Implications
The brown pelican (Pelecanus occidentalis) species is frequently impacted by human activities resulting in their need for medical care and rehabilitation. However, species variation in clinical pathology analytes hinders veterinarian and biologists’ ability to judge health in this species. We established blood chemistry and venous blood gas reference intervals for this species using point-of-care analyzers and fibrinogen reference intervals via heat precipitation methodology. The Abaxis VSPro Equine fibrinogen cartridge appears inappropriate for assessing Fibrinogen concentrations of brown pelicans. Our findings should serve as a resource for veterinarians and rehabilitators for care of this species.
Introduction
Brown pelicans (Pelecanus occidentalis) BRPE are a large, protected, piscivorous waterbird species with distribution limited to coastal areas of the Americas; that can be found along the Texas coast year round. This species was listed as federally endangered in the US based on DDT in 1970, and in Texas this species remains listed as endangered with extinction, despite federal delisting based on population recovery nationally in 2009. Along the Texas coast, this large gregarious species is often affected by human fishing, and energy based activities (petroleum) as well as a variety of zoonotic infectious diseases. Thus BRPE are often treated by veterinarians and rehabilitators, and are well suited to investigation of clinical pathology as a key to species health to include inflammatory indicators such as fibrinogen [Georgieva, 2010; Hawkey, 1988; Petzinger, 2013]. However, species specific reference ranges for brown pelicans (Pelicanus occidentalis) are few, dated, and limited in scope based on sample size or age [Zais, 2000; Ferguson, 2014; Jodice, 2022; Wolf, 1985]. In December 2014 and January 2015 a large number of juvenile brown pelicans (Pelicanus occidentalis) became stranded on the southeast coast of Texas in the Houston and Galveston area. The pelicans were brought to the Wildlife Center of Texas for medical care, rehabilitation and release. This relatively large population of rehabilitated, apparently healthy wild-caught birds being housed in a rehabilitation setting provided the perfect population for creating reference intervals that are most appropriate for use in rehabilitation settings.
Reference intervals are essential for veterinarians to determine whether abnormalities are present in bloodwork, and what is significant. Jodice [2022] established “baseline intervals” for brown pelicans, but the populations that were evaluated were trapped at nesting sites, therefore health and recent exposures was unknown. Wolf et al. [1985] evaluated the difference between captive and wild brown pelican bloodwork, but the sample populations were small. One goal of this study was to produce updated reference intervals for venous analytes using a large population of presumably healthy, adult brown pelicans. We chose to use patient-side venous blood analyzers, the Abaxis VetScan Avian/Reptile chemistry cartridge, and the iSTAT Chem8 and CG4+ cartridges to measure chemistry, electrolyte and blood gas values. These analyzers are used commonly in clinical practice and in rehabilitation efforts as these birds are commonly involved in natural and man-made disasters such as hurricanes, and oil spills, as well as zoo collections [Selman, 2012; Raynor, 2013; Kinney, 2018]. The values obtained by these panels may help the clinician to detect ailments such as renal disease, hepatic disease, fluid imbalances, and capture myopathy, among others. Another significant benefit of these testing modalities is that they deliver results quickly and may be used daily in the clinic [Rettenmund, 2014].
There are many confounding factors where reference intervals in wild or exotic species are concerned. In an ideal situation, every individual laboratory would create reference intervals using a large, healthy population of each species for each analyzer and test that is offered for that species [Rettenmund, 2014; Jodice, 2022]. Because this is not logistically reasonable for individual clinics to create, laboratory equipment comes with pre-programmed reference intervals established by the manufacturer for common species. Species specific reference values are recommended based on significant differences between species may obscure differences even within the same bird subclass such as Hispaniolan Amazon parrots and Umbrella Cockatoos [Morrisey, 2003]. In the species of concern here, the brown pelican, Wolf [1985] determined that even between wild caught vs. captive, and breeding females vs. non-breeding adults there are significant differences in normal values. In addition, the large sample size required for scientifically valid reference intervals is not often a reasonable expectation for many species of wildlife, as an adequately sized and healthy population of animals is not typically available [Geffre, 2009]. These factors should be known to wildlife and exotic animal veterinarians as reference intervals will often be either very narrow or very large due to small sample size [Kinney, 2018].
Like most avian species, brown pelicans can hide signs of disease until death is imminent. Avian clinicians are faced with cases of rapid decompensation and death on a regular basis with subtle, if any clinical signs. Apparently healthy birds can have severe inflammation as evidenced by CBC. Changes in hematologic parameters, such as heterophilia, often occur before any overt signs of disease, or merely related to stress [Cray, 1988; Kinney, 2018]. Thus, laboratory diagnostics for detection and diagnosis of disease in avian species are an essential part of avian medicine. However, CBC determination in birds remains a laborious, non-automated process which requires training and expertise, is not well standardized, and is often done at send-off laboratories, so there is a delay between sampling and results. The cost and time necessary for this test limits its usefulness in private practice. However, more rapid, automated, patient-side assays for fibrinogen and various venous analytes are available for use in the clinic which could prove invaluable for the diagnosis of acute inflammation or disease in birds at the time of presentation for care. In addition, fibrinogen analysis may be used to track disease progression and treatment success. If proven valid and useful, these more rapid testing methodologies will provide lifesaving indicators of serious inflammation in birds, hours to days earlier than that of CBC, and do so with less avian clinical proficiency or cost to the private practitioner.
Fibrinogen has a number of roles within the body. One role is as the endpoint of the coagulation cascade. Fibrinogen is cleaved by clotting factor X, thrombin, into fibrin to create a fibrin clot that helps to seal vessels, but must be removed for tissue healing to occur. Fibrinogen is not only involved with coagulation in reaction to tissue damage and inflammation, it is a pro-inflammatory mediator that stimulates chemotaxis, activation of inflammatory cells, and release of inflammatory cytokines. In mouse models, the severity of multiple inflammatory disease is decreased compared to mice with normal fibrinogen. These diseases include atherosclerosis, traumatic brain injury, Alzheimer’s, colitis associated cancer, and inflammatory bowel disease among others. In addition, fibrinogen helps to sequester bacterial infections within the body, although the fibrinogen “wall” can also be used as a ladder for bacteria to move throughout the body [Zais, 2000].
Acute phase proteins rapidly increase or decrease in concentration in the bloodstream during early inflammation. Detection of abnormal concentrations of these proteins may allow clinicians to detect illness earlier in the progression of disease than they otherwise would. Fibrinogen is a positive acute phase protein that increases drastically within the first 24-48 hours of systemic or localized inflammation or infection and has a half-life of 3-4 days in both mammals and birds. Routine measurement of fibrinogen during diagnostic testing of birds may alert the clinician to occult inflammation [Goodwin, 1982]. Increased concentrations of fibrinogen associated with numerous disease states have been documented in avian and mammalian species [Harr, 2002; Drew, 1993; Polo, 1998].
Fibrinogen has been measured in a variety of avian species including chickens, herring gulls, Caspian terns, penguins, ibis, flamingos, hawks, falcons, cranes, Amazon parrots, macaws, grey parrots and owls [Pindyck, 1977]. Only in chickens has the structure and function of fibrinogen been assessed. The chicken fibrinogen has been shown to be similar to mammalian fibrinogen, although smaller. Therefore, although many tests of fibrinogen were designed for use in humans, they may be valid to use in order to determine fibrinogen concentrations in birds. While assessment of fibrinogen concentrations may be a useful indicator of stress, subclinical, or hidden disease in brown pelicans (Pelicanus occidentalis), single test results alone are of little value without a reference interval. Reference intervals may vary based on a number of factors including assay methodology, species, and laboratory [Geffre, 2009; Hawkey, 1985; Georgieva, 2010]. Reference values for fibrinogen concentrations are not established for brown pelicans (Pelicanus occidentalis), and other reported values for fibrinogen in avian species fail to meet current standards for development of appropriate reference intervals for this analyte. Finally, the original, gold standard testing methodology, which advocated the use of fibrinogen for diagnosis of occult inflammation in birds, has become obsolete and is no longer available in most commercial laboratories.
