1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease affecting multiple systems, primarily joints [
1,
2]. Chronic inflammation replaces bone erosion through cartilage destruction [
1,
2,
3]. Recent literature suggests that various pathogenetic mechanisms of RA involve immune system cells. CD4+ memory T cells present in tissue infiltrates or ectopic germinal centers stimulate B cells to proliferate, differentiate, and produce rheumatoid factor (RF) or ACPAs. In addition, the intima undergoes significant expansion due to the increased number and activation of macrophages, which secrete pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α, which together with proteinases cause bone and cartilage damage [
2,
4,
5].
Rheumatoid arthritis (RA) has two main subtypes, which are distinguished by the presence or absence of anti-citrullinated protein antibodies (ACPAs). Other autoimmune antibodies used in the diagnosis of RA are anti-nuclear antibodies (ANA), anti-cyclic citrullinated peptide (anti-CCP) and RF [
6,
7,
8]. During the early stages of RA, anti-CCP and RF may not be detectable in a significant percentage of patients and may remain undetectable in a small number of individuals throughout the course of the disease [
9,
10,
11]. As a result, the 2010 Rheumatoid Arthritis Classification Criteria included additional markers for the early stages of RA, such as abnormal C-reactive protein (CRP) and/or abnormal erythrocyte sedimentation rate (ESR) [
1,
12,
13].
RA is associated with increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in response to inflammation, which leads to oxidative stress [
14,
15,
16]. Antioxidants regulate potentially elevated levels of ROS/RNS during oxidative stress by scavenging them and inhibiting the oxidative process in cells [
15,
17,
18]. Studies have shown that patients with active disease exhibit higher levels of ROS and lower antioxidant potential compared to healthy controls [
15,
17,
19]. These findings have been confirmed in patients with RA through increased serum malondialdehyde (MDA) levels and decreased antioxidant enzyme activity, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [
20].
In RA, inflammation is also associated with changes in lipoprotein metabolism, particularly high-density lipoprotein cholesterol (HDL) [
21,
22,
23,
24,
25,
26]. Studies have suggested that people with RA may be at higher risk for CVD due to a decreased HDL production rate and an increased TG clearance rate, resulting in a high TG to HDL ratio [
24,
25,
27,
28,
29]. Alterations in lipid metabolism may be both a cause and/or a consequence of RA. Due to conflicting results regarding lipid levels in RA caused by differences in population, study duration or analytical methods of lipid metabolism, investigating various types of lipids and their metabolites, such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and their derived oxylipins (resolvins, maresins), may clarify the interventional role of lipids in RA and guide therapeutic regimens [
24,
25].
Treatment regimens vary depending on the stage of the disease. In case of RA patients, early treatment is recommended as it has been associated with reduced progression of synovitis and bone erosions, as well as improved disability prognosis [
30]. In the first-line treatment of RA, both disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX) and/or leflunomide (LEF), and biologic DMARDs are commonly used [
2,
31]. The most commonly used DMARD, MTX, works by releasing adenosine from fibroblasts. This reduces neutrophil adhesion, inhibits neutrophil leukotriene B4 synthesis, local IL-1 production, synovial collagenase gene expression and reduces IL-6 and IL-8 levels. However, even at low doses, there is still a risk of significant liver damage as a side effect of methotrexate (MTX) treatment [
30,
31,
32,
33].
Due to the crucial and dynamic functions of calcium, magnesium and phosphorus in maintaining bone tissue balance and managing inflammation [
34,
35], numerous studies have explored the potential benefits of magnesium, calcium and vitamin D supplementation in conjunction with various therapies in RA [
36,
37,
38]. Magnesium deficiency may promote the inflammatory process by prolonging the opening of calcium channels and activating N-methyl-D-aspartate receptors [
37].
Based on the above, we conducted a study to examine the correlation between certain biochemical, hematological, and oxidative stress parameters and specific markers of RA in the context of two different therapeutic regimens. We aimed to determine the relationship between these factors and RA treatment outcomes. As further clarification is needed on the supplementation of Mg, Ca and Vit D in RA patients, we included their blood levels in our study.
