ekain Fernandez, Arash Helmi, Kai Coenen, Jon Melendez, Jose Luis Viviente, David Alfredo Pacheco Tanaka, Martin van Sint Annaland, Fausto Gallucci, Development of thin Pd–Ag supported membranes for fluidized bed membrane reactors including WGS related gases, International Journal of Hydrogen Energy, Volume 40, Issue 8, 2015, Pages 3506-3519, ISSN 0360-3199. [CrossRef]
- Mariagiovanna Minutillo, Alessandra Perna, Alessandro Sorce, Green hydrogen production plants via biogas steam and autothermal reforming processes: energy and exergy analyses, Applied Energy, Volume 277, 2020, 115452, ISSN 0306-2619. [CrossRef]
- YIN, Hang; YIP, Alex CK. A review on the production and purification of biomass-derived hydrogen using emerging membrane technologies. Catalysts, 2017, 7.10: 297. [CrossRef]
- Galvagno A, Chiodo V, Urbani F, Freni F (2013) Biogas as hydrogen source for fuel cell applications. Int J Hydrogen Energy 38:3913–3920. [CrossRef]
- Montenegro Camacho, Y.S., Bensaid, S., Piras, G. et al. Techno-economic analysis of green hydrogen production from biogas autothermal reforming. Clean Techn Environ Policy 19, 1437–1447 (2017). [CrossRef]
