Preprint
Article
Viral dynamics in the tropical Pacific Ocean: a comparison between within and outside warm eddy
Altmetrics
Downloads
122
Views
38
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
30 March 2024
Posted:
01 April 2024
You are already at the latest version
Alerts
Abstract
In mesoscale eddies, chemical properties and biological composition are different from those in surrounding water, due to their unique physical processes. It is unclear how physical-biological coupling occurs in warm-core eddies, particularly since no study has examined the relationship between bacteria and viruses. The purpose of the present study was to examine the influence of anticyclonic warm eddy on the relationship between bacterial and viral abundances as well as viral activity (viral production) in different depths. In the core of warm eddy, bacterial abundance (0.48 to 2.82 ×105 cells mL−1) fluctuated less than that of the outside eddy (1.12 to 7.03×105 cells mL−1). In particular, there was four-folds higher of viral-bacterial abundance ratio (VBR) estimated within the eddy below DCM than the outside eddy. We suggest that prevailing physical forces with downwelling at the center of the anticyclonic warm eddy, viruses can also be transmitted into the deep ocean directly through adsorption on particulate organic matter during sinking. Overall, our findings provide valuable insights into the interaction between bacterial and viral communities and their ecological mechanism within the warm eddy.
Keywords:
Subject: Environmental and Earth Sciences - Oceanography
1. Introduction
Mesoscale eddies occur often in the open ocean because of water mass turbulence [1,2,3], and include both warm-core eddies (anticyclones) and cold-core eddies (cyclones). A convergence of anticyclonic warm waters in the ocean sinks nutrient-depleted surface waters towards the subsurface, intensifying oligotrophy in the upper euphotic column [4]. The downwelling nature of warm eddies isolates nutrient-depleted water at the surface, presumably supporting the microbial food web by cycling nutrients [5]. In addition, the response of bacterial communities to eddy core water may be distinct from that of their surroundings, such as variation in bacterial abundance [6,7,8], production [9], and community composition [10,11]. Most of these studies analyze warm eddy impacts on bacterial communities and abundance, indicating that physical-chemical variations contribute to changes in bacterial dynamics. However, there is an apparent gap in the top-down control mechanisms affecting bacteria (such as viruses).
In general, viruses affect microbial mortality, as well as the ecological processes and biogeochemical cycles of the oceans of the World [12,13]. The lysis of bacteria by viruses disrupts the flow of energy and organic matter by creating a “viral loop” of bacteria, viruses, and dissolved organic matter [14]. This means that viruses in oligotrophic environments act not only as top-down agents but also as bottom-up agents regulating bacterial growth [15]. There have been numerous studies that have evaluated the spatial distribution of viruses and their hosts (such as bacteria) in estuaries, coastal waters, and open oceans, as well as in freshwater ecosystems [14,16,17,18,19]. While the influence of different layers on viral abundance and activity inside mesoscale eddies has been studied less, it is unknown how spatial patterns of bacteria affect viral abundance and activity.
There is at least an order of magnitude difference between the abundance of viruses and bacteria in most ecosystems [20]. The viral-bacterial abundance ratio (VBR) is commonly used as an indicator of the relationship between bacteria and viruses. The VBR index determines the severity of virus infection, such that high values indicate a high viral dynamic and, consequently, enhanced lysis of prokaryotic cells [14]. A low value, on the other hand, may suggest a decrease in or absence of viral activity [14,21]. In addition, differences in VBR values are also affected by physicochemical factors (e.g., temperature, salinity, and nutrients) [21]. Generally, viral abundance decreases dramatically throughout the photic zone, reaching a constant abundance at depth, and correlated with the distributions of the most abundant hosts (bacteria and phytoplankton) [14]. Taylor et al. [22] showed that the mean VBR of oxic layers (16) was significantly lower than that of anoxic layers (VBR = 31), suggesting varying relationships among viruses, hosts, and environments. There have further been reports of high VBR in bathypelagic waters in the open North Atlantic [23] and South Atlantic Ocean [24] as well as the Pacific [25]. There is still a lack of understanding of the factors controlling viral dynamics. Even though VBR is the result of a complex balance of factors that include viral production, virus transport via sinking particles, decay rates, and life strategies [20,26], However, there is scarce information on the occurrence and characteristics of viruses and bacteria in the oceanic water column of warm eddies.
To the best of our knowledge, little is known about the viral abundance and viral production response associated with warm eddies from surface to deeper waters. The purpose of the present study was to examine the influence of anticyclonic warm eddy on the relationship between bacterial and viral abundances as well as viral activity (viral production) in different depths. To achieve this goal, we conducted field experiments to measure viral production to identify the viral dynamics and relationship between bacteria and viruses inside and outside a warm eddy in the tropical Pacific Ocean during warm eddy movement in surface, DCM, 200 m and 500 m layers. Particularly, there are no studies on lytic viral infection and lysogeny in contrasting marine environments of warm eddies, investigating both viral life cycles simultaneously. To evaluate the specific role viruses play in microbial food-web processes in different environments, it is necessary to gather such information. Based on current knowledge of the effect of mesoscale eddies in the oceans, our hypothesis states that there are different viral dynamics (viral abundance, viral production) between warm eddies and the surrounding waters, and induce different relationships between bacterial and viral abundance.
2. Materials and methods
2.1. Study Site and Samplings
An investigation aboard the R/V Thompson was conducted in the west Pacific Ocean from May 29 to June 10, 2023 (Figure 1). Eddy characteristics are based on the sea surface height (SSH) and acoustic Doppler current profiler (ADCP) velocity data (Figure 1). Several large-scale circulation transport pathways cross the western equatorial Pacific Ocean, connecting the north, south, and central Pacific Oceans to the tropical Indian Ocean. The North Equatorial Current (NEC), North Equatorial Counter Current (NECC), and North Pacific Subtropical Countercurrent (STCC) form a complex current system. Our study investigated how anticyclonic eddies influence bacterial and viral abundance as well as viral production by sampling physical, chemical, and microbial parameters at two sites: out-of-eddy (OE) and eddy core (EC). We monitored the drifting positions of drifting buoys to monitor the same eddy. Furthermore, incubation experiments of viral production at OE and EC were carried out at a surface depth of 5 m, 130-140 m of DCM, 200 m, and 500 m.
2.2. Viral Production
For the purpose of estimating viral production from the experiment for 24 hours, we used the dilution technique of Wilhelm et al. [27]. Further, lytic viral infections (bacteria in a lytic stage) and lysogeny (bacteria-producing inducible prophages) were examined. The first step in obtaining virus-free water was its filtration through a 0.2 µm pore membrane, followed by tangential flow filtration (TFF) using a molecular weight cut-off of 30 kDa. Then, one liter prefiltered seawater samples (2 µm) were concentrated to obtain a volume of 100 mL using a 0.2 µm -pore-size polycarbonate filter (47 mm diameter, Millipore) under low vacuum (< 200 mm Hg), and transferred the concentrated water by a pipette. Our experiment involved diluting 100 mL of concentrated water with 400 mL of virus-free water. Consequently, the viral density in the seawater was reduced to 20% of what it had been previously, reducing the chances of contact between virus and host, and thereby preventing new infections. A chemical agent, mitomycin C (1.0 μg ml-1 final concentration; Merck), was used as an inducing agent to switch from a lysogenic to a lytic cycle for dilution samples collected, while a control dilution sample remained untreated. After that, diluted samples were incubated in triplicate in 50 mL polycarbonate bottles and a temperature-controlled chamber containing similar in situ temperatures at each depth for 24 hours in the dark. During the 24 h incubation period, subsamples of 1 mL were taken in triplicate at 6 h intervals for bacterial and viral enumeration. Briefly, lytic VP could be represented by viral accumulation in control tubes without mitomycin C addition. Based on the difference between mitomycin C-treated and control tubes, lysogenic VP was calculated [28].