2. Materials and Methods
2.1. Study Population
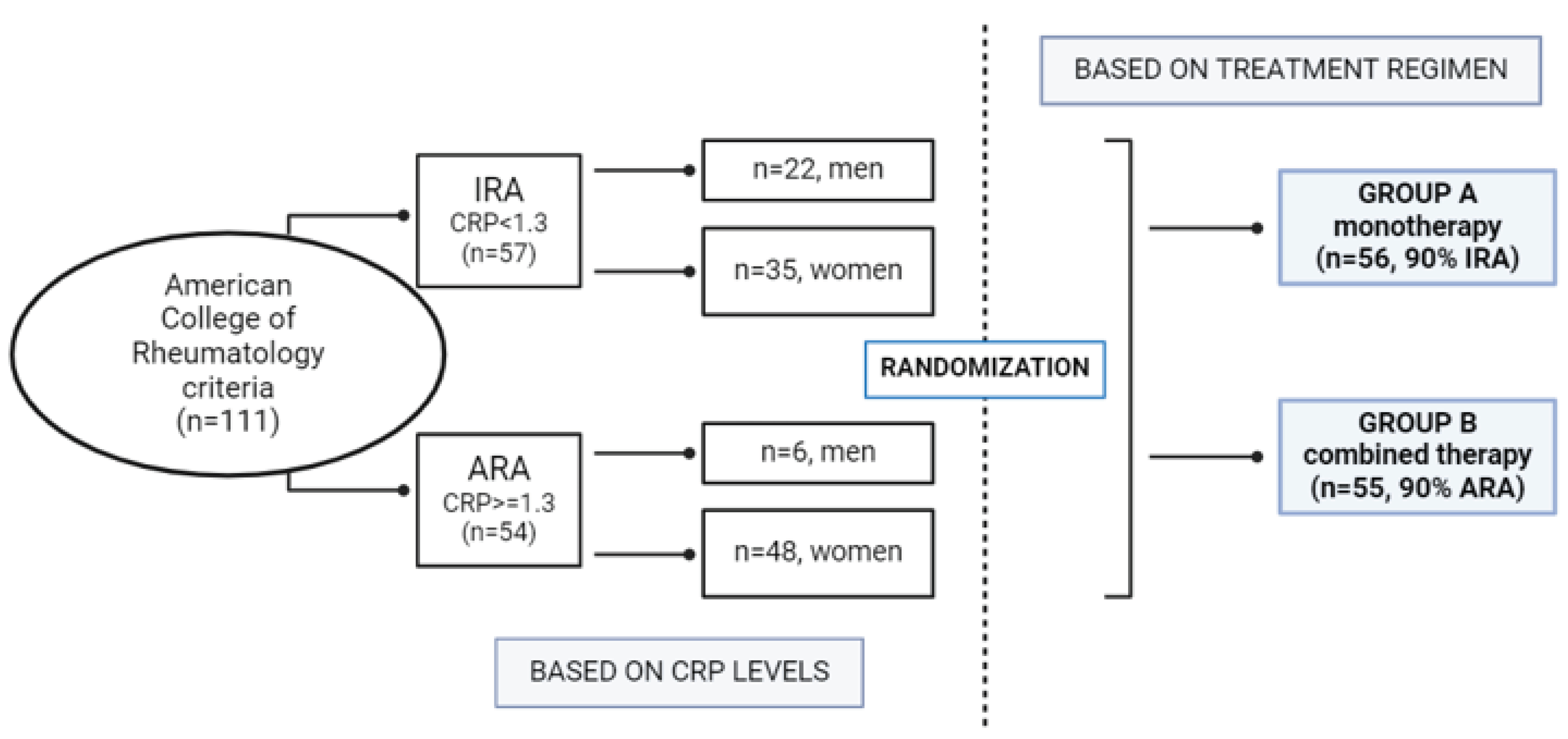
The study cohort included 111 patients (28 man, 83 women) in the early stages of RA, aged between 34 and 59 years. All patients attended the rheumatology outpatient clinic at the General Hospital and fulfilled the American College of Rheumatology criteria for the classification of RA. Written informed consent was obtained from all patients and the protocol was approved by the local ethics committee of the General Hospital. Patients were divided according to CRP levels into inactive RA patients (IRA) with CRP < 1.3 (n = 57, 22 men and 35 women) and active RA patients (ARA) with CRP ≥ 1.3 (n = 54, 6 men and 48 women). All patients (IRA and ARA patients), were divided into two groups, A and B, based on treatment regimen. Group A, consisting of 90% IRA (inactive patients), received MTX monotherapy and group B, consisting of 90% RA (active patients), receive a combination of a conventional DMARD, specifically leflunomide, with a biologic DMARD. (
Figure 1).
Blood samples were collected before and after the 12-week treatment period. Demographic and clinical information along with medical history details were collected from all patients. Our study participants were not taking any medication or dietary supplements. Blood samples were collected between 8:00 and 9:00 am, centrifuged to isolate serum and plasma samples from EDTA-treated blood, and stored at -80°C until processed. Hemolyzed samples were excluded. Laboratory analyses were performed on patients before and after treatment. These included: Erythrocyte sedimentation rate (ESR), platelets (PLT), c-reactive protein (CRP), rheumatoid factor (RF), anti-cyclic citrullinated peptide (Anti-CCP), anti-nuclear antibodies (ANA), reactive oxygen species (ROS), glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD), gamma-glutamyl transferase (γ-GT), vitamin C (Vit C), vitamin D (Vit D), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), alkaline phosphatase (ALP), amylase (AMY), phosphorus (P), magnesium (Mg) and calcium (Ca).
2.2. Determination of Significant Markers for Rheumatoid Arthritis
The specific markers for RA ˙ANA, RF and anti-CCP, were measured using the Roche Cobas E801 immunochemistry module. In particular, the determination of ANA was based on a standard indirect immunofluorescence (IIF) assay. RF was measured using a commercially available immunoturbidimetric assay.
2.3. Determination of Serum Lipoproteins, Vitamins, Liver enzymes, Amylase and Electrolytes
All biochemical parameters (TC, HDL, LDL, TG, PLT, Vit D, Vit C, γ-GT, ALP, AMY, Ca, Mg and P) were measured using colorimetric assays on the Roche Cobas 8000 analyzer series, specifically the C702 clinical chemistry module and the E801 immunochemistry module.
