Submitted:
29 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
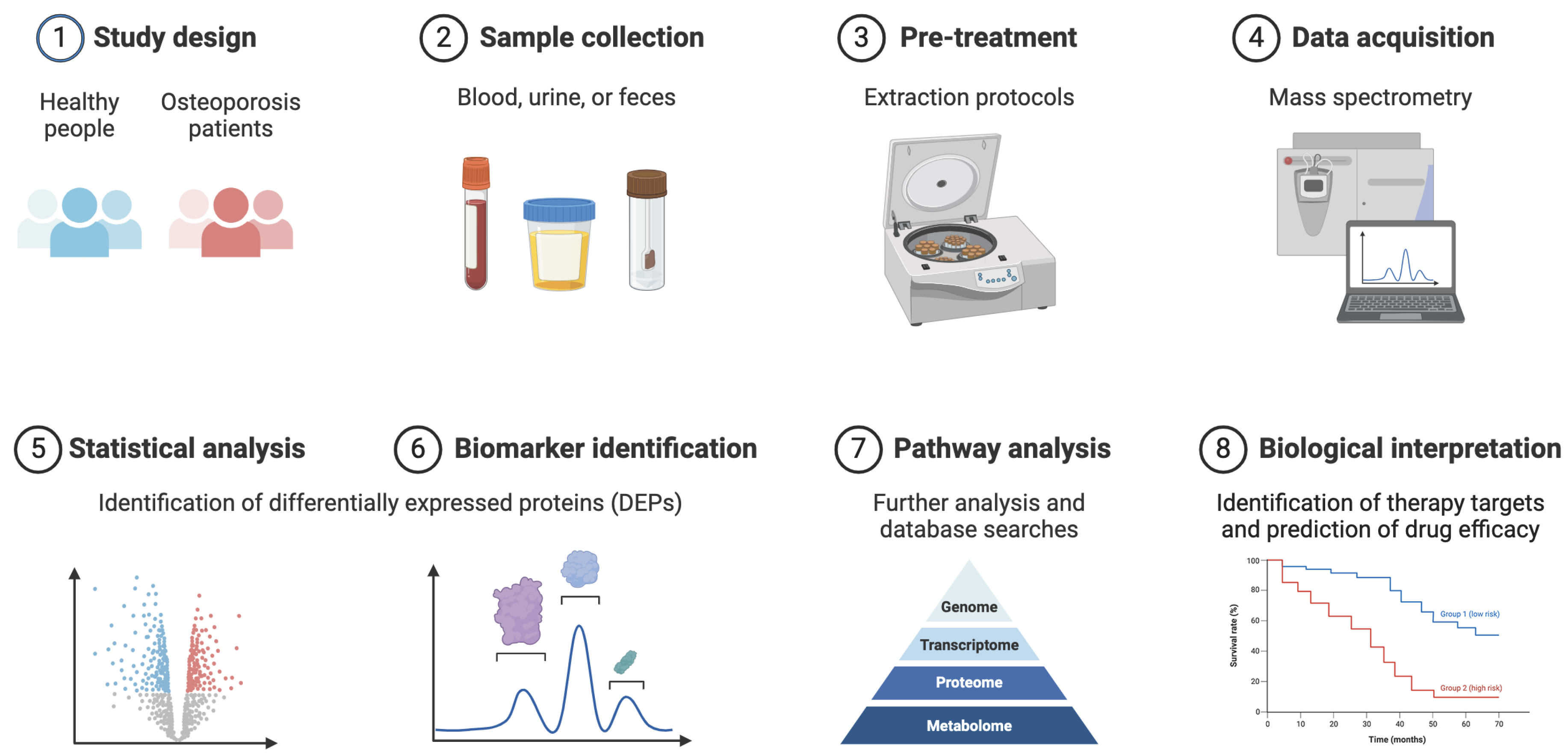
1. Introduction
2. Proteomic Technologies in Osteoporosis Research
2.1. Mass Spectrometry-Based Proteomics
2.2. Gel-Based and Gel-Free Proteomic Techniques
2.3. Bioinformatics Tools for Proteomic Data Analysis and Interpretation
3. Proteomic Insights into Bone Metabolism
3.1. Identification of Key Proteins Involved in Bone Formation and Resorption
3.2. Quantitative Proteomics in Assessing Dynamic Changes in Bone Proteome
3.3. Proteomic Studies on the Bone Extracellular Matrix
4. Diagnostic Markers in Osteoporosis
4.1. Blood and Urinary Biomarkers
4.2. Potential Integration with Imaging Techniques for Comprehensive Diagnosis
5. Therapeutic Targets and Drug Discovery
5.1. Proteomic Identification of Novel Therapeutic Targets
5.2. Evaluation of Proteomic Profiles in Drug Development and Personalized Medicine
6. Challenges and Future Directions
6.1. Limitations and Challenges in Proteomic Studies of Osteoporosis
6.2. Integration of Multi-Omics Data for a Holistic Understanding
6.3. Future Directions and Potential Impact on Clinical Practice
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos Int 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Salari, N.; Darvishi, N.; Bartina, Y.; Larti, M.; Kiaei, A.; Hemmati, M.; Shohaimi, S.; Mohammadi, M. Global prevalence of osteoporosis among the world older adults: A comprehensive systematic review and meta-analysis. J Orthop Surg Res 2021, 16, 669. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur J Rheumatol 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Gates, M.; Pillay, J.; Nuspl, M.; Wingert, A.; Vandermeer, B.; Hartling, L. Screening for the primary prevention of fragility fractures among adults aged 40 years and older in primary care: Systematic reviews of the effects and acceptability of screening and treatment, and the accuracy of risk prediction tools. Syst Rev 2023, 12, 51. [Google Scholar] [CrossRef]
- Dimai, H.P. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone 2017, 104, 39–43. [Google Scholar] [CrossRef]
- Watts, N.B. The Fracture Risk Assessment Tool (FRAX(R)): Applications in clinical practice. J Womens Health (Larchmt) 2011, 20, 525–531. [Google Scholar] [CrossRef]
- Schini, M.; Johansson, H.; Harvey, N.C.; Lorentzon, M.; Kanis, J.A.; McCloskey, E.V. An overview of the use of the fracture risk assessment tool (FRAX) in osteoporosis. Journal of Endocrinological Investigation 2024, 47, 501–511. [Google Scholar] [CrossRef]
- Zhang, H.; Recker, R.; Lee, W.N.; Xiao, G.G. Proteomics in bone research. Expert Rev Proteomics 2010, 7, 103–111. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Sai, A.J. Molecular biology of bone remodeling: Implications for new therapeutic targets for osteoporosis. Maturitas 2010, 65, 301–307. [Google Scholar] [CrossRef]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J Biol Chem 2021, 12, 57–69. [Google Scholar] [CrossRef]
- Karpov, O.A.; Stotland, A.; Raedschelders, K.; Chazarin, B.; Ai, L.; Murray, C.I.; Van Eyk, J.E. Proteomics of the Heart. Physiol Rev 2024. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Jo, H.S.; Bae, S.; Seo, Y.; Song, P.; Song, M.; Yoon, J.H. Application of Proteomics in Cancer: Recent Trends and Approaches for Biomarkers Discovery. Front Med (Lausanne) 2021, 8, 747333. [Google Scholar] [CrossRef]
- Bludau, I.; Aebersold, R. Proteomic and interactomic insights into the molecular basis of cell functional diversity. Nat Rev Mol Cell Biol 2020, 21, 327–340. [Google Scholar] [CrossRef]
- Archer, T.C.; Ehrenberger, T.; Mundt, F.; Gold, M.P.; Krug, K.; Mah, C.K.; Mahoney, E.L.; Daniel, C.J.; LeNail, A.; Ramamoorthy, D.; Mertins, P.; Mani, D.R.; Zhang, H.; Gillette, M.A.; Clauser, K.; Noble, M.; Tang, L.C.; Pierre-Francois, J.; Silterra, J.; Jensen, J.; Tamayo, P.; Korshunov, A.; Pfister, S.M.; Kool, M.; Northcott, P.A.; Sears, R.C.; Lipton, J.O.; Carr, S.A.; Mesirov, J.P.; Pomeroy, S.L.; Fraenkel, E. Proteomics, Post-translational Modifications, and Integrative Analyses Reveal Molecular Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2018, 34, 396–410 e8. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Miao, Z.; Yan, Y.; Chen, D.; Yang, Z.X.; Yue, L.; Hu, W.; Zhuo, L.; Wang, J.T.; Xue, Z.; Fu, Y.; Xu, Y.; Zheng, J.S.; Guo, T.; Chen, Y.M. Proteome-wide profiling reveals dysregulated molecular features and accelerated aging in osteoporosis: A 9.8-year prospective study. Aging Cell 2024, 23, e14035. [Google Scholar] [CrossRef]
- Liang, X.; He, M.; Zhu, B.; Zhu, Y.; He, X.; Liu, D.; Wei, Q. TMT-Based Proteomic Explores the Influence of DHEA on the Osteogenic Differentiation of hBMSCs. Front Cell Dev Biol 2021, 9, 726549. [Google Scholar] [CrossRef]
- Liang, B.C.; Shi, X.L.; Li, C.W.; Shi, Z.Y.; He, W.T.; Yao, J.L.; Kong, L.C.; Li, X.Y. Identification of human serum protein targets of Qianggu Decoction () in primary type I osteoporosis based on tandem mass tag labeling and liquid chromatography-tandem mass spectrometry technology. Chin J Integr Med 2017, 23, 747–754. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Lv, H.; Yin, P.; Zhang, L.; Tang, P. Quantitative proteomics and reverse engineer analysis identified plasma exosome derived protein markers related to osteoporosis. J Proteomics 2020, 228, 103940. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Lv, J.; Yan, Y.; Hu, Y.; Liu, C.; Zhang, F.; Wang, J.; Hao, D. Proteomic profiling analysis of postmenopausal osteoporosis and osteopenia identifies potential proteins associated with low bone mineral density. PeerJ 2020, 8, e9009. [Google Scholar] [CrossRef]
- Sasaki, K.; Ozasa, Y.; Iba, K.; Wada, T.; Imai, S.; Matsumoto, K.; Sohma, H.; Aoshima, M.; Yamashita, T.; Kokai, Y. Significant increase of plasma tetranectin in ovx mice as defined by proteomics analysis. J Orthop Sci 2014, 19, 809–819. [Google Scholar] [CrossRef]