The gold standard for fibrinogen testing in humans is the ‘clot-recovery method’, which directly quantifies fibrin generated during clotting. This test has not been assessed for use in birds and has become largely obsolete in modern laboratories based on its labor intensive method. It is routinely used at the Texas A&M University Small Animal hospital clinical pathology laboratory for avian patients. We chose not to pursue validation of PT derived methods or glutaraldehyde testing based on a number of challenges inherent to these assays and brown pelicans such as the need for species specific reagents, the lack of fibrinogen specificity or direct quantification of fibrinogen by these assays, necessary modifications based on sample size, the time and clinical technique necessary for results, and the lack of standard curves correlating to fibrinogen concentration in avian species [Metzner, 2007, Morrisey, 2003]. Instead we chose to assess quantitative tests of fibrinogen which could be directly compared, were well validated in other species, required a reasonable sample size and a readily available avian appropriate collection tube.
Our second goal in this study is to establish reference intervals for fibrinogen in Brown pelicans using the gold standard method, and a patient-side analyzer. Our third goal is to determine whether the Abaxis VSPro equine fibrinogen cartridge may be used for fibrinogen determination in avian species. We used the heat precipitation method which is the gold standard in human medicine, and gives a direct measurement of fibrinogen content. It measures the amount of fibrinogen that precipitates after incubation. We also used Abaxis VSPro equine fibrinogen cartridge which utilizes the Fibrinogen-Clauss method. The Fibrinogen-Clauss method gives an estimate of fibrinogen based on a calibration curve produced by measuring time to a detectable clot in blood containing known amounts of fibrinogen. This indirect test was chosen due to being a commonly available, relatively inexpensive test, and using a small sample size.
The purpose of this study is to provide updated reference intervals for healthy, adult Brown pelicans, to assess the agreement and usefulness of two methods of determination of fibrinogen in Brown pelicans (Pelecanus occidentalis): the heat precipitation and the Fibrinogen-Clauss method via the Abaxis VSpro equine fibrinogen cartridge. Here we propose the reevaluation and validation of assays previously advocated for use in birds to indicate inflammation.
Material and Methods
Sample Collection and Processing
One adult and 70 juvenile brown pelicans (Pelecanus occidentalis) of known gender housed at the Wildlife Center of Texas for medical care and rehabilitation were used for this study. The birds were manually restrained for pre-release physical examination, collection of cloacal swabs and venipuncture. Physical examination parameters that were recorded include cloacal temperature, body condition score, wounds or other abnormalities, Wildlife Center identification band, and when applicable, United States Fisheries and Wildlife band number. Sampling was performed on 31 December 2014; 6 January 2015; 21 January 2015; and 28 January 2015.
Blood was collected in 1.5 ml sample sizes from the right jugular vein using a three ml syringe and a 26 ga needle. The blood was immediately transferred into 500 μL lithium heparin pre-filled sampling tubes, and one ml sodium citrate microtubes[BRAND HERE] . Blood from the lithium heparin tubes was used to run Abaxis VetScan Avian/Reptile chemistry panel, iSTAT Chem8 and iSTAT CG4+ panels (
Table 1). Blood samples were transported from the collection sites in the greater Houston area to Texas A&M University on ice. Upon return to Texas A&M University’s Veterinary Teaching Hospital lithium heparinized blood was used for packed cell volume determination and plasma was separated from cells via centrifugation, and decanting and placed in a -80ºC freezer. The sodium citrate anticoagulated blood was delivered to the Texas A&M University Veterinary Teaching Hospital Clinical Pathology laboratory for fibrinogen determination by heat precipitation (
Table 1). Sodium citrated blood samples were centrifuged and cells were separated from plasma and stored in a -80 ºC freezer (
Table 2). Blood from one sampling date (21 January) was centrifuged and decanted from the cells with approximately 12 hours more delay from sampling time than all other sampling dates.
Hemolysis Assessment
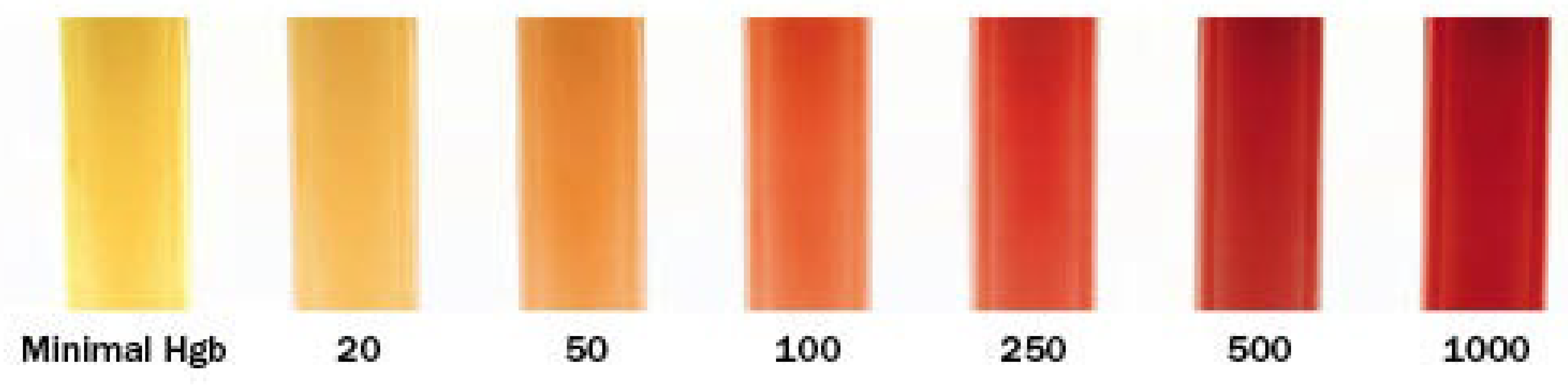
Sodium citrated plasma was removed from the -80 ºC freezer, allowed to thaw to room temperature, and assessed for degree of hemolysis prior to Abaxis VSpro fibrinogen determination via a visual assessment performed by one investigator (AGK) using the visual scale available on the Mayo Clinic Medical Laboratories website printed using a color printer [Plumhoff, 2008] (
Figure 1)
Abaxis VSpro Fibrinogen
Sodium citrated plasma was thawed to room temperature and centrifuged using a 12 cm radius centrifuge at 3000 RPM for 1 hour to obtain platelet poor plasma. VSpro fibrinogen cartridges were brought to room temperature before testing. One hundred μL of plasma was mixed with diluents in the prefilled diluent microtubes that are provided with the cartridges. The cartridges were loaded into the VSpro and testing was performed following package instructions for loading cartridge and sample into the analyzer.
Statistical Analysis
Data analysis was performed using Analyse-it
® to determine the mean, median, range, 95% confidence interval of mean, median and variance; variance, standard deviation, Coefficient of variance, skewness and kurtosis, Shapiro-Wilk W and p-values (
Table 1). The Bland-Altman plot was used to assess the agreement of analytes values determined by different analysis platforms (
Table 3). For parametrically distributed data, ANOVA tests determined if BCS had an effect on measured values. For non-parametrically distributed data, the Kruskal-Wallis test was used to determine the effect of BCS on measured values.