After correcting for the dilution of bacterial hosts between the samples and the natural communities, the lytic VP and lysogenic VP were calculated by using first-order regressions of viral abundance versus time. This was necessary to account for the loss of potentially infected cells during filtration. It was calculated using the formula proposed by Hewson and Fuhrman [29]: VP (lytic, lysogenic) = m (lytic, lysogenic) × (b/B), where m is the slope of the regression line, b is the concentration of bacteria after dilution, and B is the concentration of bacteria before dilution. In this study, the total VP (TVP) here is calculated by adding lytic VP to lysogenic VP. A viral turnover rate (VT, d-1) is calculated by dividing TVP by in situ viral abundance.
We estimated viral-induced mortality of bacteria (VMB, bacteria mL-1d-1) based on an estimate of 24 burst sizes (BS, number of viruses released per lytic event) [30]. As shown in the equation, VMB = TVP/BS. Additionally, the loss rate of bacteria from viral activity (LRB-V) was calculated as VMB / (in situ bacteria).
Calculation of the bacterial net growth rate (r) was based on the change in abundance during the exponential phase of growth during the study period (24 h). In this study, we used the equation: r = (ln Nt - ln N0)/t, where N0 and Nt are the bacterial abundances at the beginning of the study, as well as the peak values. The time when bacterial abundance reaches its peak is t. Further, the ratio of bacterial production removed by viral lysis (Viral control factor) was estimated by r/(LRB-V).
2.3. Flow Cytometric Analyses
Flow cytometry samples of viruses and bacteria were analyzed using a CytoFLEX S flow cytometer (Beckman Coulter, Indianapolis), which is equipped with an argon- Ion laser at 488 nm, a 525 nm filter, and a SYBR signal trigger. For reduction of particle density interference, virus samples were diluted 1:10 in TE buffer (pH 8.0, EM grade) before staining. Dilute the samples and incubate them with SYBR Green I for 10 minutes at 80°C (final concentration 50,000 of commercial stock). The samples were then cooled in an ice bath to 25 °C and processed through FCM in accordance with Brussaard [31]. We performed blank controls using TE buffer stained with the same concentration of SYBR Green I to detect and eliminate buffer noise. Before FCM processing, bacteria samples were stained for 15 minutes with SYBR Green I at a concentration of 1:10,000 as described by Hammes and Egli [32].
3. Results
3.1. Station Characteristics
The warm eddy had a significant effect on vertical distributions of temperature and salinity in the study (Figure 2). Based on temperature and salinity profiles for OE and EC, a well-mixed surface layer reached a depth of ca. 50 m (Figs. 2A, B). The surface water temperature was at least 1°C higher at the core (EC) than in the surrounding water (OE). As shown in Figure 2 A and B, in the upper 500 m depth of the EC, water temperatures ranged from 10.6 to 29.2°C, and salinity varied from 34.27 to 34.97 psu. Compared to sampling points in the same layers in OE, both temperature and salinity were higher in warm eddy (Figure 2). A dome-like distribution of cold and low salinity water was observed at 15–20°N at the junction of the NEC and the STCC (Figure 2B). The chlorophyll a concentration in the surface water was similar inside and outside the eddy. A deep chlorophyll maximum (DCM) at OE was centered at about 130 m, whereas a deeper DCM was found within the eddy (Figure 2C).
3.2. Bacterial and Viral Abundance
Abundance of bacteria in OE ranged from 1.12 to 7.03×105 cells mL−1 from the surface to 500 m depth with the lowest concentrations recorded from the surface and the highest concentrations recorded at 100 m depth (Figure 3A). In the EC station, bacterial abundance (0.48 to 2.82 ×105 cells mL−1) fluctuated less than that of the OE station (Figure 3A). Virus abundance was greater than bacterial abundance, viral abundance was 0.81 × 106–2.60 × 106 viruses mL−1, and 0.60 × 106–1.31 × 106 viruses mL−1 at OE and EC station, respectively (Figure 3B). There were significant differences in the bacterial and viral abundance inside and outside of the eddy (t-test, p< 0.05), with the higher abundance of bacteria and viruses were observed in the OE (Figs. 3A, B). Furthermore, a large variation of VBR was observed at the EC station with depth, and the values differed significantly between the two stations from 200 m to 500 m depth (t-test, p< 0.05), with the higher VBR values in the EC region (Figure 3C).
3.3. Viral Production
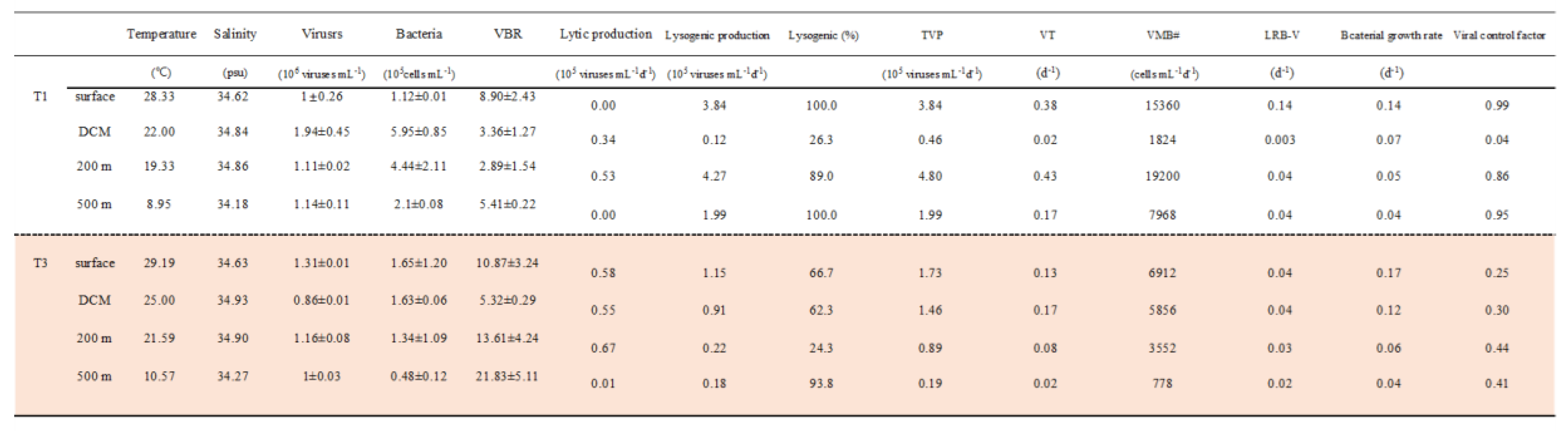
Viral abundances varied after viral reduction and during the 24-hour incubation experiment in control and mitomycin C-treated samples, as expected from inducible lysogenic bacteria (Figure 4). We analyzed the data to determine viral production and found that lytic and lysogenic bacteria were primarily detected after 12–24 hours (Figure 4). As shown in Figure 4 and Table 1, the heterotrophic bacterial community displays very high levels of lysogeny (100%) outside of the warm eddy. According to prophage induction due to mitomycin C, lysogenic production was found to range from 24 % - 94 % in bacteria communities in the EC region, with the highest values found in deep waters (500 m) (Table 1). In the OE and EC stations, the total virus population (TVP) varied widely with 0.46~4.80 and 0.19~1.73 × 105 viruses mL−1 h−1, respectively (Table 1). Viral turnover rate (VT) ranged from 0.02 to 0.43 d-1 and 0.02 to 0.17 d-1 in the OE and EC, respectively (Table 1). TVP and VT were significantly higher at experimental depths at OE than at EC, except at the DCM layer (Table 1).