- Rai, M.F.; Collins, K.H.; Lang, A.; Maerz, T.; Geurts, J.; Ruiz-Romero, C.; June, R.K.; Ramos, Y.; Rice, S.J.; Ali, S.A.; Pastrello, C.; Jurisica, I.; Thomas Appleton, C.; Rockel, J.S.; Kapoor, M. Three decades of advancements in osteoarthritis research: Insights from transcriptomic, proteomic, and metabolomic studies. Osteoarthritis Cartilage 2023. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, C.; Boll, I.; Finsen, B.; Modzel, M.; Larsen, M.R. Characterizing disease-associated changes in post-translational modifications by mass spectrometry. Expert Rev Proteomics 2018, 15, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Daswani, B.; Gupta, M.K.; Gavali, S.; Desai, M.; Sathe, G.J.; Patil, A.; Parte, P.; Sirdeshmukh, R.; Khatkhatay, M.I. Monocyte Proteomics Reveals Involvement of Phosphorylated HSP27 in the Pathogenesis of Osteoporosis. Dis Markers 2015, 2015, 196589. [Google Scholar] [CrossRef]
- Ercan, H.; Resch, U.; Hsu, F.; Mitulovic, G.; Bileck, A.; Gerner, C.; Yang, J.W.; Geiger, M.; Miller, I.; Zellner, M. A Practical and Analytical Comparative Study of Gel-Based Top-Down and Gel-Free Bottom-Up Proteomics Including Unbiased Proteoform Detection. Cells 2023, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, J.L.; Baghalabadi, V.; Rajendran, S.; Jakubec, P.J.; Said, H.; McMillen, T.S.; Dang, Z.; Doucette, A.A. Recent advances in top-down proteome sample processing ahead of MS analysis. Mass Spectrom Rev 2023, 42, 457–495. [Google Scholar] [CrossRef]
- Kim, Y.I.; Cho, J.Y. Gel-based proteomics in disease research: Is it still valuable? Biochim Biophys Acta Proteins Proteom 2019, 1867, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.; Dumas-Gaudot, E.; Renaut, J.; Sergeant, K. Gel-based and gel-free quantitative proteomics approaches at a glance. Int J Plant Genomics 2012, 2012, 494572. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.M.; Jacobs, J.M.; Orwoll, E.S. Proteomic studies of bone and skeletal health outcomes. Bone 2019, 126, 18–26. [Google Scholar] [CrossRef]
- Bernardini, G.; Braconi, D.; Spreafico, A.; Santucci, A. Post-genomics of bone metabolic dysfunctions and neoplasias. Proteomics 2012, 12, 708–721. [Google Scholar] [CrossRef]
- Shao, C.; Chen, L.; Lu, C.; Shen, C.L.; Gao, W. A gel-based proteomic analysis of the effects of green tea polyphenols on ovariectomized rats. Nutrition 2011, 27, 681–686. [Google Scholar] [CrossRef]
- Camafeita, E.; Lamas, J.R.; Calvo, E.; Lopez, J.A.; Fernandez-Gutierrez, B. Proteomics: New insights into rheumatic diseases. Proteomics Clin Appl 2009, 3, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Wakabayashi, K.; Matsuoka, T. Proteome analysis of secreted proteins during osteoclast differentiation using two different methods: Two-dimensional electrophoresis and isotope-coded affinity tags analysis with two-dimensional chromatography. Proteomics 2003, 3, 616–626. [Google Scholar] [CrossRef]
- Salkin, H.; Acar, M.B.; Korkmaz, S.; Gunaydin, Z.; Gonen, Z.B.; Basaran, K.E.; Ozcan, S. Transforming growth factor beta1-enriched secretome up-regulate osteogenic differentiation of dental pulp stem cells, and a potential therapeutic for gingival wound healing: A comparative proteomics study. J Dent 2022, 124, 104224. [Google Scholar] [CrossRef]
- Huo, C.; Li, Y.; Qiao, Z.; Shang, Z.; Cao, C.; Hong, Y.; Xiao, H. [Proteomics analysis of serum exosomes and its application in osteoporosis]. Se Pu 2019, 37, 863–871. [Google Scholar] [CrossRef]
- Bivi, N.; Picotti, P.; Muller, L.N.; Romanello, M.; Moro, L.; Quadrifoglio, F.; Tell, G. Shotgun proteomics analysis reveals new unsuspected molecular effectors of nitrogen-containing bisphosphonates in osteocytes. J Proteomics 2011, 74, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Matthiesen, R. LC-MS Spectra Processing. Methods Mol Biol 2020, 2051, 59–77. [Google Scholar] [CrossRef]
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Romero-Rodriguez, M.C.; Pascual, J.; Valledor, L.; Jorrin-Novo, J. Improving the quality of protein identification in non-model species. Characterization of Quercus ilex seed and Pinus radiata needle proteomes by using SEQUEST and custom databases. J Proteomics 2014, 105, 85–91. [Google Scholar] [CrossRef] [PubMed]
- MacCoss, M.J.; Wu, C.C.; Yates, J.R., 3rd. Probability-based validation of protein identifications using a modified SEQUEST algorithm. Anal Chem 2002, 74, 5593–5599. [Google Scholar] [CrossRef]
- Prianichnikov, N.; Koch, H.; Koch, S.; Lubeck, M.; Heilig, R.; Brehmer, S.; Fischer, R.; Cox, J. MaxQuant Software for Ion Mobility Enhanced Shotgun Proteomics. Mol Cell Proteomics 2020, 19, 1058–1069. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, P.; Skunca, N.; Hu, J.C.; Dessimoz, C. Primer on the Gene Ontology. Methods Mol Biol 2017, 1446, 25–37. [Google Scholar] [PubMed]
- Gene Ontology Consortium. Gene Ontology Consortium: Going forward. Nucleic Acids Res 2015, 43, D1049–D1056. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Rothfels, K.; Milacic, M.; Matthews, L.; Haw, R.; Sevilla, C.; Gillespie, M.; Stephan, R.; Gong, C.; Ragueneau, E.; May, B.; Shamovsky, V.; Wright, A.; Weiser, J.; Beavers, D.; Conley, P.; Tiwari, K.; Jassal, B.; Griss, J.; Senff-Ribeiro, A.; Brunson, T.; Petryszak, R.; Hermjakob, H.; D’Eustachio, P.; Wu, G.; Stein, L. Using the Reactome Database. Curr Protoc 2023, 3, e722. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; Deng, C.; Varusai, T.; Ragueneau, E.; Haider, Y.; May, B.; Shamovsky, V.; Weiser, J.; Brunson, T.; Sanati, N.; Beckman, L.; Shao, X.; Fabregat, A.; Sidiropoulos, K.; Murillo, J.; Viteri, G.; Cook, J.; Shorser, S.; Bader, G.; Demir, E.; Sander, C.; Haw, R.; Wu, G.; Stein, L.; Hermjakob, H.; D’Eustachio, P. The reactome pathway knowledgebase 2022. Nucleic Acids Res 2022, 50, D687–D692. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Ju, D. Diosgenin protects against alveolar bone loss in ovariectomized rats via regulating long non-coding RNAs. Exp Ther Med 2018, 16, 3939–3950. [Google Scholar] [CrossRef]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; Bork, P.; Jensen, L.J.; von Mering, C. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J Proteome Res 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003, 4, P3. [Google Scholar] [CrossRef]
- Piscopo, D.M.; Johansen, E.B.; Derynck, R. Identification of the GATA factor TRPS1 as a repressor of the osteocalcin promoter. J Biol Chem 2009, 284, 31690–31703. [Google Scholar] [CrossRef]