- Noureddine Hajjaji, Sylvain Martinez, Eric Trably, Jean-Philippe Steyer, Arnaud Helias, Life cycle assessment of hydrogen production from biogas reforming, International Journal of Hydrogen Energy, Volume 41, Issue 14, 2016, Pages 6064-6075, ISSN 0360-3199. [CrossRef]
- Marco Buffi, Matteo Prussi, Nicolae Scarlat, Energy and environmental assessment of hydrogen from biomass sources: Challenges and perspectives, Biomass and Bioenergy, Volume 165, 2022, 106556, ISSN 0961-9534. [CrossRef]
- Awais Ahmad, Safia Khan, Tripti Chhabra, Sadaf Tariq, Muhammad Sufyan Javed, Hu Li, Salman Raza Naqvi, Saravanan Rajendran, Rafael Luque, Ikram Ahmad, Synergic impact of renewable resources and advanced technologies for green hydrogen production: Trends and perspectives, International Journal of Hydrogen Energy, 2024, ISSN 0360-3199. [CrossRef]
- Bharati Panigrahy, K. Narayan, B. Ramachandra Rao, Green hydrogen production by water electrolysis: A renewable energy perspective, Materials Today: Proceedings, Volume 67, Part 8, 2022, Pages 1310-1314, ISSN 2214-7853. [CrossRef]
