Submitted:
30 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Etching and Characterization Methods
3. Results and Discussion
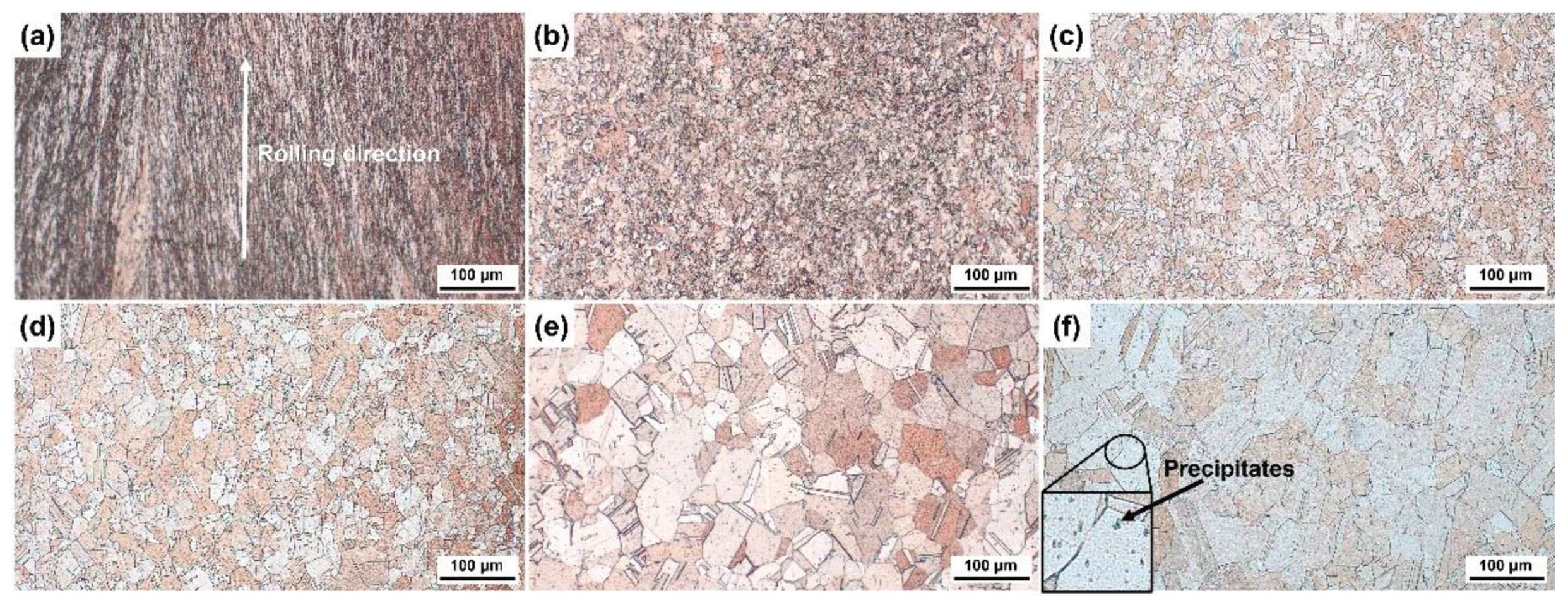
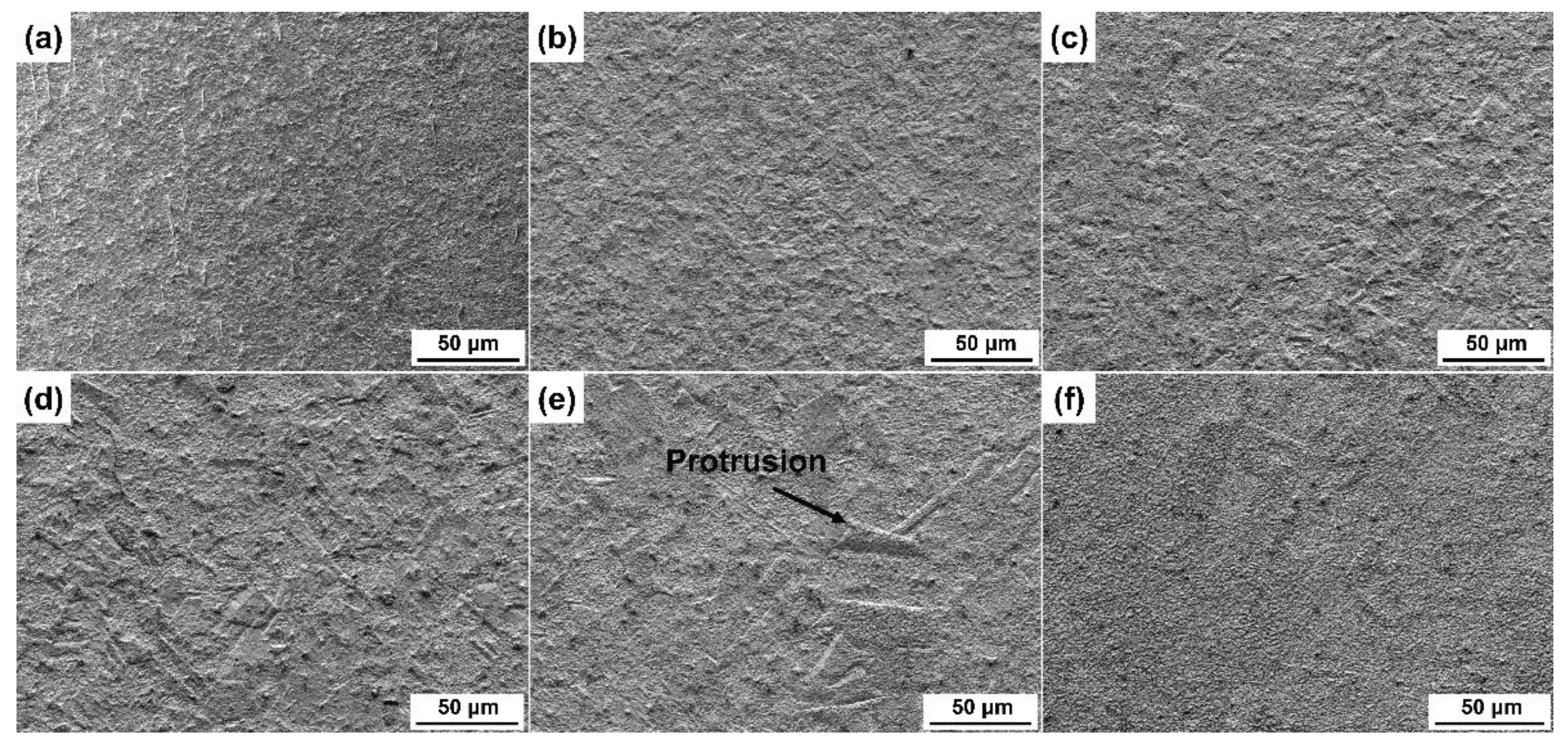
3.1. Grain Structure of Specimens Annealed at Different Temperature
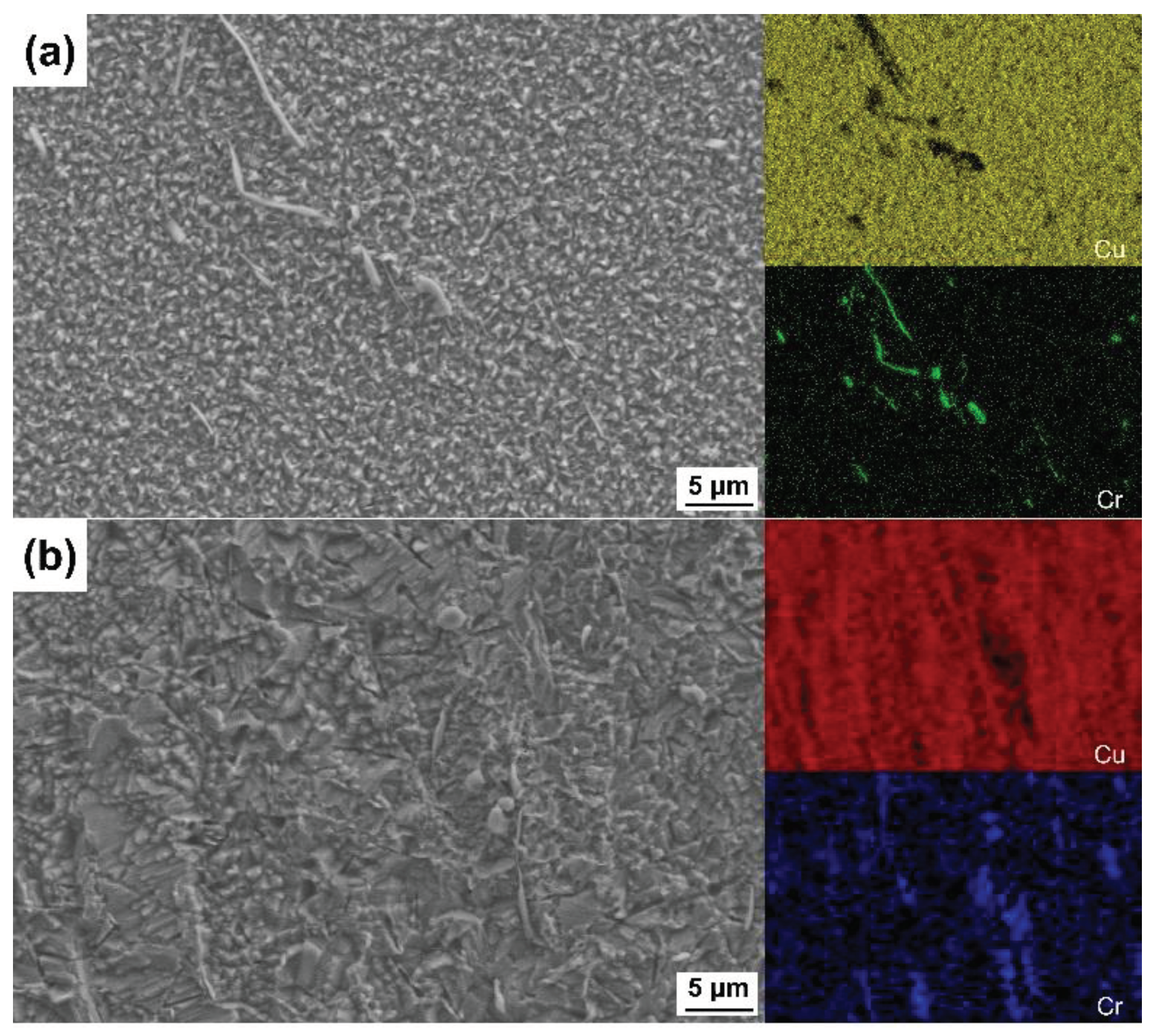
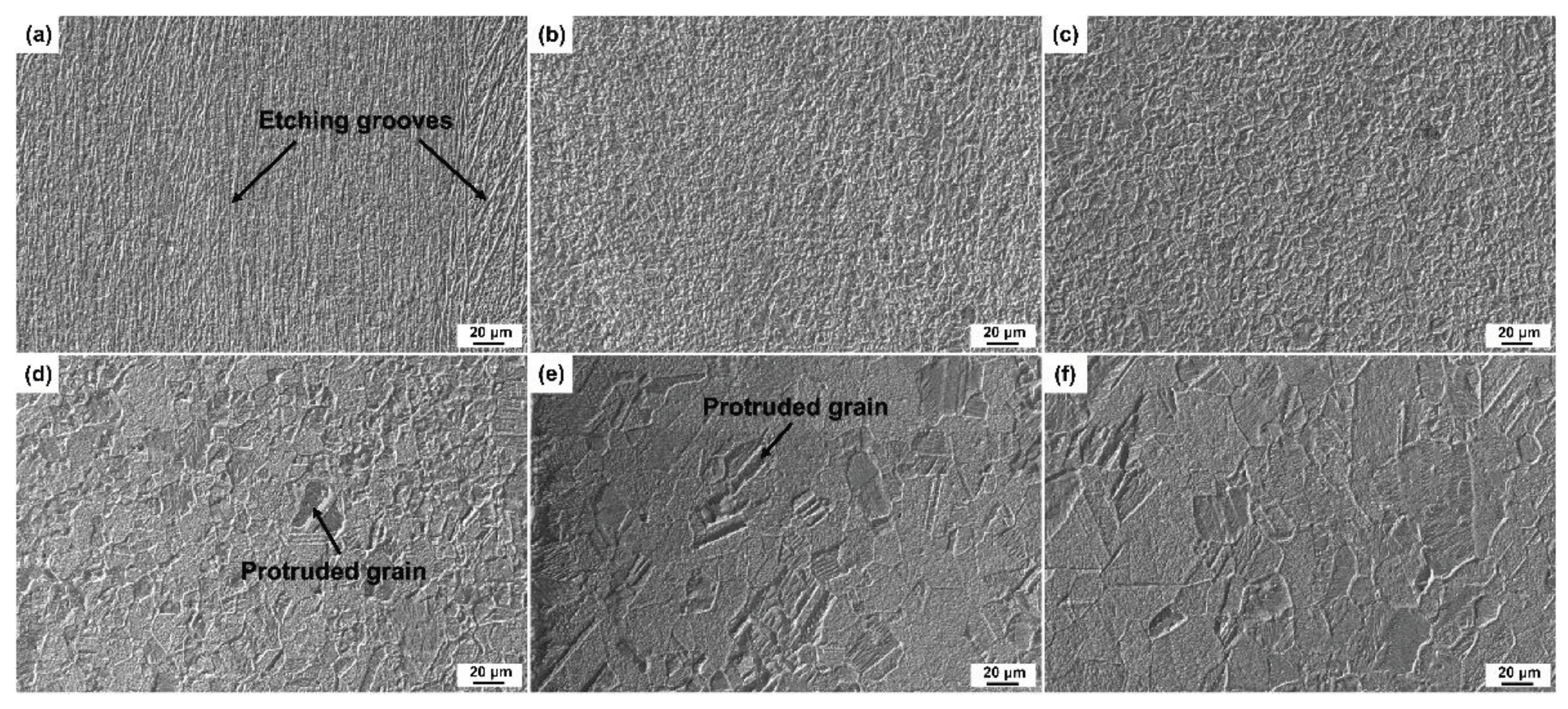
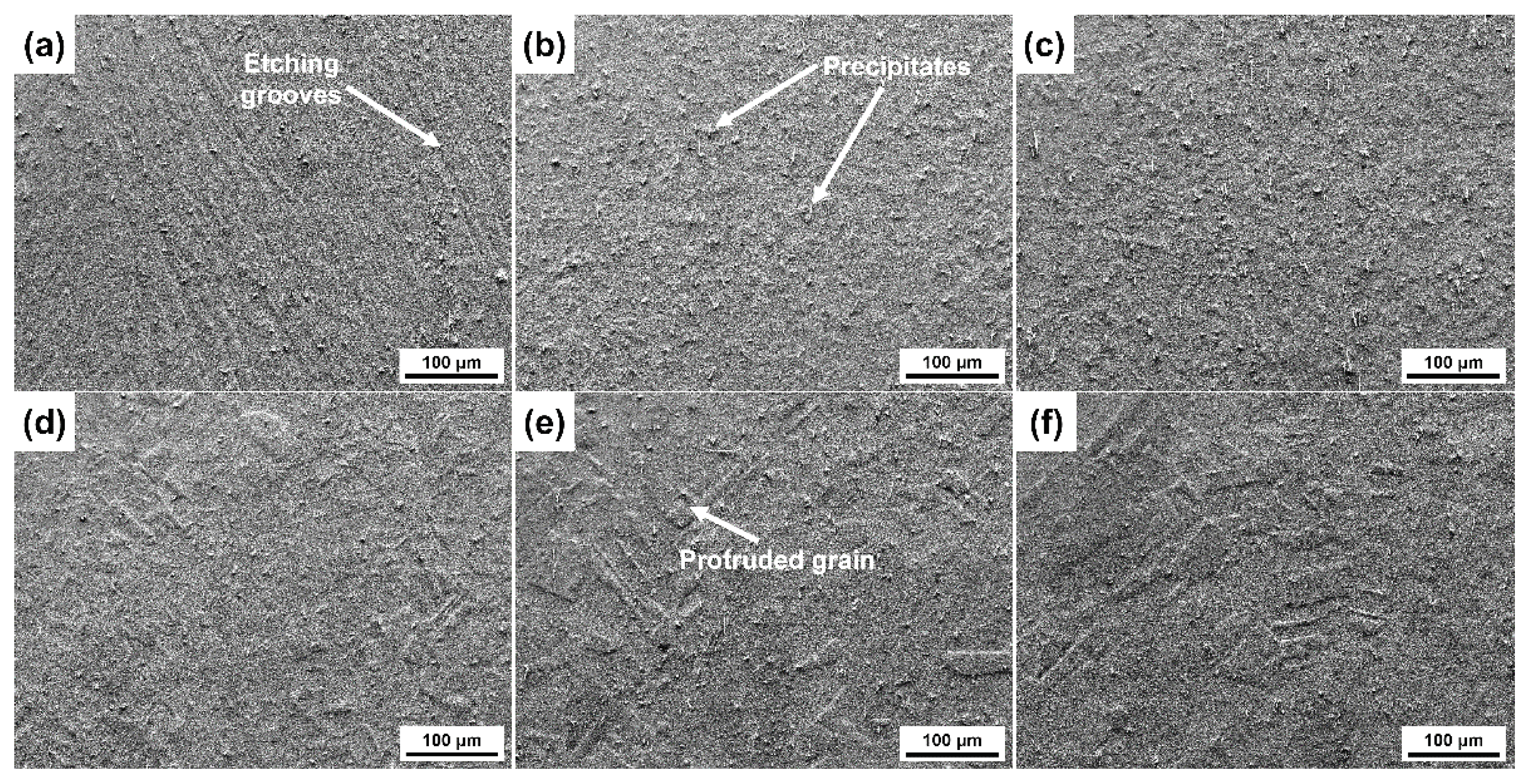
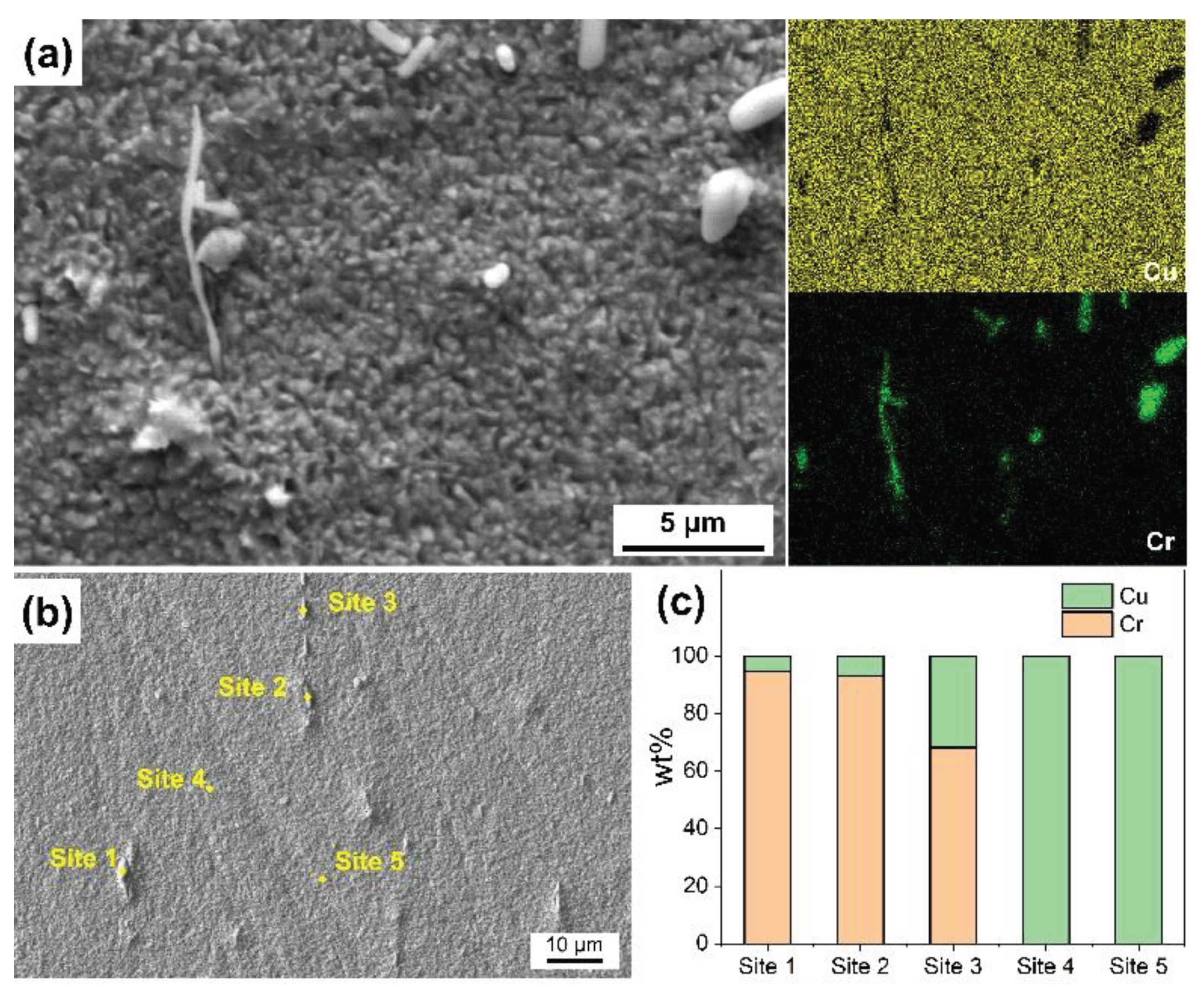
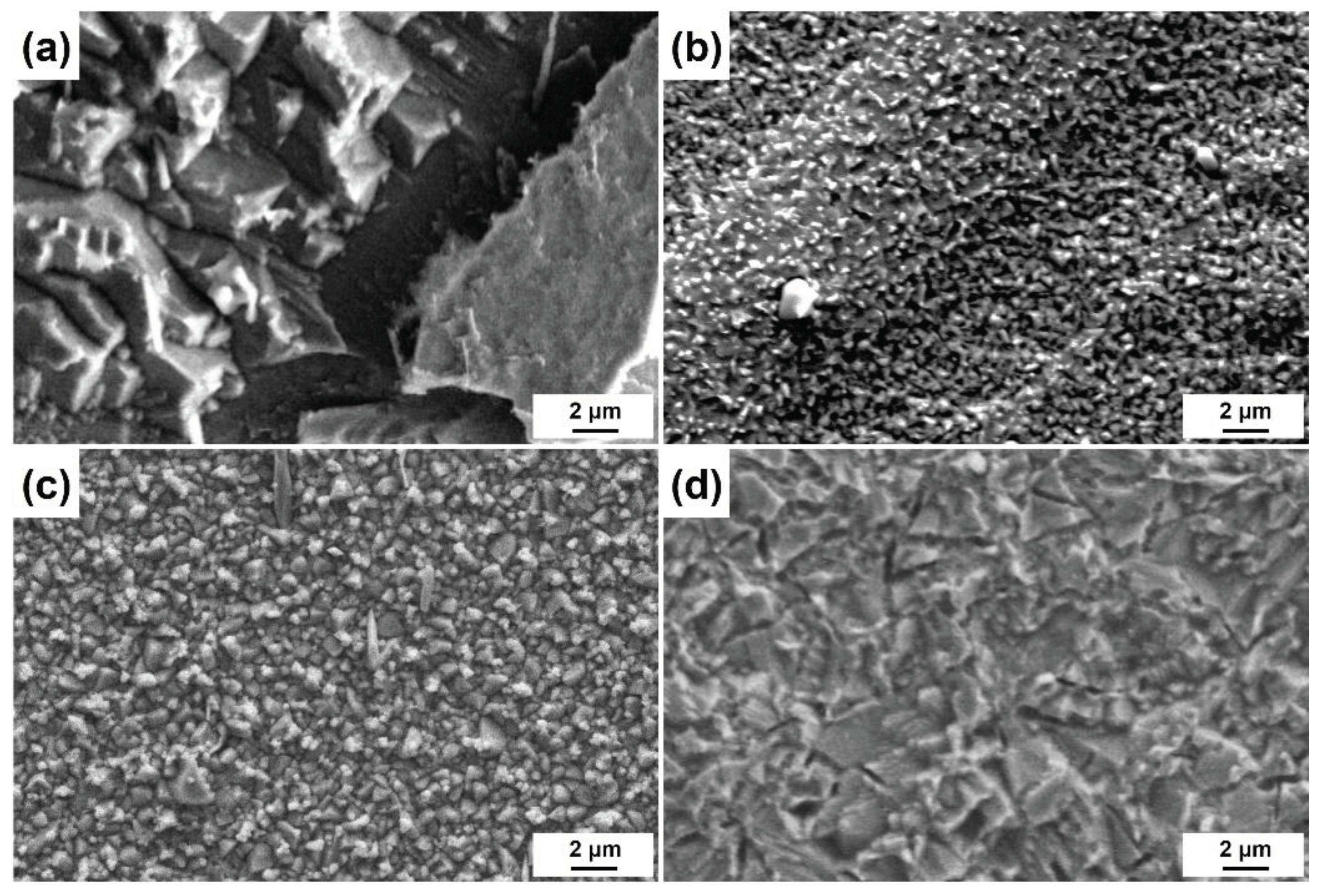
3.2. Surface Morphologies after Etching with Different Etchants
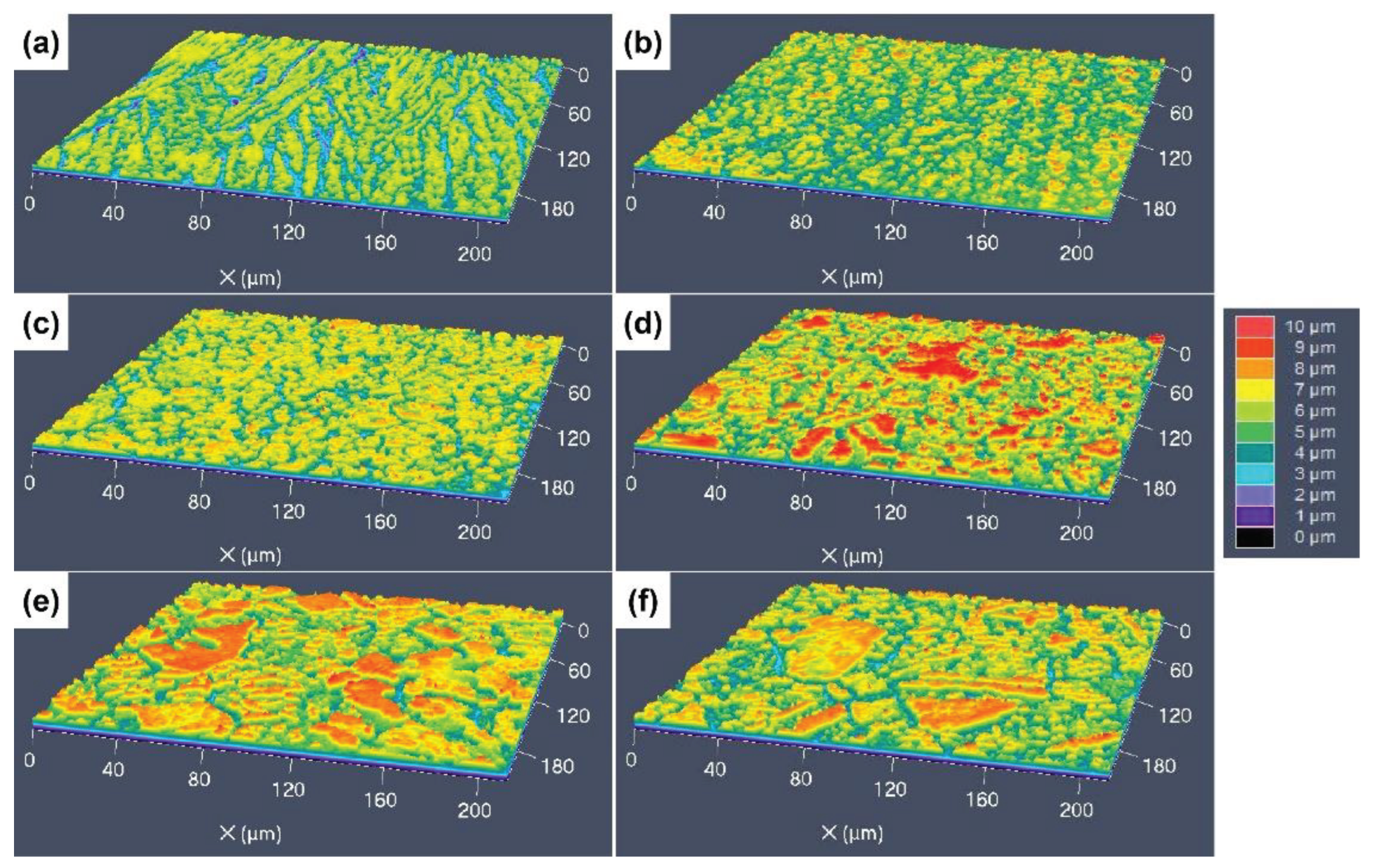
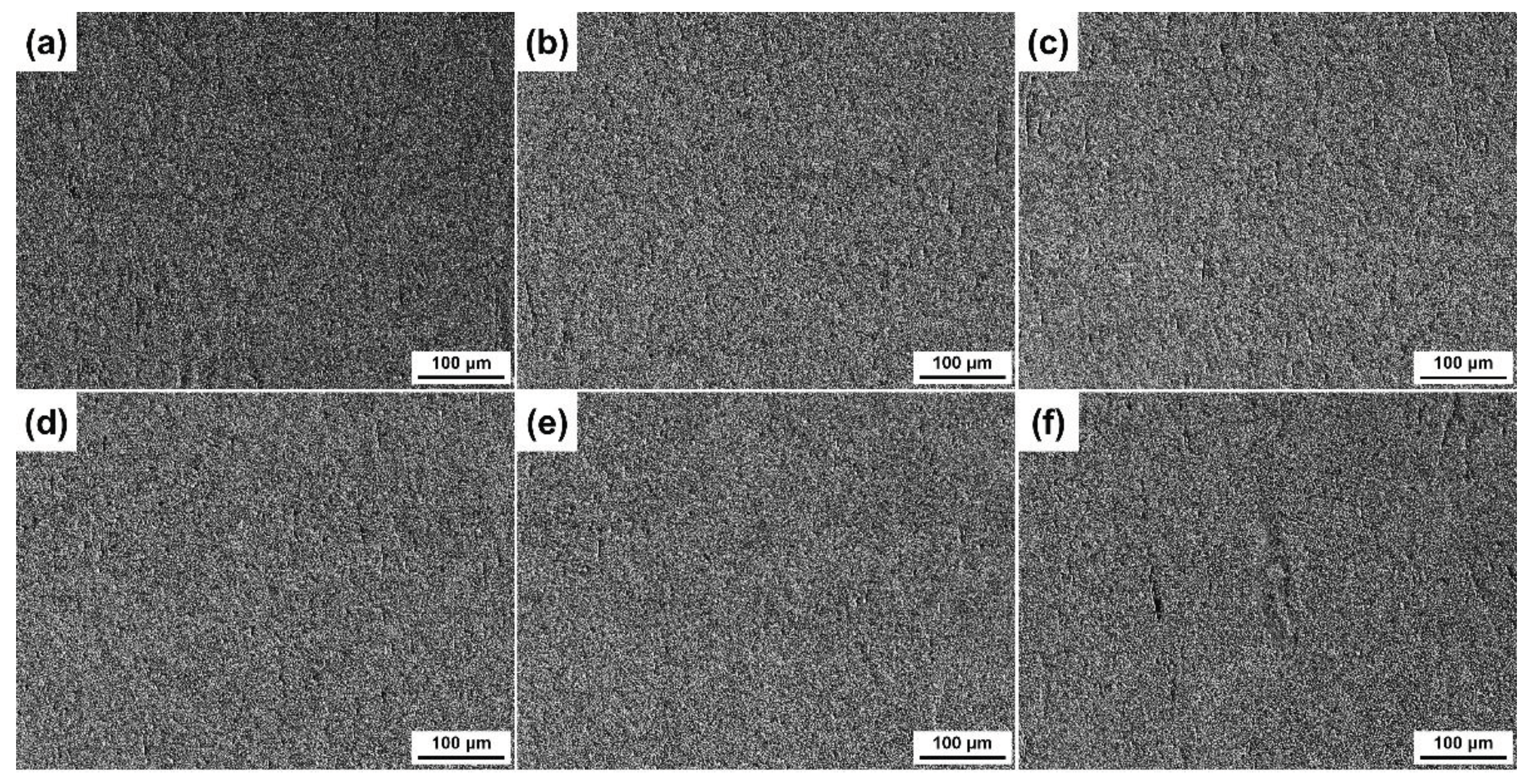
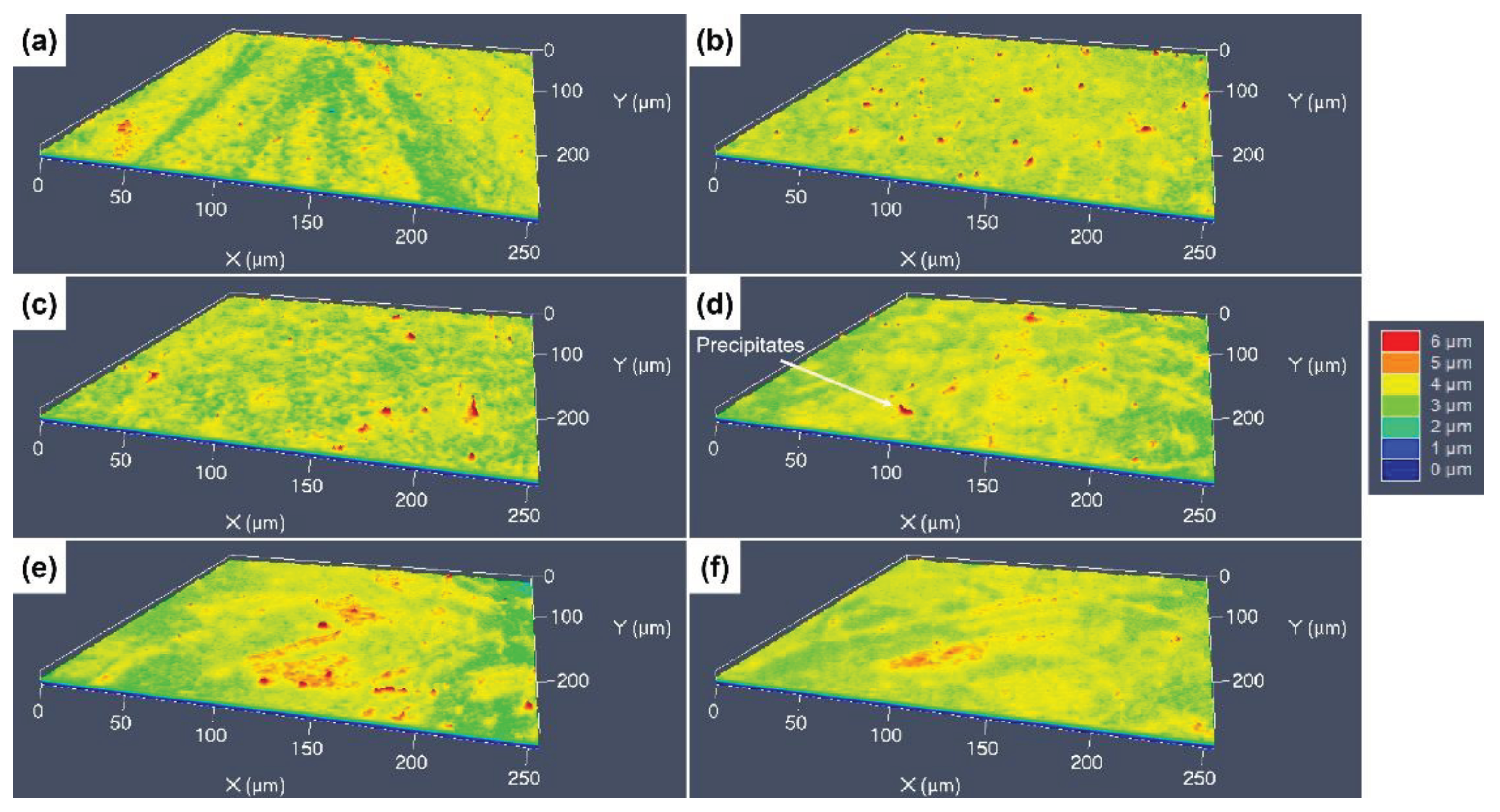
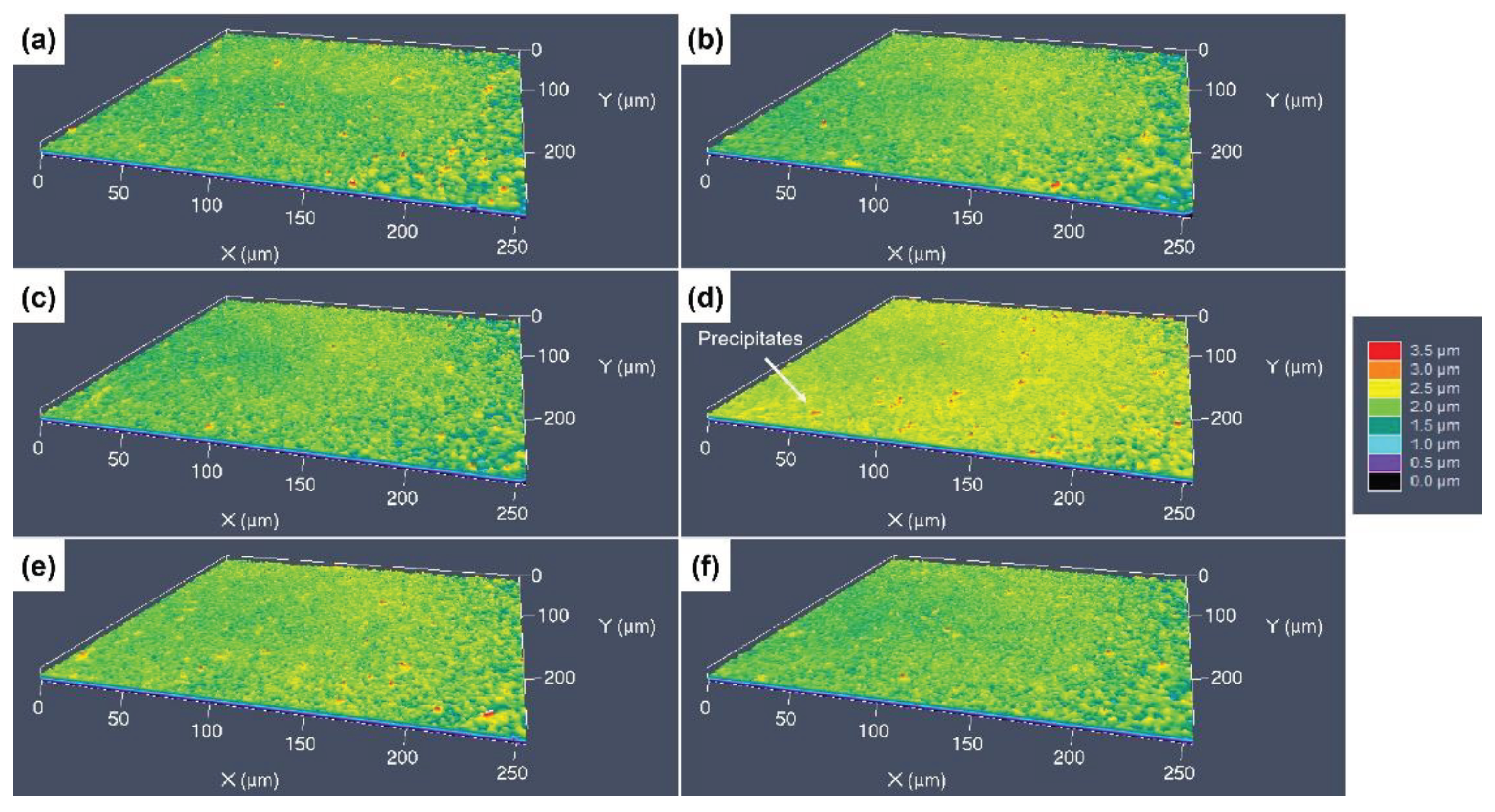
3.3. 3D Surface Undulations after Etching with Different Etchants
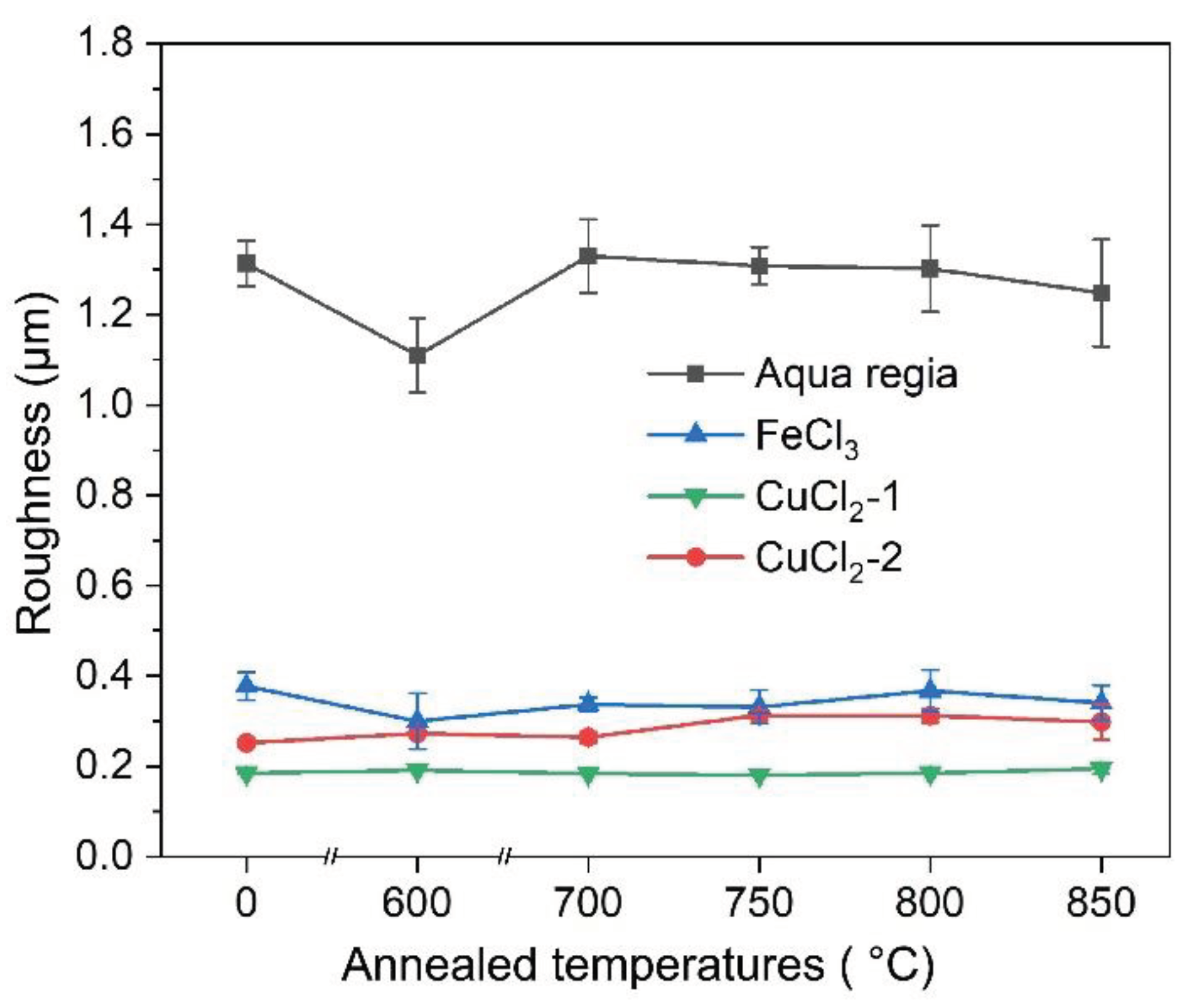
3.4. Surface Roughness