- Lei, Q.; Chen, J.; Huang, W.; Wu, D.; Lin, H.; Lai, Y. Proteomic analysis of the effect of extracellular calcium ions on human mesenchymal stem cells: Implications for bone tissue engineering. Chem Biol Interact 2015, 233, 139–146. [Google Scholar] [CrossRef]
- Xiong, Q.; Tang, P.; Gao, Y.; Zhang, L.; Ge, W. Proteomic Analysis of Estrogen-Mediated Signal Transduction in Osteoclasts Formation. Biomed Res Int 2015, 2015, 596789. [Google Scholar] [CrossRef]
- Ha, B.G.; Hong, J.M.; Park, J.Y.; Ha, M.H.; Kim, T.H.; Cho, J.Y.; Ryoo, H.M.; Choi, J.Y.; Shin, H.I.; Chun, S.Y.; Kim, S.Y.; Park, E.K. Proteomic profile of osteoclast membrane proteins: Identification of Na+/H+ exchanger domain containing 2 and its role in osteoclast fusion. Proteomics 2008, 8, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yu, H.; Liu, Y.; Ye, W.; Zhu, G.; Yan, A.; Tan, Q.; Mei, H. Serum-derived exosomes from neurofibromatosis type 1 congenital tibial pseudarthrosis impaired bone by promoting osteoclastogenesis and inhibiting osteogenesis. Exp Biol Med (Maywood) 2021, 246, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Rota, S.; Olivier, F.; Momier, D.; Guigonis, J.M.; Schaub, S.; Samson, M.; Bouler, J.M.; Scimeca, J.C.; Rochet, N.; Lagadec, P. Proteomic analysis identified LBP and CD14 as key proteins in blood/biphasic calcium phosphate microparticle interactions. Acta Biomater 2021, 127, 298–312. [Google Scholar] [CrossRef]
- Liu, J.; He, S.; Ma, B.; Li, X.; Wang, Y.; Xiong, J. TMT-based quantitative proteomic analysis revealed that FBLN2 and NPR3 are involved in the early osteogenic differentiation of mesenchymal stem cells (MSCs). Aging (Albany NY) 2023, 15, 7637–7654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, C.; Chen, X.; Cai, Y.; Li, W.; Fang, X.; Zhang, W. Bone protein analysis via label-free quantitative proteomics in patients with periprosthetic joint infection. J Proteomics 2022, 252, 104448. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, L.; Zhu, W.; Xu, C.; He, H.; Zhou, Y.; Liu, Y.Z.; Tian, Q.; Zhang, J.G.; Deng, F.Y.; Hu, H.G.; Zhang, L.S.; Deng, H.W. Quantitative proteomics and integrative network analysis identified novel genes and pathways related to osteoporosis. J Proteomics 2016, 142, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.; Eijken, M.; Swagemakers, S.; Chiba, H.; Titulaer, M.K.; Burgers, P.C.; Luider, T.M.; van Leeuwen, J.P. Proteomic analysis of human osteoblastic cells: Relevant proteins and functional categories for differentiation. J Proteome Res 2010, 9, 4688–4700. [Google Scholar] [CrossRef]
- Zecha, J.; Satpathy, S.; Kanashova, T.; Avanessian, S.C.; Kane, M.H.; Clauser, K.R.; Mertins, P.; Carr, S.A.; Kuster, B. TMT Labeling for the Masses: A Robust and Cost-efficient, In-solution Labeling Approach. Mol Cell Proteomics 2019, 18, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, N.; Yates, J.R., 3rd. Isobaric labeling-based relative quantification in shotgun proteomics. J Proteome Res 2014, 13, 5293–5309. [Google Scholar] [CrossRef]
- Velez-Bermudez, I.C.; Wen, T.N.; Lan, P.; Schmidt, W. Isobaric Tag for Relative and Absolute Quantitation (iTRAQ)-Based Protein Profiling in Plants. Methods Mol Biol 2016, 1450, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, A.; Sun, C.; Zhang, J.; Shang, J.; Tang, H.; Li, J.; Wang, M.; Kong, X.; Wei, Z.; Feng, S. Quantitative iTRAQ proteomics reveal the proteome profiles of bone marrow mesenchymal stem cells after cocultures with Schwann cells in vitro. Ann Transl Med 2022, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yan, K.; Wu, Y.; Yao, X.; Li, G.; Ge, L.; Chen, Z. Quantitative proteomics reveals the effect of Yigu decoction (YGD) on protein expression in bone tissue. Clin Proteomics 2021, 18, 24. [Google Scholar] [CrossRef]
- Gatenholm, B.; Gobom, J.; Skillback, T.; Blennow, K.; Zetterberg, H.; Brittberg, M. Peptidomic analysis of cartilage and subchondral bone in OA patients. Eur J Clin Invest 2019, 49, e13082. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhang, H.; Li, G.; Chen, B.; Li, J.; Zhang, T.; Liu, B.; Cao, Z.; Liu, G.; Jia, P.; Xu, Y. Deciphering core proteins of osteoporosis with iron accumulation by proteomics in human bone. Front Endocrinol (Lausanne) 2022, 13, 961903. [Google Scholar] [CrossRef]
- Rose, J.P.; Schurman, C.A.; King, C.D.; Bons, J.; Patel, S.K.; Burton, J.B.; O’Broin, A.; Alliston, T.; Schilling, B. Deep coverage and quantification of the bone proteome provides enhanced opportunities for new discoveries in skeletal biology and disease. PLoS ONE 2023, 18, e0292268. [Google Scholar] [CrossRef]
- Kim, J.; Yeon, A.; Parker, S.J.; Shahid, M.; Thiombane, A.; Cho, E.; You, S.; Emam, H.; Kim, D.G.; Kim, M. Alendronate-induced Perturbation of the Bone Proteome and Microenvironmental Pathophysiology. Int J Med Sci 2021, 18, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Statzer, C.; Park, J.Y.C.; Ewald, C.Y. Extracellular Matrix Dynamics as an Emerging yet Understudied Hallmark of Aging and Longevity. Aging Dis 2023, 14, 670–693. [Google Scholar] [CrossRef] [PubMed]
- Fontcuberta-Rigo, M.; Nakamura, M.; Puigbo, P. Phylobone: A comprehensive database of bone extracellular matrix proteins in human and model organisms. Bone Res 2023, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Licini, C.; Montalbano, G.; Ciapetti, G.; Cerqueni, G.; Vitale-Brovarone, C.; Mattioli-Belmonte, M. Analysis of multiple protein detection methods in human osteoporotic bone extracellular matrix: From literature to practice. Bone 2020, 137, 115363. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.; Demmers, J.A.; Bezstarosti, K.; van der Eerden, B.C.; Verhaar, J.A.; Eijken, M.; van Leeuwen, J.P. Unraveling the human bone microenvironment beyond the classical extracellular matrix proteins: A human bone protein library. J Proteome Res 2011, 10, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, T.; You, Y.; Wu, B.; Wang, X.; Wu, J. Proteoglycans and Glycosaminoglycans in Stem Cell Homeostasis and Bone Tissue Regeneration. Front Cell Dev Biol 2021, 9, 760532. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R. The extracellular matrix: An underexplored but important proteome. Expert Rev Proteomics 2010, 7, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Andrade, F.F.; Pereira, T.; Vitorio, J.G.; Diniz, M.G.; Amorim, L.S.D.; Nawrocki, A.; Felicori, L.F.; De Marco, L.; Gomes, C.C.; Larsen, M.R.; Melo-Braga, M.N.; Gomez, R.S. Quantitative proteomic study reveals differential expression of matricellular proteins between fibrous dysplasia and cemento-ossifying fibroma pathogenesis. J Oral Pathol Med 2022, 51, 405–412. [Google Scholar] [CrossRef]