3.4. Virus-Mediated Bacterial Mortality
Assuming a BS of 24 (see Methods 2.2), estimates of the virus-mediated loss rate of bacteria (VMB) varied between 1824 and 19200 cells mL-1 d−1 at the OE station, and between 778 and 6912 cells mL-1 d−1 at the EC station (Table 1). As with the experimental depths, the VMB decreased markedly with increasing depths at the EC station, but the lowest value (1824 cells mL-1 d−1) was found at the DCM layer in the OE region (Table 1). This implies that on average from 0.003 to 0.14 d—1 of the bacterial standing stock was removed daily due to viral lysis (LRB-V) in the region. Estimates of LRB-V ranged between 0.02 and 0.04 d—1 and displayed small spatial patterns at EC (Table 1). In comparison with bacterial growth, the ratio of LRB-V to bacterial growth rate (viral control factor) is higher (0.86~0.99) in the OE region, except at the DCM layer (Table 1). At the EC station, the values of the viral control factor (0.25~0.44) were significantly lower than those of OE (Table 1).
4. Discussion
In the ocean, eddies are energetic, swirling, and time-dependent circulations that are approximately 100 km wide and can be either warm-core (anticyclone) or cold-core (cyclone). A satellite-derived dataset allows researchers to study eddies in large spatiotemporal domains and over high-frequency intervals and determine their biological functions. Recently, there have been many observations of mesoscale eddies in the tropical Pacific Ocean [33,34], but little is known about their role in bacterial and viral dynamics there. It is unclear how physical-biological coupling occurs in warm-core eddies, particularly since no study has examined the relationship between bacteria and viruses.
4.1. Vertical Patterns of Bacterial Abundances
Interestingly, the OE and EC stations showed different vertical patterns of bacterial abundance and its abundances were lower in the EC station than in the OE station (Figure 3A). In different aquatic environments, different factors may limit bacterial growth [35,36]. There are, however, some studies to develop a more complete model of what limits bacterial growth in light of the effects of DOM supply, inorganic nutrients, temperature, and bacterial mortality on population abundance [37]. A tropical marine ecosystem has warm surface temperatures year-round, as shown in this study. Due to high temperatures, there is less new nutrient input in the open ocean because of stratification. It may be that tropical heterotrophic bacteria are less responsive to temperature since dissolved organic matter (DOM) supply plays a strong role in bottom-up control. In a study by Shiah and Ducklow [38], they reported that the growth rate of bacteria is limited by a temperature below 20°C in the absence of a substrate limitation. A high temperature, however, reduces this limitation, allowing bacterial populations to grow rapidly, which in turn limits substrate availability. There is also evidence from previous studies that DOM affects bacterial production in the equatorial Pacific Ocean [37]. Even though temperature played a major role in explaining the variation in bacterial properties at other study sites, spatial variability was unlikely to be explained by temperature because the temperature difference between the outside and inside of the warm eddy never exceeded 3°C along the sampling depths.
The regeneration of carbon and nutrients is particularly important in the oligotrophic aquatic environment with low DOM and inorganic nutrients to enhance bacterial growth [39,40]. With increasing emphasis on carbon and nutrient regeneration in marine systems in the last decade, a better understanding of planktonic viruses has become essential. The process of nutrient regeneration in microbial communities remains difficult to measure directly. Some studies have shown, however, that virally-mediated nutrient recycling plays an important role in some systems, including laboratory experiments with viral-host systems [39] as well as field studies [41]. Our results show that the total viral production (TVP) was higher in OE region (0.46~4.8 × 105 viruses mL−1 h−1), with higher regenerating carbon and other nutrients supporting bacterial growth, but the overall bacterial growth rates at OE were not higher than that at EC station (Table 1). We infer that higher viral production has led to greater regenerated carbon and other nutrients, but we do not observe higher bacterial growth rates in the OE region (Table 1). There is also a possibility that variations in bacterial mortality may explain the lower bacterial abundance in EC at sampling depths. In anticyclonic eddies, higher temperatures increased grazing pressure, according to some studies [3]. Similarly, Boras et al. [42] found high grazing activity at stations subjected to anticyclonic eddies, which might be associated with a higher abundance of nanoflagellates. An alternative explanation is that anticyclonic eddies have a convergence effect that drives the microzooplankton and nanoflagellates toward the core of the eddy. This can result in increased microzooplankton densities in surface waters, increasing bacterial grazing rate [43].
4.2. Percentage of Lysogenic Viral Production
The prevalence of lysogenic viral infection varied significantly over the different depths outside and inside the warm eddy (Table 1). A major mode of bacteriophage reproduction is by lytic or lysogenic infections, and the quantitative significance of these processes varies across different oceans [12]. In previous studies, lytic and lysogenic viral infections in seawater were associated with the trophic status of marine systems [26]. It is thought that productive systems favor the lytic cycle, which is dependent on frequent virus contacts, while low-abundance hosts may allow for lysogenic infection [43]. In this study, we obtain new data on a variety of viral parameters in the tropical Pacific Ocean, with a particular focus on warm eddy regions. As shown in Table 1, the percentage of lysogenic cells outside warm eddies could reach 100% at the surface and 500 m depth. We found there was lower abundance at these depths (1.1~2.1× 105 cells mL−1) and similar to other studies evidence that lysogenic infections are most effective during unfavorable conditions, particularly in waters that are low in bacteria and primary production [44,45]. Additionally, lysogeny can contribute to the resistance of host to stressors by expressing advantageous genes carried by the virus [45]. Thus, the virus can protect the host against stressors, such as UV radiation [46]. In this situation, this may be another explanation for the high percentage of lysogenic cells at the surface layer in the OE region, due to high UV radiation in the tropical ocean. Furthermore, a high percentage of lysogenic production was also observed at both stations at 500 m depth (Table 1). It is worth mentioning that viruses are resistant to low temperatures since they change their lytic life strategy into a lysogenic one when environmental conditions are unfavorable for prokaryotic growth [47].