Results
Twelve of the birds had an injury noted on physical exam. Most injuries were minor and did not preclude release. Wounds included bumblefoot, small lacerations or abrasions, and a fishhook in a toe pad. One bird had a severe injury to the patagium and was subsequently transported to the Texas A&M University Small Animal Hospital for medical care with the Zoological Medicine service. Two birds became very stressed during handling. One developed a large hematoma, and further examination was not pursued. The other died shortly after examination.
Mean body condition score was 2.8 out of 5 (Median 3.0, CI 2.6 - 3.0). Proposed reference intervals are reported in
Table 1. The majority of the data was parametrically distributed as determined using a Shapiro-Wilk method with p >0.05 indicating parametrically distributed data (
Table 1). Parametrically distributed data includes anion gap, albumin, base excess, chloride, creatine kinase, fibrinogen via the VSPro equine cartridge, glucose via both tests, hemoglobin, bicarbonate (HCO
3), hematocrit, ionized calcium, potassium via both analyzers, lactate, PCO
2, pH, phosphorus, PO
2, SO
2, sodium via both analyzers, cloacal temperature, TCO
2, and total protein. Non-parametrically distributed data (Shapiro-Wilk p <0.05) includes AST, calcium, creatinine, fibrinogen via heat precipitation, packed cell volume (PCV), total solids, BUN (All values were <3), and uric acid.