2.4. Determination of Reactive Oxygen Species
ROS activity was measured fluorometrically (infinite 200 PRO, TECAN Trading AG, Switzerland). Specifically, the assay was performed using the reagent 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), which measures the amount of H2O2 and other reactive oxygen species (ROS). The fluorescent intensity is proportional to ROS level.
2.5. Determination of antioxidants (GPx, CAT, SOD)
GPx activity was measured indirectly by a coupled reaction with glutathione reductase (GR). Oxidized glutathione, produced by the reduction of hydrogen peroxide by GPx, is converted to its reduced state by GR and NADPH. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm using the Elisa microplate reader. The decrease in NADPH measured at 340 nm is proportional to the GPx activity in the sample.
CAT activity was determined by measuring H2O2. Catalase catalyzes the decomposition of hydrogen peroxide. A decrease in the concentration of peroxide is accompanied by a decrease in absorbance at 240 nm.
A spectrophotometric method was used to determine SOD activity. The method is based on the xanthine/xanthine oxidase system, which produces superoxide which reduces nitrotetrazole blue to formazan. SOD inhibits the reaction and converts the superoxide to oxygen. The product is read at 550 nm using a Pharmacia Biotech Novaspec II spectrophotometer.
2.6. Statistical Analysis
The statistical analysis to calculate means and correlations was performed using the SPSS tool version 22.0. The data was checked for normality and appropriate statistical tests were chosen for analyses. We used the student t-test, to investigate the relationship between the two groups, before and after the implementation of the different therapeutic approaches, for each of the measured parameters. Additionally, we used the Pearson Correlation Coefficient to measure the linear correlation between the groups and the Spearman Correlation when required. In all statistical analyses, the level of significance (p-value) was set at 0.001 and 0.05.
2.7. Ethical Considerations
The study was conducted in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki and the European General Regulation 2016/679 and law Ν.2472/1997. Ethical approval to perform this study was obtained from the Administration and the Scientific Council of Hospital 638/412023. The confidentiality of the participants was strictly preserved, and personal privacy was fully respected.
3. Results
Table 1 shows the mean values of all biochemical and oxidative markers in the early stages of rheumatoid arthritis.
Participants were divided into two groups based on their CRP values: 57 participants (22 men and 35 women) with CRP < 1.3 were identified as IRA, while 54 participants (6 men and 48 women) with CRP ≥ 1.3 were identified as ARA.
All biomarkers in IRA and ARA patients showed deviant values compared to controls, except for PLTs, TC, AΝA, CAT and SOD. As expected, ARA patients had statistically significantly higher mean values of CRP (8.03mg/dL, p < 0.0001). Furthermore, ARA patients exhibited significantly higher levels of anti-CCP (17.69 U/mL, p < 0.0001), γ-GT (180.14 U/L, p < 0.01) and SOD (1.38 U/mg Hb, p < 0.05). Additionally, lower levels of TC (189.50 mg/dL, p < 0.0001) and ANA (1/360 U/mL, p < 0.0001) were also observed.
The study found no statistically significant differences between the mean values of ROS (68.71mM vs. 61.49mM, p = 0.2071), HDL (39.37mg/dL vs. 38.94mg/dL, p = 0.4145), LDL-C (197.82mg/dL vs. 193.28mg/dL, p = 0.2941), P (5.38mg/dL vs. 5.17mg/dL, p = 0.1432), Vit D (15.47ng/mL vs. 14.83ng/mL, p = 0.1995), ESR (25.12mm/h vs. 25.66mm/h, p = 0.3452), RF (43.97U/mL vs. 49.07U/mL, p = 0.1482), PLTs (301.39 103/μL vs. 316.88 103/μL, p = 0.2071), and TG (180.50mg/dL vs. 188.2 mg/dL, p = 0.21788), ALP (229.37U/L vs. 238.97U/L, p = 0.2357), AMY (202.39U/L vs. 215.11U/L, p = 0.1308), CAT (11.50U/mg Hb vs. 11.67U/mg Hb, p = 0.1099), Ca (6.76mg/dL vs. 6.83, p = 0.30546). There were no statistically significant differences between IRA and ARA patients in terms of the following parameters: 83mg/dL, p = 0.3055), GPx (7.71U/mgHb vs. 5.19U/mgHb, p = 0.4916), Mg (1.35mg/dL vs. 1.36mg/dL, p = 0.4227), and Vit C (0.34mg/dL vs. 0.34mg/dL, p = 0.4344). The p-value for each parameter was greater than 0.05, indicating no significant difference.
Table 2 presents the mean values of all parameters in group A (56 participants were treated with MTX monotherapy) and group B (55 participants were treated with a combination of a conventional DMARD). No significant differences were observed when comparing IRA and ARA patients in groups A and B. It is worth noting that in both groups A and B, the mean values of ESR, PLT, CRP, RF, Anti-CCP, ANA, GPx, CAT, SOD, γ-GT, ALP, TC, TG, LDL-C, ALP, AMY, and P decreased significantly, while ROS, Vit C, Vit D, HDL-C, Mg, and Ca values increased significantly after treatment (p < 0.0001). Also, there was no difference between the two different treatments.