- Boreum Lee, Juheon Heo, Sehwa Kim, Choonghyun Sung, Changhwan Moon, Sangbong Moon, Hankwon Lim, Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations, Energy Conversion and Management, Volume 162, 2018, Pages 139-144, ISSN 0196-8904. [CrossRef]
- S. Shiva Kumar, Hankwon Lim, An overview of water electrolysis technologies for green hydrogen production, Energy Reports, Volume 8, 2022, Pages 13793-13813, ISSN 2352-4847. [CrossRef]
- Syed Altan Haider, Muhammad Sajid, Saeed Iqbal, Forecasting hydrogen production potential in islamabad from solar energy using water electrolysis, International Journal of Hydrogen Energy, Volume 46, Issue 2, 2021, Pages 1671-1681. [CrossRef]
- N.S. Hassan, A.A. Jalil, S. Rajendran, N.F. Khusnun, M.B. Bahari, A. Johari, M.J. Kamaruddin, M. Ismail, Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society, International Journal of Hydrogen Energy, Volume 52, Part B, 2024, Pages 420-441, ISSN 0360-3199. [CrossRef]
- M. Rumayor, J. Corredor, M.J. Rivero, I. Ortiz, Prospective life cycle assessment of hydrogen production by waste photoreforming, Journal of Cleaner Production, Volume 336, 2022, 130430, ISSN 0959-6526. [CrossRef]
- M.G. Rasul, M.A Hazrat, M.A. Sattar, M.I. Jahirul, M.J. Shearer, The future of hydrogen: Challenges on production, storage and applications, Energy Conversion and Management, Volume 272, 2022, 116326, ISSN 0196-8904. [CrossRef]
- Cristina Antonini, Karin Treyer, Anne Streb, Mijndert van der Spek, Christian Bauer and Marco Mazzotti, Hydrogen production from natural gas and biomethane with carbon capture and storage – A techno-environmental analysis, Sustainable Energy Fuels, 2020, 4, 2967-2986. [CrossRef]
- N. Scarlat, J. F. Dallemand and F. Fahl, Biogas: Developments and perspectives in Europe, Renewable Energy, 2018, 129, 457–472. [CrossRef]
- Ana Susmozas, Diego Iribarren, Petra Zapp, Jochen Linβen, Javier Dufour, Life-cycle performance of hydrogen production via indirect biomass gasification with CO2 capture, International Journal of Hydrogen Energy, Volume 41, Issue 42, 2016, Pages 19484-19491, ISSN 0360-3199. [CrossRef]