To determine where there was an effect of body condition score (BCS) on the various values, ANOVA and Kruskal-Wallis tests were run for parametrically and non-parametrically distributed data, respectively. As BCS increased there was an increase in creatinine (p = 0.0106) and AST (p = 0.0117). As BCS increased there was a decrease in sodium as measured by both analyzers (VS2 p = 0.0360, iSTAT p = 0.0057), hematocrit (p = 0.0122), hemoglobin (p = 0.0130), and anion gap (p = 0.0242).
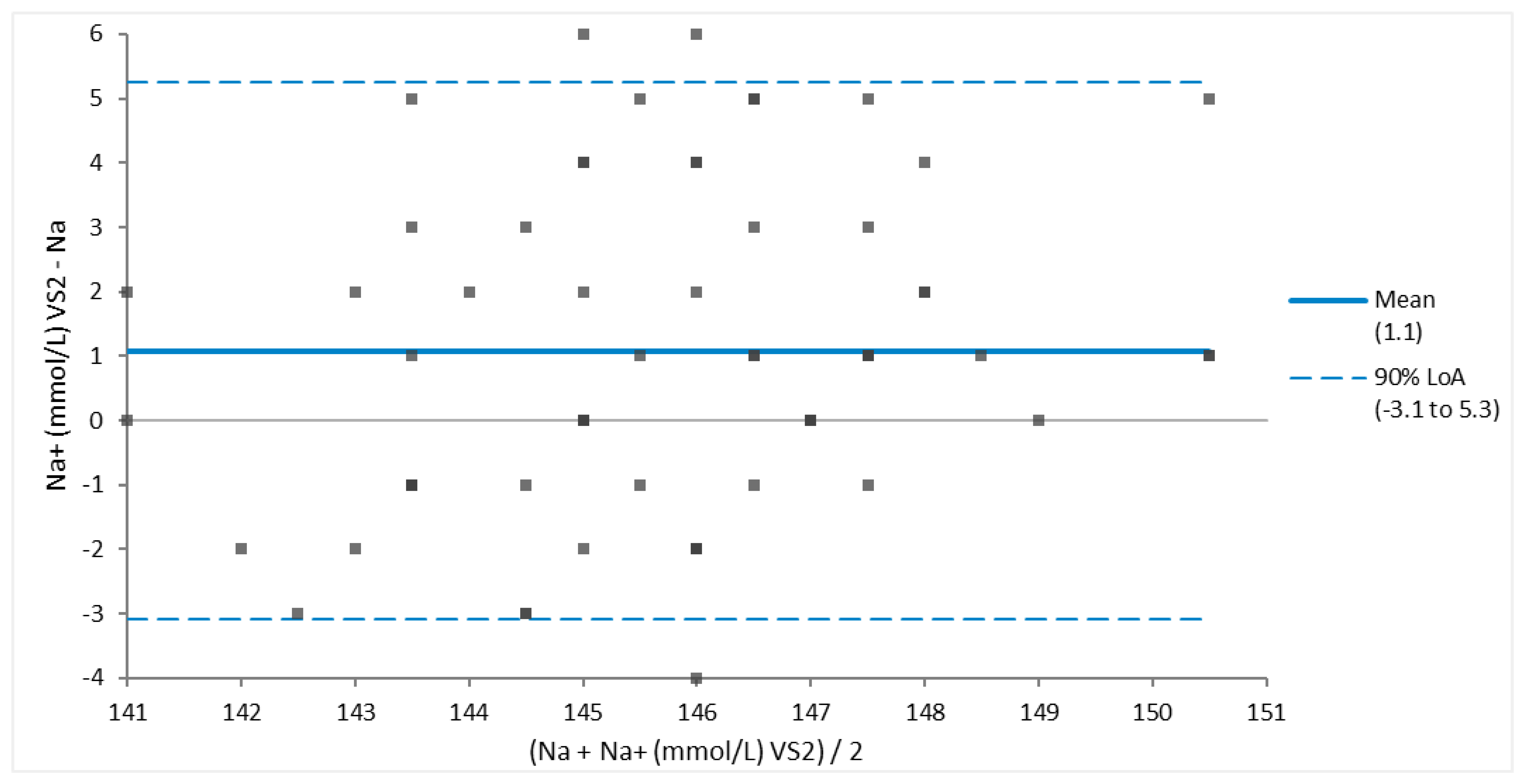
Bland Altman assessment of agreement determined that between different analyzers there was good agreement in measurements of sodium (
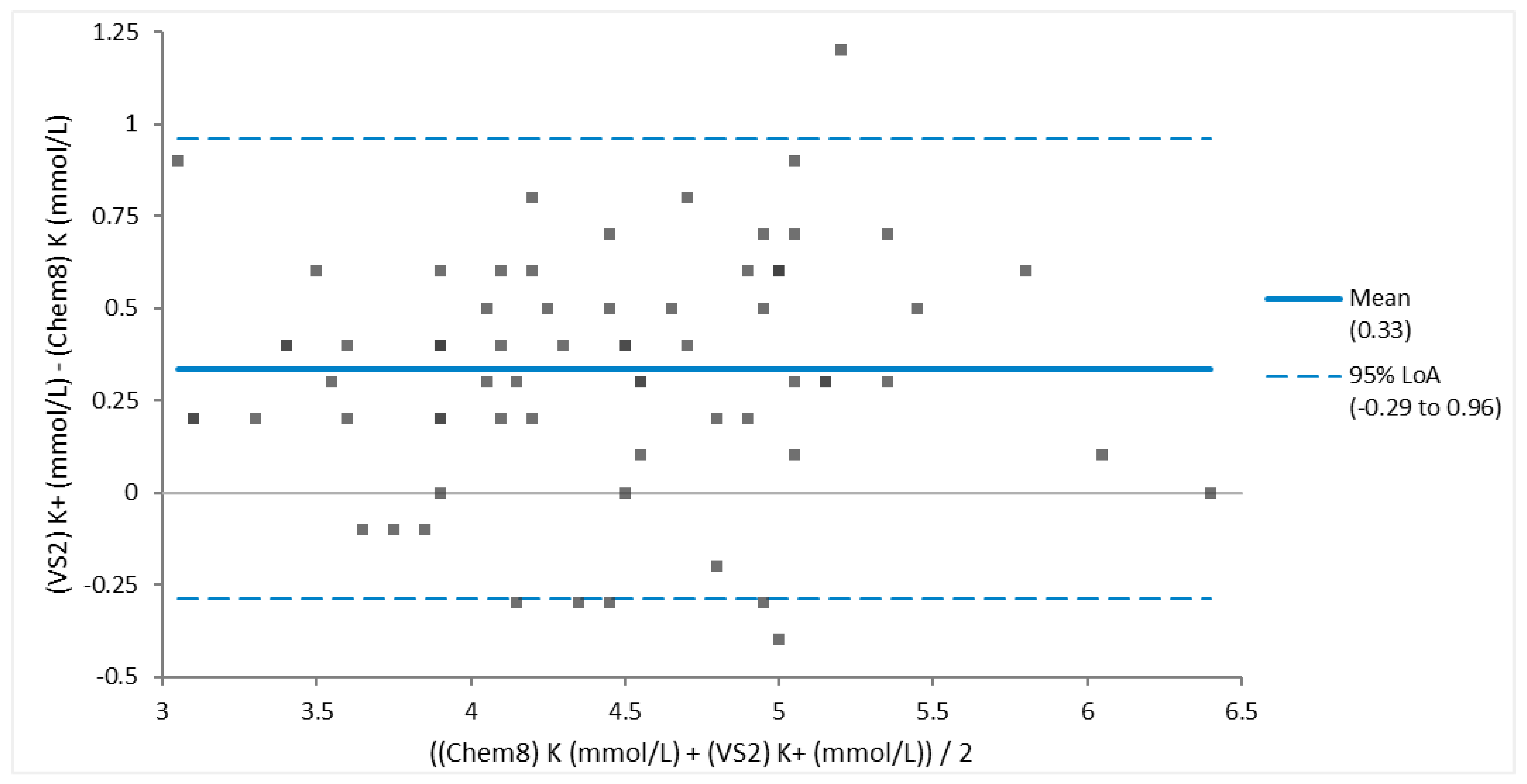
Figure 2), and potassium (
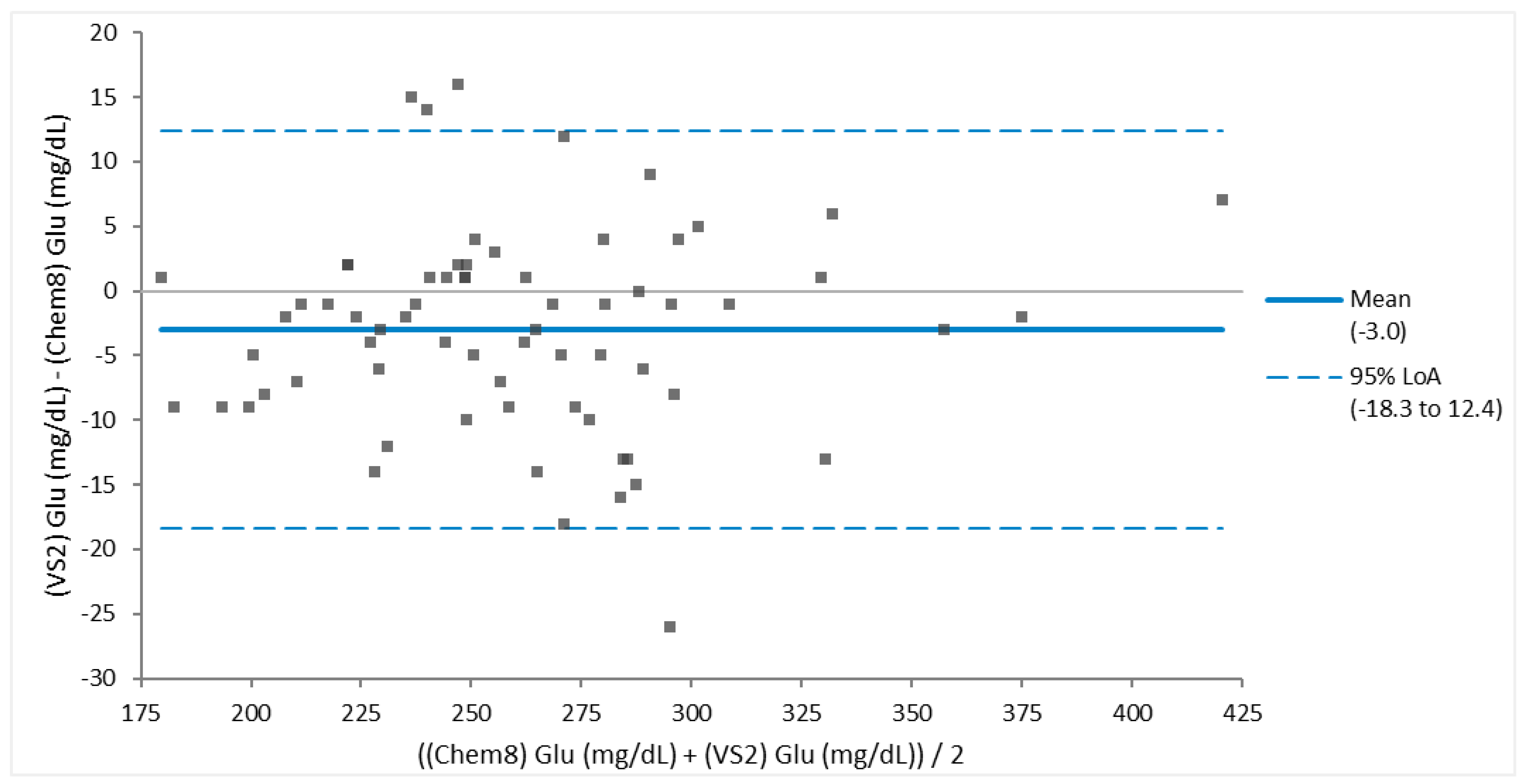
Figure 3). Glucose (
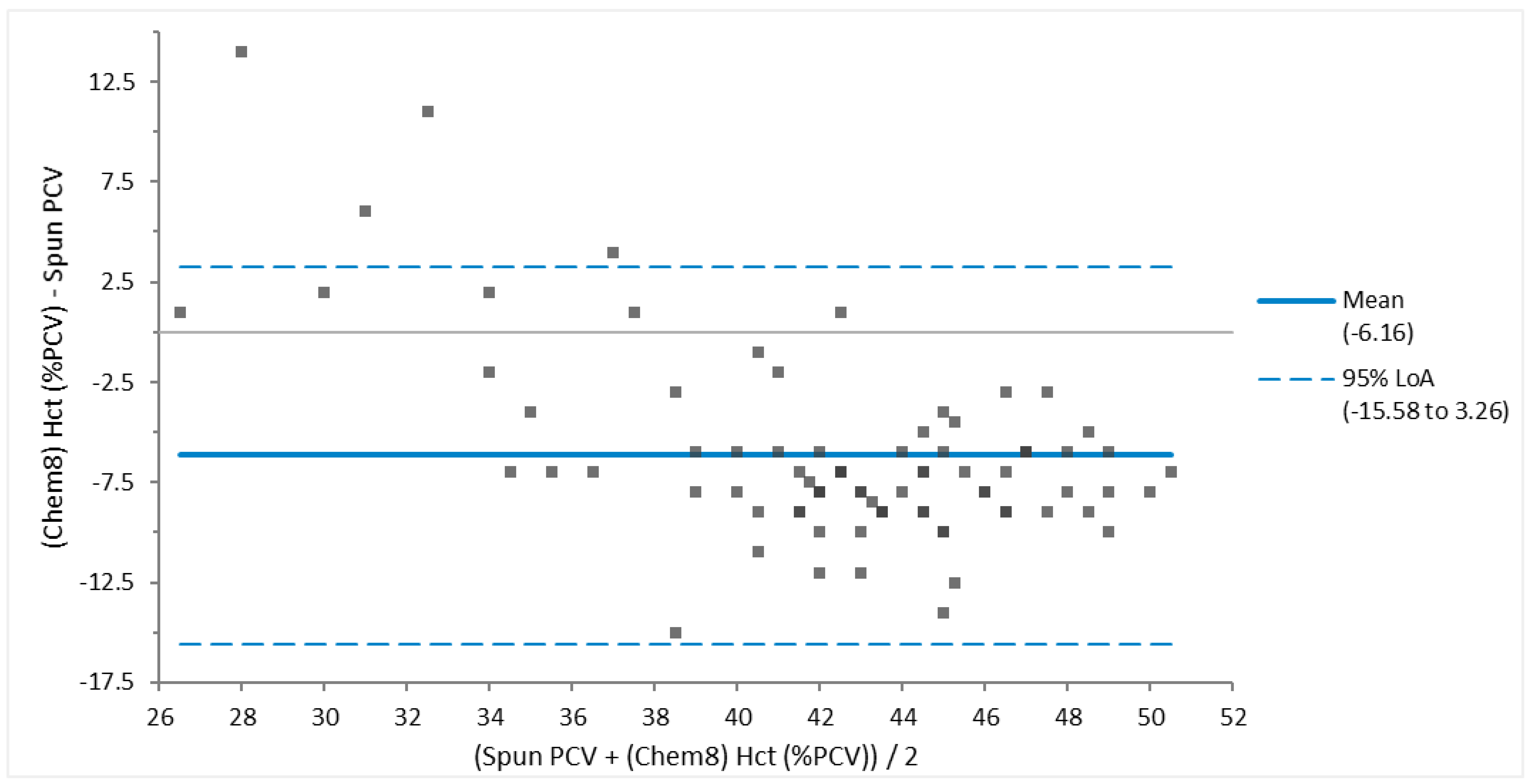
Figure 4) had fair agreement, and when one large outlier was removed, had good agreement. There was poor agreement between spun PCV and hematocrit (
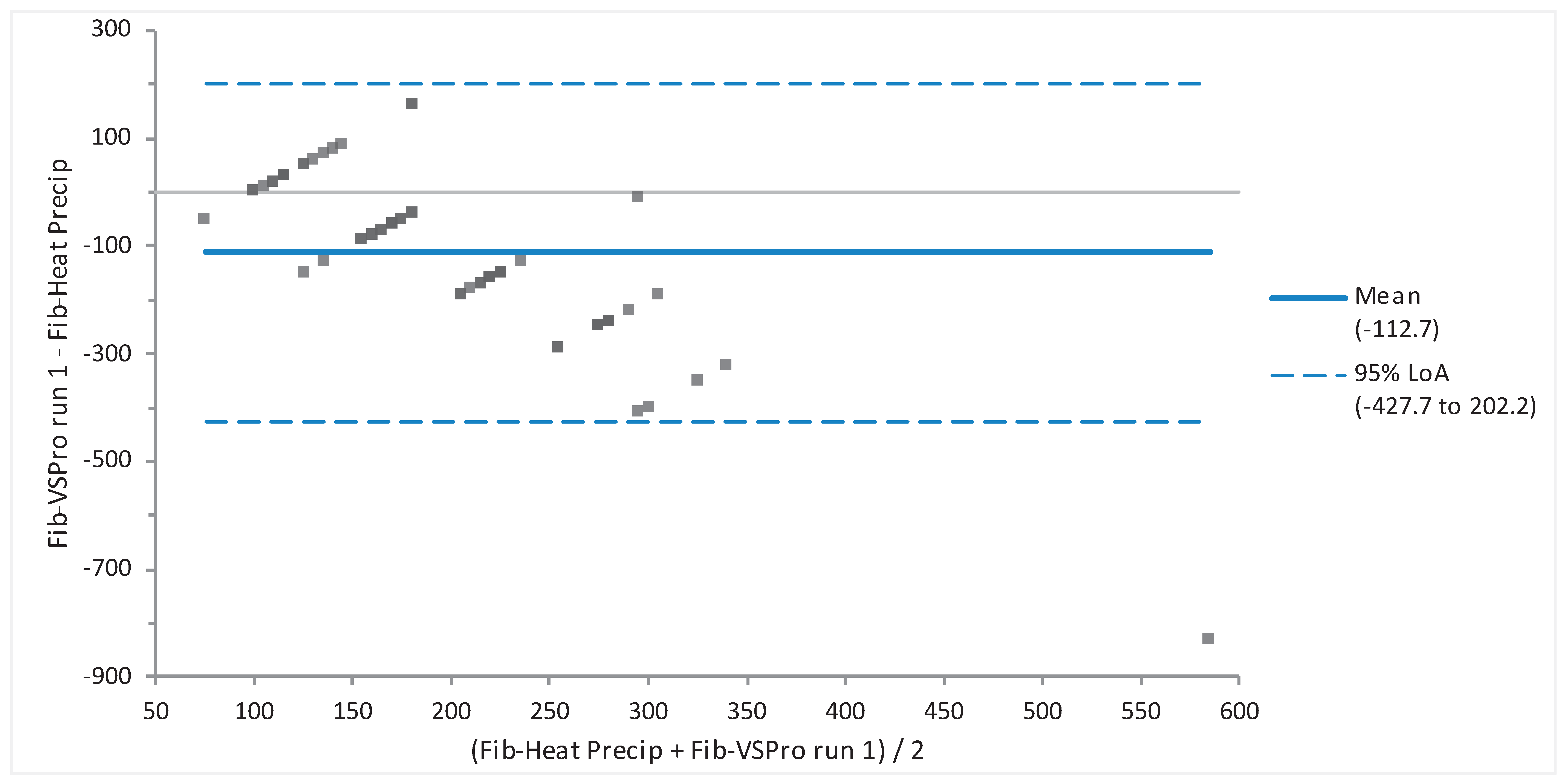
Figure 5). When the two fibrinogen testing methods (heat precipitation vs Abaxis VSPro equine Fibrinogen cartridge) were compared via Bland-Altman they showed no agreement (
Figure 6).
Blood samples collected on different sampling dates had significantly different amounts of hemolysis, determined by ANOVA (p = 0.0002). However, Kruskall-Wallis testing determined that degree of hemolysis had no statistically significant effect on fibrinogen (p = 0.2249).
Discussion
According to Geffre [2009], guidelines regarding how to create reference intervals include documenting any variation so that clinically significant factors may be controlled. Next establishing inclusion and exclusion criteria. Then determining an appropriate number of individuals. The recommended minimum number of individuals is 120. This is the minimum number that can be used to determine 90% confidence intervals using nonparametric methods. A lower number is required to use parametric methods.
In wildlife, zoo and exotic animal medicine, it is uncommon for a population of 120 healthy adult individuals to be available for reference interval determination. We were limited by our sample size of 71, however, this is a larger population size than has been used to establish reference intervals in the past for brown pelicans. [Zais, 2000; Ferguson, 2014; Wolf, 1985]. The listed mean values and accompanying reference intervals in
Table 1 are proposed as updated reference intervals for healthy juvenile and adult brown pelicans. The values obtained in this study are recommended for use as reference intervals for brown pelicans worldwide due to the large sample size, and the overall health of the birds. In addition, this population of pelicans was undergoing rehabilitation after stranding, which is frequently the reason why brown pelicans are treated by veterinarians. Stranding may be caused by injuries due to man-made disasters such as oil spills, exposure to human objects such as boats, or fishing nets and hooks, or due to natural disasters such as hurricanes, or prey shortages.