Table 3 presents the correlation between biochemical and oxidative stress markers and specific markers of RA before and after two different treatments in patients with rheumatoid arthritis. In the early stages of the disease, glutathione peroxidase (GPx) shows a positive correlation with CRP (r = 0.261), RF (r = 0.137), and anti-CCP (r = 0.162). After treatment, total cholesterol (TC) is negatively correlated with CRP (r = -0.157) and RF (r = -0.333). At this phase, HDL-C shows a positive correlation with CRP (r = 0.297) and RF (r = 0.142). Vitamin C showed a positive correlation with CRP, before (r = 0.291) and after treatment (r = 0.354). No statistically significant correlations were observed between other biomarkers and specific markers of RA.
4. Discussion
Our study showed that biochemical and oxidative stress markers in conjunction with certain RA markers, hold promise as prognostic biomarkers during the inactive phase of the disease. Our study observed the impact of two different therapeutic approaches on biomarkers, which may have implications for the management of potential outcomes of rheumatoid arthritis.
Recent studies suggest that patients with RA may experience tissue damage due to oxidative stress caused by increased ROS generation, lipid peroxidation, protein oxidation, DNA damage and decreased antioxidant activity [
18,
39,
40]. Although there are conflicting views, one study found that patients with active RA had higher levels of oxidative status and increased antioxidant activity compared to healthy subjects. However, the levels of antioxidants have been found to be insufficient to effectively mitigate oxidative damage [
11]. Our study found that participants with inactive RA had high oxidative stress and low antioxidants, as indicated by CAT and SOD levels, which promote the disease's oxidative process. Meanwhile, GPx levels were elevated possibly because of its protective role in oxidative damage especially in this phase of the disease.
Significantly elevated values of TC, TG, and LDL were also observed. The results could be attributed to lipid peroxidation and oxidized lipids, such as arachidonic acid, prostaglandin, thromboxane, and leukotriene. These are primarily considered to be substrates for pro-inflammatory process [
24]. In contrast, lipoxins are anti-inflammatory and pro-resolving molecules that help reduce inflammation and restore tissue homeostasis [
24,
41].
Moreover, our results are in line with a previous study that demonstrated increased levels of ALP and γ-GT in RA patients with inflammation [
35]. Furthermore, elevated serum levels of amylase were found in the early stages of the disease. Another study suggested that salivary AMY concentration was also elevated in RA patients, suggesting the potential use of AMY as an early diagnostic marker [
42].
The data indicates that there is a change in calcium and phosphorus metabolism in RA [
43,
44]. A recent study found that serum calcium levels and the ratio of calcium to phosphorus were reduced, whereas serum phosphorus levels were increased in patients with inactive RA [
44]. A study has suggested that serum phosphorus levels could be used as a prognostic indicator of RA disease activity, even in those with subclinical activity [
43]. This is particularly true for inactive RA patients, as higher levels of serum P and lower levels of Ca were observed in them compared to active RA patients. These findings support our results. Therefore, serum P and Ca, along with specific markers for RA, could be used as potential prognostic biomarkers. During the active phase of RA, there is a continuous inflammatory process resulting in an increase in inflammation-related biomarkers such as CRP, ESR, as well as RA-specific markers including RF, anti-CCP, and ANA. These biomarkers indicate the immune system's response to the general inflammation associated with the disease. In patients with autoimmune RA, inflammation appears to be acutely associated with an imbalance in oxidative stress status and antioxidant systems [
14,
15].
During the active phase of the disease, certain antioxidant genes are activated to neutralize high levels of ROS and preserve the redox balance. This study showed that activation of the GPx3 gene reduced extracellular ROS levels, indicating the antioxidant protective effect of GPx on cells against oxidative damage. According to a previous study, activation of the gene GPx3 reduces the levels of extracellular ROS, which ultimately proves the protective antioxidant effect of GPx on cells against oxidative damage [
45]. Gonzalez et al. (2015) investigated changes in protein carbonyl levels, superoxide dismutase, glutathione peroxidase activities, glutathione concentration, and the glutathione/oxidized glutathione ratio in RA patients and healthy participants. The study found that although patients with active RA had higher levels of SOD and GPx activity, these levels were not sufficient to prevent oxidation damage to lipids and proteins [
11]. Our study supports these findings. ROS values decreased while antioxidants such as GPx, CAT and SOD increased possibly due to the anti-inflammatory response to the primary inflammation of the disease [
46].
During this phase of the disease, we found a positive correlation between GPx and CRP (r = 0.261), RF (r = 0.137), and anti-CCP (r = 0.162) in our patients. GPx plays a protective role against oxidation and primary inflammation [
47]. It could potentially be used as a diagnostic marker in combination with specific markers for RA. Recent studies have investigated the relationship between changes in serum lipid levels in RA patients before and after treatment [
19,
24,
48]. During the active phase of the disease, some studies have observed elevated levels of LDL and TG, while HDL levels were decreased [
49,
50,
51]. These findings are consistent with our results. In particular, we found that all RA patients, had significantly high levels of TC, TG and LDL before the onset of RA, whereas the levels of HDL were low.