- Lorenzo Rosa, Marco Mazzotti, Potential for hydrogen production from sustainable biomass with carbon capture and storage, Renewable and Sustainable Energy Reviews, Volume 157, 2022, 112123, ISSN 1364-0321. [CrossRef]
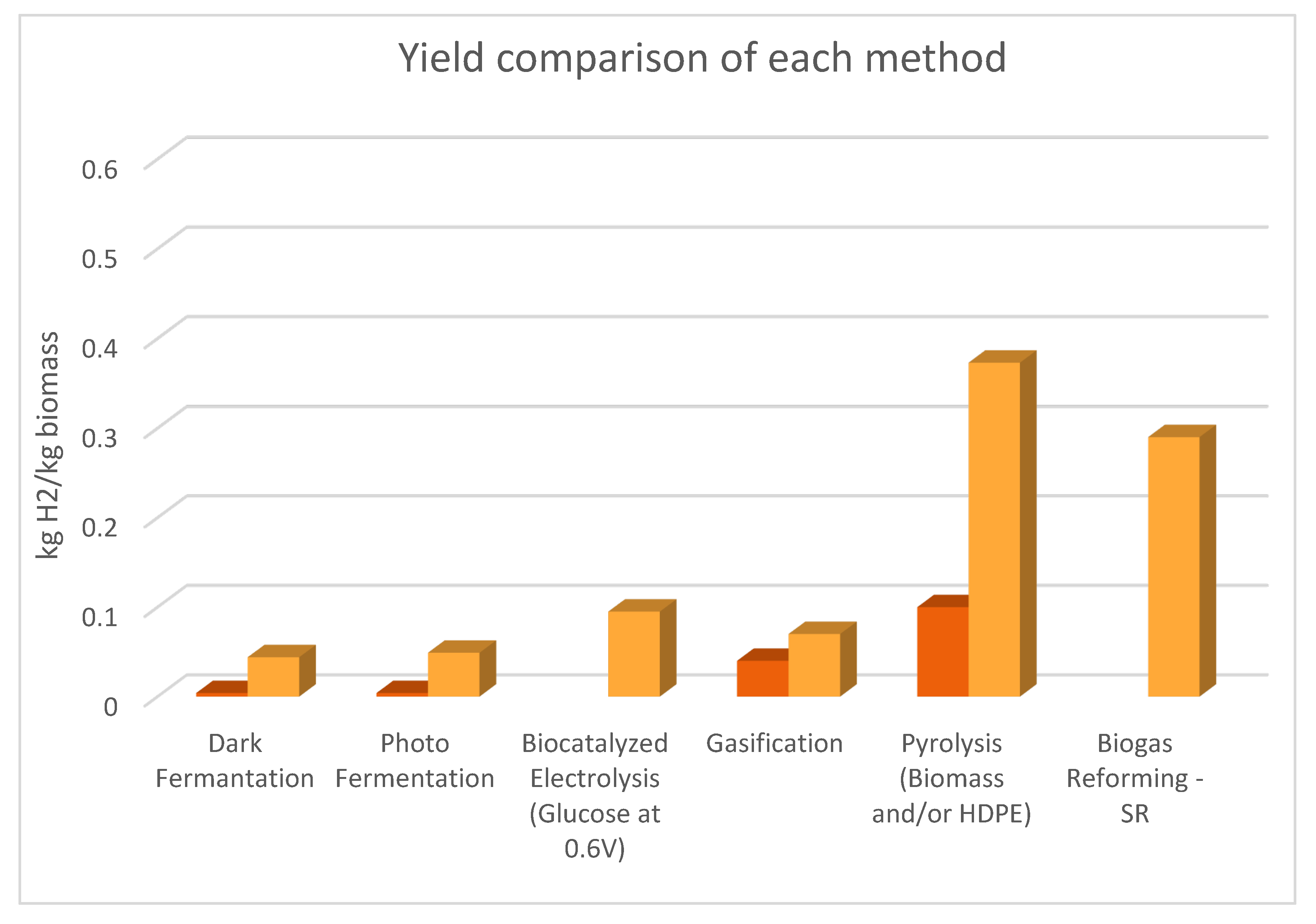
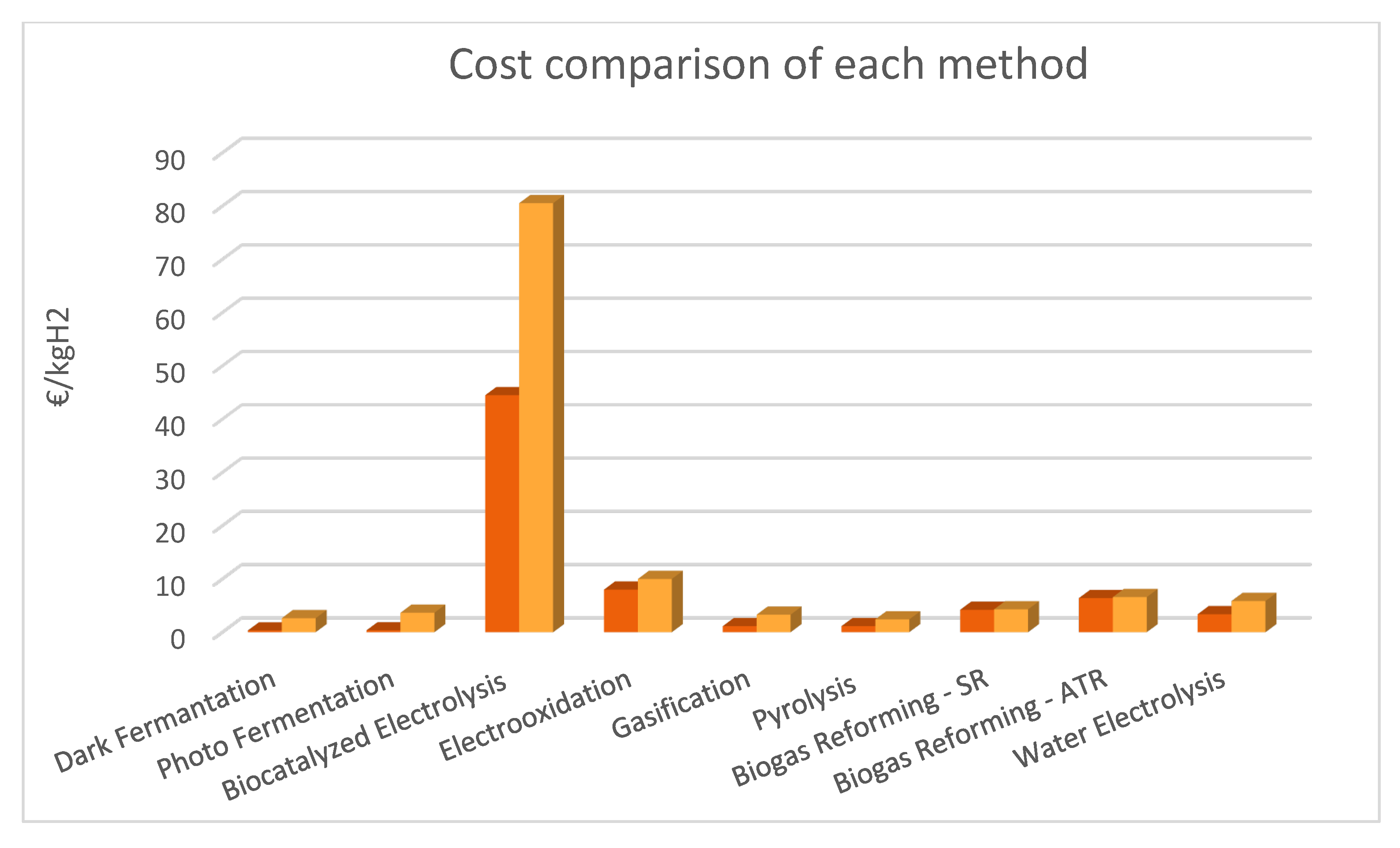
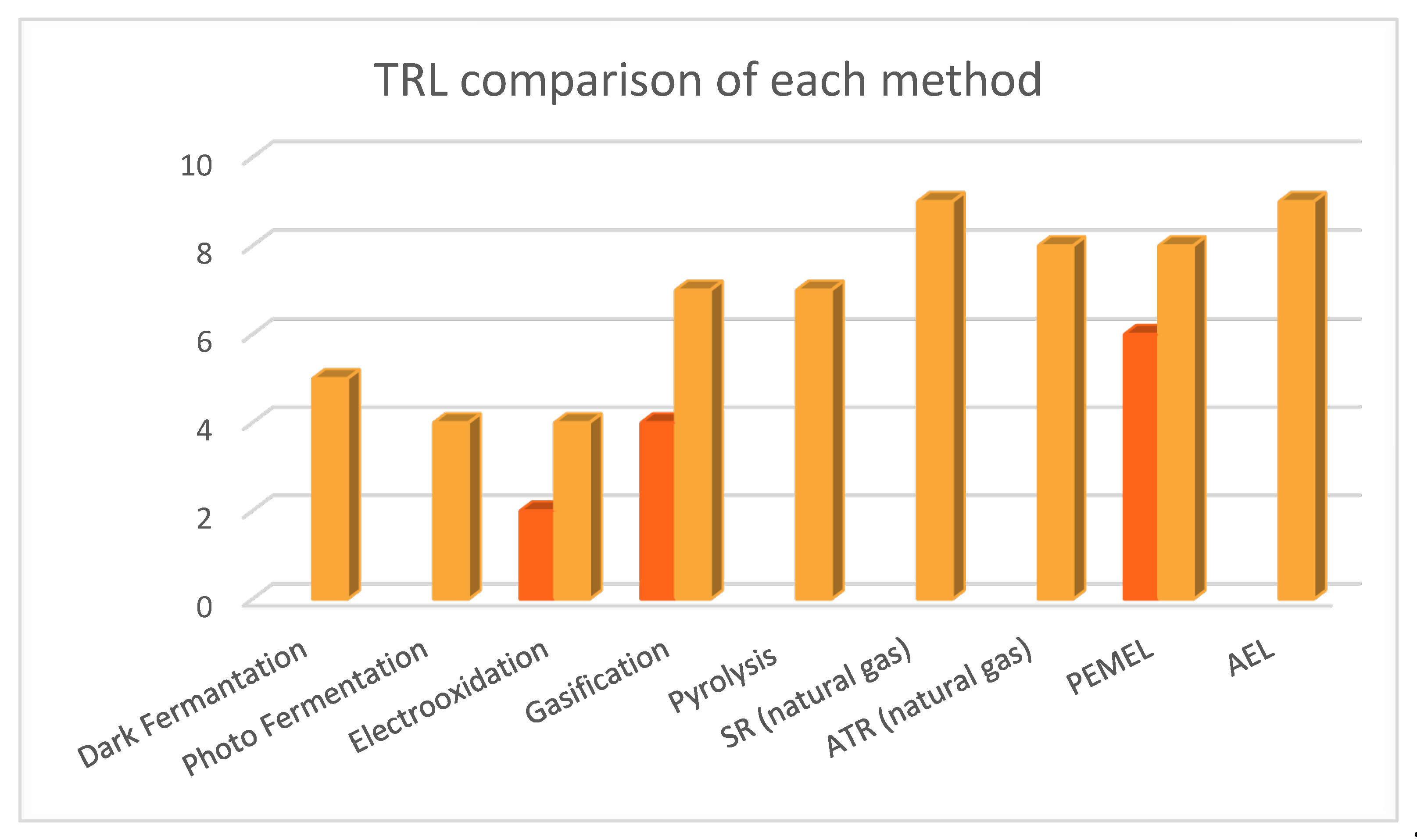
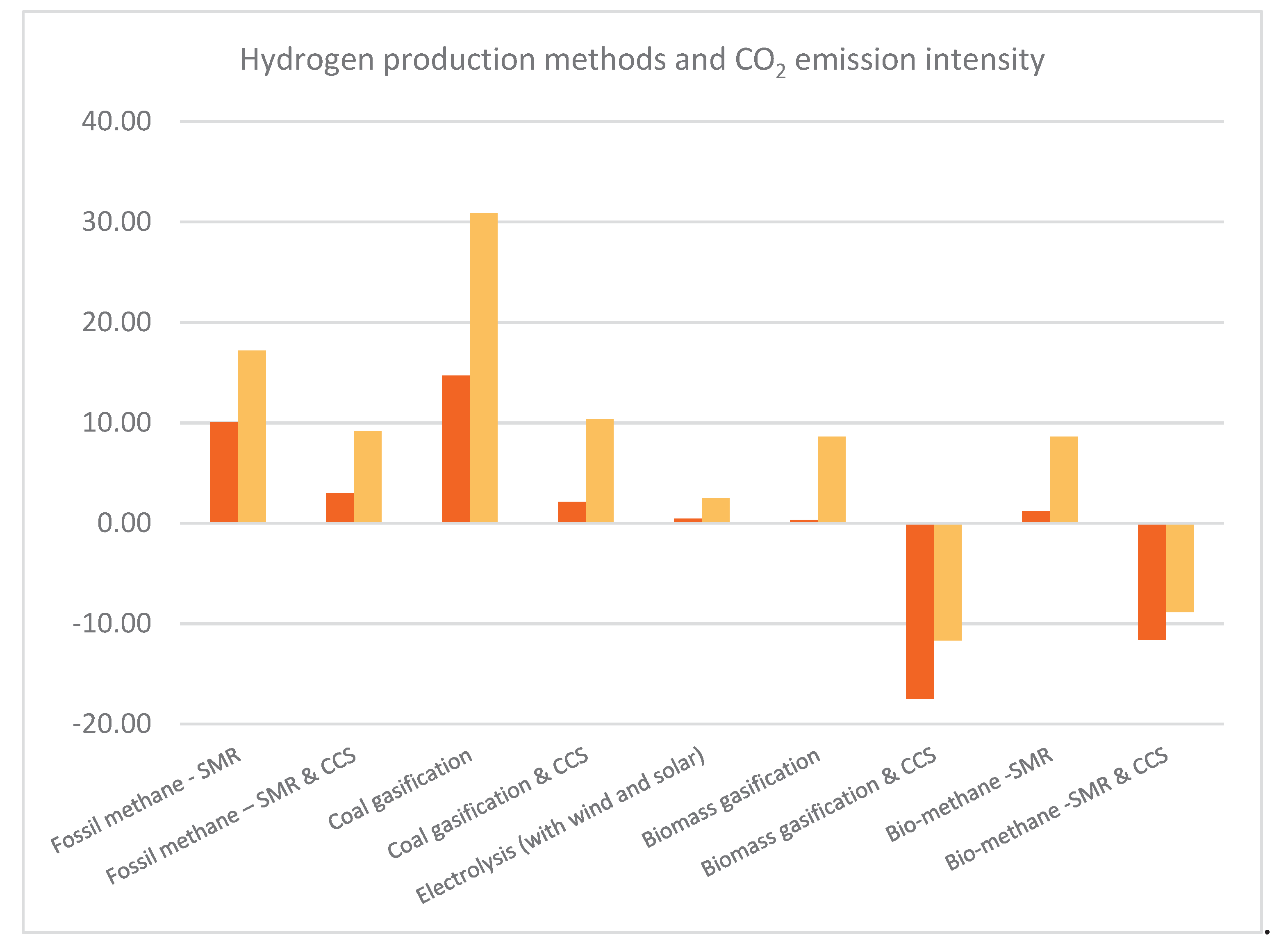
| Process | Efficiency% | Yield (kg H2/kg biomass) | Production Cost (€/ kgH2) | TRL |
|---|---|---|---|---|
| Dark fermentation | 60-80 [12] |
0.004 - 0.044 [10] | 0.332 [9] 2.42 – 2.63 [10,66] |
5 [9,10,66] |
| Photo fermentation | Light conversion efficiency 1–5 [11] | 0.004 - 0.049 [9] | 0.362 [9] 2.50 – 3.66 [10,66,69] |
4 [9,10,66] |
| Biocatalyzed electrolysis | 0.095 (kg H2/ kg glucose at 0.6 V) [84,85] |
The cathode: 44.50, ~80.55 (based on laboratory materials, not recent) [35]. |
||
| Electrooxidation | 8 - 10 [55] | 2–4 [10] |
||
| Gasification | 50 | (SG) Without catalyst: 0.040 With catalyst: 0.070 [76] | 1.14 – 3.29 [10,66,67,68] | 4-7 [10,66,67,68,112] |