There was a statistically significant increase in creatinine and AST with increasing body condition score (BCS). This is likely due to increased muscle mass in these birds providing higher levels of protein metabolites to create creatinine, and AST as it is derived from muscle as well as liver. It was also observed that hematocrit, hemoglobin, sodium and anion gap decreased as BCS decreased. It is suggested that these may be indicators of overall vitality, and as body condition score decreased, so did overall bodily reserves and health.
On the 21 January sampling date there was significantly more hemolysis than other sampling dates. This may be due to the fact that on this sampling date, the cells and plasma were centrifuged and decanted 12 to 24 hours longer after sampling than the other sampling dates. This could also be due to different personnel doing the sample collection. Regardless of cause, the hemolysis did not appear to affect the fibrinogen values, as the Kruskal-Wallis test revealed no relationship between hemolysis and fibrinogen measured by either method.
Bland-Altman analysis was used to determine whether values obtained using more than one methodology are in agreement. When assessing Bland-Altman results, one has to determine to what extent three criteria are met. First, whether all values are within the 95% limits of agreement; second, whether the bias is clinically acceptable; and third, whether the 95% limits of agreement range is clinically small. Values compared in this manner included sodium, potassium, glucose, packed cell volume (PCV) vs hematocrit (Hct), and fibrinogen. Sodium, potassium and glucose had good agreement, indicating that these different testing analyzers are comparable. The criteria for agreement were not met for the fibrinogen or PCV vs Hct. The PCV and hematocrit measurements had poor agreement. The authors recommend use of the gold standard, manual PCV, for this value. Fibrinogen values via the VSPro equine fibrinogen cartridge and the heat precipitation method had no agreement. It appeared that the VSPro often measured lower than the Heat Precipitation method to the extent that the bias was greater than -100.
The goal to establish that the Abaxis VSPro equine fibrinogen cartridge can be used in brown pelicans for measurement of fibrinogen was not achieved. The Bland-Altman analysis revealed no agreement between the two tests. This may be due to several factors. It has been reported that chicken fibrinogen is significantly smaller than human fibrinogen [Pindyck, 1977]. The Fibrinogen-Clauss method used by the Abaxis VSPro has been reported to underestimate the fibrinogen level in humans with dysfibrinogenemia, a condition where the fibrinogen molecules are smaller than normal, or abnormal in shape [Miesbach et al., 2010]. Also, the Fibrinogen-Clauss method uses a reagent that was created to test for fibrinogen in mammalian (equine in this case) blood. Comparisons have not been made between Pelicaniformes fibrinogen molecules and chicken or mammalian. It may be that all avian species’ fibrinogen is smaller than mammalian. This lack of species-specific reagent and calibration may cause a false low reading with the Fibrinogen-Clauss method.
According to the instruction manual for the Abaxis VSPro equine fibrinogen cartridges, presence of particulate matter in the blood sample can cause artifactually low readings, or failure to deliver a test result. Particulate matter may include platelets, lipemia, or hemolysis. Based on the centrifugation of plasma samples according to VSPro manual instructions for obtaining platelet poor plasma, platelets should have not been in the samples used for testing. There was a white film floating in the top layers of many serum samples that is expected to be lipemia that, while not apparent prior to centrifugation, separated out during the centrifugation process. If this layer was taken up into the pipette during sample preparation for testing, this could have introduced enough particulate matter to produce a lower test result. Many of the samples that had the highest levels of hemolysis (250-500, see
Figure 1) caused an error message on the analyzer, and no test result could be produced even with repeated testing. Our ANOVA between hemolysis and fibrinogen level did not reveal any effect of the level of hemolysis on the fibrinogen value, so any effect is expected to be minimal if present at all.
Another possibility for the discrepancy could be sample handling. In the original report where the VSPro was validated for use in equines, the blood was drawn, immediately centrifuged and decanted, and then frozen to -80ºC. The serum was thawed and used according to packaging instructions to obtain values [Epstein, 2012]. This method was used almost exactly, except that the plasma and cells were not separated until at least twenty four hours after the blood was collected. It may be that this extra time of plasma exposure to the cells caused changes to the fibrinogen so that it would not interact in the same way with the test reagents. Fibrinogen has been proven to be stable for up to 5 years when serum is frozen at -70ºC, so we suspect that if handling is the culprit, it was the extended time in which plasma was exposed to cells [Selman, 2012].
We have established that with the procedure that was used here, the Abaxis VSPro equine Fibrinogen cartridge is not suitable for measurement of fibrinogen in Brown pelicans. We cannot make an assumption that this test would not be suitable for fibrinogen determination in other species, such as psittacines or raptors, or if cells were immediately separated from plasma.
In conclusion, we have presented new reference intervals that we recommend be used when assessing health or disease status of adult brown pelicans. We have also established that the Abaxis VSPro equine Fibrinogen cartridge is not suited for use in Brown pelicans.
Ethics approval:
This project was approved by the Texas A&M University Institutional Animal Care and Use Committee.
Data and model availability statement:
None of the data were deposited in an official repository. Information on data and software used can be made available upon request.
Declaration of Generative AI:
Google Scholar was used to generate citations.
Financial support statement:
This work was funded by a grant from the Texas General Land Office.
Declaration of interest:
Abaxis provided VSPro Equine fibrinogen cartridges for use in this research.
Author Contributions
Amelia Gessner-Knepel: Investigation, Writing - Original Draft, Writing - Review and Editing, Visualization, Funding acquisition. Jordan Gentry: Investigation. Sharon Schmalz: Investigation, Resources. Karen Russell: Investigation, Resources. J. Jill Heatley: Conceptualization, Methodology, Software, Formal Analysis, Investigation, Resources, Data Curation, Writing - Review and Editing, Visualization, Supervision, Project Administration, Funding Acquisition.
Acknowledgments
Texas A&M University Veterinary Teaching Hospital, Texas General Land Office, Wildlife Center of Texas.
References
- Cray C, Tatum LM. Applications of protein electrophoresis in avian diagnostics. Journal of Avian Medicine and Surgery. 1998 Mar 1:4-10.