A decrease in serum levels of Vit C and Vit D has been observed [
52] and as they are thought to have immunomodulatory and anti-inflammatory properties, their deficiency in RA patients may contribute to the progression or severity of the disease. Low levels of vitamin D may be associated with increased immune activation, as noted in several studies [
52,
53,
54], one of which showed that patients with high disease activity had lower vitamin D levels than those with moderate or low disease activity [
55]. Vitamin C has been shown to have strong antioxidant and anti-inflammatory effects [
56,
57,
58]. The possible etiological cause of vitamin deficiency may be related to the body's use of vitamins in response to inflammatory processes. Our study provides statistical evidence that both Vitamin C and Vitamin D levels increased after both types of treatment which contributes to a reduction in the inflammation level. The mechanism of their action is currently under investigation in our laboratory. Our findings indicate an improvement in vitamin C and D deficiencies following the treatment, compared to their pre-treatment levels.
In recent years, combinations of conventional DMARD with biologic DMARD have been used to treat patients with RA. Methotrexate (MTX) is one of the most commonly used and effective conventional DMARDs due to its ability to stabilize low-level disease activity. Katturajan et al. (2021) found that MTX inhibits methionine production, leading to hyperhomocysteinemia and intracellular oxidative stress. This oxidative stress inhibits the initiation of DNA binding by nuclear factor erythroid 2-related factor 2, which encodes antioxidant genes, particularly γ-gcs, through silent mating type information regulation-2 homolog. As a result, tissue damage is prevented and glutathione synthesis is inhibited [
59]. MTX-induced toxicity is associated with glutathione depletion, resulting in oxidative stress due to reduced antioxidant capacity [
60]. In our study, after three months of treatment, the levels of ROS values continued to increase in both treatment groups, while the values of antioxidant markers (GPx, CAT, SOD) decreased. This may be due to the mechanism of MTX action, which is dependent on the generation of ROS and requires further investigation. Several studies have observed an association between thrombocytopenia and MTX treatment [
61,
62]. Increased levels of ROS are responsible for premature platelet apoptosis [
63,
64]. A recent study found that MTX caused a significant release of Ca+2 from the endoplasmic reticulum, and analysis of elF2-a immunoblots indicated that PLT were exposed to endoplasmic reticulum stress during MTX treatment [
61]. This study supports our findings, as we also observed a decrease in PLT levels in both groups after treatment with DMARD. The decrease in PLT levels appears to be related to PLT apoptosis, which is caused by the mechanism of MTX action which depends on the production of ROS.
Inflammation-related biomarkers and RA-specific markers were found to be regulated to normal levels at this stage. We also observed that the imbalanced lipid levels were under the influence of disease medications, which may be due to the important role of lipoxins in reducing inflammation. The vitamin deficiencies were also treated without the use of supplements, suggesting that both treatment options were equally effective in improving the patients' blood lipid levels and their overall health. Patients should be encouraged to test their biomarkers frequently to help prevent comorbidities, drug toxicity, and to help manage their disease.
5. Conclusions
Based on our results, GPx could be used as a potential diagnostic marker when combined with other biomarkers such as ROS, Ca, P, Vit C, Vit D, and lipid profile due to their alterations in the early inactive phase of the disease. The general state of patients' health improved after three months of treatment regimens. Both treatment options were equally effective and did not influence redox status parameters differently. After the treatment, ROS levels continued to increase while antioxidant markers decreased. This may be attributed to the mechanism of DMARD action, which depends on the generation of ROS. To ensure that the treatment remains effective in regulating patients' oxidative stress, future studies on antioxidant supplementation in personalized medicine should be conducted before or after the treatment.
Author Contributions
Conceptualization, E.L.; methodology, K.K.; validation, S.I., A.T., and E.L.; formal analysis, S.I. and E.L.; investigation, S.I., A.T., K.K., A.G., and E.L.; data curation, S.I., K.K., A.G., A.F., and M.D.; writing—original draft preparation, S.I.; writing—review and editing, K.K., A.G., A.F., M.D., and L.E.; visualization, S.I., K.K., and E.L.; supervision, E.L.; project administration, E.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the European General Regulation 2016/679 and law Ν.2472/1997. Ethical approval to perform this study was obtained from the Administration and the Scientific Council of Hospital 638/412023.).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No data is available due to privacy or ethical restrictions.