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visser, A.; Buhlert, M. Theoretical and practical aspects of the miniaturization of lead frames by double sided asymmetrical spray etching. J. Mater. Process. Technol. 2001, 115, 108–113. [Google Scholar] [CrossRef]
- Zhao, W.C.; Feng, R.; Wang, X.W.; Wang, Y.X.; Pan, Y.K.; Gong, B.K.; Han, X.J.; Feng, T.J. Effect of grain boundaries and crystal orientation on etching behavior of 12 µm thick rolled copper foil. Mater. Today Commun. 2023, 34, 105029. [Google Scholar] [CrossRef]
- Kim, B.J.; Jeon, H.I.; Kim, G.J.; Cho, N.H.; Khim, J.Y.; Kim, Y.K. Wettable flank routable thin Micro Lead Frame for automotive applications. Microelectron. Reliab. 2022, 135, 114602. [Google Scholar] [CrossRef]
- Yang, H.Y.; Ma, Z.C.; Lei, C.H.; Meng, L.; Fang, Y.T.; Liu, J.B.; Wang, H.T. High strength and high conductivity Cu alloys: A review. Sci. China Technol. Sci. 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Yang, K.; Wang, Y.H.; Guo, M.X.; Wang, H.; Mo, Y.D.; Dong, X.G.; Lou, H.F. Recent development of advanced precipitation-strengthened Cu alloys with high strength and conductivity: A review. Prog. Mater. Sci. 2023, 138, 101141. [Google Scholar] [CrossRef]
- Williams, K.R.; Gupta, K.; Wasilik, M. Etch rates for micromachining processing-Part II. J. Microelectromech. Syst. 2003, 12, 761–778. [Google Scholar] [CrossRef]
- Çakır, O. Review of etchants for copper and its alloys in wet etching processes. Key. Eng. Mater. 2008, 364, 460–465. [Google Scholar]
- Zhang, Y.; Zhang, Z.T.; Yang, J.L.; Yue, Y.K.; Zhang, H.F. Fabrication of superhydrophobic surface on stainless steel by two-step chemical etching. Chem. Phys. Lett. 2022, 797, 139567. [Google Scholar] [CrossRef]