- Drew, M.L.; Joyner, K.; Lobingier, R. Laboratory Reference Intervals for a Group of Captive Thick-Billed Parrots (Rhynchopsitta pachyrhyncha). J. Assoc. Avian Veter- 1993, 7, 35. [Google Scholar] [CrossRef]
- Epstein, K.L.; Brainard, B.M. An evaluation of the Abaxis VSPro for the measurement of equine plasma fibrinogen concentrations. Equine Veter- J. 2012, 44, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.M.; Norton, T.M.; Cray, C.; Oliva, M.; Jodice, P.G. Health assessments of brown pelican (Pelecanus occidentalis) nestlings from colonies in South Carolina and Georgia, USA. J. Zoo Wildl. Med. 2014, 45, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Geffre A, Friedrichs K, Harr K, Concordet D, Trumel C, Braun JP. Reference values: a review. Veterinary clinical pathology 2009, 38, 288–98. [Google Scholar] [CrossRef] [PubMed]
- Georgieva TM, Koinarski VN, Urumova VS, Marutsov PD, Christov TT, Nikolov J, Chaprazov T, Walshe K, Karov RS, Georgiev IP, Koinarski ZV. Effects of Escherichia coli infection and Eimeria tenella invasion on blood concentrations of some positive acute phase proteins (haptoglobin (PIT 54), fibrinogen and ceruloplasmin) in chickens. Revue de medecine veterinaire. 2010, 161, 84. [Google Scholar]
- Godwin, J.S.; Jacobson, E.R.; Gaskin, J.M. Effects of Pacheco's Parrot Disease Virus on Hematologic and Blood Chemistry Values of Quaker Parrots (Myopsitta monachus). J. Zoo Anim. Med. 1982, 13, 127. [Google Scholar] [CrossRef]
- Harr, K.E. Clinical Chemistry of Companion Avian Species: A Review. Veter- Clin. Pathol. 2002, 31, 140–151. [Google Scholar] [CrossRef]
- Hawkey, C.; Hart, M. An analysis of the incidence of hyperfibrinogenaemia in birds with bacterial infections. Avian Pathol. 1988, 17, 427–432. [Google Scholar] [CrossRef]
- Hawkey, C.; Samour, H.; Henderson, G.; Hart, M. Haematological findings in captive gentoo penguins (pygoscelis papua)with bumblefoot. Avian Pathol. 1985, 14, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Jodice, P.G.R.; Lamb, J.S.; Satgé, Y.G.; Fiorello, C. Blood biochemistry and hematology of adult and chick brown pelicans in the northern Gulf of Mexico: baseline health values and ecological relationships. Conserv. Physiol. 2022, 10, coac064. [Google Scholar] [CrossRef] [PubMed]
- Kinney, ME. The effects of capture, restraint, and transport on hematologic, plasma biochemical, and blood gas values in Dalmatian pelicans (Pelecanus crispus). Journal of avian medicine and surgery 2018, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Metzner, M.; Horber, J.; Rademacher, G.; Klee, W. Application of the Glutaraldehyde Test in Cattle. J. Veter- Med. Ser. A 2007, 54, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Miesbach, W.; Schenk, J.; Alesci, S.; Lindhoff-Last, E. Comparison of the fibrinogen Clauss assay and the fibrinogen PT derived method in patients with dysfibrinogenemia. Thromb. Res. 2010, 126, e428–e433. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, J.K.; Paul-Murphy, J.; Fialkowski, J.P.; Hart, A.; Darien, B.J. Estimation of Prothrombin Times of Hispaniolan Amazon Parrots (Amazona ventralis) and Umbrella Cockatoos (Cacatua alba). J. Avian Med. Surg. 2003, 17, 72–77. [Google Scholar] [CrossRef]
- Petzinger, C.; Larner, C.; Heatley, J.J.; Bailey, C.A.; MacFarlane, R.D.; Bauer, J.E. Conversion of a-linolenic acid to long-chain omega-3 fatty acid derivatives and alterations of HDL density subfractions and plasma lipids with dietary polyunsaturated fatty acids in Monk parrots (Myiopsitta monachus). J Anim Physiol Anim Nutr. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Pindyck J, Mosesson MW, Bannerjee D, Galanakis D. The structural characteristics of chicken fibrinogen. Biochimica et Biophysica Acta (BBA)-Protein Structure. 1977 Jun 24;492(2):377-86.
- Plumhoff EA, Masoner D, Dale JD. Preanalytic laboratory errors: identification and prevention. Mayo Clinic Communique. 2008 Dec;33(12):1-7.
- Polo FJ, Peinado VI, Viscor G, Palomeque J. Hematologic and plasma chemistry values in captive psittacine birds. Avian diseases. 1998 Jul 1:523-35.
- Raynor, E.J.; Pierce, A.R.; Owen, T.M.; Leumas, C.M.; Rohwer, F.C. Short-Term Demographic Responses of a Coastal Waterbird Community After Two Major Hurricanes. Waterbirds 2013, 36, 88–93. [Google Scholar] [CrossRef]
- Rettenmund, C.L.; Heatley, J.J.; Russell, K.E. COMPARISON OF TWO ANALYZERS TO DETERMINE SELECTED VENOUS BLOOD ANALYTES OF QUAKER PARROTS (MYIOPSITTA MONACHUS). J. Zoo Wildl. Med. 2014, 45, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Selman, W.; Hess, T.J.; Salyers, B.; Salyers, C. Short-Term Response of Brown Pelicans (Pelecanus occidentalis) to Oil Spill Rehabilitation and Translocation. Southeast. Nat. 2012, 11, G1–G16. [Google Scholar] [CrossRef]
- Wolf, S.H.; Schreiber, R.W.; Kahana, L.; Torres, J. Seasonal, sexual and age-related variation in the blood composition of the brown pelican (Pelecanus occidentalis). Comp. Biochem. Physiol. Part A: Physiol. 1985, 82, 837–846. [Google Scholar] [CrossRef]
- Zaias, J.; Fox, W.P.; Cray, C.; Altman, N.H. Hematologic, plasma protein, and biochemical profiles of brown pelicans (Pelecanus occidentalis). Am. J. Veter- Res. 2000, 61, 771–774. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Visual hemolysis scoring chart used to estimate hemolysis in samples [Plumhoff, et. al]. Hgb = Hemoglobin.
Figure 1.
Visual hemolysis scoring chart used to estimate hemolysis in samples [Plumhoff, et. al]. Hgb = Hemoglobin.
Figure 2.
Bland-Altman Plot of good agreement for 69 sodium (Na) measurements of Brown Pelicans (Pelecanus occidentalis) determined via Abaxis VS2 Avian/Reptile Chemistry vs iSTAT CG4+ cartridge. Most analyte values are within the 95% limits of agreement (LOA), the small bias is clinically acceptable, and the 95% LOA is clinically small for sodium values.
Figure 2.
Bland-Altman Plot of good agreement for 69 sodium (Na) measurements of Brown Pelicans (Pelecanus occidentalis) determined via Abaxis VS2 Avian/Reptile Chemistry vs iSTAT CG4+ cartridge. Most analyte values are within the 95% limits of agreement (LOA), the small bias is clinically acceptable, and the 95% LOA is clinically small for sodium values.
Figure 3.
Bland-Altman Plot of the blood of 68 Brown Pelicans (Pelecanus occidentalis) showing good agreement of potassium obtained via Abaxis VS2 Avian/Reptile Chemistry vs iSTAT Chem8 cartridge. Most analyte values are within the 95% limits of agreement (LoA), the small mean bias is clinically acceptable, and the 95% LoA is clinically small.
Figure 3.
Bland-Altman Plot of the blood of 68 Brown Pelicans (Pelecanus occidentalis) showing good agreement of potassium obtained via Abaxis VS2 Avian/Reptile Chemistry vs iSTAT Chem8 cartridge. Most analyte values are within the 95% limits of agreement (LoA), the small mean bias is clinically acceptable, and the 95% LoA is clinically small.
Figure 4.
Bland-Altman Plot of blood from 68 Brown Pelicans (Pelecanus occidentalis) of good agreement between glucose (Glu) measurements determined via Abaxis VS2 Avian/Reptile Chemistry vs iSTAT Chem8 cartridge. This assessment of agreement was based on the fact that all except for the one outlier lie within the 95% limits of agreement (LoA), the mean bias is clinically acceptable, and the 95% LoA is clinically small.
Figure 4.
Bland-Altman Plot of blood from 68 Brown Pelicans (Pelecanus occidentalis) of good agreement between glucose (Glu) measurements determined via Abaxis VS2 Avian/Reptile Chemistry vs iSTAT Chem8 cartridge. This assessment of agreement was based on the fact that all except for the one outlier lie within the 95% limits of agreement (LoA), the mean bias is clinically acceptable, and the 95% LoA is clinically small.
Figure 5.
Bland-Altman Plot of Packed Cell Volume (PCV) and Hematocrit (Hct) obtained via Chem 8 iSTAT cartridge for 81 Brown Pelicans (Pelecanus occidentalis). Poor agreement is demonstrated based on several values outside of the 95% limits of agreement (LoA), a large clinically unacceptable mean bias of 6.16 %, and a wide 95% LoA.
Figure 5.