Acknowledgments
We acknowledge and thank the contribution of all the doctors, nurses, and other members of the outpatient Clinic of General Hospital of Greece who were involved in the care of the patients and collection of the blood samples, but who are not listed as authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACPAs |
anti-citrullinated protein antibodies |
| ALP |
alkaline phosphatase |
| ALT |
alanine transaminase |
| AMY |
amylase |
| ANA |
anti-nuclear antibodies |
| ARA |
active rheumatoid arthritis |
| AST |
aspartate transaminase |
| Anti-CCP |
anti-cyclic citrullinated peptide |
| Ca |
calcium |
| CAT |
catalase |
| CRP |
c-reactive protein |
| CVD |
cardiovascular disease |
| DHA |
docosahexaenoic acid |
| DMARD |
disease-modifying antirheumatic drugs |
| EPA |
eicosapentaenoic acid |
| ESR |
erythrocyte sedimentation rate |
| γ-GT |
gamma-glutamyl transferase |
| GR |
glutathione reductase |
| GPx |
glutathione peroxidase |
| GSH |
glutathione |
| HDL |
high-density lipoprotein |
| H2DCFDA |
2’, 7’-dichlorodihydrofluorescein diacetate |
| H2O2 |
hydrogen peroxide |
| IIF |
indirect immunofluorescence |
| IL-1 |
interleukin-1 |
| IL-6 |
interleukin-6 |
| IL-8 |
interleukin-8 |
| IRA |
inactive rheumatoid arthritis |
| LDL |
low-density lipoprotein |
| LEF |
leflunomide |
| MDA |
malondialdehyde |
| Mg |
magnesium |
| MTX |
methotrexate |
| NADPH |
nicotinamide adenine dinucleotide phosphate |
| Ox-LDL |
oxidized low-density lipoprotein |
| P |
phosphorus |
| PLT |
platelets |
| RA |
rheumatoid arthritis |
| RADAI |
rheumatoid arthritis disease activity index |
| RF |
rheumatoid factor |
| RNS |
reactive nitrogen species |
| ROS |
reactive oxygen species |
| SOD |
superoxide dismutase |
| TC |
total cholesterol |
| TG |
triglycerides |
| TNF |
tumor necrosis factor |
| TOS |
total oxidative status |
| Vit C |
vitamin C |
| Vit D |
vitamin D |
References
- Lin YJ, Anzaghe M, Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. 8: Cells 2020; 9, 2020. [CrossRef]
- Yap HY, Tee SZY, Wong MMT, Chow SK, Peh SC, Teow SY. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018;7: 161. [CrossRef]
- Nanke, Y. The Pathogenesis of Rheumatoid Arthritis Breakthroughs in Molecular Mechanisms 1 and 2. International Journal of Molecular Sciences 2023; 24: 11060. [CrossRef]
- Jang S;, Kwon E-J;, Lee JJ, Spinelli R, Pecani A, Jang S, et al. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. International Journal of Molecular Sciences 2022; 23: 905. [CrossRef]
- Cajas LJ, Casallas A, Medina YF, Quintana G, Rondón F. Pannus and rheumatoid arthritis: Historic and pathophysiological evolution. Revista Colombiana de Reumatología (English Edition) 2019;26:118–28. [CrossRef]
- Van Boekel MAM, Vossenaar ER, Van Den Hoogen FHJ, Van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res 2002;4:87. [CrossRef]
- Conti V, Corbi G, Costantino M, De Bellis E, Manzo V, Sellitto C, et al. Biomarkers to Personalize the Treatment of Rheumatoid Arthritis: Focus on Autoantibodies and Pharmacogenetics. Biomolecules 2020; 10: 1672. [CrossRef]
- Paknikar SS, Crowson CS, Davis JM, Thanarajasingam U. Exploring the Role of Antinuclear Antibody Positivity in the Diagnosis, Treatment, and Health Outcomes of Patients With Rheumatoid Arthritis. ACR Open Rheumatol 2021;3:422. [CrossRef]
- Venables P, Maini RN. Diagnosis and differential diagnosis of rheumatoid arthritis. UpToDate 2012.
- Braschi E, Shojania K, Michael Allan G. Anti-CCP: a truly helpful rheumatoid arthritis test? Canadian Family Physician 2016;62:234.
- García-González A, Gaxiola-Robles R, Zenteno-Savín T. Oxidative stress in patients with rheumatoid arthritis. Rev Invest Clin 2015;67:46–53. [CrossRef]
- Atzeni F, Talotta R, Masala IF, Bongiovanni S, Boccassini L, Sarzi-Puttini P. Biomarkers in Rheumatoid Arthritis. Cureus 2021;13:512–6. [CrossRef]
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [CrossRef]
- Ramos-González EJ, Bitzer-Quintero OK, Ortiz G, Hernández-Cruz JJ, Ramírez-Jirano LJ. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurologia 2021. [CrossRef]
- Fonseca LJS Da, Nunes-Souza V, Goulart MOF, Rabelo LA. Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxid Med Cell Longev 2019;2019. Article ID 7536805. [CrossRef]
- Smallwood MJ, Nissim A, Knight AR, Whiteman M, Haigh R, Winyard PG. Oxidative stress in autoimmune rheumatic diseases. Free Radic Biol Med 2018;125:3–14. [CrossRef]
- Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS One 2016;11:e0152925. [CrossRef]
- Ponist S, Zloh M, Bauerova K, Ponist S, Zloh M, Bauerova K. Impact of Oxidative Stress on Inflammation in Rheumatoid and Adjuvant Arthritis: Damage to Lipids, Proteins, and Enzymatic Antioxidant Defense in Plasma and Different Tissues. Animal Models in Medicine and Biology 2019. [CrossRef]