- Eyre, D.R.; Weis, M.A. Bone collagen: New clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif Tissue Int 2013, 93, 338–347. [Google Scholar] [CrossRef]
- Sroga, G.E.; Vashishth, D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 2012, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front Cell Dev Biol 2021, 9, 770143. [Google Scholar] [CrossRef] [PubMed]
- Manninen, A.; Varjosalo, M. A proteomics view on integrin-mediated adhesions. Proteomics 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Ajeian, J.N.; Horton, E.R.; Astudillo, P.; Byron, A.; Askari, J.A.; Millon-Fremillon, A.; Knight, D.; Kimber, S.J.; Humphries, M.J.; Humphries, J.D. Proteomic analysis of integrin-associated complexes from mesenchymal stem cells. Proteomics Clin Appl 2016, 10, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Popov, C.; Alberton, P.; Aszodi, A. Integrin signaling in skeletal development and function. Birth Defects Res C Embryo Today 2014, 102, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Nakayamada, S.; Okada, Y.; Saito, K.; Tamura, M.; Tanaka, Y. Beta1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor kappaB ligand on osteoblasts and osteoclast maturation. J Biol Chem 2003, 278, 45368–45374. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, K.K.; McGuigan, F.E.; Malmgren, L.; Gerdhem, P.; Johansson, H.; Kanis, J.A.; Akesson, K.E. Bone Turnover Marker Profiling and Fracture Risk in Older Women: Fracture Risk from Age 75 to 90. Calcif Tissue Int 2022, 111, 288–299. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark Res 2017, 5, 18. [Google Scholar] [CrossRef]
- Shetty, S.; Kapoor, N.; Bondu, J.D.; Thomas, N.; Paul, T.V. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J Endocrinol Metab 2016, 20, 846–852. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X. Receptor activator of nuclear factor-kappaB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review). Mol Med Rep 2015, 11, 3212–3218. [Google Scholar] [CrossRef]
- Kurban, S.; Akpinar, Z.; Mehmetoglu, I. Receptor activator of nuclear factor kappaB ligand (RANKL) and osteoprotegerin levels in multiple sclerosis. Mult Scler 2008, 14, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.M.; Aleidi, S.M.; Masood, A.; Alnehmi, E.A.; Abdel Jabar, M.; Almogren, M.; Alshaker, M.; Benabdelkamel, H.; Abdel Rahman, A.M. Proteomics Profiling of Osteoporosis and Osteopenia Patients and Associated Network Analysis. Int J Mol Sci 2022, 23, 10200. [Google Scholar] [CrossRef] [PubMed]
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; Maccarrone, M. Human osteogenic differentiation in Space: Proteomic and epigenetic clues to better understand osteoporosis. Sci Rep 2019, 9, 8343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, C.H.; He, P.; Wu, L.F.; Lu, X.; Lei, S.F.; Deng, F.Y. Abl interactor 1: A novel biomarker for osteoporosis in Chinese elderly men. J Proteomics 2019, 207, 103440. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pan, H.; Liu, W.; Jin, G.; Liu, W.; Liang, C.; Jiang, X. Discovery of novel serum biomarkers for diagnosing and predicting postmenopausal osteoporosis patients by 4D-label free protein omics. J Orthop Res 2023, 41, 2713–2720. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Li, C.W.; Wang, J.H.; Huang, X.S.; Yuan, Y.F.; Hu, J.; Liu, K.; Liang, B.C.; Liu, Z.; Shi, X.L. Ubiquitylomes Analysis of the Whole blood in Postmenopausal Osteoporosis Patients and healthy Postmenopausal Women. Orthop Surg 2019, 11, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Huang, Q.F.; An, D.W.; Raad, J.; Martens, D.S.; Latosinska, A.; Stolarz-Skrzypek, K.; Van Cleemput, J.; Feng, Y.Q.; Mischak, H.; Allegaert, K.; Verhamme, P.; Janssens, S.; Nawrot, T.S.; Staessen, J.A. OSTEO18, a novel urinary proteomic signature, associated with osteoporosis in heart transplant recipients. Heliyon 2024, 10, e24867. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aguilar, M.M.; Aparicio-Bautista, D.I.; Ramirez-Salazar, E.G.; Reyes-Grajeda, J.P.; De la Cruz-Montoya, A.H.; Antuna-Puente, B.; Hidalgo-Bravo, A.; Rivera-Paredez, B.; Ramirez-Palacios, P.; Quiterio, M.; Valdes-Flores, M.; Salmeron, J.; Velazquez-Cruz, R. Serum Proteomic Analysis Reveals Vitamin D-Binding Protein (VDBP) as a Potential Biomarker for Low Bone Mineral Density in Mexican Postmenopausal Women. Nutrients 2019, 11, 2853. [Google Scholar] [CrossRef] [PubMed]
- Pepe, J.; Rossi, M.; Battafarano, G.; Vernocchi, P.; Conte, F.; Marzano, V.; Mariani, E.; Mortera, S.L.; Cipriani, C.; Rana, I.; Buonuomo, P.S.; Bartuli, A.; De Martino, V.; Pelle, S.; Pascucci, L.; Toniolo, R.M.; Putignani, L.; Minisola, S.; Del Fattore, A. Characterization of Extracellular Vesicles in Osteoporotic Patients Compared to Osteopenic and Healthy Controls. J Bone Miner Res 2022, 37, 2186–2200. [Google Scholar] [CrossRef]
- Cedeno-Veloz, B.A.; Lozano-Vicario, L.; Zambom-Ferraresi, F.; Fernandez-Irigoyen, J.; Santamaria, E.; Rodriguez-Garcia, A.; Romero-Ortuno, R.; Mondragon-Rubio, J.; Ruiz-Ruiz, J.; Ramirez-Velez, R.; Izquierdo, M.; Martinez-Velilla, N. Effect of immunology biomarkers associated with hip fracture and fracture risk in older adults. Immun Ageing 2023, 20, 55. [Google Scholar] [CrossRef]
- Xu, Q.; Li, C.H.; Tang, C.H.; Huang, X.L.; Wu, L.F.; Zhou, X.; Lei, S.F.; Deng, F.Y. PKM2 is a Novel Osteoporosis-Associated Protein in Chinese. Endocr Res 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Siegel, E.R.; Achenbach, S.J.; Khosla, S.; Suva, L.J. Serum biomarker profile associated with high bone turnover and BMD in postmenopausal women. J Bone Miner Res 2008, 23, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Qundos, U.; Drobin, K.; Mattsson, C.; Hong, M.G.; Sjoberg, R.; Forsstrom, B.; Solomon, D.; Uhlen, M.; Nilsson, P.; Michaelsson, K.; Schwenk, J.M. Affinity proteomics discovers decreased levels of AMFR in plasma from Osteoporosis patients. Proteomics Clin Appl 2016, 10, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Hwang, S. Identification of Osteoporosis-Associated Protein Biomarkers from Ovariectomized Rat Urine. Curr Proteomics 2017, 14, 130–137. [Google Scholar] [CrossRef]