4.3. Virus-to-Bacteria Ratios
Furthermore, we compared the surrounding and inner water columns of the warm eddy, which can be divided into two layers, above and below DCM depth (Figure 5). Using these analyses, we found no significantly difference in virus-to-bacteria ratios above DCM at both stations, with a mean of 6.1±3.9 and 8.1±3.9 in the OE and EC, respectively (Figure 5A). However, was four-folds higher the values estimated for the EC region (17.7±5.8) below DCM than the OE station (4.2±1.8) (Figure 5A). Another observation suggests that temperature can influence changes in VBR in the water column; decreasing temperatures were associated with a greater survival of viruses [47]. Due to this, a decrease in temperature of 8 ~ 10°C in the mesopelagic waters of the tropical Pacific Ocean is likely to lead to increased virus survival rates. However, temperature was not likely to explain the difference in the VBR in this study. This is due to the small temperature difference between the outside and inside of the warm eddy below DCM.
To study the relationships between viral and bacterial communities, VBR has been widely used [12,16,21]. VBR determines how viruses infect hosts, i.e., high values indicate high viral dynamics and, as a consequence, a high rate of bacterial cell lysis [12]. Alternatively, low values are interpreted as diminishing or absent viral activity [12,16,21]. The viral production measured below DCM at the EC region in our study, however, did not resemble these previous observations, a lower viral production and bacterial control factor below DCM were observed (Figs. 5C, D). In the present study, low viral production cannot result in higher viral abundance below DCM at EC and consequently higher VBR. Taking into account the variations of factors in Figure 5, we concluded that the differences between the EC and OE in VBR below the DCM region may have more to do with other factors than temperature and viral production.
Particle vertical sinking is one of the underlying mechanisms driving oceanic dynamics at a global scale in physical, chemical, and biological processes. In regards to sinking, a previous study suggests that vertical sinking may enhance both lytic and lysogenic production through the promotion of burst size, which is likely to be caused by an increased supply of nutrients, and trigger a switch from a lysogenic to a lytic strategy, probably as a result of environmental changes [48]. According to our study, this pattern differs from the previous study of Wei et al. [8], because there was no significant difference in lysogenic viral production between the above and below DCM regions at either station (Figure 5B). Aside from this, viruses can also be transmitted into the deep ocean indirectly through infected cell hosts, or directly through adsorption on particulate organic matter during sinking [49]. However, the interaction between bacteria and viruses and their role in these processes remain largely unknown. To our knowledge, this is the first report regarding the response of bacteria and viruses to the prevailing physical forces associated with the anticyclonic warm eddy in the tropical Pacific Ocean. In general, warm-core eddies are clockwise circulations that raise the sea level, usually with downwelling at the centers. In different oceanographic regimes, warm-core eddies lead to varying biological responses. Combining previous discoveries with the physical mechanisms of warm eddies, we propose that the downwelling in warm eddies enhances of sinking particles to the deeper layers. It is also possible for viruses to be transmitted into the deep ocean directly by adsorption on particulate organic matter when the particles sink. Considering the sinking process, a high VBR within a warm eddy could result from the sinking process.
There is a significant difference between the chemical properties and biological composition of water inside an anticyclonic eddy and that of surrounding water due to the unique physical processes of an eddy. This study period consisted of two field investigations (within and outside eddy) designed to demonstrate how the warm eddy affects bacterial and viral abundance distributions. There were significant differences in the bacterial and viral abundance inside and outside of the eddy, with the higher abundance of bacteria and viruses were observed outside of the eddy. In anticyclonic eddies, there appeared to be high grazing activity on bacteria, which may have controlled bacterial abundance. In particular, there was four-fold higher of VBR values estimated within the eddy below DCM than the outside eddy. We suggest that prevailing physical forces with downwelling at the center of the anticyclonic warm eddy, viruses can also be transmitted into the deep ocean directly through adsorption on particulate organic matter during sinking. Overall, our findings provide valuable insights into the interaction between bacterial and viral communities and their ecological mechanism within the warm eddy.
Author Contributions
Conceptualization: A.-Y.T.; methodology: A.-Y.T.; P.W.-Y.C.; M.O.; validation: A.-Y.T.; formal analysis: P.W.-Y.C., A.-Y.T., and M.O.; investigation: A.-Y.T.; P.W.-Y.C.; M.O.; resources: A.-Y.T.; G.C.G.; S.J.; data curation: A.-Y.T.; Writing—Original draft preparation: A.-Y.T.; P.W.-Y.C.; Writing—Review and editing: A.-Y.T.; funding acquisition: A.-Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research was conducted in the frame of the Ministry of Science and Technology, ROC (Taiwan), grant number MOST 111-2119-M-019-002.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chelton, D.B.; Gaube, P.; Schlax, M.G.; Early, J.J.; Samelson, R.M. The influence of nonlinear mesoscale eddies on near—Surface oceanic chlorophyll. Science 2011, 334, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Miyazawa, Y.; Oey, L.Y.; Kodaira, T.; Huang, S. The formation processes of phytoplankton growth and decline in mesoscale eddies in the western north pacific ocean. J. Geophys. Res. Oce. 2017, 122, 4444–4455. [Google Scholar] [CrossRef]
- An, L.; Liu, X.; Xu, F.; Fan, X.; Wang, P.; Yin, W.; Huang, B. Different responses of plankton community to mesoscale eddies in the western equatorial Pacific Ocean. Deep Sea Res. I 2024, 203, 104219. [Google Scholar] [CrossRef]
- Chelton, D.B.; Gaube, P.; Schlax, M.G.; Early, J.J.; Samelson, R.M. The influence of nonlinear mesoscale eddies on near-surface oceanic chlorophyll. Science 2011, 334, 328–332. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, D.J.Jr. Mechanisms of physical-biological-biogeochemical interaction at the oceanic mesoscale. Ann. Rev. Mar. Sci. 2016, 8, 125–159. [Google Scholar] [CrossRef] [PubMed]
- Zohary, T.; Robarts, R.D. Bacterial numbers, bacterial production, and heterotrophic nanoplankton abundance in a warm core eddy in the Eastern Mediterranean. Mar. Ecol. Prog. Ser. 1992, 84, 133–137. [Google Scholar] [CrossRef]
- Robarts, R.D.; Zohary, T.; Waiser, M.J.; Yacobi, Y.Z. Bacterial abundance, biomass, and production in relation to phytoplankton biomass in the Levantine Basin of the southeastern Mediterranean Sea. Mar. Ecol. Prog. Ser. 1996, 137, 273–281. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Li, G.; Jing, Z.; Zhou, L.; Ke, Z.; Tan, Y. Distribution of picoplankton in the northeastern South China Sea with special reference to the effects of the Kuroshio intrusion and the associated mesoscale eddies. Sci. Tot. Environ. 2017, 589, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.H.; Kim, D.; Shin, C.W.; Noh, J.H.; Yang, E.J.; Mok, J.S.; et al. Enhanced phytoplankton and bacterioplankton production coupled to coastal upwelling and an anticyclonic eddy in the Ulleung Basin, East Sea. Aquat. Microb. Ecol. 2009, 54, 45–54. [Google Scholar] [CrossRef]
- Ewart, C.S.; Meyers, M.K.; Wallner, E.R.; McGillicuddy Jr, D.J.; Carlson, C.A. Microbial dynamics in cyclonic and anticyclonic mode-water eddies in the northwestern Sargasso Sea. Deep Sea Res. Part II: Top. Stud. Oceanogra. 2008, 55, 1334–1347. [Google Scholar] [CrossRef]