| Pyrolysis | 65 using HDPE [9] | 0.100 (kg H2/kg biomass & HDPE) 0.373 (kg H2/kg HDPE) [9] |
1.14 – 2.42 [10,66,69] | 7 [10,66] |
| Biogas Reforming | 46.2-51.7 (SR) 24.5-27.8 (ATR) [92] |
0.29 (SR, kg H2/kg bio-methane) [113,116] | 4.21-4.29 (SR) 6.41-6.6 (ATR) [92] |
9 (SMR) 8 (ATR), for natural gas [105] |
| Water Electrolysis | 51-60 (AEL)46-60 (PEMEL)76-81 (SOEL) [106] |
3.38-5.45 [108] 5.87 (PEM) (including capital cost and maintenance) [107] |
9 (AEL), 6-8 (PEM) [110] |
| Process | TRL | Emission (kg CO2/kg H2) |
Source |
|---|---|---|---|
| Fossil methane - SMR | 9 | 10.09 - 17.21 | [24] |
| Fossil methane – SMR & CCS | 7-8 | 2.97 - 9.16 | [24] |
| Coal gasification | 9 | 14.72 – 30.90 | [24] |
| Coal gasification & CCS | 6-7 | 2.11 – 10.35 | [24] |
| Electrolysis (with wind and solar) | 9 | 0.47 - 2.5 | [24] |
| Biomass gasification | 5-6 | 0.31 - 8.63 | [24] |
| Biomass gasification & CCS | 3-5 | (-)17.50 - (-) 11.66 | [24] |
| Bio-methane -SMR | 9 | 1.20 - 8.60 | [113] |
| Bio-methane -SMR & CCS | 7-8 | (-)11.60 – (-)8.84 | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).