- Nielson, C.M.; Wiedrick, J.; Shen, J.; Jacobs, J.; Baker, E.S.; Baraff, A.; Piehowski, P.; Lee, C.G.; Baratt, A.; Petyuk, V.; McWeeney, S.; Lim, J.Y.; Bauer, D.C.; Lane, N.E.; Cawthon, P.M.; Smith, R.D.; Lapidus, J.; Orwoll, E.S.; Osteoporotic Fractures in Men Study Research, G. Identification of Hip BMD Loss and Fracture Risk Markers Through Population-Based Serum Proteomics. J Bone Miner Res 2017, 32, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Wang, H. Screening for specific biomarkers in the serum of postmenopausal osteoporosis patients using proteomic fingerprint techniques. Biomed Rep 2013, 1, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Q.; Li, Q.Y.; Shi, Y.; Wang, J.X.; Wu, J.S.; Ruan, H.N.; Xue, H.T. [Pathogenesis of glucocorticoid-induced osteoporosis based on label-free mass proteomics]. Zhongguo Gu Shang 2023, 36, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Montagna, G.; Pani, G.; Flinkman, D.; Cristofaro, F.; Pascucci, B.; Massimino, L.; Lamparelli, L.A.; Fassina, L.; James, P.; Coffey, E.; Rea, G.; Visai, L.; Rizzo, A.M. Long-term osteogenic differentiation of human bone marrow stromal cells in simulated microgravity: Novel proteins sighted. Cell Mol Life Sci 2022, 79, 536. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Bautista, D.I.; Becerra-Cervera, A.; Rivera-Paredez, B.; Aguilar-Ordonez, I.; Rios-Castro, E.; Reyes-Grajeda, J.P.; Salmeron, J.; Hidalgo-Bravo, A.; Velazquez-Cruz, R. Label-free quantitative proteomics in serum reveals candidate biomarkers associated with low bone mineral density in Mexican postmenopausal women. Geroscience 2024, 46, 2177–2195. [Google Scholar] [CrossRef]
- Cedeno-Veloz, B.; Lozano-Vicario, L.; Rodriguez-Garcia, A.; Zambom-Ferraresi, F.; Galbete, A.; Fernandez-Irigoyen, J.; Santamaria, E.; Garcia-Hermoso, A.; Calvani, R.; Ramirez-Velez, R.; Izquierdo, M.; Martinez-Velilla, N. Serum biomarkers related to frailty predict negative outcomes in older adults with hip fracture. J Endocrinol Invest 2024, 47, 729–738. [Google Scholar] [CrossRef]
- He, W.T.; Liang, B.C.; Shi, Z.Y.; Li, X.Y.; Li, C.W.; Shi, X.L. Weak cation exchange magnetic beads coupled with matrix-assisted laser desorption ionization-time of flight-mass spectrometry in screening serum protein markers in osteopenia. Springerplus 2016, 5, 679. [Google Scholar] [CrossRef]
- Terracciano, R.; Migliaccio, V.; Savino, R.; Pujia, A.; Montalcini, T. Association between low bone mineral density and increased alpha-defensin in salivary fluid among postmenopausal women. Menopause 2013, 20, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Austin, T.R.; Fink, H.A.; Jalal, D.I.; Tornqvist, A.E.; Buzkova, P.; Barzilay, J.I.; Lu, T.; Carbone, L.; Gabrielsen, M.E.; Grahnemo, L.; Hveem, K.; Jonasson, C.; Kizer, J.R.; Langhammer, A.; Mukamal, K.J.; Gerszten, R.E.; Nethander, M.; Psaty, B.M.; Robbins, J.A.; Sun, Y.V.; Skogholt, A.H.; Asvold, B.O.; Valderrabano, R.J.; Zheng, J.; Richards, J.B.; Coward, E.; Ohlsson, C. Large-scale circulating proteome association study (CPAS) meta-analysis identifies circulating proteins and pathways predicting incident hip fractures. J Bone Miner Res 2024. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Copeland, M.; Geiser, A.G.; Hale, L.V.; Harvey, A.; Ma, Y.L.; Powers, C.S.; Sato, M.; You, J.; Hale, J.E. Development of a highly sensitive, high-throughput, mass spectrometry-based assay for rat procollagen type-I N-terminal propeptide (PINP) to measure bone formation activity. J Proteome Res 2007, 6, 4218–4229. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Kabir, M.H.; Park, J.H.; Park, J.I.; Kang, J.S.; Ju, S.; Shin, Y.J.; Lee, S.M.; Lee, J.; Kim, S.; Lee, K.P.; Lee, S.Y.; Lee, C.; Kwon, K.S. Plasma proteomic profiling of young and old mice reveals cadherin-13 prevents age-related bone loss. Aging (Albany NY) 2020, 12, 8652–8668. [Google Scholar] [CrossRef]
- Fretwurst, T.; Tritschler, I.; Rothweiler, R.; Nahles, S.; Altmann, B.; Schilling, O.; Nelson, K. Proteomic profiling of human bone from different anatomical sites—A pilot study. Proteomics Clin Appl 2022, 16, e2100049. [Google Scholar] [CrossRef]
- Guenoun, D.; Champsaur, P. Opportunistic Computed Tomography Screening for Osteoporosis and Fracture. Semin Musculoskelet Radiol 2023, 27, 451–456. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, Y.; Hu, Y.; Zeng, F.; Wang, P.; Xu, L.; Wu, J.; Li, J.; Zhu, J.; Xiang, M.; Zeng, F. Development and validation of a machine learning-derived radiomics model for diagnosis of osteoporosis and osteopenia using quantitative computed tomography. BMC Med Imaging 2022, 22, 140. [Google Scholar] [CrossRef]
- Engelke, K. Quantitative Computed Tomography-Current Status and New Developments. J Clin Densitom 2017, 20, 309–321. [Google Scholar] [CrossRef]
- Oei, L.; Koromani, F.; Rivadeneira, F.; Zillikens, M.C.; Oei, E.H. Quantitative imaging methods in osteoporosis. Quant Imaging Med Surg 2016, 6, 680–698. [Google Scholar] [CrossRef]
- Sangondimath, G.; Sen, R.K. DEXA and Imaging in Osteoporosis. Indian J Orthop 2023, 57 (Suppl. 1), 82–93. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Shiau, S.; Kamanda-Kosseh, M.; Bucovsky, M.; Kil, N.; Lappe, J.M.; Stubby, J.; Recker, R.R.; Guo, X.E.; Shane, E.; Cohen, A. Teriparatide Followed by Denosumab in Premenopausal Idiopathic Osteoporosis: Bone Microstructure and Strength by HR-pQCT. J Bone Miner Res 2023, 38, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Hung, V.W.; Cheuk, K.Y.; Chau, W.W.; Tsoi, K.K.; Wong, R.M.; Chow, S.K.; Lam, T.P.; Yung, P.S.; Law, S.W.; Qin, L. Best Performance Parameters of HR-pQCT to Predict Fragility Fracture: Systematic Review and Meta-Analysis. J Bone Miner Res 2021, 36, 2381–2398. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Binkley, N.; Hernando, D.; Anderson, P.A. Opportunistic Use of Lumbar Magnetic Resonance Imaging for Osteoporosis Screening. Osteoporos Int 2022, 33, 861–869. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Z.; Liu, C.; Gao, Z.; Ren, Q.; Lei, L.; Ren, J. Radiomics Based on Lumbar Spine Magnetic Resonance Imaging to Detect Osteoporosis. Acad Radiol 2021, 28, e165–e171. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Muscarella, S.; Bazzocchi, A. Integrated imaging approach to osteoporosis: State-of-the-art review and update. Radiographics 2011, 31, 1343–1364. [Google Scholar] [CrossRef] [PubMed]