- Quinonez-Flores CM, Gonzalez-Chavez SA, Del Rio Najera D, Pacheco-Tena C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. Biomed Res Int 2016;2016. [CrossRef]
- Jawad Mahdi PK, Sc M, Abdulkareem Mohammed Jewad T, Mohamed Naji Kassim T, medicine I. Oxidative Stress Status In Patients With Rheumatoid Arthritis. University of Thi-Qar Journal Of Medicine 2019;17:135–44. [CrossRef]
- Navarro-Millán I, Charles-Schoeman C, Yang S, Bathon JM, Bridges SL, Chen L, et al. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum 2013;65:1430–8. [CrossRef]
- Charles-Schoeman C, Yin Lee Y, Shahbazian A, Wang X, Elashoff D, Curtis JR, et al. Improvement of High-Density Lipoprotein Function in Patients With Early Rheumatoid Arthritis Treated With Methotrexate Monotherapy or Combination Therapies in a Randomized Controlled Trial. Arthritis Rheumatol 2017;69:46–57. [CrossRef]
- Charles-Schoeman C, Wang X, Lee YY, Shahbazian A, Navarro-Millán I, Yang S, et al. Association of Triple Therapy With Improvement in Cholesterol Profiles Over Two-Year Followup in the Treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheumatol 2016;68:577–86. [CrossRef]
- Lei Q, Yang J, Li L, Zhao N, Lu C, Lu A, et al. Lipid metabolism and rheumatoid arthritis. Front Immunol 2023;14:1190607. [CrossRef]
- Liao KP, Playford MP, Frits M, Coblyn JS, Iannaccone C, Weinblatt ME, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc 2015;4. [CrossRef]
- Svenson KLG, Lithell H, Hällgren R, Selinus I, Vessby B. Serum Lipoprotein in Active Rheumatoid Arthritis and Other Chronic Inflammatory Arthritides: I. Relativity to Inflammatory Activity. Arch Intern Med 1987;147:1912–6. [CrossRef]
- Kim JY, Lee EY, Park JK, Song YW, Kim JR, Cho KH. Patients with Rheumatoid Arthritis Show Altered Lipoprotein Profiles with Dysfunctional High-Density Lipoproteins that Can Exacerbate Inflammatory and Atherogenic Process. PLoS One 2016;11:e0164564. [CrossRef]
- Fernández-Ortiz AM, Ortiz AM, Pérez S, Toledano E, Abásolo L, González-Gay MA, et al. Effects of disease activity on lipoprotein levels in patients with early arthritis: Can oxidized LDL cholesterol explain the lipid paradox theory? Arthritis Res Ther 2020;22:1–12. [CrossRef]
- Dursunoǧlu D, Evrengül H, Polat B, Tanriverdi H, Çobankara V, Kaftan A, et al. Lp(a) lipoprotein and lipids in patients with rheumatoid arthritis: serum levels and relationship to inflammation. Rheumatol Int 2005;25:241–5. [CrossRef]
- Benjamin O, Bansal P, Goyal A, Lappin SL. Disease-Modifying Antirheumatic Drugs (DMARD). StatPearls 2023.
- Hu Q, Wang H, Xu T. Predicting Hepatotoxicity Associated with Low-Dose Methotrexate Using Machine Learning. 1: Journal of Clinical Medicine 2023; 12, 2023. [CrossRef]
- Karlsson Sundbaum J, Eriksson N, Hallberg P, Lehto N, Wadelius M, Baecklund E. Methotrexate treatment in rheumatoid arthritis and elevated liver enzymes: A long-term follow-up of predictors, surveillance, and outcome in clinical practice. Int J Rheum Dis 2019;22:1226–32. [CrossRef]
- Xin D, Li H, Zhou S, Zhong H, Pu W. Effects of Anthraquinones on Immune Responses and Inflammatory Diseases. 3: Molecules 2022; 27, 2022. [CrossRef]
- Klein, GL. The Role of Calcium in Inflammation-Associated Bone Resorption. 6: Biomolecules 2018; 8, 2018. [Google Scholar] [CrossRef]
- Ciosek Ż, Kot K, Kosik-Bogacka D, Łanocha-Arendarczyk N, Rotter I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. 5: Biomolecules 2021; 11, 2021. [CrossRef]
- Wu CY, Yang HY, Luo SF, Huang JL, Lai JH. Vitamin D Supplementation in Patients with Juvenile Idiopathic Arthritis. 1: Nutrients 2022; 14, 2022. [CrossRef]
- Tarleton EK, Kennedy AG, Rose GL, Littenberg B. Relationship between Magnesium Intake and Chronic Pain in U.S. Adults. 2: Nutrients 2020; 12, 2020. [CrossRef]
- Nakamura Y, Suzuki T, Yoshida T, Yamazaki H, Kato H. Vitamin D and Calcium Are Required during Denosumab Treatment in Osteoporosis with Rheumatoid Arthritis. Nutrients 2017; 9: 428. [CrossRef]
- Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS One 2016;11. [CrossRef]
- Prakash B D, Manjunath S, Sumangala K, Chetana K, Vanishree J. Oxidative stress and enzymatic antioxidant status in rheumatoid arthritis: a case control study. Eur Rev Med Pharmacol Sci 2010;14:959–67.
- Chandrasekharan JA, Sharma-Wali N. Lipoxins: nature’s way to resolve inflammation. J Inflamm Res 2015;8:181. [CrossRef]
- Pakravan F, Isfahani MN, Ghorbani M, Salesi N, Salesi M. The salivary alpha-amylase concentration in patients with rheumatoid arthritis: A case–control study. Dent Res J (Isfahan) 2023;20:43.