- Sun, F.; Xia, X.; Simon, M.; Wang, Y.; Zhao, H.; Sun, C. , et al. Anticyclonic eddy driving significant changes in prokaryotic and eukaryotic communities in the south China Sea. Front. Mar. Sci. 2022, 9, 773548. [Google Scholar] [CrossRef]
- Weinbauer, M.G. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, W.; Cai, L.L. The fate and biogeochemical cycling of viral elements. Nat. Rev. Microbiol. 2014, 12, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Auguet, J.C.; Montanie, H.; Delmas, D.; Hartmann, H.J.; Huet, V. Dynamic of virioplankton abundance and its environmental control in the Charente estuary (France). Microb. Ecol. 2005, 50, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Berdjeb, L.; Sime-Ngando, T.; Jacquet, S. Viral abundance, production, decay rates and life strategies (lysogeny versus lysis) in Lake Bourget (France). Environ. Microbiol. 2011, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Junger, P.C.; Amado, A.M.; Paranhos, R.; Cabral, A.S.; Jacques, S.M.; Farjalla, V.F. Salinity drives the virioplankton abundance but not production in tropical coastal lagoons. Microb. Ecol. 2018, 75, 52–63. [Google Scholar] [CrossRef]
- Li, X.; He, M.; Shi, Z.; Xu, J. Nutritional Status Regulates Bacteria-Virus Interactions in the Northern South China Sea. J. Geophy. Res. 2023, 128, e2023JG007469. [Google Scholar] [CrossRef]
- Wigington, C.H.; Sonderegger, D.; Brussaard, C.P.D.; Buchan, A.; Finke, J.F.; Fuhrman, J.A.; et al. Re-examination of the relationship between marine virus and microbial cell abundances. Nat. Microbiol. 2016, 1. [Google Scholar] [CrossRef]
- Parikka, K.J.; Le Romancer, M.; Wauters, N.; Jacquet, S. Deciphering the virus-to-prokaryote ratio (VPR): insights into virus–host relationships in a variety of ecosystems. Biol. Rev. 2016, 92, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.T.; Hein, C.; Iabichella, M. Temporal variations in viral distributions in the anoxic Cariaco Basin. Aquat. Microb. Ecol. 2003, 30, 103–116. [Google Scholar] [CrossRef]
- De Corte, D.; Sintes, E.; Winter, C.; Yokokawa, T.; Reinthaler, T.; Herndl, G.J. Links between viral and prokaryotic communities throughout the water column in the (sub) tropical Atlantic Ocean. ISME J. 2010, 4, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- De Corte, D.; Sintes, E.; Yokokawa, T.; Lekunberri, I.; Herndl, G.J. Large-scale distribution of microbial and viral populations in the South Atlantic Ocean. Environ. Microbiol. Rep. 2016; 8, 305–315. [Google Scholar]
- Yang, Y.; Yokokawa, T.; Motegi, C.; Nagata, T. Large-scale distribution of viruses in deep waters of the Pacific and Southern Oceans. Aquat. Microb. Ecol. 2014, 71, 193–202. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Ingrid, B.; Manfred, G.H. Lysogeny and virus-induced mortality of bacterioplankton in surface, deep, and anoxic marine waters. Limnol. Oceanogr. 2003, 48, 1457–1465. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Brigden, S.M.; Suttle, C.A. A dilution technique for the direct measurement of viral production: A comparison in stratified and tidally mixed coastal waters. Microb. Ecol. 2002, 43, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G. , Hófle. M.G. Significance of viral lysis andflagellate grazing as factors controlling bacterioplankton pro-duction in a eutrophic lake. Appl. Environ. Microbiol. 1998, 64, 431–438. [Google Scholar]
- Hewson, I.; Fuhrman, J.A. Covariation of viral parameters with bacterial assemblage richness and diversity in the water column and sediments. Deep-Sea Res. I. 2007, 54, 811–830. [Google Scholar] [CrossRef]
- Parada, V.; Herndl, G.J.; Weinbauer, M.G. Viral burst size of heterotrophic prokaryotes in aquatic systems. J. Mar. Biol. Assoc. 2006, 86, 613–621. [Google Scholar] [CrossRef]
- Brussaard, C.P.D. Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbiol. 2004, 70, 1506–1513. [Google Scholar] [CrossRef]
- Hammes, F.; Egli, T. Cytometric methods for measuring bacteria in water : advantages, pitfalls and applications. Anal. Bioanal. Chem. 2010, 397, 1083–1095. [Google Scholar] [CrossRef]
- Yun, M.S.; Kim, Y.; Jeong, Y.; Joo, H.T.; Jo, Y.H.; Lee, C.H. Weak response of biological productivity and community structure of phytoplankton to mesoscale eddies in the oligotrophic Philippine Sea. J. Geophy. Res.: Oce. 2020. [Google Scholar]
- Wang, Y.; Zhao, F.; He, X.; Wang, W.; Chang, L.; Kang, J. Latitudinal and meridional patterns of picophytoplankton variability are contrastingly associated with Ekman pumping and the warm pool in the tropical western Pacific. Ecol. Evolut. 2023, 13, e10589. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D.L.; Malmstrom, R.R.; Cottrell, M.T. Control of bacterial growth by temperature and organic matter in the Western Arctic. Deep Sea Res. II: Top. Stud. Oceanogra. 3386. [Google Scholar]
- Morán, X.A.G.; Baltar, F.; Carreira, C.; Lønborg, C. Responses of physiological groups of tropical heterotrophic bacteria to temperature and dissolved organic matter additions: food matters more than warming. Environ. Microbiol. 2020, 22, 1930–1943. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D.; Rich, J. Regulation of Bacterial Growth Rates by Dissolved Organic Carbon and Temperature in the Equatorial Pacific Ocean. Microb. Ecol. 1997, 33, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Shiah, F.-K.; Ducklow, H.W. Temperature regulation of heterotrophic bacterioplankton abundance, production and specific growth rate in Chesapeake Bay, USA. Limnol. Oceanogr. 1994, 39, 1243–1258. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, X.; Xu, J.; Harrison, P.J.; He, L.; Yin, K. Effects of viruses on bacterial functions under contrasting nutritional conditions for four species of bacteria isolated from Hong Kong waters. Scient. Rep. 2015, 5, 14217. [Google Scholar] [CrossRef] [PubMed]
- Mine, A.H.; Coleman, M.L. Phosphorus release and regeneration following laboratory lysis of bacterial cells. Front. Microbiol. 2021, 12, 641700. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.-Y.; Gong, G.C.; Yu-Wen, H. Importance of the viral shunt in nitrogen cycling in Synechococcus spp. growth in subtropical Western Pacific coastal waters. Terr. Atmo. Oceanic Sci. 2014, 25, 839–846. [Google Scholar] [CrossRef]
- Boras, J.A.; Sala, M.M.; Baltar, F.; Arístegui, J.; Duarte, C.M.; Vaqué, D. Effect of viruses and protists on bacteria in eddies of the Canary Current region (subtropical northeast Atlantic). Limnol. Oceanogr. 2010, 55, 885–898. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Yu, J.; Wu, Q.; Sun, D. Anticyclonic mesoscale eddy induced mesopelagic biomass hotspot in the oligotrophic ocean. J. Mar. Syst. 2023, 237, 103831. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Suttle, C.A. Lysogeny and prophage induction in coastal and offshore bacterial communities. Aquat. Microb. Ecol. 1999, 18, 217–225. [Google Scholar] [CrossRef]
- Williamson, S.J.; Houchin, L.A.; McDaniel, L.; Paul, J.H. Seasonal variation in lysogeny as depicted by prophage induction in Tampa Bay, Florida. Appl. Environ. Microbiol. 2002, 68, 4307–4314. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temperate phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, R.; Peng, L.; Liang, Y.; Jiao, N. Effects of temperature and photosynthetically active radiation on virioplankton decay in the western Pacific Ocean. Sci. Rep. 2018, 8, 1525. [Google Scholar] [CrossRef]
- Wei, W.; Chen, X.; Weinbauer, M.G. Reduced bacterial mortality and enhanced viral productivity during sinking in the ocean. ISME J. 2022, 16, 1668–1675. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Bettarel, Y.; Cattaneo, R.; Luef, B.; Maier, C.; Motegi, C.; et al. Viral ecology of organic and inorganic particles in aquatic systems: avenues for further research. Aquat. Microb. Ecol. 2009, 57, 321–41. [Google Scholar] [CrossRef]
Figure 1.