- Khashayar, P.; Dimai, H.P.; Moradi, N.; Fahimfar, N.; Gharibzadeh, S.; Ostovar, A.; Nabipour, I.; Larijani, B. Protocol for a multicentre, prospective cohort study of clinical, proteomic and genomic patterns associated with osteoporosis to develop a multidimensional fracture assessment tool: The PoCOsteo Study. BMJ Open 2020, 10, e035363. [Google Scholar] [CrossRef]
- Calciolari, E.; Donos, N. Proteomic and Transcriptomic Approaches for Studying Bone Regeneration in Health and Systemically Compromised Conditions. Proteomics Clin Appl 2020, 14, e1900084. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Nuttall, M.E. Emerging techniques for the discovery and validation of therapeutic targets for skeletal diseases. Expert Opin Ther Targets 2002, 6, 679–689. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xie, N.; Sun, X.D.; Nice, E.C.; Liou, Y.C.; Huang, C.; Zhu, H.; Shen, Z. Insights and implications of sexual dimorphism in osteoporosis. Bone Res 2024, 12, 8. [Google Scholar] [CrossRef]
- Gavali, S.; Gupta, M.K.; Daswani, B.; Wani, M.R.; Sirdeshmukh, R.; Khatkhatay, M.I. Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim Biophys Acta Mol Cell Res 2019, 1866, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Tao, S.; Huang, Z.; Zheng, B.; Kong, X.; Xiang, Y.; Zhang, Q.; Song, H.; Xu, Z.; Wei, X.; Zhao, F.; Chen, J. The deubiquitinase UCHL1 negatively controls osteoclastogenesis by regulating TAZ/NFATC1 signalling. Int J Biol Sci 2023, 19, 2319–2332. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Gao, H.; Liu, J.; Zhang, M.; Leng, X.; Zhao, D. Identification of potential therapeutic targets of deer antler extract on bone regulation based on serum proteomic analysis. Mol Biol Rep 2019, 46, 4861–4872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xue, L.M.; Han, T.; Jiao, L.; Qin, L.P.; Li, Y.S.; Zheng, H.C.; Zhang, Q.Y. Antiosteoporotic effects and proteomic characterization of the target and mechanism of an Er-Xian Decoction on osteoblastic UMR-106 and osteoclasts induced from RAW264.7. Molecules 2010, 15, 4695–4710. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, X.; Wang, Y.; Gao, M.; Feng, L.; Shan, J.; Xie, T.; Cao, Y.; Cheng, F.; Bao, B. Metabolomics combined with proteomics analysis of femur provides a comprehensive interpretation of the changes in postmenopausal osteoporosis under salidroside treatment. Pharmacological Research-Modern Chinese Medicine 2022, 3, 100079. [Google Scholar] [CrossRef]
- Wei, X.; Qi, B.; Ma, R.; Zhang, Y.; Liu, N.; Fang, S.; Zhu, Y.; Xie, Y.; Dai, J.; Zhu, L. Quantitative Proteomics Revealed the Pharmacodynamic Network of Bugu Shengsui Decoction Promoting Osteoblast Proliferation. Front Endocrinol (Lausanne) 2021, 12, 833474. [Google Scholar] [CrossRef]
- Agostini, M.; Traldi, P.; Hamdan, M. Mass Spectrometry-Based Proteomics: Analyses Related to Drug-Resistance and Disease Biomarkers. Medicina (Kaunas) 2023, 59, 1722. [Google Scholar] [CrossRef]
- Qian, T.; Zhu, S.; Hoshida, Y. Use of big data in drug development for precision medicine: An update. Expert Rev Precis Med Drug Dev 2019, 4, 189–200. [Google Scholar] [CrossRef]
- Poulos, R.C.; Cai, Z.; Robinson, P.J.; Reddel, R.R.; Zhong, Q. Opportunities for pharmacoproteomics in biomarker discovery. Proteomics 2023, 23, e2200031. [Google Scholar] [CrossRef]
- Del Real, A.; Ciordia, S.; Sanudo, C.; Garcia-Ibarbia, C.; Roa-Bautista, A.; Ocejo-Vinals, J.G.; Corrales, F.; Riancho, J.A. Analysis of Serum Proteome after Treatment of Osteoporosis with Anabolic or Antiresorptive Drugs. Metabolites 2022, 12, 399. [Google Scholar] [CrossRef]
- Xue, L.; Jiang, Y.; Han, T.; Zhang, N.; Qin, L.; Xin, H.; Zhang, Q. Comparative proteomic and metabolomic analysis reveal the antiosteoporotic molecular mechanism of icariin from Epimedium brevicornu maxim. J Ethnopharmacol 2016, 192, 370–381. [Google Scholar] [CrossRef] [PubMed]
- KhalKhal, E.; Rezaei-Tavirani, M.; Rostamii-Nejad, M. Pharmaceutical Advances and Proteomics Researches. Iran J Pharm Res 2019, 18 (Suppl. 1), 51–67. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Dashatan, N.; Koushki, M.; Abbaszadeh, H.A.; Rostami-Nejad, M.; Rezaei-Tavirani, M. Proteomics Applications in Health: Biomarker and Drug Discovery and Food Industry. Iran J Pharm Res 2018, 17, 1523–1536. [Google Scholar] [PubMed]
- Duarte, T.T.; Spencer, C.T. Personalized Proteomics: The Future of Precision Medicine. Proteomes 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Hou, S.X.; Yu, B.; Jin, D.; Ferec, C.; Chen, J.M. Genetics of osteoporosis: Perspectives for personalized medicine. Per Med 2010, 7, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Enitan, S.; Adejumo, E.; Imaralu, J.; Adelakun, A.; Ladipo, O.; Enitan, C. Personalized medicine approach to osteoporosis management in women: Integrating genetics, pharmacogenomics, and precision treatments. Clin Res Commun 2023, 6, 18. [Google Scholar] [CrossRef]
- Varnavides, G.; Madern, M.; Anrather, D.; Hartl, N.; Reiter, W.; Hartl, M. In Search of a Universal Method: A Comparative Survey of Bottom-Up Proteomics Sample Preparation Methods. J Proteome Res 2022, 21, 2397–2411. [Google Scholar] [CrossRef]
- Chandramouli, K.; Qian, P.Y. Proteomics: Challenges, techniques and possibilities to overcome biological sample complexity. Hum Genomics Proteomics 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Eberl, H.C.; Kreisz, N.; Ugwu, U.J.; Starikova, T.; Kuster, B.; Wilhelm, S. An Introduction to Analytical Challenges, Approaches, and Applications in Mass Spectrometry-Based Secretomics. Mol Cell Proteomics 2023, 22, 100636. [Google Scholar] [CrossRef]
- Boschetti, E.; Righetti, P.G. Low-Abundance Protein Enrichment for Medical Applications: The Involvement of Combinatorial Peptide Library Technique. Int J Mol Sci 2023, 24, 10329. [Google Scholar] [CrossRef]