- Allen, D.M.; Almond, H.J.A. Characterization of aqueous ferric chloride etchants used in industrial photochemical machining. J. Mater. Process. Technol. 2004, 149, 238–245. [Google Scholar] [CrossRef]
- Çakır, O. Chemical etching of aluminum. J. Mater. Process. Technol. 2008, 199, 337–340. [Google Scholar] [CrossRef]
- Sheng, J.Z.; Li, H.; Shen, S.N.; Ming, R.J.; Sun, B.; Wang, J.; Zhang, D.D.; Tang, Y.G. Investigation on chemical etching process of FPCB with 18 μm line pitch. IEEE Access 2021, 9, 50872–50879. [Google Scholar] [CrossRef]
- Çakır, O.; Temel, H.; Kiyak, M. Chemical etching of Cu-ETP copper. J. Mater. Process. Technol. 2005, 162, 275–279. [Google Scholar] [CrossRef]
- Choi, J.C.; Lee, J.H. Etching behaviors of Cu and invar for metal core PCB applications. J. Nanosci. Nanotechnol. 2017, 17, 7358–7361. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Huang, C.D.; Ji, X.Q.; Wang, Y.X. A new electrolytic method for on-site regeneration of acidic copper (II) chloride etchant in printed circuit board production. Int. J. Electrochem. Sci. 2013, 8, 6258–6268. [Google Scholar] [CrossRef]
- Darchen, A.; Drissi-Daoudi, R.; Irzho, A. Electrochemical investigations of copper etching by Cu (NH3) 4Cl2 in ammoniacal solutions. J. Appl. Electrochem. 1997, 27, 448–454. [Google Scholar] [CrossRef]
- Wang, S.F.; Ding, F.; Wang, F.W.; Wang, X.; Zou, H.L. Study on reducing side etching of Copper microelectrode by multi-step etching process. Mater. Res. Express. 2019, 6, 126411. [Google Scholar] [CrossRef]
- Kondo, K.; Kurihara, H.; Murakami, H. Etching morphology of single-crystal copper. Electrochem. Solid. St. 2005, 9, C36. [Google Scholar] [CrossRef]