This map shows the sampling stations plotted against the averaged sea surface height anomaly (m) for the study period of 2023. The arrows represent sea surface currents.
Figure 1.
This map shows the sampling stations plotted against the averaged sea surface height anomaly (m) for the study period of 2023. The arrows represent sea surface currents.
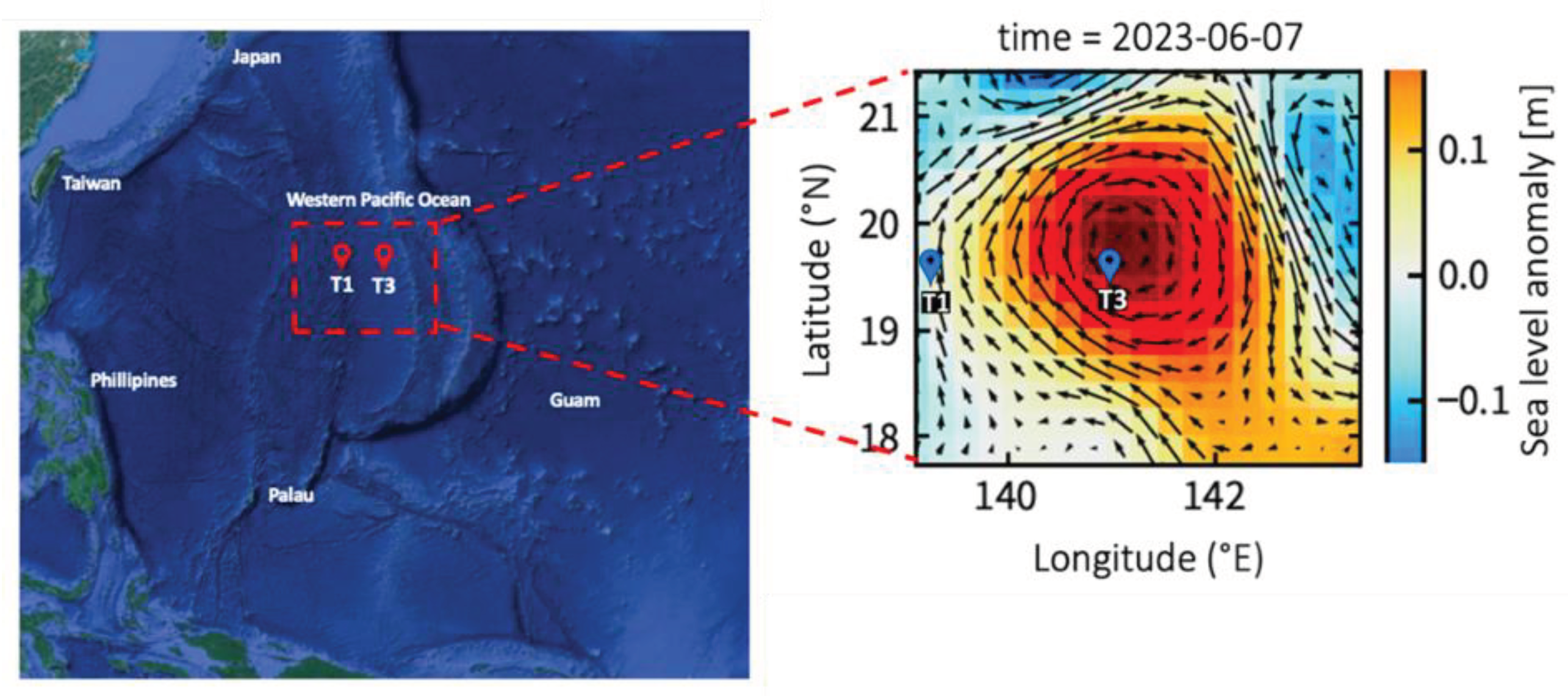
Figure 2.
Vertical profiles of temperature (A), salinity (B) and Chl a (C) out-of-eddy (OE) (white square) and eddy core (EC) (black square), respectively.
Figure 2.
Vertical profiles of temperature (A), salinity (B) and Chl a (C) out-of-eddy (OE) (white square) and eddy core (EC) (black square), respectively.
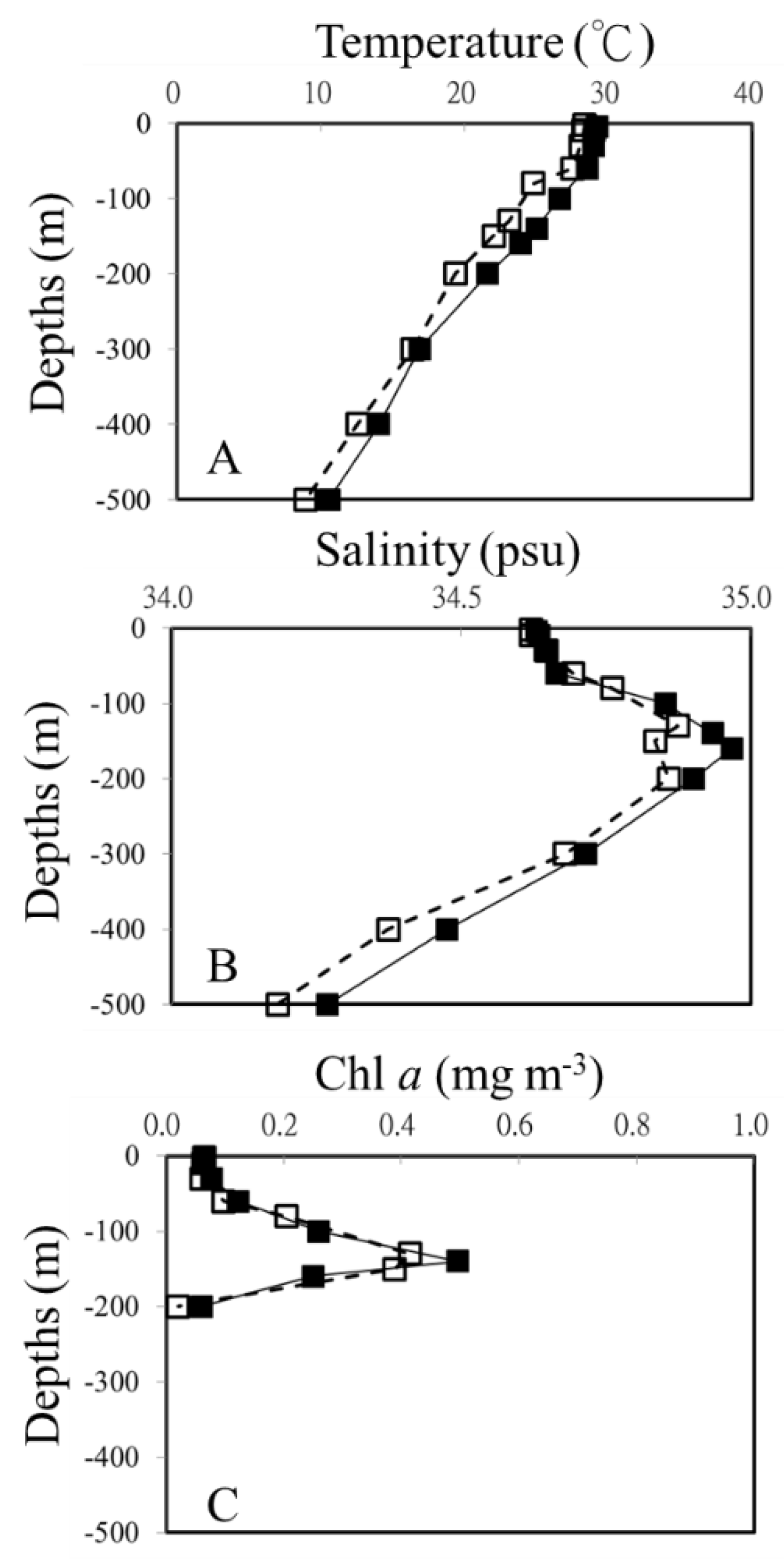
Figure 3.