Bland-Altman Plot of Packed Cell Volume (PCV) and Hematocrit (Hct) obtained via Chem 8 iSTAT cartridge for 81 Brown Pelicans (Pelecanus occidentalis). Poor agreement is demonstrated based on several values outside of the 95% limits of agreement (LoA), a large clinically unacceptable mean bias of 6.16 %, and a wide 95% LoA.
Figure 6.
Bland-Altman Plot of minimal agreement between fibrinogen of plasma from Brown Pelicans (Pelecanus occidentalis, n = 62) determined based on heat precipitation (Heat Precip) versus the Abaxis VSPro Equine Fibrinogen cartridge. While most values lie within the 95% limits of agreement, the large bias was clinically unacceptable, and 95% Limit of agreement (LoA) (-427.7 to 202.2) was unacceptably wide.
Figure 6.
Bland-Altman Plot of minimal agreement between fibrinogen of plasma from Brown Pelicans (Pelecanus occidentalis, n = 62) determined based on heat precipitation (Heat Precip) versus the Abaxis VSPro Equine Fibrinogen cartridge. While most values lie within the 95% limits of agreement, the large bias was clinically unacceptable, and 95% Limit of agreement (LoA) (-427.7 to 202.2) was unacceptably wide.
Table 1.
Reference intervals for select chemistry and venous blood gas analytes of healthy juvenile brown pelicans (Pelicanus occidentalis). Measured and calculated variables are included.
Table 1.
Reference intervals for select chemistry and venous blood gas analytes of healthy juvenile brown pelicans (Pelicanus occidentalis). Measured and calculated variables are included.
| |
Reference Intervals |
| Analyte |
N |
Mean |
95% Confidence Interval |
P-value |
95% Reference Interval |
| Anion GapI (mmol/L) |
68 |
21.5 |
20.60-22.34 |
0.588 |
12 – 29 |
| Albumin2 (g/dL) |
64 |
2.3 |
2.20-2.33 |
0.118 |
1.7-2.7 |
| Aspartate Aminotransferase23 (U/L) |
69 |
629.7 |
545.18-714.27 |
0 |
142-1872 |
| Base ExcessI (mmol/L) |
67 |
-4.4 |
-5.5- -3.3 |
0.149 |
-14 – 5 |
| Blood Urea Nitrogen2 (mg/dL) |
67 |
<0.3 |
<0.3 |
<0.3 |
<0.3 |
| Total Calcium23 (mmol/L) |
68 |
10.4 |
10.26-10.47 |
0.001 |
9.7-11.5 |
| ChlorideI (mmol/L) |
65 |
108.4 |
107.81-109.08 |
0.199 |
103-115 |
| Creatine Kinase2 (U/L) |
68 |
1263 |
1150.0-1375.0 |
0.06 |
478-2511 |
| Creatinine23 (mg/dL) |
66 |
0.34 |
0.321-0.364 |
<0.0001 |
0.2-0.5 |
| Fibrinogen-HP3 (mg/dL) |
70 |
250 |
218-279 |
<0.0001 |
100-500 |
| Fibrinogen-VS pro (mg/dL) |
59 |
140 |
133-147 |
0.636 |
70-210 |
| GlucoseI (mg/dL) |
67 |
258 |
248.9-268.0 |
0.285 |
179-376 |
| Glucose2 (mg/dL) |
69 |
258 |
248.5-267.9 |
0.358 |
178-374 |
| HemoglobinI (g/dL) |
68 |
13.4 |
13.05-13.68 |
0.338 |
10.5-16.0 |
| BicarbonateI (mmol/L) |
67 |
21 |
20.1-21.9 |
0.692 |
13.4-29.4 |
| HematocritI (%) |
68 |
39.3 |
38.38-40.24 |
0.34 |
31-47 |
| Ionized CalciumI (mmol/L) |
60 |
1.26 |
1.226-1.287 |
0.867 |
0.99-1.52 |
| PotassiumI (mmol/L) |
69 |
4.3 |
4.09-4.43 |
0.591 |
2.6-6.4 |
| Potassium2 (mmol/L) |
70 |
4.6 |
4.41-4.76 |
0.526 |
3.2-6.4 |
| LactateI (mmol/L) |
69 |
8.7 |
7.99-9.41 |
0.105 |
3.3-16.3 |
| PCO2I (mmHg) |
67 |
36.2 |
35.00-37.37 |
0.155 |
26.2-47.4 |
| PCO24 (mmHg) |
65 |
41.3 |
39.94-42.66 |
0.26 |
28.7-53.6 |
| PCV3 (%) |
66 |
46.4 |
45.23-47.59 |
0.002 |
33-54 |
| pHI5 |
63 |
7.36 |
7.350-7.379 |
0.078 |
7.24-7.49 |
| pH4 |
65 |
7.33 |
7.310-7.342 |
0.226 |
7.19-7.49 |
| Phosphorus2 (mg/dL) |
68 |
2.8 |
2.54-3.04 |
0.485 |
0.3-5.2 |
| PO2I (mmHg) |
69 |
43.3 |
42.0-45.0 |
0.127 |
30-61 |
| PO24 (mmHg) |
67 |
54.4 |
52.29-56.52 |
0.202 |
36-76 |
| sO2I (%) |
65 |
77.3 |
75.61-78.91 |
0.726 |
62-93 |
| SodiumI (mmol/L) |
68 |
145.5 |
144.90-146.02 |
0.157 |
140-150 |
| Sodium2 (mmol/L) |
70 |
146.4 |
145.70-147.01 |
0.165 |
141-153 |
| Temperature (°C) |
68 |
104.2 |
103.83-104.53 |
0.372 |
100.9-107.1 |
| TCO2I (mmol/L) |
67 |
22.1 |
21.15-22.97 |
0.667 |
14-31 |
| TCO22 (mmol/L) |
68 |
20.3 |
19.60-21.02 |
0.523 |
13-28 |
| Total Protein2 (g/dL) |
69 |
4.6 |
4.45-4.67 |
0.023 |
3.8-5.8 |
| Total Solids3 (g/dL) |
23 |
4.6 |
4.47-4.80 |
0.028 |
4.0-5.3 |
| Uric Acid2,3 (mg/dL) |
68 |
7.2 |
6.18-8.23 |
<0.0001 |
1.9-19.2 |
Table 2.
Collection dates and sample handling data.
Table 2.
Collection dates and sample handling data.
| Collection dates and sample handling timeline |
|---|
| Collection date |
Number of birds sampled |
Time after collection to sodium citrate decanting and freezing |
| 12/31/14 |
24 |
< 24 hours |
| 1/6/15 |
20 |
< 36 hours |
| 1/21/15 |
18 |
48 hours |
| 1/28/15 |
9 |
< 24 hours |
Table 3.
Results of Bland-Altman analysis to determine agreement between two different methods of measurement for selected venous analytes in brown pelicans (Pelicanus occidentalis).
Table 3.
Results of Bland-Altman analysis to determine agreement between two different methods of measurement for selected venous analytes in brown pelicans (Pelicanus occidentalis).
| |
Bland-Altman Results |
| Parameter |
n |
Bias |
95% Limits of Agreement |
Agreement level |
Glucose
VS2 vs Chem8 |
67 |
-1.6 |
-30.9 – 27.6 |
Good |
| PCV vs Hct |
64 |
-6.8 |
-13.4 - -0.1 |
Poor |
Fibrinogen
HP vs Fibrinogen VSPro |
56 |
-112.0 |
-362.1 – 138.2 |
Poor |
Sodium
VS2 vs Chem8 |
60 |
1.1 |
-3.9 – 6.1 |
Good |
Potassium
VS2 vs Chem8 |
60 |
0.31 |
-0.34 – 0.96 |
Good |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).