- Köllensperger, P.A.; Karl, W.J.; Ahmad, M.M.; Pike, W.T.; Green, M. Patterning of platinum (Pt) thin films by chemical wet etching in Aqua Regia. J. Micromech. Microeng. 2012, 22, 067001. [Google Scholar] [CrossRef]
- Seo, B.H.; Lee, S.H.; Park, I.S.; Seo, J.H.; Choe, H.H.; Jeon, J.H.; Hong, M.P. Effect of nitric acid on wet etching behavior of Cu/Mo for TFT application. Curr. Appl. Phys. 2011, 11, S262–S265. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Fang, J.Y.; Li, C.F.; Liu, F.; Hou, H.L.; Zhang, X.L.; Zhang, Q.K.; Yang, L.J.; Xu, C.; Song, Z.L. Effects of grain orientation and grain size on etching behaviors of high-strength and high-conductivity Cu alloy. Mater. Today. Commun. 2024, 38, 108111. [Google Scholar] [CrossRef]
- Peng, L.J.; Xie, H.F.; Huang, G.J.; Xu, G.L.; Yin, X.Q.; Feng, X.; Mi, X.J.; Yang, Z. The phase transformation and strengthening of a Cu-0.71 wt% Cr alloy. J. Alloy. Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Li, J.Z.; Ding, H.; Li, B.M.; Gao, W.L.; Bai, J.; Sha, G. Effect of Cr and Sn additions on microstructure, mechanical-electrical properties and softening resistance of Cu-Cr-Sn alloy. Mater. Sci. Eng. A 2021, 802, 140628. [Google Scholar] [CrossRef]
- Yamashita, M.; Mimaki, T.; Hashimoto, S.; Miura, S. Intergranular corrosion of copper and α-Cu-Al alloy bicrystals. Philos. Mag. A 1991, 63, 695–705. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yoshimura, K.; Mimaki, T.; Yamashita, M. Behavior of intergranular corrosion of< 011> tilt grain boundaries of pure copper bicrystals. Corros. Sci. 2002, 44, 1835–1846. [Google Scholar]
- Carlson, R.K.; Yang, P.; Clegg, S.M.; Batista, E.R. Mechanistic Study of the Production of NO x Gases from the Reaction of Copper with Nitric Acid. Inorg. Chem. 2020, 59, 16833–16842. [Google Scholar] [CrossRef] [PubMed]
- Atta, R.M. Effect of applying air pressure during wet etching of micro copper PCB tracks with ferric chloride. Int. J. Mater. Res. 2022, 113, 795–808. [Google Scholar] [CrossRef]
- Choi, J.C.; Lee, Y.S.; Lee, J.; Kwon, H.W.; Lee, J.H. Etching behaviors of galvanic coupled metals in PCB applications. In Proceedings of the 2018 Pan Pacific Microelectronics Symposium (Pan Pacific), Big Island, USA, 5–8 February 2018; pp. 1–4. [Google Scholar]
- Jian, C.; Jusheng, M.; Gangqiang, W.; Xiangyun, T. Effects on etching rates of copper in ferric chloride solutions. In Proceedings of the 2nd 1998 IEMT/IMC Symposium (IEEE Cat. No. 98EX225), Tokyo, Japan, 15–17 August 2002; pp. 144–148. [Google Scholar]
- Braun, M.; Nobe, K. Electrodissolution kinetics of copper in acidic chloride solutions. J. Electrochem. Soc. 1979, 126, 1666. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