Vertical profiles of bacterial abundance (A), viral abundance (B) and virus-to-bacteria ratio (VBR) (C) out-of-eddy (OE) (white bars) and eddy core (EC)(red bars), respectively.
Figure 3.
Vertical profiles of bacterial abundance (A), viral abundance (B) and virus-to-bacteria ratio (VBR) (C) out-of-eddy (OE) (white bars) and eddy core (EC)(red bars), respectively.
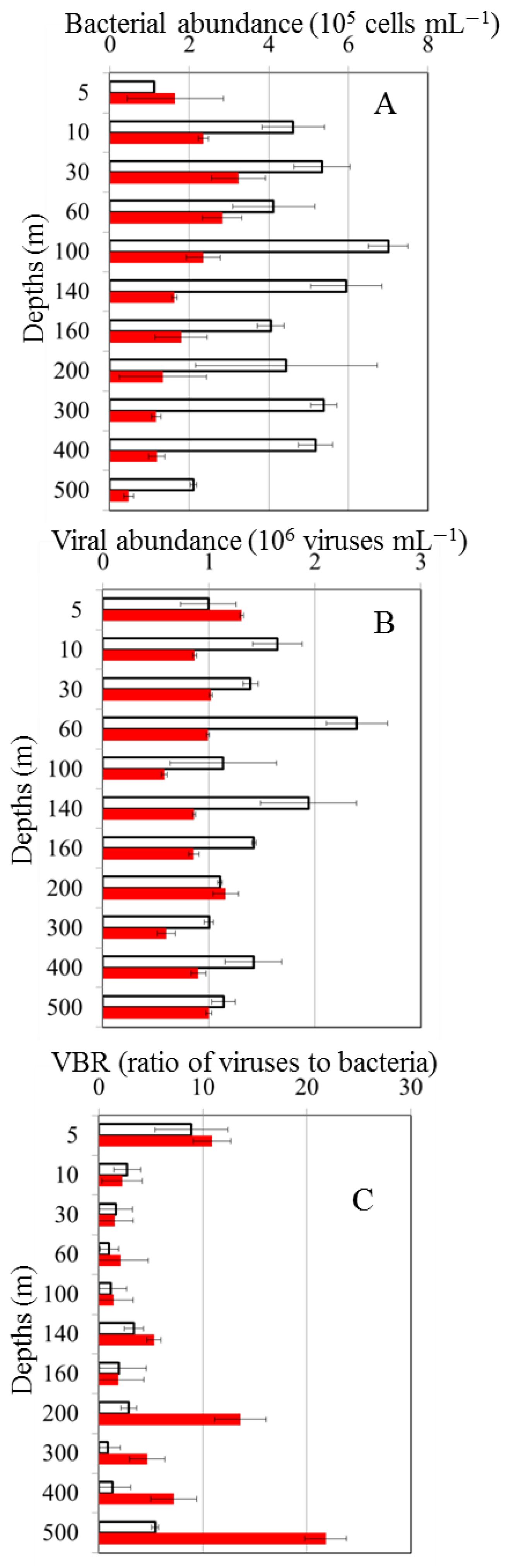
Figure 4.
Temporal variations of viral abundance during 24 h incubations for viral production in the surface water (A, E), DCM (B, F), 200 m (C, G) and 500 m depth (D, H) in OE and EC, respectively. □:control treatmenrs, ■: with mitomycin treatments.
Figure 4.
Temporal variations of viral abundance during 24 h incubations for viral production in the surface water (A, E), DCM (B, F), 200 m (C, G) and 500 m depth (D, H) in OE and EC, respectively. □:control treatmenrs, ■: with mitomycin treatments.