- Chiva, C.; Mendes Maia, T.; Panse, C.; Stejskal, K.; Douche, T.; Matondo, M.; Loew, D.; Helm, D.; Rettel, M.; Mechtler, K.; Impens, F.; Nanni, P.; Shevchenko, A.; Sabido, E. Quality standards in proteomics research facilities: Common standards and quality procedures are essential for proteomics facilities and their users. EMBO Rep 2021, 22, e52626. [Google Scholar] [CrossRef] [PubMed]
- Albar, J.P.; Canals, F. Standardization and quality control in proteomics. J Proteomics 2013, 95, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, J.; Trepte, P.; Sepke, N.; Novatchkova, M.; Schutzbier, M.; Durnberger, G.; Mechtler, K.; Knoblich, J.A. Integrated transcriptome and proteome analysis reveals posttranscriptional regulation of ribosomal genes in human brain organoids. Elife 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Langefeld, C.D.; Olivier, M.; Cox, L.A. Integrated Omics: Tools, Advances, and Future Approaches. J Mol Endocrinol 2018. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Pal, R. Integrated analysis of transcriptomic and proteomic data. Curr Genomics 2013, 14, 91–110. [Google Scholar] [CrossRef]
- Li, C.; Lin, X.; Lin, Q.; Lin, Y.; Lin, H. Jiangu granules ameliorate postmenopausal osteoporosis via rectifying bone homeostasis imbalance: A network pharmacology analysis based on multi-omics validation. Phytomedicine 2024, 122, 155137. [Google Scholar] [CrossRef]
- Yang, T.L.; Shen, H.; Liu, A.; Dong, S.S.; Zhang, L.; Deng, F.Y.; Zhao, Q.; Deng, H.W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat Rev Endocrinol 2020, 16, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Mullin, B.H.; Ribet, A.B.P.; Pavlos, N.J. Bone Trans-omics: Integrating Omics to Unveil Mechanistic Molecular Networks Regulating Bone Biology and Disease. Curr Osteoporos Rep 2023, 21, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Reppe, S.; Datta, H.K.; Gautvik, K.M. Omics analysis of human bone to identify genes and molecular networks regulating skeletal remodeling in health and disease. Bone 2017, 101, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, A.G. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clin Proteomics 2023, 20, 32. [Google Scholar] [CrossRef]
- Shi, T.; Song, E.; Nie, S.; Rodland, K.D.; Liu, T.; Qian, W.J.; Smith, R.D. Advances in targeted proteomics and applications to biomedical research. Proteomics 2016, 16, 2160–2182. [Google Scholar] [CrossRef] [PubMed]
- Mermelekas, G.; Vlahou, A.; Zoidakis, J. SRM/MRM targeted proteomics as a tool for biomarker validation and absolute quantification in human urine. Expert Rev Mol Diagn 2015, 15, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Vlahou, A. Implementation of Clinical Proteomics: A Step Closer to Personalized Medicine? Proteomics Clin Appl 2019, 13, e1800088. [Google Scholar] [CrossRef] [PubMed]
- Alyass, A.; Turcotte, M.; Meyre, D. From big data analysis to personalized medicine for all: Challenges and opportunities. BMC Med Genomics 2015, 8, 33. [Google Scholar] [CrossRef] [PubMed]
| Biomarkers | Sample Type | Proteomic Technology | Summary | Refs |
|---|---|---|---|---|
| MYH14, IGLC1, MEX3B, and FBLN1 | Serum | LC-MS | This cohort study identified potential protein biomarkers associated with osteopenia (ON) and OP using LC-MS proteomics. Notably, MYH14, IGLC1, MEX3B, and FBLN1 were highlighted as key markers showing dysregulation in low BMD progression, with a focus on inflammatory pathways such as TNF, TLR4, and IFNG. | [92] |
| Lysozyme C, Glucosidase, Disulfide Isomerase A5 | Plasma | LC–MS/MS, PRM | The expression of protein Lysozyme C was negatively related to BMD, while the expression of Glucosidase and Disulfide Isomerase A5 was positively related to BMD values. | [19] |
| Sox2, Oct3/4, Nanog, and E-cadherin | Blood-derived stem cells (BDSCs) | Proteome Profiler Array | Embryonic markers: Sox2, Oct3/4, Nanog, and E-cadherin, which showed decreased expression during osteoblastic differentiation induced by rapamycin under microgravity conditions. | [93] |
| ABI1 | Peripheral blood monocytes (PBMs), Plasma | LC-MS/MS, Western Blotting (WB), ELISA | ABI1 was significantly down-regulated in PBM in Chinese elderly men with extremely low vs. high BMD, as well as in osteoporotic fracture (OF) patients vs. non-fractured (NF) subjects; the plasma ABI1 protein has superior performance in discriminating osteopenia and healthy subjects. | [94] |
| 17 proteins | Serum exosomes | LC–MS | A total of 188, 224, and 185 proteins were identified in the normal, ON, and OP groups, respectively. There were 17 proteins significantly dysregulated in the ON and OP groups. | [34] |
| CHD1, PNP | Serum | 4-D label-free proteomics, ELISA | Serum-level CHD1 and PNP have the potential power as effective indicators for the diagnosis of postmenopausal osteoporosis (PMOP). | [95] |
| Ubiquitylomes | Whole blood | high-performance liquid chromatography (HPLC), LC-MS/MS | This study identified differential ubiquitination patterns in whole blood between healthy postmenopausal women and PMOP patients, revealing potential biomarkers associated with PMOP. Key findings include dysregulation in ubiquitin conjugating enzyme activity, enrichment in pathways such as ubiquitin-mediated proteolysis, and identification of potential diagnostic targets in whole blood. | [96] |
| An 18-peptide multidimensional OP urinary proteomic profile biomarker | Urine | capillary electrophoresis coupled with MS (CE-MS) | This study developed and validated an 18-peptide multidimensional urinary proteomic profile (OSTEO18) biomarker for osteoporosis in heart transplant recipients, showing promising diagnostic performance with improved accuracy compared to known risk factors. | [97] |
| VDBP | Serum | 2-D gel electrophoresis, ELISA | This study identified 27 spots of interest when comparing low BMD versus normal BMD postmenopausal women; and low serum vitamin D-binding protein (VDBP) levels correlate with low BMD. | [98] |
| PSMB9, AARS, PCBP2, and VSIR | Plasma exosome | LC-nano-MS/MS, PRM | This study identified 45 differentially expressed proteins and 4 of them (PSMB9, AARS, PCBP2, and VSIR) associated with osteoporosis were further verified. | [18] |