- Mohamed SB, Elkenawy MF, Elsaid TO, Mashaly GES. Serum substance P level as a marker for subclinical rheumatoid arthritis activity. Egyptian Journal of Basic and Applied Sciences 2023;10:1–10. [CrossRef]
- Jambale TA, Halyal S. Study of serum calcium/ phosphorus in rheumatoid arthritis patients. International Journal of Clinical Biochemistry and Research 2017;4:103–5. [CrossRef]
- Chen T, Zhou Z, Peng M, Hu H, Sun R, Xu J, et al. Glutathione peroxidase 3 is a novel clinical diagnostic biomarker and potential therapeutic target for neutrophils in rheumatoid arthritis. Arthritis Res Ther 2023;25:1–13. [CrossRef]
- Phillips DC, Woollard KJ, Griffiths HR. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol 2003;138:501–11. [CrossRef]
- Jacobson GA, Ives SJ, Narkowicz C, Jones G. Plasma glutathione peroxidase (GSH-Px) concentration is elevated in rheumatoid arthritis: a case-control study. Clin Rheumatol 2012;31:1543–7. [CrossRef]
- Dessie G, Tadesse Y, Demelash B, Genet S. Assessment of serum lipid profiles and high-sensitivity c-reactive protein among patients suffering from rheumatoid arthritis at tikur anbessa specialized hospital, addis ababa, ethiopia: A cross-sectional study. Open Access Rheumatol 2020;12:223–32. [CrossRef]
- Mullick OS, Bhattacharya R, Bhattacharyya K, Sarkar RN, Das A, Chakraborty D, et al. Lipid profile and its relationship with endothelial dysfunction and disease activity in patients of early Rheumatoid Arthritis. Indian J Rheumatol 2014;9:9–13. [CrossRef]
- Dessie, G. Association of atherogenic indices with C-reactive protein and risk factors to assess cardiovascular risk in rheumatoid arthritis patient at Tikur Anbessa Specialized Hospital, Addis Ababa. PLoS One 2022;17:e0269431. [CrossRef]
- Chavan VU, Ramavataram D, Patel PA, Rupani MP. Evaluation of Serum Magnesium, Lipid Profile and Various Biochemical Parameters as Risk Factors of Cardiovascular Diseases in Patients with Rheumatoid Arthritis. J Clin Diagn Res 2015;9:BC01. [CrossRef]
- Meena N, Chawla SPS, Garg R, Batta A, Kaur S. Assessment of Vitamin D in Rheumatoid Arthritis and Its Correlation with Disease Activity. J Nat Sci Biol Med 2018;9:54. [CrossRef]
- Bragazzi NL, Watad A, Neumann SG, Simon M, Brown SB, Abu Much A, et al. Vitamin D and rheumatoid arthritis: an ongoing mystery. Curr Opin Rheumatol 2017;29:378–88. [CrossRef]
- Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum 2004;50:72–7. [CrossRef]
- Vitamin D deficiency in rheumatoid arthritis. Prevalence and association with disease activity in Western Saudi Arabia - Saudi Med J 2012; 33: 5 https://pubmed.ncbi.nlm.nih. 2258.
- Gęgotek A, Skrzydlewska E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022;11. [CrossRef]
- G, GT. Effects of Vitamin C and Vitamin E in rheumatoid arthritis-A randomized, open label, and comparative study in a tertiary care hospital. Natl J Physiol Pharm Pharmacol 2022;12:9. [CrossRef]
- Pattison DJ, Silman AJ, Goodson NJ, Lunt M, Bunn D, Luben R, et al. Vitamin C and the risk of developing inflammatory polyarthritis: prospective nested case-control study. Ann Rheum Dis 2004;63:843. [CrossRef]
- Katturajan R, S V, Rasool M, Evan Prince S. Molecular toxicity of methotrexate in rheumatoid arthritis treatment: A novel perspective and therapeutic implications. Toxicology 2021;461:152909. [CrossRef]
- Schmidt S, Messner CJ, Gaiser C, Hämmerli C, Suter-Dick L. Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. Int J Mol Sci 2022;23:15116. [CrossRef]
- Paul M, Hemshekhar M, Thushara RM, Sundaram MS, NaveenKumar SK, Naveen S, et al. Methotrexate Promotes Platelet Apoptosis via JNK-Mediated Mitochondrial Damage: Alleviation by N-Acetylcysteine and N-Acetylcysteine Amide. PLoS One 2015;10. [CrossRef]
- Franck H, Rau R, Herborn G. Thrombocytopenia in patients with rheumatoid arthritis on long-term treatment with low dose methotrexate. Clin Rheumatol 1996;15:266–70. [CrossRef]
- Wang Z, Cai F, Chen X, Luo M, Hu L, Lu Y. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PLoS One 2013;8. [CrossRef]
- Girish KS, Paul M, Thushara RM, Hemshekhar M, Shanmuga Sundaram M, Rangappa KS, et al. Melatonin elevates apoptosis in human platelets via ROS mediated mitochondrial damage. Biochem Biophys Res Commun 2013;438:198–204. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).