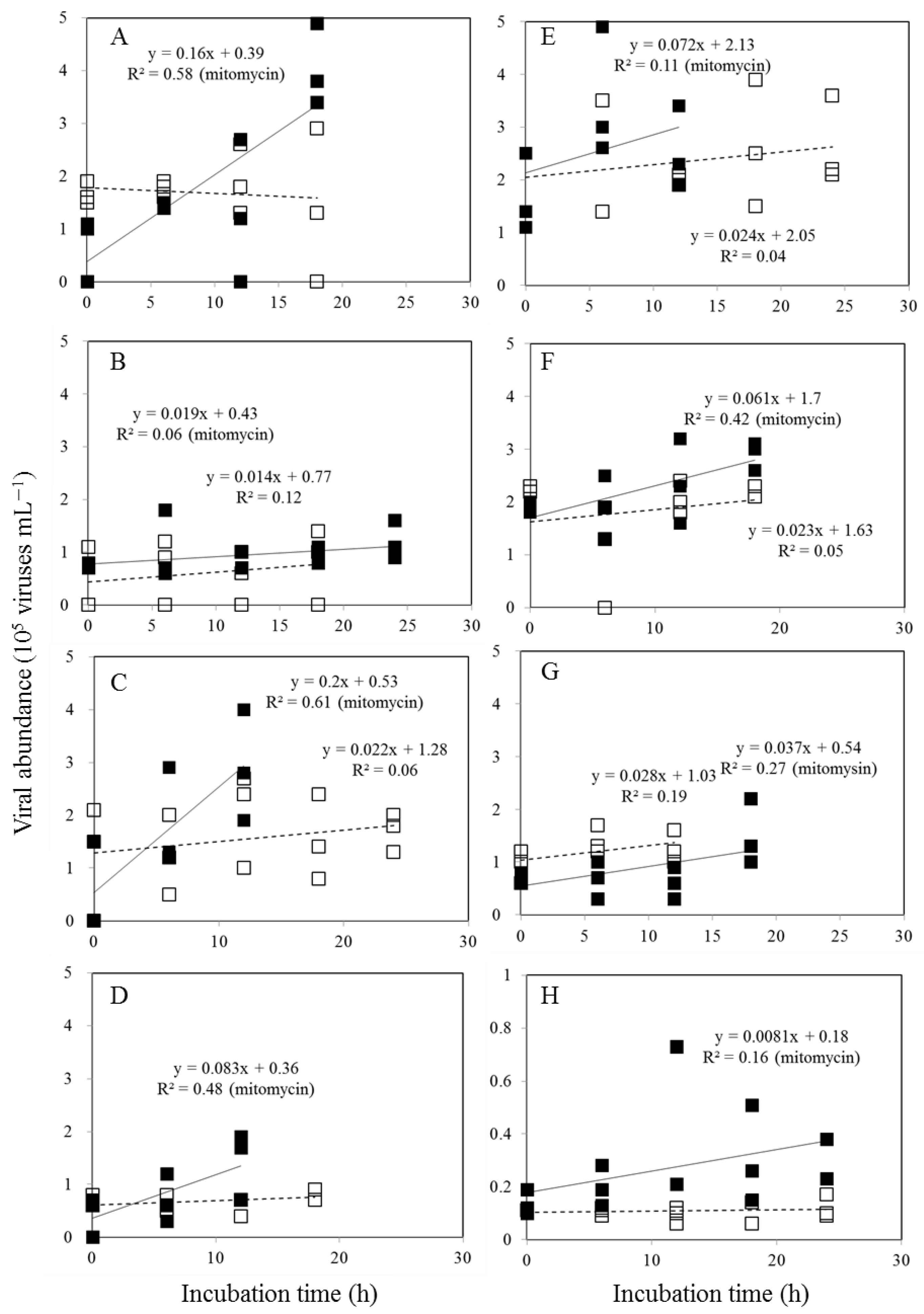
Figure 5.
Average of virus-to-bacteria ratio (VBR) (A), the percentage of lysogenic to total viral production (%) (B), total viral production (C) and viral control factor (D) above and below DCM region in OE (white bars) and EC (red bars), respectively.
Figure 5.
Average of virus-to-bacteria ratio (VBR) (A), the percentage of lysogenic to total viral production (%) (B), total viral production (C) and viral control factor (D) above and below DCM region in OE (white bars) and EC (red bars), respectively.
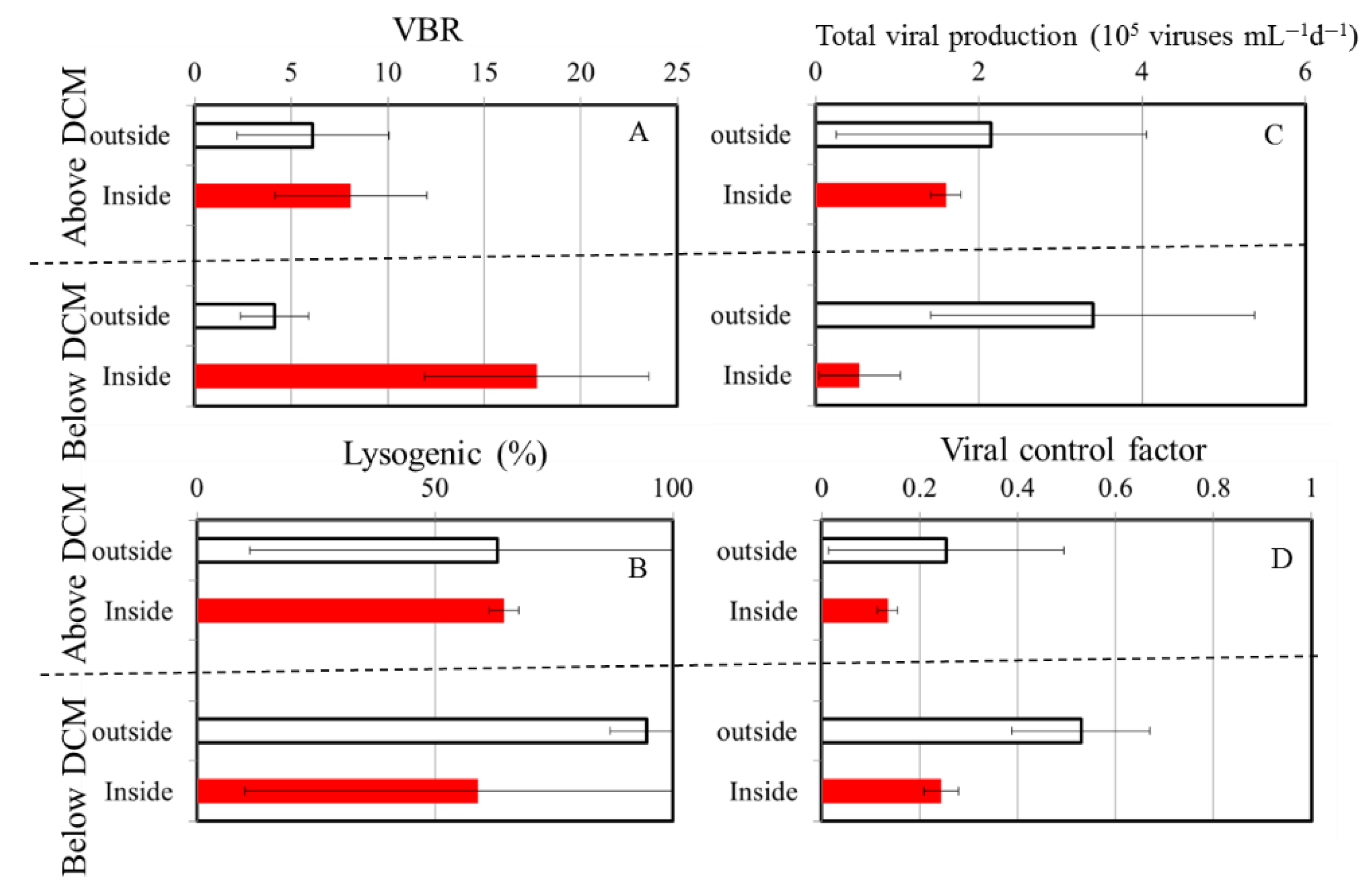
Table 1.
Temperature, salinity, bacterial, and viral abundance, virus-to-bacteria ratio (VBR), and viral activity (lytic production, lysogenic production, lysogenic (%), total viral production (TVP), viral turnover rate (VT), viral-induced mortality of bacteria (VMB), loss rate of bacteria from viral activity (LRB-V), Viral control factor) and bacterial growth rates at the sampling stations and depths. #: 24 burst sizes (BS, number of viruses released per lytic event).
Table 1.
Temperature, salinity, bacterial, and viral abundance, virus-to-bacteria ratio (VBR), and viral activity (lytic production, lysogenic production, lysogenic (%), total viral production (TVP), viral turnover rate (VT), viral-induced mortality of bacteria (VMB), loss rate of bacteria from viral activity (LRB-V), Viral control factor) and bacterial growth rates at the sampling stations and depths. #: 24 burst sizes (BS, number of viruses released per lytic event).
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