| Fibrinogen, vitronectin, clusterin, coagulation factors, and apolipoprotein | Extracellular vesicles (EVs) | nano-HPLC-ESI-MS/MS | The proteomic comparison between osteopenic and healthy controls EVs evidenced a decrease in fibrinogen, vitronectin, and clusterin and an increase in coagulation factors and apolipoprotein, which was also upregulated in OP EVs. | [99] |
| IL-6, LT-α, FLT3LG, CSF1, and CCL7 | Serum | Target 48 Cytokine Panel | This observational study identified several serum cytokines, including Interleukin 6 (IL-6), Lymphotoxin-alpha (LT-α), Fms-related tyrosine kinase 3 ligand (FLT3LG), Colony stimulating factor 1 (CSF1), and Chemokine (C-C motif) ligand 7 (CCL7), as potential markers associated with hip fracture status in older adults. | [100] |
| PKM2 | PBMs | LC-MS/MS | This study discovered 59 DEPs, and validated the significant up-regulation of pyruvate kinase isozyme 2 (PKM2) with OP. | [101] |
| ITIH4 | Serum | Protein chip SELDI TOF-MS | This study identified specific serum protein peaks, notably fragments of interalpha-trypsin-inhibitor heavy chain H4 precursor (ITIH4), as potential biomarkers for discriminating between postmenopausal women with high or low/normal bone turnover. | [102] |
| AMFR | Plasma | Protein microarray, WB | Decreased levels of autocrine motility factor receptor (AMFR) was identified and validated in the blood plasma of female osteoporosis patients. | [103] |
| SOD, A1AT | Urine (Rats) | 2-D gel, MS spectrometry | This study identified superoxide dismutase (SOD) as a down-regulated protein and alpha-1-antitrypsin (A1AT) as an up-regulated protein in the urine of ovariectomized rats. | [104] |
| 20 proteins | Serum | LC-IMS-MS | This study identified 20 proteins associated with accelerated BMD loss in older men, with five proteins also linked to incident hip fracture. Notable proteins included CD14, SHBG, B2MG, TIMP1, CO7, CO9, and CFAD, suggesting their potential as biomarkers for future research in bone biology and fracture prediction. | [105] |
| Four proteins | Serum | MALDI-TOF MS combined with WCX magnetic beads | This study identified four potential serum protein biomarkers for PMOP, including m/z peaks at 3167.4, 4071.1, 7771.7, and 8140.5, using MALDI-TOF MS combined with weak cationic exchange (WCX) magnetic beads. | [106] |
| NPM1, APMAP, COX6A1 and ACP5 | Femur (Rats) | LC-MS/MS | A total of 47 differentially expressed proteins (DEPs) were identified in glucocortocoid-induced osteoporosis (GIOP) rats. Protein NPM1, APMAP, COX6A1 and ACP5 showed a close relationship with pathogenesis of GIOP, which could serve as potential biomarkers of GIOP. | [107] |
| CSC1-like protein, PTPN11, SLC44A1, and MME | Human bone marrow stromal cells(BMSCs) | LFQ nLC-MS/MS | This study identified dysregulated proteins, including CSC1-like protein, PTPN11, SLC44A1, and MME, in human bone marrow stromal cells exposed to simulated microgravity. | [108] |
| 12 candidate biomarkers | Serum | Label-free LC-MS/MS | A panel of 12 candidate biomarkers was selected, of which 1 DEP (RYR1) was found upregulated in the osteopenia and OP groups, 8 DEPs (APOA1, SHBG, FETB, MASP1, PTK2B, KNG1, GSN, and B2M) were upregulated in OP and 3 DEPs (APOA2, RYR3, and HBD) were downregulated in osteopenia or OP. | [109] |
| IL-7, CXCL-12, CXCL-8 | Serum | Olink® Target 48 Cytokine Panel | This study identified IL-7 and CXCL-12 as biomarkers associated with better functional recovery at three months after discharge, while CXCL-8 was associated with an increased risk of readmission in older adults with hip fracture. | [110] |
| HSP27 | PBMs | 4-plex iTRAQ coupled with LC-MS/MS | Levels of heat shock protein 27 (HSP27) was elevated in low BMD conditions in both premenopausal and postmenopausal women. | [23] |
| Two proteins | Serum | MALDI-TOF-MS | This study identified two potential serum protein markers, with mass-to-charge ratios of 1699 Da and 3038 Da, for screening osteopenia in postmenopausal women. | [111] |
| HNP-1 | Salivary fluid | MALDI TOF MS | Higher concentrations of α-defensin human neutrophil peptide-1 (HNP-1, a peptide released by neutrophils) were associated with lower BMD in postmenopausal women. | [112] |
| Four proteins | Plasma (Rats) | ESI-Q-TOF-MS, ESI-QqLIT-MS | This study identified four plasma proteins, including mannose-binding lectin-C, major urinary protein 2, type I collagen alpha 2 chain, and tetranectin, as significantly elevated in ovariectomized mice (ovx) compared to sham mice. Among these proteins, tetranectin showed a marked upregulation of almost 50 times in the ovx mice. | [20] |
| GHR, IGFBP2, GDF15, EGFR, CD14, CXCL12, MMP12, and ITIH3 | Plasma | 5 K SomaScan version 4.0 aptamer-based assay | This study identified several circulating proteins associated with incident hip fractures, including proteins related to the growth hormone/insulin growth factor system (GHR and IGFBP2), as well as GDF15, EGFR, CD14, CXCL12, MMP12, and ITIH3. | [113] |
| PINP | Plasma or serum (Rats) | LC-MS/MS | Circulating PINP levels in rats showed age-dependent changes, decreased with prednisolone treatment, and increased with parathyroid hormone (PTH) treatment, suggesting its potential as a biomarker for bone physiology in rat models of osteoporosis. | [114] |
| 22 proteins | Serum | LC–MS/MS | 22 proteins including PHLD, SAMP, PEDF, HPTR, APOA1, SHBG, CO6, A2MG, CBPN, RAIN APOD, and THBG were found to significantly correlate with BMD in OP. | [15] |
| CDH-13 | Plasma (Mice) | MS | This study identified seven circulating proteins, including ANTXR2, CDH-13, CD163, COMP, DKK3, periostin, and secretogranin-1, which decrease with age in mice. Among these, CDH-13 was found to inhibit osteoclast differentiation and delay age-related bone loss in aged mice. | [115] |
| Proteomic profiling of human bone from different anatomical sites | Bone | LC-MS/MS | Results from this study revealed distinct protein profiles between alveolar bone (AB), iliac cortical (IC) bone, and iliac spongiosa (IS). AB exhibited an ECM protein-related fingerprint, while IS and IC displayed an immune-related proteome fingerprint. | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




